- 1Scancell Limited, Biodiscovery Institute, University of Nottingham, Nottingham, United Kingdom
- 2The Cancer Vaccine Group, Biodiscovery Institute, University of Nottingham, Nottingham, United Kingdom
Homocitrullination is the post translation modification (PTM) of the amino acid lysine to homocitrulline also referred to as carbamylation. This PTM has mainly been studied in relation to autoimmune diseases including rheumatoid arthritis. Homocitrullination of lysines alters their charge which can lead to generation of neoepitopes that are differentially presented by MHC-II and induce modification-specific immune responses. Homocitrullination is often considered a process which triggers autoimmune disease by bypassing self-tolerance however, we suggest that homocitrullination may also have an alternative role in immune responses including protection against cancer. Here we demonstrate that immune responses to homocitrullinated peptides from three different proteins can be induced via multiple HLA-types. Immunization of Balb/c or HLA-transgenic DR4 and DR1 mice can induce modification-specific CD4 mediated IFNγ responses. Healthy human donors show a clear repertoire for the homocitrullinated Vimentin peptide (Vim116-135Hcit), with modification-specific and oligoclonal responses. Importantly, in vivo homocitrulline specific Vim116-135Hcit,Cyk8 371-388Hcit and Aldo 140-157Hcit responses are able to confer an anti-tumor effect in the murine B16 melanoma model. The Vim116-135Hcit anti-tumor response was dependent upon tumor expression of MHC-II suggesting the direct recognition of PTMs on tumor is an important anti-tumor mechanism. Cancer patients also have a CD4 repertoire for Vim116-135Hcit. Together these results suggest that homocitrulline-specific immune responses can be generated in healthy mice and detected in human donors through a variety of HLA-restrictions. Immunization can induce responses to Vim116-135Hcit,Aldolase 140-157Hcit and Cyk8 371-388Hcit which provide anti-tumor therapy across several HLA-types. Our results advance our understanding of homocitrulline-specific immune responses, with implications for a number of fields beyond autoimmunity, including tumor immune surveillance.
Introduction
Post translation modifications (PTM) are important for many cellular processes including altering the expression, function, signaling and cellular localization of proteins. PTMs also play a key role in epitope processing and presentation via MHC through altering protein cleavage (1, 2) increasing peptide processing and promoting epitope binding to MHC class I (MHC-I) or class II (MHC-II). Interestingly, they can also be induced as part of the antigen processing pathways (3–5). Inflammation or stress-related processes such as viral or oncogenic transformation of cells induces PTMs and an altered repertoire of MHC-associated peptides and has implications for many autoimmune-mediated diseases (6–9). Since the cellular stress associated with cancer cells can also lead to changes in PTMs (10). Therefore, inducing immune responses to PTMs will also have therapeutic applications in this field. PTMs which result in the generation of new epitopes that can be distinguished from native sequences include phosphorylation (11–13), glycosylation (14), deamidation of glutamine (8), citrullination (cit) of arginine (15–18) and homocitrullination (Hcit) of lysine (19). The latter three are particularly evident in autoimmunity where PTM epitopes are presented on MHC-II and recognized by modification specific CD4 T cells.
Homocitrullination, also known as carbamylation, results in the conversion of a positively charged amino acid lysine into a neutral amino acid homocitrulline. Like citrullination, this process can result in altered MHC binding properties (5). The conversion of lysine to homocitrulline is driven by the accumulation of cyanate (19, 20). In inflammatory conditions cyanate accumulation is driven by the myeloperoxidase (MPO) enzyme which is released by neutrophils (21). In addition to neutrophils, we have previously demonstrated a role for MPO-producing tumor-associated myeloid derived suppressor cells (MDSCs) in MPO production and ultimately in causing homocitrullination within the tumor microenvironment (22).
HLA restriction of T cell responses is important in determining the sensitivity of response to PTMs. Some HLA alleles have previously been identified as favoring the binding of PTM peptides and permitting stimulation of PTM-specific T cell responses. In the case of citrullination and deamidation of glutamine the HLA-DRB1 shared epitope and HLA-DQ2 or HLA-DQ8 have been shown to be favorable alleles, respectively (8, 23–25). We have recently shown that the HLA-DP4 allele also efficiently presents citrullinated epitopes (26). To date, there is little known regarding the HLA preference of homocitrulline-specific CD4 cells; although, due to overlap between citrulline and homocitrulline responses seen in patients, it is likely that homocitrullinated molecules are also presented by members of the HLA-DRB1 shared epitope alleles. Mouse MHC II from Balb/c and C57Bl/6 strains can mediate homocitrulline specific responses (19, 27), while for human HLA alleles, responses to the synthetic JED homocitrullinated peptide can be induced in transgenic HLA-DR4 mice (27). Furthermore, we have previously shown responses to several homocitrullinated peptides in transgenic HLA-HHDII/DP4 mice (22).
Here, we identify homocitrullinated peptides from three different antigens that stimulate homocitrulline-specific immune responses, restricted through multiple HLA-alleles. We characterized the response to Vim116-135Hcit peptide and show stimulation of CD4 responses in Balb/c, HLA-HHDII/DR1 and HLA-DR4 transgenic mice and healthy human donors. We also show that these epitopes can induce effective tumor therapy in mouse models. Crucially, cancer patients have a repertoire that recognizes the Vim116-135Hcit peptide, suggesting that immune responses to homocitrullinated peptides are not restricted to autoimmune disease. We suggest that the cross-restriction of homocitrullinated peptide specific CD4 responses may pave the way to designing an effective universal anti-tumor vaccine.
Material and Methods
Unless otherwise stated reagents were obtained from Sigma-Aldridge and antibodies from eBioscience.
Peptides
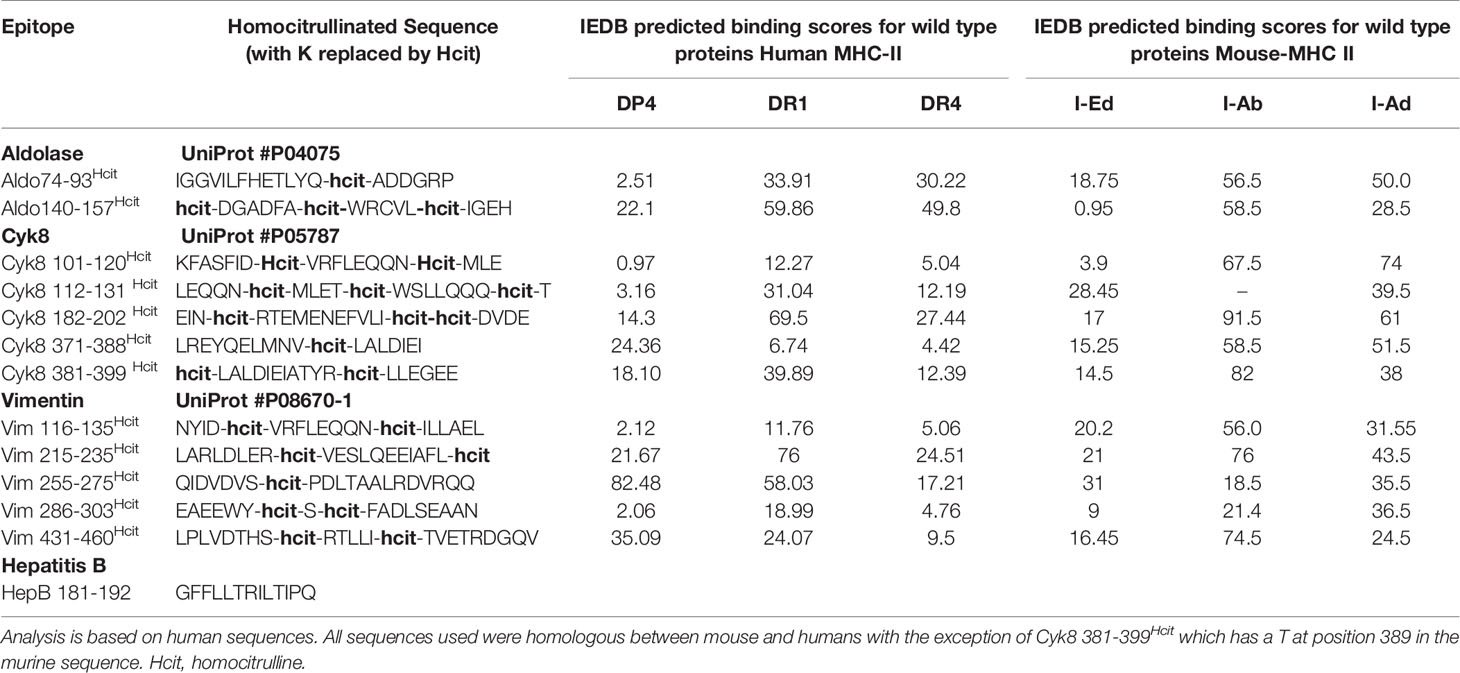
Peptides were analyzed for potential MHC-II binding using immune epitope database (IEDB) (http://www.iedb.org/) (28–30). Peptides (Table 1) were synthesized at >90% purity (Genscript), stored lyophilized at −80°C and then reconstituted in PBS on day of use.
Animals and Cell Lines
Animal experiments were carried out with ethical approval under a Home Office approved project license. HLA-DR4 mice (Model #4149, Taconic), HLA-A2/DR1 (HLA-HHDII/DR1, Pasteur Institute), HLA-A2.1+/+ HLADP4+/+ hCD4+/+ (HLA-HHDII/DP4; EM:02221, European Mouse Mutant Archive) transgenic mice and C57Bl/6J mice or Balb/c mice (Charles River) aged 8-12 weeks were used.
The murine melanoma B16F1 cell line (ATCC-CRL-6323) obtained from ATCC was engineered with mouse MHC K/O and transfected with HHDII (A2) alone or in combination with either constitutive HLA-DP4, HLA-DR1, HLA-DR4 or IFNγ-inducible HLA-DR4 (iDR4) using plasmids as previously described (17, 26). Cells were cultured in RPMI medium 1640 with L-glutamine (2 mmol/L), 10% FCS and appropriate antibiotics to maintain plasmids. Cell lines utilized were mycoplasma free, authenticated by suppliers (STR profiling), and used within ten passages.
Immunization Protocol
Mice were injected subcutaneously (s.c.) with 25 μg of each peptide either as a pool or individually in combination with 5μg of CpG ODN 1826 (CpG) and Monophosphoryl Lipid A (MPLA) (InvivoGen) on days 1, 8 and 15 and responses analyzed on day 21.
For anti-tumor experiments, mice were implanted s.c. with tumor cells three days prior to immunization regime above. Implant doses were 5.0 x 105 cells/mouse for B16 HHDII/DR1, 2.5x104 cells/mouse for B16F1DR4 and 5x104 cells/mouse for B16F1 iDR4 and B16F1 h2Ab1 B2M dKO cell lines (17, 31, 32). Tumor growth was monitored twice weekly and mice were humanely euthanized once tumors approach the license limit of 15 mm in diameter.
ELISpot Assays
Murine ELISpot kits (Mabtech) were used with 5 × 105 splenocytes/well and 10μg/ml synthetic peptide added to triplicate wells. 20μg/ml anti-CD8, or anti-CD4 antibodies were added to appropriate wells for blocking studies. Plates were incubated at 37°C for 40 hours in an atmosphere of 5% CO2. Example plate images are shown in Supplementary Figure 1. Spots were analyzed using an automated plate reader (Cellular Technologies Ltd).
Peripheral Blood Mononuclear Cells (PBMC) Isolation and Processing
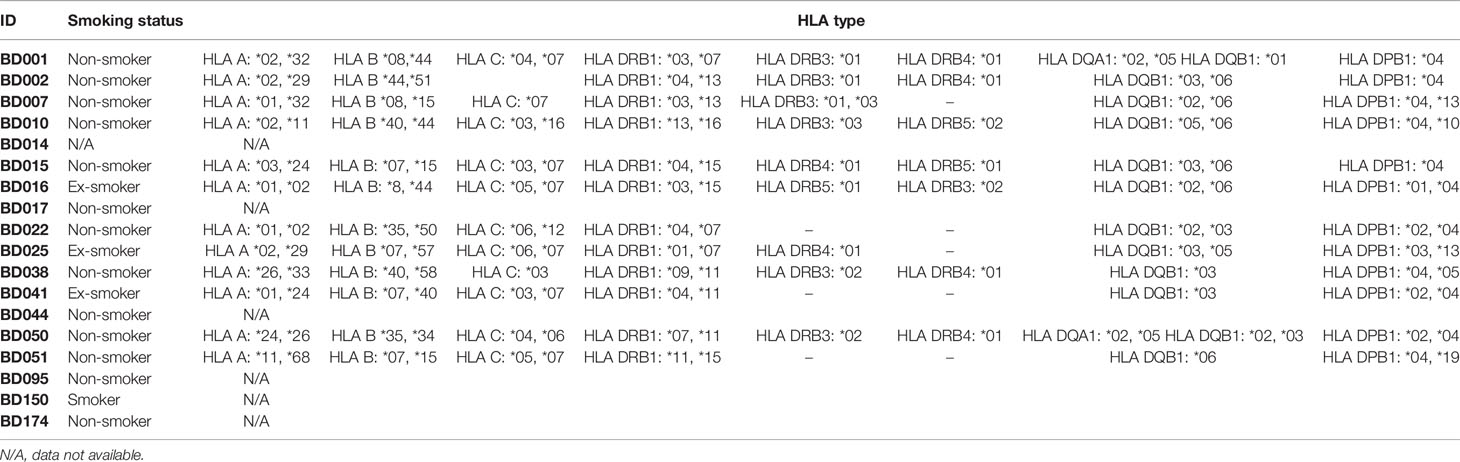
PBMCs were isolated from healthy donors and cancer patients (Table 2) using histopaque-1077 then depleted using anti-CD25 microbeads and MACS Cell Separation Columns (Miltenyi). Isolated CD25-depleted PBMCs were loaded with 5µM carboxyfluorescein succinimidyl ester (CFSE) (Thermofisher) (32). Cells were resuspended at 1.5×106/ml in RPMI with autologous serum as previously described in full (32), plated and stimulated with vehicle, PHA (10 µg/ml), soluble anti-CD3 (positive control, 30ng/ml) or peptide (10µg/ml). Peptide concentration was selected based on reports in the literature using similar post-translationally modified epitopes (16, 18, 33). On day 10, 500µl of cells were stained with anti-CD4 (Thermofisher clone RPA-T4, 1/50 dilution) and anti-CD8 (Miltenyi Biotec clone REA734, 1/50 dilution) antibodies for 30 minutes and then samples were washed, fixed and analyzed immediately. For restimulation assays, cells were cultured in media with IL-2 IL-7 and IL-15 cytokines (Peprotech) and 10ug/ml of homocitrullinated peptides. On day 14, cells were harvested. Human IFNγ ELISpot kits (Mabtech) were used with 1 x 105 cells/well with 10ug/ml peptide or PHA (10 µg/ml) in quadruplicate wells. Where appropriate, 7.6 µg/ml blocking antibodies (Isotype control clone MOPC-173 IgG2a kappa Biolegend, Anti-DR,DP,DQ clone Tu39 Biolegend, Anti-DP clone B7/21 Leinco, Anti-A,B,C cloneW6/32 Biolegend, Anti-DR clone G46-6 BD) were added for 30 minutes prior to peptides. ELISpot plates were incubated at 37°C for 24 hours in an atmosphere of 5% CO2 and then developed following the manufacturer’s instructions. Spots were analyzed using an automated plate reader (Cellular Technologies Ltd). All samples showed responses to PHA (data not shown).
TCR α and β Chains Analysis of Proliferating PBMCs
Stained CD4+/CFSEhigh and CD4+/CFSElow cell populations were bulk sorted into RNA protect (Qiagen) using a MoFlo XDP Sorter (Beckman Coulter). RNA was purified from sorted cells, RT-PCR performed, the cDNA was subjected to Amplicon rescued multiplex PCR (ARM-PCR) using human TCR α and β 250 PER primers (iRepertoire Inc., Huntsville, AL, USA) (26). Ten assessed sample libraries were pooled and sequenced using the Illumin//a MiSeq platform (Illumina, USA). Tree maps and D50 diversity values generated using iRweb software. Tree maps show each unique CDR3 as a colored rectangle, the size of each rectangle corresponds to each CDR3s abundance within the repertoire and the positioning is determined by the V region usage. Diversity was measured using D50 immune repertoire diversity index. The D50 index is a quantitative measure of the degree of diversity of T cells within a sample. The D50 is the percentage of T-cell clones that account for the cumulative 50% of the total CDR3s counted in the sample. The more diverse a library, the closer the value will be to 50. Low diversity values are associated with decreased diversity. Data are presented as non-normalized (which takes into account the frequency of each unique CDR3).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 9. Comparative analysis of the ELISpot results was performed by applying paired or unpaired ANOVA or Student t test as appropriate with P values calculated accordingly. Comparison of tumor survival was assessed by log-rank test. Human responses were analyzed by Wilcoxon matched-paired test. P<0.05 values were considered statistically significant.
Results
Homocitrulline-Peptide Responses Can Be Induced Through Multiple HLA Alleles
We have previously shown that homocitrullinated peptides can stimulate responses restricted through the HLA-DP4 allele (22). In this study we first aimed to determine whether peptide immunization can induce homocitrulline-specific responses via HLA types other than the HLA-DP4 allele. The vimentin (Vim), cytokeratin 8 (Cyk8) and aldolase A (Aldo) sequences were analyzed by IEDB for sequences containing lysine within the predicted core binding region and since homocitrulline modification may influence the binding affinity, peptides with variable predicted binding scores for HLA-DR4 or HLA-DR1 alleles were selected. Predicted binding scores are shown in Table 1. Responses to five homocitrullinated peptides were assessed in HLA-transgenic mice expressing human HLA-DR1 along with chimeric human HLA-A2 (HHDII) or HLA-DR4 (Figures 1A, B). This screening identified significant responses to Vim116-135Hcit in both strains. The homocitrullinated peptide Vim116-135Hcit was synthesized with the lysine residues at positions 120 and 129 both converted to homocitrulline (Table 1).
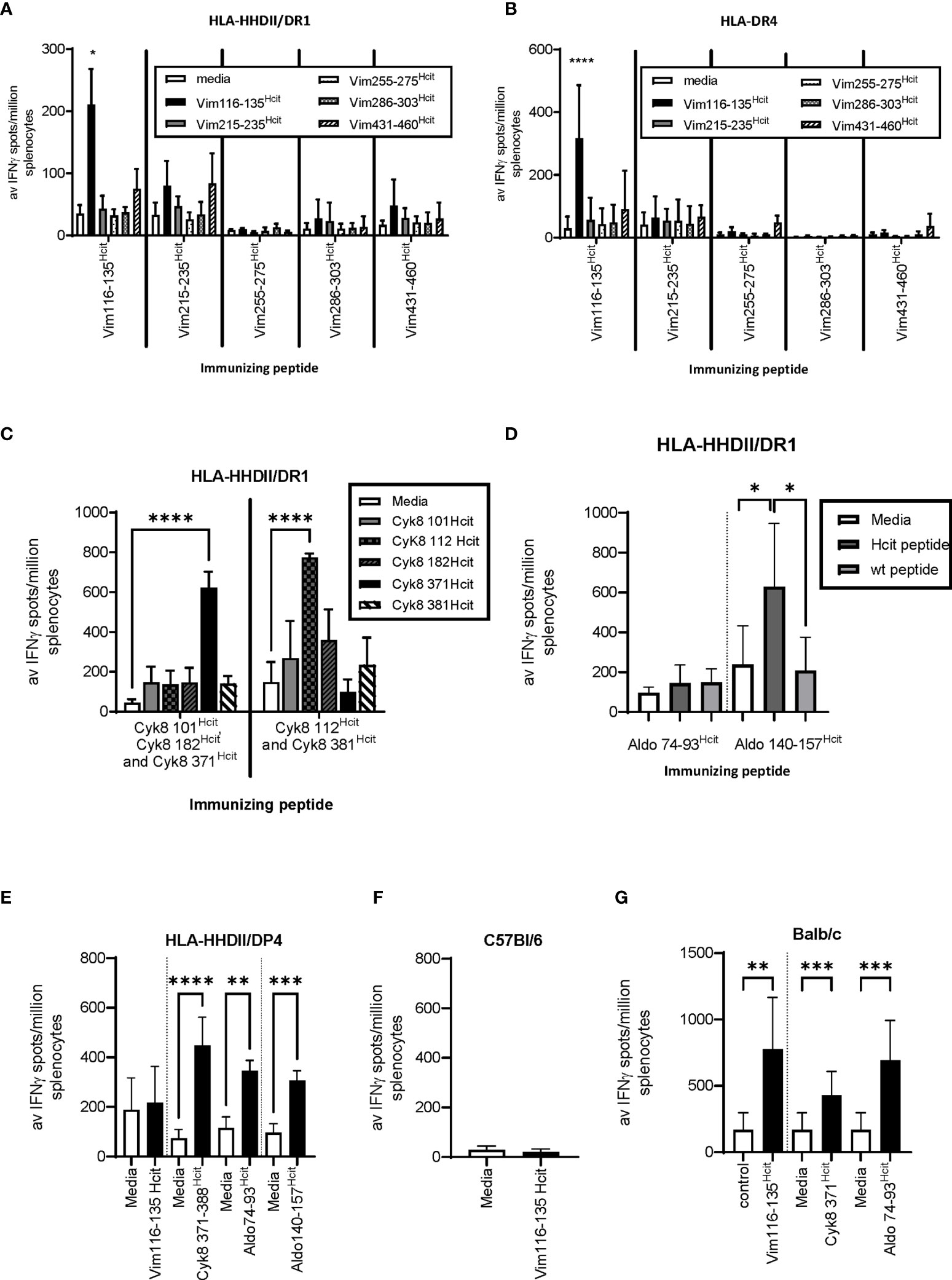
Figure 1 Homocitrullinated peptide responses in HLA-HHDII/DR1, HLA-DR4 and Balb/c mice. Mice were immunized with three doses of homocitrullinated peptides with the adjuvants CpG and MPLA. Responses to homocitrullinated vimentin (Vim) peptides were determined in HLA-HHDII/DR1 (A) and HLA-DR4 (B) mice. Responses to cytokeratin 8(Cyk8) peptides were determined in HLA-HHDII/DR1 mice (C). Aldolase (Aldo) responses were screened in HLA-HHDII/DR1 mice (D). Responding peptides were then also tested in HLA-HHDII/DP4 (E), C57Bl/6 (F) and Balb/c (G) mice. For each study n≥3. Mean and standard deviations and significant p values are shown; ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05.
Responses were also screened for five Cyk8 peptides (Figure 1C) and two Aldo peptides (Figure 1D) in HLA-transgenic mice expressing human HLA-DR1 and chimeric HLA-A2 (HLA-HHDII/DR1) mice. This screening identified Cyk8 371-388Hcit, Cyk8 112-131Hcit and Aldo140-157Hcit as inducing immune responses in the HLA-HHDII/DR1 mice. Cyk8 371-388Hcit was synthesized with the lysine converted to homocitrulline at position 381 and Aldo140-157Hcit contained three homocitrulline residues at positions 140, 147 and 153 (Table 1).
We have previously observed that both Aldo140-157Hcit and Cyk8 371-388Hcit can induce immune responses in HLA-HHDII/DP4 mice (22) suggesting an additional HLA restriction. The peptides were therefore tested in additional mouse strains. In HLA-HHDII/DP4 mice, the previously described responses were seen for Cyk8 371-388Hcit, Aldo74-93Hcit and Aldo140-157Hcit (Figure 1E). No immune responses to Vim116-135Hcit were observed in HLA-HHDII/DP4 (Figure 1E) nor C57Bl/6 mice (Figure 1F). However, significant Vim116-135Hcit (p=0.0018), Cyk8 371-388Hcit (p=0.0005) as well as Aldo 74-93Hcit (p=0.0001) responses were seen in Balb/c mice (Figure 1G).
This data shows that immunization with homocitrullinated peptides can induce responses in mice expressing the shared epitope human HLA alleles DR4 and DR1 as well as human HLA-DP4 and murine H-2d haplotype. However, cross reactivity between HLA alleles varies between peptides.
Homocitrulline Responses Are Modification Specific and CD4 Mediated
Immunization with long peptides has the potential to stimulate both CD4 and CD8 responses, therefore we further characterized the immune responses to three peptides to determine if responses were CD4 mediated and homocitrulline specific. Mice were immunized with the Vim116-135Hcit peptide and immune responses determined by IFNγ ELISpots. In HLA-HHDII/DR1 (Figure 2A), HLA-DR4 (Figure 2B) and Balb/c (Figure 2C) mice responses to the modified homocitrulline peptide were significantly greater than the responses to the wild type (wt) peptide (p<0.0001 for all strains). Anti-CD4 blocking antibodies significantly reduced the IFNγ response to Vim116-135Hcit (p<0.0001, p=0.0054 and p=0.0143 for HLA-HHDII/DR1, HLA-DR4 and Balb/c respectively) but anti-CD8 antibody had no significant effect on the immune response. Example ELISpot images are shown in Supplementary Figure 1.
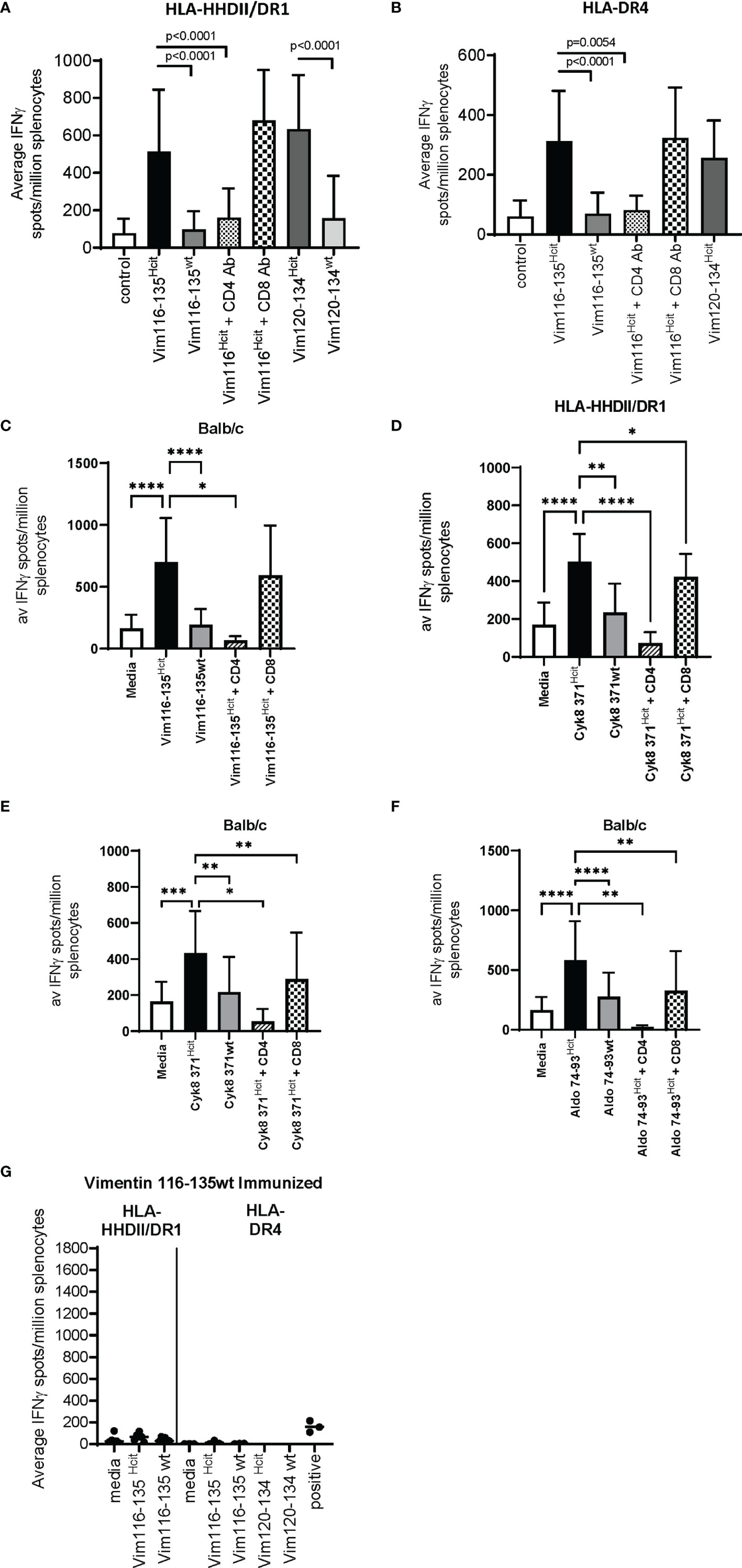
Figure 2 Characterization of the homocitrulline-mediated immune responses. Mice were immunized with homocitrullinated peptides then assessed for responses using IFNγ ELISpot assays. ELISpots were then performed with homocitrullinated (Hcit) and wild type (wt) peptides or homocitrullinated peptides in the presence of anti-CD4 or anti-CD8 blocking antibodies. Vim116-135Hcit responses were assessed in HLA-HHDII/DR1 (A), HLA-DR4 (B) and Balb/c (C) mice. Cyk8 371-388Hcit responses were assessed in HLA-HHDII/DR1 (D) and Balb/c (E) mice. Aldo 74-93Hcit responses were only assessed in Balb/c mice (F). Mice were also immunized with wild type Vim116-135 peptide and responses were assessed in HLA-HHDII/DR1 and HLA-DR4 mice (G). For each study n≥3, Homocitrulline immunized mice were assessed in ≥3 independent studies. Mean and standard deviations and significant p values are shown; ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05.
The Cyk8 371-388Hcit response in HLA-HHDII/DR1 (Figure 2D) and Balb/c (Figure 2E) mice was also assessed. IFNγ responses to Cyk8 371-388Hcit showed minimal cross reactivity to the wt peptide with the Hcit responses significantly greater than the wt response in both strains (p=0.0045 and p=0.0073 for HLA-HHDII/DR1 and Balb/c respectively). In both strains the IFNγ response to Cyk8 371-388Hcit was partially blocked by anti-CD8 antibodies but fully blocked by anti-CD4 antibodies. In HLA-HHDII/DR1 mice responses were reduced from a mean of 503 spots/million splenocytes to 73 spots/million splenocytes (p<0.0001) after blocking with the anti-CD4 antibody and only slightly reduced to a mean of 423 spots/million splenocytes in responses to anti-CD8 blocking antibodies (p=0.0148). In Balb/c mice the mean response of 433 spots/million splenocytes was reduced to 53 spots/million splenocytes after CD4 blocking (p=0.0117) and 289 spot/million splenocytes after CD8 blocking (p=0.0048), suggesting that the Cyk8 371-388Hcit responses were also CD4 and CD8 mediated.
Finally, the Aldo74-93Hcit response was characterized in Balb/c mice (Figure 2F). IFNγ responses to Aldo74-93Hcit were significantly greater than wild type responses (p<0.0001). The Aldo74-93Hcit response was partially blocked by anti-CD8 antibodies but fully blocked by anti-CD4 antibodies. Responses were reduced from a mean of 583 spots/million splenocytes to 24 spots/million splenocytes (p=0.0020) after blocking with the anti-CD4 antibody and partially reduced to a mean of 255 spots/million splenocytes in responses to anti-CD8 blocking antibodies (p=0.0059).
To further confirm that the Vim116-135Hcit response is modification specific immunizations with the wild type Vim116-135wt peptide were performed (Figure 2G). No immune responses to Vim116-135Hcit were observed in either HLA-HHDII/DR1 or HLA-DR4 mice. This confirms that only the modified peptide can induce an immune response.
Together these results confirm that the immune responses to Vim 116-135Hcit, Cyk8 371-388Hcit and Aldo 74-93Hcit were CD4 and possibly CD8 mediated and homocitrulline specific in mice with the appropriate MHC alleles.
Humans Have a Repertoire That Can Recognize the Vim116-135Hcit Peptide
Having demonstrated that immunized transgenic mice expressing human HLA types show homocitrulline-specific immune responses, we next determined whether healthy human donors exhibit a repertoire for homocitrullinated peptides. For this we concentrated on the Vim116-135Hcit peptide.
PBMCs were isolated from 17 healthy donors and assessed for proliferative responses to homocitrullinated peptides. Donor details including HLA-types are shown in Table 2. Flow cytometry staining was used to assess CFSE proliferation in the CD4+ or CD8+ cell populations. Proliferation was assessed at day 10 post-stimulation as this timepoint showed clear peptide-induced proliferative responses. An example staining profile is shown in Figure 3A and additional example CFSE profiles for day 7 and day 10 are shown in Supplementary Figure 2. CFSE proliferation to the positive control PHA was seen in both the CD4 and CD8 populations, however, Vim116-135Hcit proliferation was predominantly seen in the CD4 population. Collated data from healthy donors is shown in Figure 3B whilst CD4 proliferative responses in individual healthy donors are shown in Figure 3Ci. In PBMCs from healthy donors, 15/17 showed CD4 T-cell proliferation in response to Vim116-135Hcit peptide. Across all donors, Vim116Hcit induced a significant increase in CD4 proliferation compared to media only (p<0.0001) (Figure 3Cii).
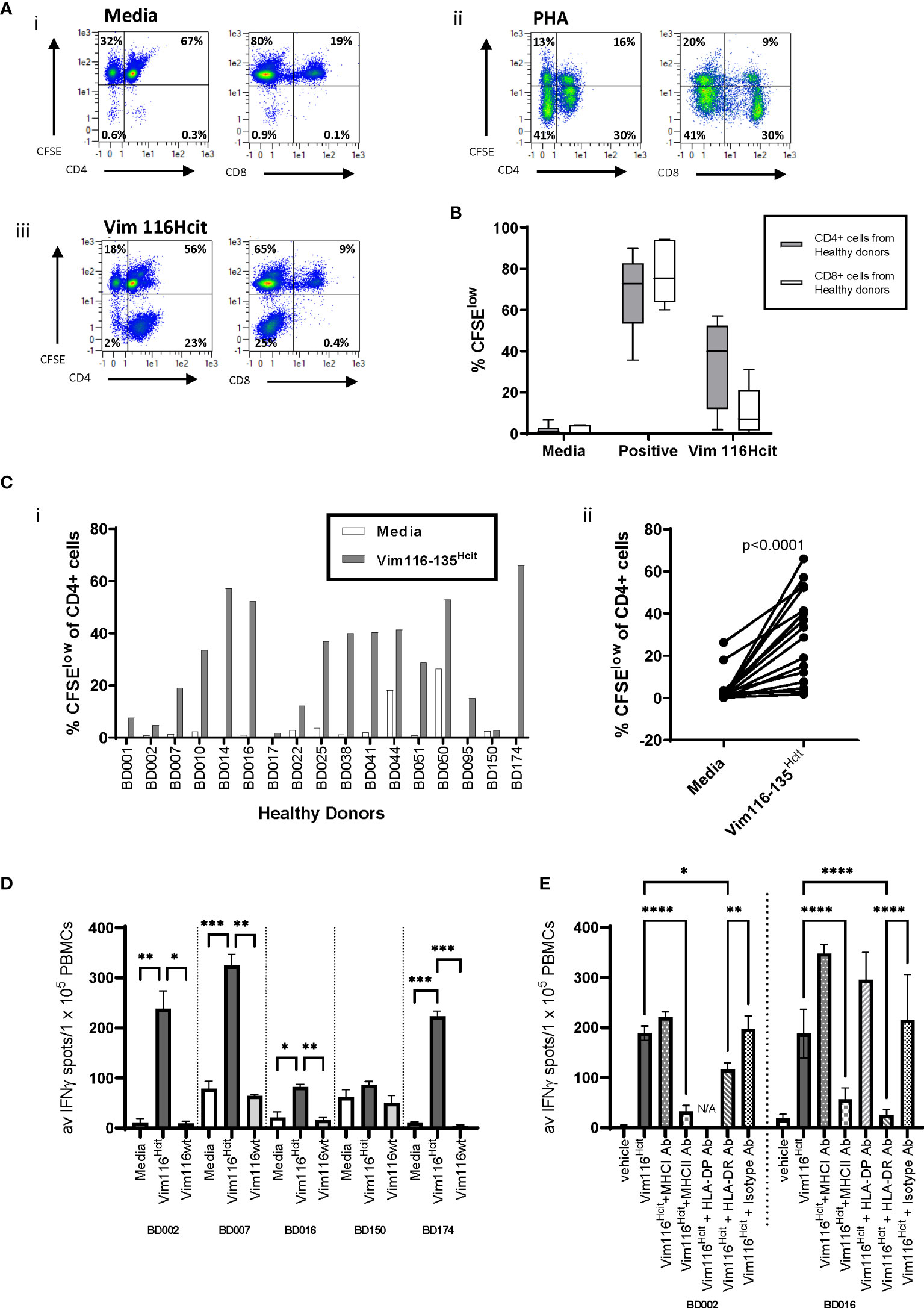
Figure 3 Heathy Human Donors have a repertoire that can recognize the Vim116-135Hcit peptide. PBMCs were isolated from 17 healthy donors and assessed for proliferative responses to homocitrullinated peptides using a CFSE dilution assay. Example plots from healthy donor BD0051 (A) show proliferation (CFSE) vs CD4 or CD8 in media control (i) PHA stimulated (ii) or Vim116-135Hcit stimulated (iii) cells after gating on the total lymphocyte population. CFSE proliferation in the gated CD4 or CD8 populations was assessed (B). Given that the majority of proliferation in response to Vim116-135Hcit was found in the CD4+ population, the percentage of the CD4+ proliferation was assessed in individual healthy donors (Ci) and paired analysis comparing media control to peptide stimulation (Cii). Restimulation assays were performed on PMBCs from 5 healthy donor that had been cultured with Vim116-135Hcit peptide for 14 days. IFNγ ELISpot showed restimulation responses to homocitrullinated (hcit) and wild type (wt) peptides (D). Restimulation to Vim116-135Hcit was also performed for two donors in the presence of anti-MHC-I, MHC-II, DP, DR or isotype blocking antibodies (E). Summary data with significant p values are shown; ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05.
To determine whether the Hcit responses seen in healthy donors were modification-specific restimulation assays were performed on 5 healthy donor PMBCs that had been cultured with modified peptide for 14 days (Figure 3D). IFNγ ELISpot showed restimulation responses to modified but not wt peptide. All responding donors showed a significantly higher response to Vim116-135Hcit compared to Vim116-135wt peptide. For two donors restimulation assays were also performed in the presence of blocking antibodies (Figure 3E). In both donors’ responses to Vim116-135Hcit were significantly reduced by addition of pan-MHC-II blocking antibodies or anti-HLA-DR blocking antibodies indicating the involvement of the HLA-DR allele. Together this data confirms that healthy human PBMCs have a modification specific repertoire for this peptide that is likely restricted through HLA-DR alleles.
The Human Responses to Vim116-135Hcit Are Oligoclonal
To determine the clonality, TCR Vα and Vβ sequencing was performed on proliferating and non-proliferating CD4 T-cells isolated from healthy donor that had been stimulated ex vivo. TCRα and β CDR3 tree maps were used to display clonality (Figure 4). Stimulation with vehicle showed minimal proliferative responses and no consistent differences in the clonality of the non-proliferative population (Figure 4A). Polyclonal CD3 stimulation also led to no clear changes in the diversity of clonotypes, whereas responses to the HLA-DP4 restricted Hepatitis B peptide showed decreased diversity after proliferation (Figure 4B). Vim116-135Hcit stimulated samples also showed decreased CDR3 diversity in the proliferating CD4+CFSElow cells with a dramatic increase in the relative frequency of subsets of CDR3 sequences suggesting a focused repertoire of cells responding to Vim116-135Hcit (Figure 4C). The oligoclonal nature of the proliferating CD4 T cells was confirmed by the lower diversity index (D50) of the CDR3 sequences from the proliferating (CD4+CFSElow) compared to non-proliferating (CD4+CFSEhigh) populations (Figure 4D).
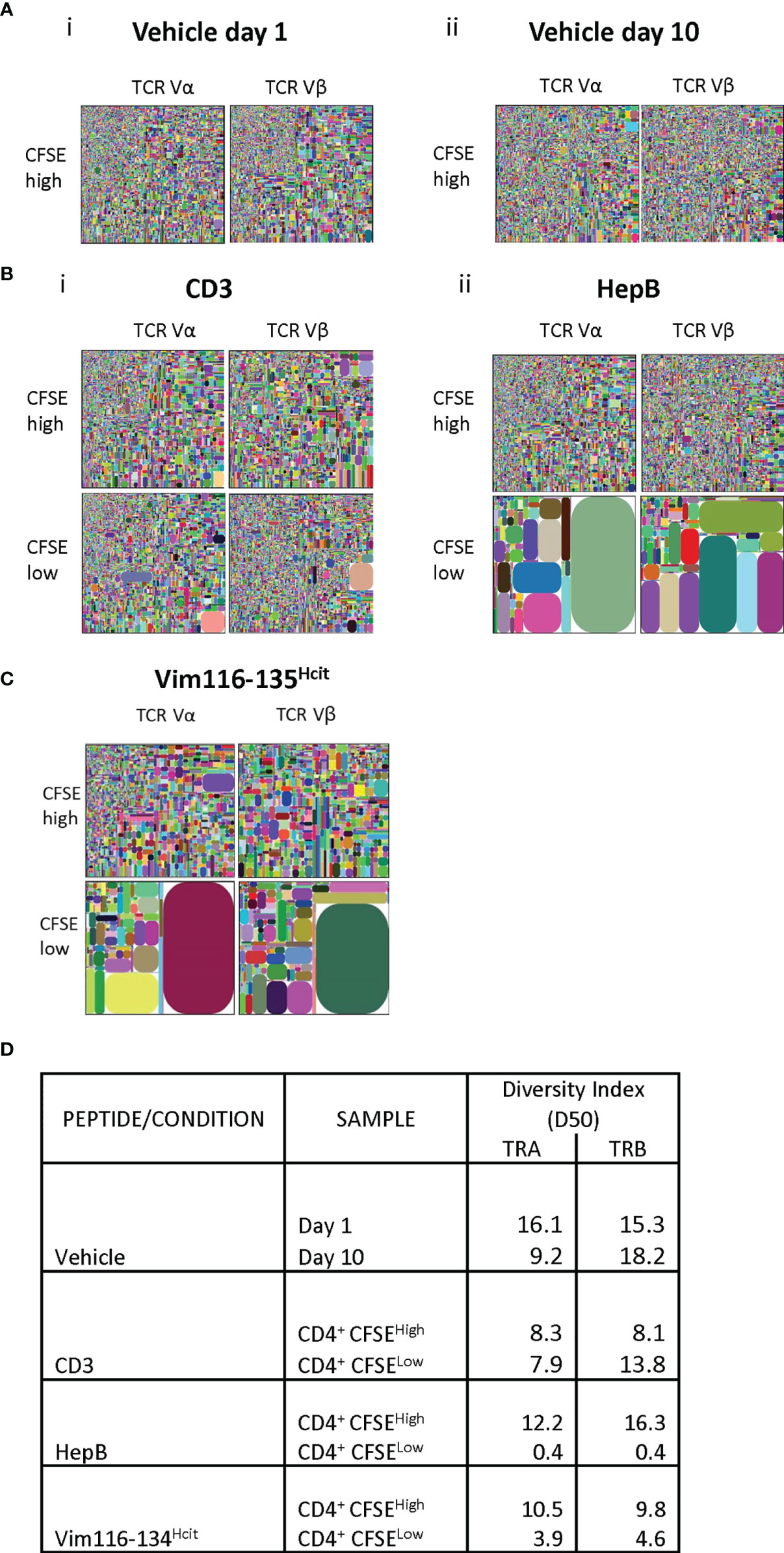
Figure 4 Human response to Vim116-135Hcit is oligoclonal. TCR α and β repertoire diversity was assessed in the CD4+ CFSEhigh/low cells in one healthy donor (BD016). Responses to vehicle (A), positive control CD3 and HepB (B) or Vim116-135Hcit peptide stimulation (C) were assessed. Tree maps depict TCR α and β chain CDR3 clonotype usage in relation to repertoire size. Each rectangle represents a unique CDR3 nucleotide sequence and rectangle size denotes the relative frequency of an individual sequence. Colors are randomly assigned and thus do not represent the same CDR3 sequence between plots. Diversity Index for each population is shown in (D).
Vim116-135Hcit Immunization Is Associated With Improved Anti-Tumor Response In Vivo
We have identified HLA DR-allele associated homocitrulline-specific immune responses. Our previous studies have shown that MDSC-produced MPO can mediate homocitrullination in the tumor microenvironment making homocitrullinated epitopes good targets for tumor therapy (22). However, not all homocitrulline-specific immune responses show anti-tumor responses suggesting that not all lysines are naturally homocitrullinated or presented. Therefore, we next determined if the immune response to Vim116-135Hcit was sufficient to provide tumor therapy in vivo. Transgenic mice were implanted with HLA-matched B16 F1 cells and immunized with Vim116-135Hcit peptide. Immunization schedule is presented in Supplementary Figure 3A. In mice containing the HLA-HHDII/DR1 allele (Figure 5A), Vim116-135Hcit immunization provided significantly better survival than mice from the unimmunized control (p<0.0001), Vim116-135wt peptide immunized (p=0.0030) or CpG/MPLA adjuvant only (p-=0.0266) groups. Tumor growth curves are presented in Supplementary Figure 3B. In HLA-DR4 mice (Figure 5B) Vim116-135Hcit immunization was also associated with a significant increase in survival when compared to unimmunized control mice (p=0.0042) or CPG/MPLA control (p=0.0235).
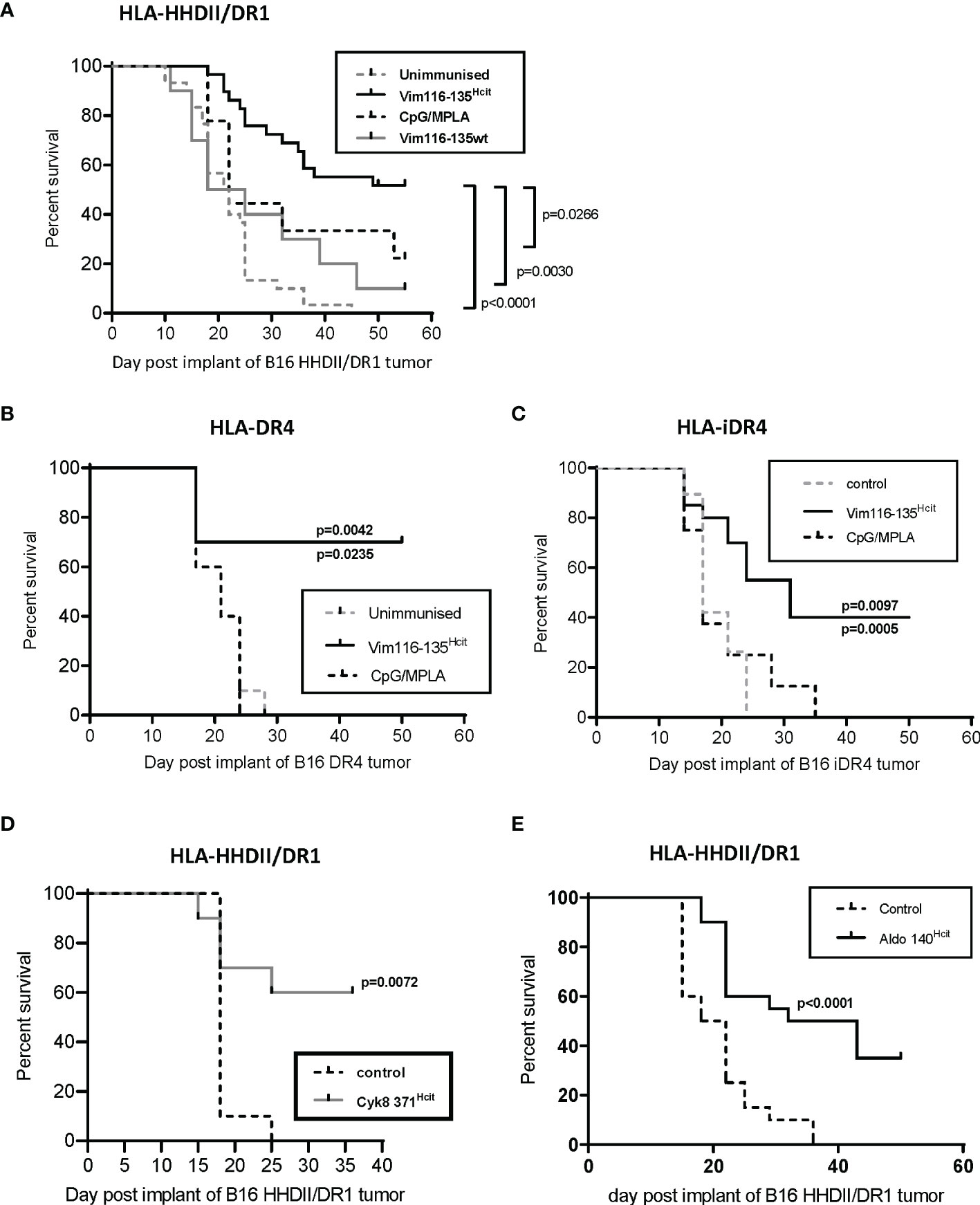
Figure 5 Vim116-135Hcit responses are associated with an anti-tumor response. HLA-HHDII/DR1 (A) and HLA-DR4 (B) mice were implanted with HLA-matched B16 F1 cells expressing MHC-II under a constitutive (A, B, D, E) or inducible (C) promoter on day 1. On day 4, 11 and 18 mice were immunized with homocitrullinated (Hcit) or wild type (wt) Vim116-135 peptides or adjuvant only controls. Anti-tumor survival was compared to unimmunized control mice. Anti-tumor studies were also performed with Cyk8 371Hcit (D) and Aldo140-157Hcit (E) in HLA-HHDII/DR1 mice implanted with HLA-matched B16 cells. For each group and study n≥10, apart from CpG/MPLA control in panel B where n=5. significant p values are shown.
Since, both these cell lines were engineered to express MHC-II constitutively and expression of MHC-II in non-APCs, including most cancer cells, is not constitutive but driven by the IFNγ-induced CIITA promoter pIV (34) we engineered B16 cells to express transgenic MHC-II under an IFNγ-inducible promoter (iDR4. Mice implanted with B16 iDR4 (Figure 5C) and immunized with Vim116-135Hcit showed a significant survival advantage over mice immunized with adjuvant only (p=0.0097) or unimmunized mice (p=0.0005). Tumor growth curves are shown in Supplementary Figure 3C. Together this data shows that immunization with Vim116-135Hcit confers a potent anti-tumor response.
To confirm whether other Homocitrullinated peptides can also induce an anti-tumor response the Cyk8 371-388Hcit and Aldo 140-157Hcit peptides were also tested in vivo. In HLA-HHDII/DR1 mice implanted with B16 HHDII/DR1 tumor cells immunization with Cyk8 371-388Hcit induced a significant survival advantage when compared to unimmunized control (p=0.0072) (Figure 5D). In this same model, immunization with Aldo 140-157Hcit also resulted in a significant anti-tumor effect (p<0.0001) when compared to unimmunized control (Figure 5E).
Expression of MHC II Is Critical for the Vim116-135Hcit Induced Anti-Tumor Response
CD4 T cells recognize homocitrullinated epitopes but the anti-tumor effect may be mediated by a bystander effect of CD4 activation of other effector cells such as CD8, NK cells or macrophages or, CD4s may act directly via induction of MHC-II on tumor cells. We therefore investigated if the expression of MHC-II on tumor cells was required for the efficacy of the vaccine. HLA-HHDII/DR1 mice were implanted with B16 cells expressing matched MHC-I but no MHC-II (B16 HHDII/MHC-I/II KO). In this model APCs express MHC-II and CD8 T-cells can recognize the tumor via MHC-I. The anti-tumor effect of Vim116-135Hcit peptide immunization was completely lost (Figure 6A) in this model, suggesting therapy was not mediated by other effector cells. In contrast, HLA-DR4 mice implanted with B16 MHC-II K/O cells and immunized with Vim116-135Hcit (Figure 6B). did demonstrate a significant survival advantage over control unimmunized mice (p=0.0010). HOWEVER, this advantage was still reduced compared to the model expressing MHC-II shown Figure 5B an suggesting that, in this case, both bystander and direct CD4 recognition of tumor may play a role in the vaccine efficacy(p=0.0010).
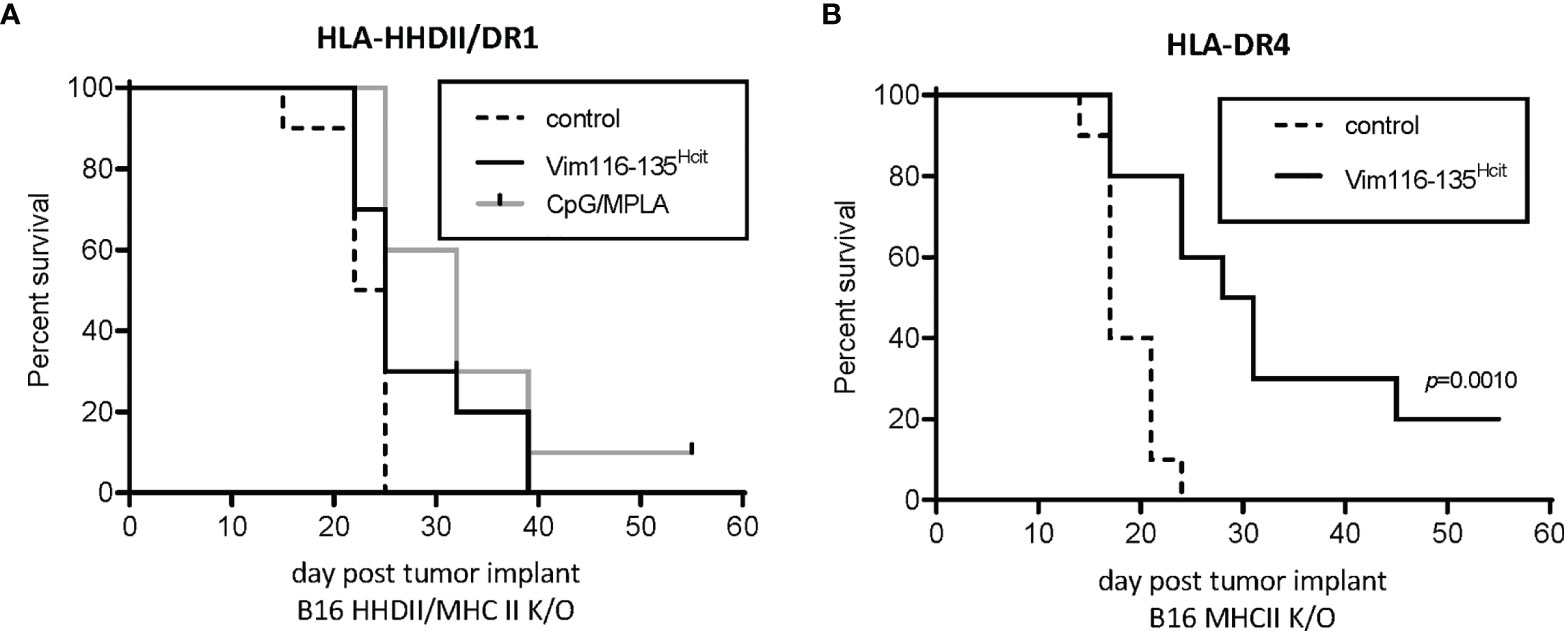
Figure 6 Anti-tumor response requires direct presentation on MHC-II by tumor. Anti-tumor studies were performed using tumor which expressed MHC-I but were knocked out for MHC-II. HLA-HHDII/DR1 (A) and HLA-DR4 (B) mice were challenged with B16 K/O MHC-II cell lines on day 1. On day 4, 11 and 18 mice were immunized with Vim116-135Hcit peptide or adjuvant only controls. Anti-tumor survival was compared to unimmunized control mice. For each group n=10, significant p values are shown.
Cancer Patients Have a Repertoire That Can Recognize the Vim116-135Hcit Peptide
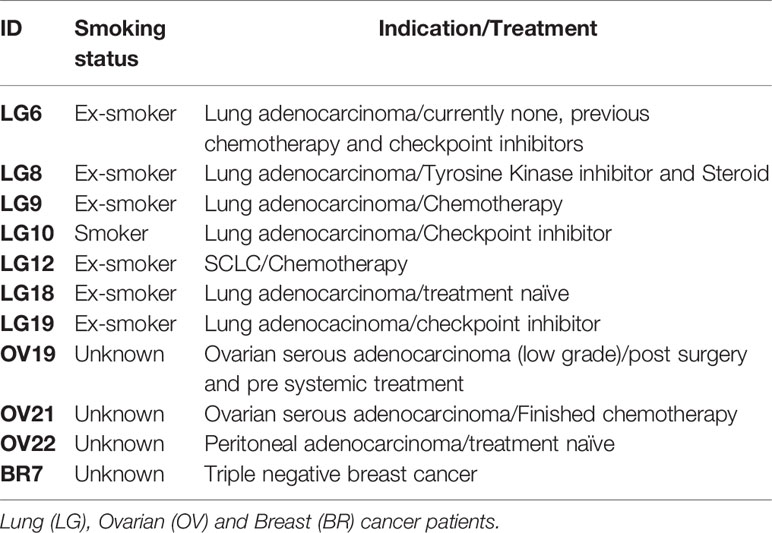
Given that Vim116-135Hcit shows a strong anti-tumor response in vivo in mouse models, we next determined if cancer patients had an immune repertoire capable of responding to this peptide. PBMCs were isolated from seven lung cancer patients, two ovarian cancer patients and one breast cancer patient (n=11, Table 3) and assessed for proliferative responses to Vim116-135Hcit. PBMCs from 4/11 cancer patients showed responses to Vim116-135Hcit (Figure 7A). Across all donors, Vim116-135Hcit induced a significant increase in CD4 proliferation compared to media only (p=0.0420) (Figure 7B). This suggests that some cancer patients may be able to respond to immunization making this a potential anti-cancer therapy. Overall, this study has identified three homocitrullinated peptides that have different but overlapping HLA-restrictions. The responses are modification specific and CD4-mediated. The results suggest that these responses can be utilized to target tumors and that they can induce responses in healthy donors and cancer patients.
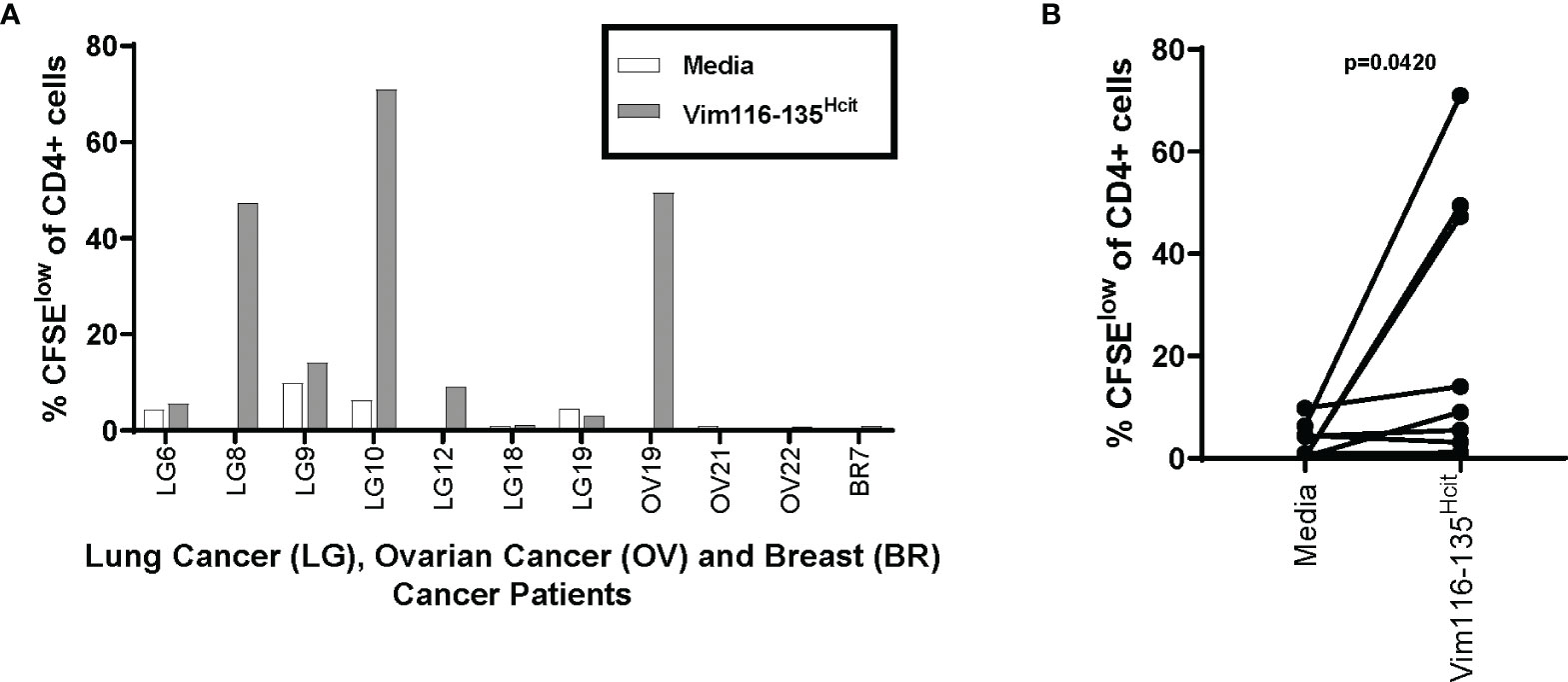
Figure 7 Cancer Patients also have a repertoire that can recognize the Vim116-135Hcit peptide. PBMCs were isolated from 11 cancer patients (7 Lung cancer patients, 2 Ovarian patients and 1 Breast cancer patient) and assessed for proliferative responses by CFSE dilution assay to homocitrullinated peptide. Graph shows CD4 proliferation in individual patients (A) and paired analysis comparing media control to Vim16-135Hcit stimulation (B).
Discussion
The stress associated PTMs citrullination (cit) and homocitrullination are known to produce autoantigens in some disease situations. Autoreactive antibodies and T cells that recognize homocitrullinated epitopes have been described alongside citrullinated epitopes in rheumatoid arthritis (RA) (35–39). Anti-Hcit responses have also been seen in other autoimmune conditions such as systemic lupus erythematosus (SLE) (9, 40). Expansion of the repertoire of T cells responding to post-translationally modified peptides is believed to contribute to disease severity (41, 42). In RA responses have been linked to expression of the shared epitope (SE) MHC-II alleles (43). SE alleles are DRB1 molecules including DR4 and DR1 with a shared amino acid motif that predisposed to RA (44, 45). The SE can act as a signal transduction ligand to increase the abundance of citrullinated peptides and bone damage (46). Although interestingly, Lac et al. showed in transgenic mice that while anti-cit responses were confined to the shared epitope, anti-Hcit responses can be induced outside of the shared epitope alleles (27). These findings have resulted in Hcit modifications being known primarily as a side effect of inflammation that leads to aberrant immune responses and the breaking of tolerance.
In this study we have shown that normal human donors have a repertoire of CD4 T cells that can respond to Hcit peptides. This anti-Hcit response was observed in both SE and other HLA types. Human data demonstrated that healthy donors with a variety of HLA types showed immune responses to Vim116-135Hcit. In fact, characterization of the restimulation response in donor BD016 showed a donor that does not express DR4 or DR1 alleles can induce a DR-dependent, Hcit specific immune response. Data from our previous study shows that responses in healthy donors are also seen to other Hcit peptides (22). These healthy donors have no diagnosis of RA despite the presence of Hcit-reactive T cells in the majority of donors tested.
This study also showed that in human SE allele transgenic and non-SE allele Balb/c mice modification specific Hcit T cell responses can be induced via immunization. In mice, responses were seen in HLA-DR4 and HLA-DR1 transgenic strains but were also observed in Balb/c mice. Other studies have also shown responses in Balb/c and C57Bl/6 mice and transgenic mice expressing the non-SE allele HLA-DP4 (19, 22, 27). This confirms that the ability to differentiate between homocitrullinated and wild type peptides is not a phenomenon restricted to the SE allele but is in fact integrated into several MHCII alleles across species.
These results align with the hypothesis that presentation of homocitrullinated epitopes is a normal response to cellular stress, inflammation and tissue damage. The accumulation of homocitrullinated proteins has been associated with normal aging (47). Homocitrullination of specific proteins are also known to have physiological effects. For example, homocitrullination of haemoglobin plays a role in the response to hypoxia and homocitrullination of erythropoietin maintains neuroprotective effects without any erythropoiesis (48). Homocitrullination can result from the accumulation of urea (21) but in inflammatory conditions, hydrogen peroxide breakdown by the myeloperoxidase (MPO) enzyme leads to the conversion of thiocyanate to cyanate and isocyanic acid which is turn results in homocitrullination of lysine residues (21, 49). MPO driven homocitrullination has been associated with inflammation and smoking (49, 50). Studies in fibroblasts show that exposure to cyanate or urea can drive accumulation of intracellular homocitrullinated peptides and that these modified peptides can then be removed via proteasomal degradation (51). As seen in the context of autoimmune disease, homocitrullination of proteins can alter proteolytic cleavage and lead to MHC presentation of neoepitopes (27, 38, 52). However, the presence of diverse alleles that can recognize Hcit-specific peptides and the widespread presence of homocitrullinated peptides leads us to believe that this system may have a role in normal cell surveillance (10).
The theory of immune surveillance suggests that the immune system can recognize and kill some precancerous cells (53–55). However, in established tumors the tumor microenvironment (TME) is highly evolved to suppress pro-inflammatory responses and therefore the immune response is attenuated. The success of checkpoint inhibitors is to re-establish immune attack in order to eliminate cancer cells. In the TME chronic cellular stress occurs, and there can be high levels of myeloid derived suppressor cells (MDSCs). We have previously shown that the MDSCs can provide a source of MPO and drive homocitrullination (22). Tumor cells can also produce high levels of H2O2, required for MPO driven homocitrullination, as a result of the oncogenic transformation associated with antioxidant imbalances (56). Therefore, vaccination to recover and expand pro-inflammatory Hcit specific T cell responses may be highly effective in some tumors. In this study we showed that Hcit-peptide immunization in Balb/c and HLA transgenic mice can provide tumor therapy. The responses were dependent on the tumors presenting peptide via MHCII showing the importance of direct tumor recognition and inflammation in upregulating MHC-II. Our previously published work demonstrates that increased immune infiltrate is correlated with successful tumor regression following homocitrullinated peptide vaccination (22). However, future studies in vivo and in patients should seek to further characterized the tumor microenvironment following immunization with homocitrullinated peptides including the peptides discussed in this paper. Interestingly we also demonstrate that unimmunized cancer patients have a repertoire of cells that can recognize the Vim116-135Hcit peptide. Despite the detection of responding CD4 T cells, there is some evidence that the cancer patients showed a lower response rate to this peptide (4/11) than healthy donors (15/17) suggesting these responses have been switched to a regulatory phenotype. Clinical trials will need to be carried out to determine whether immunization can reverse the suppression or stimulate a de novo response.
In contrast to the situation in cancer where an immunosuppressive tumor environment is prevalent, some conditions including those seen in RA patients, lead to chronic inflammation and autoimmune disease. These aberrant responses may be exacerbated in individuals with an SE allele due to better binding of modified peptides to HLA or other pro-inflammatory factors. One mouse study showed that immunization with Hcit peptides induced Hcit and cit responses in HLA-DR4 transgenic mice but only Hcit responses in C57Bl/6 mice providing further evidence that HLA alleles effect the type of immune responses generated (27). SE alleles DRB1*0401 and *0402 transgenic mice were shown to be predisposed to inducing Th17 responses which are effective at removing acute infection but that can drive autoimmune disease in the presence of chronic inflammation (57). However, Balb/c mice exhibited signs of RA disease after immunization with homocitrullinated peptides suggesting autoimmune disease is not completely restricted to individuals with SE alleles (19). The situation is complicated and the conditions which drive pro-inflammatory disease are not fully understood. Analysis of RA patients and matched first degree relatives without inflammation showed that both groups have similar levels of anti-Hcit antibodies, suggesting that homocitrulline targeted immunity alone is not always associated with disease (58). However, these autoimmune responses will need to be monitored extensively in any clinical trials aiming to use homocitrullinated peptide vaccination as an anti-tumor therapy. It may mean autoimmune disease would be a primary exclusion criterion for trials and that levels of autoantibodies would need to be continually measured in all patients.
Overall, this study identifies Vim116-135Hcit as a peptide that can induce Hcit-specific CD4 T cell responses in healthy humans and mice. It also identified a number of other homocitrullinated peptides that can stimulate immune responses via various HLA types. In vivo studies show that these responses can provide a strong anti-tumor effect that is dependent on tumor expression of MHCII. The results contribute to our understanding of Hcit as a cellular mechanism beyond the scope of autoimmune disease and may open exciting new avenues for the treatment of cancer in a diverse HLA-background.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee, University of Nottingham. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by University of Nottingham ethics committee Nottingham Trent university ethics committee.
Author Contributions
LD and VB directed the study. KC, WX, SA, PS, AA, SS, RC, and ID performed experiments and analyzed data. RM provided reagents and support. KC, WX, and VB designed experiments, analyzed the data and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from Scancell Ltd. The funder was involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Conflict of Interest
KC, WX, VB, and LD have ownership interest in a patent WO2020053304A2. LD is a director and shareholder in Scancell Ltd. All authors are employees of Scancell Ltd.
This study received funding from Scancell Ltd. The funder had the following involvement with the study: design, data collection and analysis, decision to publish and preparation of the manuscript.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would also like to thank Dr. Samantha Paston and Dr. Mireille Vankemmelbeke for help in proofreading the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.873947/full#supplementary-material
Supplementary Figure 1 | Example ELISpot images. Example ELISpot images show HLA-HHDII/DR1 mice were immunized with the Vim116-135Hcit peptide. Responses for individual mice were assessed in triplicate to media only control, 10ug/ml peptides or positive control LPS. Responses to peptide were also assessed in the presence of anti-CD4 or anti-CD8 blocking antibodies.
Supplementary Figure 2 | Additional CFSE example plots. PBMCs were isolated from healthy donors and assessed for proliferative responses to homocitrullinated peptides using a CFSE dilution assay. Example plots from healthy donor BD0051 at day 7 (A) show proliferation (CFSE) vs CD4 or CD8 in media control (i) PHA stimulated (ii) or Vim116-135Hcit stimulated (iii) cells after gating on the total lymphocyte population. Example CFSE proliferation profiles for donor BD051 on day 7 and day 10 in the gated CD4 or CD8 populations (B). Peptide responses were superior on day 10.
Supplementary Figure 3 | Tumor growth curves. For all tumor studies mice were implanted with tumor on day 1 and then immunized on days 4, 11 and 18 (A). For some mice the home office license agreed humane end point was reached prior to final immunization and mice were terminated. HLA-HHDII/DR1 (B) and HLA-DR4 (C) mice were implanted with HLA-matched B16 F1 HHDII/DR1 (B) or HLA-iDR4 (C) cells. Mice were immunized with homocitrullinated (Hcit) or wild type (wt) Vim116-135 peptides or adjuvant only controls. Tumor growth for each group is shown. Boxed number represents the number of mice that were tumor-free at the end of the study for each group.
References
1. Sadegh-Nasseri S, Kim A. Exogenous Antigens Bind MHC Class II First, and Are Processed by Cathepsins Later. Mol Immunol (2015) 68:81–4. doi: 10.1016/j.molimm.2015.07.018
2. Clancy KW, Weerapana E, Thompson PR. Detection and Identification of Protein Citrullination in Complex Biological Systems. Curr Opin Chem Biol (2016) 30:1–6. doi: 10.1016/j.cbpa.2015.10.014
3. Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL. Post-Translational Modifications of Naturally Processed MHC-Binding Epitopes. Curr Opin Immunol (2006) 18:92–7. doi: 10.1016/j.coi.2005.11.015
4. Margulies DH. How MHC Molecules Grab Citrullinated Peptides to Foster Rheumatoid Arthritis. J Biol Chem (2018) 293:3252–3. doi: 10.1074/jbc.H118.001829
5. Ting YT, Petersen J, Ramarathinam SH, Scally SW, Loh KL, Thomas R, et al. The Interplay Between Citrullination and HLA-DRB1 Polymorphism in Shaping Peptide Binding Hierarchies in Rheumatoid Arthritis. J Biol Chem (2018) 293:3236–51. doi: 10.1074/jbc.RA117.001013
6. Anderton SM. Post-Translational Modifications of Self Antigens: Implications for Autoimmunity. Curr Opin Immunol (2004) 16:753–8. doi: 10.1016/j.coi.2004.09.001
7. Balakrishnan S, Kumar P, Prabhakar BS. Post-Translational Modifications Contribute to Neoepitopes in Type-1 Diabetes: Challenges for Inducing Antigen-Specific Tolerance. Biochim Biophys Acta Proteins Proteom (2020) 1868:140478. doi: 10.1016/j.bbapap.2020.140478
8. Sollid LM. The Roles of MHC Class II Genes and Post-Translational Modification in Celiac Disease. Immunogenetics (2017) 69:605–16. doi: 10.1007/s00251-017-0985-7
9. Li Y, Jia R, Liu Y, Tang S, Ma X, Shi L, et al. Antibodies Against Carbamylated Vimentin Exist in Systemic Lupus Erythematosus and Correlate With Disease Activity. Lupus (2020) 29:239–47. doi: 10.1177/0961203319897127
10. Brentville VA, Vankemmelbeke M, Metheringham RL, Durrant LG. Post-Translational Modifications Such as Citrullination are Excellent Targets for Cancer Therapy. Semin Immunol (2020) 47:101393. doi: 10.1016/j.smim.2020.101393
11. Cobbold M, de la Pena H, Norris A, Polefrone JM, Qian J, English AM, et al. MHC Class I-Associated Phosphopeptides Are the Targets of Memory-Like Immunity in Leukemia. Sci Transl Med (2013) 5:203ra125. doi: 10.1126/scitranslmed.3006061
12. Depontieu FR, Qian J, Zarling AL, McMiller TL, Salay TM, Norris A, et al. Identification of Tumor-Associated, MHC Class II-Restricted Phosphopeptides as Targets for Immunotherapy. Proc Natl Acad Sci USA (2009) 106:12073–8. doi: 10.1073/pnas.0903852106
13. Zarling AL, Polefrone JM, Evans AM, Mikesh LM, Shabanowitz J, Lewis ST, et al. Identification of Class I MHC-Associated Phosphopeptides as Targets for Cancer Immunotherapy. Proc Natl Acad Sci USA (2006) 103:14889–94. doi: 10.1073/pnas.0604045103
14. Malaker SA, Penny SA, Steadman LG, Myers PT, Loke JC, Raghavan M, et al. Identification of Glycopeptides as Posttranslationally Modified Neoantigens in Leukemia. Cancer Immunol Res (2017) 5:376–84. doi: 10.1158/2326-6066.CIR-16-0280
15. Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting Edge: The Conversion of Arginine to Citrulline Allows for a High-Affinity Peptide Interaction With the Rheumatoid Arthritis-Associated HLA-DRB1*0401 MHC Class II Molecule. J Immunol (2003) 171:538–41. doi: 10.4049/jimmunol.171.2.538
16. Feitsma AL, van der Voort EI, Franken KL, El Bannoudi H, Elferink BG, Drijfhout JW, et al. Identification of Citrullinated Vimentin Peptides as T Cell Epitopes in HLA-DR4-Positive Patients With Rheumatoid Arthritis. Arthritis Rheum (2010) 62:117–25. doi: 10.1002/art.25059
17. Brentville VA, Metheringham RL, Gunn B, Symonds P, Daniels I, Gijon M, et al. Citrullinated Vimentin Presented on MHC-II in Tumor Cells Is a Target for CD4+ T-Cell-Mediated Antitumor Immunity. Cancer Res (2016) 76:548–60. doi: 10.1158/0008-5472.CAN-15-1085
18. Rims C, Uchtenhagen H, Kaplan MJ, Carmona-Rivera C, Carlucci P, Mikecz K, et al. Citrullinated Aggrecan Epitopes as Targets of Autoreactive CD4+ T Cells in Patients With Rheumatoid Arthritis. Arthritis Rheumatol (2019) 71:518–28. doi: 10.1002/art.40768
19. Mydel P, Wang Z, Brisslert M, Hellvard A, Dahlberg LE, Hazen SL, et al. Carbamylation-Dependent Activation of T Cells: A Novel Mechanism in the Pathogenesis of Autoimmune Arthritis. J Immunol (2010) 184:6882–90. doi: 10.4049/jimmunol.1000075
20. Burska AN, Hunt L, Boissinot M, Strollo R, Ryan BJ, Vital E, et al. Autoantibodies to Posttranslational Modifications in Rheumatoid Arthritis. Mediators Inflamm (2014) 2014:492873. doi: 10.1155/2014/492873
21. Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, et al. Myeloperoxidase-Derived Chlorinating Species Induce Protein Carbamylation Through Decomposition of Thiocyanate and Urea: Novel Pathways Generating Dysfunctional High-Density Lipoprotein. Antioxid Redox Signal (2012) 17:1043–52. doi: 10.1089/ars.2011.4403
22. Cook KW, Xue W, Symonds P, Daniels I, Gijon M, Boocock D, et al. Homocitrullination of Lysine Residues Mediated by Myeloid-Derived Suppressor Cells in the Tumor Environment Is a Target for Cancer Immunotherapy. J Immunother Cancer (2021) 9:e.001910–e.2100. doi: 10.1136/jitc-2020-001910
23. Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A New Model for an Etiology of Rheumatoid Arthritis: Smoking may Trigger HLA-DR (Shared Epitope)-Restricted Immune Reactions to Autoantigens Modified by Citrullination. Arthritis Rheum (2006) 54:38–46. doi: 10.1002/art.21575
24. Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, et al. A Molecular Basis for the Association of the HLA-DRB1 Locus, Citrullination, and Rheumatoid Arthritis. J Exp Med (2013) 210:2569–82. doi: 10.1084/jem.20131241
25. Law SC, Street S, Yu C-HA, Capini C, Ramnoruth S, Nel HJ, et al. T-Cell Autoreactivity to Citrullinated Autoantigenic Peptides in Rheumatoid Arthritis Patients Carrying HLA-DRB1 Shared Epitope Alleles. Arthritis Res Ther (2012) 14:R118–R. doi: 10.1186/ar3848
26. Brentville VA, Symonds P, Cook KW, Daniels I, Pitt T, Gijon M, et al. T Cell Repertoire to Citrullinated Self-Peptides in Healthy Humans Is Not Confined to the HLA-DR SE Alleles; Targeting of Citrullinated Self-Peptides Presented by HLA-DP4 for Tumour Therapy. Oncoimmunology (2019) 8:e1576490. doi: 10.1080/2162402X.2019.1576490
27. Lac P, Saunders S, Tutunea-Fatan E, Barra L, Bell DA, Cairns E. Immune Responses to Peptides Containing Homocitrulline or Citrulline in the DR4-Transgenic Mouse Model of Rheumatoid Arthritis. J Autoimmun (2018) 89:75–81. doi: 10.1016/j.jaut.2017.12.002
28. Zhang Q, Wang P, Kim Y, Haste-Andersen P, Beaver J, Bourne PE, et al. Immune Epitope Database Analysis Resource (IEDB-Ar). Nucleic Acids Res (2008) 36:W513–8. doi: 10.1093/nar/gkn254
29. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: Database for MHC Ligands and Peptide Motifs. Immunogenetics (1999) 50:213–9. doi: 10.1007/s002510050595
30. Schuler MM, Nastke MD, Stevanovikc S. SYFPEITHI: Database for Searching and T-Cell Epitope Prediction. Methods Mol Biol (2007) 409:75–93. doi: 10.1007/978-1-60327-118-9_5
31. Xue W, Brentville VA, Symonds P, Cook KW, Yagita H, Metheringham RL, et al. SCIB1, a Huigg1 Antibody DNA Vaccination, Combined With PD-1 Blockade Induced Efficient Therapy of Poorly Immunogenic Tumors. Oncotarget (2016) 7:83088–100. doi: 10.18632/oncotarget.13070
32. Cook K, Daniels I, Symonds P, Pitt T, Gijon M, Xue W, et al. Citrullinated Alpha-Enolase is an Effective Target for Anti-Cancer Immunity. Oncoimmunology (2018) 7:e1390642. doi: 10.1080/2162402X.2017.1390642
33. Markovics A, Ocsko T, Katz RS, Buzas EI, Glant TT, Mikecz K. Immune Recognition of Citrullinated Proteoglycan Aggrecan Epitopes in Mice With Proteoglycan-Induced Arthritis and in Patients With Rheumatoid Arthritis. PloS One (2016) 11:e0160284. doi: 10.1371/journal.pone.0160284
34. Seliger B, Kloor M, Ferrone S. HLA Class II Antigen-Processing Pathway in Tumors: Molecular Defects and Clinical Relevance. Oncoimmunology (2017) 6:e1171447. doi: 10.1080/2162402X.2016.1171447
35. Trouw LA, Huizinga TW, Toes RE. Autoimmunity in Rheumatoid Arthritis: Different Antigens–Common Principles. Ann Rheum Dis (2013) 72Suppl 2:ii132–6. doi: 10.1136/annrheumdis-2012-202349
36. Scinocca M, Bell DA, Racape M, Joseph R, Shaw G, McCormick JK, et al. Antihomocitrullinated Fibrinogen Antibodies are Specific to Rheumatoid Arthritis and Frequently Bind Citrullinated Proteins/Peptides. J Rheumatol (2014) 41:270–9. doi: 10.3899/jrheum.130742
37. Sahlstrom P, Hansson M, Steen J, Amara K, Titcombe PJ, Forsstrom B, et al. Different Hierarchies of Anti-Modified Protein Autoantibody Reactivities in Rheumatoid Arthritis. Arthritis Rheumatol (2020) 72:1643–57. doi: 10.1002/art.41385
38. Becart S, Whittington KB, Prislovsky A, Rao NL, Rosloniec EF. The Role of Posttranslational Modifications in Generating Neo-Epitopes That Bind to Rheumatoid Arthritis-Associated HLA-DR Alleles and Promote Autoimmune T Cell Responses. PloS One (2021) 16:e0245541. doi: 10.1371/journal.pone.0245541
39. Kissel T, Reijm S, Slot LM, Cavallari M, Wortel CM, Vergroesen RD, et al. Antibodies and B Cells Recognising Citrullinated Proteins Display a Broad Cross-Reactivity Towards Other Post-Translational Modifications. Ann Rheum Dis (2020) 79:472–80. doi: 10.1136/annrheumdis-2019-216499
40. Ceccarelli F, Perricone C, Colasanti T, Massaro L, Cipriano E, Pendolino M, et al. Anti-Carbamylated Protein Antibodies as a New Biomarker of Erosive Joint Damage in Systemic Lupus Erythematosus. Arthritis Res Ther (2018) 20:126. doi: 10.1186/s13075-018-1622-z
41. Shi J, Knevel R, Suwannalai P, van der Linden MP, Janssen GM, van Veelen PA, et al. Autoantibodies Recognizing Carbamylated Proteins are Present in Sera of Patients With Rheumatoid Arthritis and Predict Joint Damage. Proc Natl Acad Sci USA (2011) 108:17372–7. doi: 10.1073/pnas.1114465108
42. Volkov M, Kampstra ASB, van Schie KA, Kawakami A, Tamai M, Kawashiri S, et al. Evolution of Anti-Modified Protein Antibody Responses can be Driven by Consecutive Exposure to Different Post-Translational Modifications. Arthritis Res Ther (2021) 23:298. doi: 10.1186/s13075-021-02687-5
43. Taneja V, Behrens M, Basal E, Sparks J, Griffiths MM, Luthra H, et al. Delineating the Role of the HLA-DR4 "Shared Epitope" in Susceptibility Versus Resistance to Develop Arthritis. J Immunol (2008) 181:2869–77. doi: 10.4049/jimmunol.181.4.2869
44. Winchester R, Dwyer E, Rose S. The Genetic Basis of Rheumatoid Arthritis. The shared epitope hypothesis. Rheum Dis Clin North Am (1992) 18:761–83. doi: 10.1016/S0889-857X(21)00150-2
45. Michou L, Croiseau P, Petit-Teixeira E, du Montcel ST, Lemaire I, Pierlot C, et al. Validation of the Reshaped Shared Epitope HLA-DRB1 Classification in Rheumatoid Arthritis. Arthritis Res Ther (2006) 8:R79. doi: 10.1186/ar1949
46. van Drongelen V, Ali WH, Holoshitz J. Uncovering a Shared Epitope-Activated Protein Citrullination Pathway. J Immunol (2020) 205:579–86. doi: 10.4049/jimmunol.1901108
47. Gorisse L, Pietrement C, Vuiblet V, Schmelzer CE, Kohler M, Duca L, et al. Protein Carbamylation is a Hallmark of Aging. Proc Natl Acad Sci USA (2016) 113:1191–6. doi: 10.1073/pnas.1517096113
48. Badar A, Arif Z, Alam K. Role of Carbamylated Biomolecules in Human Diseases. IUBMB Life (2018) 70:267–75. doi: 10.1002/iub.1732
49. Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, et al. Protein Carbamylation Links Inflammation, Smoking, Uremia and Atherogenesis. Nat Med (2007) 13:1176–84. doi: 10.1038/nm1637
50. Nakabo S, Ohmura K, Akizuki S, Murakami K, Nakashima R, Hashimoto M, et al. Activated Neutrophil Carbamylates Albumin via the Release of Myeloperoxidase and Reactive Oxygen Species Regardless of NETosis. Mod Rheumatol (2019) 30:1–5. doi: 10.1080/14397595.2019.1583819
51. Desmons A, Okwieka A, Doue M, Gorisse L, Vuiblet V, Pietrement C, et al. Proteasome-Dependent Degradation of Intracellular Carbamylated Proteins. Aging (Albany NY) (2019) 11:3624–38. doi: 10.18632/aging.102002
52. van Leeuwen T, Araman C, Pieper Pournara L, Kampstra ASB, Bakkum T, Marqvorsen MHS, et al. Bioorthogonal Protein Labelling Enables the Study of Antigen Processing of Citrullinated and Carbamylated Auto-Antigens. RSC Chem Biol (2021) 2:855–62. doi: 10.1039/D1CB00009H
53. Swann JB, Smyth MJ. Immune Surveillance of Tumors. J Clin Invest (2007) 117:1137–46. doi: 10.1172/JCI31405
54. Ribatti D. The Concept of Immune Surveillance Against Tumors. The first theories. Oncotarget (2017) 8:7175–80. doi: 10.18632/oncotarget.12739
55. Cook KW, Durrant LG, Brentville VA. Current Strategies to Enhance Anti-Tumour Immunity. Biomedicines (2018) 6:37. doi: 10.3390/biomedicines6020037
56. Benfeitas R, Uhlen M, Nielsen J, Mardinoglu A. New Challenges to Study Heterogeneity in Cancer Redox Metabolism. Front Cell Dev Biol (2017) 5:65. doi: 10.3389/fcell.2017.00065
57. Luckey D, Behrens M, Smart M, Luthra H, David CS, Taneja V. DRB1*0402 may Influence Arthritis by Promoting Naive CD4+ T-Cell Differentiation in to Regulatory T Cells. Eur J Immunol (2014) 44:3429–38. doi: 10.1002/eji.201344424
Keywords: carbamylation, cancer, MHC-II, post-translation modifications, Vimentin
Citation: Cook K, Xue W, Atabani S, Symonds P, Al Omari A, Daniels I, Shah S, Choudhury RH, Weston D, Metheringham R, Brentville V and Durrant L (2022) Vaccine Can Induce CD4-Mediated Responses to Homocitrullinated Peptides via Multiple HLA-Types and Confer Anti-Tumor Immunity. Front. Immunol. 13:873947. doi: 10.3389/fimmu.2022.873947
Received: 11 February 2022; Accepted: 14 March 2022;
Published: 08 April 2022.
Edited by:
Loredana Frasca, National Institute of Health (ISS), ItalyReviewed by:
Prabhakaran Kumar, University of Illinois at Chicago, United StatesChris W. Diehnelt, Robust Diagnostics, LLC, United States
Copyright © 2022 Cook, Xue, Atabani, Symonds, Al Omari, Daniels, Shah, Choudhury, Weston, Metheringham, Brentville and Durrant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lindy Durrant, bGluZHkuZHVycmFudEBub3R0aW5naGFtLmFjLnVr
 Katherine Cook
Katherine Cook Wei Xue1
Wei Xue1 Suha Atabani
Suha Atabani Peter Symonds
Peter Symonds Abdullah Al Omari
Abdullah Al Omari Ruhul Hasan Choudhury
Ruhul Hasan Choudhury Daisy Weston
Daisy Weston Rachael Metheringham
Rachael Metheringham Victoria Brentville
Victoria Brentville Lindy Durrant
Lindy Durrant