- 1Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
- 2Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia
- 3Belozersky Institute of Physico-Chemical Biology Lomonosov Moscow State University, Moscow, Russia
- 4German Rheumatism Research Center (DRFZ), Leibniz Institute, Berlin, Germany
- 5Winogradsky Institute of Microbiology, Research Centre of Biotechnology Russian Academy of Sciences (RAS), Moscow, Russia
- 6Faculty of Biology, Lomonosov Moscow State University, Moscow, Russia
- 7Division of Immunobiology and Biomedicine, Sirius University of Science and Technology, Federal Territory Sirius, Krasnodarsky Krai, Russia
Akkermansia muciniphila is a gram-negative anaerobic bacterium, which represents a part of the commensal human microbiota. Decline in the abundance of A. muciniphila among other microbial species in the gut correlates with severe systemic diseases such as diabetes, obesity, intestinal inflammation and colorectal cancer. Due to its mucin-reducing and immunomodulatory properties, the use of probiotics containing Akkermansia sp. appears as a promising approach to the treatment of metabolic and inflammatory diseases. In particular, a number of studies have focused on the role of A. muciniphila in colorectal cancer. Of note, the results of these studies in mice are contradictory: some reported a protective role of A. muciniphila in colorectal cancer, while others demonstrated that administration of A. muciniphila could aggravate the course of the disease resulting in increased tumor burden. More recent studies suggested the immunomodulatory effect of certain unique surface antigens of A. muciniphila on the intestinal immune system. In this Perspective, we attempt to explain how A. muciniphila contributes to protection against colorectal cancer in some models, while being pathogenic in others. We argue that differences in the experimental protocols of administration of A. muciniphila, as well as viability of bacteria, may significantly affect the results. In addition, we hypothesize that antigens presented by pasteurized bacteria or live A. muciniphila may exert distinct effects on the barrier functions of the gut. Finally, A. muciniphila may reduce the mucin barrier and exerts combined effects with other bacterial species in either promoting or inhibiting cancer development.
Introduction
Gut microbiota plays an important role in maintaining intestinal homeostasis. Among a huge variety of gut colonizing bacteria, Akkermansia muciniphila (A. muciniphila) deserves special attention. A. muciniphila is a non-motile gram-negative mucin-degrading bacterium of the phylum Verrucomicrobiota first isolated from human faeces by Derrien et al. (1, 2). A strict anaerobe, A. muciniphila adapted to living in human intestine by producing mucin-degrading enzymes (α- and β-D-galactosidase, α-L fucosidase and other) to utilize mucins as a source of nitrogen and carbon (3, 4). In mucin-depleted culturing conditions A. muciniphila is capable of switching to glucose-driven glycolysis (5), thus utilizing the excess of glucose. Also, it was demonstrated that A. muciniphila utilizes circulating host lactate and urea (6), reshaping host systemic metabolism.
A. muciniphila is localized mostly in the colon mucus layer of healthy individuals with relative abundance of 3% (7). Akkermansia-like sequences were found in other anatomical regions of the human digestive tract and even in breast milk (7). A. muciniphila was reported as a part of commensal microbiota in other animal species, including mice (8–10), making mice a convenient animal model to study the in vivo functions of this microorganism (11, 12).
Over the past ten years, numerous studies addressed the role of A. muciniphila in health and disease. Reduced amounts of A. muciniphila were reported in obesity and type 2 diabetes (13) and are associated with Western-type diet. The same correlation was shown for a high-fat (14, 15) and high-sucrose diet in mice (16). It was also established that the decline in A. muciniphila abundance correlated with the development of intestinal inflammation, colorectal cancer, and even with cognitive disorders such as depression and anxiety (17). Thus, a promising therapeutic potential of A. muciniphila as a probiotic or postbiotic and gut microbiota modulator is widely recognized (18–20). However, several studies suggest that A. muciniphila over representation may correlate with negative prognosis of anti-cancer therapy (21). Animal studies aimed to elucidate the specific molecular mechanisms of A. muciniphila effects in colorectal cancer remain contradictory. In this regard, we hypothesized that the introduction of high doses of Akkermansia can lead to disruption of homeostasis and increased tumor growth, while moderate and gentle introduction of bacteria has a protective effect. In present article we are attempting to explain how the differences in experimental settings may affect the results in these earlier reported studies.
Regulatory of effects of A. muciniphila on gut homeostasis
Microbiota interacts with the immune system either directly by activating the immune cells or via production of immunomodulatory metabolites and other molecules. Recent studies suggested that A. muciniphila acquired mechanisms to control host metabolism in the gut and, therefore, may contribute to healthy niche maintenance. For example, protein P9 secreted by A. muciniphila was reported to directly promote the production of GLP-1 by the human primary intestinal epithelial cells, stimulating insulin production and fat browning (22). The most abundant outer membrane pili protein of A. muciniphila, Amuc_1100, was shown to provide beneficial effects in HFD-mice (23). As TLR2 activator, Amuc_1100 demonstrated effects on immune cells (24–26). Another study reported that Amuc_1100 synthesis was increased in mucin-depleted conditions (5), while Khan et al. found that increased sugar consumption in mice may lead to overgrowth of A. muciniphila within 1 week and its mucin-degrading activity may result in thinning of the mucus layer (27).
Bae et al. identified a lipid from A. muciniphila’s cell membrane, diacyl phosphatidylethanolamine with two branched chains (a15:0-i15:0 PE), that can contribute to immunomodulatory activity of bacteria in TLR2-TLR1 dependent manner. Interestingly, in high doses it triggers the release of TNF and IL-6 but not IL-10 or IL-12p70 by mouse BMDCs, while in low doses it resets activation thresholds and responses for immune signaling, so that weak activating signals are ignored and strong signals are moderated, contributing to the regulation of immune response (28).
A newly described outer membrane protein, Amuc_2172, was implicated in activation of immune cells via promotion of HSP70 production in cellular microenvironments (29). Bacterial control of the host immune system may indirectly affect barrier integrity, as well as suppression of autoimmunity against symbionts. It was shown that A. muciniphila secretes tripeptide RKH (Arg-Lys-His), which binds to TLR4 block signal transduction, rescuing mice form lethality in a model of CLP-induced sepsis (30). RKH production represents direct immune-suppressing activity of A. muciniphila. Since inflammation plays a significant role in cancer development, a proper use of the evolutionarily selected functions of A. muciniphila in its interaction with the host may represent novel therapeutic strategies to control inflammation and tumorigenesis.
A. muciniphila in gut inflammation control
Both colorectal cancer and inflammation are influenced by many factors, such as heredity, habits and nutrition, but in recent years much attention was paid to the relationship between the microbiota and the host immune system. The development of inflammatory bowel disease correlates with an increase in opportunistic microorganisms and a decrease in beneficial Bifidobacteria and Lactobacilli (31, 32). Colonization by A. muciniphila is thought to occur early in life during the induction of RORγt+Foxp3+ Tregs to ensure intestinal homeostasis (33). At the same time, a decrease in the abundance of A. muciniphila is characteristic for inflammatory bowel diseases (34–36), as well as for dysbiosis associated with cancer (25).
Studies of A. muciniphila in mouse models of intestinal inflammation suggested a protective role of this bacterium or its derivatives (Table 1). For example, the administration of viable A. muciniphila in both low (108 CFU) and high (3×109 CFU) doses reduced the severity of colitis, increased mucus production (37, 38, 40), reduced the intensity of inflammation (40, 41), and also compensated for dysbiosis associated with inflammation (39). Furthermore, administration of pasteurized bacterium, which exemplifies the concept of “postbiotic” or a preparation of inanimate microorganisms and their components that confers a health benefit on the host (49), as well as recombinant Amuc_1100 or Amuc_2109, also reduced cytotoxic cell accumulation in the intestine and NRLR3 activation, suggesting strong antigenic properties of this bacterium (25, 42). Other data, on the contrary, indicate that the introduction of live bacteria aggravates the symptoms of colitis, however, the same studies showed the protective role of extracellular vesicles of A. muciniphila (29, 44). Since inflammation is the key factor in the development of colorectal cancer, and A. muciniphila has been shown to be an effective anti-inflammatory agent, the bacterium is considered a promising probiotic that can reduce the development of cancer.
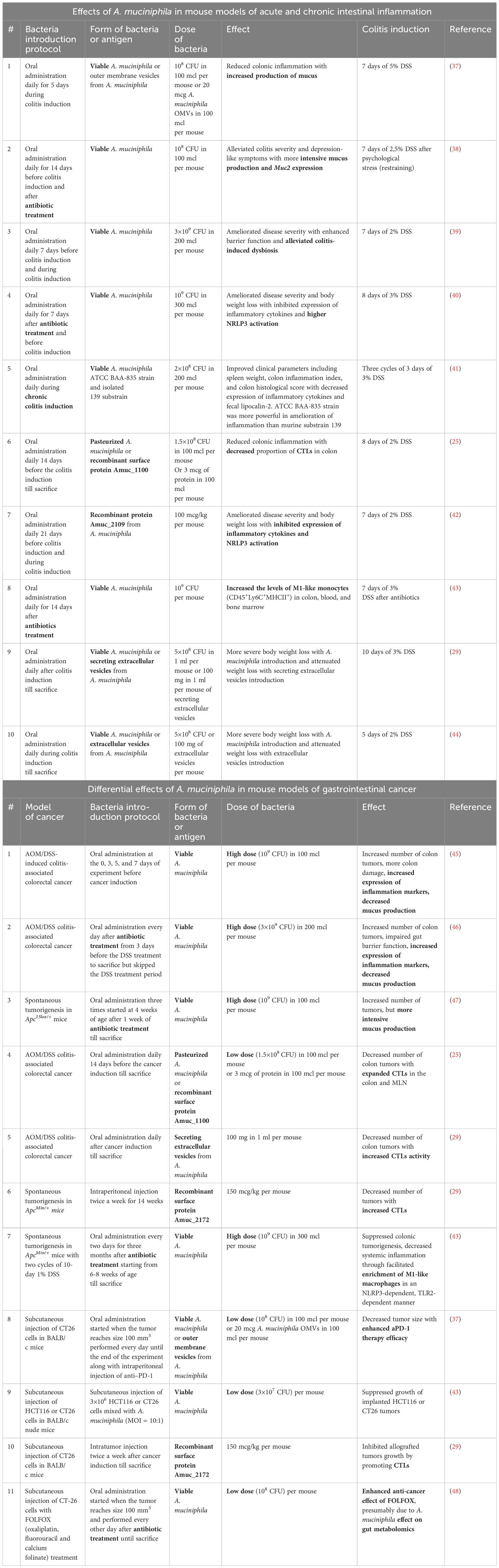
Table 1 Effects of A. muciniphila in mouse models of acute and chronic intestinal inflammation and gastrointestinal cancer.
A. muciniphila in mouse models of colorectal cancer
Studies on the role of A. muciniphila in mouse models of gut cancer provided contradictory results (Table 1). Several reports indicated that administration of A. muciniphila may aggravate the development of intestinal cancer. For example, Wang F. et al. found that administration of A. muciniphila prior to induction of colorectal cancer increased the number of intestinal tumors in the AOM/DSS model in correlation with a decrease in mucus production (45). In a similar study by Wang K. et al, oral gavage with A. muciniphila after a course of antibiotics led to increased tumor formation with a decrease in mucin expression (46). Finally, in a model of spontaneous tumor formation in Apc15lox/+ mice, oral gavage with A. muciniphila after a course of antibiotics also increased the number of tumors, but, in contrast to the data of Wang F. and Wang K., it increased mucus production (47).
At the same time, other numerous studies confirm the protective effect of the enrichment with these bacteria on colorectal cancer. In particular, oral gavage with pasteurized A. muciniphila, surface antigen Amuc_1100 (25) or A. muciniphila secretory extracellular vesicles (29) protected mice in the AOM/DSS model by increasing cytotoxic lymphocyte activity. An increase in the activity of cytotoxic lymphocytes was also shown for another A. muciniphila protein - Amuc_2172 in ApcMin/+ mice (29). Interestingly, in the ApcMin/+ model therapeutic administration of live A. muciniphila following antibiotics in the context of DSS-induced inflammation also reduced tumor burden, apparently due to a TLR2-mediated, NRLP3-dependent increase in the activity of antitumor M1 macrophages (43). The protective role of A. muciniphila was also shown in a number of studies using a transplantable tumor model. Therapeutic administration of bacteria or bacterial secretory vesicles per os reduced the growth of grafted CT26 and also enhanced the effect of anti-PD-1 therapy (37), while intratumoral administration of A. muciniphila (43) or Amuc_2172 (29) reduced the growth of allografts due to the activation of CD8+IFNy+ cytotoxic cells. Finally, it was established that administration of A. muciniphila per os enhanced the effect of the antitumor drug FOLFOX (oxaliplatin, fluorouracil and calcium folinate), and, conversely, the use of FOLFOX led to a significant increase in the A. muciniphila abundance in the gut (48).
Taken together, there is a major controversy over the effects of A. muciniphila on the development of intestinal cancer.
The molecular form of A. muciniphila shapes the outcome
One of the factors potentially explaining the different effects of A. muciniphila in colorectal cancer may be related to different protocols of bacterial administration - from live or pasteurized A. muciniphila to recombinant peptides and extracellular vesicles derived from these bacteria. Thus, in all studies in which A. muciniphila aggravated tumor growth, live bacteria was used at high concentration of 109 CFU (45–47), and in some studies, this bacteria was introduced following a course of antibiotics (46, 47). It was shown that the high dose of A. muciniphila after a course of antibiotics in colorectal cancer model dramatically changed the composition of the microbiota. There was no expansion of A. muciniphila itself, but rather an increase in the opportunistic bacteria, including Clostridia. This resulted in aggravation of dysbiosis and disturbance in the metabolic profile as indicated by a decrease of bile acids and short-chain fatty acids (46). Presumably, a high dose of A. muciniphila, especially after the depletion of gut microbiota with antibiotics, can be interpreted by the immune system as an infection and leads to an increased inflammation due to disrupted microbiota composition and increased opportunistic pathogens in the gut. Moreover, it was shown that in antibiotic treated mice, some phylogroups of A. muciniphila may outcompete others, affecting the outcome of the A. muciniphila colonization. Distinct phylogroup-specific phenotypes of the A. muciniphila modulate oxygen tolerance, iron and sulfur metabolism, and bacterial aggregation differently, therefore, the genetic variations of A. muciniphila’s strains may influence the effect of bacterial colonization after antibiotic treatment (50). Recently, it was suggested that A. muciniphila phylogroups, which bear mutations in mul gene-cluster, lack immunomodulatory effects, but are able to colonize gut in germ-free conditions (4). It can be proposed that under antibiotic treatment, “weak” variants of the microorganism can take root and mask the immunomodulatory effects (4). Assumption about the detrimental effect of the microbiota composition disruption after antibiotics is further supported by the observed decrease in mucin expression (45, 46) upon administration of A. muciniphila following antibiotics. The thinning of the mucin layer allows other microorganisms to penetrate the tissue more actively and aggravate cancer-promoting intestinal inflammation.
A. muciniphila has a direct effect on mucus production in the intestine. In this context, it appears important to establish whether different forms of bacteria - viable bacteria or pasteurized bacteria, providing distinct sets of antigens, affect the production of major mucins in the intestine. It turned out that both forms of A. muciniphila differently increased the expression of mucins in the gut (Figure 1). For example, in the colon, only pasteurized bacteria caused a significant upregulation in the expression of Muc1 and Muc4, while administration of viable A. muciniphila alone increased the expression of Muc2. In the small intestine, viable, but not pasteurized, bacteria caused a slight increase in Muc1 expression, and only pasteurized A. muciniphila affected the expression of Muc2. The expression level of Muc3 was not affected by either form of the bacterium. Thus, the thickness of the mucus layer following A. muciniphila administration was significantly influenced by the form in which bacteria were administered, as well as by the tissue specificity. In the context of colorectal cancer, the thickness of the mucus layer in the large intestine is important, and the significant upregulation of mucin expression observed with the introduction of viable and pasteurized A. muciniphila may provide protection to tissues from microbiota invasion and inflammation (52).
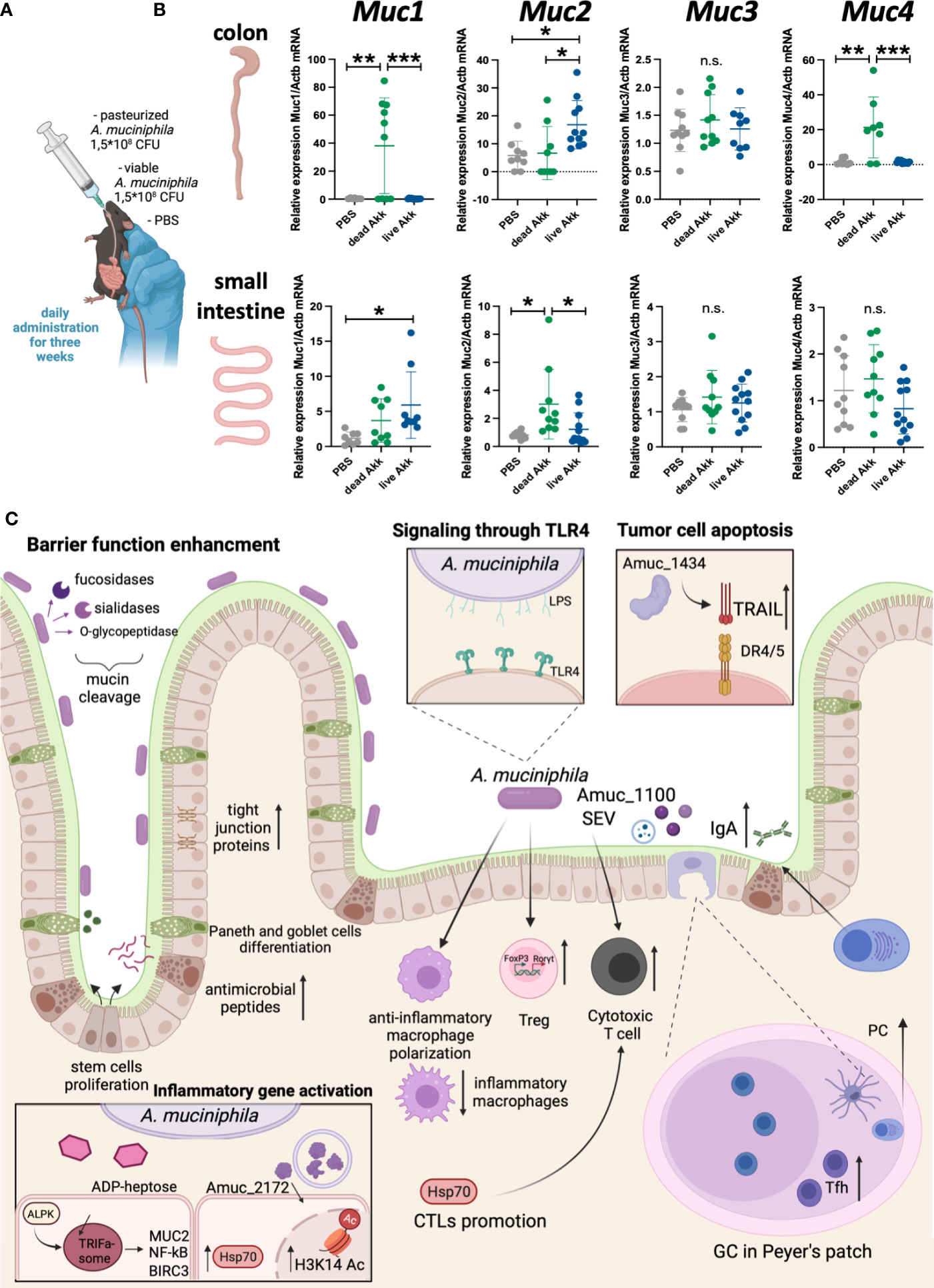
Figure 1 Live and pasteurized A. muciniphila differentially upregulate mucins expression in the gut. C57Bl/6 mice were housed in SPF conditions at the Animal Facility of the Center for Precision Editing and Genetic Technologies for Biomedicine, EIMB RAS (under the contract #075-15-2019-1660 from the Ministry of Science and Higher Education of the Russian Federation). At the age of 5-6 weeks animals of both sexes were randomly distributed between the groups and used in the experiments described below. All manipulations with animals were carried out in accordance with the protocol approved by the Bioethics Committee of the EIMB RAS (Protocol No. 3 from 27/10/22). A. muciniphila was grown anaerobically in the medium supplemented with porcine mucin (Sigma) and hemin (Sigma). The bacterial solution was collected at the concentration 7-8×107 CFU/mL, aliquoted by 1 mL and frozen at -80°C. (A) Scheme of experiment. To analyze the effect of bacteria inoculation on the gene expression at steady state C57Bl/6 WT mice were randomized into three groups of 7-9 individuals and then subjected to daily per os administration with PBS, 1.5×108 CFU of pasteurized (70°C, 30 min) A. muciniphila or 1.5×108 CFU of live A. muciniphila during 3 weeks. Fresh frozen in liquid nitrogen small intestine and colon were mechanically homogenized and lysed in ExtractRNA reagent (Evrogen, Russia). RNA was isolated by guanidinium thiocyanate-phenol-chloroform method following the manufacturer’s protocol. RNA was reverse-transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo, USA) followed by quantitative real-time PCR. qPCRmix-HS SYBR+LowROX (5X) (Evrogen, Russia). Gene expression analysis was performed using Quant Studio 6 (Applied Biosystems. USA) and the following primer set: Actb (F: GCGCTCTTTCAGCCTTCTTT; R: TGGCATAGAGGTCCTTGCG), Muc1 (F: TCGTCTATTTCCTTGCCCTG; R: ATTACCTGCCGAAACCTCCT), Muc2 (F: CCCAGAAGGGACTGTGTATG; R: TTGTGTTCGCTCTTGGTCAG), Muc3 (F: TGGTCAACTGCGAGAATGGA; R: TACGCTCTCCACCAGTTCCT), Muc4 (F: GTCTCCCATCACGGTTCAGT; R: TGTCATTCCACACTCCCAGA). Reactions were run using the following program on the Applied Biosystems 7500: 95°C for 10 min, 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. (B) Relative expression level of Muc1, Muc2, Muc3 and Muc4 in colon and small intestine was normalized using Actb and calculated as 2-ddCt fold change in experimental to control group (51). Each point in a diagram represents a single mouse; mean ± SD. *P < 0,05; **P < 0,01; ***P < 0,001; ns - not significant. One-way ANOVA test was used. (C) A. muciniphila in the gut inflammation and homeostasis.
In most studies administration of A. muciniphila protected mice from colorectal cancer. Interestingly, these studies employed experimental protocols with lower dose of A. muciniphila (107-108 CFU) (37, 43, 45, 48) and without the course of antibiotics. Some studies utilized recombinant bacterial proteins (25, 29) or secreted extracellular vesicles from A. muciniphila (29, 37) while Wang L. et al. used pasteurized bacterium (25).Only one study, which used a high dose of A. muciniphila after antibiotics, reported a protective effect of the bacterium (43). Thus, we propose that administration of the lower dose of A. muciniphila either in viable or pasteurized forms, as well as bacterial proteins or peptides, while maintaining the native composition of gut microbiota, has a clear protective effect on intestinal cancer, regardless of the carcinogenesis model.
Moderation of A. muciniphila is the key to inflammation control
Although the mechanisms by which A. muciniphila controls intestinal inflammation and colorectal cancer are not fully understood, much is known about the immunomodulatory effects of the bacterium (Figure 1С). A. muciniphila is known for its mucin-reducing activity, which determines its effect on the structural components of the intestine - epithelial cells, as well as Paneth cells and goblet cells. This bacterium can enhance intestinal barrier function: A. muciniphila increases the expression of tight junction proteins in response to disruption of epithelial integrity in vivo (23, 39) and in vitro (24, 53). In addition, A. muciniphila increases the proliferation of intestinal stem cells, as well as the differentiation of Paneth and goblet cells (54) with increased antimicrobial peptides (54) and mucus production (55). In addition to accelerating the renewal of the mucus in the intestine, A. muciniphila activates the differentiation of Tregs in the large intestine (56) and mesenteric lymph nodes (41). Not unexpectedly, induction of protective RORγt+ Tregs by A. muciniphila is dependent on TLR4 (33, 57). A. muciniphila, its secretory vesicles and antigens activate a cytotoxic response in the intestine (25, 29), and, at the same time, suppress the proliferation of inflammatory macrophages (25), activate the polarization of anti-inflammatory macrophages (58). It was shown that the Amuc_1434 protein can modulate the death of tumor cells through activation of tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL) (59). A. muciniphila upregulates genes involved in the maintanance of intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway (60). Finally, A. muciniphila may regulate IgA production by plasma cells by affecting the number of Tfh in Peyer’s patches (61), and thus influencing the microbiota composition.
Dysbiosis is a hallmark of inflammation and intestinal cancers. A. muciniphila is an important component of the normal microbiota, and changes in its abundance affect the course of the disease. A. muciniphila is capable of inducing both proinflammatory and anti-inflammatory mechanisms. Studies on the role of A. muciniphila in intestinal inflammation show its protective properties in barrier restoration and control of inflammation, while data obtained in colorectal cancer models remain contradictory. Some studies indicate a decrease in tumor burden, while others report an increase in tumor growth when the bacterium is introduced. We attempted to directly compare different experimental protocols using A. muciniphila in various models of intestinal cancer and concluded that the introduction of large amounts of A. muciniphila, especially after a course of antibiotics, provokes dysbiosis, disrupts the intestinal barrier functions (62), and aggravates the inflammation that provokes cancer (46). At the same time, lower doses of the bacterium or its derivatives without prior depletion of the microbiota have a positive effect on the course of the disease. This assumption is supported by the clinical study on the correlation between the presence of A. muciniphila and the effectiveness of checkpoint therapy. The results of this study demonstrated that moderate, but not high A. muciniphila load in the stool correlated with a good prognosis (21). Thus, delicate modulation of the microbiota by A. muciniphila may become a promising strategy for adjunctive therapy of inflammatory bowel diseases and colorectal cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by the Bioethics Committee of EIMB RAS Protocol No. 3 from 21.09.2023. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EG: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Funding acquisition, Project administration. EG: Investigation, Writing – original draft, Methodology. MB: Resources, Writing – review & editing. OP: Resources, Validation, Writing – review & editing. AS: Methodology, Writing – original draft. AY: Methodology, Writing – original draft. EB: Conceptualization, Writing – review & editing. SN: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. AK: Conceptualization, Resources, Supervision, Validation, Writing – review & editing. MD: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by RSF grant No 22-25-00534.
Acknowledgments
The authors are especially grateful to Ekaterina Bulekova for help with text editing and Pavel Matveev for assistance with figure preparation. Figure 1C was created using Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Derrien M, Vaughan EE, Plugge CM, De Vos WM. Akkermansia muciniphila gen. Nov., sp. Nov., A human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol (2004) 54:1469–76. doi: 10.1099/ijs.0.02873-0
2. Derrien M, Collado MC, Ben-Amor K, Salminen S, De Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol (2008) 74:1646–8. doi: 10.1128/AEM.01226-07
3. Derrien M, Van Passel MW, Van De Bovenkamp JH, Schipper RG, De Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes (2010) 1:254–68. doi: 10.4161/gmic.1.4.12778
4. Davey LE, Malkus PN, Villa M, Dolat L, Holmes ZC, Letourneau J, et al. A genetic system for Akkermansia muciniphila reveals A role for mucin foraging in gut colonization and host sterol biosynthesis gene expression. Nat Microbiol (2023) 8:1450–67. doi: 10.1038/s41564-023-01407-w
5. Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol (2019) 10:1137. doi: 10.3389/fmicb.2019.01137
6. Zeng X, Xing X, Gupta M, Keber FC, Lopez JG, Lee YJ, et al. Gut bacterial nutrient preferences quantified in vivo. Cell (2022) 185:3441–3456.E19. doi: 10.1016/j.cell.2022.07.020
7. Geerlings SY, Kostopoulos I, De Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms (2018) 6. doi: 10.3390/microorganisms6030075
8. Sonoyama K, Ogasawara T, Goto H, Yoshida T, Takemura N, Fujiwara R, et al. Comparison of gut microbiota and allergic reactions in Balb/C mice fed different cultivars of rice. Br J Nutr (2010) 103:218–26. doi: 10.1017/S0007114509991589
9. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Muller M, De Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila. Front Microbiol (2011) 2:166. doi: 10.3389/fmicb.2011.00166
10. Berry D, Schwab C, Milinovich G, Reichert J, Ben Mahfoudh K, Decker T, et al. Phylotype-level 16s Rrna analysis reveals new bacterial indicators of health state in acute murine colitis. Isme J (2012) 6:2091–106. doi: 10.1038/ismej.2012.39
11. Howe C, Kim SJ, Mitchell J, Im E, Kim YS, Kim YS, et al. Differential expression of tumor-associated genes and altered gut microbiome with decreased Akkermansia muciniphila confer A tumor-preventive microenvironment in intestinal epithelial Pten-deficient mice. Biochim Biophys Acta Mol Basis Dis (2018) 1864:3746–58. doi: 10.1016/j.bbadis.2018.10.006
12. Yang Y, Zheng X, Wang Y, Tan X, Zou H, Feng S, et al. Human fecal microbiota transplantation reduces the susceptibility to dextran sulfate sodium-induced germ-free mouse colitis. Front Immunol (2022) 13:836542. doi: 10.3389/fimmu.2022.836542
13. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during A dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut (2016) 65:426–36. doi: 10.1136/gutjnl-2014-308778
14. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U.S.A. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
15. Wang B, Kong Q, Li X, Zhao J, Zhang H, Chen W, et al. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference. Nutrients (2020) 12. doi: 10.3390/nu12103197
16. Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci (Weinh) (2021) 8:E2100536. doi: 10.1002/advs.202100536
17. Pellegrino A, Coppola G, Santopaolo F, Gasbarrini A, Ponziani FR. Role of Akkermansia in human diseases: from causation to therapeutic properties. Nutrients (2023) 15. doi: 10.3390/nu15081815
18. Zhang C, Wang J, Sun Z, Cao Y, Mu Z, Ji X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci (2021) 112:3005–17. doi: 10.1111/cas.14979
19. Lee KA, Thomas AM, Bolte LA, Bjork JR, De Ruijter LK, Armanini F, et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med (2022) 28:535–44. doi: 10.1038/s41591-022-01695-5
20. Rodrigues VF, Elias-Oliveira J, Pereira IS, Pereira JA, Barbosa SC, MaChado MSG, et al. Akkermansia muciniphila and gut immune system: A good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front Immunol (2022) 13:934695. doi: 10.3389/fimmu.2022.934695
21. Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, et al. Intestinal Akkermansia muciniphila predicts clinical response to Pd-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med (2022) 28:315–24. doi: 10.1038/s41591-021-01655-5
22. Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, et al. Akkermansia muciniphila secretes A glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol (2021) 6:563–73. doi: 10.1038/s41564-021-00880-5
23. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med (2017) 23:107–13. doi: 10.1038/nm.4236
24. Ottman N, Reunanen J, Meijerink M, Pietila TE, Kainulainen V, Klievink J, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PloS One (2017) 12:E0173004. doi: 10.1371/journal.pone.0173004
25. Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of Cd8(+) T cells in mice. Gut (2020) 69:1988–97. doi: 10.1136/gutjnl-2019-320105
26. Zheng X, Huang W, Li Q, Chen Y, Wu L, Dong Y, et al. Membrane protein amuc_1100 derived from Akkermansia muciniphila facilitates lipolysis and browning via activating the Ac3/Pka/Hsl pathway. Microbiol Spectr (2023) 11:E0432322. doi: 10.1128/spectrum.04323-22
27. Khan S, Waliullah S, Godfrey V, Khan MAW, Ramachandran RA, Cantarel BL, et al. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Sci Transl Med (2020) 12. doi: 10.1126/scitranslmed.aay6218
28. Bae M, Cassilly CD, Liu X, Park SM, Tusi BK, Chen X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature (2022) 608:168–73. doi: 10.1038/s41586-022-04985-7
29. Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, et al. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut (2023) 72:1308–18. doi: 10.1136/gutjnl-2022-327853
30. Xie S, Li J, Lyu F, Xiong Q, Gu P, Chen Y, et al. Novel tripeptide Rkh derived from Akkermansia muciniphila protects against lethal sepsis. Gut (2023). doi: 10.1136/gutjnl-2023-329996
31. Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol (2012) 30:759–95. doi: 10.1146/annurev-immunol-020711-074937
32. Sobhani I, Bergsten E, Couffin S, Amiot A, Nebbad B, Barau C, et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc Natl Acad Sci U.S.A. (2019) 116:24285–95. doi: 10.1073/pnas.1912129116
33. Liu Y, Yang M, Tang L, Wang F, Huang S, Liu S, et al. Tlr4 regulates rorgammat(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome (2022) 10:98. doi: 10.1186/s40168-022-01296-x
34. Png CW, Linden SK, Gilshenan KS, Zoetendal EG, Mcsweeney CS, Sly LI, et al. Mucolytic bacteria with increased prevalence in Ibd mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol (2010) 105:2420–8. doi: 10.1038/ajg.2010.281
35. Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, et al. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol (2020) 104:10203–15. doi: 10.1007/s00253-020-10948-7
36. Lo Sasso G, Khachatryan L, Kondylis A, Battey JND, Sierro N, Danilova NA, et al. Inflammatory bowel disease-associated changes in the gut: focus on Kazan patients. Inflammation Bowel Dis (2021) 27:418–33. doi: 10.1093/ibd/izaa188
37. Wang X, Lin S, Wang L, Cao Z, Zhang M, Zhang Y, et al. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci Adv (2023) 9:Eade5079. doi: 10.1126/sciadv.ade5079
38. Chen T, Wang R, Duan Z, Yuan X, Ding Y, Feng Z, et al. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Front Cell Infect Microbiol (2021) 11:723856. doi: 10.3389/fcimb.2021.723856
39. Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol (2019) 10:2259. doi: 10.3389/fmicb.2019.02259
40. Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, et al. Akkermansia muciniphila alleviates dextran sulfate sodium (Dss)-induced acute colitis by Nlrp3 activation. Microbiol Spectr (2021) 9:E0073021. doi: 10.1128/Spectrum.00730-21
41. Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol (2019) 9:239. doi: 10.3389/fcimb.2019.00239
42. Qian K, Chen S, Wang J, Sheng K, Wang Y, Zhang M. A beta-N-acetylhexosaminidase amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct (2022) 13:2216–27. doi: 10.1039/D1FO04094D
43. Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing Tlr2/Nlrp3-mediated M1-like tams. Cancer Immunol Res (2021) 9:1111–24. doi: 10.1158/2326-6066.CIR-20-1019
44. Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS One (2013) 8:E76520. doi: 10.1371/journal.pone.0076520
45. Wang F, Cai K, Xiao Q, He L, Xie L, Liu Z. Akkermansia muciniphila administration exacerbated the development of colitis-associated colorectal cancer in mice. J Cancer (2022) 13:124–33. doi: 10.7150/jca.63578
46. Wang K, Wu W, Wang Q, Yang L, Bian X, Jiang X, et al. The negative effect of Akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front Microbiol (2022) 13:932047. doi: 10.3389/fmicb.2022.932047
47. Dingemanse C, Belzer C, Van Hijum SA, Gunthel M, Salvatori D, Den Dunnen JT, et al. Akkermansia muciniphila and Helicobacter typhlonius modulate intestinal tumor development in mice. Carcinogenesis (2015) 36:1388–96. doi: 10.1093/carcin/bgv120
48. Hou X, Zhang P, Du H, Chu W, Sun R, Qin S, et al. Akkermansia muciniphila potentiates the antitumor efficacy of Folfox in colon cancer. Front Pharmacol (2021) 12:725583. doi: 10.3389/fphar.2021.725583
49. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The international scientific association of probiotics and prebiotics (Isapp) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
50. Becken B, Davey L, Middleton DR, Mueller KD, Sharma A, Holmes ZC, et al. Genotypic and phenotypic diversity among human isolates of Akkermansia muciniphila. Mbio (2021) 12. doi: 10.1128/mBio.00478-21
51. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative Pcr and the 2(-Delta Delta C(T)) method. Methods (2001) 25:402–8. doi: 10.1006/meth.2001.1262
52. Earley H, Lennon G, Balfe A, Coffey JC, Winter DC, O'connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep (2019) 9:15683. doi: 10.1038/s41598-019-51878-3
53. Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol (2015) 81:3655–62. doi: 10.1128/AEM.04050-14
54. Kim S, Shin YC, Kim TY, Kim Y, Lee YS, Lee SH, et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes (2021) 13:1–20. doi: 10.1080/19490976.2021.1892441
55. Shuoker B, Pichler MJ, Jin C, Sakanaka H, Wu H, Gascuena AM, et al. Sialidases and fucosidases of Akkermansia muciniphila are crucial for growth on mucin and nutrient sharing with mucus-associated gut bacteria. Nat Commun (2023) 14:1833. doi: 10.1038/s41467-023-37533-6
56. Kuczma MP, Szurek EA, Cebula A, Chassaing B, Jung YJ, Kang SM, et al. Commensal epitopes drive differentiation of colonic T(Regs). Sci Adv (2020) 6:Eaaz3186. doi: 10.1126/sciadv.aaz3186
57. Wen X, Xie R, Wang HG, Zhang MN, He L, Zhang MH, et al. Fecal microbiota transplantation alleviates experimental colitis through the toll-like receptor 4 signaling pathway. World J Gastroenterol (2023) 29:4657–70. doi: 10.3748/wjg.v29.i30.4657
58. Kim SM, Park S, Hwang SH, Lee EY, Kim JH, Lee GS, et al. Secreted Akkermansia muciniphila threonyl-Trna synthetase functions to monitor and modulate immune homeostasis. Cell Host Microbe (2023) 31:1021–1037.E10. doi: 10.1016/j.chom.2023.05.007
59. Meng X, Zhang J, Wu H, Yu D, Fang X. Akkermansia muciniphila aspartic protease Amuc_1434* Inhibits human colorectal cancer Ls174t cell viability via trail-mediated apoptosis pathway. Int J Mol Sci (2020) 21. doi: 10.3390/ijms21093385
60. Martin-Gallausiaux C, Garcia-Weber D, Lashermes A, Larraufie P, Marinelli L, Teixeira V, et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via Adp-heptose-dependent activation of the Alpk1/Tifa pathway. Gut Microbes (2022) 14:2110639. doi: 10.1080/19490976.2022.2110639
61. Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science (2019) 364:1179–84. doi: 10.1126/science.aaw7479
Keywords: intestinal inflammation, colorectal cancer, mucin-reducing bacteria, Akkermanisa municiphila, probiotic
Citation: Gubernatorova EO, Gorshkova EA, Bondareva MA, Podosokorskaya OA, Sheynova AD, Yakovleva AS, Bonch-Osmolovskaya EA, Nedospasov SA, Kruglov AA and Drutskaya MS (2023) Akkermansia muciniphila - friend or foe in colorectal cancer? Front. Immunol. 14:1303795. doi: 10.3389/fimmu.2023.1303795
Received: 28 September 2023; Accepted: 17 November 2023;
Published: 06 December 2023.
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Monica Barone, University of Bologna, ItalyYin Yuan, Zhejiang University, China
Le Liu, Southern Medical University, China
Copyright © 2023 Gubernatorova, Gorshkova, Bondareva, Podosokorskaya, Sheynova, Yakovleva, Bonch-Osmolovskaya, Nedospasov, Kruglov and Drutskaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ekaterina O. Gubernatorova, ZWthdGVyaW5hLmd1YmVybmF0b3JvdmE0MTJAZ21haWwuY29t; Marina S. Drutskaya, bWFyaW5hZHJ1QGdtYWlsLmNvbQ==
 Ekaterina O. Gubernatorova
Ekaterina O. Gubernatorova Ekaterina A. Gorshkova
Ekaterina A. Gorshkova Marina A. Bondareva
Marina A. Bondareva Olga A. Podosokorskaya5
Olga A. Podosokorskaya5 Anastasia S. Yakovleva
Anastasia S. Yakovleva Elizaveta A. Bonch-Osmolovskaya
Elizaveta A. Bonch-Osmolovskaya Sergei A. Nedospasov
Sergei A. Nedospasov Andrey A. Kruglov
Andrey A. Kruglov Marina S. Drutskaya
Marina S. Drutskaya