- 1Department of Public Health Sciences, Penn State College of Medicine, Hershey, PA, United States
- 2Division of Gastroenterology, Hepatology, and Nutrition, College of Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 3Department of Nutrition and Food Hygiene, School of Public Health, Institute of Nutrition, Fudan University, Shanghai, China
- 4Department of Medicine, Division of Endocrinology, Penn State College of Medicine, United States, Hershey, PA, United States
Introduction: Acute pancreatitis (AP) is an inflammatory disease of the exocrine pancreas characterized by tissue damage and sometimes necrosis. However, whether severe AP is associated with an increased risk of incident diabetes remains unclear based on real-world data. This study aims to examine the relationship between severe AP and new-onset diabetes after hospitalization.
Methods: We conducted a retrospective cohort study using the Merative™ MarketScan® claims database (2016–2023), identifying patients with AP and no prior history of diabetes at baseline. The exposure, severe AP, was defined by any of the following during hospitalization: pancreatic necrosis, hemodialysis, organ failure, or mechanical ventilation. We used multivariable stratified Cox proportional hazards regression models with propensity score strata to assess the association between severe AP and incident diabetes.
Results: The matched study population consisted of 2,046 patients with severe AP and 2,046 patients with mild AP, with baseline characteristics well balanced between groups. Individuals with severe AP had a higher risk of developing diabetes compared with those with mild AP [adjusted hazard ratio (aHR) = 1.64, 95% confidence interval (CI): 1.30–2.06], after accounting for propensity score matching. The association between severe AP and incident diabetes was stronger in men (aHR = 2.03, 95% CI: 1.50–2.74) than in women (aHR = 1.06, 95% CI: 0.69–1.64; P-interaction = 0.02).
Discussion: In this large real-world data study, severe AP was associated with an increased risk of developing diabetes. These findings underscore the importance for glycemic surveillance and the need to consider proactive management of severe AP patients to mitigate their risk of poor health outcomes.
Introduction
Acute pancreatitis (AP) is an inflammatory disease of the exocrine pancreas characterized by tissue damage and sometimes necrosis (1). AP is one of the most common gastrointestinal diseases requiring hospitalization in the United States (US) (2, 3). AP and diabetes often share a bidirectional relationship (4). Pancreatogenic diabetes (or type 3c diabetes) is an underdiagnosed form of secondary diabetes that occurs in the setting of a disease of the exocrine pancreas; AP is likely the most common cause (5, 6). It is likely the most common complication following an episode of AP and may develop more often than previously recognized, with a cumulative incidence ranging from 23% to 40% (7). Stress hyperglycemia is a common early feature in patients with AP. While the likelihood of persistent or worsening hyperglycemia is not well understood, in many cases, it is not a transient phenomenon (8), as patients with AP are at a higher risk of developing diabetes than those without AP. However, it remains unclear whether the severity of AP during hospitalization influences this risk. The revised Atlanta classification of AP is a clinically based categorization of AP severity into three degrees (mild, moderately severe, and severe), with severe AP being defined mainly by the presence of persistent systemic organ failure that is often accompanied by pancreatic necrosis (9). Previous research studies have frequently combined all cases of AP, regardless of severity during hospitalization, and their long-term influence on diabetes risk following discharge (10, 11).
Several epidemiological studies have reported the incidence of diabetes following an episode of AP. Two recent meta-analyses comprising 31 cohort studies indicated that approximately 23% of patients developed diabetes within 3 years following hospital discharge for AP (10, 11). However, many prior studies have been limited by small sample sizes, short follow-up, and lack of adjustment for confounders. Leveraging a large, real-world US claims database with longitudinal follow-up, the objective of this study was to investigate the association between severe AP during hospitalization and the risk and timing of new-onset diabetes following discharge. We hypothesized that, even after rigorous propensity score matching and adjustment for confounding variables, patients with severe AP would exhibit a significantly higher incidence and earlier onset of diabetes compared to those with mild AP. This study aims to provide more definitive evidence on this association by addressing key methodological limitations in the existing literature.
Methods
Study design
A retrospective cohort study with propensity score matching was conducted to examine the association between AP severity and the risk of new-onset diabetes following AP. All data were collected retrospectively. However, individuals with AP were followed prospectively in time to determine the risk of new-onset diabetes following discharge. The Penn State University Institutional Review Board considered this study not human participants’ research; thus, informed consent was not needed. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies (12).
Data source
This study used the Merative™ MarketScan® Commercial (MarketScan) database, a commercially available health insurance claims database. MarketScan is one of the largest and oldest nationwide longitudinal claims databases used for healthcare research, containing data on over 300 million unique, de-identified patients (13). The database consists of privately insured employees and their family members from large employers and health plans across all 50 US states and the District of Columbia (14). Longitudinal tracking of detailed patient-level healthcare claims information provides comprehensive data, including key demographic characteristics, healthcare utilization, inpatient and outpatient medical information with diagnosis codes, procedure codes, detailed prescription drugs, and financial information (15). MarketScan is fully compliant with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) (15).
Cohort derivation and assessment of exposure
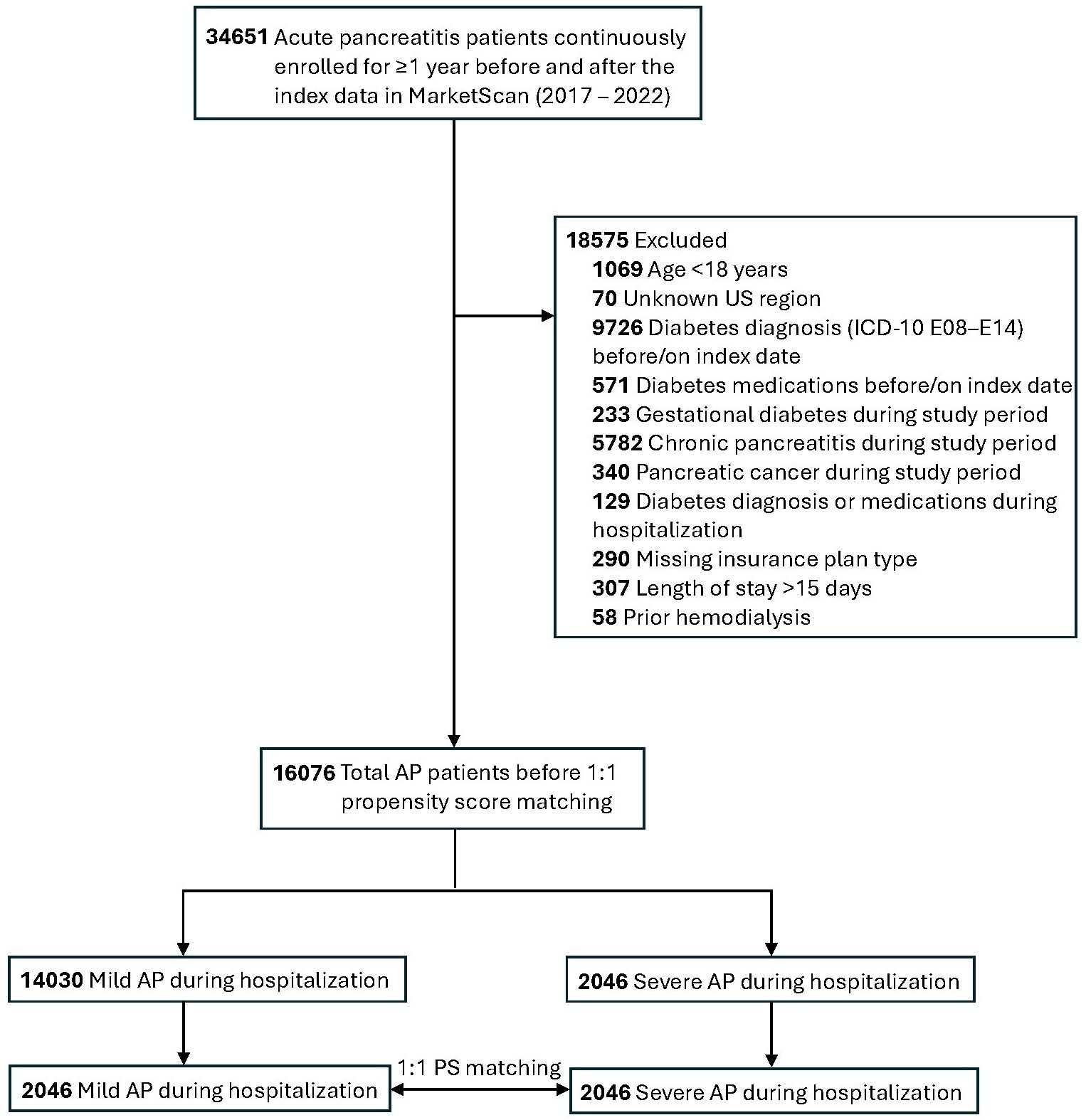
The study population included patients aged 18 to 64 years who were admitted to the hospital with a diagnosis of AP (International Classification of Diseases, Tenth Revision, Clinical Modification, ICD-10-CM code: K85) and were continuously enrolled for at least 12 months before the index admission date and at least 12 months after. The earliest inpatient admission diagnosis of AP in the database during the study period was defined as the index date of AP for each study participant. AP patients with prevalent diabetes or who were taking diabetes medication (Supplementary Table 1) before or on the index date were excluded from the study. Patients staying in the hospital longer due to primary or concurrent illnesses, which may inflate the rate of incident diabetes following hospitalization, were also excluded. To avoid confounding from severe comorbid diseases or nosocomial complications and reducing heterogeneity, we also excluded individuals with prolonged AP hospitalization (>15 days), gestational diabetes, chronic pancreatitis, and pancreatic cancer diagnosed during the study period. A complete list of exclusions is presented in the study population flow diagram (Figure 1). Data from 2017 to 2022 were primarily used for the study cohort derivation. The year prior to the index date was used to define baseline covariates, and 1 year following the index date ensured continuous insurance coverage. The overall study period covered 1 January 2016 to 31 December 2023. We conducted a propensity score matching using a greedy nearest neighbor approach in conjunction with a 1:1 matching ratio without replacement using the R package MatchIt.
The primary exposure of interest, severe AP, was defined using any of the following proxies in reference to the revised Atlanta classification during hospitalization: pancreatic necrosis, hemodialysis, shock, or organ failure, including renal, cardiac, respiratory, and mechanical ventilation, tracheal intubation, and vasopressor support (Supplementary Table 2). Mild AP was defined as the absence of all the above codes during the same period (4, 9).
Assessment of outcomes
The main outcome of interest was the incidence of diabetes, defined as the presence of one of the following ICD-10 codes in the database during the follow-up period: E08–E14 (16–18). As in previous studies, E08, E09, and E14 are included to avoid missing incident cases of diabetes due to errors in coding (17–20).
Assessment of covariates
Data on age (years), sex (men/women), and US region (South, West, North Central, Northeast) were extracted directly from the MarketScan database. Based on a comprehensive literature review, the following potential confounders, representing risk factors for AP and diabetes, were also captured using their corresponding ICD-10-CM, or Current Procedural Terminology (CPT) codes (Supplementary Table 3): obesity, hypertension, dyslipidemia, coronary artery disease (CAD), liver disease, alcohol abuse, smoking status, gallstones, number of office visits, use of glucocorticoids, statins, and antihypertensive medications, prediabetes, social determinants of health (SDOH), acute cholecystitis, chronic obstructive pulmonary disease (COPD), depression, and use of HIV-related medications (each, yes/no). The 12 months preceding the index date of AP were established as the baseline period.
Statistical analysis
We performed univariable analysis to summarize the baseline characteristics of study participants in the mild versus severe AP groups using counts (percentages) for categorical variables and means (standard deviations) for continuous variables. These characteristics were presented both before and after 1:1 propensity score matching. Standardized mean difference (SMD) was used to assess covariate balance between the two groups, with an SMD greater than 0.1 indicating potential imbalance. Propensity scores were estimated from a logistic regression model using the covariates in the full model to balance baseline data between severe AP and mild AP. The goodness-of-fit of the multivariable logistic regression models was assessed using the Hosmer–Lemeshow test. A non-significant result (P > 0.05) indicates that the model fits the data well. Person‐time of follow-up for each participant was calculated from the index AP discharge date to the first occurrence of an outcome of interest, diabetes, maximum follow-up date (i.e., the latest date in the diagnosis records), end of enrollment, or end of the study period (31 December 2023), whichever took place first.
An initial Cox proportional hazards regression model was performed before propensity matching, adjusted for the aforementioned covariates. The final model was conducted using a stratified Cox proportional hazards regression model using matching IDs constructed from the propensity scores as the strata with the demographic, comorbidities, and medication factors, which provided adjusted hazard ratios (aHRs) and their 95% confidence interval (CIs). We performed several sensitivity analyses to test the robustness of our results. First, we analyzed to account for different follow-up periods. Second, the pathophysiology of alcoholic AP often differs from non-alcoholic AP. Therefore, we further excluded individuals with an alcoholic etiology of AP. Third, models including interactions with severe AP status, specifically age (years), sex, prediabetes, tobacco use, and alcohol abuse with new-onset diabetes risk, were assessed by the −2-log likelihood ratio (−2LL), stratified by the propensity score matching IDs. Subgroup analyses were further conducted following the interaction tests. Lastly, to assess how unmeasured confounding could have affected the observed association between severe AP and new-onset diabetes, we calculated the E-value using the methods of VanderWeele and Ding (21).
A log–log survival curve was used to assess the violation of the proportional hazards (PHs) assumption. Data were analyzed using SAS Software version 9.4 (SAS Institute Inc., Cary, NC) and R software version 4.5.1 (R Foundation for Statistical Computing, Vienna, Austria) with a two-sided alpha level of 0.05.
Results
In univariable analyses, individuals with severe AP were older, were more likely to be female, reside in the Southern US, and have a history of hypertension, CAD, liver disease, COPD, depression, alcohol abuse, tobacco use, and dyslipidemia. They were also more likely to use glucocorticoids, statins, and antihypertensive medications. However, after propensity score matching, baseline characteristics were well balanced between groups, as indicated by standardized mean differences (SMD < 0.1; Table 1; Supplementary Figure 1).
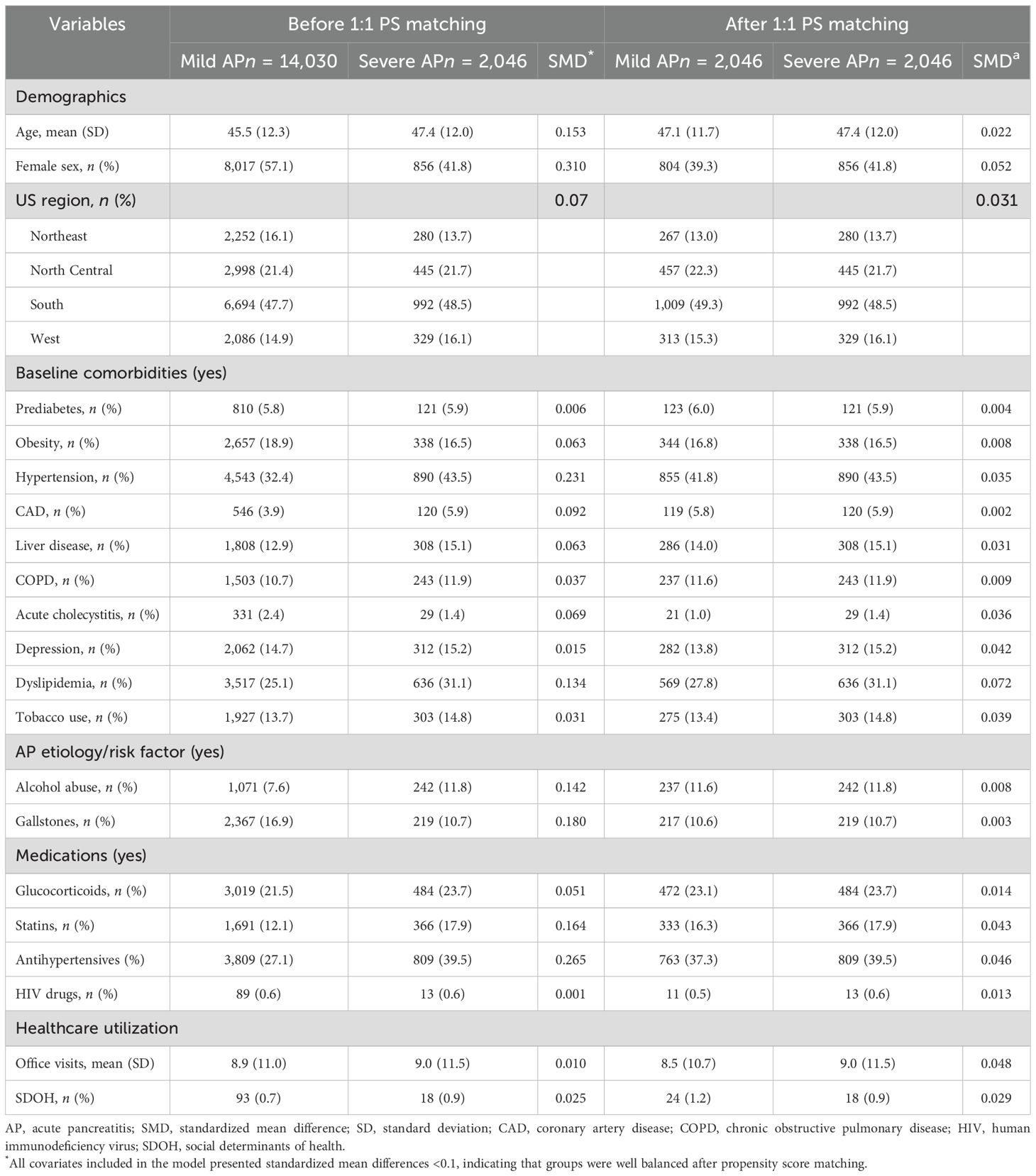
Table 1. Baseline characteristics before and after propensity score matching (PS) stratified by severe AP status.
The Hosmer–Lemeshow test yielded a P-value of 0.86, which confirmed that the models fit the data well. There was no evidence of violation of the proportional hazards assumption (PHA) (P = 0.45). Additionally, log–log survival curves for checking the PHA for the two groups were parallel, suggesting no violation (Supplementary Figure 2).
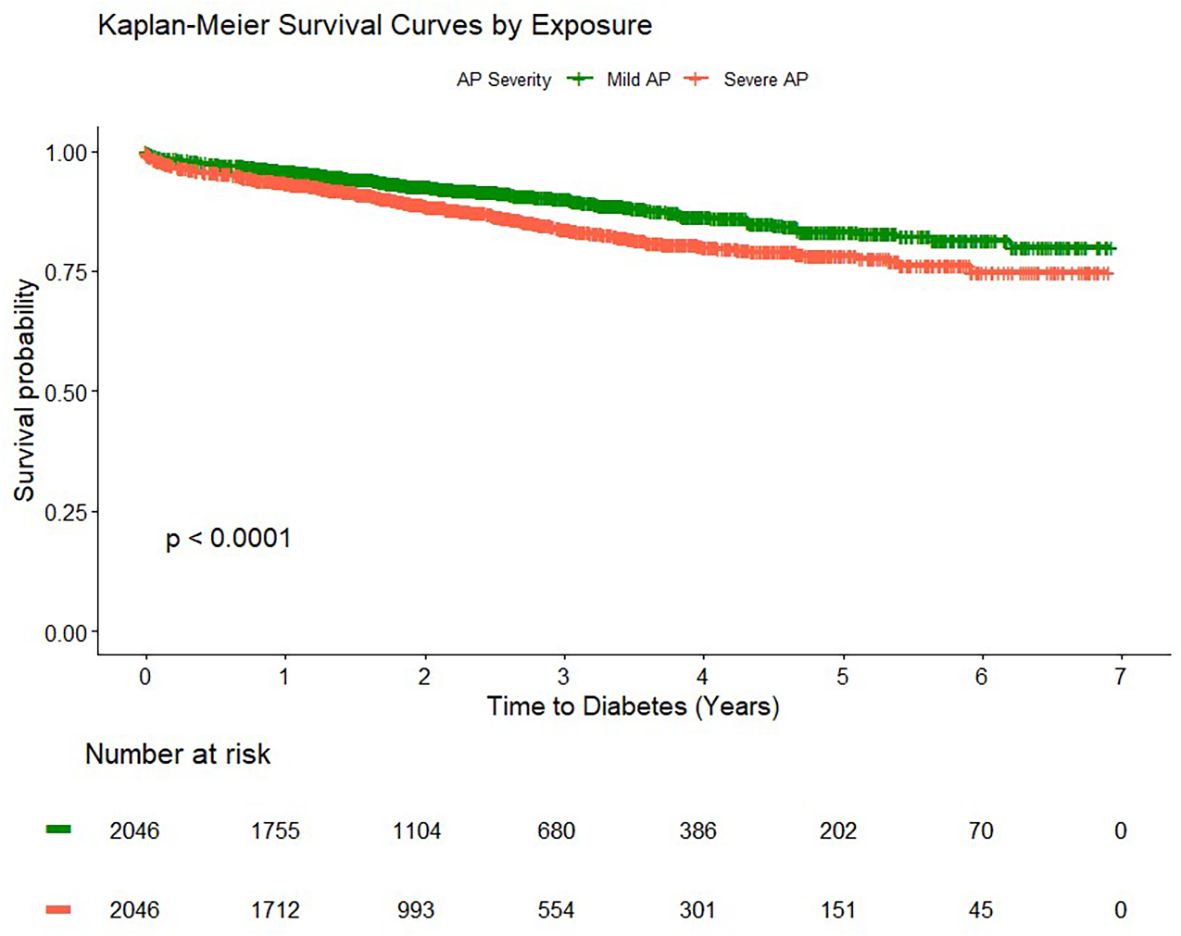
The overall unadjusted cumulative incidence and incidence density rate of diabetes were higher among participants with severe AP (56.6 per 1,000 person-years) compared to those with mild AP (36.8 per 1,000 person-years) (Table 2; Figure 2). In the stratified multivariable Cox regression model by the propensity score matching strata, severe AP was associated with a higher risk of incident diabetes (aHR = 1.64, 95% CI: 1.30–2.06) (Table 2). The association was stronger after excluding those with alcohol-related AP (aHR = 1.67, 95% CI: 1.26–2.22) (Table 2). Suggesting a strong early effect, severe AP was associated with a two-fold increased risk of diabetes within 90 days following discharge (aHR = 2.06, 95% CI: 1.36–3.12). Similar effects were observed at 6 months and 3 years of follow-up, with some attenuation over time.
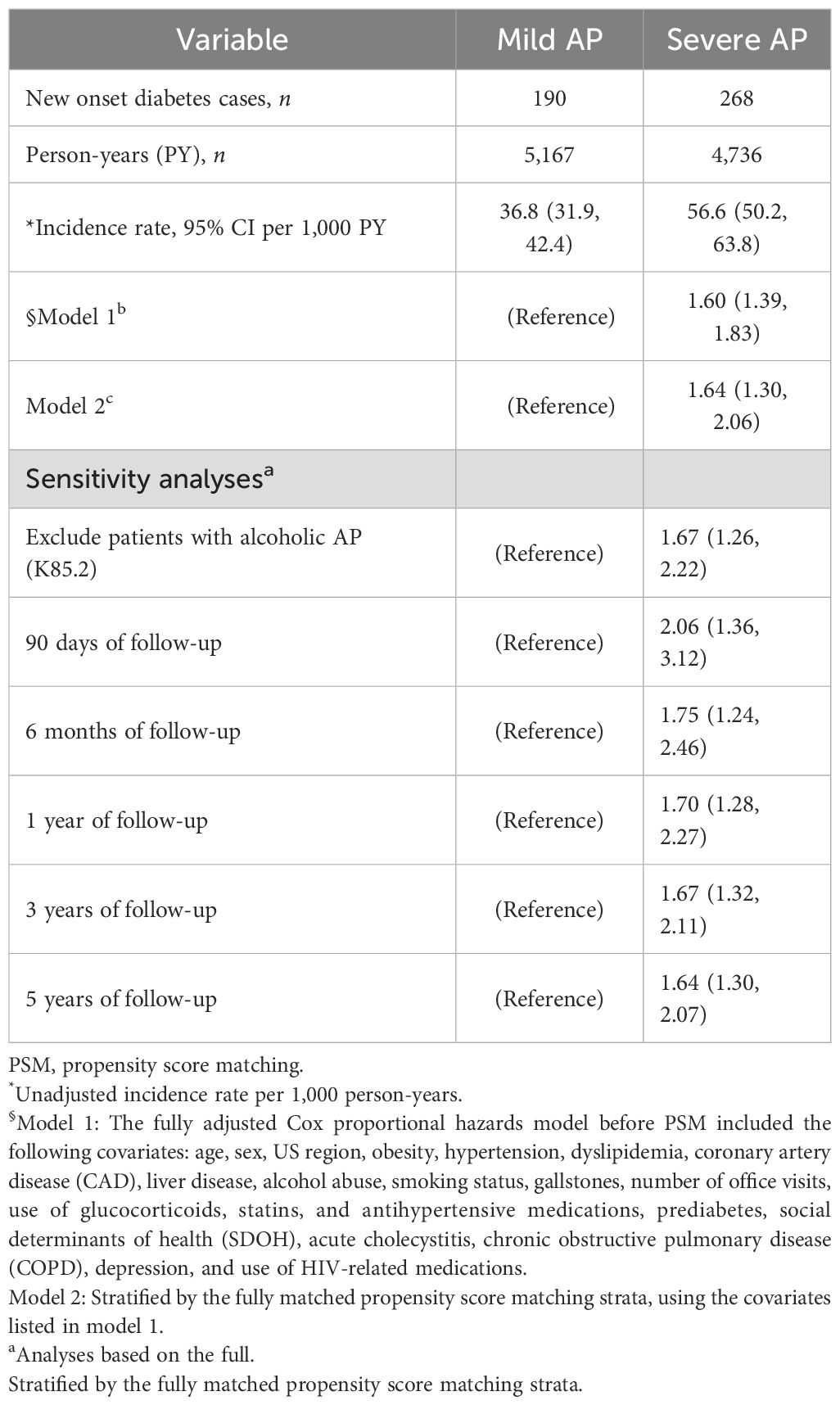
Table 2. Cox proportional hazards models showing hazard ratios (HRs) and 95% confidence intervals (CIs) before and after PSM, assessing the association between severe AP during hospitalization and incident diabetes mellitus.
In comparing the modifying effect of patient demographics and comorbidities, a stronger association between severe AP and diabetes was observed in men (aHR = 2.03, 95% CI: 1.50–2.74) than in women (aHR = 1.06, 95% CI: 0.69–1.64; Pinteraction = 0.02; Supplementary Figure 3). No significant interactions between severe AP and diabetes were found for age, prediabetes, tobacco use, and alcohol abuse (Pinteraction > 0.05 for all; Supplementary Figure 3). An E-value of 2.66 with a lower confidence limit (LL) of 1.92 indicates that unmeasured confounders in the MarketScan® database, such as race/ethnicity or imaging-related biomarkers, are unlikely to fully account for the observed association between severe AP and new-onset diabetes.
Discussion
In this real-world evidence study involving more than four thousands of AP patients with no prior history of diabetes, we found that patients with severe AP had a higher incidence rate of diabetes compared to those with mild AP (56.6 per versus 36.0 per 1,000 person-years). Adjusted analyses demonstrated a two-fold increased risk of diabetes within 90 days following hospital discharge. The risk remained elevated, albeit to a smaller degree, for up to 3 years of follow-up. The robustness of our findings was illustrated using propensity score matching and was independent of demographics, major chronic medical conditions, and medications. To our knowledge, this is the first largest study using causal inference and time-to-event approaches to evaluate the association between severe AP during hospitalization and the risk of new-onset diabetes using real-world data from a large, insured US population.
While the risk of developing diabetes is higher in the first months after AP, it does not appear to return to the risk of the general population, maintaining a cumulative incidence reported to be as high as 40% (22, 23). The resolution of stress hyperglycemia following an episode of AP is not quantified. However, one study showed that 95.8% and 68.4% of patients with mild and severe AP had normalization of serum glucose after pancreatitis treatment (24). In contrast, concurrent clinical conditions that affect the red blood cell turnover, as in the case of hemolytic anemia or blood loss, may result in falsely low HbA1c values regardless of glycemic status (25–27).
The findings of this study have both public health and clinical implications, emphasizing the importance of surveillance and screening after hospitalization, especially among high-risk patients after an episode of severe AP (28). Monitoring for dysglycemia and early intervention may prevent or slow progression to diabetes, with the ultimate goal of preventing future complications. Routine monitoring of diabetes-related biomarkers, including fasting glucose and HbA1c, is recommended within 3 months after discharge from severe AP (29). Ideally, continuity of care after discharge may also include multidisciplinary care with endocrinologists, gastroenterologists, surgeons, and dietitians/nutritionists, as required. These integrated services would focus on risk reduction tailored to pancreatitis etiology, surveillance, nutritional optimization, care of complications, and coordinated follow-up.
Our findings are consistent with previous studies that have investigated the association between severe AP using the 2012 revised Atlanta classification criteria (4, 23, 30–32). A recent meta-analysis including 50 studies found that severe AP was associated with significantly increased odds of developing diabetes (OR: 1.86; 95% CI: 1.27, 2.73) (23). Furthermore, another study from our group using the Nationwide Readmission Database found that severe AP was independently associated with higher odds of AP-related diabetes (4).
The plausible biological mechanisms for the observed association between severe AP and the development of incident diabetes may include beta-cell death, as in severe cases of AP and persistent organ failure, or islet cell destruction in the affected part of the pancreas caused by inflammation or pancreatitis-related necrosis (23, 30, 33). In addition to loss of beta cells resulting from pancreatic necrosis or resection, systemic inflammation may also promote insulin resistance and impair islet function. Furthermore, exocrine pancreatic insufficiency disrupts incretin signaling, reducing insulin secretion and worsening hyperglycemia (6). Islet cell autoimmunity appears to develop in a subset of patients, although the mechanism remains unknown (34). Lastly, there are likely overlapping and shared risk factors, including obesity and genetic predisposition to the development of hyperglycemia. Collectively, diabetes following AP is likely multifactorial in nature, related to islet cell destruction as well as a consequence of genetic predisposition, systemic and local inflammation, and hormonal dysregulation.
Consistent with prior findings, the relationship between severe AP and new-onset diabetes differed significantly between men and women, with a stronger association observed in men (35). This variation may reflect underlying biological and hormonal influences. Differences in hormone activity affecting pancreatic beta-cell function, body fat distribution, and metabolic regulation likely contribute. Men generally exhibit greater central adiposity, in contrast to women who preferentially store fat subcutaneously. Furthermore, greater insulin resistance in men can amplify pancreatic inflammation and metabolic dysfunction following AP. In addition, lifestyle and comorbid factors such as alcohol use, hypertension, and obesity may further elevate diabetes risk in men (36). These findings highlight the need to account for potential biological and behavioral differences between men and women when developing surveillance and diabetes prevention strategies after AP.
Study strengths and limitations
The strengths of our study include the use of propensity score matching combined with time-to-event analysis of longitudinal data from a large cohort of patients with AP. This approach accounts for both timing and censoring of events, offering advantages over traditional logistic regression models that do not consider these factors. While some data fields lack precision in this dataset, such as imaging-related biomarkers, our study’s relatively high E-values indicate robustness against potential unmeasured confounding. Nonetheless, our study has several limitations that should be considered when interpreting the results. This observational study used medical claims data; therefore, causality cannot be established. In addition, our study included only AP patients who had continuous enrollment in their private insurance plan from 12 months before to 12 months after the index AP date, which may introduce some selection bias. However, continuous enrollment was necessary to understand the contribution of pre-existing illnesses and ensure sufficient follow-up for the key outcome. We also acknowledge that claims-based databases may misclassify patients due to misreporting or underreporting of diagnoses or medications. Additionally, the MarketScan database does not include data on race or ethnicity, income, laboratory results, or imaging-based biomarkers. Therefore, we could not directly assess severity based on the extent of imaging abnormalities or pancreatic morphological characteristics (e.g., fat infiltration and fibrosis). There is an ongoing prospective cohort study of AP participants that is obtaining a comprehensive characterization of laboratory, metabolic, and imaging parameters to determine their contributions to incident diabetes (37–39). Finally, MarketScan includes few, if any, individuals aged 65 years. Therefore, while we cannot think of biological reasons why this would be the case, our findings may not be generalizable to older adults with AP and those without health insurance. Future studies could address this gap in the literature by using alternative real-world databases.
Conclusions
In this large US real-world study, we found that severe AP was associated with increased risk of incident diabetes. The association between the severity of AP and diabetes was two-fold higher within 90 days following hospital discharge. These findings highlight the importance for glycemic surveillance and the need to consider proactive management of severe AP patients to mitigate their risk of poor health outcomes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data used in this study were obtained from a third party, Merative MarketScan, although access is restricted because they were used under a Penn State College of Medicine license for this study and are not publicly available. The data can be accessed from Merative (https://www.merative.com/products/marketscan-research-databases). Requests to access these datasets should be directed to https://www.merative.com/products/marketscan-research-databases.
Author contributions
DMB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PH: Conceptualization, Investigation, Supervision, Writing – review & editing. TQ: Software, Visualization, Writing – review & editing. SK: Conceptualization, Investigation, Resources, Supervision, Writing – review & editing. XG: Methodology, Writing – review & editing. DL: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – review & editing. DB: Writing – review & editing. JM: Writing – review & editing. KF: Writing – review & editing. VC: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. AP-L: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
We thank the Center for Applied Studies in Health Economics (CASHE) at Penn State College of Medicine for facilitating access to the MarketScan database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1704688/full#supplementary-material
References
1. Habtezion A, Gukovskaya AS, and Pandol SJ. Acute pancreatitis: A multifaceted set of organelle and cellular interactions. Gastroenterology. (2019) 156:1941–50. doi: 10.1053/j.gastro.2018.11.082
2. Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, and Camargo CA Jr. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. (2007) 35:302–7. doi: 10.1097/MPA.0b013e3180cac24b
3. Peery AF, Murphy CC, Anderson C, Jensen ET, Deutsch-Link S, Egberg MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2024. Gastroenterology. (2025) 168:1000–24. doi: 10.1053/j.gastro.2024.12.029
4. Firkins SA, Hart PA, Papachristou GI, Lara LF, Cruz-Monserrate Z, Hinton A, et al. Identification of a risk profile for new-onset diabetes after acute pancreatitis. Pancreas. (2021) 50:696–703. doi: 10.1097/mpa.0000000000001818
5. Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (Type 3c): A retrospective cohort study. Diabetes Care. (2017) 40:1486–93. doi: 10.2337/dc17-0542
6. Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, et al. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. (2021) 6:668–75. doi: 10.1016/s2468-1253(21)00019-4
7. García-Compeán D, Jiménez-Rodríguez AR, Muñoz-Ayala JM, González-González JA, Maldonado-Garza HJ, and Villarreal-Pérez JZ. Post-acute pancreatitis diabetes: A complication waiting for more recognition and understanding. World J Gastroenterol. (2023) 29:4405–15. doi: 10.3748/wjg.v29.i28.4405
8. Cho IR, Han KD, Lee SH, Choi YH, Chung KH, Choi JH, et al. Association between glycemic status and the risk of acute pancreatitis: a nationwide population-based study. Diabetol Metab Syndr. (2023) 15:104. doi: 10.1186/s13098-023-01086-x
9. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
10. Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, and Li L. Incidence of new onset diabetes mellitus secondary to acute pancreatitis: A systematic review and meta-analysis. Front Physiol. (2019) 10:637. doi: 10.3389/fphys.2019.00637
11. Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, and Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. (2014) 63:818–31. doi: 10.1136/gutjnl-2013-305062
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, and Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
13. Merative™. The merative™ MarketScan® Research databases (2023). Available online at: https://www.merative.com/documents/brief/Marketscan_explainer_general (Accessed May 1, 2023).
14. IBM® MarketScan®. Commercial claims and encounters database. IBM Watson Health (2020). Available online at: https://www.ibm.com/products/marketscan-research-databases (Accessed May 1, 2023).
15. Adamson D, Chang S, and Hansen LG. Health research data for the real world: The MarketScan databases. Thomson Healthcare (2008) p. 1–32.
16. Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for newly diagnosed diabetes >30 days after SARS-coV-2 infection among persons aged <18 years - United States, march 1, 2020-june 28, 2021. MMWR Morb Mortal Wkly Rep. (2022) 71:59–65. doi: 10.15585/mmwr.mm7102e2
17. Xie Y and Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21. doi: 10.1016/S2213-8587(22)00044-4
18. Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, Peterson AC, et al. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-coV-2 infection. Diabetes Care. (2022) 45:782–8. doi: 10.2337/dc21-1686
19. Barrett CE, Koyama AK, Alvarez P, Chow W, Lundeen EA, Perrine CG, et al. Risk for newly diagnosed diabetes >30 days after SARS-coV-2 infection among persons aged <18 years — United states, march 1, 2020–june 28, 2021. MMWR Morb Mortal Wkly Rep. (2022) 71:59–65. doi: 10.15585/mmwr.mm7102e2
20. Kim JY, Lee J, Moon JH, Park SE, Ko SH, Choi SH, et al. Prevalence, incidence, and metabolic characteristics of young adults with type 2 diabetes mellitus in South Korea (2010-2020). Diabetes Metab J. (2025) 49:172–82. doi: 10.4093/dmj.2024.0826
21. VanderWeele TJ and Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. (2017) 167:268–74. doi: 10.7326/m16-2607
22. Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, et al. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol. (2020) 55:775–88. doi: 10.1007/s00535-020-01682-y
23. Zahariev OJ, Bunduc S, Kovács A, Demeter D, Havelda L, Budai BC, et al. Risk factors for diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Front Med (Lausanne). (2023) 10:1257222. doi: 10.3389/fmed.2023.1257222
24. Sun YF, Song Y, Liu CS, and Geng JL. Correlation between the glucose level and the development of acute pancreatitis. Saudi J Biol Sci. (2019) 26:427–30. doi: 10.1016/j.sjbs.2018.11.012
25. Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med. (2014) 29:388–94. doi: 10.1007/s11606-013-2595-x
26. Kutter D and Thoma J. Hereditary spherocytosis and other hemolytic anomalies distort diabetic control by glycated hemoglobin. Clin Lab. (2006) 52:477–81.
27. Starkman HS, Wacks M, Soeldner JS, and Kim A. Effect of acute blood loss on glycosylated hemoglobin determinations in normal subjects. Diabetes Care. (1983) 6:291–4. doi: 10.2337/diacare.6.3.291
28. Petrov MS and Olesen SS. Metabolic sequelae: the pancreatitis zeitgeist of the 21st century. Gastroenterology. (2023) 165:1122–35. doi: 10.1053/j.gastro.2023.07.025
29. Committee ADAPP. 2. Diagnosis and classification of diabetes: standards of care in diabetes—2025. Diabetes Care. (2024) 48:S27–49. doi: 10.2337/dc25-S002
30. Vipperla K, Papachristou GI, Slivka A, Whitcomb DC, and Yadav D. Risk of new-onset diabetes is determined by severity of acute pancreatitis. Pancreas. (2016) 45:e14–5. doi: 10.1097/mpa.0000000000000536
31. Man T, Seicean R, Lucaciu L, Istrate A, and Seicean A. Risk factors for new-onset diabetes mellitus following acute pancreatitis: a prospective study. Eur Rev Med Pharmacol Sci. (2022) 26:5745–54. doi: 10.26355/eurrev_202208_29511
32. Durmuş ET, Akdağ İ, and Yıldız M. Diabetes is an independent predictor of severe acute pancreatitis. Postgrad Med. (2022) 134:711–6. doi: 10.1080/00325481.2022.2105613
33. CioChina M, Balaban DV, Manucu G, Jinga M, and Gheorghe C. The impact of pancreatic exocrine diseases on the β-cell and glucose metabolism-A review with currently available evidence. Biomolecules. (2022) 12(5):618. doi: 10.3390/biom12050618
34. Yadav D, Whitcomb DC, Tang G, Slivka A, and Bellin M. Autoimmunity may explain diabetes in a subset of patients with recurrent acute and chronic pancreatitis: A pilot study. Clin Gastroenterol Hepatol. (2023) 21:226–8.e1. doi: 10.1016/j.cgh.2021.11.011
35. Shen HN, Yang CC, Chang YH, Lu CL, and Li CY. Risk of diabetes mellitus after first-attack acute pancreatitis: A national population-based study. Am J Gastroenterol. (2015) 110:1698–706. doi: 10.1038/ajg.2015.356
36. Kim B, Park E-S, Lee J-S, and Suh JG. Sex-specific differences in glucose metabolism and pancreatic function in streptozotocin-induced diabetic mice: The protective role of estrogen. Biochem Biophys Res Commun. (2025) 775:152176. doi: 10.1016/j.bbrc.2025.152176
37. Tirkes T, Chinchilli VM, Bagci U, Parker JG, Zhao X, Dasyam AK, et al. Design and rationale for the use of magnetic resonance imaging biomarkers to predict diabetes after acute pancreatitis in the diabetes RElated to acute pancreatitis and its mechanisms study: from the type 1 diabetes in acute pancreatitis consortium. Pancreas. (2022) 51:586–92. doi: 10.1097/mpa.0000000000002080
38. Dungan KM, Hart PA, Andersen DK, Basina M, Chinchilli VM, Danielson KK, et al. Assessing the pathophysiology of hyperglycemia in the diabetes RElated to acute pancreatitis and its mechanisms study: from the type 1 diabetes in acute pancreatitis consortium. Pancreas. (2022) 51:575–9. doi: 10.1097/mpa.0000000000002074
39. Hart PA, Papachristou GI, Park WG, Dyer A-M, Chinchilli VM, Afghani E, et al. Rationale and design for the diabetes RElated to acute pancreatitis and its mechanisms study: A prospective cohort study from the type 1 diabetes in acute pancreatitis consortium. Pancreas. (2022) 51:568–74. doi: 10.1097/mpa.0000000000002079
Keywords: AP, new-onset diabetes, real-world data, MarketScan, US
Citation: Ba DM, Hart PA, Qiu T, Krishna SG, Gao X, Leslie DL, Bradley D, Maranki J, Fofana K, Chinchilli VM and Pichardo-Lowden AR (2025) Association between severe acute pancreatitis and new-onset diabetes: a propensity score-matched real-world study. Front. Endocrinol. 16:1704688. doi: 10.3389/fendo.2025.1704688
Received: 13 September 2025; Accepted: 22 October 2025;
Published: 14 November 2025.
Edited by:
Qingqiang Ni, Shandong Provincial Hospital, ChinaReviewed by:
Tiago Santos, Hospital de Santo António, PortugalSrilatha Dampetla, Royal Lancaster Infirmary, United Kingdom
Copyright © 2025 Ba, Hart, Qiu, Krishna, Gao, Leslie, Bradley, Maranki, Fofana, Chinchilli and Pichardo-Lowden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Djibril M. Ba, ZGppYnJpbGJhQHBlbm5zdGF0ZWhlYWx0aC5wc3UuZWR1
†These authors share senior authorship
 Djibril M. Ba
Djibril M. Ba Phil A. Hart2
Phil A. Hart2 Somashekar G. Krishna
Somashekar G. Krishna David Bradley
David Bradley Vernon M. Chinchilli
Vernon M. Chinchilli Ariana R. Pichardo-Lowden
Ariana R. Pichardo-Lowden