- 1Department of Medicine, Karolinska Institutet (KI), Solna, Sweden
- 2Cell Therapy Institute, Nova Southeastern University, Fort Lauderdale, FL, United States
- 3Department of Oncology-Pathology, Karolinska Institutet (KI), Solna, Sweden
Chemokines govern leukocyte migration by attracting cells that express their cognate ligands. Many cancer types show altered chemokine secretion profiles, favoring the recruitment of pro-tumorigenic immune cells and preventing the accumulation of anti-tumorigenic effector cells. This can ultimately result in cancer immune evasion. The manipulation of chemokine and chemokine-receptor signaling can reshape the immunological phenotypes within the tumor microenvironment in order to increase the therapeutic efficacy of cancer immunotherapy. Here we discuss the three chemokine-chemokine receptor axes, CXCR1/2–CXCL1-3/5-8, CXCR3–CXCL9/10/11, and CXCR4-CXCL12 and their role on pro-tumorigenic immune cells and anti-tumorigenic effector cells in solid tumors. In particular, we summarize current strategies to target these axes and discuss their potential use in treatment approaches.
Introduction
Immune evasion is a hallmark of carcinogenesis (1). Tumor cells interact closely with stromal cells, immune cells and the extracellular matrix (ECM). Via complex mechanisms these communications support tumor growth, metastatic spread, and immune escape (2). A family of small chemotactic proteins, called chemokines, has key roles in these interactions. Depending on their protein sequence, and more specifically, the location of the cysteine (C) residues at their N-terminus, chemokines are subdivided into four main classes: the C-, the CC-, the CXC-, and the CX3C-chemokines (3). Irrespective of their class, chemokines signal through binding to cognate seven-transmembrane spanning G protein-coupled receptors (GPCRs), found on the migratory cells. To date, 48 chemokines and 18 signal-transducing receptors have been identified in humans. Each chemokine can activate several different receptors. Immune cell subsets differentially express chemokine receptors, which results in their selective recruitment, according to the special needs of each environment. Within the tumor microenvironment (TME), chemokine ligand secretion is often altered compared to healthy tissue. This facilitates recruitment of pro-tumorigenic immune cells such as myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils (TAN), tumor-associated macrophages (TAM), and regulatory T cells (Treg). These cells expand during tumor progression, suppress effector lymphocytes, and are associated with worse prognosis in patients with various solid malignancies (4–7). Several studies demonstrate that tumor cells secrete chemokines in an autocrine and paracrine fashion to directly promote cancer cell growth, survival and metastasis (8). Here we focus on the impact of the CXCR1/2, CXCR3, and CXCR4 chemokine axes on recruitment of pro-tumorigenic and anti-tumorigenic immune cells in solid malignancies. We highlight the role of the CXCR1/2 axis on promoting immunosuppressive cells and the impact of CXCR3 and CXCR4 axes on increasing effector cell recruitment. Furthermore, we summarize preclinical and clinical studies that shape the therapeutic potential of chemokine-targeting and their implication in combinatorial immunotherapeutic treatment approaches.
The Role of CXCR1 and CXCR2 in Solid Malignancies
CXCR1 and CXCR2 are expressed by several cell types, especially neutrophils, fibroblasts and vascular endothelial cells. CXCR1 and CXCR2 bind the ligands CXCL6 and CXCL8 (IL-8) with similar affinity, while binding of CXCL1, CXCL2, CXCL3, CXCL5, and CXCL7 is mediated by CXCR2 (9). Mice do not have a CXCL8 (IL-8) gene. Moreover, the gene product of murine CXCL5, called LIX, is homologous to human CXCL6 and binds both CXCR1 and CXCR2 (10). High levels of these chemokine receptors and ligands in tumor tissues and serum are correlated with worse prognosis in several tumor types, including ovarian cancer, lung adenocarcinoma, colorectal carcinoma and pancreatic ductal adenocarcinoma (PDA) (11–15). One explanation for the poor prognosis could be the preferential recruitment of pro-tumorigenic immune cells via the CXCR1/ 2 axis (summarized in Table 1). Altered signaling pathways in tumor cells can increase chemokine secretion. For instance, overexpression of the transcription factor Snail in ovarian cancer cells upregulated CXCL1, CXCL2, and CXCL5 through the NF-kB pathway and promoted MDSC recruitment (11). Snail depletion or antibody-mediated CXCR2 targeting diminished MDSC cell numbers within tumors and increased T cell and NK cell numbers (11). Similarly, CXCL1 and CXCL2 secretion by breast cancer cells resulted in increased infiltration of pro-tumorigenic myeloid cells and was further augmented by chemotherapeutic treatment, leading to chemoresistence (16). The role of CXCL5 in recruiting CXCR2+ MDSC and TAN has also been shown in models of renal cell carcinoma (RCC) (17), PDA (18), melanoma (19, 20), and hepatocellular carcinoma (HCC) (21). In patients with RCC, intratumoral CXCL5 and CXCL8 levels correlated with increased MDSC infiltration (17). Targeting CXCR2 reduced MDSC numbers and increased effector T cells (17). While targeting CXCR2 alone only modestly decreased tumor burden in a murine RCC model, combination with immune checkpoint inhibition significantly reduced tumor weight (17). Similarly, high CXCL5 expression was found in PDA and mediated recruitment of CXCR2+ neutrophils (18). Abrogation of CXCR2 diminished neutrophil infiltration and increased the ratio of effector T cells (18). In genetically modified mice that expressed human CXCL8, MDSC were efficiently recruited to the tumor site and suppressed T cell activity (22). Collectively, these data indicate that CXCR1/2 blockade reduces pro-tumorigenic immune cell infiltration and increases T and NK cell recruitment. This supports attempts to combine CXCR1/2 blockade with other immunotherapies, such as checkpoint inhibition or adoptive cell therapy. CXCR1/2 blockade also helps to overcome chemoresistance mediated by pro-tumorigenic immune cells (16, 23). It was recently shown that chemokine signaling within the TME displays high plasticity: CXCR2+ TAN numbers within tumor biopsies increased in PDA patients that were previously treated with an inhibitor of CCR2 (23). Inversely, depletion of TANs resulted in increased TAM numbers and only dual inhibition of both the CXCR1, CXCR2, and CCR2 axis disrupted myeloid infiltration and improved responses to chemotherapeutic treatment (23).
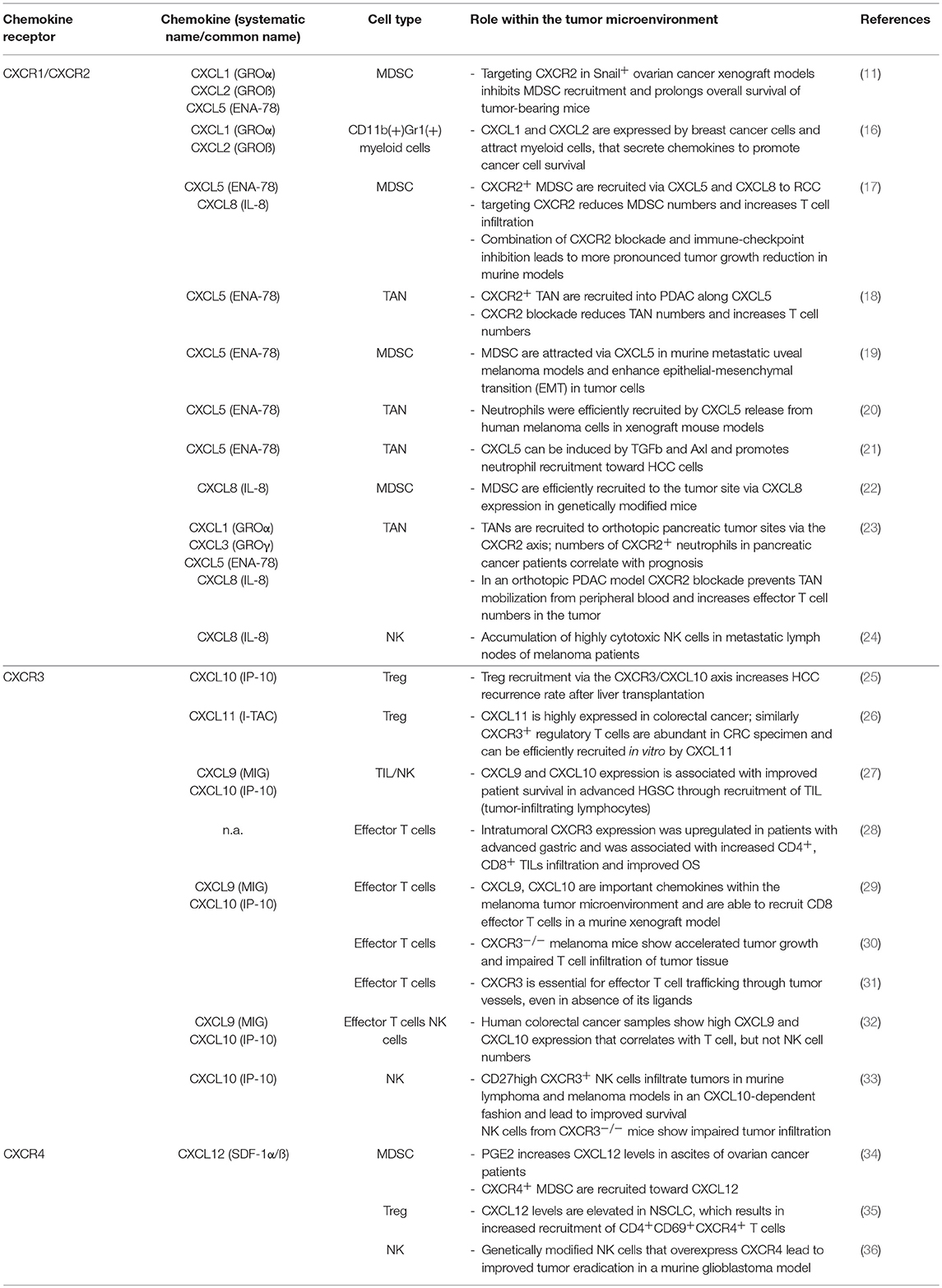
Table 1. The effect of chemokine ligands and their receptors on immune cells within the tumor microenvironment.
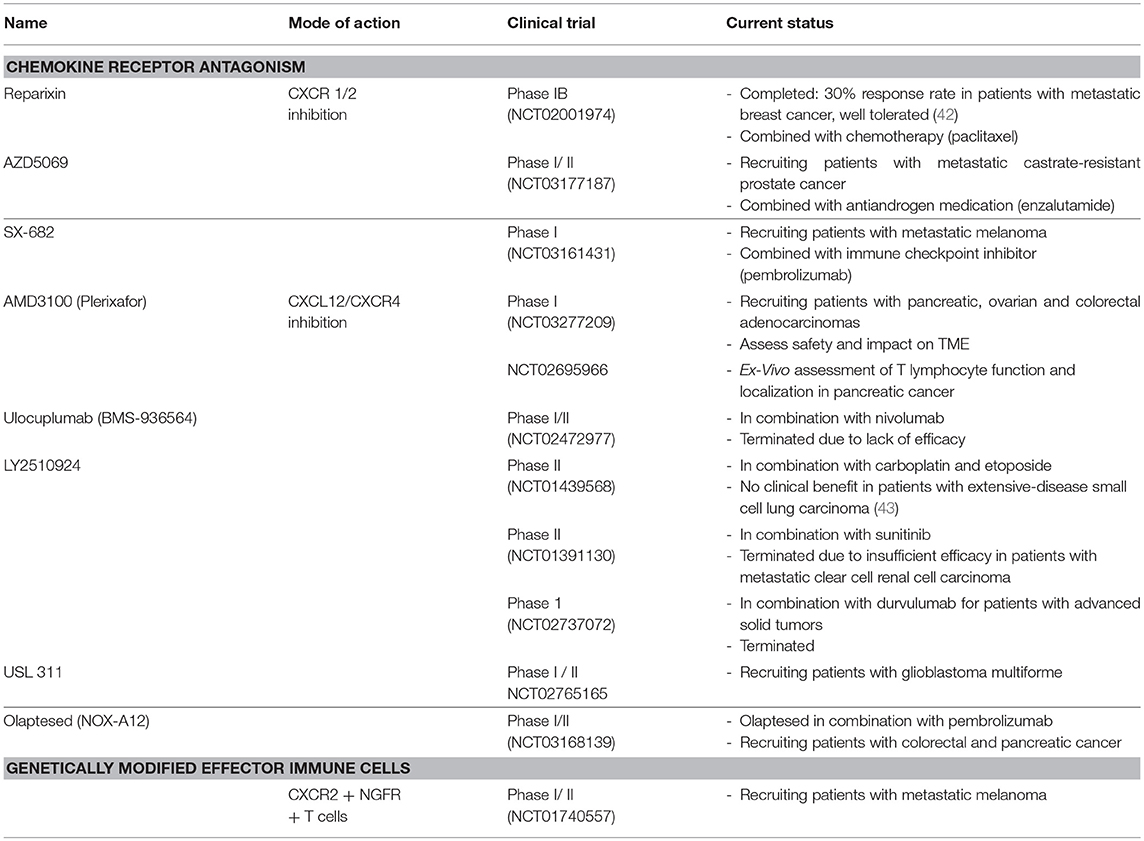
CXCR1 and CXCR2 are highly expressed by cytotoxic CD56dim NK cells (37, 38). We recently showed that CXCR2 expression is downregulated on tumor-infiltrating NK cells in RCC and genetic modification to re-express CXCR2 enhanced recruitment of NK cells to the tumor site (39). Similarly, Ali et al. showed that CXCL8 was released within the TME of melanoma-infiltrated lymph nodes and could efficiently recruit highly cytotoxic NK cells (24). The percentage of this NK cell population among all NK cells within the affected lymph node was associated with improved prognosis among patients with stage III melanoma. Likewise, genetically modified CXCR2+ T cells displayed increased in vivo migration in murine melanoma models (40, 41). A clinical phase I/II trial in patients with metastatic melanoma infused with genetically modified CXCR2+ T cells has been initiated (Table 2).
Findings from pre-clinical studies have already been translated into clinical phase studies (summarized in Table 2). The combination of paclitaxel with reparixin—a CXCR1 and CXCR2 inhibitor—was well tolerated in patients with metastatic breast cancer and resulted in 30% response rate (42). Based on these findings, a phase II study was initiated (NCT02370238). Combination therapies with CXCR1/2 inhibitors are also in clinical phase trials for prostate cancer and metastatic melanoma.
The Role of CXCR3 and Its Ligands in Solid Tumors
CXCR3 is expressed on different subtypes of T and NK cells (37, 44) and binds to CXCL9, CXCL10, and CXCL11. During homeostasis, CXCL9, CXCL10 and CXCL11 are expressed at low levels by monocytes, endothelial cells and fibroblasts, but are upregulated upon cytokine stimulation, especially by IFNγ and TNFα (45, 46). CXCR3 and its ligands are expressed by various solid tumors, although their prognostic role greatly differs among the entities. This underlines a role in tumor suppression as well as tumor growth promotion and metastasis. While high CXCR3 expression in glioblastoma, colorectal, and breast cancer is associated with poor prognosis, it correlated with better outcomes in patients with gastric cancer (28, 47, 48). In contrast, high CXCL9, CXCL10, and CXCL11 expression in the TME of patients with colorectal, oesophageal, non-small cell lung (NSCL) and ovarian cancer is an indicator of improved overall survival (27, 49–51), while it is a poor prognostic marker in patients with localized clear-cell RCC (52).
CXCR3 is a key receptor in recruitment of activated T cells as it is absent in naïve T cells, but highly expressed on activated effector and memory T cells (44). CXCR3 expression on Tregs, however, can hamper effector immune cell functions due to competitive recruitment. In HCC, Treg infiltration in the liver after liver transplantation was associated with higher rates of recurrence (25). Patients with higher numbers of circulating Tregs and increased levels of CXCL10 within the graft were more susceptible. Similarly, high expression of CXCL11 in a colorectal cancer model was shown to recruit CXCR3+ Tregs (26). In contrast, in ovarian cancer, high CXCL9 and CXCL10 expression doubled the overall survival time due to improved recruitment of tumor-infiltrating lymphocytes (27). Enhanced effector T cell recruitment via the CXCR3 axis has also been confirmed in the case of gastric cancer and melanoma (28, 29). Tumor growth was accelerated in CXCR3−/− melanoma-bearing mice and T cell infiltration was severly impaired (30). Anti-programmed death receptor (Anti-PD1) therapy was not beneficial in CXCR3−/− tumor-bearing mice due to failure of efficient T cell recruitment (30). Importantly, CXCR3 has been shown to be indispensable for CD8+ effector T cell trafficking across tumor vasculature due to its role in intravascular adhesion, even in the absence of its ligands. CCR2 and CCR5, in contrast, promoted tumor site infiltration only in a chemokine ligand dependent manner (31). CXCR3 expression plays an important role in recruiting NK cells to the tumor site: We showed that CXCR3 expression on human NK cells increased during ex vivo culture (53). In xenograft mice models, these expanded NK cells could be efficiently recruited toward CXCL10+ melanomas (53). However, the sole presentation of CXCR3 ligands within the TME does not always predict efficient effector cell recruitment. In a mouse model of uveal melanoma that leads to spontaneous metastasis into the skin and viscera, application of the chemotherapeutic drug temozolomide increased CXCL9 and CXCL10 levels within the metastatic sites (54). Nonetheless, increased T cell infiltration was only observed in the visceral sites and not in the cutaneous tumors due to altered matrix architecture and mode of CXCL9/10 presentation (54). Interestingly, high expression levels of CXCL9 and CXCL10 in colorectal cancer samples correlated with T cell infiltration, but not with NK cell infiltration that was scarce in the analyzed samples (32). The expression level of CXCR3 was not measured on NK cells versus T cells. In contrast, CXCR3+ NK cells infiltrated tumor tissue in murine lymphoma and melanoma models in a CXCL10-dependent manner (33). CXCL10 was augmented via application of IFNγ (33). Several factors can modify CXCR3 expression on T cells and NK cells. For instance, elevated CXCR3 ligands in patients with cutanenous T cell lymphoma lead to CXCR3 downregulation on cytotoxic T cells (55). Soluble HLA-G was also shown to downregulate CXCR3 expression on cytotoxic T cells and inhibit migration along CXCL9 and CXCL10 gradients (56). In another study, STAT3 signaling in CD8+ T cells was shown to downregulate IFNγ production, leading to decreased CXCL10 expression by tumor-associated macrophages. Additionally, STAT3 diminished CXCR3 expression on CD8+ T cells (57). Collectively, these data underline not only the importance of the CXCR3 axis in recruitment of effector immune cells, but also reveal complex relationships of receptor-ligand interactions in a TME-specific context.
To enhance effector cell recruitment, efforts are made to increase CXCL9 and CXCL10 expression within the TME. Several enzymes can modulate CXCR3 ligands such as dipeptidyl peptidase-4 (DPP-4/CD26) (58, 59), furin (60) as well as certain peptidylarginine deaminases and matrix metalloproteinases (61). For instance, DPP-4 was shown to cleave CXCL9, 10 and 11, which in turn reduced their chemotactic activity on lymphocytes, while not affecting their antiangiogenic activities (59). In breast cancer cell lines, Prostaglandin E2 (PGE2) impaired IFN-γ mediated CXCL9 and CXCL10 release (62). Inhibition of the cyclooxygenase (COX) isoenzymes with indomethacin and acetylsalicylic acid suppressed the downregulatory functions of PGE2 and increased CXCL9 and CXCL10 levels in vitro (62). Evidence for the role of CXCL9 in attracting NK and cytotoxic T cells was shown in a murine model of breast cancer (63). Gene transfer of CXCL10 by pCLNCX retroviral vectors in melanoma xenograft models decreased angiogenesis and tumor growth (64). Similarly, murine-leukemia virus (MLV)-derived replication-competent retroviruses were used to stably express CXCL10 in fibrosarcoma, melanoma and Lewis lung cancer models and were shown to inhibit tumor growth in vivo (65). However, the effect of CXCL10 on T or NK cell recruitment and functionality was not investigated in these early studies. Only recently, an oncolytic poxvirus was armed with CXCL11 in order to attract CXCR3+ cytotoxic T cells and NK cells to the site of the malignancy in a murine mesothelioma model (66). Besides improving effector cell homing, the virus enhanced the systemic antitumor activity by inducing the proliferation of IFNγ-producing CD8+ T cells.
Targeting the CXCR3 axis to improve efficient effector cell recruitment is hampered by the opposing role on tumor cells: CXCR3 expression can be found on tumor cells, especially at later stages of tumorigenesis and in patients with advanced disease, where it is positively correlated with the formation of metastasis (67–69). Thus, blocking CXCR3 on tumor cells might also impair the ability of CXCR3+ NK and T cells to efficiently kill tumor cells. Interestingly, ACKR3 (formerly CXCR7) is an atypical receptor of CXCL11 and CXCL12, that is not expressed on peripheral blood leukocytes but upregulated by various tumor types, including breast, esophageal and lung squamous cell cancer (70, 71). Targeting of ACKR3 with a monoclonal antibody in mice models of glioblastoma leads to increased tumor cell death via NK-cell mediated antibody-dependent cytotoxicity (ADCC) (72). Combination with temozolomide prolonged survival in tumor-bearing mice and resulted in enhanced infiltration of anti-tumorigenic M1 macrophages (72). CXCR3 and ACKR3 inhibitors are in preclinical testing for different solid tumors (72–74). Currently there are no registered clinical phase trials employing either CXCR3 or ACKR3 inhibitors in solid malignancies.
CXCR4 and Its Ligand CXCL12
CXCR4 and its ligand CXCL12 are ubiquitously expressed under physiological conditions and are important for hematopoiesis, cardiogenesis, and neurogenesis. The CXCR4-CXCL12 axis is involved in HSC maintenance and homing within the bone marrow as well as during the development of B, T, and NK cells (75, 76). In the context of cancer, CXCR4 expression is found on tumor cells, where it promotes tumor cell growth, migration, and invasiveness (77, 78). Moreover, CXCL12 produced within the tumor can attract CXCR4+ Treg, MDSC and plasmacytoid dendritic cells (pDC), potentiating the tumor-promoting effect (34, 79–81). High CXCR4 expression in biopsies of solid tumors is generally correlated with worse prognosis. In particular, CXCR4 expression in breast cancer was significantly associated with lymph node and distant metastasis and worse overall survival (82). Similar conclusions could be drawn for prostate cancer, melanoma and lung cancer (83–85).
The expression levels of CXCR4 on NK and T cells varies according to their maturation stage and subset, whereas their recruitment to the different organs is often dependent on the co-expression of other chemokines (86, 87). High CXCR4 expression on NK cells is associated with accumulation within the bone marrow compartment, whereas CXCR4 desensitization is important to enable NK cells to leave the bone marrow (88, 89). Several factors can modulate the chemokine receptor repertoire on immune cells: For instance, conditioning human NK cells with TGFβ1, derived from neuroblastoma cells, significantly upregulated CXCR4 and CXCR3 expression and downregulated CX3CR1 on NK cells (90). This generated an NK cell phenotype that is retained in the bone marrow, rather than recruited to peripheral organs and tumor tissue (91). Another study suggested that PGE2 regulates CXCL12 levels in malignant ascites from ovarian cancer patients and CXCR4 expression on MDSC (34). Blockade of PGE2 abrogated migration of MDSC toward the malignant ascites. In line with this, non-small cell lung cancer (NSCLC) express high CXCL12 levels and especially recruits CD4+CD69+CXCR4+ T cells with an increased ratio of regulatory T cells (35). Although the percentage of CD8+ T cells was not altered, NK cell numbers within the tumor tissue decreased. In accordance, regulatory T cells are maintained within the bone marrow and can migrate along the CXCR4-CXCL12 axis (92). Regarding modulation of CXCR4 expression using pharmacological agents, tyrosine kinase inhibitors (TKIs) imatinib and nilotinib have been shown to selectively increase the cell surface of CXCR4 on NK cells and monocytes, in vitro experiments using NK cells derived from neuroblastoma patients (93).
Multiple approaches to target this axis have been explored, some of which have entered clinical trials with varying outcomes (summarized in Table 2). On a preclinical level, TN14003 and AMD3100 (Plerixafor), two anti-CXCR4 inhibitors, have been tested in patient-derived xenografts (PDX) of breast cancer showing antitumor activity in the HER2 subtype (94). Interestingly, in triple-negative PDX, both inhibitors appeared neither to control tumor growth nor to impede metastatic spread, which highlights the complexity of breast cancer subtypes and their respective TMEs. AMD3100 has also been tested in a murine model of human pancreatic cancer, alone or in combination with immunological checkpoint antagonists (95). In this study, AMD3100 was able to successfully block CXCR4 signaling and promote T-cell mobilization in vivo. More importantly, AMD3100 showed improved anticancer activity when combined with an anti-PD-L1 monoclonal antibody (96). CXCR4 is also highly expressed in colorectal cancer, building a therapeutic rationale for CXCR4 targeting (97). Blocking colon carcinoma cells with a CXCL12-KDEL retention protein in vitro, resulted in the inhibition of CXCR4-mediated signaling and a subsequent dramatic decrease in metastatic cancer outgrowth (98). AMD3100 has also been tested in the particular model, exhibiting similar promising preclinical results (99). Other means of modulating the CXCR4-CXCL12 axis include oncolytic viruses and gene-engineered NK cells. In particular, introducing an oncolytic virus equipped with a CXCR4 antagonist restored the pathologic signaling in a murine model of ovarian cancer, reduced metastatic spread and diminished regulatory T cell recruitment (100). On the other hand, NK cells engineered to co-express a chimeric antigen receptor (CAR) and the chemokine receptor CXCR4 enhanced NK cell infiltration and tumor cell killing in a glioblastoma tumor model (36). Last but not least, Spiegelmer aptamers, such as the CXCL12-targeting NOX-A12, hold great potential in modulating the TME of solid tumors. Although clinical trials are still ongoing (Table 2), NOX-A12 (Olaptesed pegol) is thought to increase immune cell infiltration, sensitize tumors to checkpoint inhibitors and obstruct tumor repair mechanisms in metastatic pancreatic and colorectal cancers (Noxxon Pharma). Examples of additional types of solid tumors that may benefit from inhibition of the CXCR4-CXCL12 axis are oesophageal (101)and gastric cancer (102).
Concluding Remarks
Although our current understanding of solid tumor microenvironment and its chemokine networks is more detailed, a lot remains unexplored. The future of chemokine modulation for therapeutic purposes is very much dependent on efforts to elucidate the complex pro-tumor and antitumor roles of chemokines in the TME. The current preclinical approaches have demonstrated some promising results and defined rational immunotherapeutic combinations. The results from the eagerly awaited clinical trials, in combination with investigations on new chemokine targets and advances in drug discovery, immunotherapy and cell therapy, are expected to shape the landscape of chemokine-based therapy further in the years to come.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was supported by The Swedish Cancer Society (CAN 2015/421), The Cancer Society in Stockholm (161192) and The Swedish Childhood Cancer Foundation (PR2017-0049) for AL.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
2. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
3. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. (2014) 32:659–702. doi: 10.1146/annurev-immunol-032713-120145
4. Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C. Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment. Cancer Res. (2017) 77:1271–82. doi: 10.1158/0008-5472.CAN-16-2490
5. Zhang S, Ma X, Zhu C, Liu L, Wang G, Yuan X. The role of myeloid-derived suppressor cells in patients with solid tumors: a meta-analysis. PLoS ONE (2016) 11:e0164514. doi: 10.1371/journal.pone.0164514
6. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
7. Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ. et al Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE (2012) 7:e50946. doi: 10.1371/journal.pone.0050946
8. Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget (2013) 4:2171–85. doi: 10.18632/oncotarget.1426
9. Alfaro C, Sanmamed MF, Rodriguez-Ruiz ME, Teijeira A, Onate C, Gonzalez A, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. (2017) 60:24–31. doi: 10.1016/j.ctrv.2017.08.004
10. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity (2012) 36:705–16. doi: 10.1016/j.immuni.2012.05.008
11. Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. (2018) 9:1685. doi: 10.1038/s41467-018-03966-7
12. Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. (2013) 73:571–82. doi: 10.1158/0008-5472.CAN-12-0263
13. Zhao J, Ou B, Feng H, Wang P, Yin S, Zhu C, et al. Overexpression of CXCR2 predicts poor prognosis in patients with colorectal cancer. Oncotarget (2017) 8:28442–54. doi: 10.18632/oncotarget.16086
14. Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P, et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. (2014) 4:5911. doi: 10.1038/srep05911
15. Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. (2013) 19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525
16. Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell (2012) 150:165–78. doi: 10.1016/j.cell.2012.04.042
17. Najjar YG, Rayman P, Jia X, Pavicic PG Jr, Rini BI, Tannenbaum C, et al. Myeloid-derived suppressor cell subset accumulation in renal cell carcinoma parenchyma is associated with intratumoral expression of IL1beta IL8, CXCL5, and Mip-1alpha. Clin Cancer Res. (2017) 23:2346–55. doi: 10.1158/1078-0432.CCR-15-1823
18. Chao, T, Furth EE, Vonderheide RH. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. (2016) 4:968–82. doi: 10.1158/2326-6066.CIR-16-0188
19. Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. (2011) 9:e1001162. doi: 10.1371/journal.pbio.1001162
20. Soler-Cardona A, Forsthuber A, Lipp K, Ebersberger S, Heinz M, Schossleitner K, et al. CXCL5 facilitates melanoma cell-neutrophil interaction and lymph node metastasis. J Invest Dermatol. (2018) 138:1627–35. doi: 10.1016/j.jid.2018.01.035
21. Haider C, Hnat J, Wagner R, Huber H, Timelthaler G, Grubinger M, et al. Transforming growth Factor-beta and Axl Induce CXCL5 and Neutrophil Recruitment in Hepatocellular Carcinoma. Hepatology (2018). doi: 10.1002/hep.30166. [Epub ahead of print].
22. Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res. (2016) 22:3924–36. doi: 10.1158/1078-0432.CCR-15-2463
23. Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut (2018) 67:1112–3. doi: 10.1136/gutjnl-2017-313738
24. Ali TH, Pisanti S, Ciaglia E, Mortarini R, Anichini A, Garofalo C, et al. Enrichment of CD56(dim)KIR + CD57 + highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat Commun. (2014) 5:5639. doi: 10.1038/ncomms6639
25. Li CX, Ling CC, Shao Y, Xu A, Li XC, Ng KT, et al. CXCL10/CXCR3 signaling mobilized-regulatory T cells promote liver tumor recurrence after transplantation. J Hepatol. (2016) 65:944–52. doi: 10.1016/j.jhep.2016.05.032
26. Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, et al. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. (2011) 89:85–91. doi: 10.1189/jlb.0910506
27. Bronger H, Singer J, Windmuller C, Reuning U, Zech D, Delbridge C, et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer (2016) 115:553–63. doi: 10.1038/bjc.2016.172
28. Li K, Zhu Z, Luo J, Fang J, Zhou H, Hu M, et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int J Clin Exp Pathol. (2015) 8:14725–32.
29. Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. (2009) 69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281
30. Chheda ZS, Sharma RK, Jala VR, Luster AD, Haribabu B. Chemoattractant Receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors. J Immunol. (2016) 197:2016–26. doi: 10.4049/jimmunol.1502376
31. Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. (2015) 6:7458. doi: 10.1038/ncomms8458
32. Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. (2011) 17:678–89. doi: 10.1158/1078-0432.CCR-10-2173
33. Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. (2008) 68:8437–45. doi: 10.1158/0008-5472.CAN-08-1440
34. Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. (2011) 71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449
35. Wald O, Izhar U, Amir G, Avniel S, Bar-Shavit Y, Wald H, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J Immunol. (2006) 177:6983–90. doi: 10.4049/jimmunol.177.10.6983
36. Muller N, Michen S, Tietze S, Topfer K, Schulte A, Lamszus K, et al. Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1alpha-secreting glioblastoma. J Immunother. (2015) 38:197–210. doi: 10.1097/CJI.0000000000000082
37. Lima M, Leander M, Santos M, Santos AH, Lau C, Queiros ML, et al. Chemokine receptor expression on normal blood CD56(+) NK-cells elucidates cell partners that comigrate during the innate and adaptive immune responses and identifies a transitional NK-cell population. J Immunol Res. (2015) 2015:839684. doi: 10.1155/2015/839684
38. Morohashi H, Miyawaki T, Nomura H, Kuno K, Murakami S, Matsushima K, et al. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J Leukoc Biol. (1995) 57:180–7. doi: 10.1002/jlb.57.1.180
39. Kremer V, Ligtenberg MA, Zendehdel R, Seitz C, Duivenvoorden A, Wennerberg E, et al. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J Immunother Cancer (2017) 5:73. doi: 10.1186/s40425-017-0275-9
40. Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. (2010) 16:5458–68. doi: 10.1158/1078-0432.CCR-10-0712
41. Idorn M, Skadborg SK, Kellermann L, Halldórsdóttir HR, Holmen Olofsson G, Met, Ö, et al. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. OncoImmunology (2018) 2018:e1450715. doi: 10.1080/2162402X.2018.1450715
42. Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. (2017) 23:5358–65. doi: 10.1158/1078-0432.CCR-16-2748
43. Salgia R, Stille JR, Weaver RW, McCleod M, Hamid O, Polzer J, et al. A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer (2017) 105:7–13. doi: 10.1016/j.lungcan.2016.12.020
44. Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. (1998) 187:875–83. doi: 10.1084/jem.187.6.875
45. Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. (1997) 272:14899–907. doi: 10.1074/jbc.272.23.14899
46. Ohmori Y, Wyner L, Narumi S, Armstrong D, Stoler M, Hamilton TA. Tumor necrosis factor-alpha induces cell type and tissue-specific expression of chemoattractant cytokines in vivo. Am J Pathol. (1993) 142:861–70.
47. Zhang Y, Xu L, Peng M. CXCR3 is a prognostic marker and a potential target for patients with solid tumors: a meta-analysis. Onco Targets Ther. (2018) 11:1045–54. doi: 10.2147/OTT.S157421
48. Li L, Chen J, Lu ZH, Yu SN, Luo YF, Zhao WG, et al. Significance of chemokine receptor CXCR3 expression in breast cancer. Zhonghua Bing Li Xue Za Zhi (2011) 40:85–8.
49. Kistner L, Doll D, Holtorf A, Nitsche U, Janssen KP. Interferon-inducible CXC-chemokines are crucial immune modulators and survival predictors in colorectal cancer. Oncotarget (2017) 8:89998–90012. doi: 10.18632/oncotarget.21286
50. Sato Y, Motoyama S, Nanjo H, Wakita A, Yoshino K, Sasaki T, et al. CXCL10 expression status is prognostic in patients with advanced thoracic esophageal squamous cell carcinoma. Ann Surg Oncol. (2016) 23:936–42. doi: 10.1245/s10434-015-4909-1
51. Cao Y, Huang H, Wang Z, Zhang G. The Inflammatory CXC Chemokines, GROalpha(high), IP-10(low), and MIG(low), in tumor microenvironment can be used as new indicators for non-small cell lung cancer progression. Immunol Invest. (2017) 46:361–74. doi: 10.1080/08820139.2017.1280052
52. Klatte T, Seligson DB, Leppert JT, Riggs SB, Yu H, Zomorodian N, et al. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. (2008) 179:61–6. doi: 10.1016/j.juro.2007.08.148
53. Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. (2015) 64:225–35. doi: 10.1007/s00262-014-1629-5
54. Tan KW, Evrard M, Tham M, Hong M, Huang C, Kato M, et al. Tumor stroma and chemokines control T-cell migration into melanoma following Temozolomide treatment. Oncoimmunology (2015) 4:e978709. doi: 10.4161/2162402X.2014.978709
55. Winter D, Moser J, Kriehuber E, Wiesner C, Knobler R, Trautinger F, et al. Down-modulation of CXCR3 surface expression and function in CD8+ T cells from cutaneous T cell lymphoma patients. J Immunol. (2007) 179:4272–82. doi: 10.4049/jimmunol.179.6.4272
56. Morandi F, Ferretti E, Bocca P, Prigione I, Raffaghello L, Pistoia V. A novel mechanism of soluble HLA-G mediated immune modulation: downregulation of T cell chemokine receptor expression and impairment of chemotaxis. PLoS ONE (2010) 5:e11763. doi: 10.1371/journal.pone.0011763
57. Yue C, Shen S, Deng J, Priceman SJ, Li W, Huang A, et al. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol Res. (2015) 3:864–70. doi: 10.1158/2326-6066.CIR-15-0014
58. Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. (2001) 276:29839–45. doi: 10.1074/jbc.M103106200
59. Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood (2001) 98:3554–61. doi: 10.1182/blood.V98.13.3554
60. Hensbergen PJ, Verzijl D, Balog CI, Dijkman R, van der Schors RC, van der Raaij-Helmer EM, et al. Furin is a chemokine-modifying enzyme: in vitro and in vivo processing of CXCL10 generates a C-terminally truncated chemokine retaining full activity. J Biol Chem. (2004) 279:13402–11. doi: 10.1074/jbc.M312814200
61. Metzemaekers M, Vanheule V, Janssens R, Struyf S, Proost P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 Ligands. Front Immunol. (2017) 8:1970. doi: 10.3389/fimmu.2017.01970
62. Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stockel A, Avril S, et al. Modulation of CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. (2012) 14:R30. doi: 10.1186/bcr3115
63. Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. (2007) 30:490–8. doi: 10.1097/CJI.0b013e318031b551
64. Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, et al. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer (2002) 99:149–53. doi: 10.1002/ijc.10292
65. Sun Y, Finger C, Alvarez-Vallina L, Cichutek K, Buchholz CJ. Chronic gene delivery of interferon-inducible protein 10 through replication-competent retrovirus vectors suppresses tumor growth. Cancer Gene Ther. (2005) 12:900–12. doi: 10.1038/sj.cgt.7700854
66. Liu Z, Ravindranathan R, Li J, Kalinski P, Guo ZS, Bartlett DL. CXCL11-Armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology (2016) 5:e1091554. doi: 10.1080/2162402X.2015.1091554
67. Zhu G, Yan HH, Pang Y, Jian J, Achyut BR, Liang X, et al. CXCR3 as a molecular target in breast cancer metastasis: inhibition of tumor cell migration and promotion of host anti-tumor immunity. Oncotarget (2015) 6:43408–19. doi: 10.18632/oncotarget.6125
68. Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H, Knoll C, et al. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. Br J Cancer (2011) 104:469–79. doi: 10.1038/sj.bjc.6606056
69. Windmuller C, Zech D, Avril S, Boxberg M, Dawidek T, Schmalfeldt B, et al. CXCR3 mediates ascites-directed tumor cell migration and predicts poor outcome in ovarian cancer patients. Oncogenesis (2017) 6:e331. doi: 10.1038/oncsis.2017.29
70. Berahovich RD, Zabel BA, Penfold ME, Lewen S, Wang Y, Miao Z, et al. CXCR7 protein is not expressed on human or mouse leukocytes. J Immunol. (2010) 185:5130–9. doi: 10.4049/jimmunol.1001660
71. Behnam Azad B, Lisok A, Chatterjee S, Poirier JT, Pullambhatla M, Luker GD, et al. Targeted imaging of the atypical chemokine receptor 3 (ACKR3/CXCR7) in Human Cancer Xenografts. J Nuclear Med. (2016) 57:981–8. doi: 10.2967/jnumed.115.167932
72. Salazar N, Carlson JC, Huang K, Zheng Y, Oderup C, Gross J, et al. A chimeric antibody against ACKR3/CXCR7 in combination with TMZ activates immune responses and extends survival in mouse GBM Models. Mol Ther. (2018) 26:1354–65. doi: 10.1016/j.ymthe.2018.02.030
73. Pradelli E, Karimdjee-Soilihi B, Michiels JF, Ricci JE, Millet MA, Vandenbos F, et al. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. Int J Cancer (2009) 125:2586–94. doi: 10.1002/ijc.24665
74. Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, et al. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. Br J Cancer (2009) 100:1755–64. doi: 10.1038/sj.bjc.6605078
75. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity (2006) 25:977–88. doi: 10.1016/j.immuni.2006.10.016
76. Noda M, Omatsu Y, Sugiyama T, Oishi S, Fujii N, Nagasawa T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood (2011) 117:451–8. doi: 10.1182/blood-2010-04-277897
77. Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature (2001) 410:50–6. doi: 10.1038/35065016
78. Scotton CJ, Wilson JL, Scott K, Stamp G, Wilbanks GD, Fricker S, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. (2002) 62:5930–8.
79. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer (2005) 5:263–74. doi: 10.1038/nrc1586
80. Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. (2001) 7:1339–46. doi: 10.1038/nm1201-1339
81. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. (2006) 6:295–307. doi: 10.1038/nri1806
82. Zhang Z, Ni C, Chen W, Wu P, Wang Z, Yin J, et al. Expression of CXCR4 and breast cancer prognosis: a systematic review and meta-analysis. BMC Cancer (2014) 14:49. doi: 10.1186/1471-2407-14-49
83. Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. (2003) 89:462–73. doi: 10.1002/jcb.10522
84. Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. (2005) 11:1835–41. doi: 10.1158/1078-0432.CCR-04-1887
85. Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Mol Cancer Res. (2014) 12:38–47. doi: 10.1158/1541-7786.MCR-12-0334
86. Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J Immunol. (2014) 192:3805–15. doi: 10.4049/jimmunol.1301889
87. Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity (2006) 25:213–24. doi: 10.1016/j.immuni.2006.06.015
88. Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood (2011) 118:4863–71. doi: 10.1182/blood-2011-06-362574
89. Bernardini G, Sciume G, Bosisio D, Morrone S, Sozzani S, Santoni A. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood (2008) 111:3626–34. doi: 10.1182/blood-2007-08-106203
90. Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F, et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol. (2013) 190:5321–8. doi: 10.4049/jimmunol.1202693
91. Sciume G, De Angelis G, Benigni G, Ponzetta A, Morrone S, Santoni A, et al. CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood (2011) 117:4467–75. doi: 10.1182/blood-2010-07-297101
92. Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. (2004) 64:8451–5. doi: 10.1158/0008-5472.CAN-04-1987
93. Bellora F, Dondero A, Corrias MV, Casu B, Regis S, Caliendo F, et al. Imatinib and Nilotinib Off-Target Effects on Human NK Cells, Monocytes, and M2 Macrophages. J Immunol. (2017) 199:1516–25. doi: 10.4049/jimmunol.1601695
94. Lefort S, Thuleau A, Kieffer Y, Sirven P, Bieche I, Marangoni E, et al. CXCR4 inhibitors could benefit to HER2 but not to triple-negative breast cancer patients. Oncogene (2017) 36:1211–22. doi: 10.1038/onc.2016.284
95. Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. (2013) 110:20212–7. doi: 10.1073/pnas.1320318110
96. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
97. Ottaiano A, di Palma A, Napolitano M, Pisano C, Pignata S, Tatangelo F, et al. Inhibitory effects of anti-CXCR4 antibodies on human colon cancer cells. Cancer Immunol Immunother. (2005) 54:781–91. doi: 10.1007/s00262-004-0636-3
98. Zeelenberg ISL. Ruuls-Van Stalle and Roos E The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. (2003) 63:3833–9.
99. Li JK, Yu L, Shen Y, Zhou LS, Wang YC, Zhang JH. Inhibition of CXCR4 activity with AMD3100 decreases invasion of human colorectal cancer cells in vitro. World J Gastroenterol. (2008) 14:2308–13. doi: 10.3748/wjg.14.2308
100. Gil M, Komorowski MP, Seshadri M, Rokita H, McGray AJ, Opyrchal M, et al. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol. (2014) 193:5327–37. doi: 10.4049/jimmunol.1400201
101. Wu J, Wu X, Liang W, Chen C, Zheng L, An H. Clinicopathological and prognostic significance of chemokine receptor CXCR4 overexpression in patients with esophageal cancer: a meta-analysis. Tumour Biol. (2014) 35:3709–15. doi: 10.1007/s13277-013-1490-8
Keywords: chemokines, cancer immunotherapy, metastasis, NK cells, T cells, myeloid cells
Citation: Susek KH, Karvouni M, Alici E and Lundqvist A (2018) The Role of CXC Chemokine Receptors 1–4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 9:2159. doi: 10.3389/fimmu.2018.02159
Received: 29 May 2018; Accepted: 31 August 2018;
Published: 25 September 2018.
Edited by:
Giovanni Bernardini, Università degli Studi di Roma La Sapienza, ItalyReviewed by:
Roberta Castriconi, Università degli studi di Genova, ItalyPaul Proost, KU Leuven, Belgium
Copyright © 2018 Susek, Karvouni, Alici and Lundqvist. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Lundqvist, QW5kcmVhcy5MdW5kcXZpc3RAa2kuc2U=
 Katharina Helene Susek
Katharina Helene Susek Maria Karvouni
Maria Karvouni Evren Alici
Evren Alici Andreas Lundqvist
Andreas Lundqvist