- 1Department of Oncology, Anhui Chest Hospital, Hefei Anhui, China
- 2Anhui Medical University Clinical College of Chest, Hefei Anhui, China
- 3Department of Urology, Anhui Provincal Children’s Hospital, Hefei Anhui, China
Background: This study investigates the relationship between the systemic immune-inflammation index (SII) and all-cause mortality (ACM) risk in individuals with stages IIIB–IV epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma.
Methods: The clinical data of 187 individuals with stages IIIB–IV EGFR-mutated lung adenocarcinoma from Anhui Chest Hospital, collected from June 2017 to December 2023, were retrospectively analyzed. SII was calculated as platelet count × neutrophil count/lymphocyte count. The receiver operating characteristic (ROC) curve was employed to determine the optimal threshold SII, and individuals were classified as low and high SII groups. ACM serves as the primary endpoint. Univariate and multivariate analyses were conducted using Cox proportional hazards models. The robustness of the findings was tested by subgroup and sensitivity analyses.
Results: The ACM risk was notably elevated in the high SII group (p = 0.001) compared with the low SII group. Multivariate Cox analysis demonstrated that SII can independently predict poor prognosis. In the fully adjusted model, compared with the low SII group, the ACM risk was 1.985 times higher in the high SII group (hazard ratio [HR] = 1.985; 95% confidence interval [CI] = 1.216–3.240; p = 0.006). Subgroup analyses showed that SII was more strongly associated with ACM risk in men (HR = 3.245; p = 0.005), and this relationship was also significant among female patients (HR = 2.036; p = 0.048). In individuals aged ≥ 65 years, a high SII was significantly associated with an elevated ACM risk (HR = 2.675; p = 0.004). No such relationship was observed in individuals aged under 65. Sensitivity analyses indicated that high SII remained significantly correlated with elevated ACM risk after excluding individuals with special types of adenocarcinoma, stage III lung adenocarcinoma, or diabetes (all p< 0.05), supporting its potential as an independent prognostic indicator. ROC curve analysis demonstrated that SII had a moderate predictive ability for ACM, with an AUC of 0.669 (95% CI = 0.527–0.812; p = 0.021).
Conclusion: Elevated SII is an independent biomarker for predicting ACM in individuals with stages IIIB–IV EGFR-mutated lung adenocarcinoma, with a stronger predictive value in male and older populations.
1 Introduction
Lung adenocarcinoma is a prevalent malignancy worldwide (1). Due to its high mortality rate and complex biological characteristics, the treatment and prognosis assessment of this disease remain a major focus of clinical research. With rapid advances in immunotherapies and precision medicine, the role of epidermal growth factor receptor (EGFR) mutations in lung adenocarcinoma has become increasingly recognized. Consequently, EGFR has emerged as a key therapeutic target (2). However, substantial differences exist in treatment responses and survival outcomes among patients with stages IIIB–IV EGFR-mutated lung adenocarcinoma. Consequently, more reliable biomarkers are needed to guide treatment decisions and prognostic assessments in clinical practice. Previous studies (3) have shown that inflammation can significantly influence tumor angiogenesis, metastasis, and overall cancer progression. The systemic immune-inflammation index (SII) has emerged as a valuable biomarker for predicting outcomes in various cancer types (4). This indicator can assess the body’s immune-inflammatory status by integrating platelet, neutrophil, and lymphocyte counts (5). Existing research has demonstrated that SII is both sensitive and specific for assessing lung cancer (LC) prognosis. It provides a comprehensive measure of the balance between inflammatory cytokines and immune status. Moreover, it can effectively predict recurrence, metastasis, and overall prognosis in various malignancies, including pancreatic, breast, and bladder cancers (6).
By analyzing the relationship between SII and ACM in patients with stages IIIB–IV EGFR-mutated lung adenocarcinoma, this study aims to provide a foundation for developing a prognostic tool to guide individualized treatment. Evaluating the interplay between SII, patient clinical characteristics, treatment responses, and survival outcomes may offer valuable guidance for clinical management and further support the use of SII as a potential biomarker for assessing prognosis in this population.
2 Materials and methods
2.1 Study population
This study consecutively enrolled patients with stages IIIB–IV EGFR-mutated lung adenocarcinoma who received systemic anticancer therapy at Anhui Chest Hospital between June 2017 and December 2023, aiming to minimize potential selection bias. Cases were identified from the institutional cancer registry and electronic medical records by two independent investigators. Records that were missing or incomplete were excluded from the analysis.
The clinical data of 187 individuals with stages IIIB–IV EGFR-mutated lung adenocarcinoma from Anhui Chest Hospital between 28 June 2017 and 18 December 2023 were retrospectively collected. Inclusion criteria were as follows: (i) lung adenocarcinoma diagnosed cytologically or histologically; (ii) tumor–node–metastasis (TNM) stages IIIB–IV; (iii) EGFR gene mutation. Exclusion criteria included: (i) presence of other cancers; (ii) severe infection, autoimmune diseases, or other diseases affecting inflammatory markers; (iii) use of medications that might influence SII (such as corticosteroids and non-steroidal anti-inflammatory drugs); (iv) missing SII data prior to chemotherapy or targeted therapy; (v) incomplete follow-up data. Approval from the Ethics Committee of Anhui Chest Hospital was obtained. Given that the data used were anonymized, informed consent was waived.
Patients with histologically or cytologically confirmed lung adenocarcinoma harboring EGFR mutations (stages IIIB–IV) were consecutively enrolled. EGFR mutation status was determined by polymerase chain reaction/amplification refractory mutation system (PCR/ARMS) or next-generation sequencing (NGS) performed in our hospital or reported from external certified laboratories. Any primary EGFR mutation identified (including common sensitizing mutations such as exon 19 deletion and L858R, as well as rare or complex variants) was categorized as EGFR-mutated. However, detailed mutation subtype data were incomplete for some patients; therefore, subgroup analyses by mutation subtype (e.g., exon 19 deletion, L858R, T790M, or others) were not conducted.
2.2 Data collection and definitions
Clinical data of eligible individuals were derived from their admission records and electronic medical records. Collected data included disease-related information, demographic characteristics, lifestyle factors, medical history, physical measurements, and laboratory test results. Demographics included gender (woman or man) and age (at the time of diagnosis). Disease-related information were pathological type (adenocarcinoma [ADC] or special types of ADC), clinical stage (stage III or IV), metastatic sites (intrapulmonary metastasis only or extrapulmonary metastasis included), treatment type (targeted therapy alone or combination therapy), treatment response (partial response or stable or progressive disease based on response evaluation criteria in solid tumors, version 1.1 [RECIST 1.1]), and disease progression (categorized as progressed or not according to follow-up data).
In terms of lifestyle factors, smoking history was defined as a history of sustained smoking, regardless of current smoking status. A family history was determined as the presence of LC or other cancers in first-degree relatives. Comorbid conditions, including hypertension (HP) (according to previous diagnosis or current use of antihypertensive medications), chronic obstructive pulmonary disease (COPD) (according to established diagnosis or pulmonary function tests), diabetes mellitus (DM) (according to clinical diagnosis or current use of antidiabetic medications), and tuberculosis (based on prior diagnosis and antituberculosis treatment), were recorded as present or absent.
Body measurements included weight and height. The body mass index (BMI) was calculated as weight/height². Moreover, diastolic blood pressure (DBP) and systolic blood pressure (SBP) were documented in millimeters of mercury (mmHg) at admission.
Laboratory test parameters encompassed (i) blood cell counts, including neutrophils, white blood cells (WBC), lymphocytes, platelets, and monocytes; (ii) metabolic indicators, including fasting blood glucose (FBG), total cholesterol (TC), uric acid (UA), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C); (iii) renal function indicators, such as blood urea nitrogen (BUN) and serum creatinine (Cr); (iv) electrolytes, such as serum calcium (SC), serum sodium (SS), and serum potassium (SP); (v) hepatic function indicators, encompassing aspartate aminotransferase (AST), total bilirubin (TBil), and alanine aminotransferase (ALT).
Two researchers separately extracted and cross-checked the data to ensure accuracy and consistency. Categorical variables were defined and classified according to standardized criteria to ensure the scientific validity and reproducibility of findings.
2.3 SII definition
The SII, an emerging immune-inflammatory marker, reflects the intensity of both inflammatory status and immune response. It was calculated as platelet count × neutrophil count/lymphocyte count (unit: × 109/L). The first blood test result before systemic anti-cancer treatment was used to calculate the SII value. Given the right-skewed distribution of the original SII data, log transformation (Log10SII) was applied in the Cox proportional hazards (CPH) models to improve normality and enhance model stability.
In this study, SII was analyzed both as a categorical variable—by dividing individuals into low- and high-SII groups based on the optimal cutoff value (411.29)—for survival comparison, and as a continuous variable (SII or log10SII) in the Cox proportional hazards (CPH) models to comprehensively explore its association with all-cause mortality (ACM).
Categorization based on a cutoff value enhances clinical interpretability and facilitates risk stratification. The optimal cutoff value of SII (411.29) was determined using receiver operating characteristic (ROC) curve analysis combined with Youden’s index, which identifies the point achieving the best balance between sensitivity and specificity. This objective and data-driven method reflects the most effective threshold for distinguishing survival risk within our cohort.
Moreover, the use of ROC-based cutoff determination for SII is consistent with previous studies. For instance, Deng et al. (7) and Tong et al. (8) applied similar ROC approaches in patients with non-small cell lung cancer (NSCLC) and reported cutoff values ranging from approximately 400 to 660. The cutoff value identified in our study falls within this range, demonstrating methodological consistency and external comparability.
2.4 Follow-up
This study adopted a retrospective cohort design with long-term follow-up through multiple channels, including electronic medical records, discharge summaries, outpatient follow-ups, and telephone interviews. Follow-up began on the date the patient first received systemic anticancer treatment (such as targeted therapy or combination therapy), and ended on 1 January 2025, or at the time of death. The primary endpoint was ACM. All death events were verified by two researchers based on hospital records, death certificates, or reports from family members to maintain the accuracy of the endpoint.
2.5 Statistical analysis
SPSS 26.0 software was employed to carry out data analysis. Baseline characteristics were first summarized using descriptive statistics. Continuous variables that were normally distributed were represented by mean ± standard deviation and compared between groups using independent-samples t-tests. Nonnormally distributed continuous variables were presented as median (interquartile range) and compared using the Mann–Whitney U test. Categorical variables were reported as frequencies (percentages) and analyzed using either the χ² test or Fisher’s exact test, as appropriate.
The ROC curve was performed to determine the optimal cutoff value of the SII, based on which patients were classified into low and high SII groups. Kaplan–Meier (KM) survival curves for ACM were constructed for both groups, and the log-rank test was used to compare survival distributions. Univariate and multivariate CPH models were employed to examine the association between SII and ACM. Potential confounding factors, including demographics, comorbidities, and laboratory markers, were progressively included in three adjusted models. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated to quantify associations.
Subgroup analyses stratified by age and sex, along with sensitivity analyses excluding specific patient groups, were carried out to examine the robustness of the results. A two-sided p-value below 0.05 was considered statistically significant. ROC curve analysis was conducted to evaluate the discriminative ability of the SII for predicting ACM.
To ensure precision, HRs and 95% confidence intervals were reported to three decimal places. The multivariate Cox proportional hazards model was adjusted for variables significant in univariate analyses (p < 0.1), including diabetes, pathological type, fasting glucose, WBC, and serum calcium.
3 Results
3.1 Baseline characteristics according to SII cutoff value
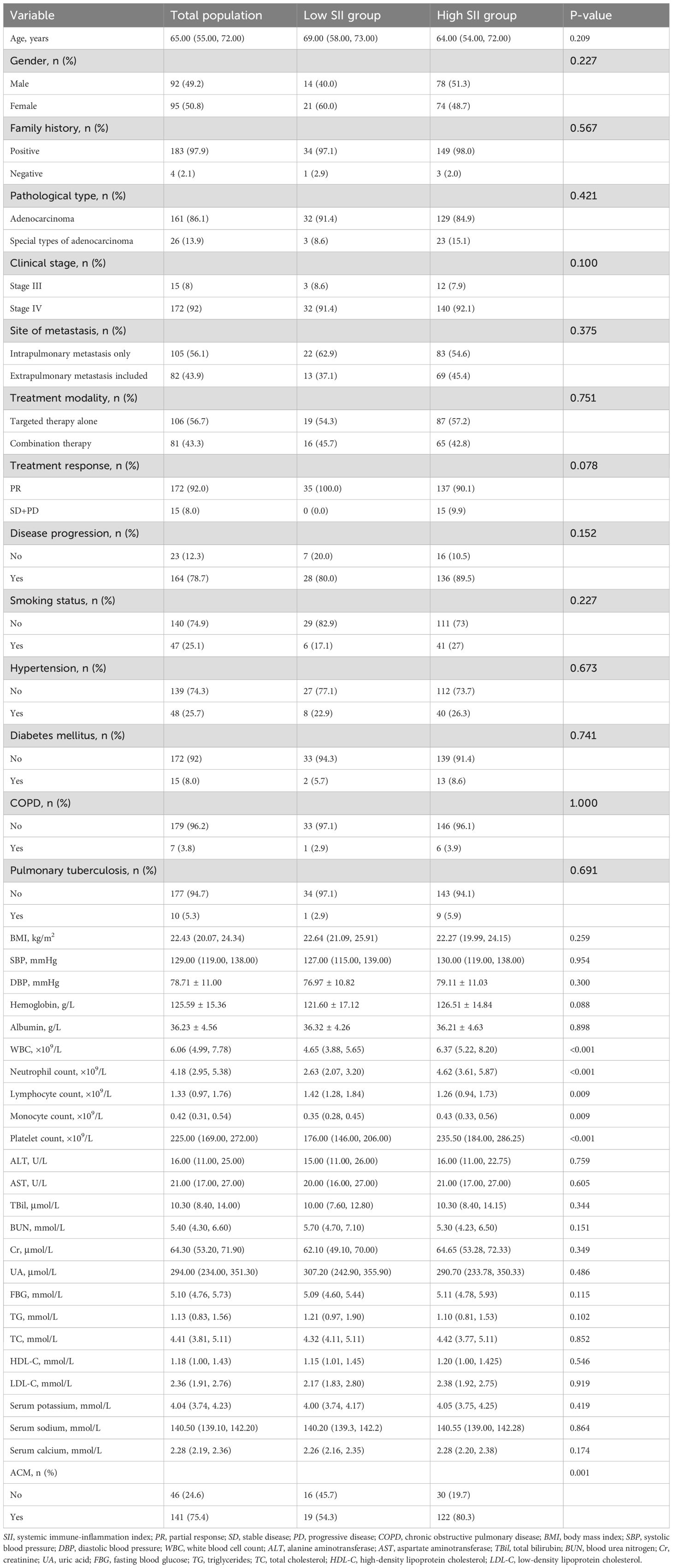
As illustrated in Table 1, compared with the low SII group, a notably greater ACM risk (p = 0.001), elevated levels of platelet count (p < 0.001), neutrophil count (p < 0.001), WBC (p < 0.001), monocyte count (p = 0.009), and lower level of lymphocyte count (p = 0.009) was found in the high SII group. However, there was no substantial differences between the two groups regarding clinical stage, gender, metastasis site, treatment modality, pathological type, family history, age, treatment response, disease progression, smoking status, HP, DM, COPD, pulmonary tuberculosis (PTB), BMI, SBP, DBP, hemoglobin, BUN, albumin, ALT, HDL-C, AST, TBil, UA, TC, TG, Cr, LDL-C, FBG, SP, SS, and SC (p > 0.05).
Additionally, the cumulative ACM risk was significantly higher in the high SII group in comparison with the low SII group. KM survival curves demonstrated a persistent notable difference between the two groups from the early stages of follow-up (log-rank p = 0.003) (Figure 1).
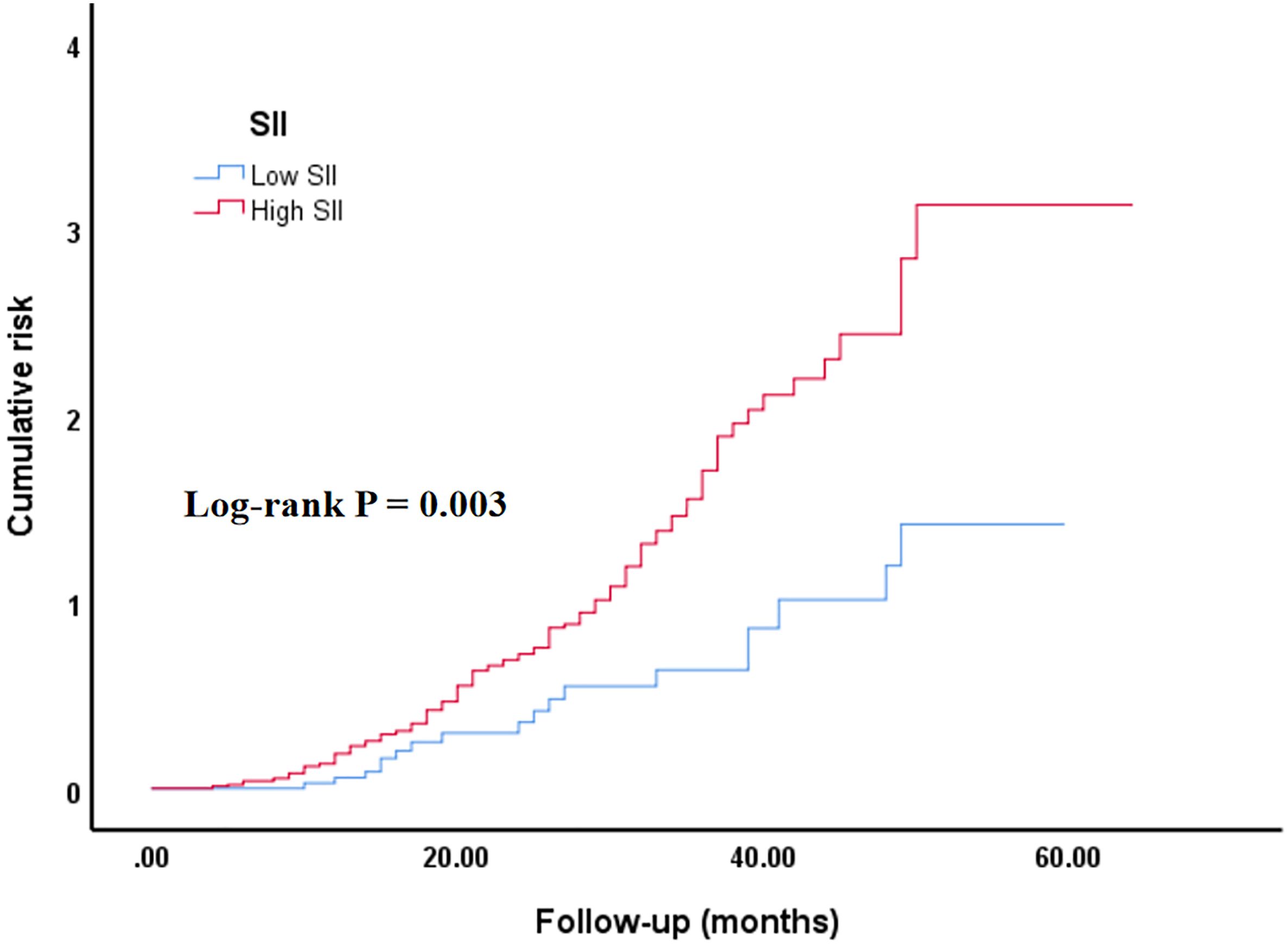
Figure 1. Kaplan–Meier curve of the cumulative risk of ACM stratified by SII level. Low SII group: SII ≤ 411.29; high SII group (SII > 411.29). SII, systemic immune-inflammation index. The cumulative risk represents the estimated probability of ACM over time, derived from the KM survival analysis.
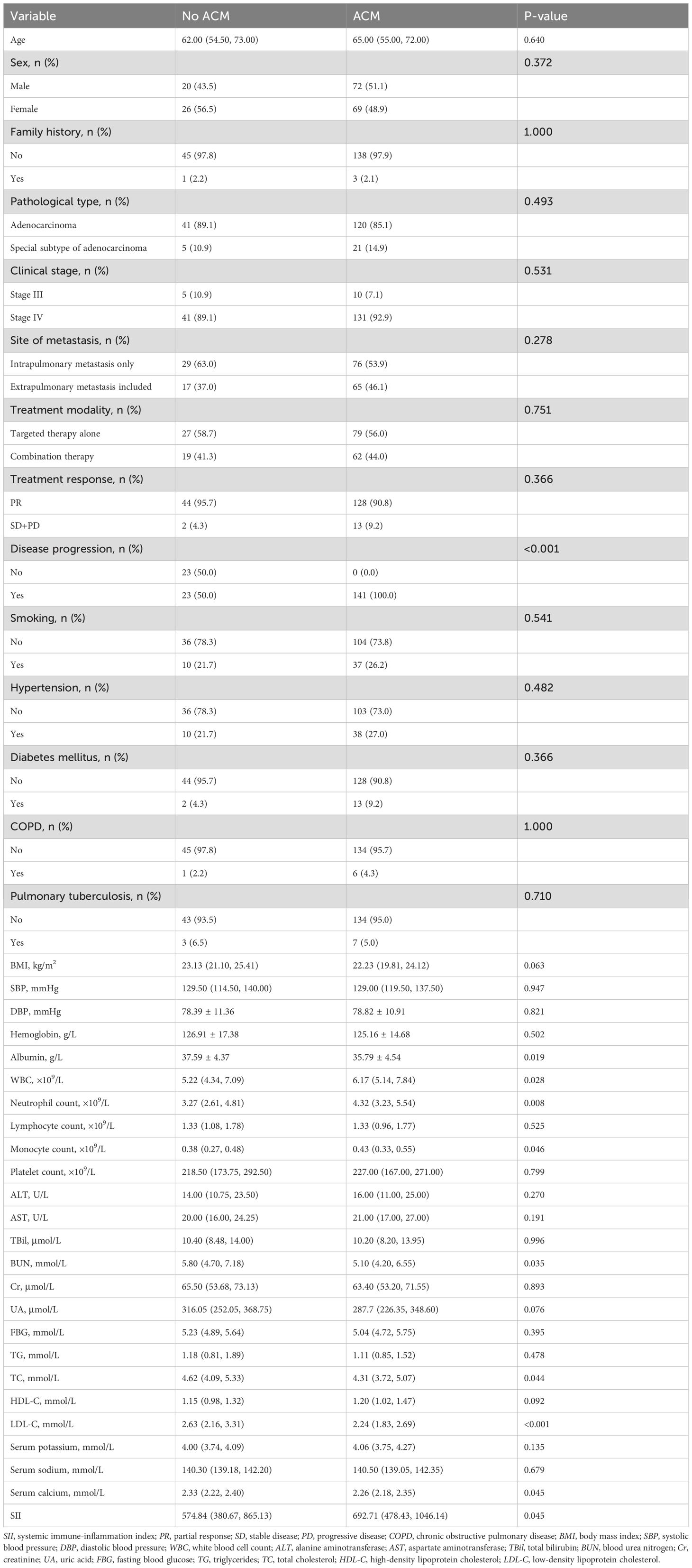
3.2 Baseline characteristics according to ACM
As illustrated in Table 2, compared with the non-ACM group, the ACM group had notably reduced levels of LDL-C (p < 0.001), BUN (p = 0.035), TC (p = 0.044), albumin (p = 0.019), SC (p = 0.045), and elevated levels of disease progression (p < 0.001), WBC (p = 0.028), neutrophil count (p = 0.008), and monocyte count (p = 0.046). Additionally, no substantial differences between the two groups were found in clinical stage, site of metastasis, treatment modality, family history, treatment response, age, smoking status, pathological type, HP, DM, COPD, PTB, BMI, SBP, DBP, hemoglobin, lymphocyte count, platelet count, HDL-C, Cr, ALT, FBG, AST, TBil, UA, TG, SP, SS, and SII (p > 0.05).
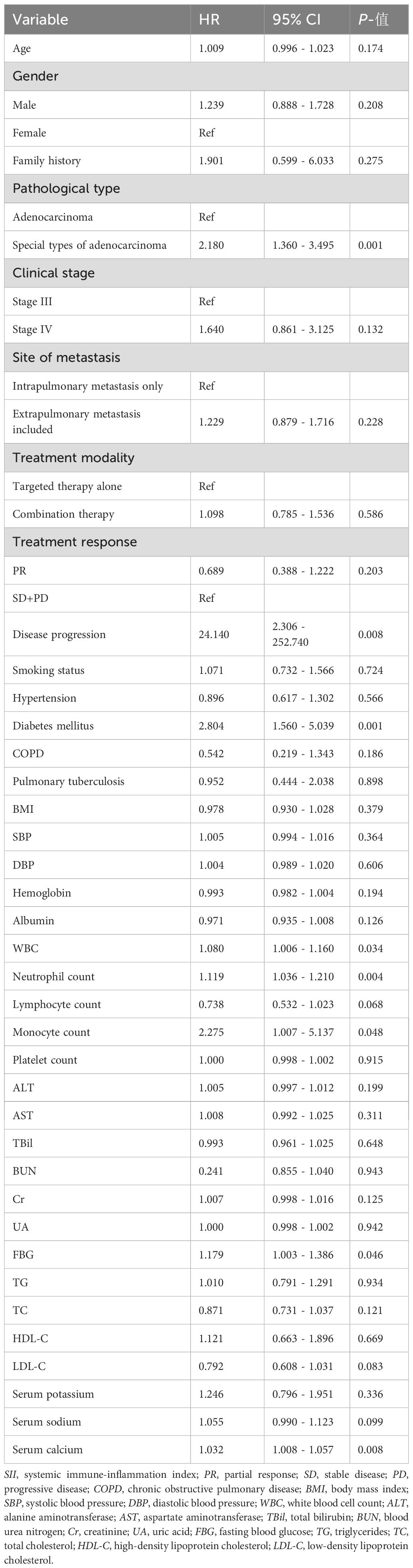
3.3 Univariate Cox analysis of ACM
As illustrated in Table 3, univariate Cox analysis demonstrated that DM, the special types of ADC, WBC, neutrophil count, monocyte count, FBG, and SC were notably linked to ACM (p < 0.05). In contrast, other variables were not significantly related to ACM (≥ 0.05).
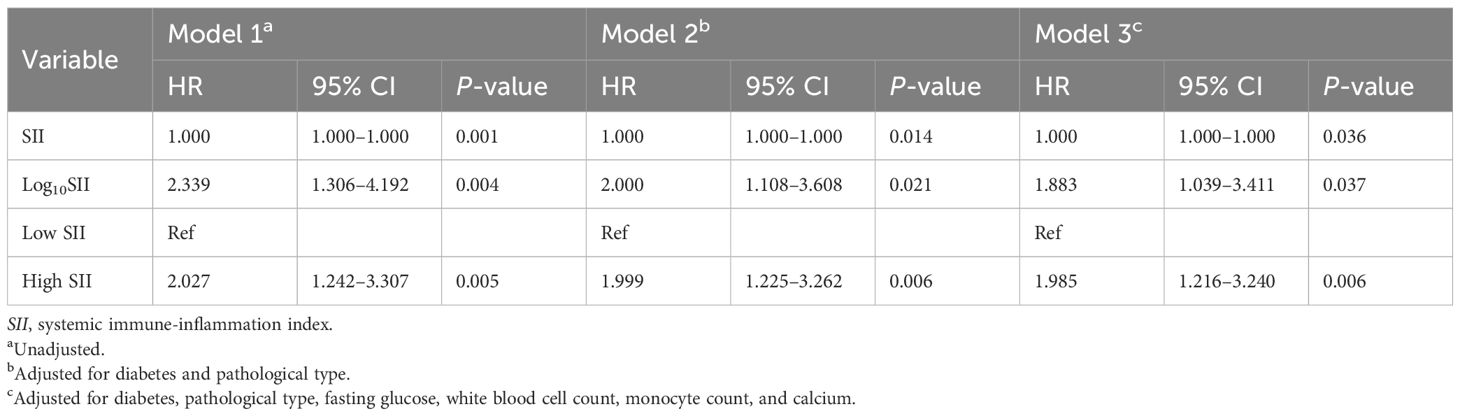
3.4 Multivariate Cox regression analysis of SII and ACM
As illustrated in Table 4, SII, analyzed both as a categorical and continuous variable, was notably associated with ACM in the unadjusted model 1. Compared with the low SII group, a 2.027-fold increase in ACM risk was found in the high SII group (HR = 2.027; 95% CI = 1.242–3.307; p = 0.005). In model 2, which controlled for DM and pathological type, a 1.999-fold elevated ACM risk was observed in the high SII group (HR = 1.999; 95% CI = 1.225–3.262; p = 0.006) in contrast to the low SII group. Furthermore, a higher SII remained correlated with an elevated ACM risk in model 3, which controlled for DM, pathological type, FBG, WBC, neutrophil count, and SC. In this model, in contrast to the low SII group, a 1.985-fold greater ACM risk was found in the high SII group (HR = 1.985; 95% CI = 1.216–3.240; p = 0.006). These findings suggested that SII may act as an independent biomarker for assessing unfavorable prognosis.
3.5 Subgroup analysis
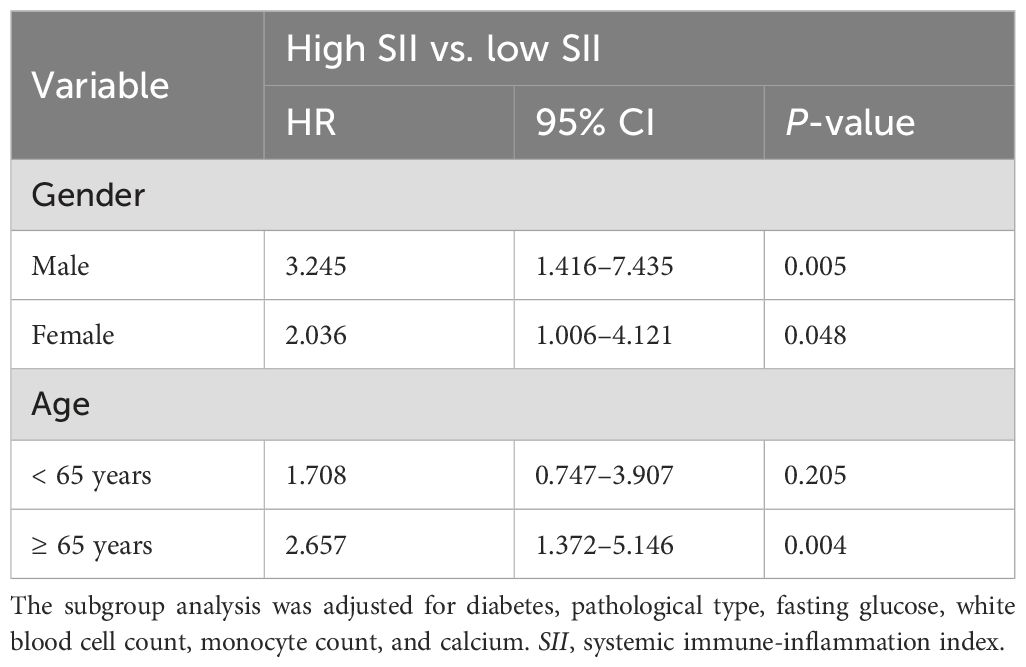
As illustrated in Table 5, multivariate Cox analysis suggested that an elevated SII was associated with a higher ACM risk among both women and men. The association was more pronounced in the male subgroup (HR = 3.245; 95% CI = 1.416–7.435; p = 0.005). Compared with the low SII group, the ACM risk in the high SII group was 2.036 times higher in the female subgroup (HR = 2.036; 95% CI = 1.006–4.121; p = 0.048). In the age subgroup, a high SII was notably associated with an elevated ACM risk among older patients aged above 65 (HR = 2.675; 95% CI = 1.372–5.146; p = 0.004), indicating that SII may be a stronger indicator for predicting mortality risk in older populations.
3.6 Sensitivity analysis
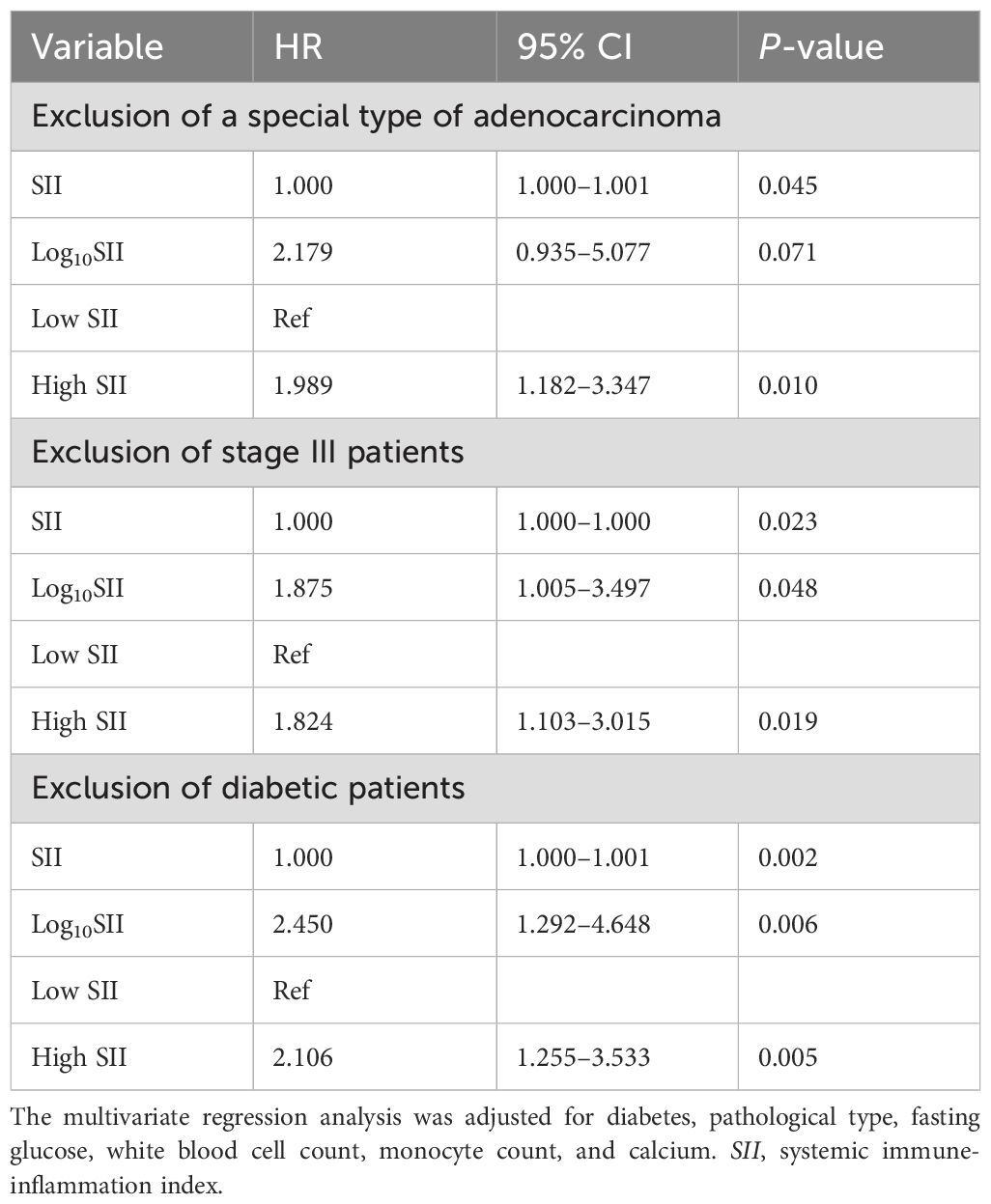
As illustrated in Table 6, after excluding individuals with special types of ADC, a higher SII was still associated with an elevated ACM risk. Compared with the low SII group, a 1.989-fold increase in ACM risk was observed in the high SII group (HR = 1.989; 95% CI = 1.182–3.347; p = 0.010). After excluding stage III LC patients, SII—whether analyzed as a categorical or continuous variable—remained notably associated with ACM, with the high SII group having a 1.824-fold increased ACM risk (95% CI = 1.103–3.015; p = 0.019) compared with the low SII group. After excluding diabetic patients, a higher SII was notably linked to an elevated ACM risk, with the high SII group having a 2.106-fold greater ACM risk (95% CI = 1.255–3.533; p = 0.005) compared with the low SII group. These sensitivity analyses indicated that the association between SII and ACM remained significant after excluding individuals with specific types of ADC, stage III disease, or DM. These results confirm the robustness of SII as an independent indicator for predicting ACM.
3.7 Prognostic value of SII for all-cause mortality
As shown in Figure 2, ROC curve analysis was performed to evaluate the ability of SII to predict ACM in patients with stages IIIB–IV EGFR-mutated lung adenocarcinoma. The area under the curve (AUC) was 0.669 (95% CI = 0.527–0.812; p = 0.021), indicating a modest but statistically significant predictive value.
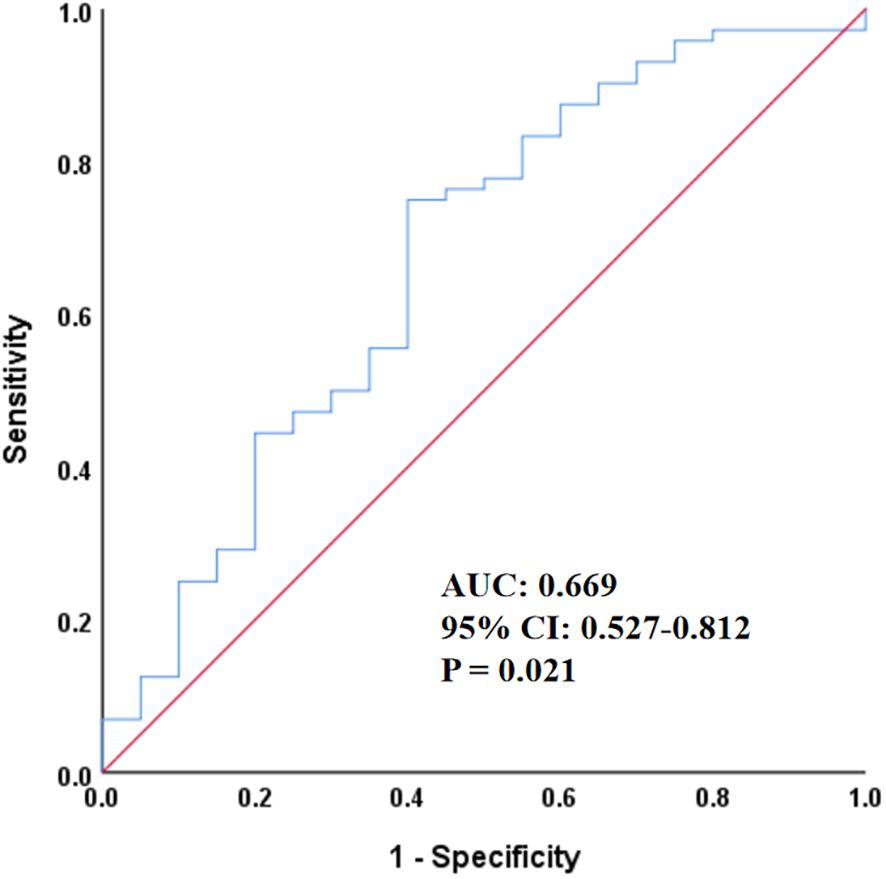
Figure 2. Receiver operating characteristic curve of SII for predicting all-cause mortality. SII, systemic immune-inflammation index; AUC, area under the curve; CI, confidence interval.
4 Discussion
Lung cancer ranks first in both incidence and mortality worldwide, and adenocarcinoma is its most common histological subtype, characterized by complex biological features. The early symptoms of lung adenocarcinoma are typically unnoticeable, so a majority of individuals are diagnosed only after clinical signs appear, usually at an advanced stage. As a result, these patients often have poor overall survival (OS) outcomes. Therefore, exploring the factors influencing lung adenocarcinoma prognosis holds great clinical significance.
Increasing evidence has revealed that inflammation plays a crucial role in the tumor biology of EGFR-mutated NSCLC. Chronic inflammatory signaling can promote tumor initiation, clonal evolution, and therapeutic resistance through multiple mechanisms. EGFR activation has been shown to interact with inflammatory pathways such as NF-κB and IL-6/STAT3, leading to enhanced tumor proliferation, angiogenesis, and an immunosuppressive microenvironment (9, 10). These interactions create a feed-forward loop in which EGFR signaling induces the release of proinflammatory cytokines, while inflammation, in turn, sustains EGFR pathway activation and tumor progression.
SII, which consists of lymphocyte, platelet, and neutrophil counts, can comprehensively and objectively assess the dynamic balance between immune status and inflammatory response (11). Hu et al. (12) first proposed the concept of SII and found that a higher SII in individuals with liver cancer was notably associated with an elevated risk of recurrence. Existing evidence has demonstrated that SII is linked to the prognosis of multiple solid tumors, including pancreatic cancer, esophageal cancer, and gastric cancer (13–15). Moreover, systemic inflammatory indices such as the SII reflect the dynamic balance between host immunity and tumor-related inflammation. Elevated SII indicates increased neutrophil- and platelet-driven inflammatory activity, along with suppressed lymphocyte-mediated immune surveillance, which may further enhance the aggressiveness of EGFR-mutant tumors (8). Recent studies suggest that chronic inflammation contributes to epithelial–mesenchymal transition (EMT), resistance to EGFR tyrosine kinase inhibitors (EGFR-TKIs), and metastatic potential in EGFR-mutant NSCLC (16). Berardi et al. (17) found that individuals with advanced NSCLC and high pretreatment SII had significantly shorter OS following first-line chemotherapy or targeted therapy. Their finding suggests that SII can independently predict unfavorable outcomes. Our results align with those of Berardi et al. (17), indicating that SII is notably related to ACM, whether treated as a continuous or categorical variable. Biswas et al. (18) have demonstrated that SII is a critical marker for predicting OS and progression-free survival (PFS) among stage III locally advanced NSCLC patients. Other researchers (19) suggested that individuals with stage IIIA/N2 NSCLC and elevated SII before treatment may require more intensive adjuvant therapies. Moreover, a recent study (20) identified SII as the strongest predictor of pulmonary complications following LC resection. Tong et al. (8) demonstrated that high SII is associated with chemoresistance among individuals with advanced NSCLC undergoing first-line platinum-based chemotherapy. Patients with SII ≥ 660 tend to have lower response rates to chemotherapy and more advanced clinical stages than those with SII< 660. These findings indicate that chemoresistance remains a major challenge in cancer treatment. SII ≥ 660 can predict poor prognosis in advanced NSCLC, and pretreatment SII is an independent indicator for predicting OS, outperforming other peripheral blood biomarkers. Therefore, pretreatment SII may help predict patients’ responsiveness to chemotherapy. A retrospective study (7) examined the performance of the blood-based SII in 203 EGFR-mutant advanced lung ADC patients who received first-line EGFR-TKIs. Their results indicated that individuals with SII ≥ 1,066.935 have a greater likelihood of poor Eastern Cooperative Oncology Group performance status (ECOG-PS). In their multivariate analysis, SII was an independent biomarker for assessing OS (HR = 2.802; 95% CI = 1.659–4.733; p < 0.001) and PFS (HR = 2.577; 95% CI = 1.677–3.958; p < 0.001). Their findings suggest that SII is notably related to prognosis in EGFR-mutant lung ADC patients receiving first-line EGFR-TKIs, with higher SII indicating greater tumor burden, more profound immunosuppression, and poorer outcomes.
The specific mechanisms by which elevated SII leads to lower survival in cancer patients remain unestablished. It is generally believed that higher SII reflects a decline in lymphocyte count and an increase in platelet and neutrophil counts. Neutrophils induce DNA damage, promote angiogenesis, and activate endothelial and stromal cells by secreting a series of proangiogenic factors and proinflammatory molecules. These processes can enhance tumor cell invasion and metastatic potential, ultimately leading to distant metastasis (21). Platelets may impact tumor progression and metastasis by secreting platelet-derived growth factor, transforming growth factor beta, and vascular endothelial growth factor (22). As major effector cells of the immune system, lymphocytes are essential for suppressing tumor cell invasion, proliferation, and migration, and are critical for tumor immune surveillance (23). Impaired lymphocyte function or reduced lymphocyte counts can compromise immune surveillance, thereby weakening tumor control and ultimately resulting in a worse prognosis. Therefore, a high SII reveals an enhanced inflammatory response and a weakened immune defense, suggesting a poorer clinical outcome.
However, some limitations should be acknowledged. Firstly, selection bias may be present, and the generalizability of the findings requires further validation, as this is a single-center retrospective study with a small sample size. Secondly, because the data were derived from real-world clinical records, some potential confounders—including nutritional status and unmeasured infections affecting inflammatory markers—are not fully accounted for. Due to incomplete information on resistance mechanisms for some patients, treatment-related variables—including the line of EGFR-TKI therapy (first, second, or third line), mutation subtype (e.g., exon 19 deletion, L858R, T790M), and the presence of acquired resistance mutations upon disease progression—were not analyzed in the subgroup models. Thirdly, as SII was assessed only at baseline, longitudinal changes and dynamic monitoring were not available, which may limit temporal interpretation. External validation using independent cohorts is required to confirm generalizability. Future prospective multicenter studies integrating serial SII measurements and molecular biomarkers are warranted. Additionally, since this study was retrospectively designed, ACM was defined as death events that occurred by the end of follow-up (1 January 2025). It was not feasible to restrict ACM to specific time intervals (e.g., 2-, 3-, or 5-year postdiagnosis) because many patients had been discharged for more than 5 years when data collection began. This temporal limitation arises inherently from the retrospective nature of the study. Future prospective cohort studies with planned follow-up at fixed time points after diagnosis (such as 2, 3, and 5 years) are warranted to provide more granular prognostic evidence and to minimize recall bias.
Future larger prospective studies conducted across multiple centers are required to further explore its prognostic value. Although subject to certain limitations, this study revealed the value of SII in independently predicting stages IIIB–IV EGFR-mutated lung adenocarcinoma. In clinical practice, weighing the cost–benefit ratio is a critical factor in formulating cancer treatment plans. Notably, SII, derived from routine laboratory parameters, is a readily accessible and cost-effective marker that may assist in guiding clinical decision-making. It holds potential as a valuable indicator for assessing prognosis in clinical practice.
5 Conclusion
Our findings suggest that SII is associated with all-cause mortality in patients with stages IIIB–IV EGFR-mutated lung adenocarcinoma, regardless of mutation subtype, indicating the potential of SII as a prognostic blood biomarker. It could provide valuable references for risk assessment and individualized treatment planning. Larger prospective studies conducted across multiple centers are required to verify the value of SII and to develop more accurate prognostic models incorporating dynamic monitoring and multiomics indicators, thereby enhancing risk stratification and guiding clinical decision-making in LC management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Approval from the Ethics Committee of Anhui Chest Hospital was obtained, Ethical Ship is KJ2025-020. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CZ: Data curation, Writing – original draft, Project administration, Conceptualization, Visualization, Writing – review & editing, Funding acquisition, Validation, Software, Methodology, Resources. CY: Writing – review & editing, Supervision, Writing – original draft, Investigation, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the 2024 Anhui Provincial Health Research Project (Grant No. AHWJ2024BAg30003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, and Jemal A. Cancer statistics, 2023. Ca-Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, and Bharat A. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Canc Ne. (2023) 21:340–50. doi: 10.6004/jnccn.2023.0020
3. Galdiero MR, Marone G, and Mantovani A. Cancer inflammation and cytokines. Cold Spring Harb Perspect Biol. (2018) 10(8):a028662. doi: 10.1101/cshperspect.a028662
4. Greten FR and Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
5. Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic immune-inflammation index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. (2020) 24:610–8. doi: 10.1007/s11605-019-04187-z
6. Wang P, Yang W, Guo H, Dong HP, Guo YY, Gan H, et al. IL-36γ and IL-36Ra reciprocally regulate NSCLC progression by modulating GSH homeostasis and oxidative stress-induced cell death. Adv Sci (Weinh). (2021) 8:e2101501. doi: 10.1002/advs.202101501
7. Deng C, Zhang N, Wang Y, Jiang S, Lu M, Huang Y, et al. High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine. (2019) 98:e16875. doi: 10.1097/MD.0000000000016875
8. Tong YS, Tan J, Zhou XL, Song YQ, and Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. (2017) 15:221. doi: 10.1186/s12967-017-1326-1
9. Taniguchi K and Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. (2018) 18:309–24. doi: 10.1038/nri.2017.142
10. Lou W, Chen Y, Zhu KY, Deng H, Wu T, and Wang J. Polyphyllin I overcomes EMT-associated resistance to erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition. Biol Pharm Bull. (2017) 40:1306–13. doi: 10.1248/bpb.b17-00271
11. Zhang Y, Chen B, Wang L, Wang R, and Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine. (2019) 98:e13788. doi: 10.1097/MD.0000000000013788
12. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
13. Zhu Z, Cong X, Li R, Yin X, Li C, and Xue Y. Preoperative systemic immune-inflammation index (SII) for predicting the survival of patients with stage I-III gastric cancer with a signet-ring cell (SRC) component. BioMed Res Int. (2020) 2020:5038217. doi: 10.1155/2020/5038217
14. Murthy P, Zenati MS, Al Abbas AI, Rieser CJ, Bahary N, Lotze MT, et al. Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Ann Surg Oncol. (2020) 27:898–906. doi: 10.1245/s10434-019-08094-0
15. Gao Y, Guo W, Cai S, Zhang F, Shao F, Zhang G, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. (2019) 10:3188–96. doi: 10.7150/jca.30281
16. Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, et al. Acquired resistance to EGFR inhibitors is associated with a manifestation of stem cell-like properties in cancer cells. Cancer Res. (2013) 73:3051–61. doi: 10.1158/0008-5472.CAN-12-4136
17. Berardi R, Santoni M, Rinaldi S, Bower M, Tiberi M, Morgese F, et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Ann Transl Med. (2019) 7:572. doi: 10.21037/atm.2019.09.18
18. Biswas T, Kang KH, Gawdi R, Bajor D, Machtay M, Jindal C, et al. Using the systemic immune-inflammation index (SII) as a mid-treatment marker for survival among patients with stage-III locally advanced non-small cell lung cancer (NSCLC). Int J Environ Res Public Health. (2020) 17(21):7995. doi: 10.3390/ijerph17217995
19. Yang H, Wang K, Li B, Li S, Li Y, and Yuan L. The prognostic role of blood inflammatory biomarkers and EGFR mutation status in stage IIIA/N2 non-small cell lung cancer patients treated with trimodality therapy. Front Oncol. (2021) 11:707041. doi: 10.3389/fonc.2021.707041
20. Mao X, Zhang W, Wang Q, Yiqian N, Niu Y, and Jiang L. Assessment of systemic immune-inflammation index in predicting postoperative pulmonary complications in patients undergoing lung cancer resection. Surgery. (2022) 172:365–70. doi: 10.1016/j.surg.2021.12.023
21. Powell DR and Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. (2016) 37:41–52. doi: 10.1016/j.it.2015.11.008
22. Li T, Guo T, Liu H, Jiang H, and Wang Y. Platelet−derived growth factor−BB mediates pancreatic cancer Malignancy via regulation of the Hippo/Yes−associated protein signaling pathway. Oncol Rep. (2020) 45:83–94. doi: 10.3892/or.2020.7859
Keywords: lung adenocarcinoma, systemic immune-inflammation index, epidermal growth factor receptor mutation, prognosis, all-cause mortality
Citation: Zhang C and Yang C (2025) Relationship between systemic immune-inflammation index and all-cause mortality in stages IIIB–IV epidermal growth factor receptor-mutated lung adenocarcinoma. Front. Endocrinol. 16:1698317. doi: 10.3389/fendo.2025.1698317
Received: 05 September 2025; Accepted: 23 October 2025;
Published: 05 November 2025.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Genlin Lu, Longyou People’s Hospital, ChinaÖkkeş Zortuk, TC Saglik Bakanligi Bandirma Egitim ve Arastirma Hastanesi, Türkiye
Copyright © 2025 Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi Zhang, aGFrdV9uYW1hX3RhdGFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share authorship
 Chi Zhang
Chi Zhang Chao Yang
Chao Yang