- 1Department of General Practice, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Medicine, Division of Rheumatology & Immunology, Medical University of South Carolina, Charleston, SC, United States
- 3Department of Rheumatology, Fukushima Medical University School of Medicine, Fukushima, Japan
The transcription factor Fli-1, a member of the ETS family of transcription factors, is implicated in the pathogenesis of lupus disease. Reduced Fli-1 expression in lupus mice leads to decreased renal Cxcl10 mRNA levels and renal infiltrating CXCR3+ T cells that parallels reduced renal inflammatory cell infiltration and renal damage. Inflammatory chemokine CXCL10 is critical for attracting inflammatory cells expressing the chemokine receptor CXCR3. The CXCL10/CXCR3 axis plays a role in the pathogenesis of various inflammatory diseases including lupus. Our data here demonstrate that renal CXCL10 protein levels are significantly lower in Fli-1 heterozygous MRL/lpr mice compared to wild-type MRL/lpr mice. Knockdown of Fli-1 significantly reduced CXCL10 secretion in mouse and human endothelial cells, and human mesangial cells, upon LPS or TNFα stimulation. The Fli-1 inhibitor, Camptothecin, significantly reduced CXCL10 production in human monocyte cells upon interferon stimulation. Four putative Ets binding sites in the Cxcl10 promoter showed significant enrichment for FLI-1; however, FLI-1 did not directly drive transcription from the human or mouse promoters, suggesting FLI-1 may regulate CXCL10 expression indirectly. Our results also suggest that the DNA binding domain of FLI-1 is necessary for regulation of human hCXCR3 promotor activity in human T cells and interactions with co-activators. Together, these results support a role for FLI-1 in modulating the CXCL10-CXCR3 axis by directly or indirectly regulating the expression of both genes to impact lupus disease development. Signaling pathways or drugs that reduce FLI-1 expression may offer novel approaches to lupus treatment.
1 Introduction
Lupus nephritis (LN) is a major cause of mortality in both animal lupus-prone strains and human patients with systemic lupus erythematosus (SLE) (1, 2). Patients with LN have significantly higher mortality compared with patients with non-lupus end stage renal diseases (3, 4). Accumulations of immune complexes and inflammatory cells in kidney are the hallmark of LN (5). At present, the major pathogenesis of LN in renal damage is not fully understood (1). Also, existing data show that current treatments of LN, cytotoxic drugs and corticosteroids, are imperfect with significant side-effects (6, 7). Hence, a better understanding of the complex pathogenesis of LN and identification of novel targets are needed.
Friend leukemia virus integration 1 (Fli-1), a member of the E26 transformation-specific (Ets) transcription factor family, plays a key role in the pathogenesis and development of various diseases including lupus, cancer, systemic sclerosis and sepsis (8–11) (12). In particular, Fli-1 is implicated in the development of lupus prone mouse strains, and elevated expression of Fli-1 is associated with active human lupus nephritis (13–17). Reduced expression of Fli-1 in two lupus mouse strains significantly attenuated renal disease evidenced by decreased infiltrating inflammatory cells and prolonged survival (14, 15). Furthermore, Fli-1 is a key regulator of several inflammatory cytokines and chemokines including monocyte chemotactic protein 1 (MCP-1), chemokine (C-C motif) ligand 5 (CCL5), interleukin 6 (IL-6), granulocyte colony stimulating factor (G-CSF), chemokine ligand 2 (CXCL2) and IL-17A (10, 18–22). Therefore, Fli-1 may affect the pathogenesis of LN as a global modulator of inflammatory mediators.
CXCL9/MIG (monokine induced by interferonγ), CXCL10/IP-10 (interferon inducible 10 protein) and CXCL11/I-TAC (inducible T cell-α chemoattractant), inflammatory chemokines belonging to the CXC chemokine family, and exert their effects by binding to chemokine (C-X-C motif) receptor 3 (CXCR3) (23). These three chemokines lack glutamic acid-leucine-arginine (ELR) and are closely related to each other, with about 40% similarity of amino acid sequence (23). CXCL10 is chemotactic for inflammatory cells including macrophages, monocytes and activated T and NK cells that express CXCR3 (24). Moreover, overexpression of CXCL10 is associated with clinical lupus nephritis (25). Previous reports demonstrated that CXCR3+ cells are recruited into inflamed kidneys in lupus prone mice and human lupus patients (26, 27). We showed that globally reducing FLI-1 levels in a lupus prone mouse strain reduced renal Cxcl9 and Cxcl10 gene expression and renal-infiltrating CXCR3+ T cells, and that FLI-1 can upregulate Cxcr3 promoter activity in T cells (27). However, the underlying mechanisms by which FLI-1 modulates CXCL10 or CXCR3 expression remain unknown.
Our present study reports for the first time that FLI-1 impacts CXCL10 protein expression in kidneys of lupus mice and inflammatory cells. Although FLI-1 could directly bind to the Cxcl10 promoter, it did not independently drive transcription in vitro. These results suggest FLI-1 may regulate Cxcl10 through an indirect mechanism. Camptothecin (CPT), a clinically used chemotherapeutic drug, inhibited Fli-1 expression and reduced CXCL10 secretion in human renal endothelial cells. Fli-1 also drove CXCR3 promoter activity in T cells, which required the -89 to +200 region of the promoter and the DNA-binding domain of FLI-1. Our results also suggest that although the DNA-binding domain of FLI-1 is necessary for upregulating hCXCR3 promoter activity, interaction with co-activators also may play a key role. Together, these results demonstrate a novel role for FLI-1 in modulating the CXCL10-CXCR3 inflammatory axis in lupus that may involve direct and indirect mechanisms of transcriptional regulation. Targeting signaling pathways or using drugs to reduce FLI-1 levels could be further explored as potential novel therapeutic approaches for LN.
2 Materials and methods
2.1 Mice
Wild-type MRL/lpr (Fli-1+/+) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and Fli-1+/− littermates used in this study were bred from a colony maintained at our animal facility at Medical University of South Carolina. Generation of MRL/lpr Fli-1+/− mice was described previously (14). Mice were housed in a pathogen-free environment and all procedures complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocol for all animal studies was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Kidneys were harvested from WT (Fli-1+/+) or Fli-1+/− MRL/lpr mice at the age of 22 weeks, minced and lysed in RIPA lysis buffer. After lysis, the supernatant was collected. Total protein concentration was detected by BCA kit (Thermo Fisher Scientific, Waltham, MA) and CXCL10, CXCL9, and CXCL11 chemokine expression levels were measured by ELISA.
2.2 ELISA
CXCL10, CXCL9, and CXCL11 protein concentrations in the supernatants collected from the kidneys of MRL/lpr mice, human umbilical vein endothelial cells (HUVECs), MS1 cells, and human renal glomerular endothelial cells (HRGECs) were measured by mouse or human CXCL10, CXCL9 and CXCL11 ELISA kits (R&D Systems Inc., Minneapolis, MN) according to the manufacturer’s instructions.
2.3 Cells
The primary human umbilical vein endothelial cells (HUVECs) and primary human renal glomerular endothelial cells (HRGECs) were purchased from ScienCell Research Laboratories (Carlsbad, CA) and maintained in endothelial cell medium (Sciencell Research Laboratories, Carlsbad, CA) according to the manufacturer’s instructions. The murine endothelial MS1 cells and human Jurkat T cells were purchased from the American Type Culture Collection (ATCC). MS1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech Inc., Manassas, VA) with 10% fetal bovine serum and 1% penicillin/streptomycin. Jurkat T cells were cultured in RPMI 1640 with L-glutamine medium [Thermo Fisher Scientific (27)], 10 mM HEPES, 10% fetal bovine serum, and 1% penicillin/streptomycin. All cells were maintained at 37°C with 5% CO2.
2.4 Cell transfection and stimulation
HUVECs, HRGECs and MS1 cells were transfected with negative control or Fli-1 specific siRNA (Integrated DNA Technologies, Coralville, IA) for 24 hours and further stimulated with LPS (Sigma-Aldrich, St. Louis, MO; 0.025, 0.5, 1, 5, 10 µg/ml for MS1 cells, 0.1 µg/ml for HUVECs and HRGECs) or TNFα (10 ng/ml, Sigma-Aldrich, St. Louis, MO) for another 6 or 24 hours. Lipofectamine 2000 (Invitrogen, Waltham, MA) and serum-free Opti-MEM (Thermo Fisher Scientific, Waltham, MA) were used for the transfection. In addition, HRGECs were treated with Camptothecin (CPT, 0.25 µM, Selleckchem) for 24 hours and further stimulated with interferon α (IFNα, 100 ng/ml, Biolegend, San Diego, CA) or interferon γ (IFNγ, 100 ng/ml, Biolegend, San Diego, CA); for another 1, 4 or 24 hours. The cell supernatants were collected at different time points after stimulation.
Jurkat T cells were transfected with a pSG promoter/reporter (P/R) vector containing the human CXCR3 (hCXCR3) proximal promoter (-435 to +284) driving Renilla luciferase (27). hCXCR3 deletion constructs (-89/+284, +101/+284, and +200/+282) were generated by restriction digests on the 5’ end of the pSG hCXCR3 -435/+284 vector to remove sequences followed by re-ligation. All deletion constructs were confirmed through direct sequencing. The P/R constructs were co-transfected into Jurkat T cells with a pcDNA vector expressing wild-type FLI-1 (pcDNA Fli-1) described previously (27), or a pSG vector expressing wild-type FLI-1 (pSG Fli-1), DNA binding mutant (pSG Fli-1 DBM), C-terminal transactivation domain truncation (pSG Fli-1 ΔCTA), acetylation K380 mutant (pSG Fli-1 Acet), or phosphorylation T312 mutant (pSG Fli-1 Phos) described previously (28, 29) using FuGENE (Promega, Madison, WI) according to manufacturer directions. Briefly, Jurkat cells were plated in 6-well plates and transfected with 0.5 μg hCXCR3 P/R vector and the amount of Fli-1 expression vector indicated in figures legends. pGL3 Control (Promega, Madison, WI) expressing firefly luciferase at 0.1 μg was co-transfected and used to normalize for transfection efficiency. Transfected cells were incubated at 37°C, 5% CO2 for four hours, stimulated by addition of 10 ng/ml PMA and 100 ng/ml ionomycin, and incubated for another 16 hrs. Molar amount of transfected DNA was kept constant across wells by the addition of empty pSG or pcDNA expression vectors as appropriate. Renilla and firefly luciferase levels were measured by the Dual luciferase assay kit (Promega) as described previously (30). Transfections were performed in duplicate within an experiment and each experiment was performed twice with similar statistically significant differences observed across experiments. Representative results are presented. Significant differences were determined as described.
2.5 Western blotting
Cells were lysed in Radioimmunoprecipitation assay (RIPA) lysis buffer. After lysis, the supernatant was collected. Equal amounts of protein (20 µg-40 µg) were run for 1.5 hours at 130V on a 4–20% Criterion TGX Stain-Free Protein Gel (Bio-Rad, Hercules, CA) and electrotransferred to a PVDF membrane by Iblot2 transfer stacks (Invitrogen, Waltham, MA). Transferred proteins were probed with both a Fli-1 polyclonal antibody described previously (20) and an antibody to β-actin (Cell Signaling, Beverly, MA). The results were visualized using the Odyssey Imaging System (LI-COR, Lincoln, NE).
2.6 Chromatin immunoprecipitation sssay
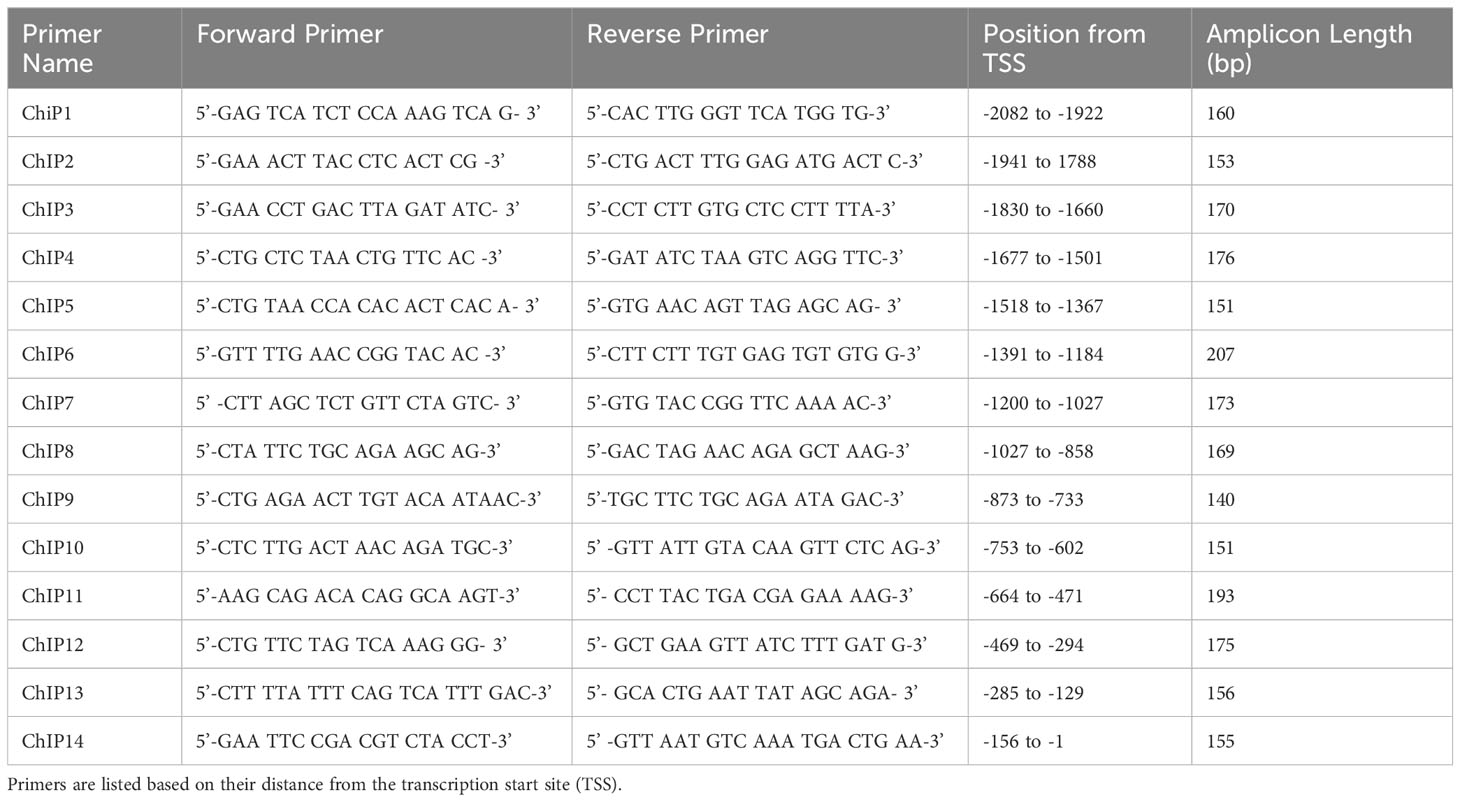
The CXCL10 promoter was screened for putative Ets-1 DNA binding sites (EBSs) through visual inspection and utilizing the MatInspector software tool (Genomatix, Ann Arbor, MI) (31). Fourteen primer pairs were designed to cover the 46 putative EBSs identified (Table 1). ChIP assay was performed using an anti-Fli-1 rabbit polyclonal antibody or normal rabbit IgG control using EpiTect ChIP OneDay Kit (Qiagen, Germantown, MD) as described (32). Briefly, chromatin was isolated from cross-linked MS1 cells and immunoprecipitated (IP) by antibodies specific for Fli-1 or normal rabbit IgG. After IP, the DNA was purified and amplified by PCR according to the manufacturer’s instructions (Qiagen, Germantown, MD). Fold change was calculated using the comparative Ct method 2−(ΔΔCT) where delta Ct was the difference between IgG and Fli-1 Ct values.
2.7 DNA transfection
The mouse embryonic fibroblast NIH3T3 cell line was grown in DMEM (Mediatech, Manassas, VA) with 10% FBS and 1% penicillin/streptomycin. The NIH3T3 cell line was used for all transient transfection experiments because it is a commonly used cell line for DNA transfection, and the expression level of Fli-1 in these cells is undetectable. Twenty four hours prior to DNA transfection the NIH3T3 cells were seeded at a concentration of 4 × 105 cells in 6 well plates. Cells were transfected using the FuGENE 6 transfection reagent (Promega, Madison, WI) following the manufacturer’s instructions. Reporter constructs were transfected into the cells at a concentration of 2 μg (pGL3/basic or pGL3/CXCL10) for all experiments. Increasing concentrations of the pcDNA/Fli-1 expression construct were used for the dose response study (0.05 μg, 0.1 μg, 0.2 μg, 0.25 μg, 0.5 μg, 1 μg and 2µg). Transfection of pcDNA/NF-κB p65 expression construct was used as positive control for driving Cxcl10 promoter activity. In all experiments empty expression vectors were used to ensure that an equivalent amount of total DNA was transfected into the cells. The Renilla luciferase construct pRL/TK (Promega) was co-transfected into the cells at a concentration of 200 ng to control for transfection efficiency. The NIH3T3 cells were harvested 48 h after the transfection experiment was completed. To measure the luciferase activity for the transient transfection experiments the Dual-luciferase reporter assay system (Promega) was employed. The Luminoskan Ascent Microplate Luminometer (Thermo Fisher Scientific) was used to detect luminescence. All experiments were normalized using the Renilla luciferase expression. Fold activation is reported as the mean + standard error compared to the empty pGL3/basic vector.
2.8 Statistics
The data are expressed as the means ± SEM or SD as indicated in figure legends. GraphPad Prism 7 software (GraphPad Software, San Diego, CA) was used for statistical analysis. Two-group Student t tests (paired or unpaired as appropriate) were applied. Differences between the means of multiple groups were compared by the one-way analysis of variance (ANOVA), followed by a Tukey multiple comparisons test, and adjusted p-values presented. A value of p<0.05 was considered statistically significant.
3 Results
3.1 Renal CXCL10 and CXCL9 concentrations are significantly reduced in Fli-1+/− MRL/lpr mice
Genetically reducing the expression of Fli-1 in two lupus mouse models significantly reduced renal damage with profoundly decreased infiltrating inflammatory cells, and prolonged survival (14, 15, 20, 33). We previously showed that the gene expression of Cxcl9 and Cxcl10 was reduced in the kidney of the MRL/lpr Fli-1+/- mice (27). To assess if CXCL9, or CXCL10 protein expression are also reduced in these mice, we measured the concentrations of these chemokines in the kidney of Fli-1+/− and wild-type littermates at the age of 22 weeks. Renal levels of CXCL10 (Figure 1A) and CXCL9 (Figure 1B), but not CXCL11 (Figure 1C), in Fli-1+/− MRL/lpr mice were significantly lower compared to wild-type littermates. Thus, renal CXCL9 and CXCL10 are decreased at the transcript and protein levels in lupus prone MRL/lpr Fli-1+/−- mice and suggests FLI-1 modulates CXCL10, CXCL9 expression in the kidneys during lupus nephritis.
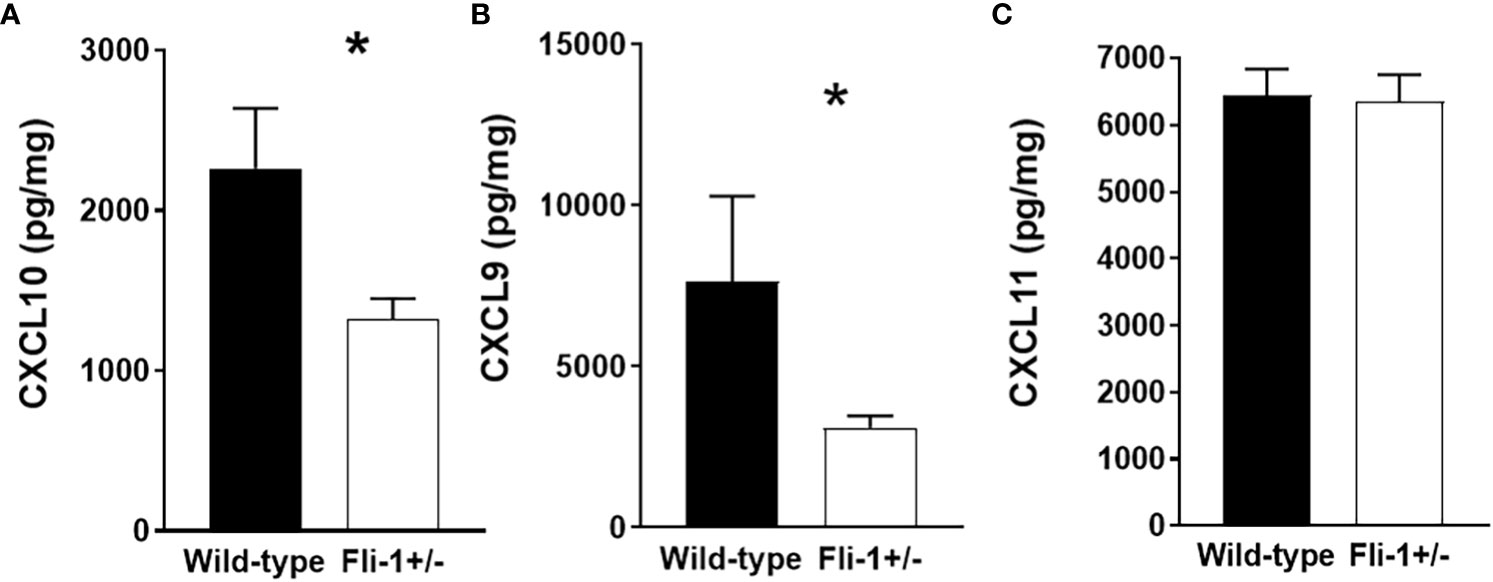
Figure 1 Decreased expression of CXCL10 and CXCL9 in kidneys from Fli-1+/- MRL/lpr mice compared to wild-type littermates. The total protein was extracted and prepared from kidneys of Fli-1+/- MRL/lpr mice and wild-type littermates at the age of 22 weeks (n = 12-13 in each group). Expressions of CXCL10 (A), CXCL9 (B), and CXCL11 (C) in kidneys were measured by ELISA. Data were shown as mean ± SEM. * indicates p < 0.05.
3.2 Reduced Fli-1 expression results in decreased secretion of CXCL10 in human and mouse endothelial cells
Kidney endothelial cells produce significant inflammatory cytokines and are implicated in nephritis development, and we demonstrated that FLI-1 is highly expressed in renal endothelial cells (20, 34). To assess if FLI-1 regulates the expression of CXCL9, CXCL10, or CXCL11, the primary human umbilical vein endothelial cells (HUVECs) were employed. HUVECs transfected with FLI-1 specific siRNA to knockdown FLI-1 expression produced significantly lower levels of CXCL10 compared to HUVECs transfected with control siRNA in response to LPS or TNF-α stimulation (Figure 2A). CXCL9 was not elevated by LPS or TNFα stimulation, and reducing FLI-1 expression had no significant effect on CXCL9 levels (Figure 2A). CXCL11 was significantly reduced in cells transfected with FLI-1 siRNA compared to cells transfected with control siRNA following LPS stimulation only. Reduction of FLI-1 protein in the cells transfected with FLI-1 siRNA was confirmed by immunoblotting (Supplementary Figure S1A).
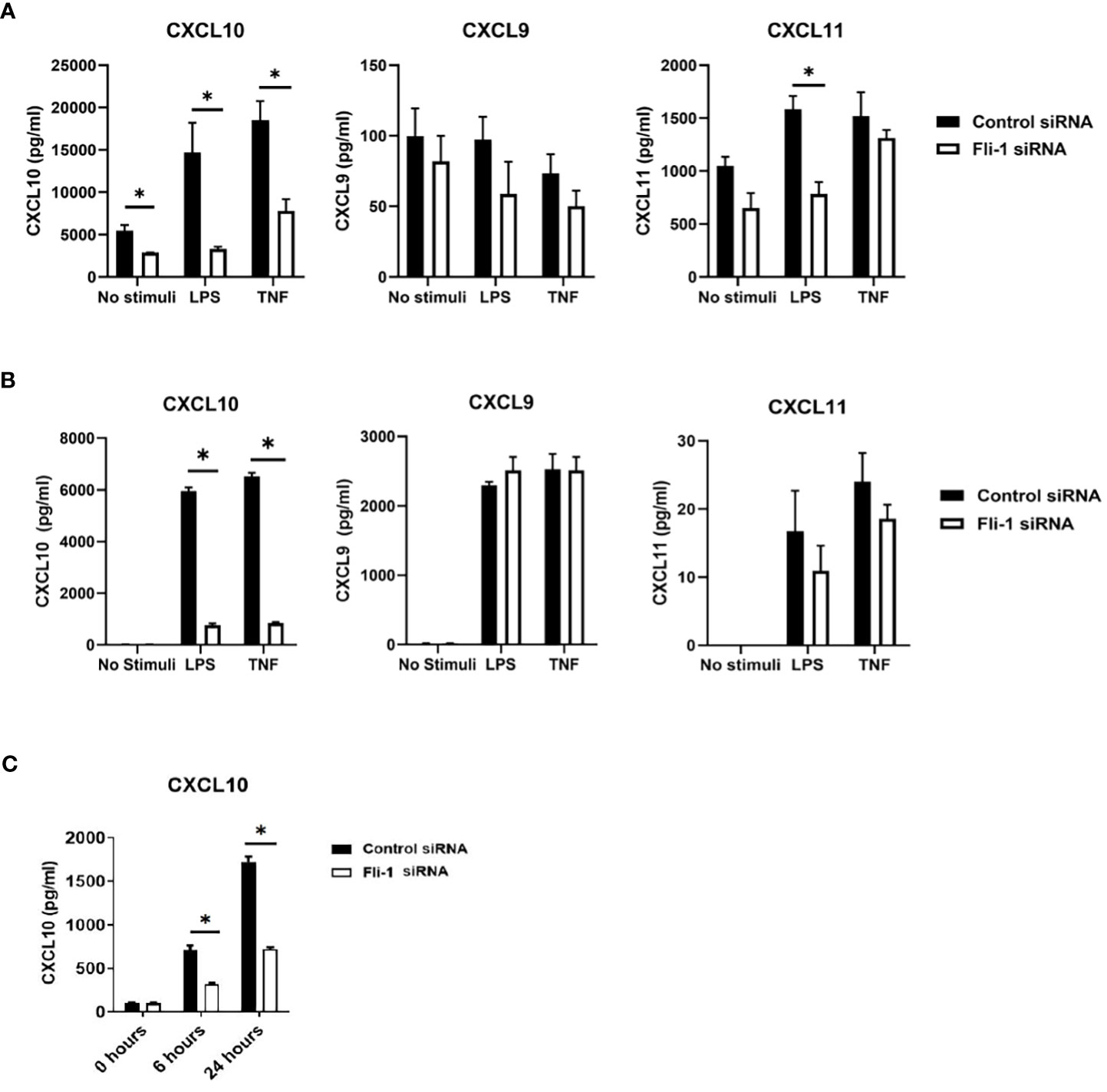
Figure 2 Inhibition of Fli-1 expression decreased production of CXCL10 in human umbilical vein endothelial cells (HUVECs), human renal glomerular endothelial cells (HRGECs), and mouse endothelial MS1 cells. HUVECs (A), or HRGECS (B) were transfected with Fli-1 specific or negative control siRNA for 24 hours and further stimulated with LPS (0.2 µg/ml) or TNFα (10 ng/ml) 24 hrs. Secretion of CXCL10, CXCL9, and CXCL11 in supernatant were measured by ELISA. N=3-4 in each group. The data were represented as mean ± SEM. * indicates p < 0.05. (C) MS1 endothelial cells were transfected with Fli-1 specific or negative control siRNA for 24 hours and stimulated with LPS (0.1 µg/ml) for another 6 or 24 hours. Secreted CXCL10, CXC9 and CXCL11 concentrations were measured by ELISA. CXCL9 and CXCL11 were undetectable. N=4 in each group. The data were represented as mean ± SEM. * indicates p< 0.05.
To confirm the results above and demonstrate relevance to lupus nephritis, the effects of FLI-1 on the production of CXCL10 in primary human renal glomerular endothelial cells (HRGECs) were assessed. HRGECs transfected with FLI-1 specific siRNA had reduced FLI-1 protein levels (Supplementary Figure S1B). LPS and TNF-α induced CXCL10 production at 24 hours, which were significantly suppressed by knockdown of FLI-1 (Figure 2B). CXCL9 and CXCL11 were also induced by LPS and TNFα, but were not significantly impacted by knockdown of FLI-1 (Figure 2B). These results suggest FLI-1 modulates CXCL10, but not CXCL9 or CXCL11 in primary HRGECs.
To determine if FLI-1 also regulates CXCL10 expression in mouse endothelial cells, Fli-1 specific siRNA or negative control siRNA was transfected into the murine endothelial cell line MS1. Reduced FLI-1 protein in the cells transfected with FLI-1 siRNA was confirmed by immunoblotting (Supplementary Figure S1C). LPS significantly elevated CXCL10 production in a dose-dependent manner (data not shown) in MS1 cells, and transfection with Fli-1 siRNA significantly decreased production of CXCL10 compared to the cells transfected with negative control siRNA at 6 and 24 hours (Figure 2C). CXCL9 and CXCL11 production from MS1 cells was undetectable in response to LPS (data not shown). Together, these data demonstrate that FLI-1 modulates the production of CXCL10 in both human and mouse endothelial cells.
3.3 FLI-1 binds to the Cxcl10 promoter
To determine if FLI-1 directly regulates CXCL10 production by binding to its promoter, we identified 46 putative ETS binding sites on the mouse Cxcl10 promoter and designed 14 primer pairs to cover all 46 sites (Figure 3A and Table 1). ChIP assays using these 14 primer pairs with MS1 cells were performed. Our previous study showed that FLI-1 regulates several chemokine genes through binding to their promoters, including MCP1 (32). Therefore, 1 pair of primers from the MCP1 promoter was used as a positive control for ChIP (ChIP Con, Figure 3B). Promoter regions ChIP3, ChIP4, ChIP7, and ChIP8 were significantly enriched more than 4-fold by FLI-1 specific antibodies compared to the IgG negative control. These results indicate that FLI-1 can bind directly to the Cxcl10 promoter and likely modulates CXCL10 production by upregulating its transcription in LPS- and TNFα-stimulated endothelial cells.
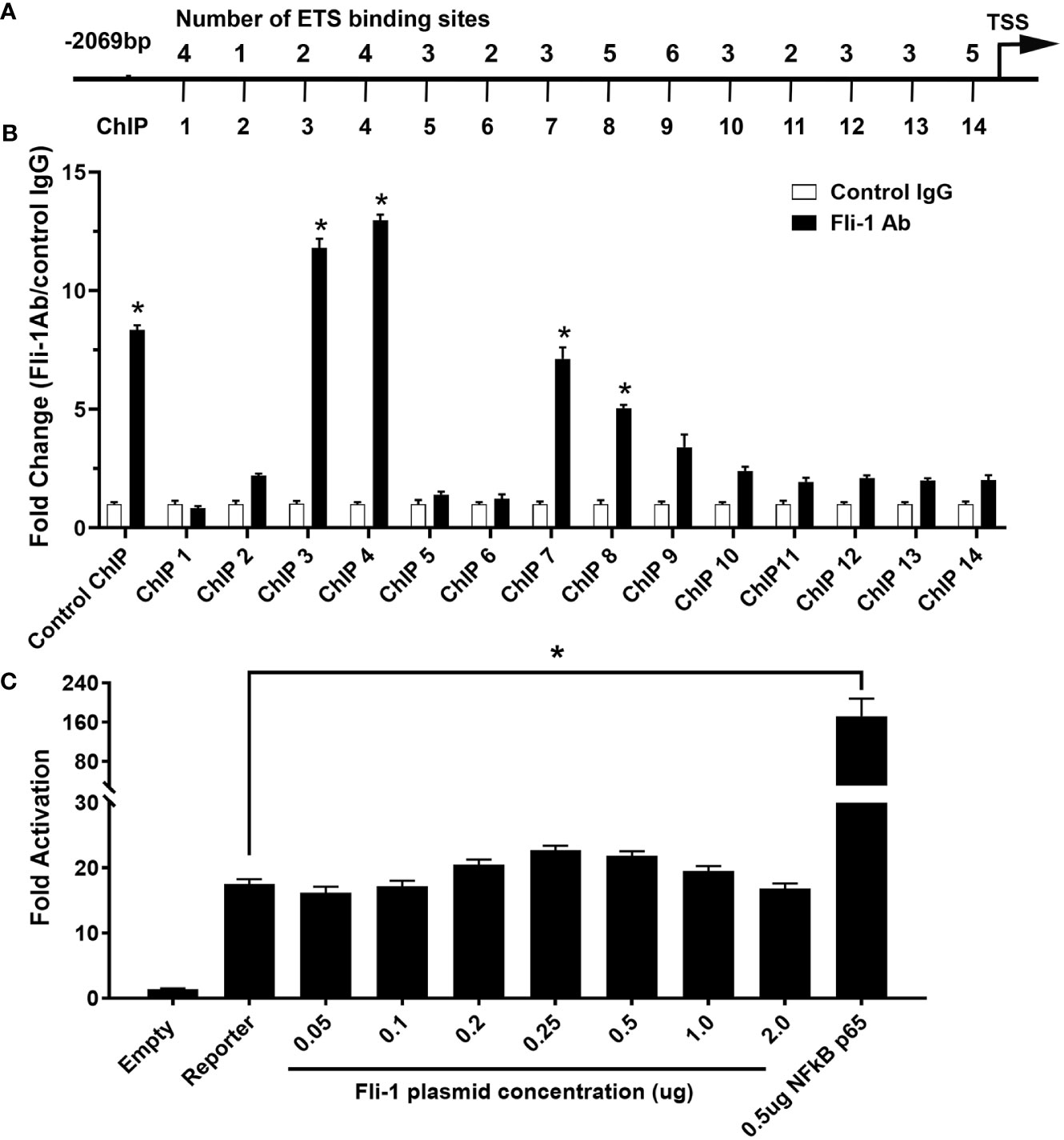
Figure 3 ChIP analysis of Fli-1 binding to the CXCL10 promoter, but Fli-1 failed to drive transcription from the CXCL10 promoter. (A) Putative Fli-1 binding sites within the murine CXCL10 promoter and the location of the primers used for the ChIP assay. The number of potential binding sites for each primer set is noted above. (B) FLI-1 binding in the ChIP assay was determined by enrichment of the indicated CXCL10 regions by IP with anti-Fli-1 antibody relative to enrichment of regions by IP with a control IgG antibody. A pair of primers for the MCP1 promoter previously shown to be enriched in a ChIP assay by anti-Fli-1 antibody was used as ChIP Control. * Indicates p<0.05. Data were represented as mean ± SEM from three independent experiments. (C) Human CXCL10 promoter cloned into the pGL3 vector, upstream of the luciferase reporter gene co-transfected with increasing amounts of a Fli-1 expression vector (0.05, 0.1, 0.2, 0.25, 0.5, 1 and 2 μg). Co-transfection of a NFkBp65 expression vector (0.5µg) was used as positive control. Values shown are fold activation over the empty vector control (mean + SEM for three replicate experiments; n = 9), * Indicates p<0.05.
3.5 FLI-1 failed to directly drive transcription from the human CXCL10 promoter
Transient transfection assays were performed to determine if FLI-1 can regulate the human CXCL10 gene. A pGL3/hCXCL10 promoter/reporter construct was used to determine if FLI-1 can drive transcription from the hCXCL10 promoter. The pGL3/hCXCL10 construct co-transfected with increasing amounts of a FLI-1 expression vector into the NIH3T3 cell line. FLI-1 failed to drive transcription from the human CXCL10 promoter even at high doses of the FLI-1 expression vector (Figure 3C). Co-transfection of a NF-κB p65 expression vector with the pGL3/hCXCL10 reporter construct was able to upregulate hCXCL10 promoter activity as reported previously (Figure 3C) (35). FLI-1 also failed to drive transcription from the mouse Cxcl10 promotor in the HUVECS (data not shown). These results indicate that FLI-1 may regulate the expression of CXCL10 through an indirect or novel mechanism. Alternatvely, the cell lines used in these studies lacked an important co-activator necessary for FLI-1 to drive, or contained a corepressor that inhibited FLI-1 from driving, CXCL10 expression.
3.6 CPT, a potential Fli-1 inhibitor, reduced CXCL10 production in human renal glomerular endothelial cells
Camptothecin (CPT) was identified as an effective Fli-1 inhibitor in a screen of known small molecule/compound libraries (36). Thus, CPT was tested to determine if it inhibits endogenous FLI-1 expression in HRGECs. HRGECs treated with CPT for 24 hours at 0.25 µM demonstrated that CPT inhibited FLI-1 protein expression (Supplementary Figure 2). Moreover, 0.25 µM CPT significantly reduced IFNγ-induced CXCL10 secretion in HRGECs at 4 and 24 hours (Figure 4).

Figure 4 CPT inhibits Fli-1 expression and reduces CXCL10 production in HRGECs. HRGECs were treated with CPT at 0.25 µM or a DMSO vehicle control for 24 hours and CXCL10 concentrations in media from HRGECs were measured by ELISA at 1, 4 and 24 hours after stimulation with 100 ng/ml of IFN-γ N=4 in each group. *p<0.05.
3.7 The DNA-binding domain of FLI-1 is necessary for its regulation of CXCR3 promoter activity in T cells
CXCL10 expression helps recruit cells expressing its receptor CXCR3. A previous study from our lab demonstrated that reducing FLI-1 levels in MRL/lpr mice significantly reduced the numbers of renal infiltrating CXCR3+ T cells and that FLI-1 regulates the promoter activity of the human CXCR3 (hCXCR3) promoter in T cells (27). To further examine the mechanism by which FLI-1 regulates hCXCR3 promoter activity, Jurkat T cells were co-transfected with a pcDNA vector expressing wild-type FLI-1 and either the full-length hCXCR3 (-485/+284) or various hCXCR3 5’ deletion promoter/reporter (P/R) constructs (Figure 5A). Results indicate that most of the FLI-1 regulated hCXCR3 promoter activity is located between -89 and +101 (Figure 5A). Our previous results also suggested that although the DNA binding region of FLI-1 was required for upregulating hCXCR3 promoter activity, FLI-1 binding to three conserved putative ETS binding sites with high homology for FLI-1 was not detected in in vitro binding assays (27). Two of these three putative binding sites are located within the -89 to +100 region. The third site, and least homologous for FLI-1, is located between +200 and +284 region of the hCXCR3 promoter.

Figure 5 FLI-1 directly regulates promoter activity of the human CXCL10 receptor hCXCR3. Jurkat T cells were transiently transfected with hCXCR3 promoter/reporter (P/R) constructs without or with FLI-1 expression vectors and stimulated by addition of PMA and ionomycin. (A) hCXCR3 deletion P/R constructs indicated in the graph were co-transfected with 0.8 μg of the expression vector pcDNA without (Empty) or with wild-type FLI-1 (WT). Locations of putative FLI-1 binding sites in hCXCR3 identified previously (27) are indicated as hatched boxes on the constructs depicted to the left of the graph. The full-length -485/+284 construct co-transfected with the Empty expression vector was set to 1 with all other values relative to its expression. *p-values on the graph compares transfection with WT to Empty for each construct and the other p-values on the graph compares the WT transfected deletion hCXCR3 constructs to the WT transfected -485/+284 construct. (B) hCXCR3 -89/+284 P/R vector was co-transfected with a pSG WT FLI-1 (WT) expression vector or the pSG FLI-1 mutant expression vectors indicated on the graph. DNA-binding mutant (DBM); deletion of the C-terminal transactivation domain (ΔCTA); acetylation mutant (Acet); phosphorylation mutant (Phos). Transfection with the pSG WT vector was set to 1 and expression with the FLI-1 mutants is relative to WT. p-values are comparison of each mutant to WT FLI-1. (C) hCXCR3 -89/+284 P/R vector was co-transfected with pcDNA Empty (0.8 μg) +pSG Empty (1.6 μg), pcDNA WT FLI-1 (0.8 μg) +pSG Empty (1.6 μg), or pcDNA WT FLI-1 (0.8 μg) +increasing amounts (0.4, 0.8, 1.6 μg) of the pSG FLI-1 mutants indicated. All values are presented relative to expression of transfection with the Empty pcDNA and pSG vectors, which was set to 1. p-values on the graph include comparison on WT +pSG Empty to pcDNA/pSG Empty, and global p-values for WT +increasing amounts of each mutant compared to WT +pSG Empty. Transfections were performed in duplicate, and each transfection was performed twice with similar results. Data presented as relative promoter activity and are the average of means ±SD. p-values were calculated as described in materials and methods.
To further determine whether FLI-1 regulates hCXCR3 by directly binding and transactivating the promoter, Jurkat T cells were transfected with P/R construct hCXCR3 -89/+284 containing all three putative FLI-1 binding sites and similar activity to the full-length -435/+284 construct along with expression vectors for wild-type (WT) or various mutants of FLI-1. As we demonstrated previously with the full-length -485/+284 promoter construct (27), the FLI-1 DNA binding mutant (DBM) does not upregulate -89/+284 hCXCR3 promoter activity (Figure 5B). Interestingly, a FLI-1 mutant missing the C-terminal transactivation domain (ΔCTA) does not impact the ability of FLI-1 to upregulate hCXCR3 promoter activity while mutations preventing FLI-1 acetylation (Acet) or phosphorylation (Phos) upregulated the activity of the hCXCR3 promoter better than wild-type FLI-1. We then used a competition assay to further assess the mechanism by which FLI-1 upregulates -89/+284 hCXCR3 promoter activity. For this experiment, WT FLI-1 was co-transfected without or with increasing amounts of each FLI-1 mutant. The FLI-1 DBM significantly and dose-dependently inhibited, while the Acet and Phos FLI-1 mutants further increased, hCXCR3 promoter activity stimulated with WT FLI-1 (Figure 5C). These results show that although FLI-1 requires its DNA binding domain to upregulate hCXCR3, it can block WT FLI-1 from activating the hCXCR3 promoter. This suggests the FLI-1 DBM likely inhibits WT FLI-1 by interacting with and sequestering co-activators needed for FLI-1 to upregulate hCXCR3 promoter activity. Furthermore, the acetylation and phosphorylation status appear to modulate FLI-1 activity in regulating hCXCR3.
Discussion
Chemokines, such as CXCL10, play a critical role in the inflammatory/autoimmune response, particularly in recruiting inflammatory/immune cells expressing receptors for chemokines, such as CXCR3, to areas of disease or injury (24, 37, 38). A recent report demonstrated that CXCR3 deficiency decreases autoantibody production and inhibits aberrant activated T follicular helper cells and B cells in lupus mice (39). Elevated expression of CXCL10 is strongly correlated with the pathogenesis of clinical LN, neuropsychiatric lupus (NPSLE), and the disease activity of SLE (24, 25, 40). A majority of renal infiltrating T cells, a hallmark of nephritis in SLE patients, express CXCR3, the receptor for CXCL10 (26, 41). CXCR3+ T cells are mainly observed in the interstitial infiltrate located in periglomerular and perivascular regions of the kidney. In these regions, CXCR3 mRNA expression correlated with CXCL10 mRNA expression, CXCR3+ T cells co-localized with CXCL10-producing cells, and both expression levels and numbers of cells expressing CXCR3 correlated with clinical markers of disease (26, 41). Loss of CXCR3 in the MRL/lpr lupus strain results in significantly reduced renal T cell infiltration and improved disease (42). Our previous studies demonstrated that MRL/lpr lupus mice with reduced FLI-1 levels exhibited decreased renal Cxcl9 and Cxcl10 mRNA levels and reduced numbers of CXCR3+ T cells infiltrating the kidneys (27). Our present study confirmed that renal CXCL9 and CXCL10 expression at the protein level are significantly decreased in Fli-1+/− MRL/lpr mice. We also demonstrated that FLI-1 impacts the expression of CXCL10 in human and mouse endothelial cells, and CXCR3, the receptor for CXCL10, in human T cells. Together, the combined results strongly suggest an important role for FLI-1 in modulating the CXCL10-CXCR3 axis and thus, in promoting nephritis, further supporting FLI-1 as potential therapeutic target.
FLI-1 impacts glomerulonephritis development by regulating inflammatory chemokines in endothelial cells, as well as inflammatory cell infiltration in the kidneys during lupus (10, 14, 18, 27, 32, 33, 43, 44). Here we showed that FLI-1 knockdown reduced production of CXCL10 in all endothelial cells tested and under all stimulatory conditions while production of CXCL9 and CXCL11 was not impacted or was impacted in only certain cells/conditions. Hence, the impact of FLI-1 on CXCL10 expression was consistent between the mouse kidney tissue and mouse or human endothelial cells. CXCL10 is regulated by nuclear factor kappa B (NF kappa B) protein, cRel, through direct binding to the promoter in mouse embryo fibroblasts (45). Signaling pathways p38, JNK, ERK and Akt also were reported to regulate the transcription of CXCL10 (38), highlighting the complex mechanisms involved in the transcriptional regulation of CXCL10. Epithelial cells were found to increase their expression of CXCL10 in response to IFN stimulation (46). However, it should be noted that Fli-1 is not detectable in epithelial cells (data not shown), and therefore, we have not yet investigated the impact of Fli-1 on CXCL10 expression in these cells. While our results show that FLI-1 can directly bind to the CXCL10 promoter, FLI-1 did not drive transcription from the human or mouse CXCL10 promoters. There are several potential explanations to interpret this result. First, a co-factor not expressed by NIH3T3 cells may be required for Fli-1 to drive transcription from the CXCL10 promoter, or these cells express a corepressor that inhibits Fli-1 activation of the CXCL10 promoter. We attempted to address this by transfecting the Fli-1 expression vector into HUVEC cells, along with the full-length CXCL10 promoter. However, Fli-1 also failed to drive transcription in HUVEC cells. Another possible explanation is that the binding of Fli-1 to the CXCL10 promoter alters the chromatin structure, which could either prevent or enhance the binding of other factors involved in regulating CXCL10. In fact, a recent report concluded that FLI-1 modulates the expression of many genes by influencing chromatin accessibility (47). Thus, FLI-1 may be indirectly regulating CXCL10 expression by altering the chromatin structure of the gene. Understanding the mechanisms by which CXCL10-CXCR3 axis is regulated could assist in the generation of therapeutic interventions for human diseases.
We also demonstrated previously that in T cells, FLI-1 drives transcription from the promoter of CXCR3, the receptor for CXCL10 (27). In this study, we further determined that maximal upregulation of CXCR3 promoter activity by FLI-1 requires the -89 to +100 region of the promoter, which contains two putative ETS binding sites. Additionally, we demonstrated that although a DNA-binding mutant of FLI-1 could not upregulate CXCR3 promoter activity, it significantly inhibited activation of the CXCR3 promoter by wild-type FLI-1, likely by sequestering a required co-factor. The FLI-1 construct lacking the C-terminal transactivation (CTA) domain retained its ability to activate the CXCR3 promoter. Together, these results suggest FLI-1 activates the CXCR3 promoter by binding to the promoter and interacting with transcriptional co-activators outside of its DNA-binding or CTA domains. We were unsuccessful in demonstrating direct binding of FLI-1 to the CXCR3 promoter in vitro (27); however, the conditions may not have been ideal for FLI-1 binding in vitro. Future studies aimed at assessing in vivo binding will likely be more informative. In addition, based on our results, the phosphorylation and acetylation status appears to regulate FLI-1 function in transcriptional activation of the CXCR3 promoter. Thus, post-translational modifications of FLI-1 also may play a role in regulating CXCR3, which was previously demonstrated for its regulation of the G-CSF or COL1A2 promoters (21, 28, 48).
Certain regimens incorporating cytotoxic drugs, especially cyclophosphamide, provide a significant therapeutic advantage over corticosteroids alone in the management of lupus disease (49). Cytotoxic drugs frequently have adverse effects and vary from person to person during the lupus treatment process. Since reducing FLI-1 decreases expression of CXCL10, FLI-1 may be an alternative drug target for lupus, an ongoing focus in our lab. Cytotoxic drug CPT is a FLI-1 inhibitor screened from known small molecule/compound libraries (36). CPT has been widely used in cancer therapy since the late 20th century (50). Herein we demonstrated that CPT significantly inhibited FLI-1 protein levels in a dose-dependent manner and reduced IFNγ-induced CXCL10 secretion in human renal endothelial cells. Indeed, low dose CPT markedly ameliorated lupus nephritis in (NZB × NZW)F1 mice (51). The role of CXCL10/CXCR3 in anti-GBM nephritis has been well established (52). Future studies are aimed at investigating the impact of CPT on nephritis by inhibiting Fli-1 modulation of the CXCL10/CXCR3 axis.
In summary, our studies support FLI-1 as a novel transcription factor modulating gene expression of inflammatory chemokine CXCL10 in endothelial cells and its receptor CXCR3 in T cells. Considering the emerging evidence that the CXCL10-CXCR3 axis plays a critical role in lupus and other inflammatory/autoimmune diseases, our results provide additional insight in defining the mechanisms by which FLI-1 modulates inflammation and disease development. Inhibition of FLI-1 may serve as a therapeutic approach for reducing inflammatory diseases by preventing upregulation of the CXCL10-CXCR3 axis in addition to multiple other cytokines, all of which contribute to renal inflammation and injury in LN (10, 18, 20, 32). Thus, we provide additional evidence that FLI-1 may serve as a global key regulator of inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by The Institutional Animal Care and Use Committee/Medical University of South Carolina. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
Study conception and design: XZ, TN: Acquisition of data: XW, XZ, TN, ML, TSC, KS, SS, Analysis and interpretation of data: XW, XZ, TN, ML, TSC, KS, SS. All authors contributed to the article and approved the submitted version.
Funding
This research was supported in part by the pilot project from the South Carolina Clinical & Translational Research (SCTR) Institute (SCTR 2303) and Bridge Funds from the College of Medicine at Medical University of South Carolina.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1219279/full#supplementary-material
References
1. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers (2020) 6(1):7. doi: 10.1038/s41572-019-0141-9
2. Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis Res Ther (2011) 13(6):250. doi: 10.1186/ar3528
3. Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrology dialysis Transplant (2012) 27(8):3248–54. doi: 10.1093/ndt/gfs073
4. Sule S, Fivush B, Neu A, Furth S. Increased hospitalizations and death in patients with ESRD secondary to lupus. Lupus (2012) 21(11):1208–13. doi: 10.1177/0961203312451506
5. Bhargava R, Li H, Tsokos GC. Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr Opin Rheumatol (2023) 35(2):107–16. doi: 10.1097/BOR.0000000000000887
6. Belmont HM. Treatment of systemic lupus erythematosus - 2013 update. Bull Hosp Joint Dis (2013) (2013) 71(3):208–13.
7. Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmun Rev (2012) 12(2):174–94. doi: 10.1016/j.autrev.2012.08.018
8. Zhang L, Lemarchandel V, Romeo PH, Ben-David Y, Greer P, Bernstein A. The Fli-1 proto-oncogene, involved in erythroleukemia and Ewing's sarcoma, encodes a transcriptional activator with DNA-binding specificities distinct from other Ets family members. Oncogene (1993) 8(6):1621–30.
9. Watson DK, Smyth FE, Thompson DM, Cheng JQ, Testa JR, Papas TS, et al. The ERGB/Fli-1 gene: isolation and characterization of a new member of the family of human ETS transcription factors. Cell Growth Differ (1992) 3(10):705–13.
10. Sato S, Lennard Richard M, Brandon D, Jones Buie JN, Oates JC, Gilkeson GS, et al. A critical role of the transcription factor fli-1 in murine lupus development by regulation of interleukin-6 expression. Arthritis Rheumatol (2014) 66(12):3436–44. doi: 10.1002/art.38818
11. Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I, et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun (2014) 5:5797. doi: 10.1038/ncomms6797
12. Kovar H. Blocking the road, stopping the engine or killing the driver? Advances in targeting EWS/FLI-1 fusion in Ewing sarcoma as novel therapy. Expert Opin Ther Targets (2014) 18(11):1315–28. doi: 10.1517/14728222.2014.947963
13. Zhang L, Eddy A, Teng YT, Fritzler M, Kluppel M, Melet F, et al. An immunological renal disease in transgenic mice that overexpress Fli-1, a member of the ets family of transcription factor genes. Mol Cell Biol (1995) 15(12):6961–70. doi: 10.1128/MCB.15.12.6961
14. Zhang XK, Gallant S, Molano I, Moussa OM, Ruiz P, Spyropoulos DD, et al. Decreased expression of the Ets family transcription factor Fli-1 markedly prolongs survival and significantly reduces renal disease in MRL/lpr mice. J Immunol (2004) 173(10):6481–9. doi: 10.4049/jimmunol.173.10.6481
15. Mathenia J, Reyes-Cortes E, Williams S, Molano I, Ruiz P, Watson DK, et al. Impact of Fli-1 transcription factor on autoantibody and lupus nephritis in NZM2410 mice. Clin Exp Immunol (2010) 162(2):362–71. doi: 10.1111/j.1365-2249.2010.04245.x
16. Georgiou P, Maroulakou I, Green J, Dantis P, ROmanospica V, Kottaridis S, et al. Expression of ets family of genes in systemic lupus erythematosus and Sjogren's syndrome. Int J Oncol (1996) 9(1):9–18. doi: 10.3892/ijo.9.1.9
17. Mackay M, Oswald M, Sanchez-Guerrero J, Lichauco J, Aranow C, Kotkin S, et al. Molecular signatures in systemic lupus erythematosus: distinction between disease flare and infection. Lupus Sci Med (2016) 3(1):e000159. doi: 10.1136/lupus-2016-000159
18. Lennard Richard ML, Sato S, Suzuki E, Williams S, Nowling TK, Zhang XK. The Fli-1 transcription factor regulates the expression of CCL5/RANTES. J Immunol (2014) 193(6):2661–8. doi: 10.4049/jimmunol.1302779
19. Lou N, Lennard Richard ML, Yu J, Kindy M, Zhang XK. The Fli-1 transcription factor is a critical regulator for controlling the expression of chemokine C-X-C motif ligand 2 (CXCL2). Mol Immunol (2017) 81:59–66. doi: 10.1016/j.molimm.2016.11.007
20. Suzuki E, Karam E, Williams S, Watson DK, Gilkeson G, Zhang XK. Fli-1 transcription factor affects glomerulonephritis development by regulating expression of monocyte chemoattractant protein-1 in endothelial cells in the kidney. Clin Immunol (2012) 145(3):201–8. doi: 10.1016/j.clim.2012.09.006
21. Lennard Richard ML, Brandon D, Lou N, Sato S, Caldwell T, Nowling TK, et al. Acetylation impacts Fli-1-driven regulation of granulocyte colony stimulating factor. Eur J Immunol (2016) 46(10):2322–32. doi: 10.1002/eji.201646315
22. Lou N, Lennard Richard ML, Yu J, Kindy M, Zhang XK. The Fli-1 transcription factor is a critical regulator for controlling the expression of chemokine C-X-C motif ligand 2 (CXCL2). Mol Immunol (2016) 81:59–66. doi: 10.1016/j.molimm.2016.11.007
23. Clark-Lewis I, Mattioli I, Gong JH, Loetscher P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J Biol Chem (2003) 278(1):289–95. doi: 10.1074/jbc.M209470200
24. Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev (2014) 13(3):272–80. doi: 10.1016/j.autrev.2013.10.010
25. Zhang CX, Cai L, Shao K, Wu J, Zhou W, Cao LF, et al. Serum IP-10 is useful for identifying renal and overall disease activity in pediatric systemic lupus erythematosus. Pediatr Nephrol (Berlin Germany) (2018) 33(5):837–45. doi: 10.1007/s00467-017-3867-1
26. Enghard P, Humrich JY, Rudolph B, Rosenberger S, Biesen R, Kuhn A, et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis rheumatism (2009) 60(1):199–206. doi: 10.1002/art.24136
27. Sundararaj KP, Thiyagarajan T, Molano I, Basher F, Powers TW, Drake RR, et al. FLI1 levels impact CXCR3 expression and renal infiltration of T cells and renal glycosphingolipid metabolism in the MRL/lpr lupus mouse strain. J Immunol (2015) 195(12):5551–60. doi: 10.4049/jimmunol.1500961
28. Asano Y, Czuwara J, Trojanowska M. Transforming growth factor-beta regulates DNA binding activity of transcription factor Fli1 by p300/CREB-binding protein-associated factor-dependent acetylation. J Biol Chem (2007) 282(48):34672–83. doi: 10.1074/jbc.M703907200
29. Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol (2009) 29(7):1882–94. doi: 10.1128/MCB.01320-08
30. Svenson JL, Chike-Harris K, Amria MY, Nowling TK. The mouse and human Fli1 genes are similarly regulated by Ets factors in T cells. Genes Immun (2010) 11(2):161–72. doi: 10.1038/gene.2009.73
31. Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, et al. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinf (Oxford England) (2005) 21(13):2933–42. doi: 10.1093/bioinformatics/bti473
32. Lennard Richard ML, Nowling TK, Brandon D, Watson DK, Zhang XK. Fli-1 controls transcription from the MCP-1 gene promoter, which may provide a novel mechanism for chemokine and cytokine activation. Mol Immunol (2015) 63(2):566–73. doi: 10.1016/j.molimm.2014.07.013
33. Sato S, Zhang XK. The Friend leukaemia virus integration 1 (Fli-1) transcription factor affects lupus nephritis development by regulating inflammatory cell infiltration into the kidney. Clin Exp Immunol (2014) 177(1):102–9. doi: 10.1111/cei.12310
34. Dimou P, Wright RD, Budge KL, Midgley A, Satchell SC, Peak M, et al. The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis. Sci Rep (2019) 9(1):8348. doi: 10.1038/s41598-019-44868-y
35. Brownell J, Bruckner J, Wagoner J, Thomas E, Loo YM, Gale M Jr., et al. Direct, interferon-independent activation of the CXCL10 promoter by NF-κB and interferon regulatory factor 3 during hepatitis C virus infection. J Virol (2014) 88(3):1582–90. doi: 10.1128/JVI.02007-13
36. Li YJ, Zhao X, Vecchiarelli-Federico LM, Li Y, Datti A, Cheng Y, et al. Drug-mediated inhibition of Fli-1 for the treatment of leukemia. Blood Cancer J (2012) 2(1):e54. doi: 10.1038/bcj.2011.52
37. Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev (2009) 8(5):379–83. doi: 10.1016/j.autrev.2008.12.002
38. Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth factor Rev (2011) 22(3):121–30. doi: 10.1016/j.cytogfr.2011.06.001
39. Wang G, Sun Y, Jiang Y, Li S, Liu Y, Yuan Y, et al. CXCR3 deficiency decreases autoantibody production by inhibiting aberrant activated T follicular helper cells and B cells in lupus mice. Mol Immunol (2023) 156:39–47. doi: 10.1016/j.molimm.2023.02.009
40. Narumi S, Takeuchi T, Kobayashi Y, Konishi K. Serum levels of ifn-inducible PROTEIN-10 relating to the activity of systemic lupus erythematosus. Cytokine (2000) 12(10):1561–5. doi: 10.1006/cyto.2000.0757
41. Segerer S, Banas B, Wörnle M, Schmid H, Cohen CD, Kretzler M, et al. CXCR3 is involved in tubulointerstitial injury in human glomerulonephritis. Am J Pathol (2004) 164(2):635–49. doi: 10.1016/S0002-9440(10)63152-5
42. Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol (2009) 183(7):4693–704. doi: 10.4049/jimmunol.0802626
43. Sato S, Zhang XK, Temmoku J, Fujita Y, Matsuoka N, Yashiro-Furuya M, et al. Ets family transcription factor Fli-1 promotes leukocyte recruitment and production of IL-17A in the MRL/lpr mouse model of lupus nephritis. Cells (2020) 9(3):714–25. doi: 10.3390/cells9030714
44. Suzuki E, Williams S, Sato S, Gilkeson G, Watson DK, Zhang XK. The transcription factor Fli-1 regulates monocyte, macrophage and dendritic cell development in mice. Immunology (2013) 139(3):318–27. doi: 10.1111/imm.12070
45. Wei L, Fan M, Xu L, Heinrich K, Berry MW, Homayouni R, et al. Bioinformatic analysis reveals cRel as a regulator of a subset of interferon-stimulated genes. J Interferon Cytokine Res (2008) 28(9):541–51. doi: 10.1089/jir.2007.0136
46. Nakaya I, Wada T, Furuichi K, Sakai N, Kitagawa K, Yokoyama H, et al. Blockade of IP-10/CXCR3 promotes progressive renal fibrosis. Nephron Exp Nephrol (2007) 107(1):e12–21. doi: 10.1159/000106505
47. Chen Z, Arai E, Khan O, Zhang Z, Ngiow SF, He Y, et al. In vivo CD8(+) T cell CRISPR screening reveals control by Fli1 in infection and cancer. Cell (2021) 184(5):1262–80.e1222. doi: 10.1016/j.cell.2021.02.019
48. Asano Y, Markiewicz M, Kubo M, Szalai G, Watson DK, Trojanowska M. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol Cell Biol (2009) 29(2):425–34. doi: 10.1128/MCB.01278-08
49. Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol (2014) 10(2):97–107. doi: 10.1038/nrrheum.2013.157
50. Martino E, Della Volpe S, Terribile E, Benetti E, Sakaj M, Centamore A, et al. The long story of camptothecin: From traditional medicine to drugs. Bioorganic medicinal Chem Lett (2017) 27(4):701–7. doi: 10.1016/j.bmcl.2016.12.085
51. Wang X, Oates JC, Helke KL, Gilkeson GS, Zhang XK. Camptothecin and topotecan, inhibitors of transcription factor Fli-1 and topoisomerase, markedly ameliorate lupus nephritis in (NZB × NZW)F1 mice and reduce the production of inflammatory mediators in human renal cells. Arthritis Rheumatol (2021) 73(8):1478–88. doi: 10.1002/art.41685
Keywords: CXCL10, CXCR3, chemokine, friend leukemia virus integration 1 transcription factor, inflammation, lupus nephritis
Citation: Wang X, Richard ML, Caldwell TS, Sundararaj K, Sato S, Nowling TK and Zhang XK (2023) Role of the transcription factor Fli-1 on the CXCL10/CXCR3 Axis*. Front. Immunol. 14:1219279. doi: 10.3389/fimmu.2023.1219279
Received: 08 May 2023; Accepted: 29 August 2023;
Published: 15 September 2023.
Edited by:
Jillian M. Richmond, University of Massachusetts Medical School, United StatesReviewed by:
Hege Lynum Pedersen, UiT The Arctic University of Norway, NorwayFlorencia Rosetti, National Institute of Medical Sciences and Nutrition Salvador Zubirán, Mexico
Copyright © 2023 Wang, Richard, Caldwell, Sundararaj, Sato, Nowling and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian K. Zhang, emhhbmdqb0BtdXNjLmVkdQ==; Tamara K. Nowling, bm93aW5nQG11c2MuZWR1
 Xuan Wang
Xuan Wang Mara Lennard Richard2
Mara Lennard Richard2 Tamara K. Nowling
Tamara K. Nowling Xian K. Zhang
Xian K. Zhang