- 1Quantitative and Systems Biology Graduate Program, University of California, Merced, Merced, CA, United States
- 2Department of Molecular Cell Biology, School of Natural Sciences, University of California, Merced, Merced, CA, United States
CD8 T cells are infrequently considered part of germinal center reactions. Yet, a distinct CXCR5+ CD8 T cell subset identified within the B cell follicle and germinal center in situations of chronic antigen has recently been defined. CXCR5+ CD8 T cells maintain transcriptional and phenotypic features consistent with the CD8 T cell nomenclature of a non-exhausted, effector memory population. CD8 T cell localization to the B cell follicle suggests a functional profile similar to CD4 T follicular helper cells that are licensed to promote B cell responses. The functional mechanisms defined under different immune settings, while largely similar, differentially control disease pathogenesis. CXCR5+ CD8 T cells control viral load during infection, and also promote antibody-mediated autoimmune disease progression. The existence of this novel CXCR5+ CD8 T cell subset in human and murine models of disease may provide a paradigm shift in our understanding of germinal center reactions.
Introduction
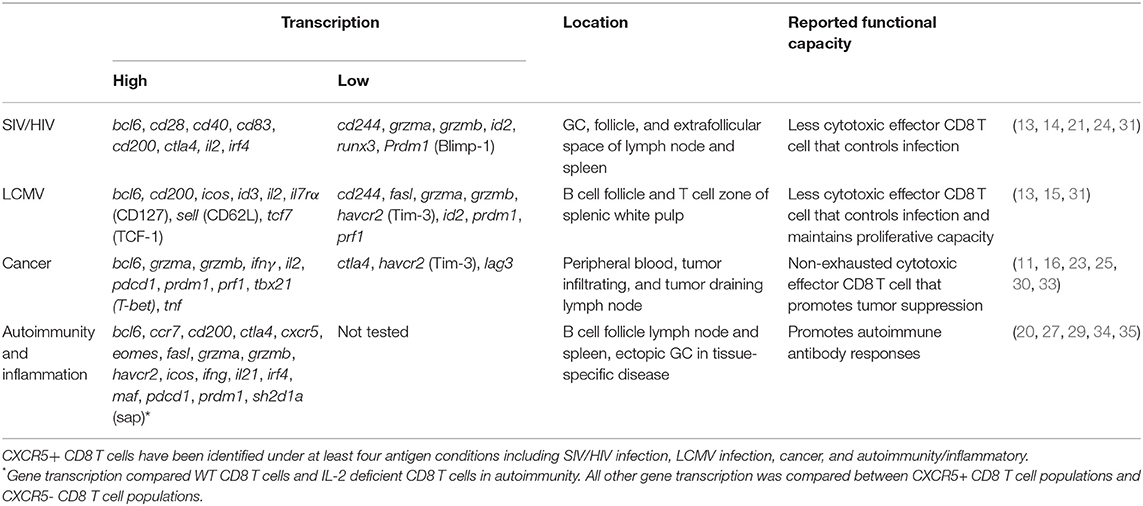
As CD8 T cells have been identified both phenotypically and functionally into distinct subsets beyond that of the classical cytotoxic CD8 T cells (CTL), it follows that novel CD8 T cell subsets may yet still emerge. A recent focus on CD8 T cells has highlighted a diversity of functional responses. Like CD4 T cells, CD8 T cells differentiate into multiple subsets that are customized to a specific infection and immune settings (1, 2) (Figure 1). CD8 Tc1 cells comprise the canonical CTL subset, producing IFNγ, perforin, and granzymes involved in targeted cell killing (3). CD8 Tc1 cells arise predominately in response to viral and intracellular infections but also in some autoimmune diseases to induce pathogenic tissue destruction. CD8 Tc2 cells are implicated in response to specific allergens and typically exhibit reduced CTL function and produce IL-4 and IL-5. CD8 T regulatory cells (Tregs) identified in the context of self-reactive responses are less well-defined and may have multiple phenotypes (4, 5). Some CD8 Tregs localize to the B cell zone but are also found in circulation (6–8). Beyond these effector subsets, at least three memory CD8 T cell types [T effector memory (Tem), tissue resident memory, and central memory] have been extensively described (9).
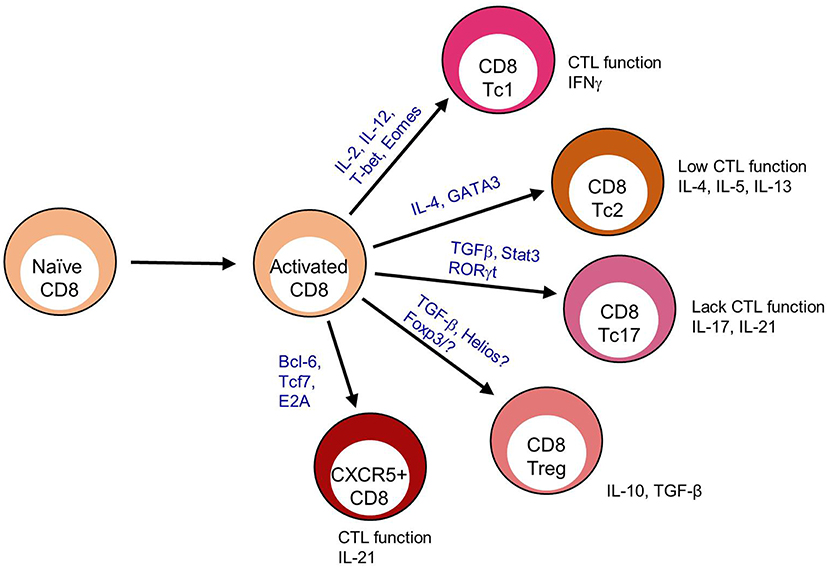
Figure 1. CD8 T cell differentiation subsets. After antigen recognition, activated CD8 T cells that receive specific TCR interactions, cytokine signaling, and dendritic cell signals upregulate transcription factors that program terminal differentiation outcomes including CD8 T cytotoxic (Tc) 1, Tc2. Tc17, Treg, and CXCR5+ subsets.
This review characterizes a novel subset of CXCR5+ CD8 T cells capable of infiltrating the B cell follicle in settings of chronic antigen exposure and inflammation. CXCR5+ CD8 T cells maintain an independent phenotype from their CXCR5- CD8 T cell counterparts. Their functional role largely depends on the immune setting, yet they maintain a cytotoxic capacity that aids in the control of viral infection, tumor growth inhibition, or the promotion of inflammation and autoimmune responses. Finally, CXCR5+ CD8 T cells have a unique developmental profile utilizing genes similar to both CD4 T follicular helper (Tfh) cell development and CD8 effector memory or memory-stem cell differentiation. Some of the gene variation found in CXCR5+ CD8 T cells seems to be dependent upon the conditions under which these cells arise.
A Novel T Cell Subset: CXCR5+ CD8 T Cells
CXCR5+ CD8 T cells develop under several conditions of chronic antigen and inflammation. They are transcriptionally and phenotypically distinct from other CD8 T cell subsets. Most studies find that CXCR5+ CD8 T cells gain entry into the B cell follicle. Yet, there is no clear consensus defining the function of this CXCR5+ CD8 T cell subset.
A Distinct Phenotype
Transcriptional and phenotypic profiling, in combination with a variety of functional responses, indicate several possible classifications for CXCR5+ CD8 T cells including: cytolytic, Tem/stem cell, exhausted and follicular helper CD8 T cells. The differences leading to these classifications likely depend on the particular immune setting and the subsequent functional responsiveness of CXCR5+ CD8 T cells.
During chronic viral infections CD8 T cells are frequently associated with an exhausted profile including reductions in IL-2 production, cytolytic function and proliferation. This shift toward exhaustion is associated with increased expression of the co-inhibitory molecules PD-1, 2B4, Tim3, KLRG1, CD160, and Lag3 among others (10). When CXCR5+ CD8 T cells wereevaluated for an exhausted phenotype, gene expression profiles reveal that CXCR5+ CD8 T cells have increased pdcd1 (PD-1) expression but reduced faslg, ctla4, lag3, havcr2 (Tim-3), and cd244 (2B4) in some studies (11–14). While in other studies, CXCR5+ CD8 T cells express elevated PD-1 and FasL with variable CTLA-4, Lag3 and Tim-3, but reduced 2B4 expression (12, 13, 15–17) (Figure 2A). Cytolytic functionality as measured by granzyme B, perforin, and CD107a, provides a mixed picture for CXCR5+ CD8 T cells as a non-exhausted population. CXCR5+ CD8 T cells express decreased grzma, grzmb, and prf1 gene expression when compared to CXCR5- CD8 T cells in viral infection (12). Yet, tumor-infiltrating and virus-specific CXCR5+ CD8 T cells appear to maintain cytolytic capacity upon ex vivo stimulation (13, 17, 25). However, considering the variability in exhaustion marker expression as well and the maintenance of cytolytic capacity (described in section II of this review), CXCR5+ CD8 T cells are likely not functionally exhausted. Specifically, CXCR5+ CD8 T cells express elevated KLRG1, CD44, T-bet, and Blimp-1 compared to CXCR5- and naïve CD8 T cells indicative of an activated, fully differentiated cytolytic subset (12, 13, 15) (Figure 2B).
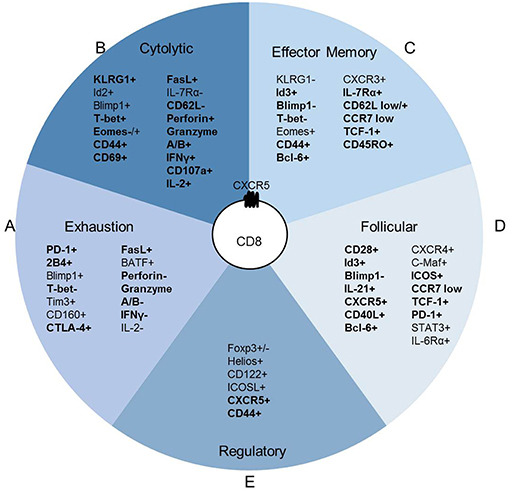
Figure 2. CXCR5+ CD8 T cells maintain a distinct expression pattern. CXCR5+ CD8 T cell protein expression relative to T cell subsets; (A) CD8 T cell exhaustion (10), (B) CD8 cytotoxic T cell, (C) CD8 T effector memory (Tem) (9), (D) CD4 T follicular helper (Tfh) (18), and (E) CD8 T regulatory cell (Treg) (4). Bold indicates literature confirmed protein expression in CXCR5+ CD8 T cells.
While CXCR5+ CD8 T cells appear to maintain a cytolytic phenotype, this phenotype does not account for the upregulation of cd127 (IL-7Rα), tcf7 (TCF-1), id3, eomes, and cd44 that are commonly associated with an effector memory phenotype (12, 13) (Figure 2C). Im et al. defined lymphocytic choriomeningitis virus (LCMV)-specific CXCR5+ CD8 T cells as stem-like CD8 Tem that proliferated into both CXCR5+ and CXCR5- CD8 T cell subsets (12). Similarly, CXCR5+ CD8 T cells isolated from PBMCs of cancer patients proliferate more than CXCR5- CD8 T cells after TCR stimulation (16, 25). Leong et al. defined CXCR5+ CD8 T cells in LCMV infection as an effector memory-like (CD62L+ IL-7R+) population by RNA sequencing (13). Perhaps, most convincingly, in simian immunodeficiency virus (SIV) infection CXCR5+ CD8 T cells in comparison to SIV-specific CXCR5- CD8 T cells, and CD8 T cells under autoimmune conditions compared to naïve CD8 T cells express significantly more bcl6 and less prmd1 (Blimp-1) (14, 29). The Tem phenotypic description attributed to CXCR5+ CD8 T cells is probably indicative of the chronic antigen exposure under which these cells have thus far shown to arise.
Alternatively, although not completely counter to evidence of an effector memory subset, CXCR5+ CD8 T cells share a transcriptional profile similar to that of CD4 Tfh cells in SIV infection by RNA sequencing of virus specific CXCR5+ CD8 T cells (14). CXCR5 is most commonly associated with B cell zone migration and homing, and has been described extensively on B cells and CD4 Tfh cells (32). CXCR5+ CD8 T cells express costimulatory, transcription factors, inhibitory genes, and proteins similar to CD4 Tfh, including: increased cd200, icos, cd28, bcl6, id3, ctla4, pdcd1, and faslg and reduced prdm1, id2, and havcr2 (Tim-3) (18) (Figure 2D). These data are supported by research in the inflammatory environment of human nasal polyps, in which a CXCR5+ CD8 T cell population arises and express FasL, CD28, OX-40, and ICOS post-ex vivo stimulation (20). In an autoimmune disease setting, CXCR5+ CD8 T cells express cytolytic molecules associated with canonical CD8 T cell function, but also express cxcr5, icos, bcl6, pdcd1, cd40l, and il21 (29). In Hogdkin's lymphoma, CXCR5+ ICOS+ CD8 T cells are more closely related to CD4 Tfh and not other T cell subsets based on gene expression profiles (33).
A CD8 Treg population that maintains germinal center (GC) reactions and controls autoimmune disease has been described within the B cell follicle (7). CD8 Tregs can express FoxP3, or associate with the transcription factor Helios (4, 6). When identified as CXCR5+, CD8 Tregs express ICOSL, CD44, and CD122 (5, 7) (Figure 2E). He et al. reported that in LCMV infection, CXCR5+ CD8 T cells were ICOSL and Helios negative but CD44+ (15). Similarly, in the context of autoimmune disease, CXCR5+ CD8 T cells largely lack ICOSL, FoxP3, and Helios expression (unpublished data). It is possible that CXCR5+ CD8 T cells, in some situations, are CD8 Treg cells (8); but most reports suggest an effector phenotype for these cells. Together, transcriptional profiling and subsequent validation by flow cytometric analysis, identify a CXCR5+ CD8 T cell population with the potential to behave as cytotoxic canonical CD8 T cells, promote B cell responses and respond as CD8 Tem (Table 1).
CXCR5+ CD8 T cells have been described in both humans and mice and may account for the variation in cytolytic capacity, homing, and function attributed to CXCR5+ CD8 T cells in multiple immunologic settings. Here we endeavor to summarize relevant data from humans and mice from multiple disease settings. As with CD4 Tfh cells, early descriptions of CXCR5+ CD8 T cells have stemmed from human samples (20, 32, 36) and augmented by transcription factor knockout and reporter mice (13, 15, 37). While multiple studies have characterized CXCR5+ CD8 T cells in humans and mice, (13, 15) CXCR5+ CD8 T cell development in chronic, but not acute settings promotes investigations primarily in human immunodeficiency virus (HIV) and SIV. Continued characterization of CXCR5+ CD8 T cells will require mechanistic studies better facilitated in mice. Describing CXCR5+ CD8 T cell transcriptional pathways using reporter and knockout mice (13, 15), evaluating CXCR5+ CD8 T cell population kinetics during disease across multiple organs (29), and evaluating the independent role of CXCR5+ CD8 T cells in controlling disease via animal transfers (12) are benefits of studies in mice. These findings once resolved with cross-species variation, will provide rationale designs for CXCR5+ CD8 T cells as therapeutic targets for human disease.
Antigen-Specific CXCR5+ CD8 T Cell Responses and Localization
As the principal chemokine receptor that facilitates entry into the B cell zone, CXCR5 expression on CD8 T cells instigated investigation into CXCR5+ CD8 T cell homing. CD8 T cells, by CCR7 upregulation and not CXCR5 expression are excluded from the B cell follicle. However, of the total CD8 T cell population only the small frequency that upregulates CXCR5 during SIV infection localize in and around the follicle (21). In addition to observations in SIV infection, CXCR5 expression on human CD8 T cells in HIV is closely associated with proximity and responsiveness to CXCL13 in the lymph node (17, 20). CXCR5 expression on CD4 Tfh is required to migrate toward CXCL13 and facilitate GC development (38). Some CD4 Tfh developmental signals do not require B cell help initially to induce the CD4 Tfh transcription factor, Bcl6 (39, 40). However, once at the B-T border, CD4 Tfh cells interact with B cells to gain entry into the GC and solidify their transcriptional profile via Bcl6 using ICOS and PD-1 interactions (37, 41, 42). In murine LCMV, CXCR5+ CD8 T cells may also require B cell interactions to enter the follicle as CXCR5+ CD8 T cells that maintained ccr7 gene expression retain their capacity to localize to the T cell zone and are excluded from the GC (12). A requirement for B-T cell interaction has yet to be directly investigated in CXCR5+ CD8 T cell development and function.
CXCR5+ CD8 T cell accumulation in the follicle does not appear to be dictated by antigen concentration or immune setting but rather by conditions of chronic inflammation and immune activation (17, 20, 29). In chronic infections, both high and low viral load correlate with expanded CXCR5+ CD8 T cell populations throughout the follicle, including the GC (21, 24, 43). Chronic HIV, SIV and LCMV infection studies identified antigen-specific CXCR5+ CD8 T cells (12–15, 31). Peripheral blood isolated HIV-specific CD8 T cells are more cytolytic than lymphoid CD8 T cell populations. Additionally, within the lymphoid CD8 T cell populations, CXCR5+ CD8 T cells maintain a robust cytolytic phenotype compared to CXCR5- CD8 T cells (19). In models of chronic viral infections, LCMV-specific CXCR5+ CD8 T cells identified within the extrafollicular space and germinal center also display a cytolytic phenotype compared to CXCR5- CD8 T cells (12, 15, 22, 31). CXCR5- CD8 T cells are likely exhausted (12, 13, 15, 19) which may account for the described increases in CXCR5+ CD8 T cell cytotoxicity that is higher than CXCR5- CD8 T cells but lower than peripheral blood CD8 T cell populations, at least in humans. Thus, irrespective of viral infection and host species, CXCR5+ CD8 T cells maintain cytolytic capacity in both the blood and lymph node in humans and mice (Figure 3A). The localization or direct cell killing capacity of CXCR5+ CD8 T cells requires continued investigation.
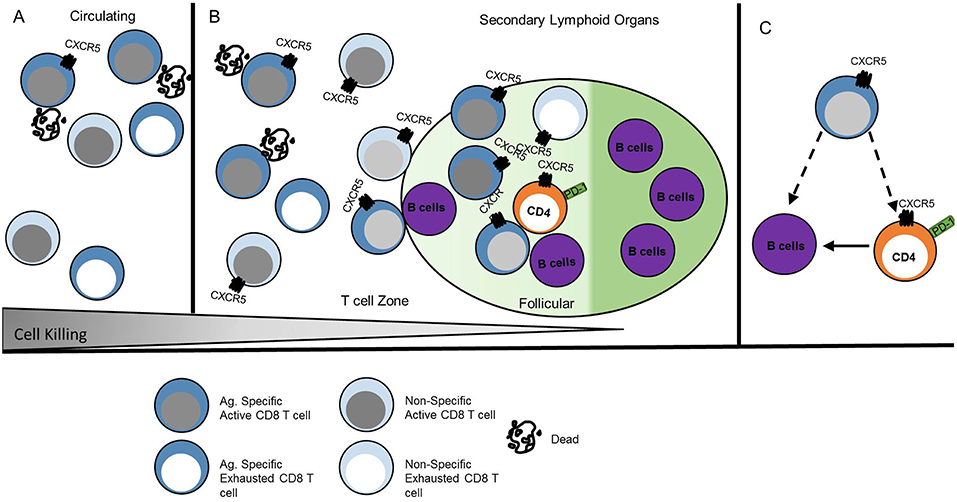
Figure 3. CXCR5+ CD8 T cell function is anatomically instructed. (A) Antigen-specific CXCR5+ CD8 T cells in peripheral blood circulation induce direct cell killing, whereas other CD8 T cells may be functionally exhausted. (B) CXCR5+ CD8 T cells within secondary lymphoid organs that localize to the extrafollicular space maintain higher direct cell killing capacity than CXCR5+ CD8 T cells within the follicle. Antigen-specific CXCR5+ CD8 T cells are maintained at similar frequency in the extrafollicular space and follicle. (C) Follicular CXCR5+ CD8 T cells maintain the capacity for cytotoxicity; different antigen scenarios likely elicit various functional outcomes that promote direct B cell or CD4 Tfh cell interactions. Dark blue cells indicate antigen specific T cells. Dashed lines indicate potential interactions.
Interaction time and specific signals during GC interactions may redirect the transcription of CXCR5+ CD8 T cells and alter effector functions as described for CD4 Tfh cells (44). In HIV infection, some CXCR5+ CD8 T cells demonstrate high lytic potential in GC regardless of antigen specificity (17), while other antigen-specific CXCR5+ CD8 T cells are less lytic in patients without strong immune responses (22). The frequency of CXCR5+ CD8 T cells isolated from pancreatic and colorectal cancer tumor masses correlate with improved patient outcomes suggestive of tumor control (11, 30). In humans at least, viral-specific or tumor infiltrating CXCR5+ CD8 T cells likely utilize cytolytic mechanisms to control viral infection and tumor growth in secondary lymphoid organs, ectopic GC, and the tumor microenvironment. The presence of antigen-specific CD8 T cells in the extrafollicular space and within the follicle suggests that CXCR5+ CD8 T cells directly interact with virally infected CD4 Tfh cells. The potential for cytolytic responses may explain the negative correlation observed between the frequency of CXCR5+ CD8 T cells and reduced CD4 Tfh cell frequency and viral load (13, 14, 17, 19, 24) (Figure 3B).
In the context of chronic inflammation and autoimmune disease, CXCR5+ CD8 T cells likely employ diverse mechanisms to promote inflammatory responses and advance disease pathogenesis at the site of autoreactive responses within ectopic GCs or lymphoid tissue. Influenza-specific murine CD8 T cells migrate to lung ectopic GCs and interact with B cells following intranasal infection (16, 45). CD40L+ CD8 T cells within human synovial fluid, that are likely antigen-specific for joint proteins, are required for the formation and maintenance of ectopic GCs in rheumatoid arthritis inflammation (27, 34). Murine autoimmune CXCR5+PD-1hi CD8 T cells expressing CD40L and GL-7 promote antibody responses (29). CXCR5+ CD8 T cells in human nasal polyps that localize to B cells promote inflammatory damage (35). Together, these data reveal a pattern of CXCR5+ CD8 T cell homing related to antigen accumulation and the site of local immunological responses (Figure 3C).
CXCR5+ CD8 T Cell Function
Viral Infection
CXCR5+ CD8 T cells have been predominately explored in the context of chronic viral infections. In chronic LCMV and SIV infection, CXCR5+ CD8 T cell frequency inversely correlates with viral load and associates with a reduction in virus-producing cells attributing cytolytic function to CXCR5+ CD8 T cells (13, 15, 21, 24, 31). However, in similar studies of chronic SIV and LCMV infection, CXCR5+ CD8 T cells display reduced cytolytic protein expression coupled with a more stem-like effector memory phenotype (12).
There are a number of possible explanations for differences in cytolytic activity across the existing viral CXCR5+ CD8 T cell literature (46) including the comparison across species of CXCR5+ CD8 T cell populations, population heterogeneity, differentiation states and subsequent cellular interactions potentially dependent on the disease model. Immerging evidence indicates an effector memory-like CD8 T cell population, that develops in situations of chronic antigen and cell exhaustion, with the capacity for cytolytic function. Inhibitory receptors such as PD-1, frequently used to describe exhausted CD8 T cells, may also denote a follicular-helper like subset of CD8 T cells that maintains its cytotoxic effector function and elicits GC entry (17, 22). Adoptively transferred LCMV-specific CXCR5+ CD8 T cells rapidly expanded to reseed the exhausted CXCR5- CD8 T cell niche (12) and significantly reduced viral load following PD-1 blockade (15). Yet, during short PD-1 blockade treatments in HIV infection, a PD-1+ subset of CXCR5+ CD8 T cells, instead produced less TNFα and IFNγ cytokines (22). Within the CXCR5+ population, there likely exists multiple effector functions similar to differences observed in CD4 Tfh cell function as it relates to PD-1 expression, follicular localization, and terminal differentiation.
In addition to a cytolytic role in controlling infection, direct interactions with infected B cells and CD4 Tfh cells may also facilitate CD8 T helper-like functions in the follicle. Human CXCR5+ CD8 T cells from chronic hepatitis B viral (HBV) infection produce IFNγ and influence IgG and IgA production when co-cultured with naïve B cells or memory B cells (28). CD8 T cells infiltrate influenza infected lungs and promote IL-21 dependent antibody class-switching and prolonged B cell survival (45). This follicular helper type function may also act to promote a tissue specific antiviral response on CD4 Tfh cells differing from the cytolytic response facilitated by chronic viral infection reservoirs in secondary lymphoid organs.
Cancer
Cancer represents a situation of chronic, low-level self-antigen much like the situation induced by chronic viral infection. In B cell lymphoma-bearing mice and diffuse large B cell lymphoma patients, CXCR5+ CD8 T cells likely arise to directly target cancer cells (13, 23). Whereas, in HBV-related hepatocellular carcinoma, viral responses may initially induce CXCR5+ CD8 T cells that then target cancer cells (16). In colorectal and pancreatic cancer, CXCR5+ CD8 T cells arise and respond to cancer cells (11, 25, 30) suggesting a prevalent role for chronic antigen exposure in the development of tumor-specific CXCR5+ CD8 T cells.
CXCR5+ CD8 T cells isolated during immune responses to cancer maintain cytolytic potential toward tumor cells despite protein expression typically indicative of exhaustion. Circulating CXCR5+ CD8 T cells isolated from patients with HBV-related hepatocellular carcinoma and diffuse large B cell lymphoma expressed granzyme B and CD107a that likely contributed to tumor cell and B cell lysis (16). Circulating, tumor infiltrating, and lymphoid CXCR5+ CD8 T cells also express PD-1 and Tim-3 but are functionally less exhausted than CXCR5- CD8 T cells (11, 16). Yet, combined blockade of Tim-3 and PD-1 augment CXCR5+ CD8 T cell specific lysis of tumor cell targets indicating reduced lytic potential (16, 30). Further, CXCR5+ CD8 T cells in colorectal cancer maintain a cytolytic capacity to directly lyse tumor cells but can also influence B cell secretion of IgG, suggesting multiple mechanisms for tumor control by these cells (25).
In spite of the fairly robust cytolytic potential and activity by CXCR5+ CD8 T cells, tumor cells likely employ inhibitory mechanisms to suppress CXCR5+ CD8 T cell function. In vitro neutralization of IL-10 or IL-10R pathway improved granzyme A, granzyme B, and perforin-mediated cytotoxicity by CXCR5+ CD8 T cells (23). IL-10 or PD-1L blockade induced CXCR5+ CD8 T cell targeted specific cell lysis of autologous tumor cells (16). Enhancing specific cell lysis by preventing tumor suppression of CXCR5+ CD8 T cells or by improved CXCR5+ CD8 T cell function provides a new potential target for existing cancer therapeutics. As pancreatic and colorectal cancer disease-free survival time is positively correlated with CXCR5+ CD8 T cell frequency (30), the maintenance of a CXCR5+ CD8 T cell population may prolong cancer treatment efficacy.
Inflammation and Autoimmune Disease
The mechanisms by which CD8 T cells mediate autoimmune disease pathology remain largely unresolved, but inflammation and autoimmune disease studies suggest a helper function for CXCR5+ CD8 T cells. In the absence of CD8 T cells, GC formation is prevented in rheumatoid arthritis and disease is delayed in spontaneous auto-antibody mediated disease (27, 29). Differential synovial ectopic GC formation is associated with CD8 T cell recruitment in a CD40L dependent manner (34), and follicular dendritic cells could not be retained in synovial GCs grafted into NOD-SCID mice in the absence of CD8 T cells (27).
While CD40L CD8 T cells appear to have a role in mediating ectopic GC formation, they do not produce the cytolytic proteins perforin and granzyme A, but maintain expression of IFNγ and TNFα (27). CXCR5+ CD8 T cells identified in human tonsils express IFNγ, TNFα, granzyme A, and IL-2 (20). Human tonsil CD8 T cells co-cultured with B cells promoted B cell survival like that of CXCR5+ CD4 T cells and induced IgG class-switching (20). IL-21-producing CD8 T cells from human nasal polyps co-express IFNγ and IL-21 to induce B cell class-switch to IgG when co-cultured with B cells (35). IFNγ is a known mediator of B class-switch to IgG2a/c, yet CXCR5+ CD8 T cells that arise in spontaneous autoimmune disease induced B cell class-switch to predominately IgG1. When transferred into TCRα deficient mice, IL-2-deficient CD8 T cells alone did not induce B cell differentiation or class-switching. Instead, CD8 T cells together with CD4 T cells enhanced plasma cell differentiation and induced IgG1 and IgG2b (29).
The primary location of CD8 T: B cell interactions within the GC, mantle zone, or extrafollicular foci, and, whether the mechanisms promoting antibody class-switch are via direct contact or secreted cytokines are predominately unexplored. Although yet untested, CXCR5+ CD8 T cell function in autoimmune disease likely includes canonical cytotoxic mechanisms in addition to acquired Tfh mechanisms. In contrast to most chronic viral infections and cancer, autoimmune and inflammatory CXCR5+ CD8 T cells likely promote the disease state, although the mechanisms that alter or advance GC reactions, in addition to direct cell lysis, may be similar.
Distinct Developmental Pathways for CXCR5+ CD8 T Cells
The similarity of CXCR5+ CD8 T cell phenotype and function to other CD8 T cell subsets described in Figure 2 likely provide overlapping, if not identical models, for differentiation and function of these cells described in other excellent reviews (9, 10, 47). Here we propose a transcriptional network that explains the gene expression and function observed in CXCR5+ CD8 T cells (Figure 4).
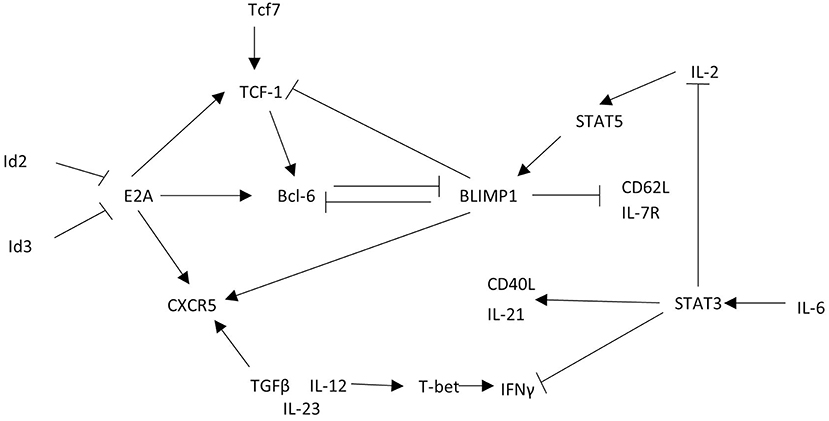
Figure 4. CXCR5+ CD8 T cells acquire a unique transcriptional profile from other CD8 T cells. CXCR5+ CD8 T cells express the transcription factor Bcl6 that is maintained by TCF-1 and E2A. This transcriptional interaction allows for CXCR5 upregulation and expression of functional proteins. Arrows indicate promoting interactions. Blunted lines indicate inhibitory interactions.
To explore CXCR5+ CD8 T cell regulation, the cxcr5 promoter has been evaluated by chromatin immunoprecipitation deep sequencing. In response to a chronic viral infection the cxcr5 promoter in CXCR5+ CD8 T cell contains two Blimp-1 binding sites and one E2A binding site, in addition to binding sites at the bcl6 and tcf7 promoter similar to that of CD8 effector memory T cells and CD4 Tfh cells (13, 15, 48). Retroviral Bcl6 induction of LCMV-specific donor cells increased CXCR5+ CD8 T cell frequency with a corresponding TCF-1 upregulation and Blimp-1 repression (13), suggesting a regulatory connection between TCF-1 and Bcl6 antagonism of Blimp-1 expression (39, 49). Further, CXCR5+ CD8 T cells do not arise in the absence of tcf7 (TCF-1) during LCMV infection (12, 13). Experiments to test the significance of Blimp-1 regulation in CXCR5+ CD8 T cells preferentially expand from cells deficient in Blimp-1 using mixed chimeras of Blimp-1 deficient and WT bone marrow indicating that, like CD4 Tfh cells, CXCR5 expression is, in part, suppressed by Blimp-1 mediated transcription (13).
Id2 and Id3 regulate E2A and other e-family proteins responsible for regulating gene transcription in CD4 T cells (48, 50). E2A overexpression enhances CXCR5 expression increasing cytotoxic responses via CD107a expression and PD-1 downregulation, producing a less exhausted phenotype (15). In CXCR5+ CD8 T cells Id2 is downregulated and Id3 is upregulated relative to CXCR5- CD8 T cells (12, 13, 15). T cell specific deletion of Id2 results in a dramatic expansion of CXCR5+ CD8 T cells (13, 15, 51). Because Id2 is significantly downregulated in CXCR5+ CD8 T cells, the expression of Id2 may block the development of CXCR5+ CD8 T cells during early CD8 T cell activation. Id3 upregulation in CXCR5+ CD8 T cells may restrain CXCR5+ CD8 T cell development, perhaps after initial subset differentiation. Thus, Id2 contains, and Id3 maintains, E2A induction of Bcl6, TCF-1, and CXCR5 to stabilize the CXCR5+ CD8 T cell phenotype (Figure 4).
Early immunological signals that prompt development of a CXCR5+ CD8 T cell population remain in question. Some evidence for specific cytokine and cellular interactions exists but is largely circumstantial via in vitro culture assays. In vitro cultures of SIV+ CD8 T cells with IL-12, IL-23, and TGFβ promote CXCR5+ CD8 T cell expansion relative to IL-12 or IL-23 alone (14). When cultured with IL-6, CD8 T cells produce IL-21 similar to CD4 T cells cultured with IL-6 (45). However, in CD8 T cells IL-6 induction of IL-21 via STAT3, inhibits IFNγ, and IL-2 production (45), unlike the robust IFNγ responses in LCMV-specific CXCR5+ CD8 T cells. Although, thus far, CXCR5+ CD8 T cells respond to similar stimuli as CD4 Tfh cells (52–54), these stimuli may induce a context specific response in CXCR5+ CD8 T cells that has yet to be carefully resolved.
In the context of chronic antigen, CD4 Tregs control inappropriate self-reactive responses (55). Within the GC, follicular Tregs maintain T-B interactions to promote B cell differentiation (56). Foxp3+ Tregs localize in close proximity to follicular and extrafollicular CXCR5+ CD8 T cells but with higher frequency to extrafollicular CXCR5+ CD8 T cells (31). During low SIV viremia CXCR5+ CD8 T cell frequency negatively correlated with viral load and positively correlated with follicular Tregs. Whereas, in high SIV viremia, the frequency of CXCR5+ CD8 T cells negatively correlated with follicular Tregs. Together, this suggests that Treg control of CXCR5+ CD8 T cells inhibits function rather than development within the GC, and the efficacy of that inhibition likely relates to viral control (24).
CD8 T cells can be found within the GC of several murine models of spontaneous autoimmune disease including in IL-2-deficient and scurfy mutant autoimmune disease. In these mice, both CD4 Tfh and CXCR5+ PD-1+ CD8 T cells are significantly expanded (29). One common feature of these autoimmune models is a defect in functional Tregs. In the absence of functional Tregs or in conditions of high chronic antigen and inflammation, CXCR5+ CD8 T cells have the capacity to expand and maintain robust effector function by cytokine secretion or direct B cell interactions.
Conclusions
CXCR5+ CD8 T cells have been found under a number of pathogenic conditions with varied functional capacity. CXCR5+ CD8 T cells promote cell lysis in viral infection and in some cancers, while in inflammation and autoimmunity CXCR5+ CD8 T cells function as helper cells, thus promoting disease pathogenesis. The presence of CD8 T cells within the B cell zone, in combination with their cytolytic and helper functionality, provides the potential for unique interactions with CD4 Tfh cells, B cells and follicular dendritic cells and access to infected CD4 T cells and cancerous B cells that have yet to be fully explored.
Treatments to influence effector responses require a clear analysis of CXCR5+ CD8 T cell function in multiple immune settings that facilitate specific cell interactions. Engineering CD8 T cells to express CXCR5 promotes migration to the B cell follicle (57). While the use of bispecific antibodies optimizes CXCR5+ CD8 T cell targeting of HIV-infected cells via cell specific lysis (17). A combination therapy to optimize CXCR5+ CD8 T cell responses in a patient specific manner will address challenges currently identified in immune non-responding patients to existing HIV treatments. CXCR5+ CD8 T cell activities within and near the follicle provide clues about the immune response that may explain class-switch choices, the development of broadly neutralizing antibodies, and promote a paradigm shift in the nuances of GC reactions.
Author Contributions
KMV conceptualization, literature evaluation, original draft writing, generated and visualized figures. KKH conceptualization, writing and review, visualization, funding acquisition, and supervision.
Funding
This work was supported by the National Institutes of Health Grant R15HL146779-01 to KKH, the University of California (UC) Presidential Dissertation Year Fellowship to KMV, and the University of California.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank UC Merced Graduate Division and UC Merced's Dissertation Bootcamp for dedicated writing time and support.
References
1. Gravano DM, Hoyer KK. Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun. (2013) 45:68–79. doi: 10.1016/j.jaut.2013.06.004
2. Mittrucker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch Immunol Ther Exp. (2014) 62:449–58. doi: 10.1007/s00005-014-0293-y
3. Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. (2008) 26:389–420. doi: 10.1146/annurev.immunol.26.021607.090404
4. Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. (2015) 350:334–9. doi: 10.1126/science.aad0616
5. Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA. (2011) 108:2010–5. doi: 10.1073/pnas.1018974108
6. Churlaud G, Pitoiset F, Jebbawi F, Lorenzon R, Bellier B, Rosenzwajg M, et al. Human and mouse CD8(+)CD25(+)FOXP3(+) regulatory T cells at steady state and during interleukin-2 therapy. Front Immunol. (2015) 6:171. doi: 10.3389/fimmu.2015.00171
7. Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. (2010) 467:328–32. doi: 10.1038/nature09370
8. Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, et al. Follicular regulatory CD8 T cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog. (2016) 12:e1005924. doi: 10.1371/journal.ppat.1005924
9. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. (2012) 12:749–61. doi: 10.1038/nri3307
11. E J, Yan F, Kang Z, Zhu L, Xing J, Yu E. CD8(+)CXCR5(+) T cells in tumor-draining lymph nodes are highly activated and predict better prognosis in colorectal cancer. Hum Immunol. (2018) 79:446–52. doi: 10.1016/j.humimm.2018.03.003
12. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. (2016) 537:417–21. doi: 10.1038/nature19330
13. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. (2016) 17:1187–96. doi: 10.1038/ni.3543
14. Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci USA. (2017) 114:1976–81. doi: 10.1073/pnas.1621418114
15. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. (2016) 537:412–28. doi: 10.1038/nature19317
16. Jin Y, Lang C, Tang J, Geng J, Song HK, Sun Z, et al. CXCR5(+)CD8(+) T cells could induce the death of tumor cells in HBV-related hepatocellular carcinoma. Int Immunopharmacol. (2017) 53:42–8. doi: 10.1016/j.intimp.2017.10.009
17. Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med. (2017) 9:eaag2285. doi: 10.1126/scitranslmed.aag2285
18. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. (2011) 29:621–63. doi: 10.1146/annurev-immunol-031210-101400
19. Reuter MA, Del Rio Estrada PM, Buggert M, Petrovas C, Ferrando-Martinez S, Nguyen S, et al. HIV-Specific CD8(+) T cells exhibit reduced and differentially regulated cytolytic activity in lymphoid tissue. Cell Rep. (2017) 21:3458–70. doi: 10.1016/j.celrep.2017.11.075
20. Quigley MF, Gonzalez VD, Granath A, Andersson J, Sandberg JK. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol. (2007) 37:3352–62. doi: 10.1002/eji.200636746
21. Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol. (2014) 193:5613–25. doi: 10.4049/jimmunol.1401161
22. Jiao YM, Yang HG, Huang HH, Tu B, Xing SJ, Mao L, et al. Dichotomous roles of programmed cell death 1 on HIV-specific CXCR5(+) and CXCR5(-) CD8(+) T cells during chronic HIV infection. Front Immunol. (2017) 8:1786. doi: 10.3389/fimmu.2017.01786
23. Tang J, Zha J, Guo X, Shi P, Xu B. CXCR5(+)CD8(+) T cells present elevated capacity in mediating cytotoxicity toward autologous tumor cells through interleukin 10 in diffuse large B-cell lymphoma. Int Immunopharmacol. (2017) 50:146–51. doi: 10.1016/j.intimp.2017.06.020
24. Rahman MA, McKinnon KM, Karpova TS, Ball DA, Venzon DJ, Fan W, et al. Associations of simian immunodeficiency virus (SIV)-specific follicular CD8(+) T cells with other follicular T cells suggest complex contributions to SIV viremia control. J Immunol. (2018) 200:2714–26. doi: 10.4049/jimmunol.1701403
25. Xing J, Zhang C, Yang X, Wang S, Wang Z, Li X, et al. CXCR5(+)CD8(+) T cells infiltrate the colorectal tumors and nearby lymph nodes, and are associated with enhanced IgG response in B cells. Exp Cell Res. (2017) 356:57–63. doi: 10.1016/j.yexcr.2017.04.014
26. Jiang J, Champion CI, Wei B, Liu G, Kelly KA. CD8(+)CXCR5(+) T cells regulate pathology in the genital tract. Infect Dis Obstet Gynecol. (2013) 2013:813238. doi: 10.1155/2013/813238
27. Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, et al. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. (2002) 195:1325–36. doi: 10.1084/jem.20011565
28. Jiang H, Li L, Han J, Sun Z, Rong Y, Jin Y. CXCR5(+) CD8(+) T cells indirectly offer B cell help and are inversely correlated with viral load in chronic hepatitis B infection. DNA Cell Biol. (2017) 36:321–7. doi: 10.1089/dna.2016.3571
29. Valentine KM, Davini D, Lawrence TJ, Mullins GN, Manansala M, Al-Kuhlani M, et al. CD8 follicular T cells promote B cell antibody class switch in autoimmune disease. J Immunol. (2018) 201:31–40. doi: 10.4049/jimmunol.1701079
30. Bai M, Zheng Y, Liu H, Su B, Zhan Y, He H. CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Exp Cell Res. (2017) 361:39–45. doi: 10.1016/j.yexcr.2017.09.039
31. Li S, Folkvord JM, Rakasz EG, Abdelaal HM, Wagstaff RK, Kovacs KJ, et al. SIV-producing cells in follicles are partially suppressed by CD8+ cells in vivo. J Virol. (2016) 90:11168–80. doi: 10.1128/JVI.01332-16
32. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. (2000) 192:1545–52. doi: 10.1084/jem.192.11.1545
33. Le KS, Ame-Thomas P, Tarte K, Gondois-Rey F, Granjeaud S, Orlanducci F, et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Adv. (2018) 2:1889–900. doi: 10.1182/bloodadvances.2018017244
34. Wagner UG, Kurtin PJ, Wahner A, Brackertz M, Berry DJ, Goronzy JJ, et al. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. (1998) 161:6390–7.
35. Xiao L, Jia L, Bai L, He L, Yang B, Wu C, et al. Phenotypic and functional characteristics of IL-21-expressing CD8(+) T cells in human nasal polyps. Sci Rep. (2016) 6:30362. doi: 10.1038/srep30362
36. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. (2000) 192:1553–62. doi: 10.1084/jem.192.11.1553
37. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. (2009) 325:1001–5. doi: 10.1126/science.1176676
38. Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. (2005) 106:1924–31. doi: 10.1182/blood-2004-11-4494
39. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. (2011) 34:932–46. doi: 10.1016/j.immuni.2011.03.023
40. Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. (2010) 185:313–26. doi: 10.4049/jimmunol.0904023
41. Shi J, Hou S, Fang Q, Liu X, Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. (2018) 49:264–74.e264. doi: 10.1016/j.immuni.2018.06.012
42. Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol. (2015) 295:46–51. doi: 10.1016/j.cellimm.2015.01.014
43. Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ. Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci USA. (2016) 113:6248–53. doi: 10.1073/pnas.1604765113
44. Liu X, Yan X, Zhong B, Nurieva RI, Wang A, Wang X, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med. (2012) 209:s1841–24. doi: 10.1084/jem.20120219
45. Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casas N, Silberger DJ, et al. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells. J Exp Med. (2016) 213:2281–91. doi: 10.1084/jem.20160417
46. Perdomo-Celis F, Taborda NA, Rugeles MT. Follicular CD8(+) T Cells: origin, function and importance during HIV infection. Front Immunol. (2017) 8:1241. doi: 10.3389/fimmu.2017.01241
47. Yu D, Ye L. A Portrait of CXCR5(+) follicular cytotoxic CD8(+) T cells. Trends Immunol. (2018) 39:965–79. doi: 10.1016/j.it.2018.10.002
48. Shaw LA, Bélanger S, Omilusik KD, Cho S, Scott-Browne JP, Nance JP, et al. Id2 reinforces TH1 differentiation and inhibits E2A to repress TFH differentiation. Nat Immunol. (2016) 17:834–43. doi: 10.1038/ni.3461
49. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. (2009) 325:1006–10. doi: 10.1126/science.1175870
50. Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, Murre C. The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat Immunol. (2011) 12:992–1001. doi: 10.1038/ni.2086
51. Correction for, Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci USA. (2017) 114:E3366. doi: 10.1073/pnas.1703867114
52. Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. (2011) 6:e17739. doi: 10.1371/journal.pone.0017739
53. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. (2008) 29:138–49. doi: 10.1016/j.immuni.2008.05.009
54. Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. (2014) 15:856–65. doi: 10.1038/ni.2947
55. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. (2011) 17:983–8. doi: 10.1038/nm.2426
56. Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. (2016) 271:246–59. doi: 10.1111/imr.12411
Keywords: B cell follicle, germinal center, chronic viral infection, cancer, autoimmune disease, CD8 T cells
Citation: Valentine KM and Hoyer KK (2019) CXCR5+ CD8 T Cells: Protective or Pathogenic? Front. Immunol. 10:1322. doi: 10.3389/fimmu.2019.01322
Received: 15 March 2019; Accepted: 24 May 2019;
Published: 18 June 2019.
Edited by:
Peter Katsikis, Erasmus University Rotterdam, NetherlandsReviewed by:
Constantinos Petrovas, Vaccine Research Center (NIAID), United StatesPamela J. Skinner, University of Minnesota Twin Cities, United States
Copyright © 2019 Valentine and Hoyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katrina K. Hoyer, a2hveWVyMkB1Y21lcmNlZC5lZHU=; orcid.org/0000-0001-5443-0733
 Kristen M. Valentine
Kristen M. Valentine Katrina K. Hoyer
Katrina K. Hoyer