- 1St. Vincent’s Clinical School, UNSW, Darlinghurst, NSW, Australia
- 2Centre for Applied Medical Research, St Vincent’s Hospital, Sydney, NSW, Australia
- 3Kirby Institute, UNSW Sydney, Sydney, NSW, Australia
- 4IDMIT Department/IBFJ, Immunology of Viral Infections and Autoimmune Diseases (IMVA), INSERM U1184, CEA, Université Paris Sud, Paris, France
- 5South Australian Health and Medical Research Institute (SAHMRI), Adelaide, SA, Australia
- 6Faculty of Science, Flinders University, Adelaide, SA, Australia
- 7Microbiology and Infectious Diseases, South Australia (SA) Pathology, Adelaide, SA, Australia
- 8Immunology Division Garvan Institute of Medical Research, Sydney, NSW, Australia
- 9Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, SA, Australia
- 10Department of Gastroenterology, St. Vincent’s Hospital, Sydney, NSW, Australia
Background: Despite successful ART in people living with HIV infection (PLHIV) they experience increased morbidity and mortality compared with HIV-negative controls. A dominant paradigm is that gut-associated lymphatic tissue (GALT) destruction at the time of primary HIV infection leads to loss of gut integrity, pathological microbial translocation across the compromised gastrointestinal barrier and, consequently, systemic inflammation. We aimed to identify and measure specific changes in the gastrointestinal barrier that might allow bacterial translocation, and their persistence despite initiation of antiretroviral therapy (ART).
Method: We conducted a cross-sectional study of the gastrointestinal (GIT) barrier in PLHIV and HIV-uninfected controls (HUC). The GIT barrier was assessed as follows: in vivo mucosal imaging using confocal endomicroscopy (CEM); the immunophenotype of GIT and circulating lymphocytes; the gut microbiome; and plasma inflammation markers Tumour Necrosis Factor-α (TNF-α) and Interleukin-6 (IL-6); and the microbial translocation marker sCD14.
Results: A cohort of PLHIV who initiated ART early, during primary HIV infection (PHI), n=5), and late (chronic HIV infection (CHI), n=7) infection were evaluated for the differential effects of the stage of ART initiation on the GIT barrier compared with HUC (n=6). We observed a significant decrease in the CD4 T-cell count of CHI patients in the left colon (p=0.03) and a trend to a decrease in the terminal ileum (p=0.13). We did not find evidence of increased epithelial permeability by CEM. No significant differences were found in microbial translocation or inflammatory markers in plasma. In gut biopsies, CD8 T-cells, including resident intraepithelial CD103+ cells, did not show any significant elevation of activation in PLHIV, compared to HUC. The majority of residual circulating activated CD38+HLA-DR+ CD8 T-cells did not exhibit gut-homing integrins α4ß7, suggesting that they did not originate in GALT. A significant reduction in the evenness of species distribution in the microbiome of CHI subjects (p=0.016) was observed, with significantly higher relative abundance of the genus Spirochaeta in PHI subjects (p=0.042).
Conclusion: These data suggest that substantial, non-specific increases in epithelial permeability may not be the most important mechanism of HIV-associated immune activation in well-controlled HIV-positive patients on antiretroviral therapy. Changes in gut microbiota warrant further study.
Introduction
Chronic HIV-1 infection is associated with persistent elevated systemic immune activation, including increases in levels of pro-inflammatory cytokines (1), lymph node germinal centre activity, immunoglobulin secretion by B-cells (2, 3) and activation and increased turnover of T-cells (4), particularly including target CCR5+ CD4 T-cells (5). A proposed cause for this is gut microbial translocation, which is the pathological translocation of luminal micro-organisms from the gastrointestinal tract (GIT) to the portal and systemic circulation as a consequence of depletion and impaired reconstitution of gut-associated lymphoid tissue (GALT) CD4 T-cells (6, 7).
Effects of HIV in the GIT include epithelial apoptosis (8, 9) and loss of epithelial barrier integrity (10, 11) with evidence of increased epithelial tight junction permeability (12, 13). Focal loss of CD4 T-cells in the mucosa (14–16) and dysregulation of T-cell subtypes (17–20) have also been implicated. Microbial translocation is believed to lead to systemic immune activation, seen as a correlation between plasma LPS levels and circulating activated CD38+HLA-DR+ CD8 T-cells (7). Furthermore, gut microbiome composition correlates with increased immune activation in HIV-infected individuals (21–23). The effect of antiretroviral therapy (ART) on the gut microbiome and mucosal and systemic lymphocytes suggests partial but not complete normalization of the dysbiosis resulting from HIV-1 infection (24). Therefore, there is a need to further study the relationship between enduring changes in the microbiome, the mucosal barrier and systemic immune responses during ART.
Studies to date directly assessing the functional integrity of the intestinal barrier in HIV-infected individuals have generally investigated impairment using immunohistochemical or transcriptional analysis of biopsies (8, 11–13). Conventional techniques such as mannitol and lactulose permeability measuring intestinal barrier function (25) have also been used to study HIV-1 infected subjects (8, 9).
Confocal endomicroscopy (CEM) shows promise for accurate, focal analysis of the intestinal barrier in vivo. CEM is a novel technique utilising a laser confocal endomicroscope integrated into a colonoscope, gathering images at 1000x magnification (26, 27). CEM has been successful in identifying gastrointestinal barrier changes in inflammatory bowel disease (28, 29), in which microbial translocation is thought to play a role (30). To the best of our knowledge, this technique has only been used once to study the gastrointestinal barrier of PLHIV. This was done in a set of mainly elite controllers, with evidence of permeability, but no quantitative comparison to HIV-uninfected controls (31).
Our study aimed to confirm and quantify the increased permeability of the gut mucosal barrier, as directly observed in vivo using CEM, compared to HIV-uninfected controls, and explore potential relationships between gut microbiome, mucosal immune function, intestinal barrier integrity and markers of immune activation in PLHIV who commenced ART during either primary or established chronic HIV infection. All parameters were compared to HIV-uninfected controls.
Methods
Subjects
This cross-sectional pilot cohort study enrolled PLHIV who initiated ART during primary (PHI) and chronic (CHI) infection and had maintained virological control for >2 years, and a control group of HIV-uninfected controls (HUC). The HUC were matched for age and sex. PLHIV were considered treated in primary HIV infection (PHI) if ART was initiated within six months of HIV infection (HIV), and in chronic HIV infection (CHI) if treatment was initiated at least 12-months after HIV infection, as defined in the PINT study (32). Volunteers were excluded if they were unfit for colonoscopy, had a fluorescein allergy, or had specific inflammatory gastrointestinal conditions associated with colitis. This study was approved by the St Vincent’s Human Research Ethics Committee (HREC 14/214). All participants provided written informed consent.
Confocal Endomicroscopy (CEM)
Colonoscopy with confocal endomicroscopy was conducted by a single colonoscopist using an Optiscan CIS-2 prototype confocal laser endomicroscope (Notting Hill, VIC, Australia). This device replaces one of two air/water channels of a conventional Olympus CF-H180AL endoscope (Tokyo, Japan) with a 488nm laser microscope. The endoscopic probe was applied perpendicular to the mucosal surface, and serial microscopic images of the terminal ileum were captured at scanning depths of 15-70µm during and after intravenous administration of 5mL 10% fluorescein sodium contrast (Alcon, Australia) in 1mL increments.
Two blinded observers reviewed the images with patient grouping identifiers removed. Images where villi and lumen were indistinguishable were discarded. The two observers then independently analysed the remaining images for evidence of cell junction enhancement and fluorescein leak (see Figure 1). Cell junction enhancement was defined as an area of increased, equal fluorescence between two epithelial cells, extending from the basal to the apical surface of the cell layer. Fluorescein leak was characterised by a distinct plume of contrast leakage into the lumen stemming from the epithelial luminal border. Both features have previously been used in studies to gauge mucosal barrier function and permeability in gastrointestinal pathologies (28, 29, 33, 34).
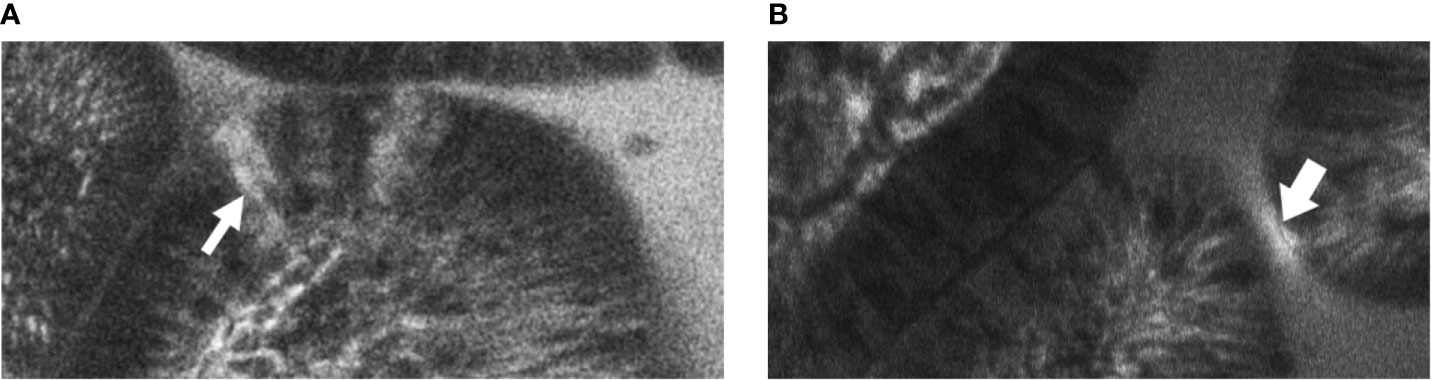
Figure 1 Example images of terminal ileal villi exhibiting (A) fluorescein leak and (B) cell junction enhancement (A) Cell junction enhancement, characterised by an area of increased fluorescence from the basal to the apical surface between two epithelial cells. (B) Fluorescein leak, characterised by increased fluorescence in the epithelial cell layer, and a distinct plume of contrast leakage into the lumen.
Flow Cytometry
Ten pinch biopsies each were taken from the terminal ileum and left colon using endoscopic biopsy forceps during colonoscopy and separately stored in containers of media solution containing 10mL Roswell Park Memorial Institute (RPMI) culture media (Invitrogen, USA) with 10% foetal bovine serum (Bovogen, Australia) and 100U PenStrep (Invitrogen). Blood was concurrently collected in Vacutainer tubes with sodium heparin anti-coagulant (Becton Dickinson, NJ, USA).
Biopsy samples were weighed, then minced with sterile scissors, before enzymatic digestion using collagenase type III (Sigma-Aldrich) and DNase (Sigma-Aldrich), in order to prepare single cell suspensions for flow cytometry, as previously described (35). All samples were stained for surface markers according to manufacturer instructions, as previously described (35). Additionally, samples from the terminal ileum and left colon were stained for epithelial cells using the monoclonal antibody EpCam-FITC (BD Biosciences, CA, USA). Samples were washed with PBS (Dulbecco’s Phosphate Buffered Saline (DPBS) with 0.5% BSA and 0.1% sodium azide) and fixed in a solution of 0.5% paraformaldehyde in DPBS. Peripheral blood samples were stained, lysed and fixed as previously described (35).
Cells were analysed using a four-laser LSR-II flow cytometer (BD Biosciences) and BD FACSDiva version 8.0 (BD Biosciences), then further analysed using FlowJo version 10.7.1 (Ashland, OR). The gating strategy for lymphocytes isolated from gastrointestinal biopsies has been previously described (35), and is shown in Supplementary Figure 1A. Gating of peripheral blood lymphocytes utilised a similar strategy as shown in Supplementary Figure 1B.
Microbiome Analysis
Stool samples were collected and preserved using OMNIgene GUT microbial stabilisation kit (DNA Genotek, Ontario, Canada). Genomic DNA was extracted using DNeasy PowerLyzer PowerSoil DNA isolation kits (Qiagen, Hilden, Germany) as per manufacturer’s instructions. 16S rRNA sequencing was performed by first preparing the amplicon library using a previously described protocol (36), and sequenced on the Illumina Miseq sequencing platform using Illumina Miseq v3 kit with 2 x 300 bp cycle (Illumina Inc., CA, USA). Downstream processing of the amplicon sequencing reads was carried out as described (36). Briefly, reads were quality filtered and merged, followed by assigning operational taxonomic units (OTUs) using the Quantitative Insights into Microbial Ecology (37) software. No samples were eliminated following subsampling to the depth of 8,079 reads.
Plasma Marker Analysis
The plasma level of soluble CD14 (sCD14) was used as a marker of monocyte activation by lipopolysaccharide and microbial translocation. Other cytokines included TNF and IL6. EDTA anti-coagulated blood was centrifuged at 1600rpm for 15 minutes to obtain plasma for testing in ELISAs using commercially available kits for sCD14, IL-6 and TNF-α (all R&D systems, MN, USA) according to manufacturer’s instructions.
Data Analysis
CD4 and CD8 T-cells in gastrointestinal biopsies at each sample site were counted as absolute cell numbers as previously described (35), but were also normalised by weight of biopsies and by the number of epithelial cells in the biopsies (35). Data was analysed using Prism software version 9.0 (GraphPad, La Jolla, CA). Quantitative analysis was performed using Kruskal-Wallis one-way ANOVA, with two-tailed Mann-Whitney U post-hoc analysis. Spearman’s correlation was used to compare two continuous variables. Cohen’s kappa score was used to measure inter-observer agreement in confocal endomicroscopy image interpretation. Faecal microbiota variation was analysed using Shannon and Simpson diversity indices, while group diversity as calculated using Bray-Curtis dissimilarity distance was illustrated using non-metric multidimensional scaling (NMDS) ordination, and tested using permutational multivariate analysis of variance (PERMANOVA). Kruskal-Wallis with Benjamini-Hochberg false-discovery rate adjustment was employed to assess significant difference in specific bacterial taxa between groups.
Results
Participants
Of the 16 HIV-positive participants from the PINT study, which prospectively studied the effect of commencing a raltegravir-containing regimen during either primary HIV-1 infection (PHI) or chronic infection (CHI) (32), five primary and two chronic HIV participants re-enrolled into this study. A further six HIV-positive participants and six HIV-uninfected controls (HUC) were recruited from outpatient clinics at St Vincent’s Hospital, Sydney. One patient in the PHI group did not attend for study procedures. Hence, a total of six HUC, five PHI and seven CHI subjects attended their allocated study session (Figure 2). All participants were male, with baseline characteristics outlined in Table 1. The CHI subjects had ART for a median 7 years (range 4-23) and the PHI subjects for 7 years (range 7-7).
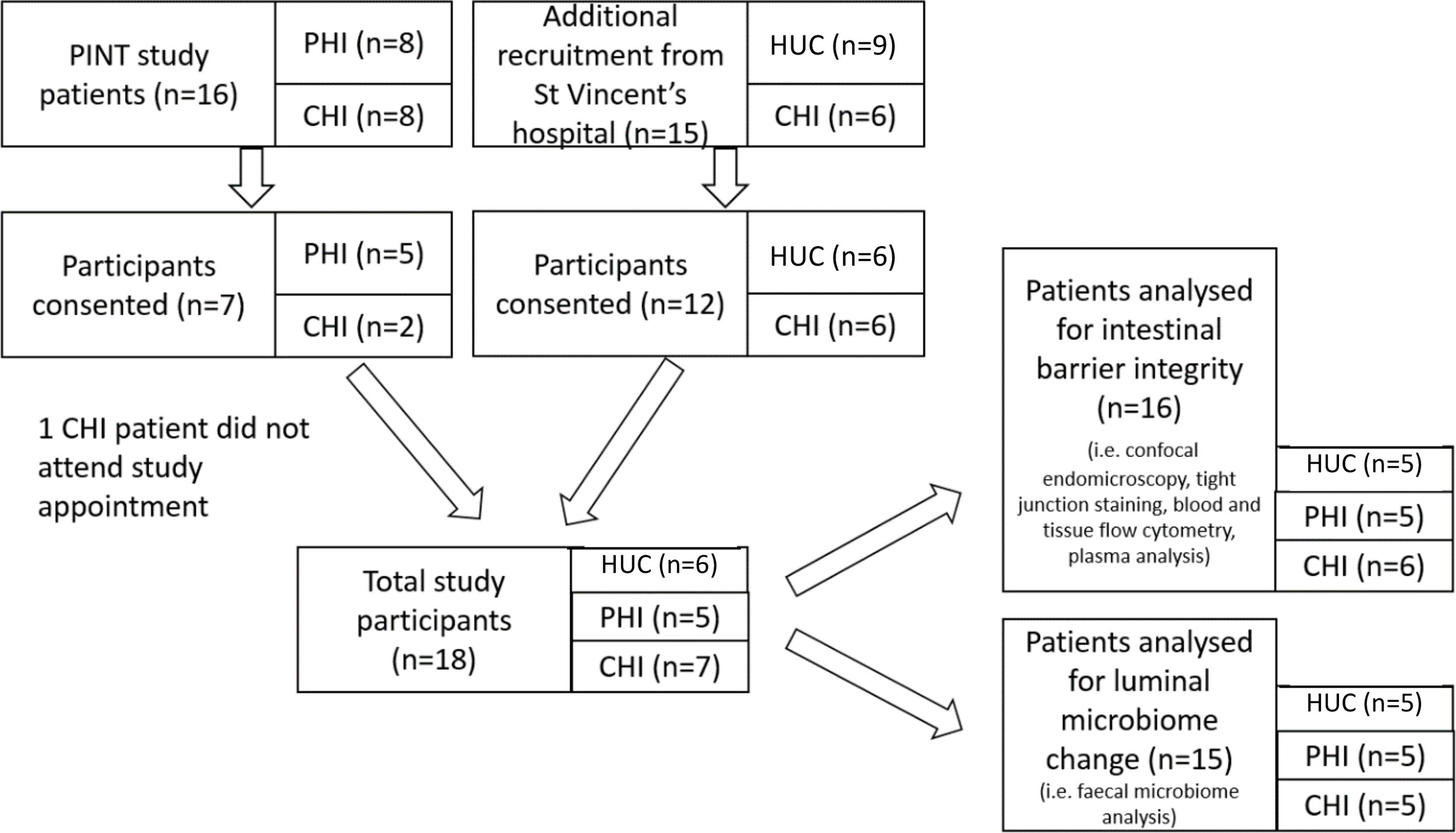
Figure 2 Patient recruitment flowchart outlining selection of patients for confocal endomicroscopy imaging, tight junction, blood and tissue lymphocyte, plasma marker and faecal microbiome analyses. HUC, HIV-uninfected control; PHI, HIV-positive participant who initiated antiretroviral therapy during primary HIV infection; CHI, HIV-positive participant who initiated antiretroviral therapy during chronic HIV infection.
One patient from the CHI group and one patient from the HUC group were excluded from confocal endomicroscopy and lymphocyte analysis due to inadequate visualisation of the terminal ileum, confocal imaging calibration issues, or non-attendance. One patient in the HUC group and two patients in the CHI group were excluded from microbiome analysis due to inadequate faecal sample.
Preservation of Gastrointestinal Mucosal Barrier Integrity Using Confocal Endomicroscopy (CEM)
The median time taken to examine and capture the CEM images in the terminal ileum per patient was 9 minutes (range 6-20). A median of 889 (382-2046) images taken per patient were analysed after removal of 282 (22-993) unfocused images per patient. Fluorescein leak (Figure 1A) and cell junction enhancement (Figure 1B) were able to be identified by the two observers. Of images identified by one or the other observer to contain fluorescein leak or cell junction enhancement, 29.8% were identified by the other observer. Inter-observer agreement for the presence of features identified per patient was moderate (κ=0.43) for cell junction enhancement and substantial (κ=0.75) for fluorescein leak. There was also a strong correlation between the total number of features identified by each observer separately and features identified by both observers (Spearman r 0.81; 95%CI 0.51-0.93; p<0.01).
The number of images with fluorescein leak and cell junction enhancement was small as a proportion of the total number of images (median 0.15%, range 0-0.54%). There was no statistically significant difference in the median percentage of CEM features seen in participants across the three groups (HUC=0.26% vs PHI 0% vs CHI 0.11%; p=0.51) (Figure 3). No significant differences were found when fluorescein leak and cell junction enhancement were analysed separately (data not shown).
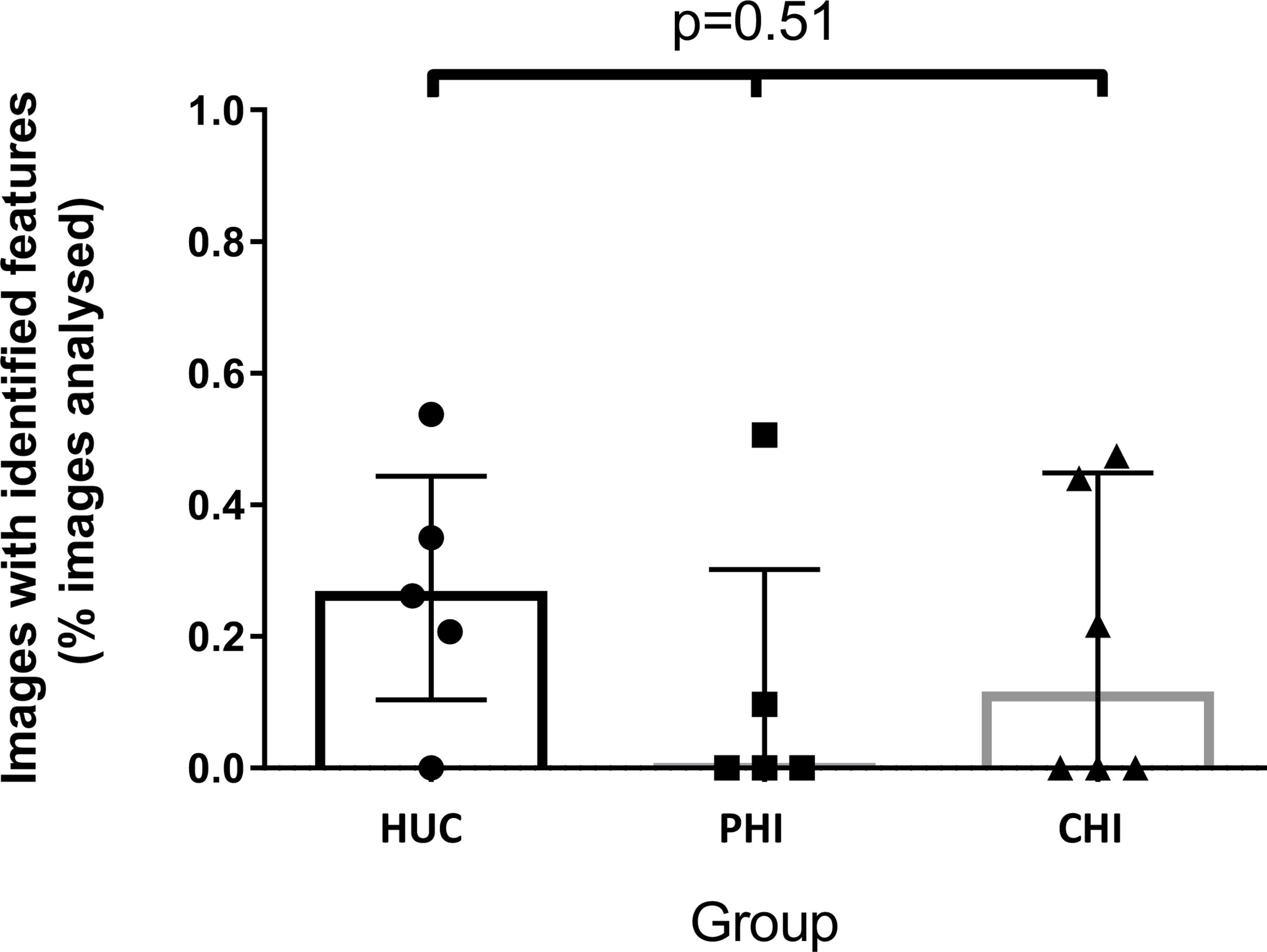
Figure 3 Images with identified confocal endomicroscopy features (fluorescein leak and cell junction enhancement) as a percentage of total images analysed for the participant, across three groups. Median percentage of images was 0.26% for HIV-uninfected controls (HUC), 0.00% for HIV-positive participants treated in primary infection (PHI), and 0.11% for HIV-positive participants treated in chronic infection (CHI). Statistical analysis was conducted using Kruskal Wallis one-way analysis of variation.
Characterisation of CD4 and CD8 T-Cells in Gastrointestinal Tissue
In addition to absolute counts of CD4 and CD8 T-cells in gastrointestinal biopsies at each sample site, as previously described (35), numbers of these cells were also normalised by weight of biopsies and also by the number of epithelial cells in the biopsies (35). This revealed similar weights and epithelial cell counts between groups, but no correlation between the weight and epithelial cell count.
By absolute count from biopsies, there was no significant difference between CD4 T-cell counts in terminal ileum (TI) biopsies across the three study groups by Kruskal-Wallis test, but there was a significant difference between HUC and CHI groups in left colon (LC) biopsies (Figure 4A). By weight, there was an absolute decrease in CD4 T-cell numbers per mg in PLHIV in the LC compared with HUC participants, (HUC 1434.5 vs PHI 392.56 vs CHI 515.6 cells/mg; p=0.03) (Figure 4B). A similar decreasing trend was found in the TI samples (HUC 2987.8 vs PHI 751.8 vs CHI 577.9 cells/mg; p=0.20) (Figure 4B). There was no significant change in the CD4 T-cell count:epithelial cell number ratio in the TI (HUC 0.027 vs PHI 0.014 vs CHI 0.016; p=0.56) or LC (HUC 0.026 vs PHI 0.013 vs CHI 0.008; p=0.17) (Figure 4C).
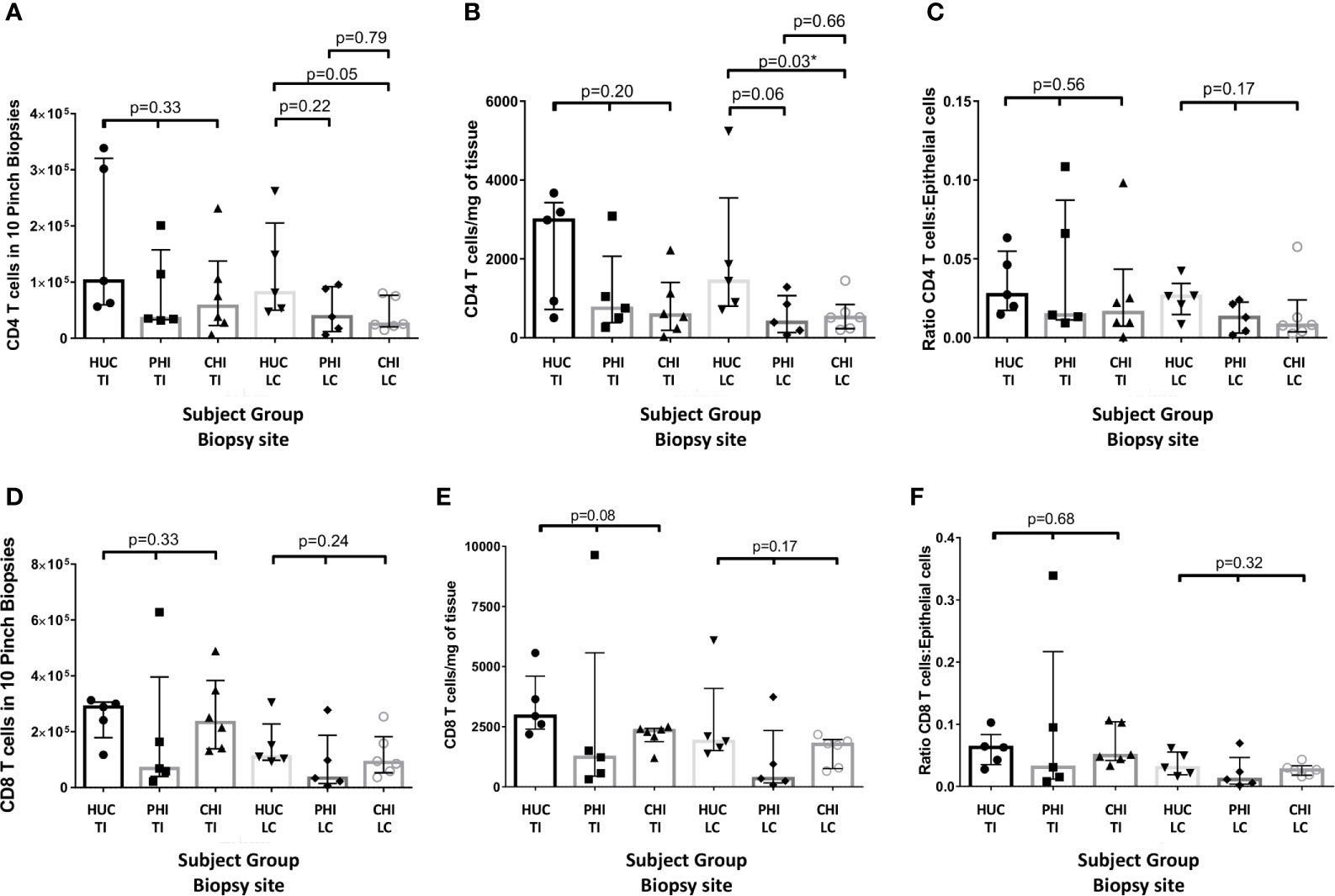
Figure 4 Changes in CD4 panels (A–C) and CD8 (D–F) cell numbers in the terminal ileum and left colon between patient groups as: (A, D) an absolute count from ten biopsies; (B, E) standardised per milligram of tissue; and (C, F) standardized by epithelial cell number HUC, HIV-uninfected controls; PHI, HIV-positive patients treated during primary infection; CHI, HIV-positive patients treated during chronic infection; TI, terminal ileum; LC, left colon, *statistically significant (p ≤ 0.05). Statistical analysis was conducted using Kruskal Wallis one-way analysis of variance with pairwise post-hoc testing using Mann Whitney U.
There were no significant changes in the CD8 T-cell count by biopsy, weight or epithelial cell ratio, in either the TI or LC across the three groups (Figures 4D–F).
Association Between Circulating and Gastrointestinal Lymphocyte Numbers
When analysing the samples of all volunteers in our study, a significant positive correlation was found between the CD4 T-cell count in peripheral blood and the number of CD4 T-cell count in gastrointestinal tissue in both the TI (p<0.01 r=0.76) and LC (p=0.02 r=0.57) (Table 2); the degree of correlation was higher in the TI than in the LC. A significant positive correlation was also found between peripheral blood CD8 T-cell count and CD8 T-cell number in the TI (p<0.01 r=0.69) (Table 3).
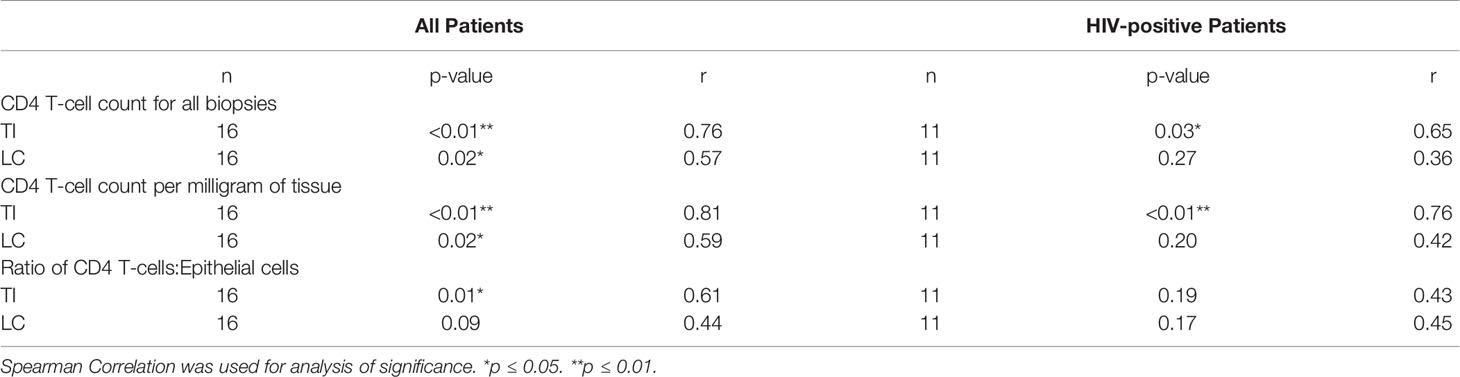
Table 2 Relationship between systemic CD4 T-cell concentration measured using peripheral blood and measured gastrointestinal lymphocyte numbers, for all study groups combined, and for HIV-positive study participants only.
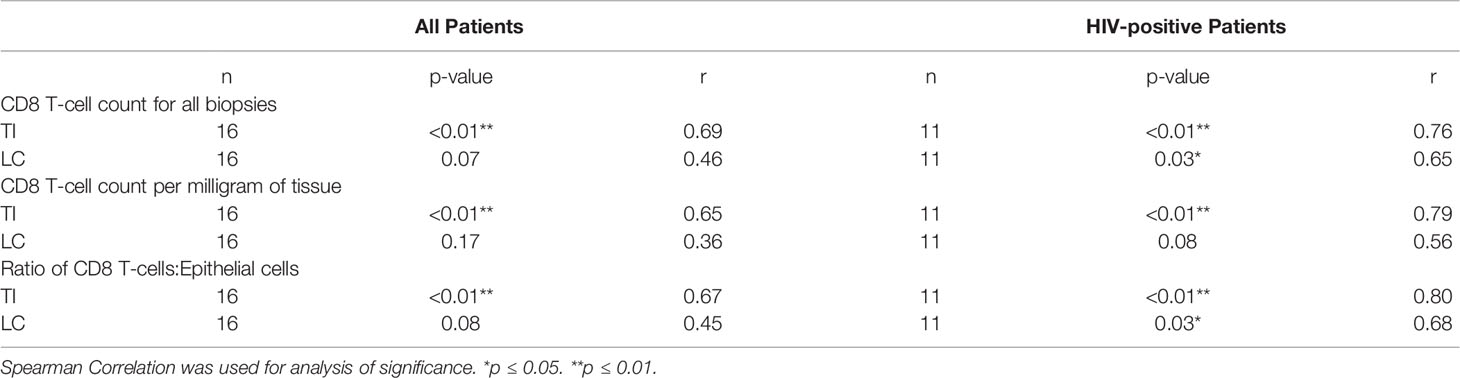
Table 3 Relationship between systemic CD8 T-cell concentration measured in peripheral blood and measured gastrointestinal lymphocyte numbers, for all study groups combined, and for HIV-positive study participants only.
CD8 T Cell Activation in Peripheral Blood and Biopsies
Circulating activated CD38+HLA-DR+ CD8 T-cells, as a proportion of total CD8 T-cells in the peripheral blood, was measured as a marker of systemic immune activation. No statistically significant differences were found across the percentages in the three subject groups of the current study (medians: HUC 2.75% PHI 3.53% CHI 3.45% Kruskal-Wallis test p=0.69) (Figure 5A left). As comparator data, a previous larger sample of HUC and all longitudinal data from the PINT study (38) showed that there was a small but significant elevation of activated CD38+HLA-DR+ CD8 T-cells in PLHIV on long-term suppressive ART (medians: HUC 1.5%, HIV+ PINT subjects 4.0%; Mann-Whitney test p<0.0001) (38) (Figure 5A, right). For the PLHIV in PINT, 25/67 observations were above 4.6%, which was the 95th percentile of the normal range.
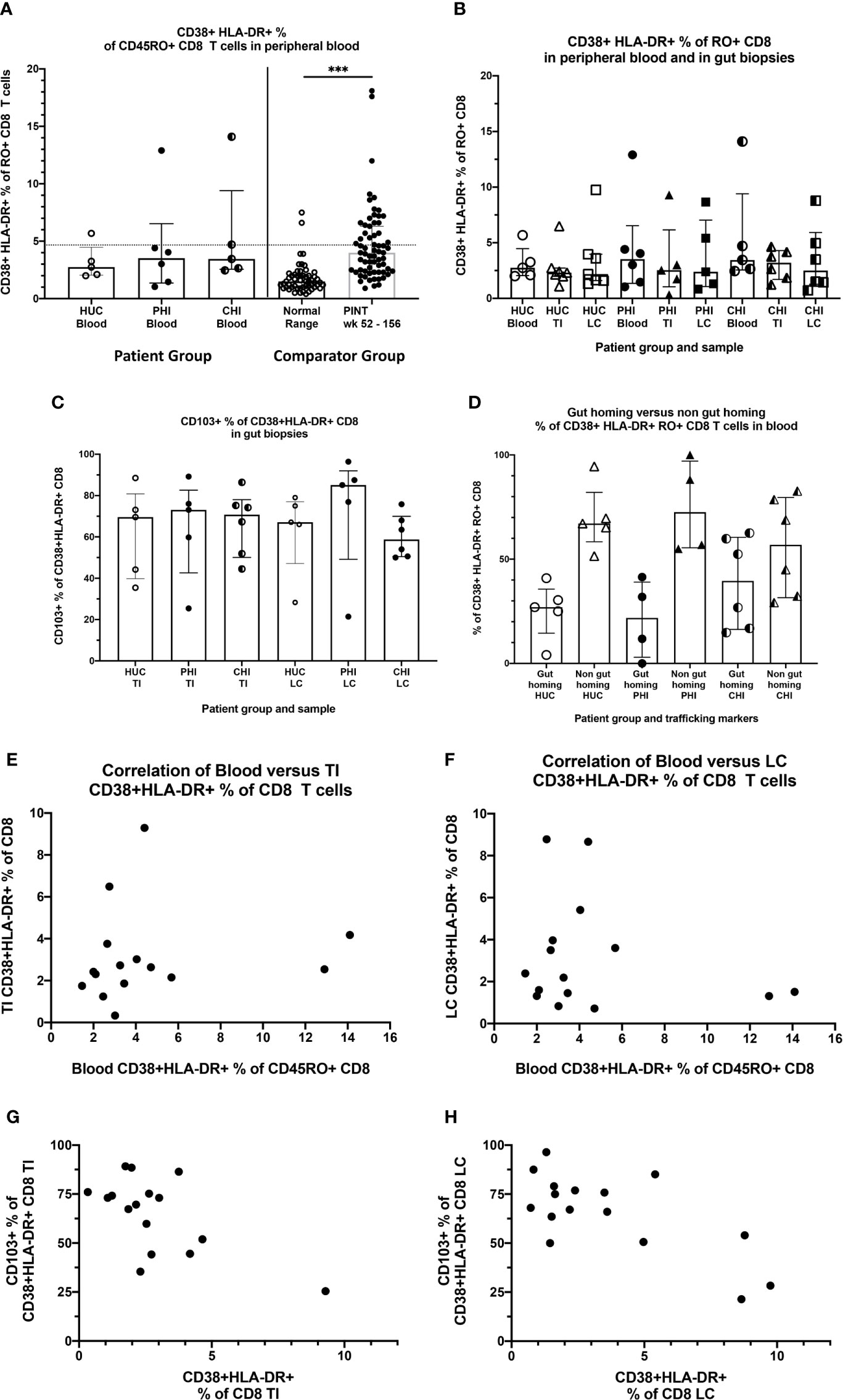
Figure 5 Activated CD38+HLA-DR+ CD8 T-cells in blood and tissues. (A) Activated CD38+HLA-DR+ as % of CD45RA- CD8 T cells in peripheral blood, by study group. HUC, HIV-uninfected controls; PHI, HIV-positive patients treated during primary infection; CHI, HIV-positive patients treated during chronic infection. The Comparator Groups are (left) normal range for HUC subjects from reference (38) and the 95th percentile (4.6%) is shown as the dotted horizontal line, and (right) PINT subjects longitudinal observations from reference (38). Statistical analysis between HUC, PHI and CHI groups was conducted using Kruskal Wallis one-way analysis of variance. Statistical analysis between comparator normal range and PINT groups was done using Mann-Whitney test. (B) Comparison of activated CD38+HLA-DR+ as % of CD45RA- CD8 T-cells in peripheral blood, TI biopsies and LC biopsies, respectively by study group. (C) Percentage of activated CD38+HLA-DR+ CD8 T-cells in TI and LC biopsies that are also CD103+. (D) Percentage of activated CD38+HLA-DR+ CD8 T-cells in peripheral blood that are either CD49d+integrin ß7+ gut-homing or CD49d+integrin ß7+-negative non-gut-homing, by study group. (E) Correlation of activated CD38+HLA-DR+ as % of CD8 T-cells in peripheral blood with activated CD38+HLA-DR+ as % of CD8 T cells in TI biopsies. (F) Correlation of activated CD38+HLA-DR+ as % of CD8 T-cells in peripheral blood with activated CD38+HLA-DR+ as % of CD8 T cells in LC biopsies. (G) Correlation of CD103+ as % of activated CD38+HLA-DR+ CD8 T-cells in TI biopsies with activated CD38+HLA-DR+ as % of CD8 T cells in TI biopsies. (H) Correlation of CD103+ as % of activated CD38+HLA-DR+ CD8 T-cells in LC biopsies with activated CD38+HLA-DR+ as % of CD8 T cells in LC biopsies. ***p < 0.0001.
We also measured the activated CD38+HLA-DR+ % of CD8 T-cells in the gut biopsies and compared them to the corresponding peripheral blood levels (Figure 5B). In general, the level of activation was in the same range as for circulating CD8 T-cells, and no significant elevations were seen in gut biopsies from PLHIV, compared to the HUC gut biopsies (Figure 5B).
When we further subdivided the activated CD38+HLA-DR+ CD8 T-cells in the gut biopsies into CD103+ resident intraepithelial cells (39) versus CD103- presumptively migratory cells (Supplementary Figure 1A), we found that, in most biopsies, the majority of CD38+HLA-DR+ CD8 T-cells were CD103+ tissue resident cells that are unlikely to recirculate (Figure 5C).
Conversely, we examined the expression of integrins α4ß7, that determine whether the cells traffic through GALT, on CD38+HLA-DR+ CD8 T-cells in peripheral blood (Supplementary Figure 1B). The results show that the majority of circulating activated CD38+HLA-DR+ CD8 T-cells do not express integrins α4ß7 (Figure 5D). We also did not see any correlation between the levels of circulating activated CD38+HLA-DR+ CD8 T-cells with the corresponding cells in either TI biopsies (Figure 5E) or LC biopsies (Figure 5F). Finally, when there were larger percentages of activated CD38+HLA-DR+ CD8 T-cells in gut biopsies, they were mostly CD103- in TI (Figure 5G) and in LC (Figure 5H), suggesting that they were migratory.
Overall, the results indicate that while it appears that there are slightly more circulating activated CD38+HLA-DR+ CD8 T-cells in PLHIV on fully suppressive ART, they appear to be more systemic in origin and not directly associated with activation within GALT.
We also used an alternative CD38+CD127- phenotype of activated CD8 T cells since it has been reported that levels of this phenotype differed between HIV+ subjects and uninfected controls, in both rectal mucosa and blood (40). When we studied these cells (Supplementary Figure 1B) we did not find any significant differences in CD38+CD127- CD8 T-cells between PLHIV and HIV-uninfected subjects in either blood or gut biopsies (Supplementary Figure 2).
Plasma Markers of Immune Activation
There were no significant differences between the levels of sCD14 (pg/mL) found in the three study groups (HUC 1.78x106 vs PHI 1.32x106 vs CHI 1.68x106 pg/ml; p=0.55) (Figure 6A). The majority of study participants (68.75%) had undetectable concentrations of TNF-α in plasma (Figure 6B). A higher number of patients in the CHI group had detectable plasma TNF-α levels compared with those in both the HIV-uninfected and PHI groups (n=3, n=1, n=1 respectively), but this was not statistically significant. Of the five participants with detectable TNF-α concentrations, 4 (80%) had detectable cell junction enhancement or fluorescein leak seen on confocal endomicroscopy (HUC n=1, PHI n=1, CHI n=2). There were no significant differences in IL-6 concentrations across the three groups (HUC 1.49pg/mL vs PHI 1.79pg/mL vs CHI 1.69pg/mL; p=0.22) (Figure 6C). Finally, the relationship between epithelial integrity and both local immune parameters in the TI as well as systemic parameters was explored. No significant differences were found between the presence of confocal endomicroscopy features and the immune parameters measured (Table 4).
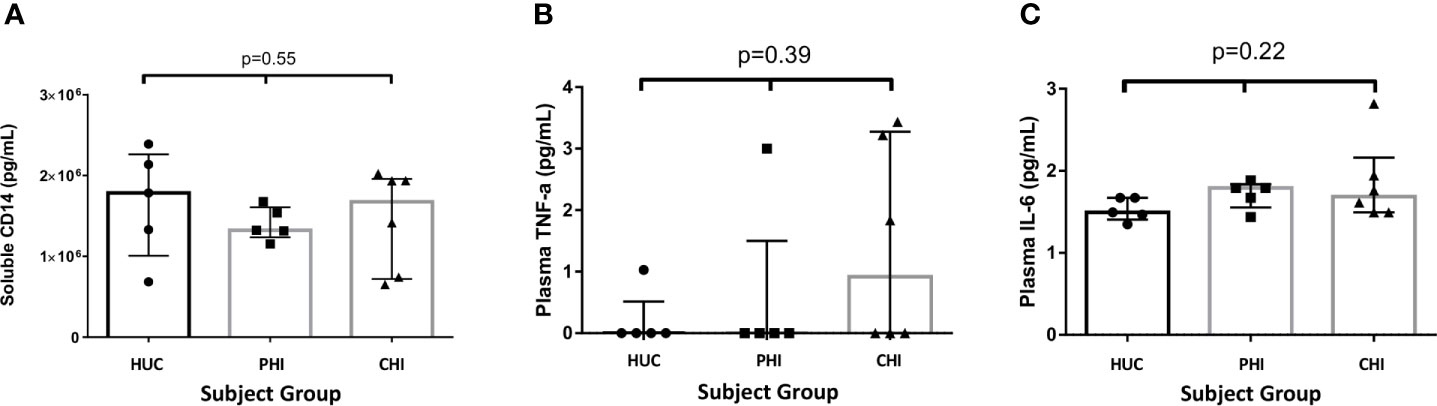
Figure 6 Levels of soluble proteins measured in plasma between the study groups. (A) Levels of soluble CD14 measured in plasma between the study groups. (B) Levels of TNF-α measured in plasma between the study groups. (C) Levels of IL-6 measured in plasma between the study groups. Statistical significance was calculated using the Kruskal Wallis test.
Table 4 Relationship of confocal endomicroscopy features identified in patients and measured parameters.
Characterisation of Gut Microbiota
Microbiota composition across all patients was dominated by fermentative bacterial taxa, including members of the Faecalibacterium (median 0.10; interquartile range [IQR] 0.05-0.12), Prevotella (median 0.21; IQR 0.09-0.38) and Bacteroides (median 0.01, IQR 0.003-0.12) genera (Figure 7A). There was no significant difference between groups in overall microbiota composition based on Bray Curtis dissimilarity, as assessed by PERMANOVA (P(perm)=0.202, pseudo-F=1.272, 9551 permutations). A non-significant decreasing trend in taxa diversity was also found using the Shannon diversity index H’ (Figure 7B). However, a significant difference (p=0.03) was found between PLHIV and HIV-uninfected participants in the distribution evenness of taxa, as measured by the Simpson diversity index (Figure 7C). The relative abundance of the Spirochaeta genus was significantly higher in the PHI group (p=0.042; Figure 7D), while the Acidaminococcus genus showed a non-significant trend towards increased relative abundance in the CHI group (Figure 7E). Patients in the CHI group exhibited a trend of increasing Prevotella relative abundance, and a trend of decreasing Faecalibacterium relative abundance. There was no correlation between the microbiota and the CEM or the cytokines (data not shown).
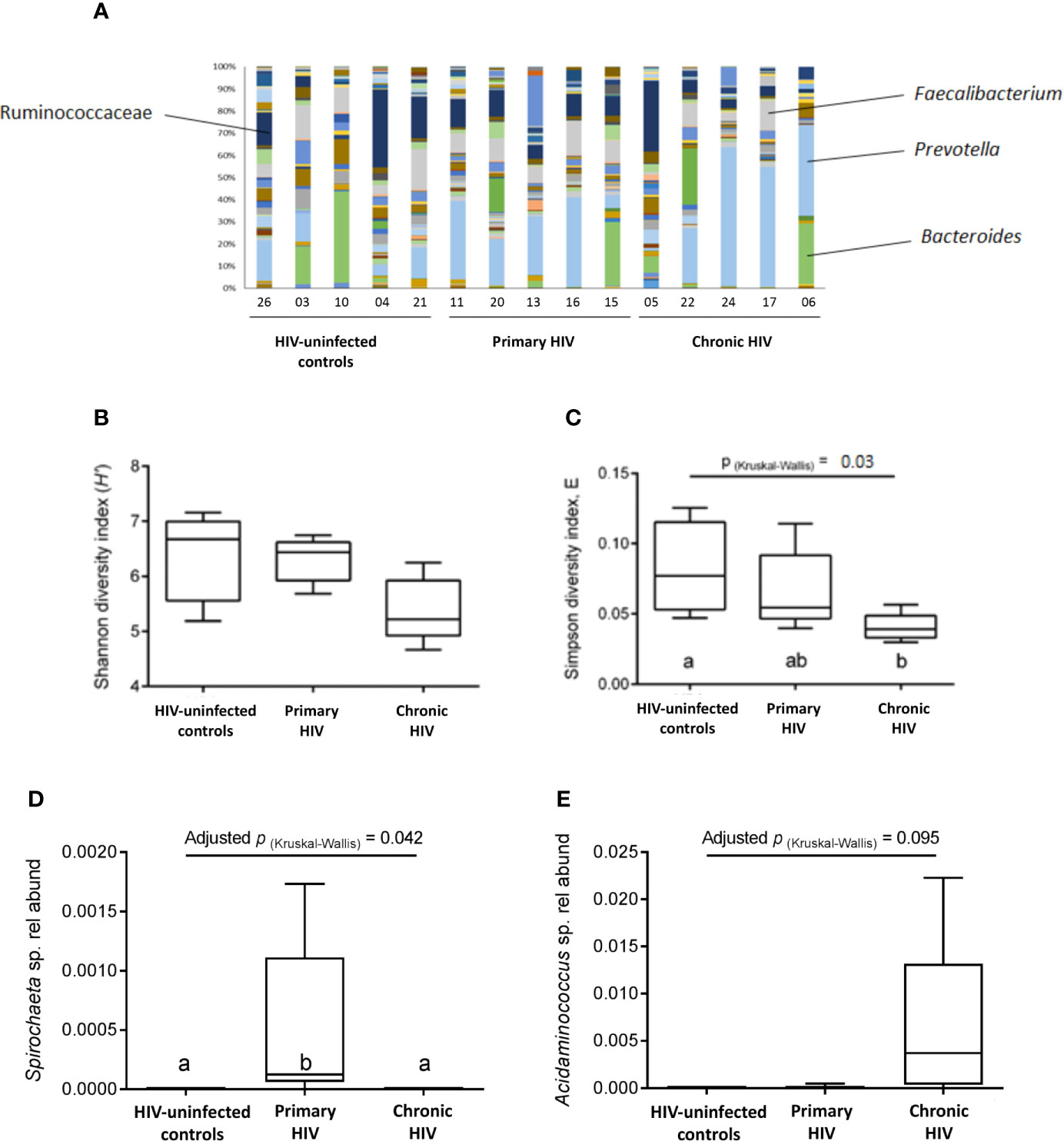
Figure 7 Faecal microbiota composition. (A) Bacterial composition from analysis of faecal samples from study participants prior to bowel preparation. A total of 145 types of taxa are represented, with increased representation of fermentative taxa (i.e., Ruminococcaceae, Faecalibacterium, Prevotella, Bacteroides) compared to other taxa. (B) Species diversity and taxa distribution in study participants as measured by Shannon diversity index (H’) (C) Species diversity and taxa distribution in study participants as measured by Simpson diversity index E (D) Relative abundance of the Spirochaeta genus between groups. (E) Relative abundance of the Acidaminococcus genus between groups.
Discussion
In this study, we aimed to confirm and characterise the in vivo intestinal permeability of study participants with treated HIV infection, compared to HIV-uninfected controls, using CEM, as well as define associations with parameters of mucosal barrier integrity and systemic immune activation and inflammation. Contrary to expectations, however, we could not confirm significant differences for in vivo intestinal permeability between study groups.
In our study methodology, the amount of fluorescein used was matched with other studies which successfully identified changes in gastrointestinal villi, determining the timeframe in which contrast-enhanced images could be taken with the confocal microscope (28, 29). Fluorescein leak and cell junction enhancement were features chosen as indicative of increased epithelial permeability in intestinal villi as interpretation could be easily standardised and due to their previous association with damage to epithelial barrier integrity in other contexts (41). These features were identifiable in our images, but, importantly, there were no significant quantitative differences between the subject groups.
A recent study had also utilized in vivo microscopic imaging of rectal mucosa and reported that there was increased fluorescein leakage and intramucosal bacteria in most of the 10 HIV+ subjects studied (31). However, unlike our study, there was no quantitative comparison to HIV-uninfected controls, and 7/10 HIV+ subjects were Elite Controllers (31), who are not representative of PLHIV on long-term ART.
Although in vitro studies have reported increased tight junction permeability, epithelial inflammation and apoptosis (10) on direct exposure to the HIV-1, this effect could be reduced in vivo due to the fast turnover rate of epithelial cells (42) and sustained suppression of HIV replication with long term ART. Certainly, this would be consistent with both this small study’s results and results from an in vitro study of intestinal permeability by Epple et al. in 2009 (12). Although inter-observer agreement on cell junction enhancement was low compared to other studies (28, 41, 43), the comparable agreement on presence of fluorescein leak with other studies, and the high percentage of study participants with CEM features suggest the methodology is sufficiently sensitive. Larger studies utilising this method in treated and untreated PLHIV with comparison to other inflammatory gastrointestinal diseases, such as ulcerative colitis or Crohn’s disease, would help place these findings in a clearer context.
Ours is the first study to directly explore the effect of early and late initiation of effective ART on intestinal permeability. A secondary hypothesis was that gastrointestinal lymphocyte depletion would be more profound in PLHIV who commenced ART during CHI. Consistent with this hypothesis, our study found a significant decrease in LC CD4 T-cell counts and a decreasing trend in TI CD4 T-cell counts of CHI participants compared to HIV-uninfected controls, and is consistent with our previous studies (35). This result is supported in previous studies on T-lymphocyte cell counts in gastrointestinal tissue (14, 16, 44) in untreated and treated (6, 44–46) PLHIV, but the effect of early ART initiation in the literature is unclear. While studies have reported limited reconstitution of the proportion of CD4 T-cells in intestinal tissue even when treated during primary infection (6, 47), a study by Allers et al. on absolute CD4 T-cell numbers reported treatment during early HIV infection led to complete preservation of CD4 T-cells in duodenal tissue (45).
Our study found a decreasing trend in LC CD4 T-cell numbers of PLHIV treated in primary infection. The association was less clear in the TI. Data from a study by Yukl et al. (48) previously showed a larger quantifiable decrease in the proportion of CD4 T-cells in ileal samples of HIV-positive volunteers than in rectal samples. It has been suggested that the effect of HIV infection on T-cell numbers is variable depending on the gastrointestinal sample site due to different frequencies of gut-homing and regulatory T cells (48). A possible identified contributor to the variability of lymphocyte counts was the nature of lymphoid follicles being concentrated in Peyer’s patches in the TI (49), making representative sampling of this site difficult. Our group’s larger analysis of gastrointestinal lymphocyte counts have revealed significant decreases in CD4 T-cells in both the LC and TI, using both absolute cell counts and epithelial cell ratio, although in that study samples were not analysed by weight (35).
Earlier studies quantified the CD4 T-cells as a percentage of total CD3+ T-cells. However, absolute CD8+ CD3+ T-cell levels may be increased in HIV-positive patients with untreated infection, with high variability in the levels in patients on ART (14, 45), resulting in either a decrease in, or increased variability, in mucosal CD4 T-cell percentages. This study is one of few to use absolute lymphocyte counts to preclude the effects of CD8 T-cell proliferation. As a standard method of normalisation is not documented, both epithelial cell counts and biopsy weight were used as normalisation methods due to possible variations in size of biopsy samples. However, we found stronger correlations between peripheral blood and tissue lymphocytes when normalising by weight. This suggests that lymphocyte loss may be more concentrated in areas of lymphoid aggregation, which are deeper in the tissue, compared to tissue-resident lymphocytes near the epithelial border. Weight may be more representative of biopsy volume, and epithelial cells representative of total biopsy tissue area. This might be a useful area of investigation, which could involve comparison of immuno-histochemistry and flow cytometry methods.
Despite systemic CD4 activation being central to HIV pathogenesis (50), we have concentrated on the study of activated CD38+HLA-DR+ CD8 T-cells, rather than activation of CD4 T-cells for several reasons. Firstly, levels of activated CD38+HLA-DR+ CD8 T-cells in blood samples from untreated patients have been clearly correlated with disease progression (51). Secondly, in patients receiving ART, we and others have documented residual elevation of activated CD8 T-cells (38, 52, 53) despite suppression of HIV replication; these levels have been correlated with plasma LPS levels, consistent with the microbial translocation hypothesis (7). Finally, the frontline CD8 T-cells in GALT can be identified as CD103+ intraepithelial resident memory cells, and these cells should be most activated by barrier dysfunction. In contrast, levels of CD4 activation in gut biopsy samples are complicated by the presence of germinal centres containing activated CD4 T follicular helper cells (35), and these cells do not leave GALT.
Instead, we have found that there was no difference in CD8 T-cell activation in gut biopsy samples between PLHIV on ART compared to HIV-uninfected controls, and in particular, we documented for the first time that CD103+ CD8 T-cells were generally more activated than CD103- migratory CD8+ T-cells. Furthermore, there was only a minority of activated CD8 T-cells in peripheral blood that expressed the gut-homing integrins α4ß7, and there was no obvious correlation of levels of activation of CD8 T-cells between the GALT and blood compartments. Altogether, our detailed results suggest that it is unlikely that the main source of activated CD8 T-cells in blood is due to gastrointestinal barrier dysfunction.
We have previously found that crucial CD4 T follicular helper cells in GALT are not significantly depleted in HIV+ subjects on ART, nor are their important downstream effector cells, IgA+ B-cells (35), which will help maintain microbial homeostasis of this tissue. Instead, in these GALT germinal centres as well as in other lymphoid tissues, it is possible that CD4 T follicular helper cells act as a residual underlying HIV reservoir (3, 54) and may contribute to lingering CD8 T-cell activation. Undoubtedly, GALT CD4 T-cells continue to contribute to the HIV reservoir under ART (55, 56). However, we have previously found that in the circulation, gut-homing CD4 T cells only contain a small proportion of the HIV-1 DNA in PBMC (57). Other tissues, despite fully suppressed plasma viremia, are likely to contain HIV-1 reservoirs that activate CD8 T-cells, such as CD4 T follicular helper cells in peripheral lymph nodes (3, 54), infected alveolar macrophages (58) and infected cells in the CNS (59, 60).
An increased relative abundance of Spirochaeta, Prevotella, Acidaminococcus species in those with HIV, and a reduced abundance of Faecalibacterium species, as suggested by our data, are consistent with gut microbiome changes described in PLHIV in other studies (22, 23, 61). Faecalibacterium prausnitzii, a member of clostridial cluster IV, is one of a number of obligate anaerobic gut commensal bacteria that are responsible for the production of the short chain fatty acid butyrate through the fermentation of carbohydrates in the colon by inducing (62). Butyrate, in turn, contributes to gut barrier function by inducing tight junction assembly through an AMPK-dependent pathway (63). In contrast, increased prevalence of Prevotella species is associated with increased susceptibility to gut inflammation through indirect suppression of IL-18 production (64). However, while it has been suggested that an increased presence of potentially pathogenic bacteria in the gastrointestinal lumen may induce expression of pro-inflammatory cytokines, thereby increasing epithelial layer permeability (65, 66), these changes in microbiota composition were not reflected in the confocal imaging results.
There are a number of reasons why interpretation of the microbiome data is difficult. First, the cohort was small, and the study was cross-sectional, not permitting longitudinal microbiome follow-up. Three participants were treated for a sexually transmitted infection with antibiotics before enrolment. Finally, it has been recognised that ART has a variable impact on the composition of the microbiome, and this could not be assessed in this study (67, 68).
Plasma sCD14 concentration is a measure of monocyte activation in response to lipopolysaccharide from the cell walls of gram-negative bacteria (45, 69). We did not find, however, an increasing trend in levels of sCD14 in CHI patients. Recent studies have revealed differing findings on the effect of ART on levels of sCD14 (45, 70, 71). This variability may be due to a number of confounding factors, such as the duration of ART, although variability persists when individual patients are followed longitudinally (71); behavioural factors such as smoking may also be implicated (72, 73). The measured levels of sCD14 varied highly between previously published studies, varying from 0.7x106pg/mL (74) to 4x106pg/mL (45) in HIV-uninfected controls. Our study’s median of 1.8x106 pg/mL for HIV-uninfected controls and 1.4x106 pg/mL for PLHIV is consistent with results from other studies (45, 74). No increasing trend in levels of TNF-α, IL-6 or systemic activated HLA-DR+CD38+ CD8+ T-cells was found in the CHI patients.
Presentation of intraluminal antigens from the intestinal lumen is part of normal physiology in the regulation of immune responses and establishment of immunotolerance (75, 76), and it is possible for increased bacterial products to enter the portal and systemic circulation due to dysregulation of immune lymphocytes despite maintenance of epithelial barrier integrity. HIV-1 can infect hepatic Kupffer cells, which express CD14 and other receptors responding to LPS, altering measured levels of these markers (77). However, partial restoration of Kupffer cells has been found following initiation of ART (78).
The strength of this study was the detailed comparison with HIV-uninfected controls, including not only the confocal imaging, but also the detailed study of immune activation locally and systemically, as well as of the microbiome. This study was however limited by its cross-sectional design. Hence, we were unable to demonstrate whether the lack of changes in intestinal permeability was due to recovery following ART initiation. A more significant limitation of this study was the small sample size, which increases the possibility of type II errors. Finally, the small sample including only men makes generalisability difficult. As a pilot study, the main aim was to identify trends in measures of the gastrointestinal barrier that could contribute to microbial translocation and contribute exploratory data to support further larger studies of barrier dysfunction and microbial dysbiosis.
In conclusion, our results indicate that despite slightly impaired CD4+ T-cell recovery in the gastrointestinal tissue of CHI patients, no changes to physical epithelial integrity were found, nor significantly increased activation of CD8 T-cells within GALT. Furthermore, microbial translocation and inflammatory markers trended towards return to baseline. Our study raises doubts about the significance of microbial translocation and systemic inflammation in HIV-positive participants on effective ART. Analyses have suggested that there is little difference in life expectancy in PLHIV compared to HIV-uninfected individuals when controlling for other risk factors (79), although a more recent study suggests that PLHIV who commenced ART in the US between 2011-2016 with high CD4 counts still had about 7 years less life expectancy, plus more years of comorbidities. Several issues remain, such as whether dysregulation of intestinal CD4+ lymphocytes alone facilitates increased passage of bacterial by-products into the portal circulation. In summary, our study suggests that the importance of microbial translocation and its contribution to systemic immune activation in well-controlled HIV-positive patients may not be as significant as widely reported and believed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the St Vincent’s Human Research Ethics Committee (HREC 14/214). All participants provided written informed consent. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Experimental work and data analysis: GM, JZ, MB, NS, GR, LL, and MD. Project conception: MD, MAB, KK, AK, and TP. Patient recruitment and procedures: MD, GM, KK, and AK. Manuscript written by GM, JZ, and MD. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by a St Vincent’s Clinic Foundation Project Grant. Cancer Institute NSW Equipment Grant 10REG114, Australia Research Council (ARC) Linkage Project LP10020080. TP is supported by National Health and Medical Research Council (NHMRC) Senior Research Fellowship (APP1155678) and the Ernest Heine Family Foundation. JZ was supported by NHMRC Fellowship 1063422 and ADK by NHMRC Program Grant 1052979.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor has declared a shared affiliation, though no other collaboration with one of the authors NS at the time of review.
Acknowledgments
The authors would like to thank Dr Yin Xu for expert technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.688886/full#supplementary-material
Supplementary Figure 1 | – Representative flow plots and gating of CD8 T-cell subsets. (A)– Top row: Gating of CD45+ SSClow lymphocytes, then CD3+ and EpCAM-negative, then CD45RA-negative, then CD8 T-cells (CD3+ CD4 negative). -Bottom row: CD8 T-cells then analysed for CD38 vs HLA-DR and CD38 vs CD127. -Then CD38+HLA-DR+ and CD38+CD127low cells, respectively, were analysed for CD103 expression. (B)– Top row: Gating of CD45+ SSClow lymphocytes, then CD3+ and CD20-negative, then CD45RA-negative, then CD8 T-cells (CD3+ CD4 negative). -Bottom row: CD8 T cells then analysed for CD38 vs HLA-DR, and CD49d vs integrin ß7, and CD38+CD127low cells. -Then CD38+HLA-DR+ cells were analysed for CD49d vs integrin ß7 expression
Supplementary Figure 2 | Activated CD38+CD127low CD8 T-cells in blood and tissues. Activated CD38+CD127low as % of CD45RA- CD8 T cells in peripheral blood, and in TI and LC biopsies, by study group. HUC: HIV-uninfected controls, PHI: HIV-positive patients treated during primary infection, CHI: HIV-positive patients treated during chronic infection.
References
1. Esser R, Von Briesen H, Brugger W, Ceska M, Glienke W, Müller S, et al. Secretory Repertoire of HIV-Infected Human Monocytes/Macrophages. Pathobiology (1991) 59(4):219–22. doi: 10.1159/000163649
2. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell Activation and Immunoregulation in Patients With the Acquired Immunodeficiency Syndrome. New Engl J Med (1983) 309(8):453–8. doi: 10.1056/NEJM198308253090803
3. Hey-Nguyen WJ, Xu Y, Pearson CF, Bailey M, Suzuki K, Tantau R, et al. Quantification of Residual Germinal Center Activity and HIV-1 DNA and RNA Levels Using Fine Needle Biopsies of Lymph Nodes During Antiretroviral Therapy. AIDS Res Hum Retroviruses (2017) 33(7):648–57. doi: 10.1089/aid.2016.0171
4. Hazenberg MD, Stuart JWC, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, et al. T-Cell Division in Human Immunodeficiency Virus (HIV)-1 Infection is Mainly Due to Immune Activation: A Longitudinal Analysis in Patients Before and During Highly Active Antiretroviral Therapy (HAART). Blood (2000) 95(1):249–55. doi: 10.1182/blood.V95.1.249.001k40_249_255
5. Zaunders JJ, Kaufmann GR, Cunningham PH, Smith D, Grey P, Suzuki K, et al. Increased Turnover of CCR5+ and Redistribution of CCR5- Cd4 T Lymphocytes During Primary Human Immunodeficiency Virus Type 1 Infection. J Infect Dis (2001) 183(5):736–43. doi: 10.1086/318827
6. Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL, et al. Viral Suppression and Immune Restoration in the Gastrointestinal Mucosa of Human Immunodeficiency Virus Type 1-Infected Patients Initiating Therapy During Primary or Chronic Infection. J Virol (2006) 80(16):8236–47. doi: 10.1128/JVI.00120-06
7. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial Translocation is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat Med (2006) 12(12):1365–71. doi: 10.1038/nm1511
8. Epple HJ, Allers K, Tröger H, Kühl A, Erben U, Fromm M, et al. Acute HIV Infection Induces Mucosal Infiltration With CD4+ and CD8+ T Cells, Epithelial Apoptosis, and a Mucosal Barrier Defect. Gastroenterology (2010) 139(4):1289–300. e2. doi: 10.1053/j.gastro.2010.06.065
9. Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut Epithelial Barrier and Systemic Inflammation During Chronic HIV Infection. AIDS (2015) 29(1):43–51. doi: 10.1097/QAD.0000000000000511
10. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation. PloS Pathog (2010) 6(4):e1000852. doi: 10.1371/journal.ppat.1000852
11. Tincati C, Merlini E, Braidotti P, Ancona G, Savi F, Tosi D, et al. Impaired Gut Junctional Complexes Feature Late-Treated Individuals With Suboptimal CD4+ T-Cell Recovery Upon Virologically Suppressive Combination Antiretroviral Therapy. AIDS (2016) 30(7):991–1003. doi: 10.1097/QAD.0000000000001015
12. Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, et al. Impairment of the Intestinal Barrier is Evident in Untreated But Absent in Suppressively Treated HIV-Infected Patients. Gut (2009) 58(2):220–7. doi: 10.1136/gut.2008.150425
13. Chung CY, Alden SL, Funderburg NT, Fu P, Levine AD. Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals. PloS Pathog (2014) 10(6):e1004198. doi: 10.1371/journal.ppat.1004198
14. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. Cd4+ T Cell Depletion During All Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med (2004) 200(6):749–59. doi: 10.1084/jem.20040874
15. Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-Cell Depletion in Gut Lymphoid Tissue During Primary Human Immunodeficiency Virus Type 1 Infection and Substantial Delay in Restoration Following Highly Active Antiretroviral Therapy. J Virol (2003) 77(21):11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003
16. Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, et al. Primary HIV-1 Infection is Associated With Preferential Depletion of CD4+ T Lymphocytes From Effector Sites in the Gastrointestinal Tract. J Exp Med (2004) 200(6):761–70. doi: 10.1084/jem.20041196
17. DaFonseca S, Niessl J, Pouvreau S, Wacleche SV, Gosselin A, Cleret-Buhot A, et al. Impaired Th17 Polarization of Phenotypically Naive CD4+ T-Cells During Chronic HIV-1 Infection and Potential Restoration With Early ART. Retrovirology (2015) 12(1). doi: 10.1186/s12977-015-0164-6
18. D’Ettorre G, Baroncelli S, Micci L, Ceccarelli G, Andreotti M, Sharma P, et al. Reconstitution of Intestinal CD4 and Th17 T Cells in Antiretroviral Therapy Suppressed HIV-Infected Subjects: Implication for Residual Immune Activation From the Results of a Clinical Trial. PloS One (2014) 9(10):e109791. doi: 10.1371/journal.pone.0109791
19. Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections. PloS Pathog (2010) 6(8):e1001052. doi: 10.1371/journal.ppat.1001052
20. Kim CJ, McKinnon LR, Kovacs C, Kandel G, Huibner S, Chege D, et al. Mucosal Th17 Cell Function is Altered During HIV Infection and is an Independent Predictor of Systemic Immune Activation. J Immunol (2013) 191(5):2164–73. doi: 10.4049/jimmunol.1300829
21. Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, et al. Early Impairment of Gut Function and Gut Flora Supporting a Role for Alteration of Gastrointestinal Mucosa in Human Immunodeficiency Virus Pathogenesis. J Clin Microbiol (2008) 46(2):757–8. doi: 10.1128/JCM.01729-07
22. Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An Altered Intestinal Mucosal Microbiome in HIV-1 Infection is Associated With Mucosal and Systemic Immune Activation and Endotoxemia. Mucosal Immunol (2014) 7(4):983–94. doi: 10.1038/mi.2013.116
23. Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the Gut Microbiota is Associated With HIV Disease Progression and Tryptophan Catabolism. Sci Trans Med (2013) 5(193):193ra91. doi: 10.1126/scitranslmed.3006438
24. Liu J, Williams B, Frank D, Dillon SM, Wilson CC, Landay AL. Inside Out: HIV, the Gut Microbiome, and the Mucosal Immune System. J Immunol (2017) 198(2):605–14. doi: 10.4049/jimmunol.1601355
25. Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S, et al. Acute Gastrointestinal Permeability Responses to Different non-Steroidal Anti-Inflammatory Drugs. Gut (2001) 49(5):650–5. doi: 10.1136/gut.49.5.650
26. Buchner AM, Wallace MB. In-Vivo Microscopy in the Diagnosis of Intestinal Neoplasia and Inflammatory Conditions. Histopathology (2015) 66(1):137–46. doi: 10.1111/his.12597
27. Goetz M, Malek NP, Kiesslich R. Microscopic Imaging in Endoscopy: Endomicroscopy and Endocytoscopy. Nat Rev Gastroenterol Hepatol (2014) 11(1):11–8. doi: 10.1038/nrgastro.2013.134
28. Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, et al. Local Barrier Dysfunction Identified by Confocal Laser Endomicroscopy Predicts Relapse in Inflammatory Bowel Disease. Gut (2012) 61(8):1146–53. doi: 10.1136/gutjnl-2011-300695
29. Lim LG, Neumann J, Hansen T, Goetz M, Hoffman A, Neurath MF, et al. Confocal Endomicroscopy Identifies Loss of Local Barrier Function in the Duodenum of Patients With Crohn’s Disease and Ulcerative Colitis. Inflammatory Bowel Dis (2014) 20(5):892–900. doi: 10.1097/MIB.0000000000000027
30. Caradonna L, Amati L, Magrone T, Pellegrino N, Jirillo E, Caccavo D. Invited Review: Enteric Bacteria, Lipopolysaccharides and Related Cytokines in Inflammatory Bowel Disease: Biological and Clinical Significance. J Endotoxin Res (2000) 6(3):205–14. doi: 10.1177/09680519000060030101
31. Etcheverry-Rufino F, Lucero C, Lopez-Ceron M, Alenar-Gelabert Y, Fernandez I, Ugarte A, et al. Local Barrier Dysfunction Identified by Confocal Laser Endomicroscopy Predicts Bacterial Translocation in HIV Infection. AIDS (2020) 34(2):328–31. doi: 10.1097/QAD.0000000000002415
32. Koelsch KK, Boesecke C, McBride K, Gelgor L, Fahey P, Natarajan V, et al. Impact of Treatment With Raltegravir During Primary or Chronic HIV Infection on RNA Decay Characteristics and the HIV Viral Reservoir. Aids (2011) 25(17):2069–78. doi: 10.1097/QAD.0b013e32834b9658
33. Turcotte JF, Kao D, Mah SJ, Claggett B, Saltzman JR, Fedorak RN, et al. Breaks in the Wall: Increased Gaps in the Intestinal Epithelium of Irritable Bowel Syndrome Patients Identified by Confocal Laser Endomicroscopy (With Videos). Gastrointestinal Endoscopy (2013) 77(4):624–30. doi: 10.1016/j.gie.2012.11.006
34. Li C-Q, Xie X-J, Yu T, Gu X-M, Zuo X-L, Zhou C-J, et al. Classification of Inflammation Activity in Ulcerative Colitis by Confocal Laser Endomicroscopy. Am J Gastroenterol (2010) 105(6):1391–6. doi: 10.1038/ajg.2009.664
35. Zaunders J, Danta M, Bailey M, Mak G, Marks K, Seddiki N, et al. Cd4+ T Follicular Helper and Iga+ B Cell Numbers in Gut Biopsies From HIV-infected Subjects on Antiretroviral Therapy are Comparable to HIV-uninfected Individuals. Front Immunol (2016) 7(438):438. doi: 10.3389/fimmu.2016.00438
36. Jervis-Bardy J, Leong L, Marri S, Smith RJ, Choo JM, Smith-Vaughan HC, et al. Deriving Accurate Microbiota Profiles From Human Samples With Low Bacterial Content Through Post-Sequencing Processing of Illumina MiSeq Data. Microbiome (2015) 3(1):1–11. doi: 10.1186/s40168-015-0083-8
37. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat Methods (2010) 7(5):335–6. doi: 10.1038/nmeth.f.303
38. Hey-Cunningham WJ, Murray JM, Natarajan V, Amin J, Moore CL, Emery S, et al. Early Antiretroviral Therapy With Raltegravir Generates Sustained Reductions in HIV Reservoirs But Not Lower T-cell Activation Levels. AIDS (2015) 29(8):911–9. doi: 10.1097/QAD.0000000000000625
39. Schenkel JM, Masopust D. Tissue-Resident Memory T Cells. Immunity (2014) 41(6):886–97. doi: 10.1016/j.immuni.2014.12.007
40. Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Short Communication: HIV+ Viremic Slow Progressors Maintain Low Regulatory T Cell Numbers in Rectal Mucosa But Exhibit High T Cell Activation. AIDS Res Hum Retroviruses (2013) 29(1):172–7. doi: 10.1089/aid.2012.0268
41. Chang J, Ip M, Yang M, Wong B, Power T, Lin L, et al. The Learning Curve: Inter-Observer and Intra-Observer Agreement of Endoscopic Confocal Laser Endomicroscopy in the Assessment of Mucosal Barrier Defects. Gastrointestinal Endoscopy (2015) 83(4):785–91. doi: 10.1016/j.gie.2015.08.045
42. Lipkin M. Growth and Development of Gastrointestinal Cells. Annu Rev Physiol (1985) 47(1):175–97. doi: 10.1146/annurev.ph.47.030185.001135
43. Neumann H, Vieth M, Atreya R, Grauer M, Siebler J, Bernatik T, et al. Assessment of Crohn’s Disease Activity by Confocal Laser Endomicroscopy. Inflammatory Bowel Dis (2012) 18(12):2261–9. doi: 10.1002/ibd.22907
44. Gordon SN, Cervasi B, Odorizzi P, Silverman R, Aberra F, Ginsberg G, et al. Disruption of Intestinal CD4+ T Cell Homeostasis is a Key Marker of Systemic CD4+ T Cell Activation in HIV-infected Individuals. J Immunol (2010) 185(9):5169–79. doi: 10.4049/jimmunol.1001801
45. Allers K, Puyskens A, Epple HJ, Schurmann D, Hofmann J, Moos V, et al. The Effect of Timing of Antiretroviral Therapy on CD4 T-Cell Reconstitution in the Intestine of HIV-infected Patients. Mucosal Immunol (2016) 9(1):265–74. doi: 10.1038/mi.2015.58
46. Hayes TL, Asmuth DM, Critchfield JW, Knight TH, McLaughlin BE, Yotter T, et al. Impact of Highly Active Antiretroviral Therapy Initiation on CD4+ T-Cell Repopulation in Duodenal and Rectal Mucosa. AIDS (London England) (2013) 27(6):867. doi: 10.1097/QAD.0b013e32835d85b4
47. Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, et al. Lack of Mucosal Immune Reconstitution During Prolonged Treatment of Acute and Early HIV-1 Infection. PloS Med (2006) 3(12):2335–48. doi: 10.1371/journal.pmed.0030546
48. Yukl SA, Shergill AK, Girling V, Li Q, Killian M, Epling L, et al. Site-Specific Differences in T Cell Frequencies and Phenotypes in the Blood and Gut of HIV-uninfected and ART-treated HIV+ Adults. PloS One (2015) 10(3):e0121290. doi: 10.1371/journal.pone.0121290
49. Van Kruiningen HJ, West AB, Freda BJ, Holmes KA. Distribution of Peyer’s Patches in the Distal Ileum. Inflammatory Bowel Dis (2002) 8(3):180–5. doi: 10.1097/00054725-200205000-00004
50. Zaunders J, Xu Y, Kent SJ, Koelsch KK, Kelleher AD. Divergent Expression of CXCR5 and CCR5 on CD4+ T Cells and the Paradoxical Accumulation of T Follicular Helper Cells During HIV Infection. Front Immunol (2017) 8:495. doi: 10.3389/fimmu.2017.00495
51. Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated Levels of CD38+ Cd8+ T Cells in HIV Infection Add to the Prognostic Value of Low CD4+ T Cell Levels: Results of 6 Years of Follow-Up. The Los Angeles Center, Multicenter Aids Cohort Study. J Acquir Immune Defic Syndr (1993) 6(8):904–12.
52. Younas M, Psomas C, Reynes J, Corbeau P. Immune Activation in the Course of HIV-1 Infection: Causes, Phenotypes and Persistence Under Therapy. HIV Med (2016) 17(2):89–105. doi: 10.1111/hiv.12310
53. Zaunders JJ, Cunningham PH, Kelleher AD, Kaufmann GR, Jaramillo AB, Wright R, et al. Potent Antiretroviral Therapy of Primary Human Immunodeficiency Virus Type 1 (HIV-1) Infection: Partial Normalization of T Lymphocyte Subsets and Limited Reduction of HIV-1 DNA Despite Clearance of Plasma Viremia. J Infect Dis (1999) 180(2):320–9. doi: 10.1086/314880
54. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. Pd-1(+) and Follicular Helper T Cells are Responsible for Persistent HIV-1 Transcription in Treated Aviremic Individuals. Nat Med (2016) 22(7):754–61. doi: 10.1038/nm.4113
55. Yukl SA, Shergill AK, McQuaid K, Gianella S, Lampiris H, Hare CB, et al. Effect of Raltegravir-Containing Intensification on HIV Burden and T-cell Activation in Multiple Gut Sites of HIV-positive Adults on Suppressive Antiretroviral Therapy. Aids (2010) 24(16):2451–60. doi: 10.1097/QAD.0b013e32833ef7bb
56. Anderson JL, Khoury G, Fromentin R, Solomon A, Chomont N, Sinclair E, et al. Human Immunodeficiency Virus (Hiv)-Infected CCR6+ Rectal Cd4+ T Cells and HIV Persistence On Antiretroviral Therapy. J Infect Dis (2020) 221(5):744–55. doi: 10.1093/infdis/jiz509
57. McBride K, Xu Y, Bailey M, Seddiki N, Suzuki K, Gao Y, et al. The Majority of HIV-1 DNA in Circulating CD4+ T Lymphocytes is Present in non-Gut Homing Resting Memory CD4+ T Cells. AIDS Res Hum Retroviruses (2013) 29(10):1330–9. doi: 10.1089/aid.2012.0351
58. Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. Healthy HIV-1-infected Individuals on Highly Active Antiretroviral Therapy Harbor HIV-1 in Their Alveolar Macrophages. AIDS Res Hum Retroviruses (2015) 31(1):64–70. doi: 10.1089/aid.2014.0133
59. Spudich S, Robertson KR, Bosch RJ, Gandhi RT, Cyktor JC, Mar H, et al. Persistent HIV-infected Cells in Cerebrospinal Fluid are Associated With Poorer Neurocognitive Performance. J Clin Invest (2019) 129(8):3339–46. doi: 10.1172/JCI127413
60. Suzuki K, Zaunders J, Levert A, Butterly S, Liu Z, Ishida T, et al. Neuron Damage and Reservoir are Secondary to HIV Transcripts Despite Suppressive ART. Topics in Antiviral Medicien. (2021) 29(1):49
61. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A Compositional Look At the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects. PloS Pathog (2014) 10(2):e1003829. doi: 10.1371/journal.ppat.1003829
62. Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol (2016) 7:979. doi: 10.3389/fmicb.2016.00979
63. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly Via Activation of AMP-activated Protein Kinase in Caco-2 Cell Monolayers. J Nutr (2009) 139(9):1619–25. doi: 10.3945/jn.109.104638
64. Iljazovic A, Roy U, Galvez EJC, Lesker TR, Zhao B, Gronow A, et al. Perturbation of the Gut Microbiome by Prevotella Spp. enhances Host susceptibility to Mucosal inflammation Mucosal Immunol (2021) 14(1):113–24. doi: 10.1038/s41385-020-0296-4
65. Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. Tnf-α-Induced Increase in Intestinal Epithelial Tight Junction Permeability Requires NF-κb Activation. Am J Physiol Gastrointest Liver Physiol (2004) 286(3):G367–G76. doi: 10.1152/ajpgi.00173.2003
66. Al-Sadi RM, Ma TY. Il-1β Causes an Increase in Intestinal Epithelial Tight Junction Permeability. J Immunol (2007) 178(7):4641–9. doi: 10.4049/jimmunol.178.7.4641
67. BenMarzouk-Hidalgo OJ, Torres-Cornejo A, Gutierrez-Valencia A, Ruiz-Valderas R, Viciana P, Lopez-Cortes LF. Differential Effects of Viremia and Microbial Translocation on Immune Activation in HIV-Infected Patients Throughout Ritonavir-Boosted Darunavir Monotherapy. Medicine (2015) 94(17):e781. doi: 10.1097/MD.0000000000000781
68. Li Z, Li W, Li N, Jiao Y, Chen D, Cui L, et al. γδ T Cells are Involved in Acute HIV Infection and Associated With AIDS Progression. PloS One (2014) 9(9):e106064. doi: 10.1371/journal.pone.0106064
69. Rajasuriar R, Booth D, Solomon A, Chua K, Spelman T, Gouillou M, et al. Biological Determinants of Immune Reconstitution in HIV-infected Patients Receiving Antiretroviral Therapy: The Role of Interleukin 7 and Interleukin 7 Receptor α and Microbial Translocation. J Infect Dis (2010) 202(8):1254–64. doi: 10.1086/656369
70. Cioe PA, Baker J, Kojic EM, Onen N, Hammer J, Patel P, et al. Elevated Soluble CD14 and Lower D-Dimer Are Associated With Cigarette Smoking and Heavy Episodic Alcohol Use in Persons Living With Hiv. JAIDS J Acquired Immune Deficiency Syndromes (2015) 70(4):400–5. doi: 10.1097/QAI.0000000000000759
71. Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D. Tobacco Smoking Increases Immune Activation and Impairs T-cell Function in HIV Infected Patients on Antiretrovirals: A Cross-Sectional Pilot Study. PloS One (2014) 9(5):e97698. doi: 10.1371/journal.pone.0097698
72. Chege D, Sheth PM, Kain T, Kim CJ, Kovacs C, Loutfy M, et al. Sigmoid Th17 Populations, the HIV Latent Reservoir, and Microbial Translocation in Men on Long-Term Antiretroviral Therapy. Aids (2011) 25(6):741–9. doi: 10.1097/QAD.0b013e328344cefb
73. Miron N, Cristea V. Enterocytes: Active Cells in Tolerance to Food and Microbial Antigens in the Gut. Clin Exp Immunol (2012) 167(3):405–12. doi: 10.1111/j.1365-2249.2011.04523.x
74. Mowat AM. Anatomical Basis of Tolerance and Immunity to Intestinal Antigens. Nat Rev Immunol (2003) 3(4):331–41. doi: 10.1038/nri1057
75. Hufert FT, Schmitz J, Schreiber M, Schmitz H, Rácz P, von Laer DD. Human Kupffer Cells Infected With HIV-1 In Vivo. JAIDS J Acquired Immune Deficiency Syndromes (1993) 6(7):772–7.
76. Balagopal A, Ray SC, De Oca RM, Sutcliffe CG, Vivekanandan P, Higgins Y, et al. Kupffer Cells are Depleted With HIV Immunodeficiency and Partially Recovered With Antiretroviral Immunereconstitution: HIV, Kupffer Cells and Antiretroviral Therapy. AIDS (London England) (2009) 23(18):2397. doi: 10.1097/QAD.0b013e3283324344
77. Sabin CA. Do People With HIV Infection Have a Normal Life Expectancy in the Era of Combination Antiretroviral Therapy? BMC Med (2013) 11(1):251. doi: 10.1186/1741-7015-11-251
78. Justice A, Falutz J. Aging and HIV: An Evolving Understanding. Curr Opin HIV AIDS (2014) 9(4):291–3. doi: 10.1097/COH.0000000000000081
Keywords: HIV, CD4, antiretroviral therapy (ART), gut-associated lymphoid tissues (GALT), microbiome
Citation: Mak G, Zaunders JJ, Bailey M, Seddiki N, Rogers G, Leong L, Phan TG, Kelleher AD, Koelsch KK, Boyd MA and Danta M (2021) Preservation of Gastrointestinal Mucosal Barrier Function and Microbiome in Patients With Controlled HIV Infection. Front. Immunol. 12:688886. doi: 10.3389/fimmu.2021.688886
Received: 31 March 2021; Accepted: 04 May 2021;
Published: 31 May 2021.
Edited by:
Remi Cheynier, U1016 Institut Cochin (INSERM), FranceReviewed by:
Jean-Pierre Routy, McGill University, CanadaStephen Kent, The University of Melbourne, Australia
Copyright © 2021 Mak, Zaunders, Bailey, Seddiki, Rogers, Leong, Phan, Kelleher, Koelsch, Boyd and Danta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark Danta, bS5kYW50YUB1bnN3LmVkdS5hdQ==
†These authors have contributed equally to this work
 Gerald Mak
Gerald Mak John J. Zaunders
John J. Zaunders Michelle Bailey3
Michelle Bailey3 Nabila Seddiki
Nabila Seddiki Geraint Rogers
Geraint Rogers Tri Giang Phan
Tri Giang Phan Anthony D. Kelleher
Anthony D. Kelleher Mark A. Boyd
Mark A. Boyd Mark Danta
Mark Danta