- 1Department of Ultrasound Medicine, Shapingba Hospital, Chongqing University (Shapingba District People’s Hospital, Chongqing), Chongqing, China
- 2Department of Scientific Research, Shapingba Hospital, Chongqing University (Shapingba District People’s Hospital, Chongqing), Chongqing, China
Background: A growing number of risk prediction models for cervical lymph node metastasis (CLNM) in papillary thyroid microcarcinoma (PTMC) have been developed, but their performance and methodological rigor remain unclear. This study systematically reviews these models to evaluate their predictive performance and critically appraise their risk of bias.
Methods: We conducted a systematic search of seven databases up to July 29, 2025. The methodological quality of the included studies was assessed using PROBAST. Model performance, measured by the area under the curve (AUC), was pooled using a random-effects meta-analysis.
Results: A total of 15 studies, comprising 24 predictive models, were included. The pooled AUC was 0.794 (95% CI: 0.769–0.820), but with substantial heterogeneity (I2 = 89.6%). Subgroup analysis revealed a performance drop from the training set (pooled AUC, 0.812) to the validation set (pooled AUC, 0.774). The PROBAST assessment revealed that 12 of the 15 studies (80%) were critically at a high risk of bias, primarily due to flaws in participant selection.
Conclusion: Although existing CLNM prediction models for PTMC show moderate to good discrimination on average, their clinical utility is severely limited by widespread methodological weaknesses and a high risk of bias. The current evidence is not robust enough to recommend any specific model for routine clinical use, and future research must prioritize methodological rigor and independent external validation.
Introduction
Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer (1, 2), accounting for approximately 85% to 90% of all thyroid malignancies (3, 4). In recent years, the incidence of PTC has been steadily increasing, making it one of the fastest-growing malignant tumors worldwide (5–9). A significant portion of these diagnoses are for papillary thyroid microcarcinoma (PTMC), defined as PTC with a diameter of not more than 10 mm (10). Although PTMC is often considered a low-risk disease, it has a notable tendency to metastasize to cervical lymph nodes relatively early (11), with reported metastasis rates ranging from 30% to 82% (12–16). This creates a significant clinical dilemma, as the presence of cervical lymph node metastasis (CLNM) is a key factor in determining the appropriate management strategy, which can range from active surveillance to surgical intervention (17–22).
To address this clinical uncertainty, risk prediction models have emerged as a promising tool to help stratify patients and guide treatment decisions. The traditional detection of CLNM primarily relies on ultrasound examination, but this modality has relatively low sensitivity in identifying metastatic lymph nodes (23–25). Consequently, there is an urgent need to identify CLNM risk more accurately to inform surgical planning (26). In response, a growing number of studies have developed multivariable prediction models, often presented as nomograms, that combine clinical, pathological, and imaging features to estimate the probability of CLNM in patients with PTMC.
Despite the proliferation of these models, their performance, reliability, and ultimate clinical utility have not been systematically and critically appraised. Many prediction model studies are known to suffer from methodological shortcomings, such as biased participant selection, inadequate handling of data, and insufficient validation, which can lead to overly optimistic performance estimates. The adoption of a poorly developed or validated model into clinical practice could lead to incorrect patient stratification, resulting in either unnecessary overtreatment or dangerous undertreatment. A comprehensive evaluation of the existing evidence is therefore essential.
Therefore, this study was conducted to systematically review existing prediction models for CLNM in PTMC, evaluate their predictive performance through meta-analysis, and critically appraise their methodological quality using Prediction model Risk Of Bias ASsessment Tool (PROBAST). The goal is to summarize the current evidence, highlight methodological limitations in the field, and provide guidance for both clinical practice and the development of future, more robust prediction models.
Methods
This systematic review and meta-analysis was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement.
Literature search
We searched the Chinese Biomedical Literature Database, China National Knowledge Infrastructure (CNKI), Wanfang, PubMed, EMbase, Web of Science, and Cochrane Library databases to collect studies on the risk prediction model of CLNM in PTMC published from the establishment of the databases to July 29, 2025. We combined keywords with related free words. The search terms included thyroid microcarcinoma, ultrasound, radiomic, lymph node, transfer, nomogram, etc. Detailed search strategies for each database, including the specific combinations of medical subject headings (MeSH), Emtree terms, and free-text words, are provided in Supplementary Appendix 1.
Inclusion and exclusion criteria
Based on the PICOTS framework, the inclusion and exclusion criteria for this study were as follows:
P (population): Patients with a postoperative pathological diagnosis of PTMC (defined as papillary thyroid carcinoma with tumor diameter ≤ 10 mm).
I (intervention model): Studies developing and/or validating a multivariable risk prediction model for CLNM.
C (comparator): Not applicable for this type of review.
O (outcome): Model performance metrics, primarily the area under the receiver operating characteristic curve (AUC).
T (timing): At the time of initial diagnosis, prior to surgical intervention.
S (setting): Eligible studies included observational study designs (e.g., retrospective or prospective cohort studies, case–control studies) that reported on the development or validation of a prediction model.
According to the qualification criteria, the included studies must be original research involving the CLNM risk prediction model of PTMC (excluding reports, reviews, conference papers, and meta-analyses), including both Chinese and English literature, with a focus on the training set and validation set of the prediction model. The exclusion criteria are studies with only training set but no validation set and studies with incomplete data or where the original text cannot be obtained.
Literature screening and data extraction
Two researchers independently conducted a preliminary screening of the titles and abstracts of the articles based on preset inclusion and exclusion criteria. Subsequently, the full-text reading of the literature that passed the initial screening was conducted to further screen whether it met the inclusion criteria. Data extraction uses standardized tables based on CHARMS (Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies) checklist. The extracted data were as follows: author, publication year, country, sample source, sample size, study type, age, lymph node metastasis rate, diagnostic basis, modeling sample size, model performance, model validation, main predictors, clinical applicability assessment, and lymph node metastasis risk stratification. During the process of article screening and data extraction, if there are any differences, a third researcher was introduced for discussion.
Risk assessment of bias
The risk of bias and suitability of the included studies were evaluated using the PROBAST checklist (27, 28). Two researchers independently assessed the risk of bias and applicability. The PROBAST checklist contains 20 signaling questions across four evaluation domains: participants, predictors, results, and analysis. The answer options for each signaling question are “yes”, “probably yes”, “no”, “probably no”, or “no information”. If the answer to any question in a certain field is “no” or “probably no”, that field is judged to have a high risk of bias. Only when all domains are determined to have a low risk of bias can the overall risk of bias be recognized as low.
Data synthesis and statistical analysis
Meta-analysis of the area under the curve (AUC) and 95% confidence interval (CI) of the model was conducted using Stata 14. Heterogeneity among studies was evaluated by using Q test and I2. If P >0.1 and I2 <50%, it is considered that the heterogeneity among studies is relatively small, and a fixed-effect model is selected. If P ≤0.1 and I2 ≥50%, the random-effects model is selected. Subgroup analysis was conducted based on the training set, validation set, and sources of predictors. Sensitivity analysis was performed using the elimination method one by one. Egger’s test was used to assess publication bias.
Result
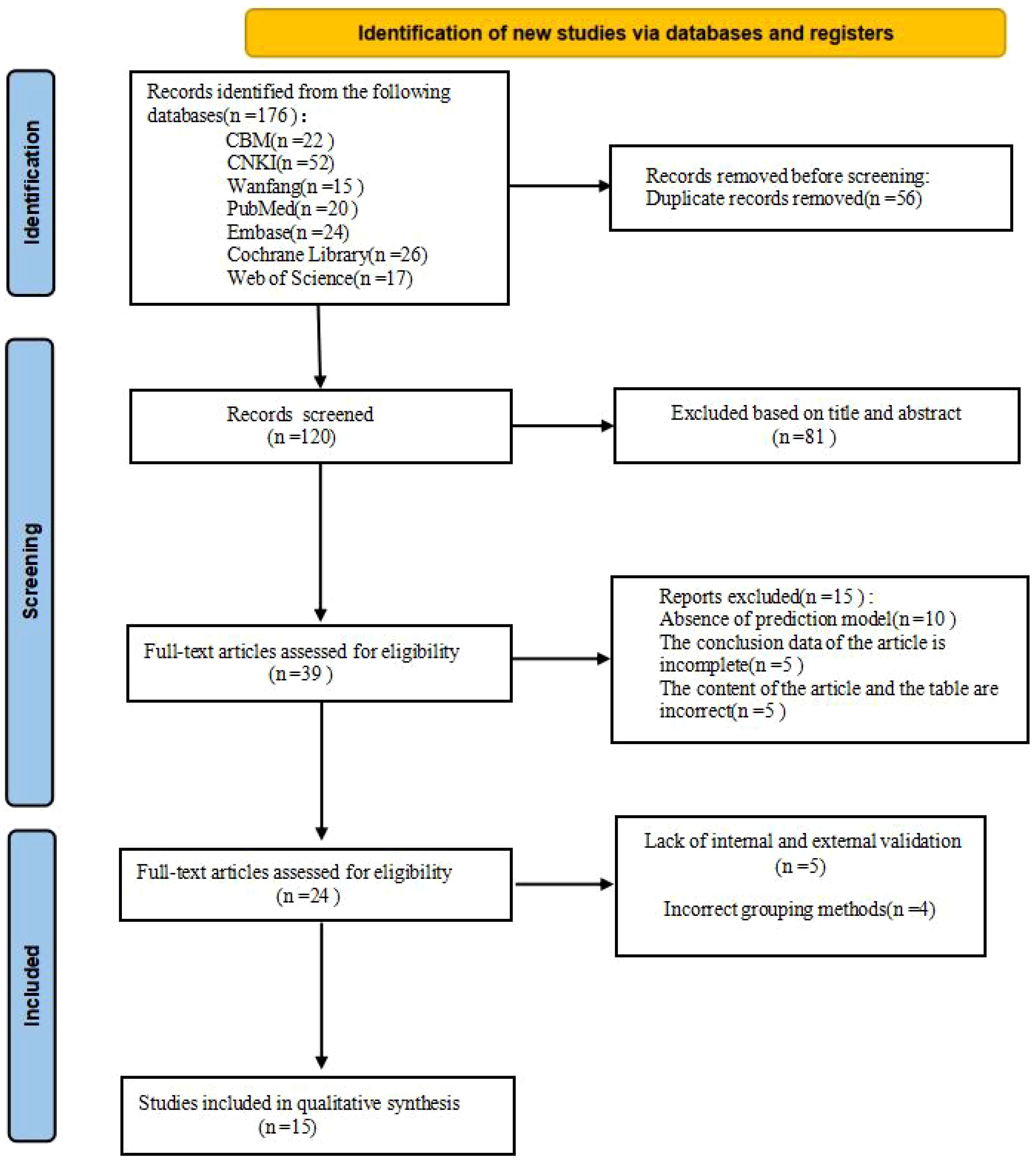
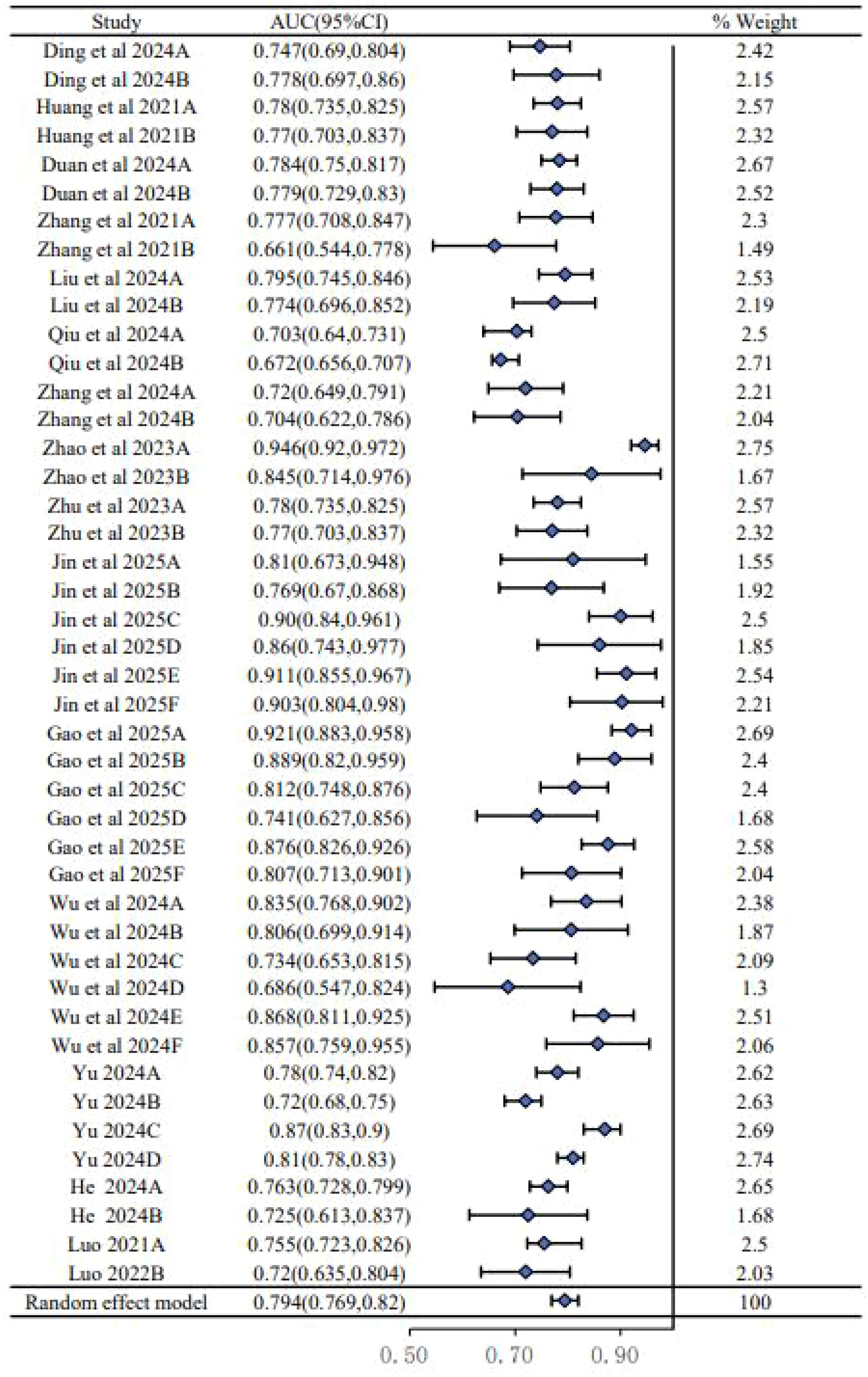
A total of 176 studies were identified through database retrieval. After removing 56 duplicate records, the titles and abstracts of the remaining studies were screened, and 81 studies were excluded. Subsequently, full-text evaluations were conducted on 39 articles. During this process, 15 studies were excluded due to the lack of a predictive model or incomplete data. Nine studies were excluded due to the lack of internal and external validation or improper grouping methods. Ultimately, 15 studies (1, 2, 4–6, 10, 11, 14, 15, 17, 18, 22, 23, 25, 29) and 24 models were included in this meta-analysis (Figure 1).
Basic characteristics included in the study
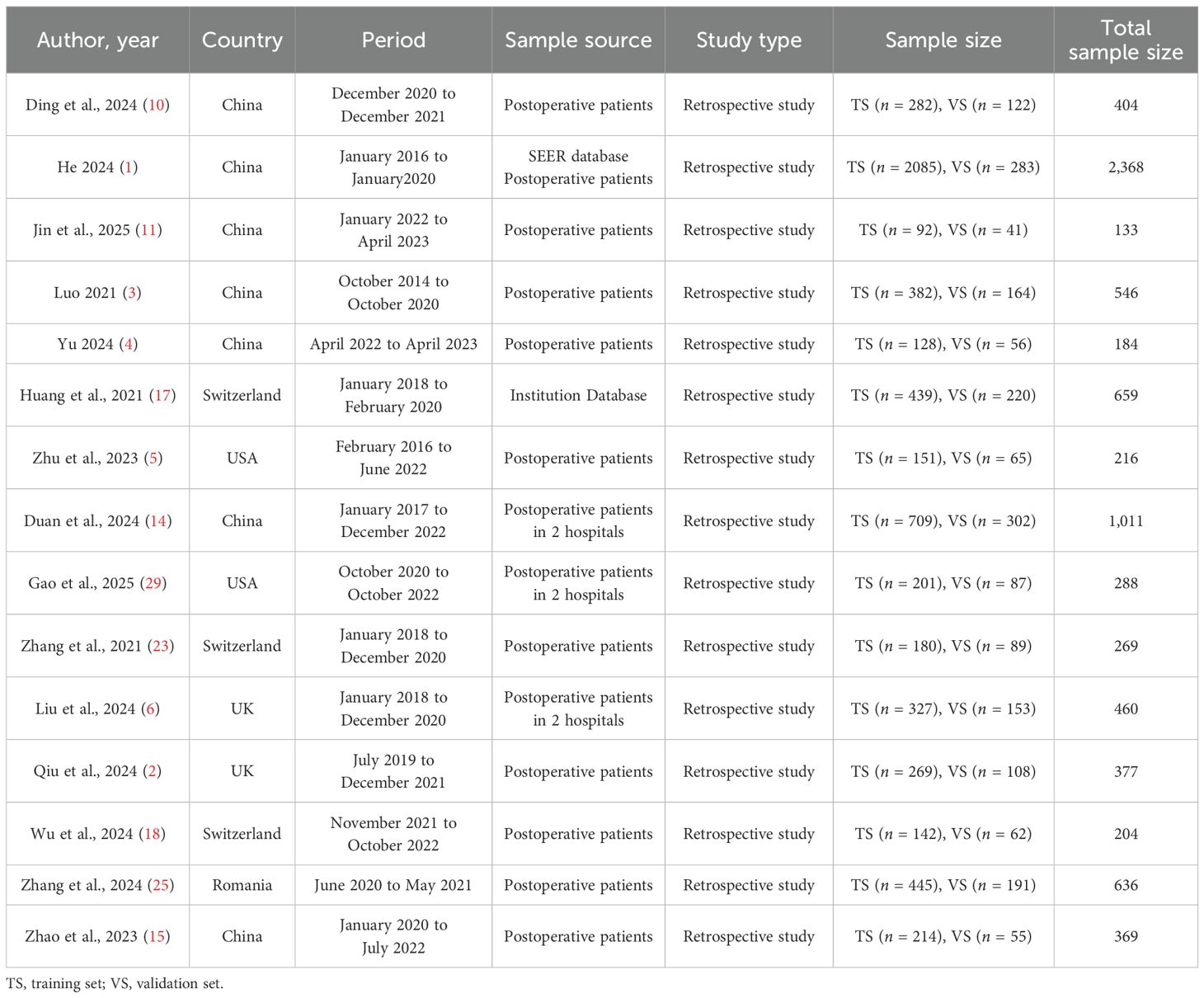
This meta-analysis included 15 retrospective studies published between 2021 and 2025, involving a total of 24 predictive models. As detailed in Table 1, the studies showed significant geographical diversity, with seven conducted in China and eight in Western countries. The total sample sizes varied considerably, ranging from 133 to 2,368 participants.
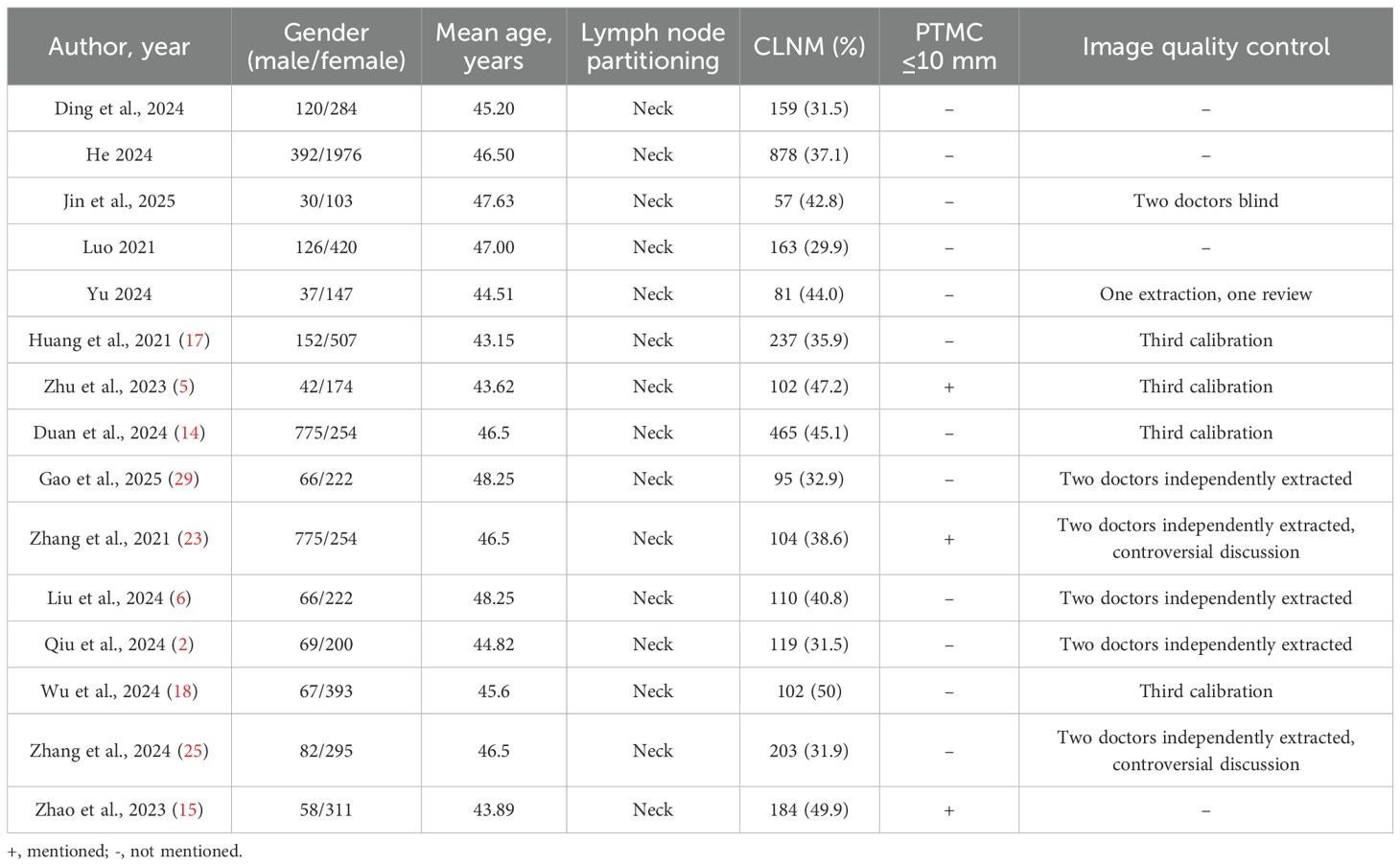
Further details on participant demographics are summarized in Table 2. The mean age of the patient cohorts was consistent across studies, ranging from 43 to 48 years. The reported rate of cervical lymph node metastasis (CLNM) was substantial, falling between 30% and 50%. A critically key methodological point highlighted in Table 2 is that only three studies explicitly confirmed that their population met the strict definition of PTMC (tumor diameter ≤10 mm), and quality control for ultrasound imaging was not reported in four of the studies.
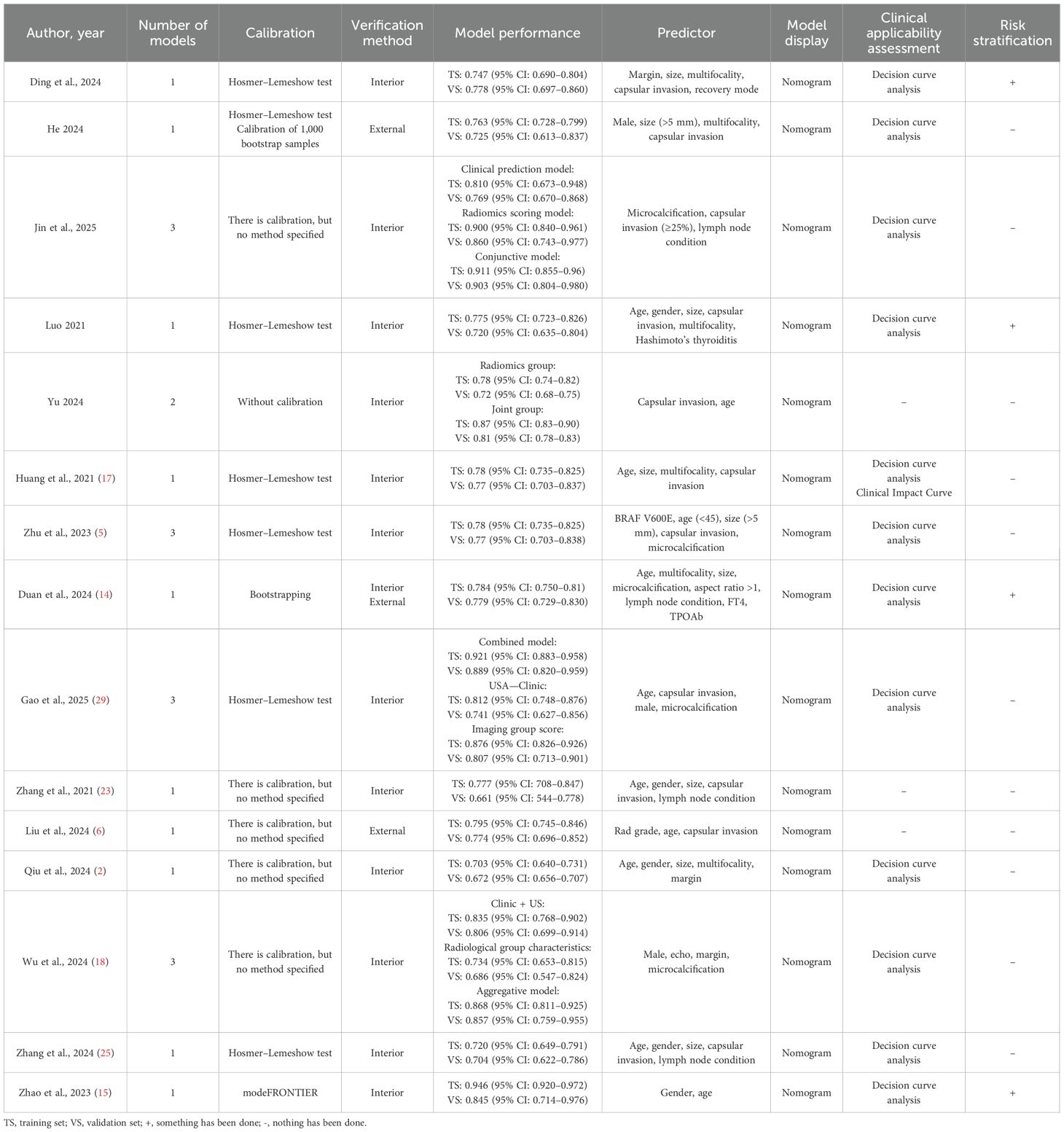
A detailed overview of the 24 prediction models is presented in Table 3. The performance of these models, as measured by the area under the curve (AUC), varied widely, with validation set AUCs ranging from a modest 0.661 to a highly discriminative 0.921. The predictors used to build these models were diverse but frequently included age, gender, tumor size, capsular invasion, and microcalcification. As shown in Table 3, while most models were presented as nomograms, their methodological follow-through varied. Although 14 studies reported some form of model calibration, the method was often unspecified. Furthermore, while 12 studies assessed clinical utility using decision curve analysis, only four attempted to define risk strata for clinical application, highlighting a common gap between model development and practical implementation.
Quality assessment results
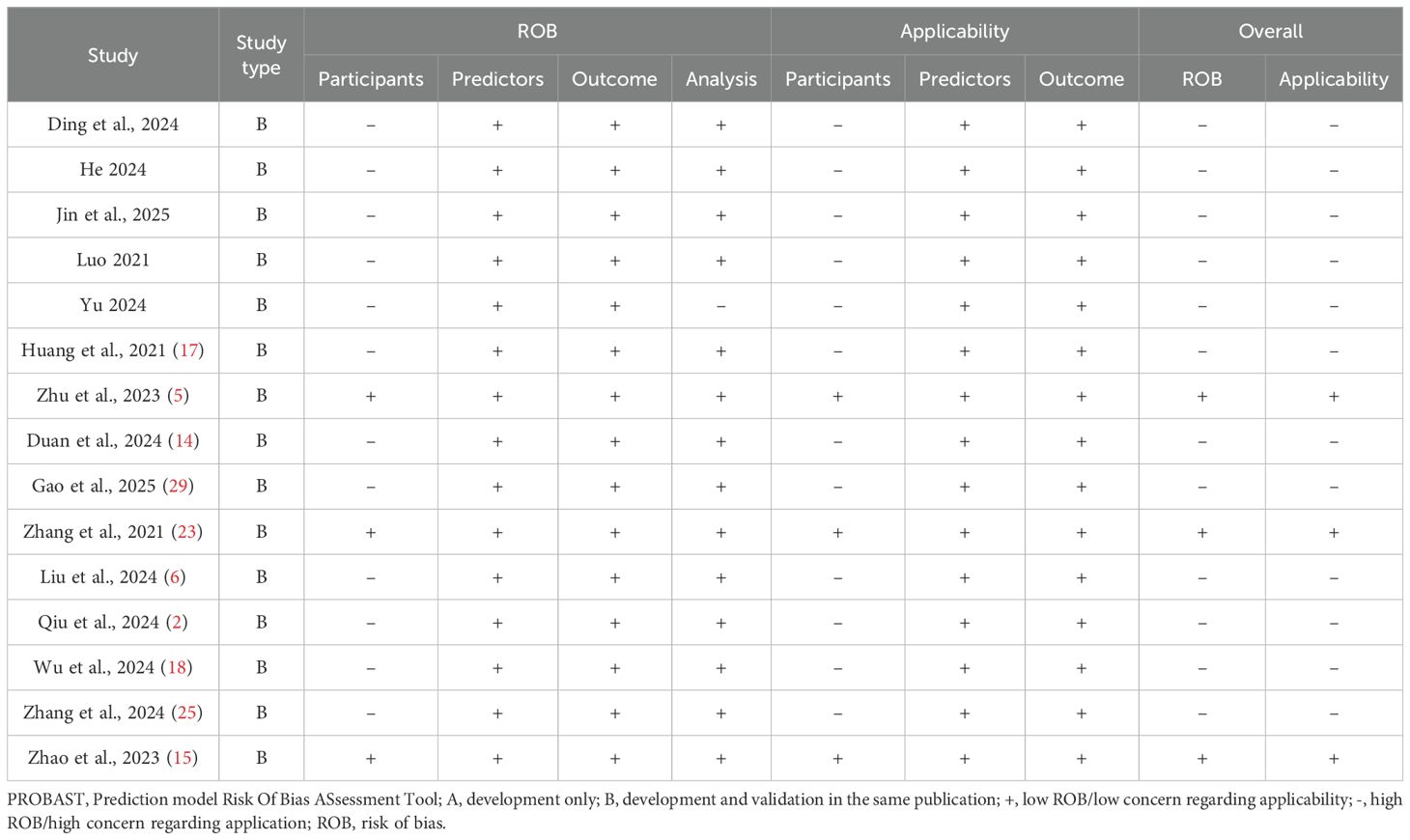
The methodological quality of the 15 included studies was assessed using PROBAST. A detailed, item-by-item evaluation of the risk of bias and applicability concerns across the four key domains for each study is presented in Table 4.
As detailed in Table 4, our analysis found that only three studies demonstrated an overall low risk of bias, while the remaining 12 were judged to have a high risk of bias. The specific domain responsible for the high risk of bias varied, namely: (1) Participant domain: This was the most common source of bias. A total of 12 studies were rated as having a high risk of bias in this domain, primarily due to the use of inappropriate inclusion criteria that did not strictly limit the tumor diameter to ≤10 mm; (2) Analysis domain: Only one study was rated as having a high risk of bias in this area. This was due to a failure to account for potential model overfitting or to assess the optimism of the model’s performance; (3) Predictors and outcome domains: All 15 studies were consistently rated as having a low risk of bias in these domains, indicating robust methods to define and measure predictors and outcomes.
In summary, the granular results in Table 4 reveal that while predictor and outcome assessments were methodologically sound, critical issues in participant selection were the predominant reason for the high risk of bias observed in the majority of the included literature.
Meta-analysis
We conducted a meta-analysis of the AUC values from 24 models reported in the 15 included studies. As shown in the forest plot (Figure 2), there was substantial heterogeneity among the studies (I2 = 89.6%, P < 0.00001), necessitating the use of a random-effects model. This high level of heterogeneity suggests a significant variability in model performance across different study populations, predictor definitions, and modeling techniques. The pooled AUC was 0.794 (95% CI: 0.769–0.820), indicating moderate to good overall discriminatory performance, though this result must be interpreted with caution given the aforementioned heterogeneity and high risk of bias in the primary studies.
Subgroup analysis
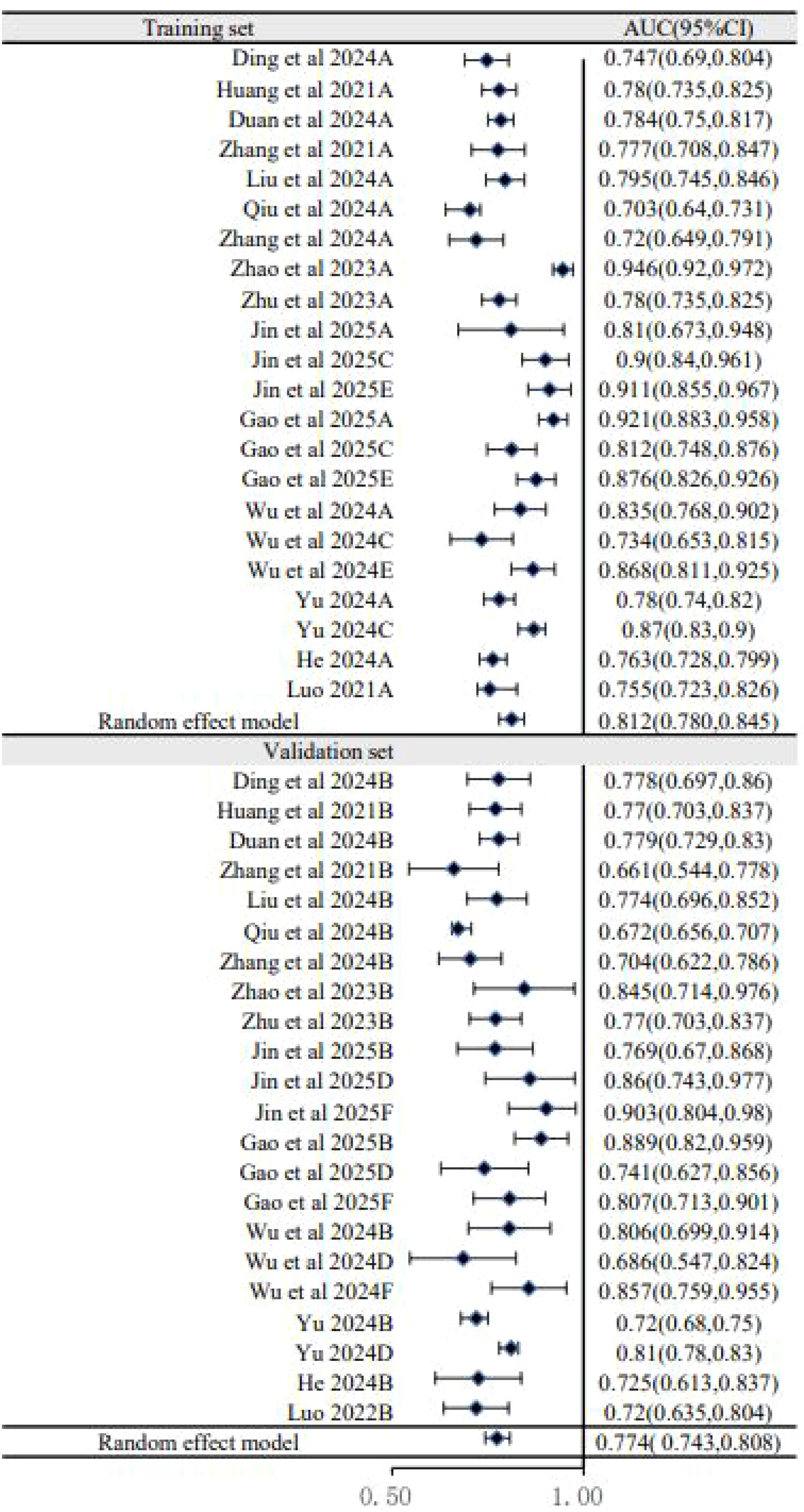
To explore sources of heterogeneity, subgroup analyses were performed. The analysis based on dataset type (Figure 3) showed that the pooled AUC of models in the training sets was 0.812 (95% CI: 0.780–0.845), which was higher than the pooled AUC in the validation sets (0.774, 95% CI: 0.743–0.808). This performance drop-off, or “optimism,” is common in prediction model studies and underscores the critical importance of external validation to assess a model’s true generalizability. A second subgroup analysis based on predictor types (Figure 4) showed that models combining US, clinical, and pathology features had a pooled AUC of 0.796 (95% CI: 0.767–0.826), while the further addition of biomarkers did not improve performance (AUC = 0.782, 95% CI: 0.763–0.802). This suggests that the biomarkers included in current models may have limited incremental predictive value over more established factors.
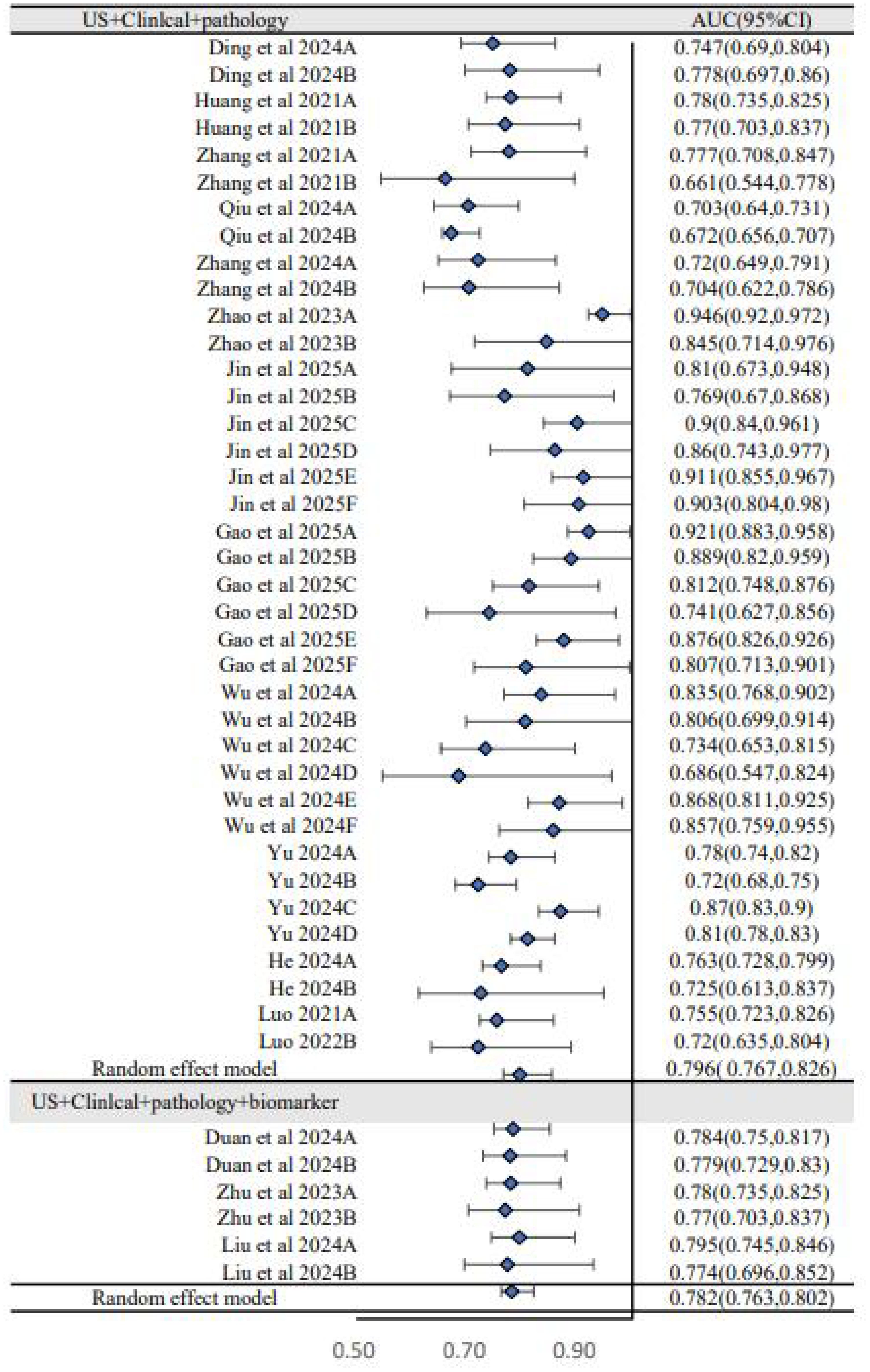
Figure 4. Subgroup analysis of forest plot (US+clinical+pathology versus US+clinical+pathology+biomarker).
Sensitivity analysis
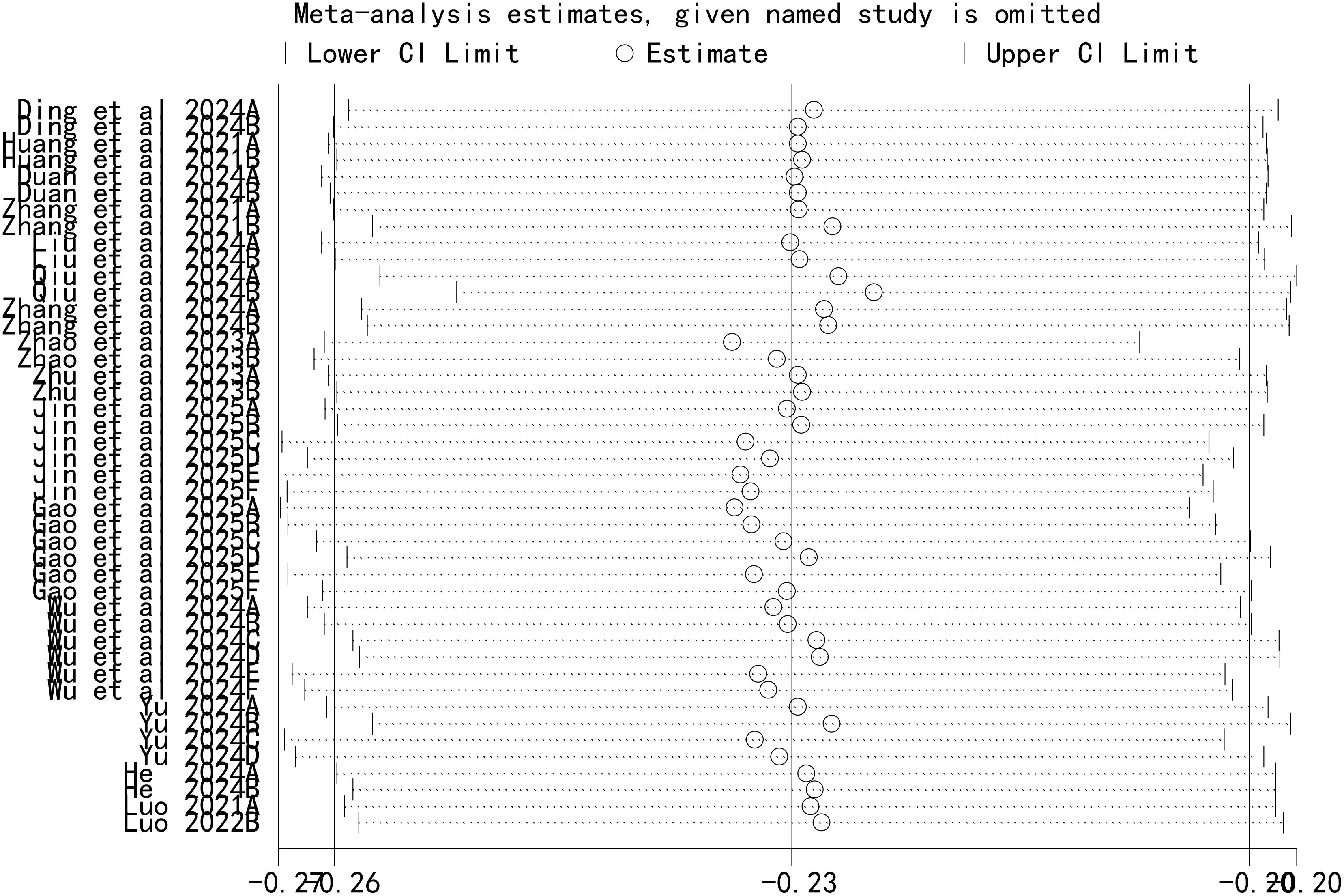
A leave-one-out sensitivity analysis was performed to assess the stability of our findings, with the results shown in Figure 5. This analysis demonstrated that the sequential removal of each individual study did not significantly alter the overall pooled AUC. This confirms that our meta-analysis results are robust and not unduly influenced by any single study.
Publication bias
Publication bias was evaluated using Egger’s test. The results showed that there was no significant publication bias in the included studies (P = 0.103).
Discussion
This systematic review and meta-analysis synthesized evidence from 15 studies involving 24 prediction models for CLNM in patients with PTMC. The principal findings are threefold, namely: first, the models demonstrate moderate to good discriminatory ability on average, with a pooled AUC of 0.794; second, this overall performance estimate is subject to considerable statistical heterogeneity (I2 = 89.6%) across studies; and third and most critically, the validity of these findings is severely threatened by the methodological quality of the underlying evidence, as 12 of the 15 included studies were judged to be at a high risk of bias according to PROBAST.
The pooled AUC of 0.794 suggests that, on paper, these models can distinguish between patients with and without CLNM reasonably well. However, this figure likely represents an overestimation of true performance. The subgroup analysis revealed a consistent drop in performance from the training sets (pooled AUC 0.812) to the validation sets (pooled AUC 0.774), a phenomenon known as “optimism” that is characteristic of models that have not been robustly validated on independent data. This performance degradation, combined with the high risk of bias identified in most studies—particularly related to participant selection and inadequate handling of potential overfitting—means that the reported performance metrics should be interpreted with extreme caution.
A significant challenge in synthesizing these models is the inconsistent role and definition of certain predictors. In terms of predictors, US and clinicopathological features such as age, gender, tumor size, and capsular invasion are the most common inclusion indicators (30). However, the contribution of some biomarkers is contentious—for instance, TPOAb was identified as a protective factor in some studies (14, 31–33), potentially through an antibody-mediated cytotoxic effect. This contradicts other reports, such as those by Sun et al. (34) and Wang et al. (35), where TPOAb was associated with an increased risk of CLNM. These discrepancies may arise from differences in study populations (e.g., underlying rates of Hashimoto’s thyroiditis), variations in TPOAb detection assays, or failure to adjust for key confounders. The predictive value of the BRAF V600E mutation likewise remains debated. While some included studies (5) found it to be a strong predictor of metastasis, consistent with a large meta-analysis by Attia et al. (36), another research by Virk et al. (37) suggests that it is not a reliable predictor in the specific context of PTMC. This highlights the need for further research to clarify the role of molecular markers before they can be reliably incorporated into clinical prediction models.
The high risk of bias found in 80% of the included studies is a major concern that limits the clinical applicability of these models. As detailed in Table 4, the primary issue was in the participant domain, where many studies failed to strictly define PTMC (≤10 mm) or used retrospective designs with inadequate sampling strategies, which can introduce selection bias. Such methodological flaws not only inflate performance metrics but also render the models unreliable for use in the specific clinical population they are intended for. Furthermore, the lack of calibration assessment in many studies is a critical omission, as a model with good discrimination (AUC) can still be clinically useless if its predicted probabilities are poorly calibrated with observed frequencies.
The strengths of our review include a comprehensive search strategy across multiple databases, duplicate data extraction and quality assessment to minimize error, and a formal risk of bias assessment using the recommended PROBAST. However, this study also has its shortcomings. Firstly, all of the included studies were retrospective studies, and there was a certain degree of bias in the included population. Secondly, most of the studies were single-center studies, and some had relatively small sample sizes. Finally, some of the included studies did not strictly control the quality of ultrasound images, and some results might have been influenced by the experience of the operators and instruments used.
Conclusion
In conclusion, while numerous CLNM risk prediction models for PTMC exist and demonstrate moderate discriminatory performance on average, their reliability and clinical utility are severely hampered by widespread methodological weaknesses and a high risk of bias. The current evidence base is not robust enough to recommend any single model for routine clinical use. Future research must prioritize methodological rigor, adhering strictly to development and reporting guidelines such as TRIPOD and PROBAST. Emphasis should be placed on large, multi-center prospective studies with independent external validation to develop truly reliable and well-calibrated models that can safely guide individualized treatment for patients with PTMC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. QZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Chongqing Joint Medical Research Project of Science and Health 2026MSXM135.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1709773/full#supplementary-material
References
1. Hualin H. To Develop and Validate a Nomogram Model for Predicting High Volume (>5) Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Hualin He: North China University of Science and Technology (2024). doi: 10.27108/d.cnki.ghelu.2024.001051.
2. Qiu P, Guo Q, Pan K, and Lin J. Development of a nomogram for prediction of central lymph node metastasis of papillary thyroid microcarcinoma. BMC Cancer. (2024) 24:235. doi: 10.1186/s12885-024-12004-3
3. Zhaoten L. Development and Validation of a Nomogram Predictive Model for Central Lymph Node Metastasis Risk in the Papillary Thyroid Microcarcinoma. Zhaoten Luo: University Of South China (2023). doi: 10.27234/d.cnki.gnhuu.2023.000555.
4. Yixing Y. Gray-scale Ultrasound-based Radiomics for Prediction of Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Yixing Yu: Henan University (2024). doi: 10.27114/d.cnki.ghnau.2024.002830.
5. Zhu D, Wu X, Zhang L, and Chen Z. Predictive value of ultrasound imaging characteristics and a BRAF V600E nomogram for central lymph node metastasis risk in papillary thyroid microcarcinoma. Altern Ther Health Med. (2023) 29:139–43.
6. Liu J, Yu J, Wei Y, Li W, Lu J, Chen Y, et al. Ultrasound radiomics signature for predicting central lymph node metastasis in clinically node-negative papillary thyroid microcarcinoma. Thyroid Res. (2024) 17:4. doi: 10.1186/s13044-024-00191-x
7. Cabanillas ME, McFadden DG, and Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/s0140-6736(16)30172-6
8. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. (2015) 136:2187–95. doi: 10.1002/ijc.29251
9. Möller M, Gustafsson U, Rasmussen F, Persson G, and Thorell A. Natural course vs interventions to clear common bile duct stones: data from the Swedish Registry for Gallstone Surgery and Endoscopic Retrograde Cholangiopancreatography (GallRiks). JAMA Surg. (2014) 149:1008–13. doi: 10.1001/jamasurg.2014.249
10. Jiaojiao D, Wei H, Junxi G, and Tao S. Risk of cervical lymph node metastasis of thyroid micropapillary carcinoma predicted by constructing a nomogram based on contrast-enhanced ultrasound features. J Xinjiang Med University. (2024) 47:39–45 + 50. doi: 10.3969/j.issn.1009-5551.2024.01.007
11. Bin J, Feng C, Lingling M, and Chenying L. Development and validation of an ultrasound radiomics-based nomogram for preoperative prediction of central lymph node metastasis in patients with papillary thyroid microcarcinoma. Zhejiang Medical. (2025) 47:1166–72. doi: 10.12056/j.issn.1006-2785.2025.47.11.2024-1739
12. Gao X, Luo W, He L, Cheng J, and Yang L. Predictors and a prediction model for central cervical lymph node metastasis in papillary thyroid carcinoma (cN0). Front Endocrinol (Lausanne). (2021) 12:789310. doi: 10.3389/fendo.2021.789310
13. Shindo M, Wu JC, Park EE, and Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. (2006) 132:650–4. doi: 10.1001/archotol.132.6.650
14. Duan S, Yang Z, Wei G, Chen S, Hu X, Ryu YJ, et al. Nomogram for predicting the risk of central lymph node metastasis in papillary thyroid microcarcinoma: a combination of sonographic findings and clinical factors. Gland Surg. (2024) 13:1016–30. doi: 10.21037/gs-24-154
15. Zhao Y, Fu J, Liu Y, Sun H, Fu Q, Zhang S, et al. Prediction of central lymph node metastasis in patients with papillary thyroid microcarcinoma by gradient-boosting decision tree model based on ultrasound radiomics and clinical features. Gland Surg. (2023) 12:1722–34. doi: 10.21037/gs-23-456
16. Zheng X, Peng C, Gao M, Zhi J, Hou X, Zhao J, et al. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: a study of 1,587 patients. Cancer Biol Med. (2019) 16:121–30. doi: 10.20892/j.issn.2095-3941.2018.0125
17. Huang C, Cong S, Shang S, Wang M, Zheng H, Wu S, et al. Web-based ultrasonic nomogram predicts preoperative central lymph node metastasis of cN0 papillary thyroid microcarcinoma. Front Endocrinol (Lausanne). (2021) 12:734900. doi: 10.3389/fendo.2021.734900
18. Wu L, Zhou Y, Li L, Ma W, Deng H, and Ye X. Application of ultrasound elastography and radiomic for predicting central cervical lymph node metastasis in papillary thyroid microcarcinoma. Front Oncol. (2024) 14:1354288. doi: 10.3389/fonc.2024.1354288
19. Hong C and Tingyue Q. Application progress of contrast-enhanced ultrasonography in thyroid carcinoma diagnosis and treatment. J Ultrasound Clin Med. (2018) 20:478–80. doi: 10.16245/j.cnki.issn1008-6978.2018.07.013
20. Zhan J and Ding H. Application of contrast-enhanced ultrasound for evaluation of thyroid nodules. Ultrasonography. (2018) 37:288–97. doi: 10.14366/usg.18019
21. Aifang B, Jianlei Z, Nini Z, Yi Y, and Yan Q. The diagnostic value of ultrasonography for the nodular goiter combined with papillary thyroid microcarcinoma. Prog Modern Biol. (2015) 15:6538–41. doi: 10.13241/j.cnki.pmb.2015.33.037
22. Qianwen L. Development and Validation of a Nomogram Predictive Model for Central Lymph Node Metastasis Risk in the Papillary Thyroid Microcarcinoma. Qianwen Luo: North China University of Science and Technology (2021). doi: 10.27108/d.cnki.ghelu.2021.000200.
23. Zhang L, Ling Y, Zhao Y, Li K, Zhao J, and Kang H. A nomogram based on clinicopathological and ultrasound imaging characteristics for predicting cervical lymph node metastasis in cN0 unilateral papillary thyroid microcarcinoma. Front Surg. (2021) 8:742328. doi: 10.3389/fsurg.2021.742328
24. Khokhar MT, Day KM, Sangal RB, Ahmedli NN, Pisharodi LR, Beland MD, et al. Preoperative high-resolution ultrasound for the assessment of Malignant central compartment lymph nodes in papillary thyroid cancer. Thyroid. (2015) 25:1351–4. doi: 10.1089/thy.2015.0176
25. Zhang X, Zhu J, Ai X, Dang M, and Huang P. An ultrasound-based nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma. Med Ultrason. (2024) 26:369–75. doi: 10.11152/mu-4411
26. Kaliszewski K, Zubkiewicz-Kucharska A, Kiełb P, Maksymowicz J, Krawczyk A, and Krawiec O. Comparison of the prevalence of incidental and non-incidental papillary thyroid microcarcinoma during 2008-2016: a single-center experience. World J Surg Oncol. (2018) 16:202. doi: 10.1186/s12957-018-1501-8
27. Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. (2019) 170:51–8. doi: 10.7326/m18-1376
28. Chen R, Wang SF, Zhou JC, Sun F, Wei WW, and Zhan SY. Introduction of the Prediction model Risk Of Bias ASsessment Tool: a tool to assess risk of bias and applicability of prediction model studies. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:776–81. doi: 10.3760/cma.j.cn112338-20190805-00580
29. Gao L, Wen X, Yue G, Wang H, Lu Z, Wu B, et al. The predictive value of a nomogram based on ultrasound radiomics, clinical factors, and enhanced ultrasound features for central lymph node metastasis in papillary thyroid microcarcinoma. Ultrason Imaging. (2025) 47:93–103. doi: 10.1177/01617346251313982
30. Sun W, Lan X, Zhang H, Dong W, Wang Z, He L, et al. Risk factors for central lymph node metastasis in CN0 papillary thyroid carcinoma: A systematic review and meta-analysis. PloS One. (2015) 10:e0139021. doi: 10.1371/journal.pone.0139021
31. Noel JE, Thatipamala P, Hung KS, Chen J, Shi RZ, and Orloff LA. Pre-operative antithyroid antibodies in differentiated thyroid cancer. Endocr Pract. (2021) 27:1114–8. doi: 10.1016/j.eprac.2021.06.014
32. Huang DM, Zhi JT, Zhang JM, Zheng XQ, Zhao JZ, Wei SF, et al. Correlations of serum TgAb and TPOAb and clinicopathological features of PTC in children and adolescents. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 57:1418–25. doi: 10.3760/cma.j.cn115330-20220927-00581
33. Li X, Zhang H, Zhou Y, and Cheng R. Risk factors for central lymph node metastasis in the cervical region in papillary thyroid carcinoma: a retrospective study. World J Surg Oncol. (2021) 19:138. doi: 10.1186/s12957-021-02247-w
34. Sun GH, Qu N, Hu JQ, Shi RL, Zhang TT, Wen D, et al. Risk for metastasis of lymph node between sternocleidomastoid and sternohyoid muscle in papillary thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 52:253–8. doi: 10.3760/cma.j.issn.1673-0860.2017.04.003
35. Wang Y, Zheng J, Hu X, Chang Q, Qiao Y, Yao X, et al. A retrospective study of papillary thyroid carcinoma: Hashimoto’s thyroiditis as a protective biomarker for lymph node metastasis. Eur J Surg Oncol. (2023) 49:560–7. doi: 10.1016/j.ejso.2022.11.014
36. Attia AS, Hussein M, Issa PP, Elnahla A, Farhoud A, Magazine BM, et al. Association of BRAF(V600E) mutation with the aggressive behavior of papillary thyroid microcarcinoma: A meta-analysis of 33 studies. Int J Mol Sci. (2022) 23:15626. doi: 10.3390/ijms232415626
Keywords: papillary thyroid microcarcinoma, lymph node metastasis, prediction model, systematic review, meta-analysis
Citation: He X, Zhang Q, Yang K, Li J, Luo M, Liu R and Yang X (2025) Risk prediction model for cervical lymph node metastasis of papillary thyroid microcarcinoma: a systematic review and meta-analysis. Front. Endocrinol. 16:1709773. doi: 10.3389/fendo.2025.1709773
Received: 21 September 2025; Accepted: 29 October 2025;
Published: 18 November 2025.
Edited by:
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, BrazilReviewed by:
Glaucia M. F. S. Mazeto, São Paulo State University, BrazilYuanyuan Yue, Chengdu First People’s Hospital, China
Copyright © 2025 He, Zhang, Yang, Li, Luo, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Yang, eWFuZ3hpXzIxMEAxMjYuY29t; Ruihan Liu, cnVpaGFuX2xAMTYzLmNvbQ==
†These authors share first authorship
 Xiaoli He
Xiaoli He Qiang Zhang
Qiang Zhang Kaiju Yang1
Kaiju Yang1 Ruihan Liu
Ruihan Liu