- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Republic of Korea
- 2Office of Biostatistics, Ajou Research Institute for Innovative Medicine, Ajou University Medical Center, Suwon, Republic of Korea
- 3Department of Gastroenterology, Ajou University School of Medicine, Suwon, Republic of Korea
- 4Division of Rheumatology, Department of Internal Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Republic of Korea
Background & aims: The faecal immunochemical test (FIT), a non-invasive test for screening colorectal cancer (CRC), is being increasingly understood to reflect heightened inflammation. We aimed to investigate the association between abnormal FIT results and onset of inflammatory bowel disease (IBD), a disease characterized with chronic gut mucosal inflammation.
Methods: Participants in the Korean National Cancer Screening Program for CRC between 2009–2013 were analysed and divided into positive and negative FIT result groups. The incidence rates of IBD after screening were calculated after excluding cases of haemorrhoids, CRC, and IBD at baseline. Cox proportional hazard analyses were used to identify independent risk factors for IBD occurrence during follow-up, and 1:2 propensity score matching was performed as a sensitivity analysis.
Results: In total, 229,594 and 815,361 participants were assigned to the positive and negative FIT result groups, respectively. The age- and sex-adjusted incidence rates of IBD in participants with positive and negative test results were 1.72 and 0.50 per 10,000 person-years, respectively. Adjusted Cox analysis revealed that FIT positivity was associated with a significantly higher risk of IBD (hazard ratio 2.93, 95% confidence interval: 2.46, 3.47, P <.001), which was consistent for both disease subtypes of ulcerative colitis and Crohn’s disease. The results of Kaplan–Meier analysis in the matched population yielded identical findings.
Conclusions: Abnormal FIT results could be a preceding sign of incident IBD in the general population. Those with positive FIT results and suspected IBD symptoms could benefit from regular screening for early disease detection.
Introduction
Inflammatory bowel disease (IBD) is a chronic, potentially life-threatening disorder that affects the digestive system and presents with recurrent episodes of abdominal pain, diarrhoea, haematochezia, fever, and weight loss (1). According to the inflamed regions, their pattern, and pathologic findings in the gastrointestinal tract, IBD is classified into two different diseases: ulcerative colitis (UC) and Crohn’s disease (CD). The underlying pathogenesis of IBD involves a complex interplay of factors, including dysregulation of intestinal microbiota, host genetic susceptibility, and environmental triggers, resulting in an immunological imbalance, which remains largely unknown (2–4). While IBD has been traditionally considered more common in Western countries, a continuous rise in the incidence of IBD has been reported in recent years, especially in Asia (5, 6). Therefore, there is a need for diagnostic tests that are useful for early detection of IBD.
Immunologic perturbation, particularly in the adaptive immune system, is thought to be a crucial element responsible for the impairment of bowel equilibrium and causing chronic gut inflammation in IBD (7–9). Consequently, mucosal inflammation documented by endoscopy or imaging of the gastrointestinal tract is a typical finding in patients with IBD (10). The faecal immunochemical test (FIT) is a non-invasive test that measures faecal haemoglobin concentrations using an antibody specific for human haemoglobin, and has been widely used for colorectal cancer (CRC) screening by detecting blood in the faeces (11, 12); however, since a characteristic feature observed in IBD is the presence of mucosal injury, it is possible that a positive FIT may be an early sign of IBD (13). Additionally, growing evidence suggests that abnormal FIT results, without a definite focus on bleeding, may reflect underlying systemic inflammation and could be correlated with chronic inflammatory diseases (14). However, the association between positive FIT results and incident IBD has not yet been determined in the general population. Because of this, we aimed to evaluate whether abnormalities of the gut mucosa, defined as a positive FIT result, are associated with the development of IBD, and to evaluate the risk factors in those who participated in the national program for CRC screening.
Materials and methods
Data source
The Korean National Cancer Screening Program (KNCSP) is a national program operated by the South Korean government and is designed to screen for cancers in the stomach, liver, colorectum, breast, and cervix according to specific recommendations (15). The results of the KNCSP are stored in the National Health Insurance Sharing Service-National Health Information Database (NHIS-NHID); the use of this data is approved for authorised researchers. In South Korea, the NHIS provides medical services covered by the national health insurance for >50 million individuals (approximately 97% of the entire population) (16).
In the KNCSP for screening colorectal cancer (CRC), the government provides an annual faecal immunochemical test (FIT) for individuals aged ≥50 years to screen for CRC. In addition, for those with positive FIT results, the NHIS provides subsequent examinations by either double-contrast barium enema or colonoscopy, based on the preference of the individual. In this study, data of the population who participated in the KNCSP for CRC between 2009–2013, including data from the NHIS, were utilised. and participants were followed up until December 21, 2019. Details of the study design, participants, and data acquisition have been described previously (17). This study was approved by the institutional review board of Ajou University Hospital (approval No. AJIRB-MED-EXP-20-479). The requirement for obtaining individual informed consent was waived because the entire dataset was anonymised.
Study design and selection of eligible participants
In total, 9,161,668 subjects participated in the KNCSP for CRC between the year of 2009–2013. Among them, those who did not undergo a FIT, had a history of colorectal cancer, and had immune-mediated inflammatory diseases (IBD, rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus) that could influence FIT results were excluded. Of the 8.646,887 participants, FIT positive (FIT [+]) and FIT-negative (FIT [−]) groups were separated by applying a 1:1 matched random sampling according to age and sex.
In the FIT (+) group, participants who had undergone colonoscopy as a subsequent evaluation were selected, and colonoscopy findings were categorised according to reports submitted to the KNCSP. Colonoscopy-positive findings were defined as documented gross abnormal mucosal lesions of suspected colon cancer, colon cancer, polyps, diverticulosis, and inflammatory lesions. We excluded subjects diagnosed with haemorrhoids, IBD, or CRC according to the colonoscopy results. In addition, those who were diagnosed with IBD and CRC within 6 and 12 months after undergoing a FIT, based on the tenth revision codes of the International Statistical Classification of Diseases (ICD-10 codes), were excluded, as they could be a missed IBD and CRC (18). In the FIT (−) group, those diagnosed with IBD within 6 months and CRC within 12 months after screening were also excluded (Figure 1).
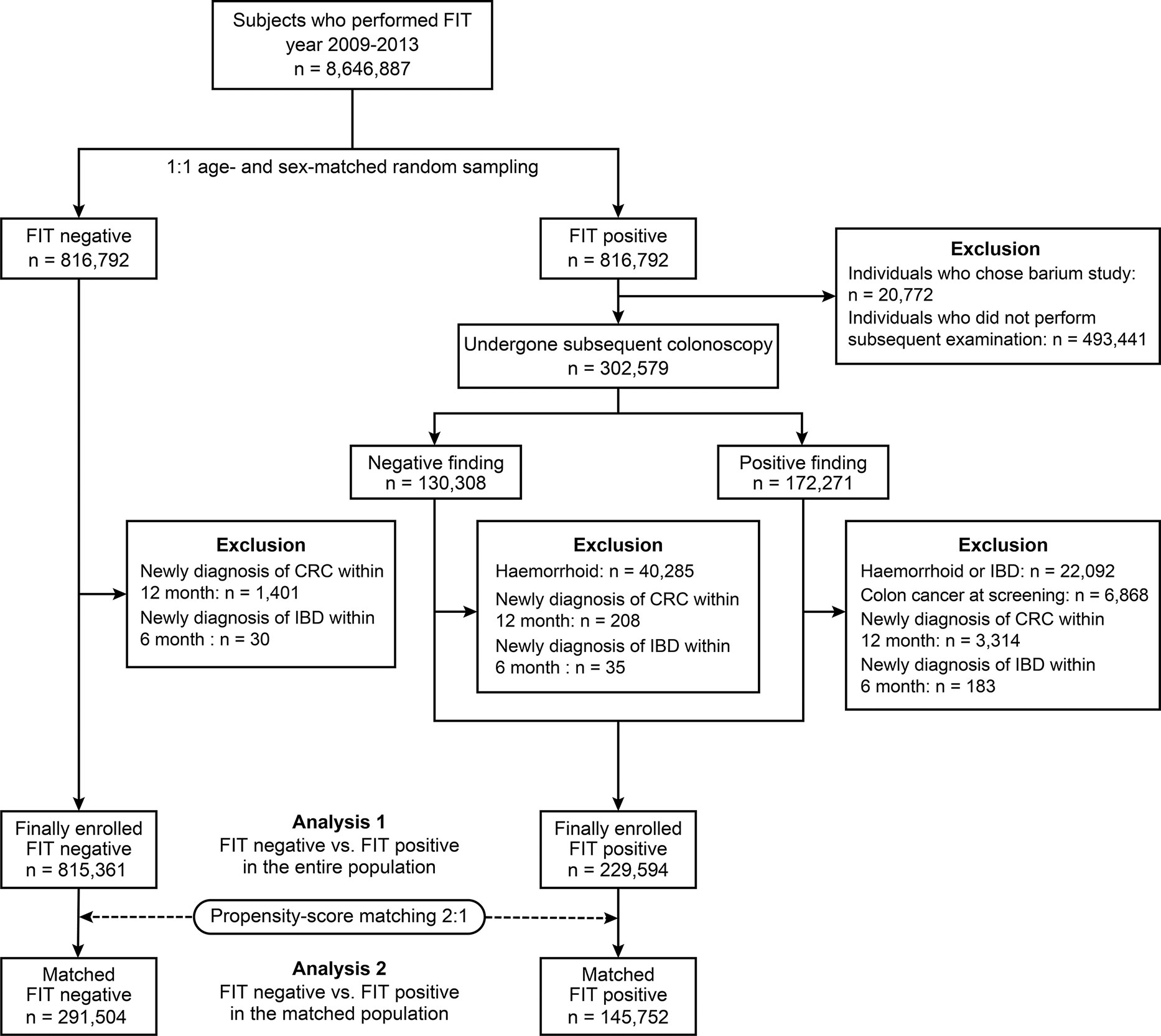
Figure 1 A Flow Diagram of Selecting the FIT (+) and FIT (-) Group. FIT, faecal immunochemical test; CRC, colorectal cancer; IBD, inflammatory bowel disease.
Faecal immunochemical tests of the participants
Faecal samples from subjects participating in the KNCSP for CRC were collected according to the general instructions provided, and were sent to an assigned centre for analyses, which were reported as positive or negative. Faecal immunochemical test assessment was conducted using qualitative and quantitative methods. For the qualitative method, a commercially available kit was used according to the cut-off values provided in the kit as follows: FOBtest, Humasis Co., Korea (50 ng/mL [10 ug/g]), SD Bioline FOB, SD Co., Korea (30 ng/mL [6 ug/g]), ASAN Easy Test FOB, Asan Pharm Co., Korea (50 ng/mL [10 ug/g]), and OC-Hemocatch Lignt™, Eiken Chemical Co., Japan (50 ng/mL [10 ug/g]). The faecal haemoglobin value was determined by latex agglutination nephelometric immunoassay in a quantitative assay (Eiken Chemical Co.), in which the cut-off value of the corresponding institution was also reported (19, 20).
Covariates
Those who participated in the CRC screening program completed questionnaires on smoking, alcohol drinking, and physical exercise, and submitted them to the responsible institutions. In addition, data on age, sex, anthropometric measurements including weight, height, and body mass index (BMI), medical and family history, socioeconomic status, and clinical information were collected. The variables used in our analysis were as follows: sex; age; BMI; smoking status (no or yes); alcohol consumption (no or yes); insurance type (medical aid or national health insurance); comorbidities (hypertension, diabetes, or dyslipidaemia); and laboratory results of haemoglobin, total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, aspartate aminotransferase (AST), and alanine aminotransferase (ALT). The normal values of laboratory data were set according to the predefined cut-off values: haemoglobin (male: ≥13 g/dL, female: ≥12 g/dL), total cholesterol (<200 mg/dL), triglyceride (<150 mg/dL), HDL cholesterol (≥60 mg/dL), LDL-cholesterol (<130 mg/dL), AST (≤40 IU/L), and ALT (≤35 IU/L) (21).
Definition of inflammatory bowel disease and CRC
The outcome of interest was the incidence of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). In South Korea, when an individual utilises a medical service covered by national insurance, all medical institutions must provide a record of their healthcare usage, which is collected in the National Health Insurance Sharing Service-National Health Information Database (NHIS-NHID) containing diagnosis codes (22). To search for cases of IBD and CRC, we first identified CRC and IBD using primary and secondary codes of the ICD-10 codes (C18–20 for CRC and K50–51 for IBD). Furthermore, as the Korean government grants special exemption codes for patients with rare and intractable diseases to subsidise their healthcare expenses for those fulfilling the criteria defined by the National Health Insurance, we used a special exemption code (V code) to ensure an accurate diagnosis of IBD. Thus, in this study, IBD cases that were designated with a special exemption code of V130 (CD) and V131 (UC) were selected (23).
Statistical analysis
Continuous and categorical variables were presented as a mean (SD) and a number (frequency), respectively. Differences between groups were evaluated using Student’s t-test and chi-squared test for continuous and categorical variables. The follow-up duration was set as the date of initial screening to the diagnosis date of IBD in those diagnosed as IBD, whereas it was defined as the last follow-up in those who did not develop IBD. Crude and age-and sex-adjusted incidence rates per 10,000 person-years were calculated using the number of incident IBD cases and person-time of those at risk. Furthermore, Cox proportional hazard analyses were used to identify independent risk factors for IBD during follow-up. As a sensitivity analysis, 1:2 propensity score matching was performed in order to adjust for the differences in baseline characteristics. Kaplan–Meier analysis and the log-rank test were used to compare the differences in IBD incidence. Statistical analyses were conducted using SAS statistical software (SAS Institute, Cary, NC, USA), and a two-tailed P <.05 was considered significant.
Results
Comparison of baseline characteristics between the FIT (+) and FIT (−) groups
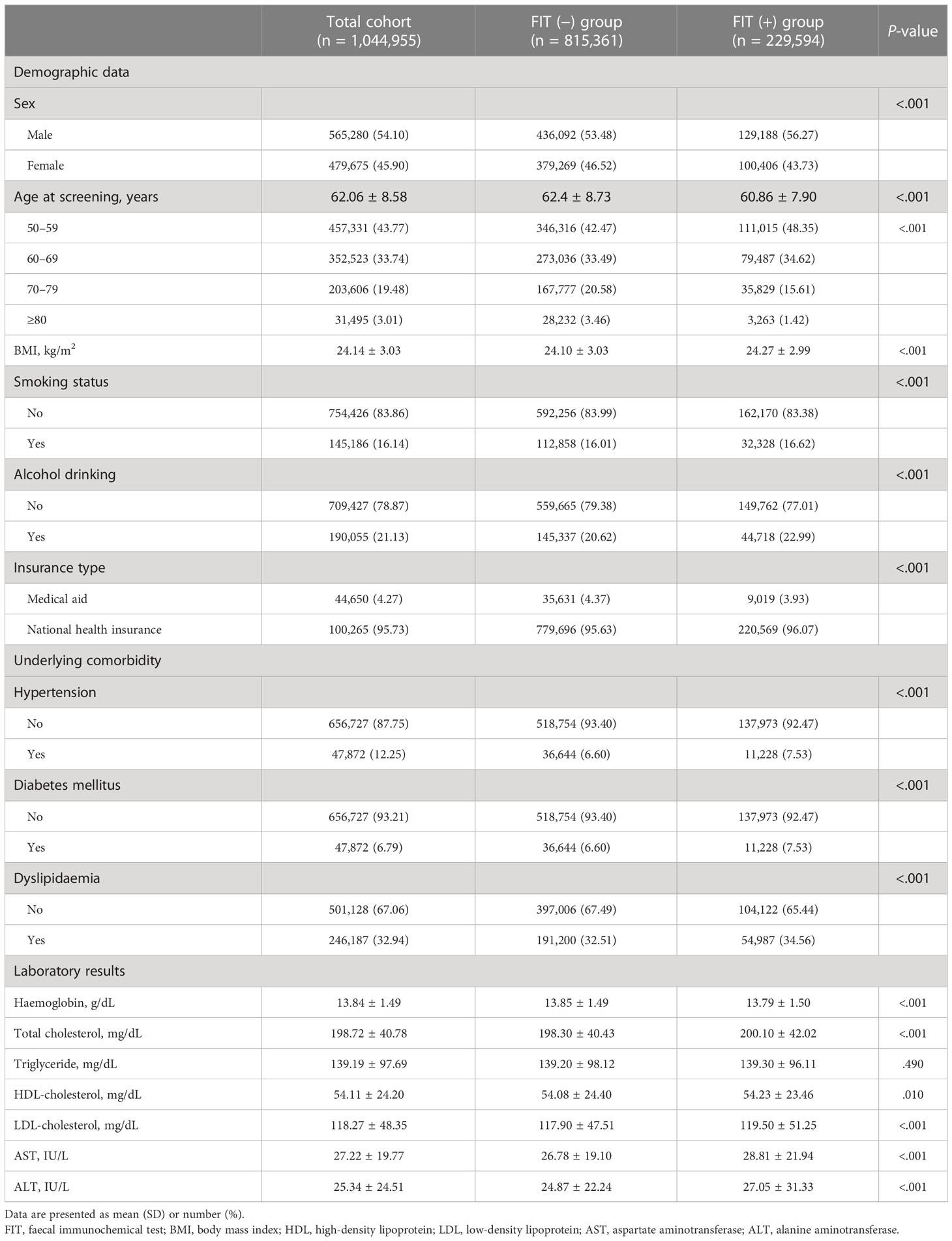
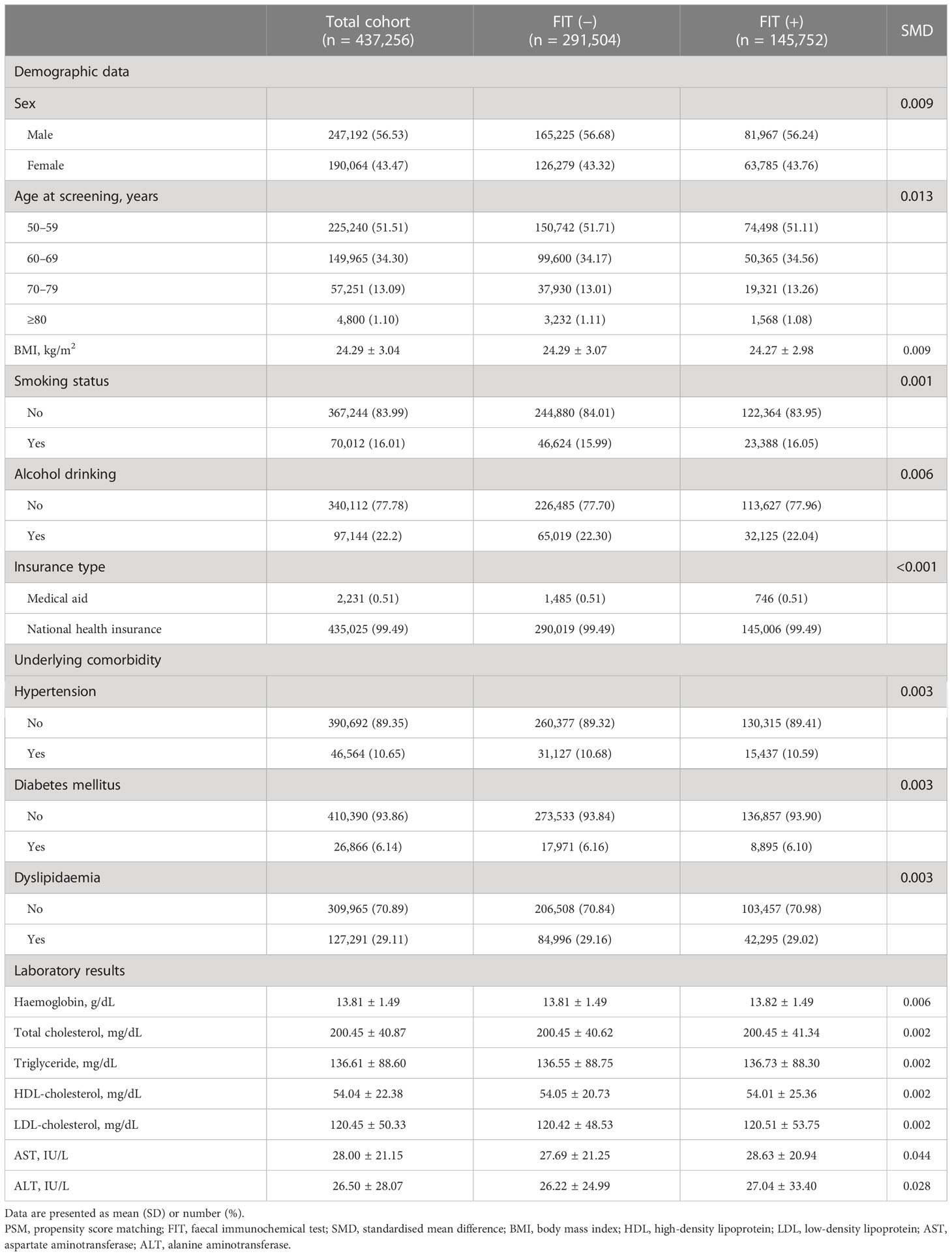
Among the 1,044,955 participants, 815,361 and 229,594 individuals were divided into FIT (−) and FIT (+) groups, respectively (Table 1). Of the total number of participants, 565,280 (54.10%) were male, the overall mean (SD) age was 62.06 (8.58) years, and the proportion of those aged 50–59 years was the highest, accounting for 43.77% of the participants. In addition, the proportions of current smokers and alcohol drinkers in the total population were 16.1% and 21.13%, respectively. There were significant differences between the FIT (−) and FIT (+) groups in the baseline characteristics investigated, including demographic data and laboratory results, with the exception of triglyceride levels (P = .490).
Incidence of IBD during the follow-up period according to FIT positivity
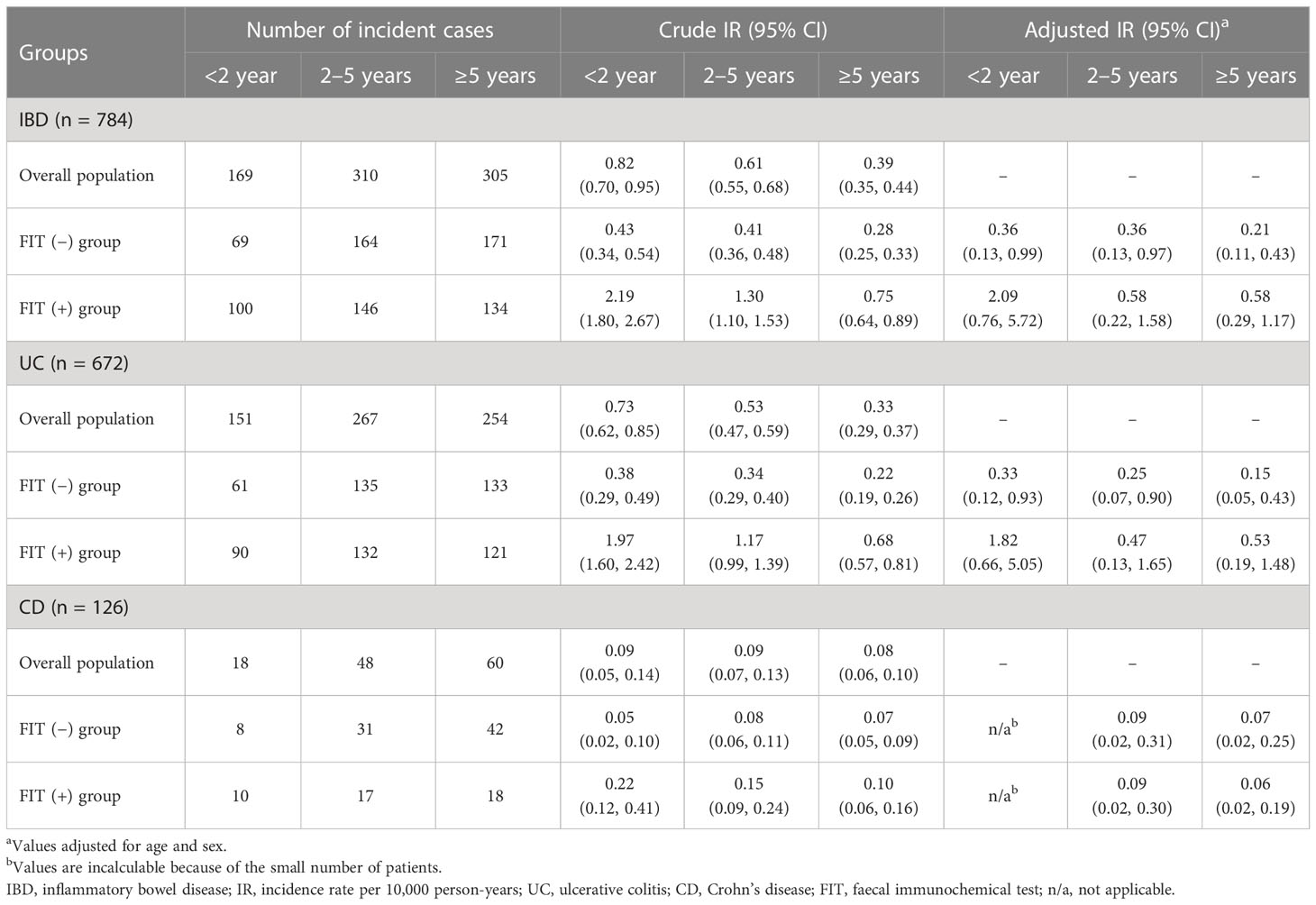
A total of 784 participants (incidence rates [IR] 0.98/10,000 person-years [PY]) were diagnosed with IBD during a mean follow-up period of 7.59 years (SD 1.81). Among those who developed IBDs, 672 (IR 0.85/10,000 PY) and 126 (IR 0.16/10,000 PY) were diagnosed with UC and CD, respectively, and 14 patients were diagnosed with both UC and CD. The incidence of UC and CD was higher in the FIT (+) group than in the FIT (−) group, even after adjusting for age and sex (Figure 2). Moreover, the cumulative incidence of IBD in the FIT (+) group was also significantly higher than that in the FIT (−) group in a Kaplan–Meier analysis among subjects with normal haemoglobin values (all P <.001) (Figure 3).

Figure 2 Comparison of IBD incidence rates between the FIT (−) and FIT (+) groups. *Values adjusted for age and sex. IBD, inflammatory bowel disease; FIT, faecal immunochemical test; UC, ulcerative colitis; CD, Crohn’s disease; IR, incidence rate per 10,000 person-years.
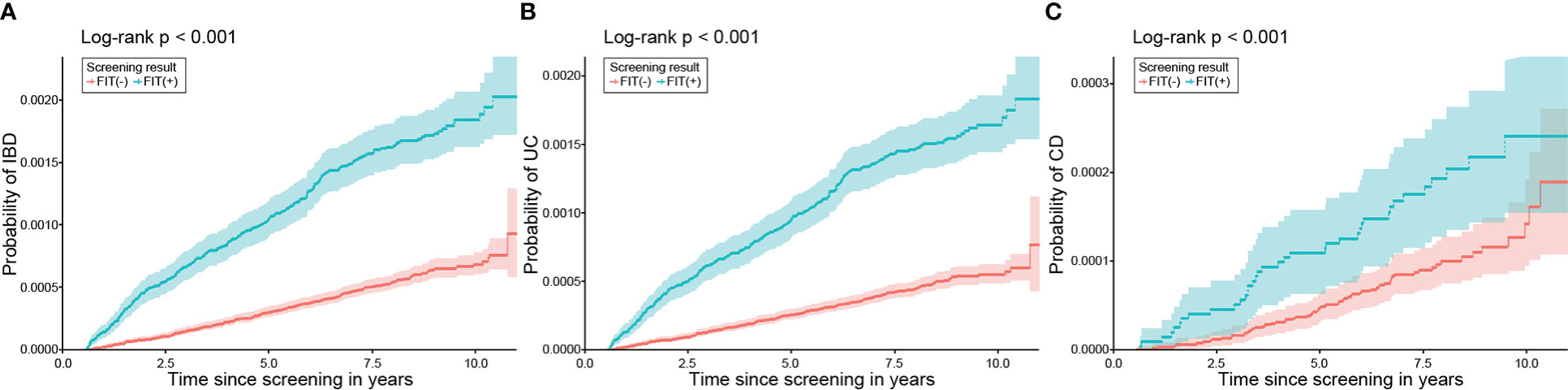
Figure 3 Cumulative incidence of IBD, UC, and CD according to FIT results in those with normal haemoglobin. The incidences of (A) IBD, (B) UC, and (C) CD were significantly higher in the positive FIT group than in the negative FIT group. IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; FIT, faecal immunochemical test.
The occurrence of IBD according to different time intervals, sex, and age
When the incidence of IBD was categorised according to three different time intervals of <2 years, 2–5 year, and ≥5 years, the incidence of IBD was observed to be the highest within the second year of screening (IR 0.82/10,000 PY, 95% CI: 0.70, 0.95), which was not affected by disease subtypes. In addition, a trend of decreasing incidence of IBD after screening was demonstrated for both UC and CD. In particular, those in the FIT (+) group were more frequently diagnosed with IBD, as well as UC, during follow-up in the adjusted analyses, but this was not evident in CD (Table 2).
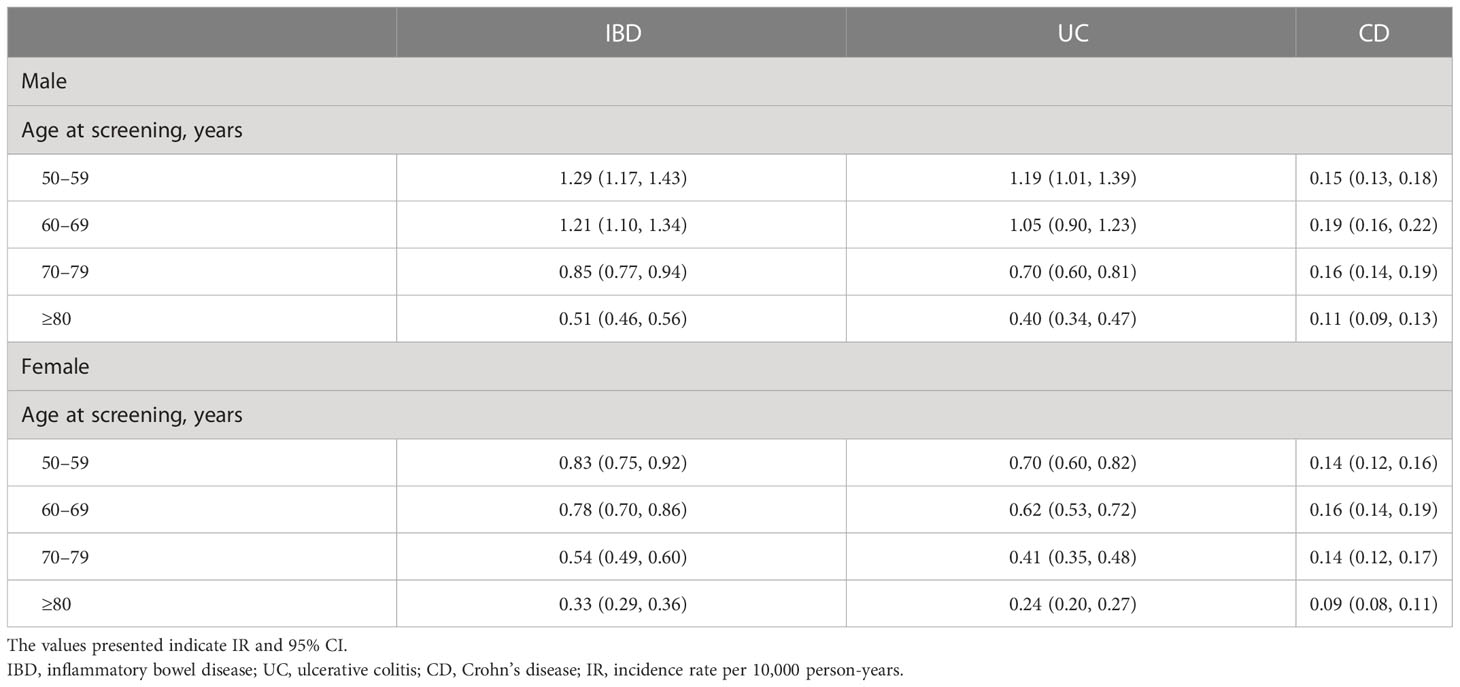
Analysis of the incidence of IBD based on sex and age showed that the overall incidence was the highest in those aged 50–59 years in both sexes (IR 1.29/10,000 PY for males and 0.83/10,000 PY for females), which decreased gradually with age. For disease subtypes, UC was most common in the age group of 50–59 years, CD was most frequently diagnosed in the age group of 60–69 years, and the incidence of UC and CD was consistently higher in males than in females across all age groups (Table 3).
Factors associated with the occurrence of IBD
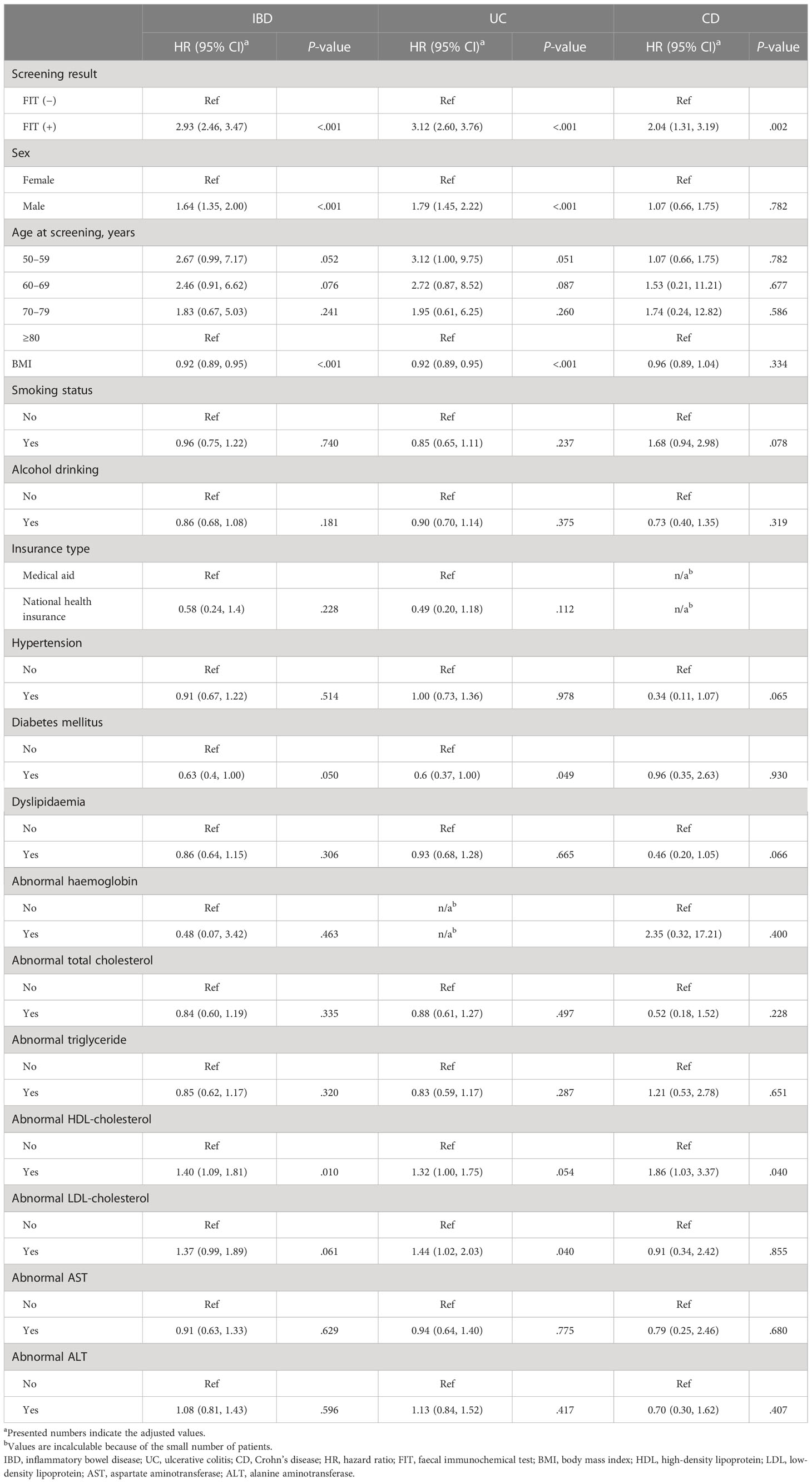
The Cox proportional hazard analysis indicated that a positive FIT result (hazard ratio [HR] 2.93, 95% CI: 2.46, 3.47, P <.001), male sex (HR 1.64, 95% CI: 1.35, 2.00, P <.001), and abnormal high-density lipoprotein (HDL) cholesterol level (HR 1.40, 95% CI: 1.09, 1.81, P = .010) increased the risk of developing IBD, while an increase in body mass index (BMI; HR 0.92, 95% CI: 0.89, 0.95, P <.001) and diabetes mellitus (HR 0.63, 95% CI: 0.40, 1.00, P = .050) had a negative association with the incidence of IBD (Table 4).
In subgroup analyses based on disease subtypes, FIT positivity (HR 3.12, 95% CI: 2.60, 3.76, P <.001), male sex (HR 1.79, 95% CI: 1.45, 2.22, P <.001), and abnormal low-density lipoprotein (LDL)-cholesterol level (HR 1.44, 95% CI: 1.02, 2.03; P = .040) were associated with a greater risk of UC; however, the risk of UC decreased following an increase in BMI (HR 0.92, 95% CI: 0.89, 0.95, P <.001) and diabetes mellitus (HR 0.60, 95% CI: 0.37, 1.00, P = .049). In terms of CD, both FIT positivity (HR 2.04, 95% CI: 1.31, 3.19, P = .002) and abnormal HDL levels (HR 1.86, 95% CI: 1.03, 3.37, P = .040) exhibited an increased risk of developing CD (Table 4).
Furthermore, we performed an additional adjusted Cox analysis in participants who underwent colonoscopy after FIT (+) to evaluate the influence of colonoscopy results on IBD occurrence. However, positive colonoscopy results did not significantly influence the occurrence of IBD (Table 5), and only male sex (HR 1.38, 95% CI: 1.03, 1.84; P = .030) and BMI (HR 0.92, 95% CI: 0.88, 0.96; P <.001) were associated with the incidence of IBD.
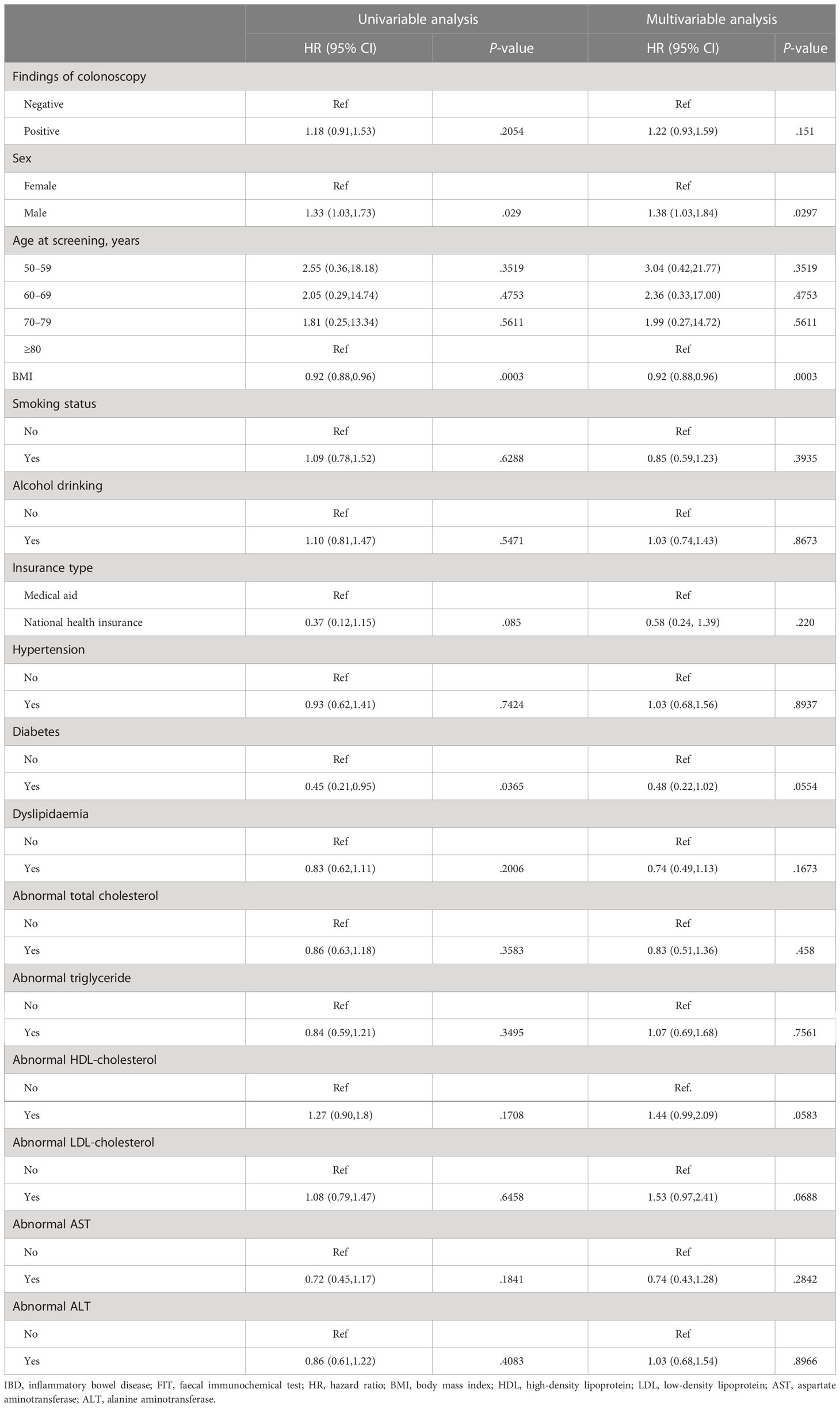
Table 5 Predictive factors of developing IBD among participants with positive FIT who underwent colonoscopy.
The incidence of IBD in the matched population
Given the considerable difference between the characteristics of the FIT (+) and FIT (−) groups, a 1:2 propensity score matching (PSM) was conducted to eliminate the difference. The demographic data and laboratory results were found to be comparable after PSM (Table 6). Kaplan–Meier analysis in this population also demonstrated an elevated risk of IBD in the FIT (+) group compared to that in the FIT (−) group (HR 2.85, 95% CI: 2.34, 3.48, P <.001). FIT positivity increased the risk of both incident UC (HR 3.17, 95% CI: 2.55, 3.94, P <.001) and CD (HR 1.68, 95% CI: 1.03, 2.74, P = .037) (Figure 4).
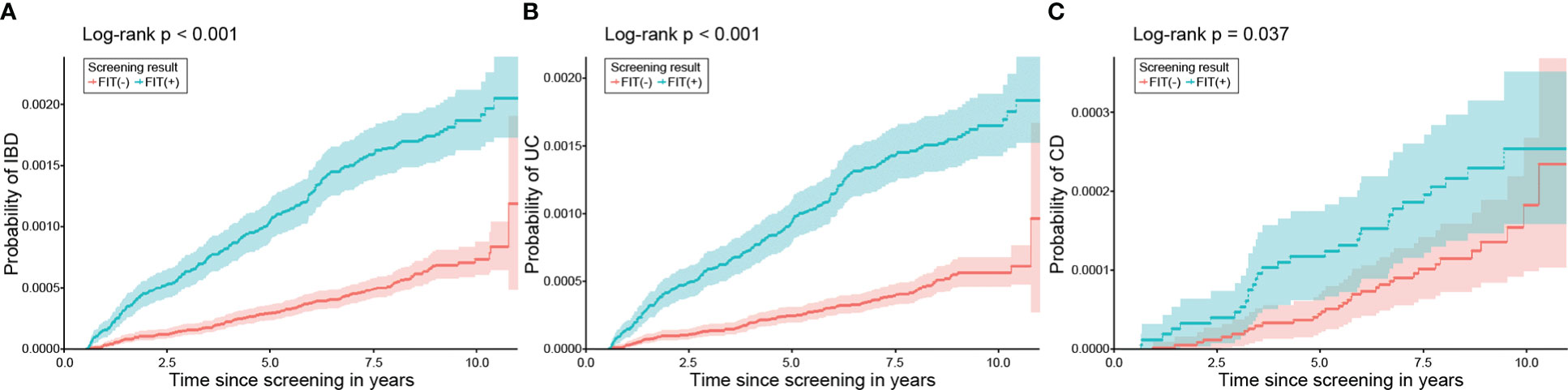
Figure 4 Probability of developing IBD, UC, and CD according to FIT results after PSM. In the matched population, patients with positive FIT results demonstrated a significantly higher risk of (A) IBD, (B) UC, and (C) CD than those with negative FIT results. IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; FIT, faecal immunochemical test; PSM, propensity score matching.
Discussion
Detection of faecal haemoglobin is a widely used method to screen for CRC, and FIT is recommended as a CRC screening test because it is considered to have higher diagnostic performance than the guaiac-based method (24). There is accumulating evidence linking positive faecal blood with diseases unrelated to CRC, indicating that this finding could reflect greater inflammation in the human body; this has been replicated in a number of studies (14, 25). Moreover, a previous investigation revealed that the risk of immune-mediated inflammatory disorders, particularly rheumatoid arthritis, is increased in patients with positive FIT results (17). In our study, by using the data of those who participated in a national CRC screening program, we identified a group of people who had positive FIT results but without evidence of apparent gastrointestinal bleeding. Importantly, we found that positive FIT results were independently associated with the occurrence of UC and CD, which was reproduced in the sensitivity analysis, indicating that FIT abnormalities in the general population could predict the onset of IBD.
The association between positive FIT and IBD occurrence observed in our study could be explained by the disruption of local bowel homeostasis and changes in the gut microbiome, which play significant roles in alterations of the immune landscape and imbalance of cytokines found in IBD (26, 27). First, the breakdown of localised gut homeostasis could shift the balance between pro- and anti-inflammatory mediators, contributing to the maintenance of intestinal mucosal integrity and inducing an abnormal systemic immune response (28). Second, interference of the balance in the gut microbiota is regarded as essential in inducing intestinal barrier damage and triggering inflammatory responses in IBD (29). Supporting this, in vivo studies have identified gut dysbiosis as a crucial component leveraging the development of IBD (30). Collectively, it could be assumed that abnormalities in the gut mucosa, defined as positive FIT results in our study, are associated with the evolution of IBD.
Predicting individuals at risk for IBD in the general population prior to disease development is highly challenging, although various risk factors for IBD have been identified (31). In our study, we demonstrated that FIT positivity confers an increased risk of IBD in the general population, even after excluding cases of IBD that were diagnosed after 6 months of screening. This finding implies that a positive FIT could be a preceding sign of IBD and could be applied to determine the high-risk population for developing IBD prior to the onset of overt disease. Notably, previous studies have reported that FIT can anticipate mucosal healing and has equivalent performance compared to faecal calprotectin (32, 33), which is the most widely adopted test for the identification of disease and quantification of inflammation in the bowel (34–36). In this context, those with a positive FIT result and symptoms that raise a suspicion of IBD might be candidates for regular screening for the presence of intestinal inflammation. Also, FIT could be advantageous as a screening test for IBD in the general population compared to faecal calprotectin, in terms of cost-effectiveness.
Our results revealed that 0.06% and 0.01% of participants were diagnosed with UC and CD, respectively, during the mean follow-up of 7.61 years. Although a large variation in the incidence rates of IBD has been reported in the literature, it is generally understood that the incidence of IBD is higher in European countries than in regions of the Eastern world (37). While the exact incidence of IBD in South Korea is not well understood, a previous study has shown that the estimated incidence of UC and CD is approximately 4/100,000 and 2/100,000, respectively, each year (38). Considering that IBD is relatively common in younger individuals, the incidence of IBD in our study (crude annual incidence rate, 0.98/10,000) was higher than that reported in the literature. Nonetheless, a higher proportion of participants developing UC than those with CD was also demonstrated, in agreement with current evidence. Notably, the discrepancies in UC and CD incidence compared with the previous study could be relevant to the study design. First, we identified those with positive FIT results and matched them with those showing a negative FIT result, which might have influenced the higher incidence of IBD. Second, the incidence of UC is relatively higher in the elderly than that of CD (39); because participants enrolled in the national CRC screening program were all aged >50 years, this would have resulted in a substantially higher UC annual incidence rate than CD.
The Cox proportional hazard analysis indicated that positive FIT, male sex, low BMI, presence of diabetes mellitus, and abnormal HDL cholesterol level were associated with the risk of subsequent IBD. The positive and negative relationship between age groups and female sex is consistent with the knowledge that IBD frequently occurs in men aged 30–40 years, with a decline in its incidence in the older population (40). In addition, the association of abnormalities in HDL cholesterol with IBD implies that changes in HDL cholesterol reflect alterations in the immune system, other than cardiovascular events (41). Also, BMI was inversely associated with the incidence of IBD. While it remains inconclusive whether low BMI confers a risk of developing IBD, population-based studies from Denmark have indicated an increase in IBD among participants with low BMI (42, 43), which may be partly explained as a consequence of chronic inflammation causing cachexia or representing a pre-clinical manifestation of IBD. Interestingly, we confirmed a negative correlation between diabetes mellitus and IBD, particularly UC. While there is a lack of studies exploring the association between diabetes mellitus and IBD, it has been reported that the use of metformin and dipeptidyl peptidase-4 inhibitors, which are the most frequently prescribed drugs for the management of diabetes mellitus in South Korea (44), could mitigate the risk of IBD in patients with diabetes mellitus (45, 46). In particular, as both drugs inhibit the activation of pro-inflammatory cytokines and improve insulin resistance, it is possible that they could decrease local inflammatory reactions in the intestine (47, 48). Therefore, the decreased risk of IBD in patients with diabetes mellitus could have been accounted for by the effects of these drugs, although we could not confirm the types of medication in The Korean National Cancer Screening Program (KNCSP) database. Lastly, the lack of association with smoking, a known environmental trigger for IBD, could be related to the fact that smoking status was categorised into current and non-current; additionally, the percentage of smokers was low, and the potential association of smoking may not have been evident because of other confounders (49).
An important strength of our study was that we demonstrated in a nationwide cohort that a positive FIT is associated with an increased the risk of future IBD development. However, our study has some limitations. First, although this was a large-scale study involving those who participated in a CRC screening program, we only had baseline information for the analysis of IBD incidence and the primary aim of CRC screening program was not to screen for IBD. Second, because the enrolled participants were exclusively >50 years of age, the risk of developing IBD among those with positive FIT results in the younger population could not be evaluated. Third, detailed colonoscopic findings could not be utilised in our analyses, and it could not be clarified whether a complete endoscopic examination was performed to exclude other potential causes. This was because full colonoscopy results were not provided because of the potential identification of individuals. Furthermore, whether other imaging tests were undertaken subsequently - such as magnetic resonance imaging small bowel study/a capsule study – could not be confirmed. Fourth, although various genetic and environmental factors, including dietary intake, could affect IBD incidence, such data are not available in the KNCSP and could not be analysed. Fifth, 14 patients were diagnosed with UC and CD simultaneously; although the number of patients was small, this may be an unclassified type of IBD showing overlapping features of UC and CD (50). However, the details of these patients could not be queried owing to the limitations of the National Health Insurance Sharing Service (NHIS). Finally, while measuring faecal calprotectin levels may be a useful strategy for early detection of IBD in patients with positive FIT and normal colonoscopy, these results were not available in the KNCSP database.
Conclusion
In conclusion, by utilising the data of those who participated in the nationwide screening program for CRC, it was found that a positive FIT is associated with the onset of IBD in the general population. Abnormal FIT results could be a preceding sign of incident IBD, and regular screening may be beneficial, especially in patients with suspected symptoms of IBD.
Data availability statement
The datasets presented in this article are not readily available because the datasets analysed in this study cannot be shared publicly because of national legislation for protection of personal information. However, data are available from the Korea National Health Insurance Sharing Service (contact via https://nhiss.nhis.or.kr, contact: +82-33-736-2432, 2433) for those authorised to access the confidential data. Requests to access the datasets should be directed to https://nhiss.nhis.or.kr.
Ethics statement
The studies involving human participants were reviewed and approved by Ajou University Hospital (approval No. AJIRB-MED-EXP-20-479). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SSA and C-KN: Conceptualisation. EL, SSA, and C-KN: Methodology. EL, GHL, SSA, and C-KN: Software. EL, SSA, and C-KN: Validation. EL and BP: Formal analysis. EL, SSA, and C-KN: Investigation. EL, GHL, SSA, and C-KN: Resources. EL: Data curation. GHL, SSA and C-KN: Writing—original draft preparation. EL, GHL, BP, SSA, and C-KN: Writing—review and editing. EL and C-KN: Visualisation. BP, SSA and C-KN: Supervision. EL, GHL, BP, SSA, and C-KN: Project administration. All authors contributed to the article and approved the submitted version.
Acknowledgments
Woohyun Cho, MFA (Medical Information & Media Centre, Ajou University School of Medicine), provided editing services for images and illustrations. She did not receive compensation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1128736/full#supplementary-material
References
1. Podolsky DK. Inflammatory bowel disease. N Engl J Med (2002) 347(6):417–29. doi: 10.1056/NEJMra020831
2. Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature (2020) 578(7796):527–39. doi: 10.1038/s41586-020-2025-2
3. Bancil AS, Sandall AM, Rossi M, Chassaing B, Lindsay JO, Whelan K. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: mechanistic insights in inflammatory bowel disease. J Crohns Colitis (2021) 15(6):1068–79. doi: 10.1093/ecco-jcc/jjaa254
4. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res (2019) 2019:7247238. doi: 10.1155/2019/7247238
5. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0
6. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol (2020) 35(3):380–9. doi: 10.1111/jgh.14872
7. Korzenik JR, Podolsky DK. Evolving knowledge and therapy of inflammatory bowel disease. Nat Rev Drug Discovery (2006) 5(3):197–209. doi: 10.1038/nrd1986
8. Choy MC, Visvanathan K, De Cruz P. An overview of the innate and adaptive immune system in inflammatory bowel disease. Inflamm Bowel Dis (2017) 23(1):2–13. doi: 10.1097/MIB.0000000000000955
9. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med (2009) 361(21):2066–78. doi: 10.1056/NEJMra0804647
10. Ford AC. Overlap between irritable bowel syndrome and inflammatory bowel disease. Gastroenterol Hepatol (N Y). (2020) 16(4):211–3.
11. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. (2005) 129(2):422–8. doi: 10.1016/j.gastro.2005.05.056
12. Park DI, Ryu S, Kim YH, Lee SH, Lee CK, Eun CS, et al. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol (2010) 105(9):2017–25. doi: 10.1038/ajg.2010.179
13. Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne). (2021) 8:765474. doi: 10.3389/fmed.2021.765474
14. Barnett KN, Clark GRC, Steele RJC, Fraser CG. Faecal haemoglobin estimated by faecal immunochemical tests–an indicator of systemic inflammation with real clinical potential. Diagnostics (Basel). (2021) 11(11):2093. doi: 10.3390/diagnostics11112093
15. Hong S, Lee YY, Lee J, Kim Y, Choi KS, Jun JK, et al. Trends in cancer screening rates among Korean men and women: results of the Korean national cancer screening survey, 2004–2018. Cancer Res Treat (2021) 53(2):330–8. doi: 10.4143/crt.2020.263
16. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J (2014) 38(5):395–403. doi: 10.4093/dmj.2014.38.5.395
17. Noh CK, Lee E, Park B, Ahn SS. A positive faecal immunochemical test result and its association with the incidence of rheumatoid arthritis, systemic lupus erythematosus, and psoriatic arthritis: an analysis of one-million national colorectal cancer screening programme results. BMC Med (2022) 20(1):226. doi: 10.1186/s12916-022-02416-y
18. Lee DW, Koo JS, Choe JW, Suh SJ, Kim SY, Hyun JJ, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol (2017) 23(35):6474–81. doi: 10.3748/wjg.v23.i35.6474
19. Shin A, Choi KS, Jun JK, Noh DK, Suh M, Jung KW, et al. Validity of fecal occult blood test in the national cancer screening program, Korea. PLos One (2013) 8(11):e79292. doi: 10.1371/journal.pone.0079292
20. Jeon CH, Lee AJ, Kim KD. Urinalysis & routine microscopy subcommittee, Korean association of external quality assessment service. Annu Rep external Qual Assess scheme urinalysis faecal occult Blood testing Korea (2014). J Lab Med Qual Assur (2015) 37(4):179–89.
21. Lee SH, Park YM, Kim TJ, Kim MJ, Kim HW, Ham JH, et al. A study on the standardization of judgment criteria and findings for national health examination. Article Korean. Goyang Korea: Res Institute Natl Health Insurance Service Ilsan Hosp (2015) 20.
22. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean national health insurance service (NHIS) and health insurance review & assessment (HIRA) database for research. J Lipid Atheroscler (2022) 11(2):103–10. doi: 10.12997/jla.2022.11.2.103
23. Lee J, Im JP, Han K, Kim J, Lee HJ, Chun J, et al. Changes in direct healthcare costs before and after the diagnosis of inflammatory bowel disease: A nationwide population-based study. Gut Liver. (2020) 14(1):89–99. doi: 10.5009/gnl19023
24. Young GP, Symonds EL, Allison JE, Cole SR, Fraser CG, Halloran SP, et al. Advances in fecal occult blood tests: the FIT revolution. Dig Dis Sci (2015) 60(3):609–22. doi: 10.1007/s10620-014-3445-3
25. Lee HJ, Han K, Soh H, Koh SJ, Im JP, Kim JS, et al. Occult blood in feces is associated with increased risk of psoriasis. Dermatology (2022) 238(3):571–8. doi: 10.1159/000518625
26. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes (2012) 3(1):4–14. doi: 10.4161/gmic.19320
27. Kayama H, Takeda K. Regulation of intestinal homeostasis by innate and adaptive immunity. Int Immunol (2012) 24(11):673–80. doi: 10.1093/intimm/dxs094
28. Zundler S, Tauschek V, Neurath MF. Immune cell circuits in mucosal wound healing: clinical implications. Visc Med (2020) 36(2):129–36. doi: 10.1159/000506846
29. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature (2011) 474(7351):298–306. doi: 10.1038/nature10208
30. Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol (2017) 14(10):573–84. doi: 10.1038/nrgastro.2017.88
31. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol (2015) 12(4):205–17. doi: 10.1038/nrgastro.2015.34
32. Takashima S, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical Test Am J Gastroenterol (2015) 110(6):873–80. doi: 10.1038/ajg.2015.66
33. Nakarai A, Kato J, Hiraoka S, Kuriyama M, Akita M, Hirakawa T, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol (2013) 108(1):83–9. doi: 10.1038/ajg.2012.315
34. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ (2010) 341:c3369. doi: 10.1136/bmj.c3369
35. Gisbert JP, Bermejo F, Perez-Calle JL, Taxonera C, Vera I, McNicholl AG, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflammation Bowel Dis (2009) 15(8):1190–8. doi: 10.1002/ibd.20933
36. Aomatsu T, Yoden A, Matsumoto K, Kimura E, Inoue K, Andoh A, et al. Fecal calprotectin is a useful marker for disease activity in pediatric patients with inflammatory bowel disease. Dig Dis Sci (2011) 56(8):2372–7. doi: 10.1007/s10620-011-1633-y
37. Burisch J, Jess T, Martinato M, Lakatos PL. ECCO -EpiCom. burden inflammatory bowel Dis Europe. J Crohns Colitis. (2013) 7(4):322–37. doi: 10.1016/j.crohns.2013.01.010
38. Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, et al. Emerging trends of inflammatory bowel disease in south Korea: a nationwide population-based study. J Gastroenterol Hepatol (2019) 34(6):1018–26. doi: 10.1111/jgh.14542
39. Kaplan GG, Bernstein CN, Coward S, Bitton A, Murthy SK, Nguyen GC, et al. The impact of inflammatory bowel disease in Canada 2018: epidemiology. J Can Assoc Gastroenterol (2019) 2(Suppl 1):S6–S16. doi: 10.1093/jcag/gwy054
40. Shah SC, Khalili H, Gower-Rousseau C, Olen O, Benchimol EI, Lynge E, et al. Sex-based differences in incidence of inflammatory bowel diseases–pooled analysis of population-based studies from western countries. Gastroenterology (2018) 155(4):1079–89.e3. doi: 10.1053/j.gastro.2018.06.043
41. Connelly MA, Shalaurova I, Otvos JD. High-density lipoprotein and inflammation in cardiovascular disease. Transl Res (2016) 173:7–18. doi: 10.1016/j.trsl.2016.01.006
42. Mendall M, Harpsoe MC, Kumar D, Andersson M, Jess T. Relation of body mass index to risk of developing inflammatory bowel disease amongst women in the Danish national birth cohort. PLos One (2018) 13(1):e0190600. doi: 10.1371/journal.pone.0190600
43. Mendall MA, Jensen CB, Sorensen TIA, Angquist LH, Jess T. Body mass index in young men and risk of inflammatory bowel disease through adult life: a population-based Danish cohort study. Sci Rep (2019) 9(1):6360. doi: 10.1038/s41598-019-42642-8
44. Ko SH, Kim DJ, Park JH, Park CY, Jung CH, Kwon HS, et al. Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002-2013: nationwide population-based cohort study. Med (Baltimore). (2016) 95(27):e4018. doi: 10.1097/MD.0000000000004018
45. Tseng CH. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J Crohns Colitis. (2021) 15(1):64–73. doi: 10.1093/ecco-jcc/jjaa136
46. Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis (2015) 74(11):1968–75. doi: 10.1136/annrheumdis-2014-205216
47. Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) (2012) 122(6):253–70. doi: 10.1042/CS20110386
48. Bank U, Heimburg A, Helmuth M, Stefin S, Lendeckel U, Reinhold D, et al. Triggering endogenous immunosuppressive mechanisms by combined targeting of dipeptidyl peptidase IV (DPIV/CD26) and aminopeptidase n (APN/ CD13)–a novel approach for the treatment of inflammatory bowel disease. Int Immunopharmacol. (2006) 6(13-14):1925–34. doi: 10.1016/j.intimp.2006.09.014
49. Carlens C, Hergens MP, Grunewald J, Ekbom A, Eklund A, Hoglund CO, et al. Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am J Respir Crit Care Med (2010) 181(11):1217–22. doi: 10.1164/rccm.200909-1338OC
Keywords: faecal immunochemical test, inflammatory bowel disease, incidence, prediction, risk
Citation: Lee E, Lee GH, Park B, Ahn SS and Noh C-K (2023) Positive faecal immunochemical test predicts the onset of inflammatory bowel disease: A nationwide, propensity score-matched study. Front. Immunol. 14:1128736. doi: 10.3389/fimmu.2023.1128736
Received: 21 December 2022; Accepted: 27 January 2023;
Published: 13 February 2023.
Edited by:
Hani S. Mousa, University of Cambridge, United KingdomReviewed by:
Shahida Din, NHS Lothian, United KingdomDevis Benfaremo, Marche Polytechnic University, Italy
Copyright © 2023 Lee, Lee, Park, Ahn and Noh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Soo Ahn, c2FuZXRoQHl1aHMuYWM=; Choong-Kyun Noh, Y2tub2gyM0BnbWFpbC5jb20=
†These authors contributed equally to this work and share first authorship
‡These authors contributed equally to this work and share senior authorship
 Eunyoung Lee
Eunyoung Lee Gil Ho Lee
Gil Ho Lee Bumhee Park
Bumhee Park Sung Soo Ahn
Sung Soo Ahn Choong-Kyun Noh
Choong-Kyun Noh