- 1Laboratory of Aquaculture & Artemia Reference Center, Faculty of Bioscience Engineering, Ghent University, Gent, Belgium
- 2Department of Animal Nutrition and Management, Faculty of Veterinary Medicine and Animal Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 3Laboratory of Immunology, Faculty of Veterinary Medicine, Ghent University, Merelbeke, Belgium
- 4Laboratory of Immunology and Animal Biotechnology, Faculty of Bioscience Engineering, Ghent University, Gent, Belgium
β-Glucans are a heterogeneous group of glucose polymers with a common structure comprising a main chain of β-(1,3) and/or β-(1,4)-glucopyranosyl units, along with side chains with various branches and lengths. β-Glucans initiate immune responses via immune cells, which become activated by the binding of the polymer to specific receptors. However, β-glucans from different sources also differ in their structure, conformation, physical properties, binding affinity to receptors, and thus biological functions. The mechanisms behind this are not fully understood. This mini-review provides a comprehensive and up-to-date commentary on the relationship between β-glucans' structure and function in relation to their use for immunomodulation.
Introduction
β-Glucans are a group of naturally occurring polysaccharides which are widely distributed in bacteria, fungi, algae, and cereals, in which they are part of the cell wall structure and have many other biological activities (1). Structurally, β-glucans are long or short-chain polymers of β-(1,3) or β-(1,4) linked glucose subunits which may be branched, with the side chains branching from the six-position of the backbone (2, 3). For example, β-glucans of mushrooms have short β-(1,6)-linked branches whereas those of yeast have β-(1,6)-side branches with additional β-(1,3) regions (4). Supplementary Figure 1 summarizes different chemical structures of β-glucans (5). Furthermore, β-glucans could also form secondary structures and the possibility of various structural forms could lead to differences in the mechanisms behind the immunomodulating activities (6, 7). The literature on immune responses to glucans can be quite confusing as what is observed for one preparation of glucan is often inappropriately extrapolated to all glucans. When discussing the immune-modulator functions of glucans, here we mostly considered β-1,3-glucan purified from fungal cell walls.
The immunomodulatory properties of β-glucans have long been recognized (8). The activation of the immune system through modulation by β-glucans is rather complex and depends on many factors that have not yet been fully revealed. β-Glucan is a key pathogen-associated molecular pattern (PAMP) that is detected upon fungal infection to trigger the host's immune responses in both vertebrates and invertebrates (9). The induction of cellular responses by β-glucans is a result of their specific interaction with several pattern recognition receptors (PRRs), such as Dectin-1, complement receptor 3 (CR3), selected scavenger receptors, and lactosylceramide (LacCer). Receptor binding triggers a signal transduction in monomorphonuclear phagocytes (e.g., macrophages, monocytes, dendritic cells, and natural killer cells) and neutrophils (10–12). The activity of these β-glucan receptors seems to be highly dependent on the cell types. Research demonstrated that neutrophil modulation by β-glucan is predominantly CR3 dependent while Dectin-1 is the most important β-glucan receptor on macrophages (13–15). Upon β-glucan binding to the lectin site of the CR3 on phagocytes and NK cells, the receptor was activated to enhance the cytotoxicity against iC3b-opsonized target cells, including tumors (16, 17). Recognition of β-glucan by Dectin-1 on macrophages activates the downstream signaling pathway. As a consequence of these signaling activations, Dectin-1 triggers phagocytosis, ROS generation, microbial killing, and cytokine production (18, 19). Moreover, recent studies demonstrated that pre-administration of β-glucans resulted in innate immune memory, protecting the mice against re-infection with a lethal Escherichia coli (20). Increased protection was related to the function of “trained” monocytes (21). Innate immune memory is defined as a heightened response to a secondary infection that can be exerted toward both homologous and heterologous microorganisms. For the underlying mechanisms, epigenetic modifications and metabolic reprogramming do play crucial roles (22–24). It is acknowledged that particulate β-glucans may be the optimal preparation to induce innate immune memory, whereas low molecular weight β-glucans (e.g., laminarin) do not favor a high response (25, 26). Figure 1 presents the different consequences of β-glucan recognition by monomorphonuclear phagocytes.
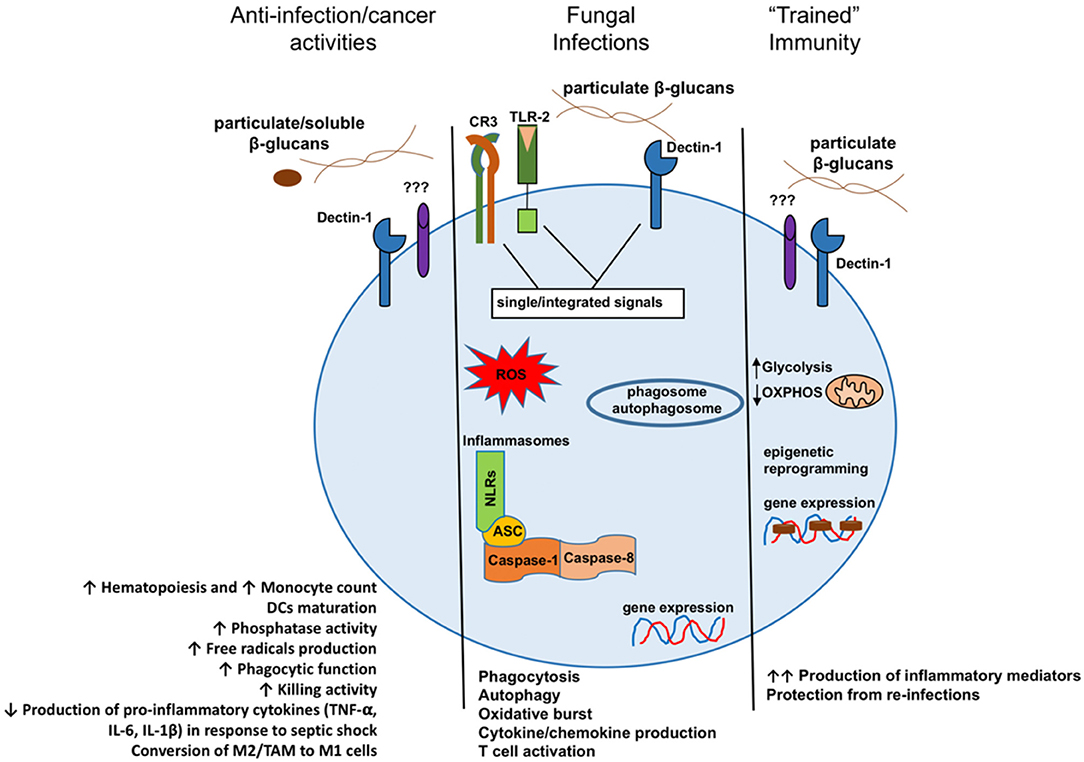
Figure 1. Model presenting the different consequences of β-glucan recognition by monomorphonuclear phagocytes in the context of antitumoral activities, fungal infection recognition, or the creation of innate immune memory. DCs, dendritic cells; M2, alternatively activated macrophages; TAM, tumor-associated macrophages; M1, classically activated macrophages. Reproduced from Camilli et al. (27) with permission and under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
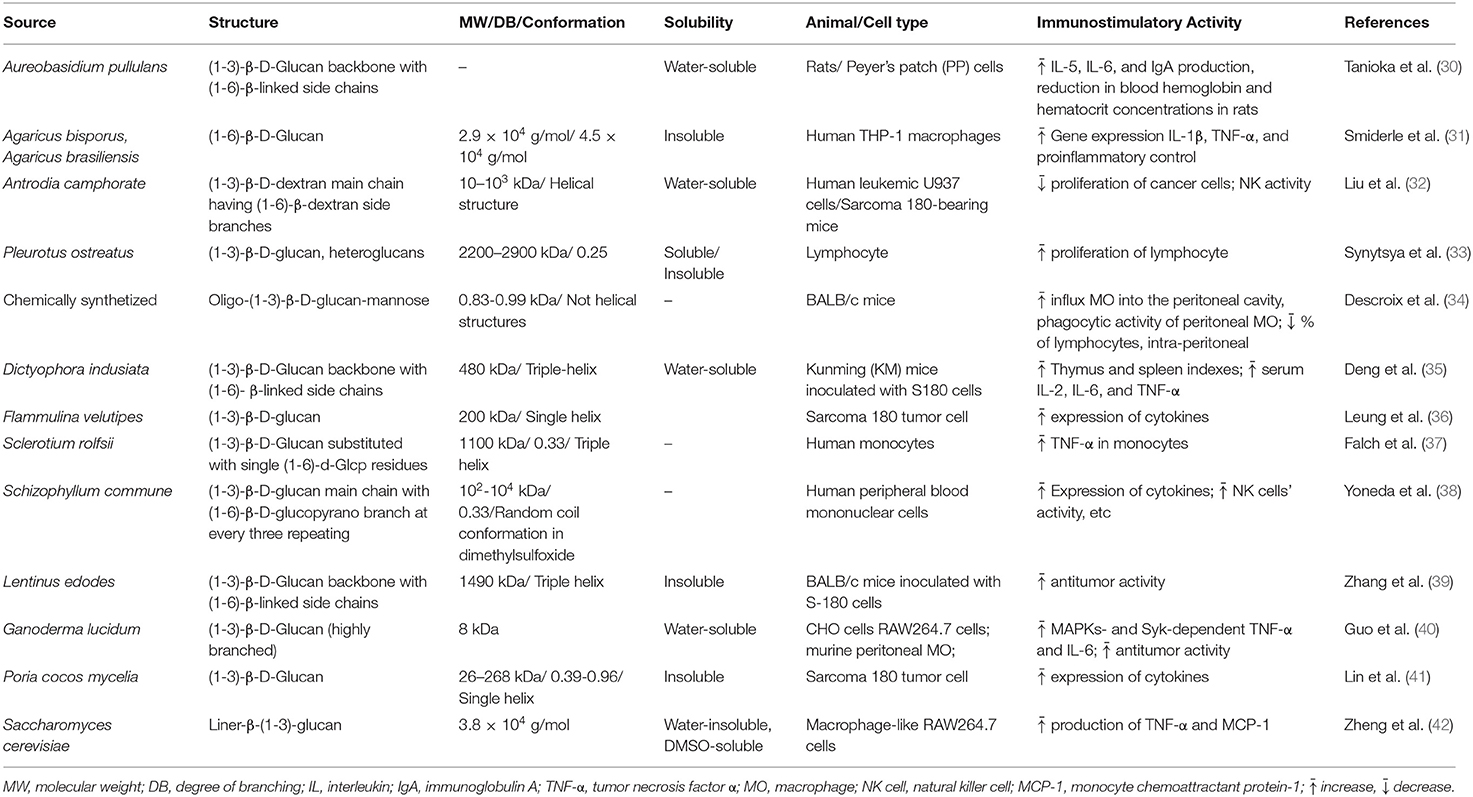
Previous reports indicate that immunomodulatory effects of glucans could be influenced by differences in their structural characteristics such as branching frequency, solubility, molecular weight, polymer charge, and conformation in solution (7, 28, 29). It is still unclear how structural differences of glucans might affect their biological functions. This is partly due to the use of β-glucan polymers with different structural characteristics. Research studies on β-glucan have continued to grow since the 1950s when studies on these biomolecules began to be published. According to the Scopus Database (see Supplementary Figure 2), as of 2019, 9,652 papers had been related to immunological activities of β-glucan while <1 fifth of the papers included the term “structure” in the title and/or abstract. Systematic studies providing structure-function relationships for immunostimulation by β-glucans are generally lacking. Different immunological effects have been described and this might be related to the use of untreated, denatured, and renatured β-(1,3)-D-glucans which all differ in their structure. Thus, incorrect conclusions can be easily drawn when comparing results obtained by using such diverse β-glucans. In Table 1, the relationship between β-glucan structure and observed immunomodulatory properties has been summarized. This mini-review will try to summarize up-to-date information on the structure-functional relationship of β-glucans in relation to immunomodulation.
Molecular Weight
Some evidences suggest that the immunomodulating activities of glucans are related to their molecular weight (MW), with higher MW glucans having more effect on the immune system. This is perhaps not so surprising as, in general, antigens with a higher MW are more immunogenic. However, maybe glucans with a high MW have a more stabilized structure and can be recognized directly by specific receptors on the surface of immune cells (43). Another influencing factor is the retention time in the intestinal system, where glucans are degraded and metabolized slowly. Differences in uptake from the intestinal lumen are dependent on their MW (44).
β-Glucans with a low MW and a short side chain (<5,000–10,000 MW) are commonly regarded as inactive (45). Besides, Brown and Gordon (44) demonstrated that immune cells can be directly activated by β-glucans with a high MW (such as zymosan), stimulating their phagocytic, cytotoxic, and antimicrobial activities, while low MW β-glucans need cytokines to modulate the immune response. The biological activities of a β-(1,3)-glucan isolated from Grifola frandosa changed with its MW, with the highest MW glucan always showing the most potent immunomodulatory effect (46). The same can also be applied to polysaccharide-K (Krestin, PSK,), a protein-bound polysaccharide obtained from basidiomycetes showing the strongest immunestimulating activities for PSK with the highest MW (>200 KDa) (47). However, controversial data on immunomodulating capacities of high molecular weight β-glucans vs. low molecular weight β-glucans still exist. For example, lentinan and schizophyllan, both pure β-(1,3)-glucans extracted from Lentinus edodes and Schizophyllum commune, respectively, exhibit the same antitumor activity against a murine cancer cell (Sarcoma 180) regardless of the use of a high or low MW form (48). Lei et al. (49), reported that a yeast β-glucan with low MW was better as an antioxidant and immunostimulant compared to the high MW form.
Molecular Structure
Backbone
In vitro, the murine Dectin-1 binding capacity of glucans in relation to their structural features was investigated by Adams et al. (50). It was demonstrated that the β-(1,3)-D-glucopyranosyl backbone of the glucans is essential for Dectin-1 recognition. Dectin-1 cannot recognize non-β-linked glucans (e.g., mannan or pullulan) and glucans isolated from plants (e.g., oats, barley, and wheat) who have a backbone of linear D-glucopyranosyl residues with a mixture of β-(1,3) and β-(1,4) linkages (51). Also, Dectin-1 did not interact with linear β-(1,3)-D-glucan oligosaccharides shorter than seven glucose subunits and β-1,3-glucans without any side chains (branches). Summarized, the minimal glucan subunit structure for Dectin-1 activation is a β-(1,3)-D-glucan oligosaccharide containing a backbone with at least seven glucose subunits and a single (1,6)-β-linked side-chain branch at the non-reducing end (50).
Sidechain
The side chain length and branching frequency are also crucial for the immunomodulating ability of β-glucans (45). A previous study showed that glucans with only one single glucose molecule in the side chain had a lower macrophage activating ability than glucans extracted from the same yeast but with more glucose in the side chain (52). It has been reported that β-glucans with a branching ratio between 0.2 (1:5 branching)-−0.33 (1:3 branching) are most potent immunomodulators (9, 53). However, exceptions do exist as the binding affinity for CR3 between schizophyllan and scleroglucan differs greatly although they have a similar branching frequency. Also, when comparing the binding affinity of laminarin (1:10 branching) and schizophyllan (1:3 branching) for CR3, an insignificant difference was observed (21 μM vs. 11 μM) (1). Moreover, a branched β-glucan named pachyman, obtained from Poria cocos, has no anti-tumor activity, while debranched pachyman exhibits significant anti-tumor activity (54). A possible explanation might be that the glucans with a higher degree of branching could stereochemically interfere with each other, leading to less binding by specific receptors (15). The discrepancy of these results remains to be clarified.
Conformation
Glucans can also form secondary structures, and this depends on the conformation of sugar residues, MW, and the inter- and intra-chain hydrogen-bonding (6). β-glucans exist in three conformations: single helix, triple helix and random coil. Whether a single helix or triple-helix β-(1-3)-D-glucan conformation has the highest biological activity is still an unresolved issue. The literature data appear inconsistent and are often contradictory. However, a single helix conformation is usually less stable than the triple helix conformation. In vivo, β-1,3-glucan assumes a triple-helical structure in which one β-1,3-glucan chain forms inter-strand hydrogen bonds with two other strands perpendicular to the axis of the triple helix. Thus, the triple helix conformation is the main structure in the cell wall of most fungi and recognition of the triple-helical β-1,3-glucan by an immune receptor is important for immune signaling (1, 55). Glucans with a single helix conformation showed a lower ability to suppress tumor growth (37) than glucans with a triple helix conformation. On the other hand, Saitô et al. (56), verified that β-(1–3)-D-glucans with a single strand chain showed a higher bioactivity than the β-glucans with a helix structure. The relationship between conformation and immunomodulatory properties of β-glucans suggest the existence of a biological system which can recognize the different conformations in the host's body. Hence, the relationship between glucan conformation and bioactivity still needs further study.
Solubility
The physical properties of β-glucan, such as solubility, can also be impacted by molecular features, such as linkage pattern and molecular weight (57). Both soluble and particulate glucans have been reported to stimulate the immune response. For example, human whole blood incubated with soluble glucan isolated from yeast (Biotec Pharmacon ASA, Tromsø, Norway) showed an increased production of tumor necrosis factor alpha (TNF-α), Interleukin-6 (IL-6), the chemokine CXCL8 and the monocyte tissues factor (TF) (58). To date, soluble glucans, for their ease of delivery in vivo, have been widely used in clinical applications, whereas particulate glucans may be more effective in a local rather than systemic immunomodulatory effect (59). The difference can be explained by the use of different receptors by soluble and particulate β-glucans. Particulate β-glucans directly stimulate immune cell activation through Dectin-1 pathways while soluble glucans require a complement and CR3-dependent pathway activation for their antitumor activities (60). Moreover, Goodridge et al. (25) demonstrated that Dectin-1, in contrast to other PRRs, discriminates between soluble and particulate β-glucans. Phagocytosis and cytokine production by macrophages are only induced when Dectin-1 is bound to particulate β-glucan through the formation of a “phagocytic synapse” and the exclusion of regulatory phosphatases. This process represents a unique mechanism to discriminate PAMPs associated with a microbial surface.
Particle Size
Particulate β-glucans can also be used as adjuvants for chemotherapy as well as adjuvants in vaccines for their additional effects on the immune system (61, 62). They could enhance hematopoietic responses in animal models under chemotherapy, by increasing interleukin-6 levels (63). Particulate β-glucans isolated from yeast are hollow, porous 2–4 μm spheres with an outer shell capable of mediating uptake by phagocytic cells. Therefore, the high payload of therapeutic agents, such as DNA, siRNA, protein/ peptide, and small molecules could be reduced by encapsulating these agents into the particles using a core-polyplex and layer-by-layer synthetic strategies and be applied to optimize the tumor microenvironment for cancer immunotherapy (64). For example, an in situ layer-by-layer syntheses of DNA-caged yeast β-glucan particles was shown to not only effectively protect the caged DNA from degradation but also facilitate the systemic delivery of the DNA content to macrophages in vivo (65, 66). The particle size of glucan matters and its generally known that nanoparticles with a diameter 1–2 μm are better absorbed by macrophages than large-size particles (67). However, a recent study showed that large (curdlan, up to 0.2 mm diameter) β-glucan-stimulated human dendritic cells (DCs) generate significantly more IL-1β, IL-6, and IL-23 compared to those stimulated with the smaller β-glucans (glucan microparticles; 1–5 μm diameter) (68). Additional studies are needed to investigate how the β-glucan size mediates the immune response.
Conclusions and Perspectives
The structural and physical features of β-glucans determine their way of acting on the immune system. So, while describing the results of different experiments on the immunomodulatory properties of glucans, one should ideally provide a thorough description of the structural features of the glucans under study. Information on solubility, particle size, molecular weight, sidechain branching frequency and conformation should be provided. Also, we need well-characterized (1,3)-β-glucan polymers with varying structural characteristics when studying the influence of carbohydrates on the biological activity of glucans. Synthetic glucans could provide a unique opportunity to investigate the immunomodulating activities of glucans (50, 69). Also, a standardized commercial glucan with assured quality control (such as for instance a commercial glucan extracted from Saccharomyces cerevisiae) could be systematically used as a positive control, to compare the immunomodulatory activities with experimental glucans being tested. In addition, differences in reactivity of β-glucans in individuals and between different strains of test animals should also be taken into consideration when comparing studies (70).
It should also be noted that the isolation method may influence the characteristics of β-glucans and differences can be expected when glucans are isolated from the same sources (71). The most appropriate isolation method, dependent on the source of the β-glucan and the extraction procedure, must not affect the molecular integrity of the glucan and needs to guarantee product purity and optimal yield (72). Furthermore, chemical modification could be an effective way to enhance the biological activities of glucans. Several methods have been applied to change the physical properties of β-glucans (e.g., solubility) in order to improve their functional properties via chemical and physical cross-linking effects (such as carboxymethylation, sulfidation, and oxidation) (73, 74).
Author Contributions
The review results from the discussion and the consensus of all authors listed BH, KB, EC, DV, and PB. The review was written by BH.
Funding
The authors acknowledge financial support from China Scholarship Council and the Belgian Science Policy Office (BELSPO) project entitled AquaStress, project number: IUAPVII/64/Sorgeloos.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.00658/full#supplementary-material
References
1. Mueller A, Raptis J, Rice PJ, Kalbfleisch JH, Stout RD, Ensley HE, et al. The influence of glucan polymer structure and solution conformation on binding to (1–>3)-beta-D-glucan receptors in a human monocyte-like cell line. Glycobiology. (2000) 10:339–46. doi: 10.1093/glycob/10.4.339su
2. Kapteyn JC, Hoyer LL, Hecht JE, Muller WH, Andel A, Verkleij AJ, et al. The cell wall architecture of Candida albicans wild-type cells and cell wall-defective mutants. Mol Microbiol. (2000) 35:601–11. doi: 10.1046/j.1365-2958.2000.01729.x
3. Kim YT, Kim E-H, Cheong C, Williams DL, Kim C-W, Lim S-TJCR. Structural characterization of β-D-(1 →3, 1 →6)-linked glucans using NMR spectroscopy. Carbohyd Res. (2000) 328:331–41. doi: 10.1016/S0008-6215(00)00105-1
4. Meng X, Liang H, Luo L. Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr Res. (2016) 424:30–41. doi: 10.1016/j.carres.2016.02.008
5. Barsanti L, Passarelli V, Evangelista V, Frassanito AM, Gualtieri P. Chemistry, physico-chemistry and applications linked to biological activities of beta-glucans. Nat Prod Rep. (2011) 28:457–66. doi: 10.1039/c0np00018c
6. Cooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. J Current medicinal chemistr. (2000) 7:715–29. doi: 10.2174/0929867003374705
7. Chen J, Seviour R. Medicinal importance of fungal beta-(1–>3), (1–>6)-glucans. Mycol Res. (2007) 111:635–52. doi: 10.1016/j.mycres.2007.02.011
8. Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. (2009) 230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x
9. Soltanian S, Stuyven E, Cox E, Sorgeloos P, Bossier P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit Rev Microbiol. (2009) 35:109–38. doi: 10.1080/10408410902753746
10. Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. (2001) 413:36–7. doi: 10.1038/35092620
11. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nature. (2007) 8:31. doi: 10.1038/ni1408
12. Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. (2009) 2:25. doi: 10.1186/1756-8722-2-25
13. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 is a major β-glucan receptor on macrophages. J Exp Med. (2002) 196:407–12. doi: 10.1084/jem.20020470
14. Van Bruggen R, Drewniak A, Jansen M, Van Houdt M, Roos D, Chapel H, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol Immunol. (2009) 47:575–81. doi: 10.1016/j.molimm.2009.09.018
15. Baert K, Sonck E, Goddeeris BM, Devriendt B, Cox E. Cell type-specific differences in beta-glucan recognition and signalling in porcine innate immune cells. Dev Comp Immunol. (2015) 48:192–203. doi: 10.1016/j.dci.2014.10.005
16. Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. (1996) 98:50–61. doi: 10.1172/JCI118777
17. Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. (1999) 42:61–74. doi: 10.1016/S0162-3109(99)00013-2
18. Rice PJ, Kelley JL, Kogan G, Ensley HE, Kalbfleisch JH, Browder IW, et al. Human monocyte scavenger receptors are pattern recognition receptors for. (1–>3)-beta-D-glucans. J Leukoc Biol. (2002) 72:140–6. doi: 10.1189/jlb.72.1.140
19. Sato T, Iwabuchi K, Nagaoka I, Adachi Y, Ohno N, Tamura H, et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J Leukoc Biol. (2006) 80:204–11. doi: 10.1189/jlb.0106069
20. Quintin, J. Fungal mediated innate immune memory, what have we learned? Semin Cell Dev Biol. (2019) 89, 71–7. doi: 10.1016/j.semcdb.2018.05.023
21. Netea MG, Quintin J, Van Der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. (2011) 9:355–61. doi: 10.1016/j.chom.2011.04.006
22. Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR-and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. (2014) 345:1250684. doi: 10.1126/science.1250684
23. Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. (2014) 345:1251086. doi: 10.1126/science.1251086
24. Norouzitallab P, Baruah K, Vanrompay D, Bossier P. Can epigenetics translate environmental cues into phenotypes? Sci Total Environ. (2019) 647:1281–93. doi: 10.1016/j.scitotenv.2018.08.063
25. Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. (2011) 472:471–5. doi: 10.1038/nature10071
26. Zhang Z, Chi H, Dalmo RA. Trained innate immunity of fish is a viable approach in larval aquaculture. Front Immunol. (2019) 10:42. doi: 10.3389/fimmu.2019.00042
27. Camilli G, Tabouret G, Quintin J. The complexity of fungal beta-glucan in health and disease: effects on the mononuclear phagocyte system. Front Immunol. (2018) 9:673. doi: 10.3389/fimmu.2018.00673
28. Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by beta-glucans. Physiol Behav. (2008) 94:276–84. doi: 10.1016/j.physbeh.2007.11.045
29. Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structure-function relationships of immunostimulatory polysaccharides: a review. Carbohydr Polym. (2015) 132:378–96. doi: 10.1016/j.carbpol.2015.05.079
30. Tanioka A, Tanabe K, Hosono A, Kawakami H, Kaminogawa S, Tsubaki K, et al. Enhancement of intestinal immune function in mice by β-D-glucan from aureobasidium pullulans ADK-34. Scand J Immunol. (2013) 78:61–8. doi: 10.1111/sji.12067
31. Smiderle FR, Alquini G, Tadra-Sfeir MZ, Iacomini M, Wichers HJ, Van Griensven LJ. Agaricus bisporus and Agaricus brasiliensis (1→ 6)-β-d-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohydr Polym. (2013) 94:91–9. doi: 10.1016/j.carbpol.2012.12.073
32. Liu J-J, Huang T-S, Hsu M-L, Chen C-C, Lin W-S, Lu F-J, et al. Antitumor effects of the partially purified polysaccharides from Antrodia camphorata and the mechanism of its action. Toxicol Appl Pharmacol. (2004) 201:186–93. doi: 10.1016/j.taap.2004.05.016
33. Synytsya A, Míčková K, Synytsya A, Jablonský I, Spěváček J, Erban V, et al. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr Polym. (2009) 76:548–56. doi: 10.1016/j.carbpol.2008.11.021
34. Descroix K, Větvička V, Laurent I, Jamois F, Yvin J-C, Ferrières V. New oligo-β-(1, 3)-glucan derivatives as immunostimulating agents. Bioorg Med Chem. (2010) 18:348–57. doi: 10.1016/j.bmc.2009.10.053
35. Deng C, Fu H, Teng L, Hu Z, Xu X, Chen J, et al. Anti-tumor activity of the regenerated triple-helical polysaccharide from Dictyophora indusiata. Int J Biol Macromol. (2013) 61:453–8. doi: 10.1016/j.ijbiomac.2013.08.007
36. Leung M, Fung K, Choy Y. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacology. (1997) 35:255–63. doi: 10.1016/S0162-3109(96)00157-9
37. Falch BH, Espevik T, Ryan L, Stokke BT. The cytokine stimulating activity of (1→ 3)-β-D-glucans is dependent on the triple helix conformation. J Carbohydr Res. (2000) 329:587–96. doi: 10.1016/S0008-6215(00)00222-6
38. Yoneda K, Ueta E, Yamamoto T, Osaki T. Immunoregulatory effects of Sizofiran. (SPG) on lymphocytes and polymorphonuclear leukocytes. Clin Exp Immunol. (1991) 86:229–35. doi: 10.1111/j.1365-2249.1991.tb05801.x
39. Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr Res. (2005) 340:1515–21. doi: 10.1016/j.carres.2005.02.032
40. Guo L, Xie J, Ruan Y, Zhou L, Zhu H, Yun X, et al. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int Immunopharmacol. (2009) 9:1175–82. doi: 10.1016/j.intimp.2009.06.005
41. Lin Y, Zhang L, Chen L, Jin Y, Zeng F, Jin J, et al. Molecular mass and antitumor activities of sulfated derivatives of α-glucan from Poria cocos mycelia. Int J Biol Macromol. (2004) 34:231–6. doi: 10.1016/j.ijbiomac.2004.08.001
42. Zheng X, Zou S, Xu H, Liu Q, Song J, Xu M, et al. The linear structure of β-glucan from baker's yeast and its activation of macrophage-like RAW264.7 cells. Carbohydr Polym. (2016) 148:61–8. doi: 10.1016/j.carbpol.2016.04.044
43. Sletmoen M, Stokke BT. Higher order structure of (1,3)-beta-D-glucans and its influence on their biological activities and complexation abilities. Biopolymers. (2008) 89:310–21. doi: 10.1002/bip.20920
44. Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. (2003) 19:311–5. doi: 10.1016/S1074-7613(03)00233-4
45. Bohn JA, Bemiller JN. (1→ 3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydr Polym. (1995) 28:3–14. doi: 10.1016/0144-8617(95)00076-3
46. Adachi Y, Ohno N, Ohsawa M, Oikawa S, Yadomae T. Change of biological activities of (1→ 3)-β-D-glucan from Grifola frondosa upon molecular weight reduction by heat treatment. J Chem Pharm Bull. (1990) 38:477–81. doi: 10.1248/cpb.38.477
47. Kim F, Sakagami H, Tanuma S, Konno K. Stimulation of interferon-gamma-induced human myelogenous leukemic cell differentiation by high molecular weight PSK subfraction. Anticancer Res. (1990) 10:55–8.
48. Ogawa T, Kaburagi T. Glucan Synthesis.2. Synthesis of a branched d-glucotetraose, the repeating unit of the extracellular polysaccharides of grifola-umbellate, sclerotinia-libertiana, porodisculus-pendulus, and schizophyllum-commune fries. Carbohyd Res. (1982) 103:53–64. doi: 10.1016/S0008-6215(82)80007-4
49. Lei N, Wang M, Zhang L, Xiao S, Fei C, Wang X, et al. Effects of low molecular weight yeast β-glucan on antioxidant and immunological activities in mice. Int J Mol Sci. (2015) 16:21575–90. doi: 10.3390/ijms160921575
50. Adams EL, Rice PJ, Graves B, Ensley HE, Yu H, Brown GD, et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther. (2008) 325:115–23. doi: 10.1124/jpet.107.133124
51. Aman P, Graham H. Mixed-linked β-(1→ 3), (1→ 4)-D-glucans in the cell walls of barley and oats-chemistry and nutrition. J Scand J Gastroenterol. (1987) 22:42–51. doi: 10.3109/00365528709095849
52. Raa J. Immune modulation by non-digestible and non-absorbable beta-1,3/1,6-glucan. Microb Ecol Health Dis. (2015) 26:27824. doi: 10.3402/mehd.v26.27824
53. Miyazaki T, Oikawa N, Yadomae T, Yamada H, Yamada Y, Hsu H-Y, et al. Relationship between the chemical structure and anti-tumour activity of glucans prepared from Grifora umbellata. Carbohydr Res. (1979) 69:165–70. doi: 10.1016/S0008-6215(00)85761-4
54. Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Antitumour polysaccharide derived chemically from natural glucan (Pachyman). Nature. (1970) 225:943–4. doi: 10.1038/225943a0
55. Kapteyn JC, Montijn RC, Dijkgraaf GJ, Van Den Ende H, Klis FM. Covalent association of beta-1,3-glucan with beta-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J Bacteriol. (1995) 177:3788–92. doi: 10.1128/JB.177.13.3788-3792.1995
56. Saitô H, Yoshioka Y, Uehara N, Aketagawa J, Tanaka S, Shibata Y. Relationship between conformation and biological response for (1→ 3)-β-d-glucans in the activation of coagulation Factor G from limulus amebocyte lysate and host-mediated antitumor activity. Demonstration of single-helix conformation as a stimulant. Carbohydr Res. (1991) 217:181–90. doi: 10.1016/0008-6215(91)84128-2
57. Lazaridou A, Biliaderis CJJOCS. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J Cereal Sci. (2007) 46:101–18. doi: 10.1016/j.jcs.2007.05.003
58. Engstad CS, Engstad RE, Olsen JO, Osterud B. The effect of soluble beta-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int Immunopharmacol. (2002) 2:1585–97. doi: 10.1016/S1567-5769(02)00134-0
59. Cleary JA, Kelly GE, Husband AJ. The effect of molecular weight and beta-1,6-linkages on priming of macrophage function in mice by (1,3)-beta-D-glucan. Immunol Cell Biol. (1999) 77:395–403. doi: 10.1046/j.1440-1711.1999.00848.x
60. Qi C, Cai Y, Gunn L, Ding C, Li B, Kloecker G, et al. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood. (2011) 117:6825–36. doi: 10.1182/blood-2011-02-339812
61. Zhong W, Hansen R, Li B, Cai Y, Salvador C, Moore GD, et al. Effect of yeast-derived beta-glucan in conjunction with bevacizumab for the treatment of human lung adenocarcinoma in subcutaneous and orthotopic xenograft models. J Immunother. (2009) 32:703–12. doi: 10.1097/CJI.0b013e3181ad3fcf
62. De Smet R, Allais L, Cuvelier CA. Recent advances in oral vaccine development: yeast-derived beta-glucan particles. Hum Vaccin Immunother. (2014) 10:1309–18. doi: 10.4161/hv.28166
63. Cramer DE, Wagner S, Li B, Liu J, Hansen R, Reca R, et al. Mobilization of hematopoietic progenitor cells by yeast-derived beta-glucan requires activation of matrix metalloproteinase-9. Stem Cells. (2008) 26:1231–40. doi: 10.1634/stemcells.2007-0712
64. Zhang M, Kim JA, Huang AY-C. Optimizing tumor microenvironment for cancer immunotherapy: β-glucan-based nanoparticles. Front Immunol. (2018) 9:341. doi: 10.3389/fimmu.2018.00341
65. Soto E, Kim YS, Lee J, Kornfeld H, Ostroff G. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers. (2010) 2:681–9. doi: 10.3390/polym2040681
66. Soto ER, Caras AC, Kut LC, Castle MK, Ostroff GR. Glucan particles for macrophage targeted delivery of nanoparticles. J Drug Deliv. (2012) 2012:143524. doi: 10.1155/2012/143524
67. Tabata Y, Ikada Y. Macrophage phagocytosis of biodegradable microspheres composed of L-lactic acid/glycolic acid homo- and copolymers. J Biomed Mater Res. (1988) 22:837–58. doi: 10.1002/jbm.820221002
68. Elder MJ, Webster SJ, Chee R, Williams DL, Hill Gaston JS, Goodall JC. β-glucan size controls dectin-1-mediated immune responses in human dendritic cells by regulating IL-1β production. Front Immunol. (2017) 8:791. doi: 10.3389/fimmu.2017.00791
69. Yu H, Williams DL, Ensley HE. 4-Acetoxy-2,2-dimethylbutanoate: a useful carbohydrate protecting group for the selective formation of beta-(1–>3)-d-glucans. Tetrahedron Lett. (2005) 46:3417–21. doi: 10.1016/j.tetlet.2005.03.099
70. Wasser, SP. Medicinal mushroom science: current perspectives, advances, evidences and challenges. Biomed J. (2014) 37, 345–356.
71. Zhu F, Du B, Xu B. A critical review on production and industrial applications of beta-glucans. J Food Hydrocolloids. (2016) 52:275–88. doi: 10.1016/j.foodhyd.2015.07.003
72. Brennan CS, Cleary LJJJOCS. The potential use of cereal (1→ 3, 1→ 4)-β-D-glucans as functional food ingredients. J Cereal Sci. (2005) 42:1–13. doi: 10.1016/j.jcs.2005.01.002
73. Silveira ML, Smiderle FR, Moraes CP, Borato DG, Baggio CH, Ruthes AC, et al. Structural characterization and anti-inflammatory activity of a linear beta-D-glucan isolated from Pleurotus sajor-caju. Carbohydr Polym. (2014) 113:588–96. doi: 10.1016/j.carbpol.2014.07.057
Keywords: β-glucans, structure-function relationship, immunomodulation, molecular structure, molecular weight, solubility
Citation: Han B, Baruah K, Cox E, Vanrompay D and Bossier P (2020) Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 11:658. doi: 10.3389/fimmu.2020.00658
Received: 20 December 2019; Accepted: 23 March 2020;
Published: 22 April 2020.
Edited by:
Philip Calder, University of Southampton, United KingdomReviewed by:
Frederick J. Sheedy, Trinity College Dublin, IrelandVaclav Vetvicka, University of Louisville, United States
Copyright © 2020 Han, Baruah, Cox, Vanrompay and Bossier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Han, Ymlhby5oYW5AVUdlbnQuYmU=
 Biao Han
Biao Han Kartik Baruah1,2
Kartik Baruah1,2 Eric Cox
Eric Cox Daisy Vanrompay
Daisy Vanrompay Peter Bossier
Peter Bossier