- 1Department of Endocrinology, Beijing Chaoyang Hospital of Capital Medical University, Beijing, China
- 2Department of Endocrinology, The People’s Hospital of Dingzhou, Dingzhou, China
- 3Department of Endocrinology, The People’s Hospital of Zhuji, Zhuji, China
- 4Department of Endocrinology, Shenzhen Hospital, Southern Medical University, Shenzhen, China
- 5Department of Endocrinology, The Second People’s Hospital of Changshu, Changshu, China
- 6Department of Endocrinology, The Second People’s Hospital of Hefei, Hefei, China
- 7Department of Endocrinology, The First People’s Hospital of Nantong, Nantong, China
- 8Department of Endocrinology, The People’s Hospital of Jiangyin, Jiangyin, China
Introduction: The combination of dapagliflozin and metformin is commonly used as an initial therapy for patients with type 2 diabetes mellitus (T2DM) with high glycated hemoglobin (HbA1c) levels. Although bioequivalence has been established for the fixed-dose combination (FDC) of dapagliflozin/metformin extended-release (XR) compared to the co-administration of dapagliflozin and metformin XR, it remains uncertain whether the efficacy of dapagliflozin/metformin FDC is comparable to that of co-administration. Additionally, it is still unclear whether fixed-dose combinations offer advantages in terms of patient adherence and satisfaction. This study aims to compare the dapagliflozin/metformin XR FDC with co-administration of dapagliflozin and metformin XR for efficacy in terms of glycemic control, patient satisfaction, quality of life and adherence in Chinese patients with newly diagnosed T2DM.
Methods and analysis: This multicenter, randomized, non-inferiority, open-label clinical trial enrolled 632 patients with T2DM (HbA1c 7.5–10%) in 35 research centers in China. After enrollment, the patients will be randomly assigned to receive either FDC treatment (10 mg dapagliflozin and 1000 mg metformin XR) or co-administered 10 mg dapagliflozin and 1000 mg metformin XR tablets for 24 weeks. The primary endpoint was the change in HbA1c level from baseline after 24 weeks of treatment. Secondary endpoints included the proportion of patients achieving HbA1c below 7.0%, absolute changes in fasting plasma glucose and postprandial glucose from baseline to week 24, the difference in patient satisfaction measured with the diabetes treatment satisfaction questionnaire, medication usage measured with adherence to refills and medications scale for diabetes between the two groups at week 24, change from baseline in diabetes quality of life questionnaire score at week 12 and week 24, and safety. Continuous glucose monitoring will also be used to evaluate the benefits of FDC compared with co-administration on glycemic variability.
Discussion: This study, as the first of its kind, will provide comparative data on the efficacy of the dapagliflozin/metformin XR FDC and co-administration of dapagliflozin and metformin XR in terms of glycemic control, patient satisfaction, quality of life and adherence. These data will help clinicians make more informed decisions for patients with type 2 diabetes and may improve medication burden, treatment adherence, and satisfaction.
1 Introduction
The global prevalence of diabetes poses a significant threat to public health, with China having the highest number of patients with diabetes worldwide. From 2015 to 2017, the prevalence of diabetes among adults in China was 12.8% (1). Long-term blood glucose control is fundamental for diabetes management, and it is important to focus on cardiovascular and renal protection during the treatment process (2).
Metformin has firmly established itself as a cornerstone in the treatment of type 2 diabetes mellitus (T2DM) and remains the first-line medication in most clinical guidelines (3). It effectively lowers blood glucose by reducing hepatic glucose output and improving peripheral insulin resistance. It has benefits such as weight reduction, reduced risk of cardiovascular diseases associated with T2DM, synergistic effects in combination therapy, a good safety profile, and is cost-effective and widely available (3, 4). Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a class of oral antidiabetic agents that have gained widespread application and have been consistently recommended by guidelines in recent years, include dapagliflozin, empagliflozin, canagliflozin, and ertugliflozin (5). These agents lower blood glucose levels by inhibiting SGLT2 in the proximal renal tubules, blocking glucose reabsorption, and promoting urinary glucose excretion (6). In addition to their glucose-lowering effects, SGLT2 inhibitors confer important non-glycemic benefits including renal hemodynamic regulation, decreased urinary protein excretion, blood pressure reduction, and weight loss (7). These non-glycemic pathways have a positive effect on patient treatment, with multiple large-scale clinical trials demonstrating the efficacy of SGLT2 inhibitors in reducing the risk of major adverse cardiovascular events, cardiovascular death, myocardial infarction, heart failure hospitalization, and all-cause mortality, as well as improving renal outcomes in T2DM patients with cardiovascular disease or at high cardiovascular risk (8–11). In addition, SGLT2 inhibitors have been shown to reduce cardiorenal and cardiovascular risks in patients with chronic kidney disease or heart failure, regardless of the presence or absence of diabetes (12, 13).
Glycemic control in patients with diabetes typically requires combination therapies. Studies have shown that initial combination therapy could achieve faster glycemic control and maintain its effects for a longer duration than sequential add‐on therapy (14, 15). The American Diabetes Association (ADA) 2025 Standards of Medical Care in Diabetes and Guidelines for the Prevention and Treatment of Diabetes Mellitus in China (2024 edition) recommended considering initial combination therapy, including fixed-dose combination (FDC), for patients whose glycated hemoglobin (HbA1c) is over 1.5% above the target level (2, 16). Furthermore, combination therapy was recommended for T2DM patients with the HbA1c ≥7.5%, according to the American Association of Clinical Endocrinology (AACE) Consensus Statement (2023) and Metabolic Disease Management Guideline for National Metabolic Management Center (MMC) (17, 18). Nowadays, low adherence to diabetes medication remains an ongoing problem (19). Studies have shown that medication adherence is inversely related to the number of medications (20), and compared to using separate drugs, fixed-dose combination therapy may significantly improve patient adherence to treatment (21). XIGDUO® XR tablet is a FDC of dapagliflozin and metformin hydrochloride XR tablets, and studies have shown bioequivalence between FDC and co-administered dapagliflozin and metformin XR tablets (22). However, it remains uncertain whether the efficacy of dapagliflozin/metformin FDC is comparable to that of co-administration.
Currently, studies comparing FDC to co-administration are generally rare, and there are no head-to-head comparative studies between dapagliflozin/metformin FDC and the co-administration of dapagliflozin and metformin hydrochloride. Additionally, there is no direct evidence of the glycemic control efficacy of the FDC of dapagliflozin 10 mg and metformin XR 1000 mg in patients with T2DM, which is the only available dosage for dapagliflozin/metformin XR FDC in clinical use in China. Therefore, we designed a multicenter, randomized, non-inferiority, open-label clinical trial to compare the effects of dapagliflozin/metformin XR FDC with those of co-administration of dapagliflozin and metformin XR on glycemic control, patient satisfaction, quality of life, adherence, and safety. This study is the first of its kind and has the potential to provide crucial evidence supporting the use of FDCs, including dapagliflozin/metformin XR FDC. Additionally, it may offer valuable insights for clinical practice regarding the selection of treatment regimens for diabetes and improvement of patient adherence.
2 Methods and analysis
2.1 Study design
This was a 24-week, multicenter, randomized, non-inferiority, open-label clinical trial designed to compare the FDC regimen of dapagliflozin/metformin XR with dapagliflozin administered alongside metformin XR, based on glycemic control, patient satisfaction, and adherence in Chinese patients with T2DM. The study was conducted at 35 sites in China.
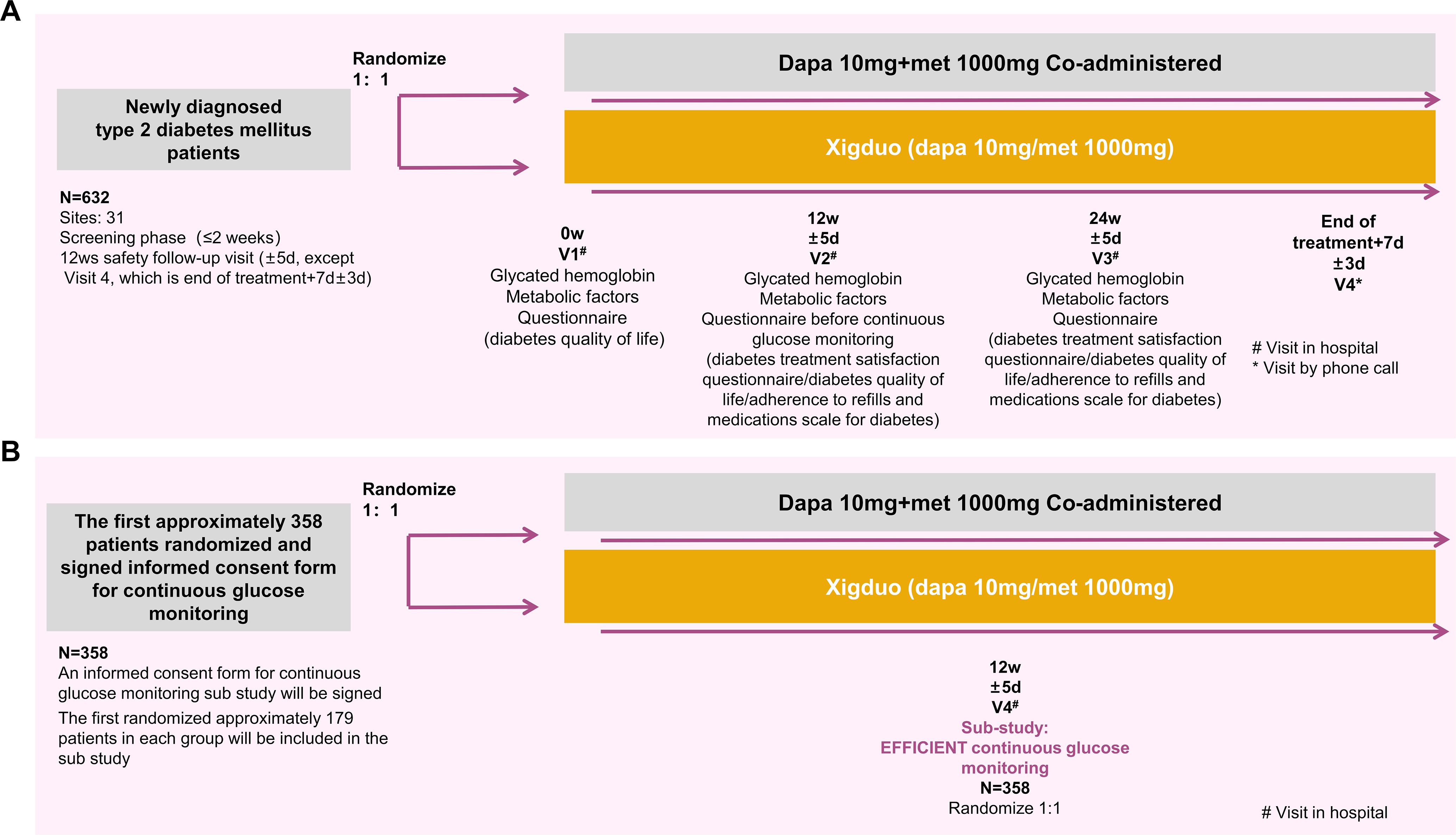
The flowchart of the study is shown in Figure 1. After obtaining written informed consent during the screening period, the patients will be assessed for eligibility according to all applicable inclusion and exclusion criteria, and laboratory samples will be collected and submitted. Once the investigator confirms the patient’s eligibility, an HbA1c blood sample will be collected and tested by the central laboratory, with results available within two weeks. Eligible patients will undergo re-evaluation of their inclusion and exclusion criteria based on the test results. Eligible patients will be randomly assigned 1:1 to one of two treatment groups. From week 0, patients in the FDC group will receive treatment with an FDC regimen consisting of 10 mg dapagliflozin and 1000 mg metformin XR tablets for 24 weeks, while the co-administration group will receive 10 mg dapagliflozin tablets and 1000 mg metformin XR tablets for 24 weeks. Among the 632 randomly assigned subjects, the first approximately 179 subjects per group who signed the continuous glucose monitoring (CGM) informed consent will be included in the CGM sub-study, totaling approximately 358 patients. After completing all scheduled tests, examinations, and questionnaires at week 12, participants in the sub-study will undergo CGM (for approximately five days). Follow-up visits will be conducted every 12 weeks (± 5 days) during the treatment period, with an additional follow-up visit at 1 week (± 3 days) after the end of the treatment to evaluate efficacy and safety.
2.2 Participants
To be eligible for the study, participants must be 18 years of age or older at the time of signing the informed consent form and must be capable of signing the written informed consent voluntarily. They had to be newly diagnosed with T2DM (WHO diagnostic criteria 1999), diagnosed for no more than 1 year, and have not received any previous medical treatment. Additionally, participants must have an HbA1c level of 7.5–10% at screening, as assessed by a local laboratory and at the pre-randomization visit, as assessed by a central laboratory, and a body mass index (BMI) between 19 and 40 kg/m² at screening.
Patients were excluded from the study if they met any of the following exclusion criteria: (1) congestive heart failure of New York Heart Association (NYHA) class III or IV or major cardiovascular events within 6 months before screening (significant cardiovascular history within the past 6 months prior to screening was defined as myocardial infarction, coronary angioplasty or bypass graft(s), valvular disease or repair, unstable angina pectoris, transient ischemic attack, or cerebrovascular accident). (2) Clinically apparent hepatobiliary disease, including but not limited to chronic active hepatitis and/or severe hepatic insufficiency. Alanine transaminase (ALT) or aspartate transaminase (AST), > 3x the upper limit of normal (ULN), or serum total bilirubin (TB) >34.2 μmol/L (2 mg/dL). (3) Estimated glomerular filtration rate (eGFR) < 45 mL/min per 1.73 m². (4) Diagnosis or history of acute metabolic diabetic complications, such as ketoacidosis and hyperglycemic hyperosmolar state, or diabetes insipidus within the past 6 months. (5) For women only, current pregnancy (confirmed by a positive pregnancy test) or breastfeeding. (6) Participation in any other study that included drug treatment in the past three months before enrollment. (7) Known hypersensitivity to dapagliflozin, metformin, or any of the excipients of the product. (8) Diagnosis or history of: a) chronic pancreatitis within the past 6 months or idiopathic acute pancreatitis within the past 4 weeks; b) gastrointestinal disease, for example, gastroenterostomy, enterectomy, Roemheld syndrome, severe hernia, intestinal obstruction, intestinal ulcer within the past 6 months; c) genetic galactose intolerance, LAPP lactase deficiency, and glucose-galactose malabsorption; d) organ transplantation or acquired immunodeficiency syndrome (AIDS) within the past 6 months; e) alcohol abuse or illegal drug abuse within the past 12 months; f) laser treatment for proliferative retinopathy within 6 months; g) stress condition, for example, surgery, serious trauma, etc., within the past 6 months, or planned surgery during the study period; h) chronic oxygen deficiency diseases, for example, pulmonary emphysema, pulmonary heart disease, within the past 6 months; i) type 1 diabetes mellitus (T1DM), diabetes resulting from pancreatic injury or secondary forms of diabetes, for example, acromegaly or (9) The subject being, in the judgment of the investigator, is unlikely to comply with the study protocol or has any severe concurrent medical or psychological conditions that may affect the interpretation of study results. (10) Involvement in planning and conducting the study.
This study protocol was approved by the ethical review board of Beijing Chaoyang Hospital of Capital Medical University with the certificate number of 2023-ke-772-3. This trial was registered at www.clinicaltrials.gov (NCT06327815). The trial protocol was in accordance with the SPIRIT guideline.
2.3 Endpoints
The primary endpoint of the study was the change in HbA1c level from baseline after 24 weeks of treatment. The secondary endpoints included the following: (1) The proportion of patients achieving HbA1c below 7.0%, absolute change in fasting plasma glucose/postprandial glucose (FPG/PPG) from baseline to week 24, and (2) the difference in satisfaction scores between the two groups measured using the diabetes treatment satisfaction questionnaire (DTSQ) at week 24. (3) Change from baseline in the diabetes quality of life (DQOL) questionnaire scores at weeks 12 and 24. (4) The differences in patient medication usage between the two groups were measured with adherence to refills and medications scale for diabetes (ARMS-D) at week 24. (5) Adverse events (AE), serious adverse events (SAE), and adverse drug reactions (ADRs). (6) Vital signs. (7) Physical examination. (8) Clinical laboratory indices. (9) Electrocardiogram
In the exploratory substudy, the primary exploratory endpoint was the proportion of time in the tight target range (TITR, 3.9-7.8 mmol/L). Secondary exploratory endpoints included time in range (TIR, i.e., the percentage of readings and time that a person spends with their blood glucose levels in a target range of 3.9–10 mmol/L), time below range (TBR, i.e., the percentage of readings and time that a person spends with their blood glucose levels below the target range), time above range (TAR, i.e., the percentage of readings and time that a person spends with their blood glucose levels above the target range), mean amplitude of glycemic excursions (MAGE, the mean blood glucose values exceeding one standard deviation (SD) from the 24‐hour mean blood glucose, which is used as an index of glycemic variability), and standard deviation of blood glucose (SDBG), which reflects the amount of variation or dispersion of a series of glucose values.
2.4 Randomization and study interventions
Patients were centrally randomized (1:1) to one of the two treatment groups using an Interactive Response Technology/Randomization and Trial Supply Management (IRT/RTSM). Prior to the study initiation, contact information and login directions for the system were provided to each site. Following randomization, patients in the FDC group received a single tablet containing 10 mg of dapagliflozin and 1000 mg of metformin hydrochloride XR once daily. Patients in the co-administration group received one tablet of 10 mg dapagliflozin once daily, and metformin hydrochloride XR, administered as two 500 mg tablets once daily with meals, or as one 500 mg tablet twice daily with meals if the patient is intolerant to the two-tablet dose. This study did not involve dose adjustments for study medications. The duration of treatment was 24 weeks.
The rescue treatment criteria from week 4 to week 12 were based on an FPG level exceeding 13.32 mmol/L, confirmed by a second measurement 3–5 days later. The rescue treatment criteria from weeks 12 to 24 were based on an FPG level exceeding 11.10 mmol/L (23). Rescue medications include but are not limited to metformin, GLP-1 receptor agonists, DPP-4 inhibitors, thiazolidinediones, sulfonylureas, and insulin. Patients administered rescue medications will continue to participate in the study and the results will be independently analyzed.
Subjects had to discontinue the use of prescription or over-the-counter medications (including vitamins, recreational drugs, dietary supplements, or herbal supplements) 7 days prior to the start of the study intervention (or 14 days in case the medication is a potential enzyme inducer) or 5 half-lives of the medication (whichever is longer) until the end of follow-up, unless the investigator and sponsor believe the medication will not interfere with the study. Acetaminophen/paracetamol with a dosage of ≤2 g/day was allowed at any time during the study period.
2.5 Data collection and management
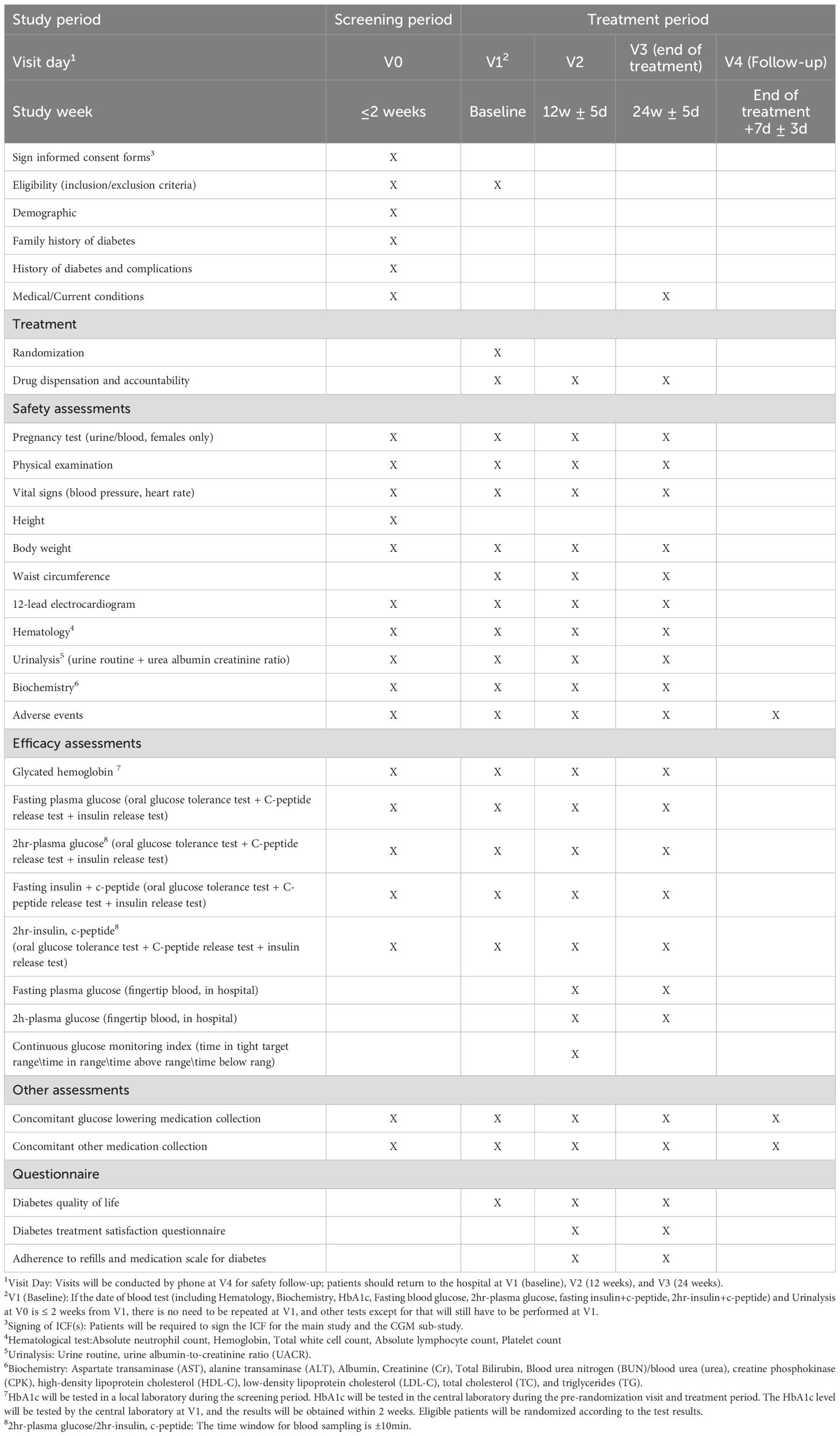
As shown in Table 1, medical history and demographic information were collected during the screening phase. In addition, urine or blood pregnancy tests (for females only), physical examinations, vital signs, height, weight, waist circumference, 12-lead electrocardiogram (ECG), routine blood test, routine urine test, blood biochemistry, adverse events, HbA1c, fasting blood glucose, fasting insulin + C-peptide, postprandial 2h blood glucose, and postprandial 2h insulin + C-peptide levels will be assessed, and concomitant medications (antidiabetic and other drugs) will be recorded.
During the treatment period, patients will visit the hospital for follow-up at weeks 0, 12 (± 5 days), and 24 (± 5 days). The follow-up will include medication dispensing and management, urine or blood pregnancy test (for females only), physical examinations, vital signs, weight, waist circumference, 12-lead ECG, blood routine test, urine routine test, blood biochemistry, adverse events, HbA1c, fasting blood glucose, fasting insulin + C-peptide, postprandial 2h blood glucose, postprandial 2h insulin + C-peptide levels, and recording of concomitant medications (antidiabetic and other drugs). At weeks 12 (± 5 days) and 24 (± 5 days), fasting fingertip blood glucose and postprandial 2h fingertip blood glucose levels were measured. At week 12 (± 5 days), CGM was performed for approximately five days. One week (± 3 days) after the end of treatment, a telephonic follow-up will be conducted to record adverse events and concomitant medications (antidiabetic and other drugs).
Patient satisfaction, quality of life, and adherence were evaluated using DTSQ, DQOL questionnaire, and ARMS-D. The patients completed the DQOL questionnaire at week 0 and the DTSQ, DQOL questionnaire, and ARMS-D at weeks 12 and 24.
All patient data related to the study will be recorded on case report forms (CRFs), unless electronically transmitted to AstraZeneca or a designee (e.g., central laboratory). The investigator must maintain an accurate documentation (source data) to support the information entered in each CRF. The investigator must allow for monitoring, auditing, Institutional Review Board (IRB)/Independent Ethics Committee (IEC) review, and regulatory agency inspections related to the study, thereby providing direct access to source data files. Records and documents related to this study, including signed informed consent forms (ICFs), must be retained by the investigator for at least 25 years after study completion or as required by local regulations. Each subject was assigned a unique identifier to ensure privacy of the included participants. The data transmitted will not include the participant’s name or any personally identifiable information; only identifiers to distinguish the subjects will be used.
2.6 Sample size
The primary objective of this study was to demonstrate the non-inferiority of the FDC regimen of dapagliflozin/metformin XR to co-administered dual therapy of dapagliflozin and metformin for changes from baseline to week 24 in HbA1c within a non-inferiority margin of 0.30%, assuming a standard deviation of 1.2% (24). At a 1-sided significance level of 0.025, 253 patients per arm will be needed to provide 80% power (given a true difference of zero between the FDC regimen of dapagliflozin/metformin and XR and co-administered dual therapy of dapagliflozin and metformin). Assuming a dropout rate of 20%, 632 patients were randomized.
For the CGM sub-study, though exploratory in nature, the sample size was calculated based on exploratory hypothesis testing of the primary exploratory endpoint, which is the proportion of TITR (3.9-7.8 mmol/L) at week 12. Assuming a mean difference of 10%, standard deviation of 30%, and 2-sided significance level of 0.05, 143 patients per arm will provide 80% power for exploratory superiority testing. Assuming a dropout rate of 20%, approximately 358 patients were needed for the sub-study.
2.7 Statistical analysis
The full analysis set (FAS) included all randomized patients with documented baseline assessment and at least one post-baseline assessment for the primary efficacy endpoint. The per-protocol analysis set (PPS) will include patients without an important protocol deviation that might affect the primary efficacy analyses. The CGM analysis set will include patients from the full analysis set administered at least one dose of the study treatment prior to CGM performance, as well as CGM-evaluable data. The safety analysis set (SS) included all randomized patients who were administered at least one dose of the study treatment.
Primary efficacy analysis will be performed on the PPS, excluding HbA1c measurements after receiving rescue medications or permanent discontinuation of the study intervention. Missing data at week 24 will be handled using the multiple-imputation approach. Analysis of covariance (ANCOVA) will be used for the primary analysis. Point estimates and 2-sided 95% confidence intervals for the mean change within each treatment group, as well as the difference in mean change between the two groups, will be provided. The dapagliflozin/metformin FDC regimen was considered non-inferior to dapagliflozin and metformin co-administered if the upper limit of the 2-sided 95% (or 1-sided 97.5%) confidence interval of the difference in mean change in HbA1c from baseline to week 24 between the groups was below 0.3%.
The secondary endpoints will be analyzed in the PPS. An ANCOVA model will be used to analyze the absolute changes in FPG and PPG from baseline to week 24, reporting the point estimates of the average changes and two-sided 95% confidence intervals (CIs) within each treatment group as well as the differences in mean changes between groups. The difference in the proportion of patients achieving HbA1c levels below 7.0% at week 24 will be analyzed using logistic regression, adjusting for baseline HbA1c values. Participants with missing HbA1c measurements at week 24 will have the values imputed by dichotomizing the imputed HbA1c values at week 24. Odds ratios (ORs) and 95% confidence intervals will be reported. Descriptive statistics will be used for DQOL, DTSQ, and ARMS-D scores. An ANCOVA model will analyze the changes in the DQOL score from baseline to weeks 12 and 24. Point estimates of mean changes and 95% CIs within each group were calculated along with the differences in mean changes between the two groups. Differences in DTSQ and ARMS-D scores at week 24 between the two groups will be compared using the t-test or Wilcoxon rank-sum test, based on the normality and homogeneity of variances of the data.
The analyses for the primary and secondary endpoints will be repeated using FAS to examine the robustness of the results.
Exploratory endpoints will be analyzed based on the CGM analysis set. Point estimates and 95% CIs for all exploratory outcomes within each group were calculated along with estimates and 95% CIs for the differences between the two groups.
Safety analysis will be based on SS, summarizing the numbers and incidence rates of AEs, SAEs, and ADRs in each treatment group. At each scheduled time point, descriptive statistics will be used to summarize the values and changes from the baseline in vital signs, physical examinations, and clinical laboratory parameters within the different groups.
3 Discussion
T2DM is a significant global health issue, and poor glycemic control can lead to severe complications including chronic kidney disease, retinopathy, neuropathy, stroke, and myocardial infarction (25, 26). Therefore, controlling blood glucose levels and delaying the onset of complications is crucial for diabetes treatment. Recent clinical guidelines suggest that metformin and SGLT2 inhibitors can be used as initial dual therapy in patients with high HbA1c levels (2).
To date, studies comparing FDC to coadministration are rare. XIGDUO® XR was launched in China in June 2023, and head-to-head clinical trials comparing the initial combination treatment with metformin and SGLT2 inhibitors with FDC therapy are still lacking. This study is a multicenter, non-inferiority, open-label randomized controlled trial designed to compare dapagliflozin/metformin XR FDC with co-administration of dapagliflozin and metformin XR in terms of glycemic control, patient satisfaction, quality of life, adherence to treatment, and safety in patients with newly diagnosed type 2 diabetes mellitus (T2DM). The primary endpoint focused on glycemic control efficacy, specifically the change in HbA1c from baseline after 24 weeks of treatment. Patient satisfaction, quality of life, and adherence to treatment were measured using the DTSQ, DQOL, and ARMS-D, respectively. The DTSQ has proven valuable for understanding and measuring patients’ treatment satisfaction in assessments of new treatments and strategies (27). DQOL, specifically developed to evaluate the quality of life of individuals with diabetes, is widely used and helps to understand how diabetes and its treatment impact the overall well-being and day-to-day experiences of patients (28, 29). The ARMS-D is a reliable and valid questionnaire designed to assess medication adherence in individuals with diabetes and helps healthcare providers identify adherence barriers and tailor interventions to improve compliance and health outcomes (30).
The multicenter design of this study enhances the generalizability and representativeness of the results. The change in HbA1c was used as the primary endpoint, as HbA1c reflects the patient’s blood glucose levels over the past 3–4 months and is considered the gold standard for blood glucose control. Therefore, the study endpoint reflects the effect of glycemic control. Additionally, CGM was employed as an exploratory study to assess glucose variability in more detail, offering deeper insights into future treatment strategies.
This study had several limitations. First, the open-label design may introduce a potential positive bias toward the FDC group, particularly concerning the DTSQ and ARMS-D scores, given the known convenience of a single tablet regimen. Second, the 24-week duration of the trial limited our ability to assess long-term outcomes, including the trajectory of β-cell function, sustainability of glycemic control, and more comprehensive safety profile. Third, the recruitment of participants exclusively from Chinese centers may limit the generalizability of our findings to other ethnic groups and healthcare settings. While this focus provides highly relevant data for the Chinese population, future multinational studies are warranted to confirm the global applicability of the results.
In conclusion, as the first study to directly compare the dapagliflozin/metformin XR FDC with the separate administration of dapagliflozin and metformin hydrochloride XR, this scientifically designed trial has the potential to provide crucial evidence supporting the use of FDCs. Additionally, it may offer valuable insights for physicians in selecting treatment regimens for diabetes and in improving patient adherence.
Ethics statement
The studies involving humans were approved by Beijing Chaoyang Hospital of Capital Medical University with the certificate number of 2023-ke-772-3. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JW: Conceptualization, Methodology, Data curation, Writing – review & editing. YW: Data curation, Writing – review & editing. PS: Writing – review & editing. JC: Writing – review & editing. LX: Writing – review & editing. YK: Writing – review & editing. JY: Writing – review & editing. XW: Writing – review & editing. JX: Writing – review & editing. GW: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the AstraZeneca. AstraZeneca provided funding for medical writing, editorial support, and publication fees in accordance with Good Publication Practice (GPP3) guidelines. AstraZeneca also played a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Acknowledgments
The authors would like to thank the participants, their families, and all investigators and teams involved in this study. The authors also thank Dr. Paul Zhang of MedSci for providing medical writing assistance funded by AstraZeneca, China. The authors would also like to thank L. Li (MM, AstraZeneca) for the review and critical suggestions for improvement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. (2020) 369:m997. doi: 10.1136/bmj.m997
2. ElSayed NA, McCoy RG, Aleppo G, Bajaj M, Balapattabi K, Beverly EA, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2025. Diabetes Care. (2025) 48:S181–206. doi: 10.2337/dc25-S009
3. Foretz M, Guigas B, and Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. (2023) 19:460–76. doi: 10.1038/s41574-023-00833-4
4. Pernicova I and Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. (2014) 10:143–56. doi: 10.1038/nrendo.2013.256
5. Li S, Vandvik PO, Lytvyn L, Guyatt GH, Palmer SC, Rodriguez-Gutierrez R, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. (2022) 377:o1080. doi: 10.1136/bmj.o1080. doi: 10.1136/bmj.o1080. Erratum for: BMJ. 2021 May 11;373:n1091. doi: 10.1136/bmj.n1091.
6. Perry RJ and Shulman GI. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. (2020) 295:14379–90. doi: 10.1074/jbc.REV120.008387
7. Heerspink HJL, Perkins BA, Fitchett DH, Husain M, and Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus. Circulation. (2016) 134:752–72. doi: 10.1161/circulationaha.116.021887
8. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. (2019) 380:2295–306. doi: 10.1056/NEJMoa1811744
9. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
11. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
12. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. (2020) 383:1436–46. doi: 10.1056/NEJMoa2024816
13. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
14. Phung OJ, Sobieraj DM, Engel SS, and Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. (2013) 16:410–7. doi: 10.1111/dom.12233
15. Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, and Del Prato S. Glycemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicenter, randomized, double-blind trial. Lancet. (2019) 394:1519–29. doi: 10.1016/s0140-6736(19)32131-2
16. Society CD, Zhu D, and Guo L. Guideline for the prevention and treatment of diabetes mellitus in China (2024 edition). Chin J Diabetes Mellitus. (2025) 17:16–139. doi: 10.3760/cma.j.cn115791-20241203-00705
17. Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, et al. American association of clinical endocrinology consensus statement: comprehensive type 2 diabetes management algorithm – 2023 update. Endocr Pract. (2023) 29:305–40. doi: 10.1016/j.eprac.2023.02.001
18. Academic Committee of Metabolic Management Center, Expert Advisory Committee of Metabolic Management Center, and Council of Metabolic Management Center. Metabolic disease management guideline for national metabolic management center (2nd edition). Chin J Endocrinol Metab. (2023) 39:538–54. doi: 10.3760/cma.j.cn311282-20230216-00072
19. Krass I, Schieback P, and Dhippayom T. Adherence to diabetes medication: a systematic review. Diabetes Med. (2015) 32:725–37. doi: 10.1111/dme.12651
20. Benner JS, Chapman RH, Petrilla AA, Tang SSK, Rosenberg N, and Schwartz JS. Association between prescription burden and medication adherence in patients initiating antihypertensive and lipid-lowering therapy. Am J Health Syst Pharm. (2009) 66:1471–7. doi: 10.2146/ajhp080238
21. Bangalore S, Kamalakkannan G, Parkar S, and Messerli FH. Fixed-dose combinations improve medication compliance: A meta-analysis. Am J Med. (2007) 120:713–9. doi: 10.1016/j.amjmed.2006.08.033
22. Chang M, Liu X, Cui D, Liang D, LaCreta F, Griffen SC, et al. Bioequivalence, Food Effect, and Steadyadyce: A Meta-Analysis. n adherence in patin Extended‐release Fixed‐dose Combination Tablets Relative to Single‐component Dapagliflozin and Metformin Extended‐release Tablets in Healthy Subjects. Clin Ther. (2015) 37:1517–28. doi: 10.1016/j.clinthera.2015.05.004
23. Yang W, Han P, Min KW, Wang B, Mansfield T, T’Joen C, et al. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial. J Diabetes. (2016) 8:796–808. doi: 10.1111/1753-0407.12357
24. Vilsbøll T, Ekholm E, Johnsson E, Dronamraju N, Jabbour S, and Lind M. Dapagliflozin plus saxagliptin add-on therapy compared with insulin in patients with type 2 diabetes poorly controlled by metformin with or without sulfonylurea therapy: A randomized clinical trial. Diabetes Care. (2019) 42:1464–72. doi: 10.2337/dc18-1988
25. Maranta F, Cianfanelli L, and Cianflone D. Glycemic control and vascular complications in diabetes mellitus type 2. Adv Exp Med Biol. (2020) 1307:129–52. doi: 10.1007/5584_2020_514
26. Zheng Y, Ley SH, and Hu FB. Global etiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. (2017) 14:88–98. doi: 10.1038/nrendo.2017.151
27. Bradley C and Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes Metab Res Rev. (2002) 18(Suppl 3):S64–9. doi: 10.1002/dmrr.279
28. Díaz-Soto G, Pérez-López P, Férnandez-Velasco P, Bahillo-Curieses P, de la O Nieto de la Marca M, Jimenez R, et al. Quality of life, diabetes-related stress and treatment satisfaction are correlated with glycemia risk index (GRI), time in range and hypoglycemia/hyperglycemia components in type 1 diabetes. Endocrine. (2024) 86:186–93. doi: 10.1007/s12020-024-03846-9
29. Kueh YC, Morris T, and Ismail A-A-S. The effect of diabetes knowledge and attitudes on self-management and quality of life among people with type 2 diabetes. Psychol Health Med. (2016) 22:138–44. doi: 10.1080/13548506.2016.1147055
Keywords: T2DM, newly diagnosed, dapagliflozin/metformin, FDC, co-administered dual therapy
Citation: Wang J, Wei Y, Shi P, Chen J, Xu L, Kong Y, Ye J, Wang X, Xu J and Wang G (2025) Study protocol of a multicenter, randomized, non-inferiority, open-label study investigating the efficacy of a fixed-dose combination regimen of dapagliflozin/metformin versus co-administered dual therapy based on glycemic control, satisfaction and adherence in patients with newly diagnosed type 2 diabetes mellitus. Front. Endocrinol. 16:1690339. doi: 10.3389/fendo.2025.1690339
Received: 21 August 2025; Accepted: 22 October 2025;
Published: 04 November 2025.
Edited by:
Feng Zhang, Third Hospital of Hebei Medical University, ChinaReviewed by:
Qian Wu, Chongqing Medical University, ChinaXiaoxue Huang, St. John’s University, United States
Priyanka Joshi, Bharat Serums and Vaccines Limited, India
Copyright © 2025 Wang, Wei, Shi, Chen, Xu, Kong, Ye, Wang, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Wang, ZHJ3ZzY2ODhAYWxpeXVuLmNvbQ==
 Jiaxuan Wang
Jiaxuan Wang Ying Wei
Ying Wei Ping Shi2
Ping Shi2 Lingling Xu
Lingling Xu Xueqin Wang
Xueqin Wang Guang Wang
Guang Wang