- 1College of Pharmacy, Gachon University, Incheon, Republic of Korea
- 2Department of Pharmacy, Chonnam National University Hwasun Hospital, Hwasun, Republic of Korea
- 3Department of Pharmacy, Samsung Medical Center, Seoul, Republic of Korea
- 4College of Pharmacy, Yeungnam University, Gyeongsan, Republic of Korea
- 5Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Republic of Korea
- 6Department of Internal Medicine, Gachon University School of Medicine, Incheon, Republic of Korea
- 7Department of Pharmacy, Gachon University Gil Medical Center, Incheon, Republic of Korea
Background: Antidiabetic drugs lower blood glucose levels and may also have neuroprotective and vascular protection effects. In particular, sodium–glucose cotransporter 2 inhibitors (SGLT2is) and incretin mimetics have demonstrated dementia-reducing effects. We evaluated whether SGLT2is reduce dementia risk compared with incretin mimetics in patients with type 2 diabetes (T2D).
Methods: Systematic review and meta-analysis were performed by searching the PubMed, Embase, and Cochrane Library databases through February 2025. Both randomized trials and cohort studies were identified and qualitatively assessed, but only cohort studies were included in the quantitative meta-analysis. We also compared the effects of SGLT2is with those of dipeptidyl peptidase-4 inhibitors (DPP-4i) or glucagon-like peptide-1 receptor agonists (GLP-1RA) on dementia incidence.
Results: Nine studies were identified for analysis. Compared with incretin mimetics, SGLT2is significantly reduced the overall dementia risk [hazard ratio (HR) 0.82, 95% CI: 0.73-0.91], and SGLT2is had stronger effects than DPP-4i (HR 0.67, 95% CI: 0.59-0.77) and GLP-1RA (HR 0.93, 95% CI: 0.86-1.00). SGLT2i also reduced the risks of vascular dementia and Alzheimer’s disease (HR 0.49, 95% CI: 0.35–0.70 vs. HR 0.68, 95% CI: 0.52–0.88, respectively). The results of subgroup analyses revealed increased benefits for patients aged older than 65 years. Empagliflozin was the most consistently protective among the SGLT2i agents.
Conclusion: SGLT2is may provide neuroprotective benefits beyond glycemic control in patients with T2D, particularly in older populations at higher risk of cognitive decline. These findings support consideration of SGLT2is as a preferred therapeutic option for patients with T2D at increased risk of dementia, although randomized controlled trials would further strengthen this evidence base.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024567890 identifier PROSPERO (CRD420251037959).
1 Introduction
Type 2 diabetes (T2D) is a chronic metabolic disorder; its prevalence is rapidly increasing worldwide, particularly given the aging of the population (1). Characterized by insulin resistance and chronic hyperglycemia, T2D can lead to a wide range of complications such as cardiovascular disease, kidney dysfunction, and neuropathy (2). Beyond these well-known complications, T2D substantially increases the risk of neurodegenerative disorders, such as dementia (3), which occurs approximately 1.5 times more frequently in patients with diabetes than in those without diabetes (4, 5). Globally, more than 500 million people are currently living with T2D, and the number is projected to rise to 643 million by 2030, with dementia affecting over 55 million people worldwide. This imposes significant healthcare and economic burdens on aging societies (6, 7).
Sodium-glucose cotransporter 2 inhibitors (SGLT2is) are antidiabetic agents that provide cardiovascular and renal protective effects beyond glycemic control (8, 9), and emerging evidence suggests potential neuroprotective benefits, reducing the risk of dementia and mortality in older adults (10, 11). Similarly, incretin-based therapies, including dipeptidyl peptidase-4 inhibitors (DPP-4i) and glucagon-like peptide-1 receptor agonists (GLP-1RA), have also shown potential cognitive benefits in some studies (12, 13).
Several retrospective cohort studies have investigated the association between SGLT2i use and dementia incidence, with most studies demonstrating a lower risk of dementia in SGLT2i users than in non-SGLT2i users, although some studies failed to achieve statistical significance (11, 14–22). A recent meta-analysis of these observational studies further supported the association between SGLT2i use and lower incidence of dementia compared with other antidiabetic medications (23, 24). However, these comparisons with non-SGLT2i users limit direct drug-to-drug comparative interpretation and the ability to draw robust conclusions about drug-specific neuroprotective effects (24, 25). To address this limitation, incretin-based therapies were selected as suitable references because they are known to possess potential neuroprotective properties (26), making them more clinically relevant for comparison than placebo or mixed control groups.
Our aim in this study was to conduct a systematic review and meta-analysis of the literature to evaluate the effects of SGLT2is on cognitive decline and preventing dementia in patients with T2D, using incretin mimetics for comparison. This comparative approach not only advances our scientific understanding of drug-specific neuroprotective effects but also offers practical insights that may guide therapeutic decision-making and optimization of treatment strategies in routine clinical practice.
2 Methods
2.1 Literature search
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (27) for systematic reviews and meta-analyses. The PRISMA checklist is provided in Supplementary Table S1, and the study protocol was registered in the PROSPERO database (Registration No: CRD420251037959). Eligibility criteria were defined according to the PICO (Population, Intervention, Comparator, Outcome) framework. The target population comprised adults (≥18 years) with T2D. The intervention of interest was treatment with SGLT2is, and the comparators were incretin-based therapies, including DPP-4i or GLP-1RAs; notably, intervention and comparator groups were interchangeable in this analysis. Primary outcomes included the incidence of all-cause dementia, while secondary outcomes comprised Alzheimer’s disease, vascular dementia, and all-cause mortality. The literature was searched using three databases, PubMed, Embase, and the Cochrane Library, to identify all relevant publications up to February 2025. Based on the PICO framework, the search terms included “sodium glucose transporter 2 inhibitors,” “glucagon-like peptide-1 receptor agonists,” “ dipeptidyl-peptidase IV inhibitors,” “dementia,” “cognitive impairment,” and “Alzheimer’s disease”. Medical Subject Heading (MeSH) terms and free text were used in parallel, and a detailed search strategy was developed to incorporate various synonyms and trade names related to antidiabetic medication and cognitive disorders, with the complete search strings for each database provided in Supplementary Table S2.
2.2 Literature selection
The inclusion criteria were as follows: First, the study population consisted of individuals aged 18 years or older who were diagnosed with T2D. Second, the intervention group included patients treated with SGLT2is, and the comparison group comprised patients receiving incretin mimetics, specifically GLP-1RA or DPP-4i. Eligible studies reported either the incidence of all-cause dementia or changes in cognitive function scores as their primary outcomes. The study designs included were randomized controlled trial (RCT) and prospective or retrospective cohort study.
The following studies were excluded: those with only the abstract available and those lacking full-text access. In addition, studies that did not involve human subjects, reported outcomes related to cognitive function or dementia, lacked sufficient data for calculating effect size, or were published in languages other than English, were also excluded.
2.3 Study selection, data extraction, and quality assessment
The studies were selected, data were extracted, and quality was assessed independently by two researchers (JC and KS). A third researcher (KC) mediated the final decision in cases of disagreement. The data were extracted from all studies meeting the inclusion criteria using a standardized form to obtain information on study characteristics, population demographics, cognitive assessment tools, types of interventions, baseline scores, types and dosages of medications used, comparison group settings, covariate adjustment, statistical analysis methods, and study results. Studies derived from the same national database were screened for inclusion in the analysis based on the study period or number of study participants to minimize the possibility of participant duplication among the selected studies. The risk of bias in RCTs was assessed using RoB 2.0 (28), whereas the ROBINS-I tool (29) was used for nonrandomized studies.
2.4 Outcomes
The primary outcome was the incidence of dementia. In addition, changes in cognitive function scores were considered in studies reporting this outcome. However, due to the limited number of such studies, these results were described qualitatively. The secondary outcomes included the incidence of dementia subtypes and all-cause mortality for the overall incretin mimetic group and individually for DPP-4i and GLP-1RA. Additionally, subgroups were analyzed to compare SGLT2is with DPP-4i and GLP-1RA, stratified by patient age, geographic region, follow-up treatment duration, and individual SGLT2i agents. Studies with longer durations were preferentially included in cases where duplication was suspected considering the possibility of overlapping participants among studies based on the same national databases (National Health Insurance Service, TriNex).
2.5 Statistical analysis
The effect sizes for the incidence of dementia were synthesized using HRs, and a meta-analysis was conducted using a random-effects model based on the inverse-variance method. The heterogeneity among studies was assessed using Cochran’s Q statistic and the I² value, with I² values exceeding 50% indicating significant heterogeneity. Sensitivity was analyzed using a leave-one-out approach by sequentially excluding individual studies, alternative analyses to account for duplicate data, and exclusion of studies with a high risk of bias. Publication bias was assessed using funnel plots, focusing on the primary outcome of dementia incidence. All statistical analyses were conducted using RevMan 5.4 (Review Manager version 5.4; Cochrane Collaboration).
3 Results
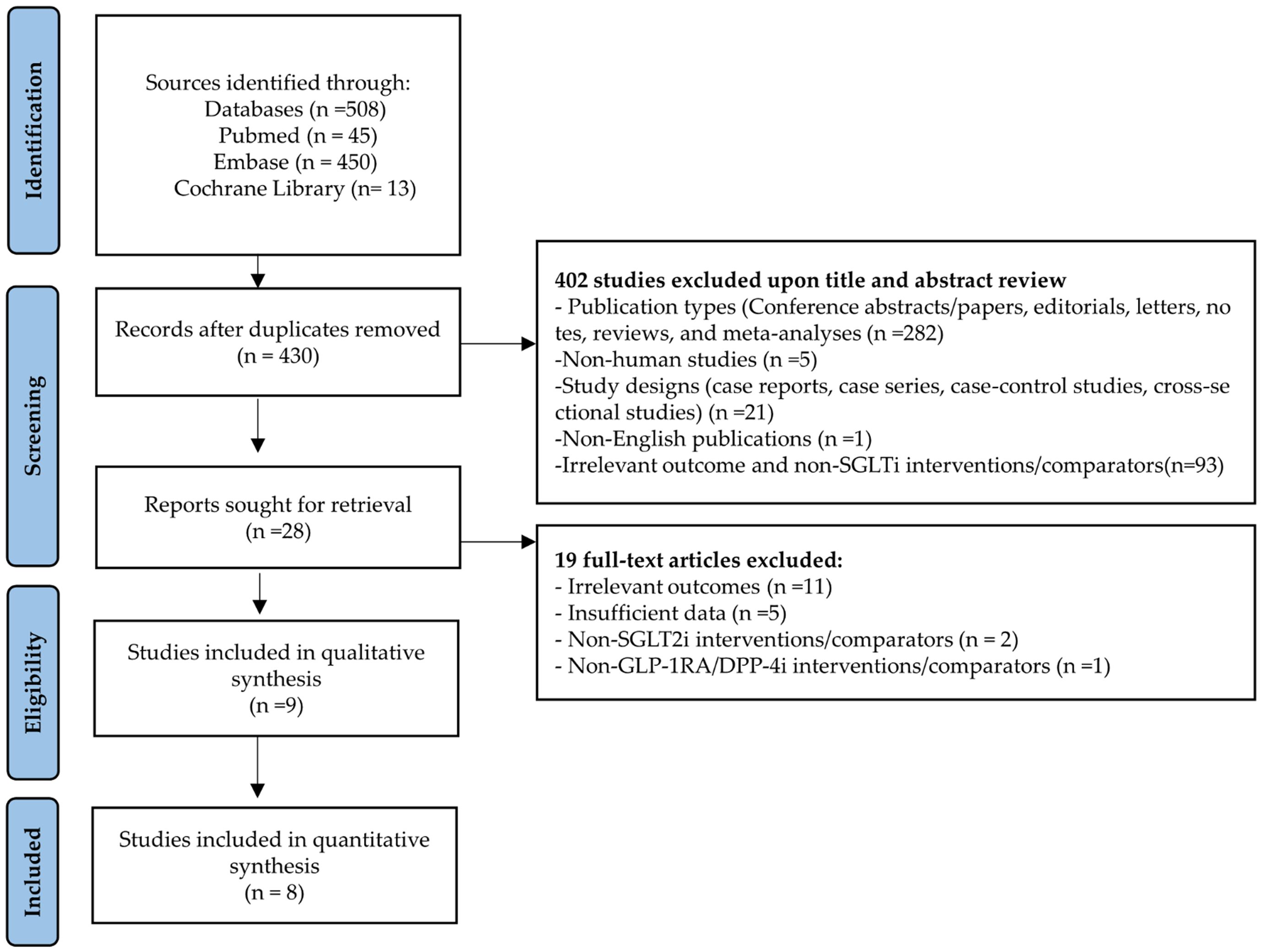
3.1 Literature selection
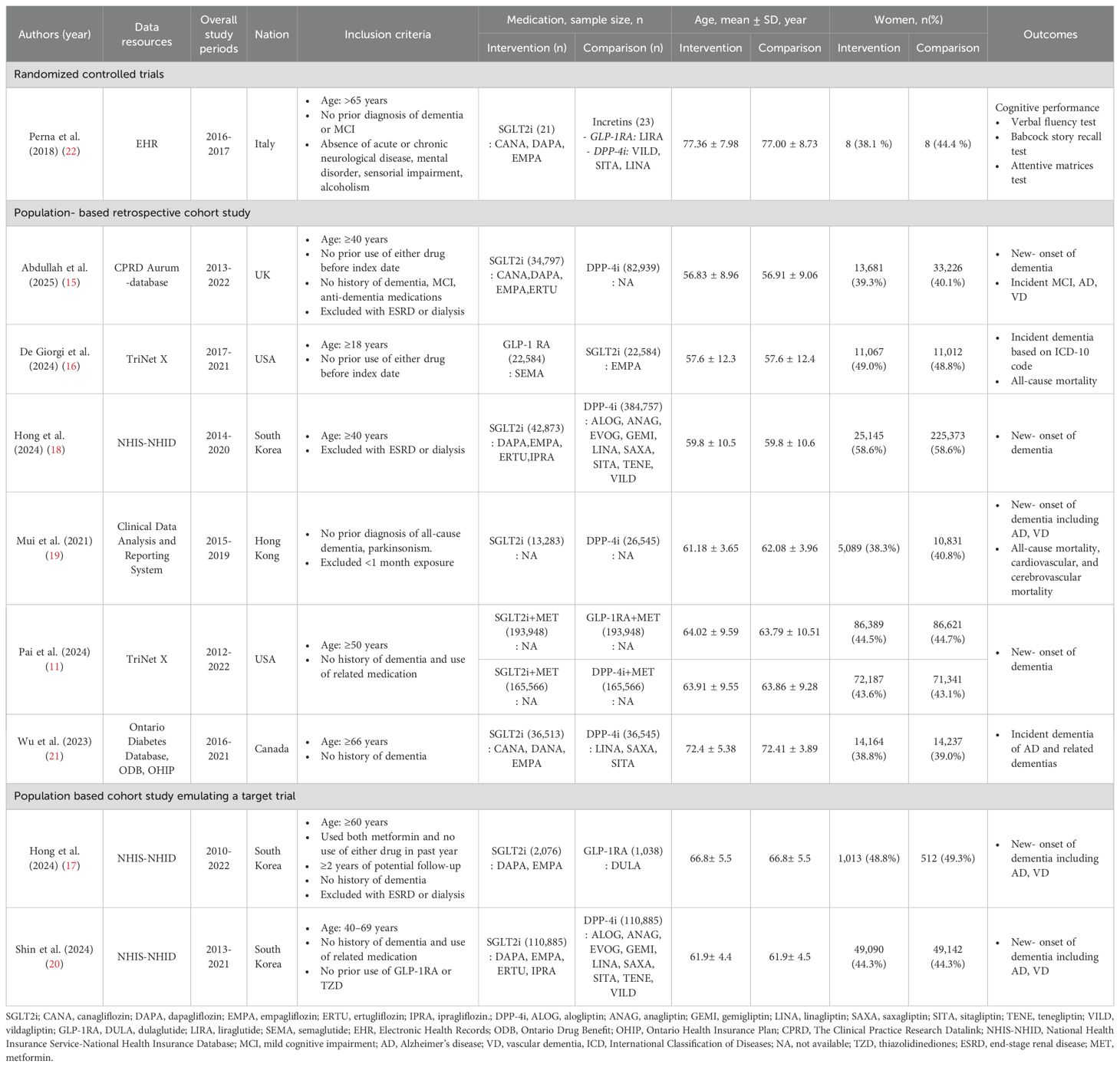
We identified 508 publications from the initial literature search. A total of 78 duplicates and 421 studies did not meet the eligibility criteria; nine studies were thus included in this analysis (Figure 1). Details of the 19 excluded full-text articles are provided in Supplementary Table S3. Of these, eight studies were included in the quantitative synthesis. Perna et al. assessed cognitive function using neuropsychological tests, including the Verbal Fluency Test, Babcock Story Recall Test, and Attentive Matrices Test [19].
3.2 Characteristics of included studies
The included studies primarily evaluated dementia risk in patients with T2D by comparing SGLT2is with incretin mimetics, including DPP-4i and GLP-1RA. The included studies comprised one RCT, six population-based retrospective cohort studies, and two cohort studies emulating a target trial. The characteristics of these studies are summarized in Table 1; Supplementary Table S4. Most study populations consisted of adults aged 50 years and older, with a predominance of older adults aged over the age of 65 years in most cohorts. The proportion of female participants ranged from approximately 40% to 60%. The number of study participants varied substantially, ranging from fewer than 50 to over 390,000 individuals.
Five studies compared SGLT2is with DPP-4 inhibitors only, two with GLP-1 receptor agonists only, and one (11) with both. One RCT (22) compared SGLT2is with incretin-based therapies as a combined comparator group. The mean follow-up period among all studies ranged from two to five years, suggesting the need for longer observational studies for evaluating the long-term dementia-prevention effects of SGLT2is.
3.3 New-onset dementia
To avoid overlap, only the study by Shin et al., which used a longer study period, was included among those using the same national database (excluding the study by Hong et al.), resulting in a total of seven studies included in the comparison between SGLT2is and incretin mimetics. SGLT2i use was associated with a significantly reduced overall risk of dementia (hazard ratio [HR] 0.82, 95% CI: 0.73–0.91, p = 0.0004, I2 = 85%). Upon separately examining each class of incretin mimetics, SGLT2is were found to be associated with a significantly lower risk of dementia than DPP-4is were (HR 0.67, 95% CI: 0.59–0.77, p <0.00001, I2 = 79%). Additionally, SGLT2is showed a trend toward a lower risk of dementia compared with GLP-1RA did (HR 0.93, 95% CI: 0.86–1.00, p = 0.04, I2 = 4%). As shown in Figure 2, Most studies consistently showed directional effects associating SGLT2is with a reduced risk of dementia.
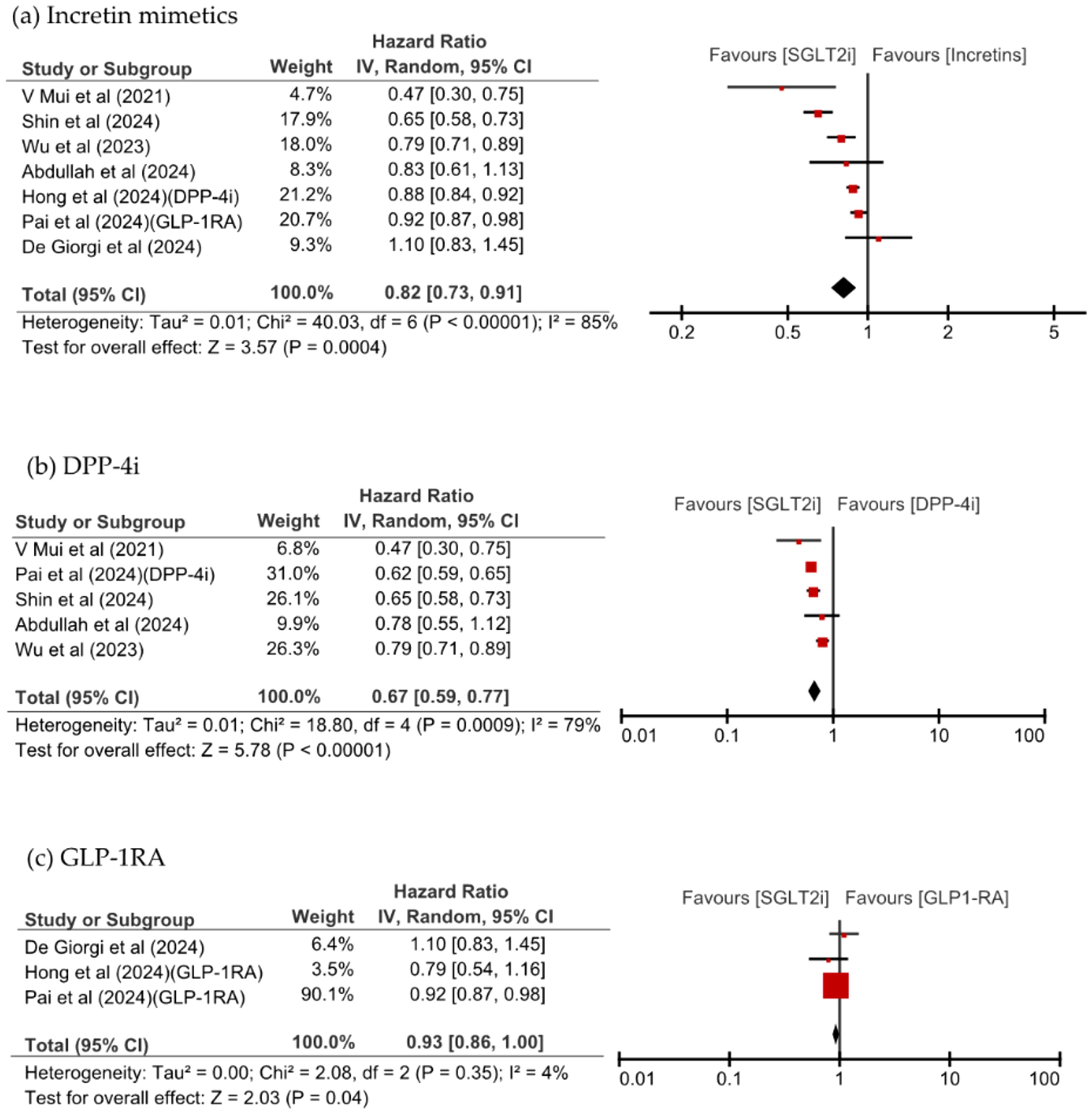
Figure 2. Forest plot of new-onset dementia with SGLT2is compared with (a) incretin mimetics, (b) DPP-4i, and (c) GLP-1RA.
3.4 Vascular dementia, Alzheimer’s disease, and all-cause mortality
Figure 3 shows that SGLT2i use was associated with a significant reduction of VD and AD risks, in the analysis of dementia risk by specific dementia types comparing SGLT2is and incretin mimetics (HR 0.49, 95% CI: 0.35–0.70, p <0.0001, I2 = 0% vs. HR 0.68, 95% CI: 0.52–0.88, p = 0.003, I2 = 33%). SGLT2is use numerically lower all-cause mortality than incretins use; however, this difference was not significant (HR 0.77, 95% CI: 0.47–1.27, p = 0.30, I2 = 98%). SGLT2i use significantly reduced the risk of VD compared with DPP-4i use (HR 0.48, 95% CI: 0.34–0.68, p <0.0001, I2 = 0%) and significantly reduced the risk of all-cause mortality (HR 0.62, 95% CI: 0.46–0.84, p = 0.002, I2 = 92%). All-cause mortality was comparable between patients taking SGLT2is and those taking GLP-1RA, as shown in Figure 4.
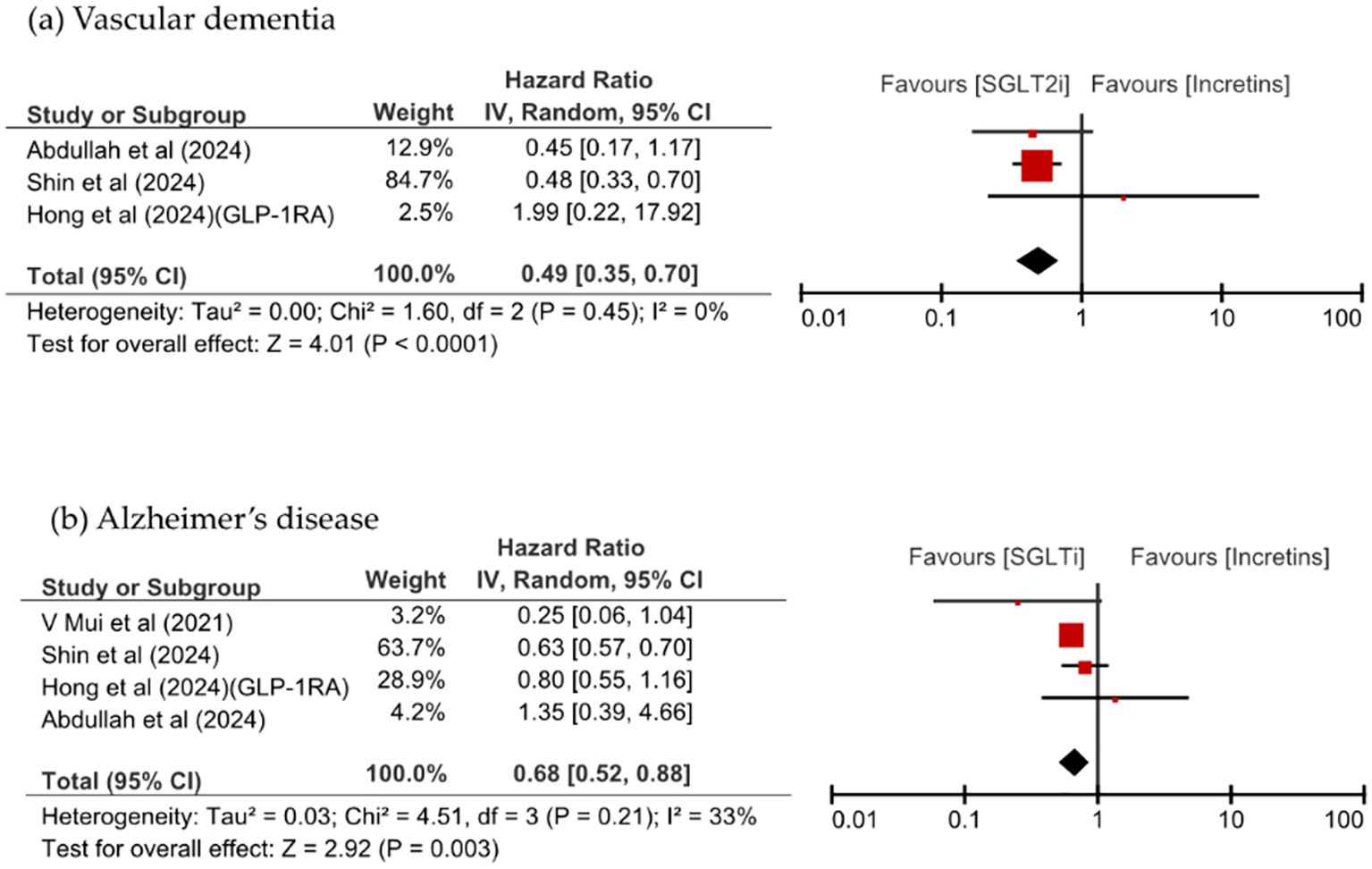
Figure 3. Comparison of dementia incidence between SGLT2is and incretin mimetics, stratified by dementia subtype: (a) vascular dementia, (b) Alzheimer’s disease.
![Three forest plots comparing drug classes with SGLT2 inhibitors. (a) Incretin mimetics: Hazard Ratio 0.77, 95% CI [0.47, 1.27]. Heterogeneity I² = 98%. (b) DPP-4i: Hazard Ratio 0.62, 95% CI [0.46, 0.84]. Heterogeneity I² = 92%. (c) GLP-1RA: Hazard Ratio 1.03, 95% CI [0.82, 1.28]. Heterogeneity I² = 89%. The plots show effect estimates and confidence intervals. Red squares represent individual studies, diamonds for pooled estimates.](https://www.frontiersin.org/files/Articles/1695075/fendo-16-1695075-HTML/image_m/fendo-16-1695075-g004.jpg)
Figure 4. Forest plot of all-cause mortality with SGLT2is compared with (a) incretin mimetics, (b) DPP-4i, and (c) GLP-1RA.
3.5 Subgroup analysis
3.5.1 Patient and study characteristics
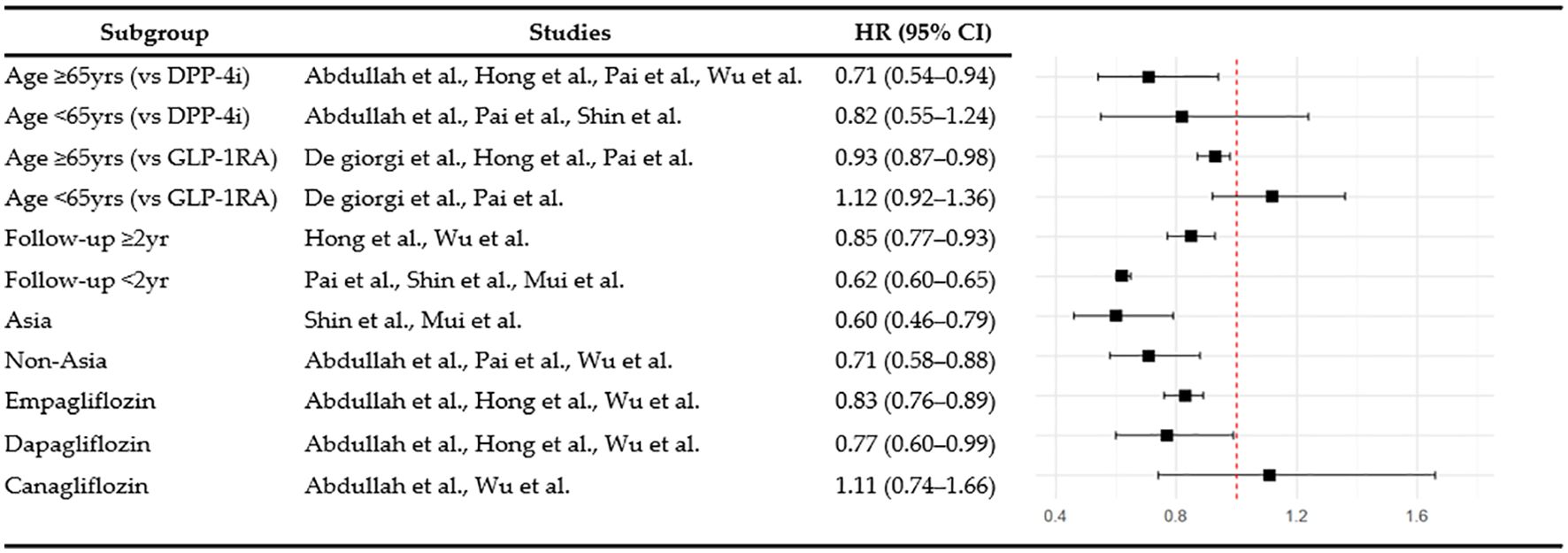
Subgroup analyses were performed to examine dementia risk according to age, follow-up duration, and study region. Among individuals aged 65 and over, SGLT2is use was associated with a significantly lower risk of dementia than GLP-1RA use (HR 0.93, 95% CI: 0.87–0.98, p = 0.008, I2 = 0%), whereas no significant difference was observed in the under-65 age group (HR 1.12, 95% CI: 0.92–1.36, p = 0.26, I2 = 0%). When stratified by follow-up duration, the dementia risk associated with SGLT2is compared with DPP-4i was 0.62 (95% CI: 0.60–0.65, p < 0.00001, I2 = 0%) for less than two years and 0.85 (95% CI: 0.77–0.93, p = 0.0006, I2 = 62%) for two or more years. In the regional subgroup analysis, SGLT2is use was associated with a significantly lower risk of dementia in Asian populations (HR 0.60, 95% CI: 0.46–0.79, p = 0.0002, I2 = 42%), and also significant among non-Asian populations (HR 0.71, 95% CI: 0.58-0.88, p = 0.002, I2 = 88%). The overall findings from these subgroup analyses are summarized in Figure 5.
3.5.2 Individual SGLT2i agents
Empagliflozin significantly reduced the risk of dementia compared with DPP-4i (HR 0.83, 95% CI: 0.76–0.89, p <0.00001, I2 = 6%) based on the results of a subgroup analysis by individual agents within the SGLT2i class. Dapagliflozin (HR 0.77, 95% CI: 0.60–0.99, p = 0.05, I2 = 73%) showed borderline significance for the risk of dementia. In contrast, the effect of canagliflozin did not reach statistical significance (HR 1.11, 95% CI: 0.74–1.66, p = 0.61, I2 = 56%).
3.6 Quality and sensitivity analyses
The results of the quality assessment of the final selected studies are presented in Supplementary Tables S5, S6. In the included RCT, the study by Perna et al. (22) was rated as having “some concerns,” primarily due to issues related to the randomization process and outcome measurement. One non-randomized study by Abdullah et al. (15) was assessed as having a critical risk of bias, mainly due to the presence of confounding effects. All other non-RCTs were evaluated as having a moderate risk of bias. Furthermore, no evidence of publication bias was detected based on visual inspection of the funnel plot (Supplementary Figure S4). Although a meta-regression was considered to explore potential sources of heterogeneity, it could not be performed because of the limited number of studies within each subgroup (30, 31). We reanalyzed the data by including studies that were previously excluded because of the potential overlap in study populations to assess the robustness of the main findings, (Pai et al. and Hong et al. [GLP-1RA]). The direction and significance of these effects were consistent. The leave-one-out analysis, in which each study was sequentially removed, also yielded consistent results: SGLT2is significantly reduced the risk of dementia compared with incretin mimetics across all analyses, as shown in Supplementary Figures S2, S3. Additional sensitivity analyses using intention-to-treat and as-treated approaches further supported the robustness of the results (Supplementary Figure S1). Although a meta-regression was considered to explore potential sources of heterogeneity, it could not be performed because of the limited number of studies within each subgroup.
Perna et al. administered SGLT2is (n=21) and incretin mimetics (n=18) for 12 months to older adults with T2D with a mean age of 77 years; no significant changes were observed in various cognitive function assessments, including the Verbal Fluency Test, Attentive Matrices Test, and Babcock Story Recall Test in either group (p > 0.05).
4 Discussion
The pathophysiological mechanisms underlying the association between T2D and dementia involve multiple interconnected pathways. Chronic hyperglycemia promotes the accumulation of advanced glycation end-products, which trigger β-amyloid deposition and neurofibrillary tangle formation, ultimately leading to neuronal damage (32). Furthermore, insulin resistance in neural cells disrupts normal insulin signaling, and impairs the suppression of β-amyloid production and tau protein hyperphosphorylation, promoting cognitive decline (33). Moreover, diabetes-associated microvascular dysfunction, oxidative stress, and low-grade systemic inflammation may exacerbate blood–brain barrier (BBB) disruption and neuroinflammatory responses, further contributing to the progression of dementia (34, 35).
We found that compared with incretin mimetics, SGLT2is significantly reduced the risk of overall incidence of dementia, VD, and AD. These findings suggest neuroprotective effects beyond glucose control, supported by preclinical and clinical studies (10, 11, 23, 36). Preclinical studies demonstrate that SGLT2is prevent structural damage and inhibit ultrastructural changes associated with cognitive decline in the neurovascular units of a diabetic mouse model (37). Reductions in β-amyloid deposition and tau protein phosphorylation were observed in the brain tissues of mice with both AD and T2D (38). Additionally, SGLT2is may modulate the AMPK/mTOR pathway and promote autophagy (39). Beyond these direct neuroprotective effects, SGLT2is exert anti-inflammatory effects that mitigate systemic and neuroinflammatory processes that contribute to cognitive decline (40, 41). Subsequently, multiple clinical studies have reported the effects of SGLT2is on dementia (9, 42, 43).
Both DPP-4i and GLP-1RA modulate the incretin hormone GLP-1 but differ in their neuroprotective mechanisms. GLP-1RA directly acts on GLP-1 receptors and can cross the BBB enabling direct central nervous system effects including anti-inflammatory, antioxidant, and β-amyloid clearance effects (44). In contrast, DPP-4i indirectly increases GLP-1 and glucose-dependent insulinotropic polypeptide levels without direct brain penetration (45). These mechanistic differences are reflected in clinical outcomes. GLP-1RAs have demonstrated consistent and robust cognitive benefits (46–48), with up to 53% in RCT and 27% in case–control studies, compared with placebo (49), while DPP-4i exhibited mixed results (50–53). Our finding that SGLT2is was more effective than DPP-4i but comparable to GLP-1RA (HR 0.93, 95% CI: 0.86–1.00, p = 0.04, I2 = 4%) align with these mechanistic differences. The pronounced neuroprotective effect of GLP-1RA may have attenuated the observed relative effect size of SGLT2is in direct comparisons, indirectly suggesting that SGLT2is are likely to exert neuroprotective effects of comparable clinical relevance.
Perna et al. observed no deterioration in cognitive function following at least one year of treatment with either SGLT2is or incretin mimetics, as assessed using standardized neuropsychological measures. These findings suggest that at least 12 months of treatment with these agents does not detrimentally affect cognitive function. Although the study was conducted in a population with normal baseline cognitive function, these findings suggest that these agents maintain cognitive stability.
In subgroup analysis, SGLT2i use significantly reduced the risk of dementia in patients aged 65 and older, whereas no significant effect was observed in those under 65 years, when compared with DPP-4i use. The age-related benefit may reflect physiological differences that may enhance the effects of the drugs in older adults, who are at high risk of dementia. Age-related deterioration in insulin sensitivity, elevated oxidative stress, and compromised mitochondrial function collectively increase susceptibility to cognitive impairment in older populations (54–56), potentially making older adults more responsive to the protective mechanism of SGLT2is. This finding aligns with those of prior studies, suggesting that the cardiovascular and metabolic benefits of SGLT2is are more evident in older patients (57–59). SGLT2is showed consistent protective effects against dementia regardless of the follow-up period. Notably, a significant reduction in dementia risk was observed even in the short-term follow-up group of less than 2 years, suggesting that SGLT2is may exert cognitive protective effects within a relatively short period. This early benefit may be attributable to the rapid improvements in inflammatory responses, vascular function, and oxidative stress that provide immediate neuroprotective effects. Longer follow-up studies have shown cumulative protective effects, underscoring the importance of both early and sustained SGLT2i use. However, some cases of dementia diagnosed within a short period after drug administration cannot exclude the possibility of reverse causality; thus, additional research is required considering the slow progression of dementia and importance of lag-time settings. The results of subgroup analysis by region showed that SGLT2is had significant dementia-prevention effects in Asian and non-Asian populations, suggesting that the cognitive benefits of SGLT2is may extend across diverse ethnic and regional groups.
Distinct trends in the reduction of dementia risk emerged when individual SGLT2i agents were compared with DPP-4i. Empagliflozin had a consistent protective effect with low heterogeneity, whereas dapagliflozin had a protective effect with borderline significance and canagliflozin did not achieve statistical significance. These findings suggest that empagliflozin may be relatively more effective in preserving cognitive function. Although canagliflozin’s lack of significance may partly reflect its substantially smaller sample size across studies, differences in pharmacological properties among SGLT2i agents may also contribute to the varying neuroprotective effects. Empagliflozin’s superior cognitive protective effects may be related to its high SGLT2 selectivity and BBB permeability, which enables effective inhibition of microglial overactivation and subsequent protection of neurons and glial cells (60). In support of this, empagliflozin reduced the levels of both the neuronal damage marker neurofilament light chain and glial damage marker S100BB. In contrast, canagliflozin’s lower SGLT2 selectivity and dual inhibition of SGLT1 and SGLT2 may have limited its central nervous system penetration. While canagliflozin reduced neurofilament light chain levels, its inconsistent effects on S100BB suggest limited glial protection (61). This distinction has clinical relevance because glial cell hyperactivation induces central nervous system inflammation in major neurodegenerative disorders, including AD and Parkinson’s disease (62). These findings suggest that the neuroprotective potential of individual SGLT2i agents depends on their BBB permeability as well as their capacity for neuroglial protection, factors that should be considered in clinical decision-making.
This study has a few limitations. First, to avoid data duplication, only one study was selected from those using the same database when researcher overlap was possible. Although this approach helped maintain data independence, it may have reduced the total number of studies included, potentially limiting the representativeness of our findings. Second, because this meta-analysis included only cohort studies, causal relationships could not be definitively established. Although most of the included studies were of high quality and adjusted for major confounders such as age, sex, body mass index, glycemic parameters, hypertension, and other cardiometabolic factors, these adjustments were not fully consistent across studies. To address these limitations and assess the robustness of our findings, we conducted extensive subgroup and sensitivity analyses—including leave-one-out testing and reanalysis of previously excluded studies—which consistently confirmed both the direction and statistical significance of the association between SGLT2is use and reduced dementia risk. Thirdly, potential publication bias was evaluated using funnel plots. Visual inspection revealed no substantial asymmetry, suggesting a low likelihood of publication bias. However, given that fewer than ten studies were included in the meta-analysis, this finding should be interpreted with caution. Finally, although one RCT was identified in our systematic review, it was not included in the meta-analysis due to differences in outcome measures. Nevertheless, the findings from this RCT provide meaningful insights into the cognitive outcomes associated with these agents.
This study is meaningful in its comparison of SGLT2i users with those using other antidiabetic agents known for their cognitive benefits. SGLT2is were found to be noninferior to GLP-1RA, which have cognitive effects; we also identified differences in effects among SGLT2i agents. Furthermore, we observed an association between SGLT2i use and a reduced risk of dementia in older adults aged 65 years and above, with no evidence of racial disparities. These results have important clinical implications for T2D management in patients at risk of cognitive decline. First, the demonstrated neuroprotective effects support expanding treatment selection criteria beyond glycemic control to include cognitive outcomes, particularly in older adults. Second, empagliflozin’s superior performance suggests that individual agent selection may be clinically meaningful when cognitive protection is a treatment goal. Third, the early onset of protective effects observed within two years indicates that timely initiation of SGLT2is therapy may provide immediate cognitive benefits alongside long-term cumulative effects. For clinical practice, these findings suggest incorporating cognitive risk assessment into diabetes care frameworks and considering SGLT2is as preferred agents for patients with elevated dementia risk. While awaiting confirmatory RCTs, the present evidence supports SGLT2is as a rational choice for comprehensive diabetes management that addresses both metabolic and cognitive outcomes. Future prospective studies should employ rigorous comparative designs with extended observation periods and careful adjustment for baseline patient factors to establish definitive causal relationships.
5 Conclusions
The results of this meta-analysis suggest that compared with incretin-based therapies, the use of SGLT2is was associated with a potential reduction in overall risk of dementia, with additional benefits observed in both VD and AD. Among SGLT2is, empagliflozin appeared to show a relatively stronger association, although this finding should be interpreted with caution due to the limited number of studies and potential differences in study design. The observed cognitive effects of SGLT2is were consistent across various subgroups, including older age groups, all follow-up durations, and diverse racial groups. Given the limitations of observational studies including heterogeneity and potential bias, future RCT are warranted. Large-scale, head-to-head studies with standardized methodologies and long-term follow-up will be required, specifically accounting for patient characteristics, comorbidities, concomitant medications, and age-specific effects to confirm the cognitive protective effects of SGLT2is.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
KS: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft. JC: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft. DJ: Formal Analysis, Methodology, Writing – original draft. DS: Funding acquisition, Validation, Writing – review & editing. YA: Validation, Writing – review & editing. KL: Data curation, Writing – review & editing. KC: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF; grant numbers: 2021R1G1A1012790 and 2020R1A6A1A03043708).
Acknowledgments
Thanks are due to all the authors of the studies included in this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1695075/full#supplementary-material
Supplementary Table 1 | Checklist of preferred reporting items for systematic reviews and meta-analyses.
Supplementary Table 2 | Search strategy.
Supplementary Table 3 | List of excluded full-text articles with reasons for exclusion.
Supplementary Table 4 | Detailed study protocol and baseline population characteristics of included studies.
Supplementary Table 5 | Detailed information on the risk of bias assessment according to the Risk of Bias 2 (ROB2) criteria.
Supplementary Table 6 | Detailed information regarding the risk of bias assessment according to the Risk of Bias in Non-randomized Studies-of Interventions (ROBINS-I).
Supplementary Figure 1 | Subgroup analysis of dementia risk: Intention-to-Treat (ITT) vs. As-Treated (AT) approaches.
Supplementary Figure 2 | Sensitivity analysis of new-onset dementia outcome.
Supplementary Figure 3 | Sensitivity analysis excluding studies with high risk of bias.
Supplementary Figure 4 | Funnel plot of included studies evaluating SGLT2is versus incretin-based therapies for incident dementia.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Hauwanga WN, Abdalhamed TY, Ezike LA, Chukwulebe IS, Ko Oo A, Wilfred A, et al. The pathophysiology and vascular complications of diabetes in chronic kidney disease: A comprehensive review. Cureus. (2024) 16:e76498. doi: 10.7759/cureus.76498
3. Beeri MS and Bendlin BB. The link between type 2 diabetes and dementia: from biomarkers to treatment. Lancet Diabetes Endocrinol. (2020) 8:736–8. doi: 10.1016/S2213-8587(20)30267-9
4. Damanik J and Yunir E. Type 2 diabetes mellitus and cognitive impairment. Acta Med Indonesiana. (2021) 53:213.
5. Cao F, Yang F, Li J, Guo W, Zhang C, Gao F, et al. The relationship between diabetes and the dementia risk: a meta-analysis. Diabetol Metab Syndr. (2024) 16:101. doi: 10.1186/s13098-024-01346-4
6. Schwarz P. IDF global clinical practice recommendations for managing type 2 diabetes - 2025. Diabetes Res Clin Pract. (2025) 222 Suppl 1:112158. doi: 10.1016/j.diabres.2025.112158
7. Xu L, Wang Z, Li M, and Li Q. Global incidence trends and projections of Alzheimer disease and other dementias: an age-period-cohort analysis 2021. J Glob Health. (2025) 15:04156. doi: 10.7189/jogh.15.04156
8. Salvatore T, Galiero R, Caturano A, Rinaldi L, Di Martino A, Albanese G, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. (2022) 23:3651. doi: 10.3390/ijms23073651
9. Hung CH and Lu LY. New insights into the role of SGLT-2 inhibitors in the prevention of dementia. Neurol Int. (2024) 16:1717–30. doi: 10.3390/neurolint16060124
10. Lardaro A, Quarta L, Pagnotta S, Sodero G, Mariani S, Del Ben M, et al. Impact of sodium glucose cotransporter 2 inhibitors (SGLT2i) therapy on dementia and cognitive decline. Biomedicines. (2024) 12:1750. doi: 10.3390/biomedicines12081750
11. Pai YW, Chen IC, Lin JF, Chen XH, Chen HH, Chang MH, et al. Association of sodium-glucose cotransporter 2 inhibitors with risk of incident dementia and all-cause mortality in older patients with type 2 diabetes: A retrospective cohort study using the TriNetX US collaborative networks. Diabetes Obes Metab. (2024) 26:5420–30. doi: 10.1111/dom.15918
12. Chai S, Liu F, Yu S, Yang Z, and Sun F. Cognitive protection of incretin-based therapies in patients with type 2 diabetes mellitus: A systematic review and meta-analysis based on clinical studies. J Diabetes Investig. (2023) 14:864–73. doi: 10.1111/jdi.14015
13. Yuan Y, Zhang Y, Lei M, Guo X, Yang X, Ouyang C, et al. Effects of DPP4 inhibitors as neuroprotective drug on cognitive impairment in patients with type 2 diabetes mellitus: A meta-analysis and systematic review. Int J Endocrinol. (2024) 2024:9294113. doi: 10.1155/2024/9294113
14. Wang W, Wang Q, Qi X, Gurney M, Perry G, Volkow ND, et al. Associations of semaglutide with first-time diagnosis of Alzheimer's disease in patients with type 2 diabetes: Target trial emulation using nationwide real-world data in the US. Alzheimers Dement. (2024) 20:8661–72. doi: 10.1002/alz.14313
15. Abdullah Z, Cui Y, Platt RW, Renoux C, Azoulay L, Xia C, et al. Association between use of sodium-glucose co-transporter-2 inhibitor and the risk of incident dementia: a population-based cohort study. BMJ Open Diabetes Res Care. (2025) 13:e004541. doi: 10.1136/bmjdrc-2024-004541
16. De Giorgi R, Koychev I, Adler AI, Cowen PJ, Harmer CJ, Harrison PJ, et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. EClinicalMedicine. (2024) 74:102726. doi: 10.1016/j.eclinm.2024.102726
17. Hong B, Bea S, Ko HY, Kim WJ, Cho YM, and Shin JY. Sodium-glucose cotransporter-2 inhibitors, dulaglutide, and risk for dementia: A population-based cohort study. Ann Intern Med. (2024) 177:1319–29. doi: 10.7326/M23-3220
18. Hong B, Lee H, Choi A, Kim WJ, Cho YM, Yon DK, et al. Sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase IV inhibitors and risk of dementia among patients with type 2 diabetes and comorbid mental disorders: A population-based cohort study. Diabetes Metab. (2024) 50:101581. doi: 10.1016/j.diabet.2024.101581
19. Mui JV, Li L, Chou OHI, Azfar N, Lee A, Hui J, et al. Comparing sodium-glucose cotransporter 2 inhibitors and dipeptidyl peptidase-4 inhibitors on new-onset depression: a propensity score-matched study in Hong Kong. Acta Diabetol. (2023) 60:917–27. doi: 10.1007/s00592-023-02063-6
20. Shin A, Koo BK, Lee JY, and Kang EH. Risk of dementia after initiation of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in adults aged 40–69 years with type 2 diabetes: population based cohort study. BMJ. (2024) 386:e079475. doi: 10.1136/bmj-2024-079475
21. Wu CY, Iskander C, Wang C, Xiong LY, Shah BR, Edwards JD, et al. Association of sodium-glucose cotransporter 2 inhibitors with time to dementia: A population-based cohort study. Diabetes Care. (2023) 46:297–304. doi: 10.2337/dc22-1705
22. Perna S, Mainardi M, Astrone P, Gozzer C, Biava A, Bacchio R, et al. 12-month effects of incretins versus SGLT2-Inhibitors on cognitive performance and metabolic profile. A randomized clinical trial in the elderly with Type-2 diabetes mellitus. Clin Pharmacol. (2018) 10:141–51. doi: 10.2147/CPAA.S164785
23. Youn YJ, Kim S, Jeong HJ, Ah YM, and Yu YM. Sodium-glucose cotransporter-2 inhibitors and their potential role in dementia onset and cognitive function in patients with diabetes mellitus: a systematic review and meta-analysis. Front Neuroendocrinol. (2024) 73:101131. doi: 10.1016/j.yfrne.2024.101131
24. Gunawan PY, Gunawan PA, and Hariyanto TI. Risk of dementia in patients with diabetes using sodium-glucose transporter 2 inhibitors (SGLT2i): A systematic review, meta-analysis, and meta-regression. Diabetes Ther. (2024) 15:663–75. doi: 10.1007/s13300-024-01538-1
25. Sun M, Wang X, Lu Z, Yang Y, Lv S, Miao M, et al. Comparative study of SGLT2 inhibitors and metformin: Evaluating first-line therapies for dementia prevention in type 2 diabetes. Diabetes Metab. (2025) 51:101655. doi: 10.1016/j.diabet.2025.101655
26. Bader M, Li Y, Tweedie D, Shlobin NA, Bernstein A, Rubovitch V, et al. Neuroprotective effects and treatment potential of incretin mimetics in a murine model of mild traumatic brain injury. Front Cell Dev Biol. (2019) 7:356. doi: 10.3389/fcell.2019.00356
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). (2021) 74:790–9. doi: 10.1016/j.recesp.2021.06.016
28. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
29. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
30. Mikolajewicz N and Komarova SV. Meta-analytic methodology for basic research: A practical guide. Front Physiol. (2019) 10:203. doi: 10.3389/fphys.2019.00203
31. Choi GJ and Kang H. Heterogeneity in meta-analyses: an unavoidable challenge worth exploring. Korean J Anesthesiol. (2025) 78:301–14. doi: 10.4097/kja.25001
32. Li H, Ren J, Li Y, Wu Q, and Wei J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front Endocrinol (Lausanne). (2023) 14:1134025. doi: 10.3389/fendo.2023.1134025
33. Wei Z, Koya J, and Reznik SE. Insulin resistance exacerbates alzheimer disease via multiple mechanisms. Front Neurosci. (2021) 15:687157. doi: 10.3389/fnins.2021.687157
34. Li Y, Liu Y, Liu S, Gao M, Wang W, Chen K, et al. Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal Transduct Target Ther. (2023) 8:152. doi: 10.1038/s41392-023-01400-z
36. Tang H, Shao H, Shaaban CE, Yang K, Brown J, Anton S, et al. Newer glucose-lowering drugs and risk of dementia: A systematic review and meta-analysis of observational studies. J Am Geriatr Soc. (2023) 71:2096–106. doi: 10.1111/jgs.18306
37. Hayden MR, Grant DG, Aroor AR, and DeMarco VG. Empagliflozin ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci. (2019) 9:57. doi: 10.3390/brainsci9030057
38. Hierro-Bujalance C, Infante-Garcia C, Del Marco A, Herrera M, Carranza-Naval MJ, Suarez J, et al. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer's disease and type 2 diabetes. Alzheimers Res Ther. (2020) 12:40. doi: 10.1186/s13195-020-00607-4
39. Yang CC, Chen KH, Yue Y, Cheng BC, Hsu TW, Chiang JY, et al. SGLT2 inhibitor downregulated oxidative stress via activating AMPK pathway for cardiorenal (CR) protection in CR syndrome rodent fed with high protein diet. J Mol Histol. (2024) 55:803–23. doi: 10.1007/s10735-024-10233-1
40. Ceasovschih A, Balta A, Aldeen ES, Bianconi V, Barkas F, Sener YZ, et al. Sodium-glucose cotransporter 2 inhibitors and atherosclerosis. Am J Prev Cardiol. (2025) 23:101061. doi: 10.1016/j.ajpc.2025.101061
41. Scisciola L, Cataldo V, Taktaz F, Fontanella RA, Pesapane A, Ghosh P, et al. Anti-inflammatory role of SGLT2 inhibitors as part of their anti-atherosclerotic activity: Data from basic science and clinical trials. Front Cardiovasc Med. (2022) 9:1008922. doi: 10.3389/fcvm.2022.1008922
42. Tran J, Parekh S, Rockcole J, Wilson D, and Parmar MS. Repurposing antidiabetic drugs for Alzheimer's disease: A review of preclinical and clinical evidence and overcoming challenges. Life Sci. (2024) 355:123001. doi: 10.1016/j.lfs.2024.123001
43. Zhang Y, Liao X, Xu J, Yin J, Li S, Li M, et al. The promising potency of sodium-glucose cotransporter 2 inhibitors in the prevention of and as treatment for cognitive impairment among type 2 diabetes patients. Biomedicines. (2024) 12:2783. doi: 10.3390/biomedicines12122783
44. Ferrari F, Moretti A, and Villa RF. Incretin-based drugs as potential therapy for neurodegenerative diseases: current status and perspectives. Pharmacol Ther. (2022) 239:108277. doi: 10.1016/j.pharmthera.2022.108277
45. Jiang X, Li J, Yao X, Ding H, Gu A, and Zhou Z. Neuroprotective effects of dipeptidyl peptidase 4 inhibitor on Alzheimer's disease: a narrative review. Front Pharmacol. (2024) 15:1361651. doi: 10.3389/fphar.2024.1361651
46. Cheng H, Zhang Z, Zhang B, Zhang W, Wang J, Ni W, et al. Enhancement of impaired olfactory neural activation and cognitive capacity by liraglutide, but not dapagliflozin or acarbose, in patients with type 2 diabetes: a 16-week randomized parallel comparative study. Diabetes Care. (2022) 45:1201–10. doi: 10.2337/dc21-2064
47. Li Q, Jia M, Yan Z, Li Q, Sun F, He C, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J Am Heart Assoc. (2021) 10:e020734. doi: 10.1161/JAHA.120.020734
48. Vadini F, Simeone PG, Boccatonda A, Guagnano MT, Liani R, Tripaldi R, et al. Liraglutide improves memory in obese patients with prediabetes or early type 2 diabetes: a randomized, controlled study. Int J Obes. (2020) 44:1254–63. doi: 10.1038/s41366-020-0535-5
49. Hui EK, Mukadam N, Kohl G, and Livingston G. Effect of diabetes medications on the risk of developing dementia, mild cognitive impairment, or cognitive decline: A systematic review and meta-analysis. J Alzheimers Dis. (2025) 13872877251319054:627–648. doi: 10.1177/13872877251319054
50. Chiang PC, Hsieh CY, and Sung SF. Comparative risk of dementia in diabetic stroke patients prescribed SGLT2 vs. DPP-4 inhibitors: A propensity-matched retrospective cohort study. J Stroke Cerebrovasc Dis. (2025) 34:108276. doi: 10.1016/j.jstrokecerebrovasdis.2025.108276
51. Piatkowska-Chmiel I, Gawronska-Grzywacz M, PawLowski K, Dudka J, Slaska B, Tkaczyk-Wlizlo A, et al. Restoring brain pathways involved in diabetes-associated neurocognitive disorders: the potential of dipeptidyl peptidase 4 inhibitors as a therapeutic strategy. Curr Neuropharmacol. (2025) 23:426–38. doi: 10.2174/1570159X22666240517094428
52. Lei M, Guo X, Yao Y, Shu T, Ren Z, Yang X, et al. Trelagliptin relieved cognitive impairment of diabetes mellitus rats: Involvement of PI3K/Akt/GSK-3β and inflammation pathway. Exp Gerontol. (2023) 182:112307. doi: 10.1016/j.exger.2023.112307
53. Osman ST, Purba W, Daramola O, Amin Bhuiyan MMA, Nwaiwu J, Fowowe M, et al. Positive impact of DPP-4 or SGLT2 inhibitors on mild cognitive impairment in type 2 diabetes patients on metformin therapy: A metabolomic mechanistic insight. Biomedicine Pharmacotherapy. (2025) 182:117771. doi: 10.1016/j.biopha.2024.117771
54. Bhat AA, Moglad E, Goyal A, Afzal M, Thapa R, Almalki WH, et al. Nrf2 pathways in neuroprotection: Alleviating mitochondrial dysfunction and cognitive impairment in aging. Life Sci. (2024) 357:123056. doi: 10.1016/j.lfs.2024.123056
55. Singh P, Barman B, and Thakur MK. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front Aging Neurosci. (2022) 14:944697. doi: 10.3389/fnagi.2022.944697
56. Willmann C, Brockmann K, Wagner R, Kullmann S, Preissl H, Schnauder G, et al. Insulin sensitivity predicts cognitive decline in individuals with prediabetes. BMJ Open Diabetes Res Care. (2020) 8. doi: 10.1136/bmjdrc-2020-001741
57. Yabe D. Harnessing the therapeutic potential of SGLT2 inhibitors in type 2 diabetes: challenges and opportunities. JMA J. (2024) 7:401–2. doi: 10.31662/jmaj.2024-0083
58. Wu Y, Yang Z, and Cao Q. Efficacy and safety of GLP-1 receptor agonists combined with SGLT-2 inhibitors in elderly patients with type 2 diabetes: a meta-analysis. Am J Transl Res. (2024) 16:6852–66. doi: 10.62347/MCEE4840
59. Jeon JY and Kim DJ. Benefit and safety of sodium-glucose co-transporter 2 inhibitors in older patients with type 2 diabetes mellitus. Diabetes Metab J. (2024) 48:837–46. doi: 10.4093/dmj.2024.0317
60. Pawlos A, Broncel M, Wozniak E, and Gorzelak-Pabis P. Neuroprotective effect of SGLT2 inhibitors. Molecules. (2021) 26:7213. doi: 10.3390/molecules26237213
61. Murasheva A, Fuks O, Timkina N, Mikhailova A, Vlasov T, Samochernykh K, et al. SGLT-2 inhibitors' and GLP-1 receptor agonists' Influence on neuronal and glial damage in experimental stroke. Biomedicines. (2024) 12:2797. doi: 10.3390/biomedicines12122797
Keywords: sodium-glucose transporter 2 inhibitors, incretins, type 2 diabetes, dementia, cognitive impairment
Citation: Song K, Choi J, Jeong D, Shin D, Ah Y-M, Lee KY and Choi KH (2025) Comparative effects of SGLT2 inhibitors and incretin-based therapies on dementia risk in type 2 diabetes: a systematic review and meta-analysis. Front. Endocrinol. 16:1695075. doi: 10.3389/fendo.2025.1695075
Received: 02 September 2025; Accepted: 17 October 2025;
Published: 29 October 2025.
Edited by:
Lixin Li, Central Michigan University, United StatesReviewed by:
Desh Deepak Singh, Amity University Jaipur, IndiaAlvin Kuate Defo, Montreal General Hospital, Canada
Copyright © 2025 Song, Choi, Jeong, Shin, Ah, Lee and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung Hee Choi, a2hjaG9pQGdhY2hvbi5hYy5rcg==
†These authors have contributed equally to this work
 Kirim Song
Kirim Song Jiwon Choi
Jiwon Choi Dayeon Jeong
Dayeon Jeong Dongyun Shin
Dongyun Shin Young-Mi Ah
Young-Mi Ah Ki Young Lee
Ki Young Lee Kyung Hee Choi
Kyung Hee Choi