- 1Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 2Department of Laboratory Medicine, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 3Department of Cardiology, Central Hospital of Dalian University of Technology, Dalian, China
Objective: This study aims to assess the impact of plasma corin levels on left atrial reverse remodeling (LARR) in patients with atrial fibrillation (AF) after catheter ablation (CA).
Methods: This prospective, single-center, observational study included 335 patients undergoing first CA of AF. All patients underwent at least two echocardiographic examinations at intervals of ≥3 months. Corin was measured before and 1 day after CA. LARR was defined as a reduction of ≥20% in left atrial volume index.
Results: After a median of 29.53 months of follow-up, 94 patients exhibited LARR. The plasma corin concentrations drawn pre- and post-procedure in the LARR group were 442.85 [241.25, 631.68] and 516.83 [362.4,697.84] pg/mL, and those were significantly higher than those in the non-LARR group (393.82 [207.09, 558.11] and 473.77 [320.88, 665.75] pg/mL; P = 0.036 and P = 0.047). The Kaplan–Meier survival curve showed high baseline corin concentrations which indicated a higher rate of LARR than low corin concentrations (P = 0.014). The Cox regression found plasma corin levels before or after the procedure were not associated with LARR in AF patients after CA, with or without adjusting for confounding factors. Absence of hypertension (P = 0.001, OR = 0.33) and diabetes mellitus (P = 0.007, OR = 0.269) was associated with a greater likelihood of LARR in AF patients after CA.
Conclusions: Although plasma corin concentrations were increased in the LARR group, it provided minimal prognostic utility in LARR after CA of AF.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia that increases mortality and morbidity worldwide (1) and which promotes left atrial (LA) electrical and structural remodeling. However, atrial remodeling has led to atrial cardiomyopathy, setting the stage for AF development. Expert consensus on atrial cardiomyopathies focuses on definition, characterization, and clinical implications (2). Catheter ablation (CA) for AF is an important rhythm-control strategy in symptomatic patients with paroxysmal or persistent AF (PeAF) who are refractory or intolerant to antiarrhythmic drugs; it is, by far, the most common cardiac ablation procedure performed worldwide (3). The restoration of sinus rhythm (SR) contributes to the amelioration of LA structural changes, namely, LA reverse remodeling (LARR), which is an important surrogate marker for AF-free survival (4–6). In addition, LARR could also improve cardiac function and reduce the risk of thromboembolism (5, 7, 8). Therefore, LARR has had an important clinical influence (9). Echocardiography is the most widely used method to evaluate LA dimension and function; LA volume (LAV) is a marker of LA structural remodeling. However, LARR following CA varies substantially among individuals (6, 10).
It has been shown that some biomarkers, such as atrial natriuretic peptide (ANP) (9, 10), B-type natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP) (11, 12), C-reactive protein (13), suppression of tumorigenicity 2/interleukin 33-receptor (14), and high-sensitivity cardiac troponin T (15), have been evaluated as predictors of LARR. Corin is a type II transmembrane serine protease highly expressed in cardiomyocytes and converts proANP/proBNP into mature ANP/BNP (16, 17). Recently, a series of studies has demonstrated that corin is associated with cardiovascular diseases, including hypertension, heart failure (HF), acute myocardial infarction, AF, and stroke (18). Soluble corin levels are increased in AF patients (19), and elevated plasma corin levels at baseline have been strongly associated with an increased incidence of AF recurrence after CA (20). However, whether soluble corin levels can predict LARR after CA of AF has not been studied. Therefore, the aim of this study was to investigate the impact of plasma corin levels (measured before and after CA for AF) on LARR after the initial CA of AF.
Methods
We conducted a single‐center, observational study at the First Affiliated Hospital of Dalian Medical University from April 2019 through May 2023. The study protocol was approved by the hospital’s ethics committee (no. PJ-KS-KY-2025-510) and adhered to the guidelines set forth by the Declaration of Helsinki. All patients signed informed consent forms prior to enrollment.
The study population, clinical measurements, definition of explanatory variables, ablation procedure, medications, and plasma corin measurements were described in a previous study (20). The participants’ peripheral venous blood samples were collected into sodium citrate coagulation test tubes before and 1 day after CA.
Echocardiographic measurements
Transthoracic echocardiography (TTE) was performed using a commercially available system, EPIQ 7 (Koninklijke Philips N.V.) or Vivid E95 (GE Vingmed Ultrasound). Each patient was analyzed by the same operator blinded to baseline characteristics and clinical outcomes at baseline and follow-up evaluations. TTE measurements were performed according to the recommendations of the American Society of Echocardiography (21, 22). In the case of irregular rhythm, the parameters were measured over 10 beats to avoid bias given by beat-to-beat variability. Left ventricular end‐diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF) were assessed using the biplane Simpson’s rule. LAV was assessed by the ellipsoid method (23), which requires three LA orthogonal diameters in end-systole just before mitral valve opening. LA diameter (LAD) is the anteroposterior diameter of the LA and was defined in the parasternal long-axis view. LA left-right diameter (LALRD) and LA superior–inferior diameter (LASID) were measured in the apical four-chamber view (24).
The formula for the ellipsoid method is LAV = π/6 × LAD × LALRD × LASID (25, 26). LAV index (LAVI) was calculated as (LAV)/body surface area (BSA), where BSA (m2) = 0.007184 × height (cm)0.725 × weight (kg)0.425. The LA sphericity index (LASI) was calculated as the ratio of LALRD and LASID, where body mass index (BMI) = weight (kg)/height(m)2. All patients underwent at least two echocardiographic examinations. When more than two tests were available, the first and last assessments were used to calculate the LARR. The time interval between the two examinations was at least 3 months (5).
Follow‐up and assessment of LARR
All patients were followed up at regular predefined intervals at our cardiology clinic or with their referring physician, with additional visits as required. Patients who did not attend regular visits were contacted by telephone. AF recurrence was defined as after a 3-month blanking period from the CA following a documented (electrocardiogram, Holter monitoring) episode of AF, atrial tachycardia, or atrial flutter (AFL) lasting >30 s (21). The study population was divided into two groups according to the extent of decrease in the LAVI during a follow-up period calculated from the second echocardiography examination until AF recurrence or until December 31, 2024. The LARR group was defined as patients who exhibited ≥20% decrease in the LAVI, inversely as the non-LARR group (27, 28).
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS), version 27.0 (IBM, Armonk, NY, USA), and GraphPad Prism, version 9 (GraphPad Software, San Diego, CA, USA). Categorical variables [sex, PaAF, hypertension, type 2 diabetes mellitus (T2DM), coronary artery disease, HF, left ventricular hypertrophy, and medications] were expressed as percentages and were compared using Pearson’s chi-square (χ2). Continuous variables (age, BMI, BSA, estimated glomerular filtration rate, lipid factors, BNP, corin, interval time, electrocardiogram parameters, and TTE parameters) were expressed as median [25th percentile (quartile 1)–75th percentile (quartile 3)] and compared using Mann–Whitney test. Receiver operating characteristic (ROC) curves and Youden index were constructed to identify the threshold of corin that best predicted LARR. The patients were categorized based on their corin concentrations according to whether the concentration was above or below the threshold value. The threshold values differed according to sex and AF type. Survival curves were generated using Kaplan–Meier analysis, with a log-rank test assessing the differences.
Cox regression analyses—adjusted for hypertension, AF type, PR interval, corrected QT (QTc) interval, T2DM, HF, and corin levels before or after CA, and TTE parameters, which were statistically different at baseline between the LARR group and non-LARR group—were performed for the primary end point with corin as continuous and dichotomous variables (low vs. high concentrations). The continuous variables, including corin values, were normalized by Z-score normalization; one standard deviation was used to calculate the hazard ratio (HR). Binary logistic regression analysis (also adjusted for the variables) was performed for the independent predictors of LARR after CA. The statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
This study initially recruited 681 inpatients who intended to undergo their first CA for AF. Of these, 343 cases were excluded because they met the exclusion criteria. Patients with valvular heart disease (n = 6), hyperthyroidism (n = 2), and severe kidney dysfunction (n = 2) were excluded. Patients who refused to undergo the CA procedure (n = 37), have undergone pacemaker implantation (n = 6), and have undergone concurrent pacemaker implantation and atrioventricular nodal ablation (n = 3) were further excluded. Also excluded were one patient diagnosed with acute MI, one patient diagnosed with acute pulmonary embolism, 266 patients with missing echocardiography data, 19 patients who did not complete follow‐up, and three patients who died during follow‐up. Consequently, 335 patients remained, with 94 cases in the LARR group and 241 in the non-LARR group. The flowchart of identification, inclusion, and exclusion is shown in Figure 1.
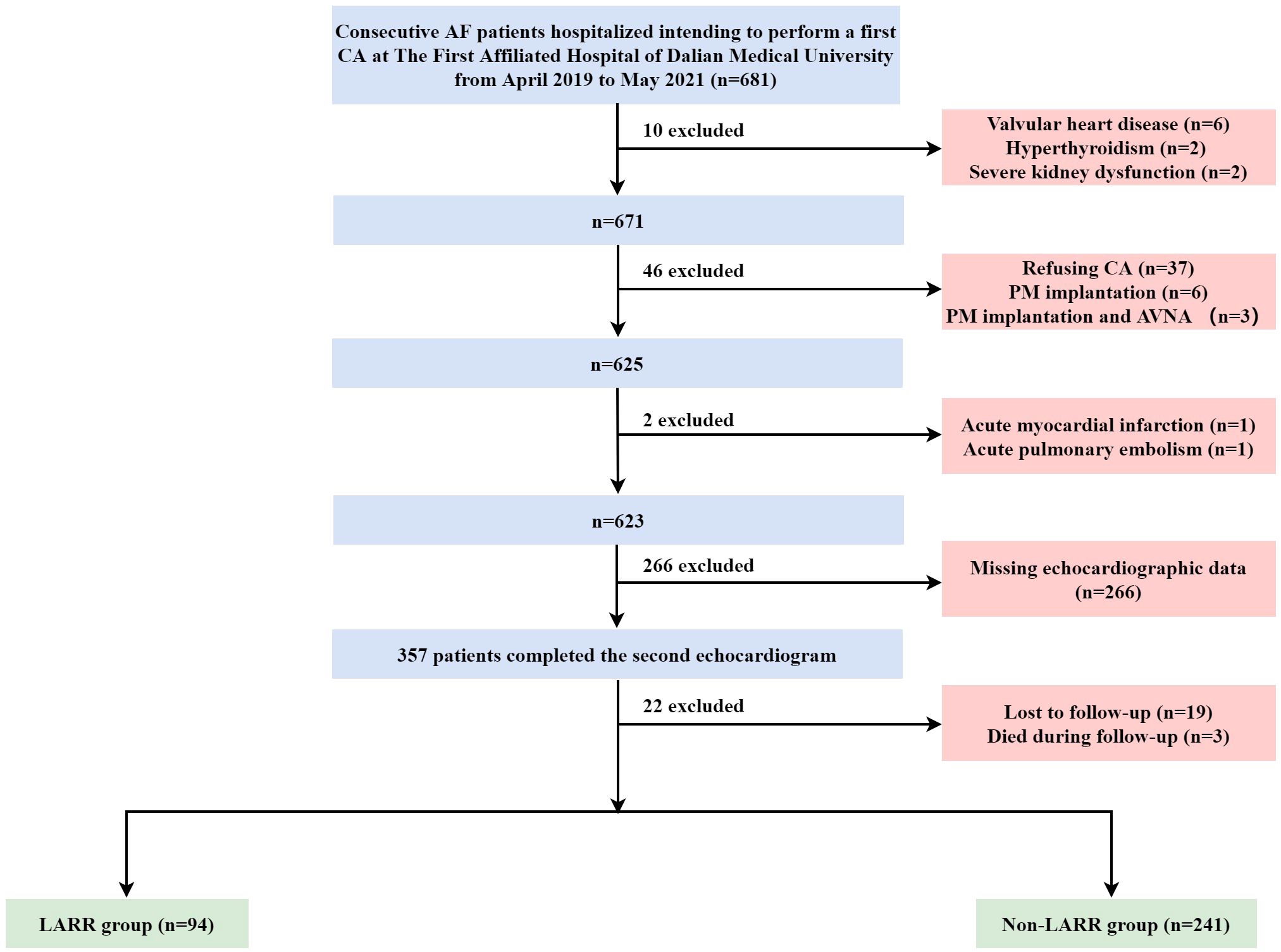
Figure 1. Flow diagram of the inclusion and exclusion of study subjects. AF, atrial fibrillation; CA, catheter ablation; PM, pacemaker; AVNA, atrioventricular nodal ablation; LARR, left atrial reverse remodeling.
The baseline characteristics of the study population are displayed in Table 1. The average age was 65 (57, 69) years; 59.4% were men, and 58.21% had paroxysmal AF (PaAF). During the follow-up period, 105 patients (31.34%) had recurrent AF. Based on a LAVI reduction ≥20%, 94 (28.06%) patients were assigned to the LARR group and 241 (71.94%) to the non-LARR group. Overall, compared with the non-recurrence group, patients in the recurrence group were predominantly male, had higher BSA and plasma corin levels before and after CA, and had a lower frequency of left ventricular hypertrophy. Compared with the non-LARR group, patients in the LARR group had lower frequencies of PaAF, hypertension, and T2DM. In addition, they had a higher frequency of HF, longer PR and QTc intervals, and higher plasma corin levels before and after CA. Moreover, they showed higher LAD, LALRD, LASID, LAV, LAVI, and LVEDD and lower LVEF than the non-LARR group before CA. However, LAD, LALRD, LASID, LAV, and LAVI were reversed in the LARR group after CA. Notably, the interval between the two TTE tests was shorter for the LARR group.
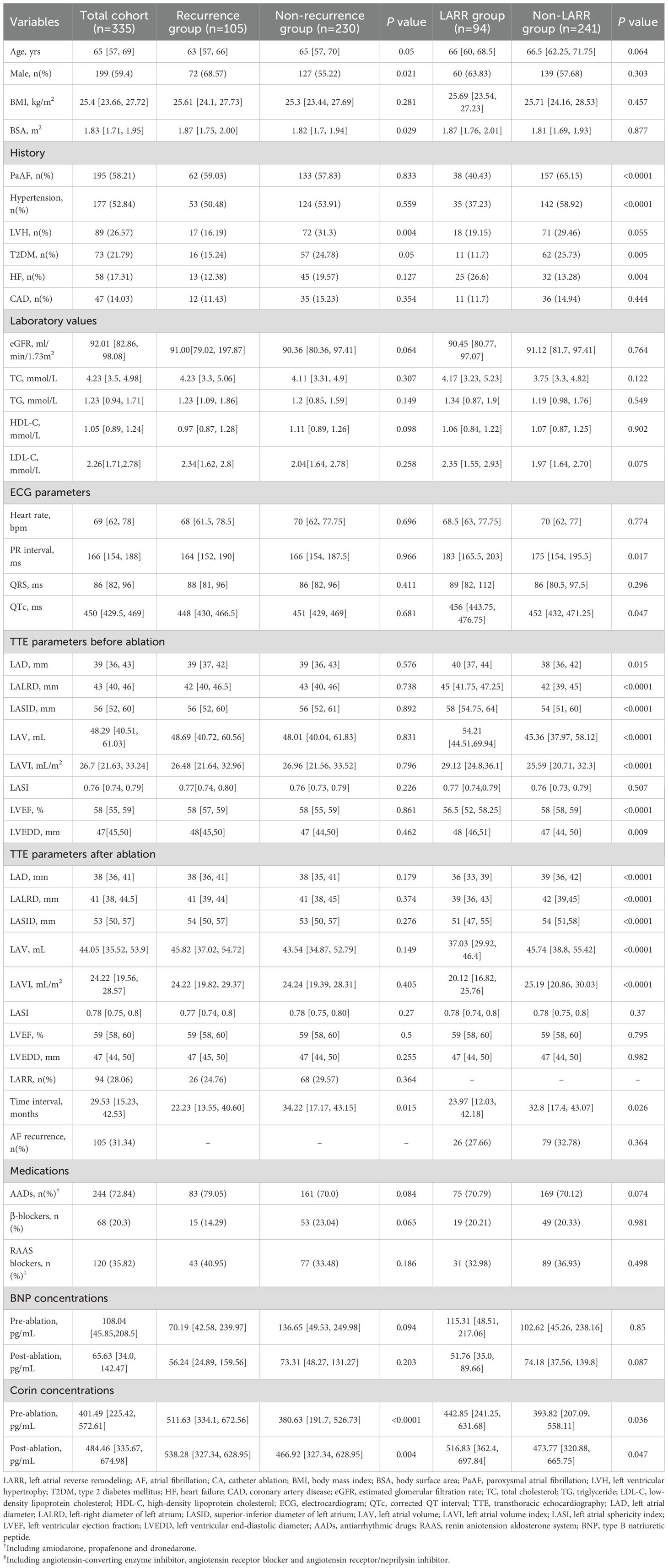
Table 1. Baselines and corin concentrations of the total cohort, non-recurrence or recurrence group, non-LARR or LARR group of AF after CA.
Pre-/post-procedural plasma corin levels in LARR and non-LARR patients treated with CA for AF
In the total cohort, the plasma corin concentrations drawn pre- and post-procedure in the LARR group were 442.85 (241.25, 631.68) and 516.83 (362.4, 697.84) pg/mL, respectively, and those were significantly higher than those in the non-LARR group [393.82 (207.09, 558.11) and 473.77 (320.88, 665.75) pg/mL; P = 0.036 and P = 0.047, respectively] (Table 2, Figure 2A).
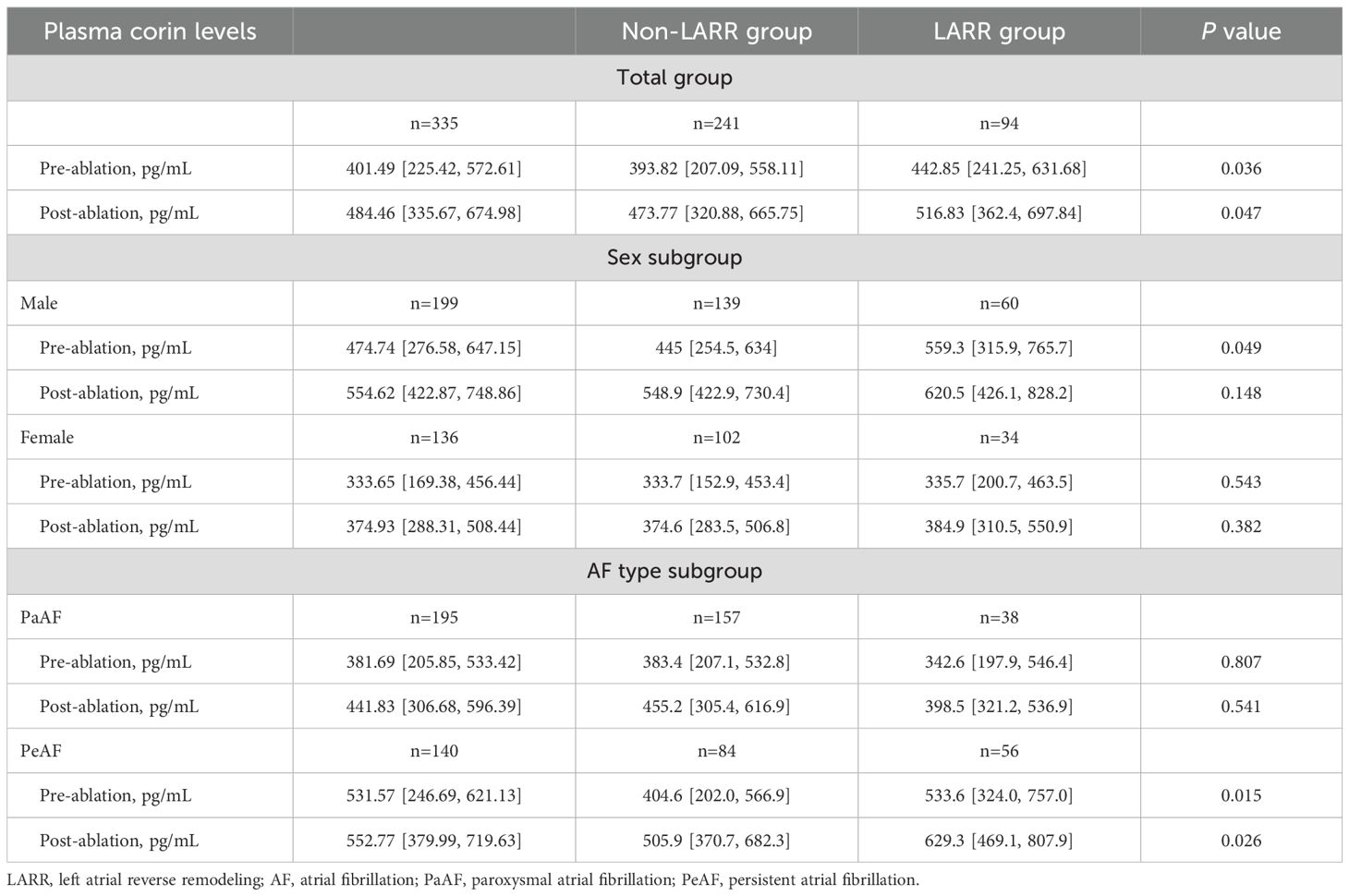
Table 2. Pre/postprocedural plasma corin levels in LARR and non-LARR patients treated with catheter for atrial fibrillation.
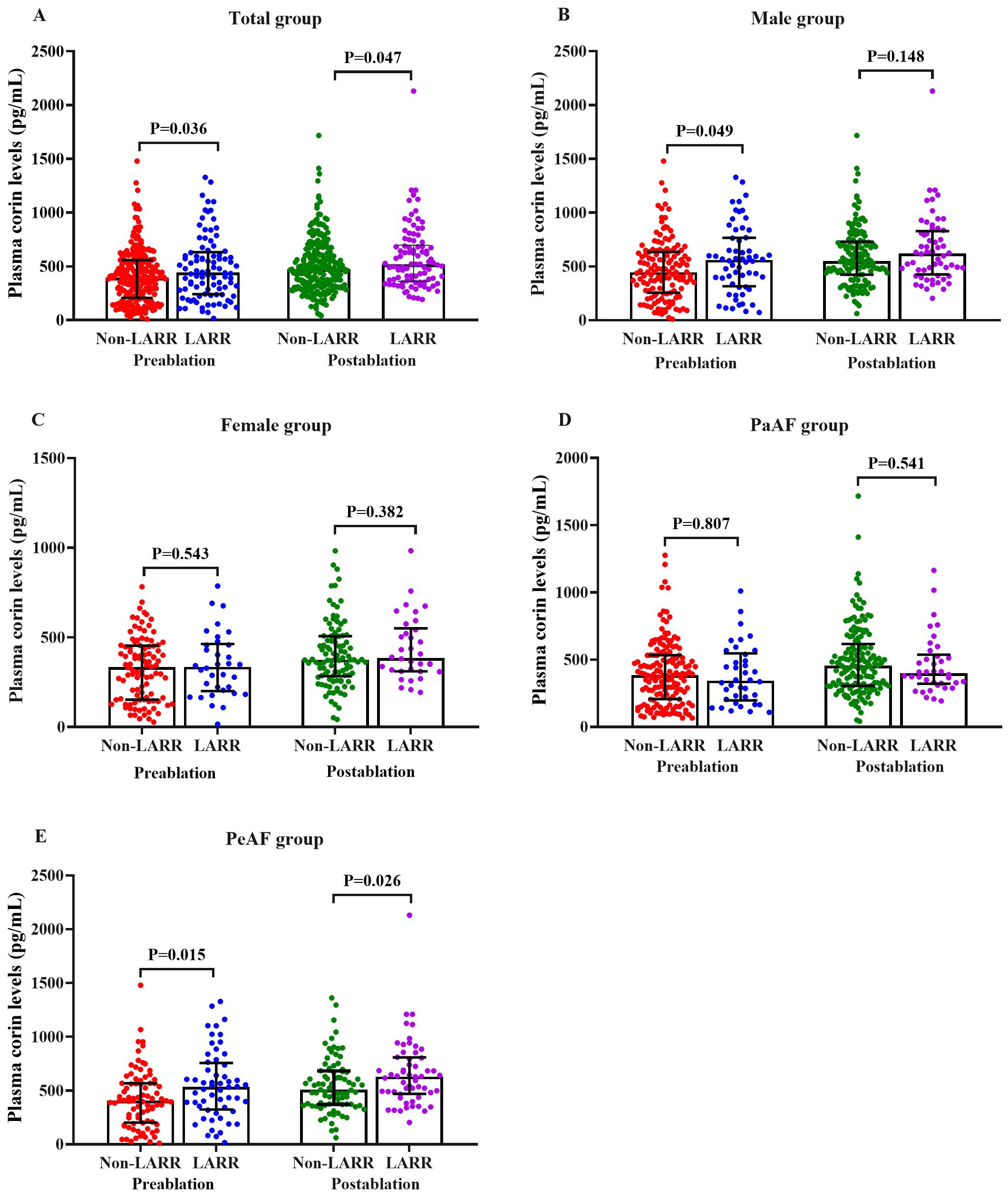
Figure 2. Pre- and post-procedural plasma corin levels in LARR and non-LARR patients treated with catheter ablation for atrial fibrillation. (A) Total group, (B) male subgroup, (C) female subgroup, (D) PaAF subgroup, and (E) PeAF subgroup. LARR, left atrial reverse remodeling; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation. The bar represents the 25%–75% range of values.
In a subgroup analysis of male patients, the plasma corin concentrations before CA were significantly higher in patients with LARR than in those without LARR [559.3 (315.9, 765.7) vs. 445 (254.5, 634) pg/mL; P = 0.049], but this was not found in post-ablation corin concentrations [620.5 (426.1, 828.2) vs. 548.9 (422.9, 730.4) pg/mL; P = 0.148; Table 2, Figure 2B]. However, in female patients, there was no difference between the LARR group and the non-LARR group both in pre- and post-ablation corin concentrations [pre-ablation: 335.7 (200.7, 463.5) vs. 333.7 (152.9, 453.4) pg/mL, P = 0.543; post-ablation: 384.9 (310.5, 550.9) vs. 374.6 (283.5, 506.8) pg/mL, P = 0.382; Table 2, Figure 2C].
In the PeAF subgroup, the corin concentrations were significantly higher in patients with LARR than in those without LARR [pre-ablation: 533.6 (324.0, 757.0) vs. 404.6 (202.0, 566.9) pg/mL, P = 0.015; post-ablation: 629.3 (469.1, 807.9) vs. 505.9 (370.7, 682.3) pg/mL, P = 0.026; Table 2, Figure 2E]. However, the corin levels between the LARR group and the non-LARR group was not found in PaAF patients [pre-ablation: 342.6 (197.9, 546.4) vs. 383.4 (207.1, 532.8) pg/mL; P = 0.807; post-ablation: 398.5 (321.2, 536.9) vs. 455.2 (305.4, 616.9) pg/mL, P = 0.541; Table 2, Figure 2D].
Plasma corin threshold of best predictive value for LARR in AF patients treated with CA
The pre-ablation ROC curve analysis of plasma corin drawn was >535.22 pg/mL, and that of post-ablation was >307.69 pg/mL. These represented the best predictive values for LARR in AF patients after CA. The pre-ablation sensitivity was 40.4%, specificity was 73.4%, and area under the curve (AUC) was 0.574. At post-ablation, sensitivity was 90.4%, specificity was 21.6%, and AUC was 0.570 (P = 0.036, P = 0.047, respectively) (Table 3, Figure 3A).
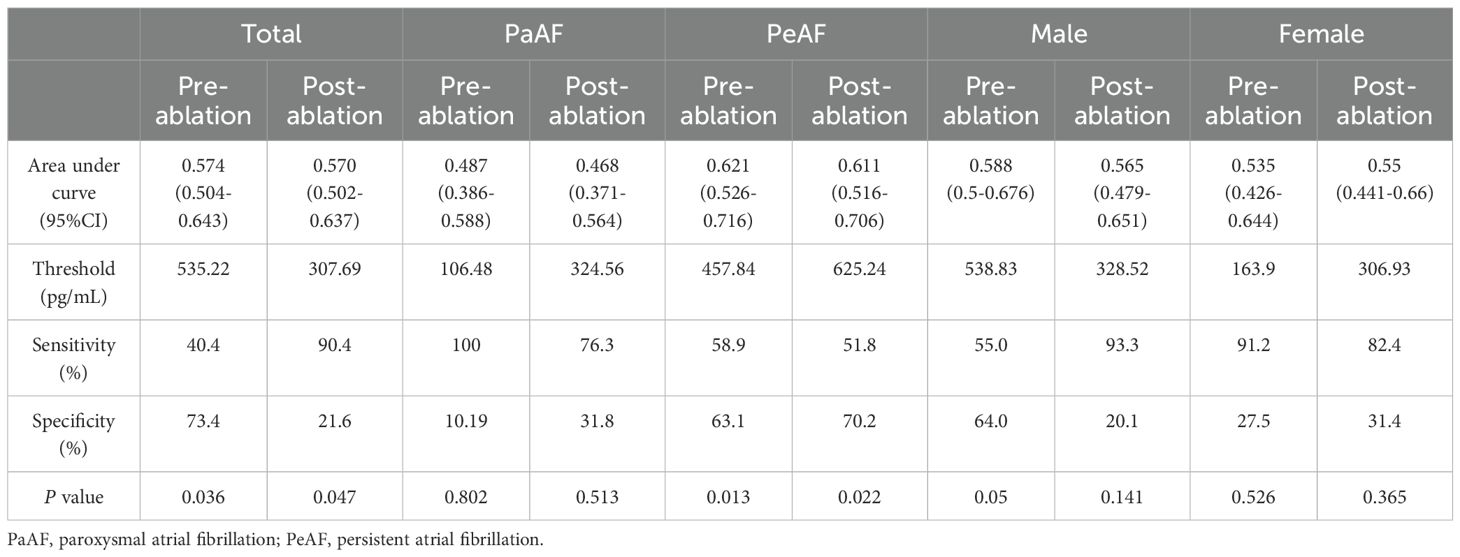
Table 3. Plasma corin threshold of best predictive value for LARR in atrial fibrillation patients treated with catheter ablation.
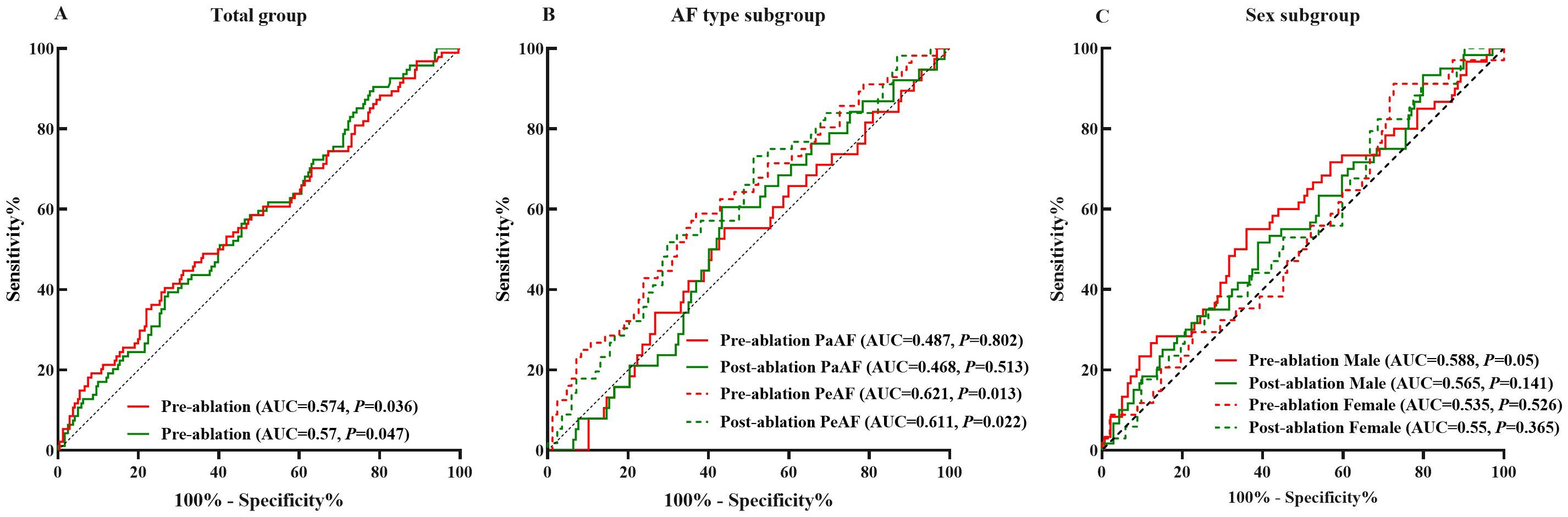
Figure 3. Plasma corin threshold of best predictive value for LARR in atrial fibrillation treated with catheter ablation. (A) Total group, (B) subgroup analysis of atrial fibrillation type, and (C) subgroup analysis of sex. LARR, left atrial reverse remodeling; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; AUC, area under the curve.
In PeAF patients, the pre-ablation threshold was >457.84 pg/mL and the post-ablation threshold was >625.24 pg/mL. The pre-ablation sensitivity was 58.9%, and specificity was 63.1% (P = 0.013). In contrast, the post-ablation sensitivity was 51.8%, and specificity was 70.2% (P = 0.022). The pre-ablation AUC was 0.621, and the post-ablation AUC was 0.611 (Table 3, Figure 3B). However, the predictive value of corin levels in PaAF, male, or female subgroups, respectively, before and after ablation was not identified (Table 3, Figures 3B, C).
Relationship between plasma corin levels and LARR in AF patients treated with CA
We classified low and high corin levels according to thresholds in different groups. The Kaplan–Meier survival curve showed that high corin concentrations measured before the procedure indicated a significantly higher LARR than low corin concentrations in the total group, PeAF group, male group, and female group (P = 0.014, 0.036, 0.018, and 0.046, respectively, Figures 4A, C–E), other than the PaAF group (P = 0.069, Figure 4B). However, post-ablation corin concentrations were not associated with LARR, whether in total group, different types of AF, or different sex (Figures 4B–J).
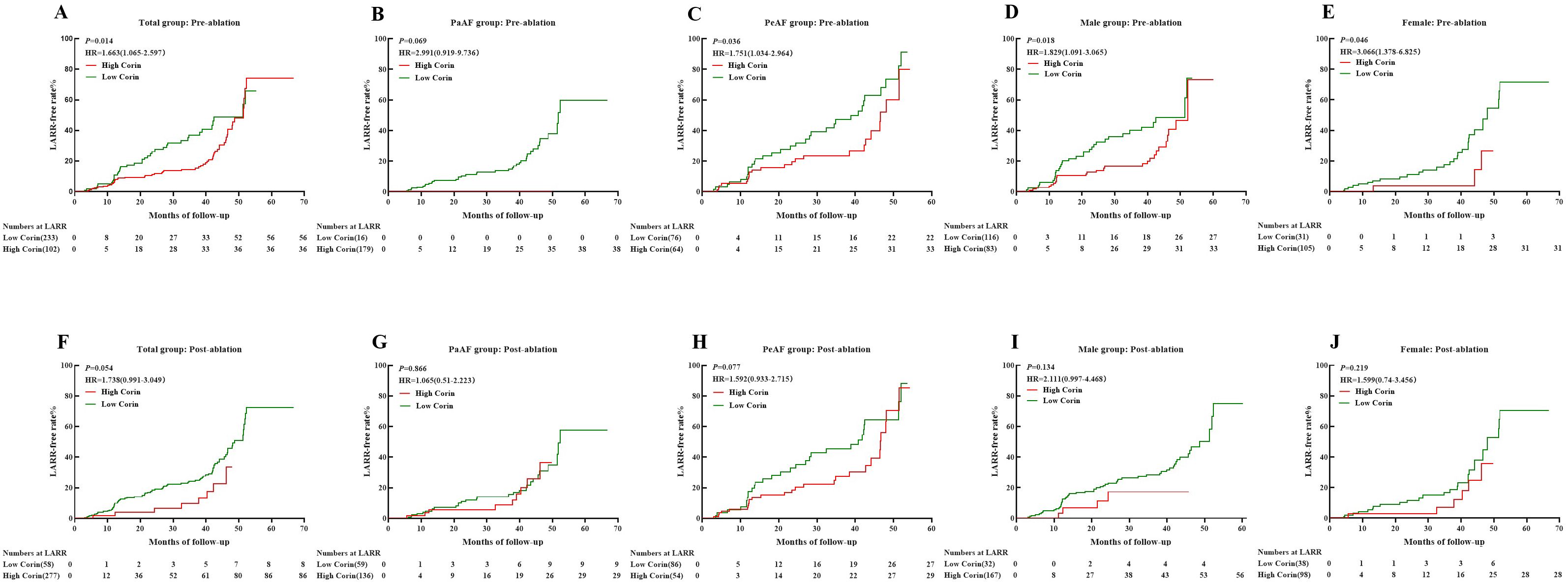
Figure 4. Kaplan–Meier curves showing LARR-free in atrial fibrillation treated with catheter ablation for different corin levels. (A–E) Corin levels pre-ablation in total, PaAF, PeAF, male, and female groups; (F–J) Corin levels post-ablation in total, PaAF, PeAF, male, and female. LARR, left atrial reverse remodeling; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; HR, hazard ratio.
Cox regression and forest plot found that the plasma corin levels before or after the procedure, whether in total group, PaAF, PeAF, male, or female, were not associated with LARR in AF patients after CA with or without adjusting for confounding factors (Supplementary Table S1, Supplementary Figure S1).
Independent predictors of LARR in AF patients treated with CA
In adjusted models, plasma corin levels before or after CA, whether in the total group, PaAF, PeAF, male, or female, did not predict LARR in AF patients after CA (Figure 5). In the total group, absence of hypertension (P = 0.001, OR = 0.33, 95% CI: 0.168–0.649) and T2DM (P = 0.007, OR = 0.269, 95% CI: 0.104–0.701) was associated with a greater likelihood of LARR in AF patients after CA (Figure 5A). The same results were found in the PeAF group (absence of hypertension: P = 0.008, OR = 0.229, 95% CI: 0.077–0.684, and T2DM: P = 0.009, OR = 0.136, 95% CI: 0.031–0.606; Figure 5C) and the male group (absence of hypertension: P = 0.011, OR = 0.306, 95% CI: 0.122–0.764, and T2DM: P = 0011, OR = 0.207, 95% CI: 0.061–0.702; Figure 5D). In the female group, only absence of hypertension was associated with a greater likelihood of LARR in AF patients after CA (P = 0.048, OR = 0.314, 95% CI: 0.099–0.99; Figure 5E). In the PaAF group, absence of hypertension (P = 0.041, OR = 0.353, 95% CI: 0.13–0.957), higher LALRD (P = 0.029, OR = 8.408, 95% CI: 1.239–57.071), and lower LVEF (P = 0.016, OR = 0.367, 95% CI: 0.161–0.832) baseline were associated with a greater likelihood of LARR in AF patients after CA (Figure 5B).
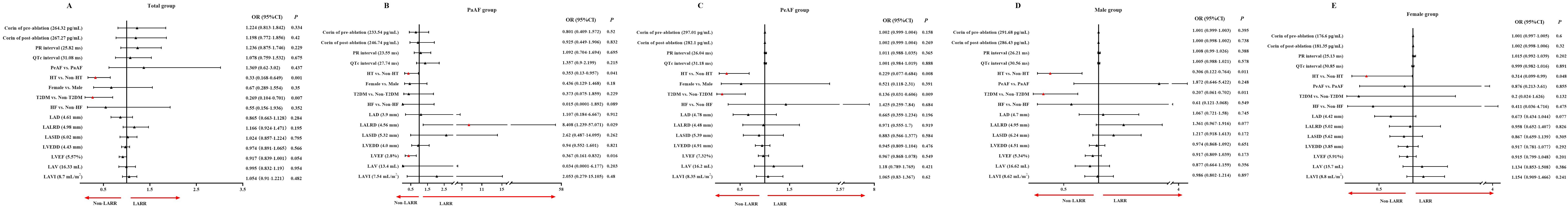
Figure 5. (A–E) Predictors of left atrial reverse remodeling in atrial fibrillation patients after catheter ablation in adjusted models. The continuous variables were normalized by Z-score, and one standard deviation was used for odds ratio calculation. OR, odds ratio; CI, confidence interval; LARR, left atrial reverse remodeling; AF, atrial fibrillation; PaAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; QTc, corrected QT; HT, hypertension; T2DM, type 2 diabetes mellitus; HF, heart failure; LAD, left atrial diameter; LALRD, left-right diameter of left atrium; LASID, superior–inferior diameter of left atrium; LAV, left atrial volume; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter. All ultrasound indicators are from baseline.
Discussion
This is the first study to investigate the association between plasma corin levels before and after CA for AF and LARR. Our primary findings are as follows: (1) Plasma corin concentrations pre- or post-ablation were significantly higher in patients with LARR than in those without LARR in general population; (2) the Kaplan–Meier survival curve showed that high corin concentrations measured before CA indicated a significantly higher rate of LARR than low corin concentrations in the total, PeAF, male, and female groups, except for the PaAF group; (3) whether or not adjusted for confounding factors, the predictive value of plasma corin levels in LARR before or after ablation was not evident; and (4) absence of hypertension and T2DM was associated with a greater likelihood of LARR in AF patients after CA.
LARR and AF ablation
LA structural remodeling, characterized by atrial dilation and progressive fibrosis, is a key pathological feature in the development and maintenance of AF. The concept of LARR has emerged as a therapeutic goal aimed at restoring atrial structure and function (29). Various studies have assessed the effects of AF ablation on LA structure and function. Most studies noted a significant reduction in LAV only in the SR group, with a significant improvement in LA active and reservoir functions but not in the AF recurrence group (8, 30–34). Our study also confirmed LAVI reduction in the entire sample after the first CA of AF. However, a difference in LAVI reduction was observed regardless of AF recurrence. Several studies have also found no association between success in ablation and atrial reduction (35–37), which is consistent with our findings. This may be explained by AF burden reduction using CA despite AF recurrence (36, 37), undetected AF recurrent patients categorized into the success group (36), the baseline LA size, AF type, and the extent of scarring following CA (38).
Plasma corin levels and LARR in AF patients treated with CA
The atrial remodeling process is associated with levels of ANP and BNP (39, 40), reflecting neurohormonal activation in response to atrial stress. Natriuretic peptides increase in the event of AF due to atrial stretch. However, longstanding AF leads to atrial structural remodeling, resulting in reduced NP production capacity due to cardiomyocyte loss and fibrosis progress (10). Several studies have reported that LARR after CA likely occurred in AF patients with higher pre-ablation ANP/BNP levels (9, 10, 41). In addition, the preprocedural ANP/BNP ratio was a robust predictor of LARR after SR restoration using rhythm-control therapy in PeAF patients (9). Circulating ANP/N-terminal proANP/mid-regional proANP levels are valuable in predicting AF development and recurrence as well as LARR after CA (10, 42–45). Corin has been identified as the only physiological proANP convertase (46). ProANP is co-localized with corin in cardiomyocytes (47, 48), and for it, cleavage by corin is dominant (29). Therefore, the role of corin in atrial diseases such as atrial arrhythmias, atrial enlargement, or mitral stenosis, may be more pivotal than in HF or coronary heart disease (18). Based on this speculation, one study by our group demonstrated that plasma corin concentrations were significantly higher in AF patients than in controls, and those in PeAF patients were much higher than those in PaAF patients (19). Therefore, the increasing trend of plasma corin levels in AF patients is consistent with that of ANP levels (19, 29). Furthermore, corin and ANP levels are also significantly elevated in patients with a higher AF burden compared with those with a lower AF burden (10).
Another prospective observational study involving 616 patients undergoing first CA of AF found that the pre-ablation corin concentration of the recurrence group was significantly higher than that of the non-recurrence group (20). Elevated pre-ablation corin levels were significantly associated with an increased risk of AF recurrence after CA (20). In our study, although plasma corin concentrations drawn pre- and post-ablation in the LARR group were higher than those in the non-LARR group, the difference was reduced after adjusting for confounding factors. This indicates that plasma corin levels provide minimal prognostic utility in LARR after CA of AF. There are several possible explanations. First, several studies enrolled AF patients with LA enlargement (LAVI ≥ 34 mL/m2) (9, 49, 50), whereas the baseline median LAVI in our study was 26.7 mL/m2. Our patients had less pronounced atrial remodeling compared to other studies. Therefore, we defined LARR as a ≥20% reduction in LAVI, differing from the 15% cutoff used in prior studies (9, 10, 49). Besides that, we did not find a significant difference in BNP concentrations of baseline before and after CA. This may occur because less atrial remodeling reduces atrial wall stretch, resulting in diminished shedding of corin from atrial cardiomyocytes and natriuretic peptide secretion. Second, numerous studies have demonstrated LARR due to restoration and maintenance of SR after CA (10, 38, 41, 50–53). Meta-analysis revealed that LAD, LAV, and LAVI were significantly decreased by ablation (54). The degree of LARR depends on the difference in LA compliance (55). LA is more compliant and stretched, with preserved LA myocytes and less fibrosis, resulting in greater reversibility from structural remodeling (10). However, we failed to demonstrate reduced AF recurrence rates among patients exhibiting LARR after CA. A plausible explanation is that AF burden decreased significantly after ablation despite AF recurrence (37). LARR may have shown a pronounced correlation with a reduction in AF burden rather than AF recurrence. Third, majority of the studies employed follow-up periods ranging from 6 to 12 months, whereas 29.53 months was used to assess extended-term LARR post-ablation in our study. Plasma corin levels may have limited predictive utility for long-term LARR following AF ablation. Atrial cardiomyopathy (ACM) is increasingly recognized as a key contributor to the development and perpetuation of AF and involves complex structural, electrical, and functional remodeling of the atrial myocardium (56, 57). Future studies concurrently consider ACM and other emerging biomarkers which can predict atrial remodeling and may help to enhance the predictive value of corin for LARR.
Hypertension
We found that absence of hypertension was associated with a higher rate of LARR, which is consistent with most studies. One study showed that absence of hypertension was an independent predictor of significant LAV reduction following ablation (35). Another study found that younger patients without hypertension are most likely to experience a reduction in LAVI following cryoballoon-based pulmonary vein isolation (58). Furthermore, anti-hypertensive therapy has been shown to prevent LA dilatation and favorable reverse remodeling (59). Hypertension induces atrial remodeling and represents an independent risk factor for AF. Hypertension increases left ventricular afterload and end-diastolic pressure (60), leading to atrial enlargement and dysfunction, interstitial fibrosis, inflammation, heterogeneous conduction, and a greater propensity for AF (61, 62). Therefore, hypertension plays a central role in initiating and perpetuating adverse LA remodeling.
Diabetes mellitus
We found that absence of T2DM was also associated with a higher rate of LARR. A study including 204 consecutive AF patients who underwent first CA found that patients with T2DM had significantly reduced LA function and increased LA stiffness compared with those without T2DM (63). T2DM was associated with reduced LA function in patients with hypertrophic cardiomyopathy (64), hypertension (65), and significant aortic regurgitation (66).
The underlying mechanisms by which T2DM contributes to atrial remodeling are not completely understood. Several hypotheses have been proposed. First, abnormal glucose metabolism activates enhanced angiotensin II and transforming growth factor-β signaling, fostering a pro-fibrotic environment that ultimately leads to LAR (67). Second, autonomic nervous system imbalance, characterized by sympathetic nervous system predominance in individuals with hyperglycemia, can contribute to LAR. Autonomic dysfunction, which is highly prevalent in diabetes patients, impacts atrial size via alterations in sympathetic and parasympathetic neural activity (67). Third, enhanced inflammation in abnormal glucose metabolism can be associated with LA remodeling (63). Furthermore, microvascular ischemia resulting from the metabolic derangements of diabetes represents an additional contributing factor (65).
Therefore, diabetes contributes to LAR through a complex interplay of metabolic disturbances, oxidative stress, inflammation, mitochondrial dysfunction, and neurohumoral activation. These mechanisms collectively promote structural and functional alterations in LA, including fibrosis, impaired compliance, and dysfunctional contractility (67).
Study limitations
Several limitations of this study should be noted. First, as a single-center observational study, our findings may be influenced by residual confounding factors inherent in this design paradigm. External validation through multicenter trials with larger cohorts is warranted to confirm these observations. Second, information on post-procedural AF burden is not uniformly available, which can affect LARR. Third, using cardiac magnetic resonance imaging to evaluate LAV is more accurate than TTE, and it can also determine LA scarring. Fourth, the study focused exclusively on LAVI quantification without LA reservoir function and strain analysis, which may provide deeper insights into post-ablation structural changes. Fifth, the potential correlation between ANP levels and LARR was not explored. The combined evaluation of circulating ANP and corin levels may serve as a new panel for LARR post-ablation, warranting future investigation. Sixth, thermal procedures may lead to ischemia, coagulation necrosis, edema, and local inflammation of atrial tissue, which may result in the increased shedding of corin from atrial myocytes. However, we did not dynamically monitor the changes in corin level after ablation, for example, at the first, third, sixth, ninth, or 12th month after ablation. Serial measurements at later time points could potentially yield different and more meaningful insights into the relationship between corin and reverse remodeling. Seventh, it is important to note that the relatively low AUC values (0.57–0.62) indicate poor discriminatory power, and the use of dichotomized variables in survival analysis based on weak cutoffs has some limitations and can reduce statistical power. Eighth, AF duration was not be analyzed in this retrospective cohort. The lack may introduce residual confounding into our multivariate models. Future studies should include the parameter to validate and extend our findings.
Conclusions
Although plasma corin concentrations drawn pre- and post-ablation in the LARR group were higher than those in the non-LARR group, the difference was reduced after adjusting for confounding factors. Plasma corin levels provide minimal prognostic utility in LARR after CA of AF. The absence of hypertension and T2DM is associated with a greater likelihood of LARR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by First Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Data curation, Writing – original draft. YL: Data curation, Methodology, Writing – original draft. XY: Investigation, Methodology, Writing – original draft. YG: Methodology, Validation, Writing – review & editing. KS: Formal analysis, Writing – original draft. CL: Data curation, Methodology, Writing – original draft. JL: Funding acquisition, Project administration, Writing – original draft. YZ: Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. FC: Conceptualization, Funding acquisition, Investigation, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Major Project of the National Science and Technology Innovation 2030: Research on Prevention and Treatment of “Cancer, Cardiovascular and Cerebrovascular Diseases, Respiratory Diseases, and Metabolic Disorders” (grant number 2023ZD0504502), National Natural Science Foundation of China (grant number 81700301), Science and Technology Project of Education Department of Liaoning Province (grant number LZ2020058 and LJ212510161069), Scientific Research Project of Dalian Medical Key Specialty “Climbing Peak Plan” (grant number 2022DF016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1717422/full#supplementary-material
References
1. Staerk L, Sherer JA, Ko D, Benjamin EJ, and Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. (2017) 120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732
2. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. (2016) 18:1455–90. doi: 10.1093/europace/euw161
3. Parameswaran R, Al-Kaisey AM, and Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. (2021) 18:210–25. doi: 10.1038/s41569-020-00451-x
4. Hirose K, Nakanishi K, Daimon M, Iwama K, Yoshida Y, Mukai Y, et al. Body fat distribution and left atrial reverse remodeling after catheter ablation for atrial fibrillation. JACC Adv. (2024) 3:100973–89. doi: 10.1016/j.jacadv.2024.100973
5. Reant P, Lafitte S, Jaïs P, Serri K, Weerasooriya R, Hocini M, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. (2005) 112:2896–903. doi: 10.1161/CIRCULATIONAHA.104.523928
6. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. (2011) 57:324–31. doi: 10.1016/j.jacc.2010.05.063
7. Buber J, Luria D, Sternik L, Raanani E, Feinberg MS, Goldenberg I, et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol. (2011) 58:1614–21. doi: 10.1016/j.jacc.2011.05.051
8. Machino-Ohtsuka T, Seo Y, Ishizu T, Yanaka S, Nakajima H, Atsumi A, et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ J. (2013) 77:1695–1704. doi: 10.1253/circj.CJ-12-1518
9. Hasegawa Y, Okada S, Sanada A, Tomii A, Sugiura H, Higuchi K, et al. The atrial natriuretic peptide-to-brain natriuretic peptide ratio predicts left atrial reverse remodeling after rhythm control therapy in patients with persistent atrial fibrillation. Intern Med. (2023) 62:3283–90. doi: 10.2169/internalmedicine.1478-22
10. Nakanishi K, Fukuda S, Yamashita H, Kosaka M, Shirai N, Tanaka A, et al. Pre-procedural serum atrial natriuretic peptide levels predict left atrial reverse remodeling after catheter ablation in patients with atrial fibrillation. JACC Clin Electrophysiol. (2016) 2:151–8. doi: 10.1016/j.jacep.2015.12.010
11. Solheim E, Off MK, Hoff PI, De Bortoli A, Schuster P, Ohm OJ, et al. N-terminal pro-B-type natriuretic peptide level at long-term follow-up after atrial fibrillation ablation: a marker of reverse atrial remodelling and successful ablation. J Interv Card Electrophysiol. (2012) 34:129–36. doi: 10.1007/s10840-011-9629-2
12. Li YG, Gong CQ, Zhao MZ, Sun J, Wang QS, Zhang PP, et al. Determinants of postoperative left atrial structural reverse remodeling in patients undergoing combined catheter ablation of atrial fibrillation and left atrial appendage closure procedure. J Cardiovasc Electrophysiol. (2019) 30:1868–76. doi: 10.1111/jce.14094
13. Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, et al. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension. (2011) 57:208–15. doi: 10.1161/HYPERTENSIONAHA.110.158915
14. Ferreira AF, Azevedo MJ, Morais J, Trindade F, Saraiva F, Diaz SO, et al. Cardiovascular risk factors during pregnancy impact the postpartum cardiac and vascular reverse remodeling. Am J Physiol Heart Circ Physiol. (2023) 325:H774–89. doi: 10.1152/ajpheart.00200.2023
15. Murphy SP, Prescott MF, Maisel AS, Butler J, Piña IL, Felker GM, et al. Association between angiotensin receptor-neprilysin inhibition, cardiovascular biomarkers, and cardiac remodeling in heart failure with reduced ejection fraction. Circ Heart Fail. (2021) 14:e008410–e008424. doi: 10.1161/CIRCHEARTFAILURE.120.008410
16. Dong N, Niu Y, Chen Y, Sun S, and Wu Q. Function and regulation of corin in physiology and disease. Biochem Soc Trans. (2020) 48:1905–16. doi: 10.1042/BST20190760
17. Zhou Y and Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep. (2014) 16:415–32. doi: 10.1007/s11906-013-0415-7
18. Gong Y, Zhao Y, Li Y, Wang Q, Li C, Song K, et al. Corin in cardiovascular diseases and stroke. Clin Chim Acta. (2025) 574:120343–56. doi: 10.1016/j.cca.2025.120343
19. Chen F, Xia Y, Liu Y, Zhang Y, Song W, Zhong Y, et al. Increased plasma corin levels in patients with atrial fibrillation. Clin Chim Acta. (2015) 447:79–85. doi: 10.1016/j.cca.2015.05.017
20. Zhao Y, Yuan X, Xie Y, Yin X, Liu Y, Sun Y, et al. Association of preablation plasma corin levels with atrial fibrillation recurrence after catheter ablation: a prospective observational study. J Am Heart Assoc. (2024) 13:e031928–e031945. doi: 10.1161/JAHA.123.031928
21. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
22. Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance American College of Chest Physicians. J Am Soc Echocardiogr. (2011) 24:229–67. doi: 10.1016/j.echo.2010.12.008
23. Canciello G, de Simone G, Izzo R, Giamundo A, Pacelli F, Mancusi C, et al. Validation of left atrial volume estimation by left atrial diameter from the parasternal long-axis view. J Am Soc Echocardiogr. (2017) 30:262–9. doi: 10.1016/j.echo.2016.11.017
24. Havranek S, Fiala M, Bulava A, Sknouril L, Dorda M, Bulkova V, et al. Multivariate analysis of correspondence between left atrial volumes assessed by echocardiography and 3-dimensional electroanatomic mapping in patients with atrial fibrillation. PloS One. (2016) 11:e0152553–e0152576. doi: 10.1371/journal.pone.0152553
25. Cimadevilla C, Nadia B, Dreyfus J, Perez F, Cueff C, Malanca M, et al. Echocardiographic measurement of left atrial volume: Does the method matter? Arch Cardiovasc Dis. (2015) 108:643–9. doi: 10.1016/j.acvd.2015.07.001
26. Zhang W, Zhou Y, Dong Y, Liu W, Li H, and Song W. Correlation between N-terminal pro-atrial natriuretic peptide, corin, and target organ damage in hypertensive disorders of pregnancy. J Clin Hypertens (Greenwich). (2022) 24:644–51. doi: 10.1111/jch.14450
27. Moon MG, Hwang IC, Lee HJ, Kim SH, Yoon YE, Park JB, et al. Reverse remodeling assessed by left atrial and ventricular strain reflects treatment response to Sacubitril/Valsartan. JACC Cardiovasc Imaging. (2022) 15:1525–41. doi: 10.1016/j.jcmg.2022.03.019
28. Mathias A, Moss AJ, McNitt S, Zareba W, Goldenberg I, Solomon SD, et al. Clinical implications of complete left-sided reverse remodeling with cardiac resynchronization therapy: a MADIT-CRT substudy. J Am Coll Cardiol. (2016) 68:1268–76. doi: 10.1016/j.jacc.2016.06.051
29. Thomas L and Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. (2017) 10:65–77. doi: 10.1016/j.jcmg.2016.11.003
30. Marsan NA, Tops LF, Holman ER, Van de Veire NR, Zeppenfeld K, Boersma E, et al. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. (2008) 102:847–53. doi: 10.1016/j.amjcard.2008.05.048
31. Sasaki N, Okumura Y, Watanabe I, Mano H, Nagashima K, Sonoda K, et al. Increased levels of inflammatory and extracellular matrix turnover biomarkers persist despite reverse atrial structural remodeling during the first year after atrial fibrillation ablation. J Interv Card Electrophysiol. (2014) 39:241–9. doi: 10.1007/s10840-013-9867-6
32. La Meir M, Gelsomino S, Lucà F, Pison L, Rao CM, Wellens F, et al. Improvement of left atrial function and left atrial reverse remodeling after minimally invasive radiofrequency ablation evaluated by 2-dimensional speckle tracking echocardiography. J Thorac Cardiovasc Surg. (2013) 146:72–7. doi: 10.1016/j.jtcvs.2012.05.068
33. Santos SN, Henz BD, Zanatta AR, Barreto JR, Loureiro KB, Novakoski C, et al. Impact of atrial fibrillation ablation on left ventricular filling pressure and left atrial remodeling. Arq Bras Cardiol. (2014) 103:485–92. doi: 10.5935/abc.20140152
34. Kawaji T, Aizawa T, Seto I, Yamano S, Naka M, Bao B, et al. Remodeling and reverse-remodeling of left atrium and appendage after catheter ablation for atrial fibrillation. Am J Cardiol. (2025) 253:23–31. doi: 10.1016/j.amjcard.2025.06.001
35. Fredersdorf S, Ucer E, Jungbauer C, Dornia C, Eglmeier J, Eissnert C, et al. Lone atrial fibrillation as a positive predictor of left atrial volume reduction following ablation of atrial fibrillation. Europace. (2014) 16:26–32. doi: 10.1093/europace/eut152
36. Müller H, Noble S, Keller PF, Sigaud P, Gentil P, Lerch R, et al. Biatrial anatomical reverse remodelling after radiofrequency catheter ablation for atrial fibrillation: evidence from real-time three-dimensional echocardiography. Europace. (2008) 10:1073–8. doi: 10.1093/europace/eun187
37. Bisbal F, Guiu E, Cabanas P, Calvo N, Berruezo A, Tolosana JM, et al. Reversal of spherical remodelling of the left atrium after pulmonary vein isolation: incidence and predictors. Europace. (2014) 16:840–7. doi: 10.1093/europace/eut385
38. Arana-Rueda E, Pedrote A, García-Riesco L, Arce-León A, Gómez-Pulido F, Durán-Guerrero JM, et al. Reverse atrial remodeling following pulmonary vein isolation: the importance of the body mass index. Pacing Clin Electrophysiol. (2015) 38:216–224. doi: 10.1111/pace.12560
39. Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. (2005) 68:8–17. doi: 10.1016/j.cardiores.2005.06.008
40. Masuda M, Matsuda Y, Uematsu H, Sugino A, Ooka H, Kudo S, et al. Clinical impact of left atrial remodeling pattern in patients with atrial fibrillation: Comparison of volumetric, electrical, and combined remodeling. J Cardiovasc Electrophysiol. (2024) 35:171–81. doi: 10.1111/jce.16129
41. Yoshida K, Tada H, Ogata K, Sekiguchi Y, Inaba T, Ito Y, et al. Electrogram organization predicts left atrial reverse remodeling after the restoration of sinus rhythm by catheter ablation in patients with persistent atrial fibrillation. Heart Rhythm. (2012) 9:1769–78. doi: 10.1016/j.hrthm.2012.06.033
42. Levin ER, Gardner DG, and Samson WK. Natriuretic peptides. N Engl J Med. (1998) 339:321–8. doi: 10.1056/NEJM199807303390507
43. Pizon M, Friedel N, Pizon M, Freundt M, Weyand M, and Feyrer R. Impact of epicardial ablation of concomitant atrial fibrillation on atrial natriuretic peptide levels and atrial function in 6 months follow-up: does preoperative ANP level predict outcome of ablation? J Cardiothorac Surg. (2013) 8:218–34. doi: 10.1186/1749-8090-8-218
44. Mandalenakis Z, Eriksson H, Welin L, Caidahl K, Dellborg M, Rosengren A, et al. Atrial natriuretic peptide as a predictor of atrial fibrillation in a male population study. The Study of Men Born in 1913 and 1923. Int J Cardiol. (2014) 171:44–8. doi: 10.1016/j.ijcard.2013.11.042
45. Jiang H, Wang W, Wang C, Xie X, and Hou Y. Association of pre-ablation level of potential blood markers with atrial fibrillation recurrence after catheter ablation: a meta-analysis. Europace. (2017) 19:392–400. doi: 10.1093/europace/euw335
46. Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. (2007) 12:4179–90. doi: 10.2741/2379
47. Hooper JD, Scarman AL, Clarke BE, Normyle JF, and Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. (2000) 267:6931–7. doi: 10.1046/j.1432-1033.2000.01806.x
48. Gladysheva IP, Robinson BR, Houng AK, Kováts T, and King SM. Corin is co-expressed with pro-ANP and localized on the cardiomyocyte surface in both zymogen and catalytically active forms. J Mol Cell Cardiol. (2008) 44:131–42. doi: 10.1016/j.yjmcc.2007.10.002
49. Yoshimoto I, Ichiki H, Miyata M, Kamada H, Ninomiya Y, Yoshimura A, et al. Cardio-ankle vascular index and left atrial reverse remodeling after ablation for atrial fibrillation. Int Heart J. (2023) 64:623–31. doi: 10.1536/ihj.23-072
50. Kawakami H, Inoue K, Nagai T, Fujii A, Sasaki Y, Shikano Y, et al. Persistence of left atrial abnormalities despite left atrial volume normalization after successful ablation of atrial fibrillation. J Arrhythm. (2021) 37:1318–1329. doi: 10.1002/joa3.12624
51. Sugumar H, Prabhu S, Voskoboinik A, Young S, Gutman SJ, Wong GR, et al. Atrial remodeling following catheter ablation for atrial fibrillation-mediated cardiomyopathy: long-term follow-up of CAMERA-MRI sudy. JACC Clin Electrophysiol. (2019) 5:681–8. doi: 10.1016/j.jacep.2019.03.009
52. Kagawa Y, Fujii E, Fujita S, and Ito M. Association between left atrial reverse remodeling and maintenance of sinus rhythm after catheter ablation of persistent atrial fibrillation. Heart Vessels. (2020) 35:239–45. doi: 10.1007/s00380-019-01475-1
53. Lin M, Hao L, Cao Y, Zhao Y, Rong B, Han W, et al. Successful catheter ablation of atrial fibrillation improves but not reverses the abnormalities of left atrial mechanics and energy loss. Echocardiography. (2019) 36:752–60. doi: 10.1111/echo.14304
54. Xiong B, Li D, Wang J, Gyawali L, Jing J, and Su L. The effect of catheter ablation on left atrial size and function for patients with atrial fibrillation: an updated meta-analysis. PloS One. (2015) 10:e0129274–e0129287. doi: 10.1371/journal.pone.0129274
55. Yoshida K, Yui Y, Kimata A, Koda N, Kato J, Baba M, et al. Troponin elevation after radiofrequency catheter ablation of atrial fibrillation: relevance to AF substrate, procedural outcomes, and reverse structural remodeling. Heart Rhythm. (2014) 11:1336–42. doi: 10.1016/j.hrthm.2014.04.015
56. Pierucci N, Mariani MV, Iannetti G, Maffei L, Coluccio A, Laviola D, et al. Atrial cardiomyopathy: new pathophysiological and clinical aspects. Minerva Cardiol Angiol. (2025). doi: 10.23736/s2724-5683.25.06725-0
57. Weerts J, Țica O, Aranyo J, Basile C, Borizanova-Petkova A, Borovac JA, et al. Atrial cardiomyopathy: from healthy atria to atrial failure. A clinical consensus statement of the Heart Failure Association of the ESC. Eur J Heart Fail. (2025). doi: 10.1002/ejhf.3782
58. Yalcin MU, Gurses KM, Kocyigit D, Evranos B, Yorgun H, Sahiner L, et al. Predictors of left atrial volume index reduction following cryoballoon-based pulmonary vein isolation. Europace. (2016) 18:392–7. doi: 10.1093/europace/euv102
59. Pathak R, Lau DH, Mahajan R, and Sanders P. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Atr Fibrillation. (2013) 6:986–95. doi: 10.4022/jafib.986
60. Matsuda M and Matsuda Y. Mechanism of left atrial enlargement related to ventricular diastolic impairment in hypertension. Clin Cardiol. (1996) 19:954–9. doi: 10.1002/clc.4960191211
61. Staszewsky L, Wong M, Masson S, Raimondi E, Gramenzi S, Proietti G, et al. Left atrial remodeling and response to valsartan in the prevention of recurrent atrial fibrillation: the GISSI-AF echocardiographic substudy. Circ Cardiovasc Imaging. (2011) 4:721–8. doi: 10.1161/CIRCIMAGING.111.965954
62. Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Worthington M, Rajendram A, et al. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Heart Rhythm. (2010) 7:396–404. doi: 10.1016/j.hrthm.2009.11.031
63. Nakanishi K, Daimon M, Fujiu K, Iwama K, Yoshida Y, Hirose K, et al. Prevalence of glucose metabolism disorders and its association with left atrial remodelling before and after catheter ablation in patients with atrial fibrillation. Europace. (2023) 25:1–23. doi: 10.1093/europace/euad119
64. Yu SQ, Shi K, Li Y, Wang J, Gao Y, Shi R, et al. The impact of diabetes mellitus on cardiac function assessed by magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Cardiovasc Diabetol. (2024) 23:293–309. doi: 10.1186/s12933-024-02384-y
65. Guida CM, Souza JB, Cesena FY, Laurinavicius AG, Gonçalves Sousa M, Vilela AA, et al. Association between diabetes mellitus and left atrial strain in individuals with hypertension. Echocardiography. (2025) 42:e70226–41. doi: 10.1111/echo.70226
66. Tan Y, Zhang Y, Cai Q, Zhao R, Luo W, Jiang J, et al. Impact of diabetes mellitus on myocardial function and clinical outcomes in patients with significant aortic regurgitation. Cardiovasc Diabetol. (2025) 24:290–312. doi: 10.1186/s12933-025-02843-0
Keywords: atrial fibrillation, corin, catheter ablation, left atrial reverse remodeling, left atrial volume index
Citation: Wang Q, Li Y, Yuan X, Gong Y, Song K, Li C, Liu J, Zhao Y and Chen F (2025) Plasma corin levels provide minimal prognostic utility in left atrial reverse remodeling after catheter ablation of atrial fibrillation: an observational study. Front. Endocrinol. 16:1717422. doi: 10.3389/fendo.2025.1717422
Received: 02 October 2025; Accepted: 31 October 2025;
Published: 24 November 2025.
Edited by:
Noha M. Shawky, University of Mississippi Medical Center, United StatesReviewed by:
Giuseppe Giunta, Sapienza University of Rome, ItalyAngelina Borizanova, Medical University Sofia, Bulgaria
Copyright © 2025 Wang, Li, Yuan, Gong, Song, Li, Liu, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichang Zhao, eWljaGFuZ3poYW9rb2ZAMTYzLmNvbQ==; Feifei Chen, c2RhcWNoZW5mZWlAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qianqian Wang1†
Qianqian Wang1† Jinqiu Liu
Jinqiu Liu Feifei Chen
Feifei Chen