- Department of Immunology and Parasitology, Graduate School of Medicine, Tokushima University, Tokushima, Japan
Cancer immunosurveillance is critical for the elimination of neoplastic cells. In addition, recent advances in immunological checkpoint blockade drugs have revealed the importance of the immune system in cancer treatment. As a component of the immune system, CD8+ T cells have important roles in suppressing tumors. CD8+ T cells can kill tumor cells with cytotoxic molecules, such as granzymes and perforin. IFNγ, which is produced by CD8+ T cells, can increase the expression of MHC class I antigens by tumor cells, thereby rendering them better targets for CD8+ T cells. IFNγ also has crucial functions in enhancing the antitumor abilities of other immune cells. Therefore, it has been hypothesized that antitumor immunity could be improved by modulating the activity of CD8+ T cells. The Notch pathway regulates CD8+ T cells in multiple ways. It directly upregulates mRNA expression of granzyme B and perforin, enhances differentiation toward short-lived effector cells, and maintains memory T cells. Intriguingly, CD8+ T cell-specific Notch2 deletion impairs antitumor immunity, whereas the stimulation of the Notch pathway can increase tumor suppression. In this review, we will summarize the roles of the Notch pathway in CD8+ T cells and discuss issues and implications for its use in antitumor immunity.
Introduction
To suppress tumor cell growth, animals use their cell-intrinsic antitumor system, which is regulated by tumor suppressor genes. A second line of defense against tumors includes the immune system itself (1, 2). Acquired immune cells, especially CD8+ T cells, can detect and kill tumors through the latter’s expression of abnormal antigens derived from mutated, overexpressed or ectopically expressed molecules. Innate immune cells also have important roles in the antitumor system. For example, NK cells can target tumors by recognizing the expression of MHC class Ib proteins induced by cellular transformation or the lack of MHC class I molecules. Many efforts have been devoted to treating cancer by enhancing immunosurveillance.
Many efforts have been made to enhance antitumor immunity. For example, administration of cytokines, such as type I interferon, IL-2, and IL12, or TLR agonists such as BCG and imiquimod is employed to non-specifically stimulate immune system (3). Vaccine against tumors is also examined to treat them; irradiated tumor cells or selected antigens specifically expressed in tumors are used to increase tumor-specific T cell response (4). In addition, in vitro activated and expanded T cells, which can recognize tumors, are adoptively transferred to patients to increase tumor-specific immunity (5). Notably, recent advances in the development of checkpoint blockade drugs, such as antibodies to PD-1 and CTLA-4, indicate that this field of research is indeed promising (6, 7). To further improve immunotherapy, we need a better understanding of the antitumor immune system.
The Notch pathway is an evolutionarily conserved signaling pathway that regulates various biological systems, including a wide variety of functions of peripheral T cells (8–10). In mammals, the Notch system consists of four receptors (Notch1 to 4) and five ligands (Dll1, 3, 4, and Jagged1, 2). When the receptor is stimulated by the ligand, it is cleaved by an ADAM-family metalloprotease and subsequently the γ-secretase complex, and its cytoplasmic domain is translocated into the nucleus. The cytoplasmic domain then binds to DNA binding protein RBPJκ (encoded by Rbpj) and co-activator MAML, leading to transcriptional regulation of specific target genes.
Research into the physiological roles of the Notch pathway in peripheral T cells has mainly focused on CD4+ T cells. The Notch pathway regulates CD4+ T cell differentiation, cytokine production, proliferation, and/or survival, although some of the data among the papers are in disagreement (8, 9). For example, Tanigaki et al. reported that Rbpj-deficient CD4 T cells showed decreased Th2 and increased Th1 in in vivo and in vitro experiments (11). Similarly, Amsen et al. reported Th2 differentiation was dependent on the Notch pathway by using Notch1/2-double deficient mice in addition to Rbpj (12). On the other hand, Auderset et al. reported that Notch1 and 2 were required for Th1 differentiation in anti-Leishmania major immunity, while Rbpj-deficiency did not show any significant effects (13). The causes of these apparent differences have not been resolved. It is possible that the functions of Notch pathway are highly context-dependent in T cells. In this review, we will summarize research into the physiological roles of the Notch pathway in CD8+ T cells and discuss its potentials for antitumor immunity (Figure 1).
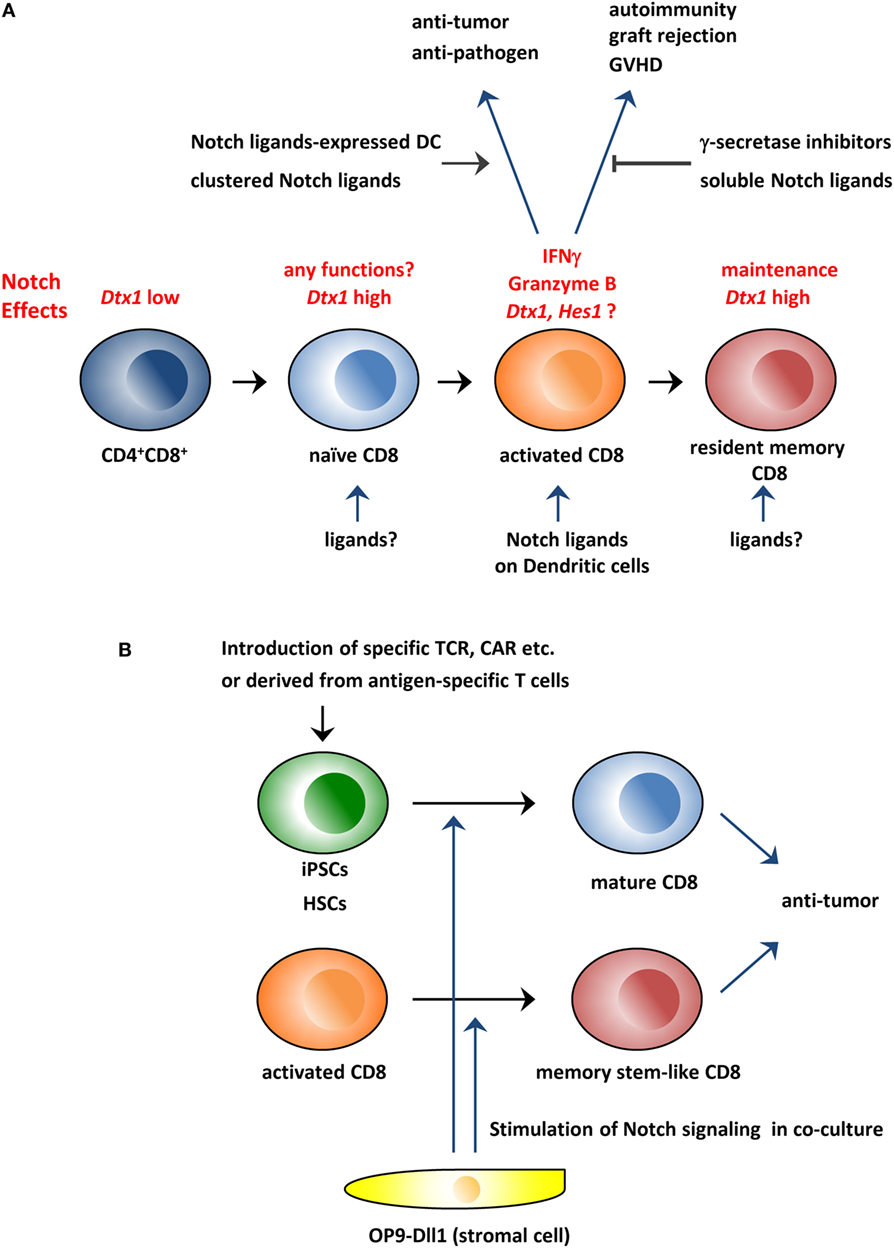
Figure 1. Schematic overview of the roles of the Notch pathway in CD8+ T cells and its application to immunotherapy. (A) The Notch pathway is stimulated during CD8+ T cell activation and is required for the production of effector molecules, such as IFNγ and granzyme B. Therefore, the modulation of the Notch pathway could be used to treat various diseases in which CD8+ T cells are involved. In addition, studies indicate that the Notch pathway is active in resting naïve and memory T cells in which the pathway is reportedly needed for the maintenance of these cells. (B) Coculture with Dll1-expressing OP9 stromal cells can generate CD8+ T cells from hematopoietic stem cells or iPSCs in vitro. In addition, the coculture system can generate memory stem cell-like T cells from activated CD8+ T cells. These in vitro generated CD8+ T cells could be superior reagents for antitumor immunity. GVHD, graft-versus-host disease; CAR, chimeric antigen receptor; iPSCs, induced pluripotent stem cells; HSCs, hematopoietic stem cells.
The Physiological Roles of the Notch Pathway in CD8+ T Cells
To elucidate the roles of Notch in CD8+ T cells, studies have analyzed mice in which the Notch pathway has been knocked out. Maekawa et al. reported that CD8+ T cell-specific (E8I-cre) Notch2 deletion led to decreased expression of Gzmb (encoding granzyme B) and increased sensitivity to Trypanosoma cruzi infection (14). This mouse also showed a significant loss of CTL activity against antigen-pulsed cells in vivo. They further showed that Notch2 and RBPJκ directly bound to Gzmb and Prf1 (encoding perforin) promoters in combination with the transcription factor CREB and activated their transcription.
Backer et al. described an influenza virus infection model in which T cell-specific (CD4-cre) Notch1/2-double KO mice showed almost complete loss of short-lived effector CD8+ T cells (SLECs) that possess the KLRG1+CD127− phenotype. On the other hand, the overall ratio of antigen-specific CD8+ T cells to that of KLRG1−CD127+ memory precursor effector cells (MPECs) was moderately increased (15). They also confirmed this phenotype was present in Rbpj KO mice. Then, they analyzed the transcriptome of activated CD8+ T cells, and showed that more than 40% of SLEC-specific genes were decreased in Notch1/2 KO cells, indicating that the Notch pathway was a critical regulator of SLEC differentiation. In addition, they also found that the Notch pathway was required for the upregulation of CD25 (IL-2Rα chain) and T-bet proteins, both of which are critical regulators of SLEC differentiation. Furthermore, they showed that T-bet overexpression enhanced SLEC differentiation in Notch1/2 KO CD8+ T cells, while the active form of Notch1 could not do so in Tbx21 (encoding T-bet) KO cells, suggesting that T-bet is a critical regulator downstream in the Notch pathway.
Similar results were reported by another laboratory. Mathieu et al. used CD8 T cell-specific Notch1/2 KO mice and showed a reduction of the ratio of SLECs after Listeria monocytogenes infection (16). However, they found that the absolute cell number of SLECs was not reduced, and the reduction of the ratio was instead due to an increased number of MPECs and early effector cells (EECs; KLRG1−CD127− cells). On the other hand, when they immunized mice with peptide-pulsed dendritic cells (DCs), they found a severe reduction of SLEC cell number, while MPEC cell numbers were not affected. The reason for this difference was not clear, but it might indicate that the roles of the Notch pathway in CD8+ T cells are context-dependent as seen in CD4+ T cells (8). As reported in the paper by Backer et al. above, they found that CD25 protein expression was diminished in Notch1/2 KO cells. However, the expression of T-bet was not affected. Instead, they found that Eomes, which is a paralog of T-bet, was moderately decreased in Notch1/2 KO cells. Eomes is reportedly required for MPEC differentiation but not for SLEC (17). Thus, the importance of the Eomes reduction in Notch1/2 KO cells for SLEC differentiation remains to be investigated.
Instead of KO mice, Maillard and colleagues used the dominant negative form of MAML (DN-MAML)-expressing mice and analyzed its effects on CD4+ and CD8+ T cells in a graft-versus-host disease (GVHD) model (18, 19). They reported that DN-MAML profoundly suppressed GVHD, with reduced production of IFNγ in CD4+ and CD8+ T cells. In contrast to KO mouse experiments, DN-MAML-expressing CD8+ T cells preserved their T-bet and Eomes protein expression. In addition, those cells showed a defect in the activation of Ras/MAPK and NF-κB pathways. Those cells also expressed higher amounts of negative regulators of T cell activation, such as Dgka, Cblb, and Pdcd1, suggesting that these factors might suppress GVHD.
In addition to the genetic approaches described above, γ-secretase inhibitors, blocking antibodies and soluble Notch ligands have been used to investigate the roles of the Notch pathway in CD8+ T cells (20–26). The consensus of these experiments is that the Notch pathway is required for IFNγ production during CD8+ T cell activation. On the other hand, the effect on the cell number after the activation of CD8+ T cells was controversial. Several papers indicated that γ-secretase inhibitors or soluble Notch ligand (Dll4) suppressed proliferation of CD8+ T cells, while their viability was not affected (22–24). Other papers showed that the inhibitors or membrane-bound Notch ligands (Jagged1) did not affect the CD8 T cell number or proliferation after activation (25, 27, 28). In addition, Notch1/2-double KO mice showed that the CD8+ T cell number was not affected or even increased when activated in vivo, although their differentiation was altered (15, 16). What caused these differences remains elusive. Further examination of the experiment-conditions and the methods of the Notch inhibition should be required in future researches.
Other studies showed that the cell surface expression of Notch1 and 2 was upregulated soon after T cell activation (14, 15, 22, 23, 29, 30). In addition, expression of its ligands (Dll1, Dll4, and/or Jagged1) was also upregulated in activated DCs (15, 21–23, 31). Based on these observations, many researchers have concluded that the Notch pathway is activated early in the process of T cell activation by the ligands on DCs. In fact, it was reported that Hes1 and/or Dtx1 (encoding Deltex1), which are well-known targets of the Notch pathway, were upregulated after T cell activation (23, 32). Other papers reported that TCR stimulation caused the cleavage of Notch receptors, indicating that the Notch pathway was activated after T cell activation (20, 33). However, transcriptome analyses clearly show that Dtx1 is upregulated during the differentiation of CD4+CD8+ thymocytes to peripheral naïve CD4+ and CD8+ T cells (Immunological genome project1; RCAI RefDIC2). We confirmed that this upregulation was dependent on Notch1/2 and Rbpj (unpublished data). Unexpectedly, Dtx1 is moderately downregulated after TCR activation, according to transcriptome data. Subsequently, its expression returns to a high level during the differentiation to memory cells. On the other hand, Hes1 expression remains low during activation of naïve and activated cells. These results suggest that the Notch pathway is active in resting T cells. The reason why Hes1 and Dtx1 were not upregulated during T cell activation remains unclear. The Notch pathway might not be activated under the conditions of T cell activation used in these studies. Alternatively, the epigenetic status of these gene loci or unknown inhibitor(s) might affect their expression during T cell activation.
Interestingly, recent papers support the hypothesis that the Notch pathway is operational in resting CD4+ and CD8+ T cells. Maekawa et al. reported that Rbpj-deficient CD4+ T cells normally expanded after antigen stimulation, but could not survive during the contraction phase. They also found that the injection of γ-secretase inhibitor to mice decreased the number of resting memory T cells (34). Hombrink et al. also reported that Notch1/2-deficiency or the treatment with γ-secretase inhibitor decreased CD103+ lung-resident memory CD8+ T cells in mice (35). These results suggest that the Notch pathway has important roles not only in activating T cells but also in resting cells.
Although some data disagree, an increasing number of reports have demonstrated that the Notch pathway was required for CD8+ T cell activation and homeostasis. When and how the Notch pathway works remains to be further investigated, but it is very probable that the manipulation of this pathway could be useful in the treatment of diseases in which the immune system is involved.
The Notch Pathway in Antitumor Immune Responses
CD8+ T cells have important roles in antitumor immunity (1, 7), some of which are dependent upon the Notch pathway. Sugimoto et al. reported that CD8-specific deletion of Notch2, but not Notch1, led to increased tumor size and decreased survival after tumor-inoculation into mice (36). Zhao et al. reported that ovarian cancer imposed glucose restriction on T cells, leading to high expression of microRNAs miR-101 and miR26a, leading to constrained expression Ezh2. Ezh2 is a suppressor of Notch pathway inhibitors Numb and Fbxw7. As a consequence, the cancer-induced glucose restriction led to the suppression of the Notch pathway. They also showed that downregulation of Ezh2 elicited poor antitumor immunity, implying that the Notch pathway was important for antitumor immunity (37). Dai et al. found that 1810011o10Rik (Tcim) was upregulated in intratumoral activated CD8+ T cells. They also showed that overexpression of Tcim blocked nuclear translocation of the intracellular domain of Notch2 and inhibited the cytotoxic efficacy of CD8+ T cells on hepatocellular carcinoma (38). All of these papers confirm that the Notch pathway in CD8+ T cells has a critical role in antitumor immunity.
Considering these reports, the manipulation of the Notch pathway in T cells could be a good approach to suppress tumors. Several papers pursued the idea in mouse models. Sugimoto et al. showed that injection of agonistic antibody to Notch2 or Dll1-overexpression in DC augmented antitumor immunity (36). Sierra et al. used intracellular Notch1-expressing mice driven by a granzyme B promoter-cre and flox system. They found that such activation of the Notch pathway in CD8+ T cells increased the cytotoxic effects and antitumor response with higher production of IFNγ and granzyme B (39). Thounaojam et al. showed that treatment with the proteasome inhibitor bortezomib caused higher expression of IFNγ in CD8+ T cells in tumor-bearing mice, probably through the upregulation of Notch receptors (40). Biktasova et al. reported that administration of clustered Dll1 enhanced IFNγ-producing CD8+ T cells and suppressed tumor growth (41). These reports reveal that Notch-targeted immune modulation could be promising. However, Notch receptors are broadly expressed in various types of cells, and the modulation of Notch might be highly context-dependent. In addition, Notch receptors are known as proto-oncogenes themselves (42). Therefore, it is possible that the activation of the pathway could exacerbate some types of tumors. Detailed investigations will be needed to examine the possibility of antitumor treatment targeting this pathway.
The therapy by immune checkpoint blockade is recent advance in antitumor immunotherapy (43). The blocking antibodies to PD-1/PD-L1 and CTLA-4 are broadly used to treat melanoma and other types of tumors. Mathieu et al. reported that Notch directly bound to the promoter region of Pdcd1 (encoding PD-1) gene and upregulated its mRNA expression in activated CD8+ T cells (23). In addition, Yu et al. indicated that γ-secretase inhibitor activated tumor-infiltrating CD8+ T cell probably through the downregulation of PD-1 expression (44). These results indicated that the Notch pathway might also have negative effect during CD8+ T cell activation. Therefore, it is expected that the antitumor therapy by Notch activation would be more efficient in combination with the blocking antibodies to PD-1 and other inhibitory receptors.
Generating Antitumor CD8+ T Cells In Vitro Using the Notch Pathway
In addition to efforts to modulate the Notch pathway in vivo to enhance antitumor immunity, there have been in vitro attempts to create cytotoxic T cells against tumors. CD8+ memory stem cells are reported to have naïve markers, but have self-renewal capacity and can rapidly respond to antigens (45, 46). In addition, they have antitumor capacities exceeding those of central and effector memory T cells (47). Kondo et al. reported that activated CD4+ or CD8+ T cells could be converted to memory stem cell-like cells when cocultured with Dll1-expressing OP9 stromal cells (OP9-Dll1) (48). They also showed that the resultant memory stem cell-like CD4+ and CD8+ T cells had superior antitumor abilities relative to naïve, activated or memory T cells when injected into mice.
In addition to peripheral T cells, the Notch pathway is well known for its role in defining the fate of T cells in early stages of differentiation. By coculturing with Dll1-expressing cells, some types of stem cells can be differentiated to T cells in vitro (49). There have been several attempts to create large number of tumor-specific CD8+ T cells through use of this in vitro system. Zhao et al. introduced a tumor antigen-specific TCR into human umbilical cord blood-derived hematopoietic cells and generated T cells by coculture with OP9-Dll1 (50). They showed that those T cells could recognize and kill antigen-pulsed antigen-presenting cells. Vizcardo et al. generated induced pluripotent stem cells (iPSCs) from melanoma antigen-specific human cytotoxic T cells and cultured them on OP9-Dll1 cells. They subsequently stimulated the differentiated CD4+CD8+ T cells with anti-CD3 antibody to create CD8+ single positive T cells (51). They found that those CD8+ T cells could respond to the specific melanoma antigen, and had antitumor ability. Themeli et al. introduced a chimeric antigen receptor into iPSCs and generated human T cells targeted against CD19 by using OP9-Dll1 (52). Although the generated T cells showed an innate T cell-like phenotype, those cells had potent antitumor capability specific for CD19-expressing lymphoma cells.
Conclusion and Future Directions
Emerging evidence indicates that the Notch pathway has important physiological roles in CD8+ T cell functions, especially in the production of effector molecules. In addition, recent research points out that the Notch pathway probably works in resting T cells to promote homeostasis. On the other hand, the presence of apparently conflicting data suggests that the roles of the Notch pathway might be highly stage and context dependent. Therefore, it is critical to clarify what determines the functions of the Notch pathway under each condition. Comprehensive analyses of Notch signaling by transcriptomic, proteomic, and ChIP-seq analyses would be helpful to elucidate the differences under each condition.
Given the physiological importance of the Notch pathway, it could prove useful in the optimization of antitumor immunotherapy. However, the manipulation of the pathway should be carefully examined because the roles of the pathway could be context-dependent even in peripheral T cells. Furthermore, Notch receptors and ligands are broadly expressed in many tissues, and the manipulation of the pathway could cause unpredicted outcomes.
As well as the administration of cytokines, TLR agonists and immune checkpoint inhibitors, the activation of the Notch pathway induces non-specific activation of immune system, which could lead to autoimmunity or unwanted inflammation. Tumor-specific activation of immune response has been tried by using vaccination against tumor antigens or adoptive transfer of tumor-specific T cells generated or expanded in vitro. As described in this minireview, the Notch pathway is an excellent tool to create large amount of CD8+ T cells from iPSCs derived from tumor-specific T cells in vitro. In addition, the Notch pathway also can induce memory stem cell-like cells from peripheral T cells. Tuning the culture conditions as well as genetic modification of the cells could be used to create various types of CD8+ T cells for cancer immunotherapy. The best combination of non-specific and specific activation of immune responses should be carefully investigated to fight against tumors in various conditions.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
1. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol (2011) 29:235–71. doi:10.1146/annurev-immunol-031210-101324
2. Tran E, Robbins PF, Rosenberg SA. ‘Final common pathway’ of human cancer immunotherapy: targeting random somatic mutations. Nat Immunol (2017) 18(3):255–62. doi:10.1038/ni.3682
3. Liu R, Luo F, Liu X, Wang L, Yang J, Deng Y, et al. Biological response modifier in cancer immunotherapy. Adv Exp Med Biol (2016) 909:69–138. doi:10.1007/978-94-017-7555-7_2
4. Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol (2017). doi:10.1038/nri.2017.131
5. Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol (2012) 12(4):269–81. doi:10.1038/nri3191
6. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol (2017) 14(8):463–82. doi:10.1038/nrclinonc.2017.43
7. Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol (2017) 47(5):765–79. doi:10.1002/eji.201646875
8. Amsen D, Helbig C, Backer RA. Notch in T cell differentiation: all things considered. Trends Immunol (2015) 36(12):802–14. doi:10.1016/j.it.2015.10.007
9. Tindemans I, Peeters MJW, Hendriks RW. Notch signaling in T helper cell subsets: instructor or unbiased amplifier? Front Immunol (2017) 8:419. doi:10.3389/fimmu.2017.00419
10. Kovall RA, Gebelein B, Sprinzak D, Kopan R. The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell (2017) 41(3):228–41. doi:10.1016/j.devcel.2017.04.001
11. Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity (2004) 20(5):611–22. doi:10.1016/S1074-7613(04)00109-8
12. Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity (2007) 27(1):89–99. doi:10.1016/j.immuni.2007.05.021
13. Auderset F, Schuster S, Coutaz M, Koch U, Desgranges F, Merck E, et al. Redundant Notch1 and Notch2 signaling is necessary for IFNgamma secretion by T helper 1 cells during infection with Leishmania major. PLoS Pathog (2012) 8(3):e1002560. doi:10.1371/journal.ppat.1002560
14. Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol (2008) 9(10):1140–7. doi:10.1038/ni.1649
15. Backer RA, Helbig C, Gentek R, Kent A, Laidlaw BJ, Dominguez CX, et al. A central role for Notch in effector CD8(+) T cell differentiation. Nat Immunol (2014) 15(12):1143–51. doi:10.1038/ni.3027
16. Mathieu M, Duval F, Daudelin JF, Labrecque N. The Notch signaling pathway controls short-lived effector CD8+ T cell differentiation but is dispensable for memory generation. J Immunol (2015) 194(12):5654–62. doi:10.4049/jimmunol.1402837
17. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol (2012) 12(11):749–61. doi:10.1038/nri3307
18. Sandy AR, Chung J, Toubai T, Shan GT, Tran IT, Friedman A, et al. T cell-specific Notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol (2013) 190(11):5818–28. doi:10.4049/jimmunol.1203452
19. Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood (2011) 117(1):299–308. doi:10.1182/blood-2010-03-271940
20. Cho OH, Shin HM, Miele L, Golde TE, Fauq A, Minter LM, et al. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol (2009) 182(6):3380–9. doi:10.4049/jimmunol.0802598
21. Ito T, Allen RM, Carson WF, Schaller M, Cavassani KA, Hogaboam CM, et al. The critical role of Notch ligand Delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog (2011) 7(11):e1002341. doi:10.1371/journal.ppat.1002341
22. Kuijk LM, Verstege MI, Rekers NV, Bruijns SC, Hooijberg E, Roep BO, et al. Notch controls generation and function of human effector CD8+ T cells. Blood (2013) 121(14):2638–46. doi:10.1182/blood-2012-07-442962
23. Mathieu M, Cotta-Grand N, Daudelin JF, Thebault P, Labrecque N. Notch signaling regulates PD-1 expression during CD8(+) T-cell activation. Immunol Cell Biol (2013) 91(1):82–8. doi:10.1038/icb.2012.53
24. Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-production in peripheral T cells. J Immunol (2003) 171(6):3019–24. doi:10.4049/jimmunol.171.6.3019
25. Sauma D, Ramirez A, Alvarez K, Rosemblatt M, Bono MR. Notch signalling regulates cytokine production by CD8+ and CD4+ T cells. Scand J Immunol (2012) 75(4):389–400. doi:10.1111/j.1365-3083.2012.02673.x
26. Wong KK, Carpenter MJ, Young LL, Walker SJ, McKenzie G, Rust AJ, et al. Notch ligation by Delta1 inhibits peripheral immune responses to transplantation antigens by a CD8+ cell-dependent mechanism. J Clin Invest (2003) 112(11):1741–50. doi:10.1172/JCI18020
27. Kijima M, Iwata A, Maekawa Y, Uehara H, Izumi K, Kitamura A, et al. Jagged1 suppresses collagen-induced arthritis by indirectly providing a negative signal in CD8+ T cells. J Immunol (2009) 182(6):3566–72. doi:10.4049/jimmunol.0803765
28. Okamoto M, Takeda K, Joetham A, Ohnishi H, Matsuda H, Swasey CH, et al. Essential role of Notch signaling in effector memory CD8+ T cell-mediated airway hyperresponsiveness and inflammation. J Exp Med (2008) 205(5):1087–97. doi:10.1084/jem.20072200
29. Coutaz M, Hurrell BP, Auderset F, Wang H, Siegert S, Eberl G, et al. Notch regulates Th17 differentiation and controls trafficking of IL-17 and metabolic regulators within Th17 cells in a context-dependent manner. Sci Rep (2016) 6:39117. doi:10.1038/srep39117
30. Fu T, Zhang P, Feng L, Ji G, Wang XH, Zheng MH, et al. Accelerated acute allograft rejection accompanied by enhanced T-cell proliferation and attenuated Treg function in RBP-J deficient mice. Mol Immunol (2011) 48(5):751–9. doi:10.1016/j.molimm.2010.11.016
31. Tindemans I, Lukkes M, de Bruijn MJW, Li BWS, van Nimwegen M, Amsen D, et al. Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable. J Allergy Clin Immunol (2017) 140(4):1079–89. doi:10.1016/j.jaci.2016.11.046
32. Bhuyan ZA, Asanoma M, Iwata A, Ishifune C, Maekawa Y, Shimada M, et al. Abrogation of Rbpj attenuates experimental autoimmune uveoretinitis by inhibiting IL-22-producing CD4+ T cells. PLoS One (2014) 9(2):e89266. doi:10.1371/journal.pone.0089266
33. Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity (2012) 36(4):623–34. doi:10.1016/j.immuni.2012.01.020
34. Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med (2015) 21(1):55–61. doi:10.1038/nm.3758
35. Hombrink P, Helbig C, Backer RA, Piet B, Oja AE, Stark R, et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol (2016) 17(12):1467–78. doi:10.1038/ni.3589
36. Sugimoto K, Maekawa Y, Kitamura A, Nishida J, Koyanagi A, Yagita H, et al. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol (2010) 184(9):4673–8. doi:10.4049/jimmunol.0903661
37. Zhao E, Maj T, Kryczek I, Li W, Wu K, Zhao L, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol (2016) 17(1):95–103. doi:10.1038/ni.3313
38. Dai K, Huang L, Huang YB, Chen ZB, Yang LH, Jiang YA. 1810011o10 Rik inhibits the antitumor effect of intratumoral CD8+ T cells through suppression of Notch2 pathway in a murine hepatocellular carcinoma model. Front Immunol (2017) 8:320. doi:10.3389/fimmu.2017.00320
39. Sierra RA, Thevenot P, Raber PL, Cui Y, Parsons C, Ochoa AC, et al. Rescue of Notch-1 signaling in antigen-specific CD8+ T cells overcomes tumor-induced T-cell suppression and enhances immunotherapy in cancer. Cancer Immunol Res (2014) 2(8):800–11. doi:10.1158/2326-6066.CIR-14-0021
40. Thounaojam MC, Dudimah DF, Pellom ST Jr, Uzhachenko RV, Carbone DP, Dikov MM, et al. Bortezomib enhances expression of effector molecules in anti-tumor CD8+ T lymphocytes by promoting Notch-nuclear factor-kappaB crosstalk. Oncotarget (2015) 6(32):32439–55. doi:10.18632/oncotarget.5857
41. Biktasova AK, Dudimah DF, Uzhachenko RV, Park K, Akhter A, Arasada RR, et al. Multivalent forms of the Notch ligand DLL-1 enhance antitumor T-cell immunity in lung cancer and improve efficacy of EGFR-targeted therapy. Cancer Res (2015) 75(22):4728–41. doi:10.1158/0008-5472.CAN-14-1154
42. Brzozowa-Zasada M, Piecuch A, Michalski M, Segiet O, Kurek J, Harabin-Slowinska M, et al. Notch and its oncogenic activity in human malignancies. Eur Surg (2017) 49(5):199–209. doi:10.1007/s10353-017-0491-z
43. Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol (2016) 34:539–73. doi:10.1146/annurev-immunol-032414-112049
44. Yu W, Wang Y, Guo P. Notch signaling pathway dampens tumor-infiltrating CD8(+) T cells activity in patients with colorectal carcinoma. Biomed Pharmacother (2017) 97:535–42. doi:10.1016/j.biopha.2017.10.143
45. Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med (2009) 15(7):808–13. doi:10.1038/nm.1982
46. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med (2011) 17(10):1290–7. doi:10.1038/nm.2446
47. Flynn JK, Gorry PR. Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies. Clin Trans Immunol (2014) 3(7):e20. doi:10.1038/cti.2014.16
48. Kondo T, Morita R, Okuzono Y, Nakatsukasa H, Sekiya T, Chikuma S, et al. Notch-mediated conversion of activated T cells into stem cell memory-like T cells for adoptive immunotherapy. Nat Commun (2017) 8:15338. doi:10.1038/ncomms15338
49. de Pooter RF, Zuniga-Pflucker JC. Generation of immunocompetent T cells from embryonic stem cells. Methods Mol Biol (2007) 380:73–81. doi:10.1007/978-1-59745-395-0_5
50. Zhao Y, Parkhurst MR, Zheng Z, Cohen CJ, Riley JP, Gattinoni L, et al. Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling. Cancer Res (2007) 67(6):2425–9. doi:10.1158/0008-5472.CAN-06-3977
51. Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell (2013) 12(1):31–6. doi:10.1016/j.stem.2012.12.006
Keywords: Notch, T cells differentiation, tumor immunity, CD8+ T cells, granzyme B
Citation: Tsukumo SI and Yasutomo K (2018) Regulation of CD8+ T Cells and Antitumor Immunity by Notch Signaling. Front. Immunol. 9:101. doi: 10.3389/fimmu.2018.00101
Received: 01 December 2017; Accepted: 12 January 2018;
Published: 30 January 2018
Edited by:
Barbara A. Osborne, University of Massachusetts Amherst, United StatesReviewed by:
Haidong Dong, Mayo Clinic Minnesota, United StatesAmorette Barber, Longwood University, United States
Copyright: © 2018 Tsukumo and Yasutomo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Koji Yasutomo, eWFzdXRvbW9AdG9rdXNoaW1hLXUuYWMuanA=
 Shin-ichi Tsukumo
Shin-ichi Tsukumo Koji Yasutomo
Koji Yasutomo