- Department of Internal Medicine 2, Rheumatology/Clinical Immunology, University Hospital Würzburg, Würzburg, Germany
Autologous hematopoietic stem cell transplantation (aHSCT) represents an effective treatment option in patients with severe forms of systemic sclerosis (SSc) by resetting the immune system. Nevertheless, secondary autoimmune disorders and progressive disease after aHSCT might necessitate renewed immunosuppressive treatments. This is particularly challenging when organ dysfunction, i.e., end-stage kidney failure, is present. In this case report, we present the unique case of a 43-year-old female patient with rapidly progressive diffuse systemic sclerosis who underwent aHSCT despite end-stage renal failure as consequence of SSc-renal crisis. Therefore, conditioning chemotherapy was performed with melphalan instead of cyclophosphamide with no occurrence of severe adverse events during the aplastic period and thereafter. After aHSCT, early disease progression of the skin occurred and was successfully treated with secukinumab. Thereby, to the best of our knowledge, we report the first case of successful aHSCT in a SSc-patient with end-stage kidney failure and also the first successful use of an IL-17 inhibitor to treat early disease progression after aHSCT.
1 Introduction
Since the ASSIST, ASTIS, and SCOT trials, autologous hematopoietic stem cell transplantation (aHSCT) is an established and proven highly effective treatment of progressive and refractory systemic sclerosis (SSc) (1–5). Despite this effective treatment, progressive disease and secondary autoimmune diseases after aHSCT might necessitate renewed immunosuppressive treatments in up to 60% of the cases (6, 7). So far, there are no recommendations for managing this scenario, especially when further challenging aspects like organ dysfunctions, i.e. kidney failure, are present (7).
In Japanese trials, inhibition of the IL-17 pathway has recently been described as an effective and promising new target in treating SSc (8, 9). In this context, we want to report our case of a 43-years old female patient with end stage kidney disease and hemodialysis after renal crisis and early skin progress after aHSCT, effectively treated with the IL-17A inhibitor secukinumab.
2 Case presentation
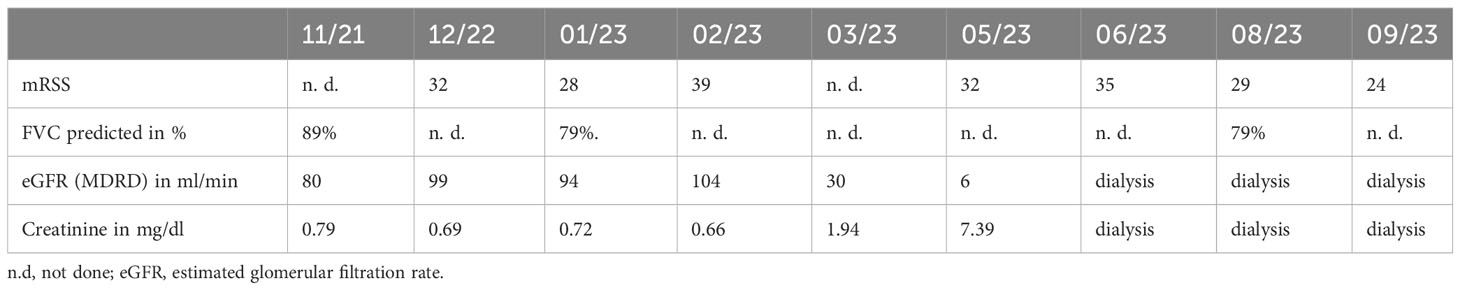
In December 2022, a 43-year-old female patient with Scl-70 positive and rapidly-progressive SSc was admitted to our clinic for initiation of immunosuppressive treatment. Diagnosis of SSc with pulmonary and cardiac involvement had been established one year before. Initial modified Rodnan skin score (mRSS) was 32, forced vital capacity (FVC) was 79% predicted, and estimated glomerular filtration rate (eGFR, MDRD) was 99 ml/min (see Table 1). Assessment of mRSS was routinely performed by the same senior consultant in rheumatology during the case, an experienced investigator of the former ASTIS trial. Due to progressive skin manifestation, treatment with cyclophosphamide (cyc) 750mg/m² was initiated. After two cycles with initial treatment response, a flare with rapid skin progression occurred in January 2023 (see Figure 1) associated with stiffness, joint pain and severe impairment in daily activities. In this situation, the indication for aHSCT was set. Rituximab was administered in a dose of 1x 1000mg as a bridging therapy to transplant. Analogous to the ASTIS protocol, mobilization chemotherapy for stem cell apheresis containing cyc in a dose of 2000mg/m² per day on two consecutive days was used followed by stimulation with granulocyte colony-stimulating factor (G-CSF) in February 2023. After successful apheresis of CD34-selected stem cells, the patient was discharged with a slightly reduced eGFR of 72 ml/min.

Figure 1 Modified Rodnan skin score in dependence on the treatment regimen from December 2022 until September 2023. Assessment of mRSS was always performed by the same investigator. The extent of the mRSS on the y-axis is shown over time. Early disease progress after aHSCT made treatment with SEC necessary. SEC achieved a rapid and sustained skin response. For the exact values, see Table 1. CYC, cyclophosphamide; RTX, rituximab; HD, high dosage chemotherapy; PBSCT, peripheral blood stem cell transplant; SEC, secukinumab.
At re-admission for aHSCT three weeks later, the patient presented progressive renal failure with an eGFR of 30ml/min, mild hemolysis, 0,7% schistocytes, and severe arterial hypertension (mean blood pressure values of 190/90 mmHg). After the exclusion of other causes, we interpreted this complication as a renal crisis, a known complication of SSc (10, 11). Therefore, we administered high-dose of an ACE inhibitor (captopril) and calcium channel inhibitor (amlodipine) to control blood pressure (10, 11). Due to aggressive and extensive skin involvement (mRSS 39 at that time), we decided to perform high-dosage chemotherapy with aHSCT after an ineffective trial with upadacitinib for two weeks. The JAK inhibitor (JAKi) upadacitinib was given over 2 weeks because the JAKi tofacitinib had been shown to be effective in prior data with respect to SSc skin involvement (12). Upadacitinib was chosen instead of tofacitinib due to severe kidney failure. Regarding the further deterioration of kidney function (eGFR 16 ml/min), cyclophosphamide was replaced by melphalan (50mg/m² on two consecutive days) accompanied by reduced ATG dose (total dosage of 15mg/kg grafalon) for four days as conditioning regimen. Engraftment of leucocytes, defined as ANC>500/µl, took place on day 14 and a total of 7 red blood cell and 4 platelet transfusions were necessary. Besides a mucositis, the aplastic period proceeded without further complication so that the patient could be discharged on day 28 after aHSCT. Unfortunately, kidney function did not improve and hemodialysis became necessary in Mai 2023. After initial response of skin involvement (mRSS declined from 39 to 32 after aHSCT (see Figure 1)), SSc started to flare up again with skin tenderness and re-increase of diffuse scleroderma accompanied by reduced range of motion, (mRSS 35) in June 2023. Because the last rituximab infusion was only five month ago as well as aHSCT was only two month ago the peripheral blood flow cytometry consecutively revealed no peripheral B cells (see Figure 2). Since the patient showed disease progression after rituximab in the past, re-therapy with rituximab was no promising option in our opinion and other immunosuppressive treatments were contra-indicated due to end-stage kidney failure with the necessity of hemodialysis. Thus, we decided to administrate the IL-17A inhibitor secukinumab (300mg on the days 0, 7, 14, 21, 28 and then monthly), starting in June 2023. At re-assessment in August 2023, the patient presented with significant clinical improvement of the skin (mRSS 29 (Figure 1)). Notably in this context, the patient was able to stretch her elbows for the first time since onset of SSc due to the improvement of the skin sclerosis. At the follow up visit in September 2023, the skin thickening had still further improved compared to August with reaching a mRSS score of 24 now. Furthermore, pulmonary involvement remained stable at FVC 79% predicted with no further deterioration (FVC January 2023: 79%). No adverse events due to secukinumab administration have been observed so far.

Figure 2 Peripheral blood flow cytometry in June 2023. No B cells were detectable in the peripheral blood in June 2023 when the systemic sclerosis relapsed. At that time, rituximab pre-treatment was only 5 month ago as well as autologous hematopoietic stem cell transplant was only 2 month ago. This finding leads together with the increased risk of severe infectious complications by re-treatment with rituximab right after transplant to the decision against re-treatment with rituximab to address the early skin relapse after autologous hematopoietic stem cell transplant.
3 Discussion
In this case report, we present the unique case of a 43-year-old female patient with rapidly progressive diffuse cutaneous systemic sclerosis who underwent aHSCT despite end-stage renal failure and was successfully treated with secukinumab for early skin relapse.
In our view, this case is remarkable for several reasons. Firstly, melphalan was used for the conditioning regimen instead of cyclophosphamide or fludarabine or thiotepa due to the patient’s end-stage kidney disease. High dosage myeloablative chemotherapy with melphalan is well established in treating multiple myeloma but is not routinely used in aHSCT in the context of SSc (1, 4, 5, 13). Secondly, to the best of our knowledge, aHSCT for SSc has never been successfully performed in a patient with end-stage kidney failure before. Regarding the use of melphalan in this context, there is some evidence that it can be safely used in high-dosage chemotherapy in patients with end-stage kidney disease in the context of multiple myeloma and amyloidosis (14). To further reduce toxicity, we decided to use half of the standard dose for treating multiple myeloma (100mg/m²) (13). Strikingly, besides a mucosits grade III no severe adverse events were seen during and after aHSCT indicating that high-dosage chemotherapy with melphalan instead of cyc might be a relatively safe treatment option for SSc-patients with end-stage kidney disease undergoing aHSCT, too.
Furthermore, we describe the first case of successful use of IL-17A inhibition in an SSc-patient with progressive skin disease after aHSCT. IL-17 inhibition with brodalumab has recently been described in Japanese trials as an effective treatment option in SSc with achievement of quick and sustained skin response (8, 9). Evidence for the optimal immunosuppressive treatment after aHSCT is generally low and no standardized treatment recommendations are available. Best evidence exists for the use of rituximab in this scenario (7). However, since the CAST trial, rituximab is increasingly used in the conditioning regimen (15). In case of early disease progression after aHSCT in already rituximab pre-treated patients, re-treatment with rituximab might not be a promising strategy, especially, when the last rituximab infusion is less than 6 months ago. In our patient, we therefore decided against the use of rituximab due to her rituximab pre-treatment only five months ago, the lack of response to that, her absent peripheral B cells at the time of disease progression, and the short latency of two months to aHSCT with increased risk of serious infectious complications like progressive multifocal leukoencephalopathy by reinfusion of rituximab. In this scenario, inhibition of the IL-17 pathway might represent an interesting new and promising treatment option. Especially due to the lack of clear significant efficacy of tocilizumab and abatacept on skin involvement in the faSScinate and focuSSed trial and the ASSET trial, respectively, we decided against treatment with tocilizumab or abatacept (16–18). Thus, we made the decision to use the IL-17A inhibitor secukinumab because of the above-mentioned evidence of the efficacy of IL-17A inhibition and the more favorable risk profile of secukinumab with an overall low risk of severe infectious complications (19). Additionally, there are no contraindications for the use of secukinumab in patients with end-stage kidney disease undergoing hemodialysis. In the three-month follow-up period, no significant adverse events could have been observed so far. The blockade of the IL-17 signaling pathway might therefore be considered a promising new and safe therapy option for SSc-patients in the challenging situation of the necessity of an immunosuppressive treatment after aHSCT. Of course, these observations need to be confirmed by further studies to make general recommendations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Due to the retrospective nature of the study, ethical approval is not needed by German legislation. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
P-PS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. HL: Writing – review & editing. L-KN: Writing – review & editing. JP: Writing – review & editing. MF: Writing – review & editing. MG: Writing – review & editing. MS: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
PPS received speaker’s fees and travel grants from Janssen-Cilag Galapagos, Eli Lilly, Boehringer/Ingelheim and AbbVie (less than $10,000 each) as well as research funding from Chugai (25000$). HL received travel grants from UCB, Boehringer Ingelheim, Pfizer, and Abbvie as well as compensations for consulting activity from Pfizer and for lecturing activities from Janssen. LKN received travel grants from Abbvie, UCB and Medac. JP received travel grants from CSL Behring, Galapagos, AbbVie. MF received speaker’s fees, travel grants or compensation for board memberships from AbbVie, Novartis, Janssen, and Eli Lilly. MG received travel grants, compensation for advisory boards or speaker’s fees from AbbVie, Chugai, Eli Lilly, Hexal, Janssen, Novartis, Pfizer, Takeda. MS received speaker’s fees, travel grants, research funding, or compensation for consultancies or board memberships from AbbVie, Actelion, AstraZeneca, BMS, Boehringer/Ingelheim, Celgene, Chugai/Roche, Eli Lilly, Genzyme, Gilead, Hexal/Sandoz, Janssen-Cilag, MSD, Novartis, Pfizer, Sanofi Pasteur, Takeda (Shire), UCB (less than $ 10,000 each).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA (2014) 311(24):2490–8. doi: 10.1001/jama.2014.6368
2. Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med (2018) 378(1):35–47. doi: 10.1056/nejmoa1703327
3. Burt RK, Shah SJ, Dill K, Grant T, Gheorghiade M, Schroeder J, et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet (2011) 378(9790):498–506. doi: 10.1016/S0140-6736(11)60982-3
4. Del Papa N, Pignataro F, Zaccara E, Maglione W, Minniti A. Autologous hematopoietic stem cell transplantation for treatment of systemic sclerosis. Front Immunol (2018) 9:2390. doi: 10.3389/fimmu.2018.02390
5. Henes J, Oliveira MC, Labopin M, Badoglio M, Scherer HU, Del Papa N, et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. Haematologica (2020) pii:haematol.2019.230128. doi: 10.3324/haematol.2019.230128
6. Strunz PP, Froehlich M, Gernert M, Schwaneck EC, Fleischer A, Pecher AC, et al. Immunological adverse events after autologous hematopoietic stem cell transplantation in systemic sclerosis patients. Front Immunol (2021) 12:723349. doi: 10.3389/fimmu.2021.723349
7. Gernert M, Tony HP, Fröhlich M, Schwaneck EC, Schmalzing M. Immunosuppressive therapy after autologous hematopoietic stem cell transplantation in systemic sclerosis patients-high efficacy of rituximab. Front Immunol (2022) 12:817893. doi: 10.3389/fimmu.2021.817893
8. Fukasawa T, Yoshizaki A, Ebata S, Fukayama M, Kuzumi A, Norimatsu Y, et al. Interleukin-17 pathway inhibition with brodalumab in early systemic sclerosis: Analysis of a single-arm, open-label, phase 1 trial. J Am Acad Dermatol (2023) 89(2):366–9. doi: 10.1016/j.jaad.2023.02.061
9. Fukasawa T, Yoshizaki A, Kagebayashi H, Sato S. POS0881 Efficacy and safety of subcutaneous brodalumab, a fully human anti–IL-17RA monoclonal antibody, for systemic sclerosis with moderate-to-severe skin thickening: a multicenter, randomized, placebo-controlled, double-blind phase 3 study. Ann Rheumatic Dis (2022) 81:736. doi: 10.1136/annrheumdis-2022-eular.2519
10. Steen VD, Medsger TA Jr. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum (1998) 41(9):1613–9. doi: 10.1002/1529-0131(199809)41:9<1613::AID-ART11>3.0.CO;2-O
11. Teixeira L, Mouthon L, Mahr A, Berezné A, Agard C, Mehrenberger M, et al. Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis (2008) 67(1):110–6. doi: 10.1136/ard.2006.066985
12. Khanna D, Bush E, Nagaraja V, Koenig A, Khanna P, Young A, et al. Tofacitinib in early diffuse cutaneous systemic sclerosis— Results of phase I/II investigator-initiated, double-blind randomized placebo-controlled trial [abstract]. Arthritis Rheumatol (2019) 71(suppl 10). Available at: https://acrabstracts.org/abstract/tofacitinib-in-early-diffuse-cutaneous-systemic-sclerosis-results-of-phase-i-ii-investigator-initiated-double-blind-randomized-placebo-controlled-trial/
13. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2021) 32(3):309–22. doi: 10.1016/j.annonc.2020.11.014
14. Casserly LF, Fadia A, Sanchorawala V, Seldin DC, Wright DG, Skinner M, et al. High-dose intravenous melphalan with autologous stem cell transplantation in AL amyloidosis-associated end-stage renal disease. Kidney Int (2003) 63(3):1051–7. doi: 10.1046/j.1523-1755.2003.00813.x
15. Burt RK, Han X, Quigley K, Arnautovic I, Shah SJ, Lee DC, et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transplant (2021) 56(1):50–9. doi: 10.1038/s41409-020-0978-2
16. Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet (2016) 387(10038):2630–40. doi: 10.1016/S0140-6736(16)00232-4
17. Khanna D, Lin CJF, Furst DE, Goldin J, Kim G, Kuwana M, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med (2020) 8(10):963–74. doi: 10.1016/S2213-2600(20)30318-0
18. Khanna D, Spino C, Johnson S, Chung L, Whitfield ML, Denton CP, et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol (2020) 72(1):125–36. doi: 10.1002/art.41055
19. Gottlieb AB, Deodhar A, Mcinnes IB, Baraliakos X, Reich K, Schreiber S, et al. Long-term safety of secukinumab over five years in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis: update on integrated pooled clinical trial and post-marketing surveillance data. Acta Derm Venereol (2022) 102:adv00698. doi: 10.2340/actadv.v102.563
Keywords: scleroderma, IL-17 inhibition, melphalan, high-dose chemotherapy, renal failure, hemodialysis, stem cell transplantation, renal crisis
Citation: Strunz P-P, Labinsky H, Nagler L-K, Portegys J, Froehlich M, Gernert M and Schmalzing M (2023) Case Report: Effectiveness of secukinumab in systemic sclerosis with early skin progress after autologous hematopoietic stem cell transplantation and end-stage kidney disease. Front. Immunol. 14:1294496. doi: 10.3389/fimmu.2023.1294496
Received: 14 September 2023; Accepted: 03 November 2023;
Published: 17 November 2023.
Edited by:
Axel Hueber, Klinikum Nürnberg, GermanyReviewed by:
Corrado Campochiaro, San Raffaele Hospital (IRCCS), ItalyMarco Zeeck, Medizinicum Hamburg, Germany
Copyright © 2023 Strunz, Labinsky, Nagler, Portegys, Froehlich, Gernert and Schmalzing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick-Pascal Strunz, U3RydW56X1BAdWt3LmRl
 Patrick-Pascal Strunz
Patrick-Pascal Strunz Hannah Labinsky
Hannah Labinsky Jan Portegys
Jan Portegys Matthias Froehlich
Matthias Froehlich Michael Gernert
Michael Gernert Marc Schmalzing
Marc Schmalzing