- 1Laboratory of Clinical Immunology and Microbiology (LCIM), Epithelial Therapeutics Unit, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda MD, United States
- 2Unit of Integrative Metabolomics, Institute of Environmental Medicine Karolinska Institute, Stockholm, Sweden
Chronic inflammatory diseases such as autoimmune disorders, cancer, cardiovascular diseases and neurodegenerative disorders are a significant cause of morbidity and mortality in the industrialized world. Socioeconomically disadvantaged communities bear a disproportionately high burden of these inflammatory diseases. This review synthesizes evidence linking various domains of the Social Determinants of Health (SDoH)—economic stability, education access and quality, healthcare access and quality, neighborhood and built environment, and social and community context—to inflammatory pathways and mechanisms. Across domains, biological mechanisms such as cytokine dysregulation, toll-like receptor (TLR) activation, hypothalamic-pituitary-adrenal (HPA) axis alterations and gut microbiome disruption act together to sustain proinflammatory states that drive adverse health outcomes in marginalized communities. Although causality is obscured by interrelated determinants, identifying inflammation as a shared pathway between various determinants highlights the need for structural interventions to reduce chronic disease burden.
Introduction
Incidence of Inflammatory diseases are rapidly rising and the prevalence of such diseases is anticipated to continue increasing in the coming decades (1). Chronic inflammatory diseases are recognized as the most significant causes of death in the industrialized world, as more than 50% of all deaths are attributed to diseases associated with inflammation (2). These conditions include allergies (3), metabolic disorders (4), cancer (5), autoimmune diseases (6), and neurodegenerative diseases (7). However, the burden of chronic inflammatory diseases is disproportionately bore by communities with higher rates of socioeconomic disadvantage and barriers to healthcare access (8). These broad social factors have been conceptualized by Healthy People 2,030 as social determinants of health (SDoH), which are organized into five domains: economic stability, education access and quality, health care access and quality, neighborhood and built environment, and social and community context (9). This review aims to highlight inflammatory exposures across the five SDoH domains and mechanisms by which social determinants have been proposed to influence chronic low-grade inflammation.
Systemic inflammation and socioeconomic status overview
Inflammation is essential for fighting pathogens and malignant cells, alongside promoting tissue repair. However, systemic and chronic activation of the immune system underpins the development of inflammatory disease (10). Sub-clinical, systemic inflammation describes elevated expression of inflammatory molecules and heightened immune activity that may not yet manifest with overt clinical symptoms. The prolonged and systemic expression of inflammatory molecules is associated with harmful effects including oxidative stress, fibrosis, mitochondrial dysfunction, and cellular senescence (11). The sustained effects of inflammatory responses in the absence of a pathogenic target are largely associated with several mechanisms of chronic disease development. For instance, the prolonged production of reactive oxygen species by immune cells and corresponding oxidative stress are associated with further stimulation of an inflammatory response, creating damage associated with organ dysfunction and metabolic dysregulation.
In measuring systemic inflammation, Interleukin-6 (IL-6) and C-reactive protein (CRP) are frequently used biomarkers (12). IL-6 is synthesized at the beginning of many immune responses and has several effects ranging from promoting antibody production to inducing acute-phase protein synthesis (13). C-reactive protein is an acute-phase protein released by the liver in response to IL-6 production, and is commonly used as a non-specific inflammation marker (14). Furthermore, the systemic, heightened presence of leukocytes and expression of inflammatory cytokines in the absence of overt clinical symptoms are suggestive of chronic inflammation. Given the broad functions and triggers of inflammation, mechanisms and biomarkers of inflammation can vary vastly and have a wide range of effects (12). CRP and IL-6 have traditionally facilitated insights into the association between systemic inflammation and chronic disease but have been recently joined by many other measurements of inflammation (15). As an example, differential white blood cell counts, were shown to be predictive of all-cause mortality, alongside cancer, cardiovascular and cerebrovascular specific mortality (16).
A large body of evidence highlights the association of socioeconomic status (SES) with low-grade inflammation (summarized in Figure 1). For instance, a meta-analysis of 43 studies which assessed the relationship between socioeconomic status, during both childhood and adulthood, and biomarkers of systemic inflammation found a significant negative correlation between SES and CRP and IL-6 (17). Although the effect was attenuated after controlling for BMI and smoking, the correlation remained significant. Another systemic review investigating the relationship between childhood socioeconomic status and chronic inflammation revealed a significant association between parental finance and inflammation, as measured by CRP (18). Transcriptional profiling of placental biopsies and umbilical blood collected at birth suggested that both elevated immune activation and decreased fetal maturation are inversely associated with maternal deprivation (19). Maternal disadvantage was determined by income receipt of federal benefits, and education level. The significant association between SES and inflammation, reported across several studies, after adjustment for factors including BMI, suggests more research is necessary to further interrogate the relationship as to understand if and which causal mechanisms exist.
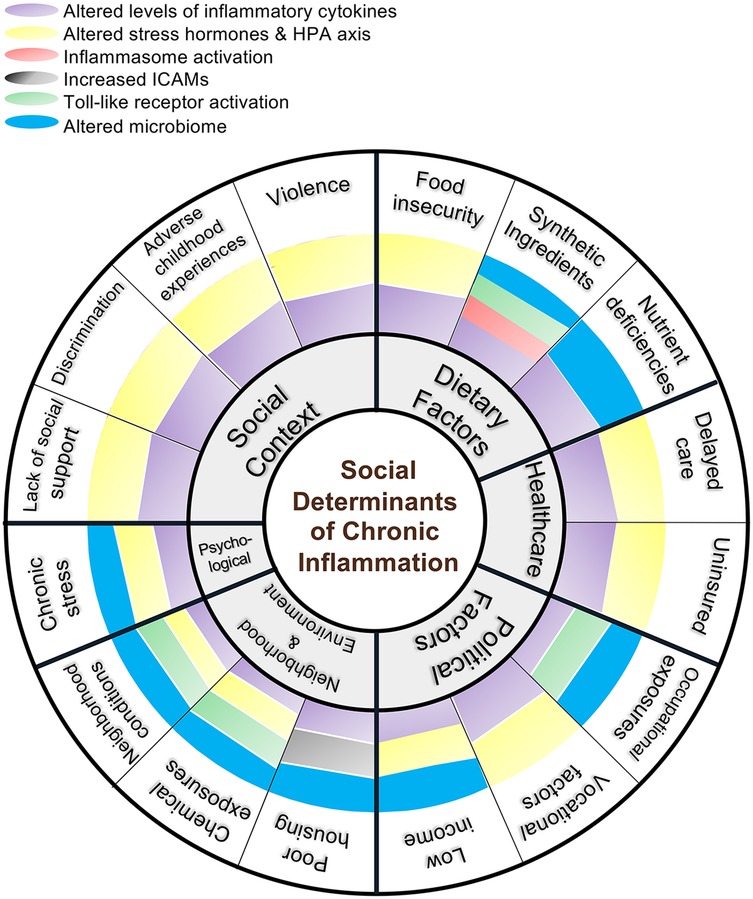
Figure 1. Integrative framework for social and biological determinants of chronic inflammation. This diagram maps social, environmental, and dietary conditions to specific immunologic pathways promoting inflammation and highlighting converging mechanisms—cytokine dysregulation, HPA axis alteration, innate immune receptor activation, and microbiome shifts.
Specific social determinants of health
Dietary determinants
Food insecurity
Food insecurity and diet quality have been proposed to mediate the association between economic stability and low-grade inflammation (20). Low income is consistently associated with lower dietary quality scores, and socioeconomic disparities in diet quality have widened over time (21). A study examining diet quality among residents of disadvantaged neighborhoods found that a minority of residents met dietary guidelines (22). The low affordability of healthy food has been shown to explain substantial portions of the association between SES and diet quality (23, 24). However, beyond high costs of healthy food, researchers have also proposed biological mechanisms by which low SES further influences poor diet quality. The associated stressors and uncertainty of poverty, especially extreme poverty, have been shown to affect stress, appetite, and the hunger-related hormones which shaping eating habits (25). Furthermore, food insecurity has been suggested to promote dependence on energy dense, palatable diets and alter metabolism through stress-related mechanisms, including the hypothalamus-pituitary-adrenal (HPA) axis (26).
Nutrient deficiencies
Poor diet contributes to low-grade inflammation through several mechanisms, including the absence of necessary nutrients that are associated with healthy immune regulation. A study using National Health and Nutrition Examination Survey (NHANES) data compared nutrient and food group intake among children aged 2–5 years with the family income to poverty ratio (PIR). The results suggested children in the low PIR cohort had lower dietary fiber, dairy, and calcium intakes, alongside lower Healthy Eating Index (HEI) scores overall (27). Dietary fiber is associated with several processes that mitigate low-grade inflammation as it metabolized by intestinal microbial species into short chain fatty acids (SCFAs). The resulting SCFAs regulate the immune response through several mechanisms, including increased gene expression of NF-κB mediators (28), generation of regulatory T cells (29), and improving epithelial barrier integrity (30). Insufficient fiber intake may promote heightened inflammation by facilitating the translocation of damage-associated molecular patterns (DAMPs) due to compromised barrier integrity, as well as promote immune dysregulation by reducing SCFA signaling (31).
Alongside fiber, several notable anti-inflammatory micronutrients are less abundant in low-income diets. Anthocyanins and quercetin, for example, are flavonoids that are predominantly found in fresh foods. Flavonoids have been shown to decrease NF-κB expression (32) and attenuate expression of pro-inflammatory cytokines (33). However, flavonoid intake has been reported to be lower in low-income cohorts than in high-income counterparts (34). Additionally, vitamin D is a key nutrient associated with maintaining healthy immune function through its anti-inflammatory properties. In cell lines and peripheral blood mononuclear cells (PBMCs), treatment with vitamin D provided strong evidence of its anti-inflammatory properties, with marked decreases in anti-inflammatory cytokines including IL-6, TNF-α, MCP-1, and IL-10. Potential mechanisms of anti-inflammatory action by vitamin D included decreased protein expression of Toll-like receptors (TLR), lower levels of phosphorylated p38, and decreased reactive oxygen species (35). However, there is some evidence that vitamin D may be a reverse acute-phase reactant; meaning, that low levels of vitamin D may result from the presence of inflammation rather than be the cause of inflammation (36). However, there are some data from randomized controlled trials showing vitamin D supplementation resulted in improved regulation of inflammation, with reductions in CRP and TNF-α, alongside increased signaling of Regulatory T cells (37, 38). Like deficiencies in other key anti-inflammatory nutrients, vitamin D deficiency is positively associated with low income in the United States (39).
Intake of foods with synthetic ingredients
In addition to insufficient intake of key anti-inflammatory nutrients, intake of highly-processed and ultra-processed foods (UPF) is largely associated with increased inflammation and dysregulation of the immune system. Evidence suggests ultra-processed food consumption is on the rise, with increased availability in low-income communities (40). However, the definition of UPF however is controversial. Sugar, salt, and cooking oils—each of which have known inflammatory effects (41)—are not considered ultra-processed (42). Consuming a meal of bread, cheese, and tomato sauce would constitute consuming a processed diet but, combining them into a cheese pizza would be defined as an ultra-processed diet. While this manuscript cannot settle this debate, for purposes of simplicity we will consider UPF as those containing ingredients which are either synthetic or require industrial processing to extract because such foods are nearly always defined as ultra-processed. For example, adding hydrogenated oils or high-fructose corn syrup to an otherwise minimally processed recipe will beget an UPF designation.
Ultra-processed diets overwhelmingly lack previously described nutrients which maintain healthy functioning of the immune system (43). Alongside their low nutritional value, many UPF have artificially heightened levels of inflammatory ingredients, such as cholesterol and processed saturated fatty acids. While naturally occurring, saturated fats tend to include several different lipid moieties, whereas processed fats are typically restricted to the highly inflammatory palmitic acid and steric acid (41). Given that these specific saturated fats are components of bacterial lipopolysaccharide (LPS), they can activate anti-bacterial mechanisms through activation of TLR4 (44). Industrial processing and storage mechanisms necessary for UPF development have also been implicated in the formation of dietary cholesterol oxidation products, which are associated with several disease etiologies (45).
In in-vivo models, high cholesterol diets led to inflammasome activation in the intestinal epithelium, resulting in both local and systemic inflammation (46). There is also a well-established role of cholesterol and its oxidation products in atherosclerosis development. High cholesterol intake is associated with higher circulating quantities of low-density lipoprotein (LDL), as opposed to high-density lipoprotein (HDL) which promotes cellular efflux of cholesterol (47). Elevated levels of LDL lead to deposition in the arterial wall, where it is oxidized and aggregated, both of which further trigger immune activity. Modified LDL also activates TLRs on macrophages, triggering TLR signaling to mount an immune response, and are engulfed by macrophages to further amplify the immune response and trigger downstream cytokine production and inflammasome activation (48). Alongside the known mechanism in atherosclerosis development, the formation of aggregated cholesterol crystals and resulting NLRP3 inflammasome signaling has been implicated in the development of colon cancer (49). Both cholesterol and processed saturated fatty acids have been shown to modify the gut microbiota with effects on TLR4 (50); the resulting oxidative stress has also been shown to promote oxidized modifications of LDL.
Abundant evidence in animals and limited evidence in humans also suggests chemical additives in highly processed food, such as emulsifiers and sweeteners, directly contribute to a pro-inflammatory state (51). Results from an in-vivo study showed exposure to saccharin, a commonly used artificial sweetener, induced inflammation by elevating expression of proinflammatory cytokines, TNF-α and iNOS, and inducing changes to the microbiota (52). Another in-vivo study determined commonly used dietary emulsifiers, carboxymethylcellulose (CMC) and polysorbate-80 (P80), increased expression of genes pertaining to virulence of otherwise mutualistic bacteria and inflammation (53). Exposure to CMC and P80 has also been shown to increase intestinal permeability and result in changes in the gut microbiome that are associated with low-grade inflammation (54).
Furthermore, a study in a cohort of nearly six hundred people found a positive association between both emulsifier and highly processed food consumption and heightened inflammation and intestinal permeability; the association persisted after controlling for energy intake, BMI, and red and processed meat intake (55). The impact of CMC consumption on microbial composition has also been highlighted in humans using a randomized controlled-feeding study. Relative to control subjects, CMC consumption increased postprandial abdominal discomfort, reduced microbial diversity and led to changes in their metabolome, including a decrease in short-chain fatty acids (56). When looking at sweeteners, short-term and long-term results showed non-caloric artificial sweetener consumption induced alteration in the gut microbiota and glycemic response (57).
Psychological determinants
Chronic stress
Chronic stress is another proposed mechanism by which low income is associated with low-grade inflammation. Chronic stress has been linked with low-grade inflammation and chronic disease across several studies (58–61). Researchers have proposed that the activation of the HPA axis links chronic stress with low-grade inflammation. This phenomenon was supported across two viral-challenge studies, in which participants with recent exposure to a long-term threatening stressful experience displayed higher glucocorticoid resistance and increased production of inflammatory cytokines (62).
A randomized controlled trial in which participants were subjected to stress reduction interventions observed significant decreases in levels of CRP (63). Furthermore, in a study concerning childhood SES, investigators hypothesized early-life social adversity contributes to defensive biological programming which involves a heightened inflammatory state. The study measured CRP and IL-6 levels and conducted transcriptional profiling of healthy volunteers; the volunteers had no history of chronic disease but differed in spending their first 5 years of life in either low or high SES environments. The results suggested that participants raised in low SES environments had increased IL-6 production, which persisted after controlling for levels of perceived stress, smoking, adiposity, exercise, alcohol use, and sleep quality. Furthermore, transcriptional profiling revealed low SES was associated with an upregulation of genes involved in translating adrenergic signals to leukocyte transcription and genes with NF-κB response elements, which are characterized as proinflammatory genes. However, in the low SES cohort, there was also a downregulation of genes with response elements for the glucocorticoid receptor, which carry out the anti-inflammatory action of cortisol, commonly considered as the “stress hormone” (64). Both the inflammatory markers and transcriptional profiling results suggest a heightened inflammatory state among participants reared in low SES environments, which was hypothesized to act through resistance to cortisol output. While chronic stress has been consistently linked with low-grade inflammation, multifaceted analyses of social stressors, such as social strain and living in a single-parent family, and CRP have produced inconclusive results, suggesting that more research is necessary to characterize specific stressors that contribute to low-grade inflammation (65).
Chronic stress has also been proposed to contribute to low-grade inflammation through alterations to the microbiome. Abundant mouse and human studies have suggested a link between several types of stress and changes in the microbiome (66). In mice subjected to social disruption stress, levels of gut Bacteroides and Parabacteroides increased. Additionally, levels of Coprococcus, Dorea, and Pseudobutyrivibrio decreased, which were inversely correlated with IL-6 levels, and antibiotic administration blocked the stress-induced IL-6 increase (67). Similarly, a study assessing the effects of acute stress on pregnant women similarly observed stress-induced increases in IL-6, as well as TNF-α, were positively associated with abundance of Bacteroides. Increased IL-6 levels were also associated with abundance of Prevotella (68). Furthermore, in a cohort of Belgian children, high stress, as defined by negative life events and low parasympathetic activity, was associated with lower alpha diversity (69). Low alpha diversity has been linked to low-grade inflammation, with a potential mechanism being reduced access to complex carbohydrates and less production of short chain fatty acids (70). However, reverse causation is possible given that chronic stress may alter the habitat of the gut in ways that benefit survival of some microbes over others.
Political determinants
The term “political determinants of health” was coined by Professor Daniel Dawes (71). The term is meant to contrast the more standard “social determinants of health” to communicate that many of the issues which fall under SDOH are not an innate part of a society. Rather, the cause and solution to these problems would require alterations in political decision making.
Education access and quality
Educational attainment both captures early childhood determinants of health and largely predicts occupational and subsequently economic attainment (72). A gradient has emerged in the past 50 years in which higher levels of schooling are increasingly linked with better health outcomes and increased lifespan, partly through pathways influencing systemic inflammation. Individuals with lower educational levels may experience greater exposure to chronic psychosocial stress, reduced access to preventive healthcare, and higher rates of poor dietary and lifestyle factors. These factors are all associated with elevated inflammatory markers such as CRP and IL-6. The education-health gradient has been observed regardless of gender or race, although the health effects are stronger for women and white individuals (73). Here to, the association may be through reverse causation as greater health may better facilitate school performance.
Childhood health
Educational context shapes childhood health outcomes, as most children spend up to 40 h of their week in school and receive approximately 35% of their daily nutrient intake while at school (74). As such, the school environment is a critical setting for interventions which reduce health inequities present in early childhood. Children with marginalized backgrounds commonly experience nutritional inequities that promote low-grade inflammation, including a lower consumption of fruits and vegetables and higher consumption of sodium, added sugars, and processed saturated fats (75, 76). Federally assisted meal programs such as the National School Lunch Program (NSLP) feed over 30 million children daily and have been considered essential in improving diet quality of underserved populations (75). NSLP lunches are required to meet nutritional standards laid out by the Dietary Guidelines for Americans (DGA). Children who participate in the NSLP have been shown to eat a healthier lunch than nonparticipants in the program regardless of income level or race (77). Even so, NSLP lunches allow for the selection of foods which meet DGA intake requirements for all major nutrients except for dietary fiber, which has been previously discussed to have strong implications for regulation of the immune system. Despite this requirement, students' average daily consumption of selected lunches did not meet intake recommendations for calcium, iron, fiber, and vitamins A and C (75). Nationally less than half of elementary students meet intake requirements for iron and vitamins A and C and very few consume the recommended amount of dietary fiber (78). However, the extent of these deficiencies is strongly shaped by the SDoH. Children from low-income households and under-resourced school districts are more likely to depend on school-provided meals as their primary source of nutrition and may have limited access to nutrient-rich foods outside of school. Socioeconomic status, neighborhood food environments, and school funding policies intersect to magnify nutritional inequities and their downstream inflammatory consequences. Increases in dietary fiber intake has been found to lower concentrations of serum CRP and fibrinogen in overweight and average weight adolescents, suggesting that dietary fiber may have protective effects against systemic inflammation (79, 80). Intake of vitamins A, C, and E have also been shown to have an inverse correlation with levels of CRP and IL-6 in children (81).
There is also evidence to suggest that higher education in a school environment which promotes attitudes of self-efficacy regarding personal health produces healthier behaviors throughout life (76). This relationship is potentially mediated by increased exposure to health education and interventions in schools, which are not offered equitably. Students at Title 1 schools, which are schools that serve a large percentage of low-income students, scored significantly lower when surveyed on nutritional knowledge and dietary behaviors compared to students of non-Title 1 schools. However, Title 1 students also demonstrated higher scores of self-efficacy when it came to selecting healthy meals which proved to be a more significant predictor of healthy dietary behaviors (82). School-based health centers (SBHC) provide another avenue for access to health services and the acquisition of healthy behaviors. Studies have shown that students with access to SBHCs showed greater participation in physical activity and met more nutritional intake guidelines compared to peers not using these facilities (83).
Vocational differences
Over the past several decades, increasing globalization and automation in the workforce has increased the economic returns of higher education while reducing demand for less skilled labor. As income inequality has become more stratified by education level, correlations between education and mortality have become stronger (84). Higher education is also associated with increased likelihood of jobs providing non-wage related benefits, such as employer provided healthcare, paid leave, and retirement funds, all of which contribute to positive employment outcomes (85). Conversely, lower educational attainment coincides with increased occupational stress and precarity of employment conditions which encompass several inflammatory exposures (86).
Higher educational attainment has been inversely correlated with an individual's likelihood of participating in shift work (87). Shift work is broadly defined as a working schedule in which takes place outside of traditional daytime hours of 7 AM–6 PM (88). This type of work is commonly characterized by an irregular work schedule which includes regular evening and nighttime work, rotating shifts, or split shifts (89). Shift work has been identified as a risk factor for systemic inflammation which predisposes this class of employees to a host of other chronic disorders such as cardiovascular disease, cancer, and metabolic syndrome(88–91). A study of shift workers in Atlanta found a 93% higher concentration of CRP in collected blood samples compared to day workers. In addition, concentrations of the pro-inflammatory cytokines IL-1β and TNF-α were 96% and 20% higher among this cohort (92). Both IL-1β and TNF-α also have been shown to circulate in the body at higher levels following periods of sleep deprivation (93). In the same study, IL-6 concentrations in the blood were 190% higher among shift workers, while plasma cortisol levels were 39% higher (92).
The correlation between shift work and inflammation has been hypothesized to be related to the misalignment between workers' endogenous circadian rhythm and the sleep wake cycles shift work demands of employees (91). On average shift workers report sleeping 30–70 min less per day than dayworkers (90, 92). Furthermore, 10%–20% of shift workers experience shift work disorder (SWD), a condition characterized by insomnia or excessive daytime sleepiness in the context of a work schedule which interferes with one's endogenous sleep-wake cycle (90). A study of full-time shift workers found that conditions of short-term circadian misalignment consisting of a 12 hour inversion of the behavioral to environmental cycles resulted in an 11% increase to CRP produced within 24 h of misalignment (91).
Occupational exposures
Employment precarity is informed by multiple factors such as low SES and minority status in a population (94, 95). Research has shown that decreasing education level is associated with increasing occupational precariousness. As the quality and stability of available employment declines, the risk that employees encounter occupational hazards increases (95). Lower educational attainment has been most strongly associated with heightened risk of exposure to chemical hazards and, to a lesser extent, physical and ergonomic hazards (95).
Lead exposure remains a common hazard of industrial jobs such as construction, often via inhalation of fumes or dust contaminated by the heavy metal. Although the American Conference of Governmental Industrial Hygienists (ACGIH) states that individuals may experience blood lead levels of 20 µg/dl without adverse effect, chronic exposure to lower levels of lead is associated with dysfunction to multiple organ systems (96). Chronic lead exposure has been correlated with significant increases to serum levels of TNF-α, IL-1β, and IL-6. Furthermore, levels of these pro-inflammatory cytokines are positively correlated with levels of angiogenic factors in lead exposed individuals, suggesting this inflammatory response may also promote cancer progression (97).
Ergonomic occupational hazards refer to working conditions which can cause strain on the body through repetitive motion, high exertion activities, or awkward posture (98). Notably, although moderate leisure time physical activity correlates with benefits to physical health and anti-inflammatory effects, occupational physical activity does not confer the same benefits. This phenomenon is referred to as the physical activity paradox (99, 100). Studies show occupational physical activity has a positive association with levels of CRP, suggesting this type of physical exertion has inflammatory effects (99, 100). Higher intensity of occupational physical activity was also associated with lower income levels (100).
Neighborhood and built environment
There is a wealth of evidence that shows the neighborhoods and homes in which people live are strong determinants of health outcomes (101). Studies have displayed associations between low-grade inflammation and factors ranging from the physical qualities of the home itself (102) to broader neighborhood exposures like air pollution (103). The hypothesized mechanisms behind these associations range from microbial dysbiosis to activation of classic inflammatory pathways.
Chemical exposures
Marginalized and low-income communities are disproportionately exposed to several classes of toxic chemicals, which have well-established harmful effects through widely encompassing sources of exposure(104–106). Inhalable particulate matter with a diameter of <2.5 μm (PM2.5), disproportionately affect marginalized communities, as higher concentrations of air pollution containing PM2.5 positively correlates with the percentage of Black residents, historical redlining score, and low-income census tracts(107–110) resulting in greater overall mortality (109, 110). Indoor air pollution is an especially significant risk factor, which sources of exposure including improper temperature control, poor indoor ventilation, smoking, and gas stove use (111, 112). Birth cohorts have displayed longitudinal associations between ambient PM2.5, PM10, and NO2 with the inflammation-association proteins IFN- γ and IL-12B (113). In experimental settings, PM2.5 exposure resulted in increased macrophage-mediated IFN-γ, IL-17, and IL-21 expression by T cells, as well as the formation of reactive oxygen species and secretion of IL-1β and TNF-α by macrophages (114, 115).
Additionally, heavy metal exposure, such as through contaminated water or food sources, disproportionately effects low-income and non-white communities (116–118). Heavy metals including lead, arsenic, and cadmium have been linked with chronic disease and immune dysregulation in both human and experimental settings (119). High ambient exposure to arsenic, chromium, cadmium, and nickel has been linked with increased rates of breast and colon cancers in marginalized communities (120). In animal models, lead exposure resulted in gut dysbiosis and an increase in opportunistic pathogens, as well as altered metabolism of key microbial species (121). Similarly, human studies showed that, depending on the route of exposure, lead exposure was associated with enhanced inflammatory responses including several proinflammatory cytokines and increased NF-κB signaling (122). Cadmium exposure has also been shown to result in lower abundance of SCFA-producing bacteria and increased TNF-α expression (123). Similarly, arsenic exposure increased proinflammatory cytokine expression, production of reactive oxygen species, and NF-κB signaling in experimental models (124–126).
There are also significant neighborhood and community-based disparities in exposure to persistent organic pollutants (POPs), which have been shown to promote inflammation. Housing conditions such as peeling paint, water leaks, cigarette smoke, pesticide residues, and old furniture expose residents to harmful chemicals and disproportionately affect urban communities with low SES (127). Exposures to POPs, including polybrominated diphenyl ethers (PBDEs), organochlorine pesticides (OCPs), polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs), are significantly higher in social housing multi-family units than in single family dwellings (128), as well as in old homes (129, 130), both of which are correlated with race and low income (131). Several epidemiologic studies have shown significant positive associations between serum concentrations of OCP and inflammatory biomarkers, including CRP, TNF-α, and IFN-γ . In in-vivo experimental studies, exposure to OCPs and PCBs through several routes increased expression of proinflammatory cytokines including IL-6, IL-10, TNF-α, and MCP-1 (132). Exposure to PCBs was also shown to promote leukocyte infiltration, activate NF-κB through the NEMO pathway, and alter metabolic processes of gut microbiota, resulting in decreases of SCFA-producing species(133–135). Likewise, exposure to PBDEs in experimental settings led to activation of NF-κB, NLRP3 inflammasome activation, and the production of reactive oxygen species due to mitochondrial dysfunction (136). Furthermore, high exposure to PAH's was significantly correlated with CRP and biomarkers of oxidative stress, as well as increased proinflammatory cytokine expression, in cohorts of pregnant women (137, 138). Benzo(α)pyrene, one of the most common PAH, induced oxidative stress, inflammatory cytokine expression, and NF-κB activation upon exposure to human endothelial cells and keratinocytes (139, 140). Given their persistence in the environment, the inflammatory effects of persistent organic pollutants span over long periods of time.
Alongside previously discussed exposures, phthalates, which are known endocrine disrupting chemicals, are additional sources of pro-inflammatory chemical exposures. There are well-established racial disparities in phthalate exposure (141–143). Weathering of construction materials in low-income housing (104, 144, 145), certain personal care products (146), and discount retailers (147), all of which have a greater presence in low-income and marginalized communities, are highly associated with high phthalate exposure (105). Several studies have suggested a causal link between phthalates and inflammation, showing phthalate exposure increased production of TNF-α by monocytes and macrophages, which has been observed in both in-vitro and in-vivo studies (148). Phthalate exposure has also been linked with heightened inflammation and oxidative stress in pregnant women (149).
Housing conditions
In addition to abundant harmful chemical exposures, marginalized communities are disproportionately exposed to other inflammatory triggers due to poor housing quality (150). The quality of one's home physical environment has been linked with low-grade inflammation through exposures such as mold and poor temperature control. Moisture damage, for example, has been linked with increased CRP levels, as well as increased expression of IL-1β, IL-6, and TNF-α (151). A pilot study further observed an increase in innate immune activity, specifically pattern recognition receptor expression and cytokine release, among subjects exposed to moisture damage, with similar findings from in-vitro stimulation with model fungal substances (152). Exposure to isolated components of fungal specimens and mold from damp building environments also increased leukocyte infiltration in the bronchial space and gene expression of TNF-α in alveolar cell lines (153). Furthermore, an interventional study showed that repairing moisture damage decreased expression of IL-6, IL-4, and TNF-α (154).
Evidence suggests that improper temperature control and indoor ventilation, which are also associated with low-income housing, have implications for low-grade inflammation (155, 156). Short term temperature effects have been observed to correlate with IL-6 expression, plasminogen activator inhibitor-1 (PAI-1) levels, and CRP levels (157). Improper indoor temperature control has also resulted in increased airway inflammation, with increased expression of IgE and IgG, leukocytes, and inflammatory cytokines in in-vivo models (158), as well Th2 responses in asthmatic mice (159). Furthermore, in a randomized crossover trial, residence in homes with poor air conditioning in hot environments lead to increased intestinal fatty-acid binding protein (I-FABP), indicating decreased barrier integrity (160).
Overcrowded housing is another factor thought to contribute to chronic stress and related health outcomes in low-income communities (161). A population-based study observed early life household overcrowding, determined by number of people per room, to be associated with several markers of inflammation, including CRP and ICAM (162). In in-vivo models, mice subjected to high density housing conditions displayed increases in colonic CXCL1, TNF-α and IL22, hyperglycemia, and low-grade gut inflammation, alongside increases in corticosterone levels (163).
Neighborhood conditions
Alongside material home characteristics, poor neighborhood conditions have also been implicated in low-grade inflammation. For instance, noise pollution is a significant environmental threat which disproportionately affects low-income and minoritized communities (164). An in-vivo study observed chronic noise exposure led to increased intestinal inflammation in rats with persistent elevation of TNF-α and IL1β, as well as alternation of the gut microbiome (165). Additional studies have observed an increase in IL-6 and other proinflammatory monocytes in response to noise, which has been hypothesized to occur in response to increases in stress hormone release upon noise exposure (166).
Longitudinal studies suggest the aspects of the built environment which influence walking and exercise habits may also contribute to the association with inflammation (167). A study investigating the association between walking behavior and built environment suggested that leisure walking was associated with retail zone walkability whereas commuter walking was associated with the number of walkable social destinations and street connectivity (167). Additional studies have observed correlations between gross population density, intersection density, and walkability indexes with physical activity (168). A cross-sectional survey of individual health survey responses also reported greater walking behavior in neighborhoods with more green space (169). Walking and exercise behaviors are key regulators of inflammation. Regular exercise has been shown to promote PGC1α, which has been shown to increase detoxification of ROS, promote vascularization, and suppress the production of inflammatory cytokines, including TNF-a and IL-6, in multiple in-vitro settings (170). Additionally, in a randomized controlled trial, increasing the steps per day reduced IL-6 levels, even after adjusting for obesity (171). A recent meta-analysis also reported lifelong exercise was associated with reduced levels of CRP and IL-6 (172).
Alongside the suggested influence on walking behavior, there are several reported health outcomes influenced by green space. Using multiple metrics of green space availability, including park cover, Normalized Different Vegetation Index (NDVI), and NatureScore, green space is positively associated with SES and percentages of non-Hispanic white residents (173). The presence of green space has also been linked with regulation of low-grade inflammation. Multiple metrics of greenness have been associated with lower CRP and IL-6, as well as white blood cell counts, B-cells and monocytes (174). In a cross-sectional study, residential greenness was also inversely associated with isoprostanes, which are robust indicators of systemic oxidative stress. Participants who lived in greener areas also had lower levels of sympathetic activation, supporting the hypothesis that stress levels may partially mediate the effect of green space on disease outcomes (175).
Neighborhood access
Beyond intrinsic factors such as the built environment, neighborhoods affect other factors that are relevant to inflammation regulation. For instance, several neighborhoods within the United States are considered as “food deserts” or “food swamps.” Food deserts are regions in which people live more than 1 mile from a supermarket and lack healthy food options, while food swamps describe regions that are more than 1 mile form a supermarket and have a greater proportion of proinflammatory food options than fresh food (176). Food desert severity has been suggested to mediate the relationship between income and inflammation (177). Although assessment of neighborhood access to green space or health food is subject to limitations, including the fact that people are not necessarily limited to nearby grocery stores and green spaces, new methods to assess urban access beyond proximity measurements are emerging and should be incorporated in further inquiry (178).
Alongside neighborhoods with predominantly low-income residents and marginalized communities having reduced access to healthy food, there is greater availability of tobacco products (179). In marginalized communities tobacco products are more widely advertised, in both the frequency and nature of advertisement (180). Furthermore, a cross-sectional study of tobacco retailers in Washington, D.C. found predatory tobacco advertising tactics, specifically more appealing descriptors of tobacco, are more prevalent in census tracts with a greater proportion of Black residents. Similar findings were observed among Hispanic/Latino residents (181). Moreover, abundant evidence suggests flavored tobacco products are associated with increased initiation and prolonged use among youth and young adult tobacco users, compared to nonflavored products (182). Several studies have established a strong association between tobacco products and cigarette smoke with immune dysfunction, ranging from both inflammatory to suppressive effects (183). Namely, cigarettes contain known immunomodulatory toxins, including nicotine, carbon monoxide, acrolein, reactive oxidant substances, and more (183). In in-vitro models, cigarette smoke activates epithelial cells and induces chemokine expression but simultaneously impairs innate immune responses to pathogens by inhibiting secretion of key antimicrobial peptides (184). Results from several case-control studies also displayed increases in inflammatory markers including CRP, CCL17, and CCL11 (185).
Social and community context
Social support and cohesion
Social and community context broadly encompasses the intertwined relationship and community dynamics including social cohesion, discrimination, and relationships in the home, workplace, and community (186). Across diverse metrics of social support, perceived social support was inversely correlated with CRP, TNF-α, and IL-6 (187). While global measures of social support did not correlate with CRP or IL-6 in a different study, the frequency of positive social interactions associated with lower CRP in middle-aged adults (188). Perceived social cohesion, assessed by survey responses, has also been suggested to moderate the relationship between SES and CRP levels (189). While further studies are necessary to understand mechanisms by which this association may occur, studies hypothesize the influence of stress from poor cohesion or lack of social support, as well as the role of social support in encouraging other healthy behaviors, have implications for chronic inflammation. A cross-sectional study using results from the Healthy Aging in Neighborhoods of Diversity Across the Lifespan Study, a longitudinal study led by the National Institutes of Health, showed neighborhood social cohesion was associated with healthier behaviors, such as increased physical activity, less cigarette use, and healthier diets; social cohesion was more pronounced in white participants (190).
Discrimination
The effects of discrimination based on race, ethnicity, migratory status, religion, class, and other factors on health outcomes have been increasingly studied, including in the context of inflammation (191). A longitudinal study assessing persistent exposure to various types of racial discrimination, including disrespectful treatment from co-workers, negative police encounters, or racial slurs, found that persistent exposure to discriminatory events was positively associated with a composite measure of inflammatory cytokines IL-1β, IL-2, IL-5, IL-6, IL-17, TNFα, and MIP-1b in a cohort of Black women (192). This association persisted after controlling for exposure to childhood adversity, BMI, and health behaviors including diet and exercise. Similar findings were concluded in a longitudinal study assessing the effects of self-report discrimination and community segregation on inflammatory cytokine expression in a cohort of 400 Black participants (193). The physiological processes hypothesized to link chronic social and economic disadvantage with racial and economic disparities have been describe as the “weathering hypothesis” (194, 195). Discrimination has been shown to over activate stress pathways, with investigators observing constructs of discrimination to be predictors and correlates of alterations in HPA axis activity (196). Several studies have also implicated the conserved transcriptional respond to adversity (CTRA), which describes increased transcription of pro-inflammatory immune response genes, as well as reduced expression of antiviral genes (197–200).
Exposure to discrimination and related stressors vary in nature and length of exposure, both of which have been suggested to influence the related effects on inflammation. Future inquiries should distinguish the effects of discrimination across various forms of chronic, low-grade inflammation. Discrimination and social disadvantage are also intertwined with previously described social conditions, such as occupational or air pollution exposures, which are associated with inflammation themselves. However, racial disparities across inflammatory markers and chronic disease are consistently observed even after controlling for several social conditions including educational attainment, household wealth, various health behaviors, usage of medication, and marriage (201–203).
Exposure to violence
Among adolescents, home neighborhood murder rate and exposure to violence have been shown to interact to predict counts of classical monocytes (204). In a cohort of 1,391 adolescents followed up to 18 years of age, childhood exposure to violence was associated with elevated levels of soluble urokinase plasminogen activator receptor (suPAR) and IL-6 (205). Furthermore, a longitudinal study involving 236 children from the Chicago area concluded neighborhood violence was associated with increased signaling of NF-κB and activator protein 1 (AP-1) control pathways, as well as greater beta-adrenergic and lower glucocorticoid signaling (206).
Adverse childhood experiences
Adverse childhood experiences (ACE), which are traumatic events that occur before the age of 18, have also been associated with higher inflammatory profiles. Specifically, among school-aged children, those who experienced parental substance abuse displayed higher levels of pro-inflammatory markers including IL-6 and IL-1β (207). The presence of ACE was also associated with an altered gut microbiota composition and response to cortisol in a cohort of pregnant women (68). Similarly, in a cohort of healthy adults, those with early childhood adversity demonstrated less response to cortisol, enrichment of inflammatory gene expression in stress responses, and increased activity of pro-inflammatory signaling overall in comparison to adults without trauma experience (208). Adolescents exposed to adversity also demonstrated elevated transcription of genes pertaining to myeloid lineage immune cells and CREB transcriptional activity, which has also been previously implicated in increased immune-related gene expression in the context of adverse experiences (209, 210).
Healthcare access and quality
Healthcare access and quality describes the availability and accessibility of quality, timely, comprehensive, and respectful healthcare services and resources. Although limited research directly examines the association between this social determinant and inflammatory markers, particularly through mechanistic pathways, there are several notable findings to report.
Delayed care
Insurance status, encompassed within healthcare access, has previously been associated with control of chronic conditions. Additionally, in a study utilizing NHANES participants from 1988–1994, those in the public/no insurance group had significantly elevated CRP compared to those with private insurance (211). Proposed mechanisms underlying this association include the observation that underinsured individuals often delay their care, resulting in worsening disease and inflammation for these individuals as they receive treatment only when their disease presents in a severe stage (212). A lack of awareness of disease may also contribute to chronic inflammation, as patients with lower insurance reimbursements also have significantly higher CRP/IL-6 levels post-surgery (213). In the case of a chronic inflammatory conditions such as lupus, those with public insurance have also been shown to have higher rates of hospitalization and readmissions compared to those on private insurance. Even among those who receive care, there is greater healthcare fragmentation, with patients often receiving services across multiple locations. Fragmented care is known to results in increased risk of comorbidities, hospitalizations, and overall healthcare costs (214). Together, these studies suggest that individuals without insurance are more likely to delay seeking care, which can lead to more advanced disease at presentation and a sustained inflammatory state that may remain undetected or unmanaged.
Medication availability
Unsurprisingly, insurance status also affects an individual's ability to adhere to their medication regimen. A 2012 study found that patients hospitalized for cardiovascular disease were more likely to be incapable of staying adherent to their medications (215). A later study also showed that those on public insurance or uninsured had elevated CRP, which was associated with functional limitations (216). This association is clinically relevant, given that functional limitations have been shown to predict poor medication adherence in prior studies (217). This highlights a potential feedback loop where poor insurance coverage leads to delays in care and worse medication adherence, which leads to elevated inflammation and promotes functional impairments, which in turn makes it more difficult for patients to manage their chronic conditions.
Discussion
There are several mechanisms by which SDoH contribute to a heightened inflammatory state that elevates the risk of chronic disease development. Both an absence of resources and reduced frequency of lifestyle factors that mitigate inflammation, as well as toxic and stress-inducing exposures actively contribute to heightened inflammation. Placing these inflammatory triggers within the context of Bronfenbrenner's social-ecological model (218) identifies the exosystem as enriched for harmful exposures (Figure 2). However, in nearly every context where people are born, live, learn, work, play, and age, lifestyle factors and exposures influence regulation of the immune system. Furthermore, exposures beginning in childhood, and even in utero, can influence chronic low-grade inflammation later in life, as evidenced by fetal impacts of maternal deprivation, anti-inflammatory behaviors developed in school age, and the inflammatory impacts of adverse childhood experiences.
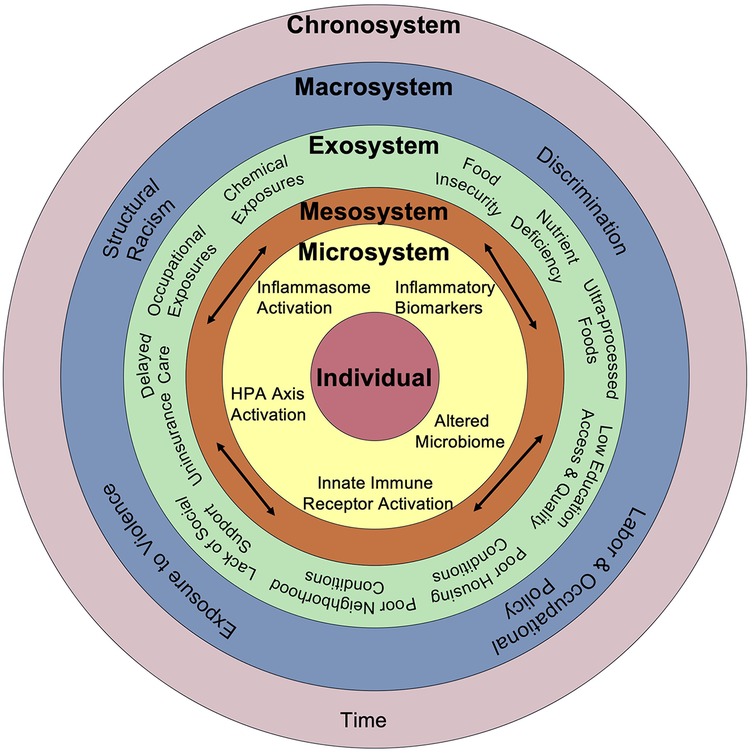
Figure 2. Comprehensive framework connecting social and biological drivers of chronic inflammation. Adapted from Bronfenbrenner's Social Ecological Model, this framework illustrates how social, environmental, and structural factors interact across nested levels—from individual biology to broader societal systems—to drive chronic inflammation through pathways including HPA axis activation, inflammasome signaling, altered microbiome composition, and innate immune receptor activation over time.
There are limitations of this review which should be further addressed in future inquiries. Firstly, chronic low-grade inflammation describes a broad condition. While measuring certain biomarkers, such as CRP and IL-6 is a common approach to assess this condition, levels of inflammatory cytokines, reactive oxygen species, and proxies for gut permeability have also been assessed to show evidence of systemic inflammation. The broad characterization of inflammation may also generalize more specific underlying processes. However, as there is strong evidence suggesting chronic low-grade inflammation, described broadly, underpins the development of several chronic diseases, this broader framework to conceptualize the large influence of SDoH on inflammatory disease risk may guide further inquiry into more specific pathways and mechanisms, as well as possible interventions to mitigate the burden of chronic disease.
Additionally, inflammatory conditions across social determinants of health often do not operate independently but are rather intertwined. For instance, the neighborhoods people live in can shape not only their built environment, but their opportunities for and access to economic prosperity, education, healthcare, and social cohesion. Researchers note deriving causal conclusions from observational studies is obscured by the interconnection between these factors, although experiments similarly present limitations in generalizability considering the cumulative effects of factors in real world settings (101).
Likewise, the directionality of reported observations is not fully clear from observational studies. For example, while lower educational attainment has been shown to be associated with greater CRP levels, further studies are necessary to confirm whether lack of education might contribute to elevated inflammation or greater inflammation might interfere with one's ability to attain higher education levels. Regardless of directionality, biomarkers of SDoH may still inform research, especially if the connections can be elucidated through multi-variant assessments of the SDoH against the specific biomarkers. For example, identifying specific biomarkers of the various SDoH parameters could reduce reliance on survey data and better determine which determinant is most impactful for each given individual. Ultimately, careful conclusions should be made from the presented research and when considering the application of these findings to interventions. For example, the noted biomarkers of inflammation present plausible mechanisms for the resultant harms to population health, however the solutions will require political and societal interventions rather than pharmacologic blockade of inflammatory pathways.
The abundant exposures that contribute to a proinflammatory state thought to underpin the development of several chronic diseases are largely connected to disparities in health outcomes. Several of the discussed mechanisms by which social contexts contribute to inflammation, from ultra-processed food to deteriorating housing conditions to discrimination predominantly affect low-income and historically marginalized communities. Through discriminatory practices such as historical redlining, Black and non-white communities were sequestered to neighborhoods which continue to be those with the highest inflammatory exposures (219). Persistent barriers across the five domains of SDoH for low-income and marginalized communities have been consistently shown to shape disparities in health outcomes (220), and the presented framework highlights inflammation as a key mechanism of this association.
Author contributions
AV: Methodology, Validation, Conceptualization, Writing – original draft, Investigation, Visualization, Writing – review & editing. CK: Writing – original draft, Methodology, Conceptualization, Investigation, Validation, Writing – review & editing. JJ: Writing – original draft, Methodology, Investigation, Conceptualization, Writing – review & editing, Validation. AE: Validation, Visualization, Writing – review & editing. KO-K: Visualization, Validation, Writing – review & editing. GR: Writing – review & editing, Validation, Visualization. IM: Writing – review & editing, Project administration, Writing – original draft, Conceptualization, Visualization, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Intramural Research Program of the National Institutes of Health (NIH). The contributions of the NIH authors(s) were made as part of their official duties as NIH federal staff, are in compliance with agency policy requirements, and are considered works of the United States Government. However, the findings and conclusions presented in this paper are those of the authors(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH). The contributions of the NIH authors(s) were made as part of their official duties as NIH federal staff, are in compliance with agency policy requirements, and are considered works of the United States Government. However, the findings and conclusions presented in this paper are those of the authors(s) and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7
3. Chawes BL, Stokholm J, Schoos AM, Fink NR, Brix S, Bisgaard H. Allergic sensitization at school age is a systemic low-grade inflammatory disorder. Allergy. (2017) 72(7):1073–80. doi: 10.1111/all.13108
4. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444(7121):860–7. doi: 10.1038/nature05485
5. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420(6917):860–7. doi: 10.1038/nature01322
6. Xiang Y, Zhang M, Jiang D, Su Q, Shi J. The role of inflammation in autoimmune disease: a therapeutic target. Front Immunol. (2023) 14:1267091. doi: 10.3389/fimmu.2023.1267091
7. Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. (2023) 8(1):267. doi: 10.1038/s41392-023-01486-5
8. Benavidez GA, Zahnd WE, Hung PY, Eberth JM. Chronic disease prevalence in the US: sociodemographic and geographic variations by zip code tabulation area. Prev Chronic Dis. (2024) 21:E14. doi: 10.5888/pcd21.230267
9. Hahn RA. What is a social determinant of health? back to basics. J Public Health Res. (2021) 10(4):jphr-2021. doi: 10.4081/jphr.2021.2324
10. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25(12):1822–32. doi: 10.1038/s41591-019-0675-0
11. Cifuentes M, Verdejo HE, Castro PF, Corvalan AH, Ferreccio C, Quest AFG, et al. Low-grade chronic inflammation: a shared mechanism for chronic diseases. Physiology (Bethesda). (2025) 40(1):4–25. doi: 10.1152/physiol.00021.2024
12. Liu CH, Abrams ND, Carrick DM, Chander P, Dwyer J, Hamlet MRJ, et al. Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nat Immunol. (2017) 18(11):1175–80. doi: 10.1038/ni.3828
13. Grebenciucova E, VanHaerents S. Interleukin 6: at the interface of human health and disease. Front Immunol. (2023) 14:1255533. doi: 10.3389/fimmu.2023.1255533
14. Clyne B, Olshaker JS. The C-reactive protein. J Emerg Med. (1999) 17(6):1019–25. doi: 10.1016/S0736-4679(99)00135-3
15. Menzel A, Samouda H, Dohet F, Loap S, Ellulu MS, Bohn T. Common and novel markers for measuring inflammation and oxidative stress ex vivo in research and clinical practice-which to use regarding disease outcomes? Antioxidants (Basel). (2021) 10(3):414. doi: 10.3390/antiox10030414
16. Proctor MJ, McMillan DC, Horgan PG, Fletcher CD, Talwar D, Morrison DS. Systemic inflammation predicts all-cause mortality: a glasgow inflammation outcome study. PLoS One. (2015) 10(3):e0116206. doi: 10.1371/journal.pone.0116206
17. Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic status and inflammation: a meta-analysis. Mol Psychiatry. (2020) 25(9):2189–99. doi: 10.1038/s41380-018-0259-2
18. Milaniak I, Jaffee SR. Childhood socioeconomic status and inflammation: a systematic review and meta-analysis. Brain Behav Immun. (2019) 78:161–76. doi: 10.1016/j.bbi.2019.01.018
19. Miller GE, Borders AE, Crockett AH, Ross KM, Qadir S, Keenan-Devlin L, et al. Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav Immun. (2017) 64:276–84. doi: 10.1016/j.bbi.2017.04.014
20. Deverts DJ, Cohen S, Kalra P, Matthews KA. The prospective association of socioeconomic status with C-reactive protein levels in the CARDIA study. Brain Behav Immun. (2012) 26(7):1128–35. doi: 10.1016/j.bbi.2012.07.017
21. Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. (2014) 174(10):1587–95. doi: 10.1001/jamainternmed.2014.3422
22. Wilcox S, Sharpe PA, Liese AD, Dunn CG, Hutto B. Socioeconomic factors associated with diet quality and meeting dietary guidelines in disadvantaged neighborhoods in the Southeast United States. Ethn Health. (2020) 25(8):1115–31. doi: 10.1080/13557858.2018.1493434
23. Monsivais P, Aggarwal A, Drewnowski A. Are socio-economic disparities in diet quality explained by diet cost? J Epidemiol Community Health. (2012) 66(6):530–5. doi: 10.1136/jech.2010.122333
24. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. (2015) 73(10):643–60. doi: 10.1093/nutrit/nuv027
25. Laraia BA, Leak TM, Tester JM, Leung CW. Biobehavioral factors that shape nutrition in low-income populations: a narrative review. Am J Prev Med. (2017) 52(2S2):S118–S26. doi: 10.1016/j.amepre.2016.08.003
26. Laraia BA. Food insecurity and chronic disease. Adv Nutr. (2013) 4(2):203–12. doi: 10.3945/an.112.003277
27. Fadeyev K, Nagao-Sato S, Reicks M. Nutrient and food group intakes among U.S. children (2–5 years) differ by family income to poverty ratio, NHANES 2011–2018. Int J Environ Res Public Health. (2021) 18(22):11938. doi: 10.3390/ijerph182211938
28. Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochem Pharmacol. (2005) 70(3):394–406. doi: 10.1016/j.bcp.2005.04.030
29. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. (2015) 8(1):80–93. doi: 10.1038/mi.2014.44
30. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J Nutr. (2009) 139(9):1619–25. doi: 10.3945/jn.109.104638
31. Maki KA, Sack MN, Hall KD. Ultra-processed foods: increasing the risk of inflammation and immune dysregulation? Nat Rev Immunol. (2024) 24(7):453–4. doi: 10.1038/s41577-024-01049-x
32. Karlsen A, Retterstol L, Laake P, Paur I, Bohn SK, Sandvik L, et al. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. (2007) 137(8):1951–4. doi: 10.1093/jn/137.8.1951
33. Overman A, Chuang CC, McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int J Obes (Lond). (2011) 35(9):1165–72. doi: 10.1038/ijo.2010.272
34. Vieux F, Maillot M, Rehm CD, Drewnowski A. Flavonoid intakes in the US diet are linked to higher socioeconomic status and to tea consumption: analyses of NHANES 2011–16 data. J Nutr. (2020) 150(8):2147–55. doi: 10.1093/jn/nxaa145
35. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. (2015) 10(11):e0141770. doi: 10.1371/journal.pone.0141770
36. Silva MC, Furlanetto TW. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. (2015) 35(2):91–6. doi: 10.1016/j.nutres.2014.12.008
37. Mousa A, Naderpoor N, Teede H, Scragg R, de Courten B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2018) 76(5):380–94. doi: 10.1093/nutrit/nux077
38. Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J Nutr. (2016) 146(11):2388–97. doi: 10.3945/jn.116.231480
39. Wei J, Zhu A, Ji JS. A comparison study of vitamin D deficiency among older adults in China and the United States. Sci Rep. (2019) 9(1):19713. doi: 10.1038/s41598-019-56297-y
40. Baraldi LG, Martinez Steele E, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. (2018) 8(3):e020574. doi: 10.1136/bmjopen-2017-020574
41. Myles IA. Fast food fever: reviewing the impacts of the western diet on immunity. Nutr J. (2014) 13:61. doi: 10.1186/1475-2891-13-61
42. Braesco V, Souchon I, Sauvant P, Haurogne T, Maillot M, Feart C, et al. Ultra-processed foods: how functional is the NOVA system? Eur J Clin Nutr. (2022) 76(9):1245–53. doi: 10.1038/s41430-022-01099-1
43. Martínez Steele E, Popkin BM, Swinburn B, Monteiro CA. The share of ultra-processed foods and the overall nutritional quality of diets in the US: evidence from a nationally representative cross-sectional study. Popul Health Metr. (2017) 15(1):6. doi: 10.1186/s12963-017-0119-3
44. Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. (2013) 191(6):3200–9. doi: 10.4049/jimmunol.1301057
45. Liu Y, Yang X, Xiao F, Jie F, Zhang Q, Liu Y, et al. Dietary cholesterol oxidation products: perspectives linking food processing and storage with health implications. Compr Rev Food Sci Food Saf. (2022) 21(1):738–79. doi: 10.1111/1541-4337.12880
46. Progatzky F, Sangha NJ, Yoshida N, McBrien M, Cheung J, Shia A, et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat Commun. (2014) 5:5864. doi: 10.1038/ncomms6864
47. Vincent MJ, Allen B, Palacios OM, Haber LT, Maki KC. Meta-regression analysis of the effects of dietary cholesterol intake on LDL and HDL cholesterol. Am J Clin Nutr. (2019) 109(1):7–16. doi: 10.1093/ajcn/nqy273
48. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. (2015) 15(2):104–16. doi: 10.1038/nri3793
49. Du Q, Wang Q, Fan H, Wang J, Liu X, Wang H, et al. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem Pharmacol. (2016) 105:42–54. doi: 10.1016/j.bcp.2016.02.017
50. Rocha DM, Caldas AP, Oliveira LL, Bressan J, Hermsdorff HH. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis. (2016) 244:211–5. doi: 10.1016/j.atherosclerosis.2015.11.015
51. Pat Y, Yazici D, D'Avino P, Li M, Ardicli S, Ardicli O, et al. Recent advances in the epithelial barrier theory. Int Immunol. (2024) 36(5):211–22. doi: 10.1093/intimm/dxae002
52. Bian X, Tu P, Chi L, Gao B, Ru H, Lu K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem Toxicol. (2017) 107(Pt B):530–9. doi: 10.1016/j.fct.2017.04.045
53. Viennois E, Bretin A, Dube PE, Maue AC, Dauriat CJG, Barnich N, et al. Dietary emulsifiers directly impact adherent-invasive E. coli gene expression to drive chronic intestinal inflammation. Cell Rep. (2020) 33(1):108229. doi: 10.1016/j.celrep.2020.108229
54. Bellanco A, Requena T, Martinez-Cuesta MC. Polysorbate 80 and carboxymethylcellulose: a different impact on epithelial integrity when interacting with the microbiome. Food Chem Toxicol. (2025) 196:115236. doi: 10.1016/j.fct.2025.115236
55. Um CY, Hodge RA, Tran HQ, Campbell PT, Gewirtz AT, McCullough ML. Association of emulsifier and highly processed food intake with circulating markers of intestinal permeability and inflammation in the cancer prevention study-3 diet assessment sub-study. Nutr Cancer. (2022) 74(5):1701–11. doi: 10.1080/01635581.2021.1957947
56. Chassaing B, Compher C, Bonhomme B, Liu Q, Tian Y, Walters W, et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology. (2022) 162(3):743–56. doi: 10.1053/j.gastro.2021.11.006
57. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. (2014) 514(7521):181–6. doi: 10.1038/nature13793
58. Miller ES, Apple CG, Kannan KB, Funk ZM, Plazas JM, Efron PA, et al. Chronic stress induces persistent low-grade inflammation. Am J Surg. (2019) 218(4):677–83. doi: 10.1016/j.amjsurg.2019.07.006
59. Wirtz PH, von Kanel R. Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep. (2017) 19(11):111. doi: 10.1007/s11886-017-0919-x
60. Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. (2014) 76(3):181–9. doi: 10.1097/PSY.0000000000000049
61. Knight EL, Jiang Y, Rodriguez-Stanley J, Almeida DM, Engeland CG, Zilioli S. Perceived stress is linked to heightened biomarkers of inflammation via diurnal cortisol in a national sample of adults. Brain Behav Immun. (2021) 93:206–13. doi: 10.1016/j.bbi.2021.01.015
62. Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. (2012) 109(16):5995–9. doi: 10.1073/pnas.1118355109
63. Villalba DK, Lindsay EK, Marsland AL, Greco CM, Young S, Brown KW, et al. Mindfulness training and systemic low-grade inflammation in stressed community adults: evidence from two randomized controlled trials. PLoS One. (2019) 14(7):e0219120. doi: 10.1371/journal.pone.0219120
64. Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. (2009) 106(34):14716–21. doi: 10.1073/pnas.0902971106
65. Gough M, Godde K. A multifaceted analysis of social stressors and chronic inflammation. SSM Popul Health. (2018) 6:136–40. doi: 10.1016/j.ssmph.2018.09.005
66. Hantsoo L, Zemel BS. Stress gets into the belly: early life stress and the gut microbiome. Behav Brain Res. (2021) 414:113474. doi: 10.1016/j.bbr.2021.113474
67. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. (2011) 25(3):397–407. doi: 10.1016/j.bbi.2010.10.023
68. Hantsoo L, Jasarevic E, Criniti S, McGeehan B, Tanes C, Sammel MD, et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav Immun. (2019) 75:240–50. doi: 10.1016/j.bbi.2018.11.005
69. Michels N, Van de Wiele T, Fouhy F, O'Mahony S, Clarke G, Keane J. Gut microbiome patterns depending on children’s psychosocial stress: reports versus biomarkers. Brain Behav Immun. (2019) 80:751–62. doi: 10.1016/j.bbi.2019.05.024
70. van den Munckhof ICL, Kurilshikov A, Ter Horst R, Riksen NP, Joosten LAB, Zhernakova A, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. (2018) 19(12):1719–34. doi: 10.1111/obr.12750
71. Dawes DE. The Political Determinants of Health. Baltimore: Johns Hopkins University Press (2020).
72. Suiter SV, Meadows ML. Educational attainment and educational contexts as social determinants of health. Prim Care. (2023) 50(4):579–89. doi: 10.1016/j.pop.2023.04.007
73. Zajacova A, Lawrence EM. The relationship between education and health: reducing disparities through a contextual approach. Annu Rev Public Health. (2018) 39:273–89. doi: 10.1146/annurev-publhealth-031816-044628
74. Briefel RR, Crepinsek MK, Cabili C, Wilson A, Gleason PM. School food environments and practices affect dietary behaviors of US public school children. J Am Diet Assoc. (2009) 109(2 Suppl):S91–107. doi: 10.1016/j.jada.2008.10.059
75. Adams EL, Raynor HA, Thornton LM, Mazzeo SE, Bean MK. Nutrient intake during school lunch in title I elementary schools with universal free meals. Health Educ Behav. (2022) 49(1):118–27. doi: 10.1177/10901981211011936
76. Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet. (2013) 113(2):297–306. doi: 10.1016/j.jand.2012.08.011
77. Gearan EC, Monzella K, Jennings L, Fox MK. Differences in diet quality between school lunch participants and nonparticipants in the United States by income and race. Nutrients. (2020) 12(12):3891. doi: 10.3390/nu12123891
78. Smith SL, Cunningham-Sabo L. Food choice, plate waste and nutrient intake of elementary- and middle-school students participating in the US national school lunch program. Public Health Nutr. (2014) 17(6):1255–63. doi: 10.1017/S1368980013001894
79. Miller SJ, Batra AK, Shearrer GE, House BT, Cook LT, Pont SJ, et al. Dietary fibre linked to decreased inflammation in overweight minority youth. Pediatr Obes. (2016) 11(1):33–9. doi: 10.1111/ijpo.12017
80. Parikh S, Pollock NK, Bhagatwala J, Guo DH, Gutin B, Zhu H, et al. Adolescent fiber consumption is associated with visceral fat and inflammatory markers. J Clin Endocrinol Metab. (2012) 97(8):E1451–7. doi: 10.1210/jc.2012-1784
81. Bujtor M, Turner AI, Torres SJ, Esteban-Gonzalo L, Pariante CM, Borsini A. Associations of dietary intake on biological markers of inflammation in children and adolescents: a systematic review. Nutrients. (2021) 13(2):356. doi: 10.3390/nu13020356
82. Hall E, Chai W, Albrecht JA. Relationships between nutrition-related knowledge, self-efficacy, and behavior for fifth grade students attending Title I and non-Title I schools. Appetite. (2016) 96:245–53. doi: 10.1016/j.appet.2015.09.033
83. McNall MA, Lichty LF, Mavis B. The impact of school-based health centers on the health outcomes of middle school and high school students. Am J Public Health. (2010) 100(9):1604–10. doi: 10.2105/AJPH.2009.183590
84. Bor J, Cohen GH, Galea S. Population health in an era of rising income inequality: uSA, 1980–2015. Lancet. (2017) 389(10077):1475–90. doi: 10.1016/S0140-6736(17)30571-8
85. Schudde L, Bernell K. Educational attainment and nonwage labor market returns in the United States. AERA Open. (2019) 5(3):2332858419874056. doi: 10.1177/2332858419874056
86. Assari S, Bazargan M. Unequal associations between educational attainment and occupational stress across racial and ethnic groups. Int J Environ Res Public Health. (2019) 16(19):3539. doi: 10.3390/ijerph16193539
87. Daghlas I, Richmond RC, Lane JM, Dashti HS, Ollila HM, Schernhammer ES, et al. Selection into shift work is influenced by educational attainment and body mass index: a Mendelian randomization study in the UK biobank. Int J Epidemiol. (2021) 50(4):1229–40. doi: 10.1093/ije/dyab031
88. Kim SW, Jang EC, Kwon SC, Han W, Kang MS, Nam YH, et al. Night shift work and inflammatory markers in male workers aged 20–39 in a display manufacturing company. Ann Occup Environ Med. (2016) 28:48. doi: 10.1186/s40557-016-0135-y
89. Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, et al. Shift work and vascular events: systematic review and meta-analysis. Br Med J. (2012) 345:e4800. doi: 10.1136/bmj.e4800
90. Moreno CRC. Shift work sleep disorder. Handb Clin Neurol. (2025) 206:89–92. doi: 10.1016/B978-0-323-90918-1.00015-0
91. Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. (2017) 32(2):154–64. doi: 10.1177/0748730417697537
92. Atwater AQ, Immergluck LC, Davidson AJ, Castanon-Cervantes O. Shift work predicts increases in lipopolysaccharide-binding protein, interleukin-10, and leukocyte counts in a cross-sectional study of healthy volunteers carrying low-grade systemic inflammation. Int J Environ Res Public Health. (2021) 18(24):13158. doi: 10.3390/ijerph182413158
93. Jewett KA, Krueger JM. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam Horm. (2012) 89:241–57. doi: 10.1016/B978-0-12-394623-2.00013-5
94. Baek SU, Kim MS, Lim MH, Kim T, Won JU, Yoon JH. Multidimensional employment precariousness mediates the association between low educational attainment and poor subjective well-being: results from a nationwide cross-sectional study in South Korea. Scand J Work Environ Health. (2023) 49(7):506–17. doi: 10.5271/sjweh.4109
95. Bonney T, Rospenda KM, Forst L, Conroy LM, Castaneda D, Avelar S, et al. Employment precarity and increased risk of hazardous occupational exposures among residents of high socioeconomic hardship neighborhoods. Ann Work Expo Health. (2022) 66(9):1122–35. doi: 10.1093/annweh/wxac062
96. Viramgami A, Shah RK, Dhatrak S, Sheth A, Singh DP, Sivaperumal P, et al. Impact of occupational lead exposure on the comprehensive health status of gas cutter workers. Clin Epidemiol Glob. (2024) 30:101820. doi: 10.1016/j.cegh.2024.101820
97. Machon-Grecka A, Dobrakowski M, Boron M, Lisowska G, Kasperczyk A, Kasperczyk S. The influence of occupational chronic lead exposure on the levels of selected pro-inflammatory cytokines and angiogenic factors. Hum Exp Toxicol. (2017) 36(5):467–73. doi: 10.1177/0960327117703688
98. Tak S, Buchholz B, Punnett L, Moir S, Paquet V, Fulmer S, et al. Physical ergonomic hazards in highway tunnel construction: overview from the construction occupational health program. Appl Ergon. (2011) 42(5):665–71. doi: 10.1016/j.apergo.2010.10.001
99. Lee J, Kim HR, Jang TW, Lee DW, Lee YM, Kang MY. Occupational physical activity, not leisure-time physical activity, is associated with increased high-sensitivity C reactive protein levels. Occup Environ Med. (2021) 78(2):86–91. doi: 10.1136/oemed-2020-106753
100. Feinberg JB, Moller A, Siersma V, Bruunsgaard H, Mortensen OS. Physical activity paradox: could inflammation be a key factor? Br J Sports Med. (2022) 56(21):1224–9. doi: 10.1136/bjsports-2022-105429
101. Diez Roux AV. Neighborhoods and health: what do we know? What should we do? Am J Public Health. (2016) 106(3):430–1. doi: 10.2105/AJPH.2016.303064
102. Schmeer KK, Yoon AJ. Home sweet home? Home physical environment and inflammation in children. Soc Sci Res. (2016) 60:236–48. doi: 10.1016/j.ssresearch.2016.04.001
103. Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. (2007) 176(4):370–6. doi: 10.1164/rccm.200611-1627OC
104. Ruiz D, Becerra M, Jagai JS, Ard K, Sargis RM. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. (2018) 41(1):193–205. doi: 10.2337/dc16-2765
105. Nguyen VK, Kahana A, Heidt J, Polemi K, Kvasnicka J, Jolliet O, et al. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999–2014. Environ Int. (2020) 137:105496. doi: 10.1016/j.envint.2020.105496
106. Howarth MV, Eiser AR. Environmentally mediated health disparities. Am J Med. (2023) 136(6):518–22. doi: 10.1016/j.amjmed.2023.02.008
107. Collins TW, Grineski SE, Shaker Y, Mullen CJ. Communities of color are disproportionately exposed to long-term and short-term PM(2.5) in metropolitan America. Environ Res. (2022) 214(Pt 4):114038. doi: 10.1016/j.envres.2022.114038
108. Bramble K, Blanco MN, Doubleday A, Gassett AJ, Hajat A, Marshall JD, et al. Exposure disparities by income, race and ethnicity, and historic redlining grade in the greater seattle area for ultrafine particles and other air pollutants. Environ Health Perspect. (2023) 131(7):77004. doi: 10.1289/EHP11662
109. Boing AF, deSouza P, Boing AC, Kim R, Subramanian SV. Air pollution, socioeconomic status, and age-specific mortality risk in the United States. JAMA Netw Open. (2022) 5(5):e2213540. doi: 10.1001/jamanetworkopen.2022.13540
110. Josey KP, Delaney SW, Wu X, Nethery RC, DeSouza P, Braun D, et al. Air pollution and mortality at the intersection of race and social class. N Engl J Med. (2023) 388(15):1396–404. doi: 10.1056/NEJMsa2300523
111. Rabito FA, Werthmann DW, Straubing R, Adamkiewicz G, Reponen T, Ashley PJ, et al. A multi-city study of indoor air quality in green vs. non-green low-income housing. Environ Res. (2024) 240(Pt 2):117576. doi: 10.1016/j.envres.2023.117576
112. Colton MD, MacNaughton P, Vallarino J, Kane J, Bennett-Fripp M, Spengler JD, et al. Indoor air quality in green vs. conventional multifamily low-income housing. Environ Sci Technol. (2014) 48(14):7833–41. doi: 10.1021/es501489u
113. He S, Klevebro S, Baldanzi G, Pershagen G, Lundberg B, Eneroth K, et al. Ambient air pollution and inflammation-related proteins during early childhood. Environ Res. (2022) 215(Pt 2):114364. doi: 10.1016/j.envres.2022.114364
114. Ma QY, Huang DY, Zhang HJ, Wang S, Chen XF. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int Immunopharmacol. (2017) 50:139–45. doi: 10.1016/j.intimp.2017.06.019
115. Xu F, Shi X, Qiu X, Jiang X, Fang Y, Wang J, et al. Investigation of the chemical components of ambient fine particulate matter [PM(2.5)] associated with in vitro cellular responses to oxidative stress and inflammation. Environ Int. (2020) 136:105475. doi: 10.1016/j.envint.2020.105475
116. Kodros JK, Bell ML, Dominici F, L'Orange C, Godri Pollitt KJ, Weichenthal S, et al. Unequal airborne exposure to toxic metals associated with race, ethnicity, and segregation in the USA. Nat Commun. (2022) 13(1):6329. doi: 10.1038/s41467-022-33372-z
117. Martinez-Morata I, Bostick BC, Conroy-Ben O, Duncan DT, Jones MR, Spaur M, et al. Nationwide geospatial analysis of county racial and ethnic composition and public drinking water arsenic and uranium. Nat Commun. (2022) 13(1):7461. doi: 10.1038/s41467-022-35185-6
118. Geron M, Cowell W, Amarasiriwardena C, Andra SS, Carroll K, Kloog I, et al. Racial/ethnic and neighborhood disparities in metals exposure during pregnancy in the Northeastern United States. Sci Total Environ. (2022) 820:153249. doi: 10.1016/j.scitotenv.2022.153249
119. Teffera M, Veith AC, Ronnekleiv-Kelly S, Bradfield CA, Nikodemova M, Tussing-Humphreys L, et al. Diverse mechanisms by which chemical pollutant exposure alters gut microbiota metabolism and inflammation. Environ Int. (2024) 190:108805. doi: 10.1016/j.envint.2024.108805
120. Tomlinson MM, Pugh F, Nail AN, Newton JD, Udoh K, Abraham S, et al. Heavy-metal associated breast cancer and colorectal cancer hot spots and their demographic and socioeconomic characteristics. Cancer Causes Control. (2024) 35(10):1367–81. doi: 10.1007/s10552-024-01894-0
121. Xia J, Jin C, Pan Z, Sun L, Fu Z, Jin Y. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci Total Environ. (2018) 631:439–48. doi: 10.1016/j.scitotenv.2018.03.053
122. Harshitha P, Bose K, Dsouza HS. Influence of lead-induced toxicity on the inflammatory cytokines. Toxicology. (2024) 503:153771. doi: 10.1016/j.tox.2024.153771
123. He X, Qi Z, Hou H, Qian L, Gao J, Zhang XX. Structural and functional alterations of gut microbiome in mice induced by chronic cadmium exposure. Chemosphere. (2020) 246:125747. doi: 10.1016/j.chemosphere.2019.125747
124. Chiocchetti GM, Velez D, Devesa V. Inorganic arsenic causes intestinal barrier disruption. Metallomics. (2019) 11(8):1411–8. doi: 10.1039/c9mt00144a
125. McDermott TR, Stolz JF, Oremland RS. Arsenic and the gastrointestinal tract microbiome. Environ Microbiol Rep. (2020) 12(2):136–59. doi: 10.1111/1758-2229.12814
126. Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. (2016) 23(9):8244–59. doi: 10.1007/s11356-016-6333-x
127. Adamkiewicz G, Zota AR, Fabian MP, Chahine T, Julien R, Spengler JD, et al. Moving environmental justice indoors: understanding structural influences on residential exposure patterns in low-income communities. Am J Public Health. (2011) 101(Suppl 1):S238–45. doi: 10.2105/AJPH.2011.300119
128. Wan YC, Diamond ML, Siegel JA. Elevated concentrations of semivolatile organic compounds in social housing multiunit residential building apartments. Environ Sci Tech Let. (2020) 7(3):191–7. doi: 10.1021/acs.estlett.0c00068
129. Ortlund K, Chandler M, Dunlop AL, Barr DB, Ryan PB, Liang D, et al. Housing characteristics, dietary patterns, and sociodemographic characteristics as predictors of persistent organic pollutant exposure among African American pregnant women in Atlanta. Environ Res. (2025) 272:121172. doi: 10.1016/j.envres.2025.121172
130. Whitehead TP, Metayer C, Ward MH, Colt JS, Gunier RB, Deziel NC, et al. Persistent organic pollutants in dust from older homes: learning from lead. Am J Public Health. (2014) 104(7):1320–6. doi: 10.2105/AJPH.2013.301835
131. Reponen T, Levin L, Zheng S, Vesper S, Ryan P, Grinshpun SA, et al. Family and home characteristics correlate with mold in homes. Environ Res. (2013) 124:67–70. doi: 10.1016/j.envres.2013.04.003
132. Peinado FM, Artacho-Cordon F, Barrios-Rodriguez R, Arrebola JP. Influence of polychlorinated biphenyls and organochlorine pesticides on the inflammatory milieu. A systematic review of in vitro, in vivo and epidemiological studies. Environ Res. (2020) 186:109561. doi: 10.1016/j.envres.2020.109561
133. Tian Y, Gui W, Rimal B, Koo I, Smith PB, Nichols RG, et al. Metabolic impact of persistent organic pollutants on gut microbiota. Gut Microbes. (2020) 12(1):1–16. doi: 10.1080/19490976.2020.1848209
134. Phillips MC, Dheer R, Santaolalla R, Davies JM, Burgueno J, Lang JK, et al. Intestinal exposure to PCB 153 induces inflammation via the ATM/NEMO pathway. Toxicol Appl Pharmacol. (2018) 339:24–33. doi: 10.1016/j.taap.2017.11.027
135. Kim MJ, Pelloux V, Guyot E, Tordjman J, Bui LC, Chevallier A, et al. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ Health Perspect. (2012) 120(4):508–14. doi: 10.1289/ehp.1104282
136. Yang B, Wang Y, Fang C, Song E, Song Y. Polybrominated diphenyl ether quinone exposure leads to ROS-driven lysosomal damage, mitochondrial dysfunction and NLRP3 inflammasome activation. Environ Pollut. (2022) 311:119846. doi: 10.1016/j.envpol.2022.119846
137. Ferguson KK, McElrath TF, Pace GG, Weller D, Zeng L, Pennathur S, et al. Urinary polycyclic aromatic hydrocarbon metabolite associations with biomarkers of inflammation, angiogenesis, and oxidative stress in pregnant women. Environ Sci Technol. (2017) 51(8):4652–60. doi: 10.1021/acs.est.7b01252
138. Craig EA, Lin Y, Ge Y, Wang X, Murphy SK, Harrington DK, et al. Associations of gestational exposure to air pollution and polycyclic aromatic hydrocarbons with placental inflammation. Environ Health (Wash). (2024) 2(9):672–80. doi: 10.1021/envhealth.4c00077
139. Shi H, Liu J, Gao H. Benzo(alpha)pyrene induces oxidative stress and inflammation in human vascular endothelial cells through AhR and NF-kappaB pathways. Microvasc Res. (2021) 137:104179. doi: 10.1016/j.mvr.2021.104179
140. Tsuji G, Takahara M, Uchi H, Takeuchi S, Mitoma C, Moroi Y, et al. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J Dermatol Sci. (2011) 62(1):42–9. doi: 10.1016/j.jdermsci.2010.10.017
141. Welch BM, Keil AP, Buckley JP, Engel SM, James-Todd T, Zota AR, et al. Racial and ethnic disparities in phthalate exposure and preterm birth: a pooled study of sixteen U.S. cohorts. Environ Health Perspect. (2023) 131(12):127015. doi: 10.1289/EHP12831
142. Varshavsky JR, Zota AR, Woodruff TJ. A novel method for calculating potency-weighted cumulative phthalates exposure with implications for identifying racial/ethnic disparities among U.S. reproductive-aged women in NHANES 2001–2012. Environ Sci Technol. (2016) 50(19):10616–24. doi: 10.1021/acs.est.6b00522
143. Baker BH, Melough MM, Paquette AG, Barrett ES, Day DB, Kannan K, et al. Ultra-processed and fast food consumption, exposure to phthalates during pregnancy, and socioeconomic disparities in phthalate exposures. Environ Int. (2024) 183:108427. doi: 10.1016/j.envint.2024.108427
144. Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, et al. Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese dwellings. Sci Total Environ. (2014) 468-469:147–57. doi: 10.1016/j.scitotenv.2013.07.107
145. Bi C, Maestre JP, Li H, Zhang G, Givehchi R, Mahdavi A, et al. Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: association with season, building characteristics, and childhood asthma. Environ Int. (2018) 121(Pt 1):916–30. doi: 10.1016/j.envint.2018.09.013
146. Parlett LE, Calafat AM, Swan SH. Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol. (2013) 23(2):197–206. doi: 10.1038/jes.2012.105
148. Hansen JF, Bendtzen K, Boas M, Frederiksen H, Nielsen CH, Rasmussen AK, et al. Influence of phthalates on cytokine production in monocytes and macrophages: a systematic review of experimental trials. PLoS One. (2015) 10(3):e0120083. doi: 10.1371/journal.pone.0120083
149. van TETJ, Rosen EM, Barrett ES, Nguyen RHN, Sathyanarayana S, Milne GL, et al. Phthalates and phthalate alternatives have diverse associations with oxidative stress and inflammation in pregnant women. Environ Sci Technol. (2019) 53(6):3258–67. doi: 10.1021/acs.est.8b05729
150. Adamkiewicz G, Spengler JD, Harley AE, Stoddard A, Yang M, Alvarez-Reeves M, et al. Environmental conditions in low-income urban housing: clustering and associations with self-reported health. Am J Public Health. (2014) 104(9):1650–6. doi: 10.2105/AJPH.2013.301253
151. Mustonen K, Karvonen AM, Kirjavainen P, Roponen M, Schaub B, Hyvarinen A, et al. Moisture damage in home associates with systemic inflammation in children. Indoor Air. (2016) 26(3):439–47. doi: 10.1111/ina.12216
152. Punsmann S, Liebers V, Lotz A, Bruning T, Raulf M. Ex vivo cytokine release and pattern recognition receptor expression of subjects exposed to dampness: pilot study to assess the outcome of mould exposure to the innate immune system. PLoS One. (2013) 8(12):e82734. doi: 10.1371/journal.pone.0082734
153. Miller JD, Sun M, Gilyan A, Roy J, Rand TG. Inflammation-associated gene transcription and expression in mouse lungs induced by low molecular weight compounds from fungi from the built environment. Chem Biol Interact. (2010) 183(1):113–24. doi: 10.1016/j.cbi.2009.09.023
154. Roponen M, Meklin T, Rintala H, Hyvarinen A, Hirvonen MR. Effect of moisture-damage intervention on the immunotoxic potential and microbial content of airborne particles and on occupants’ upper airway inflammatory responses. Indoor Air. (2013) 23(4):295–302. doi: 10.1111/ina.12032
155. Gielen AC, Shields W, McDonald E, Frattaroli S, Bishai D, Ma X. Home safety and low-income urban housing quality. Pediatrics. (2012) 130(6):1053–9. doi: 10.1542/peds.2012-1531
156. Williams AA, Spengler JD, Catalano P, Allen JG, Cedeno-Laurent JG. Building vulnerability in a changing climate: indoor temperature exposures and health outcomes in older adults living in public housing during an extreme heat event in Cambridge, MA. Int J Environ Res Public Health. (2019) 16(13):2373. doi: 10.3390/ijerph16132373
157. Schauble CL, Hampel R, Breitner S, Ruckerl R, Phipps R, Diaz-Sanchez D, et al. Short-term effects of air temperature on blood markers of coagulation and inflammation in potentially susceptible individuals. Occup Environ Med. (2012) 69(9):670–8. doi: 10.1136/oemed-2011-100469
158. Wolkoff P, Azuma K, Carrer P. Health, work performance, and risk of infection in office-like environments: the role of indoor temperature, air humidity, and ventilation. Int J Hyg Environ Health. (2021) 233:113709. doi: 10.1016/j.ijheh.2021.113709
159. Liao W, Zhou L, Zhao X, Song L, Lu Y, Zhong N, et al. Thermoneutral housing temperature regulates T-regulatory cell function and inhibits ovabumin-induced asthma development in mice. Sci Rep. (2017) 7(1):7123. doi: 10.1038/s41598-017-07471-7
160. Lee B, Meade R, Davey S, Thake C, McCormick J, King K, et al. Effects of daylong exposure to indoor overheating on enterocyte damage and inflammatory responses in older adults: a randomized crossover trial. Appl Physiol Nutr Metab. (2025) 50:1–7. doi: 10.1139/apnm-2024-0368
161. Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. (1999) 896:131–44. doi: 10.1111/j.1749-6632.1999.tb08111.x
162. Packard CJ, Bezlyak V, McLean JS, Batty GD, Ford I, Burns H, et al. Early life socioeconomic adversity is associated in adult life with chronic inflammation, carotid atherosclerosis, poorer lung function and decreased cognitive performance: a cross-sectional, population-based study. BMC Public Health. (2011) 11:42. doi: 10.1186/1471-2458-11-42
163. Delaroque C, Chervy M, Gewirtz AT, Chassaing B. Social overcrowding impacts gut microbiota, promoting stress, inflammation, and dysglycemia. Gut Microbes. (2021) 13(1):2000275. doi: 10.1080/19490976.2021.2000275
164. Casey JA, Morello-Frosch R, Mennitt DJ, Fristrup K, Ogburn EL, James P. Race/ethnicity, socioeconomic status, residential segregation, and spatial variation in noise exposure in the contiguous United States. Environ Health Perspect. (2017) 125(7):077017. doi: 10.1289/EHP898
165. Cui B, Gai Z, She X, Wang R, Xi Z. Effects of chronic noise on glucose metabolism and gut microbiota-host inflammatory homeostasis in rats. Sci Rep. (2016) 6:36693. doi: 10.1038/srep36693
166. Munzel T, Molitor M, Kuntic M, Hahad O, Roosli M, Engelmann N, et al. Transportation noise pollution and cardiovascular health. Circ Res. (2024) 134(9):1113–35. doi: 10.1161/CIRCRESAHA.123.323584
167. Hirsch JA, Moore KA, Clarke PJ, Rodriguez DA, Evenson KR, Brines SJ, et al. Changes in the built environment and changes in the amount of walking over time: longitudinal results from the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2014) 180(8):799–809. doi: 10.1093/aje/kwu218
168. Grasser G, Van Dyck D, Titze S, Stronegger W. Objectively measured walkability and active transport and weight-related outcomes in adults: a systematic review. Int J Public Health. (2013) 58(4):615–25. doi: 10.1007/s00038-012-0435-0
169. Richardson EA, Pearce J, Mitchell R, Kingham S. Role of physical activity in the relationship between urban green space and health. Public Health. (2013) 127(4):318–24. doi: 10.1016/j.puhe.2013.01.004
170. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. (2008) 454(7203):463–9. doi: 10.1038/nature07206
171. Yates T, Davies MJ, Gorely T, Talbot D, Bull F, Sattar N, et al. The effect of increased ambulatory activity on markers of chronic low-grade inflammation: evidence from the PREPARE programme randomized controlled trial. Diabet Med. (2010) 27(11):1256–63. doi: 10.1111/j.1464-5491.2010.03091.x
172. Perez-Castillo IM, Rueda R, Bouzamondo H, Aparicio-Pascual D, Valino-Marques A, Lopez-Chicharro J, et al. Does lifelong exercise counteract low-grade inflammation associated with aging? A systematic review and meta-analysis. Sports Med. (2025) 55(3):675–96. doi: 10.1007/s40279-024-02152-8
173. Klompmaker JO, Hart JE, Bailey CR, Browning M, Casey JA, Hanley JR, et al. Racial, ethnic, and socioeconomic disparities in multiple measures of blue and green spaces in the United States. Environ Health Perspect. (2023) 131(1):17007. doi: 10.1289/EHP11164
174. Riggs D, Malovichko M, Yeager R, Sears C, Sithu I, Gao H, et al. Relations of residential greenness with markers of immunity and inflammation in the green heart study. ISEE Conf Abstr. (2022) 2022(1). doi: 10.1289/isee.2022.P-0277
175. Yeager R, Riggs DW, DeJarnett N, Tollerud DJ, Wilson J, Conklin DJ, et al. Association between residential greenness and cardiovascular disease risk. J Am Heart Assoc. (2018) 7(24):e009117. doi: 10.1161/JAHA.118.009117
176. Bevel MS, Tsai MH, Parham A, Andrzejak SE, Jones S, Moore JX. Association of food deserts and food swamps with obesity-related cancer mortality in the US. JAMA Oncol. (2023) 9(7):909–16. doi: 10.1001/jamaoncol.2023.0634
177. Kelli HM, Hammadah M, Ahmed H, Ko YA, Topel M, Samman-Tahhan A, et al. Association between living in food deserts and cardiovascular risk. Circ Cardiovasc Qual Outcomes. (2017) 10(9):e003532. doi: 10.1161/CIRCOUTCOMES.116.003532
178. Wood EK, Stamos G, Mitchell AJ, Gonoud R, Horgan AM, Nomura O, et al. The association between food desert severity, socioeconomic status, and metabolic state during pregnancy in a prospective longitudinal cohort. Sci Rep. (2023) 13(1):7197. doi: 10.1038/s41598-023-32783-2
179. Giovenco DP, Spillane TE, Merizier JM. Neighborhood differences in alternative tobacco product availability and advertising in New York city: implications for health disparities. Nicotine Tob Res. (2019) 21(7):896–902. doi: 10.1093/ntr/nty244
180. Lee JG, Henriksen L, Rose SW, Moreland-Russell S, Ribisl KM. A systematic review of neighborhood disparities in point-of-sale tobacco marketing. Am J Public Health. (2015) 105(9):e8–18. doi: 10.2105/AJPH.2015.302777
181. Kong AY, Westneat SC, Anesetti-Rothermel A, van de Venne JG, Debnam C, Ribisl KM, et al. Neighborhood inequities in tobacco product descriptors, Washington, DC, 2018–2019. Nicotine Tob Res. (2024) 26(Supplement_2):S73–81. doi: 10.1093/ntr/ntad226
182. Villanti AC, Johnson AL, Glasser AM, Rose SW, Ambrose BK, Conway KP, et al. Association of flavored tobacco use with tobacco initiation and subsequent use among US youth and adults, 2013–2015. JAMA Netw Open. (2019) 2(10):e1913804. doi: 10.1001/jamanetworkopen.2019.13804
183. Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. (2012) 91(2):142–9. doi: 10.1177/0022034511421200
184. Pierson T, Learmonth-Pierson S, Pinto D, van Hoek ML. Cigarette smoke extract induces differential expression levels of beta-defensin peptides in human alveolar epithelial cells. Tob Induc Dis. (2013) 11(1):10. doi: 10.1186/1617-9625-11-10
185. Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. (2014) 106(11):dju294. doi: 10.1093/jnci/dju294
186. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of Federal Policies that Contribute to Racial and Ethnic Health Inequities, Geller AB, Polsky DE, Burke SP, editors. (2023). Federal Policy to Advance Racial, Ethnic, and Tribal Health Equity. Washingdon, DC: National Academies Press (US) (2023). doi: 10.17226/26834
187. Boen CE, Barrow DA, Bensen JT, Farnan L, Gerstel A, Hendrix LH, et al. Social relationships, inflammation, and cancer survival. Cancer Epidemiol Biomarkers Prev. (2018) 27(5):541–9. doi: 10.1158/1055-9965.EPI-17-0836
188. Bajaj A, John-Henderson NA, Cundiff JM, Marsland AL, Manuck SB, Kamarck TW. Daily social interactions, close relationships, and systemic inflammation in two samples: healthy middle-aged and older adults. Brain Behav Immun. (2016) 58:152–64. doi: 10.1016/j.bbi.2016.06.004
189. Oberndorfer M, Leyland AH, Pearce J, Grabovac I, Hannah MK, Dorner TE. Unequally unequal? contextual-level status inequality and social cohesion moderating the association between individual-level socioeconomic position and systemic chronic inflammation. Soc Sci Med. (2023) 333:116185. doi: 10.1016/j.socscimed.2023.116185
190. Rosenblatt AM, Crews DC, Powe NR, Zonderman AB, Evans MK, Tuot DS. Association between neighborhood social cohesion, awareness of chronic diseases, and participation in healthy behaviors in a community cohort. BMC Public Health. (2021) 21(1):1611. doi: 10.1186/s12889-021-11633-8
191. Tamura K, Elbel B, Athens JK, Rummo PE, Chaix B, Regan SD, et al. Assessments of residential and global positioning system activity space for food environments, body mass index and blood pressure among low-income housing residents in New York city. Geospat Health. (2018) 13(2):10–4081. doi: 10.4081/gh.2018.712
192. Simons RL, Lei MK, Klopack E, Zhang Y, Gibbons FX, Beach SRH. Racial discrimination, inflammation, and chronic illness among African American women at midlife: support for the weathering perspective. J Racial Ethn Health Disparities. (2021) 8(2):339–49. doi: 10.1007/s40615-020-00786-8
193. Simons RL, Lei MK, Beach SRH, Barr AB, Simons LG, Gibbons FX, et al. Discrimination, segregation, and chronic inflammation: testing the weathering explanation for the poor health of Black Americans. Dev Psychol. (2018) 54(10):1993–2006. doi: 10.1037/dev0000511
194. Forde AT, Crookes DM, Suglia SF, Demmer RT. The weathering hypothesis as an explanation for racial disparities in health: a systematic review. Ann Epidemiol. (2019) 33:1–18 e3. doi: 10.1016/j.annepidem.2019.02.011
195. Das A. How does race get “under the skin"?: inflammation, weathering, and metabolic problems in late life. Soc Sci Med. (2013) 77:75–83. doi: 10.1016/j.socscimed.2012.11.007
196. Busse D, Yim IS, Campos B, Marshburn CK. Discrimination and the HPA axis: current evidence and future directions. J Behav Med. (2017) 40(4):539–52. doi: 10.1007/s10865-017-9830-6
197. Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci. (2019) 28:31–7. doi: 10.1016/j.cobeha.2019.01.008
198. Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. (2003) 100(4):1920–5. doi: 10.1073/pnas.0438019100
199. Thames AD, Irwin MR, Breen EC, Cole SW. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology. (2019) 106:277–83. doi: 10.1016/j.psyneuen.2019.04.016
200. Cuevas AG, Cole SW. From discrimination to disease: the role of inflammation. Harv Rev Psychiatry. (2025) 33(2):83–9. doi: 10.1097/HRP.0000000000000422
201. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15(9):505–22. doi: 10.1038/s41569-018-0064-2
202. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. (2014) 69:S4–9. doi: 10.1093/gerona/glu057
203. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. (2006) 96(5):826–33. doi: 10.2105/AJPH.2004.060749
204. Finegood ED, Chen E, Kish J, Vause K, Leigh AKK, Hoffer L, et al. Community violence and cellular and cytokine indicators of inflammation in adolescents. Psychoneuroendocrinology. (2020) 115:104628. doi: 10.1016/j.psyneuen.2020.104628
205. Rasmussen LJH, Moffitt TE, Arseneault L, Danese A, Eugen-Olsen J, Fisher HL, et al. Association of adverse experiences and exposure to violence in childhood and adolescence with inflammatory burden in young people. JAMA Pediatr. (2020) 174(1):38–47. doi: 10.1001/jamapediatrics.2019.3875
206. Miller GE, Chen E, Finegood E, Shimbo D, Cole SW. Prospective associations between neighborhood violence and monocyte pro-inflammatory transcriptional activity in children. Brain Behav Immun. (2022) 100:1–7. doi: 10.1016/j.bbi.2021.11.003
207. Heard-Garris N, Davis MM, Estabrook R, Burns J, Briggs-Gowan M, Allen N, et al. Adverse childhood experiences and biomarkers of inflammation in a diverse cohort of early school-aged children. Brain Behav Immun Health. (2020) 1:100006. doi: 10.1016/j.bbih.2019.100006
208. Schwaiger M, Grinberg M, Moser D, Zang JC, Heinrichs M, Hengstler JG, et al. Altered stress-induced regulation of genes in monocytes in adults with a history of childhood adversity. Neuropsychopharmacology. (2016) 41(10):2530–40. doi: 10.1038/npp.2016.57
209. Kuhlman KR, Cole SW, Craske MG, Fuligni AJ, Irwin MR, Bower JE. Enhanced immune activation following acute social stress among adolescents with early-life adversity. Biol Psychiatry Glob Open Sci. (2023) 3(2):213–21. doi: 10.1016/j.bpsgos.2022.03.001
210. Kuhlman KR, Tan EN, Cole SW, Rao U. Differential immune profiles in the context of chronic stress among childhood adversity-exposed adolescents. Brain Behav Immun. (2025) 127:183–92. doi: 10.1016/j.bbi.2025.03.004
211. Bittoni MA, Wexler R, Spees CK, Clinton SK, Taylor CA. Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States. Prev Med. (2015) 81:420–6. doi: 10.1016/j.ypmed.2015.09.016
212. Bergersen KV, Pham K, Li J, Ulrich MT, Merrill P, He Y, et al. Health disparities in COVID-19: immune and vascular changes are linked to disease severity and persist in a high-risk population in Riverside County, California. BMC Public Health. (2023) 23(1):1584. doi: 10.1186/s12889-023-16462-5
213. Jiang Q, Yu T, Huang K, Huang X, Zhang Q, Hu S. The impact of medical insurance reimbursement on postoperative inflammation reaction in distinct cardiac surgery from a single center. BMC Health Serv Res. (2022) 22(1):494. doi: 10.1186/s12913-022-07920-8
214. Joo JY. Fragmented care and chronic illness patient outcomes: a systematic review. Nurs Open. (2023) 10(6):3460–73. doi: 10.1002/nop2.1607
215. Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche-Orlow MK, Schnipper JL. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. (2012) 7(6):470–5. doi: 10.1002/jhm.1940
216. Dhamoon MS, Cheung YK, Moon YP, Wright CB, Willey JZ, Sacco R, et al. C-reactive protein is associated with disability independently of vascular events: the northern manhattan study. Age Ageing. (2017) 46(1):77–83. doi: 10.1093/ageing/afw179
217. Park JH, Park JH, Lee SY, Kim SY, Shin Y, Kim SY. Disparities in antihypertensive medication adherence in persons with disabilities and without disabilities: results of a Korean population-based study. Arch Phys Med Rehabil. (2008) 89(8):1460–7. doi: 10.1016/j.apmr.2007.12.045
218. Bronfenbrenner U. Ecological Models of Human Development. International Encyclopedia of Education. NY: Freeman (1994).
219. Jung KH, Argenio KL, Jackson DJ, Miller RL, Perzanowski MS, Rundle AG, et al. Home and school pollutant exposure, respiratory outcomes, and influence of historical redlining. J Allergy Clin Immunol. (2024) 154(5):1159–68. doi: 10.1016/j.jaci.2024.06.020
Keywords: social determinants of health, pollution, inflammation, environmental justice, disparities
Citation: Vijendra A, Kunkle C, Jordan J, Erickson A, Osei-Karikari K, Ratley G and Myles IA (2025) Molecular connections between inflammation and social determinants of health. Front. Epidemiol. 5:1683955. doi: 10.3389/fepid.2025.1683955
Received: 12 August 2025; Accepted: 28 October 2025;
Published: 11 November 2025.
Edited by:
Carina Ladeira, Escola Superior de Tecnologia da Saúde de Lisboa (ESTeSL), PortugalReviewed by:
Marie Stoner, RTI International, United StatesTeresa Buckner, University of Northern Colorado, United States
Copyright: © 2025 Vijendra, Kunkle, Jordan, Erickson, Osei-Karikari, Ratley and Myles. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian A. Myles, bXlsZXNpQG5pYWlkLm5paC5nb3Y=
 Aditi Vijendra1
Aditi Vijendra1 Jalin Jordan
Jalin Jordan Anna Erickson
Anna Erickson Grace Ratley
Grace Ratley Ian A. Myles
Ian A. Myles