- Institute of Experimental Immunology, Rheinische Friedrich-Wilhelms-University of Bonn, Bonn, Germany
New vaccination strategies focus on achieving CD8+ T cell (CTL) immunity rather than on induction of protective antibody responses. While the requirement of CD4+ T (Th) cell help in dendritic cell (DC) activation and licensing, and in CTL memory induction has been described in several disease models, CTL responses may occur in a Th cell help-independent manner. Invariant natural killer T cells (iNKT cells) can substitute for Th cell help and license DC as well. iNKT cells produce a broad spectrum of Th1 and Th2 cytokines, thereby inducing a similar set of costimulatory molecules and cytokines in DC. This form of licensing differs from Th cell help by inducing other chemokines, while Th cell-licensed DCs produce CCR5 ligands, iNKT cell-licensed DCs produce CCL17, which attracts CCR4+ CD8+ T cells for subsequent activation. It has recently been shown that iNKT cells do not only enhance immune responses against bacterial pathogens or parasites but also play a role in viral infections. The inclusion of iNKT cell ligands in influenza virus vaccines enhanced memory CTL generation and protective immunity in a mouse model. This review will focus on the role of iNKT cells in the cross-talk with cross-priming DC and memory CD8+ T cell formation.
Classification of Natural Killer T Cells
Natural killer T cells (NKT cells) are a subset of lymphocytes with innate and adaptive immune functions, for example, in tumor and anti-infectious defense (1). Their TCR can be either semi-invariant and encoded by a germline Valpha gene [type I invariant natural killer T cells (iNKT cells)] or may react against the self-antigen sulphatide using an oligoclonal TCR (type II NKT cells) (2–4). This review focusses on iNKT cells in dendritic cell (DC) licensing and T cell activation leading to a sustained memory response.
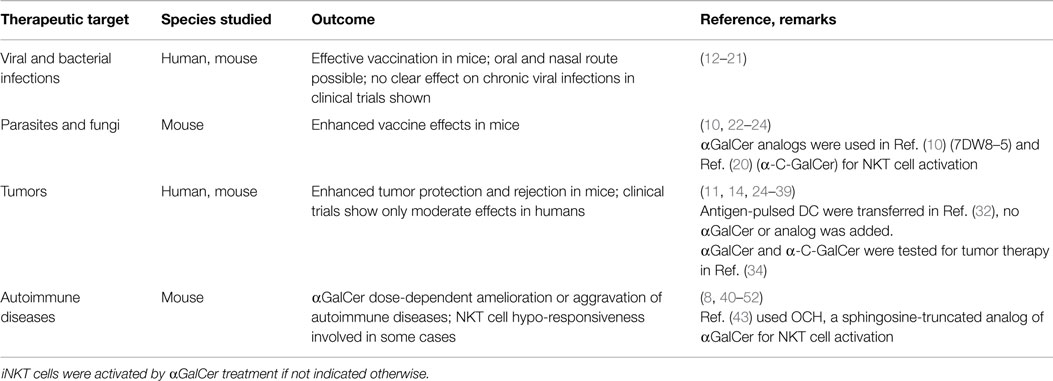
Invariant natural killer T cells respond to the marine sponge (Agelas mauritianus)-derived glycolipid alpha-galactosylceramide (αGalCer) presented by the non-polymorphic CD1d molecule and respond by rapidly producing various cytokines (5, 6). Mostly studied in mice, they represent about 0.5% of T cells in the blood, 2% in secondary lymphatic organs, and over 30% of T cells in the liver. During inflammation and infection, iNKT cell numbers can strongly increase in numerous organs, e.g., the pancreas in type I diabetes or the lung in asthma (7, 8). In human blood, only 0.1–0.2% of T cells are iNKT cells, with 5× lower numbers than in mice (9). Recently, iNKT cells came into focus as promising targets for the development of vaccine adjuvants and immunotherapies, mostly in the field of cancer treatment and in autoimmune and inflammatory diseases (Table 1). Preclinical studies using αGalCer demonstrated moderate therapeutic activity by activating DCs and providing Th-like functions, generating CD8+ cytotoxic T cell (CTL) and antibody responses. Currently, more potent αGalCer analogs for iNKT cell activation are under investigation (10–13). Applying NKT cell immunization schemes in clinical settings is a promising therapeutic opportunity, but requires detailed knowledge on how iNKT cells activate DCs.
iNKT Cell Activation, Subsets, and Cytokine Production
Most knowledge on NKT cell activation came from the use of αGalCer, a strong and prototypical CD1-restricted agonist. In the last years, additional microbial-derived glycolipid ligands were identified, including α-glucuronosylceramides (from Sphingomonas), cholesteryl α-glucoside (from Helicobacter), or diacylglycerol-containing glycolipids (from Borrelia) (53, 54). These lead to sustained iNKT cell activation with inflammatory cytokine production that is independent of TLR stimulation, IL-12, or the recognition of endogenous antigens, hence relying only on engaging the invariant TCR. α-glucuronosylceramide induces IFNγ and IL-4 release similar to αGalCer (55–57). Both glycolipid antigens are structurally similar and can be recognized by the majority of mouse and human iNKT cells (58). Synthetic iNKT cell antigens have been and continue to be studied extensively for potential therapeutic application (59). However, iNKT cell activation may also promote allergic airway inflammation, and their overstimulation can induce iNKT cell anergy (1, 60).
Most microorganisms lack cognate iNKT cell antigens, hence activation of these cells relies on cytokines, such as IL-12 or IL-18, in conjunction with endogenous antigens. Even in the absence of TCR stimulation, some bacterial and viral infections induce a robust IL-12 response by DCs thereby activating iNKT cells in vivo (61, 62). Indirect iNKT cell activation results in the release of IFNγ but usually not IL-4 and is not restricted to TLR (62–65).
Analogous to Th cells subsets, different NKT cell subsets termed NKT1, NKT2, NKT17, NKTFH, and NKT10 subsets were described with corresponding functionalities (66, 67). NKT17 cells produce the cytokines, IL-17 and IL-22, and are abundant in the lymph nodes, lungs, and skin of mice with airway neutrophilia induced by αGalCer (68). Recently, it was shown that iNKT17 cells are enriched in NOD mice, a mouse model for type I diabetes, which hint toward a possible role of those cells in disease development (69). iNKT17 cells rely on IL-7 for homeostasis and survival (70) and seem to require activation in the presence of TGF-β and IL-1β (71). The recently described NKT10 subset can dampen inflammatory responses by IL-10 production and is enriched in adipose tissue, providing protection in obesity-induced inflammation (72).
Dendritic Cell Maturation and CD8+ T Cell Cross-Priming
Dendritic cells classically gather antigens in tissues and transport them into lymphatic organs, where they orchestrate the activation and differentiation of naïve CD8+ T cells into CTL. Recent work showed that some DCs remain in tissues in order to regulate immigrating effector T cell responses, which is important in the defense against infections and may also promote the progression of many immune-mediated diseases. The cross-talk of myeloid cells with other immune cells, such as T cells and innate lymphocytes, is especially important in this context. Cellular encounters are orchestrated by chemokines, cytokines, and cell surface molecules. Some DCs, especially the XCR1+ DC subset, are specialized in cross-presentation, which allows the presentation of extracellular antigens to activate CTL, a process important for immunity against tumors, viruses, and intracellular bacteria and for vaccination (73–76). Immunogenic cross-presentation, also referred to as cross-priming, requires the presence of pathogen-derived molecules (PAMPs) and/or of specific Th cells or NKT cells that mature the cross-presenting DC (77). This process is called “licensing,” a term introduced by Lanzavecchia (78), and it aims at preventing unwanted immune answers against innocuous or self antigens. Licensing was first described by Matzinger, Heath, and Melief (79–81), and classically is mediated by CD40 ligand provided by specific CD4+ helper T cells (Th). In addition to licensing, immunogenic T cell priming requires the DCs to mature, a process that results from sensing various PAMPs, including ligands for TLR, lectins, intracellular nucleotide-binding oligomerization domain receptors, or retinoic acid-induced genes (82–85). Major consequences of DC maturation are the upregulation of costimulatory molecules like CD80 and CD86, CD40, of MHC II and the production of pro-inflammatory cytokines, especially IL-12p70 and TNF. These consequences partially can result also from CD40–CD40L interactions, but it is not clearly defined how much DC licensing and maturation functionally overlap. CD40–CD40L interactions are not only crucial for upregulation of costimulatory molecules but also for DC survival (86). Additionally, mature DCs produce chemokines to attract other immune cells and to orchestrate the ongoing immune response. In contrast to maturation-induced upregulation of MHC II, CD1 trafficking is differentially regulated during DC maturation, and CD1 molecules are already expressed on immature DCs. While human DCs express all classes of CD1 molecules, murine DCs express only CD1d (87), which is crucial for DC–iNKT cell interactions. Trafficking studies showed that antigen presentation by CD1d to iNKT cells might already occur before DC maturation and MHC II presentation (88). This notion hinted to a possible role of iNKT cells as immunological helper cells.
iNKT Cells as Immunological Helper Cells
αGalCer was found to mediate CD40-dependent activation of CTL by NKT cell-helped DC (89), directing attention to the adjuvant activity for this agent. Furthermore, αGalCer also induced resistance to tumors and intracellular pathogens (25). Compared to CD40 ligation, LPS, and CpG, αGalCer induced equally high levels of CD40, CD80, CD86, MHC II, and DEC205 in CD11c+ CD8a+ and CD11c+ CD8a− DCs, but was unable to induce DC maturation from bone marrow progenitors. Rather than acting directly on DCs, αGalCer mediated DC maturation through iNKT cells in a MyD88-independent manner. Combining αGalCer with CD40 stimulation caused DC to produce high amounts of IL12p70, while LPS and CD40 stimulation showed no such effect. IL12p70 production might explain the results of another study (90), where the simultaneous administration of OVA and αGalCer enhanced Th and CTL responses in an iNKT cell-dependent manner. A close temporal association between αGalCer and OVA-derived peptides and additional experiments with antigen-loaded DCs led to the conclusion that αGalCer and peptides must reach the same DC. Formal in vivo evidence for such a tripartite cellular interaction was provided with the use of bm1/CD1d bone marrow chimeras (91). In addition, there was synergy when Th and iNKT help were combined. The means by which iNKT cells license DCs are not fully understood but in addition to providing CD40L to DCs, iNKT cells may act by promoting cross-talk of XCR1+ DCs and plasmacytoid DC (92) or by abundant cytokine production upon activation. Whether iNKT cells play a role as helper cells when activated by less potent ligands remains to be elucidated.
iNKT Cells Help in CTL and CD8+ Memory T Cell Formation
The knowledge on mechanisms iNKT cells use to substitute CD4+ T cell help for antibody production, CTL generation, or memory formation is central for developing new vaccination strategies. An unresolved question is why some groups observed NKT cell-dependent reduction of CTL-mediated autoimmune diseases, whereas NKT cell-licensed DC induced strong CTL responses against tumors and viruses in other studies. The most obvious difference is the use of a single low dose of αGalCer for induction of protective CTL responses and the use of multiple doses or high single doses of αGalCer to inhibit unwanted T cell responses (93). In some clinical trials, αGalCer was used to treat cancer, and human CD4+ iNKT cells expanded predominantly during early stages (26). CD4+ iNKT cells can induce IL12p70 production by DC and thereby Th1 polarization (93). Double negative (DN) iNKT cells expanded later after αGalCer treatment and can induce apoptosis in αGalCer-loaded DC, thereby limiting the immune response (26). Functional differences between iNKT cell subsets in regards to cytokine production are evident both in mice and humans (94), but the effects on DC maturation, apoptosis, and CTL generation remain to be elucidated (Figure 1). A high frequency of a DN iNKT cell subset and their potential to lyse DCs may impair treatment of cancer patients and vaccination strategies. The role of iNKT cells during viral infections and the use of αGalCer as vaccine adjuvant in the context of influenza infections have been reviewed recently in Ref. (14, 95). αGalCer increased the levels of influenza-specific systemic IgG and mucosal IgA antibodies, even in the absence of Th cells and antigen-specific CTL responses (14, 95). In contrast, after combined iNKT cell activation and influenza virus vaccination, an impaired CTL response but enhanced memory CTL generation was seen (96). In line with this, enhanced CTL memory differentiation during viral infection was also shown previously (97). Another study showed that iNKT cell enrichment in the CNS during Theiler’s murine encephalomyelitis virus (TEMV) infection inhibited the antiviral CTL response and delayed the accumulation of TEMV-specific CTL. Also, the magnitude of the TEMV-specific CTL response was impaired (98). CTL memory formation was not assessed in that study. Co-administration of αGalCer with suboptimal doses of irradiated sporozoites or recombinant viruses expressing a malaria antigen enhanced protective anti-malaria immunity in mice, and co-administration of αGalCer with various immunogens enhanced antigen-specific CTL responses and Th1 responses (99). In conclusion, vaccination with αGalCer as adjuvant induced iNKT cell help for DCs, which promoted CTL memory formation but impaired primary antigen-specific CTL responses.
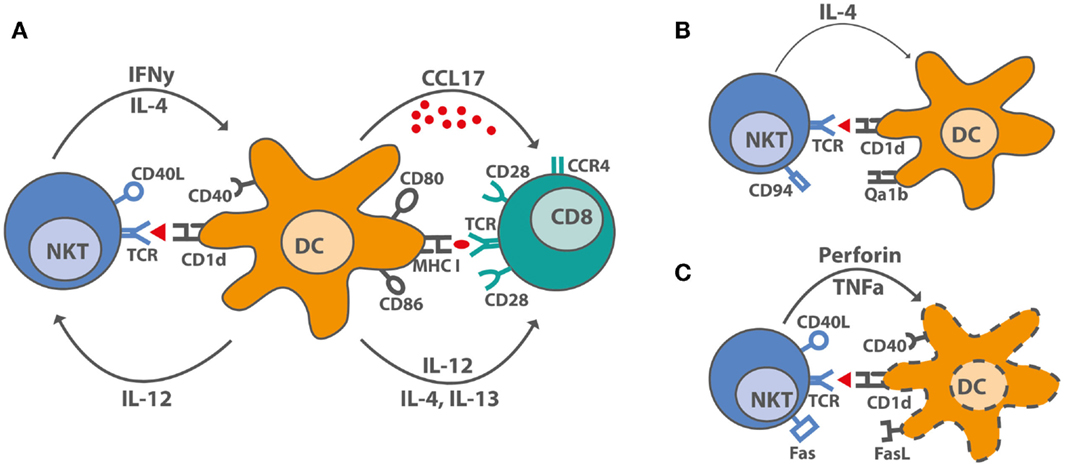
Figure 1. iNKT cell–DC interactions after stimulation with αGalCer. (A) Under optimal stimulatory conditions, iNKT cells produce IL-4, large amounts of IFNγ and upregulate CD40L, thereby inducing maturation in DC. DC maturation leads to increased costimulatory capacity through upregulation of CD80 and CD86, of MHC molecules, and by producing the pro-inflammatory cytokine, IL-12, and the chemokine, CCL17. CCL17 attracts CCR4+ cells, including CD8+ T cells, which can be activated by the licensed DC. (B) Overstimulated iNKT cells upregulate inhibitory receptors like CD94 and are incapable of producing IFNγ. DC interacing with hyporesponsive iNKT cells cannot be activated and do not induce CD8+ T cell activation. (C) Some activated iNKT cells induce DC lysis rather than maturation by yet unknown mechanisms. Proposed mechanisms suggest a role for TNFα, perforin, Fas–FasL interactions, and even CD40–CD40L.
Before we can fully understand the mechanisms of iNKT cell help in CTL formation and memory generation, it is crucial to know how “help” influences CTL responses in general. Many reports about Th help are available and most of them show a diverse picture of the requirement for CD4+ T cell help in primary and/or secondary infections. Th help seems to be crucial for the clearance of some primary virus infections like HSV or influenza that do not affect DCs directly (100, 101), while in some viral infections, Th help can be overcome (102). Additionally, CTL responses against minor H antigens, soluble proteins, tumors, and peptide-pulsed DC require Th help for the induction of optimal primary responses (79, 103, 104). Some groups disagreed whether Th help was needed during secondary responses for proper re-expansion of CTL (105, 106) or whether Th help was merely a prerequisite during primary infections for CTL memory formation (107). Additionally, Th help was dispensable for the expansion, but not for the cytotoxic capacity of CTL in tuberculosis (108).
These differential observations may be explained not only by the variance of pathogens and model antigens used but also by different experimental setups. Moreover, Th dependency was studied by using CD4−/− mice, MHC II−/− mice, or CD4-depleting antibodies, which are not biologically equivalent (109). For example, CD4-depleting antibodies also deplete regulatory T cells, CD4+ NKT cells, and CD4+ DCs. However, most older studies agreed that the requirement for Th help is not a CTL-intrinsic property but dependent on the infectious agent and DC maturation. Given the huge discrepancies in studies on Th help requirements, observations in a single model do not permit general conclusions on how CD4+ help may be substituted by iNKT cells. A deeper insight into CTL generation and memory formation is required to allow predictions for the role of iNKT cell help in CTL responses.
As reviewed previously in Ref.(110), CTL in primary responses can be divided into short-lived effector cells (SLEC) that mostly die off during the contraction phase and memory precursor cells (MPEC) that received less stimulation but more survival signals (111, 112). Even a single naive CTL can differentiate into a diverse population of effector and memory cells (113, 114) by multiple mechanisms, which have been reviewed in detail by Kaech and Cui (115). Prolonged antigen exposure and pro-inflammatory cytokines like IL-12 and IL-2 promote terminal differentiation of CTL and induce superior cytotoxic capacities (116–118). NKT cells may affect CTL differentiation other than Th cells, but this hypothesis requires further experimental exploration.
iNKT Cells and Chemokines in CTL and CD8+ T Cell Memory Formation
Chemokines play a major role in orchestrating primary and memory CTL responses. During infections, CTL upregulated CXCR3, which allowed them to enter peripheral tissues (119). Th help was required for enhanced recruitment of CTL to the site of infection in some situations (120) by promoting CXCL9 and CXCL10 production, with CXCL9 being especially important for rapid memory responses in the lymph node (121). Infections of the lung and intestine showed no requirement of Th help for migration as lung infections, e.g., by influenza induce on-site proliferation of CTL rather than recruitment (122, 123) but Th cells promoted development of lung-resident memory cells (124).
CXCR3 also drove CTL toward an effector fate rather than memory fate (125). In line with this, CXCR3 × CCR5 double-deficient mice showed a decreased contraction phase and harbored more memory CTL, which were unable to migrate into tissues and to clear infections (126). In humans, CCR5 expression was associated with effector memory T cells, whereas CCR7 was predominantly expressed on naïve and central memory T cells and CCR6 expression was found on early effector memory T cells (127–129).
iNKT cell-helped DCs produced high amounts of CCL17, thereby attracting CCR4+ lymphocytes (91). This contrasts the situation in classical Th cell-dependent cross-priming, where DCs produced CCR5 ligands to attract CTL for cross-priming. These chemokines synergically guided CTL toward those DCs that have presented relevant antigen to helper T cell subsets, and thereby facilitated the ensuing CTL response. Thus, CCR4- and CCR5-binding chemokines have been described as a new signal in T cell activation, distinct from signal 1, antigen, and signal 2, costimulation (130).
CCR4 is traditionally considered to be associated with skin homing Th2 and memory CD4+ T cells (131–134), but also with the recruitment of Treg to the inflamed liver (135). Several studies in humans showed increased CCR4 expression also on CTL in cutaneous diseases (136–138). A CCR4+ CD8+ central memory subset has been described that was generated in the presence of IL-4 and produced IL4 and IL-13 upon restimulation (139). These cells were not cytotoxic and produced little IFNγ, features associated with a so-called Tc2 subset (139, 140). Kondo and Takiguchi showed that human CCR4+ CD8+ T cells expressed less effector molecules like perforin or granzymes compared to CCR6+ early effector memory T cells, but produced more TNFα and IL-4 than CCR7+ naïve or central memory CD8+ T cells. They concluded that CCR4+CD8+ T cells are a “little more differentiated than CCR7+ central memory ones and less differentiated than CCR6+ early effector memory ones” and that they can migrate into secondary lymphoid organs where they mature after interacting with DCs expressing CCR4 ligand (141). Since iNKT cells produce IL-4 upon activation and induce CCL17 production by helped DC, they might play a role in the development or restimulation of CCR4+ CD8+ T cells. The physiological role of this subset in viral infections and tumors remains to be elucidated.
Concluding Remarks
CD4-helped DCs and NKT cell-helped DCs provide various costimulatory signals and cytokines deciding the fate of CD8+ T cells toward effector or memory. However, the set of chemokines produced by NKT cell-helped DCs attract different subsets of naïve or memory CD8+ T cells compared to chemokines produced by Th-helped DCs. Dissecting the role of those CD8+ T cells subsets in effector and memory responses directed against tumors and viral infections may facilitate developing effective NKT cell-based vaccines.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Kristina Koch for help with artwork. This work was supported by the Deutsche Forschungsgemeinschaft (grants SFB704 and 645). EM is supported by the German National Academic Foundation. CK is a member of the Excellence-Cluster ImmunoSensation.
References
1. Parekh VV, Wilson MT, Van Kaer L. iNKT-cell responses to glycolipids. Crit Rev Immunol (2005) 25(3):183–213. doi: 10.1615/CritRevImmunol.v25.i3.20
2. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol (2007) 25:297–336. doi:10.1146/annurev.immunol.25.022106.141711
3. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest (2004) 114(10):1379–88. doi:10.1172/JCI23594
4. Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology (2014) 142(3):321–36. doi:10.1111/imm.12247
5. Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science (1997) 277(5324):339–45. doi:10.1126/science.277.5324.339
6. Porcelli SA. The CD1 family: a third lineage of antigen-presenting molecules. Adv Immunol (1995) 59:1–98. doi:10.1016/S0065-2776(08)60629-X
7. Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med (2003) 9(5):582–8. doi:10.1038/nm851
8. Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med (2001) 7(9):1057–62. doi:10.1038/nm0901-1057
9. Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med (2002) 195(5):637–41. doi:10.1084/jem.20011908
10. Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U S A (2010) 107(29):13010–5. doi:10.1073/pnas.1006662107
11. Faveeuw C, Trottein F. Optimization of natural killer T cell-mediated immunotherapy in cancer using cell-based and nanovector vaccines. Cancer Res (2014) 74(6):1632–8. doi:10.1158/0008-5472.CAN-13-3504
12. Carreno LJ, Kharkwal SS, Porcelli SA. Optimizing NKT cell ligands as vaccine adjuvants. Immunotherapy (2014) 6(3):309–20. doi:10.2217/imt.13.175
13. Padte NN, Li X, Tsuji M, Vasan S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin Immunol (2011) 140(2):142–51. doi:10.1016/j.clim.2010.11.009
14. Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Alpha-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol (2005) 175(5):3309–17. doi:10.4049/jimmunol.175.5.3309
15. Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun (2002) 70(11):6302–9. doi:10.1128/IAI.70.11.6302-6309.2002
16. Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine (2008) 26(15):1807–16. doi:10.1016/j.vaccine.2008.02.002
17. Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med (2000) 192(7):921–30. doi:10.1084/jem.192.7.921
18. Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, Franck RW, et al. Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine (2009) 27(28):3766–74. doi:10.1016/j.vaccine.2009.03.090
19. Veldt BJ, van der Vliet HJ, von Blomberg BM, van Vlierberghe H, Gerken G, Nishi N, et al. Randomized placebo controlled phase I/II trial of alpha-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol (2007) 47(3):356–65. doi:10.1016/j.jhep.2007.04.018
20. Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, Scheper RJ, et al. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled phase I/II trial. Antivir Ther (2009) 14(6):809–18. doi:10.3851/1295
21. Youn HJ, Ko SY, Lee KA, Ko HJ, Lee YS, Fujihashi K, et al. A single intranasal immunization with inactivated influenza virus and alpha-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine (2007) 25(28):5189–98. doi:10.1016/j.vaccine.2007.04.081
22. Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, et al. Alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci U S A (2000) 97(15):8461–6. doi:10.1073/pnas.97.15.8461
23. Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, et al. Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun (2001) 69(1):213–20. doi:10.1128/IAI.69.1.213-220.2001
24. Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med (2003) 198(11):1631–41. doi:10.1084/jem.20031192
25. Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med (2003) 198(2):267–79. doi:10.1084/jem.20030324
26. Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med (2005) 201(9):1503–17. doi:10.1084/jem.20042592
27. Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med (2002) 196(1):119–27. doi:10.1084/jem.20020092
28. Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res (2002) 8(12):3702–9.
29. Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res (2005) 11(5):1910–7. doi:10.1158/1078-0432.CCR-04-1453
30. Kim D, Hung CF, Wu TC, Park YM. DNA vaccine with alpha-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine (2010) 28(45):7297–305. doi:10.1016/j.vaccine.2010.08.079
31. Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM, et al. Alpha-galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer (2008) 122(12):2774–83. doi:10.1002/ijc.23444
32. Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res (1995) 7(10–11):529–34.
33. Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci (2009) 100(6):1092–8. doi:10.1111/j.1349-7006.2009.01135.x
34. Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol (2009) 182(4):2492–501. doi:10.4049/jimmunol.0800126
35. Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood (2004) 103(2):383–9. doi:10.1182/blood-2003-04-1155
36. Shibolet O, Alper R, Zlotogarov L, Thalenfeld B, Engelhardt D, Rabbani E, et al. NKT and CD8 lymphocytes mediate suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells. Int J Cancer (2003) 106(2):236–43. doi:10.1002/ijc.11201
37. Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest (2004) 114(12):1800–11. doi:10.1172/JCI22046
38. Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res (2007) 67(15):7495–504. doi:10.1158/0008-5472.CAN-07-0941
39. Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother (2008) 57(3):337–45. doi:10.1007/s00262-007-0373-5
40. Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med (2001) 7(9):1052–6. doi:10.1038/nm0901-1052
41. Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, et al. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med (2001) 194(12):1789–99. doi:10.1084/jem.194.12.1789
42. Naumov YN, Bahjat KS, Gausling R, Abraham R, Exley MA, Koezuka Y, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci U S A (2001) 98(24):13838–43. doi:10.1073/pnas.251531798
43. Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med (2001) 194(12):1801–11. doi:10.1084/jem.194.12.1801
44. Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med (2001) 194(3):313–20. doi:10.1084/jem.194.3.313
45. Furlan R, Bergami A, Cantarella D, Brambilla E, Taniguchi M, Dellabona P, et al. Activation of invariant NKT cells by alphaGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-gamma. Eur J Immunol (2003) 33(7):1830–8. doi:10.1002/eji.200323885
46. Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest (2003) 112(8):1211–22. doi:10.1172/JCI17165
47. Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum (2004) 50(1):305–13. doi:10.1002/art.11489
48. Chiba A, Kaieda S, Oki S, Yamamura T, Miyake S. The involvement of V(alpha)14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum (2005) 52(6):1941–8. doi:10.1002/art.21056
49. Kim HY, Kim HJ, Min HS, Kim S, Park WS, Park SH, et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor beta1 production. J Exp Med (2005) 201(1):41–7. doi:10.1084/jem.20041400
50. Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, et al. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J Immunol (2005) 175(6):3648–55. doi:10.4049/jimmunol.175.6.3648
51. Major AS, Singh RR, Joyce S, Van Kaer L. The role of invariant natural killer T cells in lupus and atherogenesis. Immunol Res (2006) 34(1):49–66. doi:10.1385/IR:34:1:49
52. Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, et al. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum (2007) 56(4):1219–33. doi:10.1002/art.22490
53. Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol (2011) 12(10):966–74. doi:10.1038/ni.2096
54. Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest (2011) 121(1):57–69. doi:10.1172/JCI44845
55. Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature (2005) 434(7032):520–5. doi:10.1038/nature03407
56. Mattner J, Debord KL, Ismail N, Goff RD, Cantu C III, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature (2005) 434(7032):525–9. doi:10.1038/nature03408
57. Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol (2006) 7(9):978–86. doi:10.1038/ni1380
58. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science (1997) 278(5343):1626–9. doi:10.1126/science.278.5343.1626
59. Mallevaey T, Selvanantham T. Strategy of lipid recognition by invariant natural killer T cells: ‘one for all and all for one’. Immunology (2012) 136(3):273–82. doi:10.1111/j.1365-2567.2012.03580.x
60. Oki S, Miyake S. Invariant natural killer T (iNKT) cells in asthma: a novel insight into the pathogenesis of asthma and the therapeutic implication of glycolipid ligands for allergic diseases. Allergol Int (2007) 56(1):7–14. doi:10.2332/allergolint.R-06-137
61. Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol (2003) 4(12):1230–7. doi:10.1038/ni1002
62. Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog (2008) 4(7):e1000106. doi:10.1371/journal.ppat.1000106
63. Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol (2008) 181(7):4452–6. doi:10.4049/jimmunol.181.7.4452
64. Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A (2007) 104(51):20490–5. doi:10.1073/pnas.0710145104
65. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity (2007) 27(4):597–609. doi:10.1016/j.immuni.2007.08.017
66. Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med (2005) 202(9):1279–88. doi:10.1084/jem.20050953
67. Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol (2013) 14(11):1146–54. doi:10.1038/ni.2731
68. Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med (2007) 204(5):995–1001. doi:10.1084/jem.20061551
69. Li S, Joseph C, Becourt C, Klibi J, Luce S, Dubois-Laforgue D, et al. Potential role of IL-17-producing iNKT cells in type 1 diabetes. PLoS One (2014) 9(4):e96151. doi:10.1371/journal.pone.0096151
70. Webster KE, Kim HO, Kyparissoudis K, Corpuz TM, Pinget GV, Uldrich AP, et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol (2014) 7(5):1058–67. doi:10.1038/mi.2013.122
71. Monteiro M, Almeida CF, Agua-Doce A, Graca L. Induced IL-17-producing invariant NKT cells require activation in presence of TGF-beta and IL-1beta. J Immunol (2013) 190(2):805–11. doi:10.4049/jimmunol.1201010
72. Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity (2012) 37(3):574–87. doi:10.1016/j.immuni.2012.06.016
73. Schoenberger SP, van der Voort EI, Krietemeijer GM, Offringa R, Melief CJ, Toes RE. Cross-priming of CTL responses in vivo does not require antigenic peptides in the endoplasmic reticulum of immunizing cells. J Immunol (1998) 161(8):3808–12.
74. Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature (1999) 398(6722):77–80. doi:10.1038/18038
75. den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med (2000) 192(12):1685–96. doi:10.1084/jem.192.12.1685
76. Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol (2010) 10(6):403–14. doi:10.1038/nri2780
77. Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol (2004) 5(11):1143–8. doi:10.1038/ni1129
79. Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med (1997) 186(1):65–70. doi:10.1084/jem.186.1.65
80. Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature (1998) 393(6684):474–8. doi:10.1038/30989
81. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature (1998) 393(6684):480–3. doi:10.1038/31002
82. Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science (2001) 293(5528):253–6. doi:10.1126/science.1062060
83. Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol (2003) 3(5):371–82. doi:10.1038/nri1086
84. Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol (2004) 22:33–54. doi:10.1146/annurev.immunol.22.012703.104558
85. Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity (2005) 22(4):507–17. doi:10.1016/j.immuni.2005.03.004
86. Miga AJ, Masters SR, Durell BG, Gonzalez M, Jenkins MK, Maliszewski C, et al. Dendritic cell longevity and T cell persistence is controlled by CD154-CD40 interactions. Eur J Immunol (2001) 31(3):959–65. doi:10.1002/1521-4141(200103)31:3<959::AID-IMMU959>3.0.CO;2-A
87. Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol (2007) 7(12):929–41. doi:10.1038/nri2191
88. Gelin C, Sloma I, Charron D, Mooney N. Regulation of MHC II and CD1 antigen presentation: from ubiquity to security. J Leukoc Biol (2009) 85(2):215–24. doi:10.1189/jlb.0308206
89. Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol (2000) 12(7):987–94. doi:10.1093/intimm/12.7.987
90. Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol (2003) 171(10):5140–7. doi:10.4049/jimmunol.171.10.5140
91. Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol (2010) 11(4):313–20. doi:10.1038/ni.1848
92. Shimizu K, Asakura M, Shinga J, Sato Y, Kitahara S, Hoshino K, et al. Invariant NKT cells induce plasmacytoid dendritic cell (DC) cross-talk with conventional DCs for efficient memory CD8+ T cell induction. J Immunol (2013) 190(11):5609–19. doi:10.4049/jimmunol.1300033
93. Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H, et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur J Immunol (2008) 38(4):1012–23. doi:10.1002/eji.200737838
94. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A (2008) 105(32):11287–92. doi:10.1073/pnas.0801631105
95. Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A (2007) 104(10):3984–9. doi:10.1073/pnas.0700191104
96. Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, Porcelli S, et al. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A (2009) 106(9):3330–5. doi:10.1073/pnas.0813309106
97. Reilly EC, Thompson EA, Aspeslagh S, Wands JR, Elewaut D, Brossay L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS One (2012) 7(5):e37991. doi:10.1371/journal.pone.0037991
98. Mars LT, Mas M, Beaudoin L, Bauer J, Leite-de-Moraes M, Lehuen A, et al. Invariant NKT cells regulate the CD8 T cell response during Theiler’s virus infection. PLoS One (2014) 9(1):e87717. doi:10.1371/journal.pone.0087717
99. Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med (2002) 195(5):617–24. doi:10.1084/jem.20011889
100. Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol (1991) 133(1):234–52. doi:10.1016/0008-8749(91)90194-G
101. Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(-/-) mice. J Virol (2000) 74(20):9762–5. doi:10.1128/JVI.74.20.9762-9765.2000
102. Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature (2003) 421(6925):852–6. doi:10.1038/nature01441
103. Husmann LA, Bevan MJ. Cooperation between helper T cells and cytotoxic T lymphocyte precursors. Ann N Y Acad Sci (1988) 532:158–69. doi:10.1111/j.1749-6632.1988.tb36335.x
104. Guerder S, Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J Exp Med (1992) 176(2):553–64. doi:10.1084/jem.176.2.553
105. Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med (1996) 183(5):2153–63. doi:10.1084/jem.183.5.2153
106. Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. CD8 lethargy in the absence of CD4 help. Eur J Immunol (2002) 32(8):2199–207. doi:10.1002/1521-4141(200208)32:8<2199::AID-IMMU2199>3.0.CO;2-L
107. Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol (2004) 173(2):969–75. doi:10.4049/jimmunol.173.2.969
108. Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol (2001) 167(12):6991–7000. doi:10.4049/jimmunol.167.12.6991
109. Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med (2004) 199(4):559–65. doi:10.1084/jem.20031961
110. Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity (2011) 35(2):161–8. doi:10.1016/j.immuni.2011.07.010
111. Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev (2006) 211:81–92. doi:10.1111/j.0105-2896.2006.00382.x
112. Parish IA, Kaech SM. Diversity in CD8(+) T cell differentiation. Curr Opin Immunol (2009) 21(3):291–7. doi:10.1016/j.coi.2009.05.008
113. Stemberger C, Neuenhahn M, Buchholz VR, Busch DH. Origin of CD8+ effector and memory T cell subsets. Cell Mol Immunol (2007) 4(6):399–405.
114. Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med (2010) 207(6):1235–46. doi:10.1084/jem.20091175
115. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol (2012) 12(11):749–61. doi:10.1038/nri3307
116. Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol (2003) 171(10):5165–71. doi:10.4049/jimmunol.171.10.5165
117. Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol (2007) 179(4):2074–81. doi:10.4049/jimmunol.179.4.2074
118. Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity (2010) 32(1):79–90. doi:10.1016/j.immuni.2009.11.012
119. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res (2011) 317(5):620–31. doi:10.1016/j.yexcr.2010.12.017
120. Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature (2009) 462(7272):510–3. doi:10.1038/nature08511
121. Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity (2013) 38(3):502–13. doi:10.1016/j.immuni.2012.11.012
122. Bedoui S, Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: a role for antigen presentation beyond lymphoid organs? Curr Opin Immunol (2011) 23(1):124–30. doi:10.1016/j.coi.2010.11.001
123. McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J Immunol (2009) 183(7):4177–81. doi:10.4049/jimmunol.0901109
124. Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity (2014) 41(4):633–45. doi:10.1016/j.immuni.2014.09.007
125. Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med (2011) 208(8):1605–20. doi:10.1084/jem.20102101
126. Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, et al. Inflammatory chemokine receptors regulate CD8(+) T cell contraction and memory generation following infection. J Exp Med (2011) 208(8):1621–34. doi:10.1084/jem.20102110
127. Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol (2002) 168(11):5538–50. doi:10.4049/jimmunol.168.11.5538
128. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol (2004) 22:745–63. doi:10.1146/annurev.immunol.22.012703.104702
129. Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol (2004) 34(4):999–1010. doi:10.1002/eji.200324478
130. Bousso P, Albert ML. Signal 0 for guided priming of CTLs: NKT cells do it too. Nat Immunol (2010) 11(4):284–6. doi:10.1038/ni0410-284
131. Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CGA. Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol (2000) 115(4):640–6. doi:10.1046/j.1523-1747.2000.00115.x
132. Nakatani T, Kaburagi Y, Shimada Y, Inaoki M, Takehara K, Mukaida N, et al. CCR4 memory CD4+ T lymphocytes are increased in peripheral blood and lesional skin from patients with atopic dermatitis. J Allergy Clin Immunol (2001) 107(2):353–8. doi:10.1067/mai.2001.112601
133. Kusumoto M, Xu B, Shi M, Matsuyama T, Aoyama K, Takeuchi T. Expression of chemokine receptor CCR4 and its ligands (CCL17 and CCL22) in murine contact hypersensitivity. J Interferon Cytokine Res (2007) 27(11):901–10. doi:10.1089/jir.2006.0064
134. Gehad A, Al-Banna NA, Vaci M, Issekutz AC, Mohan K, Latta M, et al. Differing requirements for CCR4, E-selectin, and alpha4beta1 for the migration of memory CD4 and activated T cells to dermal inflammation. J Immunol (2012) 189(1):337–46. doi:10.4049/jimmunol.1102315
135. Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis (2010) 28(1):31–44. doi:10.1159/000282062
136. Teraki Y, Miyake A, Takebayashi R, Shiohara T. Homing receptor and chemokine receptor on intraepidermal T cells in psoriasis vulgaris. Clin Exp Dermatol (2004) 29(6):658–63. doi:10.1111/j.1365-2230.2004.01638.x
137. Wenzel J, Henze S, Worenkamper E, Basner-Tschakarjan E, Sokolowska-Wojdylo M, Steitz J, et al. Role of the chemokine receptor CCR4 and its ligand thymus- and activation-regulated chemokine/CCL17 for lymphocyte recruitment in cutaneous lupus erythematosus. J Invest Dermatol (2005) 124(6):1241–8. doi:10.1111/j.0022-202X.2005.23755.x
138. Seneviratne SL, Black AP, Jones L, Bailey AS, Ogg GS. The role of skin-homing T cells in extrinsic atopic dermatitis. QJM (2007) 100(1):19–27. doi:10.1093/qjmed/hcl132
139. Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood (2003) 101(11):4260–6. doi:10.1182/blood-2002-11-3577
140. Le Gros G, Erard F. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector functions. Curr Opin Immunol (1994) 6(3):453–7. doi:10.1016/0952-7915(94)90127-9
Keywords: natural killer T cells, dendritic cells, licensing, memory, CD8 T cells, cross-presentation
Citation: Gottschalk C, Mettke E and Kurts C (2015) The role of invariant natural killer T cells in dendritic cell licensing, cross-priming, and memory CD8+ T cell generation. Front. Immunol. 6:379. doi: 10.3389/fimmu.2015.00379
Received: 29 April 2015; Accepted: 11 July 2015;
Published: 28 July 2015
Edited by:
Elisabetta Padovan, Basel University Hospital and University of Basel, SwitzerlandReviewed by:
Francesca Di Rosa, National Research Council, ItalyAlena Donda, University of Lausanne, Switzerland
Paolo Dellabona, San Raffaele Scientific Institute, Italy
Copyright: © 2015 Gottschalk, Mettke and Kurts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Kurts, Institute of Experimental Immunology, Rheinische Friedrich-Wilhelms-University of Bonn, Sigmund-Freud-Str. 25, Bonn D-53127, Germany,Y2t1cnRzQHVuaS1ib25uLmRl
 Catherine Gottschalk
Catherine Gottschalk Elisabeth Mettke
Elisabeth Mettke Christian Kurts
Christian Kurts