Abstract
The application of immunotherapy in tumor, especially immune checkpoint inhibitors (ICIs), has played an important role in the treatment of advanced unresectable liver cancer. However, the efficacy of ICIs varies greatly among different patients, which has aroused people’s attention to the regulatory mechanism of programmed death ligand-1 (PD-L1) in the immune escape of liver cancer. PD-L1 is regulated by multiple levels and signaling pathways in hepatocellular carcinoma (HCC), including gene variation, epigenetic inheritance, transcriptional regulation, post-transcriptional regulation, and post-translational modification. More studies have also found that the high expression of PD-L1 may be the main factor affecting the immunotherapy of liver cancer. However, what is the difference of PD-L1 expressed by different types of cells in the microenvironment of HCC, and which type of cells expressed PD-L1 determines the effect of tumor immunotherapy remains unclear. Therefore, clarifying the regulatory mechanism of PD-L1 in liver cancer can provide more basis for liver cancer immunotherapy and combined immune treatment strategy. In addition to its well-known role in immune regulation, PD-L1 also plays a role in regulating cancer cell proliferation and promoting drug resistance of tumor cells, which will be reviewed in this paper. In addition, we also summarized the natural products and drugs that regulated the expression of PD-L1 in HCC.
1 Introduction
Liver cancer is the third most common cause of cancer death worldwide (1). Hepatocellular carcinoma (HCC) accounts for 75% to 85% of primary liver cancers (2). Risk factors for liver cancer include hepatitis B virus (HBV), hepatitis C virus (HCV) infection, non-alcoholic fatty liver disease, alcohol consumption, type 2 diabetes, and aflatoxin (3). Early liver cancer is mainly treated by resection, local intervention or liver transplantation. However, most patients have advanced liver cancer, and even after treatment, patients will relapse or metastasize within 5 years, so systemic therapy is still the main means of liver cancer treatment (4). In recent years, targeted therapy based on anti-angiogenic drugs has been the first-line drug in the treatment of advanced liver cancer (5). However, clinical studies have shown that sorafenib can only extend the survival of advanced HCC by 3 months, and there are adverse events such as tolerance (6).
Recently, with the continuous research on the tumor immune microenvironment and the interaction between immune cells and tumor cells. The application of immune checkpoint inhibitors (ICIs) in immune and tumor cells is a major breakthrough in the treatment of many solid tumors (7). The study found that drugs targeting programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) had significant anti-HCC effects (8). PD-1 is expressed on a variety of immune cells. By binding to ligand PD-L1 or programmed cell death 1 ligand 2 (PD-L2), PD-1 blocks the stimulation signal of T cell receptor (TCR), reduces the activity of T cells during immune response, and prevents autoimmune damage (9). In HCC, PD-L1 is mainly expressed on tumor cells, Kupffer cells, and hepatocytes (10). During immune activation, tumor antigens on cancer cells are presented to T cells by antigen-presenting cells (APC) and are recognized by binding to TCR. Activated T cells will release perforin, granzyme, interferon, and other cytokines to attack these cancer cells. And tumor cells escape T cell attack by expressing low levels of co-stimulatory immune checkpoint molecules, increasing inhibitory immune checkpoint molecules, such as PD-L1. Moreover, the increased expression of PD-L1 on tumor cells inhibits the anti-tumor effect and leads to immune tolerance of HCC (11). The expression of PD-L1 is closely related to the stage and poor prognosis of HCC (12). Therefore, it is important to clarify the regulatory mechanism of PD-L1 for HCC immunotherapy. We will review the expression and regulation of PD-L1 in different cells in the tumor microenvironment.
2 Expression of PD-L1 on host immune cells and tumor cells
Clinical patients with high expression of PD-L1 in liver tumor tissues have inconsistent responses to PD-1 inhibitors, which leads us to think about the expression of PD-L1 in tumor tissues. Through the literature research in the past decade, we found that there are new changes in the research of PD-L1. The attention of PD-L1 expression in tumor cells has gradually shifted to that of immune cells. In addition, we also found that immune cells and tumor cells are related, that is, changes in immune cell signaling regulatory factors can affect the expression of PD-L1 in tumor cells. Only by understanding the expression of PD-L1 in different types of cells and the relationship between them can immunotherapy for liver cancer be further advanced. We will summarize each of them.
2.1 Macrophages
Studies have shown that macrophages in the tumor microenvironment can promote the growth of HCC (13). Macrophage surface expression of PD-L1 promoted the formation of immunosuppressive microenvironment (14). Regulation of PD-L1 in macrophages (Figure 1). It was found that AlkB homolog 5 (ALKBH5) promoted the recruitment of PD-L1+ macrophages mediated by interleukin-8 (IL-8) through mitogen-activated protein kinase kinase kinase 8 (MAP3K8), promoting HCC cell proliferation (15). Fibronectin 1 (FN1) promoted glycolytic activation of macrophages by triggering toll-like receptor 4 (TLR4), induced macrophages to express PD-L1 (16). Ferroptosis of macrophages mediated by solute carrier family 7a member 11 (SLC7A11) significantly increased the expression of PD-L1 in macrophages and improve the anti-tumor effect of anti-PD-L1 therapy (17). Low doses of interferon-α (IFN-α) also inhibited the growth of liver cancer in mice, possibly by polarizing CD169+ macrophage populations and enhancing CD8+ T cell activity. It was worth noting that IFN-α also induced a large amount of PD-L1 expression in macrophages in vivo, blocking PD-L1 further improved the anti-tumor effect of IFN-α (18). In addition, cell division cycle 42 (CDC42) was positively correlated with M2 macrophage markers and immune checkpoints, and the expression of CDC42 was most correlated with Wnt signaling pathway (19). The study also found that CD97 was positively correlated with M2 macrophages and tumor-associated macrophage markers, and positively correlated with PD-L1 (20). Lysyl oxidase-like 4 (LOXL4) was an amine oxidase, which was highly expressed in HCC tissues. LOXL4 promoted macrophage infiltration into the liver, accelerated tumor growth, and was further eliminated by adoptive T cell metastasis or PD-L1 neutralization. The immunosuppressive function of LOXL4 on macrophages was mainly dependent on IFN-mediated signal transduction and transcription-dependent activator of PD-L1 activation. Hydrogen peroxide scavenger or copper chelate macrophages eliminated PD-L1 presentation of IFN-mediated LOXL4 (21). Oncoprotein-induced transcript 3 (OIT3) mediated the polarization of macrophages and promoted the progression of HCC (22). OIT3 increased the expression of PD-L1 in TAMs by activating the nuclear factor kB (NF-κB) signaling pathway, blocked the immunosuppressive activity of NF-κB reversal TAMs, and inhibited the tumorigenesis of HCC (23). It was found that the expression of protein tyrosine phosphatase, receptor type O (PTPRO) was significantly decreased, which was related to the increase of PD-L1 expression in peripheral blood mononuclear cells and TAMs of HCC. Serum interleukin 6 (IL-6) decreased the expression of PTPRO by activating signal transducer and activator of transcription 3 (STAT3)/c-MYC/miR-25-3p axis, leading to PD-L1-induced immunosuppression to promote tumor growth (24). Endoplasmic reticulum (ER) stress occurred in HCC cells, released exosome miR-23a-3p, and upregulated the expression of PD-L1 in macrophages via miR-23a-PTEN-AKT pathway, and inhibited T cell function (25).
Figure 1

Regulation of PD-L1 in macrophages. Tumor cells induce the polarization of macrophages and promote the expression of PD-L1 by secreting IL-8 and CCL2. The expression of PD-L1 in macrophages is also affected by the glycolysis of FN1, IFN, PTEN, NF-κB, etc.
With the research on tumor immunotherapy in recent years, macrophages have gradually become the focus of research. In macrophages expressing LysM (lysozyme M), PD-L1 gene deletion eliminate the efficacy of anti-PD-L1 antibodies in MC38 colon cancer models (26). Macrophages in liver cancer may also have similar characteristics. How to regulate the expression of PD-L1, and then affect the polarization of macrophages, from promoting tumor progression to inhibiting tumor? Regulation of metabolic reprogramming of macrophages may be the future trend. These studies provided new insights into the mechanisms of how tumor cells escape from anti-tumor immunity.
2.2 Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) may play an important role in immune regulation (27), but the immunosuppressive function of MDSCs in HCC patients has not been clarified. HCC cell lines with high expression of colony stimulating factor 1 (M-CSF) and vascular endothelial growth factor A (VEGFA) could significantly induce the expression of PD-L1 in MDSCs (28). It was found that MDSCs contributed to the formation of tumor immunosuppressive microenvironment. Tumor infiltrating CD11b+ CD33+ HLA-DR-MDSCs in HCC patients effectively inhibited the proliferation of CD8+ T cells. Studies have shown that cyclin dependent kinase 20 (CCRK) leads to MDSCs accumulation by activating the enhancer of zeste homolog 2 (EZH2)/NF-κB/IL-6 cascade (29). Notably, neoplastic CCRK depletion upregulated PD-L1 expression and increased intracellular CD8+ T cells, enhancing the effect of anti-PD-L1 in the treatment of liver cancer. Studies have found that SLC7A11 is significantly correlated with PD-L1 expression and adverse survival time (30). IL-1β-induced SLC7A11 over-expression promoted the infiltration of TAMs and MDSCs by up-regulating PD-L1 and colony stimulating factor-1 (CSF1) through the α-ketoglutarate (αKG)/hypoxia inducible factor-1α (HIF1α) axis (31).
2.3 Monocyte
Autocrine TNF-α and interleukin 10 (IL-10) released by activated monocytes stimulated the expression of PD-L1 in monocytes (32). PD-L1+ monocytes effectively inhibited tumor-specific T cell immunity and contributed to tumor growth in humans. Therefore, the expression of PD-L1 on activated monocytes/macrophages might represent a novel mechanism that links pro-inflammatory responses to immune tolerance in the tumor environment (32). IL-10 secreted by tumor monocytes was involved in the expression of PD-L1 on Treg cells through the JNK-STAT3 pathway (33). 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) mediated the expression of PD-L1 by activating NF-κB signaling in the tumor microenvironment (34).
As one of the most abundant immune cells, neutrophils in the tumor microenvironment are involved in tumor progression, including promoting tumor invasion and metastasis, inhibiting adaptive immunity, and suppressing the anti-tumor response of T cells (35). Studies have shown that PD-L1+ neutrophils in HCC patients effectively inhibit the proliferation and activation of T cells, and blocking PD-L1 partially reverse this effect (36).
2.4 Regulation of tumor PD-L1 by immune cells
Regulation of tumor PD-L1 by immune cells (Figure 2). In the tumor immune microenvironment, the carcinogenic activity of endogenous osteopontin (OPN) promoted chemotactic migration and substitution activation of macrophages. It also promoted PD-L1 expression in tumor cells by activating CSF1/CSF1R pathway in macrophages (37). Blocking CSF1/CSF1R prevents TAMs transport, enhancing the efficacy of ICIs in the treatment of HCC. Schlafen (SLFN) protein played an important role in cell proliferation and immune cell development (38). Studies have shown that macrophages induced by SLFN11 deficiency up-regulate the expression of PD-L1 in HCC cells through the NF-κB/P65 pathway. Blocking the CCL2 pathway enhanced the anti-PD-L1 efficacy of SLFN11 with low expression of HCC (39). Over-expression of e-twenty-six-specific sequence variant 4 (ETV4) in HCC cells activated the expression of PD-L1 and chemokine (C-C motif) ligand 2 (CCL2). The infiltration of tumor-associated macrophages (TAMs) and MDSCs was increased, and the accumulation of CD8+T cells was inhibited (40).
Figure 2
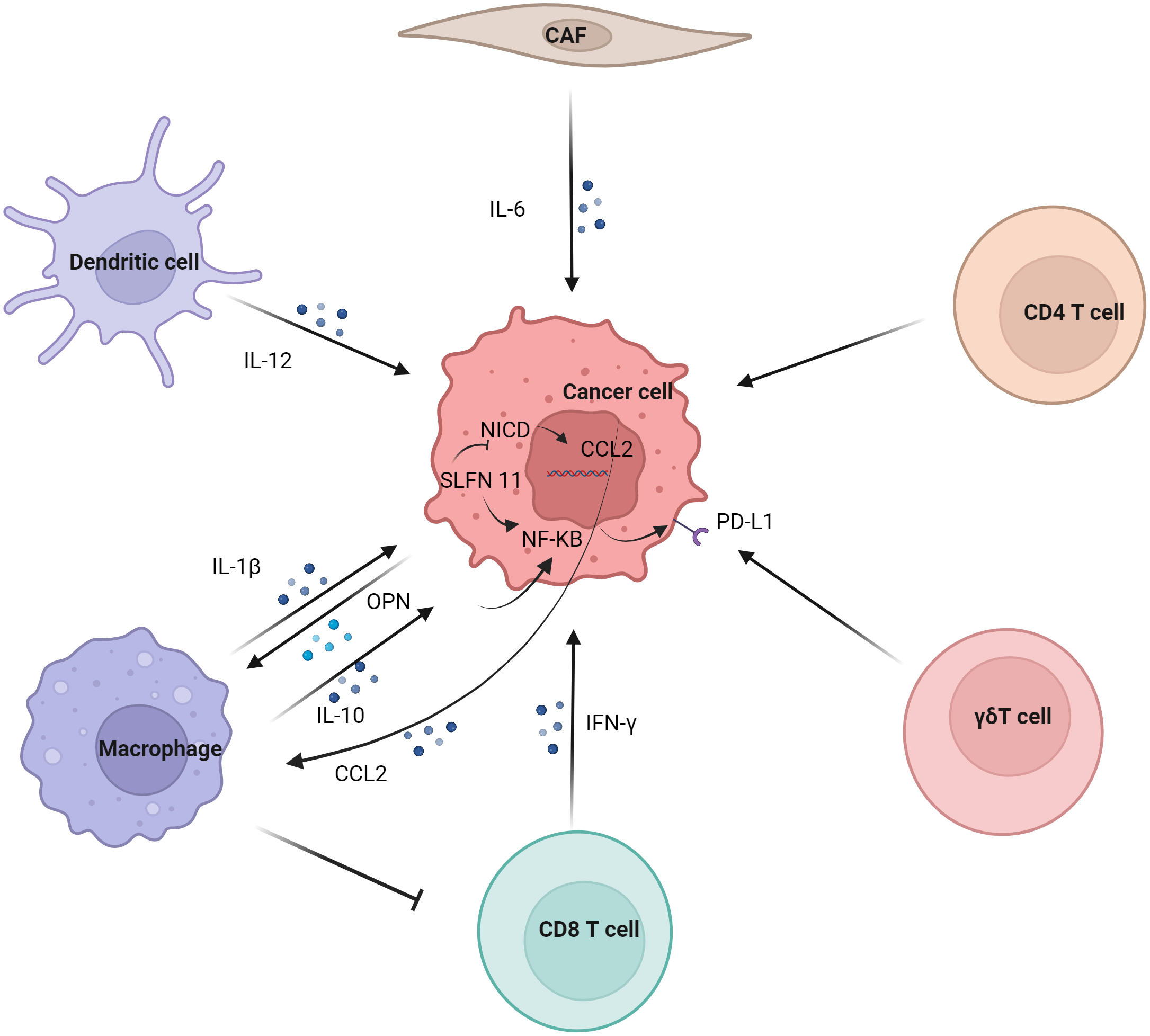
Regulation of tumor PD-L1 by immune cells. Dendritic cell, macrophage, CD8 T cell, γδT cell, CD4 T cell and CAF regulate the expression of PD-L1 on tumor cells. Macrophages have significant effect on the expression of PD-L1 in tumor cells, mainly involving IL-1β, IL10 and other cytokines on the NF-κB pathway in tumor cells.
Although M1 macrophages are generally considered to have anti-tumor effects, some studies have reported their tumor-promoting effects (41). Studies have shown that the infiltration of CD68+ HLA-DR+ M1-like macrophages is related to the expression level of PD-L1 in HCC cells. The expression of tumor cell transcription factors NF-κB p65 and interferon regulatory factor-1 (IRF-1) induced by interleukin-1β (IL-1β) secreted by M1 macrophages promoted the expression of PD-L1 (42). M2 macrophage-derived extracellular vesicles (M2-EVs) up-regulated the expression of PD-L1 through the MISP/IQGAP1/STAT3 pathway, inhibited the killing ability of CD8+T cells and promoted immune escape of HCC cells (43).
The increased level of PD-L1 might represent the adaptive immune resistance mechanism generated by tumor cells in response to endogenous anti-tumor activity. PD-L1 up-regulation was mainly induced by pre-existing activated CD8+ T cells in HCC environment (44). It has also been suggested that PD-L1 down-regulates genes related to T cell activation in TME. Co-culture of PD-L1-expressing mouse liver cancer cell line BNL-MEA with CD8+ T cells reduced the proliferation of T cell and the expression of interferon γ (IFN-γ) and TNF-α (45). Although PD-L1-expressing tumors showed a better response to anti-PD-1 therapy, CD8+ T cells exhaustion inhibited the anti-PD-1 anti-tumor effect. Studies have shown an increase in circulating PD-1+CD8+ T cells in HCC. In addition, tumor-infiltrating CD8+T cells showed a sharp increase in PD-1 expression, which was associated with poor disease progression and postoperative recurrence. CD8+ T cells induced the expression of PD-L1 on liver cancer cells in an IFN-γ-dependent manner, promoting the apoptosis of CD8+ T cells (46). γδT cells infiltrate in liver cancer and have a strong killing ability (47). It was also found that co-incubation of γδT cells increased the expression of PD-L1 in HCC cell lines (48).
Studies have shown that IL-6 is mainly secreted by cancer-associated fibroblasts (CAF). Moreover, CAF with high IL-6 expression induced immunosuppression by recruiting immunosuppressive cells, such as myeloid suppressor cells. In addition, CAF with high expression of IL-6 also disrupted the function of tumor-infiltrating T cells by up-regulating the expression of PD-L1 (49).
Studies have shown that PD-L1 expression is positively correlated with FoxP3+ Treg cell infiltration, and patients with high PD-L1 expression have poor prognosis (50). IL-12 was a cytokine naturally secreted by activated dendritic cells and mononuclear/macrophages (51). Studies have found that long-term induction of liver IL-12 expression inhibits the growth of liver cancer. In addition, the tumors of non-responsive mice expressed more FoxP3+ Treg cells and higher inhibitory immune checkpoint molecules, such as PD-1, PD-L1, vascular endothelial-derived growth factor (VEGF), cytotoxic T-lymphocyte associated protein 4 (CTLA-4), indoleamine 2,3-dioxygenase (IDO) and IL-10 (52).
In summary, what should we focus on? Studies have also demonstrated that T cell regeneration in the tumor microenvironment is insufficient to mediate the preclinical efficacy of anti-PD-L1. It has also been found in other studies that the interaction between PD-1 and PD-L1 in tumor-draining lymph nodes can predict the clinical efficacy of ICIs in patients with metastatic melanoma but not in primary tumor tissue. This also suggests that interactions between T cells and antigen-presenting cells in tumor draining lymph nodes may be critical for the efficacy of anti-PD-L1 (53). The blocking of PD-1/PD-L1 in local drainage lymph nodes may be an important direction for our future clinical and basic research. It also triggered our thinking in the treatment of liver cancer, and we may also pay attention to the relationship between PD-1 and PD-L1 in the subsequent liver cancer research. Therefore, we should first review the PD-L1 expression of immune cells and tumor cells in the local microenvironment of liver cancer tumors. Through the above review, we have a clearer understanding of the regulatory mechanism of PD-L1 in different cells, which provides more application space for our follow-up exploration of existing and newly studied targeted drugs.
3 Expression of PD-L1 in tumor cells
Most of the observed expression levels of PD-L1 on tumor cells only consider the expression levels of tumor cells in a certain time and space, and these studies are far from reflecting the true expression status of PD-L1 in tumor cells. Generally speaking, the expression of PD-L1 in tumor cells can be caused by the increase of PD-L1 caused by changes in tumor cells’ own signaling pathways (54), or by changes in the external environment, including the influence of T cells (55), macrophages, dendritic cells and tumor-related fibroblasts on tumor PD-L1 expression. And these two different causes of tumor cells PD-L1 elevation, treatment methods are completely different. The high expression of PD-L1 in tumor cells, which we are concerned about, can be either a “cause” for promoting disease progression or a “result” of immunotherapy response. Next, we will focus on the impact of changes in tumor cells themselves on PD-L1.
3.1 Genomic variation of PD-L1
The frequency and prognostic significance of PD-Ls gene alterations in liver cancer remain unknown. The clinical relevance and prognostic value of 9p24.1 gene alteration in an independent cohort of HCC patients were studied by tissue microarray analysis, and the results showed that the genetic alteration of 9p24.1 significantly promoted the upregulation of PD-L1 and PD-L2 (56). Nucleostemin (NS) promoted liver regeneration through damage repair mechanisms and protects human HCC cells from replication and drug-induced DNA damage. NS consumption in liver cancer cells increased physical DNA damage and the amount of cytoplasmic double-stranded DNA, leading to increased cytokine and PD-L1 reactivity (57).
3.2 Epigenetic regulation of PD-L1
Epigenetic regulation of PD-L1 expression (Table 1).
Table 1
| Key molecular | Regulation mechanism | PD-L1 changes | References |
|---|---|---|---|
| HDAC | HDAC up-regulates PD-L1 | Up-regulation | (58) |
| EZH2 | EZH2 can inhibit PD-L1 expression by upregulating lysine trimethylation level at N-terminal 27 of histone H3 and IRF1 on CD274 promoter | Down-regulation | (59) |
| PRMT1 | Loss of PRMT1 reduces PD-L1 expression in tumors | Up-regulation | (60) |
| DNMT1 | DNMT1 is positively correlated with PD-L1 over-expression | Up-regulation | (61) |
| PD-L1L2-SE | Activation of PD-L1L2-SE was required for the expression of PD-L1 and PD-L2 in tumor cells | Up-regulation | (62) |
| LRPPRC | LRPPRC might partially up-regulate the post-transcriptional expression of PD-L1 in an m6A-dependent manner | Up-regulation | (63) |
| ALKBH5 | ALKBH5 inhibited the expansion and cytotoxicity of T cells by sustaining tumor cell PD-L1 expression | Up-regulation | (64) |
Epigenetic regulation of PD-L1 expression.
3.2.1 Histone acetylation
Many studies have shown that histone deacetylation regulates the expression of immune checkpoints and plays an important role in cancer progression (65). Gasdermin D (GSDMD) inhibited cGAS activation by promoting autophagy through the output of potassium (K+). The expression of PD-L1 was promoted by histone deacetylase/signal transducer and activator of transcription 1 (STAT1), which induced the counter-activation of PD-L1 by input calcium (Ca2+) (66). Studies have shown that histone deacetylase (HDAC) makes cancer cells sensitive to ICIs therapy by up-regulating the expression of CTLA-4, PD-1, PD-L1, and PD-L2 on tumor cells and tumor infiltrating lymphocytes (TILs) (58). In addition, the epigenetic regulation of immune checkpoints molecules used to improve the tumor microenvironment also expands the understanding of potential therapeutic targets for improving the tumor microenvironment and restoring immune recognition and immunogenicity (67). Recently, in vitro and in vivo results have shown that epigenetic modifiers play an important role in triggering and enhancing the host immune system in the treatment of cancer (68). Two important epigenetic mechanisms in cancer included hypermethylation mediated by DNMT and histone deacetylation mediated by HDAC. Some epigenetic regulators played a negative role in the immune response, inducing immune escape in cancer cells (69). Two important epigenetic drugs, histone deacetylase inhibitor (HDACI) and DNA methyltransferase inhibitors (DNMTIs), up-regulated the expression of immune checkpoints molecules in immune cells or cancer cells (68). This provides a new mechanism for ICIs to treat cancer.
3.2.2 Histone methylation
EZH2 inhibited the expression of PD-L1 in HCC cell lines by up-regulating the promoter trimethylation on histone 3 lysine 27 (H3K27me3) (59). EZH2 might be a potential therapeutic target for the combination therapy of immune therapy for HCC. In addition, protein arginine methyltransferase 1 (PRMT1) specifically methylated the 3-site arginine of histone H4 in vitro and in vivo. Deletion of PRMT1 in mice reduced the expression of PD-L1 and PD-L2 in tumors and reduced the therapeutic effect of anti-PD-1 in HCC mice (60).
3.2.3 DNA methylation
Studies have shown that features of HCC and T cell DNA methylation are widespread in peripheral blood mononuclear cells (PBMC) and are highly enriched in genes associated with immune function. For example, PD-1 (70). Studies have shown that the significant up-regulation of DNMT1 is positively correlated with PD-L1 over-expression in sorafenib resistant HCC cells (61).
3.2.4 Super-enhancer
Super-enhancers are defined DNA regulatory elements that can be distinguished from enhancers through the size of DNA elements and epigenetic modifications such as H3K4me1, H3K4me3, and H3K27Ac (71). Super-enhancers are extremely important to maintain cell identity through inducing the expression of pivotal lineage-specific genes. By hijacking this mechanism, tumor cells often assemble new super-enhancers to trigger oncogenes such as MYC(72, 73). The SPACE prediction model also successfully predicted the super enhancer of PD-L1 (74). Activation of PD-L1L2-SE was required for the expression of PD-L1 and PD-L2 in tumor cells. Deletion of the PD-L1L2-SE gene caused tumor cells to lose immune escape and made them sensitive to T cell killing. PD-L1 and PD-L2 induced by PD-L1L2-SE were not associated with IFN-γ. Therefore, epigenetic activation of this region (PD-L1L2-SE) was associated with PD-L1 and PD-L2 (62). Studies have shown that these enhancers can predict prognosis better than nearby genes (75).
3.2.5 N6-methyladenosine
N6-methyladenosine (m6A) is a novel epigenetic modification and an important regulator of HCC progression (15). Leucine rich pentatricopeptide repeat containing (LRPPRC)-mediated M6A modification had important effect on PD-L1 mRNA and immune escape in HCC (63). LRPPRC might partially up-regulate the post-transcriptional expression of PD-L1 in an m6A-dependent manner, enhancing the stability of PD-L1 mRNA (63). In addition, tumor-intrinsic ALKBH5 inhibited the expansion and cytotoxicity of T cells by sustaining tumor cell PD-L1 expression (64).
3.3 Transcriptional regulation of PD-L1
Multiple pathways and targets regulate the transcription of PD-L1 (Figure 3).
Figure 3
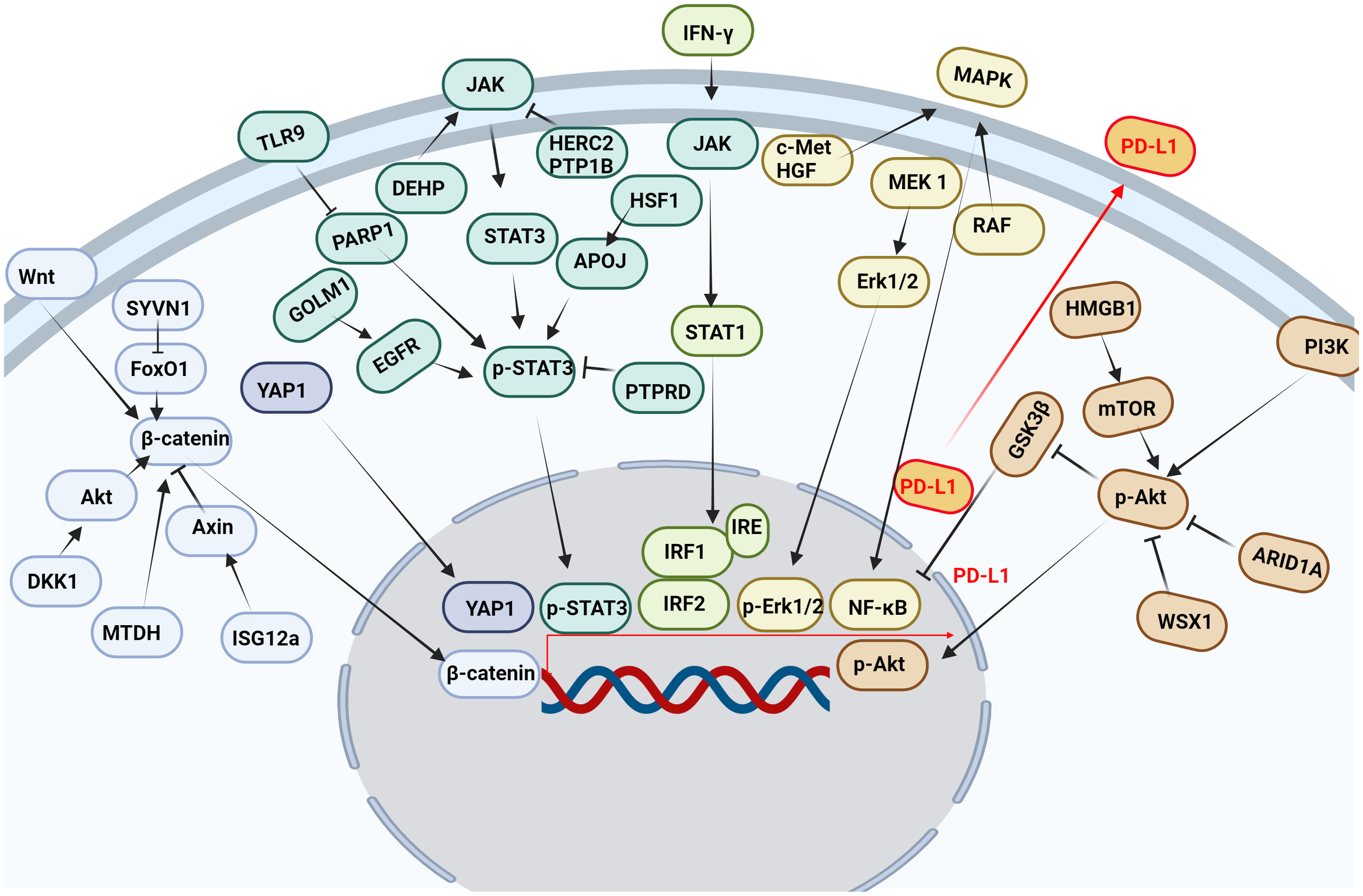
Multiple pathways and targets regulate the transcription of PD-L1. Wnt, Hippo, JAK, IFN-γ, MAPK, PI3K and other signaling pathways affect the transcription of PD-L1 in tumor cells.
3.3.1 JAK/STAT signaling pathway
The JAK/STAT signaling pathway is abnormally activated in HCC, and its downstream target genes control dysfunctions of tumor growth and angiogenesis, invasion, and metastasis (76). It was found that STAT3 bound to PD-L1 promoter and transcriptionally to regulate PD-L1 expression (77). Decreased STAT3 activity led to decreased IFN-γ-induced PD-L1 expression and restored T cell sensitivity (78). In addition, the phosphorylation of the upstream molecular pathway of STAT3 also affected the expression of PD-L1. Protein tyrosine phosphatase receptor delta (PTPRD) inhibited the expression of PD-L1 by inhibiting the phosphorylation of STAT3 (79). Golgi membrane protein 1 (GOLM1), as an oncogene, promoted the growth and metastasis of liver cancer by selectively binding with epidermal growth factor receptor (EGFR) (80). In addition, GOLM1 promoted the phosphorylation of STAT3 by enhancing the level of EGFR, up-regulating the transcriptional expression of PD-L1 (80). Toll-like receptors 9 (TLR9) negatively regulated the expression of PARP1 mediated the decrease of STAT3 Poly (ADP-ribosyl) ation (PARylation) and the increase of STAT3 Tyr705 phosphorylation, and promoted the transcription of PD-L1 (81). Studies have shown that HECT domain and RCC1-like domain 2 (HERC2) enhance cancer stemness and PD-L1-mediated immune escape of HCC cells, which is associated with activation of the STAT3 pathway during the inflammation-cancer transformation. Coupling of HERC2 with endoplasmic reticulum (ER)-resident protein tyrosine phosphatase 1B (PTP1B) restricted PTP1B transport from the ER to the ER-plasma membrane junction, improving inhibitory effect of PTP1B on phosphorylation of Janus kinase 2 (JAK2). In addition, HERC2-knocked out hepatocytes limited hepatic PD-L1 expression and improved HCC progression (82). Heat shock factor 1 (HSF1) up-regulated PD-L1 expression by inducing APOJ expression and activating STAT3 signaling pathway (83). Di (2-ethylhexyl) phthalate (DEHP) might promote the expression of PD-L1 by up-regulating JAK2/STAT3 levels, inhibiting anti-tumor immunity (84).
3.3.2 IFN-γ signaling pathway
PD-L1 expression was primarily induced by IFN-γ released from tumor-infiltrating T cells in HCC (85). IFN-γ induced PD-L1 expression by up-regulating IRF-1 expression in mouse and human HCC cells (86) (87). It has also been found that IFN-γ induces PD-L1 expression through the JAK/STAT1/IRF1 pathway in HCC cell lines (88). Both the transcription factors IRF-1 and IRF-2 signaling pathways regulated PD-L1 in HCC cells. IRF-1 antagonized IRF-2 binding to IRE promoter in PD-L1, providing new insights into the regulation of PD-L1/PD-1 pathway during ICIs therapy of HCC. In addition, over-expression of IRF-2 inhibited IFN-γ-induced PD-L1 promoter activity and protein levels (87). Studies have shown that TNF-α enhances IFN-γ signaling by up-regulating the expression of IFN-γ receptor. In addition, the expression of PD-L1 induced by TNF-α and IFN-γ promoted the growth of liver cancer (88). IFN-γ and IL-1β have a synergistic effect on PD-L1 expression (89).
3.3.3 Wnt/β-catenin signaling pathway
β-catenin was highly expressed in a variety of tumors and played an important role in tumor growth, metastasis and recurrence, especially in HCC patients. And the nuclear accumulation of β-catenin in cancer cells often predicted a poor prognosis (90). Studies have shown that interferon stimulated gene 12a (ISG12a) promotes β-catenin proteasome degradation by inhibiting ubiquitination degradation of Axin, thereby inhibiting Wnt/β-catenin signaling (91). β-catenin was considered to be a transcription factor of PD-L1.ISG12a inhibited the expression of PD-L1 by inhibiting Wnt/β-catenin signaling, rendering cancer cells sensitive to NK cell-mediated killing (92). Studies have shown that dickkopf-1 (DKK1) is positively correlated with PD-L1 and negatively correlated with CD8+ T cell infiltration in human HCC. Overexpression of DKK1 promoted PD-L1 expression by activating Akt/β-catenin signaling pathway (93). Metadherin (MTDH) increased PD-L1 expression and up-regulated PD-L1 transcriptional activity through β-catenin/LEF-1 signaling pathway. More importantly, MTDH ASO improved anti-PD-1 response in PD-1-treated malignancies and increased infiltration of cytotoxic T cells (94). PD-L1 up-regulated serum and glucocorticoid kinase 2 (SGK2), activated SGK2/β-catenin signaling pathway, and promoted the expansion of HCC cell epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) (95). Synoviolin (SYVN1) regulated FoxO1 ubiquitination and stimulated β-catenin nuclear translocation, promoting PD-L1-mediated liver cancer metastasis and immune escape (96).
3.3.4 MAPK signaling pathway
Mitogen-activated protein kinase (MAPK) signaling pathway was associated with the expression of PD-L1 in liver cancer (97). Epidermal growth factor (EGF) or IFN-γ promoted the increase of PD-L1 in HCC cell lines. While EGFR and mitogen-activated protein kinase kinase 1 (MEK1) and mitogen-activated protein kinase kinase 2 (MEK2) were blocked, EGF and IFN-γ-induced up-regulation of PD-L1 was inhibited. In addition, IFN-γ increased the transcriptional activity of PD-L1, while MAPK signaling increased the stability of PD-L1 mRNA (97). Moreover, MET proto-oncogene, receptor tyrosine kinase (c-Met) was a receptor for hepatocyte growth factor/scatter factor (HGF/SF). HGF induced c-Met activation occurs during the activation of the PD-1/PD-L1 signaling pathway (98). As the upstream target molecule of PD-L1, c-Met regulated the transcription of PD-L1 through the MAPK/NF-кBp65 pathway, promoting the progression of HCC (99). In addition, it was found that trans-activation of RAF dimer and ERK signal promoted HCC cell survival and PD-L1 expression through MAPK/NF-κB pathway (100). Studies have shown that blocking IKK complex formation leads to reduced nuclear translocation of NF-κBp65 and PD-L1 expression (101). It was found that up-regulation of alpha fetoprotein (AFP) increased the expression of PD-L1 in HCC tissues by activating P65 protein (102).
3.3.5 PI3K/AKT pathway
The PI3K/AKT signaling pathway participates in the growth and metastasis of HCC (103). Studies have shown that the RNA-RNA crosstalk network driven by high mobility group box-1 (HMGB1) promotes glutamine metabolism in HCC cells through a dual mechanism. Activation of mTORC2-AKT-C-MYC positive feedback loop up-regulated glutamine synthetase (GS) expression and induced inhibition of SIRT4 on glutamate dehydrogenase (GDH) by mTORC1 signaling pathway. At the same time, this crosstalk network may hinder the efficacy of immunotherapy through mTORC1-P70S6K-dependent PD-L1 production and PD-L1+ exosome activity (104). It was found that the absence of AT-rich interaction domain 1A (ARID1A) activated phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT signaling was significantly correlated with the high expression of PD-L1 in HCC. Low expression of ARID1A and high expression of PD-L1 were independent prognostic factors for overall survival (OS) and relapse-free survival (RFS). Patients with ARID1A deletion and high expression of PD-L1 had the worst prognosis. HCC with low expression of ARID1A was significantly associated with high levels of tumor-associated CD68-positive macrophages (105). WSX1 was down-regulated in HCC cells, and WSX1 enhanced hepatic immune surveillance by blocking the PI3Kδ/AKT/GSK3β/PD-L1pathway (106).
3.3.6 Hippo pathway
Hippo signaling pathway inactivation induces the activation of yes-associated transcriptional regulatory factor 1 (YAP1), which regulates gene transcription (107). Studies have found that YAP1 inhibitors reduce the expression of PD-L1 in tumor tissues, promote the infiltration of CD8 T cells and CD4 T cells into tumor tissue (108), which disrupt the immunosuppressive microenvironment of cancer and improve the efficacy of HCC treatment (109). Interestingly, our research group also found that PD-1/PD-L1 interaction up-regulates YAP1 expression in HepG2 cells through the MAPK/ERK pathway (110). M2-polarized macrophages stimulated by IgA complex activated YAP/TAZ mediated signaling pathway, inducing cell activation and PD-L1 up-regulation in vitro (111).
3.3.7 EMT
EMT was a malignant tumor phenotype characterized by invasion and metastasis, and TNF-α-induced EMT led to up-regulation of immunomodulators, including PD-L1 and PD-L2. Conversely, inhibition of EMT decreased the expression of PD-L1 and PD-L2 (112). In addition, TGF-β and fibroblast growth factor 2(FGF-2) effectively induced EMT through SMAD family member 3(SMAD3), MEK/Erk and mTOR pathways in HCC-827 cell line. Reversal of EMT partially restored chemical sensitivity and inhibited PD-L1 expression (113). It was found that TGF-β1 in HCC promoted EMT and induced the expression of PD-L1. TGF-β-specific inhibitor SB431542 blocked TGF-β1-mediated EMT and inhibited the expression of PD-L1 in liver cancer cells. Furthermore, down-regulation of PD-L1 inhibited EMT (114). During TGF-β1-induced EMT, the immune checkpoint molecules PD-L1 and B7-H3 were up-regulated. And reversing EMT decreased the expression of PD-L1 and B7-H3 (115).
3.3.8 AR
Studies have shown that androgen receptor (AR) negatively regulates the expression of PD-L1 by acting as a transcription suppressor of PD-L1. Thus, AR inhibited the expression of PD-L1, possibly contributing to sex differences in HCC (116, 117). In addition, it was found that hydroxysteroid 17-beta dehydrogenase 6 (HSD17B6) played an important role in the occurrence and development of HCC. HSD17B6 inhibited the expression of transforming growth factor beta 1 (TGF-β1) and PD-L1 by transforming DHT (118).
3.3.9 Other specific transcriptional regulation
SRY-box transcription factor 2 (SOX2) was a transcription factor that controls the expression of many target genes by forming trimer complexes with octamer-binding transcription factor 4 (OCT4) on DNA (119). SOX2 regulated the expression of PD-L1 by directly binding SOX2 common binding sites on the PD-L1 promoter region and regulating the promoter activity of PD-L1 (120). Y-box binding protein 1 (YB-1) 1 promoted the expression of multiple resistance genes, thus enhancing the drug resistance of tumors (121). It was found that chemotherapy induced immunosuppressive microenvironment formation and tumor immune escape through YB-1-mediated increase in PD-L1 (122). Oxidative stress responsive kinase 1 (OXSR1) was closely related to malignant progression of malignant tumors (123). The high expression of OXSR1 was positively correlated with the infiltration level of tumor-infiltrating immune cells (TIICs) and the expression of PD-L1 in HCC (124). In liver cancer cells, MYC up-regulated the expression of PD-L1 in lymphoma. Knocking down the expression of MYC promoted the increase of PD-L1 expression level (125). Down-regulation of C-X-C chemokine receptor 2 (Cxcr2) reduced PD-L1 levels and thus promoted the transformation of macrophages to the M1 type, which was mediated by down-regulation of MYC (126). The activation of the inhibitor of differentiation or DNA binding 1 (ID1)/MYC signal promoted immune escape and tumor progression of drug-resistant HCC through PD-L1 up-regulation and CCL5-induced PMN-MDSC recruitment in HCC cells (127). Methyltransferase-like 5 (METTL5) expression was elevated in HCC tissues and cells and was associated with poor prognosis. Down-regulation of METTL5 inhibited the expression of PD-L1 and the malignant cell behavior of HCC by inhibiting the MYC pathway (128). Inhibition of MYC increased the expression of STAT1, leading to increased PD-L1 expression in HCC cells exposed to IFN-γ (125). Anti-silencing function 1b (ASF1B) was highly expressed in tumor tissues, which was correlated with poor OS and progression-free survival (PFS). And ASF1B was positively correlated with PD-L1 expression (129). CKLF-like MARVEL transmembrane domain–containing 4 (CMTM4) was the main regulator of PD-L1 in HCC. CMTM4 might stabilize PD-L1 and promote the escape of T-cell-mediated cytotoxicity (130). CKLF-like MARVEL transmembrane domain-containing protein 6 (CMTM6) maintained the expression of PD-L1 by controlling its systemic circulation (131). The high expression of CMTM6/PD-L1 was associated with poorer RFS and OS in HCC patients (132). Patients with CMTM6/PD-L1 co-expressed macrotrabecular-massive (MTM) HCC had a higher risk of disease progression and death (133). Increased expression of PD-L1 and AR-VRK2 induces immune escape, development and metastasis of liver cancer (134). The expression of human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2, also known as B7-H7) was negatively correlated with PD-L1. Patients with HHLA2 and PD-L1 co-expression had the shortest survival time (135). The expression of neurotrophic factor-3 (NTF3) was negatively correlated with PD-L1, T cell immunoreceptor with Ig and ITIM domains (TIGIT) and T cell immunoglobulin and mucin domain 3 (TIM-3) (136). MMP-12 might promote the development of HCC by up-regulating PD-L1 (137). Silencing RAB42 down-regulated PD-L1 expression and inhibited immune escape by inhibiting E2F signaling pathway in hepatoma cells (138). LW6 inhibited tumor angiogenesis, down-regulated the expression of PD-L1, and promoted the apoptosis of HCC cells by inhibiting HIF-1α (139).
3.4 Regulate the expression of PD-L1 after transcription
3.4.1 Regulation of PD-L1 by microRNAs
Non-coding RNAs regulates the expression of PD-L1 (Table 2). MicroRNAs (miRNAs) were a class of endogenous non-coding RNAs that regulate cell cycle, proliferation and apoptosis, and their abnormal expression was associated with the occurrence of liver cancer (163). microRNA-1 (miR-1) was a tumor suppressor miRNA. MiR-1 directly regulated the expression of PD-L1, and the loss of miR-1 contributed to the upregulation of PD-L1 in sorafenib resistant liver cancer cells (140). Nuclear factor E2-related factor (Nrf-2) inhibited the expression of miR-1, and the regulatory axis of Nrf-2/miR-1/PD-L1 contributed to the maintenance and development of sorafenib resistance in HCC cells. In addition, it was found that miR-155-5p and miR-194-5p could up-regulate the expression of PD-L1 through X inactive specific transcript (XIST) (141). HOXA-AS3 increased PD-L1 expression. In addition, both inhibition of PD-L1 and overexpression of miR-455-5p reversed the effects of cell proliferation and invasion induced by HOXA-AS3 overexpression. (142). Olaparib enhanced the expression of PD-L1 in HCC cells by inhibiting miR-513. Inhibition of poly (ADP-ribose) polymerase (PARP) enhanced ICIs in HCC through the miR-513/PD-L1 pathway (143). Studies have shown that IRF-1 up-regulation induces HCC apoptosis by promoting miR-195 and inhibiting the expression of checkpoint kinase 1 (CHK1). IRF-1 expression or CHK1 inhibition also promoted PD-L1 expression by increasing STAT3 phosphorylation (144). MiR-329-3p inhibited the immunosuppression of tumor cells and enhanced the response of tumor cells to T-cell-induced cytotoxicity through reducing the expression of lysine-specific demethylase 1A (KDM1A, also known as LSD1).Thus, myocyte enhancer factor 2D (MEF2D) demethylation and PD-L1 expression activation were promoted (145). Studies have shown that down-regulation of myocardial infarction-associated transcript (MIAT) can enhance the cytotoxicity of T cells to HCC cells and increase the expression of miR-411-5p, STAT3 and PD-L1. Inhibition of miR-411-5p reversed the expression of STAT3 and PD-L1 in HCC cells inhibited by MIAT knockout (146). MiR-378a-3p mimics effectively reduced the expression of and inhibited the differentiation of Tregs in co-culture models. In addition, overexpression of miR-378a-3p inhibited cell proliferation and migration in HCC cells, while promoted apoptosis by inhibiting STAT3 signaling (147). β-Glucuronidase (GUSB) promoted the proliferation, invasion and migration of human HCC cells by promoting miR-513a-5p.It also down-regulated the expression of PD-L1, resulting in primary resistance to anti-PD-1 therapy (148). In HCC, p-P38 mitogen activated protein kinase (MAPK) increased activation and down-regulated miR-675-5p. Down-regulation of miR-675-5p might enhance the stability of PD-L1 mRNA through the 3’-untranslated region (3’-UTR) of PD-L1, resulting in the accumulation of PD-L1. Upregulation of HK2 enhanced aerobic glycolysis and mediated the decrease of HLA-ABC (149). These results suggested that miRNAs might play an important role in the immune microenvironment of HCC and had certain guiding significance for the clinical treatment of HCC.
Table 2
| Key molecular | Regulation mechanism | PD-L1 changes | References |
|---|---|---|---|
| MiR-1 | Inhibits the expression of PD-L1 | Down-regulation | (140) |
| MiR-155-5p and miR-194-5p | MiR-155-5p and miR-194-5p can up-regulate PD-L1 through XIST | Up-regulation | (141) |
| MiR-455-5p | Both PD-L1 inhibition and miR-455-5p overexpression reversed cell proliferation and invasion induced by HOXA-AS3 overexpression | Down-regulation | (142) |
| MiR-513 | Olaparib enhances the expression of PD-L1 in HCC cells by inhibiting miR-513 | Down-regulation | (143) |
| MiR-195 | Promotes the expression of PD-L1 | Up-regulation | (144) |
| MiR-329-3p | MiR-329-3p promotes MEF2D demethylation and activation of PD-L1 expression | Up-regulation | (145) |
| MiR-411-5p | Inhibition of miR-411-5p can reverse the expression of STAT3 and PD-L1 in HCC cells inhibited by MIAT knockout | Down-regulation | (146) |
| MiR-378a-3p | MiR-378a-3p mimics can effectively reduce the expression levels of PD-L1 mRNA and protein by regulating the expression levels of certain cytokines | Down-regulation | (147) |
| MiR-513a-5p | MiR-513a-5p promotes the proliferation, invasion and migration of human HCC cells, and down-regulated the expression of PD-L1 | Down-regulation | (148) |
| MiR-675-5p | Downregulation of miR-675-5p enhanced the stability of PD-L1 mRNA through the 3’ -UTR of PD-L1, causing the accumulation of PD-L1 | Down-regulation | (149) |
| PCED1B-AS1 | Promotes the expression of PD-L1 | Up-regulation | (150) |
| LncRNA MIAT | Promotes the expression of PD-L1 | Up-regulation | (151) |
| Lnc-RAB11B | High expression of RAB11B may lead to downregulation of PD-L1 | Down-regulation | (152) |
| LncRNA AC099850.3 | Promotes the expression of PD-L1 | Up-regulation | (153) |
| LINC00657 | LINC00657 inhibits PD-L1 expression by decreasing miR-424 | Down-regulation | (154) |
| LncRNA KCNQ1 | Promotes the expression of PD-L1 | Up-regulation | (155) |
| LINC00244 | Inhibits the expression of PD-L1 | Down-regulation | (156) |
| LINC00638 | The LINC00638/miR-4732-3p/ULBP1 axis promotes immune escape of HCC through PD-L1 | Up-regulation | (157) |
| Hsa-circ-0006852 | Hsa-circ-0006852 promotes the increase of PD-L1 expression by activating the NF-κB signaling pathway | Up-regulation | (158) |
| Hsa-circ-0003288 | Hsa-circ-0003288 acts as a miR-145 sponge and up-regulates PD-L1 expression through the PI3K/AKT signaling pathway | Up-regulation | (159) |
| Has-circ-0005239 | Inhibits the expression of PD-L1 | Down-regulation | (160) |
| CircWDR25 | CircWDR25 promotes the expression of PD-L1 in HCC cells through circWDR25/miR-4474-3p/ALOX15 and EMT axis | Up-regulation | (161) |
| CircPRDM4 | CircPRDM4 promotes PD-L1 expression by promoting HIF-1α recruitment to CD274 promoter | Up-regulation | (162) |
Non-coding RNAs regulation of PD-L1 expression.
3.4.2 Regulation of PD-L1 by lncRNAs
Long noncoding RNAs (lncRNAs) are a class of non-coding RNAs that have limited protein-coding ability and are involved in the genesis and development of tumors (164). Many studies have demonstrated that lncRNA can regulate PD-L1 expression. In HCC, the expression of PCED1B antisense RNA1 (PCED1B-AS1) and hsa-miR-194-5p was up-regulated in lncRNA. In liver cancer, PCED1B-AS1 interacted with hsa-miR-194-5p, which inhibited the expression of PD-Ls, and enhanced the expression of PD-Ls (150). Cancer susceptibility 11 (CASC11) recruited eukaryotic translation initiation factor 4A3 (EIF4A3) and enhanced the stability of E2F1 mRNA. It further affected the NF-κB signal and promoted the activation of PI3K/AKT/mTOR pathway, regulating the expression of PD-L1 (165). Studies have shown that the expression of LncRNA MIAT in liver cancer is positively correlated with the expression of inhibitory immune checkpoint molecules such as PD-1, PD-L1 and CTLA4 (151). Low expression of lnc-RAB11B-AS1 was associated with shorter OS and DFS in HCC patients. The high expression of RAB11B reduced PD-L1 expression, thereby inhibiting the progression of HCC (152). LncRNA AC099850.3 was up-regulated in HCC tissues, and its high expression was associated with poor prognosis in HCC patients. LncRNA AC099850.3 significantly improved the proliferation and invasion ability of HCC cells through the PRR11/PI3K/AKT pathway. In addition, lncRNA AC099850.3 affected the abundance of various immune cells in the tumor microenvironment, especially M2 macrophage infiltration, and was positively correlated with PD-L1 (166). LINC00657 was highly expressed in HCC and was associated with poor prognosis. LINC00657 regulated the expression of PD-L1 by decreasing miR-424. The 3’UTR of PD-L1 was highly conserved with that of miR-424, and miR-424 significantly inhibited the mRNA and protein levels of PD-L1 (154). LncRNA KCNQ1 overlapping transcript 1 (lncRNA KCNQ1OT1) was closely related to drug resistance in cancer. KCNQ1OT1 acted as a competitive endogenous RNA of miR506 and increased PD-L1 expression in sorafenib resistant HCC cells (155). LINC00244 inhibited HCC proliferation, invasion and metastasis by down-regulating the expression of PD-L1. In addition, low expression of LINC00244 activated the EMT pathway, promoting rapid growth and metastasis of HCC cells (156). Lipopolysaccharide (LPS) induced the expression of PD-1 and PD-L1 in mouse tumor and induced the expression of PD-L1 in HCC cells. LPS played a key role in immune escape of HCC through the METTL14/MIR155HG/PD-L1 axis (167). Studies have shown that patients with high ULBP1 and PD-L1 have the worst prognosis. The LINC00638/miR-4732-3p/ULBP1 axis associated with tumor mutation burden promoted immune escape in HCC via PD-L1 (157).
3.4.3 Regulation of PD-L1 by circRNAs
Circular RNAs (circRNAs) were non-coding RNAs with a closed-loop structure that regulated biological processes by acting as sponges for miRNAs or binding to proteins, and many circRNAs were involved in cell proliferation and invasion of HCC (168). In recent years, the effect of circRNAs mediated PD-L1 expression on the immune status of liver cancer has attracted much attention. Has-circ-0006852 (circCORO1C) promoted the development of HCC by activating the NF-κB signaling pathway, increasing the phosphorylation of P65, the expression of c-Myc, COX-2 and PD-L1 (158). Has-circ-0003288 acted as a miR-145 sponge and up-regulated PD-L1 expression through the PI3K/AKT signaling pathway, promoting EMT and HCC invasion (159). Targeting has-circ-0003288 might provide a therapeutic strategy for the treatment of HCC. Has-circ-0005239 promoted migration, invasion, and angiogenesis by controlling PD-L1 expression in HCC. These results revealed that has-circ-0005239 might be a potential therapeutic target for patients with advanced HCC (160). Exogenous and hepatic stellate cell (HSC) exosome derived circWDR25 promoted the proliferation and invasion of HCC cells through the circWDR25/miR-4474-3p/ALOX15 and EMT axes. It also promoted the expression of CTLA-4 and PD-L1 in HCC cells (161). CircPRDM4 was a circRNA, which was associated with hypoxia in HCC. CircPRDM4 promoted PD-L1 activation by promoting HIF-1α recruitment to the PD-L1 promoter and consolidating their interaction under hypoxic conditions. Thus, CD8+ T cell infiltration was inhibited and immune escape of HCC cells was increased (162).
These studies suggested that miRNAs, lncRNAs and circRNAs directly displayed epigenetic functions by recruiting specific protein complexes into genomic DNA, especially certain promoters that regulated the expression of corresponding genes. Studies have also shown that miRNAs, lncRNAs and circRNAs play an important role in regulating the expression of immune checkpoint molecules in various tumors (169, 170). Whether the association between miRNA expression and immune checkpoint levels in tumors can be translated into predictive markers for checkpoint inhibitor therapy in liver cancer needs further investigation. The interaction between the three RNAs was revealed in the “lncRNA-miRNA-mRNA” competitive endogenous RNA network. Some miRNAs and lncRNAs participated in the “cancer immune cycle” regulated by immune checkpoints molecules and had the potential to be the subject of future research in liver cancer.
3.5 Regulation of PD-L1 by post-translational modification
3.5.1 Phosphorylation regulation
EGF treatment enhanced H3-Thr11 phosphorylation at the PD-L1 promoter and promoted the expression of PD-L1 in HCC cells. Inhibition of EGFR reversed EGF-induced expression of PD-L1 mRNA and protein. In addition, inhibition of pyruvate kinase M2 (PKM2) also significantly inhibited EGF-induced PD-L1 expression and H3-Thr11 phosphorylation (171, 172). Studies have shown that inhibiting poly (ADP-ribose) polymerase-1 (PARP-1) activity can enhance p-glycogen synthase kinase 3 beta (p-GSK3β) up-regulate PD-L1 expression, and inhibit T cell infiltration (173).
3.5.2 Acetylation regulation
Myocyte enhancer factor 2D (MEF2D) bound to the promoter region of the PD-L1 gene (which encodes PD-L1) and activated its transcription. Over-expression of p300 or knockdown of sirtuin 7 (SIRT7) in HCC cells promoted acetylation of MEF2D and enhanced its binding to the PD-L1 promoter region. When exposed to IFN-γ, p300 acetylated MEF2D so that it bound to the PD-L1 gene promoter and upregulated PD-L1 expression. SIRT7 also reduced the acetylation of MEF2D and the expression of PD-L1 in HCC cells without exposure to IFN-γ (174).
3.5.3 Ubiquitination regulation
Ubiquitination and deubiquitination were key post-translational modifications of metabolic enzymes and contributed to the occurrence and development of various cancers, including liver cancer (175). Studies have shown that PR domain zinc finger protein 1 (PRDM1) enhances transcription of USP22 and reduces degradation of SPI1 protein through deubiquitination, thereby enhancing transcription of PD-L1 (176). GOLM1 promoted COP9 signaller-5 mediated PD-L1 deubiquitination in HCC cells and increased PD-L1 transport to exosomes by inhibiting the expression of Rab27b (177).
3.6 Other factors of PD-L1 elevation
3.6.1 Hepatitis B virus
TME for HBV-related HCC was more immunosuppressive than in a virus-free microenvironment, and HBV- related HCC was characterized by faster progression and poorer prognosis. Studies have shown that hepatitis B x protein (HBx) plays an important role in the development of HBV-related HCC. HBx promoted cell proliferation and PD-L1 expression in tumor tissues by up-regulating the expression of S100A4 (178). In addition, studies have shown that the expression of PD-L1 in tumor tissues of HCC patients with positive pre-S2 mutations is increased (179). Clinical studies have shown an acceptable safety profile in HBV-related HCC patients. However, the anti-viral activity of PD-1/PD-L1 blockers could not be determined due to the standard anti-viral therapy performed in clinical trials. In general, except for a significantly lower disease control rate (DCR) in HBV-infected HCC patients, the objective response rate (ORR) of anti-PD-1/PD-L1 did not differ significantly between virus-positive and virus-negative patients (180). The presence of PD-L1 and PD-L2 led to suppression of the immune response, which promoted viral persistence and carcinogenesis. In addition, the expressions of PD-1, PD-L1 and PD-L2 in HCC were significantly higher than those in hepatitis, and were correlated with HCC stage and the number of infiltrating lymphocytes (181).
3.6.2 Exosome
HCC cells could release exosomes containing PCED1B-AS1, which enhanced the expression of PD-Ls in HCC cells, while inhibiting the expression of T cells and macrophages (150). GOLM1 promoted the stabilization of PD-L1 and promoted the transport of PD-L1 to TAMs via exosomes, resulting in higher PD-L1 expression on TAMs than HCC cells and inducing CD8+ T cell inhibition. Zoledronic acid (ZA) combined with anti-PD-L1 reduced PD-L1+ TAM infiltration and improved CD8+ T cell inhibition (177). HCC cells released exosome-containing PCED1B-AS1, which enhanced the expression of PD-Ls in recipient HCC cells and inhibited receptor T cells and macrophages. PCED1B-AS1 induced the expression and function enhancement of PD-Ls through sponging hsa-miR-194-5p in HCC cells (182).
3.6.3 Transarterial chemoembolization
Clinical studies have found that the expression of PD-L1 in HCC pretreated by transarterial chemoembolization (TACE) is significantly higher than that of HCC without TACE (183). Further studies have shown that rat hepatic artery embolization (HAE) can promote the expression of PD-L1 through HIF-1α (184). After TACE treatment of HCC, both the number and function of CD8+ T cells were impaired, while the number of TREM2+ TAMs was increased, which was associated with a poorer prognosis (185). TREM2+ TAMs produced more Galectin-1 than TREM2- TAMs. Galectin-1 promoted the over-expression of PD-L1 in vascular endothelial cells and inhibited CD8+T cell recruitment. TREM2 deficiency also increased the infiltration of CD8+ T cells and inhibited the growth of HCC in vivo (185).
3.6.4 Aflatoxin
Aflatoxins in the diet is an important risk factor for HCC (186). Aflatoxin-related HCC tissues contained high levels of potential mutation-associated neoantigens, as well as many infiltrating lymphocytes and tumor cells expressing PD-L1. In addition to the mutation of tumor protein p53 (TP53) reported in previous studies, studies also have found that there are frequent mutations of adhesion G protein-coupled receptor B1 gene (ADGRB1). ADGRB1 mutation was closely associated with increased angiogenesis and PD-L1 expression in HCC tissues (187). The expression of aryl hydrocarbon receptor (AHR) and PD-L1 was increased in HCC patients associated with aflatoxin B1 (AFB1), and anti-PD-L1 showed greater efficacy on hepatoma xenografts derived from AHR ectopic expression cells (186).
3.6.5 Listeria HCC vaccine
Lmdd-MPFG promoted the expression of PD-L1 in HCC cells, resensitizing local tumor T cells in response to anti-PD-1 immunotherapy (188). Mechanistically, Lmdd-MPFG vaccine activated the NF-κB pathway in TAMs through toll like receptor 2 (TLR2) and myeloid differentiation primary response 88 (MyD88) pathways. SQSTM1 (sequestosome 1) was recruited to activate the autophagy pathway, tilting TAMs from M2-polarized TAMs to M1-polarized TAMs (188).
3.6.6 Autophagy
Autophagy plays a dual role in many types of cancer, such as HCC (189). In HCC, high expression of autophagy marker mRNA was associated with poor clinical status. Increased expression of LC3 in HCC cell lines promoted tumor growth. In specific Tumor types, PD-1 or PD-L1 in tumor intrinsics was associated with higher levels of autophagy. Over-expression of PD-1 or PD-L1 increased autophagy in tumor cells through autophagy-related protein 13 (ATG13) interactions (190).
All in all, factors affecting the expression of PD-L1 in tumor cells are numerous and complex. We need to pay attention to the following key points: firstly, to find key targets regulating PD-L1 and the biological or physical factors that can affect PD-L1. Secondly, in the process of liver cancer immunotherapy, mutations in PD-L1 related regulatory genes or proteins should not be ignored, and specific inhibitors combined with ICIs should eventually be targeted for clinical or preclinical studies.
4 Natural products target the expression of PD-L1
Natural products play an important role in inhibiting the expression of PD-L1 in liver cancer. Most of the natural products are derived from herbs in traditional medicine, which can be used as drugs or supplements (191). In our previous study, we found that dihydroartemisinin (DHA) can not only reduce PD-L1, sensitization chemotherapy and immunotherapy (108), but also by decreasing the expression of PD-1 in CD8+ and CD4+ T cells (108). This also raises our concerns about natural products. Based on the current understanding of PD-L1, we summarized natural products that have pharmacological effects associated with PD-L1 (Table 3).
Table 3
| Name | Regulatory mechanism | Dose | Cell line/Experiment | References |
|---|---|---|---|---|
| Astragalus polysaccharide | Inhibits PD-L1-mediated immunosuppression via miR-133a-3p | 0.1,0.5 and 1 mg/mL | SMMC-7721 and Huh-7 cells | (192) |
| Cantharidin | Reduces the expression of PD-L1 | 0.25,0.5 and 1 mg/kg | Male BALB/c mice | (193) |
| Chrysin | Inhibits the expression of PD-L1 by blocking the JAK/STAT3 and NF-κB pathways | 1,5,10,20,40 μM and 30,120 mg/kg | HepG2 cells and male BALB/c mice | (194) |
| Pentamethylquercetin | Down-regulates PD-L1 expression via IFN-γ signaling pathway | 1,3,10 μM and 5,10,20 mg/kg | HepG2 cells, 3T3-L1 preadipocytes and male KM mice | (195) |
| Quercetin | Reduces the expression of PD-L1 | 25,50,100 µM and 25,50,100 mg/kg | HepG2 cells and male BALB/c mice | (196) |
| Curcumol | Reduces the expression of PD-L1 through crosstalk between HIF-1α and p-STAT3 (T705) signaling pathways |
30 μM and 3,30 mg/kg | Hep3B cells and female athymic BALB/c nude mice |
(197) |
| Curcumin | Reduces the expression of PD-L1 | 10,20,30,40 µM and 10mg/kg | Hep3B, CSQT-2 cells and BALB/c female nude mice | (198) |
| Curcumin | Reduces the expression of PD-L1 | 200 mg/kg | Male BALB/c mice | (199) |
| Dihydroartemisinin | Reduces the expression of PD-L1 and increases CD8+ T cell infiltration | 21.5 µM and 25 mg/kg | HepG2215, shYAP1-HepG2215 cells and C57BL/6 mice | (108) |
Natural products target the expression of PD-L1 in HCC.
4.1 Astragalus polysaccharide
Astragalus polysaccharide (APS) was one of the main bioactive ingredients extracted from Astragalus membranaceus. Studies have shown that astragalus polysaccharide has anti-tumor, anti-inflammatory, antioxidant and immune-regulating effects (200). APS dose-dependent inhibited HCC growth, IFN-γ-induced PD-L1 expression and reduced PD-L1-mediated immunosuppression of HCC cells. APS attenuated PD-L1 mediated immunosuppression in HCC cells via miR-133a-3p. In addition, miR-133a-3p targeted Moesin (MSN), which inhibited the anti-tumor effects of APS by maintaining the stability of PD-L1. In addition, APS attenuated PD-L1 mediated immunosuppression through the miR-133a-3p/MSN axis and played a vital in role anti-tumor. These results suggested that APS might be an effective drug in the treatment of HCC (192).
4.2 Cantharidin
Cantharidin was an insect-derived terpenoid produced by male blister beetles. Cantharidin had anti-cancer properties due to its ability to induce cell cycle arrest, DNA damage and apoptosis (201). Cantharidin inhibited the growth of HCC, increased the proportion of CD4+/CD8+T cells and B cells, and decreased the proportion of Tregs cells. In addition, it significantly reduced the expression of inflammatory factors and immune checkpoint genes PD-1/PD-L1 (193).
4.3 Chrysin
Chrysin was a natural flavonoid found in propolis, honey, and a variety of plants. Chrysin was a phytoestrogens that could act as ligands of the endoplasmic reticulum and has anti-inflammatory, anti-viral and cancer effects (202, 203). Chrysin significantly inhibited the overexpression of PD-L1 and increased the proportion of CD4/CD8-positive T cells by blocking the JAK/STAT3 and NF-κB pathways, inhibiting the growth of liver cancer cells both in vivo and in vitro (194).
4.4 Pentamethylquercetin
Pentamethylquercetin was a natural polymethoxyflavonoid with beneficial effects such as anti-tumor, anti-obesity and heart protection (204). In H22 tumor tissue of obese mice, the expression of PD-L1 was significantly increased, and the expression of ki67 was increased, while the number of CD8+ T cells was significantly decreased. Pentamethylquercetin down-regulated adipose-cell-induced PD-L1 expression via the IFN-γ signaling pathway, at least partially inhibiting HCC progression in obese mice (195).
4.5 Quercetin
Quercetin, a member of the flavonoid family, was one of the most important anti-oxidants and was widely distributed in fruits and vegetables. Quercetin had anti-oxidant, anti-inflammatory, and immunomodulatory effects and had received a lot of attention in recent years for its anti-cancer effects in a variety of cancers (205). Quercetin significantly inhibited the proliferation, migration and invasion of HCC cells in vitro. The levels of granulocyte-macrophage and granulocyte colony-stimulating factor (GM-CSF and G-CSF) and PD-L1 were decreased. Quercetin increased the proportion of CD86+ cells and decreased the proportion of CD206+ cells, promoting the polarization of M1 macrophages. It also increased LC3 I/II expression and down-regulated p62 expression through NF-κB pathway, promoting autophagy (196).
4.6 Curcumol
Curcumol was a sesquiterpenoid compound derived from Rhizoma Curcumae (206), which had anti-tumor, anti-inflammatory, anti-oxidant, and anti-bacterial activities (207). Studies have shown that there is crosstalk between STAT3 and HIF-1α pathways, which synergistically regulate PD-L1 activation. Curcumol inhibited PD-L1 expression in liver cancer through crosstalk between HIF-1α and p-STAT3 (T705) signaling pathways, restoring cytotoxic T cell activity and the ability to kill tumor cells (197).
4.7 Curcumin
Curcumin was a natural polyphenol phytochemical derived from turmeric, which had anti-oxidant, anti-inflammatory and anti-cancer properties (208). The PD-1/PD-L1 signaling pathway promoted the differentiation of Tregs through PD-L1. Curcumin in combination with TG synergistically inhibited the expression of PD-L1 and NF-κB proteins by reducing the expression of Tregs, thus inhibiting the growth of liver cancer (198). Curcumin reduced the histone acetylation of P300-induced thrombin mediated TGF-β1 promoter region, reduced the expression of PD-L1 on the surface of tumor cells or HCC cells, impeding the proliferation of tumor cells. Curcumin also increased the activation rate of lymphocytes and the expression of immune function factors, and finally delayed immune escape (199).
4.8 Dihydroartemisinin
DHA, a derivative of artemisinin, had anti-oxidant, anti-malaria, anti-inflammatory and anti-cancer functions (209). Our previous study found that YAP1 knockdown inhibited the expression of PD-L1, which was related to the JAK1/STAT1, 3 pathways. DHA inhibited YAP1 expression and broke immune evasion in liver cancer niche, which was manifested by decreased PD-L1 level and increased CD8+ T cell infiltration in liver cancer cells. In addition, DHA combined with anti-PD-1 treatment promoted CD4+ T cell infiltration in spleen and CD8+ T cell infiltration in tumor tissue (108).
These studies suggested that natural products could target the expression of PD-L1 in liver cancer and might play an important role in the treatment of liver cancer. The anti-tumor research of natural products has become a research hotspot in recent years due to its wide available resources and certain drug-forming properties (210). Therefore, the discovery of excellent PD-L1 inhibitors from natural products has created convenience for us. But the study of natural products also has limitations. Many studies on the effects of natural products on PD-L1 in liver cancer are still in the preclinical stage, and more clinical data are needed to support the safety and efficacy of natural products. In addition, the low solubility of many natural products, such as dihydroartemisinin and curcumin leads to low bioavailability. Although natural products as chemotherapy and radiotherapy sensitizers are still some time in the clinic. However, due to its good biological activity and diverse structure, it is the best object to study the mechanism of chemotherapy and radiotherapy sensitization. However, the current research is not enough, from the type and quantity of natural product studies.
5 Combination therapy with immune checkpoint inhibitors
Meta-analysis and subgroup analysis were performed to evaluate the benefit of PD-1/PD-L1 inhibitors in combination with advanced HCC patients. A total of 29 studies with 5396 patients were included. ICIs’ combination therapy had higher ORR (26% vs 15%) and DCR (73% vs 55%), longer PFS (5.5 vs 3.1 months) and OS (15.9 vs 12.6 months). PD-1/PD-L1 inhibitors plus anti-VEGF agents had an advantage in DCR (0.80 vs 0.48, meta-regression = - 0.32, P < 0.001), but an equal ORR (0.29 vs 0.26) compared to dual ICIs. The total OS in dua-ICIs were 16.5 months (95% CI 14.2-18.7), yet not reached in the major studies of ICI plus anti-VEGF (211). There are relatively few clinical systematic studies of ICIs combination therapy, and network meta-analyses would be particularly useful. Because there are often multiple drugs available for second-line treatment, direct head-to-head comparative trials may be lacking. By comparing existing treatments with direct and indirect evidence, clinicians can gain a broader understanding of the relative efficacy and safety of these treatments. This may include looking at OS, PFS, RR, and adverse events for different treatments. Such analyses are critical to inform clinical decision-making, establish guidelines, and identify areas where further research is needed to improve outcomes for HCC patients who require second-line treatment. In addition, we reviewed some preclinical ICIs’ combination therapy in order to provide ideas for clinical treatment.
Our previous study found that anti-PD-1 reduced the expression of PD-L1 in HCC(108). ICIs has shown a durable anti-tumor response in patients with advanced HCC, but resistance to ICIs remains in most cancer patients (212). Circulating PD-L1 could be used as an independent predictor of OS and tumor recurrence survival after cryoablation in HCC patients (213). Therefore, the most effective strategy for the treatment of advanced liver cancer might be to combine ICIs with other methods for the treatment of liver cancer, for example, the combination of ICIs with other conventional ablation therapy (such as Radiofrequency ablation (RFA) or cryoablation) will be the most promising method for the treatment of HCC. However, for unresectable advanced HCC, it was more appropriate to look for other combination strategies, such as combinations with kinase inhibitors, histone deacetylase inhibitors, and anti-viral drugs, as well as dual inhibition of two immune checkpoint molecules (214).
Studies have shown that the combination of anti-angiogenic therapy and anti-PD-1. Lenvatinib inhibited the expression of PD-L1 on human umbilical vein endothelial cells. The combined treatment of lenvatinib and anti-PD-1 also led to the formation of long-term immune memory, while synergistically regulating TME and enhancing the cytotoxicity of T cells (91). Regorafenib and PD-1 inhibitors were sequentially treated in one HCC patient with HBV-induced cirrhosis with lung metastasis, with no disease progression and mild side effects (215). However, the combination of cabozantinib with the anti-PD-L1 did not show any other therapeutic benefit in the mouse HCC model tested (216). The combination with lenvatinib was twice as effective as pembrolizumab alone, promising a median OS rate of 20 months, but at the cost of increased toxicity (217). In liver cancer, the combination of cabozantinib and nivolumab showed a significant increase in response rate, extending survival, but at the cost of more frequent and more severe toxicity (218). In mouse HCC models, the combination of ICIs (anti-CTLA-4 and anti-PD-1) with histone deacetylase inhibitor Belinostat induced early upregulation of PD-L1 on tumor antigen presenting cells and late expression of PD-1 on tumor infiltrating effector T cells. The applicability of anti-PD-1 was demonstrated (219).
Atezolizumab combined with the anti-viral 2,5-dimethylcelecoxib (DMC) in ICIs increased the level of tumor-infiltrating CD8+ T cells. In addition, atezolizumab promoted the ubiquitination degradation of HBx and induced PD-L1 protein in HCC cells by activating 5’-adenosine monophosphate to activate the protein kinase pathway, which plays a more significant anti-tumor effect (91).
Double ICIs had a synergistic effect and a higher response rate and better efficacy compared to monotherapy (214). CTLA-4 and PD-1 had similar mechanisms in terms of tumor tolerance. As a result, most patients with advanced HCC using anti-PD-1/PD-L1 did not achieve lasting control, and the combination with anti-CTLA-4 improved treatment effectiveness (220). Based on current evidence, several first and second line phase 3 randomized trials in HCC patients have been initiated, although it will be several years before mature survival data are available.
6 Conclusion and future perspectives
In summary, PD-L1 is expressed on a variety of cells in the immune microenvironment of HCC, including macrophages, monocytes and tumor cells. In addition, in the tumor microenvironment, different cells will interact with each other to further promote or inhibit the expression of PD-L1, which also increases the difficulty of HCC immunotherapy. The technology of spatial omics and advanced computational methods have been developed rapidly. Therefore, we should fully consider the spatial diversity of PD-L1. A study has also shown that the spatial interaction between PD-1/PD-L1 and IDO-1/HLA-DR is closely related to anti-PD-1 clinical response (221). Spatial quantification of the tumor immune microenvironment may have better prognostic ability than existing biomarkers, and further development and application of spatial omics may promote a new revolution in the tumor immune microenvironment ecosystem (222).
In addition, in addition to studying the role of PD-L1 in immunotherapy, it has also been found that PD-L1 can also act as a proto-oncogene to regulate the conduction of other signaling pathways of tumor cells and directly promote tumor growth (223) and metastasis (224). In particular, the research on PD-L1 and tumor metastasis has become a hot topic. For example, in colorectal cancer liver metastasis, PD-L1 is still highly expressed in tumors at the site of metastasis. Moreover, the application of PD-1 inhibitors is still effective (225). In addition, there is a unique immune landscape in liver metastasis of rectal cancer, and highly metabolically activated MRC1+ CCL18+ M2-like macrophages are found at the site of metastasis (226). The expression of PD-L1 in intestinal metastasis of lung cancer also has similar characteristics (227). Metastasis is also the most serious complication in the process of tumor treatment. The influence of PD-L1 expression in metastatic cancer cells on the fragile microenvironment at the site of metastasis is still unknown. Therefore, the study of PD-L1 in tumor metastasis will also become a hot spot in the future, and corresponding technical means will continue to appear (228). Therefore, studying the regulation of PD-L1 expression in HCC is beneficial to the treatment of HCC in many ways.
Furthermore, the regulation of PD-L1 expression is regulated by many factors, including transcriptional, post-transcriptional, translational and post-translational multifaceted regulation, and cross-regulation exists among different levels and signaling pathways, and the mechanism is complex. Therefore, it is increasingly necessary for us to find the key signaling pathways regulating PD-L1. The positive expression of PD-L1 in tumor tissue is regarded as an indicator of the application of ICIs, but PD-L1 positive patients are not fully effective in ICIs therapy, on the contrary, PD-L1 negative patients can also benefit from ICIs therapy. Therefore, in the process of research, we should pay more attention to what kind of cells in the tumor microenvironment express PD-L1, which affects the tumor immunotherapy and the precise application of drugs.
In addition, we still need to deeply understand how PD-L1 plays an immunosuppressive role in liver cancer, and some studies have also brought us some inspiration. PD-1 on the surface of CD8+T cells inhibits T cell glycolysis by inhibiting PI3K/Akt/mTOR signaling pathway. On the one hand, PD-L1 on the surface of tumor cells can bind to PD-1 on the surface of CD8+T cells to inhibit T cell function. On the other hand, it can promote the translation of glycolytic enzyme GLUT1 by promoting the Akt/mTOR signaling pathway of tumor cells in the tumor microenvironment. Glucose deprivation by tumor cells affects the glucose demand of T cells. On the one hand, in the tumor microenvironment, anti-PD-L1 treatment can inhibit the Akt/mTOR pathway to reduce glucose consumption and also enhance the ability of CD8+ T cells to compete for glucose. On the other hand, it also alleviates the negative effect of PD-1 on CD8+ T cells, restores the glycolysis function of T cells, and increases the production of cytokine IFN-γ (153). Tumor cells in liver cancer glycolysis changes profoundly affect the immune cells in the tumor microenvironment (229). Understanding the relationship between PD-L1 and immunosuppression from the perspective of glycolysis may be only one aspect, and more problems need to be explored.
Therefore, the therapeutic strategy of combining ICIs has expanded a new space for the application of ICIs, and has also shown a very obvious therapeutic effect. This will ultimately lead to more choices for patients and more benefits for longer patient survival.
Statements
Author contributions
LH: Investigation, Software, Writing – original draft. SL: Conceptualization, Data curation, Writing – original draft. JD: Writing – original draft. NL: Writing – original draft. FY: Writing – original draft. ZJ: Writing – original draft. JZ: Writing – original draft. XS: Writing – original draft. XH: Funding acquisition, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was financially supported by the Science and Technology Program of Hebei (223777156D); National Natural Science Foundation of China (81973840 and 81273748); National science and Technology major projects of the 13th Five-Year Plan (2018ZX10303502); Sichuan Provincial Administration of Traditional Chinese Medicine Major science and technology projects (2021XYCZ004).
Acknowledgments
The authors acknowledge using Biorender (https://app.biorender.com/) to create the schemata (Figures 1–3).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Sung H Ferlay J Siegel RL Laversanne M Soerjomataram I Jemal A et al . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 4(10):21660. doi: 10.3322/caac.21660
2
Baecker A Liu X La Vecchia C Zhang ZF . Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev (2018) 27(3):205–12. doi: 10.1097/CEJ.0000000000000428
3
Llovet JM Kelley RK Villanueva A Singal AG Pikarsky E Roayaie S et al . Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):020–00240. doi: 10.1038/s41572-020-00240-3
4
Forner A Reig M Bruix J . Hepatocellular carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/S0140-6736(18)30010-2
5
Chen S Cao Q Wen W Wang H . Targeted therapy for hepatocellular carcinoma: Challenges and opportunities. Cancer Lett (2019) 460:1–9. doi: 10.1016/j.canlet.2019.114428
6
Meyer T . Treatment of advanced hepatocellular carcinoma: beyond sorafenib. Lancet Gastroenterol Hepatol (2018) 3(4):218–20. doi: 10.1016/S2468-1253(17)30255-8
7
Xing R Gao J Cui Q Wang Q . Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front Immunol (2021) 12:783236. doi: 10.3389/fimmu.2021.783236
8
Qin S Ren Z Meng Z Chen Z Chai X Xiong J et al . Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21(4):571–80. doi: 10.1016/S1470-2045(20)30011-5
9
Butte MJ Keir ME Phamduy TB Sharpe AH Freeman GJ . Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity (2007) 27(1):111–22. doi: 10.1016/j.immuni.2007.05.016
10
Gryziak M Wozniak K Kraj L Rog L Stec R . The immune landscape of hepatocellular carcinoma-where we are? Oncol Lett (2022) 24(5):410. doi: 10.3892/ol.2022.13530
11
Makarova-Rusher OV Medina-Echeverz J Duffy AG Greten TF . The yin and yang of evasion and immune activation in HCC. J Hepatol (2015) 62(6):1420–9. doi: 10.1016/j.jhep.2015.02.038
12
Itoh S Yoshizumi T Yugawa K Imai D Yoshiya S Takeishi K et al . Impact of immune response on outcomes in hepatocellular carcinoma: association with vascular formation. Hepatology (2020) 29(10):31206. doi: 10.1002/hep.31206
13
Yang Y Ye YC Chen Y Zhao JL Gao CC Han H et al . Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor Malignant behaviors. Cell Death Dis (2018) 9(8):018–0818. doi: 10.1038/s41419-018-0818-0
14
Shima T Shimoda M Shigenobu T Ohtsuka T Nishimura T Emoto K et al . Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci (2020) 111(2):727–38. doi: 10.1111/cas.14272
15
You Y Wen D Zeng L Lu J Xiao X Chen Y et al . ALKBH5/MAP3K8 axis regulates PD-L1+ macrophage infiltration and promotes hepatocellular carcinoma progression. Int J Biol Sci (2022) 18(13):5001–18. doi: 10.7150/ijbs.70149
16
Lu LG Zhou ZL Wang XY Liu BY Lu JY Liu S et al . PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut (2022) 71(12):2551–60. doi: 10.1136/gutjnl-2021-326350
17
Tang B Zhu J Wang Y Chen W Fang S Mao W et al . Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv Sci (2023) 10(2):28. doi: 10.1002/advs.202203973
18
Liao J Zeng DN Li JZ Hua QM Huang CX Xu J et al . Type I IFNs repolarized a CD169(+) macrophage population with anti-tumor potentials in hepatocellular carcinoma. Mol Ther (2022) 30(2):632–43. doi: 10.1016/j.ymthe.2021.09.021
19
Cao J Zhang C Jiang GQ Jin SJ Wang Q Wang AQ et al . Identification of hepatocellular carcinoma-related genes associated with macrophage differentiation based on bioinformatics analyses. Bioengineered (2021) 12(1):296–309. doi: 10.1080/21655979.2020.1868119
20
Su Q Li L Li X Li W Zhang X Dong Y et al . CD97 serves as a novel biomarker of immune cell infiltration in hepatocellular carcinoma. World J Surg Oncol (2022) 20(1):022–02829. doi: 10.1186/s12957-022-02829-2
21
Tan HY Wang N Zhang C Chan YT Yuen MF Feng Y . Lysyl oxidase-like 4 fosters an immunosuppressive microenvironment during hepatocarcinogenesis. Hepatology (2021) 73(6):2326–41. doi: 10.1002/hep.31600
22
Yang S Zhang J Xu Y Wang J Zhao H Lei J et al . OIT3 mediates macrophage polarization and facilitates hepatocellular carcinoma progression. Cancer Immunol Immunother (2022) 71(11):2677–89. doi: 10.1007/s00262-022-03188-3
23
Wen J Yang S Yan G Lei J Liu X Zhang N et al . Increased OIT3 in macrophages promotes PD-L1 expression and hepatocellular carcinogenesis via NF-κB signaling. Exp Cell Res (2023) 428(2):16. doi: 10.1016/j.yexcr.2023.113651
24
Zhang W Liu Y Yan Z Yang H Sun W Yao Y et al . IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer (2020) 8(1):2019–000285. doi: 10.1136/jitc-2019-000285
25
Liu J Fan L Yu H Zhang J He Y Feng D et al . Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology (2019) 70(1):241–58. doi: 10.1002/hep.30607
26
Oh SA Wu DC Cheung J Navarro A Xiong H Cubas R et al . PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer (2020) 1(7):681–91. doi: 10.1038/s43018-020-0075-x
27
Li K Shi H Zhang B Ou X Ma Q Chen Y et al . Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther (2021) 6(1):021–00670. doi: 10.1038/s41392-021-00670-9
28
Iwata T Kondo Y Kimura O Morosawa T Fujisaka Y Umetsu T et al . PD-L1(+)MDSCs are increased in HCC patients and induced by soluble factor in the tumor microenvironment. Sci Rep (2016) 6:39296. doi: 10.1038/srep39296
29
Zhou J Liu M Sun H Feng Y Xu L Chan AWH et al . Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut (2018) 67(5):931–44. doi: 10.1136/gutjnl-2017-314032
30
Liang Y Su S Lun Z Zhong Z Yu W He G et al . Ferroptosis regulator SLC7A11 is a prognostic marker and correlated with PD-L1 and immune cell infiltration in liver hepatocellular carcinoma. Front Mol Biosci (2022) 9:1012505. doi: 10.3389/fmolb.2022.1012505
31
He Q Liu M Huang W Chen X Zhang B Zhang T et al . IL-1β-induced elevation of solute carrier family 7 member 11 promotes hepatocellular carcinoma metastasis through up-regulating programmed death ligand 1 and colony-stimulating factor 1. Hepatology (2021) 74(6):3174–93. doi: 10.1002/hep.32062
32
Kuang DM Zhao Q Peng C Xu J Zhang JP Wu C et al . Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med (2009) 206(6):1327–37. doi: 10.1084/jem.20082173
33
Liu J Sun B Guo K Yang Z Zhao Y Gao M et al . Lipid-related FABP5 activation of tumor-associated monocytes fosters immune privilege via PD-L1 expression on Treg cells in hepatocellular carcinoma. Cancer Gene Ther (2022) 29(12):1951–60. doi: 10.1038/s41417-022-00510-0
34
Chen DP Ning WR Jiang ZZ Peng ZP Zhu LY Zhuang SM et al . Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma. J Hepatol (2019) 71(2):333–43. doi: 10.1016/j.jhep.2019.04.007
35
Gregory AD Houghton AM . Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res (2011) 71(7):2411–6. doi: 10.1158/0008-5472.CAN-10-2583
36
He G Zhang H Zhou J Wang B Chen Y Kong Y et al . Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res (2015) 34(141):015–0256. doi: 10.1186/s13046-015-0256-0
37
Zhu Y Yang J Xu D Gao XM Zhang Z Hsu JL et al . Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut (2019) 68(9):1653–66. doi: 10.1136/gutjnl-2019-318419
38
Seong RK Seo SW Kim JA Fletcher SJ Morgan NV Kumar M et al . Schlafen 14 (SLFN14) is a novel antiviral factor involved in the control of viral replication. Immunobiology (2017) 222(11):979–88. doi: 10.1016/j.imbio.2017.07.002
39
Zhou C Weng J Liu C Liu S Hu Z Xie X et al . Disruption of SLFN11 deficiency-induced CCL2 signaling and macrophage M2 polarization potentiates anti-PD-1 therapy efficacy in hepatocellular carcinoma. Gastroenterology (2023) 164(7):1261–78. doi: 10.1053/j.gastro.2023.02.005
40
Xie M Lin Z Ji X Luo X Zhang Z Sun M et al . FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol (2023) 79(1):109–25. doi: 10.1016/j.jhep.2023.02.036
41
You Y Tian Z Du Z Wu K Xu G Dai M et al . M1-like tumor-associated macrophages cascade a mesenchymal/stem-like phenotype of oral squamous cell carcinoma via the IL6/Stat3/THBS1 feedback loop. J Exp Clin Cancer Res (2022) 41(1):021–02222. doi: 10.1186/s13046-021-02222-z
42
Zong Z Zou J Mao R Ma C Li N Wang J et al . M1 macrophages induce PD-L1 expression in hepatocellular carcinoma cells through IL-1β Signaling. Front Immunol (2019) 10(1643). doi: 10.3389/fimmu.2019.01643
43
Wang X Ye X Chen Y Lin J . Mechanism of M2 type macrophage-derived extracellular vesicles regulating PD-L1 expression via the MISP/IQGAP1 axis in hepatocellular carcinoma immunotherapy resistance. Int Immunopharmacol (2023) 124(Pt A):110848. doi: 10.1016/j.intimp.2023.110848
44
Xie QK Zhao YJ Pan T Lyu N Mu LW Li SL et al . Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology (2016) 5(7):e1181252. doi: 10.1080/2162402X.2016.1181252
45
Ou DL Lin YY Hsu CL Chen CW Yu JS Miaw SC et al . Development of a PD-L1-expressing orthotopic liver cancer model: implications for immunotherapy for hepatocellular carcinoma. Liver Cancer (2019) 8(3):155–71. doi: 10.1159/000489318
46
Shi F Shi M Zeng Z Qi RZ Liu ZW Zhang JY et al . PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer (2011) 128(4):887–96. doi: 10.1002/ijc.25397
47
Xi X Guo Y Zhu M Qiu F Lei F Li G et al . Identification of new potential antigen recognized by γδT cells in hepatocellular carcinoma. Cancer Immunol Immunother (2021) 70(7):1917–27. doi: 10.1007/s00262-020-02826-y
48
Jiang H Yang Z Song Z Green M Song H Shao Q . γδ T cells in hepatocellular carcinoma patients present cytotoxic activity but are reduced in potency due to IL-2 and IL-21 pathways. Int Immunopharmacol (2019) 70:167–73. doi: 10.1016/j.intimp.2019.02.019
49
Liu H Shen J Lu K . IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun (2017) 486(2):239–44. doi: 10.1016/j.bbrc.2017.02.128
50
Gao Q Wang XY Qiu SJ Yamato I Sho M Nakajima Y et al . Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009) 15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608
51
Wang Q Chen F Peng Y Yi X He Y Shi Y . Research progress of interleukin-27 in inflammatory bowel disease. Inflammation Bowel Dis (2023) 29(11):1846. doi: 10.1093/ibd/izad153
52
Zabala M Lasarte JJ Perret C Sola J Berraondo P Alfaro M et al . Induction of immunosuppressive molecules and regulatory T cells counteracts the antitumor effect of interleukin-12-based gene therapy in a transgenic mouse model of liver cancer. J Hepatol (2007) 47(6):807–15. doi: 10.1016/j.jhep.2007.07.025
53
Chow A Perica K Klebanoff CA Wolchok JD . Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol (2022) 19(12):775–90. doi: 10.1038/s41571-022-00689-z
54
Nishida N Sakai K Morita M Aoki T Takita M Hagiwara S et al . Association between genetic and immunological background of hepatocellular carcinoma and expression of programmed cell death-1. Liver Cancer (2020) 9(4):426–39. doi: 10.1159/000506352
55
Jin H Qin S He J Xiao J Li Q Mao Y et al . New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials. Int J Biol Sci (2022) 18(7):2775–94. doi: 10.7150/ijbs.70691
56
Ma LJ Feng FL Dong LQ Zhang Z Duan M Liu LZ et al . Clinical significance of PD-1/PD-Ls gene amplification and overexpression in patients with hepatocellular carcinoma. Theranostics (2018) 8(20):5690–702. doi: 10.7150/thno.28742
57
Wang J McGrail DJ Bhupal PK Zhang W Lin KY Ku YH et al . Nucleostemin modulates outcomes of hepatocellular carcinoma via a tumor adaptive mechanism to genomic stress. Mol Cancer Res (2020) 18(5):723–34. doi: 10.1158/1541-7786.MCR-19-0777
58
Dunn J Rao S . Epigenetics and immunotherapy: The current state of play. Mol Immunol (2017) 87:227–39. doi: 10.1016/j.molimm.2017.04.012
59
Xiao G Jin LL Liu CQ Wang YC Meng YM Zhou ZG et al . EZH2 negatively regulates PD-L1 expression in hepatocellular carcinoma. J Immunother Cancer (2019) 7(1):019–0784. doi: 10.1186/s40425-019-0784-9
60
Schonfeld M Zhao J Komatz A Weinman SA Tikhanovich I . The polymorphism rs975484 in the protein arginine methyltransferase 1 gene modulates expression of immune checkpoint genes in hepatocellular carcinoma. J Biol Chem (2020) 295(20):7126–37. doi: 10.1074/jbc.RA120.013401
61
Liu J Liu Y Meng L Liu K Ji B . Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol Rep (2017) 38(2):899–907. doi: 10.3892/or.2017.5722
62
Xu Y Wu Y Zhang S Ma P Jin X Wang Z et al . A tumor-specific super-enhancer drives immune evasion by guiding synchronous expression of PD-L1 and PD-L2. Cell Rep (2019) 29(11):3435–47. doi: 10.1016/j.celrep.2019.10.093
63
Wang H Tang A Cui Y Gong H Li H . LRPPRC facilitates tumor progression and immune evasion through upregulation of m(6)A modification of PD-L1 mRNA in hepatocellular carcinoma. Front Immunol (2023) 14:1144774. doi: 10.3389/fimmu.2023.1144774
64
Qiu X Yang S Wang S Wu J Zheng B Wang K et al . M(6)A demethylase ALKBH5 regulates PD-L1 expression and tumor immunoenvironment in intrahepatic cholangiocarcinoma. Cancer Res (2021) 81(18):4778–93. doi: 10.1158/0008-5472.CAN-21-0468
65
Li T Zhang C Hassan S Liu X Song F Chen K et al . Histone deacetylase 6 in cancer. J Hematol Oncol (2018) 11(1):018–0654. doi: 10.1186/s13045-018-0654-9
66
Lv T Xiong X Yan W Liu M Xu H He Q . Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J Immunother Cancer (2022) 10(6):2022–004763. doi: 10.1136/jitc-2022-004763
67
Xu F Jin T Zhu Y Dai C . Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res (2018) 37(1):018–0777. doi: 10.1186/s13046-018-0777-4
68
Chiappinelli KB Zahnow CA Ahuja N Baylin SB . Combining epigenetic and immunotherapy to combat cancer. Cancer Res (2016) 76(7):1683–9. doi: 10.1158/0008-5472.CAN-15-2125
69
Nelson HH Kelsey KT . Epigenetic epidemiology as a tool to understand the role of immunity in chronic disease. Epigenomics. (2016) 8(8):1007–9. doi: 10.2217/epi-2016-0055
70
Zhang Y Petropoulos S Liu J Cheishvili D Zhou R Dymov S et al . The signature of liver cancer in immune cells DNA methylation. Clin Epigenet (2018) 10(8):017–0436. doi: 10.1186/s13148-017-0436-1
71
Whyte WA Orlando DA Hnisz D Abraham BJ Lin CY Kagey MH et al . Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell (2013) 153(2):307–19. doi: 10.1016/j.cell.2013.03.035
72
Herranz D Ambesi-Impiombato A Palomero T Schnell SA Belver L Wendorff AA et al . A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat Med (2014) 20(10):1130–7. doi: 10.1038/nm.3665
73
Bahr C von Paleske L Uslu VV Remeseiro S Takayama N Ng SW et al . Author Correction: A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature (2018) 558(7711):018–0113. doi: 10.1038/s41586-018-0113-3
74
Wu Y Yang Y Gu H Tao B Zhang E Wei J et al . Multi-omics analysis reveals the functional transcription and potential translation of enhancers. Int J Cancer (2020) 147(8):2210–24. doi: 10.1002/ijc.33132
75
Wu Y Zhao J Zhu H Fan Z Yuan X Chen S et al . SPACE: a web server for linking chromatin accessibility with clinical phenotypes and the immune microenvironment in pan-cancer analysis. Cell Mol Immunol (2020) 17(12):1294–6. doi: 10.1038/s41423-020-0416-9
76
Hin Tang JJ Hao Thng DK Lim JJ Toh TB . JAK/STAT signaling in hepatocellular carcinoma. Hepat Oncol (2020) 7(1):2020–0001. doi: 10.2217/hep-2020-0001
77
Ding L Chen X Xu X Qian Y Liang G Yao F et al . PARP1 suppresses the transcription of PD-L1 by poly(ADP-ribosyl)ating STAT3. Cancer Immunol Res (2019) 7(1):136–49. doi: 10.1158/2326-6066.CIR-18-0071
78
Ke M Zhang Z Xu B Zhao S Ding Y Wu X et al . Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol (2019) 75(105824):19. doi: 10.1016/j.intimp.2019.105824
79
Huang X Qin F Meng Q Dong M . Protein tyrosine phosphatase receptor type D (PTPRD)-mediated signaling pathways for the potential treatment of hepatocellular carcinoma: a narrative review. Ann Transl Med (2020) 8(18):20–4733. doi: 10.21037/atm-20-4733
80
Yan J Zhou B Guo L Chen Z Zhang B Liu S et al . GOLM1 upregulates expression of PD-L1 through EGFR/STAT3 pathway in hepatocellular carcinoma. Am J Cancer Res (2020) 10(11):3705–20.
81
Zhou B Yan J Guo L Zhang B Liu S Yu M et al . Hepatoma cell-intrinsic TLR9 activation induces immune escape through PD-L1 upregulation in hepatocellular carcinoma. Theranostics (2020) 10(14):6530–43. doi: 10.7150/thno.44417
82
Liu Y Xu Q Deng F Zheng Z Luo J Wang P et al . HERC2 promotes inflammation-driven cancer stemness and immune evasion in hepatocellular carcinoma by activating STAT3 pathway. J Exp Clin Cancer Res (2023) 42(1):023–02609. doi: 10.1186/s13046-023-02609-0
83
Cheng H Wang S Huang A Ma J Gao D Li M et al . HSF1 is involved in immunotherapeutic response through regulating APOJ/STAT3-mediated PD-L1 expression in hepatocellular carcinoma. Cancer Biol Ther (2023) 24(1):1–9. doi: 10.1080/15384047.2022.2156242
84
Xu Q Huang S Xu ZM Ji K Zhang X Xu WP et al . Promotion effects of DEHP on hepatocellular carcinoma models: up-regulation of PD-L1 by activating the JAK2/STAT3 pathway. Toxicol Res (2021) 10(3):376–88. doi: 10.1093/toxres/tfab018
85
Itoh S Yugawa K Shimokawa M Yoshiya S Mano Y Takeishi K et al . Prognostic significance of inflammatory biomarkers in hepatocellular carcinoma following hepatic resection. BJS Open (2019) 3(4):500–8. doi: 10.1002/bjs5.50170
86
Sawada Y Yoshikawa T Shimomura M Iwama T Endo I Nakatsura T . Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int J Oncol (2015) 46(1):28–36. doi: 10.3892/ijo.2014.2737
87
Yan Y Zheng L Du Q Yan B Geller DA . Interferon regulatory factor 1 (IRF-1) and IRF-2 regulate PD-L1 expression in hepatocellular carcinoma (HCC) cells. Cancer Immunol Immunother (2020) 69(9):1891–903. doi: 10.1007/s00262-020-02586-9
88
Li N Wang J Zhang N Zhuang M Zong Z Zou J et al . Cross-talk between TNF-α and IFN-γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol Immunother (2018) 67(2):271–83. doi: 10.1007/s00262-017-2086-8
89
Numata Y Akutsu N Ishigami K Koide H Wagatsuma K Motoya M et al . Synergistic effect of IFN-γ and IL-1β on PD-L1 expression in hepatocellular carcinoma. Biochem Biophys Rep (2022) 30:101270. doi: 10.1016/j.bbrep.2022.101270
90
Yu B Yang X Xu Y Yao G Shu H Lin B et al . Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol (2009) 50(5):948–57. doi: 10.1016/j.jhep.2008.11.020
91
Deng H Kan A Lyu N Mu L Han Y Liu L et al . Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer (2020) 9(3):338–57. doi: 10.1159/000505695
92
Deng R Zuo C Li Y Xue B Xun Z Guo Y et al . The innate immune effector ISG12a promotes cancer immunity by suppressing the canonical Wnt/β-catenin signaling pathway. Cell Mol Immunol (2020) 17(11):1163–79. doi: 10.1038/s41423-020-00549-9
93
Yang RH Qin J Cao JL Zhang MZ Li YY Wang MQ et al . Dickkopf-1 drives tumor immune evasion by inducing PD-L1 expression in hepatocellular carcinoma. Biochem Pharmacol (2023) 208(115378):10. doi: 10.1016/j.bcp.2022.115378
94
Wan JL Wang B Wu ML Li J Gong RM Song LN et al . MTDH antisense oligonucleotides reshape the immunosuppressive tumor microenvironment to sensitize Hepatocellular Carcinoma to immune checkpoint blockade therapy. Cancer Lett (2022) 541:215750. doi: 10.1016/j.canlet.2022.215750
95
Kong X Peng H Liu P Fu X Wang N Zhang D . Programmed death ligand 1 regulates epithelial-mesenchymal transition and cancer stem cell phenotypes in hepatocellular carcinoma through the serum and glucocorticoid kinase 2/β-catenin signaling pathway. Cancer Sci (2023) 114(6):2265–76. doi: 10.1111/cas.15753
96
Xie W Shi L Quan H Xiao H Chen J Liu J et al . SYVN1 ubiquitinates FoxO1 to induce β-catenin nuclear translocation, PD-L1-mediated metastasis, and immune evasion of hepatocellular carcinoma. Cell Oncol (2023) 26(10):023–00811. doi: 10.1007/s13402-023-00811-y
97
Xing S Chen S Yang X Huang W . Role of MAPK activity in PD-L1 expression in hepatocellular carcinoma cells. J Buon (2020) 25(4):1875–82.
98
Chun HW Hong R . Significance of PD-L1 clones and C-MET expression in hepatocellular carcinoma. Oncol Lett (2019) 17(6):5487–98. doi: 10.3892/ol.2019.10222
99
Xu R Liu X Li A Song L Liang J Gao J et al . c-Met up-regulates the expression of PD-L1 through MAPK/NF-κBp65 pathway. J Mol Med (2022) 100(4):585–98. doi: 10.1007/s00109-022-02179-2
100
Chen Y Liu YC Sung YC Ramjiawan RR Lin TT Chang CC et al . Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep (2017) 7:44123. doi: 10.1038/srep44123
101
Mo D Zhu H Wang J Hao H Guo Y Han X et al . Icaritin inhibits PD-L1 expression by targeting protein IκB kinase α. Eur J Immunol (2020) 22(10):202048905. doi: 10.1002/eji.202048905
102
Li QT Qiu MJ Yang SL Fang X Huang S . Alpha-fetoprotein regulates the expression of immune-related proteins through the NF- κ B (P65) pathway in hepatocellular carcinoma cells. J Oncol (2020) 2020:1–9. doi: 10.1155/2020/9327512
103
Chi Y Gong Z Xin H Wang Z Liu Z . microRNA-206 prevents hepatocellular carcinoma growth and metastasis via down-regulating CREB5 and inhibiting the PI3K/AKT signaling pathway. Cell Cycle (2022) 21(24):2651–63. doi: 10.1080/15384101.2022.2108275
104
Wei Y Tang X Ren Y Yang Y Song F Fu J et al . An RNA-RNA crosstalk network involving HMGB1 and RICTOR facilitates hepatocellular carcinoma tumorigenesis by promoting glutamine metabolism and impedes immunotherapy by PD-L1+ exosomes activity. Signal Transduct Target Ther (2021) 6(1):021–00801. doi: 10.1038/s41392-021-00801-2
105
Iseda N Itoh S Yoshizumi T Yugawa K Morinaga A Tomiyama T et al . ARID1A deficiency is associated with high programmed death ligand 1 expression in hepatocellular carcinoma. Hepatol Commun (2020) 5(4):675–88. doi: 10.1002/hep4.1659
106
Wu M Xia X Hu J Fowlkes NW Li S . WSX1 act as a tumor suppressor in hepatocellular carcinoma by downregulating neoplastic PD-L1 expression. Nat Commun (2021) 12(1):021–23864. doi: 10.1038/s41467-021-23864-9
107
Szulzewsky F Holland EC Vasioukhin V . YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev Biol (2021) 475:205–21. doi: 10.1016/j.ydbio.2020.12.018
108
Peng Q Li S Shi X Guo Y Hao L Zhang Z et al . Dihydroartemisinin broke the tumor immunosuppressive microenvironment by inhibiting YAP1 expression to enhance anti-PD-1 efficacy. Phytother Res (2023) 37(5):1740–53. doi: 10.1002/ptr.7695
109
Li S Ji J Zhang Z Peng Q Hao L Guo Y et al . Cisplatin promotes the expression level of PD-L1 in the microenvironment of hepatocellular carcinoma through YAP1. Mol Cell Biochem (2020) 475(1-2):79–91. doi: 10.1007/s11010-020-03861-0
110
Li S Dai M Wang F Hao L Feng C Jia Y et al . PD-1/PD-L1 interaction upregulates YAP1 expression in hepG2 cells through MAPK/ERK pathway. Natural Product Commun (2023) 18(10):1934578X231210413. doi: 10.1177/1934578x231210413
111
Sung PS Park DJ Roh PR Mun KD Cho SW Lee GW et al . Intrahepatic inflammatory IgA(+)PD-L1(high) monocytes in hepatocellular carcinoma development and immunotherapy. J Immunother Cancer (2022) 10(5):2021–003618. doi: 10.1136/jitc-2021-003618
112
Shrestha R Bridle KR Crawford DHG Jayachandran A . TNF−α−mediated epithelial−to−mesenchymal transition regulates expression of immune checkpoint molecules in hepatocellular carcinoma. Mol Med Rep (2020) 21(4):1849–60. doi: 10.3892/mmr.2020.10991
113
Kurimoto R Iwasawa S Ebata T Ishiwata T Sekine I Tada Y et al . Drug resistance originating from a TGF-β/FGF-2-driven epithelial-to-mesenchymal transition and its reversion in human lung adenocarcinoma cell lines harboring an EGFR mutation. Int J Oncol (2016) 48(5):1825–36. doi: 10.3892/ijo.2016.3419
114
Shrestha R Prithviraj P Bridle KR Crawford DHG Jayachandran A . Combined inhibition of TGF-β1-induced EMT and PD-L1 silencing re-sensitizes hepatocellular carcinoma to sorafenib treatment. J Clin Med (2021) 10(9):1889. doi: 10.3390/jcm10091889
115
Shrestha R Bridle KR Crawford DHG Jayachandran A . Immune checkpoint molecules are regulated by transforming growth factor (TGF)-β1-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Int J Med Sci (2021) 18(12):2466–79. doi: 10.7150/ijms.54239
116
Jiang G Shi L Zheng X Zhang X Wu K Liu B et al . Androgen receptor affects the response to immune checkpoint therapy by suppressing PD-L1 in hepatocellular carcinoma. Aging (2020) 12(12):11466–84. doi: 10.18632/aging.103231
117
Yang C Jin J Yang Y Sun H Wu L Shen M et al . Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity (2022) 55(7):1268–83. doi: 10.1016/j.immuni.2022.05.012
118
Lv L Zhao Y Wei Q Yi Q . Downexpression of HSD17B6 correlates with clinical prognosis and tumor immune infiltrates in hepatocellular carcinoma. Cancer Cell Int (2020) 20(210):020–01298. doi: 10.1186/s12935-020-01298-5
119
Rodda DJ Chew JL Lim LH Loh YH Wang B Ng HH et al . Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem (2005) 280(26):24731–7. doi: 10.1074/jbc.M502573200
120
Zhong F Cheng X Sun S Zhou J . Transcriptional activation of PD-L1 by Sox2 contributes to the proliferation of hepatocellular carcinoma cells. Oncol Rep (2017) 37(5):3061–7. doi: 10.3892/or.2017.5523
121
Maurya PK Mishra A Yadav BS Singh S Kumar P Chaudhary A et al . Role of Y Box Protein-1 in cancer: As potential biomarker and novel therapeutic target. J Cancer (2017) 8(10):1900–7. doi: 10.7150/jca.17689
122
Tao Z Ruan H Sun L Kuang D Song Y Wang Q et al . Targeting the YB-1/PD-L1 axis to enhance chemotherapy and antitumor immunity. Cancer Immunol Res (2019) 7(7):1135–47. doi: 10.1158/2326-6066.CIR-18-0648
123
Li Y Li L Qin J Wu J Dai X Xu J . OSR1 phosphorylates the Smad2/3 linker region and induces TGF-β1 autocrine to promote EMT and metastasis in breast cancer. Oncogene (2021) 40(1):68–84. doi: 10.1038/s41388-020-01499-2
124
Chen J Zhou J Fu H Ni X Shan Y . Upregulation of oxidative stress-responsive 1(OXSR1) predicts poor prognosis and promotes hepatocellular carcinoma progression. Bioengineered (2020) 11(1):958–71. doi: 10.1080/21655979.2020.1814659
125
Zou J Zhuang M Yu X Li N Mao R Wang Z et al . MYC inhibition increases PD-L1 expression induced by IFN-γ in hepatocellular carcinoma cells. Mol Immunol (2018) 101:203–9. doi: 10.1016/j.molimm.2018.07.006
126
Wan Y Ge K Zhou W Lu J Jia C Zhu H . C-X-C chemokine receptor 2 (Cxcr2) promotes hepatocellular carcinoma immune evasion via regulating programmed death-ligand 1 (PD-L1). Biol Chem (2021) 402(6):729–37. doi: 10.1515/hsz-2020-0328
127
Zhang F Hu K Liu W Quan B Li M Lu S et al . Oxaliplatin-resistant hepatocellular carcinoma drives immune evasion through PD-L1 up-regulation and PMN-singular recruitment. Cell Mol Gastroenterol Hepatol (2023) 15(3):573–91. doi: 10.1016/j.jcmgh.2022.12.002
128
Xu W Liu S Zhang G Liu J Cao G . Knockdown of METTL5 inhibits the Myc pathway to downregulate PD-L1 expression and inhibits immune escape of hepatocellular carcinoma cells. J Chemother (2023) 35(5):455–64. doi: 10.1080/1120009X.2022.2143614
129
Zhang S Xu L Feng J Tan D Zhu Y Hou J et al . ASF1B is a promising prognostic biomarker and correlates with immunotherapy efficacy in hepatocellular carcinoma. Front Genet (2022) 13:842351. doi: 10.3389/fgene.2022.842351
130
Chui NN Cheu JW Yuen VW Chiu DK Goh CC Lee D et al . Inhibition of CMTM4 sensitizes cholangiocarcinoma and hepatocellular carcinoma to T cell-mediated antitumor immunity through PD-L1. Hepatol Commun (2022) 6(1):178–93. doi: 10.1002/hep4.1682
131
Muranushi R Araki K Yokobori T Chingunjav B Hoshino K Dolgormaa G et al . High membrane expression of CMTM6 in hepatocellular carcinoma is associated with tumor recurrence. Cancer Sci (2021) 112(8):3314–23. doi: 10.1111/cas.15004
132
Yugawa K Itoh S Yoshizumi T Iseda N Tomiyama T Morinaga A et al . CMTM6 stabilizes PD-L1 expression and is a new prognostic impact factor in hepatocellular carcinoma. Hepatol Commun (2020) 5(2):334–48. doi: 10.1002/hep4.1643
133
Liu LL Zhang SW Chao X Wang CH Yang X Zhang XK et al . Coexpression of CMTM6 and PD-L1 as a predictor of poor prognosis in macrotrabecular-massive hepatocellular carcinoma. Cancer Immunol Immunother (2021) 70(2):417–29. doi: 10.1007/s00262-020-02691-9
134
Lin XT Zhang J Liu ZY Wu D Fang L Li CM et al . Elevated FBXW10 drives hepatocellular carcinoma tumorigenesis via AR-VRK2 phosphorylation-dependent GAPDH ubiquitination in male transgenic mice. Cell Rep (2023) 42(7):13. doi: 10.1016/j.celrep.2023.112812
135
Xu Y Huang Z Yu X Li Z Zheng L Xu J . HHLA2 expression is associated with poor survival in patients with hepatocellular carcinoma. Biologics (2021) 15:329–41. doi: 10.2147/BTT.S325019
136
Liu R Li R Yu H Liu J Zheng S Li Y et al . NTF3 correlates with prognosis and immune infiltration in hepatocellular carcinoma. Front Med (2021) 8:795849. doi: 10.3389/fmed.2021.795849
137
Gao H Zhou X Li H Liu F Zhu H Song X et al . Role of matrix metallopeptidase 12 in the development of hepatocellular carcinoma. J Invest Surg (2021) 34(4):366–72. doi: 10.1080/08941939.2019.1637975
138
Kong J Wang X Wang J Yu G . Silencing of RAB42 down-regulated PD-L1 expression to inhibit the immune escape of hepatocellular carcinoma cells through inhibiting the E2F signaling pathway. Cell Signal (2023) 108(110692):26. doi: 10.1016/j.cellsig.2023.110692
139
Xu H Chen Y Li Z Zhang H Liu J Han J . The hypoxia-inducible factor 1 inhibitor LW6 mediates the HIF-1α/PD-L1 axis and suppresses tumor growth of hepatocellular carcinoma in vitro and in vivo. Eur J Pharmacol (2022) 930(175154):19. doi: 10.1016/j.ejphar.2022.175154
140
Li D Sun FF Wang D Wang T Peng JJ Feng JQ et al . Programmed death ligand-1 (PD-L1) regulated by NRF-2/microRNA-1 regulatory axis enhances drug resistance and promotes tumorigenic properties in sorafenib-resistant hepatoma cells. Oncol Res (2020) 28(5):467–81. doi: 10.3727/096504020X15925659763817
141
Atwa SM Handoussa H Hosny KM Odenthal M Tayebi HME . Pivotal role of long non-coding ribonucleic acid-X-inactive specific transcript in regulating immune checkpoint programmed death ligand 1 through a shared pathway between miR-194-5p and miR-155-5p in hepatocellular carcinoma. World J Hepatol (2020) 12(12):1211–27. doi: 10.4254/wjh.v12.i12.1211
142
Zeng C Ye S Chen Y Zhang Q Luo Y Gai L et al . HOXA-AS3 Promotes Proliferation and Migration of Hepatocellular Carcinoma Cells via the miR-455-5p/PD-L1 Axis. J Immunol Res (2021) 27:9289719. doi: 10.1155/2021/9289719
143
Sun G Miao G Li Z Zheng W Zhou C Cao H et al . Inhibition of PARP Potentiates Immune Checkpoint Therapy through miR-513/PD-L1 Pathway in Hepatocellular Carcinoma. J Oncol (2022) 13:6988923. doi: 10.1155/2022/6988923
144
Yan Y Zheng L Du Q Cui X Dong K Guo Y et al . Interferon regulatory factor 1 (IRF-1) downregulates Checkpoint kinase 1 (CHK1) through miR-195 to upregulate apoptosis and PD-L1 expression in Hepatocellular carcinoma (HCC) cells. Br J Cancer (2021) 125(1):101–11. doi: 10.1038/s41416-021-01337-6
145
Wang Y Cao K . KDM1A promotes immunosuppression in hepatocellular carcinoma by regulating PD-L1 through demethylating MEF2D. J Immunol Res (2021) 1:9965099. doi: 10.1155/2021/9965099
146
Zhang X Pan B Qiu J Ke X Shen S Wang X et al . lncRNA MIAT targets miR-411-5p/STAT3/PD-L1 axis mediating hepatocellular carcinoma immune response. Int J Exp Pathol (2022) 103(3):102–11. doi: 10.1111/iep.12440
147
Li Y Zhou T Cheng X Li D Zhao M Zheng WV . microRNA-378a-3p regulates the progression of hepatocellular carcinoma by regulating PD-L1 and STAT3. Bioengineered (2022) 13(3):4730–43. doi: 10.1080/21655979.2022.2031408
148
Kong X Zheng Z Song G Zhang Z Liu H Kang J et al . Over-expression of GUSB leads to primary resistance of anti-PD1 therapy in hepatocellular carcinoma. Front Immunol (2022) 13:876048. doi: 10.3389/fimmu.2022.876048
149
Liu Z Ning F Cai Y Sheng H Zheng R Yin X et al . The EGFR-P38 MAPK axis up-regulates PD-L1 through miR-675-5p and down-regulates HLA-ABC via hexokinase-2 in hepatocellular carcinoma cells. Cancer Commun (2021) 41(1):62–78. doi: 10.1002/cac2.12117
150
Fan F Chen K Lu X Li A Liu C Wu B . Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging hsa-miR-194-5p induces immunosuppression in hepatocellular carcinoma. Hepatol Int (2020) 21(10):020–10101. doi: 10.1007/s12072-020-10101-6
151
Peng L Chen Y Ou Q Wang X Tang N . ). LncRNA MIAT correlates with immune infiltrates and drug reactions in hepatocellular carcinoma. Int Immunopharmacol (2020) 89(Pt A):19. doi: 10.1016/j.intimp.2020.107071
152
Wang D Hu X Chen J Liang B Zhang L Qin P et al . Bioinformatics analysis and validation of the role of lnc-RAB11B-AS1 in the development and prognosis of hepatocellular carcinoma. Cells (2022) 11(21):3517. doi: 10.3390/cells11213517
153
Zhong JX Lin XR Hu H . Research progress on targeting metabolic reprogramming of tumor microenvironment to reinvigorate exhausted CD8+T cells. Biomed Transformation (2022) 3(02):46–56. doi: 10.3389/fimmu.2023.1204363
154
Cao X Zhang G Li T Zhou C Bai L Zhao J et al . LINC00657 knockdown suppresses hepatocellular carcinoma progression by sponging miR-424 to regulate PD-L1 expression. Genes Genomics (2020) 42(11):1361–8. doi: 10.1007/s13258-020-01001-y
155
Zhang J Zhao X Ma X Yuan Z Hu M . KCNQ1OT1 contributes to sorafenib resistance and programmed death−ligand−1−mediated immune escape via sponging miR−506 in hepatocellular carcinoma cells. Int J Mol Med (2020) 46(5):1794–804.doi: 10.3892/ijmm.2020.4710
156
Sun Z Xue C Li J Zhao H Du Y Du N . LINC00244 suppresses cell growth and metastasis in hepatocellular carcinoma by downregulating programmed cell death ligand 1. Bioengineered (2022) 13(3):7635–47. doi: 10.1080/21655979.2022.2050073
157
Qi F Du X Zhao Z Zhang D Huang M Bai Y et al . Tumor mutation burden-associated LINC00638/miR-4732-3p/ULBP1 axis promotes immune escape via PD-L1 in hepatocellular carcinoma. Front Oncol (2021) 11:729340. doi: 10.3389/fonc.2021.729340
158
Wu F Sun G Zheng W Tang W Cheng Y Wu L et al . circCORO1C promotes the proliferation and metastasis of hepatocellular carcinoma by enhancing the expression of PD-L1 through NF-κB pathway. J Clin Lab Anal (2021) 35(12):22. doi: 10.1002/jcla.24003
159
Xu G Zhang P Liang H Xu Y Shen J Wang W et al . Circular RNA hsa_circ_0003288 induces EMT and invasion by regulating hsa_circ_0003288/miR-145/PD-L1 axis in hepatocellular carcinoma. Cancer Cell Int (2021) 21(1):021–01902. doi: 10.1186/s12935-021-01902-2
160
He T Huang J Liang H Zhong B Xu G Zhu X . Circular RNA hsa_circ_0005239 contributes to hepatocellular carcinoma cell migration, invasion, and angiogenesis by targeting the miR-34a-5p/PD-L1 axis. Cell Biol Int (2023) 47(9):1519–34. doi: 10.1002/cbin.12049
161
Liu L Liao R Wu Z Du C You Y Que K et al . Hepatic stellate cell exosome-derived circWDR25 promotes the progression of hepatocellular carcinoma via the miRNA-4474-3P-ALOX-15 and EMT axes. Biosci Trends (2022) 16(4):267–81. doi: 10.5582/bst.2022.01281
162
Chen ZQ Zuo XL Cai J Zhang Y Han GY Zhang L et al . Hypoxia-associated circPRDM4 promotes immune escape via HIF-1α regulation of PD-L1 in hepatocellular carcinoma. Exp Hematol Oncol (2023) 12(1):023–00378. doi: 10.1186/s40164-023-00378-2
163
Gupta M Akhtar J Sarwat M . MicroRNAs: Regulators of immunological reactions in hepatocellular carcinoma. Semin Cell Dev Biol (2022) 124:127–33. doi: 10.1016/j.semcdb.2021.05.025
164
Soghli N Yousefi T Abolghasemi M Qujeq D . NORAD, a critical long non-coding RNA in human cancers. Life Sci (2021) 264(118665):27. doi: 10.1016/j.lfs.2020.118665
165
Song H Liu Y Li X Chen S Xie R Chen D et al . Long noncoding RNA CASC11 promotes hepatocarcinogenesis and HCC progression through EIF4A3-mediated E2F1 activation. Clin Transl Med (2020) 10(7):220. doi: 10.1002/ctm2.220
166
Zhong F Liu S Hu D Chen L . LncRNA AC099850.3 promotes hepatocellular carcinoma proliferation and invasion through PRR11/PI3K/AKT axis and is associated with patients prognosis. J Cancer (2022) 13(3):1048–60. doi: 10.7150/jca.66092
167
Peng L Pan B Zhang X Wang Z Qiu J Wang X et al . Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via m6A modification of lncRNA MIR155HG to upregulate PD-L1 expression. Cell Biol Toxicol (2022) 38(6):1159–73. doi: 10.1007/s10565-022-09718-0
168
Meng H Niu R Huang C Li J . Circular RNA as a novel biomarker and therapeutic target for HCC. Cells (2022) 11(12):1948. doi: 10.3390/cells11121948
169
Ali MA Matboli M Tarek M Reda M Kamal KM Nouh M et al . Epigenetic regulation of immune checkpoints: another target for cancer immunotherapy? Immunotherapy (2017) 9(1):99–108. doi: 10.2217/imt-2016-0111
170
Jiang Y Zhao L Wu Y Deng S Cao P Lei X et al . The role of ncRNAs to regulate immune checkpoints in cancer. Front Immunol (2022) 13:853480. doi: 10.3389/fimmu.2022.853480
171
Wang X Liang C Yao X Yang RH Zhang ZS Liu FY et al . PKM2-induced the phosphorylation of histone H3 contributes to EGF-mediated PD-L1 transcription in HCC. Front Pharmacol (2020) 11:577108. doi: 10.3389/fphar.2020.577108
172
Li TE Wang S Shen XT Zhang Z Chen M Wang H et al . PKM2 drives hepatocellular carcinoma progression by inducing immunosuppressive microenvironment. Front Immunol (2020) 11(589997). doi: 10.3389/fimmu.2020.589997
173
Zhou B Guo L Zhang B Liu S Zhang K Yan J et al . Disulfiram combined with copper induces immunosuppression via PD-L1 stabilization in hepatocellular carcinoma. Am J Cancer Res (2019) 9(11):2442–55.
174
Xiang J Zhang N Sun H Su L Zhang C Xu H et al . Disruption of SIRT7 increases the efficacy of checkpoint inhibitor via MEF2D regulation of programmed cell death 1 ligand 1 in hepatocellular carcinoma cells. Gastroenterology (2020) 158(3):664–78. doi: 10.1053/j.gastro.2019.10.025
175
Ning Z Guo X Liu X Lu C Wang A Wang X et al . USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat Commun (2022) 13(1):022–29846. doi: 10.1038/s41467-022-29846-9
176
Li Q Zhang L You W Xu J Dai J Hua D et al . PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells. Nat Commun (2022) 13(1):022–35469. doi: 10.1038/s41467-022-35469-x
177
Chen J Lin Z Liu L Zhang R Geng Y Fan M et al . GOLM1 exacerbates CD8(+) T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct Target Ther (2021) 6(1):021–00784. doi: 10.1038/s41392-021-00784-0
178
Zhu K Huang W Wang W Liao L Li S Yang S et al . Up-regulation of S100A4 expression by HBx protein promotes proliferation of hepatocellular carcinoma cells and its correlation with clinical survival. Gene (2020) 749(144679):21. doi: 10.1016/j.gene.2020.144679
179
Teng CF Li TC Wang T Wu TH Wang J Wu HC et al . Increased expression of programmed death ligand 1 in hepatocellular carcinoma of patients with hepatitis B virus pre-S2 mutant. J Hepatocell Carcinoma (2020) 7:385–401. doi: 10.2147/JHC.S282818
180
Li B Yan C Zhu J Chen X Fu Q Zhang H et al . Anti-PD-1/PD-L1 blockade immunotherapy employed in treating hepatitis B virus infection-related advanced hepatocellular carcinoma: A literature review. Front Immunol (2020) 11(1037). doi: 10.3389/fimmu.2020.01037
181
Wang BJ Bao JJ Wang JZ Wang Y Jiang M Xing MY et al . Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol (2011) 17(28):3322–9. doi: 10.3748/wjg.v17.i28.3322
182
Fan F Chen K Lu X Li A Liu C Wu B . Dual targeting of PD-L1 and PD-L2 by PCED1B-AS1 via sponging hsa-miR-194-5p induces immunosuppression in hepatocellular carcinoma. Hepatol Int (2021) 15(2):444–58. doi: 10.1007/s12072-020-10101-6
183
Montasser A Beaufrère A Cauchy F Bouattour M Soubrane O Albuquerque M et al . Transarterial chemoembolization enhances programmed death 1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology (2020) 16(10):14317. doi: 10.1111/his.14317
184
Takaki H Hirata Y Ueshima E Kodama H Matsumoto S Wada R et al . Hepatic artery embolization enhances expression of programmed cell death 1 ligand 1 in an orthotopic rat hepatocellular carcinoma model: in vivo and in vitro experimentation. J Vasc Interv Radiol (2020) 31(9):1475–82. doi: 10.1016/j.jvir.2020.03.023
185
Tan J Fan W Liu T Zhu B Liu Y Wang S et al . TREM2(+) macrophages suppress CD8(+) T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatol (2023) 79(1):126–40. doi: 10.1016/j.jhep.2023.02.032
186
Zhu Q Ma Y Liang J Wei Z Li M Zhang Y et al . AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct Target Ther (2021) 6(1):021–00713. doi: 10.1038/s41392-021-00713-1
187
Zhang W He H Zang M Wu Q Zhao H Lu LL et al . Genetic features of aflatoxin-associated hepatocellular carcinoma. Gastroenterology (2017) 153(1):249–62. doi: 10.1053/j.gastro.2017.03.024
188
Xu G Feng D Yao Y Li P Sun H Yang H et al . Listeria-based hepatocellular carcinoma vaccine facilitates anti-PD-1 therapy by regulating macrophage polarization. Oncogene (2020) 39(7):1429–44. doi: 10.1038/s41388-019-1072-3
189
Yu S Wang Y Jing L Claret FX Li Q Tian T et al . Autophagy in the "inflammation-carcinogenesis" pathway of liver and HCC immunotherapy. Cancer Lett (2017) 411:82–9. doi: 10.1016/j.canlet.2017.09.049
190
Chen Z Liu S Xie P Zhang B Yu M Yan J et al . Tumor-derived PD1 and PD-L1 could promote hepatocellular carcinoma growth through autophagy induction in vitro. Biochem Biophys Res Commun (2022) 605:82–9. doi: 10.1016/j.bbrc.2022.03.075
191
Ri MH Ma J Jin X . Development of natural products for anti-PD-1/PD-L1 immunotherapy against cancer. J Ethnopharmacol (2021) 281(114370):30. doi: 10.1016/j.jep.2021.114370
192
He L Xu K Niu L Lin L . Astragalus polysaccharide (APS) attenuated PD-L1-mediated immunosuppression via the miR-133a-3p/MSN axis in HCC. Pharm Biol (2022) 60(1):1710–20. doi: 10.1080/13880209.2022.2112963
193
Yan J Deng XL Ma SQ Hui Li Y Gao YM Shi GT et al . Cantharidin suppresses hepatocellular carcinoma development by regulating EZH2/H3K27me3-dependent cell cycle progression and antitumour immune response. BMC Complement Med Ther (2023) 23(1):023–03975. doi: 10.1186/s12906-023-03975-0
194
Rong W Wan N Zheng X Shi G Jiang C Pan K et al . Chrysin inhibits hepatocellular carcinoma progression through suppressing programmed death ligand 1 expression. Phytomedicine (2022) 95(153867):2. doi: 10.1016/j.phymed.2021.153867
195
Li Z Gao WQ Wang P Wang TQ Xu WC Zhu XY et al . Pentamethylquercetin inhibits hepatocellular carcinoma progression and adipocytes-induced PD-L1 expression via IFN-γ Signaling. Curr Cancer Drug Targets (2020) 20(11):868–74. doi: 10.2174/1568009620999200730184514
196
Wu R Zhou T Xiong J Zhang Z Tian S Wang Y et al . Quercetin, the ingredient of xihuang pills, inhibits hepatocellular carcinoma by regulating autophagy and macrophage polarization. Front Biosci (2022) 27(12). doi: 10.31083/j.fbl2712323
197
Zuo HX Jin Y Wang Z Li MY Zhang ZH Wang JY et al . Curcumol inhibits the expression of programmed cell death-ligand 1 through crosstalk between hypoxia-inducible factor-1α and STAT3 (T705) signaling pathways in hepatic cancer. J Ethnopharmacol (2020) 257(112835):9. doi: 10.1016/j.jep.2020.112835
198
Deng Z Xu XY Yunita F Zhou Q Wu YR Hu YX et al . Synergistic anti-liver cancer effects of curcumin and total ginsenosides. World J Gastrointest Oncol (2020) 12(10):1091–103. doi: 10.4251/wjgo.v12.i10.1091
199
Guo L Li H Fan T Ma Y Wang L . Synergistic efficacy of curcumin and anti-programmed cell death-1 in hepatocellular carcinoma. Life Sci (2021) 279(119359):19. doi: 10.1016/j.lfs.2021.119359
200
Bamodu OA Kuo KT Wang CH Huang WC Wu ATH Tsai JT et al . Astragalus polysaccharides (PG2) enhances the M1 polarization of macrophages, functional maturation of dendritic cells, and T cell-mediated anticancer immune responses in patients with lung cancer. Nutrients (2019) 11(10):2264. doi: 10.3390/nu11102264
201
Swagatika S Tomar RS . Cantharidin downregulates PSD1 expression and inhibits autophagic flux in yeast cells. FEBS Open Bio (2022) 12(5):1017–35. doi: 10.1002/2211-5463.13196
202
Han DH Denison MS Tachibana H Yamada K . Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem (2002) 66(7):1479–87. doi: 10.1271/bbb.66.1479
203
Lim HK Kwon HJ Lee GS Moon JH Jung J . Chrysin-induced G protein-coupled estrogen receptor activation suppresses pancreatic cancer. Int J Mol Sci (2022) 23(17):9673. doi: 10.3390/ijms23179673
204
Wu J Du J Li Z He W Wang M Jin M et al . Pentamethylquercetin regulates lipid metabolism by modulating skeletal muscle-adipose tissue crosstalk in obese mice. Pharmaceutics (2022) 14(6):1159. doi: 10.3390/pharmaceutics14061159
205
Guo H Ding H Tang X Liang M Li S Zhang J et al . Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac Cancer (2021) 12(9):1415–22. doi: 10.1111/1759-7714.13925
206
Ma C Tang X Tang Q Wang S Zhang J Lu Y et al . Curcumol repressed cell proliferation and angiogenesis via SP1/mir-125b-5p/VEGFA axis in non-small cell lung cancer. Front Pharmacol (2022) 13:1044115. doi: 10.3389/fphar.2022.1044115
207
Wang J Huang F Bai Z Chi B Wu J Chen X . Curcumol Inhibits Growth and Induces Apoptosis of Colorectal Cancer LoVo Cell Line via IGF-1R and p38 MAPK Pathway. Int J Mol Sci (2015) 16(8):19851–67. doi: 10.3390/ijms160819851
208
Lu KH Lu PW Lu EW Lin CW Yang SF . Curcumin and its analogs and carriers: potential therapeutic strategies for human osteosarcoma. Int J Biol Sci (2023) 19(4):1241–65. doi: 10.7150/ijbs.80590
209
Li J Bai Y Ma K Ren Z Zhang J Shan A . Dihydroartemisinin alleviates deoxynivalenol induced liver apoptosis and inflammation in piglets. Ecotoxicol Environ Saf (2022) 241(113811):27. doi: 10.1016/j.ecoenv.2022.113811
210
Tan W Lu J Huang M Li Y Chen M Wu G et al . Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med (2011) 6(1):27. doi: 10.1186/1749-8546-6-27
211
Zheng J Shao M Yang W Ren J Chen X Yang H . Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis. Int Immunopharmacol (2022) 112(109244):18. doi: 10.1016/j.intimp.2022.109244
212
Tang H Wang Y Chlewicki LK Zhang Y Guo J Liang W et al . Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell (2016) 30(3):12. doi: 10.1007/s10555-020-09944-0
213
Zeng Z Shi F Zhou L Zhang MN Chen Y Chang XJ et al . Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PloS One (2011) 6(9):1. doi: 10.1371/journal.pone.0023621
214
Hodi FS Chesney J Pavlick AC Robert C Grossmann KF McDermott DF et al . Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol (2016) 17(11):1558–68. doi: 10.1016/S1470-2045(16)30366-7
215
Li R Jiang X Zhang Y Wang S Chen X Yu X et al . Cyclin B2 overexpression in human hepatocellular carcinoma is associated with poor prognosis. Arch Med Res (2019) 50(1):10–7. doi: 10.1016/j.arcmed.2019.03.003
216
Shang R Song X Wang P Zhou Y Lu X Wang J et al . Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut (2020) 3(320716):2020–320716. doi: 10.1136/gutjnl-2020-320716
217
Finn RS Ikeda M Zhu AX Sung MW Baron AD Kudo M et al . Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
218
Sangro B Sarobe P Hervás-Stubbs S Melero I . Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18(8):525–43. doi: 10.1038/s41575-021-00438-0
219
Llopiz D Ruiz M Villanueva L Iglesias T Silva L Egea J et al . Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol Immunother (2019) 68(3):379–93. doi: 10.1007/s00262-018-2283-0
220
Cheng H Sun G Chen H Li Y Han Z Zhang P et al . Trends in the treatment of advanced hepatocellular carcinoma: immune checkpoint blockade immunotherapy and related combination therapies. Am J Cancer Res (2019) 9(8):1536–45.
221
Johnson DB Bordeaux J Kim JY Vaupel C Rimm DL Ho TH et al . Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in metastatic melanoma. Clin Cancer Res (2018) 24(21):5250–60. doi: 10.1158/1078-0432.CCR-18-0309
222
Wu Y Cheng Y Wang X Fan J Gao Q . Spatial omics: Navigating to the golden era of cancer research. Clin Transl Med (2022) 12(1):696. doi: 10.1002/ctm2.696
223
Dong P Xiong Y Yue J Hanley SJB Watari H . Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: beyond immune evasion. Front Oncol (2018) 8:386. doi: 10.3389/fonc.2018.00386
224
Shen M Xu Z Xu W Jiang K Zhang F Ding Q et al . Inhibition of ATM reverses EMT and decreases metastatic potential of cisplatin-resistant lung cancer cells through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res (2019) 38(1):019–1161. doi: 10.1186/s13046-019-1161-8
225
Rong D Sun G Zheng Z Liu L Chen X Wu F et al . MGP promotes CD8(+) T cell exhaustion by activating the NF-κB pathway leading to liver metastasis of colorectal cancer. Int J Biol Sci (2022) 18(6):2345–61. doi: 10.7150/ijbs.70137
226
Wu Y Yang S Ma J Chen Z Song G Rao D et al . Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discovery (2022) 12(1):134–53. doi: 10.1158/2159-8290.CD-21-0316
227
Ishikawa E Nakaguro M Nakamura M Yamamura T Sawada T Mizutani Y et al . Gastrointestinal tract metastasis of lung cancer: The PD-L1 expression and correlated clinicopathological variables. Pathol Int (2021) 71(1):33–41. doi: 10.1111/pin.13048
228
Wu Y Zhang T Zhang X Gao Q . Decoding the complexity of metastasis. Cancer Biol Med (2022) 19(3):284–8. doi: 10.20892/j.issn.2095-3941.2022.0031
229
Li S Hao L Hu X . Natural products target glycolysis in liver disease. Front Pharmacol (2023) 14:1242955. doi: 10.3389/fphar.2023.1242955
Summary
Keywords
PD-L1, hepatocellular carcinoma, liver cancer, cancer, HCC
Citation
Hao L, Li S, Deng J, Li N, Yu F, Jiang Z, Zhang J, Shi X and Hu X (2023) The current status and future of PD-L1 in liver cancer. Front. Immunol. 14:1323581. doi: 10.3389/fimmu.2023.1323581
Received
18 October 2023
Accepted
27 November 2023
Published
12 December 2023
Volume
14 - 2023
Edited by
Vito Longo, National Cancer Institute Foundation (IRCCS), Italy
Reviewed by
Yingcheng Wu, Fudan University, China
Antonella Argentiero, National Cancer Institute Foundation (IRCCS), Italy
Updates
Copyright
© 2023 Hao, Li, Deng, Li, Yu, Jiang, Zhang, Shi and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyu Hu, xiaoyuhu202206@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.