- 1Center for Infectious Diseases, Beijing You’an Hospital, Capital Medical University, Beijing, China
- 2Department of Infectious Diseases, Peking Union Medical College Hospital, Beijing, China
- 3Department of Information Technology, Peking Union Medical College Hospital, Beijing, China
- 4AIDS Healthcare Foundation, Beijing, China
- 5School of Biomedical Engineering, Capital Medical University, Beijing, China
- 6Department of Infectious Diseases, Chongqing Public Health Medical Center, Chongqing, China
- 7Zunyi Medical University, Zunyi, China
Background: Western blot (WB) assay is considered the gold standard test for HIV infection confirmation. However, it requires technical expertise and is quite time-consuming. WHO recommends blood-based rapid diagnosis to achieve same-day test and treatment. However, this rapid testing strategy has not been promoted worldwide due to inadequate research evaluating the effectiveness of rapid tests (RTs) as an alternative confirmatory HIV test for WB. This study aims to compare the diagnostic performance of rapid HIV tests compared with WB.
Methods: PubMed and Web of Science were searched for publications on rapid HIV tests using blood specimen. A meta-analysis was performed to quantitatively evaluate the diagnostic performance of rapid HIV tests compared with the WB assay in terms of pooled sensitivity, specificity, area under summary receiver operating characteristic (SROC) curve, and diagnostic odds ratio (DOR).
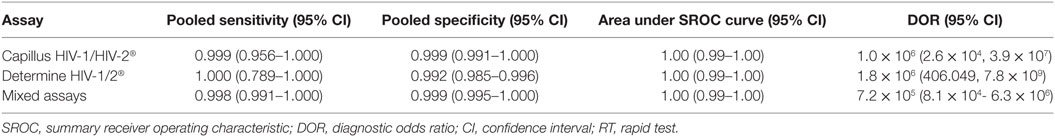
Results: Twenty articles involving 27,343 fresh specimens for rapid HIV tests were included in the meta-analysis. Regarding Capillus HIV-1/HIV-2, the pooled sensitivity, specificity, area under SROC curve, and DOR derived from six studies were 0.999 (95% CI, 0.956–1.000), 0.999 (95% CI, 0.991–1.00), 1.00 (95% CI, 0.99–1.00), and 1.0 × 106 (95% CI, 2.6 × 104–3.9 × 107) compared with the WB assay, respectively. With respect to Determine HIV-1/2, the pooled sensitivity, specificity area under SROC, and DOR derived from eight studies were 1.00 (95% CI, 0.789–1.000), 0.992 (95% CI, 0.985–0.996), 1.00 (95% CI, 0.99–1.00), and 1.8 × 106 (95% CI 406.049–7.8 × 109) compared with the WB assay, respectively. Regarding two-step serial RTs, the pooled sensitivity, specificity area under SROC, and DOR derived from eight studies were 0.998 (95% CI, 0.991–1.000), 0.998 (95% CI, 0.994–0.999), and 1.00 (95% CI 0.99–1.00) compared with the WB assay, respectively.
Conclusion: Our meta-analysis results may provide evidenced-based support for substituting RT for WB. Blood-based rapid HIV tests have comparable sensitivity and specificity to WB for HIV early therapy.
Introduction
Western blot (WB) is regarded as the gold standard for a definitive confirmatory test for HIV infection. It usually takes up to 7–14 days to get the result and costs a lot (1, 2). Moreover, WB tests can only be performed by lab-professionals, which greatly restrict the accessibility of HIV testing. Surveys indicate that roughly 40–50% of HIV cases progressed to AIDS within the first year of diagnosis largely due to late diagnosis (3, 4). According to the Chinese integrated HIV/AIDS database, about 25% of newly diagnosed HIV/AIDS cases reported in China were already AIDS patients when they were first identified (5). Such late diagnoses are mainly caused by lack of access to fast and accurate HIV testing, particularly in settings that continue to rely on traditional WB confirmatory testing (6, 7). However, simple and rapid blood-based HIV tests other than ELISA that target HIV antibodies have become available, enabling testing in outreach settings outside the laboratory at the point of client contact (8). Compared with WB assay, a rapid and accurate diagnosis in 15–30 min strategy is required. Blood-based rapid tests (RTs) targeting HIV antibody are convenient tests that require minimal laboratory infrastructure and expertise training compared with WB assay. Test results can be available in less than 30 min, so WHO recommends its serial use in different clinical settings to achieve same-day test and treatment (9, 10). These tests can offer prompt test results and receipt of a “preliminary positive” test result, and therefore have greatly enhanced both the awareness rate and result notification rate of the infected and the coverage of antiretroviral therapy (ART) (11). The FDA recommends that the sensitivity and specificity of RTs should exceed 98% (12). Health authorities have recommended diagnosis strategies to replace traditional WB confirmatory test (13–15), using either combinations of HIV antibody RT reagents or combinations of RT reagents and ELISA reagents.
China relies on a traditional HIV testing algorithm that requires WB confirmation to date. It is of great need to explore a fast and accurate strategy of HIV diagnosis by using RT centered testing. However, the effectiveness of HIV diagnosis using RTs has not been adequately explored. Therefore, the purpose of this paper is to evaluate the diagnostic performance of blood-based rapid HIV tests compared with the WB assay in terms of pooled sensitivity and specificity by meta-analysis, aiming to summarize the overall diagnostic accuracy of blood-based RTs as alternative algorithms for HIV early therapy.
Methods
This study aims to compare the diagnostic performance of rapid HIV tests compared with WB assay in terms of pooled sensitivity, specificity, area under summary receiver operating characteristic (SROC) curve, and diagnostic odds ratio (DOR) among different populations. The work was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis PRISMA guidelines [see RPISMA checklist in Supplementary Material (16)].
Search Strategy
PubMed and Web of Science were searched using combinations of the following search keywords: [(HIV or AIDS) and (“rapid test” or “fast test”)]. Reference lists of selected articles and related review articles were further screened. Additional searches in Google Scholar and manual searches of related journals (e.g., AIDS; Journal of Clinical Virology and Lancet Infectious Diseases) were also performed. Articles were limited to those published from January 2000 to May 2016. The search was limited to English language publications.
Study Selection
Studies were identified as eligible if RTs were conducted on whole blood sample.
Studies were excluded if (i) sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were not reported or could not be calculated from the information provided; (ii) RTs were conducted on saliva; (iii) RT results were not confirmed by WB; or (iv) they were the reviews of rapid HIV antibody tests.
Two independent reviewers (Jieqing Chen and Xiaojie Huang) without prior consideration of the results initially selected search results based on titles and abstracts. The remaining articles were further selected by full-text assessment. Disagreements between reviewers about eligibility were resolved by discussion.
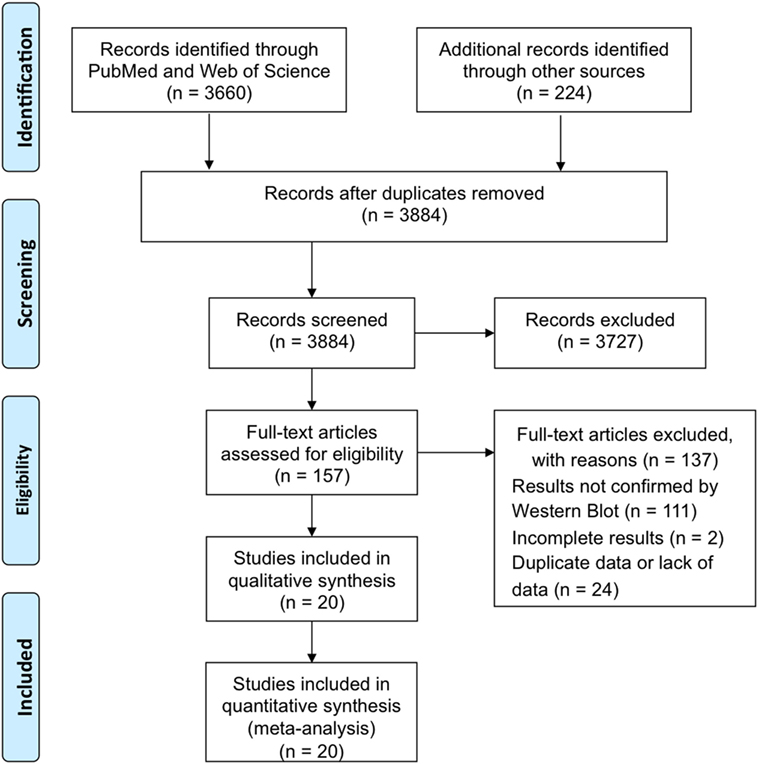
The procedure of study selection and numbers of included and excluded studies is shown in Figure 1.
Data Extraction
Two independent researchers (Bin Su and Zhiying Liu) extract data of each study into a spreadsheet. The following information was extracted from each selected study: information about the article (name of the first author, journal title, and the publication year), information about the RT (assay type, specimen type, and number of the test samples), information about the HIV-infected patients, and the performance outcomes, such as sensitivity, specificity, PPV, and NPV or other relevant data, to construct 2 × 2 tables of test results.
If there were several RTs performed on the blood samples from the same subjective populations in a study, just one RT could be included in a certain meta-analysis to ensure the independence of the included studies in one meta-analysis.
The quality of individual studies was rated by two independent reviewers (Xinchao Liu and Yaokai Chen) using a Quality Assessment of Diagnostic Accuracy Studies (QUADAS) scale (17).
Statistical Analysis
Diagnostic meta-analysis was conducted by using command “midas” in StataSE 12.0, where the command required the number of observations of greater than or equal to 4. The presence of between-study heterogeneity when combining the study results was assessed using the chi-square-based Q-test of heterogeneity and the I2 statistic (18). Heterogeneity was considered as moderate to large when P < 0.1 for Q-test or I2 > 50%. Due to the paucity of trials in each comparison, we relied primarily on the I2 statistic. Fixed-effect models were adopted to combine the performance measures derived from studies not presenting moderate to large heterogeneity, assuming that all differences in observed effects were due to sampling error, and that there was one true effect size (i.e., the fixed effect) which underlined all the studies in the analysis. Otherwise, meta-analyses were conducted by random-effects models, because the true effect could vary from study to study and the true effect sizes were assumed to represent a random sample (i.e., the random effects) of effect sizes of individual studies.
The combined performance indexes included the pooled sensitivity, specificity, summary receiver operating characteristic (SROC) curve, and diagnostic odds ratio (DOR) (19). In a single diagnostic test, sensitivity and specificity are defined as TP/(TP + FN) and TN/(TN + FP), respectively, where TP, FP, FN, and TN represent the number of true positives, false positives, false negatives, and true negatives, respectively. Then, DOR is defined as:
DOR is more suitable to compare diagnostic performance for two or more diagnostic tests. A DOR value ranges from 0 to infinity, with a higher value indicating a better discriminatory performance. DOR value of 1 means that a test does not discriminate between patients with and without the disorder. DOR also equals to the ratio of positive likelihood (LR+) and negative likelihood ratio (LR−), where a diagnostic test with LR+ >10 and LR− <0.1 (i.e. DOR > 100) is considered to be excellent discriminatory performance.
For those more used RT assays, one meta-analysis would be conducted on each of them to evaluate their performances, while one meta-analysis would be conducted on a mix of different assays that were just used in one or two RTs.
Results
General Study Information
The search strategy identified 3,884 papers from electronic databases and other sources in total. By reviewing the titles and abstracts of these papers, 3,727 irrelevant papers were ruled out. Of the remaining 157 studies that assessed rapid HIV testing, 111 articles without final retest results confirmed by WB were excluded after reviewing abstracts; 2 studies were excluded after detailed reviewing of the full text, because they did not provide sufficient information to construct the diagnostic 2 × 2 tables. Another 24 articles were excluded due to duplicate data or lack of data. Therefore, 20 papers involving 27,343 fresh specimens for RTs were included in the meta-analyses (Figure 1).
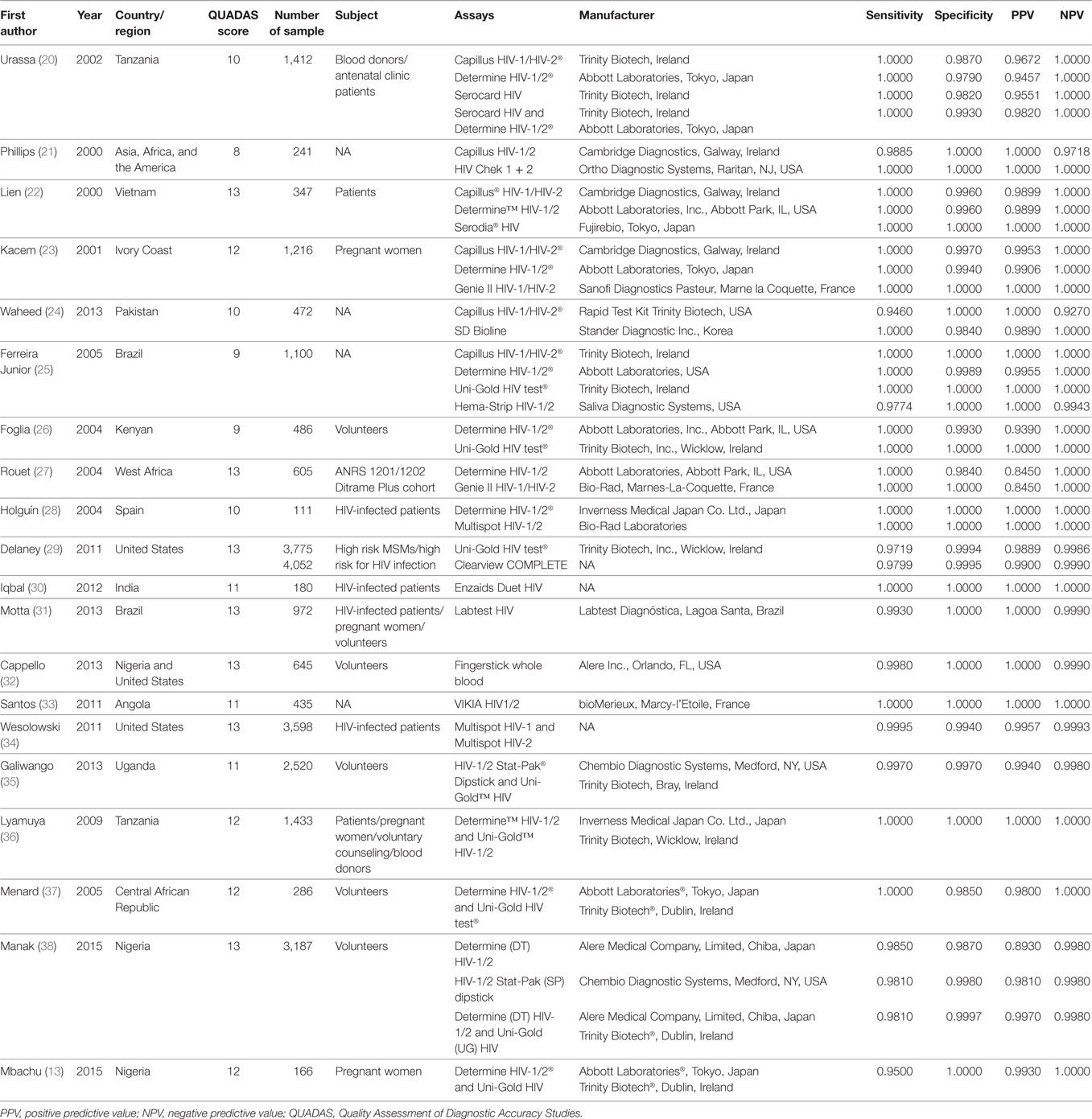
Among the 27,343 specimens from 20 studies, 6,753 specimens were HIV-positive and 20,590 were HIV-negative by WB. The rapid HIV antibody detection assays used in these studies included Capillus HIV-1/HIV-2® (Trinity Biotech, Ireland or Cambridge Diagnostics, Galway, Ireland), Determine HIV-1/2® (Abbott Laboratories, Tokyo, Japan or Inverness Medical Japan Co. Ltd., Japan), Uni-Gold HIV test® (Trinity Biotech, Inc., Wicklow, Ireland), and other independent assays. Combinations of two independent assays were also used in six studies. A summary of the studies and their key features is listed in Table 1.
Diagnostic Performance for a Single RT Assay
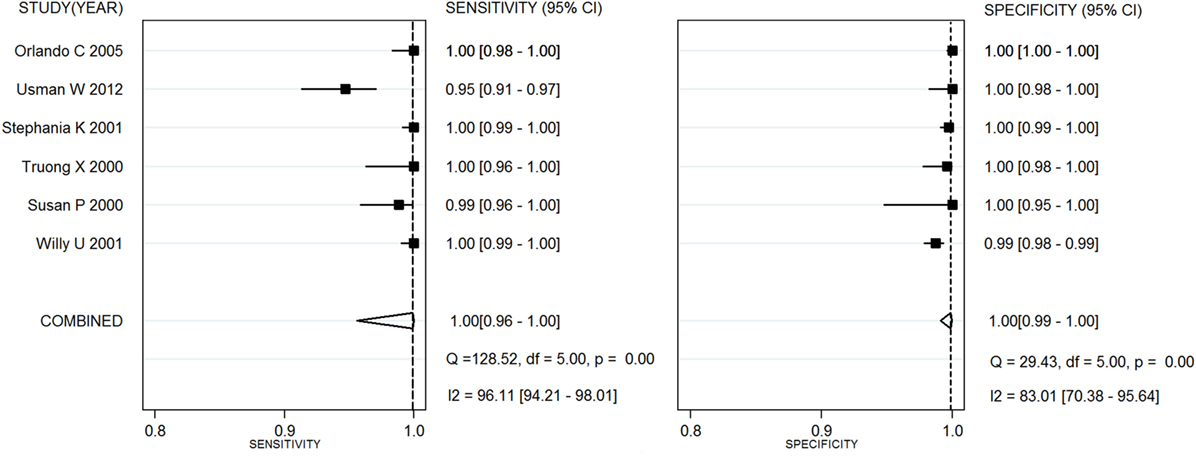
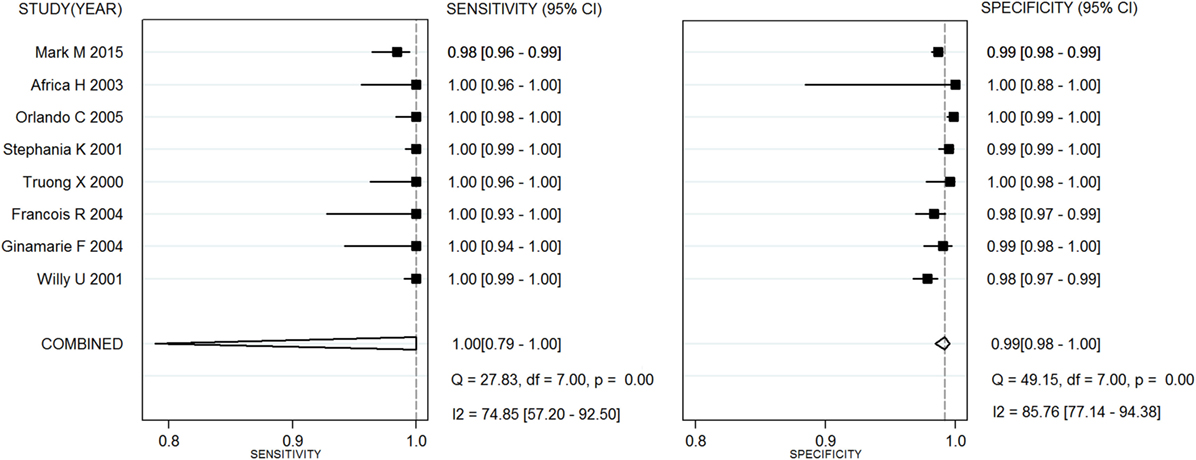
Capillus HIV-1/HIV-2®, Determine HIV-1/2®, and Uni-Gold HIV test® were used in six studies (20–25), eight studies (26–28), and three studies (25, 26, 29), respectively. Two meta-analyses were conducted on RTs with Capillus HIV-1/HIV-2® and Determine HIV-1/2®. The statistic I2s in the heterogeneity tests were 96% for the pooled sensitivity and 83% for the pooled specificity, 75% and 86% among the included studies with regard to Capillus HIV-1/HIV-2® and Determine HIV-1/2®, indicating moderate to large heterogeneity among the included studies. Therefore, random-effects models were chosen for the meta-analyses. In addition, 14 other RT assays, such as Serocard HIV, HIV Chek 1 + 2, Serodia HIV, and Genie II HIV-1/HIV-2, were used in 14 independent studies (20–25). These RTs with mixed assays were included into one single meta-analysis. Performance measures for evaluating these assays are listed in Table 2; Figures 2 and 3 depict their forest plots.
Diagnostic Performance for RT Assay Combinations
There were seven kinds of RT assay serial combinations (34–37), involving Determine HIV-1/2®, Uni-Gold HIV test®, HIV-1/2 Stat-Pak® Dipstick, Multispot HIV-1 and HIV-2, and Serocard HIV. The commonly used serial combinations are Determine HIV-1/2 and Uni-Gold HIV. The pooled sensitivity, specificity, and area under the SROC curve were 0.998 (95% CI, 0.991–1.000), 0.998 (95% CI, 0.994–0.999), and 1.00 (95% CI 0.99–1.00), respectively. DOR was 2.7 × 105 with 95% CI of 9.0 × 104–8.1 × 105.
Discussion
The prevention and control of HIV/AIDS as a chronic infectious disease should focus on timely detection and treatment. To further increase the coverage and availability of testing, traditional testing methods will not suffice (11, 39). To achieve widespread testing coverage and rapid result acquisition, RT is a promising diagnostic approach that should be promoted. Though in many studies specificity and sensitivity were reportedly high, single studies were unable to give sufficient statistical power (20, 37). While global guidance from WHO promotes a RT strategy, it has not been successfully implemented globally. One hypothesized reason for the lag in implementation is the inadequate research evaluating the effectiveness of RTs as an alternative to WB as a confirmatory HIV test. Moreover, it is difficult to validate trustworthy testing algorithms using different RTs with varying sensitivity and specificity. Currently, RT reagents can be used for blood (including whole blood, serum, or plasma) and saliva. Several surveys conclude that RT reagents have lower specificity and higher false positive rate in saliva than in blood (40). Therefore, we conducted a meta-analysis to evaluate the specificity and sensitivity of blood-based RT compared with the WB assay in terms of pooled sensitivity, specificity by meta-analysis in a large number of samples. All the studies with appropriate reference (confirmed with WB) reported excellent estimates of sensitivity and specificity. We evaluated the performance of these RT strategies in the context of multiple diagnostic algorithms. Our meta-analysis evaluated the effectiveness of using different FDA-approved RTs on diagnostic accuracy. Areas under the SROC curves for the two most popular rapid assays (Capillus HIV-1/HIV-2 and Determine HIV-1/2) were all above 0.99 with sensitivities above 99.9%. In addition to the most popular RTs, 15 other kits have been evaluated in 15 studies (see mixed assays in Table 2). The pooled sensitivity, specificity, and area under the SROC curve of these assays were 0.998, 0.999, and 1.00, respectively, which demonstrates blood-based rapid HIV test has comparable accuracy to WB for HIV early therapy.
HIV screening via successive or simultaneous RT reagents has been widely adopted in Africa (41). Two successive RT reagents have lower costs than simultaneous RT reagents and are widely used for HIV screening (24). A study in Tanzania indicates that a good pair in combination is Korean SD and US Abbott Determine (20). SD can be used for screening and Determine can be used to recheck positive results. Both the sensitivity and specificity of this combination can reach up to 100% (23). Our meta-analysis also studied serial testing strategies (the second test is done only if the first test is positive). Overall, the pooled sensitivity and specificity were 0.998 and 0.998, respectively. Therefore, a serial two-step testing strategy has comparable accuracy to single test strategies.
The FDA regulation for manufacturers seeking licensure of tests recommends that the lower bound of the one-sided 95% confidence interval for sensitivity and specificity exceed 98% (12). Our review suggests that blood-based RT have high diagnostic accuracy, with comparable estimates when using a two-step or single testing strategy. It leads to early diagnosis and treatment of HIV and better clinical results. These data have the potential to change recommendations on voluntary counseling and testing from using originally ELISA based testing to RTs, and furthermore to replace the confirmatory WB test for HIV early therapy at the same day of detection. Particularly in countries and regions with high HIV/AIDS prevalence, timely actions should be taken to develop the relevant policies, technical protocols, and quality assurance systems to ensure the widespread implementation of RT.
Although the sensitivity and specificity of RT reagents both exceed 99.5%, they could be compromised due to unstandardized operations in non-laboratory settings (42, 43). The sensitivity of RT can be reduced in the absence of quality assurance and evaluation system (21). Unstandardized operations may lead RT false negative rate of up to 5.4% (29). RT test inevitably faces other challenges, such as inability of rechecking the same sample, and relatively low sensitivity for early HIV infection (42).
Our meta-analysis has several strengths. Algorithms either using serial RT testing strategies or single FDA-approved RT have been proved with satisfactory results. In addition, there has been an expansion in suitable specimen types (finger stick whole blood). We performed a comprehensive search of sources to identify studies that adopt different kinds of RTs. Several meta-analyses addressing the efficacy of so-called “rapid HIV testing” have been published recently, some of which focused on the fourth-generation ELISA test (Ag/Ab combination) that takes several hours to get testing result instead of “real” rapid HIV tests (43–45). And small sample sized meta-analysis has showed that rapid HIV voluntary counseling and testing improves the receipt rate of HIV test results among clients who seek HIV counseling and testing (45). Therefore, our current meta-analysis contributed uniquely to the field with greater sample size and trustworthy results. In some countries and regions, traditional tests still prevail, particularly in China. Thus, it is feasible to have RT performed by trained non-medical professionals outside laboratories, which can promote HIV testing services among high risk groups such as MSM population more easily and greatly enhance both the awareness rate and result notification rate of the infected and the coverage of ART.
There also have been several limitations. For example, statistical comparison between subgroups (i.e., different populations) was not possible due to lack of data. Only English language studies were included in this meta-analysis, which may lead to a potential reporting bias. Different areas can determine the combinations based on the performance of reagents, costs, HIV prevalence, and risk behaviors of populations. Especially in low epidemic areas, indicating the need for counselors and clients to understand the limitations of RT positive results and the necessity to receive confirmatory tests. WB confirmatory test can be applied mainly for confirmation of indeterminate result.
Overall, our study indicated that RT would function as well as the WB and RT can and should be accessible and extensively used for HIV early therapy at the same day of detection if it is carried out by standardized management protocols. Quality assurance and quality control of RTs are extremely important and non-trivial in a devolved environment where such practices are not engrained in the culture, thus it is of great importance to acquire the knowledge and skills required for conducting quality control at a RT site. The time is ripe for RTs to play a more central role in increasing timely identification of HIV infections.
Conclusion
Our meta-analysis results may provide evidenced-based support for substituting rapid test for WB. Blood-based rapid HIV tests have comparable sensitivity and specificity to WB for HIV early therapy.
Author Contributions
Study conception and design: HW, HC, and XH. Acquisition of data: XL, JC, BS, ZL, XH, and YC. Preparation of figures: HC, ZL, BS, XL, and WX. Preparation of tables: HC, HX, and AS. Writing and revision of the manuscript: XL, JC, YC, and XH. All the authors have given approval to the final version of the manuscript.
Conflict of Interest Statement
The authors have declared that no competing interests exist and no manufacturers have funded this study.
Funding
This work was supported by the Chinese Government 13th Five-Year Plan (2017ZX10201101), Major Project of Beijing Municipal Science and Technology Committee (D161100000416003, D171100000517003), the National Natural Science Foundation of China (No. 81701984), the NSFC-NIH Biomedical collaborative research program (81761128001), The Capital Health Research and Development of Special Fund (2016-1-2182), Beijing Key Laboratory (No. BZ0089), and Projects of medical scientific research of Health and Family Planning Commission of Chongqing (2016HBRC008).
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01458/full#supplementary-material.
References
1. Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect (2003) 79:442–7. doi:10.1136/sti.79.6.442
2. Centers for Disease, and Prevention. HIV and AIDS – United States, 1981-2000. MMWR Morb Mortal Wkly Rep (2001) 50:430–4.
3. Centers for Disease Control and Prevention (CDC). Late versus early testing of HIV – 16 Sites, United States, 2000-2003. MMWR Morb Mortal Wkly Rep (2003) 52:581–6.
4. Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS (2001) 15:77–85. doi:10.1097/00002030-200101050-00012
5. Qiu J. China. Stigma of HIV imperils hard-won strides in saving lives. Science (2011) 332:1253–4. doi:10.1126/science.332.6035.1253
6. Wu Z, Zhao Y, Ge X, Mao Y, Tang Z, Shi CX, et al. Simplified HIV testing and treatment in China: analysis of mortality rates before and after a structural intervention. PLoS Med (2015) 12:e1001874. doi:10.1371/journal.pmed.1001874
7. Harper KN. Simplified HIV testing and treatment reduces mortality rate by over 60% in a trial in China. AIDS (2016) 30:N11. doi:10.1097/QAD.0000000000001057
8. Angotti N, Bula A, Gaydosh L, Kimchi EZ, Thornton RL, Yeatman SE. Increasing the acceptability of HIV counseling and testing with three C’s: convenience, confidentiality and credibility. Soc Sci Med (1982) 68(2009):2263–70. doi:10.1016/j.socscimed.2009.02.041
9. Shimelis T, Tadesse E. The diagnostic performance evaluation of the SD BIOLINE HIV/syphilis Duo rapid test in southern Ethiopia: a cross-sectional study. BMJ Open (2015) 5:e007371. doi:10.1136/bmjopen-2014-007371
10. World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee, Guidelines for Using HIV Testing Technologies in Surveillance: Selection, Evaluation and Implementation: 2009 Update. Geneva: World Health Organization (2009).
11. Ma Q, Xia S, Pan X, Cai G, Zhou X, Wang H, et al. Rapid HIV antibody testing among men who have sex with men who visited a gay bathhouse in Hangzhou, China: a cross-sectional study. BMJ Open (2015) 5:e008661. doi:10.1136/bmjopen-2015-008661
12. Shaw SY, Ireland L, Mcclarty LM, Loeppky C, Yu N, Wylie JL, et al. Prior history of testing for syphilis, hepatitis B and hepatitis C among a population-based cohort of HIV-positive individuals and their HIV-negative controls. AIDS Care (2016) 29:67–72. doi:10.1080/09540121.2016.1200715
13. Mbachu II, Udigwe G, Joseph I, John O, Samuel UO, Joseph U, et al. The evaluation of accuracy of serial rapid HIV test algorithm in the diagnosis of HIV antibodies among pregnant women in south east Nigeria. BMC Res Notes (2015) 8:557. doi:10.1186/s13104-015-1454-8
14. Mungrue K, Sahadool S, Evans R, Boochay S, Ragoobar F, Maharaj K, et al. Assessing the HIV rapid test in the fight against the HIV/AIDS epidemic in Trinidad. HIV AIDS (Auckl) (2013) 5:191–8. doi:10.2147/HIV.S30432
15. Cardenas AM, Baughan E, Hodinka RL. Evaluation of the Bio-Rad Multispot HIV-1/HIV-2 Rapid Test as an alternative to western blot for confirmation of HIV infection. J Clin Virol (2013) 58(Suppl 1):e97–e103. doi:10.1016/j.jcv.2013.08.021
16. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi:10.1371/journal.pmed.1000097
17. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol (2003) 3:25. doi:10.1186/1471-2288-3-25
18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J (2003) 327:557–60. doi:10.1136/bmj.327.7414.557
19. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol (2003) 56:1129–35. doi:10.1016/S0895-4356(03)00177-X
20. Urassa W, Nozohoor S, Jaffer S, Karama K, Mhalu F, Biberfeld G. Evaluation of an alternative confirmatory strategy for the diagnosis of HIV infection in Dar Es Salaam, Tanzania, based on simple rapid assays. J Virol Methods (2002) 100:115–20. doi:10.1016/S0166-0934(01)00408-6
21. Phillips S, Granade TC, Pau CP, Candal D, Hu DJ, Parekh BS. Diagnosis of human immunodeficiency virus type 1 infection with different subtypes using rapid tests. Clin Diagn Lab Immunol (2000) 7:698. doi:10.1128/CDLI.7.4.698-699.2000
22. Lien TX, Tien NT, Chanpong GF, Cuc CT, Yen VT, Soderquist R, et al. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg (2000) 62:301–9. doi:10.4269/ajtmh.2000.62.301
23. Kacem S, Ducasse O, Eichwald O, Yousfi M, Meziane M, Sarrette JP, et al. Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast. J Clin Microbiol (2001) 39:1808. doi:10.1128/JCM.39.5.1808-1812.2001
24. Waheed U, Hayat K, Ahmad B, Waheed Y, Zaheer HA. Evaluation of HIV/AIDS diagnostics kits and formulation of a testing strategy for Pakistan. J Clin Virol (2013) 56:367–9. doi:10.1016/j.jcv.2012.12.012
25. Ferreira Junior OC, Ferreira C, Riedel M, Widolin MR, Barbosajúnior A. Evaluation of rapid tests for anti-HIV detection in Brazil. AIDS (2005) 19(Suppl 4):S70. doi:10.1097/01.aids.0000191494.51489.75
26. Foglia G, Wasunna KM, Kibaya R, Malia JA, Calero EK, Sateren W, et al. Use of rapid and conventional testing technologies for human immunodeficiency virus type 1 serologic screening in a rural Kenyan reference laboratory. J Clin Microbiol (2004) 42:3850–2. doi:10.1128/JCM.42.8.3850-3852.2004
27. Rouet F, Ekouevi DK, Inwoley A, Chaix ML, Burgard M, Bequet L, et al. Field evaluation of a rapid Human Immunodeficiency Virus (HIV) serial serologic testing algorithm for diagnosis and differentiation of HIV type 1 (HIV-1), HIV-2, and dual HIV-1-HIV-2 infections in West African pregnant women. J Clin Microbiol (2004) 42:4147. doi:10.1128/JCM.42.9.4147-4153.2004
28. Holguín A. Evaluation of three rapid tests for detection of antibodies to HIV-1 non-B subtypes. J Virol Methods (2004) 115:105–7. doi:10.1016/j.jviromet.2003.09.028
29. Delaney KP, Branson BM, Uniyal A, Phillips S, Candal D, Owen SM, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis (2011) 52:257–63. doi:10.1093/cid/ciq068
30. Iqbal HS, Solomon S, Murugavel KG, Solomon SS, Balakrishnan P. Evaluation of two indigenous rapid and two ELISA assays for the diagnosis of HIV infection India. Indian J Med Microbiol (2012) 30:397–402. doi:10.4103/0255-0857.103758
31. Motta LRD, Vanni AC, Kato SK, Borges LGDA, Sperhacke RD, Ribeiro RMM, et al. Evaluation of five simple rapid HIV assays for potential use in the Brazilian national HIV testing algorithm. J Virol Methods (2013) 194:132–7. doi:10.1016/j.jviromet.2013.08.016
32. Cappello JM, Gunasekera A, Gunasekera D, Esfandiari J, Ippolito T. A multicenter performance evaluation of the DPP(®) HIV-1/2 assay for the detection of HIV antibodies in various HIV testing algorithms. J Clin Virol (2013) 58:e59–64. doi:10.1016/j.jcv.2013.08.032
33. Santos Â, Clemente S, Bártolo I, Palladino C, Cavaco-Silva P, Franco V, et al. Evaluation of the diagnostic performance of the rapid test VIKIA HIV1/2 in a highly complex HIV-1 epidemic. Diagn Microbiol Infect Dis (2011) 71:90–2. doi:10.1016/j.diagmicrobio.2011.06.002
34. Wesolowski LG, Delaney KP, Hart C, Dawson C, Owen SM, Candal D, et al. Performance of an alternative laboratory-based algorithm for diagnosis of HIV infection utilizing a third generation immunoassay, a rapid HIV-1/HIV-2 differentiation test and a DNA or RNA-based nucleic acid amplification test in persons with established HI. J Clin Virol (2011) 52:S45–9. doi:10.1016/j.jcv.2011.09.026
35. Galiwango RM, Musoke R, Lubyayi L, Ssekubugu R, Kalibbala S, Ssekweyama V, et al. Evaluation of current rapid HIV test algorithms in Rakai, Uganda. J Virol Methods (2013) 192:25–7. doi:10.1016/j.jviromet.2013.04.003
36. Lyamuya EF, Aboud S, Urassa WK, Sufi J, Mbwana J, Ndugulile F, et al. Evaluation of simple rapid HIV assays and development of national rapid HIV test algorithms in Dar es Salaam, Tanzania. BMC Infect Dis (2009) 9:19. doi:10.1186/1471-2334-9-19
37. Menard D, Mairo A, Mandeng MJ, Doyemet P, Koyazegbe T, Rochigneux C, et al. Evaluation of rapid HIV testing strategies in under equipped laboratories in the Central African Republic. J Virol Methods (2005) 126:75–80. doi:10.1016/j.jviromet.2005.01.023
38. Manak MM, Njoku OS, Ashley S, Jennifer M, Jagodzinski LL, Mark M, et al. Evaluation of performance of two rapid tests for detection of HIV-1 and -2 in high- and low-prevalence populations in Nigeria. J Clin Microbiol (2015) 53:3501–6. doi:10.1128/JCM.01432-15
39. Centers for Disease, and Prevention. Rapid HIV testing in outreach and other community settings – United States, 2004-2006. MMWR Morb Mortal Wkly Rep (2007) 56:1233–7.
40. Pavie J, Rachline A, Loze B, Niedbalski L, Delaugerre C, Laforgerie E, et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS One (2010) 5:e11581. doi:10.1371/journal.pone.0011581
41. Plate DK. Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS Res Hum Retroviruses (2007) 23:1491–8. doi:10.1089/aid.2007.0020
42. Walensky RP, Arbelaez C, Reichmann WM, Walls RM, Katz JN, Block BL, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med (2008) 149:153. doi:10.7326/0003-4819-149-3-200808050-00003
43. Pant PN, Balram B, Shivkumar S, Martinezcajas JL, Claessens C, Lambert G, et al. Head-to-head comparison of accuracy of a rapid point-of-care HIV test with oral versus whole-blood specimens: a systematic review and meta-analysis. Lancet Infect Dis (2012) 12:373–80. doi:10.1016/S1473-3099(11)70368-1
44. Smallwood M, Vijh R, Nauche B, Lebouché B, Joseph L, Pai NP. Evaluation of a rapid point of care test for detecting acute and established HIV infection, and examining the role of study quality on diagnostic accuracy: a Bayesian meta-analysis. PLoS One (2016) 11:e0149592. doi:10.1371/journal.pone.0149592
Keywords: western blot, rapid test, HIV, early therapy, meta-analysis
Citation: Huang X, Liu X, Chen J, Bao Y, Hou J, Lu X, Xia W, Xia H, Song A, Liu Z, Su B, Chen H, Chen Y and Wu H (2018) Evaluation of Blood-Based Antibody Rapid Testing for HIV Early Therapy: A Meta-Analysis of the Evidence. Front. Immunol. 9:1458. doi: 10.3389/fimmu.2018.01458
Received: 26 December 2017; Accepted: 12 June 2018;
Published: 26 June 2018
Edited by:
Mario (Mago) Clerici, Università degli Studi di Milano, ItalyReviewed by:
Yann Le Duff, National Institute for Biological Standards and Control (NIBSC), United KingdomAnthony Dominic Kelleher, University of New South Wales, Australia
Copyright: © 2018 Huang, Liu, Chen, Bao, Hou, Lu, Xia, Xia, Song, Liu, Su, Chen, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Chen, Y2hlbmh1aUBjY211LmVkdS5jbg==;
Yaokai Chen, eWFva2FpY2hlbkBob3RtYWlsLmNvbQ==;
Hao Wu, d2hkb2NAc2luYS5jb20=
†These authors have contributed equally to this work.
 Xiaojie Huang
Xiaojie Huang Xinchao Liu
Xinchao Liu Jieqing Chen3†
Jieqing Chen3† Jianhua Hou
Jianhua Hou Xiaofan Lu
Xiaofan Lu Huan Xia
Huan Xia Aixin Song
Aixin Song Zhiying Liu
Zhiying Liu Bin Su
Bin Su Hao Wu
Hao Wu