- 1Department of Nephrology, Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, China
- 2The First School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, China
Diabetic kidney disease (DKD) is the most common microvascular complication of diabetes and a leading cause of end-stage renal disease (ESRD). Traditionally, its pathogenesis has been attributed to hyperglycemia-induced metabolic disturbances, glomerular hyperperfusion and hyperfiltration, activation of the renin–angiotensin–aldosterone system (RAAS), and oxidative stress. Recent evidence, however, indicates that chronic inflammation and immune dysregulation also play critical roles in DKD progression.Impaired macrophage cholesterol efflux (MCE) has emerged as a central pathogenic mechanism in DKD. Under hyperglycemic conditions, advanced glycation end-products (AGEs) suppress the LXR/PPARγ signaling pathway and downregulate downstream transporters ABCA1 and ABCG1, thereby reducing cholesterol efflux. This disruption promotes lipid accumulation and macrophage foam cell formation, leading to the sustained release of pro-inflammatory cytokines such as TNF-α, IL-1β, and MCP-1, which accelerate glomerulosclerosis and tubulointerstitial fibrosis. MCE dysfunction thus provides a mechanistic link bridging metabolic dysregulation and immune-mediated inflammation in DKD.Therapeutic strategies targeting MCE show promising potential. Pharmacological agents such as LXR/RXR agonists, PPARγ activators, sodium-glucose cotransporter 2(SGLT2) inhibitors, and glucagon-like peptide-1 receptor agonists(GLP-1RAs) enhance cholesterol transport, promote macrophage polarization toward the M2 anti-inflammatory phenotype, and ameliorate renal injury. In addition, natural bioactive compounds and nanodelivery systems can selectively modulate ABCA1/G1-mediated cholesterol efflux, attenuating lipid accumulation.In conclusion, this study highlights the pivotal role of macrophage cholesterol efflux in DKD pathogenesis beyond traditional metabolic factors and proposes novel MCE-targeted therapeutic strategies, offering new insights for the prevention and treatment of DKD.
1 Introduction
Based on data from the IDF in 2019, it is estimated that approximately 463 million adults aged 20–79 worldwide are affected by diabetes, with projections indicating that this number will increase to 578.4 million by 2030. The global range of end-stage renal disease (ESRD) cases attributed to diabetes is between 10% and 67% (1). Diabetic kidney disease (DKD), a significant microvascular complication of diabetes, is a leading cause of ESRD. Recent studies have identified a strong correlation between the pathogenesis of DKD and inflammatory and immune responses. Macrophages, serving as the primary inflammatory cells, are integral to advancing DKD (2). The hyperglycemic milieu resultant from diabetes can prompt M1 polarization of macrophages, influence macrophage cholesterol efflux (MCE), and prompt their transformation into foam cells, thereby exacerbating DKD (3). This study delves into the issue of lipid metabolism disturbances in macrophages triggered by DKD, scrutinizes the physiological mechanisms of MCE, investigates the correlation between impaired MCE and DKD progression, and outlines potential strategies for reinstating MCE with the objective of retarding the progression of DKD.
2 The biological basis of macrophage cholesterol efflux
2.1 The mechanisms of macrophage cholesterol efflux
Macrophages are essential components in lipid metabolism within the human body, participating in various processes such as lipid uptake, storage, metabolism, and degradation. MCE is the term used to describe the mechanism by which macrophages transport cholesterol to the extracellular space, a vital process for regulating intracellular cholesterol levels and preventing lipid accumulation (4). Macrophage surface receptors, particularly scavenger receptors (SR), have the capability to internalize oxidized low-density lipoprotein (ox-LDL). The absorbed lipids are subsequently sequestered as lipid droplets within macrophages and are mobilized as necessary following enzymatic degradation by lipases and cholesterol esterases (4). Liver X receptor (LXR) and peroxisome proliferator-activated receptor gamma (PPARγ) play pivotal roles as transcription factors in the regulation of macrophage cholesterol metabolism. They enhance MCE by inducing the upregulation of ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) (5, 6). Activation of cholesterol-dependent LXR/retinoid X receptor (RXR) transcription factors occurs when there is a need to export lipids from macrophages or in cases of lipid overload. These transcription factors target the DR4 sites within the proximal promoters of the ABCA1 gene, thereby modulating the expression of lipid transport proteins like ABCA1 on the macrophage surface (7). The ABCA1-mediated pathway is the principal mechanism by which cholesterol is transported out of macrophages (8, 9). This pathway involves the creation of a plasma membrane microdomain by ABCA1, which aids in the transfer of phospholipids and cholesterol to apolipoprotein A-I (ApoA-I). Subsequently, the lipidated ApoA-I, also known as nascent high-density lipoprotein (HDL) particles, acquire additional cholesterol through the ABCG1-mediated efflux pathway, leading to the formation of mature HDL particles. These mature HDL particles then interact with the scavenger receptor class B type I (SR-BI) on the cell surface. The extracellular domain of SR-BI functions as a nonpolar channel facilitating cholesterol exchange (10–12). Studies have shown that SR-BI exhibits the greatest binding affinity for large, spherical HDL particles. In contrast, ABCA1 primarily binds and cross-links lipid-poor ApoA-I, demonstrating minimal interaction with smaller HDL3 and no interaction with larger HDL2 subtypes (13, 14). Consequently, the interaction between ABCA1 and the predominant HDL subtypes present in plasma is restricted. ABCA1 is believed to have a central role in the initiation of cholesterol efflux from macrophages and other cells to lipid-poor apolipoproteins, whereas SR-BI primarily aids in the removal of cholesterol and cholesterol esters from large HDL particles. Furthermore, mature HDL particles can also acquire cholesterol through aqueous diffusion (AD). AD involves a straightforward diffusion mechanism in which cholesterol esters disengage from the endoplasmic reticulum or HDL particles, and are transported back to the cell membrane through cholesterol transporters such as ABCA1 and ABCG1. Subsequently, these cholesterol esters are released into the extracellular milieu, gathered, and conveyed to the liver by HDL particles for the synthesis of bile acids (4).
2.2 The physiological role of macrophage cholesterol efflux
Throughout the process of monocyte differentiation into macrophages, notable alterations in lipid metabolism frequently take place. Due to the lack of regulation by cellular cholesterol levels, SR can lead to uncontrolled uptake of modified LDL particles, surpassing the cell’s ability to store and release lipids. This results in the accumulation of cholesterol within macrophages and the development of foam cells (4). An overabundance of cholesterol uptake disrupts cellular equilibrium, leading to the activation of inflammatory signaling pathways that control the generation of reactive oxygen species (ROS), oxidative cytokines, and chemokines, exacerbating the situation (15). The formation of foam cells serves as an initial indicator of the development of atherosclerotic plaques. By facilitating cholesterol efflux, macrophages can uphold intracellular homeostasis and impede the onset and advancement of this process (16). Furthermore, MCE has been shown to effectively mitigate intracellular cholesterol accumulation, attenuate the activation of inflammatory pathways, suppress the release of inflammatory cytokines, and modulate macrophage polarization, thereby exerting a significant influence on both local and systemic inflammatory processes (15). Additionally, the regulation of MCE has the potential to enhance systemic insulin sensitivity, thereby aiding in the prevention and management of diabetes and its associated complications (17).
3 The relationship between macrophage cholesterol efflux and diabetic kidney disease
3.1 The impact of a high-glucose environment on macrophage cholesterol efflux
An elevated glucose concentration in the renal blood vessels can stimulate inflammatory reactions and the secretion of cytokines and macrophage chemoattractant protein-1 (MCP-1), which attract macrophages. These macrophages utilize Toll-like receptors 2 and 4 to internalize saturated fatty acids, activating themselves and initiating inflammatory signaling cascades involving interferon regulatory factor 3 (IRF3), activator protein 1 (AP1), and nuclear factor κB (NF-κB). This ultimately results in the impairment of glomerular endothelial cells and mesangial cells (18, 19). The inflammatory mediators generated as a result can exacerbate LDL oxidation and suppress the transcription of LXR and PPARγ, consequently diminishing the levels of ABCA1 and ABCG1 expression, leading to impaired MCE. A study by Aécio Lopes de Araújo Lira et al. revealed that albumin derived from individuals with Type 2 Diabetes Mellitus and an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m² undergoes heightened carbamylation, impacting MCE facilitated by HDL2 and HDL3. This process may facilitate the accumulation of lipids in macrophages and disrupt the mechanism of reverse cholesterol transport (RCT). Prior research has demonstrated that albumin extracted from the serum of individuals with poorly controlled diabetes or from rats with induced uremia can hinder macrophage reverse cholesterol transport by decreasing the expression of ABCA1 and ABCG1 (20). Correspondingly, Joseph et al. observed a notable decrease in ABCA1 and ABCG1 levels in mesangial cells of mice with diabetic nephropathy, aligning with these results (21). Furthermore, the buildup of advanced glycation end products (AGEs) may stimulate the secretion of inflammatory cytokines such as interleukin-8 (IL-8). Xiaoer Tang et al. conducted experiments on cholesterol transport to show that IL-8 effectively suppresses ApoA-I-mediated, ABCA1-dependent MCE by upregulating miR-18322 expression (22). (Shown in Figure 1).
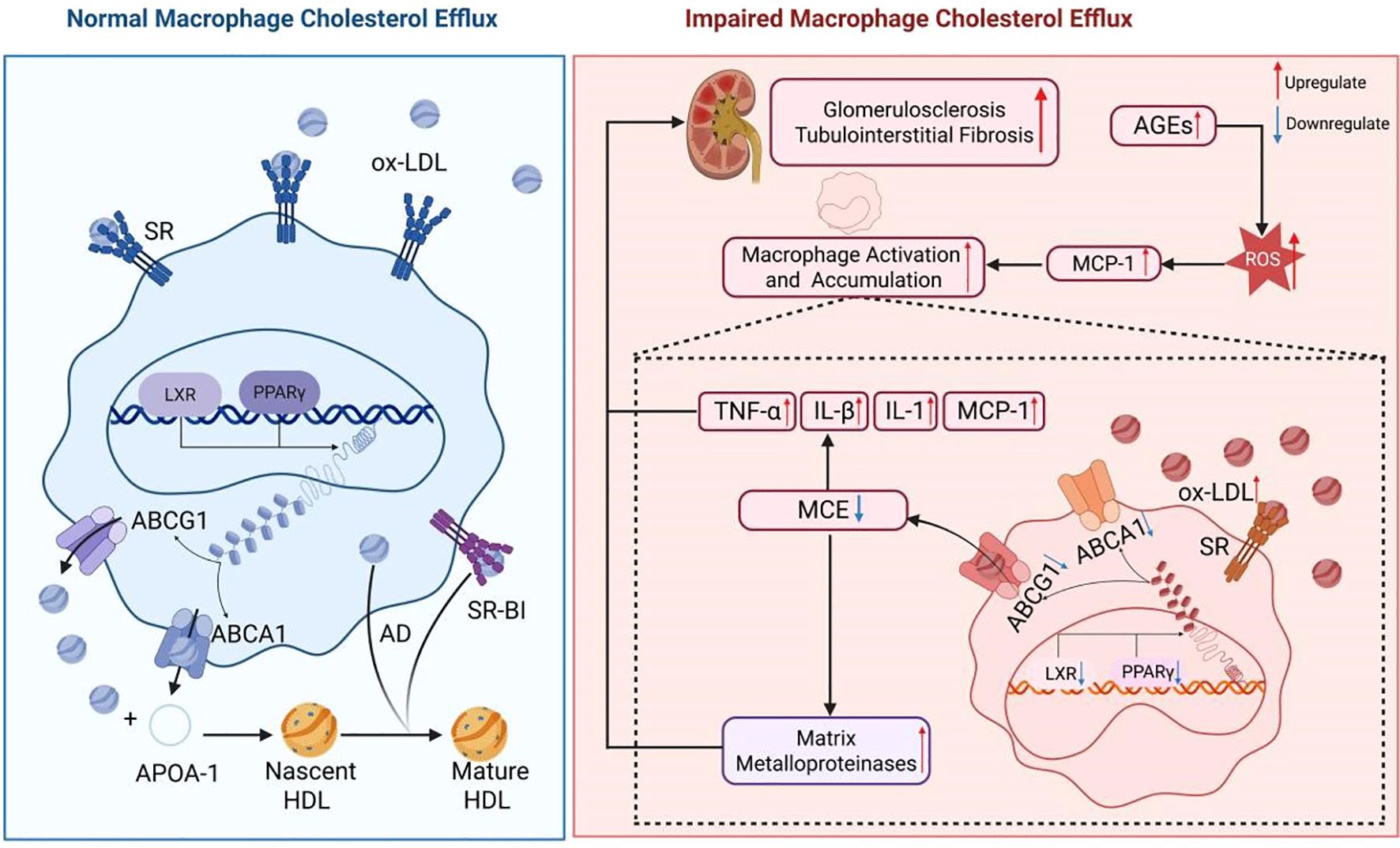
Figure 1. Effects of impaired macrophage cholesterol efflux in diabetic kidney disease. In the presence of elevated glucose levels, the body initiates an inflammatory cascade by releasing pro-inflammatory cytokines including TNFα, IL-8, IL-1β, and MCP-1. This process results in a notable influx of macrophages into renal tissue, leading to the injury of glomerular endothelial cells and mesangial cells. Furthermore, the released inflammatory mediators promote LDL oxidation by interacting with Toll-like receptors on the macrophage surface. The activation of IRF3, NF-κB, and AP1 leads to the release of inflammatory factors from macrophages and the generation of ROS, thereby exacerbating renal inflammation. Furthermore, these inflammatory factors suppress the expression of nuclear transcription factors LXR and PPARγ, impacting the expression of ABCA1 and ABCG1 genes, ultimately resulting in impaired cholesterol efflux in macrophages.
2.2 The impact of impaired macrophage cholesterol efflux on diabetic kidney disease
Renal biopsies of patients with DKD demonstrate that macrophages are the predominant infiltrating leukocytes in both the glomeruli and tubulointerstitium. The degree of macrophage infiltration is positively associated with the development of glomerulosclerosis, tubular atrophy, and interstitial fibrosis (23). Dysregulation of macrophages, characterized by increased cholesterol uptake and subsequent production of inflammatory mediators, can lead to further complications such as LDL oxidation, endothelial cell activation, and recruitment of monocytes. Ox-LDL has been shown to induce the secretion of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and MCP-1, leading to increased macrophage infiltration in renal tissue and worsening kidney damage (24, 25). Additionally, the accumulation of foam cells, originating from macrophages, in the glomeruli and tubules plays a crucial role in the development of glomerulosclerosis and interstitial fibrosis. The matrix metalloproteinases released by foam cells have the ability to break down the extracellular matrix, disrupt the glomerular basement membrane, and damage interstitial structures, thereby hastening the progression of glomerulosclerosis and tubular fibrosis (26). Furthermore, compromised MCE leads to excessive production of ROS by macrophages, resulting in direct harm to glomerular and tubular cells and exacerbating renal damage (27). The accumulation of intracellular lipids due to impaired MCE also contributes to increased cell membrane stiffness, which impairs the macrophage’s ability to recognize and bind apoptotic cells. This disruption hinders the signaling of receptors like MERTK, suppresses the expression of genes related to phagocytosis, and diminishes phagocytic effectiveness, ultimately exacerbating kidney injury and inflammatory reactions (28). (Shown in Figure 1).
2.3 Association between impaired macrophage cholesterol efflux and clinical biomarkers in diabetic kidney disease
MCE represents a critical pathological mechanism in metabolic diseases such as DKD and shows strong correlations with several clinical biomarkers. In a cohort study involving 220 patients with diabetes and 70 healthy controls, Ahmet Karatas et al. reported that the monocyte/HDL ratio (MHR) was significantly higher in patients with DKD compared with those with normoalbuminuric diabetes and healthy individuals. Moreover, MHR was positively associated with the urinary albumin-to-creatinine ratio (UACR), suggesting its potential as a biomarker for monitoring proteinuria progression in DKD (29). Similarly, Barati F et al. demonstrated that exposure of the human monocyte cell line U937 to platelets and ox-LDL(80 µg/ml) markedly upregulated the expression of CD36, ABCA1, SR-B1, ACAT1, and LXRα in macrophages, implying that elevated platelet activation and ox-LDL accumulation may contribute to cholesterol imbalance and foam cell formation (30). Therefore, assessment of ox-LDL levels and platelet activity may provide valuable insight into the progression of DKD. Furthermore, cathepsins L and S have been found to promote LDL degradation while suppressing cholesterol efflux, exacerbating lipid accumulation and foam cell development. Elevated serum levels of these proteases may thus serve as indirect indicators of MCE impairment in DKD (31).
4 Potential therapeutic strategies to restore macrophage cholesterol efflux in diabetic kidney disease
4.1 Targeting lipid transport proteins regulation
Transcription factors LXR/RXR regulate genes that mediate MCE (APOE, ABCA1, and possibly ABCG1), transport (LPL, CETP, and several genes encoding ApoC isoforms), cholesterol conversion to bile acids (CYP7A), and metabolism and excretion into bile or intestinal lumen (ABCG5 and ABCG8) (7). LXR agonists have been shown to decrease lipid accumulation in macrophages that infiltrate renal tissue, thereby mitigating the activation of multiple signaling pathways, including JNK1, JNK2, and NF-κB, resulting in decreased levels of pro-inflammatory cytokines such as IL6 and TNFα (21). Furthermore, treatment of macrophages with LXR or RXR activators can enhance ABCA1 mRNA expression and facilitate cholesterol efflux to ApoA-I (7). For example, Tall et al. demonstrated that administration of LXR agonists to Apo E KO mice resulted in increased expression of ABCA1 and ABCG1 mRNA in lesions (7). Similarly, LXR-623, an LXRβ agonist, has been shown to produce similar effects (32). Currently, two synthetic LXR agonists (T0901317 and GW3965) have been identified as potential therapeutic agents for DKD, primarily through the upregulation of sterol regulatory element-binding transcription factor 1c (SREBP-1c) to stimulate lipogenesis, leading to excessive secretion of triglycerides into the systemic circulation. Nevertheless, the clinical application of these therapies is impeded by hepatotoxicity. In response to this challenge, researchers have engineered innovative sHDL nanoplatforms featuring a KT peptide surface and a hydrophobic core incorporating LXR agonists. These nanoplatforms have the ability to circumvent the glomerular filtration barrier, facilitate mesangial retention, and augment cholesterol efflux, thereby presenting a potentially efficacious therapeutic option for individuals with DKD (21).PPARγ, a crucial transcription factor in macrophage cholesterol metabolism, has the potential to enhance ABCA1 transcription and cholesterol efflux by upregulating LXRα and ABCA1 sequentially (33). Recent studies have demonstrated that mangiferin may stimulate MCE through the PPARγ-LXRα-ABCA1/G1 pathway (34). Nevertheless, despite the identification of a genuine PPARγ response element in the LXRα gene, recent studies have not been able to validate the induction of ABCA1 mRNA and cholesterol efflux in macrophages by PPARγ agonists like troglitazone or rosiglitazone (35–37). It is proposed that the limitation in ABCA1 expression in differentiated cells may be attributed to the availability of LXR/RXR ligands rather than the abundance of LXRs. Due to the limited responsiveness of PPARγ to endogenous fatty acids, it has been observed that oxidized derivatives of fatty acids, specifically those containing circulating ox-LDL, can effectively stimulate PPARγ activation and facilitate the efflux of cholesterol (5).What’s more,the primary role of microRNA miR-33a, situated within the intron of the transcription factor sterol regulatory element-binding protein 2 (SREBP-2), is to suppress the expression of ABCA1 (38). Research conducted by Jenika D. Marshall demonstrated that lipoprotein lipase (LPL) hinders the gene expression of ABCA1, ABCG1, and SR-BI via the Akt pathway after 18 hours, establishing LPL as a crucial mediator in the process through which it inhibits cholesterol efflux (39). Consequently, the inhibition of microRNA miR-33a and Akt has the potential to enhance cholesterol efflux in macrophages. Another approach to augment efflux capacity involves the utilization of cholesterol ester transfer protein (CETP) inhibitors, which elevate HDL levels by way of the ABCG1 transporter (5). Furthermore, research has demonstrated that resolvin T4 (RvT4) can stimulate MCE through the SR-BI-neutral cholesterol ester hydrolase (NCEH) pathway (40).
Targeting ABCA1 and ABCG1 to regulate cholesterol efflux in MCE pathways is a promising strategy.While reduced ABCA1 expression alone is not enough to induce DKD, experimental manipulation through genetic or pharmacological means to increase ABCA1 levels has shown promising results in mitigating kidney disease progression, indicating ABCA1 as a potential target for therapeutic intervention. Experimental investigations have demonstrated that Tetramethylpyrazine-Paeoniflorin (TP), sorbitol A, gypenoside monomer–gypenoside XVII (GP-17), curcumin, LCBP, and 17β-estradiol estrogen receptor A can enhance MCE and inhibit lipid accumulation through the upregulation of ABCA1 and ABCG1 expression (41–46). Qianxia Yin et al. demonstrated that photobiomodulation therapy (PBMT) enhances ABCA1 expression and facilitates cholesterol efflux in lipid-loaded primary peritoneal macrophages through the activation of the phosphatidylinositol 3-kinase/protein kinase C zeta/specific protein 1 signaling cascade (47). Conversely, Min Zhang and team identified that stabilizing ABCA1 through the inhibition of protein degradation can elevate ABCA1 levels and improve cholesterol efflux (48).
4.2 Anti-inflammatory strategies
Excessive cholesterol accumulation in macrophages situated in an inflammatory microenvironment triggers the activation of the Triggering Receptor Expressed on Myeloid Cells 2(TREM2) and AMP-activated protein kinase (AMPK) signaling pathways. This activation subsequently leads to the upregulation of LXR, which in turn facilitates the expression of downstream genes such as ABCA1 and ABCG1, thereby modulating lipid metabolism in macrophages (49, 50). When the lipid burden surpasses the macrophage’s intrinsic regulatory abilities, a transformation into foam cells occurs. The collective action of inflammasomes and ox-LDL intensifies macrophage oxidative stress and inflammatory reactions, consequently impeding MCE (15). Hence, the implementation of anti-inflammatory approaches is crucial in enhancing MCE. Jun Mei et al. discovered that TP has the ability to decrease the secretion of TNFα, IL-1β, and MCP-1 induced by ox-LDL, which is a significant finding in the context of inflammation alleviation (41). Additionally, Metformin, a widely prescribed medication for diabetes, has been shown to effectively slow down the progression of DKD by enhancing cholesterol efflux and lipid metabolism via the activation of the AMPK pathway (51).As emerging therapeutic agents for DKD, sodium-glucose cotransporter 2 (SGLT2) inhibitors induce glycosuria, thereby shifting the primary cellular energy substrate from glucose to free fatty acid (FFA) oxidation. This metabolic reprogramming helps decrease intracellular levels of toxic lipid intermediates in podocytes, mesangial cells, and proximal tubular cells, ultimately mitigating kidney injury caused by excessive cholesterol accumulation (52). Moreover, glucagon-like peptide-1 receptor agonists (GLP-1RAs) have been shown to suppress activation of the NF-κB inflammatory pathway and reduce the release of proinflammatory cytokines such as IL-6, TNF-α, and MCP-1, while promoting macrophage polarization toward the anti-inflammatory M2 phenotype rather than the proinflammatory M1 phenotype—thus exerting beneficial effects on macrophage cholesterol metabolism. Clinically used GLP-1RAs, including exenatide and liraglutide, have been demonstrated to lower IL-10 levels in the kidneys of diabetic mice, an effect further confirmed in vitro in human monocytes (53).
Macrophage polarization involves the differentiation of macrophages into distinct functional states in response to various signals within the microenvironment, resulting in the classification of M1 macrophages as pro-inflammatory and M2 macrophages as anti-inflammatory (54). In a pro-inflammatory setting, M1 macrophages have been shown to suppress the expression and activity of ABCA1 and ABCG1, thereby promoting intracellular cholesterol buildup (55). Hence, enhancing the M1/M2 conversion of macrophages may lead to improved cholesterol efflux function. For instance, the activation of the miR-182-5p/HDAC9 signaling pathway by GP-17 can facilitate the transition of macrophages to the M2 phenotype (43). In order to enhance both cellular cholesterol efflux and targeted drug delivery to macrophages, researchers have developed a ROS-responsive PF/TC-AT-d-rHDL, which effectively enhances cholesterol clearance in foam cells upon exposure to ROS, inhibits intracellular lipid deposition, and promotes M2 polarization of macrophages (56). Moreover, research conducted by Saba Soltani et al. has demonstrated that PON1 and PON2, both paraoxonases implicated in HDL-mediated cholesterol efflux, play a crucial role in safeguarding cholesterol-laden foam cells. Specifically, PON1 functions to diminish ox-LDL formation, while PON2 acts as a defense mechanism against oxidative stress. Consequently, enhancing the activity of PON1 and PON2 has the potential to mitigate cholesterol accumulation and facilitate efflux (5). Additionally, lipophagy, a specialized autophagic process responsible for degrading intracellular lipid droplets (LDs), liberates free cholesterol and fatty acids. Promoting cholesterol efflux can be achieved by enhancing lipophagy through the selective knockdown of genes located on lipid droplets, including SQSTM1/p62, NBR1, and OPTN (57).
4.3 Interventions Targeting the MCE Pathway and Their Potential for Prognostic Improvement
Emerging therapeutic agents for DKD, including liraglutide and canagliflozin, have been shown to activate AMPK and downstream MCE-related signaling pathways, highlighting the central role of MCE in metabolic regulation, inflammation resolution, and renal protection (58, 59). In the SUSTAIN-6 trial, treatment with the GLP-1 receptor agonist semaglutide significantly reduced the risk of new-onset or worsening nephropathy compared with placebo (HR = 0.64, 95% CI: 0.46–0.88; P < 0.01), suggesting that its renoprotective effects may be mediated through improved metabolic homeostasis and anti-inflammatory mechanisms (60). Likewise, findings from the CANVAS program demonstrated that the SGLT2 inhibitor canagliflozin slowed the decline in eGFR, reduced UACR levels, and lowered the incidence of composite renal outcomes, further supporting its protective role against renal function deterioration (61, 62). Collectively, these data indicate that modulation of MCE may represent a pivotal mechanism by which metabolic agents confer renal benefits. In the future, therapeutic strategies targeting MCE may drive a paradigm shift in DKD management—from conventional metabolic control toward integrated immunometabolic modulation—thereby offering new opportunities for precision treatment and long-term outcome improvement. However, it is worth noting that direct pharmacological modulators of MCE remain at the preclinical research stage, and further studies are warranted to translate these findings into clinical practice (63).
5 Conclusion
Recent research has elucidated the mechanism of MCE, highlighting the importance of lipid metabolism in macrophages, particularly the role of surface proteins ABCA1 and ABCG1 in transporting cholesterol bound to ApoA-I and HDL for recycling in the liver. A stable lipid metabolism in macrophages is essential for internal homeostasis and the inhibition of inflammatory mediator release. Recent studies have shown that modulating lipid transport proteins, crucial transcription factors in cholesterol metabolism, and anti-inflammatory approaches can effectively modulate macrophages to enhance cholesterol efflux. Despite advancements in comprehending MCE, there are still constraints.Most studies have focused on the link between MCE and atherosclerosis, whereas direct investigations of MCE in the context of DKD, both in experimental models and clinical settings, remain limited. Moreover, although certain novel agents can indirectly enhance MCE, no therapeutics specifically targeting MCE have yet been developed or approved for clinical use.
Author contributions
JW: Writing – review & editing, Writing – original draft. YC: Writing – original draft. YF: Writing – review & editing. QZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of China for Young Scholars (Grant No. 82205008); Medical Scientific Research Foundation of Zhejiang Province, China (Grant No. 2023RC242); Hangzhou Municipal Health Commission Project(Grant No.A20210083); Zhejiang Traditional Medicine and Technology Program, China (Grant No. 2023ZF137); Key research project of the research project of Zhejiang University of Traditional Chinese Medicine Affiliated Hospital(Grant No.2022FSYYZZ14).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MCE, Macrophage cholesterol efflux;ESRD, End-stage renal disease;DKD, Diabetic kidney disease; RAAS, Renin–angiotensin–aldosterone system;SR, Scavenger receptors;ox-LDL, Oxidized low-density lipoprotein;LXR, Liver X receptor;PPARγ:Peroxisome proliferator-activated receptor gamma;ABCA1:ATP-binding cassette transporters A1;ABCG1:ATP-binding cassette transporters G1;RXR, Retinoid X receptor;ApoA-I:Apolipoprotein A-I;SR-BI, Scavenger receptor class B type I;HDL, High-Density Lipoprotein;AD, Aqueous diffusion;ROS, Reactive oxygen species;MCP-1:Macrophage chemoattractant protein-1;IRF3:Interferon regulatory factor 3;AP1:Activator protein 1;NF-κB:Nuclear factor κB;eFR, Estimated glomerular filtration rate;RCT, Reverse cholesterol transport;AGEs, Advanced glycation end products;IL-8:Interleukin-8;TNF-α:Tumor necrosis factor-α;IL-1β:Interleukin-1β;MHR, Monocyte/HDL ratio; TP, Tetramethylpyrazine-Paeoniflorin;GP-17:Gypenoside XVII;PBMT, Photobiomodulation therapy;SREBP-2:Sterol regulatory element-binding protein 2;LPL, Lipoprotein lipase;RvT4:Resolvin T4;NCEH, Neutral cholesterol ester hydrolase;SREBP-1c:Sterol regulatory element-binding transcription factor 1c;TREM2:Triggering Receptor Expressed on Myeloid Cells 2;AMPK, AMP-activated protein kinase;LDs, Lipid droplets; SGLT2:Sodium-glucose cotransporter 2;FFA, Free fatty acid;GLP-1RAs, Glucagon-like peptide-1 receptor agonists.
References
1. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086
2. Wada J and Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol. (2016) 12:13–26. doi: 10.1038/nrneph.2015.175
3. Lin DW, Yang TM, Ho C, Shih YH, Lin CL, and Hsu YC. Targeting macrophages: therapeutic approaches in diabetic kidney disease. Int J Mol Sci. (2024) 25:4350. doi: 10.3390/ijms25084350
4. Choi HY, Ruel I, Choi S, and Genest J. New strategies to promote macrophage cholesterol efflux. Front Cardiovasc Med. (2021) 8:795868. doi: 10.3389/fcvm.2021.795868
5. Soltani S, Boozari M, Cicero AFG, Jamialahmadi T, and Sahebkar A. Effects of phytochemicals on macrophage cholesterol efflux capacity: Impact on atherosclerosis. Phytother Res PTR. (2021) 35:2854–78. doi: 10.1002/ptr.6991
6. Ye Y, Liu J, Guo Y, Gao Y, Rao J, Su R, et al. PPARγ Ameliorates Mycobacterium tuberculosis H37Ra-Induced Foamy Macrophage Formation via the ABCG1-Dependent Cholesterol Efflux Pathway in THP-1 Macrophages. Front Microbiol. (2022) 13:829870. doi: 10.3389/fmicb.2022.829870
7. Tall AR, Costet P, and Wang N. Regulation and mechanisms of macrophage cholesterol efflux. J Clin Invest. (2002) 110:899–904. doi: 10.1172/JCI16391
8. Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. (2012) 125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589
9. Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. (2007) 48:2453–62. doi: 10.1194/jlr.M700274-JLR200
10. Genest J, Schwertani A, and Choi HY. Membrane microdomains and the regulation of HDL biogenesis. Curr Opin Lipidol. (2018) 29:36–41. doi: 10.1097/MOL.0000000000000470
11. Segrest JP, Jones MK, Catte A, Manchekar M, Datta G, Zhang L, et al. Surface density-induced pleating of a lipid monolayer drives nascent high-density lipoprotein assembly. Struct Lond Engl. (2015) 1993:23, 1214–1226. doi: 10.1016/j.str.2015.05.010
12. Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. (2008) 263:256–73. doi: 10.1111/j.1365-2796.2007.01898.x
13. Liadaki KN, Liu T, Xu S, Ishida BY, Duchateaux PN, Krieger JP, et al. Binding of high density lipoprotein (HDL) and discoidal reconstituted HDL to the HDL receptor scavenger receptor class B type I. Effect of lipid association and APOA-I mutations on receptor binding. J Biol Chem. (2000) 275:21262–71. doi: 10.1074/jbc.M002310200
14. Wang N, Silver DL, Thiele C, and Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. (2001) 276:23742–7. doi: 10.1074/jbc.M102348200
15. Zhuo S, Song S, Wang C, Wang Z, Zhang M, Lin D, et al. Inflammatory corpuscle AIM2 facilitates macrophage foam cell formation by inhibiting cholesterol efflux protein ABCA1. Sci Rep. (2024) 14:10782. doi: 10.1038/s41598-024-61495-4
16. Nyandwi JB, Ko YS, Jin H, Yun SP, Park SW, and Kim HJ. Rosmarinic acid increases macrophage cholesterol efflux through regulation of ABCA1 and ABCG1 in different mechanisms. Int J Mol Sci. (2021) 22:8791. doi: 10.3390/ijms22168791
17. Lee JH, Lee SH, Lee EH, Cho JY, Song DK, Lee YJ, et al. SCAP deficiency facilitates obesity and insulin resistance through shifting adipose tissue macrophage polarization. J Adv Res. (2023) 45:1–13. doi: 10.1016/j.jare.2022.05.013
18. Xie S and Xu X. Role of pyruvate kinase M20-regulated glycolysis in inflammatory activation of macrophages in diabetic nephropathy. J Army Med Univ. (2020) 42:2394–402. doi: 10.16016/j.1000-5404.202007015
19. Mitrofanova A, Fontanella AM, Merscher S, and Fornoni A. Lipid deposition and metaflammation in diabetic kidney disease. Curr Opin Pharmacol. (2020) 55:60–72. doi: 10.1016/j.coph.2020.09.004
20. de Araújo Lira AL, de Fátima Mello Santana M, de Souza Pinto R, Minanni CA, Iborra RT, de Lima AMS, et al. Serum albumin modified by carbamoylation impairs macrophage cholesterol efflux in diabetic kidney disease. J Diabetes Complications. (2021) 35:107969. doi: 10.1016/j.jdiacomp.2021.107969
21. He H, Halseth TA, Mei L, Shen C, Liu L, and Schwendeman A. Nanodisc delivery of liver X receptor agonist for the treatment of diabetic nephropathy. J Control Release Off J Control Release Soc. (2022) 348:1016–27. doi: 10.1016/j.jconrel.2022.06.029
22. Tang XE, Li H, Chen LY, Xia XD, Zhao ZW, Zheng XL, et al. IL-8 negatively regulates ABCA1 expression and cholesterol efflux via upregulating miR-183 in THP-1 macrophage-derived foam cells. Cytokine. (2019) 122:154385. doi: 10.1016/j.cyto.2021.155597
23. Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2017) 32:1322–9. doi: 10.1093/ndt/gfw260
24. Sasai Y, Iwakawa K, Yanagida K, Shen Y, Hosono T, Ariga T, et al. Advanced glycation endproducts stimulate renal epithelial cells to release chemokines that recruit macrophages, leading to renal fibrosis. Biosci Biotechnol Biochem. (2012) 76:1741–5. doi: 10.1271/bbb.120347
25. Galkina E and Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. (2006) 17:368–77. doi: 10.1681/ASN.2005080859
26. Zhang Q, Ye J, Zhang Z, Hu Y, Wang X, Jiang W, et al. Aristolocholic acid I promotes renal tubular epithelial fibrosis by upregulating matrix metalloproteinase-9 expression via activating the C3a/C3aR axis of macrophages. Toxicol Lett. (2023) 381:27–35. doi: 10.1016/j.toxlet.2023.04.009
27. Song Y, Zhou T, Zong Y, Gu B, Tan X, and Yang L. Arsenic inhibited cholesterol efflux of THP-1 macrophages via ROS-mediated ABCA1 hypermethylation. Toxicology. (2019) 424:152225. doi: 10.1016/j.tox.2019.05.012
28. Lai YS, Putra RBDS, Aui SP, and Chang KT. M2C polarization by baicalin enhances efferocytosis via upregulation of MERTK receptor. Am J Chin Med. (2018) 46:1899–914. doi: 10.1142/S0192415X18500957
29. Karatas A, Turkmen E, Erdem E, Dugeroglu H, and Kaya Y. Monocyte to high-density lipoprotein cholesterol ratio in patients with diabetes mellitus and diabetic nephropathy. biomark Med. (2018) 12:953–9. doi: 10.2217/bmm-2018-0048
30. Barati F, Bashash D, Mohamadi MH, Mehrpori M, and Hamidpour M. The effect of ox-LDL and platelets on macrophages, M2 macrophage polarization, and foam cell formation. ARYA Atheroscler. (2023) 19:25–33. doi: 10.48305/arya.2022.11777.2422
31. Lutgens SP, Cleutjens KB, Daemen MJ, and Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. (2007) 21:3029–41. doi: 10.1096/fj.06-7924com
32. Wang S, Yan W, Kong L, Zuo S, Wu J, Zhu C, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat Commun. (2023) 14:4367. doi: 10.1038/s41467-023-39683-z
33. Yan J and Horng T. Lipid metabolism in regulation of macrophage functions. Trends Cell Biol. (2020) 30:979–89. doi: 10.1016/j.tcb.2020.09.006
34. Ren K, Li H, Zhou HF, Liang Y, Tong M, Chen L, et al. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging. (2019) 11:10992–1009. doi: 10.18632/aging.102498
35. Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. (2001) 7:161–71. doi: 10.1016/s1097-2765(01)00164-2
36. Claudel T, Leibowitz MD, Fiévet C, Tailleux A, Wagner B, Repa JJ, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A. (2001) 98:2610–5. doi: 10.1073/pnas.041609298
37. Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. (2002) 22:2607–19. doi: 10.1128/MCB.22.8.2607-2619
38. Esobi IC, Oladosu O, Echesabal-Chen J, Powell RR, Bruce T, and Stamatikos A. miR-33a expression attenuates ABCA1-dependent cholesterol efflux and promotes macrophage-like cell transdifferentiation in cultured vascular smooth muscle cells. J Lipids. (2023) 2023:8241899. doi: 10.1155/2023/8241899
39. Marshall JD, Courage ER, Elliott RF, Fitzpatrick MN, Kim AD, Lopez-Clavijo AF, et al. THP-1 macrophage cholesterol efflux is impaired by palmitoleate through Akt activation. PloS One. (2020) 15:e0233180. doi: 10.1371/journal.pone.0233180
40. Walker ME, De Matteis R, Perretti M, and Dalli J. Resolvin T4 enhances macrophage cholesterol efflux to reduce vascular disease. Nat Commun. (2024) 15:975. doi: 10.1038/s41467-024-44868-1
41. Mei J, Xu F, Zhou Q, Zhang Y, Ji J, and Li M. Tetramethylpyrazine and paeoniflorin synergistically attenuate cholesterol efflux in macrophage cells via enhancing ABCA1 and ABCG1 expression. Evid.-based complement. Altern Med ECAM. (2022) 2022:4304790. doi: 10.1155/2022/4304790
42. Wang D, Hiebl V, Schachner D, Ladurner A, Heiss EH, Atanasov AG, et al. Soraphen A enhances macrophage cholesterol efflux via indirect LXR activation and ABCA1 upregulation. Biochem Pharmacol. (2020) 177:114022. doi: 10.1016/j.bcp.2020.114022
43. Deng WY, Zhou CL, and Zeng MY. Gypenoside XVII inhibits ox-LDL-induced macrophage inflammatory responses and promotes cholesterol efflux through activating the miR-182-5p/HDAC9 signaling pathway. J Ethnopharmacol. (2024) 319:117070. doi: 10.1016/j.jep.2023.117070
44. Tan C, Zhou L, Wen W, and Xiao N. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J Toxicol Sci. (2021) 46:209–22. doi: 10.2131/jts.46.209
45. Liu S, Sui Q, Zhao Y, and Chang X. Lonicera caerulea berry polyphenols activate SIRT1, enhancing inhibition of raw264.7 macrophage foam cell formation and promoting cholesterol efflux. J Agric Food Chem. (2019) 67:7157–66. doi: 10.1021/acs.jafc.9b02045
46. Bao Z, Liu ZQ, He PY, Adali J, Yang YC, and Wulasihan M. 17β-estradiol regulates adenosine triphosphate-binding cassette transporters A1 expression via estrogen receptor A to increase macrophage cholesterol efflux. J Physiol Pharmacol Off J Pol Physiol Soc. (2023) 74. doi: 10.26402/jpp.2023.5.05
47. Yin Q, Chang H, Shen Q, and Xing D. Photobiomodulation therapy promotes the ATP-binding cassette transporter A1-dependent cholesterol efflux in macrophage to ameliorate atherosclerosis. J Cell Mol Med. (2021) 25:5238–49. doi: 10.1111/jcmm.16531
48. Zhang M, Li L, Xie W, Wu JF, Yao F, Tan YL, et al. Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis. (2016) 248:149–59. doi: 10.1016/j.atherosclerosis.2016.03.008
49. Patterson MT, Xu Y, Hillman H, Osinski V, Schrank PR, Kennedy AE, et al. Trem2 agonist reprograms foamy macrophages to promote atherosclerotic plaque stability-brief report. Arterioscler Thromb Vasc Biol. (2024) 44:1646–57. doi: 10.1161/ATVBAHA.124.320797
50. Chen X, Zou D, Chen X, Wu H, and Xu D. Hesperetin inhibits foam cell formation and promotes cholesterol efflux in THP-1-derived macrophages by activating LXRα signal in an AMPK-dependent manner. J Physiol Biochem. (2021) 77:405–17. doi: 10.1007/s13105-020-00783-9
51. Ren H, Shao Y, Wu C, Ma X, Lv C, and Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol. (2020) 500:110628. doi: 10.1016/j.mce.2019.110628
52. Mazzieri A and Marcon LMR. Nephroprotective mechanisms of SGLT2i: beyond the glucose-lowering effect. Biomedicines. (2025) 13:2123. doi: 10.3390/biomedicines13092123
53. J C, Me C, and Mt C. Renoprotective mechanisms of glucagon-like peptide-1 receptor agonists. Diabetes Metab. (2025) 51(3):101641. doi: 10.1016/j.diabet.2025.101641
54. Wang Y, Zheng Z, Lei X, Lei Q, and Tian J. Notch signaling pathway ligand DLL4 aggravates diabetic nephropathy immune damage by promoting M1 macrophage differentiation. Mian Yi Xue Za Zhi. (2020) 36:926–34. doi: 10.13431/j.cnki.immunol.j.20200144
55. O'Reilly ME, Kajani S, Ralston JC, Lenighan YM, Roche HM, and McGillicuddy FC. Nutritionally derived metabolic cues typical of the obese microenvironment increase cholesterol efflux capacity of adipose tissue macrophages. Mol Nutr Food Res. (2019) 63:e1800713. doi: 10.1002/mnfr.201800713
56. Zhang Q, He J, Xu F, Huang X, Wang Y, Zhang W, et al. Correction : Supramolecular copolymer modified statin-loaded discoidal rHDLs for atherosclerotic anti-inflammatory therapy by cholesterol efflux and M2 macrophage polarization. Biomater Sci. (2021) 9:6153–68. doi: 10.1039/d2bm90014a
57. Robichaud S, Fairman G, Vijithakumar V, Mak E, Cook DP, Pelletier AR, et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy. (2021) 17:3671–89. doi: 10.1080/15548627.2021.1886839
58. Zhao Y, Li Y, Liu Q, Tang Q, Zhang Z, Zhang J, et al. Canagliflozin facilitates reverse cholesterol transport through activation of AMPK/ABC transporter pathway. Drug Des Devel Ther. (2021) 15:2117–28. doi: 10.2147/DDDT.S306367
59. Xuan Y, Ding TT, Mao XL, Pang S, He R, Qin L, et al. Liraglutide alleviateshigh-fat diet-induced kidney injury in mice by regulating the CaMKKβ/AMPK pathway. Ren Fail. (2024) 46:2351473. doi: 10.1080/0886022X.2024.2351473
60. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
61. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
62. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. (2018) 6:691–704. doi: 10.1016/S2213-8587(18)30141-4
Keywords: diabetic kidney disease, macrophages, lipid metabolism disorders, M1/M2 phenotype, LXR/PPARγ signaling
Citation: Wang J, Cai Y, Feng Y and Zhu Q (2025) Diabetic kidney disease macrophage cholesterol efflux: a revolution from metabolism to immune. Front. Endocrinol. 16:1714167. doi: 10.3389/fendo.2025.1714167
Received: 27 September 2025; Accepted: 24 October 2025;
Published: 06 November 2025.
Edited by:
Jean-François Tanti, INSERM U1065 Centre Méditerranéen de Médecine Moléculaire, FranceReviewed by:
Alessio Mazzieri, USL Umbria 1, ItalyJunaid Ahmad, University of Rome Tor Vergata, Italy
Copyright © 2025 Wang, Cai, Feng and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Zhu, emh1cWluZmVpZmVpQDEyNi5jb20=
†These authors have contributed equally to this work
 Jinjin Wang
Jinjin Wang Yi Cai2†
Yi Cai2† Yuxi Feng
Yuxi Feng