- 1Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
- 2Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia
- 3Laboratory of Transplantation Immunology, National Medical Research Center for Hematology, Moscow, Russia
Regulatory B lymphocytes (Bregs) are B cells with well-pronounced immunosuppressive properties, allowing them to suppress the activity of effector cells. A broad repertoire of immunosuppressive mechanisms makes Bregs an attractive tool for adoptive cell therapy for diseases associated with excessive activation of immune reactions. Such therapy implies Breg extraction from the patient’s peripheral blood, ex vivo activation and expansion, and further infusion into the patient. At the same time, the utility of Bregs for therapeutic approaches is limited by their small numbers and extremely low survival rate, which is typical for all primary B cell cultures. Therefore, extracting CD19+ cells from the patient’s peripheral blood and specifically activating them ex vivo to make B cells acquire a suppressive phenotype seems to be far more productive. It will allow a much larger number of B cells to be obtained initially, which may significantly increase the likelihood of successful immunosuppression after adoptive Breg transfer. This comparative study focuses on finding ways to efficiently manipulate B cells in vitro to differentiate them into Bregs. We used CD40L, CpG, IL4, IL21, PMA, and ionomycin in various combinations to generate immunosuppressive phenotype in B cells and performed functional assays to test their regulatory capacity. This work shows that treatment of primary B cells using CD40L + CpG + IL21 mix was most effective in terms of induction of functionally active regulatory B lymphocytes with high immunosuppressive capacity ex vivo.
1 Introduction
Regulatory B cells (Bregs), major regulators of immune system homeostasis, are known to be directly involved in the pathogenesis of rheumatoid arthritis, psoriasis, multiple sclerosis, type 1 diabetes, Sjögren’s syndrome, systemic lupus erythematosus, myocarditis, allergies, bacterial and viral infections, cancers, and graft versus host reaction (1–4). Reduced Breg pool has been shown in patients with chronic inflammatory diseases, autoimmune pathologies, and asthma (5, 6). Oliveria and colleagues demonstrated that regulatory B cells display distinct dysregulation in healthy volunteers compared to both allergic patients with and without asthma (7, 8). Elevated Bregs have been observed in cancer and acute bacterial infections (9, 10). Recently, COVID-19 patients have been shown to have increased levels of Bregs in their blood compared to healthy donors (11). In addition, the ability of certain subpopulations of Bregs to suppress effector T lymphocytes and NK cells in the tumor microenvironment has been convincingly demonstrated to facilitate tumor immune escape (9, 12).
Subpopulations of regulatory B cells can be subdivided into more than ten subgroups (13). However, when investigating the role of regulatory B cells in various pathologies, the most common subpopulations of human Bregs to be studied are memory Bregs (mBregs, CD19+CD24hiCD27+) and transitional Bregs (tBregs, CD19+CD24hiCD38hi), because of their high immunosuppressive capacity (14). One of the hypothetical models of Breg development is based on the assumption that any B cell can differentiate into Breg depending on specific stimuli (15), as suggested by experimental induction of Breg differentiation using various stimulation agents (16, 17). Differentiation of Bregs requires BCR signaling and the CD40-CD40L interaction (18). CD40-CD40L interaction occurs between B and T cells, respectively, and normally induces the formation of antibody-secreting cells. However, excessive duration of this signal inhibits the differentiation to plasma cells and leads mainly to the generation of Bregs. The role of CD40 in the induction of Bregs was confirmed in experiments on the mice model with CD40-deficient B lymphocytes. These mice exhibited a severe form of experimental autoimmune encephalomyelitis, accompanied by decreased IL10 production and increased Th1 and Th17 responses (19, 20).
Toll-like receptor (TLR) signaling is also important for Breg activation and, together with CD40, for their differentiation since TLR ligands are known to be strong promoters of Breg development (21). TLRs play a key role in the body’s defense against pathogens and may have protective value in autoimmune pathologies. Agonist stimulation through TLR4 (using LPS) and/or TLR9 (using CpG) has been shown to reduce symptoms of diabetes, EAE, and arthritis in mice, while decreased TLR9 expression in humans leads to an increased incidence of systemic lupus erythematosus (22–25). Bregs possess a unique spectrum of immunosuppressive mechanisms, which includes soluble factors (IL10, IL27, IL35, TGFβ, granzyme B) and surface molecules (PD-L1, FasL, CD39, CD73) (26–32). Such a broad repertoire of immunosuppressive mechanisms makes the adoptive transfer of Bregs a promising therapeutic approach for diseases associated with excessive activation of immune responses (33–35). At the same time, small numbers of Bregs and their extremely low survival rate in culture complicate the development of therapeutic applications.
There are various approaches for increasing the survival of B cells in vitro. For a long time, activation and proliferation of B cells in vitro were achieved by feeder cell culture systems expressing CD40L on the plasma membrane (36). CD40L binding to CD40 expressed on B cells mimics the signal from follicular T helper cells. By expressing CD40L and IL21, these cells give B lymphocytes a strong costimulatory signal. Many studies have shown feeder systems to be effective, but the standardization of assay protocols is quite complicated. Consequently, the introduction of soluble CD40L into cell culture has begun (37). CD40L can be replaced with an agonistic antibody to cross-link CD40 on the cell surface. CD40L is often used together with antibodies to the B cell receptor (BCR) in order to mimic BCR binding to an antigen (38). Activation using CD40L together with anti-BCR antibodies mostly promotes the proliferation of naive B cells (38), whereas the addition of IL21 to the activation medium promotes the proliferation of all B cell subtypes (39). It should be noted that CD40L + IL21 stimulation could promote B lymphocyte differentiation into germinal center B cells (40) which are characterized by rapid division and death ex vivo, as well as the potential ability to give rise to new CD27+ memory B cells.
IL4 and IL21 have also found application in in vitro B cell culture (41). It has been shown that the addition of IL4 promotes better survival of non-switched memory cells (42), while IL21, in contrast, promotes differentiation into a more mature state, which is accompanied by an increase in the number of plasma cells and switched memory cells (43). IL4 is considered important for B cell survival, while IL21 is more important for B cell differentiation (44). TLR antagonists can also be used to activate B cells in vitro. Stimulation of TLR9 with CpG was shown to promote the proliferation and differentiation of memory B cells, whereby cells with switched antibody isotype were more activated than non-switched ones (38).
In order to exhibit suppressive functions, B cells require inflammatory stimuli (via TLRs), costimulatory signals (via CD40), and cytokines (IL4, IL21, etc.) (45). Several approaches enhance the survival of Bregs under in vitro conditions. Glass et al. conducted a comprehensive phenotypic analysis of IL10+ B cells induced by a variety of exogenous stimuli in ex vivo cell culture experiments (46). The most popular stimulating agents for Breg culture are CpG, CD40L, and phorbol-12-myristate-13-acetate (PMA) in various combinations. Chen and colleagues, in their work on Bregs in patients with thyroid-associated ophthalmopathy, used combinations of CD40L and CpG to enrich peripheral blood mononuclear cells (PBMCs) with CD19+IL10+ Breg fraction (47). Iwata and colleagues studied the induction of PBMC differentiation into B10 cells (IL10-expressing B cells) by the following stimulating agents: lipopolysaccharide, CD40L, antibody to CD40, CpG, PMA, and ionomycin in various combinations (48). In another study, B lymphocytes isolated from PBMCs were activated with CpG and demonstrated an increased suppression of T lymphocyte activity due to secretion of the anti-inflammatory cytokine TGFβ and indoleamine-2,3-dioxygenase (27). Bankó et al. showed that B cells isolated from rheumatoid arthritis patients can be stimulated by CpG + CD40L for the induction of IL10 response. This effect can be boosted by adding IL21 to culture medium (49). This work aims to assess the applicability of such approaches to the preparation of biomedical cell products for adoptive Breg therapy.
2 Materials and methods
2.1 Human subjects
Current study was performed on healthy human subjects who donated their blood at the National Medical Research Center for Hematology (Moscow, Russia). The study was approved by the Research Ethics Committee of the National Medical Research Center for Hematology (Protocol № 126, 25.02.2022). All donors signed the informed consent form before enrollment.
2.2 Isolation of B cells from PBMCs
PBMCs were isolated by Ficoll-Hypaque (1.077 g/cm³, Paneco, Russia) density gradient centrifugation (400g, 30 min). B cells were purified by magnetic separation using the CD19-MicroBeads (Miltenyi Biotec, Germany). Cells were cultured in full RPMI-1640 medium supplemented with 2mМ glutamine (Paneco), 20% FBS (Biosera, France), 1 mМ sodium pyruvate, 10 mМ HEPES, 100 U/ml penicillin, and 100 mcg/ml streptomycin (all Paneco).
2.3 Activation of B cells
B cells were cultured in the activation medium for 7 days. Among activation agents, there were recombinant CD40L (1 mcg/ml, BioLegend, USA), IL4 (10 ng/ml), IL21 (25 ng/ml, all Miltenyi Biotec), PMA (10 ng/ml) and ionomycin (1 mcg/ml, all Sigma, USA) and CpG (2 mcM, ODN2006, 5’-tcgtcgttttgtcgttttgtcgtt-3’), in various combinations. Negative control was maintained in the growth medium only. On day 7, supernatants were collected separately.
2.4 B cell immunophenotyping
Cells were stained with antibodies to the main phenotypic molecular markers of regulatory B cells: CD24-PE (clone ML5, lot No. B273849), CD27-PE-Cy7 (clone O323, lot No. B274956), CD38-APC (clone HIT2, lot No. B255162) (all BioLegend). To determine cell viability, cells were also stained with Viability Dye-eFluor780 (Thermo Fisher Scientific, USA). Cells were then analyzed by flow cytometry using FACS Canto II (BD Biosciences, USA). FlowJo Software version 10 (TreeStar, USA) was used for analysis.
2.5 Breg proliferation assay
Magnetically separated B cells were labeled with CellTrace Violet (CTV, cat No. C34557, Thermo Fisher Scientific) and cultured (105 cells) in the activation medium for 7 days. Negative control was maintained in full RPMI-1640 without any additional stimuli. After 7 days of cultivation, cells were then phenotyped as described above.
2.6 Analysis of anti-inflammatory gene expression level
Total RNA was purified from activated B cells on day 7 with the ExtractRNA reagent (Evrogen, Russia). cDNAs were synthesized using oligo(dT)18 primers with MMLV RT kit (Evrogen), and mRNA expression was determined with the Bio-Rad Real-time CFX96 Touch (Bio-Rad, USA) using a qPCRmix-HS SYBR kit (Evrogen). The ΔΔСt method (50) was used to normalize transcription to beta-actin (ACTB). The following primer pairs were used: CD274 - forward 5’-TGCAGGGCATTCCAGAAAGA-3’ and reverse 5’-TAGGTCCTTGGGAACCGTGA-3’, EBI3 - forward 5’-GCTCCCTACGTGCTCAATGT-3’ and reverse 5’-CCCTGACGCTTGTAACGGAT-3’, ACTB - forward 5’-ACTGGGACGACATGGAGAAA-3’ and reverse 5’-GGCGTACAGGGATAGCACAG-3’.
2.7 Determination of pro- and anti-inflammatory cytokine levels secreted by B cells
Supernatants from activated B cells were collected separately on day 7. Concentrations of pro- and anti-inflammatory cytokines TNF and IL10 were determined by ELISA (cat No. 88-7346-88 and 88-7106-88, Thermo Fisher Scientific).
2.8 Effector cell proliferation assay
Before the experiment, CD19-depleted PBMCs were maintained in IL2-containing (20 ng/ml, SCI-Store, Russia) full RPMI-1640 medium. Then autologous CTV-labeled (cat No. C34557, Thermo Fisher Scientific) CD19-depleted PBMCs (105 cells) were co-cultured with previously differentially activated and non-activated B cells (105 cells). After 5 days of co-incubation, supernatants were collected separately to determine pro- and anti-inflammatory cytokine levels by ELISA (cat No. A-8752 for IFNγ, A-8756 for TNF, A-8766 for IL1β, A-8768 for IL6, and A-8774 for IL10, Vector-Best, Russia). Cells were stained with antibodies to the main phenotypic markers of NK- and T cells: CD3-FITC (Sorbent, Russia), CD4-APC (clone RPA-T4, lot No. B307926, BioLegend), CD8-PE-Cyanine7 (clone SK1, lot No. B276851, BioLegend), CD16-PE (Sorbent), cell viability was determined using Viability Dye-eFluor780 (Thermo Fisher Scientific). Frequencies of proliferating CD3-CD16+ NK-cells, CD3+CD4+ T helpers, CD3+CD8+ T killers were determined by flow cytometry following the loss of CTV signal to determine the suppressive capacity of B cells. Cells were then analyzed by flow cytometry using a FACS Canto II (BD Biosciences, USA), FlowJo software version 10 (TreeStar) was used for analysis.
2.9 Measurement of NK-mediated cytotoxic activity by monitoring cancer cell adhesion and apoptosis using the xCELLigence RTCA system
MCF7 and NKL cell lines were used for this experiment. MCF7 adhesive human breast cancer cells (provided by late Dr. E. Zabarovsky from Karolinska Institutet, Stockholm, Sweden) were cultured in full DMEM (Paneco). NKL natural killer cells (provided by Dr. M. Streltsova and Dr. A. Sapozhnikov from the Institute of Bioorganic Chemistry, Moscow, Russia) were maintained in full RPMI-1640 supplemented with IL2 (20 ng/ml, SCI-Store). MCF7 cells were seeded in each well of the E-plate (5*104 cells per well). The impedance values of individual cells were automatically monitored by the xCELLigence system (xCelligence DP, Agilent, USA) and expressed as a cell index (CI) value (51). Meantime, NKL cells were co-cultured with differentially activated and non-activated B cells overnight. When MCF7 reached the steady-state growth phase, NKL (2*105 cells) and B cells (2*105 cells) were seeded onto the E-plate at a density of 105 per well. The E-plate was then placed into the xCELLigence system. Sweeps were taken every 15 minutes for the duration of the experiment to determine the MCF7 adhesion level, which was indicative of NK-dependent cancer cell cytolysis. The cell index was normalized to 1.0 at the moment of NKL addition.
2.10 B-cell-mediated Treg-differentiation
CD4+ T cells were magnetically separated from PBMCs (CD4-MicroBeads, Miltenyi) and maintained in IL2-containing (20 ng/ml, SCI-Store) full RPMI-1640 medium until the start of the experiment. Then they were labeled with CTV (cat No. C34557, Thermo Fisher Scientific) and co-cultured (105 cells) with autologous previously differentially activated for 5 days and non-activated B cells (105 cells). After 5 days of co-incubation, supernatants were collected separately for determination of IL10 level by ELISA (cat No. 88-7106-88, Thermo Fisher Scientific). Cells were stained with antibodies to the main phenotypic molecular markers of regulatory T cells: CD4-APC (clone RPA-T4, lot No. B307926, BioLegend), CD25-PE (clone BC96, lot No. 2173867, eBioscience), and CD127-FITC (clone A019D5, lot No. E-AB-F1152L, Elabscience, USA). To determine cell viability, cells were also stained with Viability Dye-eFluor780 (Thermo Fisher Scientific). Cells were then analyzed by flow cytometry using FACS Canto II (BD Biosciences), FlowJo Software version 10 (TreeStar) was used for analysis.
2.11 Statistical analysis
All analysis was performed by GraphPad Prism, version 9.0.0. (GraphPad Software Inc., La Jolla, CA, USA). Results are expressed as mean ± SEM.
3 Results
3.1 Breg ratio, viability, and proliferation rates differ under the influence of various stimuli
Since CD24hiCD38hi and CD24hiCD27+ B lymphocytes are known to be the major Breg subsets in human peripheral blood (14), we used them as an analytical instrument to assess Breg percentage among differentially activated CD19+ lymphocytes (B cells) using flow cytometry analysis (Figure S1, Supplementary Data). mBregs show a higher survival rate compared to the tBreg subpopulation (Figures 1A, C). This feature may be explained by their memory phenotype, which makes them more persistent and ensures their longevity (52). Transitional B cells require more environmental factors to survive (53), thus showing lower survival rates without stimulation in vitro (Figure 1).
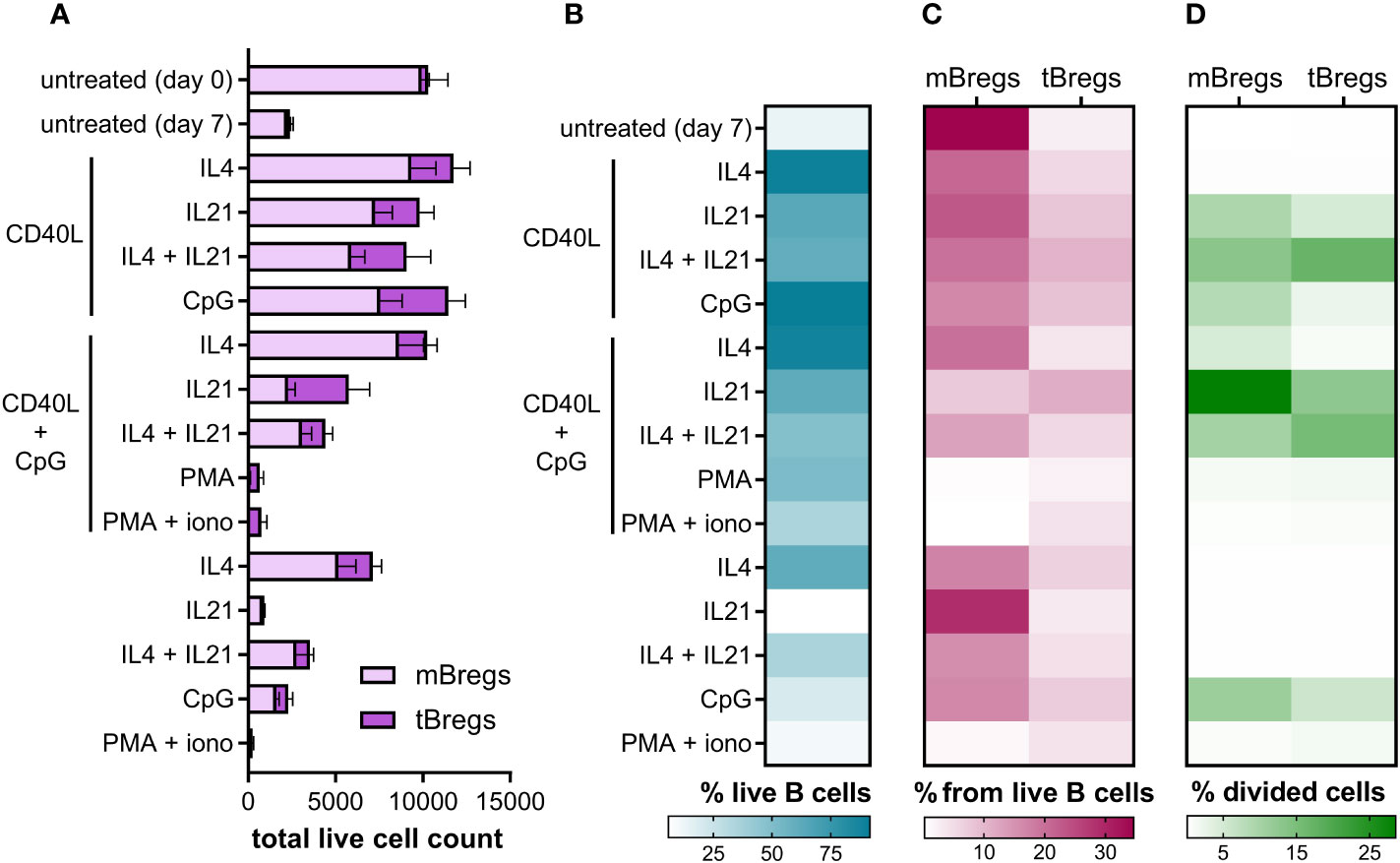
Figure 1 Differential activation of primary CD19+ lymphocytes alter the Breg subsets ratio, their viability, and proliferation rates ex vivo. (A) Total live cell count of mBregs and tBregs after various stimulation (50,000 events were acquired). Untreated control values were measured on day 0 and day 7. Mean values ± SEM of five independent experiments for each condition are shown. (B) Heatmap showing average B cell viability percentages after various stimulation. The mean values of five independent experiments for each stimulation are shown. (C) The ratio of mBregs and tBregs among live B cells after activation with various stimuli for 7 days. Untreated control values were measured on day 0 and day 7. Mean values of five independent experiments for each condition are shown. (D) Heatmap showing the average percentage of divided mBregs and tBregs induced by differential activation of primary B cells. The mean values of five independent experiments for each stimulation are shown.
We observed that the majority of activation agents (such as the combination of CD40L + IL4, CD40L + CpG, etc.) lead to a higher frequency of mBregs compared to tBregs (Figures 1A, C). At the same time, PMA or PMA + ionomycin, alone, or in combination with CD40L + CpG, abrogated the acquisition of a regulatory phenotype by peripheral blood B cells, which is especially pronounced for the mBreg subpopulation. Activation of B cells by CD40L, CpG, and IL4 in various combinations leads to increased survival, but supplementing the activation medium with IL21 partially lowers cell viability (Figure 1B).
In order to assess the rates of Breg proliferation under various stimuli, we used CellTrace Violet staining to monitor distinct generations of proliferating cells by dye dilution (Figure S1). This assay has revealed that under various stimuli mBregs and tBregs proliferate at different rates (Figure 1D). While most stimuli induced the predominant proliferation of mBregs, supplementation of the CD40L-containing activation mix with IL21 increased the growth of both mBregs and tBregs. CpG also positively influenced their proliferation rate but to a smaller extent. Some activation agents, such as PMA, ionomycin, or interleukins alone, did not induce the proliferation of Breg subpopulations. The results also revealed that IL4 in combination with CD40L + CpG, or CD40L alone, enhanced primary B cell survival, but hardly induced the proliferation of either mBregs or tBregs.
3.2 Expression of major molecules involved in Breg-mediated immunoregulation
We next analyzed the immunoregulatory molecular profile of differentially activated B cells. Regulatory B cells are known to express a variety of inhibitory molecules – both membrane-bound factors, such as PD-L1, and soluble molecules, such as IL10, IL35, etc. We tested differentially activated B cells for IL10 secretion levels and the expression of anti-inflammatory genes CD274 (coding for PD-L1) and EBI3 (coding for IL27B – a common subunit of IL35 and IL27 cytokines); TNF production by B cells was assessed to monitor inflammatory responses (Figure 2). All three inhibitory molecules tested were expressed at the highest level by B cells treated with CD40L + CpG + IL21, making this combination of stimuli the most efficient in inducing an anti-inflammatory response. IL10 was expressed at the same high level in B cells treated with CD40L in several combinations with CpG, IL21, and/or IL4. The usage of PMA and ionomycin as stimulation agents increased the level of EBI3, CD274, and IL10 expression. However, this treatment also resulted in an undesirable increase in TNF level, especially in combination with CD40L and CpG.
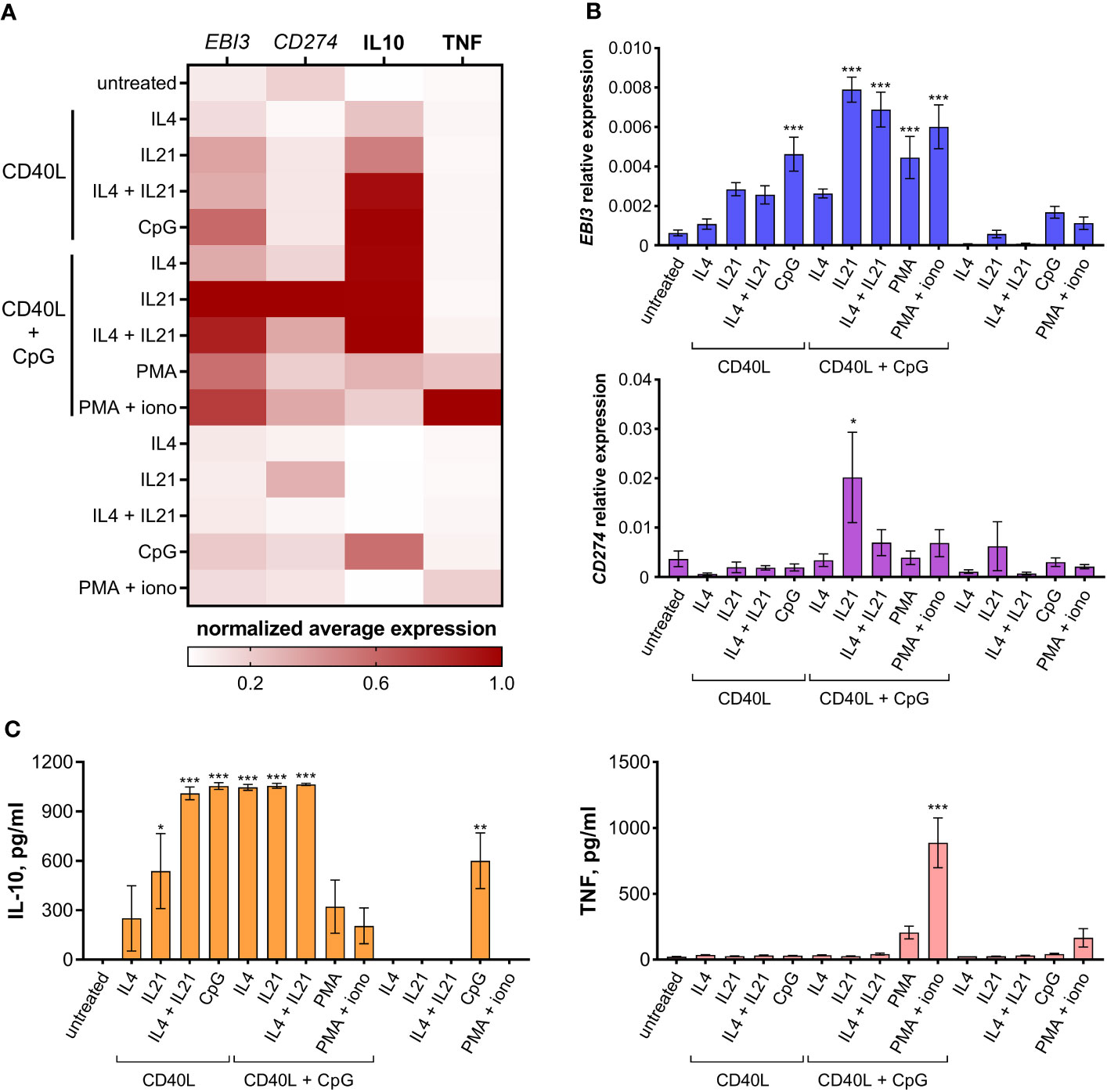
Figure 2 Immunoregulatory molecular profile of differentially activated B cells. (A) Heatmap showing EBI3, CD274, IL10, and TNF normalized average expression levels in differentially activated B cells. (B) EBI3 and CD274 relative expression levels were determined by RT-PCR using total RNA extracted from differentially activated B cells on day 7 of cultivation. (C) IL10 and TNF levels were determined by ELISA in the medium from differentially activated B cells on day 7 of cultivation. Mean values ± SEM of at least four independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control, as calculated by ANOVA.
3.3 Activation-induced regulatory B cells mediate Treg differentiation
Based on expression levels of inhibitory molecules (Figure 2) and rates of Breg proliferation (Figure 1), we have selected 5 combinations of activation stimuli for further functional analysis: CD40L + CpG, CD40L + CpG + IL4, CD40L + CpG + IL21, CD40L + CpG + IL4 + IL21, and CpG alone.
Regulatory immune cells are known to significantly influence nearby immune cells, making them acquire a suppressive phenotype. To assess the capacity of activated B cells to induce regulatory T cell (Treg) differentiation, we used a co-cultivation assay followed by an evaluation of Treg proliferation rate using CTV staining (Figure S1). These Tregs are likely to be peripherally induced Tregs (pTregs) since pTregs are inducible within suppressive microenvironments, which are precisely created by Bregs (54). Our data revealed that B cells stimulated with CD40L + CpG + IL4 efficiently induced the proliferation of Tregs (gated as CD4+CD25hi T cells) (Figure 3A). B cells treated with CD40L + CpG and CD40L + CpG + IL21 also mediated Treg proliferation, but to a lesser extent. CD4+CD25hi T cells were further verified for low expression of CD127 (Figure S2), which is characteristic of Tregs (55). IL10 levels in the culture medium demonstrated a similar pattern, however, the use of B cells treated with CD40L + CpG resulted in the highest level of this anti-inflammatory cytokine (Figure 3B). Thus, activation-induced Bregs induce the immunosuppressive response in T helper cells, as demonstrated by the increase in Treg proliferation and IL10 secretion.
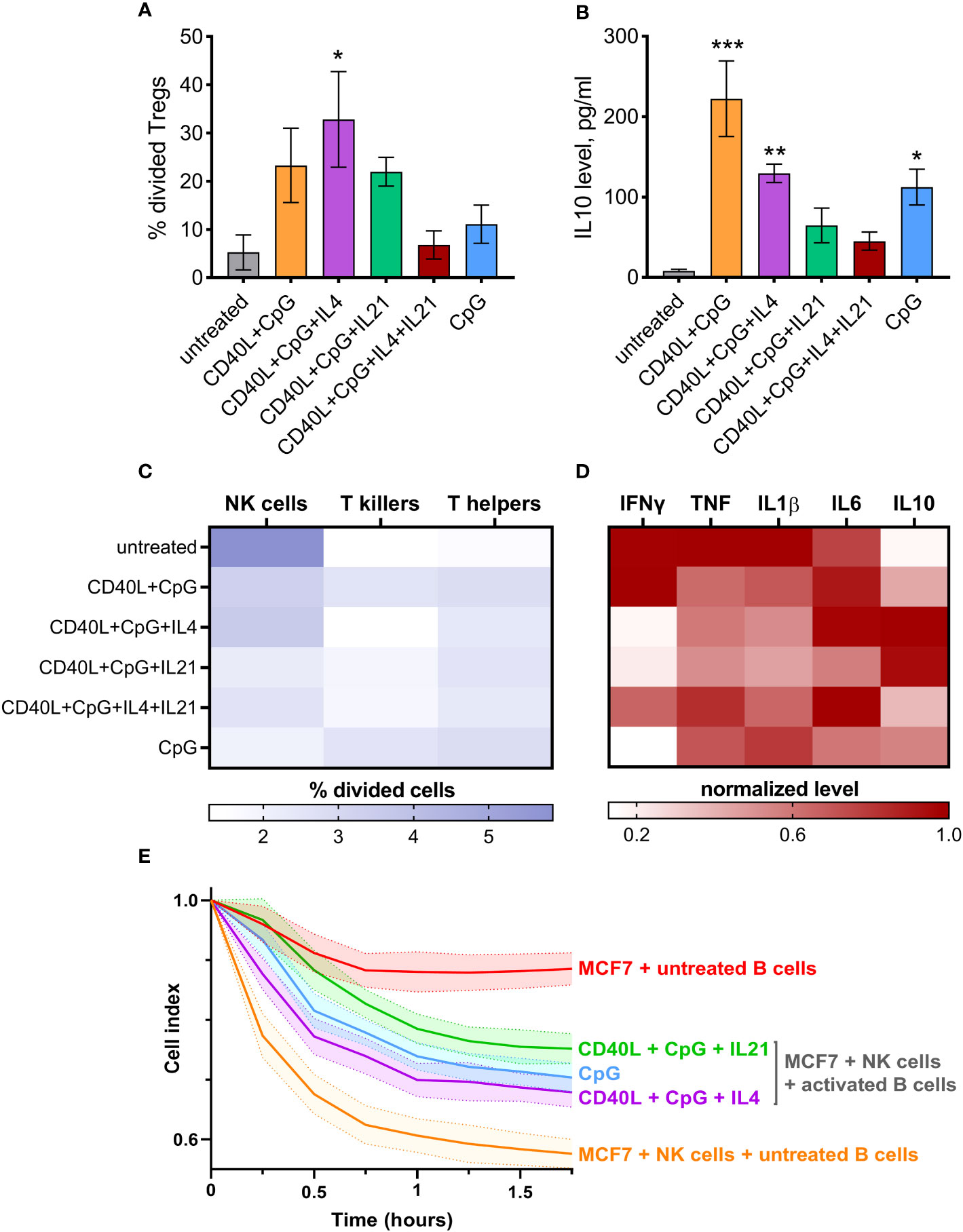
Figure 3 Differentially activated B cells suppress the activity of effector cells and mediate CD4+ T cells differentiation into Tregs (CD4+CD25hi) with simultaneous induction of IL10 secretion. (A) Tregs proliferation induced by co-cultivation of differentially activated B cells and CD4+ T cells. The percentage of divided Tregs was determined by flow cytometry using CellTrace Violet dye. (B) IL10 level was determined in the medium from CD4+ T cells co-cultivated with differentially activated B cells on day 5 of cultivation using ELISA. Mean values ± SEM of four independent experiments is shown. *P < 0.05, **P < 0.01, ***P < 0.001 compared to untreated control, as calculated by ANOVA. (C) Activated B cells influence the proliferation rate of T helpers, T killers, and NK cells from CD19-depleted PBMCs after 5 days of co-cultivation. (D) IFNγ, TNF, IL1β, IL6, IL10 normalized levels were determined using ELISA in the medium from B cell-depleted PBMCs after co-cultivation. The average values of four independent experiments are shown. (E) Activated B cells reduce NK-mediated killing of cancer cell line MCF7. Mean values ± SD of three independent experiments is shown.
3.4 Activation-induced Bregs modify effector cell functioning
We further tested the ability of in vitro-stimulated B cells to modulate proliferation rates of NK cells, CD4+ T cells (T helpers), and CD8+ T cells (T killers) (Figure S1). B cells activated using CD40L + CpG + IL21, or CpG alone, had the highest inhibitory effect on the growth of NK cells (gated as CD3–CD16+). T helper and T killer proliferation rates were not suppressed by differentially activated B lymphocytes; some B cells (such as CD40L + CpG treated cells) even promoted their proliferation (Figure 3C). With regard to the major subpopulations of T cells, a slight decrease in the percentage of T killers can be observed under the influence of activated B cells (Figure S3). This phenomenon may be attributed to the regulatory ability of B cells to suppress T killers (56). Furthermore, a minor increase in the percentage of T helpers is detected, which could be associated with the proliferation of Tregs induced by suppressive B cells (Figures 3, S2, S3). We also assessed the level of the common effector cytokine IFNγ, pro-inflammatory TNF, IL1β, IL6, and anti-inflammatory IL10 secreted by CD19-depleted PBMCs cultured with treated B cells for 5 days. B cells treated with CD40L + CpG + IL21/IL4, or with CpG alone, decreased inflammatory response, as assessed by IFNγ levels (Figure 3D). B cells stimulated with CD40L + CpG + IL21/IL4 increased production of IL10 which also helped reduce the inflammatory reaction. Production of TNF and IL1β did not change dramatically, but the pattern of their expression resembled the IFNγ production. At the same time, the level of IL6 cytokine showed no clear pattern of changes in expression, presumably due to its bipolar proinflammatory and homeostatic nature (Figure 3D). In order to test activated B cells in a more physiological setting, we used the xCELLigence system for real-time monitoring of NK cell-mediated cytotoxicity against MCF7 breast cancer cells (Figure 3E). This analysis showed that the above-mentioned combinations of activation agents induced B cells to significantly inhibit the cytotoxic activity of NK cells. Thus, Bregs generated in vitro proved to possess immunosuppressive effects on NK cells and, probably, some other effector cells from human peripheral blood, which have not been examined in this study (based on the increased level of IL10 and decreased IFNγ, TNF, IL1β expression by B cell-depleted PBMCs, Figure 3D).
4 Discussion
Our analysis demonstrated that different combinations of activators have quite diverse effects on the separate components of the regulatory phenotype of B cells in vitro. As can be seen from Figure 4, which visualizes our findings, of the five “semi-finalists” selected based on proliferation and expression data, the CD40L + CpG + IL21 activation cocktail appeared to be the most universal mix overall. The selected activation mix was a bit less effective than other combinations in terms of cell survival (where CD40L + CpG mix apparently worked better without additional cytokines, Figure 1B), and Treg induction (where IL4, a cytokine with known immunosuppressive properties, had the edge over IL21, Figure 3). At the same time, after such activation, B cells didn’t show signs of the inflammatory response: the level of TNF expression was not raised (Figure 2C). Activated B cells have also decreased the expression levels of IFNγ, TNF, IL1β and increased IL10 production by other peripheral blood cells and suppressed the proliferation of NK cells and their cytotoxic activity (Figure 3). Our findings are consistent with studies on the role of IL21 in Breg maturation, where effector Bregs could not be generated in the absence of IL21 or its receptor (57), and T follicular helper cells suggested themselves as the most probable normal source of IL21 for the induction of Breg differentiation (58). Spolski and Leonard suggested using IL21 to expand B regulatory cells in vitro prior to adoptive transfer into patients with multiple sclerosis to inhibit inflammatory response (59). Chesneau and colleagues used a mixture of CD40L + CpG + IL21 with added anti-BCR and IL2 to induce ex vivo expansion of granzyme B-expressing B cells with potent regulatory properties (60). We propose to use IL21 in combination with CD40L, a major activator of B cells, and CpG as a polyclonal innate stimulus, in order to generate Bregs with proven high broad-spectrum immunosuppressive capacities and proliferation rate (Figure 4), which could potentially allow to efficiently reduce the inflammatory response in the treatment of pathologic conditions associated with immune system overactivation using adoptive Breg therapy.
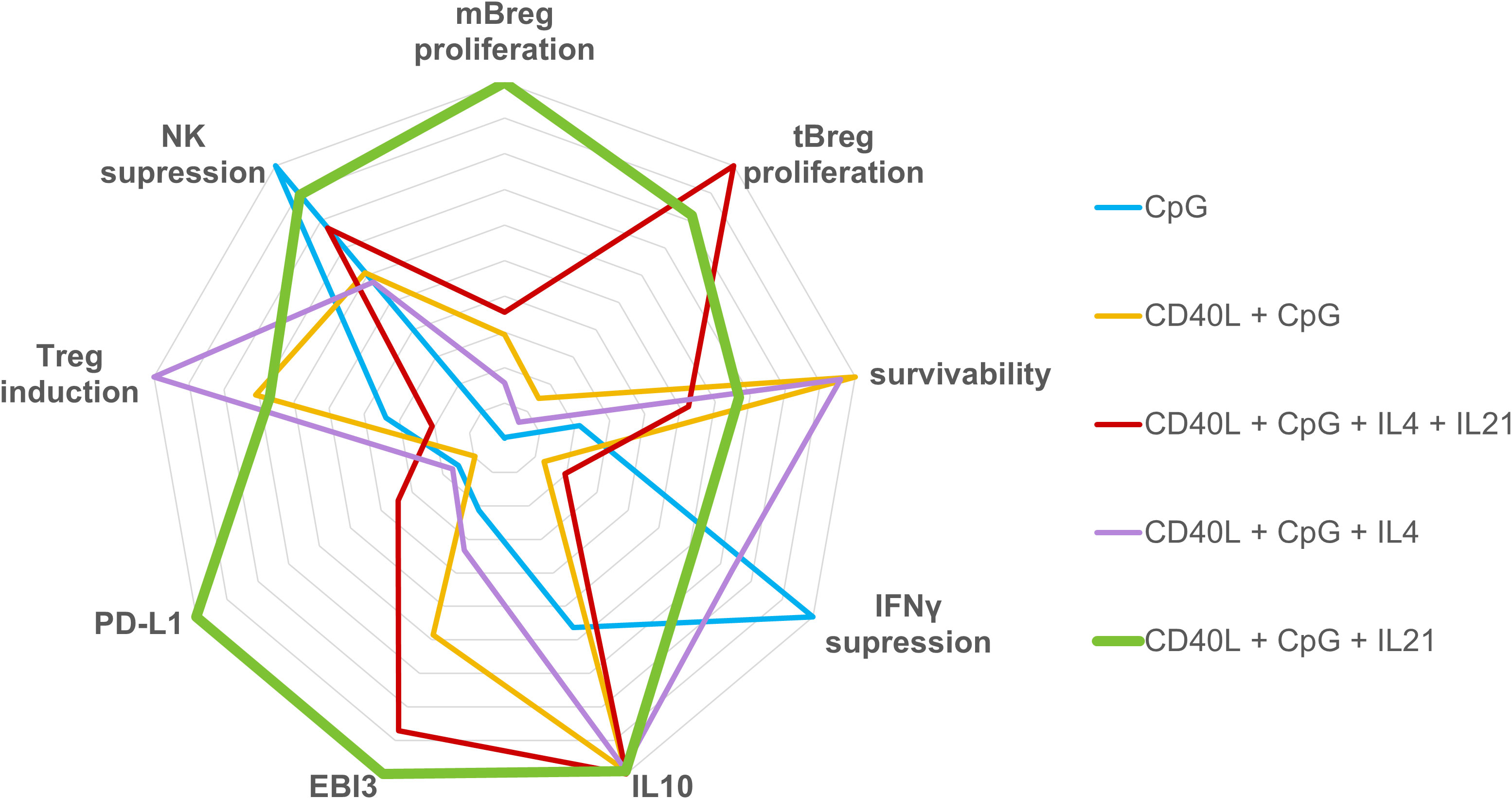
Figure 4 Radar graph representing the regulatory phenotype of B cells induced by various activation stimuli. Axes display immunosuppressive parameters of activated B lymphocytes assessed in the study. All data from Figures 1-3 used for immunogram construction were proportionally normalized to a single scale from 0 to 10. The values of each axis have been joined to form the central polygon area, which represents the general regulatory phenotype induced by various activation cocktails.
Adding IL21 to the cocktail partially lowers cell survival which is in agreement with the published data that IL21 can trigger activation-induced cell death via downregulation of anti-apoptotic Bcl-2 and Bcl-xL production, and upregulation of Cas-8 and Cas-3 expression (61–63). This may become an obstacle when preparing Bregs as a biomedical cell product for adoptive transfer. Nevertheless, under in vivo conditions, some survivability is probably worth sacrificing for the sake of optimal immunosuppressive capacity as long as this problem can be solved by enrichment of the fraction of live Bregs via cell sorting before the cell transfer. Stimulation of B cells with CD40L + CpG + IL21 resulted in an increase in the population of plasmablasts (Figure S4), which can be attributed to the potential saturation of the Breg compartment, given the regulatory function demonstrated for CD27hiCD38hi cells (64). Following activation, the number of conventional memory B cells exhibited a notable reduction, suggesting their potential differentiation into plasmablasts. Furthermore, we observed proliferation of naive B cells upon exposure to activating stimuli, in agreement with the findings reported in the study conducted by Glass et al. (46).
It is important to clarify that direct administration of CpG, CD40L and IL21 in vivo is unacceptable due to the risk of systemic immune hyperactivation and inflammatory response. Instead, we suggest utilizing these agents solely ex vivo to target B cells, and subsequently introducing the resulting purified cell product into the patient. This approach allows for targeted intervention without excessive systemic immune disturbances.
Reflecting on the reasons why this combination of activation agents has proven to induce immunosuppressive response in B cells, we have noted that there is evidence that CD40L and IL21 signaling induces STAT3 (65, 66) and STAT5 (67, 68) activation, which in their turn may promote IL10 (65, 69) and PD-L1 expression (70). IL21 signaling was also found to activate STAT1 (71). Thus, it may be leading to the increase in EBI3 (72) and PD-L1 (73) expression. TLR9 – a receptor for CpG – has also been shown to promote IL10 (65), EBI3 (74), and PD-L1 (75) expression (Figure 5).
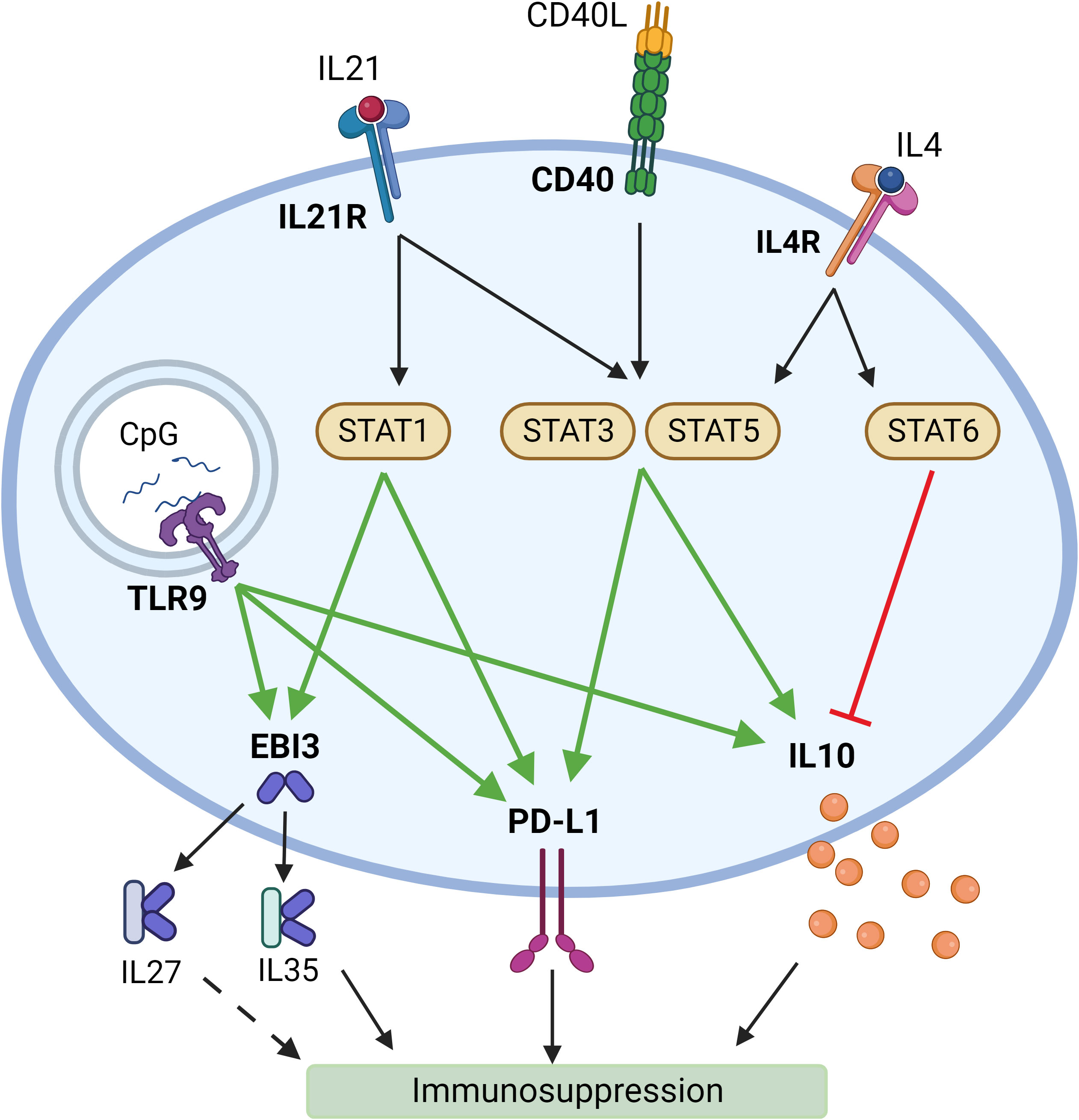
Figure 5 Potential mechanisms of induction of a suppressive phenotype in B lymphocytes by synergistic activation by different ligands. Created with BioRender.com.
However, in pathologies associated with decreased Treg activity, a combination of CD40L, CpG, and IL4 may be more useful since Bregs activated this way significantly increased Treg proliferation (Figure 3A). It is known that the IL4R signaling pathway is involved in the differentiation of naive B cells in IL4-producing B cells (76). Thus, when in vitro stimulated with IL4, B cells can be induced to produce IL4 in an autocrine manner. It has also been shown that Treg differentiation depends on this cytokine: IL4 signaling can induce STAT6-dependent Foxp3 expression and maintenance of this transcription factor in Tregs (77). When co-cultivated with T helper cells, CD40L + CpG treated B cells induced IL10 secretion at a higher level compared to those treated with CD40L + CpG + IL4 mix (Figure 3B). Yet, there are many more mechanisms of Treg-mediated immunosuppression except for IL10 (78), which is why we suppose that such parameter as Treg proliferation rate is more indicative of the efficiency of Breg-inducing activation of B cells. Interestingly, during co-cultivation with CD19+-depleted PBMCs, B cells activated with CD40L + CpG + IL21/IL4 induced highest IL10 production (Figure 3D). This can be explained by the presence of other cells in PBMC that could have acquired an anti-inflammatory phenotype after such treatment – for example, monocytes (79).
CpG seemed to be an important player in the in vitro induction of Bregs; its addition to the stimulation mix has increased the secretion of inhibitory molecules (Figure 2) and contributed to a higher rate of Breg proliferation (Figure 1D). The work of Gallego-Valle and colleagues have also shown the effectiveness of CpG stimulation in the generation of Breg-like phenotype in B cells (80). However, CpG treatment alone has shown to be less effective than stimulation cocktails, consisting of CD40L, CpG, and IL4/IL21, primarily due to its low immunosuppression-inductive capacity (Figure 3).
Regarding PMA-containing cocktails, our results showed that such activation types weren’t successful in induction of immunosuppressive response. Interestingly, PMA alone or PMA + ionomycin-containing mixes are quite often used for the generation of Bregs in vitro, but in this study, they enhanced inflammatory response in B cells (based on the level of TNF secretion, Figure 2), didn’t induce either mBreg or tBreg proliferation, and even decreased survivability of primary B cells (Figure 1).
To gain insights into the distribution of Bregs in the body following adoptive cell transfer, we can consider the existing studies that have examined the in vivo assessment of infused Treg cells and their outcomes in humans (81). In a study involving four patients with autoimmune hepatitis, Tregs infused into blood were primarily found in the liver (22-44%), spleen (11-24%), and bone marrow (9-13%) (82). Bregs can also be expected to exhibit a predominant distribution towards the site of inflammation which would be consistent with the objective to target and suppress inflammation in a specific localized area rather than pursuing a systemic approach.
There are several limitations of our study that should be mentioned. One is our gating strategy for Bregs that is well-established but not the only one possible since a canonical regulatory Breg phenotype remains elusive in the absense of a specific Breg marker (14). Another limitation is inclusion of IL21 in the activation cocktail that appears to partially lower the cell survivability. However, in the context of potential adoptive therapy, this reduced survival may not be a significant issue and could even be advantageous, as it limits prolonged cell proliferation, thereby reducing the probability of non-specific immortalization and transformation into malignant B cells.
Within the above-mentioned limitations, our comparative study sheds light on efficient ways of generating functional Bregs from bulk peripheral blood B cells. Our study shows the high efficiency of the CD40L + CpG + IL21 activation mix in the induction of functionally active regulatory B cells ex vivo. Such activation could be performed on the peripheral blood B cells prior to adoptive cell transfer. We hope this research will bring new insights into the development of new approaches to the adoptive cell therapy of pathologies caused by immune overactivation, such as allergies, graft-versus-host disease, and autoimmune diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of the National Medical Research Center for Hematology (Protocol № 126, 25.02.2022). The patients/participants provided their written informed consent to participate in this study.
Author contributions
EZ, ASU, and KK designed research and analyzed data. EZ, ASU, ANU, NK, and KK performed experiments. ES, VG, AB, NM, and DK contributed to the critical expertise, materials, and techniques. EZ wrote the manuscript. DK and KK reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by grants #21-74-00106 (Figures 1, S1, S4) and #22-14-00398 (Figures 3, S2, S3) from Russian Science Foundation, and grant #075-15-2019-1660 from the Ministry of Science and Higher Education of the Russian Federation (Figures 2, 4, 5).
Acknowledgments
BioRender (https://biorender.com/) was used to make Figure 5. EZ acknowledges the support from the Charitable Foundation for Scientific Research and Development ‘Global Impact Alliance’ with the support of LLC ‘SciStoreLab’.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
Any opinions, findings, and conclusions presented here are those of the author(s) and do not necessarily reflect the views of the Charitable Foundation for Scientific Research and Development ‘Global Impact Alliance and/or LLC ‘SciStoreLab’.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1178445/full#supplementary-material
References
1. Wu H, Su Z, Barnie PA. The role of B regulatory (B10) cells in inflammatory disorders and their potential as therapeutic targets. Int Immunopharmacol (2020) 78:106111. doi: 10.1016/j.intimp.2019.106111
2. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy (2021) 76:2699–715. doi: 10.1111/all.14763
3. Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, et al. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J Leukoc Biol (2013) 94(4):563–73. doi: 10.1189/jlb.1112562
4. Wang A, Rojas O, Lee D, Gommerman JL. Regulation of neuroinflammation by B cells and plasma cells. Immunol Rev (2021) 299:45–60. doi: 10.1111/imr.12929
5. Ray A, Dittel BN. Mechanisms of regulatory b cell function in autoimmune and inflammatory diseases beyond IL-10. J Clin Med (2017) 6(1):12. doi: 10.3390/jcm6010012
6. Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol (2015) 158:204–11. doi: 10.1016/j.clim.2015.02.016
7. Oliveria J-P, El-Gammal AI, Yee M, Obminski CD, Scime TX, Watson RM, et al. Changes in regulatory B-cell levels in bone marrow, blood, and sputum of patients with asthma following inhaled allergen challenge. J Allergy Clin Immunol (2018) 141:1495–1498.e9. doi: 10.1016/j.jaci.2017.11.013
8. Oliveria JP, Agayby R, Gauvreau GM. Regulatory and igE+ B cells in allergic asthma. Methods Mol Biol (2021) 2270:375–418. doi: 10.1007/978-1-0716-1237-8_21
9. Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol (2017) 14:662–74. doi: 10.1038/cmi.2017.35
10. Fillatreau S. Regulatory roles of B cells in infectious diseases. Clin Exp Rheumatol (2016) 34:1–5.
11. Gupta S, Su H, Narsai T, Agrawal S. SARS-CoV-2-associated T-cell responses in the presence of humoral immunodeficiency. Int Arch Allergy Immunol (2021) 182(3):195–209. doi: 10.1159/000514193
12. Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res (2006) 66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766
13. Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity (2020) 53:217–232.e5. doi: 10.1016/j.immuni.2020.06.013
14. Hasan MM, Thompson-Snipes L, Klintmalm G, Demetris AJ, O’Leary J, Oh S, et al. CD24hiCD38hi and CD24hiCD27+ Human regulatory B cells display common and distinct functional characteristics. J Immunol (2019) 203:2110–20. doi: 10.4049/jimmunol.1900488
15. Mauri C, Menon M. The expanding family of regulatory B cells. Int Immunol (2015) 27:479–86. doi: 10.1093/intimm/dxv038
16. Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat Med (2014) 20:1334–9. doi: 10.1038/nm.3680
17. Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med (2014) 20:633–41. doi: 10.1038/nm.3554
18. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol (2006) 176:705–10. doi: 10.4049/jimmunol.176.2.705
19. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3:944–50. doi: 10.1038/ni833
20. Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol (2000) 12(5):597–605. doi: 10.1093/intimm/12.5.597
21. Chayé MAM, Tontini C, Ozir-Fazalalikhan A, Voskamp AL, Smits HH. Use of toll-like receptor (TLR) ligation to characterize human regulatory B-cells subsets. (2021), 235–61. doi: 10.1007/978-1-0716-1237-8_13
22. Buenafe AC, Bourdette DN. Lipopolysaccharide pretreatment modulates the disease course in experimental autoimmune encephalomyelitis. J Neuroimmunol (2007) 182:32–40. doi: 10.1016/j.jneuroim.2006.09.004
23. Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RAA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity (2006) 25(3):417–28. doi: 10.1016/j.immuni.2006.07.013
24. Lampropoulou V, Hoehlig K, Roch T, Neves P, Gómez EC, Sweenie CH, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol (2008) 180(7):4763–73. doi: 10.4049/jimmunol.180.7.4763
25. Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or cpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol (2000) 165(11):6148–55. doi: 10.4049/jimmunol.165.11.6148
26. Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
27. Nouël A, Pochard P, Simon Q, Ségalen I, Le Meur Y, Pers JO, et al. B-Cells induce regulatory T cells through TGF-β/IDO production in A CTLA-4 dependent manner. J Autoimmun (2015) 59:53–60. doi: 10.1016/j.jaut.2015.02.004
28. Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature (2014) 507:366–70. doi: 10.1038/nature12979
29. Xu L, Liu X, Liu H, Zhu L, Zhu H, Zhang J, et al. Impairment of granzyme B-producing regulatory B cells correlates with exacerbated rheumatoid arthritis. Front Immunol (2017) 8:768. doi: 10.3389/fimmu.2017.00768
30. Figueiró F, Muller L, Funk S, Jackson EK, Battastini AMO, Whiteside TL. Phenotypic and functional characteristics of CD39 high human regulatory B cells (Breg). Oncoimmunology (2016) 5:e1082703. doi: 10.1080/2162402X.2015.1082703
31. Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell–mediated immune suppression through CD73 expression and adenosine production. J Immunol (2014) 193:5904–13. doi: 10.4049/jimmunol.1400336
32. Choi JK, Yu C-R, Bing SJ, Jittayasothorn Y, Mattapallil MJ, Kang M, et al. IL-27–producing B-1a cells suppress neuroinflammation and CNS autoimmune diseases. Proc Natl Acad Sci (2021) 118(47):e2109548118. doi: 10.1073/pnas.2109548118
33. Beckett J, Hester J, Issa F, Shankar S. Regulatory B cells in transplantation: roadmaps to clinic. Transpl Int (2020) 33:1353–68. doi: 10.1111/tri.13706
34. Bottomley MJ, Brook MO, Shankar S, Hester J, Issa F. Towards regulatory cellular therapies in solid organ transplantation. Trends Immunol (2022) 43:8–21. doi: 10.1016/j.it.2021.11.001
35. Slepicka PF, Yazdanifar M, Bertaina A. Harnessing mechanisms of immune tolerance to improve outcomes in solid organ transplantation: A review. Front Immunol (2021) 12:688460. doi: 10.3389/fimmu.2021.688460
36. Liebig TM, Fiedler A, Zoghi S, Shimabukuro-Vornhagen A, von Bergwelt-Baildon MS. Generation of human CD40-activated B cells. J Vis Exp (2009) (32):1373. doi: 10.3791/1373
37. Wagner M, Poeck H, Jahrsdoerfer B, Rothenfusser S, Prell D, Bohle B, et al. IL-12p70-dependent th1 induction by human B cells requires combined activation with CD40 ligand and cpG DNA. J Immunol (2004) 172:954–63. doi: 10.4049/jimmunol.172.2.954
38. Marasco E, Farroni C, Cascioli S, Marcellini V, Scarsella M, Giorda E, et al. B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur J Immunol (2017) 47:131–43. doi: 10.1002/eji.201646574
39. Karnell JL, Ettinger R. The interplay of IL-21 and BAFF in the formation and maintenance of human B cell memory. Front Immunol (2012) 3:2. doi: 10.3389/fimmu.2012.00002
40. Luo W, Conter L, Elsner RA, Smita S, Weisel F, Callahan D, et al. IL-21R signal reprogramming cooperates with CD40 and BCR signals to select and differentiate germinal center B cells. Sci Immunol (2023) 8:eadd1823. doi: 10.1126/sciimmunol.add1823
41. Bethke S, Singh AS, Dauben A, Vorholt D, Dzionek A. A standardized and reproducible method for expansion and activation of human primary B cells. Eur J Immunol (2019) 49:318–9. doi: 10.1002/eji.201970400
42. Banchereau J, de Paoli P, Vallé A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Sci (80- ) (1991) 251:70–2. doi: 10.1126/science.1702555
43. Kuchen S, Robbins R, Sims GP, Sheng C, Phillips TM, Lipsky PE, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol (2007) 179:5886–96. doi: 10.4049/jimmunol.179.9.5886
44. Wurster AL, Rodgers VL, White MF, Rothstein TL, Grusby MJ. Interleukin-4-mediated protection of primary B cells from apoptosis through stat6-dependent up-regulation of bcl-xL. J Biol Chem (2002) 277:27169–75. doi: 10.1074/jbc.M201207200
45. Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest (2017) 127:772–9. doi: 10.1172/JCI85113
46. Glass MC, Glass DR, Oliveria J-P, Mbiribindi B, Esquivel CO, Krams SM, et al. Human IL-10-producing B cells have diverse states that are induced from multiple B cell subsets. Cell Rep (2022) 39:110728. doi: 10.1016/j.celrep.2022.110728
47. Chen G, Ding Y, Li Q, Li Y, Wen X, Ji X, et al. Defective regulatory B cells are associated with thyroid-associated ophthalmopathy. J Clin Endocrinol Metab (2019) 104:4067–77. doi: 10.1210/jc.2018-01812
48. Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011) 117(2):530–41. doi: 10.1182/blood-2010-07-294249
49. Bankó Z, Pozsgay J, Szili D, Tóth M, Gáti T, Nagy G, et al. Induction and differentiation of IL-10–producing regulatory B cells from healthy blood donors and rheumatoid arthritis patients. J Immunol (2017) 198:1512–20. doi: 10.4049/jimmunol.1600218
50. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods (2001) 25:402–8. doi: 10.1006/meth.2001.1262
51. Bird C, Kirstein S. Real-time, label-free monitoring of cellular invasion and migration with the xCELLigence system. Nat Methods (2009) 6:v–vi. doi: 10.1038/nmeth.f.263
52. Tangye SG, Tarlinton DM. Memory B cells: Effectors of long-lived immune responses. Eur J Immunol (2009) 39:2065–75. doi: 10.1002/eji.200939531
53. Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev (2010) 237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x
54. Schmitt EG, Williams CB. Generation and function of induced regulatory T cells. Front Immunol (2013) 4:152. doi: 10.3389/fimmu.2013.00152
55. Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med (2006) 203:1693–700. doi: 10.1084/jem.20060468
56. Chen Z, Zhu Y, Du R, Pang N, Zhang F, Dong D, et al. Role of regulatory B cells in the progression of cervical cancer. Mediators Inflammation (2019) 2019:6519427. doi: 10.1155/2019/6519427
57. Tedder TF. B10 cells: A functionally defined regulatory B cell subset. J Immunol (2015) 194:1395–401. doi: 10.4049/jimmunol.1401329
58. Yang X, Yang J, Chu Y, Xue Y, Xuan D, Zheng S, et al. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PloS One (2014) 9:e88441. doi: 10.1371/journal.pone.0088441
59. Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discovery (2014) 13:379–95. doi: 10.1038/nrd4296
60. Chesneau M, Le MH, Danger R, Le Bot S, Nguyen T-V-H, Bernard J, et al. Efficient expansion of human granzyme B–expressing B cells with potent regulatory properties. J Immunol (2020) 205:2391–401. doi: 10.4049/jimmunol.2000335
61. Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol (2008) 84:348–56. doi: 10.1189/jlb.0308149
62. Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol (2003) 170:4111–8. doi: 10.4049/jimmunol.170.8.4111
63. Akamatsu N, Yamada Y, Hasegawa H, Makabe K, Asano R, Kumagai I, et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Lett (2007) 256:196–206. doi: 10.1016/j.canlet.2007.06.001
64. de Masson A, Socié G, Bagot M, Bensussan A, Bouaziz J-D. Deficient regulatory B cells in human chronic graft-versus-host disease. Oncoimmunology (2015) 4:e1016707. doi: 10.1080/2162402X.2015.1016707
65. Michée-Cospolite M, Boudigou M, Grasseau A, Simon Q, Mignen O, Pers J-O, et al. Molecular mechanisms driving IL-10- producing B cells functions: STAT3 and c-MAF as underestimated central key regulators? Front Immunol (2022) 13:818814. doi: 10.3389/fimmu.2022.818814
66. Berglund LJ, Avery DT, Ma CS, Moens L, Deenick EK, Bustamante J, et al. IL-21 signalling via STAT3 primes human naïve B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood (2013) 122:3940–50. doi: 10.1182/blood-2013-06-506865
67. Epron G, Ame-Thomas P, Le Priol J, Pangault C, Dulong J, Lamy T, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia (2012) 26:139–48. doi: 10.1038/leu.2011.179
68. Scheeren FA, Diehl SA, Smit LA, Beaumont T, Naspetti M, Bende RJ, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood (2008) 111:4706–15. doi: 10.1182/blood-2007-08-105643
69. Hedrich CM, Rauen T, Apostolidis SA, Grammatikos AP, Rodriguez Rodriguez N, Ioannidis C, et al. Stat3 promotes IL-10 expression in lupus T cells through trans- activation and chromatin remodeling. Proc Natl Acad Sci (2014) 111:13457–62. doi: 10.1073/pnas.1408023111
70. Guru SA, Sumi MP, Mir R, Waza AA, Bhat MA, Zuberi M, et al. Ectopic PD-L1 expression in JAK2 (V617F) myeloproliferative neoplasm patients is mediated via increased activation of STAT3 and STAT5. Hum Cell (2020) 33:1099–111. doi: 10.1007/s13577-020-00370-6
71. Leonard WJ, Wan C-K. IL-21 signaling in immunity. F1000Research (2016) 5:224. doi: 10.12688/f1000research.7634.1
72. Sawant DV, Hamilton K, Vignali DAA. Interleukin-35: expanding its job profile. J Interf Cytokine Res (2015) 35:499–512. doi: 10.1089/jir.2015.0015
73. Sasidharan Nair V, Toor SM, Ali BR, Elkord E. Dual inhibition of STAT1 and STAT3 activation downregulates expression of PD-L1 in human breast cancer cells. Expert Opin Ther Targets (2018) 22:547–57. doi: 10.1080/14728222.2018.1471137
74. Birkenbach M, Blumberg RS, Neurath MF. EBV-induced gene 3 transcription is induced by TLR signaling in primary dendritic cells via NF-κB activation. J Immunol (2005) 174:2814–24. doi: 10.4049/jimmunol.174.5.2814
75. Qian J, Meng H, Lv B, Wang J, Lu Y, Li W, et al. TLR9 expression is associated with PD-L1 expression and indicates a poor prognosis in patients with peripheral T-cell lymphomas. Pathol - Res Pract (2020) 216:152703. doi: 10.1016/j.prp.2019.152703
76. Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor α, and th2 cells. J Immunol (2005) 175:7103–7. doi: 10.4049/jimmunol.175.11.7103
77. Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol (2010) 87:1011–8. doi: 10.1189/jlb.1209772
78. Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol (2012) 3:51. doi: 10.3389/fimmu.2012.00051
79. Staples KJ, Smallie T, Williams LM, Foey A, Burke B, Foxwell BMJ, et al. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol (2007) 178:4779–85. doi: 10.4049/jimmunol.178.8.4779
80. Gallego-Valle J, Pérez-Fernández V, Correa-Rocha R, Pion M. Generation of human breg-like phenotype with regulatory function in vitro with bacteria-derived oligodeoxynucleotides. Int J Mol Sci (2018) 19:1737. doi: 10.3390/ijms19061737
81. Orozco G, Gupta M, Gedaly R, Marti F. Untangling the knots of regulatory T cell therapy in solid organ transplantation. Front Immunol (2022) 13:883855. doi: 10.3389/fimmu.2022.883855
Keywords: regulatory B cells, Breg induction ex vivo, mBregs, tBregs, primary B cell activation, immunosuppression, immune response regulation, adoptive Breg transfer
Citation: Zheremyan EA, Ustiugova AS, Uvarova AN, Karamushka NM, Stasevich EM, Gogoleva VS, Bogolyubova AV, Mitkin NA, Kuprash DV and Korneev KV (2023) Differentially activated B cells develop regulatory phenotype and show varying immunosuppressive features: a comparative study. Front. Immunol. 14:1178445. doi: 10.3389/fimmu.2023.1178445
Received: 02 March 2023; Accepted: 18 August 2023;
Published: 05 September 2023.
Edited by:
Michael Zemlin, Saarland University Hospital, GermanyReviewed by:
John-Paul Oliveria, Genentech Inc., United StatesIrina Grigorova, University of Michigan, United States
Copyright © 2023 Zheremyan, Ustiugova, Uvarova, Karamushka, Stasevich, Gogoleva, Bogolyubova, Mitkin, Kuprash and Korneev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elina A. Zheremyan, ZWx5YXpoZXJlbXlhbkBtYWlsLnJ1; Kirill V. Korneev, a2lya29ybmVldkBnbWFpbC5jb20=
 Elina A. Zheremyan
Elina A. Zheremyan Alina S. Ustiugova1
Alina S. Ustiugova1 Violetta S. Gogoleva
Violetta S. Gogoleva Apollinariya V. Bogolyubova
Apollinariya V. Bogolyubova Nikita A. Mitkin
Nikita A. Mitkin Dmitry V. Kuprash
Dmitry V. Kuprash Kirill V. Korneev
Kirill V. Korneev