- 1Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- 2Innovation Center for Medical Redox Navigation, Kyushu University, Fukuoka, Japan
- 3Department of Psychiatry, Graduate School of Medical Sciences, Saga University, Saga, Japan
The pathophysiology of bipolar disorder, especially the underlying mechanisms of the bipolarity between manic and depressive states, has yet to be clarified. Microglia, immune cells in the brain, play important roles in the process of brain inflammation, and recent positron emission tomography studies have indicated microglial overactivation in the brain of patients with bipolar disorder. We have recently developed a technique to induced microglia-like (iMG) cells from peripheral blood (monocytes). We introduce a novel translational approach focusing on bipolar disorder using this iMG technique. We hypothesize that immunological conditional changes in microglia may contribute to the shift between manic and depressive states, and thus we herein analyzed gene profiling patterns of iMG cells from three patients with rapid cycling bipolar disorder during both manic and depressive states, respectively. We revealed that the gene profiling patterns are different between manic and depressive states. The profiling pattern of case 1 showed that M1 microglia is dominant in the manic state compared to the depressive state. However, the patterns of cases 2 and 3 were not consistent with the pattern of case 1. CD206, a mannose receptor known as a typical M2 marker, was significantly downregulated in the manic state among all three patients. This is the first report to indicate the importance of shifting microglial M1/M2 characteristics, especially the CD206 gene expression pattern between depressive and manic states. Further translational studies are needed to dig up the microglial roles in the underlying biological mechanisms of bipolar disorder.
Bipolar Disorder and Microglia
The pathophysiology of bipolar disorder has yet to be well understood, while recent studies have indicated abnormal immunological functions may be a contributing factor (1, 2). Microglia, immune cells in the brain, play important roles in the process of brain inflammation, and recent positron emission tomography (PET) studies have shown microglial overactivation in the brain of patients with various psychiatric disorders including bipolar disorder (3–8). Based on the above evidence, microglia has been highlighted in the study of various psychiatric disorders to understand the underlying biological mechanisms (9–12).
We have recently developed a technique to induced microglia-like (iMG) cells from peripheral blood (13) and are now confirming the utilities of this technique for psychiatric research (14, 15). The underlying mechanisms of the bipolarity between manic and depressive states have yet to be clarified. Immunological conditional changes in microglia may contribute to the manic–depressive shift in bipolar disorder. In the field of immunology, M1/M2 polarization is recognized as a useful marker of macrophages and related cells including microglia. Polarization pattern is well known to distinguish functional phenotypes: pro-inflammation (M1) and anti-inflammation (M2) (16, 17). Recently, M1/M2 polarization has been highlighted in the understanding of psychiatric disorders (18–20). However, there is no research analyzing M1/M2 polarization of microglia in patients with bipolar disorder. We hypothesize that the expression profile of inflammation-related genes known as M1 (CD45, CD80, HLA-DR, TNF-α, IL-1β, and IL-23) or M2 markers (CD206, CD209, CD23, BDNF, IL-10, and CCL18) of microglia may shift between manic and depressive states. In order to clarify this hypothesis, we herein analyzed iMG cells from three patients with rapid cycling bipolar disorder during both manic and depressive states, respectively.
M1/M2 Microglia and Bipolar Disorder
Patients’ demographic data are shown in Table S1 in Supplementary Material. We produced iMG cells of each patient from both manic and depressive states and compared the gene expression profiles between both states. Relative gene expression (normalized by depressive state) of M1 and M2 markers are shown in Figures 1 and 2A,B, respectively.
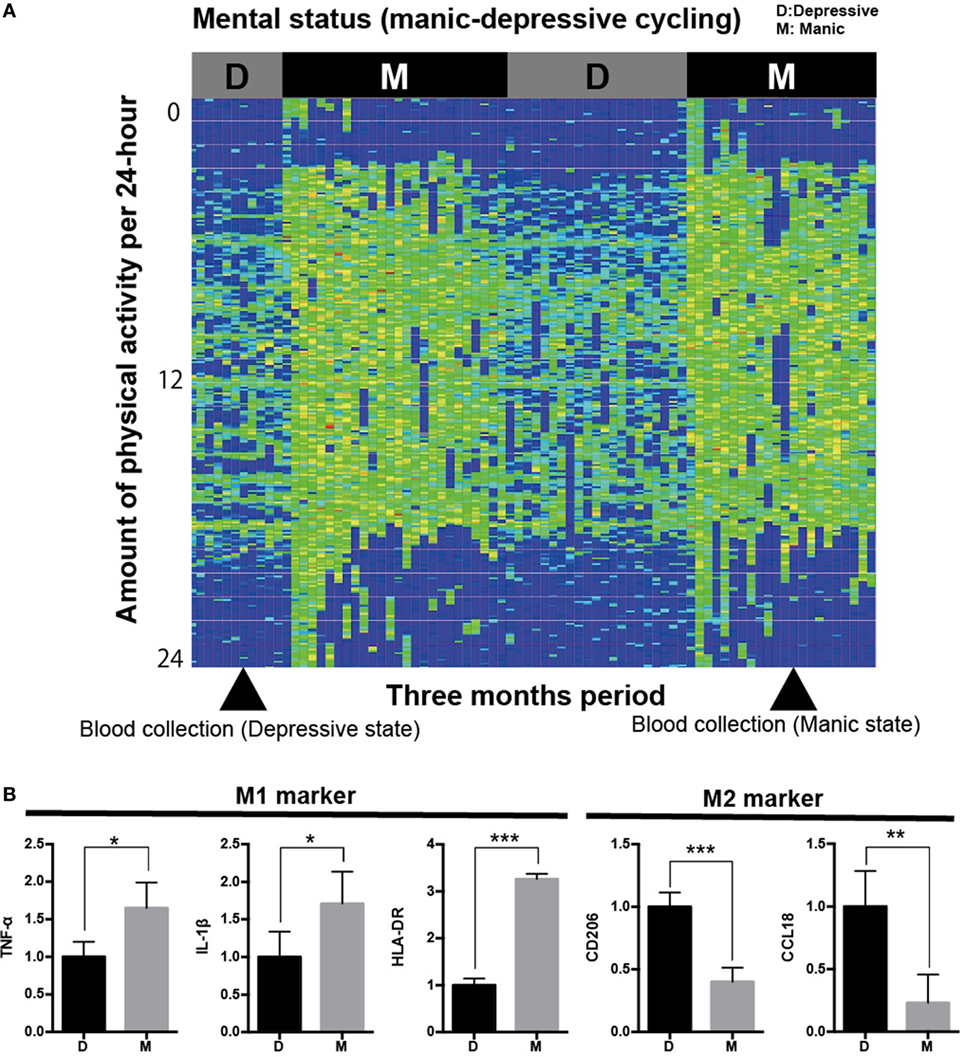
Figure 1. Analysis of iMG cells from a typical case with rapid cycling bipolar disorder. (A) Physical/mental activity of a patient with rapid cycling bipolar disorder for 3 months (case 1). (B) Gene profiling pattern of iMG cells from case 1 showed that M1 microglia is dominant in the manic state compared to the depressive state.
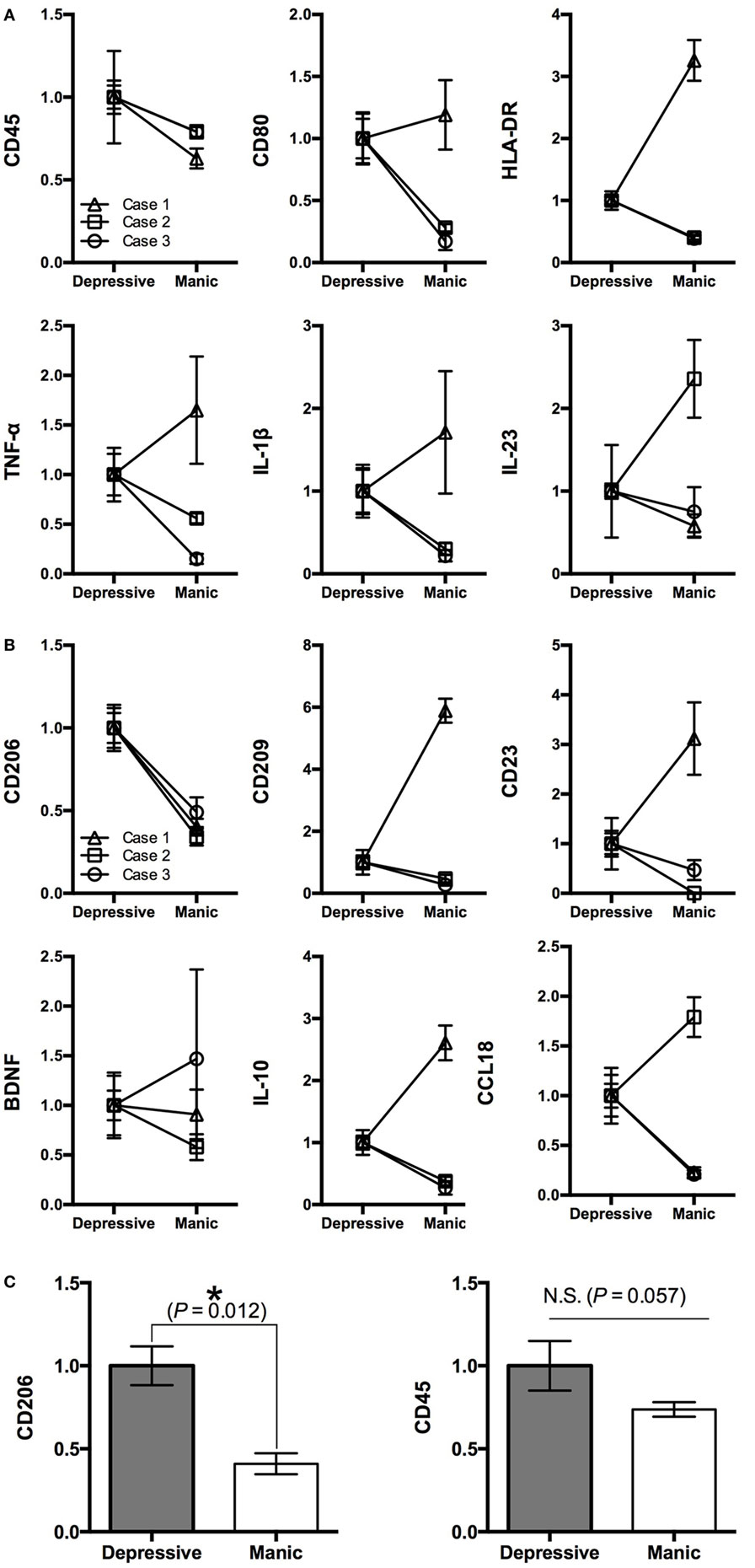
Figure 2. Gene profiling pattern of iMG cells between depressive and manic states among three patients. (A) M1 markers, (B) M2 markers, and (C) statistical analysis of CD206 and CD45 among the three patients (mean value ± SD).
We revealed that the gene profiling patterns of iMG cells are different between manic and depressive states. The profiling pattern of case 1 showed that M1 microglia is dominant in the manic state compared to the depressive state (Figure 1B). However, the patterns of cases 2 and 3 were not consistent with the pattern of case 1 (Figures 2A,B).
For M1 markers, CD45 was downregulated in the manic state among all three patients. Other M1 markers such as TNF-α, IL-1β, IL-23, CD80, and HLA-DR shifted differently among the patients (Figure 2A). For M2 markers, CD206 was downregulated in the manic state among all three patients. Other M2 markers such as BDNF, IL-10, CCL18, CD23, and CD209 shifted differently among the patients (Figure 2B). Thus, we performed statistical analysis [Student’s t-test (two-tailed)] among the three patients. As shown in Figure 2C, CD206 was significantly downregulated in the manic state (p = 0.012). On the other hand, CD45 showed no significant difference between manic and depressive states (p = 0.057).
CD206 and Microglia
In the present study, we have shown that downregulation of the CD206 gene of iMG cells in the manic state was consistent across all three patients with bipolar disorder. CD206, known as a mannose receptor, is a 175-kDa transmembrane protein, mostly expressed by macrophages, dendritic cells, and endothelial cells. This receptor selectively and efficiently captures mannosylated ligands such as microbial antigen (21). In the brain, CD206 is also expressed in microglia (22, 23) and astrocytes (24, 25). CD206 is widely recognized as a typical M2 microglial marker (23, 26, 27). CD206 has some important cellular functions especially in pinocytosis and phagocytosis on microglia (22, 25, 28–30). Therefore, CD206 may be critical as the first step in the recognition and capture of pathogens in the brain (25). To date, there are no studies focusing on microglial CD206 in psychiatric disorders including bipolar disorder. In the present study, CD206 was downregulated in the manic state. This finding might suggest that the manic state of microglia is more vulnerable to the pathogen and/or insoluble matter compared to the depressive state. Based on the present results, M2 microglia may be dominant in the depressive state in patients with bipolar disorder, especially rapid cycling patients. Further translational investigations should be conducted to clarify our hypothesis.
Limitation and Future Perspectives
One major limitation of the present study is that all three patients were on medication. Given that it took 2 weeks to induce iMG cells, the influence of medication is assumed to be minimal. Additional studies are needed in medication-free patients. On the other hand, recent PET studies have suggested microglial overactivation in patients with bipolar disorder (7, 8). Thus, human PET studies should be conducted in patients with bipolar disorder during both manic–depressive states. However, even the most advanced brain imaging techniques cannot analyze M1/M2 polarization in the human brain, and thus we believe that the iMG technique has an advantage for such analyses in translational research.
A recent study has revealed that the origin of brain microglia is primitive macrophages migrated from the yolk sac before embryonic day 8 (31). One of the limitations in the present study is that iMG cells are not actual microglial cells in the brain. However, we believe that our iMG cells are surrogate cells, which can represent some of the characteristics of brain microglial cells (13). Further comparison studies are needed to investigate the similarity and differences between brain microglial cells and iMG cells in more detail.
The life span of intravascular blood monocytes is only a few days long (32). Thus, we believe that the iMG cells from peripheral blood monocytes are useful not only as a trait marker but also as a state marker in order to assess a variety of mental states. However, an inherent limitation of our iMG analysis is the time delay between the date of blood collection and the date of analysis of iMG cells (after 14 days) due to the necessity of 14-day induction from blood monocytes. Further investigations should be conducted to clarify the impact of time delays in analysis of iMG cells.
The underlying biological mechanism shifting the expression patterns of iMG cells between depressive and manic states has not been clarified at present, while some internal and/or external factors are suggested to contribute to this shifting mechanism. Recent immunological studies have suggested that immune cell activities, including microglia, are modulated by the circadian clock system (33), methylation (34), and/or external stress (35), which may contribute to the activation patterns of microglia during clinical courses of bipolar disorder.
A previous study has shown that peripheral blood mononuclear cells from patients with rapid cycling bipolar disorder presented a different pattern of gene expression between manic and depressive states (36). In addition to the present study, this report also supports the premise that the cellular phenotype of microglia including M1/M2 state is different between depressive and manic states. Further studies are required to determine the clinical importance of these pilot findings.
Conclusion
We introduced a novel translational approach focusing on bipolar disorder using the iMG technique. To our knowledge, this is the first report to indicate the importance of shifting microglial CD206 gene expression between depressive and manic states. This study is the first step toward understanding the contribution of microglia to the pathogenesis of bipolar disorders. Further studies are needed to dig up the microglial roles in bipolar disorder.
Ethics Statement
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Graduate School of Medical Sciences, Kyushu University.
Author Contributions
All the authors contributed substantially to the scientific process leading up to the writing of the present manuscript. TK: the principal investigator of the present research; MO: the first author created the conception and design of the project and wrote the protocol. YH, TM, YM, TM-H, and AM: performed the clinical recruitment. MO, TK, YH, TM, YM, NS, and TM-H: performed the experiments and data analyses/interpretation. MO: wrote the first draft of the manuscript. TK, AM, YM, and SK: made critical revisions of the manuscript. All the authors approved this submission in its current form.
Conflict of Interest Statement
The authors report no financial relationships with commercial interests.
Acknowledgments
The authors would like to thank Ms. Aya Yamada, Ms. Miwa Irie, Ms. Yuka Matsushita, and Mr. Shogo Inamine for their technical assistances.
Funding
This work was supported in part by Grant-in-Aid for Scientific Research on (1) The Japan Agency for Medical Research and Development (AMED) (Yugo-No to TK and Syogaisya-Taisaku-Sogo-Kenkyu-Kaihatsu-Jigyo to SK), (2) The Japan Society for the Promotion of Science—KAKENHI [Grant-in-Aid 26713039 for Young Scientists (A) to TK, Grant-in-Aid 26860933 for Young Scientists (B) to MO], (3) Innovative Areas of The Ministry of Education, Culture, Sports, Science, and Technology, Japan (“Will Dynamics” 16H06403 to TK and “Glia Assembly” 25117011 to SK), (4) Young Principal Investigators’ Research Grant of Innovation Center for Medical Redox Navigation, Kyushu University (to TK), (5) Takeda Medical Research Foundation (to TK), and (6) SENSHIN Medical Research Foundation (to TK, MO, and SK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2016.00676/full#supplementary-material.
References
1. Barbosa IG, Machado-Vieira R, Soares JC, Teixeira AL. The immunology of bipolar disorder. Neuroimmunomodulation (2014) 21(2–3):117–22. doi: 10.1159/000356539
2. Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci (2014) 68(1):21–36. doi:10.1111/pcn.12089
3. Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry (2013) 70(1):49–58. doi:10.1001/jamapsychiatry.2013.272
4. Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol (2010) 13(7):943–50. doi:10.1017/S1461145710000313
5. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med (2009) 50(11):1801–7. doi:10.2967/jnumed.109.066647
6. van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry (2008) 64(9):820–2. doi:10.1016/j.biopsych.2008.04.025
7. Haarman BCM, Riemersma-Van der Lek RF, de Groot JC, Ruhe HG, Klein HC, Zandstra TE, et al. Neuroinflammation in bipolar disorder – A [C-11]-(R)-PK11195 positron emission tomography study. Brain Behav Immun (2014) 40:219–25. doi:10.1016/j.bbi.2014.03.016
8. Haarman BC, Burger H, Doorduin J, Renken RJ, Sibeijn-Kuiper AJ, Marsman JB, et al. Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder – a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun (2016) 56:21–33. doi:10.1016/j.bbi.2015.09.004
9. Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry (2013) 42:115–21. doi:10.1016/j.pnpbp.2011.12.002
10. Kato TA, Yamauchi Y, Horikawa H, Monji A, Mizoguchi Y, Seki Y, et al. Neurotransmitters, psychotropic drugs and microglia: clinical implications for psychiatry. Curr Med Chem (2013) 20(3):331–44. doi:10.2174/092986713804870800
11. Kato TA, Hayakawa K, Monji A, Kanba S. Missing and possible link between neuroendocrine factors, neuropsychiatric disorders, and microglia. Front Integr Neurosci (2013) 7:53. doi:10.3389/fnint.2013.00053
12. Monji A, Kato T, Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci (2009) 63(3):257–65. doi:10.1111/j.1440-1819.2009.01945.x
13. Ohgidani M, Kato TA, Setoyama D, Sagata N, Hashimoto R, Shigenobu K, et al. Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci Rep (2014) 4:4957. doi:10.1038/srep04957
14. Ohgidani M, Kato TA, Kanba S. Introducing directly induced microglia-like (iMG) cells from fresh human monocytes: a novel translational research tool for psychiatric disorders. Front Cell Neurosci (2015) 9:184. doi:10.3389/fncel.2015.00184
15. Sato-Kasai M, Kato TA, Ohgidani M, Mizoguchi Y, Sagata N, Inamine S, et al. Aripiprazole inhibits polyI:C-induced microglial activation possibly via TRPM7. Schizophr Res (2016) 178(1–3):35–43. doi:10.1016/j.schres.2016.08.022
16. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol (2011) 11(11):750–61. doi:10.1038/nri3088
17. Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci (2013) 16(9):1211–U75. doi:10.1038/nn.3469
18. Mikita J, Dubourdieu-Cassagno N, Deloire MSA, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler (2011) 17(1):2–15. doi:10.1177/1352458510379243
19. Nakagawa Y, Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel) (2014) 7(12):1028–48. doi:10.3390/ph7121028
20. Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience (2015) 300:141–54. doi:10.1016/j.neuroscience.2015.05.018
21. Stahl PD, Ezekowitz RAB. The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol (1998) 10(1):50–5. doi:10.1016/S0952-7915(98)80031-9
22. Marzolo MP, von Bernhardi R, Inestrosa NC. Mannose receptor is present in a functional state in rat microglial cells. J Neurosci Res (1999) 58(3):387–95. doi:10.1002/(SICI)1097-4547(19991101)58:3<387::AID-JNR4>3.0.CO;2-L
23. Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia (2012) 60(5):717–27. doi:10.1002/glia.22298
24. Burudi EME, Riese S, Stahl PD, Regnier-Vigouroux A. Identification and functional characterization of the mannose receptor in astrocytes. Glia (1999) 25(1):44–55. doi:10.1002/(SICI)1098-1136(19990101)25:1<44::AID-GLIA5>3.0.CO;2-C
25. Regnier-Vigouroux A. The mannose receptor in the brain. Int Rev Cytol (2003) 226:321. doi:10.1016/S0074-7696(03)01006-4
26. Hu XM, Li PY, Guo YL, Wang HY, Leak RK, Chen SE, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke (2012) 43(11):3063–70. doi:10.1161/Strokeaha.112.659656
27. Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis (2013) 4:e525. doi:10.1038/cddis.2013.54
28. Battelli MG, Musiani S, Monti B, Buonamici L, Sparapani M, Contestabile A, et al. Ricin toxicity to microglial and monocytic cells. Neurochem Int (2001) 39(2):83–93. doi:10.1016/S0197-0186(01)00024-9
29. Zimmer H, Riese S, Regnier-Vigouroux A. Functional characterization of mannose receptor expressed by immunocompetent mouse microglia. Glia (2003) 42(1):89–100. doi:10.1002/glia.10196
30. Broderick C, Duncan L, Taylor N, Dick AD. IFN-gamma and LPS-mediated IL-10-dependent suppression of retinal microglial activation. Invest Ophthalmol Vis Sci (2000) 41(9):2613–22.
31. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science (2010) 330(6005):841–5. doi:10.1126/science.1194637
33. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol (2013) 13(3):190–8. doi:10.1038/nri3386
34. Nardone S, Elliott E. The interaction between the immune system and epigenetics in the etiology of autism spectrum disorders. Front Neurosci (2016) 10:329. doi:10.3389/fnins.2016.00329
35. Ohgidani M, Kato TA, Sagata N, Hayakawa K, Shimokawa N, Sato-Kasai M, et al. TNF-alpha from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav Immun (2016) 55:17–24. doi:10.1016/j.bbi.2015.08.022
36. Gurvich A, Begemann M, Dahm L, Sargin D, Miskowiak K, Ehrenreich H. A role for prostaglandins in rapid cycling suggested by episode-specific gene expression shifts in peripheral blood mononuclear cells: a preliminary report. Bipolar Disord (2014) 16(8):881–8. doi:10.1111/bdi.12223
37. Kawamoto K, Kuriyama H, Tajima S. Actigraphic detection of REM sleep based on respiratory rate estimation. J Med Bioeng (2013) 2(1):20–5. doi:10.12720/jomb.2.1.20-25
Keywords: bipolar disorder, rapid cycling, microglia, CD206, induced microglia-like (iMG) cells, state marker, M1/M2 polarization, translational research
Citation: Ohgidani M, Kato TA, Haraguchi Y, Matsushima T, Mizoguchi Y, Murakawa-Hirachi T, Sagata N, Monji A and Kanba S (2017) Microglial CD206 Gene Has Potential as a State Marker of Bipolar Disorder. Front. Immunol. 7:676. doi: 10.3389/fimmu.2016.00676
Received: 30 September 2016; Accepted: 21 December 2016;
Published: 09 January 2017
Edited by:
Björn Tackenberg, University of Marburg, GermanyReviewed by:
Björn Spittau, University of Freiburg, GermanyChristiane Charriaut-Marlangue, INSERM, France
Copyright: © 2017 Ohgidani, Kato, Haraguchi, Matsushima, Mizoguchi, Murakawa-Hirachi, Sagata, Monji and Kanba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiro A. Kato, dGFrYWhpcm9AbnBzeWNoLm1lZC5reXVzaHUtdS5hYy5qcA==
 Masahiro Ohgidani
Masahiro Ohgidani Takahiro A. Kato
Takahiro A. Kato Yoshinori Haraguchi3
Yoshinori Haraguchi3 Yoshito Mizoguchi
Yoshito Mizoguchi Noriaki Sagata
Noriaki Sagata Akira Monji
Akira Monji