- 1Division of Experimental Medicine, Department of Medicine, University of San Francisco, San Francisco, CA, United States
- 2Division of Cardiology, Department of Medicine, University of San Francisco, San Francisco, CA, United States
- 3Radiopharmaceutical Research Program, Center for Molecular and Functional Imaging, University of San Francisco, San Francisco, CA, United States
A major obstacle to HIV eradication is the presence of infected cells that persist despite suppressive antiretroviral therapy (ART). HIV largely resides outside of the peripheral circulation, and thus, numerous anatomical and lymphoid compartments that have the capacity to harbor HIV are inaccessible to routine sampling. As a result, there is a limited understanding of the tissue burden of HIV infection or anatomical distribution of HIV transcriptional and translational activity. Novel, non-invasive, in vivo methods are urgently needed to address this fundamental gap in knowledge. In this review, we discuss past and current nuclear imaging approaches that have been applied to HIV infection with an emphasis on current strategies to implement positron emission tomography (PET)-based imaging to directly visualize and characterize whole-body HIV burden. These imaging approaches have various limitations, such as the potential for limited PET sensitivity and specificity in the setting of ART suppression or low viral burden. However, recent advances in high-sensitivity, total-body PET imaging platforms and development of new radiotracer technologies that may enhance anatomical penetration of target-specific tracer molecules are discussed. Potential strategies to image non-viral markers of HIV tissue burden or focal immune perturbation are also addressed. Overall, emerging nuclear imaging techniques and platforms may play an important role in the development of novel therapeutic and HIV reservoir eradication strategies.
Introduction
Despite the overwhelming success of antiretroviral therapy (ART) to achieve complete or near-complete HIV suppression, residual virus that integrates into host cell genomes prior to ART initiation persists indefinitely. Blood-derived resting CD4+ T cells comprise one of the most characterized reservoirs of latent HIV, and integrated viral DNA can exist at frequencies below one copy per million resting CD4+ T cells (1–6). However, HIV largely resides in organized lymphoid or other tissues outside of the peripheral circulation, and many anatomical regions are inaccessible to routine sampling (7–16). Only a small amount of tissue from a small number of sites can be realistically obtained from living human participants, and one of the major barriers to the successful design and implementation of HIV eradication or immune-based therapeutic strategies is the limited ability to characterize the tissue-wide burden of HIV in the setting of ART.
HIV-1 infection leads to immune activation and inflammation throughout all stages of disease. Markers of T-cell activation remain elevated in blood and lymphoid tissues in HIV-infected individuals, even in the setting of elite control or after years of suppressive ART. Certain immune privileged environments may be especially important foci of HIV persistence and viral transcriptional activity. For example, CD4+ T-follicular cells (TFH) within lymph node B cell follicles have been shown to be highly enriched in HIV-1 DNA, are very permissible to HIV infection, and are able to produce high levels of replication competent virus upon ex vivo stimulation (12, 17–19). TFH cells may be protected from various host immune responses by their location in the unique histological makeup (12, 17–19). Even outside of infected tissues, persistent HIV has lasting and often profound effects on tissues such as vascular endothelium, gut, and brain, and leads to sustained, systemic inflammatory responses. Markers of inflammation, coagulation, and immune activation remain elevated in effectively treated HIV infection and are strong predictors of mortality and non-AIDS events, which has been demonstrated in a variety of cohorts (20–23). As a result, there are direct and indirect consequences of HIV infection that are clinically relevant, even in the setting of treated and suppressed HIV. For example, HIV has been associated with increased cardiovascular disease, neurological disorders, and various hematological and solid-tumor malignancies (24).
The direct and indirect impact of persistent HIV on immune activation, systemic inflammation, and increased clinical comorbidities has led to interest in positron emission tomography (PET) and other molecular imaging techniques as tools to better understand the whole-body burden and consequences of HIV infection. Molecular imaging has been critical for the diagnosis, treatment, and management of various malignancies and other diseases. Similar modalities have the potential to provide insights into the design, implementation, and analysis of immunotherapies and other interventions to reduce HIV reservoir burden, lower inflammation, and thus reduce HIV-related morbidity.
Nuclear Imaging Approaches to HIV Persistence and HIV-Related Morbidity
The Molecular Imaging Toolbox
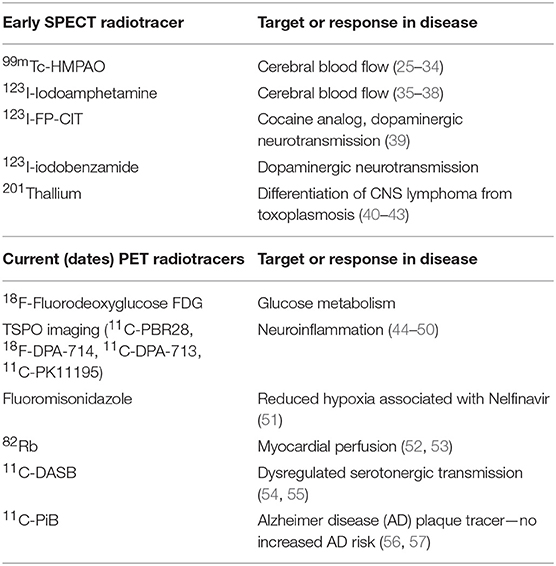
Innovative strategies to perform molecular imaging, from microscopic visualization and characterization techniques on the tissue level, to whole-body in vivo anatomical and functional imaging incorporating techniques such as SPECT and PET, are rapidly being developed for a wide range of diseases, including HIV and other chronic infections (see Table 1).
Ex vivo molecular imaging on the cellular and tissue level has already provided many important insights into HIV pathogenesis such as identifying foci of residual infected cells in the setting of ART and characterizing the immunological microenvironments of such foci (58–65). These studies have focused largely on gut, lymphoid, and central nervous system tissues but may involve a wide variety of other scenarios such as tumor microenvironments and quantifying vascular inflammation. However, the focus of this review covers in vivo nuclear medicine approaches with an emphasis on novel PET imaging approaches of HIV persistence.
Nuclear Imaging Approaches to HIV Infection
Common nuclear imaging approaches that have been applied to HIV infection for over 20 years include SPECT/CT and PET/CT imaging (44). These modalities involve the detection, anatomical location, and kinetics of radioactive tracer uptake, with SPECT involving the detection of single photon gamma emission and PET measuring positron emission. Clinically, these nuclear imaging modalities are commonly used to diagnose various malignancies and provide information on potential tumor burden or sites of metastases, disease staging, and response to various treatment strategies. They are also used to differentiate benign, metabolically quiescent tissues from metabolically active foci, which may be manifested by active infections, reactive lymphoid tissues, vascular inflammation, and more. As a result, nuclear imaging has been applied in the setting of HIV infection and HIV-related comorbidities. HIV imaging studies are diverse and have involved numerous tracers and measured outcomes. As summarized in Table 1 and below, PET imaging has been used to (1) measure cellular metabolic activity in a variety of different clinical scenarios (e.g., 18F-FDG); (2) carry out anatomical and functional neuroimaging involving various metabolic measures, cerebral fluid, dopamine transport, and cellular activation in the setting of HIV-associated neurological disease (HAND), central nervous system malignancies, and opportunistic infections; (3) determine ART-related toxicities; (4) quantify changes in various immune cell types, such as CD4+ T-cell distribution in the setting of immunomodulatory therapies in animal studies; and (5) characterize the effects of HIV on cardiovascular disease. A recent PubMed search using HIV or AIDS and PET yielded 537 references, averaging about 10 articles per year.
Over the past several years, there has been increased interest in the development of HIV-specific tracers to provide direct anatomical localization and burden of infection. In vivo studies are currently taking place using techniques such as radiolabeling monoclonal antibodies (mAbs) specific for HIV or SIV envelope proteins (66, 67). In addition, traditional nuclear medicine approaches, such as FDG-PET, have been applied to look at HIV persistence in the setting of active infection, HIV controllers (i.e., those who are able to suppress virus without ART), and ART-suppressed individuals (see discussion below). These immunoimaging approaches have the potential to significantly improve our understanding of where and how residual viral replication and HIV-related inflammation resides in the setting of suppressive therapy. More specifically, the diverse nuclear imaging toolbox may prove to be useful in people living with HIV to:
• Understand the temporal changes that occur within the whole body as a function of disease status, ART use, viral recrudescence following cessation of therapy, or foci of HIV reactivation during a “shock and kill” approach to HIV remission.
• Distinguish opportunistic infections and malignancies from direct or indirect impact of active or suppressed HIV infection.
• Assist in the development of new drugs and therapeutic paradigms.
• Aid in participant selection for various therapeutic strategies.
• Monitor individualized responses to various therapeutic interventions (including ART, immunotherapies, etc.).
Radiopharmaceutical, Pharmacokinetic, and Nuclear Imaging Considerations
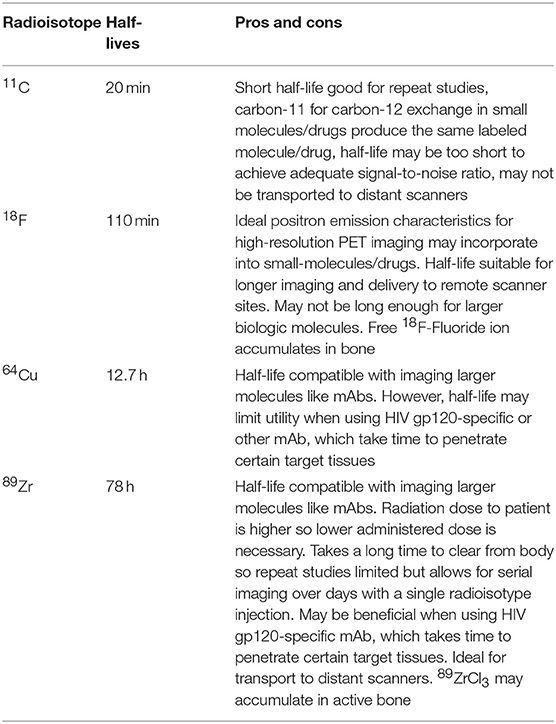
The utility of a specific nuclear imaging strategy is tightly linked with the various properties of the applied radiopharmaceutical tracer. These properties include radiologic dose, exposure, decay rates and tissue uptake, drug metabolism, and excretion. PET tracers involve a radiolabeled molecule as a source of positrons. These isotopes have a wide range of radiological half-lives (t1/2). Decay rates range widely from minutes to many days as summarized in Table 2, and ideally are in synergy with the pharmacokinetics of the radiolabeled tracer. For example, mAbs may take several days to reach target tissues and bind to specific targets, therefore requiring longer-acting isotopes such as zirconium-89 (t1/2 = 78 h), whereas FDG uptake (fluorine-18 t1/2 = 110 min) is rapid and glucose is internalized relatively quickly by metabolically active cells. Special care in matching the appropriate radioactive molecule with the target drug will be critical in the rational design and implementation of HIV-specific imaging agents. In addition, human studies are limited by the total radiation exposure to a participant, leading to challenges with administration of high enough doses for clinically meaningful target-to-background contrast, restricting the frequency of tracer administration and may limit longitudinal imaging studies. In addition, target densities may be quite low in various clinical scenarios such as ART-suppressed HIV infection, where viral proteins may be expressed in very low amounts or frequencies on cells or in tissue, if at all. As a result, there are expected to be significant challenges to increase signal-to-noise ratios in these participants, and this highlights the continued need for non-viral-specific tracers to provide information on location, burden, and immunological impact of persistent HIV infection.
PET Imaging in HIV Infection—Cellular Metabolic Activity, Immune Activation, and HIV Persistence
In the research setting, PET/CT has commonly been used in conjunction with FDG, which provides a measurement of glucose metabolism as a surrogate for inflammation, which is taken up substantially higher by inflammatory cells and macrophages in the tissue (68, 69). FDG-PET imaging has been reported for HIV in the mid to late 1980s, with monitoring of HIV pre- and post-AZT monotherapy (combination ART was not widely available until the mid-1990s), and workup of HIV-associated neurological disorders along with staging of malignancies (44, 70, 71). In addition, FDG-PET studies have involved anatomical localization of HIV-associated immune activation, correlating lymph node inflammation with disease stage, and associating high areas of FDG uptake in non-human primates with productive SIV infection (72–77). Since this time, studies in the general population have demonstrated that arterial inflammation assessed using FDG-PET/CT can predict future cardiovascular (CV) events (78). Furthermore, lipid lowering using statin therapy along with thiazolidinedione therapy has reduced arterial FDG-PET uptake in several clinical trials (79–83). Our group also has recently reported that using a mAb to IL-1β significantly reduced inflammatory markers along with arterial and bone marrow metabolic activity assessed using FDG-PET/CT in the setting of treated HIV (84). Studies involving animal models and humans showed that both relative and absolute FDG uptake within inflamed tissues (e.g., atherosclerotic plaques) correlate with the degree of immune cell infiltration (12, 17–19, 85–89). More recently, FDG-PET has been applied to assess altered glucose metabolism in HIV-associated inflammation and has demonstrated that HIV patients have higher arterial inflammation that is associated with sCD163 (87). Initiation of ART reduced bone marrow activity but did not affect arterial inflammation; furthermore, metabolic activity on FDG-PET/CT prior to ART was predictive of immune reconstitution inflammatory syndrome development (90).
Subsequently, our group showed that HIV-infected individuals on ART have higher metabolic activity as measured by FDG-PET/CT in the arterial vasculature and lymph nodes than matched uninfected controls and that these markers correlated with measures of HIV persistence in peripheral blood (91). Importantly, individuals on ART had higher FDG uptake in lymph nodes and arterial vasculature than matched uninfected controls. Overall, lymph node FDG activity was significantly associated with levels of integrated HIV DNA measured in peripheral blood mononuclear cells (91). This study suggests that PET-based imaging of inflammation or immune activation has the potential to provide information regarding regional areas of HIV persistence. However, FDG is likely taken up by immune activation/inflammation even when not in tissue with HIV-persistent foci (e.g., arterial wall, which may be influenced by monocyte activation); therefore, more specific markers of T-cell trafficking and targeting of infected tissues are needed.
Recently, advances in molecular imaging of immune activation by PET have made it possible to use non-invasive strategies to monitor immune activation with increased T-cell specificity than FDG. Increased activity of nucleoside salvage pathways has been associated with the proliferation of adaptive immune cells (92). In preclinical models, the PET probe [18F]-2-fluoro-d-(arabinofuranosyl)cytosine ([18F]-FAC), which targets the deoxycytidine salvage pathway, was shown to localize to focal sites of immune activation (93) and is predominantly accumulated in proliferative T cells (94). Recently, a radiofluorinated imaging agent [18F]F-AraG (95) was synthesized with a goal of development for human use. F-AraG is a fluorinated purine derivative with selective T-cell uptake. A water-soluble AraG prodrug, Nelarabine, is FDA-approved for the treatment of relapsed T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphomas (96, 97). [18F]F-AraG is a high-affinity substrate for deoxyguanosine kinase (dGK) and a low-affinity substrate for deoxycytidine kinase (dCK). Both dGK and dCK are over-expressed in activated T cells. Blocking the expression of either dGK or dCK causes reduction in [18F]F-AraG uptake, while over-expression of either dGK or dCK leads to increased accumulation of [18F]F-AraG. T-cell-specific tracers such as these may play an important role in imaging HIV persistence, with the potential to be more specific to regional areas of immune perturbance as a result of HIV replication or residual viral transcriptional activity.
Neuroimaging Microglia Activation in HIV Infection and Related Neurologic Disorders
PET imaging using tracers specific for activated microglial cells is another example of how non-specific markers of increased immune activation has been successfully applied to study HIV-related comorbidities in the central nervous system. More specifically, molecules have been developed that target the 18-kDa mitochondrial translocator protein (TSPO) that shuttles cholesterol into mitochondria for steroid biosynthesis (45–50). TSPO is upregulated in activated microglia, and, as a result, has been used in neuroimaging to determine differences between HIV-infected and uninfected individuals and to characterize differences between various HIV clinical disease manifestations, including HAND (50). PET imaging with TSPO-specific tracers appear to be more specific to innate immune activation than FDG (45) and have led to some important insights into central nervous system persistence of HIV. For example, ART-suppressed individuals without cognitive impairment have been observed to have chronically elevated microglial activation (48), whereas other studies showed that TSPO levels correlated with worse executive brain performance and other HIV-associated cognitive vulnerabilities (46, 49). Despite varying results complicated by various experimental designs and definitions of cognitive impairment (50), there is continued interest in using PET-based immune activation approaches to study the direct impact of residual HIV infection in the setting of suppressive ART.
Antiretroviral Drug Labeling
The question of whether or not there is ongoing replication in various tissue sanctuaries in the setting of otherwise suppressive ART remains controversial. For example, there is a paucity of robust phylogenic evidence for evolution of HIV sequences or development of resistant mutations in suppressed individuals over time and ART intensification studies have not demonstrated reductions in low-level, residual plasma HIV RNA levels (98–103). Many of these studies were performed in peripheral blood or limited by the depth of sequence coverage or tissues sampled. Other studies have shown potential indirect evidence of replication such as an increase in unintegrated episomal HIV DNA in blood and cell-associated RNA in tissue (104–106). One topic of interest is the extent to which various ART drugs reach or have activity in various anatomical tissue compartments (107), potentially creating viral sanctuaries that permit low-level replication or, at the very least, allow higher levels of viral transcriptional activity (9, 106, 108). Transcriptionally active cells may also lead to chronic immune activation and inflammation. However, sampling all of the potential sites of persistent HIV for concomitant ART concentrations and viral reservoir persistence is not practical. It is also difficult to obtain information on the kinetics of drug distribution within tissues outside of peripheral blood. As a result, PET-based imaging of radiolabeled antiretroviral drugs may play an important role in pinpointing areas of poor ART penetration and therefore important sites of persistent HIV burden and potential foci of viral rebound following ART cessation. Imaging studies using fluorine-18-labeled raltegravir (a strand-transfer integrase inhibitor) are ongoing (NCT03174977) and have the potential to locate areas of HIV persistence.
PET Immunoimaging of CD4+ T Cell Dynamics in SIV Infection
CD4+ T cells are the main target of HIV infection. Active disease leads to subsequent and profound reduction in CD4+ lymphocytes throughout the blood and tissues. While counts may improve in many individuals on ART, lasting perturbations to tissues such as the lymph nodes and gut-associated lymphoid tissues are common (8, 109–114). As a result, there has been interest in CD4+ T-cell-specific PET-based imaging techniques to follow CD4+ T-cell dynamics and recovery following various interventions. A recent investigation of the use of an α4β7 mAb in acute SIV infection in macaques demonstrated sustained virological control in mAb-treated monkeys. While these results have yet to be confirmed, the study involved PET-CT imaging using a 64Cu-labeled F(ab′)2 antibody against CD4. The study demonstrated repopulation of CD4+ T cells in a number of tissues, including gut, which was unexpected based on the original study hypothesis that the α4β7 mAb would interfere with CD4+ T-cell trafficking to these areas (67). This investigation is an example of how imaging various cell-specific markers may provide critical information regarding whole-body responses to various immune-based or other therapies for a wide variety of diseases. For example, CD8+ T-cell responses can theoretically be tracked over time in response to interventions such as vaccines or therapies that remove immune checkpoint and reverse T-cell exhaustion (e.g., anti-PD1 therapy).
PET-Based Direct Imaging of SIV Infection
As above, PET-based imaging techniques have the potential to delineate tissue burden and sequelae of HIV infection. PET/CT imaging approaches using a radiolabeled 64Cu-labeled SIV gp120 mAb-specific clone (7D3) have been recently applied to assess SIV envelope protein expression in infected macaques with varying degrees of viremic control and in the setting of early initiation of ART (66). Results from this pivotal study demonstrated that areas of active SIV replication can be visualized and distinguished from non-selective tracer uptake in uninfected animals, with some HIV-related signal detected several weeks following ART initiation. As would be expected, lymphoid-rich areas were localized predominately at sites of persistent SIV protein expression (66). The study also showed that anatomical regions that are often neglected by in vivo tissue sampling, such as nasal-associated lymph node tissue, may play an important role in initial HIV seeding and subsequent persistence. A follow-up sub-study of anti-α4β7 treatment in SIV-infected macaques incorporating the radiolabeled SIV gp120 mAb demonstrated a reduction in SIV protein expression in various tissues, including the lung, spleen, and lymph node chains (89). These data suggest that direct SIV or HIV imaging radiotracers have the potential to play a critical role in characterizing HIV persistence and response to curative strategies. As a result, there is currently a high level of interest in direct HIV imaging techniques to humans. However, immunoimaging in SIV infection does have several potential limitations. For example, mAb or antigen binding fragments may have heterogeneous tissue distribution in vivo, and humanization or simianization may lead to immunogenicity concerns (115). Finally, the SIV or HIV antigen-specific PET-imaging approaches do not allow for direct discrimination between actively viral producing cells, cells expressing SIV or HIV antigens at the surface, viral particles, or simply viral antigen trapping by non-infected cells.
Human HIV-Specific PET Imaging: Challenges and Promises
Despite the early success of direct SIV specific in the first non-human primate PET/CT imaging studies, there are several challenges in adopting these techniques to human imaging. For example:
1. Non-human primates are typically infected with a clonal SIV strain with known binding affinity to gp120-specific mAb. HIV-infected humans can be extraordinarily diverse with both minority and majority clones capable of harboring resistance mutations to the clinically available HIV-specific mAbs, which have been previously developed as therapeutic broadly neutralizing antibodies (116–122). As a result, there is expected to be a wide range of mAb binding affinities between study participants that will require implementation of mAb resistance testing and careful considerations as to data analysis and interpretation.
2. HIV gp120 expression is expected to be very low among infected tissues in participants on suppressive ART. As a result, there may be insufficient signal-to-noise ratio in order to visualize areas of persistent infection. However, PET imaging may be particularly useful during early infection and for characterizing foci of early tissue HIV recrudescence following cessation of ART; incorporating PET imaging approaches in studies involving analytical ART interruptions is of utmost importance.
3. mAbs do not readily cross the blood–brain barrier. Barring any inflammation and major perturbations of the blood–brain barrier, imaging potential foci of HIV in the central nervous system will be challenging. As a result, the development of small-molecule HIV-specific tracers with improved central nervous system or other immune privileged tissue penetration is urgently needed.
4. Longitudinal human trials are limited by radiation exposure; therefore, multiple imaging time points may be difficult to incorporate into a variety of studies. This may be a particular issue when implementing tracers conjugated with radioisotopes with longer half-lives in vivo, which are likely going to be required given the kinetics of mAb uptake as discussed above. These limitations provide the rationale to incorporate more than one radiotracer in human studies. For example, administering an HIV-specific mAb tracer following PET imaging using a non-viral specific marker of inflammation or immune activation may provide important insights into the relationship between ongoing immune perturbations and HIV persistence.
Fortunately, several strategies exist or are in development to address these challenges using radiolabeled mAbs in PET imaging. For example, smaller affibody proteins or antibody fragments (e.g., minibodies, nanobodies, and single-chain variable regions) (123–125) may have improved tissue penetration and favorable pharmacokinetics for imaging low-level HIV protein expression in various tissues. There is also a high level of interest in the development of dual or multi-targeted molecules for immunoimaging (126) or engineering antibodies to have greater anatomical barrier penetration. One exciting strategy is increasing antibody delivery across the blood–brain barrier by developing bispecific antibodies or designer molecular shuttles that bind to the transferrin receptor (127–130). Animal studies are exciting and can theoretically be applied to HIV-specific mAb or antibody fragments.
The development and implementation of very-high-sensitivity, total-body PET scanners, such as the EXPLORER platform (131–133), are also likely to overcome some of the signal-to-noise limitations of imaging HIV-infected cells in ART-suppressed individuals or those with low overall HIV envelope protein expression. These platforms are just now coming on line for in vivo use, and have the potential to revolutionize immunoPET imaging. Approximately 1% of the photons emitted during traditional PET scanning are detected given a limited axial field of view and body length that can be imaged at one time. The field of view in EXPLORER is extended to the entire individual by using a large number of parallel detectors that simultaneously detect photon emission (134). Early data suggest that EXPLORER PET provides a >40-fold gain in effective sensitivity and a >6-fold increase in signal-to-noise ratio compared with standard PET scanners (135). The first-in-human imaging studies have recently been completed (131) and offer an opportunity to significantly advance PET-based imaging of HIV reservoirs. Other emerging technologies include solid-state digital photon counting PET systems, such as those that use solid-state silicon photomultiplier technology (136). These systems have led to improvements in signal-to-noise ratios and enhancing image contrast (137, 138) and may play an important role in improving PET imaging in HIV infection.
Limitations of in vitro Modeling of HIV-Specific Immunoimaging Techniques
HIV or SIV envelope-specific PET immunoimaging strategies are likely to be semiquantitative at best. For example, PET/MR or PET/CT imaging techniques reveal relative changes in mAb tracer uptake in various tissue region of interest (e.g., lymph node tissues, gut) before or after initiation of ART or immunotherapy (66, 67). However, questions arise as to what the intensity of the PET signal means in terms of the actual number of infected, HIV or SIV envelope-expressing cells. In other words, can PET imaging be used to directly quantify the burden of HIV in vivo? One solution that is often presented is to perform ex vivo studies involving PET imaging of three-dimensional clusters of known numbers of infected and uninfected cells (either laboratory infected or derived directly from infected individuals) in order to determine the sensitivity of PET to detect various levels of HIV protein expression. While appealing, these studies are limited by the multitude of variables within living organisms that determine tracer uptake and PET detection. Modern PET scanners are sensitive and able to detect tracer-derived positron emission events above normal background radiation (139). Simply labeling a cell or a group of cells that express HIV envelope will likely lead to a detectable signal. However, regardless of what threshold in the number of infected cells can be detected (e.g., 10, 100, or 1,000 in a sub-centimeter cluster) in isolation, these types of ex vivo experiments are unable to account for many biasing factors. For example, radiotracers are often delivered in microdoses, with or without a specified amount of unlabeled antibody. The distribution of these microdoses to various tissues relies on many variables, such as blood flow dynamics, tissue fibrosis, and non-specific tracer uptake, to name just a few. In addition, there is background radiation that is given off by tracers in the macro and microcirculation and from organs involved in tracer metabolism and excretion. Coupled with the need for PET attenuation and tomographic reconstructions in image acquisition and analysis, it will likely be difficult to correlate readout of ex vivo PET sensitivity studies with actual uptake in living organisms. In addition, each individual has different metabolic and physiologic dynamics (e.g., liver function, cardiac output, body surface area and mass, renal glomerular filtration rates, local microanatomical variations, etc.). As a result, performing parallel in vivo tissue biopsy studies along with PET imaging may be the most useful strategy to provide some quantitative understanding of radiotracer uptake signal and direct cellular measures of HIV burden or cell activation state.
Conclusions
PET imaging offers several exciting strategies to characterize HIV and HIV-related comorbidities. Despite limitations of traditional of nuclear imaging techniques in identifying HIV-infected cells in vivo, proof-of-concept SIV non-human primate studies demonstrate that various immunoimaging approaches have potential to enhance HIV curative and persistence research. Signal-to-noise issues are likely to limit imaging in ART-suppressed individuals when cell-surface HIV protein expression is expected to be low. However, novel approaches such as high-sensitivity, total-body EXPLORER imaging, PET imaging during latent HIV reservoir reactivation or ATI, and development and implementation of non-viral markers of HIV persistence have the capacity to overcome these limitations and provide important tools for the development of novel therapeutic strategies. In addition, technical and data processing advancements may allow for combination imaging approaches, from tissue-level microscopy to whole-body PET imaging.
Author Contributions
TH, PH, and HV wrote the manuscript and obtained funding.
Funding
Funding was provided by grant support from the Delaney AIDS Research Consortium (DARE) UM1AI126611, amfAR Institute for HIV Cure Research, Merck & Co., and K24AI112393 (PH). Additional resources were provided by CellSight Technologies.
Conflict of Interest Statement
The authors declare that they received funding from Merck for a study discussed in this review. The funder provided grant support to perform PET-MR imaging of raltegravir. PH received honoraria unrelated to the topic of the paper from Gilead and Merck. TH has received honoraria unrelated to the topic of the paper from Merck.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DS, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. (2013) 155:540–1. doi: 10.1016/j.cell.2013.09.020
3. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. (2003) 9:727–8. doi: 10.1038/nm880
4. Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. (1997) 387:183–8. doi: 10.1038/387183a0
5. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. (1997) 278:1295–300. doi: 10.1126/science.278.5341.1295
6. Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. (1997) 278:1291–5. doi: 10.1126/science.278.5341.1291
7. Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: Implications for viral persistence. J Infect Dis. (2013) 208:1212–20. doi: 10.1093/infdis/jit308
8. Belmonte L, Olmos M, Fanin A, Parodi C, Bare P, Concetti H, et al. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. AIDS. (2007) 21:2106–8. doi: 10.1097/QAD.0b013e3282efb74b
9. Licht A, Alter G. A drug-free zone—Lymph nodes as a safe haven for HIV. Cell Host Microbe. (2016) 19:275–6. doi: 10.1016/j.chom.2016.02.018
10. Lorenzo-Redondo R, Fryer HR, Bedford T, Kim EY, Archer J, Kosakovsky Pond SL, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. (2016) 530:51–6. doi: 10.1038/nature16933
11. Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. (2015) 112:E1126–34. doi: 10.1073/pnas.1414926112
12. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. (2013) 210:143–56. doi: 10.1084/jem.20121932
13. Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, et al. HIV DNA is frequently present within pathologic tissues evaluated at autopsy from cART-treated patients with undetectable viral load. J Virol. (2016) 90:8968–83. doi: 10.1128/JVI.00674-16
14. Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol. (2016) 14:55–60. doi: 10.1038/nrmicro.2015.5
15. Kulpa DA, Chomont N. HIV persistence in the setting of antiretroviral therapy: when, where and how does HIV hide? J Virus Erad. (2015) 1:59–66.
16. Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad. (2015) 1:67–71.
17. Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. (2012) 120:4172–81. doi: 10.1182/blood-2012-06-437608
18. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. (2012) 122:3271–80. doi: 10.1172/JCI64314
19. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. (2015) 21:132–9. doi: 10.1038/nm.3781
20. Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS ONE. (2016) 11:e0155100. doi: 10.1371/journal.pone.0155100
21. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. (2014) 210:1248–59. doi: 10.1093/infdis/jiu254
22. Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. (2010) 55:316–22. doi: 10.1097/QAI.0b013e3181e66216
23. Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis. (2016) 63:984–90. doi: 10.1093/cid/ciw409
24. Wong C, Gange SJ, Moore RD, Justice AC, Buchacz K, Abraham AG, et al. Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis. (2018) 66:1230–8. doi: 10.1093/cid/cix998
25. Maini CL, Pigorini F, Pau FM, Volpini V, Galgani S, Rosci MA, et al. Cortical cerebral blood flow in HIV-1-related dementia complex. Nucl Med Commun. (1990) 11:639–48. doi: 10.1097/00006231-199009000-00007
26. Ajmani A, Habte-Gabr E, Zarr M, Jayabalan V, Dandala S. Cerebral blood flow SPECT with Tc-99m exametazine correlates in AIDS dementia complex stages. A preliminary report. Clin Nucl Med. (1991) 16:656–9. doi: 10.1097/00003072-199109000-00009
27. Garavelli PL, Villa G, Zoccola R, Scopinaro G. Single photon emission computed tomography with 99mTc-HMPAO in HIV-1 infection: a help in early diagnosis of AIDS dementia complex. Acta Neurol. (1991) 13:282–4.
28. Holman BL, Garada B, Johnson KA, Mendelson J, Hallgring E, Teoh SK, et al. A comparison of brain perfusion SPECT in cocaine abuse and AIDS dementia complex. J Nucl Med. (1992) 33:1312–5.
29. Geier SA, Schielke E, Tatsch K, Sadri I, Bogner JR, Hammel G, et al. Brain HMPAO-SPECT and ocular microangiopathic syndrome in HIV-1-infected patients. AIDS. (1993) 7:1589–94. doi: 10.1097/00002030-199312000-00007
30. Beldarrain MG, Garcia-Monco JC, Llorens V, Cortes J, Mayo J. Neuropsychological differences but comparable regional cerebral blood changes in asymptomatic HIV-1-positive and -negative drug addicts. Eur Neurol. (1994) 34:193–8. doi: 10.1159/000117037
31. Rubbert A, Bock E, Schwab J, Marienhagen J, Nusslein H, Wolf F, et al. Anticardiolipin antibodies in HIV infection: association with cerebral perfusion defects as detected by 99mTc-HMPAO SPECT. Clin Exp Immunol. (1994) 98:361–8. doi: 10.1111/j.1365-2249.1994.tb05498.x
32. Sacktor N, Van Heertum RL, Dooneief G, Gorman J, Khandji A, Marder K, et al. A comparison of cerebral SPECT abnormalities in HIV-positive homosexual men with and without cognitive impairment. Arch Neurol. (1995) 52:1170–3. doi: 10.1001/archneur.1995.00540360048015
33. Szeto ER, Freund J, Brew BJ, Loder A, Griffiths MR. Cerebral perfusion scanning in treating AIDS dementia: a pilot study. J Nucl Med. (1998) 39:298–302.
34. Christensson B, Ljungberg B, Ryding E, Svenson G, Rosen I. SPECT with 99mTc-HMPAO in subjects with HIV infection: cognitive dysfunction correlates with high uptake. Scand J Infect Dis. (1999) 31:349–54. doi: 10.1080/00365549950163761
35. Rourke SB, Dupont RM, Grant I, Lehr PP, Lamoureux G, Halpern S, et al. Reduction in cortical IMP-SPET tracer uptake with recent cigarette consumption in a young group of healthy males. San Diego HIV Neurobehavioral Research Center. Eur J Nucl Med. (1997) 24:422–7. doi: 10.1007/BF00881815
36. Harris GJ, Pearlson GD, McArthur JC, Zeger S, LaFrance ND. Altered cortical blood flow in HIV-seropositive individuals with and without dementia: a single photon emission computed tomography study. AIDS. (1994) 8:495–9. doi: 10.1097/00002030-199404000-00012
37. Pohl P, Riccabona G, Hilty E, Deisenhammer F, Rossler H, Zangerle R, et al. Double-tracer SPECT in patients with AIDS encephalopathy: a comparison of 123I-IMP with 99Tcm-HMPAO. Nucl Med Commun. (1992) 13:586–92. doi: 10.1097/00006231-199213080-00003
38. O'Connell RA, Van Heertum RL, Billick SB, Holt AR, Gonzalez A, Notardonato H, et al. Single photon emission computed tomography (SPECT) with [123I]IMP in the differential diagnosis of psychiatric disorders. J Neuropsychiatry Clin Neurosci. (1989) 1:145–53. doi: 10.1176/jnp.1.2.145
39. Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, et al. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm. (2010) 117:699–705. doi: 10.1007/s00702-010-0415-6
40. Giancola ML, Rizzi EB, Schiavo R, Lorenzini P, Schinina V, Alba L, et al. Reduced value of thallium-201 single-photon emission computed tomography in the management of HIV-related focal brain lesions in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. (2004) 20:584–8. doi: 10.1089/0889222041217446
41. Antinori A, De Rossi G, Ammassari A, Cingolani A, Murri R, Di Giuda D, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. (1999) 17:554–60. doi: 10.1200/JCO.1999.17.2.554
42. Miller RF, Hall-Craggs MA, Costa DC, Brink NS, Scaravilli F, Lucas SB, et al. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Infect. (1998) 74:258–64. doi: 10.1136/sti.74.4.258
43. Gomez MV, Gallardo FG, Cobo J, Babe J. Identification of AIDS-related tuberculosis with concordant gallium-67 and three-hour delayed thallium-201 scintigraphy. Eur J Nucl Med. (1996) 23:852–4. doi: 10.1007/BF00843714
44. Sathekge M, McFarren A, Dadachova E. Role of nuclear medicine in neuroHIV: PET, SPECT, and beyond. Nucl Med Commun. (2014) 35:792–6. doi: 10.1097/MNM.0000000000000139
45. Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol. (2014) 20:219–32. doi: 10.1007/s13365-014-0239-5
46. Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. (2014) 28:67–72. doi: 10.1097/01.aids.0000432467.54003.f7
47. Zhou T, Dang Y, Zheng YH. The mitochondrial translocator protein TSPO, inhibits HIV-1 envelope glycoprotein biosynthesis via the endoplasmic reticulum-associated protein degradation pathway. J Virol. (2014) 88:3474–84. doi: 10.1128/JVI.03286-13
48. Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology. (2016) 86:1425–32. doi: 10.1212/WNL.0000000000002485
49. Rubin LH, Sacktor N, Creighton J, Du Y, Endres CJ, Pomper MG, et al. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS. (2018) 32:1661–7. doi: 10.1097/QAD.0000000000001858
50. Boerwinkle A, Ances BM. Molecular imaging of neuroinflammation in HIV. J Neuroimmune Pharmacol. (2019) 14:9–15. doi: 10.1007/s11481-018-9823-4
51. Wilson JM, Fokas E, Dutton SJ, Patel N, Hawkins MA, Eccles C, et al. ARCII: a phase II trial of the HIV protease inhibitor Nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancer. Radiother Oncol. (2016) 119:306–11. doi: 10.1016/j.radonc.2016.03.021
52. Orbaek M, Hasbak P, Sejersten Ripa R, Kjaer A, Lebech AM, Knudsen A. Comparison of the peripheral reactive hyperemia index with myocardial perfusion reserve by (82)Rb PET/CT in HIV-infected patients. Diagnostics. (2017) 7:E31. doi: 10.3390/diagnostics7020031
53. Knudsen A, Christensen TE, Ghotbi AA, Hasbak P, Lebech AM, Kjaer A, et al. Normal myocardial flow reserve in HIV-infected patients on stable antiretroviral therapy: a cross-sectional study using rubidium-82 PET/CT. Medicine. (2015) 94:e1886. doi: 10.1097/MD.0000000000001886
54. Hammoud DA, Endres CJ, Hammond E, Uzuner O, Brown A, Nath A, et al. Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. Neuroimage. (2010) 49:2588–95. doi: 10.1016/j.neuroimage.2009.10.037
55. Endres CJ, Hammoud DA, Pomper MG. Reference tissue modeling with parameter coupling: application to a study of SERT binding in HIV. Phys Med Biol. (2011) 56:2499–513. doi: 10.1088/0031-9155/56/8/011
56. Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. (2012) 69:72–7. doi: 10.1001/archneurol.2011.761
57. Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case–control study. Neurology. (2010) 75:111–5. doi: 10.1212/WNL.0b013e3181e7b66e
58. Vasquez JJ, Hussien R, Aguilar-Rodriguez B, Junger H, Dobi D, Henrich TJ, et al. Elucidating the burden of HIV in tissues using multiplexed immunofluorescence and in situ hybridization: methods for the single-cell phenotypic characterization of cells harboring HIV in situ. J Histochem Cytochem. (2018) 66:427–46. doi: 10.1369/0022155418756848
59. Deleage C, Wietgrefe SW, Del Prete G, Morcock DR, Hao XP, Piatak M Jr, et al. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun. (2016) 1:68–106. doi: 10.20411/pai.v1i1.100
60. Jiang G, Maverakis E, Cheng MY, Elsheikh MM, Deleage C, Mendez-Lagares G, et al. Disruption of latent HIV in vivo during the clearance of actinic keratosis by ingenol mebutate. JCI Insight. (2019) 4:126027. doi: 10.1172/jci.insight.126027
61. Mudd JC, Busman-Sahay K, DiNapoli SR, Lai S, Sheik V, Lisco A, et al. Hallmarks of primate lentiviral immunodeficiency infection recapitulate loss of innate lymphoid cells. Nat Commun. (2018) 9:3967. doi: 10.1038/s41467-018-05528-3
62. Estes JD, LeGrand R, Petrovas C. Visualizing the immune system: providing key insights into HIV/SIV infections. Front Immunol. (2018) 9:423. doi: 10.3389/fimmu.2018.00423
63. Utay NS, Kitch DW, Yeh E, Fichtenbaum CJ, Lederman MM, Estes JD, et al. Telmisartan therapy does not improve lymph node or adipose tissue fibrosis more than continued antiretroviral therapy alone. J. Infect Dis. (2018) 217:1770–81. doi: 10.1093/infdis/jiy064
64. Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med. (2017) 23:1271–6. doi: 10.1038/nm.4411
65. Deleage C, Turkbey B, Estes JD. Imaging lymphoid tissues in nonhuman primates to understand SIV pathogenesis and persistence. Curr Opin Virol. (2016) 19:77–84. doi: 10.1016/j.coviro.2016.07.002
66. Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. (2015) 12:427–32. doi: 10.1038/nmeth.3320
67. Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, et al. Sustained virologic control in SIV+ macaques after antiretroviral and alpha4beta7 antibody therapy. Science. (2016) 354:197–202. doi: 10.1126/science.aag1276
68. Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: Ready for prime time? J Am Coll Cardiol. (2010) 55:2527–35. doi: 10.1016/j.jacc.2009.12.061
69. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. (2006) 48:1818–24. doi: 10.1016/j.jacc.2006.05.076
70. Sathekge M, Maes A, Kgomo M, Pottel H, Stolz A, Van De Wiele C. FDG uptake in lymph-nodes of HIV+ and tuberculosis patients: implications for cancer staging. Q J Nucl Med Mol Imaging. (2010) 54:698–703.
71. Sathekge M. Differentiation of HIV-associated lymphoma from HIV-reactive adenopathy using quantitative FDG-PET and symmetry. Eur J Nucl Med Mol Imaging. (2014) 41:593–5. doi: 10.1007/s00259-014-2701-2
72. Brust D, Polis M, Davey R, Hahn B, Bacharach S, Whatley M, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. (2006) 20:985–93. doi: 10.1097/01.aids.0000222070.52996.76
73. Lucignani G, Orunesu E, Cesari M, Marzo K, Pacei M, Bechi G, et al. FDG-PET imaging in HIV-infected subjects: relation with therapy and immunovirological variables. Eur J Nucl Med Mol Imaging. (2009) 36:640–7. doi: 10.1007/s00259-008-1023-7
74. Iyengar S, Chin B, Margolick JB, Sabundayo BP, Schwartz DH. Anatomical loci of HIV-associated immune activation and association with viraemia. Lancet. (2003) 362:945–50. doi: 10.1016/S0140-6736(03)14363-2
75. Davison JM, Subramaniam RM, Surasi DS, Cooley T, Mercier G, Peller PJ. FDG PET/CT in patients with HIV. AJR Am J Roentgenol. (2011) 197:284–94. doi: 10.2214/AJR.10.6332
76. Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med. (2013) 43:349–66. doi: 10.1053/j.semnuclmed.2013.04.008
77. Scharko AM, Perlman SB, PWn Hinds, JM Hanson, Uno H, Pauza CD. Whole body positron emission tomography imaging of simian immunodeficiency virus-infected rhesus macaques. Proc Natl Acad Sci USA. (1996) 93:6425–30. doi: 10.1073/pnas.93.13.6425
78. Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. (2013) 6:1250–9. doi: 10.1016/j.jcmg.2013.08.006
79. Emami H, Vucic E, Subramanian S, Abdelbaky A, Fayad ZA, Du S, et al. The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: a multicenter FDG-PET trial. Atherosclerosis. (2015) 240:490–6. doi: 10.1016/j.atherosclerosis.2015.03.039
80. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. (2013) 62:909–17. doi: 10.1016/j.jacc.2013.04.066
81. Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. (2006) 48:1825–31. doi: 10.1016/j.jacc.2006.03.069
82. Ishii H, Nishio M, Takahashi H, Aoyama T, Tanaka M, Toriyama T, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. (2010) 32:2337–47. doi: 10.1016/j.clinthera.2010.12.001
83. Nitta Y, Tahara N, Tahara A, Honda A, Kodama N, Mizoguchi M, et al. Pioglitazone decreases coronary artery inflammation in impaired glucose tolerance and diabetes mellitus: evaluation by FDG-PET/CT imaging. JACC Cardiovasc Imaging. (2013) 6:1172–82. doi: 10.1016/j.jcmg.2013.09.004
84. Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M, et al. IL-1beta inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. (2018) 72:2809–11. doi: 10.1016/j.jacc.2018.09.038
85. Vaidyanathan S, Patel CN, Scarsbrook AF, Chowdhury FU. FDG PET/CT in infection and inflammation—Current and emerging clinical applications. Clin Radiol. (2015) 70:787–800. doi: 10.1016/j.crad.2015.03.010
86. Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. (2014) 66:164–71. doi: 10.1097/QAI.0000000000000138
87. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. (2012) 308:379–86. doi: 10.1001/jama.2012.6698
88. Yarasheski KE, Laciny E, Overton ET, Reeds DN, Harrod M, Baldwin S, et al. 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. J Inflamm. (2012) 9:26. doi: 10.1186/1476-9255-9-26
89. Santangelo PJ, Cicala C, Byrareddy SN, Ortiz KT, Little D, Lindsay KE, et al. Early treatment of SIV+ macaques with an alpha4beta7 mAb alters virus distribution and preserves CD4(+) T cells in later stages of infection. Mucosal Immunol. (2018) 11:932–46. doi: 10.1038/mi.2017.112
90. Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. (2016) 1:474–80. doi: 10.1001/jamacardio.2016.0846
91. Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. (2017) 2:163–71. doi: 10.1001/jamacardio.2016.4728
92. Radu CG, Shu CJ, Nair-Gill E, Shelly SM, Barrio JR, Satyamurthy N, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2'-deoxycytidine analog. Nat Med. (2008) 14:783–8. doi: 10.1038/nm1724
93. Nair-Gill E, Wiltzius SM, Wei XX, Cheng D, Riedinger M, Radu CG, et al. PET probes for distinct metabolic pathways have different cell specificities during immune responses in mice. J Clin Invest. (2010) 120:2005–15. doi: 10.1172/JCI41250
94. Namavari M, Chang YF, Kusler B, Yaghoubi S, Mitchell BS, Gambhir SS. Synthesis of 2'-deoxy-2'-[18F]fluoro-9-beta-D-arabinofuranosylguanine: a novel agent for imaging T-cell activation with PET. Mol Imaging Biol. (2011) 13:812–8. doi: 10.1007/s11307-010-0414-x
95. DeAngelo DJ. Nelarabine for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia or lymphoblastic lymphoma. Hematol Oncol Clin North Am. (2009) 23:1121–35, vii–viii. doi: 10.1016/j.hoc.2009.07.008
96. Franc BL, Goth S, MacKenzie J, Li X, Blecha J, Lam T, et al. In vivo PET imaging of the activated immune environment in a small animal model of inflammatory arthritis. Mol Imaging. (2017) 16. 1536012117712638. doi: 10.1177/1536012117712638
97. Ronald JA, Kim BS, Gowrishankar G, Namavari M, Alam IS, D'Souza A, et al. A PET imaging strategy to visualize activated T cells in acute graft-versus-host disease elicited by allogenic hematopoietic cell transplant. Cancer Res. (2017) 77:2893–902. doi: 10.1158/0008-5472.CAN-16-2953
98. Evering TH, Mehandru S, Racz P, Tenner-Racz K, Poles MA, Figueroa A, et al. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. (2012) 8:e1002506. doi: 10.1371/journal.ppat.1002506
99. Capoferri AA, Bale MJ, Simonetti FR, Kearney MF. Phylogenetic inference for the study of within-host HIV-1 dynamics and persistence on antiretroviral therapy. Lancet HIV. (2019) 6:e325–33. doi: 10.1016/S2352-3018(19)30051-7
100. Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci USA. (2009) 106:9403–8. doi: 10.1073/pnas.0903107106
101. Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. (2010) 7:e1000321. doi: 10.1371/journal.pmed.1000321
102. McMahon D, Jones J, Wiegand A, Gange SJ, Kearney M, Palmer S, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. (2010) 50:912–9. doi: 10.1086/650749
103. Gandhi RT, Coombs RW, Chan ES, Bosch RJ, Zheng L, Margolis DM, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. (2012) 59:229–35. doi: 10.1097/QAI.0b013e31823fd1f2
104. Hatano H, Strain MC, Scherzer R, Bacchetti P, Wentworth D, Hoh R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis. (2013) 208:1436–42. doi: 10.1093/infdis/jit453
105. Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. (2010) 16:460–5. doi: 10.1038/nm.2111
106. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. (2016) 11:417–23. doi: 10.1097/COH.0000000000000287
107. Asahchop EL, Meziane O, Mamik MK, Chan WF, Branton WG, Resch L, et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology. (2017) 14:47. doi: 10.1186/s12977-017-0370-5
108. Kirtane AR, Langer R, Traverso G. Past, present, and future drug delivery systems for antiretrovirals. J Pharm Sci. (2016) 105:3471–82. doi: 10.1016/j.xphs.2016.09.015
109. Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. (2008) 197:126–33. doi: 10.1086/524143
110. Talal AH, Monard S, Vesanen M, Zheng Z, Hurley A, Cao Y, et al. Virologic and immunologic effect of antiretroviral therapy on HIV-1 in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr. (2001) 26:1–7. doi: 10.1097/00126334-200101010-00001
111. Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, Andersson J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. Aids. (2007) 21:565–74. doi: 10.1097/QAD.0b013e3280117204
112. Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. (2008) 82:538–45. doi: 10.1128/JVI.01449-07
113. Sheth PM, Chege D, Shin LY, Huibner S, Yue FY, Loutfy M, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal Immunol. (2008) 1:382–8. doi: 10.1038/mi.2008.23
114. Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, Albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. (2017) 64:124–31. doi: 10.1093/cid/ciw683
115. Carmon KS, Azhdarinia A. Application of Immuno-PET in antibody–drug conjugate development. Mol Imaging. (2018) 17. doi: 10.1177/1536012118801223
116. Lynch RM, Tran L, Louder MK, Schmidt SD, Cohen M, Dersimonian R, et al. The development of CD4 binding site antibodies during HIV-1 infection. J Virol. (2012) 86:7588–95. doi: 10.1128/JVI.00734-12
117. Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. (2015) 7:319ra206. doi: 10.1126/scitranslmed.aad5752
118. Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med. (2016) 375:2037–50. doi: 10.1056/NEJMoa1608243
119. Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. (2018) 15:e1002493. doi: 10.1371/journal.pmed.1002493
120. Bar-On Y, Gruell H, Schoofs T, Pai JA, Nogueira L, Butler AL, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med. (2018) 24:1701–7. doi: 10.1038/s41591-018-0186-4
121. Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. (2018) 561:479–84. doi: 10.1038/s41586-018-0531-2
122. Mayer KH, Seaton KE, Huang Y, Grunenberg N, Isaacs A, Allen M, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: results of a phase 1 randomized trial. PLoS Med. (2017) 14:e1002435. doi: 10.1371/journal.pmed.1002435
123. De Vos J, Devoogdt N, Lahoutte T, Muyldermans S. Camelid single-domain antibody-fragment engineering for (pre)clinical in vivo molecular imaging applications: adjusting the bullet to its target. Expert Opin Biol Ther. (2013) 13:1149–60. doi: 10.1517/14712598.2013.800478
124. Fu R, Carroll L, Yahioglu G, Aboagye EO, Miller PW. Antibody fragment and affibody ImmunoPET imaging agents: radiolabelling strategies and applications. ChemMedChem. (2018) 13:2466–78. doi: 10.1002/cmdc.201800624
125. Lee HJ, Ehlerding EB, Cai W. Antibody-based tracers for PET/SPECT imaging of chronic inflammatory diseases. Chembiochem. (2019) 20:422–36. doi: 10.1002/cbic.201800429
126. Ehlerding EB, Sun L, Lan X, Zeng D, Cai W. Dual-targeted molecular imaging of cancer. J Nucl Med. (2018) 59:390–5. doi: 10.2967/jnumed.117.199877
127. Sehlin D, Fang XT, Cato L, Antoni G, Lannfelt L, Syvanen S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer's disease. Nat Commun. (2016) 7:10759. doi: 10.1038/ncomms10759
128. Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. (2011) 3:84ra44. doi: 10.1126/scitranslmed.3002230
129. Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, et al. Therapeutic bispecific antibodies cross the blood–brain barrier in nonhuman primates. Sci Transl Med. (2014) 6:261ra154. doi: 10.1126/scitranslmed.3009835
130. Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. (2014) 81:49–60. doi: 10.1016/j.neuron.2013.10.061
131. Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. (2019) 60:299–303. doi: 10.2967/jnumed.119.226498
132. Lv Y, Lv X, Liu W, Judenhofer MS, Zwingenberger A, Wisner E, et al. Mini EXPLORER II: a prototype high-sensitivity PET/CT scanner for companion animal whole body and human brain scanning. Phys Med Biol. (2019) 64:075004. doi: 10.1088/1361-6560/aafc6c
133. Berg E, Zhang X, Bec J, Judenhofer MS, Patel B, Peng Q, et al. Development and evaluation of mini-EXPLORER: a long axial field-of-view PET scanner for nonhuman primate imaging. J Nucl Med. (2018) 59:993–8. doi: 10.2967/jnumed.117.200519
134. Cherry SR, Badawi RD, Karp JS, Moses WW, Price P, Jones T. Total-body imaging: Transforming the role of positron emission tomography. Sci Transl Med. (2017) 9:eaaf6169. doi: 10.1126/scitranslmed.aaf6169
135. Zeglis BM, Lewis JS. The bioconjugation and radiosynthesis of 89Zr-DFO-labeled antibodies. J Vis Exp. (2015) 96:52521. doi: 10.3791/52521
136. Zhang J, Maniawski P, Knopp MV. Performance evaluation of the next generation solid-state digital photon counting PET/CT system. EJNMMI Res. (2018) 8:97. doi: 10.1186/s13550-018-0448-7
137. Cabello J, Ziegler SI. Advances in PET/MR instrumentation and image reconstruction. Br J Radiol. (2018) 91:20160363. doi: 10.1259/bjr.20160363
138. Salvadori J, Imbert L, Perrin M, Karcher G, Lamiral Z, Marie PY, et al. Head-to-head comparison of image quality between brain (18)F-FDG images recorded with a fully digital versus a last-generation analog PET camera. EJNMMI Res. (2019) 9:61. doi: 10.1186/s13550-019-0526-5
Keywords: human immunodeficiency virus, positron emission tomography imaging, simian immunodeficiency virus, nuclear medicine, molecular imaging
Citation: Henrich TJ, Hsue PY and VanBrocklin H (2019) Seeing Is Believing: Nuclear Imaging of HIV Persistence. Front. Immunol. 10:2077. doi: 10.3389/fimmu.2019.02077
Received: 13 June 2019; Accepted: 16 August 2019;
Published: 12 September 2019.
Edited by:
Mirko Paiardini, Emory University School of Medicine, United StatesReviewed by:
Roger Le Grand, Commissariat à l'Energie Atomique et aux Energies Alternatives (CEA), FranceFrancois Villinger, University of Louisiana at Lafayette, United States
Copyright © 2019 Henrich, Hsue and VanBrocklin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy J. Henrich, dGltb3RoeS5oZW5yaWNoQHVjc2YuZWR1
 Timothy J. Henrich
Timothy J. Henrich Priscilla Y. Hsue2
Priscilla Y. Hsue2 Henry VanBrocklin
Henry VanBrocklin