- 1Department of Cardiology, Second Hospital of Jilin University, Changchun, China
- 2Key Laboratory of Myocardial Ischemia, Ministry of Education, Harbin Medical University, Harbin, China
- 3Department of Clinical Laboratory, Second Hospital of Jilin University, Changchun, China
Atherosclerosis is characterized as a chronic inflammatory response to cholesterol deposition in arteries. Low-density lipoprotein (LDL), especially the oxidized form (ox-LDL), plays a crucial role in the occurrence and development of atherosclerosis by inducing endothelial cell (EC) dysfunction, attracting monocyte-derived macrophages, and promoting chronic inflammation. However, the mechanisms linking cholesterol accumulation with inflammation in macrophage foam cells are poorly understood. Long non-coding RNAs (lncRNAs) are a group of non-protein-coding RNAs longer than 200 nucleotides and are found to regulate the progress of atherosclerosis. Recently, many lncRNAs interfering with cholesterol deposition or inflammation were identified, which might help elucidate their underlying molecular mechanism or be used as novel therapeutic targets. In this review, we summarize and highlight the role of lncRNAs linking cholesterol (mainly ox-LDL) accumulation with inflammation in macrophages during the process of atherosclerosis.
Introduction
Atherosclerosis is the major cause of cardiovascular diseases with complications such as endothelial dysfunction, lipid accumulation, and chronic inflammation (1). Uncontrolled cholesterol deposition and chronic inflammation are two key factors in the pathogenesis of atherosclerosis (2). In addition, there is a link between cholesterol and the innate immune system involving macrophages, which plays a central role at different stages of atherogenesis (3). Low-density lipoprotein (LDL), especially its oxidized form (ox-LDL), plays a crucial role in the initiation and development of atherosclerosis by inducing endothelial cell (EC) dysfunction, attracting monocyte-derived macrophages, and promoting chronic inflammation (4). Macrophages engulf ox-LDL to form lipid-laden foam cells that ultimately release pro-inflammatory cytokines and exacerbate local inflammation (5). High-density lipoprotein (HDL) opposes the process of atherosclerosis by promoting the cellular efflux of cholesterol and reducing inflammation (6). HDL has been proposed to inhibit cellular inflammatory signaling resulting in inhibition of monocyte chemoattractant protein-1 (MCP-1) and CD11b expression and monocyte transmigration (7).
Long non-coding RNAs (lncRNAs) are a group of non-protein-coding RNAs >200 nucleotides in length, which participate in diverse biological processes and pathophysiological conditions including cardiovascular disease (8). Recently, several lncRNAs that interfere with the progress of atherosclerosis have been identified. They also participate in the pathology of atherosclerosis such as EC dysfunction, cholesterol accumulation, and inflammation (9). For example, lncRNA-growth arrest-specific 5 (GAS5) expression was significantly increased in the atherosclerotic plaque and exaggerated inflammation in ox-LDL-treated macrophages (10, 11). LncRNA-DYNLRB2-2 was upregulated in ox-LDL-induced THP-1 macrophage-derived foam cells and could promote cholesterol efflux and inhibit inflammation (12). These lncRNAs might help us understand the molecular mechanism underlying the cholesterol accumulation linked with monocyte/macrophage-mediated inflammation during the development of atherosclerosis.
In this review, we summarize and highlight the role of lncRNAs as the link between cholesterol (mainly ox-LDL) accumulation and inflammation during atherosclerosis. The data summarized in the review provide helpful insights regarding further study of lncRNAs associated with atherosclerosis.
ox-LDL-Mediated Macrophage Inflammation in Atherosclerosis
Endothelial dysfunction leads to the accumulation of lipoproteins and inflammatory cells, including monocytes, in the arterial wall, where monocytes are activated by LDL and differentiated into macrophages (13). In atherosclerotic lesions, macrophages can engulf ox-LDL to form lipid-laden foam cells that ultimately release pro-inflammatory cytokines such as interleukin-6 (IL-6), MCP-1, and tumor necrosis factor alpha (TNF-α), exacerbating local inflammation (14). Macrophages display high heterogeneity, plasticity, and differentiate into two main phenotypes of macrophages, M1 and M2 macrophages, in atherosclerotic plaques (15). M1 macrophages secrete pro-inflammatory cytokines such as IL-1, IL-6, IL-12, IL-15, IL-18, and TNF-α after ox-LDL uptake (16). M2 macrophages secrete anti-inflammatory cytokines such as IL-4, IL-10, and IL-13, and are found predominantly in stable plaques (17, 18).
Macrophage Receptors Involved in Lipid Accumulation and Inflammation
In atherosclerosis, the pattern recognition receptors (PRRs) on monocyte-derived macrophages, such as scavenger receptors (SRs), are involved in lipid recognition and inflammation (19). Scavenger receptor-A1 (also known as macrophage SR-A1, MSR1, or CD204) belongs to the class A SR family and facilitates uptake of ox-LDL in macrophages (20). SR-A1 knock-down reduces the formation of foam cells and atherosclerosis progression in ApoE−/− mice (21). CD36 is a member of the SR class B family that can facilitate the endocytosis of ox-LDL in macrophages (22). CD36 and SR-A are principal contributors to cholesterol uptake, accounting for up to 90% of ox-LDL loading in macrophages (23). Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is not detectable in human monocytes, but is induced in differentiated macrophages by several stimuli, including ox-LDL, high glucose levels, and pro-inflammatory cytokines (24). LOX-1 in macrophage uptake and degradation of ox-LDL under normal conditions present in small amounts (25). The ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1) are responsible for most of the macrophage cholesterol efflux to the serum or HDL in macrophage foam cells (26). The ABCA1 is a primary cholesterol transporter, and is associated with severe HDL deficiency and premature atherosclerosis (27). ABCA1-deficient mice have low HDL cholesterol and develop foam cell lesions (28). Inhibition of ABCA1 in mouse macrophages led to cholesterol accumulation and increased secretion of pro-inflammatory cytokines (29). Similarly, ABCG1 knock-down resulted in a moderate rise of atherosclerotic plaques in LDL receptor-deficient mice (30). In contrast, inhibition of ABCG1 exerted an atheroprotective effect in LDL receptor-deficient mice (31).
In addition to cholesterol accumulation, these receptors alone or in conjunction with Toll-like receptors (TLRs) participate in macrophage-mediated inflammation (32). For example, TLR2 and TLR4 act as the PRRs for ox-LDL and can directly trigger pro-inflammatory signaling via the upregulation of fatty acid-binding protein aP2 (33). Depletion of aP2 in macrophages reduced production of TNF-a, MCP-1, and IL-6 upon exposure to ox-LDL (34). SR-A1 was also identified as a pro-inflammatory receptor in macrophages (35). SR-A−/− mice exhibit increased sensitivity to endotoxin-induced shock, related to high TNF-α production (36). CD36 has been shown to cooperate with TLR2, TLR4, and TLR6 to induce inflammation in macrophages (37). Ox-LDL triggers MyD88-dependent inflammation through the CD36-TLR4-TLR6 heterodimer in macrophages (38), accompanied by activation of mitogen-activated kinases (MAPKs) such as p38 MAPK or NF-κB (39). LOX-1 is significantly reduced in atherosclerosis and involved in inflammation; NF-κB expression and inflammatory markers are reduced in LOX-1 deficient animals (40). HDL has potent anti-inflammatory effects via regulation of ABCA1 and ABCG1 (41). ABCA1 and ABCG1 deficiency increased inflammatory cytokine production via activation of TLR4 signaling in macrophages (42). In addition, ABCA1 and ABCG1 suppress macrophage inflammatory responses through activation of TLR2 and TLR3 (9, 43, 44). Activation of TLR3 and TLR4 signaling suppresses expression of ABCA1 and ABCG1 in macrophages (43, 44).
There is a loop between cholesterol accumulation and inflammation. Pro-inflammatory cytokines such as TNF-a and IL-6 promote SR-A1 expression by activating NF-κB, which is closely related to the inflammatory immune response (45). Ox-LDL upregulates the expression of CD36 to further enhance its uptake by macrophages via activation of peroxisome proliferator–activated receptor-γ (PPAR-γ) (46). In addition, TLR agonists strongly stimulate particle uptake by macrophage via CD36. Exogenous TLR2 activation enhances CD36-mediated particle uptake. Similarly, ox-LDL or inflammatory cytokines such as TNF-a, IL-1 can upregulate LOX-1 expression (25, 47).
lncRNA Biology and Function in Atherosclerosis
Long non-coding RNAs (lncRNAs) are RNAs longer than 200 nucleotides in length, with little functional protein-coding ability as they lack open reading frames (ORFs) (48). Although lncRNAs are expressed at lower levels and are evolutionarily ill conserved, they show more tissue-specificity (49). Based on their genomic location, lncRNAs are classified into long intergenic non-coding RNAs, intronic lncRNAs, bidirectional lncRNAs, sense lncRNAs, antisense lncRNAs, and enhancer RNAs (49). According to the molecular mechanism, lncRNAs are classified into four main categories: signals, decoys, guides, and scaffolds (50). Signal lncRNAs can react to diverse stimulations and transduce relevant signals. Decoy lncRNAs can sequester other effectors, such as RNA-binding proteins and microRNAs, to negatively regulate their function. Guide lncRNAs act as molecular chaperones, localizing ribonucleoproteins to specific chromatin targets. Scaffold lncRNAs bind distinct proteins to coordinate their function. In addition, lncRNAs exhibit a unique ability to interact with target molecules and guide them to specific genomic regions in neighboring (cis) or distantly located (trans) genes to modulate gene expression. Finally, lncRNAs can bind partners, acting as a scaffold (50). In addition, lncRNAs can be further categorized into cellular or nuclear lncRNA. Cellular lncRNAs can influence the stability of mRNAs by regulating mRNA translation or interacting partners of microRNAs (51, 52). Nuclear lncRNAs are involved in epigenetic processing and can regulate gene expression at the transcriptional and post-transcriptional levels (51, 52).
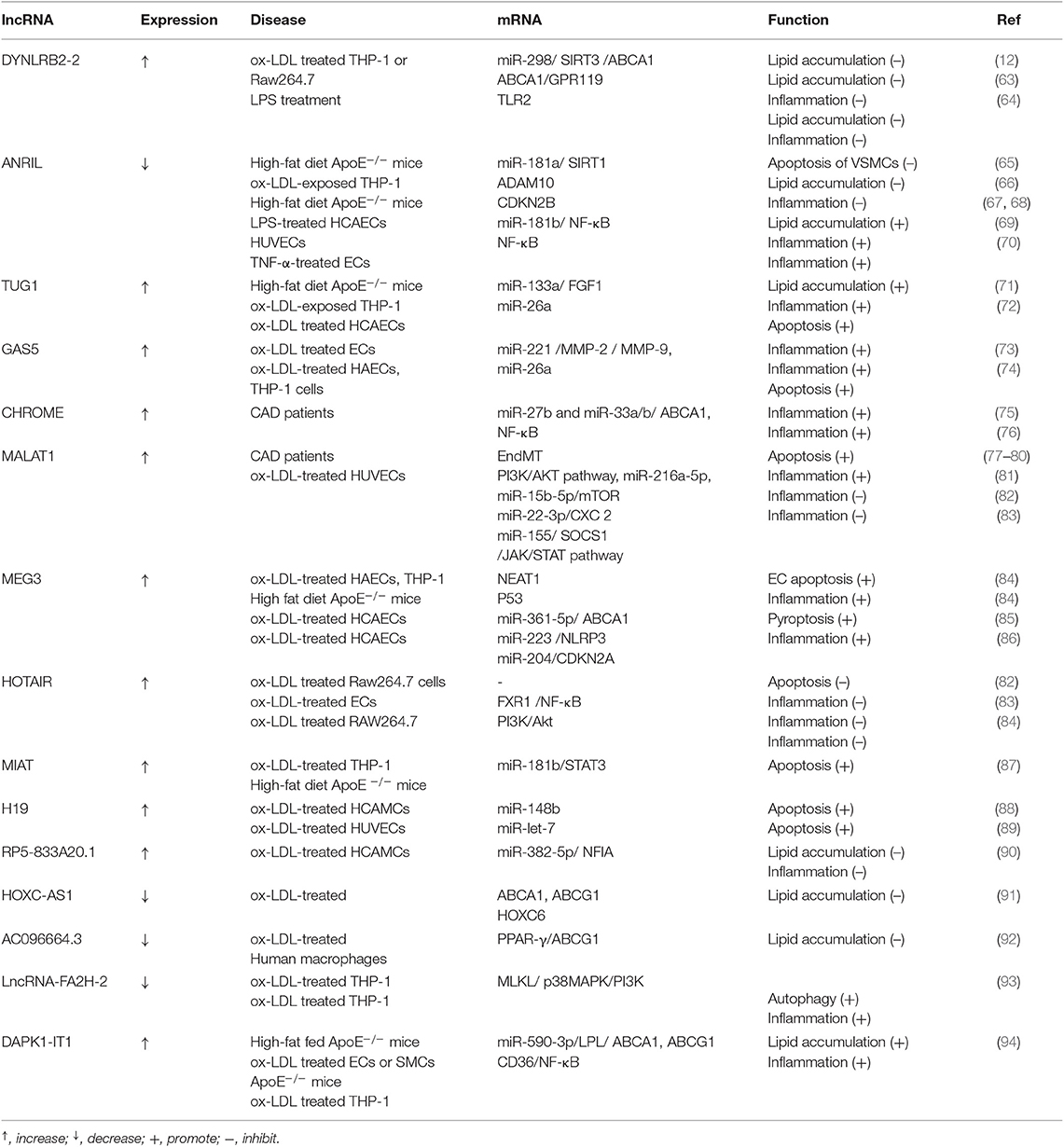
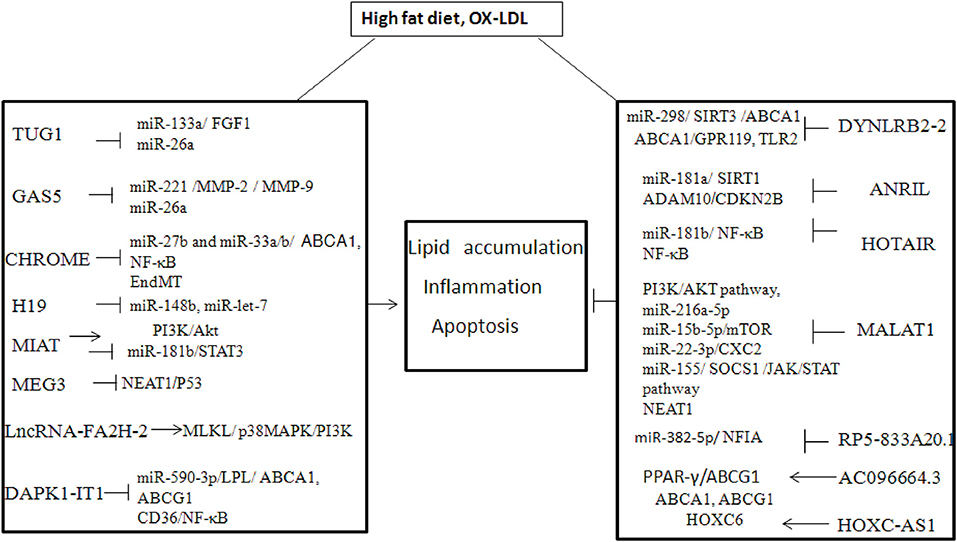
A growing number of studies have found that lncRNAs can influence the progression of atherosclerosis by regulating the function of ECs, vascular smooth muscle cells (VSMCs), vascular inflammation, macrophages, and lipid metabolism (53). The onset of atherosclerosis is partly mediated by EC dysfunction, when subjected to various noxious stimuli (54) For example, lncRNA GAS5 is highly expressed in ECs, and downregulation of lncRNA GAS5 exacerbates hypertension-induced microvascular dysfunction (55). MALAT1 is strongly upregulated in response to hypoxia and induces a switch of ECs from a migratory phenotype to a proliferative phenotype) (56) The proliferation and migration of smooth muscle cells (SMCs) play an important role in atherosclerotic lesion progression and re-stenosis, which are also regulated by lncRNAs (57). It has been shown that lncRNA p21 can repress cell proliferation and stimulate apoptosis in VSMCs by modulating transcriptional activity of p53 both in vitro and in vivo (58). Another study showed that lncRNA Ang362, induced by angiotensin II, also modulates the proliferation of VSMCs (59). In addition, hypoxia-related lncRNA HIF1a-AS1 markedly inhibited proliferation and promoted apoptosis by decreasing B-cell lymphoma 2 (Bcl2) expression and increasing the expression of caspase3 and caspase8 (or caspase9 in ECs) in VSMCs and ECs (60). Macrophages with high cholesterol accumulation can induce chronic inflammation, which facilitates the development of foam cells and atherosclerotic plaques. Many lncRNAs have been identified in atherosclerotic plaques or ox-LDL-induced macrophage-derived foam cells (9). Several lncRNAs are involved in the regulation of cholesterol metabolism and inflammation (61, 62), which might unveil the molecular mechanism of cholesterol-mediated inflammation (Table 1, Figure 1).
lncRNA-DYNLRB2-2
LncRNA DYNLRB2-2 was upregulated in ox-LDL induced THP-1 macrophage-derived foam cells and ox-LDL treated THP-1 or RAW264.7 cells in vitro (12, 95, 95). DYNLRB2-2 displayed an anti-atherosclerotic property by promoting cholesterol efflux and inhibiting inflammation. DYNLRB2-2 inhibited THP-1 macrophage foam cell formation and promoted cholesterol efflux by increasing ABCA1 expression in vitro. DYNLRB2-2 induced ABCA1 expression by sponging miR-298 targeting SIRT3, thereby activating the LKB1/AMPK/mTOR signaling pathway in vitro (12). Another study revealed that the lncRNA-DYNLRB2-2 promoted cholesterol efflux and inhibited inflammation in THP-1 macrophage-derived foam cells by regulating ABCA1 and G protein-coupled receptor 119 (GPR119), respectively (63). Moreover, DYNLRB2-2 was also found to promote cholesterol efflux and inhibit the lipopolysaccharide (LPS)-induced inflammatory cytokines including TNF-α, IL-1β, and IL-6 in macrophages by decreasing TLR2 expression (64). Over-expression of TLR2 reversed the effect of DYNLRB2-2 on cholesterol accumulation and inflammation in macrophages or ApoE−/− mice with a high-fat diet (64). These evidences show that DYNLRB2-2 has therapeutic potential in atherosclerosis.
Anril (CDKN2B-AS1)
LncRNA Antisense non-coding RNA in the INK4 locus (ANRIL), also called CDKN2B antisense RNA 1 (CDKN2B-AS1), has gene polymorphism that is associated with a risk of developing coronary artery disease (CAD) (71, 96–98). For example, the variant of rs10757274 and rs1333049 were associated with the susceptibility of the Han CAD population, indicating that ANRIL might affect the development of atherosclerosis (97). Further study showed that ANRIL could enhance the viability of VSMCs via regulating miR-181a/silent information regulator 1 (SIRT1) (65). In addition, ANRIL downregulation correlated with elevated pro-inflammatory cytokines in patients with acute ischemic stroke, indicating ANRIL modulates inflammation (99). It was suggested that ANRIL could promote cholesterol efflux and inhibit inflammatory cytokines such as IL-1β and TNF-α in ox-LDL-exposed THP-1 macrophages by silencing ADAM (a disintegrin and metalloprotease) 10 (66). ADAMs participated in a variety of metabolic and inflammatory conditions including atherosclerosis, neuro-inflammatory response, and acute lung inflammation (66). In colorectal cancer, ANRIL could sponge miR-Let-7a to enhance the expression of ATP binding cassette subfamily C member 1 (ABCC1), which promotes cholesterol efflux and inhibits inflammation in vivo (100). The circular form of ANRIL (circ-ANRIL) was also identified in human atherosclerotic plaques and conferred atheroprotection in vascular smooth muscle cells and macrophages (101). In contrast, ANRIL was also found to promote the lipid uptake and cholesterol transport in THP-1 macrophage-derived foam cells and mouse models by regulating the CDKN2B promoter (67, 68). ANRIL promotes LPS induced-inflammation in human coronary artery endothelial cells (HCAECs) and human umbilical vein endothelial cells (HUVECs) via sponging miR-181b and then activating NF-κB signaling in vitro (69). Similarly, ANRIL induced by TNF-α also promoted inflammatory cytokines such as IL-6 or IL-8 in ECs through activation of NF-κB (70). Therefore, the effect of ANRIL on lipid metabolism and inflammation needs further study.
TUG1
LncRNA taurine-upregulated gene 1 (TUG1) was upregulated in ox-LDL treated ECs, VSMCs, and macrophages and in high-fat fed ApoE−/− mice (102). The high levels of TUG1 correlated with low levels of miR-26a in high-fat diet ApoE−/− mice and a further study confirmed that TUG1 could accelerate the ox-LDL-induced the apoptosis of ECs by sponging miR-26a (72). MiR-26a was also found to attenuate hyperlipidemia and the inflammatory response in vivo, suggesting the TUG1 might play an important role in cholesterol efflux and inflammation by regulating of miR-26a (103). It was suggested that TUG1 promoted hyperlipidemia and inflammatory cytokines such as IL-6 and TNF-α by sponging miR-133a, which targeted the fibroblast growth factor 1 (FGF1) in ox-LDL-treated RAW264.7 cells (102).
lncRNA GAS5
LncRNA GAS5 was significantly increased in the atherosclerotic plaque collected from patients and in the rat model (10, 11, 104). GAS5 was found to accelerate ox-LDL-induced apoptosis of HCAECs and THP-1 cells by sponging miR-26a in vitro (74). As discussed above, miR-26a could attenuate hyperlipidemia and the inflammatory response in high fat-fed ApoE−/− mice, indicating that GAS5 might affect the development of atherosclerosis via regulation of cholesterol efflux and inflammation (104). Another study showed that GAS5 could promote monocyte migration and production of inflammatory cytokines including IL-6, IL-1β, TNF-α, and MCP-1 via sponging of miR-221, leading to upregulation of MMP-2 and MMP-9 in ox-LDL treated THP-1 cells (73). Consistently, miR-221 was involved in atherosclerotic development by regulating the function of ECs and inflammation (105, 106). It was also found that GAS5 was downregulated in M2 macrophages (107). These evidences suggest that GAS5 might promote the development of atherosclerosis via regulation of EC function, cholesterol efflux, inflammation, and might be used as a therapeutic target.
lncRNA CHROME (AC009948.5, linc-OSBPL6)
LncRNA CHROME (cholesterol homeostasis regulator of miRNA expression), also known as AC009948.5 or linc-OSBPL6, was upregulated in the plasma of patients with atherosclerotic vascular disease (75, 108). CHROME has seven variants that are transcriptionally regulated by the cholesterol-sensing liver X receptors, which are also associated with cellular cholesterol homeostasis. CHROME sponges miR-27b and miR-33a/b, which target ABCA1, promoting cholesterol efflux and HDL metabolism by gene analysis (75). CHROME was higher in the plasma of patients with inflammatory conditions such as coronary artery disease and inhibition of CHROME contributed to the decrease of inflammatory gene expression including NF-κB by transcriptome analyses (76). Therefore, CHROME might promote atherosclerosis development by regulating cholesterol efflux and inflammation.
MALAT1
Long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was first found in non-small cell lung cancer and also found to be upregulated in high-fat diet ApoE−/− mice and patients with unstable angina (109, 110). MALAT1 could protect HUVECs against ox-LDL-induced apoptosis by upregulating endothelial-to-mesenchymal transition (EndMT), which is associated with plaque instability in vitro (77). The protective effect of MALAT1 was also found in ox-LDL-treated HCAECs, partly through competing with miR-22-3p, which targeted the CXC chemokine receptor 2 (81). Other studies revealed that the protective effect of MALAT1 in HUVECs was associated with the induction of autophagy by inhibiting the PI3K/AKT pathway or sponging miR-216a-5p, respectively (78, 79). However, another study revealed that MALAT1 repressed the ox-LDL-induced apoptosis by inhibiting autophagy through sponging miR-15b-5p to activate the mTOR signaling pathway in vitro (80). In addition, MALAT1 also repressed the production of ox-LDL mediated pro-inflammatory cytokines such as IL-6 and IL-8 in HCAECs via sponging miR-155, which targeted Suppressor of cytokine signaling 1 (SOCS1) and restrained JAK/STAT signaling pathway (82). Exosomal MALAT1 released by ox-LDL-treated HUVECs promoted M2 macrophage polarization and decreased the expression of the M1 macrophage marker, IL-12 (111). In MALAT1-deficient ApoE−/− mice, the pro-inflammatory cytokines such as IFN-γ, IL6, and aortic root plaque size were increased. MALAT1 exerted anti-inflammatory effects by interacting with nuclear enriched abundant transcript (NEAT1), which enhanced lipid uptake in macrophages through the formation of par speckles as well as the inflammatory molecules including IL-6, IL-1β, and TNF-α in ox-LDL-treated RAW264.7 cells (83, 112). Collectively, MALAT1 could inhibit ox-LDL-induced EC apoptosis, inflammation and might be targeted for atherosclerosis treatment.
MEG3
Long non-coding RNA maternally expressed gene 3 (MEG3) was significantly decreased in ox-LDL-treated VSMCs and the serum of high fat-fed ApoE−/− mice 103). Inhibition of MEG3 protected VSMCs from ox-LDL-induced injury by increasing p53 expression in vitro (84). Another study showed that MEG3 promoted the ox-LDL-induced apoptosis in VSMCs by sponging the miR-361-5p, which targeted ABCA1 expression (113). As ABCA1 can regulate cholesterol metabolism and inflammation, indicating MEG3 could indirectly affect cholesterol metabolism and inflammation. It was shown that MEG3 enhanced pyroptosis, a highly inflammatory form of programmed cell death, in ox-LDL treated HCAECs by sponging miR-223 targeting NOD-like receptor protein 3 (NLRP3) (85, 114). Consistently, MEG3 was upregulated in ox-LDL treated RAW264.7 cells and increased secretion of TNF-α and IL1β by sponging miR-204, which targeted the cyclin-dependent kinase inhibitor 2A (CDKN2A) (86). Thus, MEG3 promotes the development of atherosclerosis by enhancing EC dysfunction and inflammation.
HOTAIR
Long non-coding RNA Hox transcript antisense intergenic RNA (HOTAIR) was much lower in ECs and peripheral blood lymphocytes from atherosclerotic plaques in CAD patients (115). HOTAIR protected ECs against ox-LDL-induced apoptosis (115, 116). In addition, HOTAIR was also significantly increased in ox-LDL-treated RAW264.7 cells and could reduce lipid accumulation and inhibit the inflammatory response by suppressing FXR1 via the NF-κB pathway (117). In contrast, HOTAIR also found to increase the inflammatory cytokines such as IL-6, IL-1β, in ox-LDL treated THP-1 cells by sponging miR-330-5p (118). Consistently, miR-330-5p inhibited inflammation and promoted macrophage M2 polarization in diabetic mice (119). Thus, the effect of HOTAIR on lipid accumulation and inflammation needs further study.
MIAT
Long non-coding RNA myocardial infarction associated transcript (MIAT), was highly expressed in human carotid plaques, in the serum of patients with vulnerable plaques and high-fat fed ApoE−/− mice (120, 121). MIAT significantly increased lipid content and promoted apoptosis of aortic cells as well as the production of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in ApoE−/− mice through the activation of the PI3K/Akt signaling pathway (121). MIAT promotes the phenotypic transition of SMCs to macrophage-like cells via kruppel-like factor 4 (KLF4) in vitro (122). In addition, MIAT inhibited efferocytosis of macrophages by sponging miR-149-5p, which targeted the anti-phagocytic molecule CD47 in vitro (123). In contrast, MIAT was also found to inhibit ox-LDL-induced apoptosis in HCAMCs partly by inhibition of miR-181b, which induces the upregulation of signal transducer and activator of transcription 3 (STAT3) in vitro (87). Notably, STAT3 is a critical inflammatory mediator in atherosclerosis and inhibition of STAT3 suppressed the ox-LDL induced inflammation in high-fat fed ApoE−/− mice (124). MIAT promotes inflammation and increased lipid content to accelerate atherosclerosis development, however its effect on apoptosis of SMCs needs further study.
H19
Long non-coding RNA H19 was significantly upregulated in the serum of patients with atherosclerosis and those with a high risk of coronary artery disease in a Chinese population (125, 126). In addition, H19 was also induced by ox-LDL and inhibition of H19 protected HUVECs and HAVSMCs against ox-LDL induced apoptosis via sponging miR-148b or miR-let-7, respectively (88, 89). H19 knockdown suppressed the pro-inflammation cytokines such as IL-1β, IL-6, and TNF-α in HUVECS by sponging miR-let-7 in vitro (89). Additionally, H19 was found to regulate lipid metabolism in the liver, suggesting H19 could regulate cholesterol metabolism (127). Knockdown of H19 decreased lipid accumulation and pro-inflammatory factors (TNF-α, IL-1β) and increased the level of anti-inflammatory factors (IL-4, IL-10) in ox-LDL treated RAW264.7 cells by upregulating miR-130b (90). Thus, H19 could link lipid accumulation and inflammation, and knockdown of H19 might be a therapeutic strategy in atherosclerosis.
RP5-833A20.1
Long non-coding RNA RP5-833A20.1 was upregulated in human acute monocytic leukemia macrophage–derived foam cells or induced by ox-LDL (128). RP5-833A20.1 regulated the cholesterol homeostasis and inhibited inflammatory cytokines (TNF-α, IL-1β, and IL-6) in human acute monocytic leukemia macrophages by reducing expression of nuclear factor I A (NFIA), whose genomic location overlaps with RP5-883A20.1, by upregulating miR-382-5p (128). NFIA could stimulate the expression of ABCA1 and ABCG1 but reduce the expression of SRA1 and CD36 in THP-1 macrophages (128). NFIA could be used to treat atherosclerosis by promoting cholesterol homeostasis and inhibiting inflammatory cytokines.
lncRNA HOXC-AS1
Long non-coding RNA-HOXC cluster antisense RNA 1 (HOXC-AS1) was significantly downregulated in atherosclerotic plaques and ox-LDL treated THP-1 macrophages (91). HOXC-AS1 can suppress ox-LDL-induced cholesterol accumulation in THP-1 macrophages by promoting homeobox C6 (HOXC6) expression (91).
lncRNA AC096664.3
Long non-coding RNA AC096664.3 expression was decreased in ox-LDL treated VSMCs and THP-1 macrophages. Downregulation of AC096664.3 caused an increase in total and free cholesterol and decreased ABCG1 by inhibiting the expression of PPAR-γ (92).
lncRNA FA2H-2
Long non-coding RNA FA2H-2 was significantly decreased in high-fat fed ApoE−/− mice and reduced inflammatory cytokines such as MCP-1 and IL-6 in atherosclerotic lesions. FA2H-2 was also reduced in ox-LDL treated ECs or SMCs and could inhibit the activity of NF-κB, which plays an important role -n inflammation. In addition, FA2H-2 also repressed autophagy by inhibiting mixed lineage kinase domain-like protein (MLKL), which can activate the inflammasome via the p38 MAPK/PI3K pathway (93). FA2H-2 could regulate ox-LDL induced inflammation, involved in the development of atherosclerosis.
lncRNA DAPK1-IT1
Long non-coding RNA-DAPK1-IT1 was upregulated in high-fat fed ApoE−/− mice and ox-LDL treated macrophages (94). DAPK1-IT1 decreased the levels of HDL and increased the levels of LDL in the mouse model. DAPK1-IT1 reduced ABCA1 and ABCG1 protein levels in THP-1 macrophages by sponging miR-590–3p, which targeted lipoprotein lipase (LPL). Moreover, the DAPK1-IT1/miR590-3p/LPL axis also increased the production of inflammatory cytokine via activation of CD36, and NF-κB (94). Thus, DAPK1-IT1 plays an important role in lipid metabolism and inflammation.
lncRNA LOC286367 and lncRNA ENST00000602558.1
Long non-coding RNA ENST00000602558.1 was found to promote lipid accumulation in VSMCs, along with downregulation of ABCG1-mediated cholesterol efflux to HDL, through a p65-dependent pathway (129). LncRNA LOC286367 could inhibit ABCA1 expression in macrophages and reduced expression of the LPS-induced pro-inflammatory cytokines including IL-6 and TNF-α. As ABCA1 and ABCG1 play an important role in lipid accumulation and inflammation, these data indicate that lncRNA LOC286367 and lncRNA ENST00000602558.1 link lipid accumulation with inflammation in atherosclerosis (130).
Discussion
Atherosclerosis is usually recognized as a chronic inflammatory disease arising from unbalanced lipid metabolism. Clinically, statins remain the mainstay of treatment of atherosclerosis by lowering LDL cholesterol. However, statins also exert anti-inflammatory effects, suggesting a link between cholesterol accumulation and chronic inflammation. Scavenger receptors (SRs), the ATP-binding cassette transporters A1 and G1 in macrophages, are involved in cholesterol metabolism alone or in cooperation with TLRs and play an important role in inflammation. However, cholesterol accumulation and chronic inflammation were independently investigated to unveil the pathology of atherosclerosis. Recently, lncRNAs have emerged as critical regulators of atherosclerotic processes including EC dysfunction, lipid accumulation, and inflammation and might be used to unveil the molecular mechanism of cholesterol (mainly ox-LDL) accumulation during inflammation or as targets for therapy (53, 54). For example, DYNLRB2-2 promoted cholesterol efflux and inhibited inflammation via activation of ABCA1 and inhibition of TLR2 (64). However, TUG1 promoted hyperlipidemia and inflammatory cytokines such as IL-6 and TNF-α via sponging miR-133a (103). In addition to unveiling the molecular link between cholesterol accumulation in atherosclerosis, lncRNAs might be used as therapeutic targets. For example, MIAT significantly increased lipid content and promoted apoptosis of aortic cells as well as increased inflammatory cytokines, indicating it might be used as a therapeutic target for atherosclerosis (120–122). Specific antisense oligonucleotides treatment targeting MALAT1 could inhibit the growth of mammary carcinoma or metastasis of human non-small cell lung cancer, respectively (131, 132). Thus, specific antisense oligonucleotide treatment targeting lncRNAs represent possible therapeutic strategies for cholesterol accumulation and inflammation, and might have beneficial effects in patients with atherosclerosis.
Author Contributions
YY wrote the article. DS and JWu designed the table and figure. JWa provided ideas and the initial design.
Funding
This study was supported by the National Natural Science Foundation of China (31600728) and Fund of Key Laboratory of Myocardial Ischemia, Ministry of Education.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
LDL, Low-density lipoprotein; ox-LDL, oxidized LDL; lncRNAs, Long non-coding RNAs; EC, endothelial cell; HDL, High-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; lncRNA- GAS5, growth arrest-specific 5; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; PRRs, pattern recognition receptors; SRs, scavenger receptors; LOX-1, Lectin-like oxidized low-density lipoprotein receptor-1; ABCA1, ATP-binding cassette transporters A1; ABCG1, ATP-binding cassette transporters G1; TLRs, Toll-like receptors; PPAR-γ, peroxisome proliferator–activated receptor-γ; LPS, lipopolysaccharide; SIRT1, silent information regulator 1; ANRIL, Antisense non-coding RNA in the INK4 locus; ADAM, a disintegrin and metalloprotease; HUVECs, human umbilical vein endothelial cells; TUG1, taurine-up-regulated gene 1; CHROME, cholesterol homeostasis regulator of miRNA expression; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; EndMT, endothelial-to-mesenchymal transition; NEAT1, neighboring nuclear enriched abundant transcript; MEG3, maternally expressed gene 3; CDKN2A, cyclin-dependent kinase inhibitor 2A; HOTAIR, Hox transcript antisense intergenic RNA; NLRP3, NOD-like receptor protein 3; MIAT, myocardial infarction associated transcript; STAT3, signal transducer and activator of transcription 3; KLF4, kruppel-like factor 4; SMC, smooth muscle cells; STAT3, signal transducer and activator of transcription 3; LPL, lipoprotein lipase; MLKL, mixed lineage kinase domain-like protein; HOXC6, homeobox C6; HOXC-AS1, HOXC cluster antisense RNA.
References
1. Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. (2017) 14:133. doi: 10.1038/nrcardio.2016.185
2. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. (2014) 5:927.
3. Murphy AJ, Dragoljevic D, Tall AR. Cholesterol efflux pathways regulate myelopoiesis: a potential link to altered macrophage function in atherosclerosis. Front. Immunol. (2014) 5:490. doi: 10.3389/fimmu.2014.00490
4. Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc Pharmacol. (2016) 84:1–7. doi: 10.1016/j.vph.2016.05.013
5. Remmerie A, Scott CL. Macrophages and lipid metabolism. Cell Immunol. (2018) 330:27–42. doi: 10.1016/j.cellimm.2018.01.020
6. Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Int Med. (2008) 263:256–73. doi: 10.1111/j.1365-2796.2007.01898.x
7. Rye KA, Barter PJ. Antiinflammatory actions of HDL: a new insight. Arterioscler Thromb Vasc Biol. (2008) 28:1890–1. doi: 10.1161/ATVBAHA.108.173575
8. Archer K, Broskova Z, Bayoumi A, Teoh JP, Davila A, Tang Y, et al. Long non-coding RNAs as master regulators in cardiovascular diseases. Int J Mol Sci. (2015) 16:23651–67. doi: 10.3390/ijms161023651
9. Zhou T, Ding JW, Wang XA, Zheng XX. Long noncoding RNAs and atherosclerosis. Atherosclerosis. (2016) 248:51–61. doi: 10.1016/j.atherosclerosis.2016.02.025
10. Chen L, Yao H, Hui JY, Ding SH, Fan YL, Pan YH, et al. Global transcriptomic study of atherosclerosis development in rats. Gene. (2016) 592:43–8. doi: 10.1016/j.gene.2016.07.023
11. Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J, et al. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE. (2017) 12:e0185406. doi: 10.1371/journal.pone.0185406
12. Li Y, Sun T, Shen S, Wang L, Yan J. LncRNA DYNLRB2-2 inhibits THP-1 macrophage foam cell formation by enhancing autophagy. Biol Chem. (2019) 400:1047–57. doi: 10.1515/hsz-2018-0461
13. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. (2003) 10:63–71. doi: 10.5551/jat.10.63
14. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. (2016) 20:17–28 doi: 10.1111/jcmm.12689
15. De Paoli F, Staels B, Chinetti-Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circ J. (2014) 78:1775–81. doi: 10.1253/circj.CJ-14-0621
16. Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco) bacteria. Proc Natl Acad Sci USA. (2004) 101:4560–5. doi: 10.1073/pnas.0400983101
17. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
18. Lee CW, Hwang I, Park CS, Lee H, Park DW, Kang SJ, et al. Macrophage heterogeneity of culprit coronary plaques in patients with acute myocardial infarction or stable angina. Am J Clin Pathol. (2013) 139:317–22. doi: 10.1309/AJCP7KEYGN3OBGQX
19. De Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. (1999) 66:740–6. doi: 10.1002/jlb.66.5.740
20. Nickel T, Schmauss D, Hanssen H, Sicic Z, Krebs B, Jankl S, et al. oxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiation. Atherosclerosis. (2009) 205:442–50. doi: 10.1016/j.atherosclerosis.2009.01.002
21. Dai XY, Cai Y, Mao DD, Qi YF, Tang C, Xu Q, et al. Increased stability of phosphatase and tensin homolog by intermedin leading to scavenger receptor A inhibition of macrophages reduces atherosclerosis in apolipoprotein E-deficient mice. J Mol Cell Cardiol. (2012) 53:509–20. doi: 10.1016/j.yjmcc.2012.07.006
22. Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. (2007) 75:468–77. doi: 10.1016/j.cardiores.2007.03.010
23. Mäkinen PI, Lappalainen JP, Heinonen SE, Leppänen P, Lähteenvuo MT, Aarnio JV, et al. Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res. (2010) 88:530–8. doi: 10.1093/cvr/cvq235
24. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm. (2013) 2013:152786. doi: 10.1155/2013/152786
25. Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. (2013) 70:2859–72. doi: 10.1007/s00018-012-1194-z
26. Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. (2013) 112:1456–65. doi: 10.1161/CIRCRESAHA.113.301086
27. Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. (2007) 32:172–9. doi: 10.1016/j.tibs.2007.02.001
28. Dove DE, Su YR, Swift LL, Linton MF, Fazio S. ACAT1 deficiency increases cholesterol synthesis in mouse peritoneal macrophages. Atherosclerosis. (2006) 186:267–74. doi: 10.1016/j.atherosclerosis.2005.08.005
29. Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. (2008) 283:22930–41. doi: 10.1074/jbc.M801408200
30. Meurs I, Lammers B, Zhao Y, Out R, Hildebrand RB, Hoekstra M, et al. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis. (2012) 221:41–7. doi: 10.1016/j.atherosclerosis.2011.11.024
31. Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z, et al. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. (2006) 26:2295–300. doi: 10.1161/01.ATV.0000237629.29842.4c
32. Miller YI. Toll-like receptors and atherosclerosis: oxidized LDL as an endogenous Toll-like receptor ligand. Fut Cardiol. (2005) 1:785–92. doi: 10.2217/14796678.1.6.785
33. Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR. Adipocyte fatty acid–binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. (2005) 25:1220–4. doi: 10.1161/01.ATV.0000159163.52632.1b
34. Fu Y, Luo L, Luo N, Garvey WT. Lipid metabolism mediated by adipocyte lipid binding protein (ALBP/aP2) gene expression in human THP-1 macrophages. Atherosclerosis. (2006) 188:102–11. doi: 10.1016/j.atherosclerosis.2005.10.041
35. Todt JC, Hu B, Curtis JL. The scavenger receptor SR-AI/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J Leukoc Biol. (2008) 84:510–8. doi: 10.1189/jlb.0307135
36. Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. (1997) 186:1431–9. doi: 10.1084/jem.186.9.1431
37. Erdman LK, Cosio G, Helmers AJ, Gowda DC, Grinstein S, Kain KC. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol. (2009) 183:6452–9. doi: 10.4049/jimmunol.0901374
38. Stewart CR, Stuart LM, Wilkinson K, Van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. (2010) 11:155. doi: 10.1038/ni.1836
39. Zhao M, Liu Y, Wang X, New L, Han J, Brunk UT. Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. APMIS. (2002) 110:458–68. doi: 10.1034/j.1600-0463.2002.100604.x
40. Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. (2007) 100:1634–42. doi: 10.1161/CIRCRESAHA.107.149724
41. Hafiane A, Genest J. HDL, atherosclerosis, and emerging therapies. Cholesterol. (2013) 2013:891403. doi: 10.1155/2013/891403
42. Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. (2010) 30:139–43. doi: 10.1161/ATVBAHA.108.179283
43. Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. (2010) 51:3196–206. doi: 10.1194/jlr.M006486
44. Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. (2003) 12:805–16. doi: 10.1016/S1097-2765(03)00384-8
45. Hashizume M, Mihara M. Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. Eur J Pharmacol. (2012) 689:249–54. doi: 10.1016/j.ejphar.2012.05.035
46. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. (1998) 93:229–40. doi: 10.1016/S0092-8674(00)81574-3
47. Pirillo A, Reduzzi A, Ferri N, Kuhn H, Corsini A, Catapano AL. Upregulation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) by 15-lipoxygenase-modified LDL in endothelial cells. Atherosclerosis. (2011) 214:331–7. doi: 10.1016/j.atherosclerosis.2010.11.006
48. Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. (2013) 339:159–66. doi: 10.1016/j.canlet.2013.06.013
49. Chen J, Fu Z, Ji C, Gu P, Xu P, Yu N, et al. Systematic gene microarray analysis of the lncRNA expression profiles in human uterine cervix carcinoma. Biomed Pharmacother. (2015) 72:83–90. doi: 10.1016/j.biopha.2015.04.010
50. Ounzain S, Micheletti R, Beckmann T, Schroen B, Alexanian M, Pezzuto I, et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur Heart J. (2014) 36:353–68. doi: 10.1093/eurheartj/ehu180
51. Taylor DH, Chu ETJ, Spektor R, Soloway PD. Long non-coding RNA regulation of reproduction and development. Mol Reprod Dev. (2015) 82:932–56. doi: 10.1002/mrd.22581
52. van Heesch S, van Iterson M, Jacobi J, Boymans S, Essers PB, de Bruijn E, et al. Extensive localization of long noncoding RNAs to the cytosol and mono-and polyribosomal complexes. Genome Biol. (2014) 15:R6. doi: 10.1186/gb-2014-15-1-r6
53. Aryal B, Rotllan N, Fernández-Hernando C. Noncoding RNAs and atherosclerosis. Curr Atheroscler Rep. (2014) 16:407. doi: 10.1007/s11883-014-0407-3
54. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Curr Pharm Des. (2012) 18:4266–88. doi: 10.2174/138161212802481237
55. Wang YNZ, Shan K, Yao MD, Yao J, Wang JJ, Li X, et al. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension. (2016) 68:736–48. doi: 10.1161/HYPERTENSIONAHA.116.07259
56. Lelli A, Nolan KA, Santambrogio S, Gonçalves AF, Schönenberger MJ, Guinot A, et al. Induction of long noncoding RNA MALAT1 in hypoxic mice. Hypoxia. (2015) 3:45. doi: 10.2147/HP.S90555
57. Michalik KM, You X, Manavski Y, Doddaballapur A, Zörnig M, Braun T, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. (2014) 114:1389–97. doi: 10.1161/CIRCRESAHA.114.303265
58. Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. (2012) 95:156–64. doi: 10.1093/cvr/cvs115
59. Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. (2011) 43:621. doi: 10.1038/ng.848
60. Leung A, Trac C, Jin W, Lanting L, Akbany A, Sætrom P, et al. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. (2013) 113:266–78. doi: 10.1161/CIRCRESAHA.112.300849
61. Chen Z. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol Metab. (2016) 5:164–70. doi: 10.1016/j.molmet.2015.12.003
62. Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. (2015) 29:3595–611. doi: 10.1096/fj.14-260323
63. Hu YW, Yang JY, Ma X, Chen ZP, Hu YR, Zhao JY, et al. A lincRNA-DYNLRB2–2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J Lipid Res. (2014) 55:681–97. doi: 10.1194/jlr.M044669
64. Li Y, Shen S, Ding S, Wang L. LincRNA DYN-LRB2-2 upregulates cholesterol efflux by decreasing TLR2 expression in macrophages. J Cell Biochem. (2018) 119:1911–21. doi: 10.1002/jcb.26352
65. Tan P, Guo YH, Zhan JK, Long LM, Xu ML, Ye L, et al. LncRNA-ANRIL inhibits cell senescence of vascular smooth muscle cells by regulating miR-181a/Sirt1. Biochem Cell Biol. (2019) 97:571–80. doi: 10.1139/bcb-2018-0126
66. Li H, Han S, Sun Q, Yao Y, Li S, Yuan C, et al. Long non-coding RNA CDKN2B-AS1 reduces inflammatory response and promotes cholesterol efflux in atherosclerosis by inhibiting ADAM10 expression. Aging. (2019) 11:1695. doi: 10.18632/aging.101863
67. Bochenek G, Häsler R, El Mokhtari NE, König IR, Loos BG, Jepsen S, et al. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. (2013) 22:4516–27. doi: 10.1093/hmg/ddt299
68. Ou M, Li X, Zhao S, Cui S, Tu J. Long Non-Coding RNA CDKN2B-AS1 Contributes to Atherosclerotic Plaque Formation in Apolipoprotein e Knockout Mice by Forming RNA-DNA Triplex in the CDKN2B Promoter (2019). Available online at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3351996
69. Guo F, Tang C, Li Y, Liu Y, Lv P, Wang W, et al. The interplay of LncRNA ANRIL and miR-181b on the inflammation-relevant coronary artery disease through mediating NF-κB signalling pathway. J Cell Mol Med. (2018) 22:5062–75. doi: 10.1111/jcmm.13790
70. Zhou X, Han X, Wittfeldt A, Sun J, Liu C, Wang X, et al. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol. (2016) 13:98–108. doi: 10.1080/15476286.2015.1122164
71. Beigi SSH, Ghaderian SMH, Doosti A. Investigation of the association between rs4977574 A> G polymorphism in ANRIL gene and coronary artery disease in Iranian population. Int Cardiovasc Res J. (2015) 9:139–44.
72. Chen C, Cheng G, Yang X, Li C, Shi R, Zhao N. Tanshinol suppresses endothelial cells apoptosis in mice with atherosclerosis via lncRNA TUG1 up-regulating the expression of miR-26a. Am J Transl Res. (2016) 8:2981.
73. Ye J, Wang C, Wang D, Yuan H. LncRBA GSA5, up-regulated by ox-LDL, aggravates inflammatory response and MMP expression in THP-1 macrophages by acting like a sponge for miR-221. Exp Cell Res. (2018) 369:348–55. doi: 10.1016/j.yexcr.2018.05.039
74. Liang W, Fan T, Liu L, Zhang L. Knockdown of growth-arrest specific transcript 5 restores oxidized low-density lipoprotein-induced impaired autophagy flux via upregulating miR-26a in human endothelial cells. Eur J Pharmacol. (2019) 843:154–61. doi: 10.1016/j.ejphar.2018.11.005
75. van Solingen C, Hennessy EJ, Ouimet M, Rinehold K, Hussein M, Garbedian MJ, et al. Identification of CHROME as a competing endogenous RNA that regulates cholesterol homeostasis. Arterioscler Thromb Vasc Biol. (2016) 36:A403.
76. Scacalossi KR, van Solingen C, Hennessy EJ, Rizzacasa B, Berger JS, Kazan H, et al. LncRNA CHROME is increased in cardiovascular disease and regulates inflammatory gene expression. Arterioscler Thromb Vasc Biol. (2018) 38(Suppl_1):A456. doi: 10.1161/atvb.38.suppl_1.456
77. Li H, Zhao Q, Chang L, Wei C, Bei H, Yin Y, et al. LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway. Lipids Health Dis. (2019) 18:62. doi: 10.1186/s12944-019-1006-7
78. Wang K, Yang C, Shi J, Gao T. Ox-LDL-induced lncRNA MALAT1 promotes autophagy in human umbilical vein endothelial cells by sponging miR-216a-5p and regulating Beclin-1 expression. Eur J Pharmacol. (2019) 858:172338. doi: 10.1016/j.ejphar.2019.04.019
79. Li S, Pan X, Yang S, Ma A, Yin S, Dong Y, et al. LncRNA MALAT1 promotes oxidized low-density lipoprotein-induced autophagy in HUVECs by inhibiting the PI3K/AKT pathway. J Cell Biochem. (2019) 120:4092–101. doi: 10.1002/jcb.27694
80. Zhu Y, Yang T, Duan J, Mu N, Zhang T. MALAT1/miR-15b-5p/MAPK1 mediates endothelial progenitor cells autophagy and affects coronary atherosclerotic heart disease via mTOR signaling pathway. Aging. (2019) 11:1089. doi: 10.18632/aging.101766
81. Tang Y, Jin X, Xiang Y, Chen Y, Shen CX, Zhang YC, et al. The lncRNA MALAT1 protects the endothelium against ox-LDL-induced dysfunction via upregulating the expression of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett. (2015) 589:3189–96. doi: 10.1016/j.febslet.2015.08.046
82. Li S, Sun Y, Zhong L, Xiao Z, Yang M, Chen M, et al. The suppression of ox-LDL-induced inflammatory cytokine release and apoptosis of HCAECs by long non-coding RNA-MALAT1 via regulating microRNA-155/SOCS1 pathway. Nutr Metab Cardiovasc Dis. (2018) 28:1175–87. doi: 10.1016/j.numecd.2018.06.017
83. West JA, Davis CP, Sunwoo H, Simon MD, Sadreyev RI, Wang PI, et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell. (2014) 55:791–802. doi: 10.1016/j.molcel.2014.07.012
84. Liu Y, Jia L, Min D, Xu Y, Zhu J, Sun Z. Baicalin inhibits proliferation and promotes apoptosis of vascular smooth muscle cells by regulating the MEG3/p53 pathway following treatment with ox-LDL. Int J Mol Med. (2019) 43:901–13. doi: 10.3892/ijmm.2018.4009
85. Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J, et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res. (2018) 64:e12449. doi: 10.1111/jpi.12449
86. Yan L, Liu Z, Yin H, Guo Z, Luo Q. Silencing of MEG3 inhibited ox-LDL-induced inflammation and apoptosis in macrophages via modulation of the MEG3/miR-204/CDKN2A regulatory axis. Cell Biol Int. (2019) 43:409–20 doi: 10.1002/cbin.11105
87. Zhong X, Ma X, Zhang L, Li Y, Li Y, He R. MIAT promotes proliferation and hinders apoptosis by modulating miR-181b/STAT3 axis in ox-LDL-induced atherosclerosis cell models. Biomed Pharmacother. (2018) 97:1078–85. doi: 10.1016/j.biopha.2017.11.052
88. Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL-stimulated vascular smooth muscle cells. J Biomed Sci. (2018) 25:11. doi: 10.1186/s12929-018-0418-4
89. Cao L, Zhang Z, Li Y, Zhao P, Chen Y. LncRNA H19/miR-let-7 axis participates in the regulation of ox-LDL-induced endothelial cell injury via targeting periostin. Int Immunopharmacol. (2019) 72:496–503. doi: 10.1016/j.intimp.2019.04.042
90. Han Y, Ma J, Wang J, Wang L. Silencing of H19 inhibits the adipogenesis and inflammation response in ox-LDL-treated Raw264. 7 cells by up-regulating miR-130b. Mol Immunol. (2018) 93:107–14. doi: 10.1016/j.molimm.2017.11.017
91. Huang C, Hu YW, Zhao JJ, Ma X, Zhang Y, Guo FX, et al. Long noncoding RNA HOXC-AS1 suppresses Ox-LDL-induced cholesterol accumulation through promoting HOXC6 expression in THP-1 macrophages. DNA Cell Biol. (2016) 35:722–9. doi: 10.1089/dna.2016.3422
92. Xu BM, Xiao L, Kang CM, Ding L, Guo FX, Li P, et al. LncRNA AC096664. 3/PPAR-γ/ABCG1-dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis. J Cell Biochem. (2019) 120:13775–82. doi: 10.1002/jcb.28650
93. Guo FX, Wu Q, Li P, Zheng L, Ye S, Dai XY, et al. The role of the LncRNAFA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ. (2019) 26:1670–87. doi: 10.1038/s41418-018-0235-z
94. Zhen Z, Ren S, Ji H, Ding X, Zou P, Lu J. The lncRNA DAPK-IT1 regulates cholesterol metabolism and inflammatory response in macrophages and promotes atherogenesis. Biochem Biophys Res Commun. (2019) 516:1234–41. doi: 10.1016/j.bbrc.2019.06.113
95. Hu YW, Zhao JY, Li SF, Wang Q, Zheng L. Genome-wide profiling to analyze the effects of Ox-LDL induced THP-1 macrophage-derived foam cells on gene expression. Genomics Data. (2014) 2:328–31. doi: 10.1016/j.gdata.2014.09.011
96. Congrains A, Kamide K, Oguro R, Yasuda O, Miyata K, Yamamoto E, et al. Genetic variants at the 9p21 locus contribute to atherosclerosis through modulation of ANRIL and CDKN2A/B. Atherosclerosis. (2012) 220:449–55. doi: 10.1016/j.atherosclerosis.2011.11.017
97. Wang S, Song K, Tian H, Liu S, Wang B, Sun G. Association of the CDKN2B-AS1 genetic polymorphisms with coronary artery disease. Int J Clin Exp Med. (2016) 9:23697–701.
98. Rahimi E, Ahmadi A, Boroumand MA, Soltani BM, Behmanesh M. Association of ANRIL expression with coronary artery disease in type 2 diabetic patients. Cell J. (2018) 20:41–5. doi: 10.22074/cellj.2018.4821
99. Feng L, Guo J, Ai F. Circulating long noncoding RNA ANRIL downregulation correlates with increased risk, higher disease severity and elevated pro-inflammatory cytokines in patients with acute ischemic stroke. J Clin Lab Anal. (2019) 33:e22629. doi: 10.1002/jcla.22629
100. Zhang Z, Feng L, Liu P, Duan W. ANRIL promotes chemoresistance via disturbing expression of ABCC1 by regulating the expression of Let-7a in colorectal cancer. Biosci Rep. (2018) 38:BSR20180620. doi: 10.1042/BSR20180620
101. Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. (2016) 7:12429. doi: 10.1038/ncomms12429
102. Zhang L, Cheng H, Yue Y, Li S, Zhang D, He R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc Pathol. (2018) 33:6–15. doi: 10.1016/j.carpath.2017.11.004
103. Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. (2015) 5:9401. doi: 10.1038/srep09401
104. Shen Z, She Q. Association between the deletion allele of ins/del polymorphism (rs145204276) in the promoter region of gas5 with the risk of atherosclerosis. Cell Physiol Biochem. (2018) 49:1431–43. doi: 10.1159/000493447
105. Bazan HA, Hatfield SA, O'Malley CB, Brooks AJ, Lightell D Jr, Woods TC. Acute loss of miR-221 and miR-222 in the atherosclerotic plaque shoulder accompanies plaque rupture. Stroke. (2015) 46:3285–7. doi: 10.1161/STROKEAHA.115.010567
106. Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Human miR-221/222 in physiological and atherosclerotic vascular remodeling. BioMed Res Int. (2015) 2015:354517. doi: 10.1155/2015/354517
107. Ito I, Asai A, Suzuki S, Kobayashi M, Suzuki F. M2b macrophage polarization accompanied with reduction of long noncoding RNA GAS5. Biochem Biophys Res Commun. (2017) 493:170–5. doi: 10.1016/j.bbrc.2017.09.053
108. Hennessy EJ, van Solingen C, Scacalossi KR, Ouimet M, Afonso MS, Prins J, et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primates. Nat Metab. (2019) 1:98. doi: 10.1038/s42255-018-0004-9
109. Gast M, Schroen B, Voigt A, Haas J, Kuehl U, Lassner D, et al. Long noncoding RNA MALAT1-derived mascRNA is involved in cardiovascular innate immunity. J Mol Cell Biol. (2016) 8:178–81. doi: 10.1093/jmcb/mjw003
110. Gast M, Rauch BH, Nakagawa S, Haghikia A, Jasina A, Haas J, et al. Immune system-mediated atherosclerosis caused by deficiency of long non-coding RNA MALAT1 in ApoE–/– mice. Cardiovasc Res. (2018) 115:302–14. doi: 10.1093/cvr/cvy202
111. Huang C, Han J, Wu Y, Li S, Wang Q, Lin W, et al. Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Mol Med Rep. (2018) 18:509–15. doi: 10.3892/mmr.2018.8982
112. Chen DD, Hui LL, Zhang XC, Chang Q. NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J Cell Biochem. (2019) 120:2493–501. doi: 10.1002/jcb.27541
113. Wang M, Li C, Zhang Y, Zhou X, Liu Y, Lu C. LncRNA MEG3-derived miR-361–5p regulate vascular smooth muscle cells proliferation and apoptosis by targeting ABCA1. Am J Transl Res. (2019) 11:3600.
114. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. (2019) 52:e12563. doi: 10.1111/cpr.12563
115. Avazpour N, Hajjari M, Yazdankhah S, Sahni A, Foroughmand AM. Circulating HOTAIR LncRNA is potentially up-regulated in coronary artery disease. Genomics Informatics. (2018) 16:e25. doi: 10.5808/GI.2018.16.4.e25
116. Peng Y, Meng K, Jiang L, Zhong Y, Yang Y, Lan Y, et al. Thymic stromal lymphopoietin-induced HOTAIR activation promotes endothelial cell proliferation and migration in atherosclerosis. Biosci Repo. (2017) 37:BSR20170351. doi: 10.1042/BSR20170351
117. Pang JL, Wang JW, Hu PY, Jiang JS, Yu C. Hotair alleviates ox-ldl-induced inflammatory response in raw264.7 cells via inhibiting NF-κB pathway. Eur Rev Med Pharmacol Sci. (2018) 22:6991–8. doi: 10.26355/eurrev_201810_16170
118. Liu J, Huang GQ, Ke ZP. Silence of long intergenic noncoding RNA HOTAIR ameliorates oxidative stress and inflammation response in ox-LDL-treated human macrophages by upregulating miR-330-5p. J Cell Physiol. (2019) 234:5134–42. doi: 10.1002/jcp.27317
119. Sun J, Huang Q, Li S, Meng F, Li X, Gong X. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol Immunol. (2018) 95:107–13. doi: 10.1016/j.molimm.2018.02.006
120. Tan J, Liu S, Jiang Q, Yu T, Huang K. LncRNA-MIAT increased in patients with coronary atherosclerotic heart disease. Cardiol Res Pract. (2019) 2019:6280194. doi: 10.1155/2019/6280194
121. Sun G, Li Y, Ji Z. Up-regulation of MIAT aggravates the atherosclerotic damage in atherosclerosis mice through the activation of PI3K/Akt signaling pathway. Drug Deliv. (2019) 26:641–9. doi: 10.1080/10717544.2019.1628116
122. Li Y, Jin H, Perisic L, Chernogubova E, Hansson GK, Hedin U, et al. Long non-coding RNA MIAT regulates smooth muscle cell plasticity and macrophage activity in advanced atherosclerosis lesions. Circulation. (2017) 136:A15313.
123. Ye ZM, Yang S, Xia YP, Hu RT, Chen S, Li BW, et al. LncRNA MIAT sponges miR-149–5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. (2019) 10:138. doi: 10.1038/s41419-019-1409-4
124. Wang R, Zhang Y, Xu L, Lin Y, Yang X, Bai L, et al. Protein inhibitor of activated STAT3 suppresses oxidized LDL-induced cell responses during atherosclerosis in apolipoprotein E-deficient mice. Sci Rep. (2016) 6:36790. doi: 10.1038/srep36790
125. Bitarafan S, Yari M, Broumand MA, Ghaderian SMH, Rahimi M, Mirfakhraie R, et al. Association of increased levels of lncRNA H19 in PBMCs with risk of coronary artery disease. Cell J. (2019) 20:564–8. doi: 10.22074/cellj.2019.5544
126. Zhang Z, Gao W, Long QQ, Zhang J, Li YF, Yan JJ, et al. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. (2017) 7:7491. doi: 10.1038/s41598-017-07611-z
127. Liu C, Yang Z, Wu J, Zhang L, Lee S, Shin DJ, et al. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology. (2018) 67:1768–83. doi: 10.1002/hep.29654
128. Hu YW, Zhao JY, Li SF, Huang JL, Qiu YR, Ma X, et al. RP5-833A20. 1/miR-382-5p/NFIA–dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. (2015) 35:87–101. doi: 10.1161/ATVBAHA.114.304296
129. Cai C, Zhu H, Ning X, Li L, Yang B, Chen S, et al. LncRNA ENST00000602558. 1 regulates ABCG1 expression and cholesterol efflux from vascular smooth muscle cells through a p65-dependent pathway. Atherosclerosis. (2019) 285:31–9 doi: 10.1016/j.atherosclerosis.2019.04.204
130. Ma X, Wang T, Zhao ZL, Jiang Y, Ye S. Propofol suppresses proinflammatory cytokine production by increasing ABCA1 expression via mediation by the long noncoding RNA LOC286367. Mediat Inflamm. (2018) 2018: 8907143. doi: 10.1155/2018/8907143
131. Gutschner T, Hämmerle M, Eißmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. (2013) 73:1180–9. doi: 10.1158/0008-5472.CAN-12-2850
Keywords: atherosclerosis, ox-LDL, lncRNAs, cholesterol accumulation, inflammation
Citation: Yan Y, Song D, Wu J and Wang J (2020) Long Non-Coding RNAs Link Oxidized Low-Density Lipoprotein With the Inflammatory Response of Macrophages in Atherogenesis. Front. Immunol. 11:24. doi: 10.3389/fimmu.2020.00024
Received: 09 September 2019; Accepted: 07 January 2020;
Published: 30 January 2020.
Edited by:
Sermin Genc, Dokuz Eylul University, TurkeyReviewed by:
Bunyamin Akgul, Izmir Institute of Technology, TurkeyJudith Sluimer, Maastricht University, Netherlands
Copyright © 2020 Yan, Song, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junnan Wang, amRleXdqbkAxNjMuY29t
 Youyou Yan
Youyou Yan Dandan Song3
Dandan Song3 Junnan Wang
Junnan Wang