- 1Laboratory Electrochemistry and Functional Materials, Department of Chemistry, University of New Brunswick, Fredericton, NB, Canada
- 2Department of Chemistry, Brock University, St. Catharines, ON, Canada
The recent analysis by Mora and colleagues revealed that over 277 diseases can worsen due to climatic hazards resulting from greenhouse gas emissions. Specifically, more than 58% of known human diseases can be aggravated by climate change. Furthermore, there are over 1,000 pathways through which various climatic hazards have contributed to disease outbreaks, primarily due to the diversity of pathogens. This analysis also urges immediate action to address the root of the problem—reducing greenhouse gas (GHG) emissions. Numerous climatic hazards affect the incidence of human pathogenic diseases. Unfortunately, due to the complexity and multifaceted nature of the problem, there cannot be a single comprehensive solution to minimize climate-driven outbreaks. This study seeks to identify outbreaks of specific diseases categorized as epidemics, whose incidence is strongly correlated with global warming. The focus of this analysis is on (1) organizations responding to climate-related diseases to decelerate the incidence rates; (2) to call for a new disciplines in epidemiology that focuses exclusively on climate change-related prediction for future pandemics; (3) looking at the problem from the patient's point of view—how do non-medical/health professionals contribute to minimizing the spread of climate-related diseases?; (4) to analyze outbreaks vs. urbanization/pollution/increase in population density and public health policies; also (5) to verify the vaccination coverage vs. case reduction rate.
1 Introduction
1.1 Global warming
Global warming refers to a gradual rise in the Earth's average temperature, affecting the atmosphere, oceans, and land surfaces (1). This phenomenon is generally considered to have begun in the mid-1800s, when society started to industrialize. An initial collection of temperature gathered reveals signals of temperature rising in the 1950s. However, today's hypothesis traces the start of global warming back 120 years to 1830 (2).
The earliest known theories on climate change are attributed to French scientist Joseph Fourier, who first proposed the concept of the “greenhouse effect,” whereby the sun's rays bouncing off the Earth are trapped by gases, eventually leading to warmer temperatures (3). Over the next century and a half, climate change theories continued to evolve with the contribution of other individual scientists, such as John Tyndall and Svante Arrhenius (3). However, it was not until 1988 that the first internationally recognized organization was established to address the issue of climate change. Following a series of alarming weather events, the Intergovernmental Panel on Climate Change (IPCC) was established by the United Nations Environment Programme and the World Meteorological Organization due to concerns about the potential for global warming (4).
Global warming is primarily caused by anthropogenic activity, which releases greenhouse gases such as carbon dioxide and Methane into the atmosphere. These gases act as a heat blanket, covering the Earth's surface and making it warmer than it should be (5). The burning of coal is the largest source of carbon dioxide emissions, such that “the increase in atmospheric CO2 in recent decades represents about half of the emissions from fossil fuels and changes in tropical land use” (6). The remaining CO2 in the atmosphere is absorbed by the ocean, terrestrial biosphere, and soils (6). The primary natural source of methane release is from microbial decay of organic matter in wetlands. Anthropogenic sources account for twice as much methane as natural sources and include rice cultivation, livestock, bacterial decay in landfills, and the leakage of methane during the mining of fossil fuels (6).
While human activities are the primary drivers of global warming, some argue that natural factors, such as solar activity, volcanic eruptions, and changes in ocean circulation, also play a role (7). Thermohaline circulation, for example, helps distribute heat across the planet through the ocean's large heat capacity and its ability to transport heat efficiently (8). Solar activity causes heating in the lower atmosphere, resulting from changes in the amount of sunlight and alterations in the upper atmosphere's chemical composition due to shifts in the sun's UV rays (9). Lastly, volcanic eruptions are also a source of global warming due to the ejection of sulfur gases into the atmosphere, which are oxidized to aerosols. Aerosols spread and produce chemical and radiative effects, resulting in changes in the climate system (10). The global warming effects of aerosols include increasing cloud brightness due to increased sunlight reflection and increasing cloud coverage, as the aerosols inhibit rainfall (6).
In conclusion, global warming is a result of a combination of human activities and natural variations. “Modeling experiments suggest that anthropogenic activities dominate the warming of the 20th century, while natural forcings, such as volcanic and solar activities, are the principal drivers of climate change over the past millennium” (7).
The rate of global warming has been approximately 0.06°C per decade since 1850. However, since 1982, the rate of warming has increased to 0.20°C per decade (11).
1.2 Diseases affected by global warming/climate change
Numerous diseases are linked to climate change. A recent article by Mora et al. entitled “Over half of known human pathogenic diseases can be aggravated by climate change” (12) reveals that 277 diseases can worsen due to climatic hazards resulting from greenhouse gas emissions. Climatic hazards have at some point aggravated 58% of infectious diseases confronted by humanity worldwide. Additionally, this article identifies over 1,000 pathways through which various climatic hazards have contributed to disease outbreaks, primarily due to the diversity of pathogens. The significant number of pathogenic diseases and transmission pathways underscores the severe threat that climate change poses to human health, highlighting the urgent need for decisive action to reduce greenhouse gas (GHG) emissions.
Furthermore, the focus of this study is on diseases that are epidemics in nature, which are outbreaks that are not typically present in a region or population. Still, when they occur, they affect many individuals within a short period, suddenly and widely. The most prevalent diseases in the epidemic category that are driven by climate change are Dengue, Malaria, Yellow Fever, Influenza, HIV, and Monkeypox. Equally important are other infectious diseases, such as the viral diseases Zika, Ebola, and SARS-CoV-2, as well as bacterial Lyme disease and hard-tick relapsing fever (HTRF), among many others. However, many of these diseases are classified as endemic or pandemic, and their direct correlation with climate change must be analyzed with the evaluation of their host carriers—specifically, a particular species of mosquitoes, fruit bats, and blacklegged ticks. These categories of disease will not be analyzed in this study.
1.2.1 Dengue
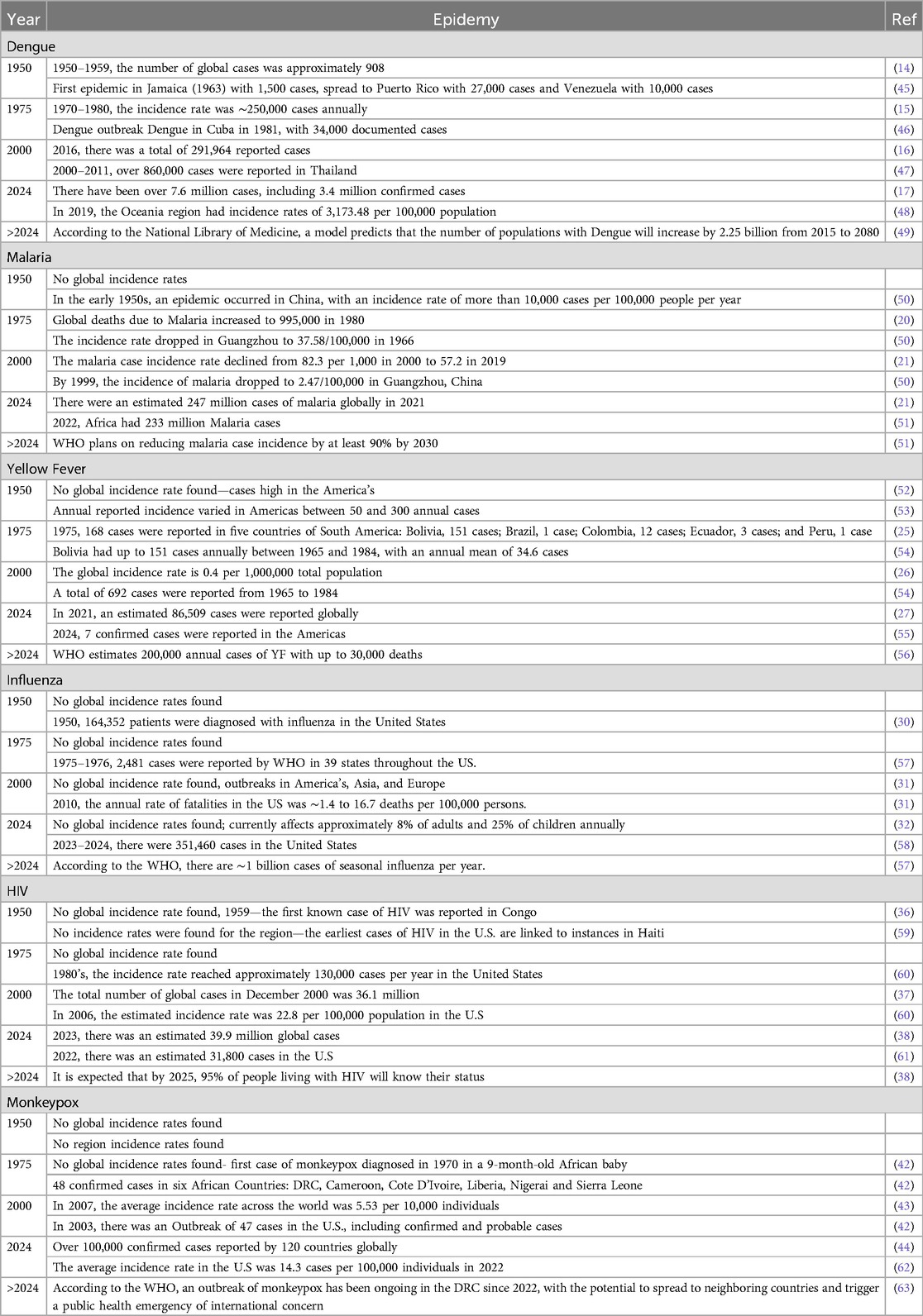
Rising global temperatures, particularly in warmer climates, influence dengue as they promote the growth and reproduction of Aedes mosquitoes, the primary vectors responsible for spreading the disease (13). “Monthly increases of 1°C in temperature increased the population risk of dengue by 1.95 times” (13). Dengue is highly concentrated in tropical areas and is therefore strongly correlated with temperature and the rainy season (13). In 1950, the number of global cases was approximately 908 (14), which increased to 250,000 annual cases between 1970 and 1980 (15). In 2016, a total of 291,964 cases were globally reported (16). As of 2024, there are over 7.6 million cases of Dengue globally (17).
1.2.2 Malaria
Malaria is also a mosquito-borne disease found in warmer climates transmitted from the female Anopheles mosquito to humans. “Each 2°C–3°C increase in ambient environmental temperature increases malaria transmission by 3%–5%” (18). Anopheles mosquitoes thrive in warmer climates at lower altitudes, as their reproductive phases are shortened and their feeding frequencies are increased (18). Additionally, heavy precipitation is favorable for Anopheles living conditions, as in dry conditions, these mosquitoes will desiccate (19). Anopheles mosquitoes are at their most reproductive when a rainy season has just come to an end, as the water level decreases and water pools in areas with deformed terrain become prime for breeding (19). Global deaths caused by Malaria increased from 995,000 in 1980 to 1,817,000 in 2004 (20). As of 2021, there were 247 million cases of malaria globally (21).
1.2.3 Yellow fever
Yellow fever is a viral hemorrhagic fever transmitted by infected A. Aegyptus mosquitoes (22). There is strong evidence that the predicted global warming will alter the international distribution of A. Aegyptus and increase the risk of the disease globally (23). Factors that influence and improve the occurrence of Yellow Fever include increased rainfall, higher air humidity and temperature, as well as non-primate human species, which serve as hosts for this disease (24). The mosquitoes carrying this disease have a peak population during a rainy summer, as increased rainfall and temperature (24) contribute to their proliferation. As of 1975, only 168 cases were reported in South America (25). In 2000, the global incidence rate of Yellow Fever was 0.4 per 1,000,000 total population (with 699 total cases reported in 2000) (26). In 2021, there were an estimated 86,509 cases reported globally (27).
1.2.4 Influenza
Influenza is an airborne, transmitted viral disease (28) that is strongly influenced by absolute humidity, which in turn is affected by environmental temperature (29). At low temperatures, drier conditions promote the spread of the influenza virus (29). This occurs because cold temperatures make the viral envelope more fragile, and dry air helps the virus remain stable, allowing it to stay airborne for more extended periods and increasing its ability to spread (29). On the other hand, at high temperatures, higher humidity levels are more favorable for the virus's survival (29). In warm conditions, the virus is more likely to dry out; however, high humidity helps prevent this, keeping the virus viable for longer periods and facilitating its spread (29). In 1950, a total of 164,352 patients were diagnosed with influenza in the United States (30). As of 2010, the annual rate of fatalities due to Influenza was∼1.4–16.7 deaths per 100,000 persons (31). Currently, as of 2024, Influenza affects approximately 8% of adults and 25% of children annually (32).
1.2.5 HIV
HIV is a viral infection caused by the human immunodeficiency virus and is spread by exposure to blood or other bodily fluids through sexual and mother-to-infant contact (33). Climate change has been linked to HIV transmission through its effects on public health, including increased food insecurity and greater human migration or climate refugees (34). Food insecurity is related to climate change as it increases substance use, resulting in a driving force for sexually risky behaviors (34). The chain of events begins with climate change-induced land degradation, followed by a reduction in food production. Then, those living in poverty may resort to desperate measures to seek food, possibly including transactional or forced sex, which raises the risk of HIV transmission (35). The first known case of HIV was reported in 1959 in Congo (36). In 2000, the total number of global cases in December 2000 was 36.1 million (37). As of 2023, there were an estimated 39.9 million global cases (38).
1.2.6 Monkeypox
Monkeypox is a viral disease caused by a pox virus, a member of the genus Orthopoxvirus (39). Climate change, resulting in a warmer climate, is predicted to lead to changes in the geographic ranges at risk of Monkeypox, caused by direct climate effects on the pathogen or indirect effects on species that harbor the pathogen or vector species (40). Warmer temperatures are found to be much more apt for human MPX (40). “Specifically, for each 1°C increase in the heat index (HI), daily cases increased by 7.7%” (41). Conversely, an increase in wind speed has been found to decrease monkeypox cases (41). The first case of monkeypox was diagnosed in 1970 in a 9-month-old African baby (42). In 2007, the average incidence rate worldwide was 5.53 per 10,000 individuals (43). As of 2024, over 100,000 confirmed cases had been reported by 120 countries worldwide (44). Table 1 summarizes the climate-related incident rate of epidemics over the last 75 years.
2 Discussion
2.1 Organizations responding to climate-related diseases to decelerate the incidence rates
2.1.1 Dengue
In response to the emergence of dengue as an epidemic in more than 100 countries, in 2024, the World Health Organization launched the Global Strategic Preparedness, Readiness and Response Plan (SPRP) (64). Their aim is, and has been, to coordinate a global response. Their plan involves 5 primary tenets: (1) Emergency coordination (setting up a team with leaders to handle the situation), (2) Collaborative surveillance: creating tools to quickly spot and control outbreaks of Dengue (within the lab), (3) Community protection (working closely with communities to share information and prevention strategies), (4) Safe and Scalable Care (ensuring health services are equipped to provide adequate care to patients with dengue, and (5) Access to countermeasures (continuing ongoing research to find better treatments and vaccines) (64). Due to the rapid global spread of dengue, the WHO has also employed a risk mapping strategy to assess which countries are currently at risk of dengue and which countries are vulnerable to the onset of dengue transmission (64). Their objective in using the risk map was to pinpoint countries where preparedness and response interventions were required, and in such cases, these countries would be registered for increased surveillance (64). WHO has also adopted the approach of using vector control strategies, including removing breeding sites and employing chemical agents to kill the larvae of Aedes Mosquitoes (64). The World Health Organization is aware of the role that climate change plays in the increase of dengue cases and is therefore adjusting dengue control methods based on different levels of risk, considering how changing climate patterns may impact the location of mosquito breeding sites (64). The SPRP is in the stages of developing an efficient diagnostic test, efficient treatments options and a vaccine for prevention (64). There have been dengue vaccines released in the past, such as Dengvaxia and Qdenga, and another more efficient dengue vaccine is currently in the middle of clinical development (64). The WHO has recommended the dengue vaccine for routine immunization programs in areas with high transmission rates and where dengue is a major public health concern, specifically for children aged 9–16 years (64).
2.1.2 Malaria
As Malaria emerges as a more prevalent disease globally, the World Health Organization administered the Global Technical Strategy for Malaria 2016–2030 (65). Their objective is to reduce the global malaria rates by 90% by 2030 (65). WHO plans to eliminate malaria from countries in which malaria was prevalent in 2015 in at least 20 of such countries by 2025, and at least 35 of those countries by 2030 (65). The malaria eradication strategy is based on five key principles: tailoring approaches to local needs, strong leadership and community involvement, enhanced data tracking, equitable access to health services, and innovation in new tools to accelerate progress towards elimination (65). WHO also emphasizes the importance of vector control strategies in eradicating malaria, utilizing tools such as long-lasting insecticidal nets and indoor spraying (65). Research is needed to figure out where and how targeting the parasite reservoir can help reduce malaria (65).
2.1.3 Yellow fever
The long-term (2017–2026) global strategy of the World Health Organization to “Eliminate Yellow Fever Epidemics” (EYE) focuses on three main goals: “(1) protecting at-risk populations, (2) preventing international spread, and (3) containing outbreaks quickly” (66). The strategy includes recommendations of vaccination, building resistant cities that are ready for emergencies and making sure international health rules are followed more effectively (66). The EYE strategy exclusively targets countries that are at the highest level of risk for a yellow fever outbreak (66). In the next ten years, vaccine makers are expected to provide 1.38 billion doses (66). Also, the WHO has adopted the Integrated Disease Surveillance and Response (IDSR) program to obtain a data count of suspected, probable, and confirmed yellow fever cases (66). By the end of 2025, WHO suspects that all high-risk countries in Africa can now test and confirm yellow fever and by the end of 2026, all high-risk countries will have completed national vaccination campaigns (66).
2.1.4 Influenza
In June 2024, the Center for Disease Control and Prevention (CDC) launched the Global Influenza Program (67). CDC is teamed up with global partners to improve their response to Influenza threats (67). Their programs core tenets are to work with partners to improve flu surveillance, develop pandemic plans (and prevention plans), support research projects, and expand flu vaccination programs (67). As well, CDC is partnered with the World Health Organization to monitor seasonal flu, detect novel flu and work on a vaccine (67). The WHO predicts that by 2030, we will have better global tools, including a specific plan with more research devoted to preventing, detecting, controlling, and treating influenza, along with stronger country capacities, where each country will have its own customized influenza program, ensuring global health preparedness (68).
2.1.5 HIV
By the end of 2025, Canada's global target is to have 95% of people living with HIV diagnosed, and 95% of those diagnosed on treatment, along with 95% of those on treatment responding positively to the treatment with reduced viral load of HIV (69) The Global Health HIV Prevention Clinic (GPC) also launched the HIV prevention strategy 2025 in which they predict to have fewer than 370,000 annual new cases of HIV by the end of 2025 (70). The road map that GPC created includes 10 steps: (1) conduct assessments and gather data on HIV prevention programs; (2) adopt a precise prevention approach focused on target populations; (3) identify the country's financial investment needs for adequate HIV responses; (4) strengthen leadership for HIV prevention by promoting collaboration, oversight, and management; (5) boost community-led HIV prevention services; (6) eradicate social and legal barriers to HIV prevention services for target populations. (7) integrate HIV prevention into key services to improve outcomes; (8) create systems to introduce new HIV prevention tools and innovations quickly; (9) set up real-time monitoring systems for prevention programs with regular updates; and (10) improve accountability for everyone involved in HIV prevention progress (70). Their actions focus on combination prevention for key groups, like adolescent girls, young women, men, and boys in high-risk areas, promoting condoms and lubricants and expanding access to antiretroviral prevention, including PrEP (Pre-exposure Prophylaxis) (70).
2.1.6 Monkeypox
As of 2024, the World Health Organization has launched the Global Strategic Preparedness and Response Plan to combat the outbreak of monkeypox (71). Their plan focuses on implementing robust surveillance, prevention, and response strategies; enhancing access to medical tools such as tests and vaccines; reducing animal-to-human transmission; and engaging communities in outbreak control (71). The WHO is actively working on an efficient vaccine for individuals who are the most vulnerable, specifically healthcare workers in contact with recent cases (71). Globally, the focus is on strong leadership, timely guidance, and providing medical tools to high-risk groups in affected countries (71). In Africa, WHO Regional Office (AFRO) and Africa CDC will lead the mpox response, using a shared plan and budget (71). At the national and local levels, health authorities will adjust strategies based on the current situation (71). Currently, there is no specific antiviral treatment for Monkeypox, which is a growing global concern (72).
2.2 The World Health Organization's response to climate-related diseases
The World Health Organization recognizes the increasing risk that global warming has on climate-related epidemics (73). They are aware that an increase in temperature and precipitation boosts the spread of vector-borne diseases and that “without preventative actions, deaths from such diseases, currently over 700,000 annually, may rise” (73). Therefore, in response to this WHO has created an approach to avert health impacts due to climate change (73). WHO's response is based on three main objectives. The first is to “promote actions that both reduce carbon emissions and improve health” (73). This centers health as the primary concern of climate change, ensuring that actions benefit both the environment and health, and utilizes the health community to advocate for policies that protect both (73). Secondly, WHO is aiming to build better and more “climate-resilient and environmentally sustainable health systems”(73). This includes helping health systems adopt more affordable, environmentally friendly solutions and ensuring that health investments support sustainability and climate adaptation, including training healthcare workers (73). The third objective of the WHO is to “protect health from the wide range of climate change” (73). This includes identifying climate-related health risks, enhancing our systems' capacity to respond to such risks, and providing funding for health systems to adapt to climate change (73). “WHO strives to embed climate change in health priorities, similar to those of United Health Care, and target carbon neutrality by 2030” (73).
2.3 New discipline in epidemiology that focuses exclusively on climate change-related prediction for future pandemics
“Climate epidemiology” appears to be a growing research discipline, driven in response to the perceived public health risks associated with climate change. In recent decades, greenhouse gases have been the primary driver of climate change (74). Furthermore, multiple studies have demonstrated that climate change influences disease transmission, although the exact nature of the causal impact is not always known (75). As global warming continues to accelerate, climate-related illnesses, such as vector-borne diseases, are expected to become an increasing threat to public health (76).
Climate epidemiology involves collaboration between epidemiologists and climate scientists to understand the connections between climate change and the emergence of infectious diseases, including the prediction of future pandemics (74). It does so by examining how climate change (including rising air, land, and sea temperatures, altered precipitation patterns, extreme weather events, and shifts in ecosystems) may affect the transmission and spread of infectious diseases (74). More specifically, scientists in this field aim to determine how the environment influences disease vectors (such as mosquitoes or ticks), how shifts in ecosystems may impact animal hosts, and how human migration or population displacement due to climate events can introduce new pathogens into different regions (74).
The ultimate goal of climate epidemiology is to provide insight into the causation and effects of disease spread through climate modeling, in a way that can be used to help predict potential issues and develop preventive strategies.
2.4 Climate-driven model of diseases
Globally, nations such as the US, Europe, and Australia are attempting to preserve the environment to combat the transmission of dengue (77). By clearing dirty environments, this makes the habitat less favorable for mosquitoes to live in, as they prefer areas with high levels of filth. Campaigns to sanitize bodies of water are being initiated by voluntary organizations such as the India Peace Youth Movement, the American Federation of Students, and the National Youth Society (77). Another strategy that Japan has adopted is spreading educational knowledge to students in preventing dengue through environmentally sustainable practices (77).
In 2021, two organizations—the World Health Organization's Strategic Advisory Group on Malaria Eradication and the Lancet Commission on Malaria Eradication—proposed strategies to combat global malaria, which is influenced by a climate-driven model (78). The first strategy outlined involves using accurate risk mapping to identify malaria transmission hotspots, specifically areas where climate conditions make the region more susceptible to transmission (78). Their other strategy is to research and find effective insecticides, which are dependent on the fact that certain insecticides are less effective in hotter climates (78). These organizations are also incorporating climate considerations into their malaria control efforts, such as investing in surveillance systems that monitor both climate and malaria (78). This is essential to understand and implement knowledge regarding the complex relationship between climate and malaria transmission.
2.5 From the patient's point of view—how do non-medical/health professionals contribute to minimizing the spread of climate-related diseases?
From the patient's perspective, non-medical and non-healthcare professionals can play a vital role in minimizing the spread of climate-related diseases and preventing new epidemics by collaborating to reduce public vulnerability. The vulnerability of a population or region to the impacts of climate change is a function of (79).
• Exposure to climate hazards
• Sensitivity to those impacts
• Adaptive capacity
2.5.1 Exposure
Reducing exposure to climate hazards can be achieved by preventing them in the first place, which starts with increased awareness and education regarding the threat of climate change. A recent study conducted on 2,273 participants in more than 30 countries revealed a strong correlation between people's belief in climate change and their willingness to make behavioral changes to mitigate it (80). Furthermore, according to the American Journal of Health, only one in five Americans reports having a strong understanding of climate change (81). Moreover, roughly half of Americans express distrust of related media coverage.
Moving beyond essential awareness, citizens can further mitigate the risk of climate change exposure by supporting government policy change, as well as reducing their carbon footprint (82). Although broad public support is not always a prerequisite for implementing policies designed to reduce greenhouse gases, it has been demonstrated to facilitate the adoption and implementation of robust policies (82).
On a personal level, individual citizens can take simple steps to reduce their carbon footprint, such as using public transportation, reducing waste, conserving water, and eating a more plant-based diet (83). Environmental studies have shown that daily household consumption is the primary contributor to greenhouse gases, accounting for two-thirds of global carbon emissions (83). When undertaken collectively, individual actions can contribute to the broader effort of mitigating the long-term impact of climate change.
2.5.2 Sensitivity
Sensitivity to the societal impacts of current climate change can be influenced by a multitude of factors directly related to health and well-being, including socioeconomic status, biology and genetic endowment, access to health services, gender, and personal health practices (79). Similar to mitigating the risk of exposure, individuals can help lower sensitivity to climate change-driven health threats by raising personal awareness and supporting community initiatives (79).
One type of public initiative to reduce climate change sensitivity is creating more resilient infrastructure to reduce the impact of climate change, such as “the designing of flood-resistant homes, improving waste management systems, or building more green spaces can limit the conditions that foster disease outbreaks, particularly in vulnerable populations” (84). Unfortunately, the current reality is that longer-term climate-related risks are not always taken into account in the shorter-term profit-focused public and private investment decisions. As a result, consequently, data shows that large amounts of capital continue to flow into hazard-prone areas (84).
Another form of public initiative to reduce climate change sensitivity is to ensure disaster preparedness. Providing immediate aid, setting up shelter, ensuring clean water, and managing sanitation can help reduce the risk of secondary diseases following a natural disaster.
2.5.3 Adaptive capacity
Adaptive capacity is “the potential or ability of a system, region, or community to adapt to the effects or impacts of climate change” (85). Communities that have established high adaptive capacity, as evidenced by comprehensive plans and responses, have been shown to provide citizens with a better ability to protect their own health (79). Human adaptation is crucial in mitigating the socio-economic impacts of climate change on ecosystems and populations (86). Non-medical citizens can also strengthen these measures through education and support.
A prime example of adaptive behavior is the water management initiatives under way in the UK (87). UK researchers are using climate change factors to predict future alterations in rainfall and river flow. These estimates are then incorporated into the long-term plans of water utility companies to ensure the sustainability of water supply (87).
As climate change influences the spread of vector-borne diseases such as malaria, dengue, and Zika, personal adaptive health measures that citizens can employ include limiting exposure (using insect repellent and wearing protective clothing when outdoors), eliminating areas of standing water, and using bed nets when sleeping (88). As an example of the effectiveness of one of these solutions, the World Health Organization (WHO) estimated that between 2000 and 2015, the annual incidence of malaria cases fell by 37% and that the malaria mortality rate fell by 60% (88) These reductions are believed to have been attained, in large part, due to the increase in the use of insecticide-treated bed nets and access to treatment. However, the WHO also indicated that only about 68% of individuals at risk of malaria slept under an insecticide-treated bed net in 2015, so the reduction in malaria could have been even more significant with improved precautions.
For viral-based or waterborne diseases, personal adaptive measures could include regular hand washing, boiling potentially unsafe water, and/or avoiding drinking from unknown sources. The U.S. Centers for Disease Control and Prevention has cited hand washing as the single most effective way to prevent the transmission of disease (89).
2.6 Outbreaks vs. urbanization/pollution/increase in population density and public health policies
2.6.1 Dengue
Although climate patterns highly influence the transmission of dengue, it has been shown that dengue cases are not always consistently distributed in cities where climate is considered uniform (90). Therefore, there are other confounding factors at play in the outbreak of dengue (90). Such factors include socioeconomic statuses (SES), population growth, mass human movements, and urbanization (90). A study done in Nouméa during two major dengue epidemics (2008–2009 and 2012–2013) reveals that the neighborhoods with lower socioeconomic statuses were correlated with higher dengue incidence rates (90). Rapid urbanization has been found to play a large role in the outbreak of dengue, as it enables easier distribution of the Aedes population (91). Urban developments are creating more suitable habitats for Aedes mosquitoes, such as highly concentrated areas of human density, which provide more opportunities for blood feeding, and increased areas of moisture, like flowerpots (91). Ultimately, it is not only climate change that plays a role in the increase of dengue, but multiple variables are at play in this disease's transmission.
2.6.2 Malaria
For malaria, it appears that areas of large human densities contribute to a decline in the spread of malaria (92). This is because urbanization and anthropogenic factors create a less suitable breeding ground for Anopheles mosquito species (92). These anthropogenic variables include fewer water surfaces for breeding and natural surfaces covered with infrastructure (92). Additionally, urbanization contributes to increased pollution, which negatively impacts breeding sites for Anopheles species (92). Other anthropogenic factors that contribute to the spread of malaria include agriculture (93). For instance, when farmers construct irrigation channels, this appears to create suitable mosquito breeding sites (93). Lastly, low socioeconomic factors also contribute to an increase in malaria cases, as those with lower socioeconomic statuses will have less access to health care and risk prevention (94).
2.6.3 Yellow fever
Other confounding factors that contribute to an increase in Yellow Fever outbreaks include social, ecological, and behavioural factors (95). Such factors include the movement of human populations into forest areas and coastal zones, where there is an increased number of breeding sites for Aedes aegypti mosquitoes (95). The number of non-vaccinated human populations also plays a role in the spread of Yellow Fever (95). It appears that urbanization has caused an increase in Yellow Fever outbreaks (96). According to WHO, an outbreak in Angola in 2016 was due to a large urban outbreak in a transportation center, which showed how Yellow Fever can rapidly spread to foreign nations (96). In Africa, growing and increasingly populated cities (increasing by 4% annually) appear to play a role in the increase of Yellow Fever, as unvaccinated populations are exposed to drinking water in large open containers—favourable breeding sites for Aedes aegypti mosquitoes (97). Fortunately, public health initiatives like Oswaldo Cruz's campaign against the mosquito vector Aedes aegypti drastically reduced Yellow Fever cases in Rio de Janeiro (95). In the early 1940s, both vaccination campaigns and the eradication of Aedes aegypti significantly contributed to the elimination of urban Yellow Fever transmission in Brazil and across the Americas (95).
2.6.4 Influenza
Aside from climate change contributing to the spread of influenza, other impacting factors like urbanization have a major effect on the intensity of influenza epidemics (98). Urbanization shapes the severity of influenza's transmission patterns due to increased human population densities, which increase human contact and population mobility (98). It appears that cities are the principal areas for human influenza transmissions, with more diffuse epidemics occurring in more populated cities, as there are higher rates of personal contact (99). It also appears that in smaller cities, surges of influenza outbreaks last shorter periods of time (99). Regarding the public health interventions that were set in place during the peak of COVID-19, this decreased the cases of influenza from 71.9 per 1,000 people to 49.8 per 1,000 people (100). Such public health interventions included remaining at home when experiencing respiratory symptoms, wearing masks in public spaces, and hand sanitization (100).
2.6.5 HIV
A strongly contributing factor to the prevalence of HIV outbreaks is in-migration into large cities (101). Migration increases opportunities for contact between humans, growing sexual networks from different areas, and ultimately increasing HIV's transmission rate (101). According to the article “HIV/AIDS and Urbanization”, “urban levels of HIV infection are typically four to ten times those of rural areas” (102). Public health policies have helped reduce HIV cases in certain parts of the world, particularly in Africa, where the President's Emergency Plan for AIDS Relief was employed (103). It is thought that through advances in treatment, the HIV epidemic in the United States could be quickly ended (103).
2.6.6 Monkeypox
Although the chances of Monkeypox outbreaks tend to increase in warmer climates, there are other variables that play a role in its transmission. The surge of Monkeypox cases in Nigeria in 2017 was driven by a combination of factors, including population growth and unvaccinated residents (104). Two key factors contributing to the outbreak were increased exposure of humans to forest animals due to deforestation and population migration, and declining vaccination immunity since the 1970s (104). Additionally, globalization has increased the likelihood that Monkeypox will spread around the world, as humans are more connected, giving way to quicker disease transmission (104).
Generally, there appears to be a strong correlation between high infection rates and urban areas (denser populations) (105). Another variable that has appeared to contribute to an increase in Monkeypox cases includes environmental pollution, such that air pollutants like CO and NO₂ have a positive association with daily Monkeypox cases (106).
2.7 Implementation effects—the vaccination coverage vs. case reduction rate
2.7.1 Dengue
2.7.1.1 TAK-003 vaccine (Asia & Latin America)
In a substantial Phase 3 trial, TAK-003 showed an overall efficacy of 80.9% in preventing virologically confirmed dengue, reporting 0.5 cases per 100 person-years in the vaccine group vs. 2.5 cases per 100 person-years in the placebo group. The vaccine group also experienced a 95.4% reduction in hospitalizations compared to the placebo (107–109). In Sri Lanka, TAK-003 led to a projected 69.1% decrease in dengue cases and a 72.7% decline in hospitalizations during an outbreak. With improved coverage and accelerated rollout, these reductions rose to over 80% (110).
2.7.1.2 CYD-TDV (dengvaxia) vaccine
In Latin America, a significant phase 3 trial reported 60.8% efficacy against symptomatic dengue and 80.3% efficacy against hospitalizations, along with a 95.5% reduction in severe cases among vaccinated children aged 9–16 (111). Mass vaccination campaigns in Brazil's Paraná State resulted in an overall effectiveness of 21.3% in reducing symptomatic dengue cases, with a 71% decrease noted among individuals with a previous dengue history. For those without such a history, effectiveness was lower at 12% (112). A 6-year cohort study conducted in southern Brazil revealed a 33.7% reduction in probable dengue cases and a 20.1% reduction in laboratory-confirmed cases, with a notable increase in effectiveness for the DENV-1 and DENV-4 serotypes (113).
Additionally, modelling studies have shown that in areas with high transmission, vaccinating 9-year-olds with an 80% coverage rate could lead to a remarkable reduction in the dengue burden by 13%–25% over the next 30 years. In moderate-to-high endemicity areas, the reductions were found to range from 6% to 25% (108). In Yucatán, Mexico, models are predicting an impressive decrease of up to 80% in annual dengue incidence within just 5 years if a durable vaccine is implemented (109).
Key factors influencing the reduction rates of dengue include serostatus—the presence or absence of specific antibodies in a person's blood serum, as identified by a serological test—and virus serotype, which refers to a serologically distinguishable strain of a virus. Considering the efficacy of vaccines based on serostatus, studies have indicated that vaccination is more effective in individuals with prior dengue exposure (i.e., seropositive). Simultaneously, some cases have shown an increased risk or reduced effectiveness in seronegative individuals (112, 114, 115). The efficacy of vaccines varies by dengue virus serotype, with lower effectiveness against DENV-2 and higher effectiveness against DENV-3 and DENV-4 (111, 113, 116).
Dengue vaccination has made a remarkable impact by significantly reducing the number of dengue cases and hospitalizations, especially among high-transmission, seropositive populations. The reduction rates vary widely, ranging from 20% to over 80%, depending on the type of vaccine used, the setting, and the characteristics of the population involved. Yet, there's still more we can do to improve these outcomes! For example, achieving higher vaccination coverage and starting campaigns promptly can lead to even greater decreases in cases and hospitalizations, as highlighted in literature (109, 110). Additionally, it's vital to assess the durability of the vaccine to address the issue of waning immunity, which could lead to larger epidemics if booster doses aren't given as needed (109).
2.7.2 Malaria
Malaria continues to pose a significant health challenge around the world, yet we've made remarkable strides in reducing both the number of cases and deaths, especially with the advent of new vaccines. The recent malaria vaccines, particularly RTS, S/AS01 and R21/Matrix-M, have been instrumental in significantly lowering malaria cases among children in regions that bear the highest burden. Between 2000 and 2015, global malaria incidence rates experienced an impressive drop of 37%, and mortality rates decreased by an incredible 60%. This success is largely due to the increased use of insecticide-treated nets, enhanced diagnostics, and effective antimalarial treatments—vaccines came into play later, as they weren't widely accessible during this period (117, 118). In sub-Saharan Africa, an astonishing 70% of the reduction in cases can be credited to these vital interventions (117).
The impact of malaria vaccines on the incidence rate is truly remarkable. One shining example is the RTS S/AS01 vaccine, highly recommended for children living in high-transmission areas. This vaccine has shown that it can prevent a median of 2,653 malaria cases for every 100,000 people each year, and it has helped avoid around 82,270 cases in fully vaccinated children per 100,000 (119–121). In long-term studies, the number of severe malaria cases in vaccinated children has stayed impressively low, ranging from just 0.004 to 0.007 cases per person-year, with no notable rise in cases observed 6–7 years after vaccination (122). Additionally, the newer R21/Matrix-M vaccine has proven to be effective as well, showcasing 75% efficacy at seasonal sites and 68% at standard sites, leading to a decrease of 868 malaria cases per 1,000 child-years at seasonal sites and 296 per 1,000 at standard sites over the course of 12 months (118).
It is encouraging to observe that acceptance rates for malaria vaccines are notably high in endemic countries, attaining an impressive rate of 95.3%. This indicates substantial potential for achieving a widespread impact as these vaccines become increasingly accessible. Prior to the introduction of vaccines, the decline in malaria cases was primarily attributed to vector control measures and treatment protocols. However, the advent of vaccines such as RTS, S/AS01 and R21/Matrix-M has resulted in even more significant reductions in malaria cases among children, particularly in Africa. Given such elevated vaccine acceptance rates, we are optimistic about the ongoing advancements we can achieve in the fight against malaria.
2.7.3 Yellow fever
Yellow fever is a serious viral disease found in various regions of Africa and South America. The introduction and expansion of yellow fever vaccination campaigns have made a remarkable difference, leading to significant reductions in cases and deaths around the world, especially in high-risk areas. Thanks to mass vaccination, we've seen a substantial decline in the incidence of yellow fever and outbreaks.
In Africa, these mass vaccination efforts have resulted in an impressive 47% reduction in yellow fever deaths in 2018 when compared to what might have happened without the vaccine, preventing around 10,000 deaths that year alone (123). Preventive mass vaccination campaigns (PMVCs) across African provinces have brought about an incredible 86% decrease in the incidence of yellow fever outbreaks (with an incidence rate ratio of 0.14), leading to a 34% reduction in outbreaks during the study period from 2005 to 2018 (124–131). Between 2005 and 2017, vaccination activities in Africa are estimated to have prevented between 3.3 and 6.1 million deaths over the lifetimes of those vaccinated, depending on the level of herd immunity considered (127).
Since 1970, global yellow fever vaccination coverage has improved significantly, yet as of 2016, 43%–52% of individuals in risk zones still needed vaccination to reach the recommended 80% coverage threshold for preventing outbreaks (125). While vaccination has been especially effective in high-risk areas, there are still gaps in some regions, particularly in certain parts of Nigeria, the Democratic Republic of Congo, and South Sudan (128).
Overall, yellow fever vaccination campaigns have had a significant impact, reducing cases, deaths, and outbreaks, particularly in Africa. However, we must continue our efforts to close these coverage gaps and maintain high immunity levels to prevent future outbreaks.
2.7.4 Influenza
Vaccines play a vital role in keeping us healthy by consistently reducing cases of influenza, hospitalizations, and severe complications. The greatest benefits are seen in children and those in high-risk groups. In the United States from 2016 to 2018, influenza vaccination helped prevent up to 46.6 cases for every 1,000 vaccinated children (ages 6 months to 8 years) and 6.9 cases for every 1,000 vaccinated adults aged 65 and older during seasons when the vaccine effectiveness ranged between 29% and 40% (132). Over in Europe during the 2022/23 season, vaccine effectiveness against influenza A varied from 27% to 44% across all ages, with children benefiting the most at 49%–77%. For influenza B, vaccine effectiveness was impressive at over 50% overall, soaring to 87%–95% in children (133). In the US during the 2019/20 season, vaccination lowered the risk of influenza-related hospitalization by 41% in adults (132). A meta-analysis reveals that vaccine effectiveness among children is 39% for reducing medical visits and an excellent 57% for cutting down hospitalizations; for the elderly, it stands at 25% for visits and 14% for hospitalizations (134, 135).
2.7.5 Monkeypox
Getting vaccinated against monkeypox, especially with the JYNNEOS vaccine, plays a vital role in minimizing the spread and impact of monkeypox outbreaks across the globe. While we don't have specific numbers showing the reduction in global cases before and after vaccination, there's clear evidence that the vaccine produces strong immune responses. These responses are expected to lead to lower case numbers and a lighter disease burden.
The JYNNEOS vaccine elicits a robust antibody response against monkeypox and related orthopoxviruses, with its peak response occurring approximately 2 weeks after the second dose, regardless of whether the individual has received prior vaccination (136). Most participants continued to have orthopoxvirus-specific antibodies for up to 2 years after vaccination, indicating that this vaccine provides long-lasting immune protection (136). It also effectively generates neutralizing antibodies, which are crucial for preventing infection and reducing transmission (136). Even though we don't have specific global case reduction rates detailed before and after vaccination, the strong immune response and the long-lasting presence of antibodies suggest that the JYNNEOS vaccination is likely making a significant impact in reducing monkeypox cases and helping to manage outbreaks.
3 Recommendations to the public to minimize the spread of climate-related disease
To minimize the spread of climate-related diseases, it's essential to take steps that both reduce exposure to the factors driving climate change and enhance personal health resilience. Here are some general recommendations:
1. Reduce carbon emissions by adopting new skills such as using public transport or carpooling, switching to renewable energy sources, and implementing energy-efficient practices in homes and workplaces (137).
2. Protect against vector-borne diseases by employing strategies like wearing insect repellent and protective clothing when going outdoors, eliminating standing water to prevent mosquito breeding, and using bed nets when sleeping in areas where diseases are common (138).
3. Stay informed about climate-related health risks in your region and get vaccinated to diminish the risk of diseases (139, 140).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AM: Writing – original draft, AI: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a Catalyst Grant from the Canada Institute for Health Research (CIHR), project no. RC2-199011.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amanda MacMillan JT. Global Warming 101, Definition, Facts, Causes and Effects of Global Warming. NRDC (the Natural Resources Defense Council) (2024). Available online at: https://www.nrdc.org/stories/global-warming-101 (Accessed March 12, 2025).
2. Harrisson T. Scientists Clarify Starting Point for Human-Caused Climate Change. CarbonBrief Clean on Climate (2019). Available online at: https://www.carbonbrief.org/scientists-clarify-starting-point-for-human-caused-climate-change/ (Accessed March 12, 2025).
6. Hansen J, Sato M, Ruedy R, Lacis A, Oinas V. Global warming in the twenty-first century: an alternative scenario. Proc Natl Acad Sci U.S.A. (2000) 97(18):9875–80. doi: 10.1073/pnas.170278997
7. Jian-Bin H, Shao-Wu W, Yong L, Zong-Ci Z, Xin-Yu W. Debates on the causes of global warming. Adv Clim Change Res. (2012) 3(1):38–44, ISSN 1674-9278. doi: 10.3724/SP.J.1248.2012.00038
8. Clark PU, Pisias NG, Stocker TF, Weaver AJ. The role of the thermohaline circulation in abrupt climate change. Nature. (2002) 415:863–9. doi: 10.1038/415863a
9. Shapiro AV, Egorova TA, Shapiro AI, Arsenovic P, Rozanov EV, Gizon L. Transition of the sun to a regime of high activity: implications for the earth climate and role of atmospheric chemistry. J Geophys Res Atmospheres. (2024) 129(15):2–3. doi: 10.1029/2023jd039894
10. Robock A. Volcanic eruptions and climate. Rev Geophys. (2000) 38(2):191–219. doi: 10.1029/1998rg000054
11. Dahlman RLAL. Climate Change: Global Temperature. NOAA. (2024). Available online at: https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (Accessed July 18, 2025).
12. (a) Mora C, McKenzie T, Gaw IM, Dean JM, von Hammerstein H, Knudson TA, et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat Clim Chang. (2022) 12:869–75. doi: 10.1038/s41558-022-01426-1; (b) Feldscher, K. The next pandemic: not if, but when. Harvard T.H. Chan School of Public Health (2024). Available online at: https://hsph.harvard.edu/news/next-pandemic-not-if-but-when/ (Accessed March 12, 2025).35968032
13. Jing Q, Wang M. Dengue epidemiology. Glob Health J. (2019) 3(2):37–45, ISSN 2414-6447. doi: 10.1016/j.glohj.2019.06.002
14. Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. (2005) 2:1. doi: 10.1186/1742-7622-2-1
15. Monath TP. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci U S A. (1994) 91(7):2395–400. JSTOR, Available online at: http://www.jstor.org/stable/2364243 8146129
16. Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. (2017) 7:317. doi: 10.3389/fcimb.2017.00317
17. Dengue—Global Situation. World Health Organization (2024). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON518 (Accessed March 13, 2025).
18. Rejeki DSS, Solikhah S, Wijayanti SPM. Risk factors analysis of malaria transmission at cross-boundaries area in menoreh hills, Java, Indonesia. Iran J Public Health. (2021) 50(9):1816–24. doi: 10.18502/ijph.v50i9.7054 PMID: 34722377; PMCID: PMC8542814; b) A.J. McMichael, D.H. Campbell-Lendrum, C.F. Corvalán, K.L. Ebi, A.K. Githeko, J.D. Scheraga, A. Woodward, Climate change and human health, RISKS AND RESPONSESWORLD HEALTH ORGANIZATION GENEVA 2003, https://iris.who.int/bitstream/handle/10665/42742/924156248X_eng.pdf?sequence=1
19. Castro MC. Malaria transmission and prospects for malaria eradication: the role of the environment. Cold Spring Harb Perspect Med. (2017) 7(10):a025601. doi: 10.1101/cshperspect.a025601
20. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. (2012) 379(9814):413–31. doi: 10.1016/S0140-6736(12)60034-8
21. Malaria. World Health Organization (2023). Available online at: https://www.who.int/data/gho/data/themes/malaria#:∼:text=Malaria%20case%20incidence%20reduced%20from,897%20000%20to%20568%20000 (Accessed March 13, 2025).
22. Yellow Fever. World Health Organization (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (Accessed March 12, 2025).
23. Gaythorpe KM, Hamlet A, Cibrelus L, Garske T, Ferguson NM. The effect of climate change on yellow fever disease burden in Africa. eLife. (2020) 9:e55619. doi: 10.7554/eLife.55619
24. Abreu FV, de Andreazzi CS, Neves MS, Meneguete PS, Ribeiro MS, Dias CM, et al. Ecological and environmental factors affecting transmission of sylvatic yellow fever in the 2017–2019 outbreak in the Atlantic forest, Brazil. Parasit Vectors. (2022) 15:23. doi: 10.1186/s13071-021-05143-0
25. World Health Organization. Weekly Epidemiological Record [Relevé Épidémiologique Hebdomadaire] (1976). Available online at: https://iris.who.int/bitstream/handle/10665/211054/WER3224_301-301bis.PDF;jsessionid=78BDE3E373F494512941A7CBD2E22ABB?sequence=1 (Accessed March 13, 2025).
26. Who Immunization Data Portal—Detail Page. Yellow Fever (YF) reported cases and incidence. World Health Organization (2025). Available online at: https://immunizationdata.who.int/global/wiise-detail-page/yellow-fever-(yf)-reported-cases-and-incidence?CODE=Global&YEAR= (Accessed March 13, 2025).
27. Liang Y, Dai X. The global incidence and trends of three common flavivirus infections (dengue, yellow fever, and Zika) from 2011 to 2021. Front Microbiol. (2024) 15:1458166. doi: 10.3389/fmicb.2024.1458166
28. Mayo Clinic. Influenza (flu). Mayo Foundation for Medical Education and Research (MFMER) (2025). Available online at: https://www.mayoclinic.org/diseases-conditions/flu/symptoms-causes/syc-20351719#:~:text=Overview,get%20better%20on%20their%20own (Accessed March 12, 2025).
29. Deyle ER, Maher MC, Hernandez RD, Basu S, Sugihara G. Global environmental drivers of influenza. Proc Natl Acad Sci U.S.A. (2016) 113(46):13081–6. doi: 10.1073/pnas.1607747113
30. Hilleman MR, Mason RP, Rogers NG. Laboratory studies on the 1950 outbreak of influenza. Public Health Reports (1896–1970). (1950) 65(24):771. doi: 10.2307/4587371
31. Update: Influenza Activity—United States and Worldwide, 1999–2000 Season, and Composition of the 2000–01 Influenza Vaccine. Centers for Disease Control and Prevention (2000). Available online at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4917a5.htm (Accessed March 13, 2025).
32. Wu H, Xue M, Wu C, Ding Z, Wang X, Fu T, et al. Estimation of influenza incidence and analysis of epidemic characteristics from 2009 to 2022 in Zhejiang province, China. Front Public Health. (2023) 11:1154944. doi: 10.3389/fpubh.2023.1154944
33. Blattner WA. HIV epidemiology: past, present, and future. FASEB J. (1991) 5(10):2340–8. doi: 10.1096/fasebj.5.10.2065886
34. Lieber M, Chin-Hong P, Whittle HJ, Hogg R, Weiser SD. The synergistic relationship between climate change and the HIV/AIDS epidemic: a conceptual framework. AIDS Behav. (2021) 25:2266–77. doi: 10.1007/s10461-020-03155-y
35. Talman A, Bolton S, Walson JL. Interactions between HIV/AIDS and the environment: toward a syndemic framework. Am J Public Health. (2013) 103(2):253–61. doi: 10.2105/AJPH.2012.300924
36. Zhu T, Korber BT, Nahmias AJ, Hooper E, Sharp PM, Ho DD. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. (1998) 391:594–7. doi: 10.1038/35400
37. AIDS epidemic update. (2000). Available online at: https://data.unaids.org/publications/irc-pub05/aidsepidemicreport2000_en.pdf (Accessed March 13, 2025).
38. HIV Data and Statistics. World Health Organization (2025). Available online at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics#:∼:text=HIV%20incidence&text=1.2%20million%20%5B950%20000%E2%80%931.5,million%5D%20people%20have%20acquired%20HIV (Accessed March 13, 2025).
39. Should I Be Concerned About Mpox? Cleveland Clinic (2025). Available online at: https://my.clevelandclinic.org/health/diseases/22371-monkeypox (Accessed March 12, 2025).
40. Thomassen HA, Fuller T, Asefi-Najafabady S, Shiplacoff JAG, Mulembakani PM, Blumberg S, et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in Central Africa. PLoS One. (2013) 8(7):1–6. doi: 10.1371/journal.pone.0066071
41. Rahman AR, Munir T, Fazal M, Cheema SA, Bhayo MH. Climatic determinants of monkeypox transmission: a multi-national analysis using generalized count mixed models. J Virol Methods. (2025) 332:115076, ISSN 0166-0934. doi: 10.1016/j.jviromet.2024.115076
42. Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, et al. The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis. (2022) 16(2):2–5. doi: 10.1371/journal.pntd.0010141
43. Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U.S.A. (2010) 107(37):16262–7. doi: 10.1073/pnas.1005769107
44. Epidemiological Update, Week 37/2024: Mpox Due to Monkeypox Virus Clade I. European Centre for Disease Prevention and Control. (2024). Available online at: https://www.ecdc.europa.eu/en/news-events/mpox-monkeypox-epidemiological-update-week-37-2024 (Accessed March 14, 2025).
45. Dick OB, Martín JLS, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg. (2012) 87(4):584–93. doi: 10.4269/ajtmh.2012.11-0770
46. Monath TP. Dengue: the risk to developed and developing countries. In: Roizman B, Editor. Infectious Diseases in an Age of Change: The Impact of Human Ecology and Behavior on Disease Transmission. Vol. 271. Washington, DC: The National Academies Press (1995). p. 48–49. doi: 10.17226/4772
47. Limkittikul K, Brett J, L’Azou M. Epidemiological trends of dengue disease in Thailand (2000–2011): a systematic literature review. PLoS Negl Trop Dis. (2014) 8(11):e3241. doi: 10.1371/journal.pntd.0003241
48. Tian N, Zheng JX, Guo ZY, Li LH, Xia S, Lv S, et al. Dengue incidence trends and its burden in major endemic regions from 1990 to 2019. Trop Med Infect Dis. (2022) 7(8):180. doi: 10.3390/tropicalmed7080180
49. Du M, Jing W, Liu M, Liu J. The global trends and regional differences in incidence of dengue infection from 1990 to 2019: an analysis from the global burden of disease study 2019. Infect Dis Ther. (2021) 10(3):1625–43. doi: 10.1007/s40121-021-00470-2
50. Chen Y, Zhang H, Chen H, Fan L, Xu C, Xu J, et al. Malaria epidemiological characteristics and control in Guangzhou, China, 1950–2022. Malar J. (2023) 22(1):265. doi: 10.1186/s12936-023-04696-y
51. Fact Sheet About Malaria. World Health Organization (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/malaria#:∼:text=The%20WHO%20African%20Region%20carries,580%20000)%20of%20malaria%20deaths (Accessed March 13, 2025).
52. Staples JE, Monath TP. Yellow fever: 100 years of discovery. JAMA. (2008) 300(8):960–2. doi: 10.1001/jama.300.8.960
53. Gianchecchi E, Cianchi V, Torelli A, Montomoli E. Yellow fever: origin, epidemiology, preventive strategies and future prospects. Vaccines (Basel). (2022) 10(3):372. doi: 10.3390/vaccines10030372
55. Pan American Health Organization / World Health Organization. Epidemiological Update. Yellow fever in the Region of the Americas, 21 March 2024, Washington, D.C. PAHO/WHO (2024). Available online at: https://www.paho.org/sites/default/files/2024-03/2024-march-21-phe-epi-updateyellow-feveren_0.pdf (Accessed March 13, 2025).
56. Canada, P.H.A. of. Government of Canada, Canada.ca. (2023). Available online at: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-25-yellow-fever-vaccine.html (Accessed March 13, 2025).
57. Influenza (seasonal). World Health Organization. (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (Accessed March 13, 2025).
58. Influenza Activity in the United States During the 2023–2024 Season and Composition of the 2024–2025 Influenza Vaccine. Centers for Disease Control and Prevention (2024). Available online at: https://www.cdc.gov/flu/whats-new/flu-summary-2023-2024.html (Accessed March 13, 2025).
59. McDow TF. A Century of HIV. Origins: Current Events in Historical Perspective (2018). Available online at: https://origins.osu.edu/article/century-hiv-world-aids-day-africa-actup-unaids (Accessed March 13, 2025).
60. Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. (2008) 300(5):520–9. doi: 10.1001/jama.300.5.52
61. U.S. statistics. HIV.gov. (2025). Available online at: https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics (Accessed March 13, 2025).
62. Payne AB, Ray LC, Kugeler KJ, Fothergill A, White EB, Canning M, et al. Incidence of monkeypox among unvaccinated persons compared with persons receiving ≥1 JYNNEOS vaccine dose—32 U.S. jurisdictions, July 31–September 3, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1278–82. doi: 10.15585/mmwr.mm7140e3
63. World Health Organization. 2022–25 Mpox Outbreak: Global Trends. Geneva: World Health Organization (2025). Available online at: https://worldhealthorg.shinyapps.io/mpx_global/ (Accessed July 23, 2025).
64. World Health Organization. Global-SPRP-for-dengue-and-other-aedes-borne-... (2024). Available online at: https://cdn.who.int/media/docs/default-source/ntds/dengue/global-sprp-for-dengue-and-other-aedes-borne-arboviruses.pdf?sfvrsn=7ab2e43b_3&download=true (Accessed March 14, 2025).
65. World Health Organization. Global technical strategy for malaria 2016-2030 (2015). Available online at: https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf (Accessed March 14, 2025).
66. World Health Organization. Global strategy to eliminate yellow fever epidemics (eye). (2016). Available online at: https://terrance.who.int/mediacentre/data/sage/SAGE_Docs_Ppt_Oct2016/11_session_Yellow-fever/Oct2016_Session11_EYE_strategy.pdf (Accessed March 14, 2025).
67. About CDC’s Global Influenza Program. Centers for Disease Control and Prevention (n.d.). Available online at: https://www.cdc.gov/flu-global/about/index.html (Accessed March 14, 2025).
68. Global Influenza Strategy 2019–2030. World Health Organization (n.d.). Available online at: https://www.who.int/publications/i/item/9789241515320 (Accessed March 14, 2025).
69. Canada, P.H.A. of. Government of Canada, Canada.ca. (2024). Available online at: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/canada-progress-towards-ending-hiv-epidemic.html (Accessed March 14, 2025).
70. HIV Prevention 2025 Roadmap. (n.d.). Available online at: https://www.unaids.org/sites/default/files/media_asset/prevention-2025-roadmap_en.pdf (Accessed March 14, 2025).
71. Global Strategic Preparedness and Response Plan Launched by WHO to Contain Mpox Outbreak. World Health Organization (2024). Available online at: https://www.who.int/news/item/26-08-2024-global-strategic-preparedness-and-response-plan-launched-by-who-to-contain-mpox-outbreak (Accessed March 14, 2025).
72. Diatta KLES, Faye O, Sall AA, Faye O, Faye M. Useful public health countermeasures to control the current multicountry outbreak of monkeypox disease. Front Public Health. (2023) 10:1060678. doi: 10.3389/fpubh.2022.1060678
73. Climate Change. World Health Organization (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health#:∼:text=Promote%20actions%20that%20both%20reduce,mobilizing%20the%20strength%20of%20the (Accessed March 14, 2025).
74. Anderson GB, Barnes EA, Bell ML, Dominici F. The future of climate epidemiology: opportunities for advancing health research in the context of climate change. Am J Epidemiol. (2019) 188(5):866–72. doi: 10.1093/aje/kwz034
75. Wu X, Lu Y, Zhou S, Chen L, Xu B. Impact of climate change on human infectious diseases: empirical evidence and human adaptation. Environ Int. (2016) 86:14–23. ISSN 0160-4120. doi: 10.1016/j.envint.2015.09.007
76. Ma J, Guo Y, Gao J, Tang H, Xu K, Liu Q, et al. Climate change drives the transmission and spread of vector-borne diseases: an ecological perspective. Biology (Basel). (2022) 11(11):1628. doi: 10.3390/biology11111628
77. Montasir F, Risha RK. Global Strategies for Combating Dengue Virus: A Review on the Control Methods (BSc thesis). Brac University, Dhaka, Bangladesh (2024). Available at: https://dspace.bracu.ac.bd/xmlui/bitstream/handle/10361/24111/19326039_MNS.pdf?sequence=1&isAllowed=y (Accessed July 23, 2025).
78. Nissan H, Ukawuba I, Thomson M. Climate-proofing a malaria eradication strategy. Malar J. (2021) 20(1):190. doi: 10.1186/s12936-021-03718-x Erratum in: Malar J. 2021 May 10;20(1):215. doi: 10.1186/s12936-021-03747-6.33865383
79. Bélanger D, Séguin J. Human Health in a Changing Climate: A Canadian Assessment of Vulnerabilities and Adaptive Capacity. Ottawa: Health Canada (2008).
80. Berger S, Wyss AM. Climate change denial is associated with diminished sensitivity in internalizing environmental externalities. Environ Res Lett. (2021) 16(7):074018. doi: 10.1088/1748-9326/ac08c0
81. Frumkin H, Hess J, Luber G, Malilay J, McGeehin M. Climate change: the public health response. Am J Public Health. (2008) 98(3):435–45. doi: 10.2105/ajph.2007.119362
82. Bostrom A, O’Connor RE, Böhm G, Hanss D, Bodi O, Ekström F. Causal thinking and support for climate change policies: international survey findings. Glob Environ Change. (2012) 22(1):210–22. ISSN 0959-3780. doi: 10.1016/j.gloenvcha.2011.09.012
83. Shigetomi Y, Kanemoto K, Yamamoto Y, Kondo Y. Quantifying the carbon footprint reduction potential of lifestyle choices in Japan. Environ Res Lett. (2021) 16(6):064022. doi: 10.1088/1748-9326/abfc07
84. Schwartz G, Fouad M, Hansen T, Verdier G. Well Spent: How Strong Infrastructure Governance Can End Waste in Public Investment. Washington, DC: International Monetary Fund (2020).
85. Intergovernmental Panel on Climate Change. IPCC (n.d.). Available online at: https://archive.ipcc.ch/ipccreports/tar/wg2/index.php?idp=643 (Accessed March 14, 2025).
86. Bartelet HA, Barnes ML, Bakti LAA, Cumming GS. Testing the reliability of adaptive capacity as a proxy for adaptive and transformative responses to climate change. Glob Environ Change. (2023) 81:102700, ISSN 0959-3780. doi: 10.1016/j.gloenvcha.2023.102700
87. Wilby RL, Dessai S. Robust Adaptation to Climate Change. CORE (2009). Available online at: https://core.ac.uk/reader/288355770 (Accessed March 14, 2025).
88. Greenwood B. How much more malaria could be prevented? Lancet Infect Dis. (2016) 16(4):393–4. doi: 10.1016/s1473-3099(15)00482-x
89. Delaney LR, Gunderman RB. Hand hygiene. Radiology. (2008) 246(1):15–9. doi: 10.1148/radiol.2461061676
90. Zellweger RM, Cano J, Mangeas M, Taglioni F, Mercier A, Despinoy M, et al. Socioeconomic and environmental determinants of dengue transmission in an urban setting: an ecological study in Nouméa, New Caledonia. PLoS Negl Trop Dis. (2017) 11(4):e0005471. doi: 10.1371/journal.pntd.0005471
91. Kolimenakis A, Heinz S, Wilson ML, Winkler V, Yakob L, Michaelakis A, et al. The role of urbanisation in the spread of aedes mosquitoes and the diseases they transmit-a systematic review. PLoS Negl Trop Dis. (2021) 15(9):e0009631. doi: 10.1371/journal.pntd.0009631
92. Kabaria CW, Gilbert M, Noor AM, Snow RW, Linard C. The impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar J. (2017) 16:49. doi: 10.1186/s12936-017-1694-2
93. Kassam NA, Kaaya RD, Damian DJ, Schmiegelow C, Kavishe RA, Alifrangis M, et al. Ten years of monitoring malaria trend and factors associated with malaria test positivity rates in lower Moshi. Malar J. (2021) 20(1):193. doi: 10.1186/s12936-021-03730-1
94. Ricci F. Social implications of malaria and their relationships with poverty. Mediterr J Hematol Infect Dis. (2012) 4(1):e2012048. doi: 10.4084/MJHID.2012.048
95. Possas C, Martins RM, Oliveira RL, Homma A. Urgent call for action: avoiding spread and re-urbanisation of yellow fever in Brazil. Mem Inst Oswaldo Cruz. (2018) 113(1):1–2. doi: 10.1590/0074-02760170361
96. Eliminate yellow fever epidemics (EYE) strategy 2017-2026. (n.d.). Available online at: https://www.who.int/initiatives/eye-strategy#:∼:text=The%202016%20Angolan%20outbreak%20showed,%2Dna%C3%AFve%20for%20yellow%20fever (Accessed July 18, 2025).
97. Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. (2010) 30(1):237–60. doi: 10.1016/j.cll.2010.01.001
98. Wang C, Yang YN, Xi L, Yang LL, Du J, Zhang ZS, et al. Dynamics of influenza-like illness under urbanization procedure and COVID-19 pandemic in the subcenter of Beijing during 2013–2021. J Med Virol. (2022) 94(8):3801–10. doi: 10.1002/jmv.27803
99. Dalziel BD, Kissler S, Gog JR, Viboud C, Bjørnstad ON, Metcalf CJ, et al. Urbanization and humidity shape the intensity of influenza epidemics in U.S. Cities. Science. (2018) 362:75–9. doi: 10.1126/science.aat6030
100. Lee H, Lee H, Song K-H, Kim ES, Park JS, Jung J, et al. Impact of public health interventions on seasonal influenza activity during the COVID-19 outbreak in Korea. Clin Infect Dis. (2021) 73(1):e132–40. doi: 10.1093/cid/ciaa672
101. Voeten HA, Vissers DC, Gregson S, Zaba B, White RG, de Vlas SJ, et al. Strong association between in-migration and HIV prevalence in urban sub-Saharan Africa. Sex Transm Dis. (2010) 37(4):240–3. doi: 10.1097/OLQ.0b013e3181c3f2d0
102. Dyson T. HIV/AIDS and urbanization. Popul Dev Rev. (2003) 29(3):427–42. doi: 10.1111/j.1728-4457.2003.00427.x
103. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA. (2019) 321(9):844–5. doi: 10.1001/jama.2019.1343
104. Nguyen PY, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017–2020. Emerg Infect Dis. (2021) 27(4):1007–14. doi: 10.3201/eid2704.203569
105. Han L, Qiang Y, Ma C, Li X, Salim Z, Bhandari R, et al. Spatial pattern and socioeconomic factors of 2022 monkeypox outbreak in the United States. Prof Geogr. (2025) 77:1–12. doi: 10.1080/00330124.2025.2474445
106. Meo SA, Al-Masri AA, Alkhliwi HTM, Alkhalifah JM. Impact of environmental pollutants particulate matter PM2.5, carbon monoxide, nitrogen dioxide and ozone on the incidence of monkeypox cases. Corrected. Eur Rev Med Pharmacol Sci. (2022) 26(21):8197–203. doi: 10.26355/eurrev_202211_30173
107. Biswal S, Reynales H, Saez-Llorens X, Lopez P, Borja-Tabora C, Kosalaraksa P, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med. (2019) 381:1–2. doi: 10.1056/NEJMoa1903869
108. Flasche S, Jit M, Rodríguez-Barraquer I, Coudeville L, Recker M, Koelle K, et al. The long-term safety, public health impact, and cost-effectiveness of routine vaccination with a recombinant, live-attenuated dengue vaccine (dengvaxia): a model comparison study. PLoS Med. (2016) 13:2–3. doi: 10.1371/journal.pmed.1002181
109. Hladish TJ, Pearson CA, Chao DL, Rojas DP, Recchia GL, Gómez-Dantés H, et al. Projected impact of dengue vaccination in Yucatán, Mexico. PLoS Negl Trop Dis. (2016) 10:1–2. doi: 10.1371/journal.pntd.0004661
110. Fernando L, Kastner R, Wickramasinghe P, Fernando AD, Gunasekera D, Nguyen VH, et al. Role of the dengue vaccine TAK-003 in an outbreak response: modeling the Sri Lanka experience. PLoS Negl Trop Dis. (2024) 18:1–2. doi: 10.1371/journal.pntd.0012376
111. Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. (2015) 372:113–6. doi: 10.1056/NEJMoa1411037
112. Diaz-Quijano FA, de Carvalho DS, Raboni SM, Shimakura SE, de Mello AM, da Costa-Ribeiro MC, et al. Effectiveness of mass dengue vaccination with CYD-TDV (dengvaxia®) in the state of Paraná. Brazil: integrating case-cohort and case-control designs. Lancet Reg Health Am. (2024) 35:1–2. doi: 10.1016/j.lana.2024.100777
113. DS C, Luhm KR, Shimakura SE, Raboni SM, da Costa-Ribeiro V, Diaz-Quijano FA, et al. Dengue vaccine effectiveness: results from a six-year population-based cohort study in southern Brazil. MedRxiv [Preprint]. (2023). doi: 10.1101/2023.12.28.23300598
114. Ndii MZ, Mage AR, Messakh JJ, Djahi BS. Optimal vaccination strategy for dengue transmission in Kupang city, Indonesia. Heliyon. (2020) 6:5–6. doi: 10.1016/j.heliyon.2020.e05345
115. Aguiar M, Stollenwerk N, Halstead SB. The impact of the newly licensed dengue vaccine in endemic countries. PLoS Negl Trop Dis. (2016) 10:3–5. doi: 10.1371/journal.pntd.0005179
116. da Silveira LT, Tura B, Santos M. Systematic review of dengue vaccine efficacy. BMC Infect Dis. (2019) 19(1):750. doi: 10.1186/s12879-019-4369-5
117. Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, et al. Malaria: global progress 2000–2015 and future challenges. Infect Dis Poverty. (2016) 5:5–7. doi: 10.1186/s40249-016-0151-8
118. Datoo MS, Dicko A, Tinto H, Ouédraogo JB, Hamaluba M, Olotu A, et al. Safety and efficacy of malaria vaccine candidate R21/matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. (2024) 403:538–9. doi: 10.1016/S0140-6736(23)02511-4
119. Topazian HM, Schmit N, Gerard-Ursin I, Charles GD, Thompson H, Ghani AC, et al. Modelling the relative cost-effectiveness of the RTS, S/AS01 malaria vaccine compared to investment in vector control or chemoprophylaxis. Vaccine. (2023) 41:3218–9. doi: 10.1016/j.vaccine.2023.04.011
120. Stanisic DI, Good MF. Malaria vaccines: progress to date. BioDrugs. (2023) 37:738–9. doi: 10.1007/s40259-023-00623-4
121. Sulaiman SK, Musa MS, Tsiga-Ahmed FI, Dayyab FM, Sulaiman AK, Bako AT. A systematic review and meta-analysis of the prevalence of caregiver acceptance of malaria vaccine for under-five children in low-income and middle-income countries (LMICs). PLoS One. (2022) 17:2–3. doi: 10.1371/journal.pone.0278224
122. Tinto H, Otieno W, Gesase S, Sorgho H, Otieno L, Liheluka E, et al. Long-term incidence of severe malaria following RTS, S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect Dis. (2019) 19:827. doi: 10.1016/s1473-3099(19)30300-7
123. Gaythorpe KAM, Hamlet ATP, Jean K, Ramos DG, Cibrelus L, Garske T, et al. The global burden of yellow fever. MedRxiv [Preprint]. (2020). doi: 10.1101/2020.10.14.20212472
124. Jean K, Raad H, Gaythorpe KAM, Hamlet A, Mueller JE, Hogan D, et al. Assessing the impact of preventive mass vaccination campaigns on yellow fever outbreaks in Africa: a population-level selfcontrolled case series study. PLoS Med. (2021) 18:1–2. doi: 10.1371/journal.pmed.1003523
125. Shearer FM, Moyes CL, Pigott DM, Brady OJ, Marinho F, Deshpande A, et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis. (2017) 17:1213–4, 1209. doi: 10.1016/S1473-3099(17)30419-X
126. Wu JT, Peak CM, Leung GM, Lipsitch M. Fractional dosing of yellow fever vaccine to extend supply: a modelling study. Lancet. (2016) 388:2904–11. doi: 10.1016/S0140-6736(16)31838-4
127. Jean K, Hamlet A, Benzler J, Cibrelus L, Gaythorpe KA, Sall A, et al. Eliminating yellow fever epidemics in Africa: vaccine demand forecast and impact modelling. PLoS Negl Trop Dis. (2020) 14:1–16. doi: 10.1371/journal.pntd.0008304
128. Shearer FM, Longbottom J, Browne AJ, Pigott DM, Brady OJ, Kraemer MU, et al. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Health. (2018) 6:e270–8. doi: 10.1016/S2214-109X(18)30024-X
129. Farnsworth MG, Khanipov K, Botnar K, Weaver SC, Barrett ADT, Golovko G. Real-world evidence of yellow fever vaccination data-driven study. Vaccine. (2025) 48:1–6. doi: 10.1016/j.vaccine.2025.126758
130. Schnyder JL, Bache BE, Welkers MRA, Spijker R, Schaumburg F, Goorhuis A, et al. Yellow fever breakthrough infections after yellow fever vaccination: a systematic review and meta-analysis. The Lance Microbe. (2024) 5:1–10. doi: 10.1016/j.lanmic.2024.06.004
131. Kimathi D, Juan A, Bejon P, Grais RF, Warimwe GM, YEFE and NIFTY vaccine trials teams. Randomized, double-blinded, controlled non-inferiority trials evaluating the immunogenicity and safety of fractional doses of yellow fever vaccines in Kenya and Uganda. Wellcome Open Res. (2019) 4:816–7. Available online at: https://wellcomeopenresearch.org/articles/4-182/v1
132. Tenforde MW, Talbot HK, Trabue CH, Gaglani M, McNeal TM, Monto AS, et al. Influenza vaccine effectiveness against hospitalization in the United States, 2019–2020. J Infect Dis. (2021) 224:813–20. doi: 10.1093/infdis/jiaa800
133. Kissling E, Maurel M, Emborg H-D, Whitaker H, McMenamin J, Howard J, et al. Interim 2022/23 influenza vaccine effectiveness: six European studies, October 2022 to January 2023. Eurosurveillance. (2023) 28:1–2. doi: 10.2807/1560-7917.ES.2023.28.21.2300116
134. Restivo V, Costantino C, Bono S, Maniglia M, Marchese V, Ventura G, et al. Influenza vaccine effectiveness among high-risk groups: a systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother. (2017) 14:724–5. doi: 10.1080/21645515.2017.1321722
135. Corder BN, Bullard BL, Poland GA, Weaver EA. A decade in review: a systematic review of universal influenza vaccines in clinical trials during the 2010 decade. Viruses. (2020) 12:1–22. doi: 10.3390/v12101186
136. Deputy NP, Deckert J, Chard AN, Sandberg N, Moulia DL, Barkley E, B. S., et al. Vaccine effectiveness of JYNNEOS against Mpox disease in the United States. N Engl J Med. (2023) 388:2434–43. doi: 10.1056/NEJMoa2215201
137. Climate Change. World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (Accessed March 14, 2025)
138. A Global Brief on Vector-Borne Diseases. World Health Organization. (n.d.). Available online at: https://iris.who.int/bitstream/handle/10665/311664/9789241550536-eng.pdf?sequence=1 (Accessed March 15, 2025).
139. Integrated Surveillance and Climate-Informed Health Early Warning Systems. World Health Organization (n.d.). Available online at: https://www.who.int/teams/environment-climate-change-and-health/climate-change-and-health/country-support/integrated-surveillance-and-climate-informed-health-early-warning-systems (Accessed March 14, 2025).
140. Vaccines and Immunization. World Health Organization. (n.d.). Available online at: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (Accessed March 14, 2025).
Keywords: climate change, dengue, yellow fever, malaria, HIV, Monkeypox, influenza, global warming
Citation: Murray A and Ignaszak A (2025) Mapping climate change-driven epidemics. Front. Epidemiol. 5:1605058. doi: 10.3389/fepid.2025.1605058
Received: 2 April 2025; Accepted: 8 July 2025;
Published: 29 July 2025.
Edited by:
Wei Wang, Capital Medical University, ChinaReviewed by:
Pengfei Zhu, Merck Sharp and Dohme (China) Ltd., ChinaRu Zhang, Capital Medical University, China
Copyright: © 2025 Murray and Ignaszak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Ignaszak, YWlnbmFzemFAdW5iLmNh; YWlnbmFzemFrQGJyb2NrdS5jYQ==
 Allyson Murray1
Allyson Murray1 Anna Ignaszak
Anna Ignaszak