- Research Laboratory and Hepatic Surgery Center, Department of Hepatic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and has a high mortality rate worldwide. The percentage of HCC patients with vascular invasion at the time of initial HCC diagnosis is 10%–40%. According to most guidelines, HCC with vascular invasion is classified as advanced stage, and resection is only suggested for a minority of such patients. Recently, advances in systemic and locoregional treatments for such patients have resulted in amazing response rates. Therefore, a “conversion therapy” strategy including systemic and locoregional treatments is proposed to select patients from an initially unresectable state to eventually undergo R0 resection. Recently, many studies have proven that conversion therapy followed by subsequent surgery is achievable in well-selected advanced HCC patients and can provide prolonged long-term outcomes. Based on published research, this review has summarized the clinical experience and evidence of conversion treatment in HCC patients with vascular invasion.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and has a high mortality rate worldwide. The percentage of HCC patients with vascular invasion at the time of initial HCC diagnosis is 10%–40% (1–3). According to the American Association for the Study of Liver Disease/Barcelona Clinic for Liver Cancer (AASLD/BCLC) staging system and treatment guidelines, HCC associated with vascular invasion or bile duct invasion is regarded as an advanced stage. The suggested treatment for such patients is systematic treatment or conservative treatment (4). However, the median survival time of these patients is very unsatisfactory. According to the China Liver Cancer Staging (CNLC) system (5), HCC with vascular invasion is regarded as advanced IIIa stage. In contrast to Western countries, hepatectomy and locoregional therapies, including transarterial chemoembolization (TACE), HAIC, or radiation therapy combined with systemic treatment, are suggested for HCC patients with macrovascular invasion. Compared with systematic treatment or conservative treatment only, combined therapy might provide a better prognosis with minimal side effects (6).
Downstaging conversion therapy is a new strategy for unresectable HCC that aims to reduce tumor burden by a combination of locoregional or systemic therapy and eventually allow patients to become amenable to surgical resection. The effects of this type of surgical resection are still under debate. Many concerns about downstaging conversion therapy remain. This review article discusses the following problems in downstaging conversion therapy. First, the target patients are chosen; second, the most commonly used conversion downstaging treatment and its conversion rate are introduced; and third, a possible hypothesis to increase the efficacy of conversion treatment is proposed.
The target population of conversion downstaging treatment for HCC patients with vascular invasion
As we have mentioned above, conversion downstaging treatment is proposed for unresectable HCC patients. The major reasons for unresectable HCC with vascular invasion can be divided into surgical and oncological causes (7). Surgical causes are common for most surgeons, which means that surgical excision cannot be performed safely because of the patient’s general condition, liver function, or insufficient remnant liver volume. Oncological causes refer to the prognosis after hepatectomy failing to surpass other nonsurgical treatments (7, 8). This part of the cause is still under debate between surgeons and medical oncologists. Vascular invasion in HCC patients includes two different types, i.e., tumor thrombosis in the portal vein (PVTT) or in the hepatic vein (HVTT) (9, 10). Depending on the different locations of the tumor thrombus, the complications and prognosis of surgery or nonsurgical treatment might be different. Based on recent reports focused on comparing the prognosis between hepatectomy and nonsurgical treatment, we tried to find the target population of HCC with vascular invasion that is not suitable for hepatectomy because of oncological reasons.
To date, the survival rate is poor for HCC patients with macrovascular invasion. Surgical treatment is generally not suggested because tumor cells might spread throughout the whole body, and the survival time is only 2.7–4 months after diagnosis if no suitable treatment is adopted (11). However, the prognosis of HCC with vascular invasion varies according to the different locations of vascular invasion. As we have mentioned above, vascular invasion in HCC patients can be divided into two different types: PVTT and HVTT. Compared with HCC patients with PVTT, HCC patients with only HVTT have different outcomes (12). The prognosis of HCC patients with either type of vascular invasion is correlated with the extent of invasion (9, 13, 14). Based on the extent of invasion, PVTT could be divided into type I–IV and HVTT could be divided into type I–III according to Chen’s study (14, 15) (Figure 1). For HCC patients with vascular invasion in the peripheral hepatic vein, the overall survival (OS) after hepatectomy might be 27.1–63 months (12–14). If vascular invasion in the hepatic vein extends into the inferior vena cava/right atrium, the OS after hepatectomy might be shortened to 5–16.8 months (13, 14, 16, 17). For HCC patients with vascular invasion in the intrahepatic portal vein, after R0 resection, the OS might be 18–50 months (2, 13, 18). If vascular invasion in the portal vein extends into the main portal vein, the OS after hepatectomy might be only 6–10 months (3, 13, 15). In some published research, resection might provide a better prognosis than nonsurgery treatment, including TACE/HAIC or sorafenib treatment, in HCC patients with PVTT (13). Nevertheless, when subgroup analysis was performed, the prognosis after hepatectomy combined with thrombectomy seemed to not surpass the prognosis after nonsurgical treatment if the tumor thrombus had already extended into the main portal vein (13, 19). Therefore, for HCC patients with PVTT, if the intrahepatic tumor lesion is resectable, main portal vein thrombus might be an indication for conversion therapy. For HCC patients with HVTT, the situation might be different. According to Kokudo’s study, the mean survival time in HCC patients with HVTT in the major hepatic vein was similar to that in patients with HVTT in the peripheral hepatic vein after hepatectomy (4.85 years vs. 4.67 years, respectively). However, in the nonsurgery group, the mean survival time was only 1.58–1.81 years. As PVTT is usually accompanied in HCC patients with HVTT, it could greatly decrease the mean survival time (9, 12). Therefore, for HCC patients with HVTT, conversion therapy might be suggested if the HVTT has already extended into the inferior vena cava or has been accompanied by PVTT.

Figure 1 (A), classification of the tumor thrombus in the portal vein; (B), classification of the tumor thrombus in the hepatic vein (14, 15).
Since treatment options for HCC have been studied, such as tyrosine kinase inhibitor (TKI) treatment combined with immune treatment, many clinical trials have been carried out in advanced HCC patients. Many amazing results have been published based on TKI treatment and immune treatment combined with or without locoregional treatment. For example, in a clinical trial (Keynote 524) (20), lenvatinib (a kind of TKI medicine) combined with pembrolizumab provided an OS of 22 months in unresectable HCC patients, among which 79% were BCLC-C stage HCC patients. When combined with locoregional treatment, including TACE/HAIC/radiation therapy, the 1-year survival rate of unresectable HCC patients is extended to 75%–100% (21–24). However, thus far, there are still no well-designed clinical trials for HCC patients with vascular invasion to compare the prognosis after hepatectomy or nonsurgical treatment.
Based on published data and the China liver cancer staging system (5), the prognosis of HCC patients with vascular invasion after hepatectomy varies according to the extent of vascular invasion. Resection cannot surpass nonsurgical treatment if vascular invasion is detected in the main portal vein (PVTT type III and IV) and inferior vena cava (HVTT type II and III). Therefore, for these patients, conversion therapy should be suggested even if the tumor and thrombus can be resected. With the improvement of systemic therapy, the target population of conversion therapy in HCC patients with vascular invasion might be further enlarged.
Conversion therapy in advanced-stage HCC with vascular invasion
The principle of conversion therapy for advanced-stage HCC with vascular invasion is to downstage HCC patients by utilizing multiple treatments. The most commonly used treatments for conversion therapy are systemic treatments combined with or without locoregional treatments. Locoregional treatments include transcatheter arterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), and radiation therapy. Systemic treatments should include TKIs, immunotherapy, and chemotherapy. To downstage HCC patients in a short time, a combination of multimodality treatment approaches is usually suggested. In the following article, we summarize the reported data concentrated on the conversion treatment of HCC patients with vascular invasion according to different multimodality combinations.
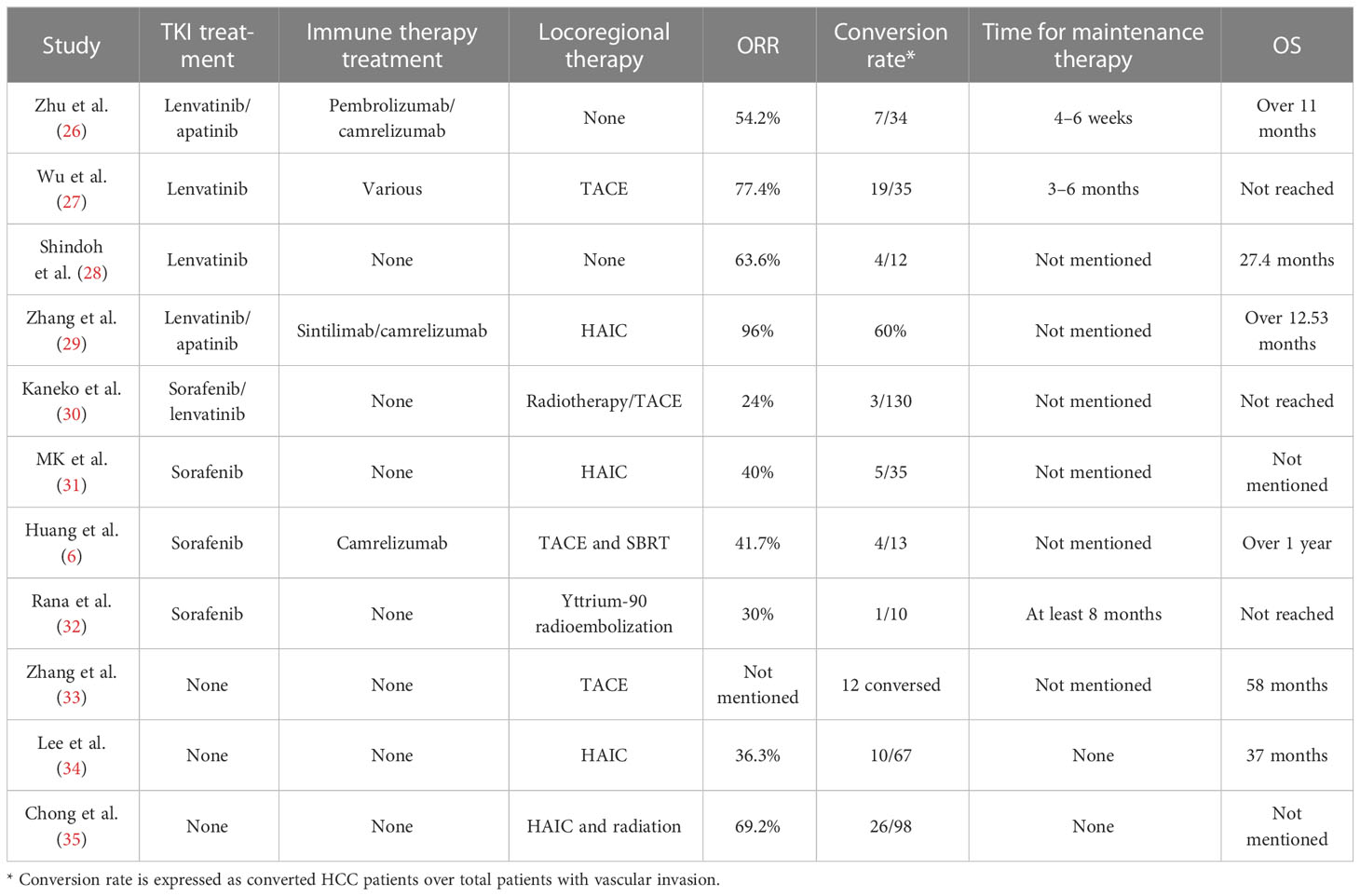
Sorafenib is the first TKI inhibitor that has been demonstrated to have a survival benefit in advanced HCC (25). However, the prognosis of HCC after sorafenib has been associated with a low objective response rate (ORR) of 3.3% (25). At the same time, locoregional treatments for HCC patients with vascular invasion, such as TACE or HAIC, have shown limited efficacy (Table 1). Although some of the reports showed that conversion could be performed in such patients, the conversion rate was lower than 15%, which is not satisfactory (34). Therefore, for a long time, neoadjuvant and downstaging therapy could not be suggested as a standard protocol for advanced HCC. In 2018, the phase 3 REFLECT trial showed that lenvatinib was associated with an amazing ORR of 40.6% in unresectable HCC patients (36). Although lenvatinib did not provide better OS than sorafenib, it brings us a new hope of conversion therapy. In the following years, some new TKI treatments, such as donafenib and regorafenib, have proven their value in advanced HCC, although no clinical studies have been reported. In a parallel-controlled phase II–III trial, donafenib showed a better ORR than sorafenib (4.6% versus 2.7%, respectively) (37). In the donafenib group, 0.3% of patients had a complete response, and 5.8% of patients had a partial response, whereas no patients had a complete response and only 3% of patients had a partial response. Regorafenib is an oral TKI shown to provide a survival benefit in HCC patients progressing on sorafenib. The ORR was 11% in patients progressing on sorafenib. One percent of patients had a complete response (38). With amazing efficacy compared with sorafenib, new TKI drugs provide new insight into conversion treatment.
Immune checkpoint inhibitors (ICIs) are now being introduced into HCC treatment. Although it has not been placed in the first-line treatment, positive outcomes have been achieved in the combination of TKI treatment and ICIs in conversion treatment when compared with sorafenib treatment (26). In one phase Ib single-arm study of 100 unresectable HCC patients, lenvatinib plus pembrolizumab yielded a confirmed response rate of 46% according to mRECIST (20). Among these patients, 20% had macroscopic vascular invasion. Although no further report on the conversion rate in patients with vascular invasion was published, the high ORR rate still provides insight into conversion for HCC patients with vascular invasion (7). Recently, a phase III study of lenvatinib plus pembrolizumab versus lenvatinib for advanced HCC patients was reported (39). The OS and progression-free survival (PFS) were not significantly different between these two groups. However, in the subgroup analysis of OS, the HR in the macrovascular invasion/extrahepatic spread or HBV etiology group indicated that advanced HCC patients could benefit from lenvatinib plus pembrolizumab (39). According to Zhu’s study, lenvatinib plus a PD-1 inhibitor showed an ORR of 54.3%, and 20% of HCC patients with vascular invasion could undergo surgery (30). Except for the combination of TKI treatment and PD-1 inhibitors, VEGFR inhibitors (bevacizumab) combined with PD-L1 inhibitors (atezolizumab) showed a better prognosis than sorafenib in unresectable patients (32). Although no reported data on bevacizumab combined with atezolizumab have been reported in HCC patients with vascular invasion, the promising ORR of 26% in unresectable HCC shows good insight into its usage in conversion therapy.
Various combinations of treatments have been used in unresectable HCC. Locoregional therapy included HAIC, TACE (40), transarterial radiotherapy (TARE) (29), and radiotherapy. TACE and HAIC are two different treatments that take advantage of the relatively selective arterial vascularization of hepatic tumors (41, 42). More than one guideline has suggested that TACE/HAIC could be applied in patients with vascular invasion (5, 43). However, because of different characteristics between HVTT and PVTT, the prognosis for these patients after TACE and HAIC might be different. To date, most reports based on HAIC and TACE have mainly focused on PVTT (33, 44–46). In HCC patients with PVTT, HAIC could provide a high response rate between 36% and 50% (45, 47). At the same time, TACE could also provide a response rate between 13% and 33% (33, 35, 48). The ORR of TACE and HAIC applied to HCC patients with PVTT is acceptable, so several studies have been proposed to check whether TACE or HAIC could be applied in conversion therapy alone (Table 1). However, the conversion rate reported was 14% to 26%, which was significantly lower than that of combined treatment (34, 49).
As TKI inhibitors or VEGFR inhibitors could inhibit the vascular endothelial growth factor receptor, whose level could be elevated after TACE or HAIC, systemic treatment is believed to enhance the efficacy of TACE or HAIC in HCC patients with PVTT (27, 31). When combined with systemic treatment, the ORR of HAIC or TACE could be elevated to 40%–77.4% (28, 48, 50). The successful conversion rate for unresectable HCC patients with vascular invasion could be increased to 14%–33% (50, 51) (Table 1). ICIs are a new kind of therapy that could enhance the efficacy of TKI inhibitors. The combination of TKI inhibitors, ICIs, and TACE or HAIC has also been introduced into unresectable patients with vascular invasion. The ORR of patients has been increased to 77%–96%, which was amazing (28, 42). The conversion rate from an unresectable state to a resectable state could be increased to 54%–60% (28, 42). As we have discussed in the former part, the study reported conversion therapy in HCC with vascular invasion primarily concentrated in portal vein invasion. To date, no specific study has focused on the efficacy of conversion therapy in unresectable HCC patients with HVTT. Because of the unique characteristics of HVTT, the efficacy of conversion therapy in HCC patients with HVTT might be different, which needs further discussion. According to Table 1, for successfully converted HCC patients, the existing studies usually recommend prolonged systemic therapy for more than 6 months. However, this suggestion was proposed only based on the experience of the surgeon. The time for maintenance therapy still needs further confirmation by well-designed clinical trials.
Hypothesis on how to increase the efficacy of conversion treatment
As we have mentioned above, HCC is usually diagnosed in advanced stages, which has limited treatment options. Before systemic therapy was introduced, conversion therapy was restricted to TACE or HAIC combined with or without sorafenib. Because of the low ORR of these treatments, the conversion rate for HCC with advanced stage is not satisfactory (Table 1). Therefore, seeking new combination treatment strategies to increase the conversion rate in advanced-stage HCC is a major challenge. Current clinical trials emphasize the breakthrough of lenvatinib combined with pembrolizumab in unresectable HCC (20). Despite the amazing ORR, some patients still have no response. To further increase the ORR of HCC patients in advanced stages, the mechanism needs to be clarified for optimal patient selection or the other adjuvant therapies need to be combined with TKI treatments and ICIs to enhance the treatment efficacy.
Various immunotherapeutic clinical trials have been conducted for HCC, but there is still limited evidence of HCC biomarkers to select HCC patients. In general, PD-1/PD-L1 expression, microsatellite instability, tumor mutation burden, and immunosuppressive cells, including tumor-associated macrophages, marrow-derived suppressor cells, and regulatory T cells (Tregs), are used as biomarkers associated with immunotherapy effects in various cancer types (52, 53). In Yi’s report (54), lenvatinib reduced tumor lesion PD-L1 levels and Treg differentiation to improve anti-PD-1 efficacy by blocking FGFR4. Levels of FGFR4 expression and Treg infiltration in HCC tissues could function as biomarkers to screen sensitive patients with lenvatinib plus anti-PD-1 combination therapy. Chronic liver injury exists in most HCC patients and can lead to liver inflammation, fibrosis, and eventually cirrhosis. Liver cirrhosis is also a state of immune dysfunction caused by excessive activation of proinflammatory cytokines (55). In patients with cirrhosis, an abnormal gastrointestinal barrier and bacterial translocation could increase the risk of infection and sepsis in the abdomen and carcinogenesis (56). Shi’s study showed that commensal probiotics combined with IL-2-based immunotherapy could enhance the antitumor immune response and tumor clearance (56). Microsatellite instability and tumor mutation burden in HCC are rare in HCC and have not yet been applied in the clinic. In recent years, genomics and bioinformatics have enabled the identification of target populations of HCC patients. Several signatures have been proposed to predict the prognosis of HCC patients based on lncRNAs, miRNAs, and mRNAs (57–59). According to Dai’s report, an immune-related gene-based prognostic index containing 11 differentially expressed immune-related genes was constructed by using a LASSO regression model to predict the infiltration of immune cells in the tumor microenvironment of HCC and predict the response to immune therapy at the same time (59). However, the application of these results in the clinic is still limited.
Portal hypertension is usually detected in HCC patients in Asia due to chronic viral hepatitis and cirrhosis (60). It often presents with gastroesophageal varices and hepatic encephalopathy, hypersplenism, thrombocytopenia, and leukopenia (Figure 2). With the complication mentioned above, many treatments could be limited in such patients. In HCC patients with vascular tumor thrombus, there is usually a higher chance of worse hepatic function and complications related to portal hypertension, which eventually affect the usage of treatment (61). For example, atezolizumab plus bevacizumab showed a better prognosis than sorafenib in unresectable patients (32). This highlights the usage of this kind of combination in conversion therapy. However, bevacizumab, a kind of VEGFR inhibitor, has a high risk of gastrointestinal bleeding (62). Therefore, key exclusion criteria for this treatment included untreated or incompletely treated esophageal or gastric varices with bleeding or a high risk of bleeding (62). In addition to bleeding, which could restrict the use of possible conversion therapy in HCC patients with vascular invasion, thrombocytopenia is another restriction for conversion therapy in HCC patients with vascular invasion. Thrombocytopenia is a common complication in HCC patients with portal hypertension and is caused by decreased levels of thrombopoietin and increased levels of platelet destruction by hypersplenism (63, 64). In HCC patients with vascular invasion, the incidence of thrombocytopenia might be increased further (65). For the most commonly used TKI inhibitors, including sorafenib or lenvatinib, a platelet count decrease is detected in nearly 20% of HCC patients (66, 67). Once a platelet count decrease is detected in HCC patients with thrombocytopenia, a dose reduction or withdrawal would be needed, which would then decrease the efficacy of conversion therapy. Several reports have reported that hypersplenism could downregulate the immune function of the spleen in the development of HCC (68). Hypersplenism could lead to an abnormal T-cell receptor CD3 complex and suppress the immune function of T cells (69). Except for the T-cell receptor CD3 complex, splenic CD11b+Gr-intLy6chi cells expand in the marginal zone of the spleen, which is associated with memory CD8+ T cells, cross-presenting tumor antigens and eventually leading to immune suppression (70). Although there is still no report of the prognosis between hypersplenism and the efficacy of immune therapy, leukopenia in HCC patients with vascular invasion and hypersplenism might restrict the efficacy of immunotherapy or conversion therapy.
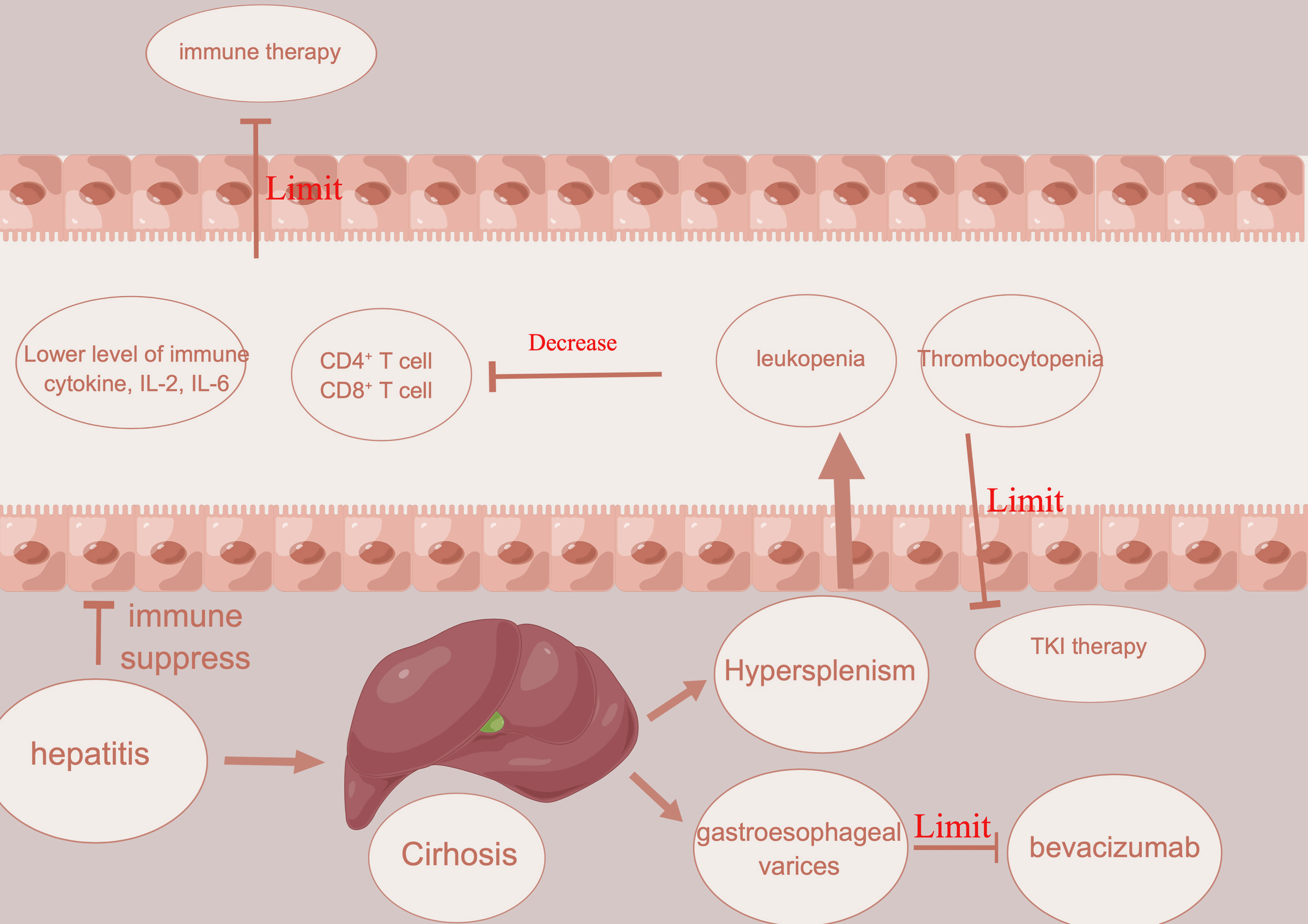
Figure 2 Illustration about how hepatitis, cirrhosis, and portal hypertension limit the usage of immune therapy and angiogenesis inhibitors.
Splenectomy has been performed in cirrhotic patients since 1950 and can address portal hypertension, thrombocytopenia, and leukopenia (71). Several studies have suggested that splenectomy could improve the prognosis of HCC in patients (70, 72). First, splenectomy could reduce portal vein pressure and increase the level of white blood cells and platelet counts, which could lower the risk of restriction of conversion therapy (73). Second, splenectomy could promote recovery of the balance between T lymphocyte subsets and improve antitumor immunology (74). After splenectomy, natural killer cells and CD4+ and CD8+ cells are increased, which could increase the immune response against HCC (75). To increase the efficacy of conversion therapy, splenectomy might be a good choice. Except for splenectomy, partial splenic embolization (PSE) can function similarly to splenectomy. After PSE, white blood cells (neutrophils, lymphocytes, and monocytes) could also increase. Th1 and Th2 cells could also be increased compared with those before PSE, which means that PSE could not only promote the recovery of leukopenia and thrombocytopenia but also induce activation of host immunity (76).
Conclusion
Compared with other treatments, surgery is still the only curative treatment (13). However, only a minority of patients with HCC receive curative treatment. Conversion therapy aimed at tumor downregulation could improve the prognosis in HCC patients with vascular invasion (77). In the era of immune therapy combined with targeted therapy, the ORR is elevated in HCC patients with vascular invasion. Despite this, an increasing number of reports have suggested that locoregional treatment, including TACE or radiotherapy combined with systemic therapy, could increase the conversion rate for advanced HCC patients. Because of the specialty of HCC patients with vascular invasion, patients with PVTT or HVTT should be considered under different situations. Since reports on conversion therapy in HCC patients with vascular invasion are limited, the conversion rate is still unknown. Hypersplenism, as a common state in HCC patients with vascular invasion, is a type of restriction in conversion therapy. To improve the conversion rate in HCC patients with vascular invasion, clarifying the mechanism for optimal patient selection and adjuvant splenectomy might be helpful. Currently, the outcomes for patients who have chosen conversion therapy are promising, and the data from well-designed large randomized controlled trials are still limited and require further investigation.
Author contributions
ZZ performed the majority of the writing and prepared the table. EZ designed the outline of this paper. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Project of the Natural Science Foundation of China, No. 81802767.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet Lond Engl (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
2. Shi J, Lai ECH, Li N, Guo W-X, Xue J, Lau WY, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol (2010) 17:2073–80. doi: 10.1245/s10434-010-0940-4
3. Chen X-P, Qiu F-Z, Wu Z-D, Zhang Z-W, Huang Z-Y, Chen Y-F, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol (2006) 13:940–6. doi: 10.1245/ASO.2006.08.007
4. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis (1999) 19:329–38. doi: 10.1055/s-2007-1007122
5. Xie D-Y, Ren Z-G, Zhou J, Fan J, Gao Q. 2019 Chinese Clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr (2020) 9:452–63. doi: 10.21037/hbsn-20-480
6. Huang Y, Zhang Z, Liao W, Hu K, Wang Z. Combination of sorafenib, camrelizumab, transcatheter arterial chemoembolization, and stereotactic body radiation therapy as a novel downstaging strategy in advanced hepatocellular carcinoma with portal vein tumor thrombus: a case series study. Front Oncol (2021) 11:650394. doi: 10.3389/fonc.2021.650394
7. Sun H-C, Zhu X-D. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front Oncol (2021) 11:772195. doi: 10.3389/fonc.2021.772195
8. Sun H-C, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese Expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr (2022) 11:227–52. doi: 10.21037/hbsn-21-328
9. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: a Japanese nationwide survey. Hepatol Baltim Md (2017) 66:510–7. doi: 10.1002/hep.29225
10. Zhang Z-Y, Zhang E-L, Zhang B-X, Chen X-P, Zhang W. Treatment for hepatocellular carcinoma with tumor thrombosis in the hepatic vein or inferior vena cava: a comprehensive review. World J Gastrointest Surg (2021) 13:796–805. doi: 10.4240/wjgs.v13.i8.796
11. Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the national cancer institute clinical trials planning meeting. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28:3994–4005. doi: 10.1200/JCO.2010.28.7805
12. Kokudo T, Hasegawa K, Yamamoto S, Shindoh J, Takemura N, Aoki T, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol (2014) 61:583–8. doi: 10.1016/j.jhep.2014.04.032
13. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol (2016) 65:938–43. doi: 10.1016/j.jhep.2016.05.044
14. Chen Z-H, Wang K, Zhang X-P, Feng J-K, Chai Z-T, Guo W-X, et al. A new classification for hepatocellular carcinoma with hepatic vein tumor thrombus. Hepatobiliary Surg Nutr (2020) 9:717–28. doi: 10.21037/hbsn.2019.10.07
15. Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology (2007) 54:499–502.
16. Liu J, Wang Y, Zhang D, Liu B, Ou Q. Comparison of survival and quality of life of hepatectomy and thrombectomy using total hepatic vascular exclusion and chemotherapy alone in patients with hepatocellular carcinoma and tumor thrombi in the inferior vena cava and hepatic vein. Eur J Gastroenterol Hepatol (2012) 24:186–94. doi: 10.1097/MEG.0b013e32834dda64
17. Wang Y, Chen H, Wu M, Sun Y, Lin C, Jiang X, et al. [Surgical treatment of hepatocellular carcinoma with tumor thrombus in the inferior vena cava]. Zhonghua Wai Ke Za Zhi (2003) 41:165–8.
18. Zhang Y-F, Wei W, Guo Z-X, Wang J-H, Shi M, Guo R-P. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with hepatic vein tumor thrombus. Jpn J Clin Oncol (2015) 45:837–43. doi: 10.1093/jjco/hyv089
19. Zhang Z-Y, Dong K-S, Zhang E-L, Zhang L-W, Chen X-P, Dong H-H. Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis: a systematic review and meta-analysis. Med (Baltimore) (2019) 98:e18362. doi: 10.1097/MD.0000000000018362
20. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
21. He M-K, Liang R-B, Zhao Y, Xu Y-J, Chen H-W, Zhou Y-M, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
22. Chiang C-L, Chan ACY, Chiu KWH, Kong F-MS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: a potential synergistic treatment strategy. Front Oncol (2019) 9:1157. doi: 10.3389/fonc.2019.01157
23. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int (2021) 15:663–75. doi: 10.1007/s12072-021-10184-9
24. Khan AR, Wei X, Xu X. Portal vein tumor thrombosis and hepatocellular carcinoma - the changing tides. J Hepatocell Carcinoma (2021) 8:1089–115. doi: 10.2147/JHC.S318070
25. Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
26. Zhao Y, Zhang Y-N, Wang K-T, Chen L. Lenvatinib for hepatocellular carcinoma: from preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer (2020) 1874:188391. doi: 10.1016/j.bbcan.2020.188391
27. Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-a specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res (2008) 103:1027–36. doi: 10.1161/CIRCRESAHA.108.181115
28. Wu J-Y, Yin Z-Y, Bai Y-N, Chen Y-F, Zhou S-Q, Wang S-J, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma (2021) 8:1233–40. doi: 10.2147/JHC.S332420
29. Rana N, Ju AW, Bazylewicz M, Kallakury B, He AR, Unger KR, et al. Yttrium-90 radioembolization in patients with hepatocellular carcinoma who have previously received sorafenib. Front Oncol (2013) 3:323. doi: 10.3389/fonc.2013.00323
30. Zhu X-D, Huang C, Shen Y-H, Ji Y, Ge N-L, Qu X-D, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer (2021) 10:320–9. doi: 10.1159/000514313
31. Tekkesin N, Taga Y, Sav A, Almaata I, Ibrisim D. Induction of HGF and VEGF in hepatic regeneration after hepatotoxin-induced cirrhosis in mice. Hepatogastroenterology (2011) 58:971–9.
32. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
33. Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib–a retrospective controlled study. Radiology (2014) 272:284–93. doi: 10.1148/radiol.14131946
34. Lee B-H, Lee D-S, Cho CW, Yun S-S. Role and limitation of neoadjuvant hepatic arterial infusion chemotherapy in advanced hepatocelluar carcinoma patients with child-pugh class a. World J Surg Oncol (2019) 17:143. doi: 10.1186/s12957-019-1685-6
35. Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? ten years of experience. Oncologist (2016) 21:1442–9. doi: 10.1634/theoncologist.2016-0094
36. Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet Lond Engl (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
37. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39:3002–11. doi: 10.1200/JCO.21.00163
38. Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl (2017) 389:56–66. doi: 10.1016/S0140-6736(16)32453-9
39. Richard SF. Primary results from the phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). LBA34 (2022).
40. Kaneko S, Tsuchiya K, Yasui Y, Tanaka Y, Inada K, Ishido S, et al. Conversion surgery for hepatocellular carcinoma after tyrosine kinase inhibitor treatment. JGH Open Open Access J Gastroenterol Hepatol (2022) 6:301–8. doi: 10.1002/jgh3.12735
41. Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg (1997) 173:358–65. doi: 10.1016/S0002-9610(96)00384-4
42. Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy: a retrospective study. Front Oncol (2021) 11:729764. doi: 10.3389/fonc.2021.729764
43. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer (2014) 3:458–68. doi: 10.1159/000343875
44. Liu M, Shi J, Mou T, Wang Y, Wu Z, Shen A. Systematic review of hepatic arterial infusion chemotherapy versus sorafenib in patients with hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol Hepatol (2020) 35:1277–87. doi: 10.1111/jgh.15010
45. Ahn YE, Suh SJ, Yim HJ, Seo YS, Yoon EL, Kim TH, et al. Comparison of sorafenib versus hepatic arterial infusion chemotherapy-based treatment for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Gut Liver (2020) 15(2):284–94. doi: 10.5009/gnl19367
46. Sung PS, Yang K, Bae SH, Oh JS, Chun HJ, Nam HC, et al. Reduction of intrahepatic tumour by hepatic arterial infusion chemotherapy prolongs survival in hepatocellular carcinoma. Anticancer Res (2019) 39:3909–16. doi: 10.21873/anticanres.13542
47. Nouso K, Miyahara K, Uchida D, Kuwaki K, Izumi N, Omata M, et al. Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the nationwide survey of primary liver cancer in Japan. Br J Cancer (2013) 109:1904–7. doi: 10.1038/bjc.2013.542
48. Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol (2021) 11:821599. doi: 10.3389/fonc.2021.821599
49. Chong JU, Choi GH, Han DH, Kim KS, Seong J, Han K-H, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol (2018) 25:3308–15. doi: 10.1245/s10434-018-6653-9
50. He M-K, Zou R-H, Li Q-J, Zhou Z-G, Shen J-X, Zhang Y-F, et al. Phase II study of sorafenib combined with concurrent hepatic arterial infusion of oxaliplatin, 5-fluorouracil and leucovorin for unresectable hepatocellular carcinoma with major portal vein thrombosis. Cardiovasc Intervent Radiol (2018) 41:734–43. doi: 10.1007/s00270-017-1874-z
51. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol (2021) 28:7663–72. doi: 10.1245/s10434-021-09974-0
52. Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci (2021) 22:5801. doi: 10.3390/ijms22115801
53. Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer (2019) 18:130. doi: 10.1186/s12943-019-1047-6
54. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatol Baltim Md (2021) 74:2544–60. doi: 10.1002/hep.31921
55. Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2011) 9:727–38. doi: 10.1016/j.cgh.2011.02.031
56. Shi L, Sheng J, Chen G, Zhu P, Shi C, Li B, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer (2020) 8:e000973. doi: 10.1136/jitc-2020-000973
57. Zhao Q-J, Zhang J, Xu L, Liu F-F. Identification of a five-long non-coding RNA signature to improve the prognosis prediction for patients with hepatocellular carcinoma. World J Gastroenterol (2018) 24:3426–39. doi: 10.3748/wjg.v24.i30.3426
58. Bing Z, Tian J, Zhang J, Li X, Wang X, Yang K. An integrative model of miRNA and mRNA expression signature for patients of breast invasive carcinoma with radiotherapy prognosis. Cancer Biother Radiopharm (2016) 31:253–60. doi: 10.1089/cbr.2016.2059
59. Dai Y, Qiang W, Lin K, Gui Y, Lan X, Wang D. An immune-related gene signature for predicting survival and immunotherapy efficacy in hepatocellular carcinoma. Cancer Immunol Immunother CII (2021) 70:967–79. doi: 10.1007/s00262-020-02743-0
60. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
61. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol (2016) 22:7289. doi: 10.3748/wjg.v22.i32.7289
62. Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
63. Ishikawa T, Ichida T, Matsuda Y, Sugitani S, Sugiyama M, Kato T, et al. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol (1998) 13:907–13. doi: 10.1111/j.1440-1746.1998.tb00760.x
64. Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int Off J Int Assoc Study Liver (2017) 37:778–93. doi: 10.1111/liv.13317
65. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet Lond Engl (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
66. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs (2019) 79:665–74. doi: 10.1007/s40265-019-01116-x
67. Endo M, Honda K, Saito T, Shiraiwa K, Sueshige Y, Tokumaru T, et al. Maximum plasma concentration of lenvatinib is useful for predicting thrombocytopenia in patients treated for hepatocellular carcinoma. World J Oncol (2021) 12:165–72. doi: 10.14740/wjon1399
68. Jasnis MA, Elján AM, Oisgold-Dagá S. Regulation of tumor growth by soluble spleen factors: effect of tumor resection. J Surg Oncol (1987) 35:139–45. doi: 10.1002/jso.2930350216
69. Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med (1995) 181:1881–6. doi: 10.1084/jem.181.5.1881
70. Zhang X, Li C, Wen T, Peng W, Yan L, Li B, et al. Synchronous splenectomy and hepatectomy for patients with small hepatocellular carcinoma and pathological spleen: neutrophil to lymphocyte ratio changes can predict the prognosis. Oncotarget (2017) 8:46298–311. doi: 10.18632/oncotarget.17758
72. Takeishi K, Kawanaka H, Itoh S, Harimoto N, Ikegami T, Yoshizumi T, et al. Impact of splenic volume and splenectomy on prognosis of hepatocellular carcinoma within Milan criteria after curative hepatectomy. World J Surg (2018) 42:1120–8. doi: 10.1007/s00268-017-4232-z
73. Amin MA, el-Gendy MM, Dawoud IE, Shoma A, Negm AM, Amer TA. Partial splenic embolization versus splenectomy for the management of hypersplenism in cirrhotic patients. World J Surg (2009) 33:1702–10. doi: 10.1007/s00268-009-0095-2
74. Cao Z-X, Chen X-P, Wu Z-D. Changes of immune function in patients with liver cirrhosis after splenectomy combined with resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int HBPD Int (2003) 2:562–5.
75. Shimada M, Hashizume M, Shirabe K, Takenaka K, Sugimachi K. A new surgical strategy for cirrhotic patients with hepatocellular carcinoma and hypersplenism. performing a hepatectomy after a laparoscopic splenectomy. Surg Endosc (2000) 14:127–30. doi: 10.1007/s004649900082
76. Matsukiyo Y, Nagai H, Matsui T, Igarashi Y. Host immunological effects of partial splenic embolization in patients with liver cirrhosis. J Immunol Res (2018) 2018:1746391. doi: 10.1155/2018/1746391
Keywords: conversion therapy, HCC, advanced stage, vascular invasion, downstage
Citation: Zhang Z and Zhang E (2023) Conversion therapy for advanced hepatocellular carcinoma with vascular invasion: a comprehensive review. Front. Immunol. 14:1073531. doi: 10.3389/fimmu.2023.1073531
Received: 18 October 2022; Accepted: 12 April 2023;
Published: 26 April 2023.
Edited by:
Songqing He, Guangxi Medical University, ChinaReviewed by:
Bálint Tamaskovics, Heinrich Heine University of Düsseldorf, GermanyXiude Fan, Shandong Provincial Hospital, China
Copyright © 2023 Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erlei Zhang, YmFpeXUxOTg2MTEwNEAxNjMuY29t
 Zunyi Zhang
Zunyi Zhang Erlei Zhang
Erlei Zhang