- 1United Arab Emirates University, Al Ain, United Arab Emirates
- 2University of Salford, Manchester, UK
- 3University of Cambridge, Cambridge, UK
- 4University of Manchester, Manchester, UK
A commentary on
Recent years have witnessed the development of highly effective immunomodulatory therapies, especially for treating cancers and autoimmune diseases. The most promising targets include immunosuppressive Tregs, characterized by FoxP3 expression, and the inhibitory pathways involving CTLA-4 and PD-1/PD-L1 molecules. A recent paper by Germanidis et al. provides interesting insights into the relevance of these immunosuppressive pathways for treating chronic hepatitis B virus (HBV) hepatitis (CHB) (1).
CHB is characterized by chronic liver damage, inflammation, and fibrosis, eventually leading to cirrhosis and hepatic carcinoma. The HBV life cycle is not cytolytic to infected hepatocytes, and the liver damage is caused by a prolonged immune response induced by the expression of HBsAg on infected hepatocytes. The immune response involves HBsAg-specific CD8+ CTLs and to a lesser extent CD4+ T effector cells (Teff) (2). CHB is managed with either interferon-based therapies that act by enhancing anti-viral immune responses or nucleos(t)ide analogs (NAs), such as entecavir, which inhibit HBV replication (3). Successful anti-viral therapy is characterized by (i) restored anti-viral immune response, (ii) HBsAg seroconversion, (iii) a decrease in covalently closed circular DNA (cccDNA), and (iv) a decrease in circulating HBV DNA (3–5). However, most patients initially experience “remission,” followed by HBV reactivation after treatment withdrawal (3). cccDNA is the HBV transcription template, it is highly stable and persists even following resolution of CHB or acute HBV infection. It enables HBV reactivation and it is a key hurdle for achieving complete remission (3).
Elevated levels of intra-hepatic and circulating FoxP3+ Tregs have been described in CHB and chronic hepatitis C (CHC) (6–8). In this study, Germanidis et al. reported down-regulated liver mRNA expression of FoxP3 and suppressive cytokines, IL-10, and TGF-β, in patients maintained on-treatment following 5 years of remission compared to patients with active disease and no prior treatment (1). CD8 was also decreased in patients on-treatment and in remission. This data suggest a decrease in both intra-hepatic FoxP3+ Tregs and CTLs following CHB resolution. Interestingly, IL-2 and IFN-γ expressions were not restored during remission; this could indicate long-term CTL impairment or else it could be due to a reduction in intra-hepatic CTLs preventing any immune response from being restored to pre-infection intensity.
The role of Tregs in CHB and other chronic viral infections is not fully clear and further analyses are required (9, 10). In vitro Treg depletion can restore the functional activity of virus-specific CTLs (11, 12). In addition, Tregs are expanded in severe CHB, but correlate with serum viral load rather than impaired HBV-specific immune responses (6, 7). In this study, Germanidis et al. proposed that FoxP3+ Tregs might be expanded non-specifically in response to chronic liver inflammation rather than as HBV-specific Tregs (1). In a previous study, the same group reported that liver FoxP3 expression is closely linked to liver inflammation regardless of the underlying cause; viral, toxic, or autoimmunity (13). The current findings confirm FoxP3 is strongly correlated with inflammation in CHB, in addition to PD-1, PD-L1, and CD8. FoxP3 expression also correlated closely with serum viral load, ALT, and AST (markers of liver injury). Apoptosis-induced inflammation is observable in CHB and other liver diseases due to sustained liver injury (14), and Tregs could prevent catastrophic pathology due to apoptosis-induced inflammation. During inflammatory expansion, HBV-specific Tregs might also be generated in response to HBsAg on infected hepatocytes explaining differing reports regarding HBV-specific and non-specific Tregs from CHB patients.
FoxP3 is essential for Treg development and it is used as the hallmark marker to identify Tregs in most studies. However, FoxP3 can be upregulated on non-suppressive Teff during inflammation and activation. More specific Treg markers have been identified, as recently reviewed (15). Therefore, more robust phenotypic and functional identification of intra-hepatic suppressive Treg subsets is critical, and future studies should utilize robust markers, in vitro suppression assays or TSDR methylation to confirm the suppressive Treg status.
This study also reported a positive correlation of PD-1, PD-L1, and the apoptotic mediators FAS and FAS-L with inflammation intensity (1). The PD-1/PD-L1 pathway is prominent in immune tolerance by promoting Treg development and T cell dysfunction (16). PD-1 and PD-L1 are upregulated, respectively, on “exhausted” CTLs in various pathological settings and on virally infected hepatocytes (17, 18). PD-1 upregulation has been characterized during chronic liver inflammation and, similar to the proposed mechanism for Treg expansion, upregulation of the PD-1/PD-L1 pathway might represent a protective mechanism against chronic inflammation in CHB (17). Tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL) was upregulated during remission and negatively correlated with inflammation. This might be representative of enhanced hepatocyte destruction during inflammation. Further investigation into TRAIL expression in CHB and chronic viral inflammation is needed. The immunosuppressive mechanisms in CHB active disease and their resolution upon remission are summarized in Figure 1.
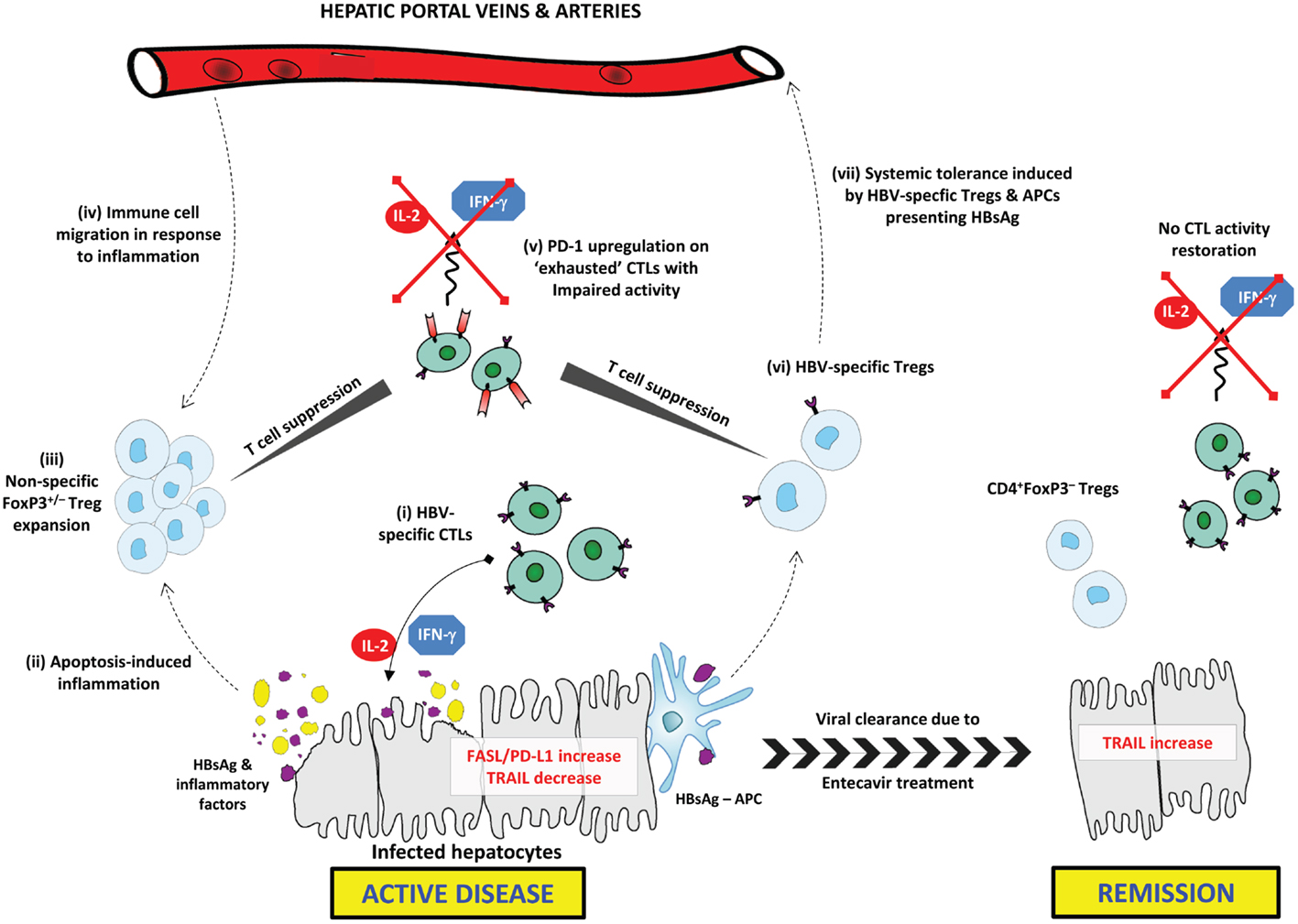
Figure 1. Immunosuppressive mechanisms in CHB active disease and their resolution upon remission. In active CHB disease, FAS-L and PD-L1 expression are increased on hepatocytes, while TRAIL is down-regulated. (i) HBsAg-specific CTLs secrete effector cytokines (IL-2 and IFN-γ) inducing apoptosis in infected hepatocytes expressing HBsAg. (ii) Excessive apoptosis and pro-inflammatory cytokine secretion induces chronic inflammation, and the release of inflammatory factors. HBsAg is released from lysed hepatocytes. (iii) Tregs are expanded in response to inflammatory factors; this may include both FoxP3+/− Treg subsets. Tregs exert non-specific CTL suppression. (iv) Circulating Tregs and other immune cells, including NK cells and MDSCs, migrate toward the site of inflammation contributing to impaired HBV-specific immune responses. (v) CTLs exhibit an “exhausted” anergic phenotype characterized by upregulated PD-1 expression. Exhausted CTLs are unable to exert any immune activity including secretion of effector cytokines. (vi) HBsAg-presenting APCs induce generation of HBV-specific Tregs that selectively suppress HBV-specific CTLs. (vii) Circulating HBV-specific Tregs and HBsAg–APCs are able to induce systemic tolerance to HBsAg, thus further delaying HBV clearance. Upon remission, FAS-L and PD-L1 expression are down-regulated, while TRAIL is upregulated. CD4+FoxP3– T cells may be expanded or persist following resolution of inflammation, while CTLs do not regain similar functional capacity to pre-infection.
It is important to take into account the significant heterogeneity of Treg subpopulations. Both FoxP3+ Tregs and FoxP3− Tregs, such as Tr1 and Th3, exist. Tissue-specific hepatic Tregs have also been described (19). Germanidis et al. note that in patients in remission, there is no corresponding decrease in CD4 with FoxP3 as might be expected with CD4+FoxP3+ Treg reduction. Peripheral Tregs (pTregs), as opposed to thymic Tregs (tTregs), are known to be expanded during chronic inflammation (20). Tregs expanded due to chronic inflammation could be FoxP3– pTregs in addition to FoxP3+ pTregs. Moreover, FoxP3 might also be reduced due to resolved inflammation.
The unique immune function of the liver should also be considered when investigating CHB immunobiology. Various subsets of immune cells continuously traffic into and out of the liver interacting with APCs, including Kupffer cells and LSECs. This complex network of APCs and immune-modulating cells maintain a tolerogenic environment and are able to induce systemic tolerance in response to intra-hepatic antigen presentation (21). Breaking liver-induced systemic HBV tolerance might be key to restoring effective anti-HBV immune responses.
The study by Germanidis et al. raises some interesting questions regarding immune regulation in CHB. What is the nature of TRAIL and the FAS/FAS-L pathway in HBV clearance? What is the role of other immune regulatory cells in CHB immune tolerance? Most importantly, are Tregs and immune-modulating pathways promoted as a result of chronic inflammation or the cause for delayed HBV clearance?
With regards to Tregs as a therapeutic target, further investigations are required. Tregs only comprise 1% intra-hepatic immune cells; expanded B regulatory cells, NK cells, and myeloid-derived suppressor cells have all been characterized in CHB and CHC (8). Immunomodulation could provide a novel therapeutic approach for CHB and chronic viral infections. Interestingly, antibody blockade of the PD-1/PD-L1 pathway restored the activity of intra-hepatic CTLs in vitro (18, 22). However, significant challenges remain in balancing immune activation to clear HBV infection without inducing further liver injury (23, 24).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Germanidis G, Argentou N, Hytiroglou P, Vassiliadis T, Patsiaoura K, Germenis AE, et al. Liver FOXP3 and PD1/PDL1 expression is down-regulated in chronic HBV hepatitis on maintained remission related to the degree of inflammation. Front Immunol (2013) 4:207. doi:10.3389/fimmu.2013.00207
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol (2006) 87:1439–49. doi:10.1099/vir.0.81920-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Fletcher SP, Delaney WE. New therapeutic targets and drugs for the treatment of chronic hepatitis B. Semin Liver Dis (2013) 33:130–7. doi:10.1055/s-0033-1345713
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology (2009) 49:S72–84. doi:10.1002/hep.22884
5. Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther (2010) 15:133–43. doi:10.3851/IMP1497
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. El-Badawy O, Sayed D, Badary MS, Abd-Alrahman ME, El-Feky MA, Thabit AG. Relations of regulatory T cells with hepatitis markers in chronic hepatitis B virus infection. Hum Immunol (2012) 73:335–41. doi:10.1016/j.humimm.2012.01.014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect Dis (2007) 7:804–13. doi:10.1016/S1473-3099(07)70289-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Wang L, Qiu J, Yu L, Hu X, Zhao P, Jiang Y. Increased numbers of CD5+CD19+CD1dhighIL-10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med (2014) 12:251. doi:10.1186/s12967-014-0251-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol (2008) 82:5043–53. doi:10.1128/JVI.01548-07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol (2008) 82:21–30. doi:10.1128/JVI.01768-07
11. Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, et al. Circulating and liver resident CD4+CD25+ regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol (2006) 177:739–47. doi:10.4049/jimmunol.177.1.739
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Zhang HH, Mei MH, Fei R, Liu F, Wang JH, Liao WJ, et al. Regulatory T cells in chronic hepatitis B patients affect the immunopathogenesis of hepatocellular carcinoma by suppressing the anti-tumour immune responses. J Viral Hepat (2010) 17(Suppl 1):34–43. doi:10.1111/j.1365-2893.2010.01269.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Speletas M, Argentou N, Germanidis G, Vassiliadis T, Mantzoukis K, Patsiaoura K, et al. Foxp3 expression in liver correlates with the degree but not the cause of inflammation. Mediators Inflamm (2011) 2011:827565. doi:10.1155/2011/827565
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut (2005) 54:1024–33. doi:10.1136/gut.2004.053850
15. Chaudhary B, Abd Al Samid M, al-Ramadi BK, Elkord E. Phenotypic alterations, clinical impact and therapeutic potential of regulatory T cells in cancer. Expert Opin Biol Ther (2014) 14:931–45. doi:10.1517/14712598.2014.900539
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev (2010) 236:219–42. doi:10.1111/j.1600-065X.2010.00923.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology (2009) 50:1625–37. doi:10.1002/hep.23173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Watanabe T, Bertoletti A, Tanoto TA. PD-1/PD-L1 pathway and T-cell exhaustion in chronic hepatitis virus infection. J Viral Hepat (2010) 17:453–8. doi:10.1111/j.1365-2893.2010.01313.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Lehtimaki S, Lahesmaa R. Regulatory T cells control immune responses through their non-redundant tissue specific features. Front Immunol (2013) 4:294. doi:10.3389/fimmu.2013.00294
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity (2009) 30:626–35. doi:10.1016/j.immuni.2009.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol (2013) 10:292–302. doi:10.1038/cmi.2013.7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology (2010) 138:682–93. doi:10.1053/j.gastro.2009.09.052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Oo YH, Sakaguchi S. Regulatory T-cell directed therapies in liver diseases. J Hepatol (2013) 59:1127–34. doi:10.1016/j.jhep.2013.05.034
24. Wang L, Zou ZQ, Liu CX, Liu XZ. Immunotherapeutic interventions in chronic hepatitis B virus infection: a review. J Immunol Methods (2014) 407:1–8. doi:10.1016/j.jim.2014.04.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: immunosuppressive, Foxp3, PD1PD-L1, chronic HBV, downregulation
Citation: Chaudhary B and Elkord E (2015) Downregulation of immunosuppressive environment in patients with chronic HBV hepatitis on maintained remission. Front. Immunol. 6:52. doi: 10.3389/fimmu.2015.00052
Received: 29 December 2014; Paper pending published: 19 January 2015;
Accepted: 27 January 2015; Published online: 11 February 2015.
Edited by:
Stephen Paul Cobbold, University of Oxford, UKReviewed by:
Paul Klenerman, University of Oxford, UKCopyright: © 2015 Chaudhary and Elkord. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:ZS5lbGtvcmRAc2FsZm9yZC5hYy51aw==;ZWVsa29yZEB1YWV1LmFjLmFl
 Belal Chaudhary
Belal Chaudhary Eyad Elkord
Eyad Elkord