- Department of Biology, University of Padova, Padova, Italy
Tunicates are the closest relatives of vertebrates, and their peculiar phylogenetic position explains the increasing interest toward tunicate immunobiology. They are filter-feeding organisms, and this greatly influences their defense strategies. The majority of the studies on tunicate immunity were carried out in ascidians. The tunic acts as a first barrier against pathogens and parasites. In addition, the oral siphon and the pharynx represent two major, highly vascularized, immune organs, where circulating hemocytes can sense non-self material and trigger immune responses that, usually, lead to inflammation and phagocytosis. Inflammation involves the recruitment of circulating cytotoxic, phenoloxidase (PO)-containing cells in the infected area, where they degranulate as a consequence of non-self recognition and release cytokines, complement factors, and the enzyme PO. The latter, acting on polyphenol substrata, produces cytotoxic quinones, which polymerize to melanin, and reactive oxygen species, which induce oxidative stress. Both the alternative and the lectin pathways of complement activation converge to activate C3: C3a and C3b are involved in the recruitment of hemocytes and in the opsonization of foreign materials, respectively. The interaction of circulating professional phagocytes with potentially pathogenic foreign material can be direct or mediated by opsonins, either complement dependent or complement independent. Together with cytotoxic cells, phagocytes are active in the encapsulation of large materials. Cells involved in immune responses, collectively called immunocytes, represent a large fraction of hemocytes, and the presence of a cross talk between cytotoxic cells and phagocytes, mediated by secreted humoral factors, was reported. Lectins play a pivotal role as pattern-recognition receptors and opsonizing agents. In addition, variable region-containing chitin-binding proteins, identified in the solitary ascidian Ciona intestinalis, control the settlement and colonization of bacteria in the gut.
Introduction
Tunicates or urochordates are marine, filter-feeding invertebrates, members of the phylum Chordata. They owe their name to the tunic that embeds the larval and adult body. Tunicates (ca 3,000 species) include Ascidiacea (benthic and sessile), Thaliacea (pelagic), and Larvacea or Appendicularia (pelagic).
Ascidians have a free-swimming, tadpole-like larva whereas adults have sac-like bodies with two siphons, allowing water flux, and a large branchial basket provided with a ventral endostyle secreting the mucous net required for filtration. They include Phlebobranchia, Aplousobranchia, and Stolidobranchia, previously grouped as Enterogona (Phlebobranchia and Aplousobranchia) and Pleurogona (Stolidobranchia).
Thaliaceans have barrel-like bodies; they include Pyrosomida (colonial), Doliolida (solitary/colonial), and Salpida (solitary/colonial). All Thaliaceans but Doliolida are devoid of larval stages. Larvaceans or appendicularians are similar to ascidian larvae, hence their name: they secrete a gelatinous house containing traps for food particles and use their tail to move water for filtration. Today, Larvaceans are considered a sister group of the remaining tunicates and Thaliaceans as a sister group of Enterogona (Figure 1).
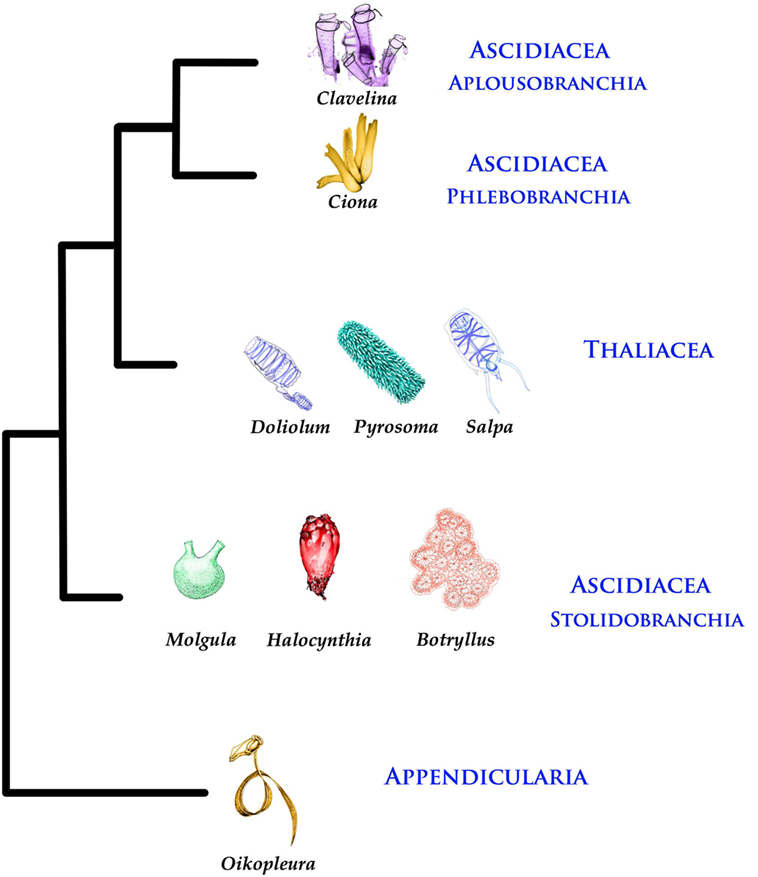
Figure 1. Phylogenetic tree of Tunicates [according to Ref. (1)].
Tunicates are the closest relatives to vertebrates (2), and this explains the increasing interest toward this group of animals. Like other invertebrates, tunicates rely only on innate immunity that lacks somatic recombination and long-term immune memory and has a limited array of effector responses.
Ascidians include about 2,300 species and are the most studied tunicates. Accordingly, the majority of the information on tunicate immune responses comes from studies on these organisms. In addition, ascidian innate immune genes did not undergo the expansions reported in other invertebrate deuterostomes, such as amphioxus and sea urchin (3, 4). This review, then, will focus mainly on the ascidian strategies of immune defense. Where available, information on immune responses of pelagic tunicates will be added.
The Sites of Immune Responses
The marine habitat contains 105–106 microbes/ml in the water column and much more in the sediments (5); the amount of viruses is 10 times higher (6). Tunicates, therefore, require an efficient immune system in order to prevent the risk of infections and select appropriate mutualistic bacterial strains for gut colonization (see below). The sites where the ascidian immune system is alerted by the contact with non-self molecules include the tunic, the hemolymph, and the digestive tract.
Tunic
The tunic represents the first outpost against pathogens and parasites and its damage, as in the soft tunic syndrome, can lead the organism to death (7). It is mainly of epidermal origin and resembles the vertebrate connective tissue in consisting of an amorphous matrix containing fibers and interspersed cells (8). The tunic can contain spicules, acting as physical defense against predators and varying in morphology, size, and mineral content (8). Molecules with antibacterial and anti-inflammatory activity are usually present in the matrix (9, 10). The tunic fibrous components include tunicin, a cellulose-like polysaccharide, collagen, and elastin (8, 11). Intermediate filaments (12) and mucopolysaccharides (9) contribute to the structural integrity of the tunic. The outermost compact layer, known as the cuticle, is continuous with the tunic matrix and frequently presents minute protrusions or spines (8, 13–15).
Tunic cells derive from both the epidermis and the hemocytes that can enter the tunic in response to infections (8, 16, 17). Hemocytes include spreading and round phagocytes, always present, cytotoxic granulocytes, widely found, and other cell types in some particular taxa, such as net cells and cells storing acid or pigments (8, 17–21), all contributing to protect the organism from predators, pathogens, or parasites. Phagocytes ingest foreign cells having entered the tunic (17, 22), and tunic phagocytes are the main effectors of allorecognition in the colonial species Aplidium yamazii (23). Granulocytes frequently contain and release antimicrobial peptides (24) and the enzyme phenoloxidase (PO) (25). Bladder cells store acid that, once released, decreases the pH of the tunic, disinfects the wounds, and exerts antifouling activity (17, 26, 27); net cells allow the shrinkage of the tunic in wound areas (17). PO-containing granulocytes can contribute to tunic formation or regeneration via degranulation and release of tunichromes, likely fragments of DOPA-containing proteins, that, once oxidized, cross-link tunicin fibers (28, 29). Tunic phagocytes and net cells are present also in Thaliaceans, although their role in defense has been poorly investigated. In pyrosomes, the density of tunic cells is comparable to that of ascidians (30), whereas doliolids and salps have a lower number of cells in their tunic (14, 17, 21). Larvaceans or appendicularians have no tunic, but tunicin is present in their house, secreted by specialized portions of the trunk epithelium (31).
Hemolymph
Ascidians have an open circulatory system and a colorless hemolymph, isotonic with seawater. The beating of a tubular heart guarantees the circulation in blood sinuses and lacunae. It periodically reverses the direction of the peristaltic waves thus inverting the hemolymph flow (8, 13). Circulating hemocyte types differ in morphology and ultrastructure. Various authors proposed unifying classification schemes [Figure 2; Table 1; references therein (32)], but uncertainties and doubts persist on terminology, hemocyte relationships, and differentiation pathways.
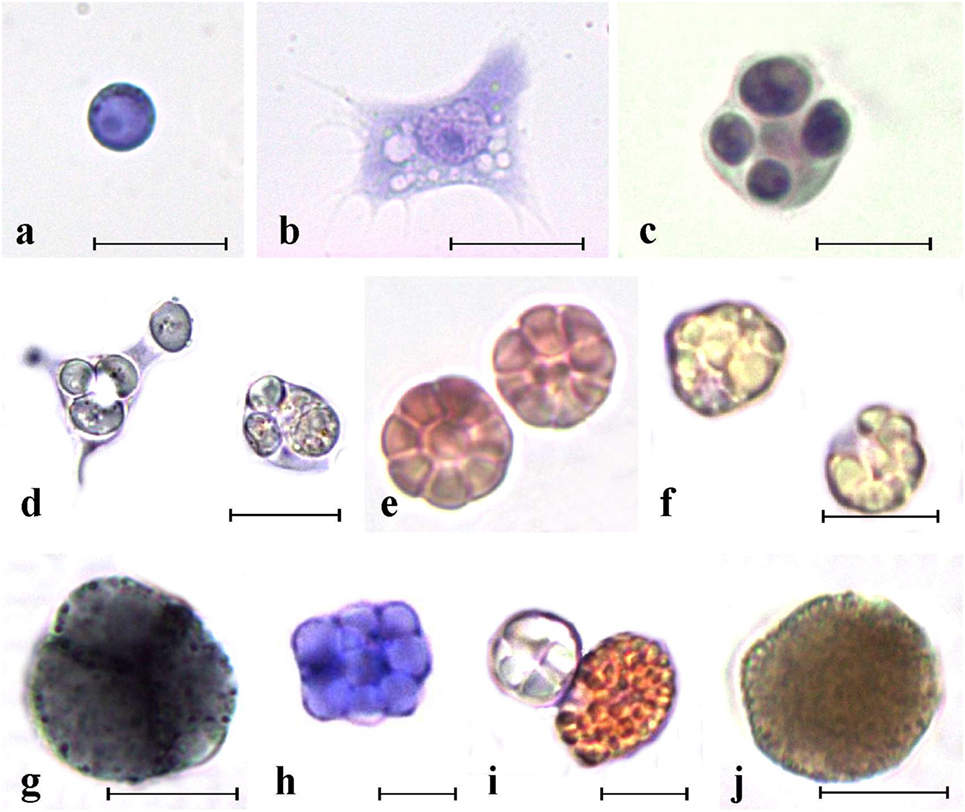
Figure 2. Main ascidian hemocytes. (A) Undifferentiated cells. (B–F) Immunocytes. (B,C) Botryllus schlosseri spreading and round phagocytes, respectively; (D) Polyandrocarpa misakiensis speading and round phagocyte with ingested yeast cells; (E) B. schlosseri morula cells (MCs); (F) P. misakiensis MCs; (G–J) storage cells. (G) B. schlosseri blue pigment cells; (H) P. misakiensis trophocyte; (I) P. misakiensis pigment cell and trophocyte; (J) B. schlosseri nephrocyte. (A–C,E,H) aldehyde-fixed cells stained with hematoxylin–eosin; (D,F,G,I,J) living cells. Scale bar: 10 µm.
Ascidian hemocytes, involved in immune responses (immunocytes), represent a relevant fraction of circulating hemocytes (32), synthesize most of the pattern-recognition receptors (Table 2) and actively transcribe genes required for immune defense (60, 61): they include phagocytes and cytotoxic cells. Phagocytes are wandering, spreading cells that actively move toward foreign cells or particles and ingest them. Upon the ingestion of foreign material, phagocytes withdraw their projections and assume a round morphology. Spreading phagocytes can reach 20 µm in length and have a well-defined actin cytoskeleton, with abundance of stress fibers (25). They contain fine cytoplasmic granules, unresolvable under the light microscope, showing positivity for lysosomal enzyme activities (32). Round phagocytes are large cells (15–20 µm in diameter) with one or more phagosomes containing the ingested material as well as hydrolytic enzymes, lipids, and lipofuscins (32). In the colonial ascidian, Botryllus schlosseri, the presence of a static and a mobile population of phagocytes was described: the former adhere to the basal lamina of the peribranchial epithelium and form the ventral islands, on both sides of the endostylar sinus (62).
Cytotoxic cells are granular cells, 10–15 µm in diameter; their cytoplasm is filled with large granules containing the inactive form of PO (34). They frequently constitute the most abundant circulating hemocyte type (32). In most of the studied species, cytotoxic cells assume a typical berry-like morphology after aldehyde fixation and are called morula cells (MCs).
As regards pelagic tunicates, Cima et al. (21) reported the characterization of circulating hemocytes of Thalia democratica oozooids: they include phagocytes that contain hydrolytic enzymes in their cytoplasm and can migrate into the tunic. Larvaceans have no hemocytes (21).
Hemocytes containing histamine and heparin inside their granules were observed in both ascidians and Thaliaceans: the molecules can either stabilize the granular content or, when released, modulate the inflammatory reaction by inducing tunic vessel-contraction and inhibition of phagocytosis (21, 63).
Digestive System
The oral and the atrial (cloacal) siphons are preferential ways of entrance of microorganisms. Here, a population of phagocytes is exposed to seawater, adhering to the internal tunic. Such sentinel or guard cells can recognize and ingest foreign particles or cells, thus preventing their entrance in the pharynx or in the atrium (64); they were found also in Thaliaceans (21).
In the solitary ascidian Ciona intestinalis, both the endostyle and the gastric epithelium constitutively transcribe genes involved in the inflammatory response triggered by the injection of LPS in the body wall (11, 65, 66), suggesting the importance of the alimentary tract in the recognition and the clearance of non-self material. This assumption is corroborated by the reported transcription of genes for Toll-like receptors (TLRs), mannose-binding lectins (MBLs), and MBL-associated serine proteases (MASPs) in both the stomach and the intestine, in addition to hemocytes, in accordance with the important immunosurveillance role of the alimentary tract (48, 58). In addition, variable region-containing chitin-binding proteins (VCBPs), secreted in the gut lumen and recognizing the surface of Gram (+) and Gram (−) bacteria (see below), probably exert a pivotal function in the maintenance of a stable commensal gut microbial flora. This is consistent with the hypothesis of a role of the immune system in both protecting host tissues from pathogenic attack and supporting the growth of the mutualistic microbiota (67). In B. schlosseri, gut epithelial cells are involved in the clearance of neighboring apoptotic cells during the generation change (68).
Humoral Defensive Repertoire
Phenoloxidase
The presence of PO activity in ascidian hemolymph has been widely reported in both solitary and colonial species [references therein (34)]. PO is assumed located as inactive proenzyme (probably, proPO), inside the granules of PO-containing hemocytes and activated by serine proteases once released outside the cells (35, 69). PO-containing hemocytes of C. intestinalis store also serine proteases that, once released, are activated by LPS and laminarin shortly before the activation of PO (70, 71). A soluble serine protease is present also in B. schlosseri hemolymph (72). This support the idea of an activation of PO mediated by serine proteases, analogous to what is reported in arthropods (73).
Phenoloxidase is involved in cytotoxic responses of ascidians. In colonial botryllid ascidians, the enzyme contributes to the formation of the necrotic spots along the border of contacting, genetically incompatible colonies (34). According to the analysis of nucleotide and predicted amino-acid sequences, ascidian PO shows high similarity with arthropod hemocyanins (74, 75).
Phenoloxidase substrates are likely represented by tunichromes, or other phenol-containing peptides, contained inside the hemocyte (mainly MC) granules (29, 35, 76–79). The enzyme produce quinones, that polymerize to melanin, and reactive oxygen species (ROS), that induce oxidative stress and related toxicity in neighboring cells (35).
Lectins
Ascidian immunocytes can synthesize and release humoral lectins with various molecular features and carbohydrate specificities (36, 80–84). Some of them have a clear role in the recognition of foreign molecules or in the modulation of immune responses (36, 65, 85–88). In most cases, they enhance the phagocytosis of microorganisms acting as opsonins (86, 87, 89, 90). Lectins can also trigger the respiratory burst and act as molecules able to influence the behavior of other immunocytes, as in the case of the Botryllus rhamnose-binding lectin (BsRBL) (37), or to activate the complement system (46).
A subset of B. schlosseri blood cells, probably phagocytes, express an ortholog of the vertebrate CD94 receptor on NK cells, a type II transmembrane protein with a C-type lectin domain (91). A second ortholog in C. intestinalis (CiCD94-1) contain a C-type lectin domain without carbohydrate-binding capability: it probably recognizes peptides instead of carbohydrates and is expressed in the same cell type engaged in the production of PO, also recognized by the anti-CiCD94-1 antibody. The fraction of cells positive to the CiCD94-1-1 antisense riboprobe increases after LPS exposure. The anti-CiCD91-1 antibody inhibits phagocytosis, suggesting that the interaction of CiCD94-1 with its ligand(s) can indirectly stimulate phagocytes (39, 92), probably through the release of cytokines (see below).
Immunoglobulin (Ig) Domain-Containing Proteins
Despite the lack of orthologs of genes for major histocompatibility complex proteins, T-cell receptors, and Igs, transcripts for putative molecules with Ig domains were identified in tunicates (93, 94). Three novel genes for VCBPs, containing two N-terminal, variable-type Ig domains, were described in C. intestinalis. VCBP-A, -B, and -C are synthesized by epithelial zymogenic cells of the stomach and the intestine, as well as by a fraction of circulating hemocytes (40, 95). VCBPs can bind Gram (+) and Gram (−) bacteria with the variable-type Ig domains and significantly increase microbe phagocytosis by hemocytes, acting as opsonins (40), whereas the chitin-binding domain interacts with the chitin-rich mucus along the intestinal wall, thus influencing the settlement of bacterial communities and the colonization of the intestinal lumen by the microbiota. Indeed, VCBP-C can enhance the in vitro production of biofilms by bacteria previously identified in the gut of Ciona (41).
Complement System
Both the alternative and lectin complement-activation pathways are present in ascidians [Figure 3; (46, 96)]. Genes for C3 were identified in all the ascidian species investigated so far (97–100). They are active in the adult (98), and their transcription rate increases after LPS injection in the tunic; similar behavior is reported for the C3-a fragment deriving from the cleavage of C3 in the presence of non-self (101). C3-a can recruit hemocytes to the inflammation site (102) via its binding to a G protein-coupled receptor, constitutively expressed in PO-containing hemocytes (103). C3b, the main C3 fragment, can adhere to the microbial surfaces and exert an opsonic role enhancing the recognition and ingestion of bacteria by phagocytes (89, 97, 98, 100, 104).
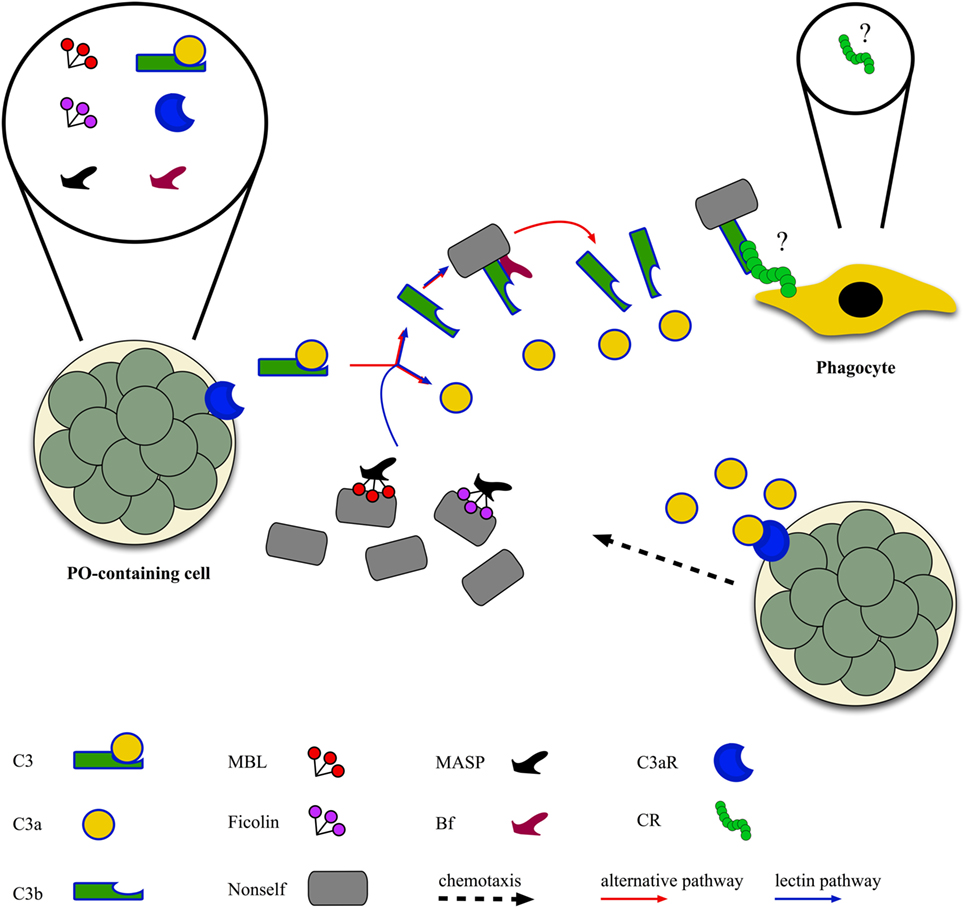
Figure 3. Ascidian complement-activation pathways: complement components of both the alternative and the lectin pathway [C3, Bf, mannose-binding lectins (MBLs), ficolins, MBL-associated serine proteases (MASPs)] are released by morula cells that also express the receptor for C3a (see text), whereas the receptor(s) for C3b [complement receptor (CR)] are probably located on the surface of phagocytes as the activation of C3 increases the phagocytic activity.
The transcription of C3 genes occurs in hemocytes, mainly PO-containing hemocytes (97, 100, 101). In Styela plicata, hemocytes secrete a protein recognized by anti-C3 antibodies, the concentration of which increases in the culture supernatant after the exposure to non-self molecules (105). In Pyura stolonifera, the incubation of hemolymph with LPS induces the release of a chemotactic protein recognized by anti-human C3 antibody (99). In Halocynthia roretzi, also cells of the stomach wall transcribe C3 (97), whereas, in Ciona, even ciliated cells bordering the branchial stigmata contain C3 mRNA (101).
Transcripts for Bf, a component of the alternative activation pathway, were identified in various ascidian species (100, 106, 107). Genes for MBLs, C-type lectins members of the collectin family and involved in the lectin pathway of complement activation, are present in the C. intestinalis genome (42, 44, 46–48) and over-transcribed during inflammatory reactions (42). Transcripts for MBLs were identified also in other ascidian species (43, 45, 49). In S. plicata (108, 109), an increase in the secretion of collectins and in the fraction of hemocytes immunopositive to anti-collectin antibody is observable during inflammatory responses (110). Transcripts for ficolins, also components of the lectin pathway, are present in H. roretzi (50), Botrylloides leachii (51), and B. schlosseri (49, 52). The transcription of H. roretzi ficolin 3 gene is significantly impaired in organisms with the soft tunic disease (7). A C-type lectin, interacting with MASP, is involved in the recognition of microbial surfaces and the activation of C3 in H. roretzi (111). Transcripts for MASPs were widely described in ascidians (43–46, 48, 49, 55, 96, 104, 112, 113).
C1q-like transcripts were found in C. intestinalis (53, 54) and B. schlosseri (55). In vertebrates, C1q, a component of the classical activation pathway, can bind pentraxins (mainly C-reactive protein). These molecules were identified in Ciona (53) and Didemnum candidum (83), suggesting the interaction with pentraxins as the original role of C1q in invertebrate chordates (53). In B. schlosseri, the transcription of genes for C1q, MASPs, Bf, and ficolins is upregulated during the allorejection reaction (55); in addition, genes for C3, Bf, ficolin, MASPs, and a putative CR1 are over-transcribed during the recurrent generation changes (113).
As regards complement regulators, in B. schlosseri, cDNAs for a putative complement-control protein (CCP), featuring CCP domains, were isolated (114). Genes for α2-macroglobulin, able to inhibit MASPs, and for various putative molecules with the CCP domain(s), were reported in C. intestinalis (44).
C6/C9-like transcripts for proteins containing the membrane-attack complex/perforin domain were described in C. intestinalis (44, 46, 47); whether or not a cytolytic pathway is present ascidians, is still a matter of debate.
In Ciona, integrin α and β subunits, part of a complement receptor (CR) and showing homology with mammalian CR3 or CR4, are expressed on the surface of hemocytes (46, 56, 57).
Chemical Defense
Ascidians are the source of a great variety of bioactive molecules of potential interest in the sanitary field; some of them have also entered human clinical trials (115). Many compounds act as antiviral or repellents against foulants, predators, and competitors (116–120). Acid substances and metals stored in vacuoles within tunic cells can contribute to additional protection (26, 121, 122). The tunic may host prokaryotes that produce many of the above-reported products (115, 121).
Ascidians produce also molecules with antimicrobial activity (123–126). Most of them are peptides; in many cases, they are synthesized by hemocytes, mostly PO-containing cells. In H. roretzi, halocyamines A and B are synthesized by MCs (127), and their cytotoxic activity is likely related to the presence of diphenol rings that render them substrates for PO. S. clava MCs produce clavanins A–D, histidine-rich, α-helix peptides, and clavaspirin (128, 129). In the same species, five styelins, cationic antimicrobial peptides, were identified and isolated from hemocyte lysates (130, 131). In C. intestinalis, PO-containing hemocytes synthesize two families of α-helix antimicrobial peptides and the injection of non-self material in the body wall enhances the transcription of the corresponding genes (24, 132–134). Anticancer derivatives were also described (135, 136), and ascidian tunichromes can exert a cytotoxic activity (28). A gene homologous to mammalian EB1, a protein with tumor suppressing effect, was described in B. schlosseri (137).
Cytokines and Cross Talk between Immunocytes
Despite the common opinion that invertebrate cytokines share no homologies with their vertebrate counterparts (138, 139), putative genes for IL1 and TNF receptors were identified in the Ciona genome (44, 61). A gene for a TNFα homolog, the transcription of which increases in Ciona hemocytes after LPS injection in the body wall, was also cloned (11, 140): it probably exerts a role in recruiting hemocytes to the inflamed area (141). Genes for a putative IL17 receptor and three IL17 homologs were identified in Ciona (3, 60, 61): their expression (in hemocytes) is also upregulated after LPS injection in the tunic (142).
In B. schlosseri, MCs are the main source of molecules recognized by antibodies raised against mammalian pro-inflammatory cytokines, secreted upon the recognition of foreign molecules (143). They induce phagocytes to synthesize and release BsRBL, with opsonic activity [Figure 4; (144)]. Anti-cytokine antibodies prevent the increase in phagocytosis observed when hemocytes are incubated in the supernatants of hemocytes cultures previously challenged with yeast (Saccharomyces cerevisiae) cells (145). In botryllid ascidians, during the allorejection reaction, MCs produce and release molecules immunopositive to anti-IL1α and anti-TNFα antibodies (25, 100, 146). They are involved in the recruitment of these cells to the ampullae of the contact region (see below), as demonstrated by the inhibition of the MC chemotaxis, induced by cell-free hemolymph from incompatible colonies, in the presence of the above-reported antibodies (146, 147). In B. schlosseri, the gene for an IL-17 ortholog is over-transcribed during the generation change: it probably modulates the cellular events occurring during this phase of the colonial life cycle and mediates the cross talk between MCs and phagocytes (113). A cooperation between MCs and phagocytes was reported also in C. intestinalis (70).
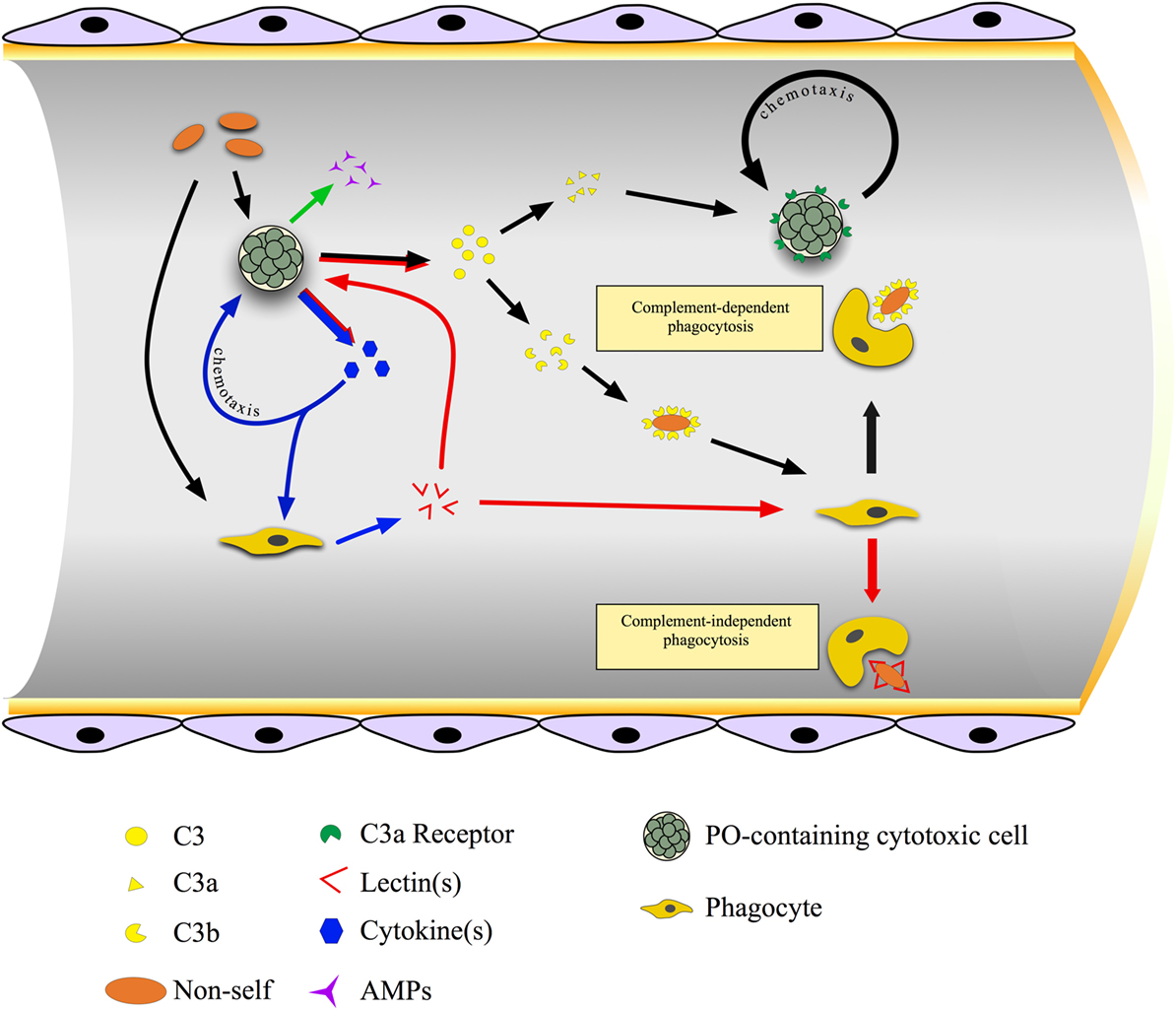
Figure 4. Cross talk between immunocytes in the colonial ascidian Botryllus schlosseri. Cytotoxic, phenoloxidase (PO)-containing cells are the first cells to sense non-self material and, as a consequence, they synthesize and release cytokines, antimicrobial peptides, and complement C3. Cytokines act on both morula cells (MCs) themselves, inducing their chemotaxis, and on phagocytes triggering the synthesis and the release of lectins, mainly rhamnose-binding lectin (RBL), that bind carbohydrates on the microbial surfaces and exert a complement-independent opsonic role. C3 is cleaved to C3a, which cooperates in recruiting MCs, and C3b, which interacts with the microbial surface and acts as opsonin.
Variety of Cell-Mediated Immune Responses in Ascidians
Hemocyte Aggregation
Tunicate lack a coagulation system and hemocytes migrate and aggregate to plug the injured sites and prevent hemolymph leakage. Hemocyte aggregation was particularly studied in the solitary ascidian H. roretzi (148) where a membrane glycoprotein, active in both phagocytosis and hemocyte aggregation was identified (149). It contains two immunoreceptor tyrosine-based activation motifs (ITAMs) and associates with phosphorylated and unphosphorylated proteins, strongly suggesting its involvement in triggering signal transduction pathways (150). Further analyses demonstrated that, during hemocyte aggregation, it induces gene transcription through the activation of phosphatidylinositol-3 kinase (PI3K) and cytosolic calcium rise (151).
Endocytosis
In ascidians, the ingestion of foreign materials occurs through either macropinocytosis or phagocytosis. In both cases, integrins and molecules containing the Arg–Gly–Asp (RGD) motif (e.g., fibronectin or fibrinogen) are involved (25). Pattern-recognition receptors allow the direct interaction of circulating professional phagocytes with potentially pathogenic foreign material. As an alternative, they recognize opsonins covering the microbial surfaces and enhancing phagocytosis. Opsonin-mediated phagocytosis can be either complement-dependent or complement-independent (Figure 4). A transient rise in cytosolic Ca2+ concentration is required for the ingestion, whereas a sustained increase lowers the extent of phagocytosis (25). The interaction of phagocytes with non-self particles triggers a respiratory burst, with the activation of both a membrane oxidase and an inducible nitric oxide (NO) synthase that leads to the production of ROS and reactive nitrogen species with microbicidal activity (152).
As for receptors involved in endocytosis, in C. intestinalis, two TLR genes were identified (60) and fully characterized: the corresponding proteins have cytoplasmic TIR, transmembrane, and extracellular LRR domains and are located in both the plasma membrane and the endosome membrane of phagocytes (58). In addition, Ciona also possesses a rich repertoire of transcripts of genes involved in signal transduction, including those for proteins with immunoreceptor tyrosine-based inhibition motifs and ITAMs, MyD88, IL1 receptor-associated kinase, TNF receptor-associated factor, nuclear factor κB (NF-κB), and inhibitor of κB (44, 53, 60). In the colonial B. schlosseri, TLRs are present on the surface and the interior of phagocytes (25). Here, the signal transduction pathways triggered by non-self recognition, include the activation of trimeric G-proteins, protein kinase A, protein kinase C, PI3K, mitogen-activated protein kinases (MAPKs), and NF-κB (25, 153, 154).
Phagocytosis of apoptotic cells is a common event in botryllid ascidians, where cyclical generation of new zooids by budding occurs, and old zooids are periodically resorbed (155). Generation change or take-over implies massive apoptosis in the tissues of old zooids and the clearance of dying cells by professional and occasional phagocytes (68, 156–158). Phagocytes recognize phosphatidylserine and the lack of sialic acid on the surface of effete cells and corpses (38, 59) and avidly ingest them: because of the sudden increase of oxygen consumption and the related oxidative stress, they undergo phagocytosis-induced apoptosis and are, in turn, ingested by other phagocytes (159). Clearance of dying cells requires also the presence of CD36, a scavenger receptor able to recognize oxidized lipids, on the phagocyte surface (59); a putative CD36 ortholog was identified in the Ciona genome (44). In B. schlosseri, the clearance of apoptotic cells by phagocytes is necessary for the completion of the take-over and the progression of bud development (160). The opposite is also true: buds are required for the clearance of cell corpses as they recycle the nutrients deriving from their digestion by phagocytes (161, 162).
Encapsulation
Foreign material too large to be ingested by phagocytosis is usually encapsulated by circulating hemocytes. The formation of multi-layered capsules was observed around parasitic crustaceans, and both phagocytes and cytotoxic MCs can be involved in capsule formation (33). In C. intestinalis, intratunical injection of mammalian erythrocytes or non-self molecules results in massive recruitment of hemocytes to the inoculum site and capsule formation (11).
In B. scalaris, unlike other botryllid ascidians (see below), encapsulation plays a pivotal role in allorecognition. Here, the circulatory systems fuse during allorejection and blood exchange begins. Phagocytes crowd inside the fused vessels and stimulate the aggregation of hemocytes into large clusters, finally encapsulated by other phagocytes, so to plug the lumen of the vessels and interrupt the hemolymph flow in a few minutes (163).
Cytotoxicity
A Ca2+-dependent cytotoxic activity against mammalian erythrocytes or tumor cells, inhibited by sphingomyelin, was described in C. intestinalis and S. plicata (164–166). In C. intestinalis, cytotoxicity against mammalian cells requires the activity of the enzyme phospholipase A2, modulated by lectins with specificity for galactosides (167). A cytotoxic reaction, called contact reaction, occurs in allogeneic or xenogeneic combinations of hemocytes from various solitary ascidians (168). In B. schlosseri, cytotoxicity can be observed in vitro by exposing hemocytes to non-self molecules or cell-free hemolymph of incompatible colonies (79). In all the above cases, cytotoxicity is consequent to the release of active PO in the medium upon degranulation of PO-containing hemocytes and the oxidation of polyphenol substrata, leading to the production of toxic quinones and ROS (34). In B. schlosseri, NO is also involved in the induction of cell death (146). The production of NO by hemocytes, after their exposure to either LPS or zymosan, was reported also in S. plicata and Phallusia nigra (169, 170).
Inflammation
Inflammation is characterized by the recruitment of circulating hemocytes, extravasation, cell degranulation, induction of cytotoxicity, and phagocytosis (or encapsulation) of the foreign material. Inflammation-related cytotoxicity requires the recruitment of PO-containing hemocytes and the release of active PO in the infected area (142, 171, 172). It was particularly studied in C. intestinalis, after the injection of foreign material in the tunic (11). Circulating hemocytes of treated animals increase the transcription of genes involved in the recognition of non-self and tissue repair (11, 173–175).
Inflammation in Tissue Transplantation
Tissue transplantation represents a cause of inflammation. In solitary species, higher recruitments of hemocytes occur in the case of allografts with respect to autografts, leading to allograft rejection. The latter is more rapid in primed animals, having previously received (and rejected) a similar graft (176–178). Graft rejection relies on PO-containing hemocytes reaching the inflamed area and the induction of cytotoxicity (179). In C. intestinalis, the products of a polymorphic gene, structurally similar to a vertebrate CR and containing CCP domains, were proposed as individuality markers. They are synthesized by hemocytes, with various splice variants and high interindividual variability (180, 181).
Inflammation in Allorecognition
In botryllid ascidians, inflammatory events are the consequence of allorecognition between incompatible colonies, probably to prevent the risk of somatic/germ cell parasitism in genetically unrelated colonies (182, 183). In Botryllus primigenus and B. schlosseri, a highly polymorphic fusibility/histocompatibility (Fu/HC) gene with codominant alleles controls the outcome of the colony contact (184, 185). When colonies share no alleles at the Fu/HC locus, partial fusion of the facing tunics occurs as well as the leakage of soluble histocompatibility factors, recognized by MCs (186). Activated MCs release chemotactic cytokines able to recruit other MCs in the peripheral blind endings of the tunic vasculature (ampullae) of the contact region (147), from which they enter the tunic and degranulate, thus releasing the enzyme PO and its polyphenol substrata. A series of melanic cytotoxic foci, called points of rejection, appear along the contact border as a result of cytotoxicity [Figure 5; (34, 79, 146, 187)]. Rejecting colonies of B. schlosseri increase the transcription rate of various immune-relevant genes (52, 55, 188). A change in the growth direction of contacting colonies occurs after the allorejection reaction (189). MCs are involved in the allorejection reaction also in Botrylloides simodensis, Botrylloides fuscus, Botrylloides violaceus, B. leachii (35), and Didemnum perlucidum (190). The unusual growth of facing ampullae during allorecognition was reported in B. leachi (35, 191).
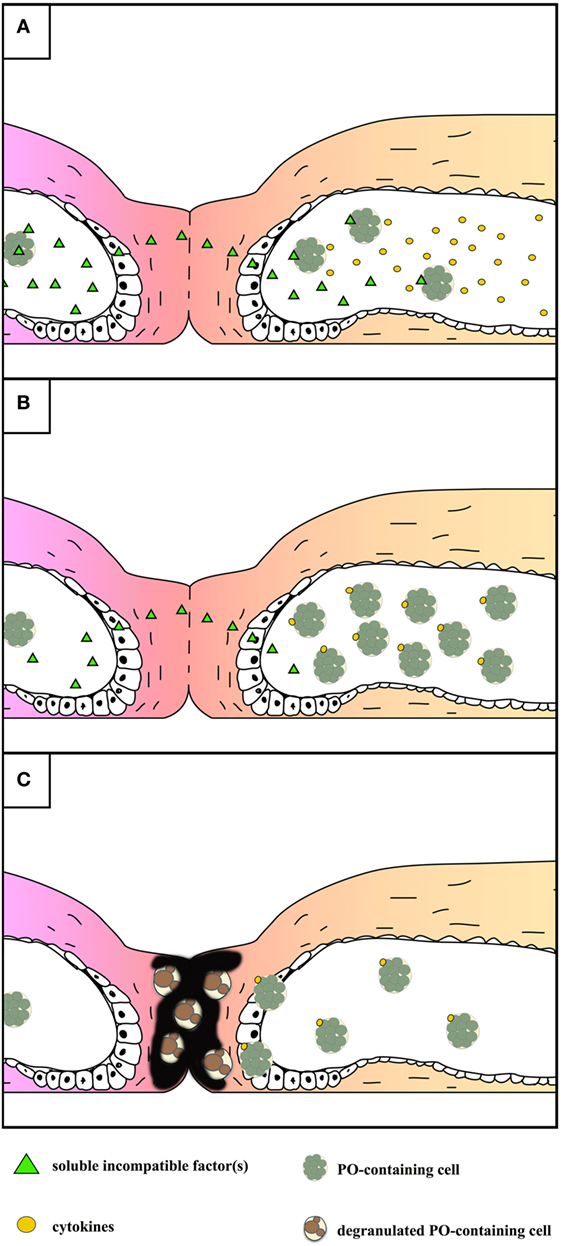
Figure 5. Schematic representation of the events occurring during the allorejection reaction of Botryllus schlosseri. For sake of simplicity, the main steps are reported on the right colony only. (A) local fusion of the contacting tunics and diffusion of soluble, incompatible factor(s) recognized by morula cells (MCs) inside the facing ampullae of the alien colony that, as a consequence, release cytokines. (B) Recruitment of MCs inside the tips of the ampullae facing the alien colony. (C) Extravasation of MCs and their degranulation in the tunic: melanin is formed as a consequence of the release of polyphenols and active phenoloxidase (PO); both melanin and reactive oxygen species contribute to the cytotoxicity observed in the contacting region.
An intense inflammatory reaction is observed when incompatible colonies of ovoviviparous botryllid ascidians are brought into contact at their cut surfaces (192, 193), whereas fusion of tunics and hemolymph vessels always occurs in the case of viviparous species. This suggests that, in the latter case, hemocytes have lost their ability of allorecognition, probably to avoid immune attacks toward the brooded embryos that share only one Fu/HC allele with the mother colony (194). In support of the above hypothesis, the PO activity of the hemolysate of viviparous species is lower than that of ovoviviparous ones (195, 196).
When Botryllus colonies share at least one allele at the Fu/HC locus, contacting colonies can fuse and form a single chimeric colony (197). However, in the case of a single shared allele, the resorption of one of the chimeric partner occurs within 30 days from the temporary fusion (198). Even in this case, MCs are directly involved as they infiltrate the tissues of the loser colony, together with phagocytes. The resorption phenomenon can be induced by the injection of enriched populations of MCs in the vasculature of recipient colonies and shares many similarities with the take-over, including apoptosis in zooid tissues, clearance of dying cells by phagocytes, and modulation by IL17 (113).
In B. schlosseri, the ampullar epithelium and hemocytes express genes for proteins involved in allorecognition, although uncertainties on the identity of the allorecognition gene still persist (199–204).
Role in Development?
Many invertebrate molecules have a role in both development and immunity. The best example is the Drosophila Toll receptor, required for the establishment of dorsal–ventral polarity early in development and switching to an immune role in adult flies (205). In Tunicates, various genes, involved in adult immune responses, are transcribed also during embryonic, larval, and asexual development, and this opens interesting perspectives on their role in development.
In B. villosa, the analysis of the transcriptome revealed the expression of immune-related genes in both the larval and juvenile development (43). In addition, MASPs are probably involved in the activation of metamorphosis (206).
In the larva of the ascidian Ascidia callosa, tunichrome, the putative substrate of PO, is required for tunic morphogenesis (207).
In C. intestinalis, a C3-like gene is transcribed during early development: it codifies a protein that, probably, does not exert a typical C3 role (208). Orthologous genes of C6 and C1 are also active during the embryonic stage (54). In addition, the gene for CiCD94-1 is transcribed in larval papillae, in cells of the larval nervous system, and in the coronet cells, the probable precursors of neural crest cells, with a role in modeling the nervous system during development (39). Furthermore, swimming larvae transcribe a gene for a CiTNFα-like protein (141), and PO gene expression is modulated in early and larval development (209). In the same organism, very low transcription levels of VCBP genes can be detected before the tailbud stage. From the larval stage onward, their mRNAs are located in gut primordia, with different distributions in defined territories, suggesting a role of VCBPs in the functional compartmentalization of the developing intestine (95, 210). VCBP mRNAs are translated after metamorphosis, with different timing of appearance and distribution (41). The transcription of VCBP genes in juveniles is differentially modulated by Gram (+) and Gram (−) bacteria, fitting the idea of their role in mediating the onset of the microbial gut colonization (95, 210).
An increase in the transcription of several immune-related genes occurs also during the whole body regeneration of B. leachii (51). In addition, signaling pathways, such as those involving MAPK and the NF-κB/Rel family members, are required in the formation of the larval notochord (211) and in the budding process of botryllid ascidians (212).
Future Perspectives
Tunicates, and ascidians in particular, are simple chordates that represent valuable models for the study of the innate immune responses and the evolutionary events that occurred in the course of invertebrate–vertebrate transition, leading to the appearance of lymphocytes and receptor diversification through somatic recombination. The progressive availability of new sequenced transcriptomes and genomes from tunicates will enable researchers to dissect the genetic and molecular processes associated with immune responses, clarify the regulatory pathways and the diversity of pattern-recognition receptors involved in immune responses, and compare them with what known in vertebrates. Ascidians offer also the possibility to study some particular aspects of the immune responses, such as the evolutionary importance of the polymorphism found in Fu/HC and other immune genes and its relationships with pathogen threats, the molecular basis of the priming phenomenon, the evolution of the complement system, and the role of lectins as immunomodulatory molecules. In addition, the possibility of synthesizing the gene products once the gene sequences are known, can render available a quantity of bioactive molecules, involved in chemical defense, testable as antimicrobial, antiviral, or anticancer compounds. Last, but not least, research on hemocytes will contribute to disentangle the unresolved aspects of hemocyte ontogeny and differentiation pathways and better elucidate their role in tunicate biology.
Author Contributions
LB set up the work plan. LB and NF equally contributed to the text of the review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the University of Padova (DOR 2016).
References
1. Swalla BJ, Smith AB. Deciphering deuterostome phylogeny: molecular, morphological and paleontological perspectives. Philos Trans R Soc (2008) 363B:1557–68. doi:10.1098/rstb.2007.2246
2. Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature (2006) 439:965–8. doi:10.1038/nature04336
3. Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso AW, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science (2002) 298:2157–67. doi:10.1126/science.1080049
4. Voskoboynik A, Neff NF, Sahoo D, Newman AM, Pushkarev D, Koh W, et al. The genome sequence of the colonial chordate, Botryllus schlosseri. Elife (2013) 2:e00569. doi:10.7554/eLife.00569
5. Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A (1998) 95:6578–83. doi:10.1073/pnas.95.12.6578
6. Suttle CA. Marine viruses – major players in the global ecosystem. Nat Rev Microbiol (2007) 5:801–12. doi:10.1038/nrmicro1750
7. Cha IS, Segovia del Castillo C, Nho SW, Hikima J, Aoki T, Jung TS. Innate immune response in the hemolymph of an ascidian, Halocynthia roretzi, showing soft tunic syndrome, using label-free quantitative proteomics. Dev Comp Immunol (2011) 35:809–16. doi:10.1016/j.dci.2011.01.011
8. Burighel P, Cloney RA. Urochordata: Ascidiacea. In: Harrison FW, Ruppert EE, editors. Microscopic Anatomy of Invertebrates. (Vol. 15), New York: Wiley-Liss Inc. (1997). p. 221–347.
9. Xu CX, Jin H, Chung YS, Shin JY, Woo MA, Lee KH, et al. Chondroitin sulfate extracted from the Styela clava tunic suppresses TNF-α-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-kB signal in JB6 cells. Cancer Lett (2008) 264:93–100. doi:10.1016/j.canlet.2008.01.022
10. Christomelba DC, Ananthan G. Chitosan derived from the tunic of ascidian Phallusia nigra (Savigny, 1816) showing antibacterial activities and its characterization. Int J Med Med Sci (2016) 8:91–5. doi:10.5897/IJMMS2014.1087
11. Parrinello N, Cammarata M, Parrinello D, Vizzini A. Inflammatory response of the ascidian Ciona intestinalis. In: Ballarin L, Cammarata M, editors. Lessons in Immunity: from Single-Cell Organisms to Mammals. London: Elsevier (2016). p. 177–92.
12. Di Bella MA, Carbone MC, D’Amato M, Alessandro R, De Leo G. The identification and localization of two intermediate filament proteins in the tunic of Styela plicata (Tunicata, Styelidae). Tissue Cell (2009) 41:381–9. doi:10.1016/j.tice.2009.04.001
13. Goodbody I. The physiology of ascidians. Adv Mar Biol (1974) 12:1–149. doi:10.1016/S0065-2881(08)60457-5
14. Hirose E, Kimura S, Itoh T, Nishikawa J. Tunic morphology and cellulosic components of pyrosomas, doliolids, and salps (Thaliacea, Urochordata). Biol Bull (1999) 196:113–20. doi:10.2307/1543173
15. Di Bella MA, Cassarà G, Russo D, De Leo G. Cellular components and tunic architecture of the solitary ascidian Styela canopus (Stolidobranchiata, Styelidae). Tissue Cell (1998) 30:352–9. doi:10.1016/S0040-8166(98)80048-7
16. Di Bella MA, Carbone MC, De Leo G. Aspects of cell production in mantle tissue of Ciona intestinalis L. (Tunicata, Ascidiacea). Micron (2005) 36:477–81. doi:10.1016/j.micron.2005.01.007
17. Hirose E. Ascidian tunic cells: morphology and functional diversity of free cells outside the epidermis. Invertebr Biol (2009) 128:83–96. doi:10.1111/j.1744-7410.2008.00153.x
19. Hirose E, Saito Y, Watanabe H. Tunic cell morphology and classification in botryllid ascidians. Zoolog Sci (1991) 8:951–8.
20. Hirose E. Pigmentation and acid storage in the tunic: protective functions of the tunic cells in the tropical ascidian Phallusia nigra. Invertebr Biol (1999) 118:414–22. doi:10.2307/3227010
21. Cima F, Caicci F, Sordino P. The haemocytes of the salp Thalia democratica (Tunicata, Thaliacea): an ultrastructural and histochemical study in the oozoid. Acta Zool (Stockh) (2014) 95:375–91. doi:10.1111/azo.12034
22. Hirose E, Ishii T, Saito Y, Taneda Y. Phagocytic activity of tunic cells in the colonial ascidian Aplidium yamazii (Polyclinidae Aplousobranchia). Zoolog Sci (1994) 11:203–8.
23. Ishii T, Hirose E, Taneda Y. Tunic phagocytes are involved in allorejection reaction in the colonial tunicate Aplidium yamazii (Polyclinidae, Ascidiacea). Biol Bull (2008) 214:145–52. doi:10.2307/25066671
24. Di Bella MA, Fedders H, De Leo G, Leippe M. Localization of antimicrobial peptides in the tunic of Ciona intestinalis (Ascidiacea, Tunicata) and their involvement in local inflammatory-like reactions. Results Immunol (2011) 1:70–5. doi:10.1016/j.rinim.2011.09.001
25. Ballarin L. Immunobiology of compound ascidians, with particular reference to Botryllus schlosseri: state of art. Invert Surviv J (2008) 5:54–74.
26. Hirose E. Acid containers and cellular networks in the ascidian tunic with special remarks on ascidian phylogeny. Zoolog Sci (2001) 18:723–31. doi:10.2108/zsj.18.723
27. Hirose E, Yamashiro H, Mori Y. Properties of tunic acid in the ascidian Phallusia nigra (Ascidiidae, Phlebobranchia). Zool Sci (2001) 18:309–14. doi:10.2108/zsj.18.309
28. Cai M, Sugumaran M, Robinson WE. The crosslinking and antimicrobial properties of tunichrome. Comp Biochem Physiol (2008) 151B:110–7. doi:10.1016/j.cbpb.2008.06.004
29. Sugumaran M, Robinson WE. Structure, biosynthesis and possible function of tunichromes and related compounds. Comp Biochem Physiol (2012) 163B:1–25. doi:10.1016/j.cbpb.2012.05.005
30. Hirose E, Ohshima C, Nishikawa J. Tunic cells in pyrosomes (Thaliacea, Urochordata): cell morphology, distribution, and motility. Invertebr Biol (2001) 120:386–93. doi:10.1111/j.1744-7410.2001.tb00047.x
31. Kimura S, Ohshima C, Hirose E, Nishikawa J, Itoh T. Cellulose in the house of the appendicularian Oikopleura rufescens. Protoplasma (2001) 186:24–33. doi:10.1007/BF01276931
32. Cima F, Franchi N, Ballarin L. Origin and function of tunicate hemocytes. In: Malagoli D, editor. The Evolution of the Immune System. London: Elsevier (2016). p. 29–49.
33. Wright RK, Cooper EL. Inflammatory reactions of the protochordata. Am Zool (1983) 23:205–11. doi:10.1093/icb/23.1.205
34. Ballarin L. Ascidian cytotoxic cells: state of the art and research perspectives. Invert Surviv J (2012) 9:1–6.
35. Franchi N, Ballarin L. Cytotoxic cells of compound ascidians. In: Ballarin L, Cammarata M, editors. Lessons in Immunity: from Single-Cell Organisms to Mammals. London: Elsevier (2016). p. 193–203.
36. Vizzini A, Parrinello D, Sanfratello MA, Salerno G, Cammarata M, Parrinello N. Inducible galectins are espressed in the inflamed pharynx of the ascidian Ciona intestinalis. Fish Shellfish Immunol (2012) 32:101–9. doi:10.1016/j.fsi.2011.10.028
37. Franchi N, Schiavon F, Carletto M, Gasparini F, Bertoloni G, Tosatto SCE, et al. Immune roles of a rhamnose-binding lectin in the colonial ascidian Botryllus schlosseri. Immunobiology (2011) 216:725–36. doi:10.1016/j.imbio.2010.10.011
38. Cima F, Manni L, Basso G, Fortunato E, Accordi B, Schiavon F, et al. Hovering between death and life: natural apoptosis in the blastogenetic cycle of the colonial ascidian Botryllus schlosseri. Dev Comp Immunol (2010) 34:272–85. doi:10.1016/j.dci.2009.10.005
39. Zucchetti I, Marino R, Pinto MR, Lambris JD, Du Pasquier L, De Santis R. CiCD94-1, an ascidian multipurpose C-type lectin-like receptor expressed in Ciona intestinalis hemocytes and larval neural structures. J Immunol (2008) 162:387–91. doi:10.1111/j.1432-0436.2007.00214.x
40. Dishaw LJ, Giacomelli S, Melillo D, Zucchetti I, Haire RN, Natale L, et al. A role for variable region-containing chitin-binding proteins (VCBPs) in host gut-bacteria interactions. Proc Nat Acad Sci U S A (2011) 108:16747–52. doi:10.1073/pnas.1109687108
41. Dishaw LJ, Leigh B, Cannon JP, Liberti A, Mueller MG, Skapura DP, et al. Gut immunity in a protochordate involves a secreted immunoglobulin-type mediator binding host chitin and bacteria. Nat Commun (2016) 7:10617. doi:10.1038/ncomms10617
42. Bonura A, Vizzini A, Salerno G, Parrinello N, Longo V, Colombo P. Isolation and expression of a novel MBL-like collectin cDNA enhanced by LPS injection in the body wall of the ascidian Ciona intestinalis. Mol Immunol (2009) 46:2389–94. doi:10.1016/j.molimm.2009.04.035
43. Davidson B, Swalla BJ. A molecular analysis of ascidian metamorphosis reveals activation of an innate immune response. Development (2002) 129:4739–51.
44. Azumi K, De Santis R, De Tomaso AW, Rigoutsos I, Yoshizaki F, Pinto MR, et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: “waiting for Godot”. Immunogenetics (2003) 55:570–81. doi:10.1007/s00251-003-0606-5
45. Vasta GR, Quesenberry MS, Ahmed H, O’Leary N. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol (1999) 23:401–20. doi:10.1016/S0145-305X(99)00020-8
46. Fujita T, Endo Y, Nonaka M. Primitive complement system – recognition and activation. Mol Immunol (2004) 41:103–11. doi:10.1016/j.molimm.2004.03.026
47. Wakoh T, Ikeda M, Uchino R, Azumi K, Nonaka M, Kohara Y, et al. Identification of transcripts expressed preferentially in hemocytes of Ciona intestinalis that can be used as molecular markers. DNA Res (2004) 11:345–52. doi:10.1093/dnares/11.5.345
48. Skjoedt MO, Palarasah Y, Rasmussen K, Vitved L, Salomonsen J, Kliem A, et al. Two mannose-binding lectin homologues and an MBL-associated serine protease are expressed in the gut epithelia of the urochordate species Ciona intestinalis. Dev Comp Immunol (2010) 34:59–68. doi:10.1016/j.dci.2009.08.004
49. Franchi N, Ballarin L. Morula cells as key hemocytes of the lectin pathway of complement activation in the colonial tunicate Botryllus schlosseri. Fish Shellfish Immunol (2017) 63:157–64. doi:10.1016/j.fsi.2017.02.003
50. Kenjo A, Takahashi M, Matsushita M, Endo Y, Nakata M, Mizuochi T, et al. Cloning and characterization of novel ficolins from the solitary ascidian Halocynthia roretzi. J Biol Chem (2001) 276:19959–65. doi:10.1074/jbc.M011723200
51. Rinkevich Y, Douek J, Haber O, Rinkevich B, Reshef R. Urochordate whole body regeneration inaugurates a diverse innate immune signaling profile. Dev Biol (2007) 312:131–46. doi:10.1016/j.ydbio.2007.09.005
52. Oren M, Escande ML, Paz G, Fishelson Z, Rinkevich B. Urochordate histoincompatible interactions activate vertebrate-like coagulation system components. PLoS One (2008) 3:e3123. doi:10.1371/journal.pone.0003123
53. Iwanaga S, Lee BL. Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol (2005) 38:128–50.
54. Azumi K, Sabau SV, Fujie M, Usami T, Koyanagi R, Kawashima T, et al. Gene expression profile during the life cycle of the urochordate Ciona intestinalis. Dev Biol (2007) 308:572–82. doi:10.1016/j.ydbio.2007.05.022
55. Oren M, Paz G, Douek J, Rosner A, Or Amar K, Rinkevich B. Marine invertebrates cross phyla comparisons reveal highly conserved immune machinery. Immunobiology (2013) 218:484–95. doi:10.1016/j.imbio.2012.06.004
56. Miyazawa S, Azumi K, Nonaka M. Cloning and characterization of integrin α subunits from the solitary ascidian Halocynthia roretzi. J Immunol (2001) 166:1710–5. doi:10.4049/jimmunol.166.3.1710
57. Miyazawa S, Nonaka M. Characterization of novel ascidian ß integrins as primitive complement receptor subunits. Immunogenetics (2004) 55:836–44. doi:10.1007/s00251-004-0651-8
58. Sasaki N, Ogasawara M, Sekiguchi T, Kusumoto S, Satake H. Toll-like receptors of the ascidian Ciona intestinalis: prototypes with hybrid functionalities of vertebrate toll-like receptors. J Biol Chem (2009) 284:27336–43. doi:10.1074/jbc.M109.032433
59. Cima F, Basso G, Ballarin L. Apoptosis and phosphatidylserine-mediated recognition during the take-over phase of the colonial life-cycle in the ascidian Botryllus schlosseri. Cell Tissue Res (2003) 312:369–76. doi:10.1007/s00441-003-0738-9
60. Shida K, Terajima D, Uchino R, Ikawa S, Ikeda M, Asano K, et al. Hemocytes of Ciona intestinalis express multiple genes involved in innate immune host defense. Biochem Biophys Res Commun (2003) 302:207–18. doi:10.1016/S0006-291X(03)00113-X
61. Terajima D, Yamada S, Uchino R, Ikawa S, Ikeda M, Shida K, et al. Identification and sequence of seventy-nine new transcripts expressed in hemocytes of Ciona intestinalis, three of which may be involved in characteristic cell-cell communication. DNA Res (2003) 10:203–12. doi:10.1093/dnares/10.5.203
62. Lauzon RJ, Brown C, Kerr L, Tiozzo S. Phagocyte dynamics in a highly regenerative urochordate: insights into development and host defense. Dev Biol (2013) 374:357–73. doi:10.1016/j.ydbio.2012.11.006
63. García-García E, Gómez-González NE, Meseguer J, García-Ayala A, Mulero V. Histamine regulates the inflammatory response of the tunicate Styela plicata. Dev Comp Immunol (2014) 46:382–91. doi:10.1016/j.dci.2014.05.017
64. Cima F, Ballarin L, Gasparini F, Burighel P. External amebocytes guard the pharynx entry in a tunicate (Ascidiacea). Dev Comp Immunol (2006) 30:463–72. doi:10.1016/j.dci.2005.07.004
65. Parrinello D, Sanfratello MA, Vizzini A, Parrinello N, Cammarata M. Ciona intestinalis galectin (Cigals-a and Cigals-b) genes are differentially expressed in endostyle zones and challenged by LPS. Fish Shellfish Immunol (2015) 42:171–6. doi:10.1016/j.fsi.2014.10.026
66. Parrinello D, Sanfratello MA, Vizzini A, Tetasecca L, Parrinello N, Cammarata M. The Ciona intestinalis immune-related galectin genes (Cigals-a and Cigals-b) are espressed by the gastric epithelium. Fish Shellfish Immunol (2017) 62:24–30. doi:10.1016/j.fsi.2016.12.027
67. Dishaw LJ, Cannon JP, Litman GW, Parker W. Immune-directed support of rich microbial communities in the gut has ancient roots. Dev Comp Immunol (2014) 47:36–51. doi:10.1016/j.dci.2014.06.011
68. Tiozzo S, Ballarin L, Burighel P, Zaniolo G. Programmed cell death in vegetative development: apoptosis during the colonial life cycle of the ascidian Botryllus schlosseri. Tissue Cell (2006) 38:193–201. doi:10.1016/j.tice.2006.02.003
69. Vizzini A, Parrinello D, Sanfratello MA, Trapani MR, Mangano V, Parrinello N, et al. Upregulated transcription of phenoloxidase genes in the pharynx and endostyle of Ciona intestinalis in response to LPS. J Invertebr Pathol (2015) 126:6–11. doi:10.1016/j.jip.2015.01.009
70. Smith VJ, Peddie CM. Cell cooperation during host defense in the solitary tunicate Ciona intestinalis (L). Biol Bull (1992) 183:211–9. doi:10.2307/1542208
71. Jackson AD, Smith VJ. LPS-sensitive protease activity in the blood cells of the solitary ascidian, Ciona intestinalis (L). Comp Biochem Physiol (1993) 106B:505–12.
72. Müller WE, Pancer Z, Rinkevich B. Molecular cloning and localization of a novel serine protease from the colonial tunicate Botryllus schlosseri. Mol Mar Biol Biotechnol (1994) 3:70–7.
73. Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol Rev (2004) 198:116–26. doi:10.1111/j.0105-2896.2004.00116.x
74. Immesberger A, Burmester T. Putative phenoloxidase in the tunicate Ciona intestinalis and the origin of the arthropod hemocyanin superfamily. J Comp Physiol (2004) 174B:169–80. doi:10.1007/s00360-003-0402-4
75. Ballarin L, Franchi N, Schiavon F, Tosatto SC, Mičetić I, Kawamura K. Looking for putative phenoloxidases of compound ascidians: haemocyanin-like proteins in Polyandrocarpa misakiensis and Botryllus schlosseri. Dev Comp Immunol (2012) 38:232–42. doi:10.1016/j.dci.2012.05.008
76. Taylor SW, Ross MM, Waite JH. Novel 3,4-di-and 3,4,5-trihydroxyphenylalanine-containing polypeptides from the blood cells of the ascidian Ascidia ceratodes and Molgula manhattensis. Arch Biochem Biophys (1995) 324:228–40. doi:10.1006/abbi.1995.0035
77. Tincu JA, Taylor SW. Tunichrome sp-1: new pentapeptide tunichrome from the hemocytes of Styela plicata. J Nat Prod (2002) 65:377–8. doi:10.1021/np010352z
78. Rao MR, Faulkner DJ. Botryllamides E-H, four new tyrosine derivatives from the ascidian Botrylloides tyreum. J Nat Prod (2004) 67:1064–6. doi:10.1021/np0499618
79. Franchi N, Cima F, Ballarin L. Insights on cytotoxic cells of the colonial ascidian Botryllus schlosseri. Invert Surviv J (2015) 12:109–17.
80. Coombe DR, Ey PL, Jenkin CR. Ascidian haemagglutinins: incidence in various species, binding specificities and preliminary characterisation of selected agglutinins. Comp Biochem Physiol (1984) 77B:811–9.
82. Vasta GR, Quesenberry MS, Ahmed H, O’Leary N. Lectins from tunicates: structure-function relationships in innate immunity. Adv Exp Med Biol (2001) 484:275–87. doi:10.1007/978-1-4615-1291-2_26
83. Quesenberry MS, Ahmed H, Elola MT, O’Leary N, Vasta GR. Diverse lectin repertoires in tunicates mediate broad recognition and effector innate immune responses. Integr Comp Biol (2003) 43:323–30. doi:10.1093/icb/43.2.323
84. Ballarin L, Cammarata M, Franchi N, Parrinello N. Routes in innate immunity evolution: galectins and rhamnose-binding lectins in ascidians. In: Kim S-W, editor. Marine Protein and Peptides. Biological Activities and Applications. Chichester: Wiley-Blackwell (2013). p. 185–205.
85. Coombe DR, Ey PL, Jenkin CR. Particle recognition by haemocytes from the colonial ascidian Botrylloides leachi: evidence that the B. leachi HA-2 is opsonic. J Comp Physiol (1984) 154:509–21. doi:10.1007/BF02515156
86. Kelly KL, Cooper EL, Raftos DA. Purification and characterization of a humoral opsonin from the solitary urochordate Styela clava. Comp Biochem Physiol (1992) 103B:749–53.
87. Pearce S, Newton RA, Nair SV, Raftos DA. Humoral opsonins of tunicate, Pyura stolonifera. Dev Comp Immunol (2001) 25:377–85. doi:10.1016/S0145-305X(01)00011-8
88. Parrinello N, Arizza V, Cammarata M, Giaramita FT, Pergolizzi M, Vazzana M, et al. Inducible lectins with galectin properties and human IL1α epitopes opsonize yeast during the inflammatory response of the ascidian Ciona intestinalis. Cell Tissue Res (2007) 329:379–90. doi:10.1007/s00441-007-0415-5
89. Azumi K, Ishimoto R, Fujita T, Nonaka M, Yokosawa H. Opsonin-independent and -dependent phagocytosis in the ascidian Halocynthia roretzi: galactose-specific lectin and complement C3 function as target-dependent opsonins. Zoolog Sci (2000) 17:625–32. doi:10.2108/zsj.17.625
90. Gasparini F, Franchi N, Spolaore B, Ballarin L. Novel rhamnose-binding lectins from the colonial ascidian Botryllus schlosseri. Dev Comp Immunol (2008) 32:1177–91. doi:10.1016/j.dci.2008.03.006
91. Khalturin K, Becker M, Rinkevich B, Bosch TCG. Urochordates and the origin of natural killer cells: identification of a CD94/NKR-P1-related receptor in blood cells of Botryllus. Proc Natl Acad Sci U S A (2003) 100:622–7. doi:10.1073/pnas.0234104100
92. Parrinello N. Focusing on Ciona intestinalis (Tunicata) innate immune system. Evolutionary implications. Invert Surviv J (2009) 6:S46–57.
93. Pancer Z, Cooper L, Müller WEG. A tunicate (Botryllus schlosseri) cDNA reveals similarity to vertebrate antigen receptor. Immunogenetics (1996) 45:69–72. doi:10.1007/s002510050169
94. Pancer Z, Diehl-Seifert B, Rinkevich B, Müller WEG. A novel tunicate (Botryllus schlosseri) putative C-type lectin features an immunoglobulin domain. DNA Cell Biol (1997) 16:801–6.
95. Liberti A, Melillo D, Zucchetti I, Natale L, Dishaw LJ, Litman GW, et al. Expression of Ciona intestinalis variable region-containing chitin-binding proteins during development of the gastrointestinal tract and their role in host-microbe interactions. PLoS One (2014) 9:e94984. doi:10.1371/journal.pone.0094984
96. Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol (2002) 2:346–53. doi:10.1038/nri800
97. Nonaka M, Azumi K, Ji X, Namikawa-Yamada C, Sasaki M, Saiga H, et al. Opsonic complement component C3 in the solitary ascidian Halocynthia roretzi. J Immunol (1999) 162:387–91.
98. Marino R, Kimura Y, DeSantis R, Lambris JD, Pinto MR. Complement in urochordates: cloning and characterization of two C3-like genes in the ascidian Ciona intestinalis. Immunogenetics (2002) 53:1055–64. doi:10.1007/s00251-001-0421-9
99. Raftos DA, Robbins J, Newton RA, Nair SV. A complement component C3a-like stimulates chemotaxis by hemocytes from an invertebrate chordate – the tunicate, Pyura stolonifera. Comp Biochem Physiol (2003) 134A:377–86. doi:10.1016/S1095-6433(02)00287-8
100. Franchi N, Ballarin L. Preliminary characterization of complement in a colonial tunicate: C3, Bf and inhibition of C3 opsonic activity by compstatin. Dev Comp Immunol (2014) 46:430–8. doi:10.1016/j.dci.2014.05.014
101. Giacomelli S, Melillo D, Lambris JD, Pinto MR. Immune competence of the Ciona intestinalis pharynx: complement system-mediate activity. Fish Shellfish Immunol (2012) 33:946–52. doi:10.1016/j.fsi.2012.08.003
102. Pinto MR, Chinnici CM, Kimura Y, Melillo D, Marino R, Spruce LA, et al. CiC3-1a-mediated chemotaxis in the deuterostome invertebrate Ciona intestinalis (Urochordata). J Immunol (2003) 171:5521–8. doi:10.4049/jimmunol.171.10.5521
103. Melillo D, Sfyroera G, De Santis R, Graziano R, Marino R, Lambris JD, et al. First identification of a chemotactic receptor in an invertebrate species: structural and functional characterization of Ciona intestinalis C3a receptor. J Immunol (2006) 177:4132–40. doi:10.4049/jimmunol.177.6.4132
104. Nonaka M, Azumi K. Opsonic complement system of the solitary ascidian Halocynthia roretzi. Dev Comp Immunol (1999) 23:421–7. doi:10.1016/S0145-305X(99)00021-X
105. Raftos DA, Fabbro M, Nair SV. Exocytosis of a complement component C3-like protein by tunicate hemocytes. Dev Comp Immunol (2004) 28:181–90. doi:10.1016/S0145-305X(03)00136-8
106. Ji X, Namikawa-Yamada M, Nakanishi M, Sasaki M, Nonaka M. Molecular cloning of complement factor B from a solitary ascidian: unique combination of domains implicating ancient exon shuffling. Immunopharmacology (2000) 49:43. doi:10.1016/S0162-3109(00)80122-8
107. Yoshizaki FY, Ikawa S, Satake M, Satoh N, Nonaka M. Structure and the evolutionary implication of the triplicated complement factor B genes of a urochordate ascidian, Ciona intestinalis. Immunogenetics (2005) 56:930–42. doi:10.1007/s00251-004-0752-4
108. Nair SV, Pearce S, Green PL, Mahajan D, Newton RA, Raftos DA. A collectin-like protein from tunicates. Comp Biochem Physiol (2000) 125B:279–89. doi:10.1016/S0305-0491(99)00180-7
109. Green P, Luty A, Nair S, Radford J, Raftos D. A second form of collagenous lectin from the tunicate, Styela plicata. Comp Biochem Physiol (2006) 144B:343–50. doi:10.1016/j.cbpb.2006.03.011
110. Green PL, Nair SV, Raftos DA. Secretion of a collectin-like protein in tunicates is enhanced during inflammatory responses. Dev Comp Immunol (2003) 27:3–9. doi:10.1016/S0145-305X(02)00067-8
111. Sekine H, Kenjo A, Azumi K, Ohi G, Takahashi M, Kasukawa R, et al. An ancient lectin-dependent complement system in an ascidian: novel lectin isolated from the plasma of the solitary ascidian, Halocynthia roretzi. J Immunol (2001) 167:4504–10. doi:10.4049/jimmunol.167.8.4504
112. Ji X, Azumi K, Sasaki M, Nonaka M. Ancient origin of the complement lectin pathway revealed by molecular cloning of mannan binding protein-associated serine protease from a urochordate, the Japanese ascidian Halocynthia roretzi. Proc Natl Acad Sci U S A (1997) 94:6340–5. doi:10.1073/pnas.94.12.6340
113. Corey DM, Rosental B, Kowarsky M, Sinha R, Ishizuka KJ, Palmeri KJ, et al. Developmental cell death programs license cytotoxic cells to eliminate histocompatible partners. Proc Natl Acad Sci U S A (2016) 113:6520–5. doi:10.1073/pnas.1606276113
114. Pancer Z, Gershon H, Rinkevich B. Cloning of a urochordate cDNA featuring mammalian short consensus repeats (SCR) of complement-control protein superfamily. Comp Biochem Physiol (1995) 111B:625–32. doi:10.1016/0305-0491(95)00025-4
115. Cooper EL, Yao D. Diving for drugs: tunicate anticancer compounds. Drug Discov Today (2012) 17:636–48. doi:10.1016/j.drudis.2012.02.006
116. Davis AR, Bremner JB. Potential antifouling natural products from ascidians: a review. In: Fingerman M, Nagabhushanam R, Thompson M-F, editors. Marine Biotechnology, Volume 3: Biofilms, Bioadhesion, Corrosion and Biofouling. Boca Raton, FL: CRC Press (1999). p. 259–308.
117. Pisut DP, Pawlik JR. Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? J Exp Mar Bio Ecol (2002) 270:203–14. doi:10.1016/S0022-0981(02)00023-0
118. Bryan PJ, McClintock JB, Slattery M, Rittschof DP. A comparative study of the non-acidic chemically mediated antifoulant properties of three sympatric species of ascidians associated with seagrass habitats. Biofouling (2003) 19:235–45. doi:10.1080/0892701031000085222
119. Núñez-Pons L, Forestieri R, Nieto RM, Varela M, Nappo M, Rodríguez J, et al. Chemical defenses of tunicates of the genus Aplidium from the Weddell Sea (Antarctica). Polar Biol (2010) 33:1319–29. doi:10.1007/s00300-010-0819-7
120. Mayzel B, Haber M, Ilan M. Chemical defense against fouling in the solitary ascidian Phallusia nigra. Biol Bull (2014) 227:232–41. doi:10.1086/BBLv227n3p232
121. Martinez-García M, Diaz-Valdés M, Ramos-Esplá A, Salvador N, Lopez P, Larriba E, et al. Cytotoxicity of the ascidian Cystodytes dellechiajei against tumor cells and study of the involvement of associated microbiota in the production of cytotoxic compounds. Mar Drugs (2007) 5:52–70. doi:10.3390/md503052
122. Odate S, Pawlik JR. The role of vanadium in the chemical defense of the solitary tunicate, Phallusia nigra. J Chem Ecol (2007) 33:643–54. doi:10.1007/s10886-007-9251-z
123. Lippert H, Brinkmeyer R, Mülhaupt T, Iken K. Antimicrobial activity in sub-Arctic marine invertebrates. Polar Biol (2003) 26:591–600. doi:10.1007/s00300-003-0525-9
124. Seleghim MHR, Lira SP, Kossuga MH, Batista T, Berlink RGS, Hajdu E, et al. Antibiotic, cytotoxic and enzyme inhibitory activity of crude extracts from Brazilian marine invertebrates. Rev Bras Farmacogn (2007) 17:287–318. doi:10.1590/S0102-695X2007000300002
125. Tadesse M, Gulliksen B, Strøm MB, Styrvold OB, Haug T. Screening for antibacterial and antifungal activities in marine benthic invertebrates from northern Norway. J Invertebr Pathol (2008) 99:286–93. doi:10.1016/j.jip.2008.06.009
126. Lu Y, Zhuang Y, Liu J. Mining antimicrobial peptides from small open reading frames in Ciona intestinalis. J Pept Sci (2014) 20:25–9. doi:10.1002/psc.2584
127. Azumi K, Yokosawa H, Ishii S. Halocyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry (1990) 29:159–65. doi:10.1021/bi00453a021
128. Lee IH, Zhao C, Nguien T, Menzel L, Waring AJ, Sherman MA, et al. Clavaspirin, an antibacterial and haemolytic peptide from Styela clava. J Pept Res (2001) 58:445–56. doi:10.1034/j.1399-3011.2001.10975.x
129. Menzel LP, Lee IH, Sjostrand B, Lehrer RI. Immunolocalization of clavanins in Styela clava hemocytes. Dev Comp Immunol (2002) 26:505–15. doi:10.1016/S0145-305X(02)00010-1
130. Zhao C, Liaw L, Lee IH, Lehrer RI. cDNA cloning of three cecropin-like antimicrobial peptides (Styelins) from the tunicate Styela clava. FEBS Lett (1997) 412:144–8. doi:10.1016/S0014-5793(97)00769-2
131. Lehrer RI, Tincu JA, Taylor SW, Menzel LP, Waring AJ. Natural peptide antibiotics from tunicates: structures, functions and potential uses. Integr Comp Biol (2003) 43:313–22. doi:10.1093/icb/43.2.313
132. Fedders H, Leippe M. A reverse search for antimicrobial peptides in Ciona intestinalis: identification of a gene family expressed in hemocytes and evaluation of activity. Dev Comp Immunol (2008) 32:286–98. doi:10.1016/j.dci.2007.06.003
133. Fedders H, Michalek M, Grötzinger J, Leippe M. An exceptional salt-tolerant antimicrobial peptide derived from a novel gene family of haemocytes of the marine invertebrate Ciona intestinalis. Biochem J (2008) 416:65–75. doi:10.1042/BJ20080398
134. Jena P, Mishra B, Leippe M, Hasilik A, Griffiths G, Sonawane A. Membrane-active antimicrobial peptides and human placental lysosomal extracts are highly active against mycobacteria. Peptides (2011) 32:881–7. doi:10.1016/j.peptides.2011.03.002
135. Cotelle N, Moreau S, Cotelle P, Catteau JP, Bernier JL, Hénichart JP. Generation of free radicals by simple pregnylated hydroquinone derivatives, natural antitumor agents from the marine urochordate Aplidium californicum. Chem Res Toxicol (1991) 4:300–5. doi:10.1021/tx00021a007
136. Ogi T, Taira J, Margiastuti P, Ueda K. Cytotoxic metabolites from the Okinawan ascidian Diplosoma virens. Molecules (2008) 13:595–602. doi:10.3390/molecules13030595
137. Pancer Z, Cooper L, Müller WEG. A urochordate putative homolog of human EB1, the protein which binds APC. Cancer Lett (1996) 109:155–60. doi:10.1016/S0304-3835(96)04440-0
138. Beschin A, Bilej M, Torreele E, De Baetselier P. On the existence of cytokines in invertebrates. Cell Mol Life Sci (2001) 58:801–14. doi:10.1007/PL00000901
139. Beschin A, Bilej M, Magez S, Lucas R, De Baetselier P. Functional convergence of invertebrate and vertebrate cytokine-like molecules based on a similar lectin-like activity. Prog Mol Subcell Biol (2004) 34:145–63. doi:10.1007/978-3-642-18670-7_6
140. Parrinello N, Vizzini A, Arizza V, Salerno G, Parrinello D, Cammarata M, et al. Enhanced expression of a cloned and sequenced Ciona intestinalis TNFα-like (CiTNFα) gene during the LPS-induced inflammatory response. Cell Tissue Res (2008) 334:305–17. doi:10.1007/s00441-008-0695-4
141. Parrinello N, Vizzini A, Salerno G, Sanfratello MA, Cammarata M, Arizza V, et al. Inflamed adult pharynx tissues and swimming larva of Ciona intestinalis share CiTNFα-producing cells. Cell Tissue Res (2010) 341:299–311. doi:10.1007/s00441-010-0993-5
142. Vizzini A, Di Falco F, Parrinello D, Sanfratello MA, Mazzarella C, Parrinello N, et al. Ciona intestinalis interleukin 17-like genes expression is upregulated by LPS challenge. Dev Comp Immunol (2015) 48:129–37. doi:10.1016/j.dci.2014.09.014
143. Ballarin L, Franchini A, Ottaviani E, Sabbadin A. Morula cells as the major immunomodulatory hemocytes in ascidians: evidences from the colonial species Botryllus schlosseri. Biol Bull (2001) 201:59–64. doi:10.2307/1543526
144. Menin A, Ballarin L. Immunomodulatory molecules in the compound ascidian Botryllus schlosseri: evidence from conditioned media. J Invertebr Pathol (2008) 99:275–80. doi:10.1016/j.jip.2008.08.001
145. Menin A, Del Favero M, Cima F, Ballarin L. Release of phagocytosis-stimulating factor(s) by morula cells in a colonial ascidian. Mar Biol (2005) 148:225–30. doi:10.1007/s00227-005-0081-7
146. Cima F, Sabbadin A, Ballarin L. Cellular aspects of allorecognition in the compound ascidian Botryllus schlosseri. Dev Comp Immunol (2004) 28:881–9. doi:10.1016/j.dci.2004.02.001
147. Cima F, Sabbadin A, Zaniolo G, Ballarin L. Colony specificity and chemotaxis in the compound ascidian Botryllus schlosseri. Comp Biochem Physiol (2006) 145A:376–82. doi:10.1016/j.cbpa.2006.07.017
148. Takahashi H, Azumi K, Yokosawa H. Hemocyte aggregation in the solitary ascidian Halocynthia roretzi: plasma factors, magnesium ion, and Met-Lys-bradykinin induce the aggregation. Biol Bull (1994) 186:247–53. doi:10.2307/1542270
149. Takahashi H, Azumi K, Yokosawa H. A novel membrane glycoprotein involved in ascidian hemocyte aggregation and phagocytosis. Eur J Biochem (1995) 233:778–83. doi:10.1111/j.1432-1033.1995.778_3.x
150. Takahashi H, Ishikawa G, Ueki K, Azumi K, Yokosawa H. Cloning and tyrosine phosphorylation of a novel invertebrate immunocyte protein containing immunoreceptor tyrosine-based activation motifs. J Biol Chem (1997) 272:32006–10. doi:10.1074/jbc.272.51.32006
151. Azumi K, Sasaki T, Okochi K, Yamasaki S, Saito T, Takayama H, et al. Differential display analysis reveals the expression of glutathione S-tranferase ω and novel genes through an ITAM-containing receptor in ascidian immunocytes. Immunogenetics (2005) 27:444–52. doi:10.1007/s00251-005-0003-3
152. Cima F, Ballarin L, Sabbadin A. New data on phagocytes and phagocytosis in the compound ascidian Botryllus schlosseri. Ital J Zool (1996) 63:357–64. doi:10.1080/11250009609356159
153. Ishikawa G, Azumi K, Yokosawa H. Involvement of tyrosine kinase and phosphatidylinositol 3-kinase in phagocytosis by ascidian hemocytes. Comp Biochem Physiol (2000) 125A:351–7. doi:10.1016/S1095-6433(00)00165-3
154. Franchi N, Schiavon F, Betti M, Canesi L, Ballarin L. Insight on signal transduction pathways involved in phagocytosis in the colonial ascidian Botryllus schlosseri. J Invertebr Pathol (2013) 112:260–6. doi:10.1016/j.jip.2012.12.001
155. Manni L, Zaniolo G, Cima F, Burighel P, Ballarin L. Botryllus schlosseri: a model ascidian for the study of asexual reproduction. Dev Dyn (2007) 236:335–52. doi:10.1002/dvdy.21037
156. Lauzon RJ, Ishizuka KJ, Weissman IL. A cyclical, developmentally-regulated death phenomenon in a colonial urochordate. Dev Dyn (1992) 194:71–83. doi:10.1002/aja.1001940109
157. Lauzon RJ, Patton CW, Weissman IL. A morphological and immunohistochemical study of programmed cell death in Botryllus schlosseri (Tunicata, Ascidiacea). Cell Tissue Res (1993) 272:115–27. doi:10.1007/BF00323577
158. Ballarin L, Burighel P, Cima F. A tale of death and life: natural apoptosis in the colonial ascidian Botryllus schlosseri (Urochordata Ascidiacea). Curr Pharm Des (2008) 14:138–47. doi:10.2174/138161208783378798
159. Franchi N, Ballin F, Manni L, Schiavon F, Basso G, Ballarin L. Recurrent phagocytosis-induced apoptosis in the ciclica generation change of the compound ascidian Botryllus schlosseri. Dev Comp Immunol (2016) 62:8–16. doi:10.1016/j.dci.2016.04.011
160. Voskoboynik A, Rinkevich B, Weiss A, Moiseeva E, Reznick AZ. Macrophage involvement for successful degeneration of apoptotic organs in the colonial urochordate Botryllus schlosseri. J Exp Biol (2004) 207:2409–16. doi:10.1242/jeb.01045
161. Lauzon RJ, Shizuka KJ, Weissman IL. Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new: a model for development and regeneration. Dev Biol (2002) 249:333–48. doi:10.1006/dbio.2002.0772
162. Lauzon RJ, Kidder SJ, Long P. Suppression of programmed cell death regulates the cyclical degeneration of organs in a colonial urochordate. Dev Biol (2007) 301:92–105. doi:10.1016/j.ydbio.2006.08.055
163. Shirae M, Hirose E, Saito Y. Behavior of hemocytes in the allorejection reaction in two compound ascidians Botryllus scalaris and Symplegma reptans. Biol Bull (1999) 197:188–97. doi:10.2307/1542614
164. Parrinello N, Cammarata M, Lipari L, Arizza V. Sphingomyelin inhibition of Ciona intestinalis (Tunicata) cytotoxic hemocytes assayed against sheep erythrocytes. Dev Comp Immunol (1995) 19:31–41. doi:10.1016/0145-305X(94)00046-I
165. Lipari L, Cammarata M, Arizza V, Parrinello D. Cytotoxic activity of Styela plicata hemocytes against mammalian cell targets: I. Properties of the in vitro reaction against erythrocytes. Anim Biol (1995) 4:131–7.
166. Cammarata M, Candore G, Arizza V, Caruso C, Parrinello N. Cytotoxic activity of Styela plicata hemocytes against mammalian cell targets: II properties of the in vitro reaction against human tumour cell lines. Anim Biol (1995) 4:139–44.
167. Arizza V, Parrinello D, Cammarata M, Vazzana M, Vizzini A, Giaramita FT, et al. A lytic mechanism based on soluble phospholypases A2 (sPLA2) and b-galactoside specific lectins is exerted by Ciona intestinalis (ascidian) unilocular refractile hemocytes against K562 cell line and mammalian erythrocytes. Fish Shellfish Immunol (2011) 30:1014–23. doi:10.1016/j.fsi.2011.01.022
168. Fuke M. “Contact reactions” between xenogeneic or allogeneic coelomic cells of solitary ascidians. Biol Bull (1980) 158:304–15. doi:10.2307/1540857
169. De Barros CM, De Carvalho DR, Andrade LR, Pavão MSG, Allodi S. Nitric oxide production by hemocytes of the ascidian Styela plicata. Cell Tissue Res (2009) 338:117–28. doi:10.1007/s00441-009-0851-5
170. De Barros CM, Emrich LC, de A Mello A, Da Fonseca RN, Allodi S. Regulation of nitric-oxide production in hemocytes of the ascidian Phallusia nigra. Nitric Oxide (2014) 38:26–36. doi:10.1016/j.niox.2014.02.007
171. Cammarata M, Arizza V, Cianciolo C, Parrinello D, Vazzana M, Vizzini A, et al. The prophenoloxidase system is activated during the tunic inflammatory reaction of Ciona intestinalis. Cell Tissue Res (2008) 333:481–92. doi:10.1007/s00441-008-0649-x
172. Trapani MR, Sanfratello MA, Mangano V, Parrinello D, Vizzini A, Cammarata M. Phenoloxidases of different sizes are modulated by LPS inoculation into Ciona intestinalis tunic and pharynx. Invert Surviv J (2015) 12:75–81.
173. Vizzini A, Parrinello D, Sanfratello MA, Mangano V, Parrinello N, Cammarata M. Ciona intestinalis peroxinectin is a novel component of the peroxidase-cyclooxygenase superfamily upregulated by LPS. Dev Comp Immunol (2013) 41:59–67. doi:10.1016/j.dci.2013.03.015
174. Vizzini A, Bonura A, Longo V, Sanfratello MA, Parrinello D, Cammarata M, et al. Isolation of a novel LPS-induced component of the ML superfamily in Ciona intestinalis. Dev Comp Immunol (2015) 53:70–8. doi:10.1016/j.dci.2015.06.018
175. Vizzini A, Di Falco F, Parrinello D, Sanfratello MA, Cammarata M. Tranforming growth factor β (CiTGF-β) gene expression is induced in the inflammatory reaction of Ciona intestinalis. Dev Comp Immunol (2016) 55:102–10. doi:10.1016/j.dci.2015.10.013
176. Reddy AL, Bryan B, Hildemann WH. Integumentary allograft versus autograft reactions in Ciona intestinalis: a protochordate species of solitary tunicata. Immunogenetics (1975) 7:584–90.
177. Raftos DA, Tait NN, Briscoe DA. Allograft rejection and alloimmune memory in the solitary urochordate Styela plicata. Dev Comp Immunol (1987) 11:343–51. doi:10.1016/0145-305X(87)90078-4
178. Raftos DA, Tait NN, Briscoe DA. Cellular basis of allograft rejection in the solitary urochordate Styela plicata. Dev Comp Immunol (1987) 11:713–25. doi:10.1016/0145-305X(87)90078-4
179. Parrinello N. Cytotoxic activity of tunicate hemocytes. In: Rinkevich B, Muller WEG, editors. Invertebrate Immunology. Berlin: Springer-Verlag (1996). p. 190–217.
180. Kürn U, Sommer F, Hemmrich G, Bosch TCG, Kalthurin K. Allorecognition in urochordates: identification of a highly variable complement receptor-like protein expressed in follicle cells of Ciona. Dev Comp Immunol (2007) 31:360–71. doi:10.1016/j.dci.2006.06.008
181. Sommer F, Awazu S, Anton-Exleben F, Jiang D, Klimovich AV, Klimovich BV, et al. Blood system formation in the urochordate Ciona intestinalis requires the variable receptor vCRL1. Mol Biol Evol (2012) 29:3081–93. doi:10.1093/molbev/mss120
182. Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell (2005) 123:1351–60. doi:10.1016/j.cell.2005.10.026
183. De Tomaso AW. Allorecognition polymorphism versus parasitic stem cells. Trends Genet (2006) 22:485–90. doi:10.1016/j.tig.2006.07.001
184. Sabbadin A. Le basi genetiche della capacità di fusione fra colonie in Botryllus schlosseri (Ascidiacea). Rend Accad Naz Lincei (1962) 32:1021–35.
185. Oka H. Colony specificity in compound ascidians. The genetic control of fusibility. In: Yukawa H, editor. Profiles of Japanese Science and Scientists. Tokyo: Kodanska (1970). p. 196–206.
186. Rinkevich B, Tartakover S, Gershon H. Contribution of morula cells to allogeneic responses in the colonial ascidian Botryllus schlosseri. Mar Biol (1998) 131:227–36. doi:10.1007/s002270050315
187. Rinkevich B. Aspects of the incompatibility nature in botryllid ascidian. Anim Biol (1992) 1:17–28.
188. Oren M, Douek J, Fishelson Z, Rinkevich B. Identification of immune-relevant genes in histoincompatible rejecting colonies of the tunicate Botryllus schlosseri. Dev Comp Immunol (2007) 31:889–902. doi:10.1016/j.dci.2006.12.009
189. Rinkevich B, Weissman IL. Retreat growth in the ascidian Botryllus schlosseri: a consequence of nonself recognition. In: Grosberg RK, Hedgecock D, Nelson K, editors. Invertebrate Historecognition. New York: Plenum Press (1988). p. 93–109.
190. Dias GM, Yokohama Q. Spatial competition induces the mobilization of morula cells in the colonial ascidian Didemnum perlucidum (Tunicata: Didemnidae). Invertebr Biol (2011) 130:186–92. doi:10.1111/j.1744-7410.2011.00222.x
191. Rinkevich B, Lilker-Levav T, Goren M. Allorecognition/xenorecognition responses in Botrylloides (Ascidiacea) subpopulations from the Mediterranean coast of Israel. J Exp Zool (1994) 270:302–13. doi:10.1002/jez.1402700309
192. Hirose E, Saito Y, Watanabe H. Surgical fusion between incompatible colonies of the compound ascidian Botrylloides fuscus. Dev Comp Immunol (1994) 18:287–94. doi:10.1016/S0145-305X(94)90354-9
193. Saito Y, Hirose E, Watanabe H. Allorecognition in compound ascidians. Int J Dev Biol (1994) 38:237–47.
194. Hirose E. Colonial allorecognition, hemolytic rejection, and viviparity in botryllid ascidians. Zoolog Sci (2003) 20:387–94. doi:10.2108/zsj.20.387
195. Shirae M, Saito Y. A comparison of hemocytes and their phenoloxidase activity among botryllid ascidians. Zoolog Sci (2000) 17:881–91. doi:10.2108/zsj.17.881
196. Okuyama M, Saito Y, Hirose E. Fusion between incompatible colonies of a viviparous ascidian Botrylloides lentus. Invertebr Biol (2002) 121:163–9. doi:10.1111/j.1744-7410.2002.tb00057.x
197. Katow H, Watanabe H. Fine structure of fusion reaction in the compound ascidian Botryllus primigenus Oka. Dev Biol (1980) 76:1–14. doi:10.1016/0012-1606(80)90358-9
198. Rinkevich B, Weissman IL. Allogeneic resorption in colonial protochordates: consequences of non-self recognition. Dev Comp Immunol (1992) 16:275–86. doi:10.1016/0145-305X(92)90002-T
199. McKitrick TR, Muscat CC, Pierce JD, Bhattacharya D, De Tomaso AW. Allorecognition in a basal chordate consists of independent activating and inhibitory pathways. Immunity (2011) 34:616–26. doi:10.1016/j.immuni.2011.01.019
200. Rinkevich B, Douek J, Rabinowitz C, Paz G. The candidate Fu/HC gene in Botryllus schlosseri (Urochordata) and ascidians’ historecognition – an oxymoron? Dev Comp Immunol (2012) 36:718–27. doi:10.1016/j.dci.2011.10.015
201. Nydam ML, Netuschil N, Sanders E, Langenbacher A, Lewis DD, Taketa DA, et al. The candidate histocompatibility locus of a basal chordate encodes two highly polymorphic proteins. PLoS One (2013) 8:e65980. doi:10.1371/journal.pone.0065980
202. Voskoboynik A, Newman AM, Corey DM, Sahoo D, Pushkarev D, Neff NF, et al. Identification of a colonial chordate histocompatibility gene. Science (2013) 341:384–7. doi:10.1126/science.1238036
203. Taketa DA, De Tomaso AW. Botryllus schlosseri allorecognition: tackling the enigma. Dev Comp Immunol (2015) 48:254–65. doi:10.1016/j.dci.2014.03.014
204. Taketa DA, Nydam ML, Langenbacher AD, Rodriguez D, Sanders E, De Tomaso AW. Molecular evolution and in vitro characterization of Botryllus histocompatibility factor. Immunogenetics (2015) 67:605–23. doi:10.1007/s00251-015-0870-1
205. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell (1996) 86:973–83. doi:10.1016/S0092-8674(00)80172-5
206. Roberts B, Davidson B, MacMaster G, Lockhart V, Ma E, Wallace SS, et al. A complement response may activate metamorphosis in the ascidian Boltenia villosa. Dev Genes Evol (2007) 217:449–58. doi:10.1007/s00427-007-0157-0
207. Robinson WE, Kustin K, Cloney RA. The influence of tunichrome and other reducing compounds on tunic and fin formation in embryonic Ascidia callosa Stimpson. J Exp Zool (1986) 237:63–72. doi:10.1002/jez.1402370110
208. Hibino T, Nonaka M. A novel third complement component C3 gene of Ciona intestinalis expressed in the endoderm at the early developmental stages. Invert Surv J (2013) 10:29–37.
209. Parrinello D, Sanfratello MA, Vizzini A, Cammarata M. The expression of an immune-related phenoloxidase gene is modulated in Ciona intestinalis ovary, test cells, embryos and larva. J Exp Zool (2015) 342B:141–51. doi:10.1002/jez.b.22613
210. Liberti A, Leigh B, De Santis R, Pinto MR, Cannon JP, Dishaw LJ, et al. An immune effector system in the protochordate gut sheds light on fundamental aspects of vertebrate immunity. Results Probl Cell Differ (2015) 57:159–73. doi:10.1007/978-3-319-20819-0_7
211. Kawai N, Takahashi H, Nishida H, Yokosawa H. Regulation of NF-kappaB/Rel by IkappaB is essential for ascidian notochord formation. Dev Biol (2005) 277:80–91. doi:10.1016/j.ydbio.2004.09.007
Keywords: tunicates, immune responses, complement, lectins, inflammation, chemical defense
Citation: Franchi N and Ballarin L (2017) Immunity in Protochordates: The Tunicate Perspective. Front. Immunol. 8:674. doi: 10.3389/fimmu.2017.00674
Received: 21 March 2017; Accepted: 24 May 2017;
Published: 09 June 2017
Edited by:
Larry J. Dishaw, University of South Florida St. Petersburg, United StatesReviewed by:
Stefano Fiorucci, University of Perugia, ItalyTaruna Madan, National Institute for Research in Reproductive Health, India
Copyright: © 2017 Franchi and Ballarin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Loriano Ballarin, bG9yaWFuby5iYWxsYXJpbkB1bmlwZC5pdA==
 Nicola Franchi
Nicola Franchi Loriano Ballarin
Loriano Ballarin