- 1Thomas E. Starzl Transplantation Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2School of Medicine, Tsinghua University, Beijing, China
- 3Department of Kidney Transplantation and Department of Organ Transplantation and General Surgery, Second Xiangya Hospital of Central South University, Changsha, China
- 4Department of Rheumatology and Clinical Immunology, First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 5Department of Medicine, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 6Evergrande Center for Immunologic Diseases, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA, United States
- 7Klarman Cell Observatory, Broad Institute of MIT and Harvard, Cambridge, MA, United States
- 8Department of Immunology, University of Pittsburgh, Pittsburgh, PA, United States
B cells can be polarized to express various cytokines. The roles of IFNγ and IL-10, expressed respectively by B effector 1 (Be1) and Bregs, have been established in pathogen clearance, tumor growth, autoimmunity and allograft rejection. However, the in vivo role of B cell IL-4, produced by Be2 cells, remains to be established. We developed B-IL-4/13 iKO mice carrying a tamoxifen-inducible B cell-specific deletion of IL-4 and IL-13. After alloimmunization, B-IL-4/13 iKO mice exhibited decreased IL-4+ Th2 cells and IL-10+ Bregs without impact on Th1, Tregs, or CD8 T cell responses. B-IL-4/13 iKO mice rejected islet allografts more rapidly, even when treated with tolerogenic anti-TIM-1 mAb. In ovalbumin-induced allergic airway disease (AAD), B-IL-4/13 iKO mice had reduced inflammatory cells in BAL, and preserved lung histology with markedly decreased infiltration by IL-4+ and IL-5+ CD4+ T cells. Hence, B cell IL-4 is a major driver of Th2 responses in vivo which promotes allograft survival, and conversely, worsens AAD.
Introduction
While B cells are central to humoral immunity, they also play an important role shaping the immune response by presenting antigen, providing T cell co-stimulation, and producing various cytokines (1–9). For example, B cell Lymphotoxin-α is required for follicular dendritic cell differentiation and the generation of B cell follicles (10, 11). Regulatory B cells (Bregs) expressing IL-10 and other inhibitory cytokines, play a critical role maintaining self-tolerance, and can inhibit autoimmunity, allograft and tumor rejection, and anti-microbial responses (3, 9, 12–16). Lund and colleagues showed that Th1 cells, IFNγ, or IL-12, can polarize B cells (termed Be1) to express pro-inflammatory cytokines such as IFNγ and IL-12 (5). In contrast, interaction with Th2 cells or IL-4, polarized B cells (termed Be2) to produce IL-2 and IL-4. Be1 and Be2 cells could then promote the in vitro differentiation of Th1 and Th2 cells, respectively (5, 17). Subsequently, B cell IFNγ was shown to play an important role in Th1 responses that promote allograft and tumor rejection, autoimmune arthritis, and antibacterial responses (8, 9, 18, 19). In contrast, the role of B cell IL-4 in promoting Th2 responses modulating disease in vivo, remains uncertain. Constitutive B cell-specific deletion of IL-4Rα reduced Th2 and increased Th1 responses, enhancing susceptibility to Schistosoma mansoni (controlled by Type 2 responses), while reducing Th2-driven cutaneous Leishmania major infection and house dust mite (HDM)-induced asthma (20, 21). These studies establish a clear role for IL-4-responsive B cells in vivo. However, the role of B cell IL-4 expression itself is less clear. In a bone marrow chimera model, loss of B cell IL-4 expression reduced Th2 and augmented Th1 responses, conferring protection to cutaneous L. major (20). However, in HDM-induced asthma, transfer of IL-4/IL-13−/− B cells into μMT mice reduced airway hyperresponsiveness but had no effect on the Th2 response or airway inflammation—suggesting that IL-4 responsiveness, rather than IL-4 production by B cells, drives Th2 responses and airway inflammation (21). In agreement, while IL-4-responsive B cells are required, B cell IL-2 and not IL-4, drove protective Th2 responses to H. polgyrus infection (22). Similarly, B cells, but not B cell IL-4, were required for Th2 responses to Nippostrongylus brasiliensis infection (7). Thus, IL-4-responsive B cells play an important role in parasitic and asthma immune responses, but the role of B cell IL-4 per se, particularly in Type 2-mediated diseases, remains unclear.
Contradictory outcomes might result from examination of different disease models, but also from different (non-physiologic) approaches used to generate mice with IL-4-deficient B cells. In both cell transfer and bone marrow chimera approaches, B cells develop in constitutively cytokine-deficient environments which might lead to compensatory changes. Given the major role of other B cell cytokines in wide-ranging immune responses, we aimed to establish whether B cell IL-4 is an important driver of in vivo Th2 responses using mice with a novel inducible, B cell-specific deletion of IL-4 and IL-13. IL-13 is a signature type 2 cytokine encoded within the Th2 cytokine locus that is coordinately expressed with IL-4 in Th2 cells, while the IL-13 receptor (IL-4Rα:IL-13Rα heterodimer), is largely confined to non-hematopoietic cells (23). Unlike the models described above, these mice are developmentally and physiologically normal until tamoxifen treatment which induces nuclear translocation of Cre which is specifically expressed in B cells. Testing the immune responses in both type I and type II-driven disease models, we determined that B cell IL-4 plays an important role driving Th2 responses in vivo, leading to exacerbation of airway inflammation in allergic airway disease (AAD; Th2-driven), and reducing islet allograft rejection (where Th2 responses may be protective). Thus, B cell IL-4 production has a major impact on Th2 cells and amplifies Type 2 immune responses.
Materials and Methods
Mice
C57BL/6 (B6), BALB/c and IL-4/IL-13fl/fl mice (BALB/c) were purchased from the Jackson Laboratories. hCD20-ERT2.Cre+/− (BALB/c; kindly provided by Mark Shlomchik, University of Pittsburgh) were used as Cre-controls (24). We crossed hCD20-ERT2.Cre and IL-4/IL-13fl/fl mice to generate hCD20-ERT2.Cre+/− IL-4/IL-13fl/fl mice (B-IL-4/13 iKO). B IL-4/13 iKO and hCD20.Cre-control mice were placed on a 1:1 mixture of normal and tamoxifen-containing chow (Envigo RMS LLC; 250 mg/kg) for 7 days to acclimate, then placed on tamoxifen chow alone (~40 mg intake/d/mouse) for 7 days prior to initiating experiments. Mice remained on tamoxifen chow for the duration of each experiment. Mice were used between 8 and 12 weeks of age. The University of Pittsburgh Animal Care and Use Committee approved all animal studies.
Immunization, Cell Stimulation, and Flow Cytometry
Mice were immunized with 30 × 106 mitomycin C-treated allogeneic (C57BL/6) splenocytes intraperitoneally (i.p.) and animals were then sacrificed at the indicated times (3, 25, 26). To determine cytokine expression, splenocytes were cultured in RPMI complete media (10% FBS, 2 mM L-glutamine, 100 U/ml Penicillin/Streptomycin, and 50 μM 2-mercaptoethanol) and stimulated for 5 h, as follows: B cell IL-4: 10 μg/mg LPS, 0.75 μg/ml ionomycin, 50 ng/ml PMA (Sigma Aldrich Co.) and monensin (eBioscience Inc.). B cell IL-10: splenocytes were stimulated as above except ionomycin was used at 50 ng/ml. T cells cytokines: 0.50 μg/ml ionomycin and 50 ng/ml PMA. Cells were stained with Aqua Live-Dead Fixable dye (ThermoFisher Scientific Inc.), washed and incubated with Fc block (anti-CD16/CD32), then stained with anti- CD3, CD4, CD8, Siglec-1 and CD19 fluorochrome conjugates (Fisher Scientific Inc. and BioLegend Inc.). For intracellular staining, cells were fixed and permeabilized using BD Fixation/Permeabilization kit (BD Biosciences Inc.). Cytokines were detected using fluorochrome conjugated anti-IL-4, IL-5, IL-10, and IFN-γ (Fisher Scientific Inc. and BioLegend Inc.). Intracellular staining controls were incubated with relevant isotype/fluorochrome controls. BD LSRII or Fortessa flow cytometers were used to collect the data and FlowJo software was used for data analysis (BD Biosciences Inc.).
Islet Transplants
B6 pancreata were digested with collagenase V (Sigma Aldrich) for 20 min at 37°C, as we described (3). Digested tissue was layered onto a Ficoll gradient and islets collected from the 11 and 20.5% interface after centrifugation at 1,800 rpm for 10 min at 4°C. Islets were washed and incubated in RPMI complete medium overnight at 37°C in 5% CO2. Islets were collected and 250 islets/mouse were placed under the kidney capsule of sex-matched allogeneic recipients with streptozotocin-induced diabetes (blood glucose levels >250 mg/dl) (3). Recipients whose glycemia normalized to <150 mg/dl within 2 days were considered successful. Grafts were considered rejected if blood glucose was >250 mg/dl for two consecutive days. Recipients with normal glycemia beyond 100 days were nephrectomized to demonstrate that euglycemia was due to transplanted islets.
Allergic Airway Disease and Analysis
Mice were immunized with 40 μg of Ova precipitated in Alum by intraperitoneal injection on days -14 and -7. On day 0, mice received 15 μg of intranasal Ova for 3 consecutive days. Bronchoalveolar lavage (BAL) was carried out by injecting 1ml of RPMI into the lungs through the trachea and recovering the injected media. This procedure was repeated 9 more times. The lungs were washed with total of 10 ml per mouse. Total lung tissue infiltrate was isolated by injecting mice with 5 μg of anti-CD45-FITC mAb (i.v.) to stain intravascular lymphocytes for exclusion (27). After 5 min, mice were perfused with 20 ml of PBS containing 2% heparin through the left ventricle while the right ventricle was cut in order to drain the blood. Lungs were removed, diced and transferred to gentle-MACS tubes containing RPMI medium, 0.25 mg/ml of Liberase TL and 0.20 mg/ml of DNAase I. The tissue was then dissociated with a gentle-MACS tissue disrupter and further digested for 30–40 min at 37°C in 5% CO2 according to instructions of the manufacturer. The digested tissue was passed through a 40 μm filter and cell suspension washed with PBS. Cells were prepared for flow cytometry as described above, with the addition of anti-CD45-APC mAb to identify leukocytes within lung tissue.
Statistical Analyses and Graphing
Statistical analyses and graphing were carried out using GraphPad Prism 9. Data were analyzed using unpaired 2-tailed Student’s t-test, ANOVA, or log-rank (Mantel–Cox) test, as indicated. Differences were considered to be significant at p-values <0.05.
Results
Acute Reduction in B Cell IL-4 Expression Decreases IL-10+ Bregs and Th2 Polarization
To examine the role of B cell IL-4 on Th2 differentiation and Th2-mediated immune responses, we crossed hCD20-ERT2.Cre and IL-4/IL-13fl/fl BALB/c mice to generate mice with a tamoxifen (Tam)-inducible B cell-specific deletion of IL-4 and IL-13 (B-IL-4/13 iKO). Mice were placed on tamoxifen chow for two weeks prior to initiating experiments. At baseline, naïve Tam-treated B-IL-4/13 iKO and hCD20.Cre-control mice did not have any significant differences in splenic CD3+, CD4+, or CD8+ T cells or B cell frequency or number (Supplemental Figures 1A, B). Further, there were no differences in cytokine expression by CD4+ T cells or B cells (Supplemental Figures 1C, D). Consistent with previous findings (28), IL-4 expression is essentially undetectable in B cells from naive hCD20.Cre-control or B-IL-4/13 iKO mice (Supplemental Figure 1D). To evoke an immune response, Tam-treated B-IL-4/13 iKO and hCD20.Cre-control mice were alloimmunized and spleens harvested on day 14. After alloimmunization, a small but reproducible fraction of B cells expresses IL-4 (Figure 1A). B cells from B-IL-4/13 iKO mice had a 60% decrease in frequency of IL-4 expression compared to those from hCD20.Cre-control B cells (Figure 1A). Of note, we did not detect IL-13 protein expression by B cells from alloimmunized B-IL-4/13 iKO or hCD20.Cre-control mice (Supplemental Figure 2A).
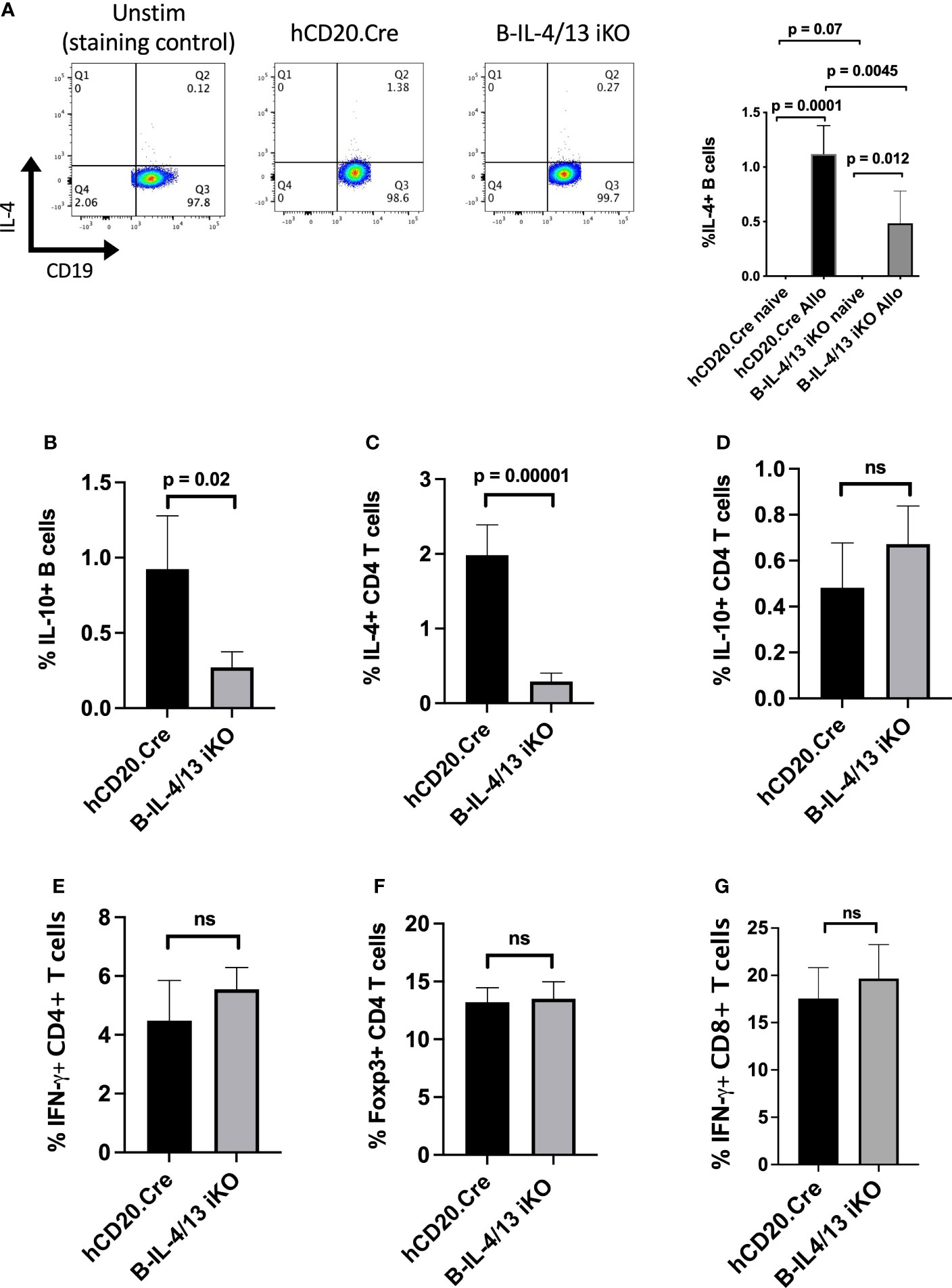
Figure 1 B cell IL-4 expression promotes Breg and Th2 expansion in response to alloantigen challenge. Splenocytes were isolated from Tam-treated hCD20.Cre-control and B-IL-4/13 iKO mice that were unimmunized (naive) or 14 days after alloimmunization (Allo). B cells were stimulated in vitro with PMA, ionomycin, LPS and monensin (LPIM) for 5 h. (A) Representative FACs plots showing frequency of IL-4 expression on CD19+ B cells from unstimulated alloimmunized hCD20.Cre-control mice (negative staining control), and LPIM-stimulated B cells from alloimmunized hCD20.Cre-control and B-IL-4/13 iKO mice. Right Panel: Bar graph shows cumulative data comparing IL-4 expression in B cells from naive vs. alloimmunized hCD20.Cre-control and B-IL-4/13 iKO mice. (IL-4 expression was not detected in naïve mice). (B–G) Bar graphs comparing cells from alloimmunized hCD20.Cre-control and B-IL-4/13 iKO mice: (B) B cell IL-10 frequency. (C) CD4 T cell IL-4 frequency. (D) CD4 T cell IL-10 frequency. (E) CD4 T cell IFNγ frequency and (F) CD4 T cell Foxp3 frequency. (G) CD8 T cell IFN-γ frequency. n = 4–6 mice per group in 3 independent experiments. Graphs show mean ± SD. An unpaired Student’s t-test was used to compare differences between the indicated groups. Numbers above each panel signify p-values where significant. ns, not significant.
We previously demonstrated that TIM-1+ B cells, a population with potent Breg activity, were highly enriched for both IL-4 and IL-10 expression (3). Moreover, B cells from constitutive IL-4−/− and IL-4Rα−/− mice exhibited markedly decreased expression of both TIM-1 and IL-10, suggesting that IL-4 signaling was important for Breg development. In alloantigen immunized B-IL-4/13 iKO mice, acute reduction in B cell IL-4 expression resulted in an ~4-fold decrease in the frequency of IL-10+ B cells (Bregs) compared to hCD20.Cre-controls (Figure 1B and Supplemental Figure 2A). Because the number of B and T cells in spleens of control and iKO mice are comparable (Supplemental Figure 1A), these changes in frequency of B and T cell subsets reflect changes in overall number. Thus, acutely reducing endogenous B cell-derived IL-4 markedly reduces Breg IL-10 expression. Examination of CD4+ T cells from the same mice revealed that reduced B cell IL-4 resulted in a 7.5-fold decrease in frequency of IL-4 expression, while the expression of IL-10, IFNγ, and Foxp3 were unchanged (Figures 1C–F and Supplemental Figure 2). Moreover, there was no difference in IFNγ expression by CD8 T cells (Figure 1G). Thus, reduction in B cell IL-4 results in a specific decrease in IL-10+ Bregs and Th2 cells. This indicates that B cell IL-4 normally promotes Th2-polarization in response to alloimmunization.
Acute Reduction in B Cell IL-4 Expression Reduces IL-4 Expression by CD4 T Cells and Promotes Allograft Rejection
Studies performed several decades ago suggested that allograft rejection is exacerbated by Th1 immune responses while Th2 responses promote allograft acceptance (29–34). Given that B cell IL-4 supports Th2 responses after alloimmunization, we examined its role in allograft survival. To accomplish this, diabetic Tam-treated B-IL-4/13 iKO versus hCD20.Cre-control mice were transplanted with fully MHC-mismatched islet allografts. B-IL-4/13 iKO transplant recipients exhibited accelerated rejection of allografts compared to hCD20.Cre-control mice (MST = 21 days vs. 31 days, respectively; p = 0.04, Figure 2A). This suggests that acute rejection is exacerbated by reduced B cell IL-4 and resultant decreased Th2 responses in B-IL-4/13 iKO mice, at least when combined with Breg deficiency. In contrast, we previously showed deficiency of either Bregs or B cell IL-10 alone, do not accelerate rejection in untreated recipients (9, 35).
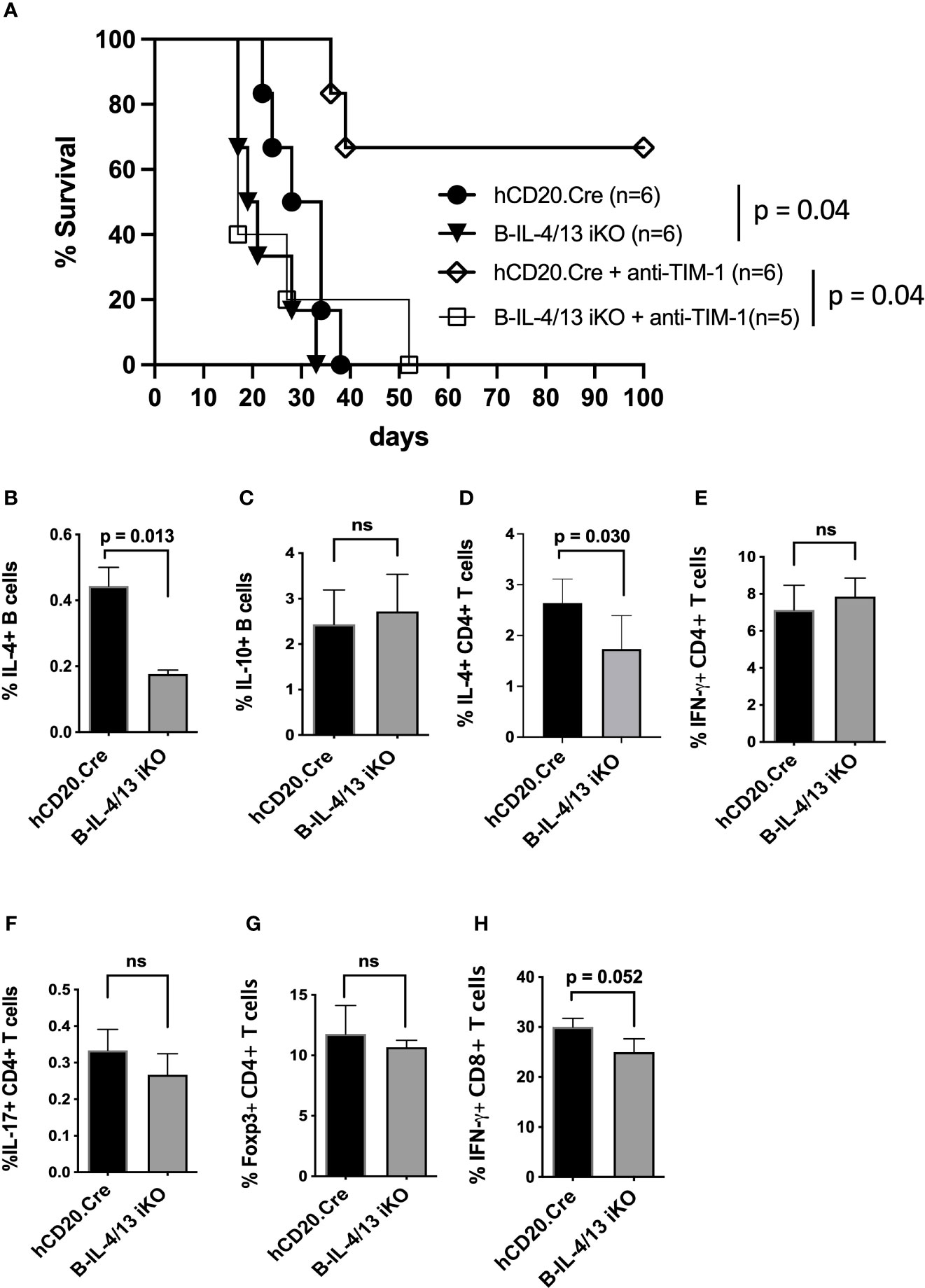
Figure 2 B cell IL-4 expression promotes islet allograft survival. (A) Kaplan–Meier plot showing islet allograft graft survival in hCD20.Cre-control and B-IL-4/13 iKO mice that were untreated or treated with anti-TIM-1 (as designated). Log-rank (Mantel–Cox) test was used to compare indicated groups. (B–H) Intracellular cytokine and Foxp3 expression by splenic B cells (B, C), CD4+ T cells (D–G) and CD8+ T cells (H) from alloimmunized anti-TIM-1 treated hCD20.Cre-control and B-IL-4/13 iKO mice after in vitro stimulation, as described in Figure 1. n = 4–6 mice per group and in 2 independent experiments. Graphs show mean ± SD. An unpaired Student’s t-test was used to compare differences between the indicated groups. ns, not significant.
Treatment of islet allograft recipients with tolerogenic anti-TIM-1 mAb (RMT1-10) was shown to increase IL-10 expression by B cells ~3–5 fold and prolongation of allograft survival was dependent on B cell IL-10 expression (3, 9, 36, 37). To determine whether anti-TIM-1 remains effective in the face of reduced B cell IL-4, diabetic Tam-treated B-IL-4/13 iKO and hCD20.Cre-control mice received islet transplants as above but were treated with anti-TIM-1. Anti-TIM-1 markedly prolonged allograft survival in hCD20.Cre-control mice, resulting in long-term survival in 66% of recipients (MST 17days vs. >100 days, respectively; p = 0.04, Figure 2A). In contrast, anti-TIM-1 did not prolong allograft survival of B-IL-4/13 iKO mice. After anti-TIM-1 treatment, alloimmunized B-IL-4/13 iKO mice maintained an ~60% reduction in splenic IL-4+ B cells compared to control hCD20.Cre mice (Figure 2B). However, in this setting, anti-TIM-1 elicited a similar increase in splenic IL-10+ Bregs in both strains (Figures 2B, C). This contrasts with the decrease in B cell IL-10 expression in alloimmunized B-IL-4/13 iKO mice in the absence of anti-TIM-1 (Figure 1B). Regardless, the frequency of splenic IL-4+ CD4 T cells was still reduced in anti-TIM-1 treated B-IL-4/13 iKO recipients compared to hCD20.Cre-control mice, while the frequency of IFNγ, IL-17 and Foxp3 expressing CD4 T cells and IFNγ expressing CD8 T cell remained unchanged (Figures 2D–H). These data support the notion that B cell IL-4 supports Th2 differentiation and that B cell IL-4 and Th2 polarization play an important role in anti-TIM-1 mediated allograft survival that is independent from the role of IL-10+ Bregs, Tregs or Th1 responses.
Acute Reduction in B Cell IL-4 Expression Reduces Th2 Expression and Markedly Inhibits Allergic Airway Disease (AAD)
IL-4 expression by Th2 cells is a major contributing factor to pathophysiology of AAD including airway inflammation (38, 39). To directly address the role of B cell IL-4 expression on Th2 polarization and airway inflammation, we subjected Tam-treated B-IL-4/13 iKO and hCD20.Cre-control mice to Ova-induced AAD. In naïve mice, ~120,000 CD45+ leukocytes were recovered in BAL from either hCD20.Cre-control or B-IL-4/13 iKO mice (Supplemental Figure 3A). After induction of AAD, there was virtually no increase in number of CD45+ leukocytes in the bronchoalveolar space of B-IL-4/13 iKO mice, whereas those from hCD20.Cre-control mice increased ~5-fold (Figure 3A). BAL fluid had 3.5× more CD3 T cell and 12.5× more eosinophils in hCD20.Cre-controls (Figures 3B, C). Very few, if any, B cells were found in the BAL of either mouse strain (data not shown).
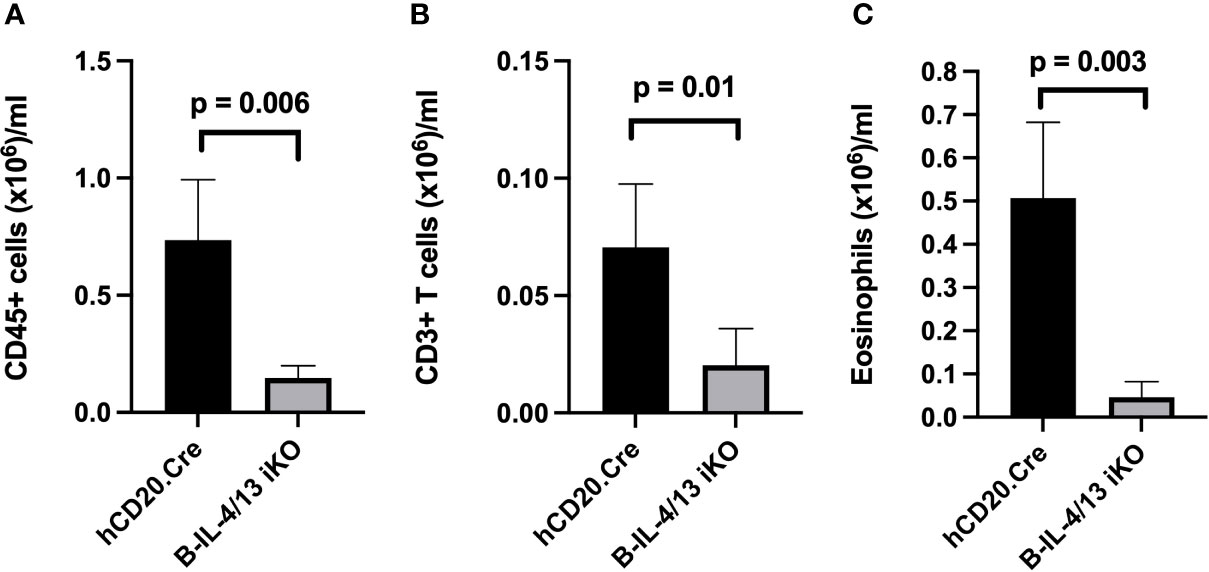
Figure 3 B cell IL-4 expression promotes airway inflammation in AAD. BAL from Ova-sensitized hCD20.Cre-control and B-IL-4/13 iKO mice was examined for (A) Total CD45+ cells; (B) Total CD3+ T cells; and (C) Eosinophils. n = 3–4 mice/group in 2 independent experiments. Graphs show number of cells/ml of lavage fluid expressed as mean ± SD. An unpaired Student’s t-test was used to compare differences between the indicated groups.
Lung histopathology in hCD20.Cre-control mice revealed marked thickening and infiltration of inflammatory cells into the alveolar septae compared to B-IL-4/13KO mice, which had preserved lung architecture (Figures 4A, B). Moreover, in hCD20.Cre-control mice, an inflammatory cell infiltrate and proteinaceous debris was observed in the alveolar space and an inflammatory infiltrate was observed around a proportion of small airways (Figure 4B). In contrast, B-IL-4/13 iKO mice had clear alveolar spaces and no infiltrate around small airways (Figures 4A, B). No differences were observed in epithelial thickening between the two groups. To quantitate and characterize the parenchymal infiltrate, lung tissue was digested and intravascular lymphocytes were gated out (see Materials and Methods; Supplemental Figure 4). As seen in alloimmunized mice, after induction of AAD, no B cell IL-13 could be detected in lung tissue from either B-IL-4/13 iKO or hCD20.Cre control mice (Supplemental Figure 5). Naïve Control and B-IL-4/13 iKO mice, had a similarly low number of resident CD45+ cells in lung tissue, of which only a small fraction was comprised of T or B lymphocytes or eosinophils (Figures 4C–E, H, I). After induction of AAD, lung tissue from hCD20.Cre-control mice exhibited a >5-fold increase in infiltration by (CD45+) leukocytes (from 4.9×106 to 21×106), ~75% of which was comprised by a marked expansion of T cells. B cell infiltration increased ~5-fold but still comprised only about 5% of the total infiltrate. Eosinophil numbers increased markedly, but their contribution to the total infiltrate was minor. Despite the preservation of lung architecture and normalization of BAL seen in B-IL-4/13 iKO mice after AAD induction, infiltration of leukocytes and lymphocytes into lung parenchyma was increased compared to baseline levels seen in naive mice (Figures 4B–F). However, in comparison to hCD20.Cre control mice, after AAD induction, B-IL-4/13 iKO mice exhibited a 2-fold decrease in the number of infiltrating (CD45+) leukocytes, including an ~2-fold decrease in total (CD3+) and CD4+ T cells (Figures 4C–E). Moreover, amongst CD4 T cells in lung tissue, there was an ~2-fold decrease in IL-4 and IL-5 expressing cells in B-IL-4/13 iKO mice (Figures 4F, G). Despite the marked differences in BAL, there was no significant difference between B-IL-4/13 iKO and hCD20.Cre control mice in eosinophil infiltration into lung tissue, nor were there differences in B cell infiltration (Figures 4H, I). Thus, acute reduction in B cell IL-4 significantly reduced Th2 polarization, lung T cell infiltration, and airway inflammation in AAD.
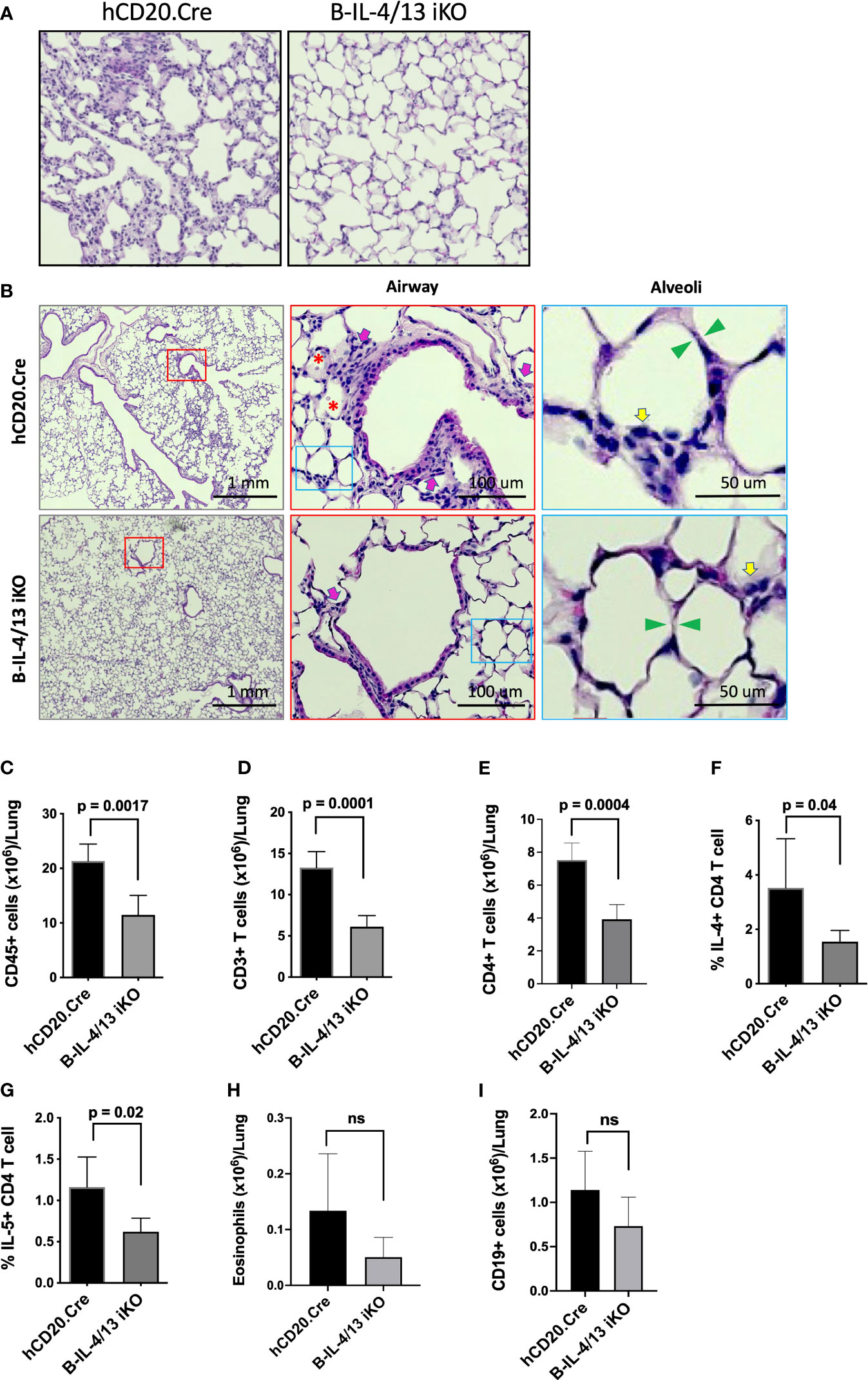
Figure 4 B cell IL-4 expression promotes lung tissue inflammatory cell infiltration and Th2 polarization in AAD. Lungs from Ova-sensitized hCD20.Cre-control and B-IL-4/13 iKO mice were harvested and either paraffin embedded or digested to examine immune cell infiltration. (A) Representative lung tissue sections stained with H&E showing degree of immune infiltrate in hCD20.Cre-control and B-IL-4/13 iKO lungs (×20 magnification). (B) Left panels: Large area scan image that was stitched together (×10 magnification) of H&E stained lungs with red boxes highlighting representative small airways shown at higher magnification in middle panels. Middle Panels: Red box inset image of area surrounding small airways. Pink arrows indicate inflammatory cells around airways. Red asterisks indicate pink proteinaceous debris and red blood cells. Blue boxes show alveoli in area of airways, further magnified in right panels. Right Panels: inset image of alveoli from airway image. Yellow arrows indicate inflammatory cells in alveolar/interstitial spaces. Green arrows show alveolar septa. (C–I) show quantification of infiltrating leukocyte populations obtained from digested lung tissue. (C) Total CD45+ cells/lung. (D) Total CD3+ T cells/lung. (E) Total CD4+ T cells/lung. (F) Frequency of lung IL-4+ CD4 T cells. (G) Frequency of lung IL-5+ CD4 T cells. (H) Total Eosinophils/lung. (I) Total CD19+ B cells/lung. n = 3–5 mice/group in 2 independent experiments. Graphs show mean ± SD. An unpaired Student’s t-test was used to compare differences between the indicated groups. *p < 0.05, **p < 0.001, ***p < 0.0001. ns, not significant.
Discussion
Despite their relative paucity, B cells expressing various cytokines have a major impact on immune responses in infection, autoimmunity, cancer and transplantation (40–43). In vitro studies first showed that Th2 cells (or IL-4) could polarize B cells to produce IL-4, which in turn, amplified Th2 responses (5, 17). Subsequently, it was shown that IL-4-responsive B cells are important for Th2 polarization, and promoted clearance of helminthic infections, while worsening asthma and leishmaniasis (20, 21). However, the in vivo role of B cell IL-4 per se was unclear, with studies showing that it was required for Th2 responses with resultant worsening of leishmaniasis, but it was not required for Th2 responses and protection from helminthic infections or pulmonary/airway inflammation in allergic asthma (7, 20–22).
To directly address the participation of B cell IL-4 in Th2 responses, we developed a novel mouse strain which is developmentally normal until tamoxifen-mediated acute deletion of B cell IL-4 (B-IL-4/13 iKO). We were unable to detect IL-13 expression by B cells obtained after immunization of hCD20.Cre control mice in either disease model, even after in vitro stimulation. Harris et al. did show that MD4 BCR transgenic B cells express low levels of IL-13 mRNA after 3-day culture with antigen and primed Th2 cells followed by PMA and ionomycin for 24 h; however, IL-13 protein was not reported (5). While we cannot eliminate a contribution from loss of IL-13 in our animal model, the lack of B cell IL-13 protein expression in AAD or allograft models suggests that this cytokine does not contribute significantly to our findings. Acute reduction of B cell IL-4 reduces B cell IL-10 expression and reduces Th2 responses, without affecting Th1 or Treg frequency. The latter is interesting since Bregs have generally been shown to correlate with both Foxp3 and IL-10 expression by CD4 cells (3, 9, 44). Our data suggests that neither Breg IL-10 nor IL-4 are required to support Treg/Tr1 subsets. The combination of reduced B cell IL-4 and Th2 cells markedly reduces pulmonary inflammation in AAD. Notably, this occurs despite the reduction in IL-10+ Bregs, which in and of themselves are protective in this model (3).
Our findings indicate that IL-4 expression by B cells promotes Th2 responses that underlie allergic diseases such as asthma. While B cells may not produce IL-13, they induce Th2 cells that secrete IL-13 which has pleiotropic effects in asthma pathobiology (45). The current results should prompt a thorough search for IL-4-expressing B cells in human BAL and biopsy material from patients with asthma or other allergic diseases. In this regard, small studies show that B cell depletion with anti-CD20 (rituximab) reduces steroid dependence in asthma associated with eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome), and significantly reduces atopic dermatitis where a reduction in IL-13 production, but not IgE, was observed (46, 47). While such insight may not impact ongoing attempts to target IL-4 and IL-13 in treatment of asthma, it could prompt further studies into B cell-directed therapies for allergic disease.
In the allograft setting, studies performed at the advent of the Th1/Th2 paradigm, suggested that Th2 cytokines might promote allograft survival. For example, STAT6−/− mice (Th2-deficient) exhibited reduced survival of minor histocompatibility antigen mismatched cardiac allografts in untreated recipients. Additionally, anti-IL-4 blocked the ability of splenocytes from anti-CD4 treated mice to adoptively transfer tolerance to new allograft recipients and prevented induction of neonatal tolerance (32, 33, 48). None of these approaches is specific for Th2 cells per se, and they should now be more broadly viewed as resulting from inhibition of type 2 responses. Ultimately, interest in the role of Th1 versus Th2 cells in allograft survival was overshadowed by other Th subsets and regulatory cells. Here, in the absence of therapy, the combined decrease in IL-10+ Bregs and Th2 cells resulting from reduced B cell IL-4, accelerates rejection in otherwise untreated fully MHC-mismatched islet allograft recipients. In comparison, we previously showed that lack of Bregs or B cell IL-10 alone do not hasten rejection (35). More telling, is that deficiency B cell IL-4 prevents induction of long-term allograft survival by anti-TIM-1 despite normal Breg induction in this setting, suggesting that deficiency in B cell IL-4 and resulting decrease in Th2 cells, prevent tolerance. Of course, it remains possible that other compensatory changes resulting from an acute decrease in B cell IL-4 also contribute to the loss of tolerance. Unfortunately, we do not yet have specific agents to target particular subsets of B cells that express harmful versus salutary cytokines. Cases in point, were attempts to use anti-CD20 in the peri-transplant period to limit subsequent antibody-mediated rejection in both renal and cardiac transplantation (49, 50). Unfortunately, both acute cellular renal allograft rejection and cardiac allograft vasculopathy were significantly increased after anti-CD20 treatment, suggesting that B cells play a critical regulatory role early after transplantation. Ultimately, a better understanding of the biology of IL-4-expressing B cells and the timing of their involvement in immune-mediated disease will help us specifically target this B cell subset in attempts to improve patient outcomes. It is interesting that anti-TIM-1 can induce Bregs in this setting while the deficiency in B cell IL-4 is maintained. This provides hope that different subsets of B cells might be individually targeted to inhibit asthma or prevent allograft rejection.
In summary, acute deficiency in B cell IL-4 reduces Th2 polarization indicating that B cell IL-4 is an important driver of Th2 responses in vivo. Acute reduction of B cell IL-4 also reduces B cell IL-10 expression, which can be overcome with antibody mediated ligation of TIM-1—a potent inducer of Bregs. As such, a reduction in B cell IL-4 markedly reduces AAD-mediated lung inflammation, while promoting allograft rejection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by the University of Pittsburgh Animal Care and Use Committee.
Author Contributions
DR conceived the study. ZS, WY, LZ, XW, and KM conducted study and contributed to experimental data collection. KM, VK, and DR contributed to experimental design. KM and DR wrote manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by NIH grants R01AI114587 (DMR) and P01129880 (DMR and VKK). KM was supported by the Joseph A. Patrick Research Fellowship in Transplantation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We also thank Dr. Mark Shlomchik for providing us with genetically altered mice developed in his laboratory.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.762390/full#supplementary-material
References
1. Malynn BA, Romeo DT, Wortis HH. Antigen-Specific B Cells Efficiently Present Low Doses of Antigen for Induction of T Cell Proliferation. J Immunol (1985) 135(2):980–8.
2. Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, et al. Costimulation via OX40L Expressed by B Cells is Sufficient to Determine the Extent of Primary CD4 Cell Expansion and Th2 Cytokine Secretion In Vivo. J Exp Med (2003) 197(7):875–83. doi: 10.1084/jem.20021290
3. Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, et al. Regulatory B Cells Are Identified by Expression of TIM-1 and can be Induced Through TIM-1 Ligation to Promote Tolerance in Mice. J Clin Invest (2011) 121(9):3645–56. doi: 10.1172/JCI46274
4. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B Cells Regulate Autoimmunity by Provision of IL-10. Nat Immunol (2002) 3(10):944–50. doi: 10.1038/ni833
5. Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal Regulation of Polarized Cytokine Production by Effector B and T Cells. Nat Immunol (2000) 1(6):475–82. doi: 10.1038/82717
6. Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, et al. Regulatory B Cell (B10 Cell) Expansion During Listeria Infection Governs Innate and Cellular Immune Responses in Mice. J Immunol (2013) 190(3):1158–68. doi: 10.4049/jimmunol.1201427
7. Liu Q, Liu Z, Rozo CT, Hamed HA, Alem F, Urban JF Jr, et al. The Role of B Cells in the Development of CD4 Effector T Cells During a Polarized Th2 Immune Response. J Immunol (2007) 179(6):3821–30. doi: 10.4049/jimmunol.179.6.3821
8. Bao Y, Liu X, Han C, Xu S, Xie B, Zhang Q, et al. Identification of IFN-Gamma-Producing Innate B Cells. Cell Res (2014) 24(2):161–76. doi: 10.1038/cr.2013.155
9. Ding Q, Mohib K, Kuchroo VK, Rothstein DM. TIM-4 Identifies IFN-Gamma-Expressing Proinflammatory B Effector 1 Cells That Promote Tumor and Allograft Rejection. J Immunol (2017) 199(7):2585–95. doi: 10.4049/jimmunol.1602107
10. Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, et al. Mature Follicular Dendritic Cell Networks Depend on Expression of Lymphotoxin Beta Receptor by Radioresistant Stromal Cells and of Lymphotoxin Beta and Tumor Necrosis Factor by B Cells. J Exp Med (1999) 189(1):159–67. doi: 10.1084/jem.189.1.159
11. Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The Sequential Role of Lymphotoxin and B Cells in the Development of Splenic Follicles. J Exp Med (1998) 187(7):997–1007. doi: 10.1084/jem.187.7.997
12. Xiao S, Bod L, Pochet N, Kota SB, Hu D, Madi A, et al. Checkpoint Receptor TIGIT Expressed on Tim-1(+) B Cells Regulates Tissue Inflammation. Cell Rep (2020) 32(2):107892. doi: 10.1016/j.celrep.2020.107892
13. Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling via the MyD88 Adaptor Protein in B Cells Suppresses Protective Immunity During Salmonella Typhimurium Infection. Immunity (2010) 33(5):777–90. doi: 10.1016/j.immuni.2010.10.016
14. Dilillo DJ, Matsushita T, Tedder TF. B10 Cells and Regulatory B Cells Balance Immune Responses During Inflammation, Autoimmunity, and Cancer. Ann N Y Acad Sci (2010) 1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x
15. Braza F, Chesne J, Durand M, Dirou S, Brosseau C, Mahay G, et al. A Regulatory CD9(+) B-Cell Subset Inhibits HDM-Induced Allergic Airway Inflammation. Allergy (2015) 70(11):1421–31. doi: 10.1111/all.12697
16. Singh A, Carson WFT, Secor ER Jr, Guernsey LA, Flavell RA, Clark RB, et al. Regulatory Role of B Cells in a Murine Model of Allergic Airway Disease. J Immunol (2008) 180(11):7318–26. doi: 10.4049/jimmunol.180.11.7318
17. Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting Edge: The Development of IL-4-Producing B Cells (B Effector 2 Cells) is Controlled by IL-4, IL-4 Receptor Alpha, and Th2 Cells. J Immunol (2005) 175(11):7103–7. doi: 10.4049/jimmunol.175.11.7103
18. Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, et al. B Cell Depletion Therapy Ameliorates Autoimmune Disease Through Ablation of IL-6-Producing B Cells. J Exp Med (2012) 209(5):1001–10. doi: 10.1084/jem.20111675
19. Olalekan SA, Cao Y, Hamel KM, Finnegan A. B Cells Expressing IFN-Gamma Suppress Treg-Cell Differentiation and Promote Autoimmune Experimental Arthritis. Eur J Immunol (2015) 45(4):988–98. doi: 10.1002/eji.201445036
20. Hurdayal R, Ndlovu HH, Revaz-Breton M, Parihar SP, Nono JK, Govender M, et al. IL-4-Producing B Cells Regulate T Helper Cell Dichotomy in Type 1- and Type 2-Controlled Diseases. Proc Natl Acad Sci USA (2017) 114(40):E8430–9. doi: 10.1073/pnas.1708125114
21. Hadebe S, Khumalo J, Mangali S, Mthembu N, Ndlovu H, Scibiorek M, et al. Deletion of IL-4Ralpha Signaling on B Cells Limits Hyperresponsiveness Depending on Antigen Load. J Allergy Clin Immunol (2020) 148:99–109. doi: 10.1016/j.jaci.2020.12.635
22. Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, et al. Cytokine-Producing Effector B Cells Regulate Type 2 Immunity to H. Polygyrus Immun (2009) 30(3):421–33. doi: 10.1016/j.immuni.2009.01.006
23. Bao K, Reinhardt RL. The Differential Expression of IL-4 and IL-13 and its Impact on Type-2 Immunity. Cytokine (2015) 75(1):25–37. doi: 10.1016/j.cyto.2015.05.008
24. Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B Cells in Murine Lupus: Efficacy and Resistance. J Immunol (2007) 179(5):3351–61. doi: 10.4049/jimmunol.179.5.3351
25. Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T Cell Differentiation and Memory T Cell Maintenance Outside Secondary Lymphoid Organs. J Immunol (2006) 176(7):4051–8. doi: 10.4049/jimmunol.176.7.4051
26. Yamada A, Salama AD, Sho M, Najafian N, Ito T, Forman JP, et al. CD70 Signaling is Critical for CD28-Independent CD8+ T Cell-Mediated Alloimmune Responses In Vivo. J Immunol (2005) 174(3):1357–64. doi: 10.4049/jimmunol.174.3.1357
27. Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, et al. Intravascular Staining for Discrimination of Vascular and Tissue Leukocytes. Nat Protoc (2014) 9(1):209–22. doi: 10.1038/nprot.2014.005
28. Ahuja A, Anderson SM, Khalil A, Shlomchik MJ, et al. Maintenance of the Plasma Cell Pool is Independent of Memory B Cells. Proc Natl Acad Sci USA (2008) 105(12):4802–7. doi: 10.1073/pnas.0800555105
29. Nickerson P, Steurer W, Steiger J, Zheng X, Steele AW, Strom TB. Cytokines and the Th1/Th2 Paradigm in Transplantation. Curr Opin Immunol (1994) 6(5):757–64. doi: 10.1016/0952-7915(94)90081-7
30. Zhai Y, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Th1 and Th2 Cytokines in Organ Transplantation: Paradigm Lost? Crit Rev Immunol (1999) 19(2):155–72. doi: 10.1615/CritRevImmunol.v19.i2.40
31. Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, et al. Th1 Cytokines, Programmed Cell Death, and Alloreactive T Cell Clone Size in Transplant Tolerance. J Clin Invest (2002) 109(11):1471–9. doi: 10.1172/JCI0214947
32. Donckier V, Wissing M, Bruyns C, Abramowicz D, Lybin M, Vanderhaeghen ML, et al. Critical Role of Interleukin 4 in the Induction of Neonatal Transplantation Tolerance. Transplantation (1995) 59(11):1571–6. doi: 10.1097/00007890-199506000-00012
33. Bushell A, Niimi M, Morris PJ, Wood KJ. Evidence for Immune Regulation in the Induction of Transplantation Tolerance: A Conditional But Limited Role for IL-4. J Immunol (1999) 162(3):1359–66.
34. Takeuchi T, Lowry RP, Konieczny B. Heart Allografts in Murine Systems. The Differential Activation of Th2-Like Effector Cells in Peripheral Tolerance. Transplantation (1992) 53(6):1281–94. doi: 10.1097/00007890-199206000-00023
35. Mohib K, Cherukuri A, Zhou Y, Ding Q, Watkins SC, Rothstein DM. Antigen-Dependent Interactions Between Regulatory B Cells and T Cells at the T:B Border Inhibit Subsequent T Cell Interactions With DCs. Am J Transplant (2020) 20(1):52–63. doi: 10.1111/ajt.15546
36. Lee KM, Kim JI, Stott R, Soohoo J, O'Connor MR, Yeh H, et al. Anti-CD45RB/anti-TIM-1-Induced Tolerance Requires Regulatory B Cells. Am J Transplant (2012) 12(8):2072–8. doi: 10.1111/j.1600-6143.2012.04055.x
37. Hosseini H, Yi L, Kanellakis P, Cao A, Tay C, Peter K, et al. Anti-TIM-1 Monoclonal Antibody (RMT1-10) Attenuates Atherosclerosis By Expanding IgM-Producing B1a Cells. J Am Heart Assoc (2018) 7(13). doi: 10.1161/JAHA.117.008447
38. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-Like Bronchoalveolar T-Lymphocyte Population in Atopic Asthma. N Engl J Med (1992) 326(5):298–304. doi: 10.1056/NEJM199201303260504
39. Lambrecht BN, Hammad H. The Immunology of Asthma. Nat Immunol (2015) 16(1):45–56. doi: 10.1038/ni.3049
40. Lund FE. Cytokine-Producing B Lymphocytes-Key Regulators of Immunity. Curr Opin Immunol (2008) 20(3):332–8. doi: 10.1016/j.coi.2008.03.003
41. Cherukuri A, Mohib K, Rothstein DM. Regulatory B Cells: TIM-1, Transplant Tolerance, and Rejection. Immunol Rev (2021) 299(1):31–44. doi: 10.1111/imr.12933
42. Mauri C. Novel Frontiers in Regulatory B Cells. Immunol Rev (2021) 299(1):5–9. doi: 10.1111/imr.12964
43. Lino AC, Dorner T, Bar-Or A, Fillatreau S. Cytokine-Producing B Cells: A Translational View on Their Roles in Human and Mouse Autoimmune Diseases. Immunol Rev (2016) 269(1):130–44. doi: 10.1111/imr.12374
44. Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, et al. Mice Lacking Endogenous IL-10-Producing Regulatory B Cells Develop Exacerbated Disease and Present With an Increased Frequency of Th1/Th17 But a Decrease in Regulatory T Cells. J Immunol (2011) 186(10):5569–79. doi: 10.4049/jimmunol.1100284
45. Doyle AD, Mukherjee M, LeSuer WE, Bittner TB, Pasha SM, Frere JJ, et al. Eosinophil-Derived IL-13 Promotes Emphysema. Eur Respir J (2019) 53(5):1801291–304. doi: 10.1183/13993003.01291-2018
46. Simon D, Hosli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (Rituximab) Treatment Improves Atopic Eczema. J Allergy Clin Immunol (2008) 121(1):122–8. doi: 10.1016/j.jaci.2007.11.016
47. Casal Moura M, Berti A, Keogh KA, Volcheck GW, Specks U, Baqir M. Asthma Control in Eosinophilic Granulomatosis With Polyangiitis Treated With Rituximab. Clin Rheumatol (2020) 39(5):1581–90. doi: 10.1007/s10067-019-04891-w
48. Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, et al. Physiological Mechanisms of Regulating Alloimmunity: Cytokines, CTLA-4, CD25+ Cells, and the Alloreactive T Cell Clone Size. J Immunol (2002) 169(7):3744–51. doi: 10.4049/jimmunol.169.7.3744
49. Starling RC, Armstrong B, Bridges ND, Eisen H, Givertz MM, Kfoury AG, et al. Accelerated Allograft Vasculopathy With Rituximab After Cardiac Transplantation. J Am Coll Cardiol (2019) 74(1):36–51. doi: 10.1016/j.jacc.2019.04.056
Keywords: B cell, IL-4, transplantation, allergic airway disease (AAD), Th2 (type-2) immune responses
Citation: Song Z, Yuan W, Zheng L, Wang X, Kuchroo VK, Mohib K and Rothstein DM (2022) B Cell IL-4 Drives Th2 Responses In Vivo, Ameliorates Allograft Rejection, and Promotes Allergic Airway Disease. Front. Immunol. 13:762390. doi: 10.3389/fimmu.2022.762390
Received: 23 August 2021; Accepted: 09 February 2022;
Published: 14 March 2022.
Edited by:
James William Wells, The University of Queensland, AustraliaReviewed by:
Tatiani Uceli Maioli, Federal University of Minas Gerais, BrazilLaura Maggi, Università degli Studi di Firenze, Italy
Copyright © 2022 Song, Yuan, Zheng, Wang, Kuchroo, Mohib and Rothstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David M. Rothstein, cm90aHN0ZWluZEB1cG1jLmVkdQ==; orcid.org/0000-0002-9455-7971
†These authors have contributed equally to this work
 Zhixing Song
Zhixing Song Wenjia Yuan1,3
Wenjia Yuan1,3 Kanishka Mohib
Kanishka Mohib David M. Rothstein
David M. Rothstein