- 1Columbus Technologies, Inc., Contractor to the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
- 2Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
Tuberculosis (TB) remains a global health threat of alarming proportions, resulting in 1.5 million deaths worldwide. The only available licensed vaccine, Bacillus Calmette–Guérin, does not confer lifelong protection against active TB. To date, development of an effective vaccine against TB has proven to be elusive, and devising newer approaches for improved vaccination outcomes is an essential goal. Insights gained over the last several years have revealed multiple mechanisms of immune manipulation by Mycobacterium tuberculosis (Mtb) in infected macrophages and dendritic cells that support disease progression and block development of protective immunity. This review provides an assessment of the known immunoregulatory mechanisms altered by Mtb, and how new interventions may reverse these effects. Examples include blocking of inhibitory immune cell coreceptor checkpoints (e.g., programed death-1). Conversely, immune mechanisms that strengthen immune cell effector functions may be enhanced by interventions, including stimulatory immune cell coreceptors (e.g., OX40). Modification of the activity of key cell “immunometabolism” signaling pathway molecules, including mechanistic target of rapamycin, glycogen synthase kinase-3β, wnt/β-catenin, adenosine monophosophate-activated protein kinase, and sirtuins, related epigenetic changes, and preventing induction of immune regulatory cells (e.g., regulatory T cells, myeloid-derived suppressor cells) are powerful new approaches to improve vaccine responses. Interventions to favorably modulate these components have been studied primarily in oncology to induce efficient antitumor immune responses, often by potentiation of cancer vaccines. These agents include antibodies and a rapidly increasing number of small molecule drug classes that have contributed to the dramatic immune-based advances in treatment of cancer and other diseases. Because immune responses to malignancies and to Mtb share many similar mechanisms, studies to improve TB vaccine responses using interventions based on “immuno-oncology” are needed to guide possible repurposing. Understanding the regulation of immune cell functions appropriated by Mtb to promote the imbalance between protective and pathogenic immune responses may guide the development of innovative drug-based adjunct approaches to substantially enhance the clinical efficacy of TB vaccines.
Introduction
Tuberculosis (TB) caused by the bacillus Mycobacterium tuberculosis (Mtb), is the most common infectious cause of death worldwide. In 2014, an estimated 1.5 million deaths were attributed to TB (1). Approximately one-third of the world’s population is infected with Mtb, and 90–95% of those will remain latently infected and asymptomatic. The other 5–10% will progress to active disease (2). Bacillus Calmette–Guérin (BCG) vaccine has been available for more than 90 years but is not sufficiently successful in preventing active TB (3). The efficacy of BCG vaccine efficacy in preventing pulmonary TB is limited, with studies showing 0–80% protective benefit (4). BCG is 70–80% efficacious against severe forms of TB in childhood, particularly in infant meningitis (5). The variations in BCG vaccine efficacy found in different studies have been attributed to geographical differences, exposure to certain endemic mycobacteria, and varied manufacturing facilities with inconsistent quality control (4, 6). The tremendous variability associated with its protective effects against TB, specifically the waning of protection in the adolescent and adult populations has resulted in intense efforts to improve its partial efficacy (7). Currently, more than a dozen vaccine candidates for TB are at different stages of clinical trial development. Recent developments of prime-boost TB candidate vaccines include subunit, multi-epitopic candidate vaccines with potentially broad immunological coverage consisting of Mtb-secreted components (ESAT-6, the antigens 85A and 85B) and the PPE family members. Newer multistage subunit vaccine strategies have included antigens from dormancy/starvation, early reactivating (resuscitation-promoting factors), and active bacilli stages (8). BCG replacement vaccines include live recombinant BCG, modified non-pathogenic mycobacteria (M. vaccae, RUTI, and M. smegmatis), and inactivated whole cell vaccine (WCV) candidates (9–11). Although successful in animal models, the viral vectored MVA85A, the first candidate TB vaccine to enter efficacy trials showed disappointing results in Phase II trials (12, 13). The protein/adjuvant candidates including M72/AS01 and H4 and H56/IC31 have shown promising immunogenicity in Phase 1/II trials and are currently in efficacy trials with results awaited (14–16). The lack of known correlates of protection associated with different stages of TB infection and disease has been a barrier to the clinical evaluation of vaccine candidates, and the TB vaccine field is now redirecting its attention to basic discovery and preclinical development (17).
New strategies are needed to improve vaccine efficacy based on both a better understanding of the mechanisms mediating protective immunity and mechanism of subversion of host immune responses by immunopathogenic components of Mtb. Opinion on the balance between protective and destructive responses to Mtb infection in humans and animal models and the functional role of granuloma is still evolving (18, 19). A better understanding of the features that contribute to protective host responses to Mtb infection will aid in the identification of drugs that can be repurposed for use to potentiate current TB vaccination strategies. The Mtb-infected host-cell microenvironment is characterized by dysregulated immunoregulation and immunometabolism signaling pathways, for example, Th1/Th17 versus Th2 balance, regulatory T (Treg) and suppressive myeloid cell populations, T cell anergy, and a shift from M1 to M2 polarized macrophages (20–24).
Immunologic responses to malignancies and TB have many similarities in terms of persistent inflammation and innate and T cell-mediated responses (20). In this review, some of the key pathways regulating the function of Mtb-infected antigen-presenting cells (APC) are explored. Improved understanding of the fundamental mechanisms of immune cell suppression and downregulation of protective immune responses by Mtb may lead to the introduction of novel interventions to promote vaccine-induced anti-Mtb immunity. The relevant immune cellular pathways are well known in various other diseases, but a cohesive picture of the affected immunoregulatory and immunometabolism machinery is yet to be established for TB vaccinology.
Potential adaption of the following two key strategies will be delineated:
1. Immuno-oncology: immune cell coreceptor checkpoints that determine the intensity of T-cell responses to particular antigens and imbalances in the numbers and activity of immune regulatory cells.
2. Immunometabolism: a relatively new concept developed in oncology, which refers to manipulation of checkpoints causing changes in cell metabolism that are required for and/or direct immune cell differentiation and function.
This review will focus on the potential of these interventions to be tested as adjunct strategies to shift the balance of TB vaccines toward protective responses and potentiate host responses induced by vaccines.
Targeting Cell Regulatory Pathways and Immune Checkpoints as Adjunct Strategies for TB Vaccination
Targeting Autophagy Regulation Pathways
Autophagy is a process for maintaining cellular homeostasis, particularly during stress conditions, by continual degradation of damaged organelles, protein aggregates, and intracellular pathogens through the autophagosome. Autophagic processes also contribute to the activation of innate and adaptive immune responses (25–27). During Mtb infection, pro-inflammatory cytokines positively regulated by autophagy induction control intracellular Mtb proliferation, and both TLR (TLR2, TLR4, and TLR9) and non-TLR pathways are triggered to degrade the bacteria (28–35). Nevertheless, Mtb in autophagosomes can escape elimination by autophagy by preventing lysosomal fusion and acidification though the underlying mechanisms of escape remain poorly understood (27, 36–38).
Experimental evidence suggests that vaccine efficacy can be improved through enhanced antigen presentation to T cells by boosting autophagy-mediated antigen presentation (39–41). Inhibition of the mechanistic target of rapamycin (mTOR) is a well-known mechanism for inducing autophagy (Table 1). Rapamycin (sirolimus) is a well-characterized inhibitor of mTOR (33, 42). The efficacy of BCG vaccine is improved in a murine model, and antigen presentation by DCs in vitro is enhanced by rapamycin with an increase in Th1 responses (39, 43). As inhaled drug and vaccine delivery platforms gain recognition and prolonged use of autophagy inducers can result in immunosuppression, rapamycin delivered in poly lactide-co-glycolide nano particles can maintain high localized intracellular drug concentration and minimize systemic side effects (40, 44). Coadministration of a DNA vaccine composed of the immunodominant mycobacterial antigen Ag85B and incorporating an autophagy-inducing mTOR-KD plasmid induced primarily a Th1 immune response (33, 39). This DNA vaccine was delivered by chitosan particles to enhance mucosal immunity.
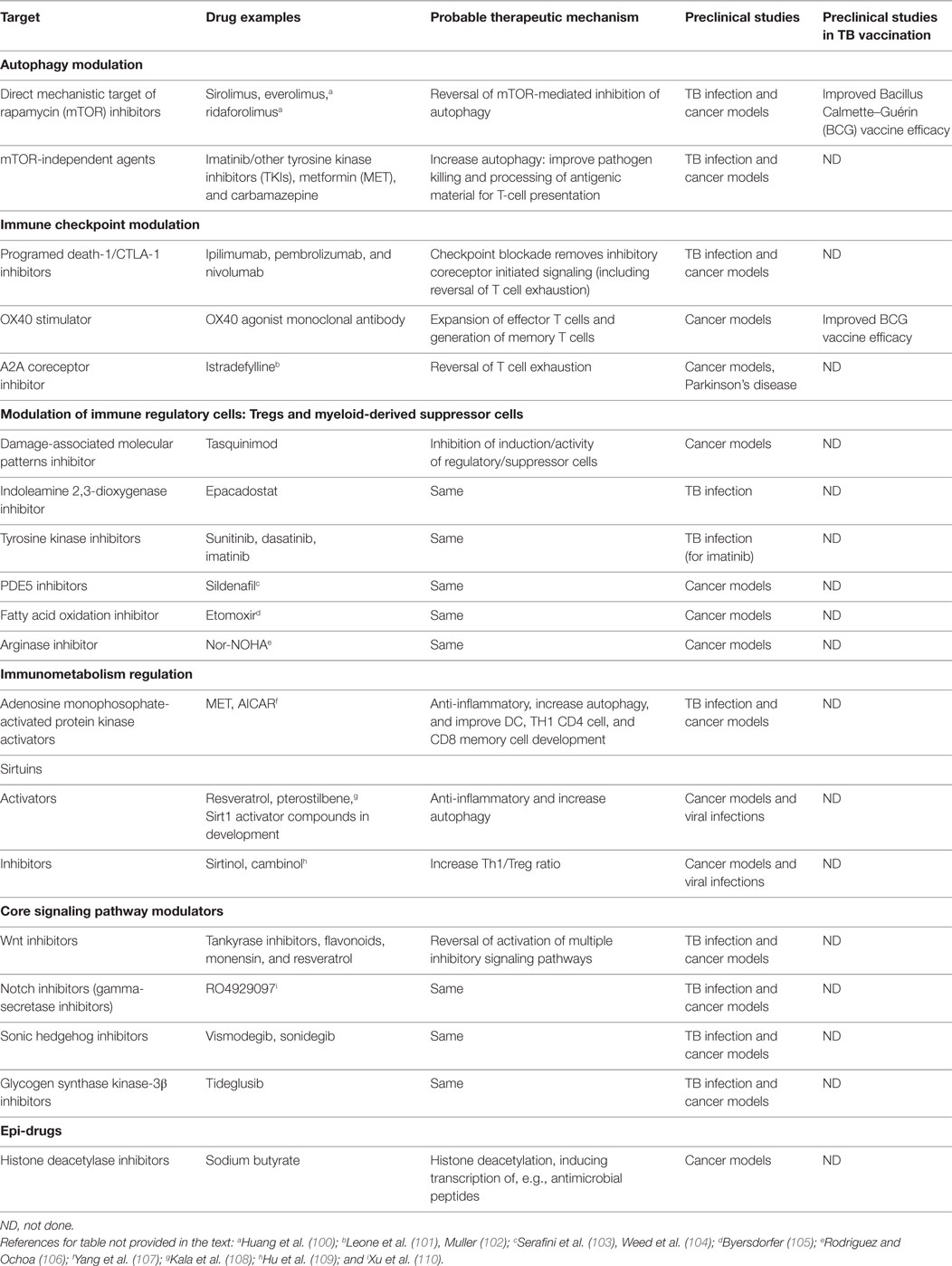
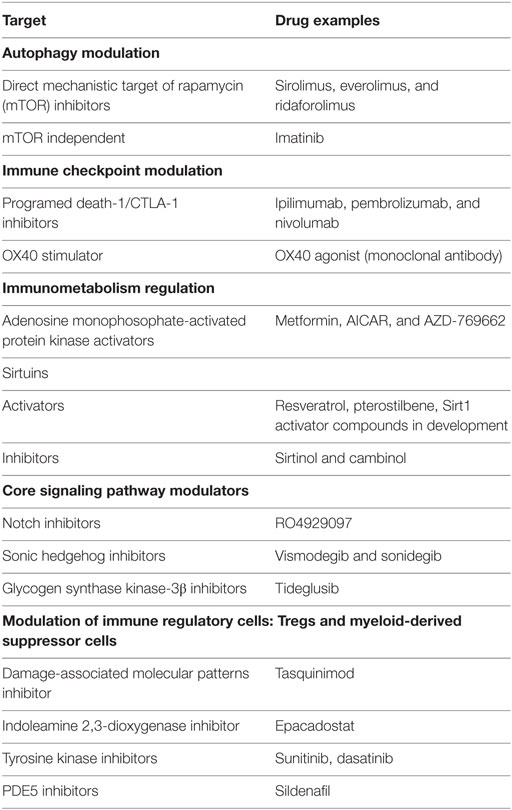
Table 1. Agents that target immune checkpoints, immune regulatory cells, and key pathways involved in Mtb pathogenesis.
Inducing autophagy by mTOR-independent methods is also being pursued. Microtubule-associated protein light chain-3 (LC3) transports Mtb lipoprotein (LpqH) to autophagosomes and is involved in autophagy activation via TLR1/2/CD14 receptors. A LC3–LpqH DNA vaccine enhanced protective efficacy against Mtb in mice (45). Screening of FDA-approved drugs has identified compounds stimulating autophagic killing of mycobacteria at therapeutic concentrations though none of these agents have been tested preclinically in conjunction with TB vaccine candidates. Carbamazepine triggers autophagy independently of mTOR and effectively targets multidrug-resistant Mtb in vivo by stimulating both innate and adaptive immunity (46). Regulation of the acidification of intracellular compartments has also shown potential to enhance host defense against pathogens. Imatinib, a tyrosine kinase inhibitor (TKI), promotes lysosome acidification and anti-mycobacterial activity in macrophages in a mouse model, and is being further tested as a potential complementary therapy for TB (47–49). TKI inhibition is a strategy for improving autophagy but may also have other mechanisms of action, including suppression of myeloid-derived suppressor cells (MDSCs) and Tregs. Statins, HMG-CoA reductase inhibitors, and autophagy-inducing agents have shown immunomodulatory properties in TB models, resulting in increased bacterial clearance and improved efficacy of first-line TB drugs with a substantial decrease in inflammation (Table 1) (50, 51).
Immune Checkpoint Modulation
Modulation of Programed Death-1 Pathway
The programed death-1 (PD-1) receptor is a member of the CD28 superfamily that negatively regulates T cell responses to antigen stimulation and enhances tolerance, triggered by interaction with its ligands PD-L1 and PD-L2 (52, 53). PD-1 is expressed on Tregs, natural killer (NK) cells, follicular T and B cells, and APCs (53). PD-1 has recently emerged as an important co-inhibitory molecule and “immune checkpoint” in a number of chronic intracellular infections, including TB, and overexpression of PD-1, cytotoxic T-lymphocyte antigen (CTLA-4) receptor, and TIM3 has been associated with T-cell exhaustion (54–56). In human TB infection, PD-1 activity induces Tregs and immunomosuppressive cytokines such as interleukin (IL)-10 and TGF-β (57, 58). HIV–TB coinfection is associated with increased expression of PD-1 on Mtb-specific cytokine-secreting CD4+ cell subsets (59).
Checkpoint blockade, i.e., removal of inhibitory coreceptor initiated signaling, is a strategy used successfully in cancer treatment for vaccine potentiation. Monoclonal antibodies (mAbs) that modulate inhibitor signaling by CTLA-4 and PD-1, e.g., ipilimumab, pembrolizumab, and nivolumab, are now in wide clinical use for several cancers (Table 1) (60). Combination of checkpoint blockade with PD-1 and CTLA-4 inhibition enhances response rates in patients with minimal responses to single checkpoint blockade with lesser toxicity compared to previous immunotherapies, e.g., IL-2 (60, 61). PD-1 blockade with the cancer vaccine TEGVAX in mice showed regression of established tumors, providing a rationale using vaccines combined with immune checkpoint blockade (62).
The overall role of PD-1 blockade in TB remains a complicated issue. PD-1-deficient mice have shown dramatically reduced survival compared with wild-type mice post-Mtb infection. Increased levels of pro-inflammatory cytokines and uncontrolled bacterial proliferation were seen in the lungs of these mice (63). These observations indicate that control of Mtb infection requires a carefully balanced immune response that contains the infection without causing pathology (64).
Interestingly, the specific role of PD1–L2 is currently of interest as a therapy for malaria. Blockade of PD1–L1 binding resulted in unfavorable outcome in a malaria mouse model, but blockade of PD1–L2 did not, suggesting different functions of the two PD-1 ligand functions in malarial infections (65). Administration of soluble multimeric PD-L2 outcompeted PD-L1 for PD-1 binding and improved CD4+ T cell responses in mice with lethal malaria, dramatically improving survival (66). The results also suggest that PD-L2 not only competes with PD-L1 but also has a stimulatory action via interaction with a different coreceptor.
Additional studies are needed to further investigate these issues that might allow tuning of TB vaccine responses to be more effective. Energy supply insufficiency due to cell metabolic alterations directed by PD-1 signaling leads to exhaustion and begins early in CD8+ T cell responses in chronic lymphocytic choriomeningitis virus infection (67). Downregulation of the glycolytic and peroxisome proliferator-activated receptor c coactivator 1α (PGC-1a) pathways leads to reduced mitochondrial energy availability. Additionally, mTOR signaling drives anabolic metabolism of effector T cells, exacerbating energy deficiency, further contributing to cell exhaustion. The reversion of these changes in a subset of exhausted CD8+ T cells by anti-PD-1 therapy suggests that targeted agents to directly inhibit these metabolic changes should be explored (67). The nuclear factor of activated T cells (NFAT) proteins is also primary in the establishment of T cell exhaustion in mouse models of tumor growth and bacterial infection (68). During persistent antigen stimulation, NFAT promotes T cell exhaustion by binding directly to regulatory regions of PD-1 and TIM3 (68). Targeting NFAT isoforms or their associated signaling molecules is being actively pursued in cancer therapy (69).
Stimulation through Coreceptors: OX40
The various checkpoint co-inhibitors may need to be combined with T-cell co-stimulators (or stimulators could be used alone), for effective CD4+ Th1 and CD8+ T-cell development and subsequent sustained T-cell memory responses (70). CD134 (OX40), a TNF-α receptor superfamily member, is expressed transiently on newly activated T cells and NK cells (71, 72). CD134–CD134 ligand interactions are crucial for the expansion of effector T cells and generation of memory T cells (73). OX40 agonist mAb administration results in a Th1-cytokine-driven response enhancing IL-2 production by effector T cells and preventing the expression of CTLA-4, FoxP3, and IL-10 (71, 74). Compared with BCG vaccination alone, OX40 ligation with BCG vaccination in a murine model provided enhanced protection against aerosol and intravenous Mtb challenge with NK cells playing a crucial role (71). In cancer therapy, the beneficial results of combination immunotherapy targeting both costimulatory and co-inhibitory molecules (combined OX40/PD-1 or OX40/CTLA-4 mAb therapy) dramatically improved survival in the prostate, ovarian carcinoma, and sarcoma models (70, 75). The adjunct potential of OX40 agonists, in conjunction with CTLA–4 mAb, has been shown for infection by the intracellular bacterium, Leishmania donovani (76). The role of activation of stimulatory coreceptors to improve vaccine responses deserves further investigation.
Modulation of Immune Regulatory Cells
Regulatory T Cells
Th1-type immunity is downregulated by a subset of immunosuppressive CD4+ T cells expressing CD25 and the transcription factor FoxP3, referred to as Tregs (77–79). As excessive inflammation related to highly activated populations of CD4+ T cells can cause tissue damage and exacerbate TB disease, a critical balance of regulatory and effector T-cell responses govern the outcome of infection (80, 81). Tregs suppress both T cells and APCs, thus enhancing persistent infection (79, 82–84). Expansion of Tregs in active TB has been observed both at organ-specific sites and in blood (80, 85, 86). Multiple studies suggest Tregs delay the arrival of potential effector T cells into the pulmonary lymph nodes during early infection, play a role in reactivation of latent infection, and have a positive correlation with bacterial burden and extent of active disease (80, 81, 87, 88). Contrasting results have been observed in the TB treatment phase, with some studies reporting declining levels of Tregs and others showing a transient increase (86, 89, 90).
Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells are another group of regulatory cells controlling TB inflammation. MDSCs are a diverse population of monocyte, granulocyte, and DC precursors showing features of immune suppressors (91). Similar to macrophages, polarized MDSC lineages can be distinguished as M1 and M2 cells (92). Their numbers are expanded significantly in cancer and in pleural effusions and blood of TB patients (93, 94). MDSCs have been shown to become infected by Mtb, accumulate within the inflamed lung, interact with granuloma-residing cells, and decrease in number after TB chemotherapy (95, 96).
Strategies for Modulation of Treg and MDSC Population
Studies of Treg suppression agents in combination with TB vaccination have yielded unclear results. A heterologous BCG/DNA-heat shock protein (HSP) 65 vaccination regimen was protective, and the effect was associated with lower numbers of Tregs in murine lungs (97). Concurrent inhibition of Th2 cells and Tregs by using the small molecule inhibitors Suplatast tosylate and D4476, respectively, during BCG vaccination improved vaccine efficacy in mice, by enhancing Mtb clearance, induction of superior Th1 responses, and long-lasting protective central memory T cell responses (98). However, reduction of Tregs using anti-CD25 antibody prior to BCG vaccination and an experimental ESAT-6 subunit TB vaccine administered concurrently with IL-28B that downregulates Tregs did not enhance the protective effect in mice models, suggesting a need to refine Treg inactivation strategies (79, 99).
Table 1 lists several other agents being tested for their regulatory cell modulation properties including damage-associated molecular patterns (DAMPs), inducible indoleamine 2,3-dioxygenase (IDO), TK, phosphodiesterase-5 (PDE5) and fatty acid oxidation (FAO), and arginase inhibitors.
Damage-associated molecular patterns (also known as alarmins) are released by dying or stressed cells and include HSPs, S100, and high mobility group box (HMGB1) proteins (111). Expansion of both Treg and MDSCs in cancer involves the participation of DAMPs (112). Interestingly, HMGB1 was evaluated as an adjuvant for a TB subunit HMGB1–ESAT-6 fusion protein vaccine in a mouse model. Poly-functional CD4 T cell-mediated immune response generated by the fusion protein vaccination correlated with protection against subsequent Mtb challenge (113). Tasquinimod, an S100A9 inhibitor currently in phase III trials for prostate carcinoma, decreased accumulation and activity of MDSCs with enhanced CD8+ responses in different combination immunotherapeutic strategies including a tumor vaccine in murine cancer models (114, 115). The potential use of DAMPs and DAMP inhibitors in TB vaccination strategies needs to be resolved.
Indoleamine 2,3-dioxygenase blocks T cell proliferation and modulates immune responses to Mtb (116–118). Increased IDO expression is strongly associated with poor outcome in many cancers and is emerging as new target for potentiation of cancer vaccines (119, 120). Similarly, reduced immunogenicity seen following MVA85A vaccination in South Africans compared to UK subjects has been attributed to increased baseline IDO activity (118). Use of IDO inhibitor drugs like epacadostat that significantly decrease Treg proliferation and increase CTL activity in vitro provide rationale for improving vaccine responses by combining with IDO inhibitors (121). A first-in-human, phase I clinical study, combining ipilimumab (anti CTLA-4) with a single-epitope peptide vaccine targeting IDO showed detectable T-cell responses in a subset of metastatic melanoma patients (120). New strategies targeting the IDO enzyme in cancer, either through vaccine components directed against IDO epitopes or silencing the IDO gene using small hairpin RNA plasmids, are some of the strategies with potential application in the context of TB vaccination (118, 122).
Tyrosine kinase inhibitors can also reduce the Tregs and MDSCs (123, 124), probably through multiple mechanisms, including cKIT inhibition. Sunitinib, currently used in cancer therapy, depletes MDSCs and synergized with a cancer vaccine to enhance antigen-specific immune responses and tumor eradication in mice (125). Similarly, dasatinib exhibits modest single-agent clinical efficacy but showed superior efficacy associated with reduced levels of MDSC and Treg populations in a combination treatment regimen with a DC vaccine candidate in a murine melanoma model (126). Imatinib, used as an approved therapeutic for chronic myelogenous leukemia, reduced bacterial load and associated pathology in mice infected with Mtb and acted in a synergistic manner with anti-TB drugs (47). Several mechanisms are possible for this effect, including inhibition of suppressor cells, and imatinib is being considered for study as a vaccine adjunct for infections.
Manipulation of Immunometabolism by Mtb and Potential Interventions
Adenosine Monophosophate-Activated Protein Kinase
Adenosine monophosophate-activated protein kinase (AMPK) is an energy sensor kinase that regulates cellular energy homeostasis and acts as a negative regulator of inflammation (127–129). AMPK-dependent control of T cell metabolism (as an immunometabolic checkpoint) is a crucial contributing factor to determine T cell development and effector responses. AMPK also has an essential role in memory T-cell differentiation by regulating the metabolic switch from primarily aerobic glycolysis to oxidative phosphorylation of lipids (129, 130). During Mtb infection, AMPK activation enhances autophagy, leading to phagosomal maturation and improved antimicrobial response (107, 129, 131).
The antidiabetic drug metformin (MET) is an AMPK activator that inhibits intracellular Mtb growth, restricts disease immunopathology, and induces expansion of Mtb-specific effector and memory T cells (Table 1) (128). Additionally, MET-treated diabetic patients with LTBI have increased numbers of Mtb-specific T cells (128). Although issues of dosage and incorporation into current TB treatments remain, MET is under consideration for evaluation as an adjunctive agent for TB treatment (132). Therapeutic cancer vaccination combined with MET improved tumor-infiltrating lymphocytes’ multifunctionality and protection from apoptosis and exhaustion (133). Additionally, MET is able to block M2-like macrophage polarization (partially through AMPK) in cancer. The Notch signaling pathway is a potentially important mechanism in this polarization (134, 135).
Cross Talk of AMPK with Other Signaling Pathways, Particularly mTOR and HIF-1α
Increasing evidence indicates that T cells have metabolic checkpoints impacting their development and functions, including final phenotypes and numbers. AMPK and mTOR are chief players in the sensing and control of metabolic state and determination of subsequent immune cellular fates (136). mTOR inhibition is among the downstream effects of AMPK signaling. As a result, rapamycin, an inhibitor of mTOR, may share mechanistic effects with MET, an AMPK activator that subsequently inhibits mTOR (133).
Similarly, mTOR signaling, primarily through activating the transcription factor hypoxia-inducible factor-1 (HIF-1α), regulates T cell effector and memory differentiation (137). HIF-1α increases aerobic glycolysis (opposed by AMPK) and regulates the differentiation of CD4+ T cells, favoring differentiation into Th17 cells versus Tregs and production of pro-inflammatory cytokines in response to T cell receptor activation (136, 138). Elevated levels of HIF result in the differentiation of cytotoxic CD8+ lymphocytes (CTLs) with a high glycolytic activity, increased effector capacity, and expression of costimulatory receptors like OX40 (139). These HIF-dependent effects enhanced and sustained T cell effector function in tumor and persistent infection models (139, 140). However, HIF-1α may be a key factor for Mtb infection control, as mice lacking HIF-1α in the myeloid lineage were more susceptible to infection and exhibited defective production of inflammatory cytokines and microbicidal effectors (141). The role of HIF-1 activity in the regulation of immune responses for infections in general remains an area in need of investigation before any assessment can be made regarding potential clinical applications.
Sirtuins
Silent information regulator 2 proteins or sirtuins (Sirts) are seven NAD-dependent protein deacetylases involved in stress adaption, cell survival, aging, and apoptosis (142–144). Sirts, specifically Sirt1, Sirt2, Sirt6, and Sirt7, are also emerging as important players in epigenetic changes (145). Sirt1 activates the key autophagy proteins ATG5, ATG7, ATG8/LC3, and Sirt1 and Sirt2 have anti-inflammatory roles, such as inhibition of NF-kB signaling and NLRP3 inflammasome activity (146–149). Sirt1 has recently emerged as a key regulator in immune regulation modulating both innate and adaptive responses.
Sirt1 inhibitors (Table 1) are able to block virus infections including HCMV, influenza A virus, and adenovirus Ad5 (150). In a tumor microenvironment, Sirt1 deficiency impacted immune suppression by switching MDSCs to a pro-inflammatory M1 phenotype through HIF-1α-dependent glycolysis (92). This suggests that Sirt1 inhibitors should be considered for evaluation as vaccine adjuncts. Conversely, the enzymatic activity of Sirt1 can be enhanced by small molecule activators including the natural polyphenol, resveratrol, and synthetic Sirt1 activator compounds (151). In respiratory syncytial virus infection, Sirt1 promotes an effective antiviral response through DC cytokine secretion and autophagy-mediated processes, providing an option for a novel vaccination strategy, such as a Sirt1-activating viral vaccine (152).
Sirtuins have multiple effects on immune regulation based on integration of immune cell metabolic and immune function regulation including potentially modulating T cell exhaustion. Sirt1 activates PGC-1a and negatively regulates NFAT, mTOR, and HIF-1a activity (149, 153, 154). This effect could reduce metabolic cell stresses and improve the effectiveness of PD-1 blockade therapy. Overall, the role of Sirt family in Mtb infection and its potential combined use with vaccination requires further exploration.
Immunomodulatory Potential of Core Signaling Pathways
Wnt/β-Catenin Signaling
The canonical Wnt/β-catenin pathway plays an important role in the regulation of various cellular processes, including cell differentiation, apoptosis, polarity, motility, embryonic development, adult tissue homeostasis, and carcinogenesis (155–158). Infection with BCG activates Wnt/β-catenin signaling, resulting in the expression of a host of genetic signatures important for subsequent regulatory responses, including cyclooxygenase (COX)-2, suppressor of cytokine signaling-3 (SOCS-3), and Jagged1 (ligand for Notch1 signaling) (159–162). Additionally, wnt/β-catenin signaling in DCs induces production of various anti-inflammatory cytokines that induce Treg cells and suppress TH1/Th17 responses (163).
The wnt/β-catenin pathway has been suggested as a therapeutic target for adjunct treatments along with vaccination in the ovarian tumor microenvironment (164). Agents for targeting the wnt/β-catenin pathway include flavonoids, monensin, and resveratrol, and the use of antibodies against the Notch receptor or ligand (Table 1) (165). Additionally, telomere protection enzymes, the tankyrase (Tnks) subfamily of PARPs, control transcriptional response to secreted Wnt signaling molecules. Tnks inhibitors are currently being developed as therapeutic agents for targeting Wnt-related cancers (166). Despite recent reports of studies implicating Wnt signaling pathway in the development and progression of TB, the utilization of potential signaling inhibitors as effective adjuncts in TB vaccination remains to be explored.
Notch Signaling
Notch signaling regulates various developmental stages of T and B cells and T cell activation and differentiation (167). This highly conserved pathway also integrates further inputs from other signaling pathways. This cross talk includes upregulation of ligands or receptors (e.g., Wnt regulating Notch ligand levels) and co-regulation of shared target genes [e.g., Hes1 and Gsk3β regulation by Notch, Wnt, and sonic hedgehog (SHH)] (168, 169). Notch signaling directly controls PD-1 transcription in activated CD8+ T cells, as blocking Notch signaling leads to the inhibition of PD-1 expression (170). BCG infection of murine macrophages induced upregulation of Notch1 expression. Activation of Notch1 signaling was demonstrated in granulomatous lesions in brains of humans with TB meningitis in comparison to healthy individuals (167, 171). Several notch inhibitors, mainly gamma-secretase inhibitors, are now in clinical trials, e.g., RO4929097 (Table 1) (172).
Sonic Hedgehog
Sonic hedgehog, a pleotropic member of the hedgehog family of signaling molecules, plays a key role in cellular homeostasis and development (173). Aberrant activation of the HH signaling pathway has been implicated in the pathogenesis of several types of cancer (174). SHH signaling constitutes one of the networks that macrophages use to tailor differential immune responses to infections with pathogenic mycobacteria. In addition to BCG triggering a robust activation of SHH signaling in macrophages, SHH signaling is heightened in humans with PTB and TBM (175). Additionally, TNFα-driven SHH signaling downregulates M. bovis-specific TLR2 responses through micro RNA-31 and -150 targeting MyD88 (175). These changes lead to modulation of a battery of genes that regulate various functions of macrophages genes, including SOCS-3, COX-2, MMP-9, and M1 and M2 genes and emphasize a novel role for SHH signaling in host immune responses to mycobacterial infections. Vismodegib and sonidegib are small molecule inhibitors targeting the SHH pathway in cancer (Table 1) (174, 176).
Glycogen Synthase Kinase-3β
The binding of Wnt ligands secreted from various cell types to transmembrane receptors of the Frizzled family activates a signaling cascade resulting in the inhibition of a negative regulator of β-catenin levels, glycogen synthase kinase-3β (GSK3β) (177). GSK3β plays a pivotal role in regulating many cellular functions, including cell survival and apoptosis (165, 178–180). M. bovis BCG induces phosphorylation of GSK3 by the PI3K–Akt signaling pathway, switching the transcriptional activity to an anti-inflammatory CREB-driven cytokine response instead of the pro-inflammatory NF-κB transcription (181, 182). GSK3β is an upstream kinase regulating PD-1 transcription, and the use of GSK3β inhibitors in vivo downregulates PD-1 and enhances CTL clearance of viral infections (183). Several inhibitors of GSK3β are in various stages of development, e.g., tideglusib (Table 1) (184). Additionally, in a DC-based tumor vaccination murine model, inhibition of GSK3β by siRNA enhanced CTL activity via suppression of IDO expression (185).
Epigenetic Changes and Trained Immunity
Trained immunity (TI) is a new paradigm for vaccination referring to a prolonged, enhanced functional innate response after adequate priming. While the specificity and memory of innate immunity is not as sophisticated as adaptive immunity, TI could potentially contribute to BCG, yellow fever, influenza, vaccinia, and measles vaccine-induced responses (186). The molecular basis of innate immune memory is cell type-specific epigenetic modifications, including DNA methylation and histone modifications, such as acetylation, methylation, and phosphorylation (187). Specifically, after BCG vaccination, the TI response in human monocytes and NK cells appears to be mediated through autophagy and regulatory pathway signaling changes leading to epigenetic reprograming (188, 189). Following histone modification profiles and genome-wide transcriptomics to study trained human monocytes, an mTOR- and HIF-1α-mediated aerobic glycolysis pathway has been suggested as the metabolic basis for TI (190). The identification of glycolysis as a fundamental process in TI defines potential targets that inhibit TI including inhibition of mTOR/HIF-1a axis and use of MET and sirtuin activators (190).
Whether training of innate immunity to exhibit adaptive features can significantly potentiate vaccine responses is an important research question. Possible epigenetic-based interventions may include enhancement of TI development with sirtuin inhibition and stabilizing the activity of the mTOR/HIF axis. The knowledge that modification of epigenetic changes can potentially improve immune responses has led to development of “epi-drugs” now approved for use in targeted oncologic treatment strategies (191, 192). These drugs, inhibitors of DNA methyltransferases and histone deacetylases (HDACs) (Table 1), for example, sodium butyrate can modulate expression of immune regulatory molecules to improve immune responses in murine models, including in therapeutic combinations with cancer vaccines (193).
Testing of Potential Agents with Candidate TB Vaccines in Appropriate Animal Models
Table 2 is a prioritized list of candidate-potentiating agents that are either approved or currently being tested for other diseases. These include activators of effector T cell expansion and generation of memory T cells responses (MET and OX40 agonists) and agents targeting specific initiators of regulatory pathway signaling (PD1, wnt/notch, and mTOR).
Testing the wide range of candidate TB vaccine in conjunction with immunoregulatory and immunometabolic interventions as adjuncts requires developing appropriate preclinical models. Due to the remarkable amount of cross talk between the cellular immune pathways (for example, AMPK and mTOR are chief players in the controlled sensing of metabolic state and subsequent immune cell fates), in vitro testing with particular focus on macrophages and DCs will be important to ascertain that specific cellular interactions are targeted without affecting other pathways. Following in vitro testing of new candidates, assessment of their combinatorial effect and safety interactions in small animal models including transgenic mice models and non-human primates (NHPs) will be essential evaluations. While experimental studies in mouse models have provided direct insight into Mtb- and BCG-induced changes in Wnt, Gsk3b, Notch1, SHH, and mTOR signaling (194–196), many differences in human versus murine immune cell regulation are known. In view of these differences, the use of NHPs to determine the role of regulatory pathway disruptions and the resulting effects on essential immune functions can be potentially illuminating.
As shown in the Tables 1 and 2, many of the proposed agents have been studied in the context of TB disease and are yet to be tested with candidate TB vaccines. Due to the highly complex patterns of host responses to TB infection/disease, it is probable that what relates to TB infection/disease may not be effective in the context of vaccination, and this possibility requires careful exploration. Also, differences in specific antigens and corresponding immune responses for the numerous classes of candidate TB vaccines complicate the evaluation of these adjunct agents for vaccination. Among the whole cell, viral-vectored, and adjuvanted subunit candidate vaccines, attenuated WCV may present a viable opportunity to be tested first. The antigenic complexity associated with WCV most probably will modulate key signaling pathways and/or immune cell functions that can be targeted for modification with drugs.
Timing of vaccine administration with these potential adjunct therapies remains to be determined. A balanced regulatory and effector T-cell response is required for optimal control of bacterial replication while simultaneously limiting effector cell-mediated tissue damage (197). For example, Sirt1 inhibiting drugs could potentiate vaccine responses and improve outcomes in very early stages of infection when enhanced immunity is essential. Conversely, Sirt1 activators may potentially be useful for the control of inflammatory damage and control of pathogen spread during later stages of infections. Similarly, timing of GSK3β inhibition, for instance, has to be determined as this inhibition suppresses pro-inflammatory cytokine expression, including TNF-α (198, 199). The use of inhibitors of mTOR/HIF-1a axis and MET can inhibit TI, suggesting that studies to determine the timing of use of these complementary therapies will also be needed.
Path Forward
Tuberculosis is an ancient human disease and has very effectively adapted to persist in its hosts despite the multiple robust immune responses directed against it. While antigen-specific CD4+ T cells and macrophage-activating cytokines are required for the control of TB, merely inducing more of these T cells or cytokines may not result in improved protection (18, 200). Both Mtb and BCG bacilli are highly immunogenic and drive robust antigen-specific T cell responses (18). The limited effectiveness of BCG and failure to improve on it, underlines the lack of understanding of why highly protective immune responses are not obtained. A potential underlying cause of ineffectiveness for some Mtb vaccines may be distortion of immune cell regulatory pathways required for development of protection by many potential mechanisms.
In cancer, innovative new strategies have been developed that include restoration of proper immune checkpoint, immunometabolism and epigenetic interventions. Most of these types of interventions have much in common, as reversal of alterations will allow affected immune cells to perform necessary immune functions, including autophagy, antigen processing and presentation, cytokine secretion, and other effector mechanisms. The potential for using both immunometabolism and co-signaling interventions in combination to balance effector and regulatory effects is beginning to be explored, to potentiate host responses induced by vaccines.
Similar to cancer, the complex regulation of immune cells and the extensive cross talk among several key regulatory pathways in response to TB infection make the therapeutic targeting and utilization of immune and metabolic checkpoints in TB very challenging. The most critical need is to determine the precise points of signaling pathway dysregulation that can be reversed by targeted intervention to result in improved immune protection. A large spectrum of innovative interventions to improve immune responses are now in clinical evaluation in other diseases, including enhancement of vaccine outcomes, and are available for adaption to TB vaccine research. Truly multidisciplinary research teams including investigators in the fields of immuno-oncology, immunometabolic, and applied epigenetic research working together with more traditional pathogen vaccine researchers will be important to develop successful new TB vaccine strategies.
Author Contributions
LJ and RH contributed equally to the development of outline and writing of the paper, reviewing relevant literature, and preparation of tables in the paper.
Disclaimer
This paper was written by one of the authors as a National Institute of Allergy and Infectious Diseases (NIAID) employee, but the views expressed in this paper do not necessarily represent those of NIAID.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Glaziou P, Floyd K, Weil D, Raviglione M. TB deaths rank alongside HIV deaths as top infectious killer. Int J Tuberc Lung Dis (2016) 20(2):143–4. doi:10.5588/ijtld.15.0985
2. da Silva MV, Massaro VJ Jr, Machado JR, Silva DA, Castellano LR, Alexandre PB, et al. Expression pattern of transcription factors and intracellular cytokines reveals that clinically cured tuberculosis is accompanied by an increase in Mycobacterium-specific Th1, Th2, and Th17 cells. Biomed Res Int (2015) 2015:591237. doi:10.1155/2015/591237
3. Singh VK, Srivastava R, Srivastava BS. Manipulation of BCG vaccine: a double-edged sword. Eur J Clin Microbiol Infect Dis (2016) 35(4):535–43. doi:10.1007/s10096-016-2579-y
4. Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin (2009) 5(2):70–8. doi:10.4161/hv.5.2.7210
5. Rodrigues LC, Mangtani P, Abubakar I. How does the level of BCG vaccine protection against tuberculosis fall over time? BMJ (2011) 343:d5974. doi:10.1136/bmj.d5974
6. Andersen P, Doherty TM. The success and failure of BCG – implications for a novel tuberculosis vaccine. Nat Rev Microbiol (2005) 3(8):656–62. doi:10.1038/nrmicro1211
7. Li M, Liu H, Zhao X, Wan K. Comparative analysis of human B cell epitopes based on BCG genomes. Biomed Res Int (2016) 2016:3620141. doi:10.1155/2016/3620141
8. Xin Q, Niu H, Li Z, Zhang G, Hu L, Wang B, et al. Subunit vaccine consisting of multi-stage antigens has high protective efficacy against Mycobacterium tuberculosis infection in mice. PLoS One (2013) 8(8):e72745. doi:10.1371/journal.pone.0072745
9. Cardona PJ, Amat I, Gordillo S, Arcos V, Guirado E, Diaz J, et al. Immunotherapy with fragmented Mycobacterium tuberculosis cells increases the effectiveness of chemotherapy against a chronical infection in a murine model of tuberculosis. Vaccine (2005) 23(11):1393–8. doi:10.1016/j.vaccine.2004.09.008
10. von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS (2010) 24(5):675–85. doi:10.1097/QAD.0b013e3283350f1b
11. Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med (2011) 17(10):1261–8. doi:10.1038/nm.2420
12. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet (2013) 381(9871):1021–8. doi:10.1016/S0140-6736(13)60177-4
13. Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med (2015) 3(3):190–200. doi:10.1016/S2213-2600(15)00037-5
14. Geldenhuys H, Mearns H, Miles DJ, Tameris M, Hokey D, Shi Z, et al. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: a randomized controlled trial. Vaccine (2015) 33(30):3592–9. doi:10.1016/j.vaccine.2015.05.036
15. Luabeya AK, Kagina BM, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine (2015) 33(33):4130–40. doi:10.1016/j.vaccine.2015.06.051
16. Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, et al. Safety and immunogenicity of candidate vaccine M72/AS01E in adolescents in a TB endemic setting. Vaccine (2015) 33(32):4025–34. doi:10.1016/j.vaccine.2015.05.088
17. Abubakar I, Lipman M, McHugh TD, Fletcher H. Uniting to end the TB epidemic: advances in disease control from prevention to better diagnosis and treatment. BMC Med (2016) 14:47. doi:10.1186/s12916-016-0599-1
18. Orme IM, Robinson RT, Cooper AM. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol (2015) 16(1):57–63. doi:10.1038/ni.3048
19. Kiran D, Podell BK, Chambers M, Basaraba RJ. Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review. Semin Immunopathol (2016) 38(2):167–83. doi:10.1007/s00281-015-0537-x
20. Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med (2014) 371(4):380–3. doi:10.1056/NEJMcibr1404664
21. Mahon RN, Hafner R. Immune cell regulatory pathways unexplored as host-directed therapeutic targets for Mycobacterium tuberculosis: an opportunity to apply precision medicine innovations to infectious diseases. Clin Infect Dis (2015) 61(Suppl 3):S200–16. doi:10.1093/cid/civ621
22. Qualls JE, Murray PJ. Immunometabolism within the tuberculosis granuloma: amino acids, hypoxia, and cellular respiration. Semin Immunopathol (2015) 38(2):139–52. doi:10.1007/s00281-015-0534-0
23. Dallenga T, Schaible UE. Neutrophils in tuberculosis – first line of defence or booster of disease and targets for host-directed therapy? Pathog Dis (2016) 74(3):pii: ftw012. doi:10.1093/femspd/ftw012
24. Kolahian S, Oz HH, Zhou B, Griessinger CM, Rieber N, Hartl D. The emerging role of myeloid-derived suppressor cells in lung diseases. Eur Respir J (2016) 47(3):967–77. doi:10.1183/13993003.01572-2015
25. Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, et al. The role of autophagy during the early neonatal starvation period. Nature (2004) 432(7020):1032–6. doi:10.1038/nature03029
26. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell (2011) 147(4):728–41. doi:10.1016/j.cell.2011.10.026
27. Yuk JM, Jo EK. Host immune responses to mycobacterial antigens and their implications for the development of a vaccine to control tuberculosis. Clin Exp Vaccine Res (2014) 3(2):155–67. doi:10.7774/cevr.2014.3.2.155
28. Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell (2004) 119(6):753–66. doi:10.1016/j.cell.2004.11.038
29. Vergne I, Singh S, Roberts E, Kyei G, Master S, Harris J, et al. Autophagy in immune defense against Mycobacterium tuberculosis. Autophagy (2006) 2(3):175–8. doi:10.4161/auto.2830
30. Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A (2007) 104(14):6031–6. doi:10.1073/pnas.0700036104
31. Jo EK, Shin DM, Choi AM. Autophagy: cellular defense to excessive inflammation. Microbes Infect (2012) 14(2):119–25. doi:10.1016/j.micinf.2011.08.014
32. Jo EK, Yuk JM, Shin DM, Sasakawa C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front Immunol (2013) 4:97. doi:10.3389/fimmu.2013.00097
33. Meerak J, Wanichwecharungruang SP, Palaga T. Enhancement of immune response to a DNA vaccine against Mycobacterium tuberculosis Ag85B by incorporation of an autophagy inducing system. Vaccine (2013) 31(5):784–90. doi:10.1016/j.vaccine.2012.11.075
34. Bento CF, Empadinhas N, Mendes V. Autophagy in the fight against tuberculosis. DNA Cell Biol (2015) 34(4):228–42. doi:10.1089/dna.2014.2745
35. Rubinsztein DC, Bento CF, Deretic V. Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J Exp Med (2015) 212(7):979–90. doi:10.1084/jem.20150956
36. Ghadimi D, de Vrese M, Heller KJ, Schrezenmeir J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol (2010) 10(6):694–706. doi:10.1016/j.intimp.2010.03.014
37. Shaler CR, Horvath C, Lai R, Xing Z. Understanding delayed T-cell priming, lung recruitment, and airway luminal T-cell responses in host defense against pulmonary tuberculosis. Clin Dev Immunol (2012) 2012:628293. doi:10.1155/2012/628293
38. Netea-Maier RT, Plantinga TS, Van De Veerdonk FL, Smit JW, Netea MG. Modulation of inflammation by autophagy: consequences for human disease. Autophagy (2015) 12(2):245–60. doi:10.1080/15548627.2015.1071759
39. Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med (2009) 15(3):267–76. doi:10.1038/nm.1928
40. Ni Cheallaigh C, Keane J, Lavelle EC, Hope JC, Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin Exp Immunol (2011) 164(3):291–300. doi:10.1111/j.1365-2249.2011.04381.x
41. Tey SK, Khanna R. Host immune system strikes back: autophagy-mediated antigen presentation bypasses viral blockade of the classic MHC class I processing pathway. Autophagy (2012) 8(12):1839–41. doi:10.4161/auto.21860
42. Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans (2013) 41(5):1103–30. doi:10.1042/BST20130134
43. Jagannath C, Bakhru P. Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol Biol (2012) 821:295–303. doi:10.1007/978-1-61779-430-8_18
44. Gupta A, Pant G, Mitra K, Madan J, Chourasia MK, Misra A. Inhalable particles containing rapamycin for induction of autophagy in macrophages infected with Mycobacterium tuberculosis. Mol Pharm (2014) 11(4):1201–7. doi:10.1021/mp4006563
45. Hu D, Wu J, Zhang R, Chen L, Chen Z, Wang X, et al. Autophagy-targeted vaccine of LC3-LpqH DNA and its protective immunity in a murine model of tuberculosis. Vaccine (2014) 32(20):2308–14. doi:10.1016/j.vaccine.2014.02.069
46. Schiebler M, Brown K, Hegyi K, Newton SM, Renna M, Hepburn L, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med (2015) 7(2):127–39. doi:10.15252/emmm.201404137
47. Napier RJ, Rafi W, Cheruvu M, Powell KR, Zaunbrecher MA, Bornmann W, et al. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe (2011) 10(5):475–85. doi:10.1016/j.chom.2011.09.010
48. Bruns H, Stegelmann F, Fabri M, Dohner K, van Zandbergen G, Wagner M, et al. Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages. J Immunol (2012) 189(8):4069–78. doi:10.4049/jimmunol.1201538
49. Steiger J, Stephan A, Inkeles MS, Realegeno S, Bruns H, Kroll P, et al. Imatinib triggers phagolysosome acidification and antimicrobial activity against Mycobacterium bovis Bacille Calmette-Guerin in glucocorticoid-treated human macrophages. J Immunol (2016) 197(1):222–32. doi:10.4049/jimmunol.1502407
50. Parihar SP, Guler R, Khutlang R, Lang DM, Hurdayal R, Mhlanga MM, et al. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis (2014) 209(5):754–63. doi:10.1093/infdis/jit550
51. Skerry C, Pinn ML, Bruiners N, Pine R, Gennaro ML, Karakousis PC. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J Antimicrob Chemother (2014) 69(9):2453–7. doi:10.1093/jac/dku166
52. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol (2001) 2(3):261–8. doi:10.1038/85330
53. Yao S, Chen L. PD-1 as an immune modulatory receptor. Cancer J (2014) 20(4):262–4. doi:10.1097/PPO.0000000000000060
54. Jurado JO I, Alvarez B, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol (2008) 181(1):116–25. doi:10.4049/jimmunol.181.1.116
55. Alvarez IB, Pasquinelli V, Jurado JO, Abbate E, Musella RM, de la Barrera SS, et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis (2010) 202(4):524–32. doi:10.1086/654932
57. Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, Bandaru A, et al. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation with Mycobacterium tuberculosis. J Infect Dis (2011) 203(9):1256–63. doi:10.1093/infdis/jir011
58. Hassan SS, Akram M, King EC, Dockrell HM, Cliff JM. PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS One (2015) 10(9):e0137646. doi:10.1371/journal.pone.0137646
59. Pollock KM, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, Kon OM, et al. PD-1 expression and cytokine secretion profiles of Mycobacterium tuberculosis-specific CD4+ T-cell subsets; potential correlates of containment in HIV-TB co-infection. PLoS One (2016) 11(1):e0146905. doi:10.1371/journal.pone.0146905
60. Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther (2015) 37(4):764–82. doi:10.1016/j.clinthera.2015.02.018
61. Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine (2015) 33(51):7377–85. doi:10.1016/j.vaccine.2015.10.057
62. Fu J I, Malm J, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res (2014) 74(15):4042–52. doi:10.1158/0008-5472.CAN-13-2685
63. Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A (2010) 107(30):13402–7. doi:10.1073/pnas.1007394107
64. Attanasio J, Wherry EJ. Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity (2016) 44(5):1052–68. doi:10.1016/j.immuni.2016.04.022
65. Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, et al. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to plasmodium-induced acute immune pathology. PLoS Pathog (2012) 8(2):e1002504. doi:10.1371/journal.ppat.1002504
66. Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, et al. Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4(+) T cell immunity. Immunity (2016) 45(2):333–45. doi:10.1016/j.immuni.2016.07.017
67. Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity (2016) 45(2):358–73. doi:10.1016/j.immuni.2016.07.008
68. Martinez GJ, Pereira RM, Aijo T, Kim EY, Marangoni F, Pipkin ME, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity (2015) 42(2):265–78. doi:10.1016/j.immuni.2015.01.006
69. Shou J, Jing J, Xie J, You L, Jing Z, Yao J, et al. Nuclear factor of activated T cells in cancer development and treatment. Cancer Lett (2015) 361(2):174–84. doi:10.1016/j.canlet.2015.03.005
70. Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol (2015) 5:34. doi:10.3389/fonc.2015.00034
71. Snelgrove RJ, Cornere MM, Edwards L, Dagg B, Keeble J, Rodgers A, et al. OX40 ligand fusion protein delivered simultaneously with the BCG vaccine provides superior protection against murine Mycobacterium tuberculosis infection. J Infect Dis (2012) 205(6):975–83. doi:10.1093/infdis/jir868
72. Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol (2015) 42(4):640–55. doi:10.1053/j.seminoncol.2015.05.014
73. Zaunders JJ, Munier ML, Seddiki N, Pett S, Ip S, Bailey M, et al. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol (2009) 183(4):2827–36. doi:10.4049/jimmunol.0803548
74. Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J Immunol (2008) 180(4):2140–8. doi:10.4049/jimmunol.180.4.2140
75. Guo Z, Wang X, Cheng D, Xia Z, Luan M, Zhang S. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One (2014) 9(2):e89350. doi:10.1371/journal.pone.0089350
76. Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur J Immunol (2004) 34(5):1433–40. doi:10.1002/eji.200324021
77. Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol (2004) 22:531–62. doi:10.1146/annurev.immunol.21.120601.141122
78. Henao-Tamayo MI, Obregon-Henao A, Arnett K, Shanley CA, Podell B, Orme IM, et al. Effect of Bacillus Calmette-Guerin vaccination on CD4+Foxp3+ T cells during acquired immune response to Mycobacterium tuberculosis infection. J Leukoc Biol (2015) 99(4):605–17. doi:10.1189/jlb.4A0614-308RR
79. Luo Y, Ma X, Liu X, Lu X, Niu H, Yu H, et al. IL-28B down-regulates regulatory T cells but does not improve the protective immunity following tuberculosis subunit vaccine immunization. Int Immunol (2015) 28(2):77–85. doi:10.1093/intimm/dxv061
80. Wergeland I, Assmus J, Dyrhol-Riise AM. T regulatory cells and immune activation in Mycobacterium tuberculosis infection and the effect of preventive therapy. Scand J Immunol (2011) 73(3):234–42. doi:10.1111/j.1365-3083.2010.02496.x
81. Lieske NV, Tonby K, Kvale D, Dyrhol-Riise AM, Tasken K. Targeting tuberculosis and HIV infection-specific regulatory T cells with MEK/ERK signaling pathway inhibitors. PLoS One (2015) 10(11):e0141903. doi:10.1371/journal.pone.0141903
82. O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med (2004) 10(8):801–5. doi:10.1038/nm0804-801
83. Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol (2008) 69(11):760–70. doi:10.1016/j.humimm.2008.07.017
84. Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol (2009) 27:393–422. doi:10.1146/annurev.immunol.021908.132703
85. Marin ND, Paris SC, Velez VM, Rojas CA, Rojas M, Garcia LF. Regulatory T cell frequency and modulation of IFN-gamma and IL-17 in active and latent tuberculosis. Tuberculosis (Edinb) (2010) 90(4):252–61. doi:10.1016/j.tube.2010.05.003
86. Feruglio SL, Tonby K, Kvale D, Dyrhol-Riise AM. Early dynamics of T helper cell cytokines and T regulatory cells in response to treatment of active Mycobacterium tuberculosis infection. Clin Exp Immunol (2015) 179(3):454–65. doi:10.1111/cei.12468
87. Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med (2010) 207(7):1409–20. doi:10.1084/jem.20091885
88. Kumar NP, Moideen K, Banurekha VV, Nair D, Sridhar R, Nutman TB, et al. IL-27 and TGFbeta mediated expansion of Th1 and adaptive regulatory T cells expressing IL-10 correlates with bacterial burden and disease severity in pulmonary tuberculosis. Immun Inflamm Dis (2015) 3(3):289–99. doi:10.1002/iid3.68
89. He XY, Xiao L, Chen HB, Hao J, Li J, Wang YJ, et al. T regulatory cells and Th1/Th2 cytokines in peripheral blood from tuberculosis patients. Eur J Clin Microbiol Infect Dis (2010) 29(6):643–50. doi:10.1007/s10096-010-0908-0
90. Jackson-Sillah D, Cliff JM, Mensah GI, Dickson E, Sowah S, Tetteh JK, et al. Recombinant ESAT-6-CFP10 fusion protein induction of Th1/Th2 cytokines and FoxP3 expressing Treg cells in pulmonary TB. PLoS One (2013) 8(6):e68121. doi:10.1371/journal.pone.0068121
91. Rossowska J, Pajtasz-Piasecka E, Anger N, Wojas-Turek J, Kicielinska J, Piasecki E, et al. Cyclophosphamide and IL-12-transduced DCs enhance the antitumor activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs number. J Immunother (2014) 37(9):427–39. doi:10.1097/CJI.0000000000000054
92. Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, et al. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis. Cancer Res (2014) 74(3):727–37. doi:10.1158/0008-5472.CAN-13-2584
93. Centuori SM, Trad M, LaCasse CJ, Alizadeh D, Larmonier CB, Hanke NT, et al. Myeloid-derived suppressor cells from tumor-bearing mice impair TGF-beta-induced differentiation of CD4+CD25+FoxP3+ Tregs from CD4+CD25-FoxP3- T cells. J Leukoc Biol (2012) 92(5):987–97. doi:10.1189/jlb.0911465
94. Yang B, Wang X, Jiang J, Zhai F, Cheng X. Identification of CD244-expressing myeloid-derived suppressor cells in patients with active tuberculosis. Immunol Lett (2014) 158(1–2):66–72. doi:10.1016/j.imlet.2013.12.003
95. du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med (2013) 188(6):724–32. doi:10.1164/rccm.201302-0249OC
96. Dorhoi A, Kaufmann SH. Versatile myeloid cell subsets contribute to tuberculosis-associated inflammation. Eur J Immunol (2015) 45(8):2191–202. doi:10.1002/eji.201545493
97. Fedatto PF, Sergio CA, Paula MO, Gembre AF, Franco LH, Wowk PF, et al. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+)/CD4(+) Foxp3(+) cells. Immunology (2012) 137(3):239–48. doi:10.1111/imm.12006
98. Bhattacharya D, Dwivedi VP, Kumar S, Reddy MC, Van Kaer L, Moodley P, et al. Simultaneous inhibition of T helper 2 and T regulatory cell differentiation by small molecules enhances Bacillus Calmette-Guerin vaccine efficacy against tuberculosis. J Biol Chem (2014) 289(48):33404–11. doi:10.1074/jbc.M114.600452
99. Quinn KM, Rich FJ, Goldsack LM, de Lisle GW, Buddle BM, Delahunt B, et al. Accelerating the secondary immune response by inactivating CD4(+)CD25(+) T regulatory cells prior to BCG vaccination does not enhance protection against tuberculosis. Eur J Immunol (2008) 38(3):695–705. doi:10.1002/eji.200737888
100. Huang Z, Wu Y, Zhou X, Qian J, Zhu W, Shu Y, et al. Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review. Future Oncol (2015) 11(11):1687–99. doi:10.2217/fon.15.70
101. Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J (2015) 13:265–72. doi:10.1016/j.csbj.2015.03.008
102. Muller T. The safety of istradefylline for the treatment of Parkinson’s disease. Expert Opin Drug Saf (2015) 14(5):769–75. doi:10.1517/14740338.2015.1014798
103. Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med (2006) 203(12):2691–702. doi:10.1084/jem.20061104
104. Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res (2015) 21(1):39–48. doi:10.1158/1078-0432.CCR-14-1711
105. Byersdorfer CA. The role of fatty acid oxidation in the metabolic reprograming of activated T-cells. Front Immunol (2014) 5:641. doi:10.3389/fimmu.2014.00641
106. Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev (2008) 222:180–91. doi:10.1111/j.1600-065X.2008.00608.x
107. Yang CS, Kim JJ, Lee HM, Jin HS, Lee SH, Park JH, et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy (2014) 10(5):785–802. doi:10.4161/auto.28072
108. Kala R, Shah HN, Martin SL, Tollefsbol TO. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer (2015) 15:672. doi:10.1186/s12885-015-1693-z
109. Hu J, Jing H, Lin H. Sirtuin inhibitors as anticancer agents. Future Med Chem (2014) 6(8):945–66. doi:10.4155/fmc.14.44
110. Xu R, Shimizu F, Hovinga K, Beal K, Karimi S, Droms L, et al. Molecular and clinical effects of notch inhibition in glioma patients: a phase 0/I trial. Clin Cancer Res (2016) 22(19):4786–96. doi:10.1158/1078-0432.CCR-16-0048
111. Li W, Li J, Sama AE, Wang H. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med (2013) 19:203–11. doi:10.2119/molmed.2013.00064
112. Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res (2014) 74(20):5723–33. doi:10.1158/0008-5472.CAN-13-2347
113. Grover A, Troudt J, Foster C, Basaraba R, Izzo A. High mobility group box 1 acts as an adjuvant for tuberculosis subunit vaccines. Immunology (2014) 142(1):111–23. doi:10.1111/imm.12236
114. Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res (2015) 3(2):136–48. doi:10.1158/2326-6066.CIR-14-0036
115. Sternberg C, Armstrong A, Pili R, Ng S, Huddart R, Agarwal N, et al. Randomized, double-blind, placebo-controlled phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J Clin Oncol (2016) 34(22):2636–43. doi:10.1200/JCO.2016.66.9697
116. Blumenthal A, Nagalingam G, Huch JH, Walker L, Guillemin GJ, Smythe GA, et al. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS One (2012) 7(5):e37314. doi:10.1371/journal.pone.0037314
117. Mehra S, Alvarez X, Didier PJ, Doyle LA, Blanchard JL, Lackner AA, et al. Granuloma correlates of protection against tuberculosis and mechanisms of immune modulation by Mycobacterium tuberculosis. J Infect Dis (2013) 207(7):1115–27. doi:10.1093/infdis/jis778
118. Tanner R, Kakalacheva K, Miller E, Pathan AA, Chalk R, Sander CR, et al. Serum indoleamine 2,3-dioxygenase activity is associated with reduced immunogenicity following vaccination with MVA85A. BMC Infect Dis (2014) 14:660. doi:10.1186/s12879-014-0660-7
119. Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, et al. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep (2015) 13(2):412–24. doi:10.1016/j.celrep.2015.08.077
120. Bjoern J, Iversen TZ, Nitschke NJ, Andersen MH, Svane IM. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy (2016) 18(8):1043–55. doi:10.1016/j.jcyt.2016.05.010
121. Jochems C, Fantini M, Fernando RI, Kwilas AR, Donahue RN, Lepone LM, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget (2016) 7(25):37762–72. doi:10.18632/oncotarget.9326
122. Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, et al. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res (2012) 72(24):6447–56. doi:10.1158/0008-5472.CAN-12-0193
123. Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol (2011) 77(1):12–9. doi:10.1016/j.critrevonc.2010.02.004
124. Nishioka Y, Aono Y, Sone S. Role of tyrosine kinase inhibitors in tumor immunology. Immunotherapy (2011) 3(1):107–16. doi:10.2217/imt.10.79
125. Draghiciu O, Nijman HW, Hoogeboom BN, Meijerhof T, Daemen T. Sunitinib depletes myeloid-derived suppressor cells and synergizes with a cancer vaccine to enhance antigen-specific immune responses and tumor eradication. Oncoimmunology (2015) 4(3):e989764. doi:10.4161/2162402X.2014.989764
126. Lowe DB, Bose A, Taylor JL, Tawbi H, Lin Y, Kirkwood JM, et al. Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology (2014) 3(1):e27589. doi:10.4161/onci.27589
127. Araki K, Ahmed R. AMPK: a metabolic switch for CD8+ T-cell memory. Eur J Immunol (2013) 43(4):878–81. doi:10.1002/eji.201343483
128. Singhal A, Jie L, Kumar P, Hong GS, Leow MK, Paleja B, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med (2014) 6(263):263ra159. doi:10.1126/scitranslmed.3009885
129. Ong CW, Elkington PT, Brilha S, Ugarte-Gil C, Tome-Esteban MT, Tezera LB, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog (2015) 11(5):e1004917. doi:10.1371/journal.ppat.1004917
130. Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, Yurchenko E, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity (2015) 42(1):41–54. doi:10.1016/j.immuni.2014.12.030
131. Kim JK, Yuk JM, Kim SY, Kim TS, Jin HS, Yang CS, et al. MicroRNA-125a inhibits autophagy activation and antimicrobial responses during mycobacterial infection. J Immunol (2015) 194(11):5355–65. doi:10.4049/jimmunol.1402557
132. Vashisht R, Brahmachari SK. Metformin as a potential combination therapy with existing front-line antibiotics for tuberculosis. J Transl Med (2015) 13:83. doi:10.1186/s12967-015-0443-y
133. Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci U S A (2015) 112(6):1809–14. doi:10.1073/pnas.1417636112
134. Chen M, Zhang J, Hu F, Liu S, Zhou Z. Metformin affects the features of a human hepatocellular cell line (HepG2) by regulating macrophage polarization in a co-culture microenviroment. Diabetes Metab Res Rev (2015) 31(8):781–9. doi:10.1002/dmrr.2761
135. Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H, et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget (2015) 6(34):36441–55. doi:10.18632/oncotarget.5541
136. Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol (2012) 13(10):907–15. doi:10.1038/ni.2386
137. Zeng H, Chi H. mTOR signaling and transcriptional regulation in T lymphocytes. Transcription (2014) 5(2):e28263. doi:10.4161/trns.28263
138. Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell (2011) 146(5):772–84. doi:10.1016/j.cell.2011.07.033
139. Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol (2013) 14(11):1173–82. doi:10.1038/ni.2714
140. Kheshtchin N, Arab S, Ajami M, Mirzaei R, Ashourpour M, Mousavi N, et al. Inhibition of HIF-1alpha enhances anti-tumor effects of dendritic cell-based vaccination in a mouse model of breast cancer. Cancer Immunol Immunother (2016) 65(10):1159–67. doi:10.1007/s00262-016-1879-5
141. Braverman J, Sogi KM, Benjamin D, Nomura DK, Stanley SA. HIF-1alpha is an essential mediator of IFN-gamma-dependent immunity to Mycobacterium tuberculosis. J Immunol (2016) 197(4):1287–97. doi:10.4049/jimmunol.1600266
142. Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov (2012) 11(6):443–61. doi:10.1038/nrd3738
143. Vachharajani V, Liu T, McCall CE. Epigenetic coordination of acute systemic inflammation: potential therapeutic targets. Expert Rev Clin Immunol (2014) 10(9):1141–50. doi:10.1586/1744666X.2014.943192
144. Baharia RK, Tandon R, Sharma T, Suthar MK, Das S, Siddiqi MI, et al. Recombinant NAD-dependent SIR-2 protein of Leishmania donovani: immunobiochemical characterization as a potential vaccine against visceral leishmaniasis. PLoS Negl Trop Dis (2015) 9(3):e0003557. doi:10.1371/journal.pntd.0003557
145. Jing H, Lin H. Sirtuins in epigenetic regulation. Chem Rev (2015) 115(6):2350–75. doi:10.1021/cr500457h
146. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A (2008) 105(9):3374–9. doi:10.1073/pnas.0712145105
147. Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol (2010) 30(19):4712–21. doi:10.1128/MCB.00657-10
148. Cardoso F, Castro F, Moreira-Teixeira L, Sousa J, Torrado E, Silvestre R, et al. Myeloid sirtuin 2 expression does not impact long-term Mycobacterium tuberculosis control. PLoS One (2015) 10(7):e0131904. doi:10.1371/journal.pone.0131904
149. Chen X, Lu Y, Zhang Z, Wang J, Yang H, Liu G. Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology (2015) 145(4):455–67. doi:10.1111/imm.12473
150. Mellini P, Valente S, Mai A. Sirtuin modulators: an updated patent review (2012–2014). Expert Opin Ther Pat (2015) 25(1):5–15. doi:10.1517/13543776.2014.982532
151. Opal S, Ellis JL, Suri V, Freudenberg JM, Vlasuk GP, Li Y, et al. Sirt1 activation markedly alters transcription profiles and improves outcome in experimental sepsis. Shock (2016) 45(4):411–18. doi:10.1097/SHK.0000000000000528
152. Owczarczyk AB, Schaller MA, Reed M, Rasky AJ, Lombard DB, Lukacs NW. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus-induced immune responses. J Immunol (2015) 195(4):1637–46. doi:10.4049/jimmunol.1500326
153. Jia YY, Lu J, Huang Y, Liu G, Gao P, Wan YZ, et al. The involvement of NFAT transcriptional activity suppression in SIRT1-mediated inhibition of COX-2 expression induced by PMA/Ionomycin. PLoS One (2014) 9(5):e97999. doi:10.1371/journal.pone.0097999
154. Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen X, et al. Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1. Proc Natl Acad Sci U S A (2015) 112(9):E957–65. doi:10.1073/pnas.1420419112
155. Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem (2006) 281(32):22429–33. doi:10.1074/jbc.R600015200
156. Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis (2008) 4(2):68–75. doi:10.4161/org.4.2.5851
157. Luo T, Dunphy PS, Lina TT, McBride JW. Ehrlichia chaffeensis exploits canonical and noncanonical host Wnt signaling pathways to stimulate phagocytosis and promote intracellular survival. Infect Immun (2015) 84(3):686–700. doi:10.1128/IAI.01289-15
158. Nusse R. Cell signalling: disarming Wnt. Nature (2015) 519(7542):163–4. doi:10.1038/nature14208
159. Schaale K, Neumann J, Schneider D, Ehlers S, Reiling N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur J Cell Biol (2011) 90(6–7):553–9. doi:10.1016/j.ejcb.2010.11.004
160. Li Y, Shi J, Yang J, Ma Y, Cheng L, Zeng J, et al. A Wnt/beta-catenin negative feedback loop represses TLR-triggered inflammatory responses in alveolar epithelial cells. Mol Immunol (2014) 59(2):128–35. doi:10.1016/j.molimm.2014.02.002
161. Wu X, Deng G, Hao X, Li Y, Zeng J, Ma C, et al. A caspase-dependent pathway is involved in Wnt/beta-catenin signaling promoted apoptosis in Bacillus Calmette-Guerin infected RAW264.7 macrophages. Int J Mol Sci (2014) 15(3):5045–62. doi:10.3390/ijms15035045
162. Wu X, Deng G, Li M, Li Y, Ma C, Wang Y, et al. Wnt/beta-catenin signaling reduces Bacillus Calmette-Guerin-induced macrophage necrosis through a ROS-mediated PARP/AIF-dependent pathway. BMC Immunol (2015) 16:16. doi:10.1186/s12865-015-0080-5
163. Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science (2010) 329(5993):849–53. doi:10.1126/science.1188510
164. Cannon MJ, Ghosh D, Gujja S. Signaling circuits and regulation of immune suppression by ovarian tumor-associated macrophages. Vaccines (Basel) (2015) 3(2):448–66. doi:10.3390/vaccines3020448
165. Xiao YF, Yong X, Tang B, Qin Y, Zhang JW, Zhang D, et al. Notch and Wnt signaling pathway in cancer: crucial role and potential therapeutic targets (review). Int J Oncol (2016) 48(2):437–49. doi:10.3892/ijo.2015.3280
166. Kulak O, Chen H, Holohan B, Wu X, He H, Borek D, et al. Disruption of Wnt/beta-catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol Cell Biol (2015) 35(14):2425–35. doi:10.1128/MCB.00392-15
167. Kapoor N, Narayana Y, Patil SA, Balaji KN. Nitric oxide is involved in Mycobacterium bovis Bacillus Calmette-Guerin-activated Jagged1 and Notch1 signaling. J Immunol (2010) 184(6):3117–26. doi:10.4049/jimmunol.0903174
168. Manoranjan B, Venugopal C, McFarlane N, Doble BW, Dunn SE, Scheinemann K, et al. Medulloblastoma stem cells: where development and cancer cross pathways. Pediatr Res (2012) 71(4 Pt 2):516–22. doi:10.1038/pr.2011.62
169. Borggrefe T, Lauth M, Zwijsen A, Huylebroeck D, Oswald F, Giaimo BD. The Notch intracellular domain integrates signals from Wnt, Hedgehog, TGFbeta/BMP and hypoxia pathways. Biochim Biophys Acta (2016) 1863(2):303–13. doi:10.1016/j.bbamcr.2015.11.020
170. Mathieu M, Cotta-Grand N, Daudelin JF, Thebault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol (2013) 91(1):82–8. doi:10.1038/icb.2012.53
171. Palaga T, Ratanabunyong S, Pattarakankul T, Sangphech N, Wongchana W, Hadae Y, et al. Notch signaling regulates expression of Mcl-1 and apoptosis in PPD-treated macrophages. Cell Mol Immunol (2013) 10(5):444–52. doi:10.1038/cmi.2013.22
172. Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett (2015) 369(1):20–7. doi:10.1016/j.canlet.2015.07.048
173. Holla S, Ghorpade DS, Singh V, Bansal K, Balaji KN. Mycobacterium bovis BCG promotes tumor cell survival from tumor necrosis factor-alpha-induced apoptosis. Mol Cancer (2014) 13:210. doi:10.1186/1476-4598-13-210
174. Burness CB. Sonidegib: first global approval. Drugs (2015) 75(13):1559–66. doi:10.1007/s40265-015-0458-y
175. Ghorpade DS, Holla S, Kaveri SV, Bayry J, Patil SA, Balaji KN. Sonic hedgehog-dependent induction of microRNA 31 and microRNA 150 regulates Mycobacterium bovis BCG-driven toll-like receptor 2 signaling. Mol Cell Biol (2013) 33(3):543–56. doi:10.1128/MCB.01108-12
176. Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel) (2016) 8(2):pii: E22. doi:10.3390/cancers8020022
177. Bansal K, Trinath J, Chakravortty D, Patil SA, Balaji KN. Pathogen-specific TLR2 protein activation programs macrophages to induce Wnt-beta-catenin signaling. J Biol Chem (2011) 286(42):37032–44. doi:10.1074/jbc.M111.260414
178. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci (2004) 29(2):95–102. doi:10.1016/j.tibs.2003.12.004
179. Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, et al. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity (2006) 24(5):563–74. doi:10.1016/j.immuni.2006.02.014
180. Neumann J, Schaale K, Farhat K, Endermann T, Ulmer AJ, Ehlers S, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J (2010) 24(11):4599–612. doi:10.1096/fj.10-160994
181. Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol (2005) 6(8):777–84. doi:10.1038/ni1221
182. Lutay N, Hakansson G, Alaridah N, Hallgren O, Westergren-Thorsson G, Godaly G. Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response. PLoS One (2014) 9(1):e86466. doi:10.1371/journal.pone.0086466
183. Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity (2016) 44(2):274–86. doi:10.1016/j.immuni.2016.01.018
184. Wang H, Huang S, Yan K, Fang X, Abussaud A, Martinez A, et al. Tideglusib, a chemical inhibitor of GSK3beta, attenuates hypoxic-ischemic brain injury in neonatal mice. Biochim Biophys Acta (2016) 1860(10):2076–85. doi:10.1016/j.bbagen.2016.06.027
185. Noh KT, Son KH, Jung ID, Kang TH, Choi CH, Park YM. Glycogen synthase kinase-3beta (GSK-3beta) inhibition enhances dendritic cell-based cancer vaccine potency via suppression of interferon-gamma-induced indoleamine 2,3-dioxygenase expression. J Biol Chem (2015) 290(19):12394–402. doi:10.1074/jbc.M114.628578
186. Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol (2015) 98(3):347–56. doi:10.1189/jlb.5RI0315-096R
187. Lerm M, Netea MG. Trained immunity: a new avenue for tuberculosis vaccine development. J Intern Med (2015) 279(4):337–46. doi:10.1111/joim.12449
188. Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Jacobs C, Xavier RJ, et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin Immunol (2014) 155(2):213–9. doi:10.1016/j.clim.2014.10.005
189. van der Meer JW, Joosten LA, Riksen N, Netea MG. Trained immunity: a smart way to enhance innate immune defence. Mol Immunol (2015) 68(1):40–4. doi:10.1016/j.molimm.2015.06.019
190. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi:10.1126/science.1250684
191. Gherardini L, Sharma A, Capobianco E, Cinti C. Targeting cancer with epi-drugs: a precision medicine perspective. Curr Pharm Biotechnol (2016) 17(10):856–65. doi:10.2174/1381612822666160527154757
192. Venza M, Visalli M, Catalano T, Beninati C, Teti D, Venza I. Epidrugs in the immunotherapy of cutaneous and uveal melanoma. Anticancer Agents Med Chem (2016) 16:1–16. doi:10.2174/1871520616666160425110401
193. Xiong F, Mou YZ, Xiang XY. Inhibition of mouse B16 melanoma by sodium butyrate correlated to tumor associated macrophages differentiation suppression. Int J Clin Exp Med (2015) 8(3):4170–4.
194. Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics (2011) 5(5):485–96. doi:10.1186/1479-7364-5-5-485
195. Wang J, Sinha T, Wynshaw-Boris A. Wnt signaling in mammalian development: lessons from mouse genetics. Cold Spring Harb Perspect Biol (2012) 4(5):pii: a007963. doi:10.1101/cshperspect.a007963
196. Mathern DR, Laitman LE, Hovhannisyan Z, Dunkin D, Farsio S, Malik TJ, et al. Mouse and human Notch-1 regulate mucosal immune responses. Mucosal Immunol (2014) 7(4):995–1005. doi:10.1038/mi.2013.118
197. Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, et al. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med (2013) 188(4):492–502. doi:10.1164/rccm.201208-1385OC
198. Schaale K, Brandenburg J, Kispert A, Leitges M, Ehlers S, Reiling N. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J Immunol (2013) 191(10):5182–95. doi:10.4049/jimmunol.1201819
199. Poirier V, Bach H, v-Gay YA. Mycobacterium tuberculosis promotes anti-apoptotic activity of the macrophage by PtpA protein-dependent dephosphorylation of host GSK3alpha. J Biol Chem (2014) 289(42):29376–85. doi:10.1074/jbc.M114.582502
Keywords: tuberculosis, vaccines, immunometabolism, immuno-oncology
Citation: Jayashankar L and Hafner R (2016) Adjunct Strategies for Tuberculosis Vaccines: Modulating Key Immune Cell Regulatory Mechanisms to Potentiate Vaccination. Front. Immunol. 7:577. doi: 10.3389/fimmu.2016.00577
Received: 01 September 2016; Accepted: 23 November 2016;
Published: 16 December 2016
Edited by:
Shen-An Hwang, University of Texas Medical School at Houston, USAReviewed by:
Willem Hanekom, Gates Foundation, USADaniel Olive, French Institute of Health and Medical Research, France
Copyright: © 2016 Jayashankar and Hafner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard Hafner, cmhhZm5lckBuaWFpZC5uaWguZ292
 Lakshmi Jayashankar
Lakshmi Jayashankar Richard Hafner2*
Richard Hafner2*