- 1Department of Pediatrics, Division of Pediatric Endocrinology, Stanford University, Palo Alto, CA, United States
- 2AdventHealth Translational Research Institute, Orlando, FL, United States
- 3Department of Nutrition, University of North Carolina, Chapel Hill, NC, United States
Introduction
Type 1 diabetes (T1D) is caused by the autoimmune destruction of pancreatic β−cells, creating a lifelong need for exogenous insulin and glucose monitoring (1). Despite therapeutic advances in T1D, cardiovascular disease (CVD) remains high in this population with up to 45% developing ≥2 CVD risk factors within ten years after diagnosis (1, 2). Additionally, CVD-related mortality remains approximately twice as high in T1D compared to the general population, even when glycemic targets are achieved (3, 4). Individualized medical nutrition therapy (MNT) is a pillar for improving glycemic outcomes, promoting adequate growth, and reducing or delaying the development of chronic complications (such as CVD) and comorbidities (such as dyslipidemia or hypertension) in persons with T1D (5). MNT delivered by a registered dietitian is associated with improved cardiometabolic markers, including a 1.0-1.9% reduction in HbA1c in people living with T1D (5). In practice, MNT for T1D management has focused on carbohydrates as the main contributor to postprandial glycemic fluctuations, but compelling evidence and practice guidelines suggest that MNT should additionally incorporate broader eating patterns, food preferences, cultural practices, relationships with food and body, culinary skills, and food security to optimize health for people living with diabetes (5–7). In this commentary, we discuss the evolution of MNT for T1D management and opportunities to revise nutritional guidelines to reflect recent therapeutic advances and improve outcomes beyond glucose management, including those related to cardiometabolic risk and quality of life.
The road so far: the evolution of MNT for T1D management
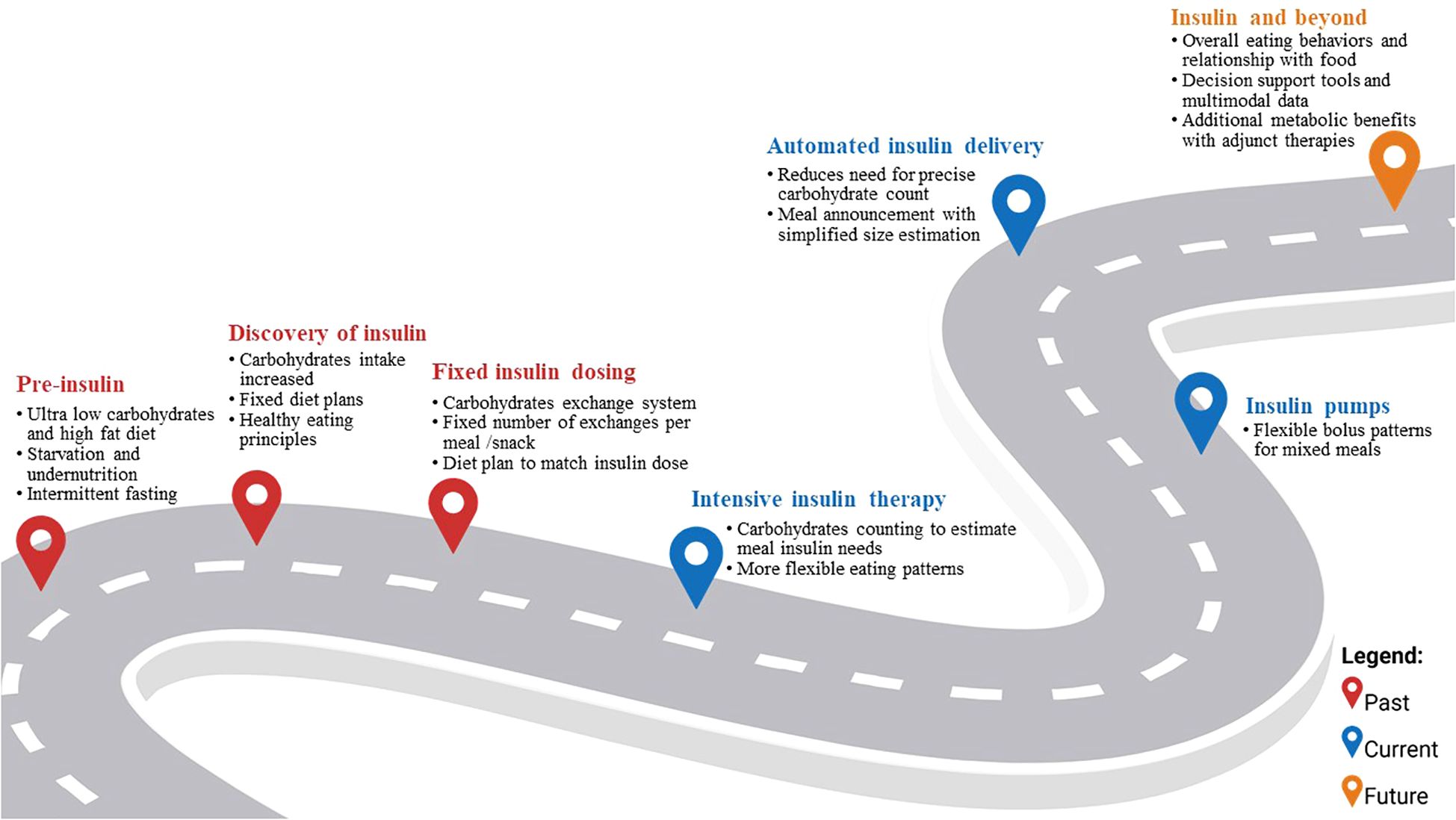
Although MNT has transformed over the past century, it has always been a cornerstone of T1D management (Figure 1). Before the discovery of insulin, life expectancy after T1D diagnosis was estimated to be less than three years (8). MNT primarily took the form of carbohydrate restriction (as low as 10g of carbohydrates per day or ≤10% of daily caloric intake) to delay mortality associated with T1D (9). In some cases, high fat diets (i.e., up to 80% caloric intake from fats) and intermittent fasting regimens (i.e., once a week complete fast with just water or bouillon) were also recommended to manage glucosuria and prolong life expectancy (10, 11). Other more drastic measures like prolonged fasting (up to ten days) and hypocaloric diets were effective in reducing ketoacidosis and glucosuria but were not sustainable as they resulted in significant nutrient deficiencies, starvation, and eventual death (10, 12).
With the discovery of insulin in the early 1920s, recommended carbohydrate intake was increased to 40 to 70% of daily intake (9). Specifically, recommendations for people with T1D were to follow fixed diet plans to match carbohydrate intake to insulin doses administered (13). By the mid-20th century, and as insulin formulations and glucose monitoring developed to better reflect physiological responses to nutrient intake, a carbohydrate exchange system (1 exchange = 10 to 16 grams of carbohydrates) was proposed (14). People with T1D were prescribed a fixed number of insulin units which dictated the number and distribution of recommended carbohydrate exchanges to consume to approach euglycemia. The exchange system allowed people with T1D to choose from a variety of carbohydrate sources while matching carbohydrate amounts to their prescribed insulin dosing (15).
The development of intensive insulin therapy (i.e., three or more daily injections of insulin) and capillary blood glucose meters transformed T1D management (15). People with T1D could monitor their glycemia at home and match their insulin dose to their carbohydrate intake instead of having to rigidly adhere to predetermined carbohydrate amounts based on a fixed insulin dose. Before this, glucose monitoring relied on urine tests as blood glucose monitoring strips only became available in 1965 for use in the clinic and 1980s for at home use (16). This advancement provided added flexibility in what, when, and how to eat (15). Specifically, in the 1990s carbohydrate counting (CC, i.e., counting the number of carbohydrates found in food and using this value to estimate prandial insulin needs) became a topic of interest in the US, solidified with the Diabetes Control and Complications Trial results (15).
Although CC has remained a mainstay of the dietary management of T1D, its efficacy is equivocal, and unintended effects on diabetes distress and disordered eating are of concern (17). While some studies showed improvement in HbA1c values (standard mean difference [95%CI]: -0.51 [-0.83, -019] %) when insulin dosing was combined with precise CC and was compared to standard diabetes education (16), evidence for CC superiority is less clear when compared to other dietary approaches of matching insulin to food intake (-0.31 [-0.99, 1.61]%) such as glycemic index or fixed carbohydrate amounts (18). Discrepancies between insulin doses estimated using CC and postprandial glycemic responses can be accounted for by both dietary and non-dietary factors including fiber and other macronutrients, previous physical activity, and time of day (7, 19–21). CC is complex, takes time, requires nutritional literacy and numerical skills, and is generally inaccurate, especially when estimating carbohydrate intake from fresh and non-prepackaged foods which are the basis of a healthy diet (19, 22–24). Additionally, parallel to the general population (25), low diet quality remains a persistent challenge for people with T1D and has implications for both glycemic outcomes and downstream cardiometabolic risk, thus supporting the need to shift from a carbohydrate-centric approach to target overall diet quality (26, 27). Current national and international guidelines state that there is no one ideal diet approach for MNT in T1D; however, dietary patterns that are rich in sources of fiber and unsaturated fatty acids such as whole grains, vegetables, and lean protein, and low in added sugars and sodium, have consistently been associated with improved cardiometabolic health outcomes, including glycemia (5, 7, 28, 29). Specifically, dietary patterns rich in fiber (daily average 35 grams) have been associated with lower HbA1c (mean difference [95%CI]: -0.18 [-0.30, -0.07]%), LDL cholesterol (−0.17 [−0.27, −0.08]mmol/L), triglycerides (−0.16 [−0.23, −0.09] mmol/L), and Body Mass Index (−0.36 [-0.55, −0.16]) compared to dietary patterns lower in fiber (daily average 19 grams) in persons with diabetes (30). Similar trends have been observed in youth (<18 years of age) with T1D. In a recent study with 120 youth participants with T1D, those reporting dietary patterns rich in ultra-processed foods had a 3.5 higher odds of having higher HbA1c levels (31). Meeting nutritional guidelines remains challenging for many. In a sample with 291 families of children with T1D aged 8–18 years, the average eating patterns had less than half the recommended amount of fruits, vegetables, whole grains, nuts and seeds (source of 17% of energy intake) while 48% of daily energy intake came from refined-grain products, desserts, chips, and sweetened beverages (32). These trends are especially concerning given the rising prevalence of overweight/obesity in the T1D population, with overweight and obesity affecting 35% and 20% of adults with T1D respectively, increasing the risk of insulin resistance and CVD (33).
Additionally, the psychological burden associated with T1D such as the fear of hypoglycemia and the demands of precise CC increases the risk of developing disordered eating behaviors (5, 34, 35). The demands for precise CC to match insulin dosing can also increase meal-related anxiety and disturb intuitive eating and satiety/hunger signaling (36). Specifically, behaviors such as under-bolusing or omitting insulin to lose weight are prevalent in people with T1D, with prevalence rates up to 40% in youth and 20% in adults (37). Although limited, the current evidence supports the theory of an association between disordered eating and T1D management outcomes. In a cross-sectional analysis with 151 adolescents, participants in the “at risk for disordered eating” group had lower diet quality compared to teens who were in the low-risk group (38). Disordered eating has also been linked to higher HbA1c levels, higher risk of ketoacidosis, and chronic T1D complications (5, 39). Thus, nutrition interventions for healthful eating should be mindful of the heightened disordered eating risk in T1D.
The road ahead: proposed strategies for adapting MNT to contemporary clinical care
Automated insulin delivery (AID) is now the standard of care as per international guidelines for youth and adults with T1D (40, 41). These systems increase time in target glucose range (TIR; i.e., 70–180 mg/dL) by 10.9% [9.4, 12.4] and reduce HbA1c by 0.37% [-0.49, -0.29] compared to non-automated insulin administration modalities (42). Currently available AID systems can ease some of the burden associated with mealtime management in T1D and could potentially reduce the need for precise CC while maintaining glycemic targets. Accordingly, the iLet™ system was designed to reduce diabetes burden and thus only allows users to indicate meal type and relative size (“usual,” “more,” or “less”) but not specific carbohydrate amounts, and decreased HbA1c from 7.9% to 7.3% and improved TIR from 51% to 65% in 219 youth and adults relative to injections, non-automated insulin pumps and hybrid closed-loop (43, 44). In another study, adolescents using Medtronic’s MiniMed™ 780G AID system maintained higher TIR (80% at 12 months follow-up) when employing carbohydrate counting versus simplified meal announcements, although those using simplified announcements still achieved TIR within recommended targets (73% at 12 months) (45). In a crossover design using the CamAPS™ system, Laesser et al. found no difference in TIR between a simplified meal announcement (69.9 ± 12.4%) and CC (70.7 ± 13.0%) (p=0.48) (46). Another team of investigators found that up to 20 grams of unannounced carbohydrates could be consumed with the MiniMed™ 780G system without significant differences in postprandial glucose compared to when the 20 grams were announced (TIR: 70.8% vs. 70.3% and time above range (TAR; i.e., >180 mg/dL): 27.6% vs. 27.1%, respectively) (47). However, larger amounts of carbohydrates (≥40 grams) resulted in significantly higher glycemic excursions when meals were not announced, such that TAR was 15-20% higher compared to when an announcement was made (47). Thus, certain AID systems allow users to achieve reasonable glycemic outcomes using a less burdensome carbohydrate announcement approach, but larger carbohydrate intakes may necessitate greater user input. Additionally, current evidence supports the glycemic impact of protein and fat on postprandial glycemia thus further complicating mixed-meal management in T1D. Recent literature reviews found that both protein and fat contribute to postprandial glycemic excursions by extending their duration and amplifying their magnitude (48, 49). Current guidelines recommend meal-time insulin adjustments for mixed meals to account for fat and protein content by modifying both dose and delivery of the insulin bolus (5, 7). Thus, parallel to the dual-wave boluses used with non-automated insulin pumps that improved postprandial glycaemia in high-fat, high-protein and low-GI meals (50), the CamAPS FX system includes a setting for “slowly absorbed meals” and the DBLG1-Diabeloop system offers a “high-fat meal” option (51). Overall, while AID systems reduce the burden of precise CC, meal announcements and a general understanding of glycemic effects of macronutrients remain important for safety and effective self-management, and thus continue to be part of the MNT. The relationships between dietary composition of meals and glycemia fluctuations are complex and vary widely between and within individuals owing to a multitude of clinical and sociodemographic factors (5). These complex interactions require the integration of multi-modal personal data to provide effective eating decision support tools for AID systems (52, 53). In order to progress the development of such tools, machine learning approaches which can automatically identify complex relationships in data should be explored. One study, proposed an eating decision support model that accounts for clinical characteristics, physical activity, insulin, glycemia, and eating behavior data in order to provide adolescents with T1D with meal timing and macronutrient composition recommendations to optimize TIR (52). The investigators’ (A.CS. and E.M-D.) propose the model as a foundation to build a mobile health application or integrate with an AID system depending on the individual’s access to diabetes technology (52). However, translation of these findings to clinical practice faces a multitude of challenges spanning issues related to affordability and access, potential for sensor or pump malfunction, cybersecurity and privacy concerns, data management, standardization and interoperability across devices, regulatory constraints, pharmacokinetic and pharmacodynamic constraints of available insulin formulations, and provider skills or bias (54).
A recent review catalogued the impact of AID systems on eating behaviors, including limited evidence from four qualitative and three quantitative studies (55). The available evidence revealed that AID systems reduce eating-related stress and increase confidence around food in people with T1D. However, some users reported increasing portion sizes and the intake of energy-dense foods, suggesting that the benefits of added food flexibility, increased quality of life, and reduced mealtime burden afforded by AID may be offset by the trend to consume more discretionary foods (55). Although the authors did not report on overall changes in diet quality, these results invite speculation that AID systems lead to changes in dietary patterns (55). As AID moves towards fully closed-loop systems that no longer require CC for every meal, nutrition education can increasingly focus on diet quality, variety, eating behaviors, and individualized meal patterns aligned with healthful eating principles (56). This shift to emphasize healthy eating patterns associated with reduction in cardiometabolic risk factors is especially important given the rising rates of overweight, obesity, and insulin resistance in T1D (57).
In addition to advancements in insulin delivery, preliminary data on adjunctive therapies like glucagon-like peptide 1 receptor agonists (GLP-1 RA) for T1D management are promising (57, 58). A recent review found an average reduction in HbA1c of 0.21% [-0.26, -0.17] and significant weight loss averaging 3.78 kg [-4.39, -3.71] with the use of GLP-1 RA (59). The delayed gastric emptying effect of GLP-1 RA could particularly benefit fully closed AID systems by creating better alignment between the delayed timing of exogenous insulin action and carbohydrate absorption (58). This mechanism mirrors the advantages seen with mixed meals containing fiber, complex carbohydrates, protein and fat, which can provide AID systems more time to respond compared to high-glycemic index or refined carbohydrate meals that cause rapid glucose spikes. While GLP-1RAs remain off-label for T1D, real-world adoption is growing, often with the prescription for obesity as an additional diagnosis. The percentage of US adults with T1D prescribed GLP-1RAs increased from 0.3% in 2010 to 6.6% in 2023 (60). Emerging evidence suggests potential for complementary benefits when combining these pharmacological approaches with advanced AID technologies (58).
Conclusion
Current nutritional recommendations should evolve in the context of recent therapeutic advancements in AID systems and adjunctive to insulin medications. As such future research is needed to design, evaluate, and implement new MNT models to meet the evolving realities of T1D management. Given AID’s ability to compensate for inaccuracies in CC, nutritional guidance should pivot from a sole focus on precise carbohydrate quantification toward practical dietary principles emphasizing overall nutritional quality. The goal is not to eliminate education around the impact of carbohydrates and other macronutrients on glycemic excursions, but to propose a more comprehensive approach that also offers cardiometabolic protection and that can be individualized, thus empowering people with T1D to make healthier food choices without added cognitive burden or stress.
Author contributions
MKT: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. FKB: Conceptualization, Writing – original draft, Writing – review & editing. DI: Writing – review & editing. KDC: Writing – review & editing. AS: Writing – review & editing. EJM-D: Writing – review & editing. DMM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. (2014) 130:1532–58. doi: 10.1161/CIR.0000000000000094
2. Ahuja A, Agrawal S, Acharya S, Reddy V, and Batra N. Strategies for cardiovascular disease prevention in type 1 diabetes: A comprehensive review. Cureus. (2024) 16:e66420. doi: 10.7759/cureus.66420
3. Colom C, Rull A, Sanchez-Quesada JL, and Pérez A. Cardiovascular disease in type 1 diabetes mellitus: epidemiology and management of cardiovascular risk. J Clin Med. (2021) 10:1798. doi: 10.3390/jcm10081798
4. Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. (2018) 392:477–86. doi: 10.1016/S0140-6736(18)31506-X
5. American Diabetes Association Professional Practice Committee. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes—2025. Diabetes Care. (2024) 48:S86–127. doi: 10.2337/dc25-S005
6. American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S59–85. doi: 10.2337/dc25-S004
7. Annan SF, Higgins LA, Jelleryd E, Hannon T, Rose S, Salis S, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. (2022) 23:1297–321. doi: 10.1111/pedi.13429
8. Barbetti F and Taylor SI. Insulin: still a miracle after all these years. J Clin Invest. (2019) 129:3045–7. doi: 10.1172/JCI130310
9. Soczewka M, Kędzia A, Skowrońska B, and Niechciał E. Dietary treatment of type 1 diabetes - once upon a time versus today. Pediatr Endocrinol Diabetes Metab. (2023) 29:184–9. doi: 10.5114/pedm.2023.132027
10. Westman EC, Yancy WS, and Humphreys M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914-1922). Perspect Biol Med. (2006) 49:77–83. doi: 10.1353/pbm.2006.0017
11. Nuttall FQ. Diet and the diabetic patient. Diabetes Care. (1983) 6:197–207. doi: 10.2337/diacare.6.2.197
12. Allen FM. Prolongd fasting in diabetes. Am J Med Sci. (1915) 150:480–4. doi: 10.1097/00000441-191510000-00002
13. Wolever TM, Hamad S, Chiasson JL, Josse RG, Leiter LA, Rodger NW, et al. Day-to-day consistency in amount and source of carbohydrate intake associated with improved blood glucose control in type 1 diabetes. J Am Coll Nutr. (1999) 18:242–7. doi: 10.1080/07315724.1999.10718858
14. Gillespie SJ, Kulkarni KD, and Daly AE. Using carbohydrate counting in diabetes clinical practice. J Am Diet Assoc. (1998) 98:897–905. doi: 10.1016/S0002-8223(98)00206-5
15. Anderson EJ, Delahanty L, Richardson M, Castle G, Cercone S, Lyon R, et al. Nutrition interventions for intensive therapy in the diabetes control and complications trial. J Am Dietetic Assoc. (1993) 93:768–72. doi: 10.1016/0002-8223(93)91750-K
16. Hirsch IB. Introduction: history of glucose monitoring. ADA Clin Compendia. (2018) 2018:1. doi: 10.2337/db20181-1
17. Rigby KR, Iturbe I, Candler T, Anderson R, Hamilton-Shield JP, and Hinton EC. A scoping review exploring research investigating the influence of carbohydrate counting on eating behaviour and/or disordered eating in type 1 diabetes. Diabetes Res Clin Pract. (2025) 222:112068. doi: 10.1016/j.diabres.2025.112068
18. Builes-Montaño CE, Ortiz-Cano NA, Ramirez-Rincón A, and Rojas-Henao NA. Efficacy and safety of carbohydrate counting versus other forms of dietary advice in patients with type 1 diabetes mellitus: a systematic review and meta-analysis of randomised clinical trials. J Hum Nutr Diet. (2022) 35:1030–42. doi: 10.1111/jhn.13017
19. Cristello Sarteau A and Mayer-Davis E. oo much dietary flexibility may hinder, not help: could more specific targets for daily food intake distribution promote glycemic management among youth with type 1 diabetes? Nutrients. (2022) 14:824. doi: 10.3390/nu14040824
20. Clerc A. Nutrition education to type 1 diabetes patients: few changes over the time. Front Clin Diabetes Healthc. (2023) 4:1243237. doi: 10.3389/fcdhc.2023.1243237
21. Evert AB. Factors beyond carbohydrate to consider when determining mealtime insulin doses: protein, fat, timing, and technology. Diabetes Spectr. (2020) 33:149–55. doi: 10.2337/ds20-0004
22. Amorim D, Miranda F, Santos A, Graça L, Rodrigues J, Rocha M, et al. Assessing carbohydrate counting accuracy: current limitations and future directions. Nutrients. (2024) 16:2183. doi: 10.3390/nu16142183
23. Gurnani M, Pais V, Cordeiro K, Steele S, Chen S, and Hamilton JK. One potato, two potato,… assessing carbohydrate counting accuracy in adolescents with type 1 diabetes. Pediatr Diabetes. (2018) 19:1302–8. doi: 10.1111/pedi.12717
24. Spiegel G, Bortsov A, Bishop FK, Owen D, Klingensmith GJ, Mayer-Davis EJ, et al. Randomized nutrition education intervention to improve carbohydrate counting in adolescents with type 1 diabetes study: is more intensive education needed? J Acad Nutr Diet. (2012) 112:1736–46. doi: 10.1016/j.jand.2012.06.001
25. Richardson LA, Basu A, Chien LC, Pang T, Alman AC, and Snell-Bergeon JK. Longitudinal associations of the alternative healthy eating index with coronary artery calcification and pericardial adiposity in US adults with and without type 1 diabetes. Nutr Metab Cardiovasc Dis. (2024) 34:1741–50. doi: 10.1016/j.numecd.2024.03.019
26. Gingras V, Leroux C, Desjardins K, Savard V, Lemieux S, Rabasa-Lhoret R, et al. Association between cardiometabolic profile and dietary characteristics among adults with type 1 diabetes mellitus. J Acad Nutr Diet. (2015) 115:1965–74. doi: 10.1016/j.jand.2015.04.012
27. Nansel TR, Lipsky LM, and Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J Clin Nutr. (2016) 104:81–7. doi: 10.3945/ajcn.115.126136
28. Diabetes Canada Clinical Practice Guidelines Expert Committee, Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, and Williams SL. Nutrition therapy. Can J Diabetes. (2018) 42 Suppl 1:S64–79. doi: 10.1016/j.jcjd.2017.10.009
29. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care. (2019) 42:731–54. doi: 10.2337/dci19-0014
30. Reynolds AN, Akerman AP, and Mann J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PloS Med. (2020) 17:e1003053. doi: 10.1371/journal.pmed.1003053
31. Souza Da Cunha LV, Carvalho RS, De Oliveira D, De Oliveira Cardoso L, Sartorelli DS, Xavier Peniche B, et al. The association between dietary pattern, obesity, and glycemic control of children and adolescents with type 1 diabetes mellitus. Nutrients. (2024) 16:364. doi: 10.3390/nu16030364
32. Nansel TR, Haynie DL, Lipsky LM, Laffel LMB, and Mehta SN. Multiple indicators of poor diet quality in children and adolescents with type 1 diabetes are associated with higher body mass index percentile but not glycemic control. J Acad Nutr Diet. (2012) 112:1728–35. doi: 10.1016/j.jand.2012.08.029
33. Lalanne-Mistrih ML, Bonhoure A, Messier V, Boudreau V, Lebbar M, Talbo MK, et al. Overweight and obesity in people living with type 1 diabetes: A cross-sectional analysis of the BETTER registry. Diabetes Metab Res Rev. (2024) 40:e3837. doi: 10.1002/dmrr.3837
34. Partridge H, Figueiredo C, Rouse L, Cross C, Pinder C, Ryder J, et al. Type 1 diabetes and disordered eating (T1DE): the ComPASSION Project – Wessex. Pract Diabetes. (2020) 37:127–32. doi: 10.1002/pdi.2286
35. Chad-Friedman E, Clary L, and Jhe G. Disordered eating in adolescents with type 1 diabetes: risk factors and screening recommendations. Curr Opin Pediatr. (2024) 36:351–7. doi: 10.1097/MOP.0000000000001353
36. Wheeler BJ, Lawrence J, Chae M, Paterson H, Gray AR, Healey D, et al. Intuitive eating is associated with glycaemic control in adolescents with type I diabetes mellitus. Appetite. (2016) 96:160–5. doi: 10.1016/j.appet.2015.09.016
37. van Duinkerken E, Snoek FJ, and de Wit M. The cognitive and psychological effects of living with type 1 diabetes: a narrative review. Diabetic Med. (2020) 37:555–63. doi: 10.1111/dme.14216
38. Tse J, Nansel TR, Haynie DL, Mehta SN, and Laffel LMB. Disordered eating behaviors are associated with poorer diet quality in adolescents with type 1 diabetes. J Acad Nutr Diet. (2012) 112:1810–4. doi: 10.1016/j.jand.2012.06.359
39. Scheuing N, Bartus B, Berger G, Haberland H, Icks A, Knauth B, et al. Clinical characteristics and outcome of 467 patients with a clinically recognized eating disorder identified among 52,215 patients with type 1 diabetes: A multicenter german/Austrian study. Diabetes Care. (2014) 37:1581–9. doi: 10.2337/dc13-2156
40. Biester T, Berget C, Boughton C, Cudizio L, Ekhlaspour L, Hilliard ME, et al. International society for pediatric and adolescent diabetes clinical practice consensus guidelines 2024: diabetes technologies - insulin delivery. Horm Res Paediatr. (2024) 97:636–62. doi: 10.1159/000543034
41. American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S146–66. doi: 10.2337/dc25-er04b
42. Godoi A, Reis Marques I, Padrão EMH, Mahesh A, Hespanhol LC, Riceto Loyola Júnior JE, et al. Glucose control and psychosocial outcomes with use of automated insulin delivery for 12 to 96 weeks in type 1 diabetes: a meta-analysis of randomised controlled trials. Diabetol Metab Syndr. (2023) 15:190. doi: 10.1186/s13098-023-01144-4
43. Lynch J, Kanapka LG, Russell SJ, Damiano ER, El-Khatib FH, Ruedy KJ, et al. The insulin-only bionic pancreas pivotal trial extension study: A multi-center single-arm evaluation of the insulin-only configuration of the bionic pancreas in adults and youth with type 1 diabetes. Diabetes Technol Ther. (2022) 24:726–36. doi: 10.1089/dia.2022.0341
44. Bionic Pancreas Research Group. Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med. (2022) 387:1161–72. doi: 10.1056/NEJMoa2205225
45. Petrovski G, Campbell J, Pasha M, Hussain K, Khalifa A, Umer F, et al. Twelve-month follow-up from a randomized controlled trial of simplified meal announcement versus precise carbohydrate counting in adolescents with type 1 diabetes using the miniMed™ 780G advanced hybrid closed-loop system. Diabetes Technol Ther. (2024) 26:76–83. doi: 10.1089/dia.2023.0429
46. Laesser CI, Piazza C, Schorno N, Nick F, Kastrati L, Zueger T, et al. Simplified meal announcement study (SMASH) using hybrid closed-loop insulin delivery in youth and young adults with type 1 diabetes: a randomised controlled two-centre crossover trial. Diabetologia. (2025) 68:295–307. doi: 10.1007/s00125-024-06319-w
47. Shalit R, Minsky N, Laron-Hirsh M, Cohen O, Kurtz N, Roy A, et al. Unannounced meal challenges using an advanced hybrid closed-loop system. Diabetes Technol Ther. (2023) 25:579–88. doi: 10.1089/dia.2023.0139
48. Al Balwi R, Al Madani W, and Al Ghamdi A. Efficacy of insulin dosing algorithms for high-fat high-protein mixed meals to control postprandial glycemic excursions in people living with type 1 diabetes: A systematic review and meta-analysis. Pediatr Diabetes. (2022) 23:1635–46. doi: 10.1111/pedi.13436
49. Furthner D, Lukas A, Schneider AM, Mörwald K, Maruszczak K, Gombos P, et al. The role of protein and fat intake on insulin therapy in glycaemic control of paediatric type 1 diabetes: A systematic review and research gaps. Nutrients. (2021) 13:3558. doi: 10.3390/nu13103558
50. Metwally M, Cheung TO, Smith R, and Bell KJ. Insulin pump dosing strategies for meals varying in fat, protein or glycaemic index or grazing-style meals in type 1 diabetes: A systematic review. Diabetes Res Clin Pract. (2021) 172:108516. doi: 10.1016/j.diabres.2020.108516
51. Di Molfetta S, Rossi A, Assaloni R, Franceschi R, Grancini V, Guardasole V, et al. Tips for successful use of commercial automated insulin delivery systems: An expert paper of the Italian working group on diabetes and technology. Diabetes Res Clin Pract. (2025) 223:112117. doi: 10.1016/j.diabres.2025.112117
52. Cristello Sarteau A. Use of a priori and a posteriori methodologies to inform the development of feasible eating strategies that promote glycemic management in young people with type 1 diabetes. (2025). doi: 10.17615/w5b4-2706
53. Talbo MK, McClure R, Bonhoure A, Molveau J, South CA, Lebbar M, et al. Exploring technology’s influence on health behaviours and well-being in type 1 diabetes: a review. Curr Diabetes Rep. (2024) 24:61–73. doi: 10.1007/s11892-024-01534-6
54. Aaron RE, Tian T, Yeung AM, Huang J, Arreaza-Rubín GA, Ginsberg BH, et al. NIH fifth artificial pancreas workshop 2023: meeting report: the fifth artificial pancreas workshop: enabling fully automation, access, and adoption. J Diabetes Sci Technol. (2024) 18:215–39. doi: 10.1177/19322968231201829
55. South CA, Talbo MK, Roy-Fleming A, Peters TM, Nielsen DE, Iceta S, et al. Does insulin delivery technology change our relationship with foods? A scoping review. Diabetes Technol Ther. (2024) 26:136–45. doi: 10.1089/dia.2023.0382
56. Lawton J, Blackburn M, Rankin D, Allen J, Campbell F, Leelarathna L, et al. The impact of using a closed-loop system on food choices and eating practices among people with Type 1 diabetes: a qualitative study involving adults, teenagers and parents. Diabetes Med. (2019) 36:753–60. doi: 10.1111/dme.13887
57. Casu A, Bilal A, and Pratley RE. Advancing Care for Type 1 Diabetes, Obesity Network (ACT1ON). Pharmacological therapies to address obesity in type 1 diabetes. . Curr Opin Endocrinol Diabetes Obes. (2020) 27:194–206. doi: 10.1097/MED.0000000000000555
58. Shah VN, Peters AL, Umpierrez GE, Sherr JL, Akturk HK, Aleppo G, et al. Consensus report on glucagon-like peptide-1 receptor agonists as adjunctive treatment for individuals with type 1 diabetes using an automated insulin delivery system. J Diabetes Sci Technol. (2025) 19:191–216. doi: 10.1177/19322968241291512
59. Park J, Ntelis S, Yunasan E, Downton KD, Yip TCF, Munir KM, et al. Glucagon-like peptide 1 analogues as adjunctive therapy for patients with type 1 diabetes: an updated systematic review and meta-analysis. J Clin Endocrinol Metab. (2023) 109:279–92. doi: 10.1210/clinem/dgad471
Keywords: type 1 diabetes, nutrition therapy, dietary patterns, eating behaviors, automated insulin delivery, adjunct-to-insulin therapy
Citation: Talbo MK, Bishop FK, Igudesman D, Corbin KD, Cristello Sarteau A, Mayer-Davis EJ and Maahs DM (2025) Nutrition therapy in the era of automated insulin delivery. Front. Endocrinol. 16:1690486. doi: 10.3389/fendo.2025.1690486
Received: 21 August 2025; Accepted: 17 October 2025;
Published: 31 October 2025.
Edited by:
Davide Tinti, Department of Public Health and Pediatric Sciences, ItalyReviewed by:
Andrea Enzo Scaramuzza,Istituti Ospitalieri di Cremona, ItalyCopyright © 2025 Talbo, Bishop, Igudesman, Corbin, Cristello Sarteau, Mayer-Davis and Maahs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meryem K. Talbo, bXRhbGJvQHN0YW5mb3JkLmVkdQ==; David M. Maahs, ZG1hYWhzQHN0YW5mb3JkLmVkdQ==
 Meryem K. Talbo
Meryem K. Talbo Franziska K. Bishop1
Franziska K. Bishop1