Abstract
Introduction:
This meta-analysis aims to evaluate the efficacy and safety of neoadjuvant PD-1 inhibitors or PD-L1 inhibitors [PD-(L)1 inhibitors] for muscle-invasive bladder carcinoma (MIBC).
Materials and methods:
Four databases (Medline, Embase, Web of Science, and 21 CENTRAL) were searched for articles studying neoadjuvant PD-(L)1 inhibitors for MIBC. The search time period was from the establishment of each database to 21 July 2023. Meta-analyses of pCR, pPR, Grade≥ 3 irAEs rate, RFS, and OS were performed.
Results:
In total, 22 studies were included for meta-analysis. The overall pooled pCR of neoadjuvant PD-(L)1 inhibitors was 0.36 (95%CI=0.30–0.42, p=0.00). In subgroup meta-analysis, the pooled PCR of PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI, and PD-(L)1 inhibitors plus chemotherapy was 0.27 (95%CI=0.19–0.35, p=0.1), 0.41 (95%CI=0.21–0.62, p=0.01), 0.43 (95%CI=0.35–0.50, p=0.06), respectively. The overall pooled pPR of neoadjuvant PD-(L)1 inhibitors was 0.53 (95%CI=0.46–0.60, p=0.00). In subgroup meta-analysis, the pooled pPR of PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI, and PD-(L)1 inhibitors plus chemotherapy was 0.36 (95%CI=0.22–0.51, p=0.01), 0.51 (95%CI=0.39–0.62, p=0.43), and 0.61 (95%CI=0.53–0.69, p=0.01), respectively. Kaplan–Meier curves for OS and RFS were reconstructed, but there was no significant difference among three groups in terms of OS or RFS. The pooled result of Grade≥ 3 irAEs rate for neoadjuvant PD-(L)1 inhibitors was 0.15 (95%CI=0.09–0.22, p=0.00%). In subgroup analysis, the pooled result of Grade≥ 3 irAEs rate for PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI, and PD-(L)1 inhibitors plus chemotherapy was 0.07 (95%CI=0.04–0.11, p=0.84), 0.31 (95%CI=0.16–0.47, p=0.06), and 0.17 (95%CI=0.06–0.31, I2 = 71.27%, p=0.01), respectively.
Conclusion:
Neoadjuvant PD-(L)1 inhibitors were feasible and safe for muscle invasive bladder cancer. Compared with PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI and PD-(L)1 inhibitors plus chemotherapy were associated with higher pCR and pPR, but higher Grade≥3 irAEs. Kaplan–Meier curves for OS and RFS indicated that neoadjuvant PD-(L)1 inhibitors had an acceptable long-term prognostic, but it was not possible to discern statistical differences between the three neoadjuvant subgroups.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023452437, identifier PROSPERO (CRD42023452437).
1 Introduction
Bladder cancer is the most common malignancy of the urinary system with high prevalence in the world (1). Approximately 30% of bladder cancers are muscle-invasive bladder carcinoma (MIBC), which are related to high risk of metastases-related death, and another 70% of bladder cancers are non-muscle-invasive bladder carcinoma (NMIBC), which is not as serious as MIBC (2). According to the risk stratification of the European Association of Urology (EAU) guidelines, NMIBC can be further classified as low-, intermediate-, and high-risk groups based on risk of recurrence and/or progression (3). Unfortunately, 60%–80% of patients with high-risk NMIBC would have a relapse, and 20%–40% of them would develop into MIBC after 5 years (4–6). The prognosis of MIBC remains poor, with the 5-year overall survival (OS) rate decreasing to 60% (7).
Neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) has been recommended for eligible patients with MIBC (8, 9). Commonly used chemotherapy regimens are platinum-based NACs, including gemcitabine and cisplatin (GC), and dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) (10, 11). NAC has obviously improved the OS of MIBC, with the 5-year OS rate approaching 90% for patients achieving a pathological partial response (pPR) at the time of RC (12). However, NAC reported frequent adverse events (AEs), and a partial of cisplatin-eligible MIBC patients have to discontinue the treatment protocol because of severe treatment-related adverse (10, 13). In addition, NAC cannot meet the needs of cisplatin-ineligible patients with MIBC (14). Thus, alternative treatment options are highly necessary.
Recently, the use of immune checkpoint inhibitors (ICIs) has reshaped the treatment paradigm and revolutionized the prognosis of several cancers, such as non-small-cell lung cancer, melanoma, and renal cell carcinoma (15–18). Antibodies against programmed cell death 1 or its ligand have been used for the treatment of advanced/metastatic urothelial cancer, and a significant clinical benefit of PD-(L)1 has been demonstrated (19, 20). At the same time, a growing number of multiple clinical trials have explored combination of PD-(L)1 inhibitors and platinum-based chemotherapy with the reduced risk of developing resistance and/or anticipation of synergistic effect (21, 22). Considering the effectiveness of PD-(L)1 inhibitors in metastatic bladder cancer, clinical trials have been developed to explored the feasibility and safety of neoadjuvant therapy using PD-(L)1 inhibitors (23–25). Basile et al. reported a 37% pathological complete response (pCR) rate and 55% pathological partial response(pPR) rate in the PURE-01 study in which three cycles of pembrolizumab were given to patients with a diagnosis of MIBC and eligible for RC, and 36-month event-free survival (EFS) and over survival (OS) were 74.4% and 83.8% (24, 26, 27). Other clinical trials have been conducted to evaluate the safety and efficacy of PD-(L)1 inhibitors combined with chemotherapy or PD-(L)1 inhibitors combined with other ICI strategies. Kim et al. reported a 35% pCR rate of RC patients after neoadjuvant nivolumab plus gemcitabine/cisplatin chemotherapy (28). The NABUCCO study investigating ipilimumab plus nivolumab reported a 45.8% pCR rate (29).
In the present study, we aimed to systematically assess the available evidence in the literature regarding the safety and efficacy of neoadjuvant PD-(L)1 inhibitors in patients with stage II–III MIBC.
2 Materials and methods
2.1 Search strategy
The present meta-analysis was conducted according to the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines. This study has been registered at PROSPERO with a registration number of CRD42023452437. Four databases including PubMed, Embase, Web of Science, and the Cochrane Library were systematically searched for literatures published up to 21 July 2023, using the following searching strategy: (“PD-1 inhibitor” OR “PD-L1 inhibitor”) AND “neoadjuvant” AND “bladder cancer” AND (“randomized controlled trial” OR “prospective” OR “retrospective”). Supplementary Material 1 presents the searching record in detail.
2.2 Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) patients diagnosed as MIBC (stage II/III); (2) neoadjuvant therapy using PD-(L)1 inhibitors was administrated, with or without chemotherapy or other ICI, and RC was performed after neoadjuvant therapy; (3) at least one of the following outcomes were reported, namely, pCR, pPR, OS, RFS, Grade≥ 3 irAEs rate, Grade≥ 3 TRAEs rate; and s(4) study types, namely, randomized controlled studies, non-randomized controlled studies, single-arm trials, prospective studies, and retrospective studies.
Exclusion criteria were as follows: (1) other types of articles, such as case reports, publications, letters, reviews, meta-analyses, editorials, pharmacological intervention, animal studies, and protocols; (3) other cancers; (4) no relative outcomes; (5) reduplicate cohort of patients; and (6) failure to extract data for meta-analysis.
2.3 Data extraction
Two independent investigators (S.H. and Y.H.) reviewed the title and abstract and then read the full text. Discrepancy were resolved by consulting with a third investigator (M.H.). Data retrieved included first author’s name, year, trial ID, study design, sample size, intervention, male ratio, age, study design, cTNM stage, cisplatin eligibility, regimen, pCR, pPR, OS, RFS, Grade≥ 3 irAEs rate, Grade≥ 3 TRAEs rate, Kaplan–Meier curves for OS, and Kaplan–Meier curves for RFS.
2.4 Risk of bias assessment
The risk of bias was assessed by two independent reviewers (L.H. and S.H.), using the modified Jadad scale (30) for RCTs while using the methodological index for non-randomized studies (MINORS) (31) for single-arm studies or non-RCTs.
2.5 Statistical analysis
The selection duplicate removal of studies included was conducted using EndNote (Version 20; Clarivate Analytics). All analyses were performed using Stata 12.0 and R version 4.3.1 [R version Copyright (C) 2023, The R Foundation for Statistical Computing]. The “meta” package and IPDformKM package were utilized in the analysis. GetData Graph Digitizer software was used to extract data from articles containing Kaplan–Meier curves, and individual data were reconstructed with IPDformKM package. The established method by Guyot et al. was used to reconstruct individual patient-level data (32). Continuous variables were compared using weighted mean difference (WMD) with a 95% confidence interval (CI). Relative ratio (RR) with 95% CI were used to compare binary variables. The medians and interquartile ranges of continuous data were converted to the mean and standard deviation. Statistical heterogeneity between included studies was calculated using the Cochrane ‘Sq test and the I2 index (I2 >50% indicating high heterogeneity). When there is high heterogeneity among studies, the random effects model is adopted, otherwise the fixed effects model is adopted (33). A p-value < 0.05 was considered statistically significant. Begg’s method was used to test the publication bias among various studies and to draw a funnel plot. Finally, a sensitivity analysis was performed to determine the impact of individual studies on the aggregated results and to test the reliability of the results.
3 Results
3.1 Search results
The process of the literature selection and inclusion is presented in Figure 1. Our initial search found a total of 577 studies. After excluding repeat studies, only 390 cases remained. By reading the full text, 295 other types of articles, 7 articles investigating other types of cancer, and 48 unrelated articles were excluded. Finally, 22 studies involving 843 patients with advanced bladder cancer were ultimately included in this meta-analysis.
Figure 1

Flow chart of literature search strategies.
3.2 Patient characteristics and quality assessment
Most of the included studies were phase II single-arm trials with a total of 22 cohorts, eight of which explored neoadjuvant PD-(L)1 inhibitors alone (two pembrolizumab (27, 34), two atezolizumab (35, 36), two nivolumab (37, 38), one durvalumab (39), and one avelumab (40)), five cohorts exploring PD-(L)1 inhibitors plus other ICI (three ipilimumab plus nivolumab (29, 37, 41) and two durvalumab plus tremelimumab (42, 43)), and PD-(L)1 inhibitors plus chemotherapy in 11 cohorts (eight gemcitabine/cisplatin [GC] plus ICI (28, 44–50), one dose-dense course of methotrexate, vinblastine, doxorubicin, and cisplatin [ddMVAC] plus ICIs (51), one gemcitabine plus ICI (52), and one paclitaxel/gemcitabine [PG] plus ICI) (40). The quality of RCT literature was evaluated using modified Jadad scale for RCTs, and both RCTs were high-quality articles. Other articles were scored using MINORS, with 15 points for 4 articles, 14 points for 8 articles, 13 points for 2 articles, 12 points for 5 articles, and 6 points for 2 articles. A total of 13 cases were recorded involving 542 patients, and the proportion of TNM stages was reported in detail: 65.7% for cT2, 33.4% for cT3-4a, and 2.0% for cN1. Details of all studies and the characteristics of the patients with bladder cancer are shown in Table 1.
Table 1
| Hor | Registration ID | Year | Study design | cTNM stage | Cis-ineligible or refusal | Study arm(s) | No. of patients | Regimen, cycles | Age (median, years) | Gender (male, %) | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim (28) | KCT0003804 CRIS | 2022 | single-arm | T2-4aN0M0 | No | GC+ Nivolumab | 51 | 3–4 | NA | NA | 14 |
| Van Dijk (29) | NCT03387761cohort I | 2020 | single-arm | T2-T4aN0-1M0, | Regardless | Nivolumab + Ipilimumab | 24 | 3 | 65 | 75% | 15 |
| Goubet (34) | NCT03212651 | 2022 | single-arm | T2-4aN0M0 | NA | Pembrolizumab | 39 | 3 | NA | NA | 12 |
| Necchi (27) | NCT02736266 | 2022 | single-arm | T2-4aN0M0 | Regardless | Pembrolizumab | 114 | 3 | 66 | 86.8% | 15 |
| Szabados (35) | NCT02662309 | 2022 | single-arm | T2–T4aN0M0 | Yes | Atezolizumab | 95 | 2 | 73 | 85% | 15 |
| Koshkin (36) | NCT02451423 | 2021 | single-arm | T2-4aN0-1M0 | Yes | Atezolizumab | 20 | 1-3 | 69 | 75% | 14 |
| Guercio (37) | NCT03520491 | 2022 | non-RCT | T2-4aN0M0, | Yes | armA: Nivolumab | armA:15 | NA | 76 | 80% | 13 |
| armB: Nivolumab + Ipilimumab | armB: 15 | ||||||||||
| Yin (38) | NCT03532451 | 2021 | non-RCT | T2-4aN0-1M0 | Yes | armA: Nivolumab | armA:13 | NA | 75 | 67% | 14 |
| Wei (39) | NCT03773666 | 2020 | single-arm | T2-4aN0M0 | Yes | Durvalumab | 10 | 3 | 67 | 80% | 14 |
| Chanza (40) | NCT03674424 | 2022 | RCT | T2-4aN0-1M0 | armA: No | armA: PG+ Avelumab | armA:28 | 4 | armA: 72 | armA: 93% | 6 |
| armB: Yes | armB: Avelumab | armB: 28 | armB: 75 | armB: 93% | |||||||
| Van Dorp (41) | NCT03387761cohort II | 2021 | single-arm | stage III | Yea | Nivolumab + Ipilimumab | 30 | 3 | NA | NA | 13 |
| Grande (42) | NCT03472274 | 2020 | RCT | cT2‐4aN0-1M0 | No | armA: Durvalumab+Tremelimumab | armA:23 | 3 | NA | NA | 6 |
| armB: GC/ddMVAC | armB: 38 | ||||||||||
| Gao (43) | NCT02812420 | 2020 | single-arm | T2-4aN0M0 | Yes | Durvalumab+ Tremelimumab | 28 | 2 | 71 | 71% | 15 |
| Xing (44) | ChiCTR2000032359 | 2023 | single-arm | T2-4aN0-1M0 | No | GC+ Camrelizumab | 19 | 3 | 69 | 73.7% | 12 |
| Rose (45) | NCT02690558 | 2021 | single-arm | T2-4aN0-1M0 | No | GC+ Pembrolizumab | 39 | 4 | NA | NA | 14 |
| Lin (46) | ChiCTR2000037670 | 2022 | single-arm | T2-4aN0M0 | No | GC+ Tislelizumab | 17 | 4 | 62 | NA | 12 |
| Kaimakliotis (47) | NCT02365766 | 2019 | single-arm | T2-4aN0M0 | No | GC+ Pembrolizumab | 40 | 4 | 65 | 75% | 14 |
| Gupta (48) | NCT03294304 | 2022 | single-arm | T2-4aN0-1M0 | No | GC+ Nivolumab | 41 | 4 | NA | NA | 14 |
| Funt (49) | NCT02989584 | 2021 | single-arm | T2-4aN0M0 | No | GC+ Atezolizumab | 44 | 4 | NA | NA | 12 |
| Cathomas (50) | SAKK 06/17 | 2020 | single-arm | T2-4aN0-1M0 | Yes | GC+ Durvalumab | 61 | 4 | 67.5 | 79% | 14 |
| Thibault (51) | NCT03549715 | 2020 | single-arm | NA | No | ddMVAC+ Durvalumab+Tremelimumab | 12 | 2 | 59.5 | 12 | |
| Hristos (52) | NCT02365766 cohort2 | 2020 | single-arm | T2-4aN0M0 | Yes | Gemcitabine+Pembrolizumab | 37 | 3 | 72 | 70% | 13 |
Characteristics of included studies and patients.
3.3 pCR
Figure 2 shows forest plot of the meta-analysis for pCR. The overall pooled pCR of neoadjuvant PD-(L)1 inhibitors was 0.36(95%CI=0.30–0.42, I2 = 57.4%, p=0.00). Results of subgroup meta-analysis are shown in Table 2.
Figure 2

Forest plot of the meta-analysis for pCR.
Table 2
| Outcomes | No. of studies |
Heterogeneity | Overall effect size |
95% CI of overall effect |
Weight(%) |
|---|---|---|---|---|---|
| I2(%) p-value | |||||
| PCR | |||||
| PD-(L)1 inhibitors alone | 8 | 41.71 0.10 | 0.27 | 0.19–0.35 | 33.83 |
| PD-(L)1 inhibitors plus other ICI | 5 | 72.73 0.01 | 0.41 | 0.21–0.62 | 17.34 |
| PD-(L)1 inhibitors plus chemotherapy | 11 | 42.80 0.06 | 0.43 | 0.35–0.50 | 48.83 |
| Overall pooled PCR | 24 | 57.40 0.00 | 0.36 | 0.30–0.42 | 100 |
| PPR | |||||
| PD-(L)1 inhibitors alone | 6 | 65.79 0.01 | 0.36 | 0.22–0.51 | 26.26 |
| PD-(L)1 inhibitors plus other ICI | 4 | 0.00 0.43 | 0.51 | 0.39–0.62 | 17.56 |
| PD-(L)1 inhibitors plus chemotherapy | 11 | 55.15 0.01 | 0.61 | 0.53–0.69 | 56.19 |
| Overall pooled PPR | 21 | 60.94 0.00 | 0.53 | 0.46–0.60 | 100 |
| Grade≥ 3 irAEs rate | |||||
| PD-(L)1 inhibitors alone | 7 | 0.00 0.84 | 0.07 | 0.04–0.11 | 44.05 |
| PD-(L)1 inhibitors plus other ICI | 4 | 59.17 0.06 | 0.31 | 0.16–0.47 | 24.36 |
| PD-(L)1 inhibitors plus chemotherapy | 5 | 71.27 0.01 | 0.17 | 0.06–0.31 | 31.59 |
| Overall pooled Grade≥ 3 irAEs rate | 16 | 69.83 0.00 | 0.15 | 0.09–0.22 | 100 |
Results of the meta-analysis for pCR, pPR, and Grade≥ 3 irAEs rate.
3.4 pPR
Figure 3 shows the forest plot of the meta-analysis for pPR. The overall pooled pPR of neoadjuvant PD-(L)1 inhibitors was 0.53 (95%CI=0.46–0.60, I2 = 60.94%, p=0.00). Results of subgroup meta-analysis are shown in Table 2.
Figure 3

Forest plot of the meta-analysis for pPR.
3.5 OS
In total, five studies reported Kaplan–Meier curves for overall survival (OS), with two studies reporting PD-(L)1 inhibitors plus chemotherapy (49, 53), two studies reporting PD-(L)1 inhibitors alone (26, 54), and one study reporting PD-(L)1 inhibitors plus other ICI (55). Using the IPDformKM package, we extracted individual data and reconstructed Kaplan–Meier curves for OS (Figure 4). The OS of neoadjuvant PD-(L)1 inhibitors was 91.67%, 86.03%, and 81.64% at 1 year, 2 years, and 3 years, respectively. Results of subgroup meta-analysis are shown in Table 3. However, there was no significant difference in OS among the three groups (p=0.25).
Figure 4

Kaplan–Meier curves for OS.
Table 3
| Outcomes | No. Of studies | 1 year | 2 years | 3 years |
|---|---|---|---|---|
| OS | ||||
| PD-(L)1 inhibitors alone | 2 | 91.7% | 84.85% | 80.28% |
| PD-(L)1 inhibitors plus other ICI | 1 | 96.05% | 96.05% | 96.05% |
| PD-(L)1 inhibitors plus chemotherapy | 2 | 92.11% | 80.07% | 80.07% |
| Neoadjuvant PD-(L)1 inhibitors | 5 | 91.67% | 86.03% | 81.64% |
| RFS | ||||
| PD-(L)1 inhibitors alone | 2 | 85.3% | 80.12% | 79.3% |
| PD-(L)1 inhibitors plus other ICI | 1 | 91.72% | 91.72% | 91.72% |
| PD-(L)1 inhibitors plus chemotherapy | 3 | 84.47% | 71.84% | 71.84% |
| Neoadjuvant PD-(L)1 inhibitors | 6 | 85.69% | 79.67% | 79.05% |
Results of OS and RFS.
3.6 RFS
Totally, six studies reported Kaplan–Meier curves for recurrence-free survival (RFS), with three studies reporting PD-(L)1 inhibitors plus chemotherapy (28, 45, 49), two studies reporting PD-(L)1 inhibitors alone (26, 54), and one study reporting PD-(L)1 inhibitors plus other ICI (55). Using the IPDformKM package, we extracted individual data and reconstructed Kaplan–Meier curves for RFS (Figure 5). The RFS of neoadjuvant PD-(L)1 inhibitors was 85.69%, 79.67%, and 79.05% at 1 year, 2 years, and 3 years, respectively. Results of subgroup meta-analysis are shown in Table 3. However, there was no significant difference in RFS among the three groups (p=0.22).
Figure 5
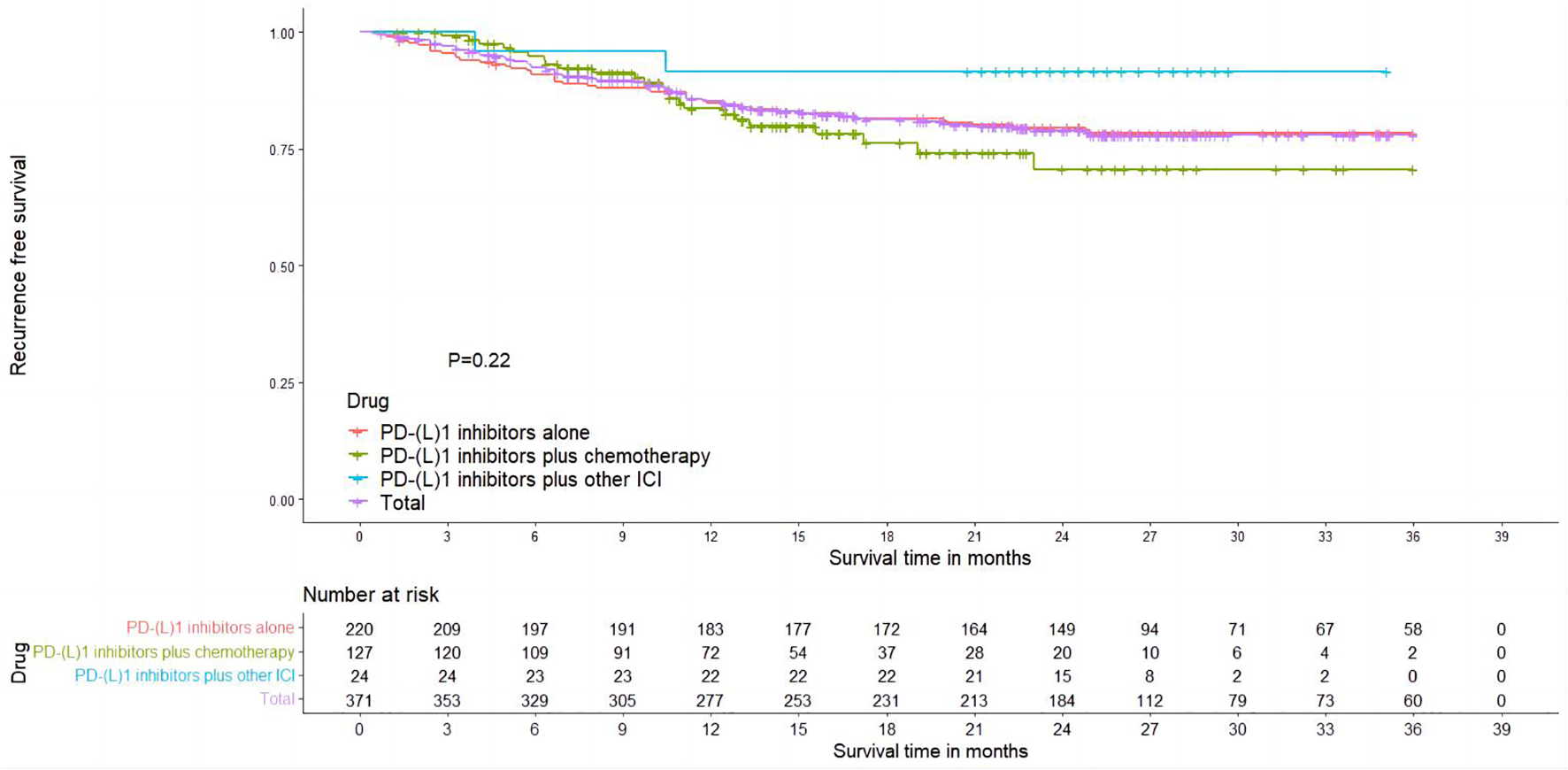
Kaplan–Meier curves for RFS.
3.7 Safety
Regarding safety, Grade≥ 3 irAEs rate was evaluated, which was reported in a total of 17 cohorts (Figure 6). The pooled result of Grade≥ 3 irAEs rate for neoadjuvant PD-(L)1 inhibitors was 0.15 (95%CI=0.09–0.22, I2 = 69.83%, p=0.00). Results of subgroup meta-analysis are shown in Table 2. The common irAEs included elevated liver enzymes, elevated amylase/lipase, imDC, hematological toxicity, skin reactions, and fatigue.
Figure 6
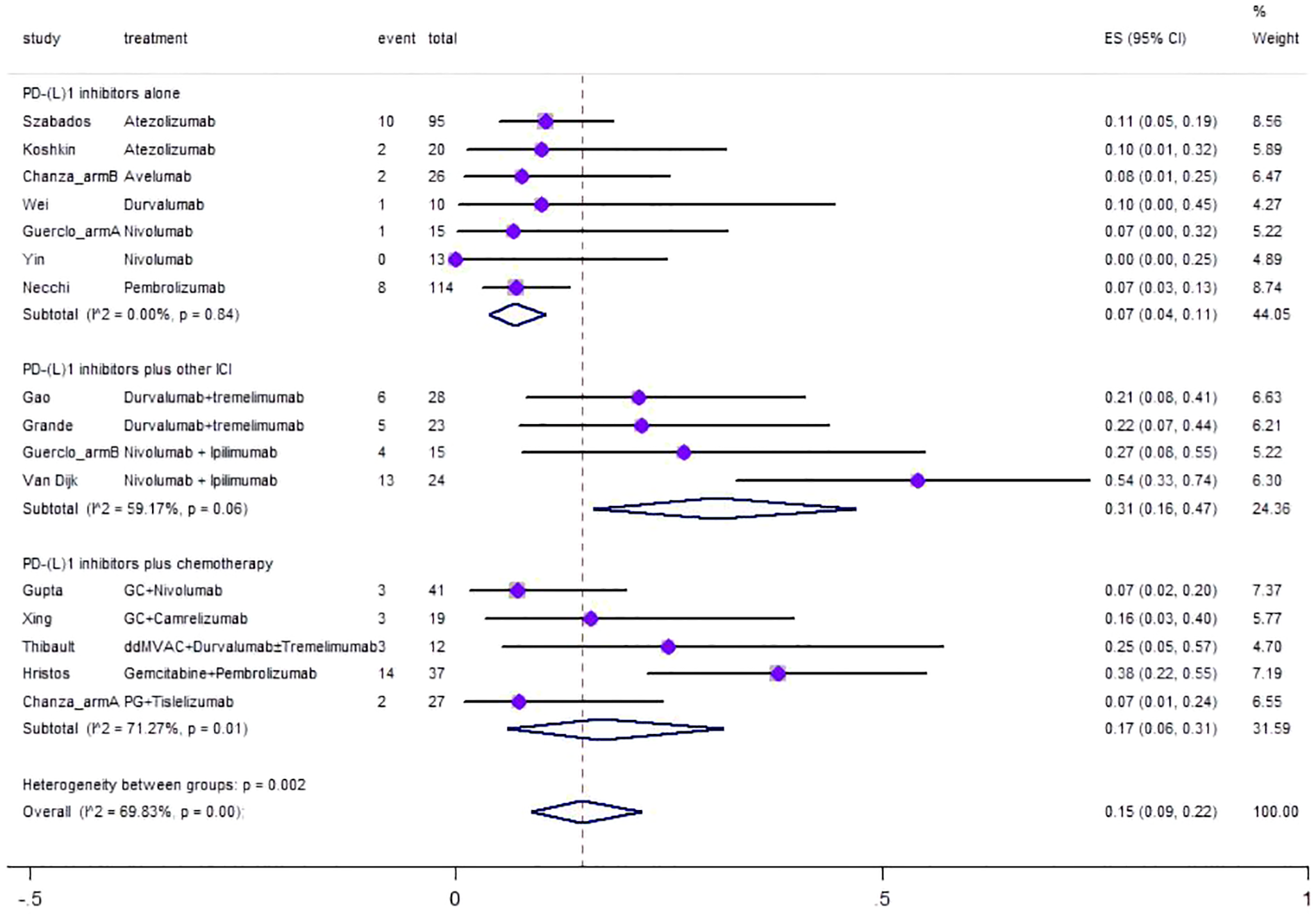
Forest plot of the meta-analysis for Grade≥ 3 irAEs rate.
3.8 Supplement oncological and safety outcomes
Supplementary Material 2 reports PCR (%), PRR (n), ≥ Grade3 irAEs, ≥ Grade 3 surgical complications, and AEs in detail.
4 Discussion
Since the significant clinical benefit of PD-(L)1 inhibitors demonstrated in patients with advanced/metastatic urothelial cancer, a growing number of clinical trials has been performed to evaluate the safety and efficacy of PD-(L)1 inhibitors in the neoadjuvant therapy for MIBC patients. In these clinical trials, PD-(L)1 inhibitors were used alone, combined with chemotherapy, or combined with other ICIs. In the present study, a systemic review and meta-analysis was conducted to evaluate the safety and efficacy of neoadjuvant PD-(L)1 inhibitors in patients with MIBC.
PD-(L)1 inhibitors alone or plus other ICI, PD-(L)1 inhibitors provided an optional treatment modality for patients who either were ineligible or refused cisplatin-based neoadjuvant chemotherapy. PD-(L)1 inhibitors plus other ICI seem to have advantage in efficacy over PD-(L)1 inhibitors alone. In the present study, the polled analysis showed that pCR of PD-(L)1 inhibitors plus other ICI was higher than that of PD-(L)1 inhibitors alone, and similar results were present regarding pRR. However, our study showed that there was no significant difference among three groups in terms of OS or RFS. Only five studies reported Kaplan–Meier curves for OS, and six studies reported Kaplan–Meier curves for RFS, with relatively short follow-up time. The statistical results of oncology outcomes were difficult to reflect the differences among three groups due to the small sample and short follow-up time. In previous literature, PD-(L)1 inhibitors were effective in the neoadjuvant therapy for non-small-cell lung cancer (NSCLC). Forde et al. conducted a phase 2 study designed to evaluate the safety and feasibility of administration of two doses of nivolumab over 4 weeks before surgery in patients with stage I–IIIA resectable NSCLC and reported a major pathological response rate of 45% with a complete pathological response rate of 10% (56). Although median DFS and OS have not yet been reached in this study, 80% of patients were alive without recurrence at 1 year. Recent clinical trials have declared the safety, feasibility, and efficacy of neoadjuvant PD-(L)1 inhibitors in solid tumors other than MIBC, including triple-negative breast cancer, melanoma, and NSCLC (57–59).
The administration of immune checkpoint inhibitors in the neoadjuvant therapy has several advantages (60). First, with neoadjuvant therapy of immune checkpoint inhibitors, the intact tumor could become the source for antigen-specific T-cell immunity with multiple antigen load. Second, the early evaluation of therapy response in individual patients by pathological analysis on the excised tumor allows for potential to adjust systemic therapy according to pathological response. Furthermore, a unique platform for relative basic and translational investigations can be provided by neoadjuvant therapy strategies with immune checkpoint inhibitors (61).
Liu et al. used two models of spontaneously metastatic breast cancers in mice to illustrate the significantly therapeutic power of neoadjuvant in the context of primary tumor resection and found that mice treated with anti-PD-1/anti-CD137 combination before surgery demonstrated a 40% long-term survival compared with 0% in the adjuvant group (62). In addition, an increase in tumor-specific CD8+ T cells was seen in the neoadjuvant group but not in the adjuvant group, which suggested that neoadjuvant ICIs with the tumor in situ contribute to a more robust T-cell response. This study highlighted the above advantages of neoadjuvant therapy with immune checkpoint inhibitors.
Regarding safety, irAEs seem to occur more frequently when PD-(L)1 inhibitors plus other ICI were administrated. In the present study, Grade≥ 3 irAEs morbidity was 0.51 in patients who were treated by PD-(L)1 inhibitors plus other ICI, while the rate was 0.36 in PD-(L)1 inhibitors alone group. Similarly, a randomized, open-label, multicenter, phase 3 trial (DANUBE) in patients with untreated, unresectable, locally advanced, or metastatic urothelial carcinoma reported that grade 3 or 4 treatment-related adverse events occurred in 47 (14%) of 345 patients in the durvalumab group while 93 (27%) of 340 patients in the durvalumab plus tremelimumab group (63). Thus, the safety profile should not be ignored when PD-(L)1 inhibitors plus other ICI were administrated.
Although NAC has been preferred by the National Comprehensive Cancer Network, only 36%–49% of MIBC patients treated by NAC can achieve non-muscle invasive downstaging (13, 64). A more effective neoadjuvant therapy is urgent for patients with MIBC. Several clinical trials has reported the efficacy of PD-(L)1 inhibitors in the treatment of platinum-resistant metastatic bladder carcinoma, which demonstrated that there is no clinical cross-resistance between NAC and PD-(L)1 inhibitors (65–67). Recent studies reported that PD-(L)1 inhibitors plus chemotherapy resulted in better RFS and OS in patients with advanced or metastatic MIBC, compared with chemotherapy alone (21, 68). Based on the above results, several clinical trials have recently been conducted to assess the efficacy of neoadjuvant PD-(L)1 inhibitors plus chemotherapy for patients with MIBC. The pooled result of the present meta-analysis showed that the pCR and pPR was 43% and 61% for neoadjuvant PD-(L)1 inhibitors plus chemotherapy, respectively, which seems to have advantage over NAC in oncological outcomes. A meta-analysis comparing oncological outcomes of ddMVAC with GC as neoadjuvant chemotherapy for muscle-invasive bladder cancer reported a pCR of 35.2% in patients treated by ddMVAC while 25.1% in patients treated by GC[50]. A recent randomized phase III trial comparing dd-MVAC with GC reported that pCR was observed in 42% of the ddMVAC group and in 36% of the GC group, respectively, and <pT2N0 rates of 63% and 49%[51]. A retrospective study reported that the mean Kaplan–Meier estimates of OS was 4.2 years in the GC group and 7.0 years in the ddMVAC group (69). A cross-sectional analysis indicated that 2-year Kaplan–Meier survival probability estimates were 73.3% for ddMVAC and 62% for GC (70). Therefore, compared with NAC alone, neoadjuvant PD-(L)1 inhibitors plus chemotherapy provided a more effective treatment modality for patients who were fit for cisplatin-based neoadjuvant chemotherapy. There is an important question that needs to be answered: is there a major advantage of the use of PD-(L)1 inhibitors over neoadjuvant cisplatin-based chemotherapy? In view of the revolution brought about by the EV 302 trial (71), the KEYNOTE-B15/EV-304 (NCT04700124) trial is now underway, which is a phase 3 trial that aims to assess the effectiveness and safety of perioperative Enfortumab vedotin (EV) plus pembrolizumab compared to neoadjuvant chemotherapy using gemcitabine/cisplatin in patients with muscle-invasive bladder cancer who are eligible for cisplatin treatment (72). The outcome of this trial is eagerly awaited to answer the above question.
Regarding safety, our results showed that the Grade≥ 3 irAEs rate was 17% after neoadjuvant PD-(L)1 inhibitors plus chemotherapy, while the Grade≥ 3 TRAEs rate was 47%. A retrospective multicenter study of a clinical database reported that the Grade≥ 3 AEs occurred in 31% patients during neoadjuvant chemotherapy for muscle invasive bladder cancer (73). A recent randomized trial reported that 52% patients had Grade≥ 3 AEs in dd-MVAC arm while 55% in GC arm (13).
In light of the potential significant negative consequences, the high expenses associated with therapy, and the emergence of alternative therapeutic options, the significance of predictive biomarkers for personalized treatment seems more crucial than ever. Several trials included in the study evaluated PD-L1 testing and the rate of positivity, and the secondary endpoints of these trials reported the pCR rate in patients who tested positive for PD-L1 (27, 29, 35, 46, 47). In the ABACUS trial, Thomas Powles et al. characterized PD-L1 positivity as the presence of ≥5% of immune cells staining using the SP142 antibody. However, some other trials have classified PD-L1 positivity as CPS>10%. Three trials demonstrated no statistically significant differences in pCR rates between patients who tested positive for PD-L1 and those who tested negative for PD-L1 (29, 35, 46). Nevertheless, the PURE-01 research found that PD-L1 positivity (OR, 1.02; 95% CI, 1.01–1.04) was a statistically significant factor (27). This suggests that the presence of PD-L1 could potentially be used to predict the response to PD-(L)1 inhibitors in terms of pathology. In addition, tumor mutational burden (TMB) enhances the amount of tumor neoantigens and the likelihood of effective T-cell identification. The field of urothelial carcinoma (UC) has observed noteworthy correlations between elevated tumor mutational burden (TMB) and positive treatment outcomes in both the neoadjuvant therapy context (PURE-01 trial) (27) and for metastatic tumors (IMvigor210, KEYNOTE-028) (74, 75). Circulating tumor DNA (ctDNA) refers to bits of DNA from tumors that are present in the bloodstream. It has been discovered that only patients who test positive for ctDNA receive a significant advantage from adjuvant atezolizumab treatment (IMvigor010), indicating that ctDNA can be used to identify individuals at a high risk of metastasis in UC. Currently, there are ongoing clinical trials (TOMBOLA, IMvigor011) that are enrolling patients who have detectable ctDNA following radical cystectomy for the purpose of receiving atezolizumab treatment. However, in this instance, ctDNA functions as a prognostic biomarker rather than a predictive one (71, 76, 77).
There were several strengths in the present study. First of all, few meta-analysis has assessed the efficacy and safety of PD-(L)1 inhibitors in the neoadjuvant therapy for MIBC, and we conducted a systemic review and meta-analysis including the latest studies on neoadjuvant PD-(L)1 inhibitors in patients with stage II–III MIBC. Second, the outcomes were pooled by PP and subgroup analyses, since discrepancies of different literature were included. Third, Kaplan–Meier curves for OS and RFS were reconstructed using the IPDformKM package, presenting an intuitive impression for oncological outcomes. Specifically, three protocols were analyzed: PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI, and PD-(L)1 inhibitors plus chemotherapy. The PP analysis contributes to represent the latest progress of each treatment regimens.
Our study has several limitations. First of all, most studies were non-randomized single-arm clinical trials with a small sample size, resulting in indirect comparisons among different treatment regimens. Second, there was significant heterogeneity in the majority of clinical outcomes. The probable reasons consist of the included population bias and the difference in drug types, dosage, and cycles of regimens. Third, most studies have not yet reached their endpoint, which failed to provide data of survival outcomes, making it difficult to assess the lasting benefits of neoadjuvant PD-(L)1 inhibitors.
In conclusion, neoadjuvant PD-(L)1 inhibitors were feasible and safe for muscle-invasive bladder cancer. Compared with PD-(L)1 inhibitors alone, PD-(L)1 inhibitors plus other ICI and PD-(L)1 inhibitors plus chemotherapy were associated with higher pCR and pPR but higher Grade≥ 3 irAEs. Kaplan–Meier curves for OS and RFS indicated that neoadjuvant PD-(L)1 inhibitors had an acceptable long-term prognostic, but it was not possible to discern statistical differences between the three neoadjuvant subgroups. To further confirm the safety and efficacy of neoadjuvant PD-(L)1 inhibitors, more multicenter, randomized controlled trials and longer follow-up time are necessary.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SH: Data curation, Software, Writing – original draft. YH: Data curation, Software, Writing – original draft. CL: Methodology, Project administration, Supervision, Writing – original draft. YL: Conceptualization, Data curation, Writing – original draft. MH: Investigation, Supervision, Writing – original draft. RL: Conceptualization, Funding acquisition, Resources, Writing – original draft. WL: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors disclose the receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Scientific Research Foundation of Guangxi University of Science and Technology (20Z13), the College students Innovation and Entrepreneurship Training Project of Guangxi University of Science and Technology (S202310594022), the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927) and the Scientific Research Foundation of Guangxi Health Commission (Z-B20220930).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1332213/full#supplementary-material
References
1
Ploeg M Aben KKH Kiemeney LA . The present and future burden of urinary bladder cancer in the world. World J Urol (2009) 27(3):289–93. doi: 10.1007/s00345-009-0383-3
2
Guillaume L Guy L . Epidemiology of and risk factors for bladder cancer and for urothelial tumors. La Rev du praticien (2014) 64(10):1372–4, 8-80.
3
Babjuk M Bohle A Burger M Capoun O Cohen D Comperat EM et al . Eau guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol (2017) 71(3):447–61. doi: 10.1016/j.eururo.2016.05.041
4
Cambier S Sylvester RJ Collette L Gontero P Brausi MA van Andel G et al . Eortc nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guerin. Eur Urol (2016) 69(1):60–9. doi: 10.1016/j.eururo.2015.06.045
5
Dyrskjot L Zieger K Real FX Malats N Carrato A Hurst C et al . Gene expression signatures predict outcome in non-muscle invasive bladder carcinoma: A multicenter validation study. Clin Cancer Res (2007) 13(12):3545–51. doi: 10.1158/1078-0432.ccr-06-2940
6
Fernandez-Gomez J Madero R Solsona E Unda M Martinez-Pineiro L Gonzalez M et al . Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus calmette-guerin: the cueto scoring model. J Urol (2009) 182(5):2195–203. doi: 10.1016/j.juro.2009.07.016
7
Edge SB Compton CC . The American joint committee on cancer: the 7th edition of the ajcc cancer staging manual and the future of tnm. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
8
Kulkarni GS Black PC Sridhar SS Kapoor A Zlotta AR Shayegan B et al . Canadian urological association guideline: muscle-invasive bladder cancer. Cuaj-Canadian Urological Assoc J (2019) 13(8):230–8. doi: 10.5489/cuaj.5902
9
Witjes JA Bruins HM Cathomas R Comperat EM Cowan NC Gakis G et al . European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol (2021) 79(1):82–104. doi: 10.1016/j.eururo.2020.03.055
10
Lee Y Kim YS Hong B Cho YM Lee JL . Comparison of clinical outcomes in patients with localized or locally advanced urothelial carcinoma treated with neoadjuvant chemotherapy involving gemcitabine-cisplatin and high dose-intensity mvac. J Cancer Res Clin Oncol (2021) 147(11):3421–9. doi: 10.1007/s00432-021-03582-x
11
Ravi P Pond GR Diamantopoulos LN Su C Alva A Jain RK et al . Optimal pathological response after neoadjuvant chemotherapy for muscle-invasive bladder cancer: results from a global, multicentre collaboration. Bju Int (2021) 128(5):607–14. doi: 10.1111/bju.15434
12
Funt SA Rosenberg JE . Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol (2017) 14(4):221–34. doi: 10.1038/nrclinonc.2016.188
13
Pfister C Gravis G Fléchon A Soulié M Guy L Laguerre B et al . Randomized phase iii trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the getug/afu V05 vesper trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol (2021) 79(2):214–21. doi: 10.1016/j.eururo.2020.08.024
14
Galsky MD Hahn NM Rosenberg JE Sonpavde G Oh WK Dreicer R et al . Defining "Cisplatin ineligible" Patients with metastatic bladder cancer. J Clin Oncol (2011) 29(7):238. doi: 10.1200/jco.2011.29.7_suppl.238
15
Choueiri TK Powles T Burotto M Escudier B Bourlon MT Zurawski B et al . Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. New Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
16
Hellmann MD Paz-Ares L Caro RB Zurawski B Kim SW Costa EC et al . Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. New Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
17
Hodi FS Chiarion-Sileni V Gonzalez R Grob JJ Rutkowski P Cowey CL et al . Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (Checkmate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/s1470-2045(18)30700-9
18
Motzer RJ Tannir NM McDermott DF Frontera OA Melichar B Choueiri TK et al . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
19
Sonpavde G . Pd-1 and pd-L1 inhibitors as salvage therapy for urothelial carcinoma. N Engl J Med (2017) 376(11):1073–4. doi: 10.1056/NEJMe1701182
20
Bellmunt J Powles T Vogelzang NJ . A review on the evolution of pd-1/pd-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev (2017) 54:58–67. doi: 10.1016/j.ctrv.2017.01.007
21
Galsky MD Arija JÁA Bamias A Davis ID De Santis M Kikuchi E et al . Atezolizumab with or without chemotherapy in metastatic urothelial cancer (Imvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet (London England) (2020) 395(10236):1547–57. doi: 10.1016/s0140-6736(20)30230-0
22
Powles T Csoszi T Ozguroglu M Matsubara N Geczi L Cheng SYS et al . Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (Keynote-361): A randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/s1470-2045(21)00152-2
23
Powles T Kockx M Rodriguez-Vida A Duran I Crabb SJ van der Heijden MS et al . Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the abacus trial. Nat Med (2019) 25(11):1706–14. doi: 10.1038/s41591-019-0628-7
24
Necchi A Anichini A Raggi D Briganti A Massa S Lucianò R et al . Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (Pure-01): an open-label, single-arm, phase ii study. J Clin Oncol (2018) 36(34):3353–60. doi: 10.1200/jco.18.01148
25
Hahn NM Necchi A Loriot Y Powles T Plimack ER Sonpavde G et al . Role of checkpoint inhibition in localized bladder cancer. Eur Urol Oncol (2018) 1(3):190–8. doi: 10.1016/j.euo.2018.05.002
26
Basile G Bandini M Gibb EA Ross JS Raggi D Marandino L et al . Neoadjuvant pembrolizumab and radical cystectomy in patients with muscle-invasive urothelial bladder cancer: 3-year median follow-up update of pure-01 trial. Clin Cancer Res (2022) 28(23):5107–14. doi: 10.1158/1078-0432.ccr-22-2158
27
Necchi A Raggi D Gallina A Madison R Colecchia M Luciano R et al . Updated results of pure-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol (2020) 77(4):439–46. doi: 10.1016/j.eururo.2019.10.026
28
Kim H Jeong BC Hong J Kwon GY Kim CK Park W et al . Neoadjuvant nivolumab plus gemcitabine/cisplatin chemotherapy in muscle-invasive urothelial carcinoma of the bladder. Cancer Res Treat (2023) 55(2):636–42. doi: 10.4143/crt.2022.343
29
van Dijk N Gil-Jimenez A Silina K Hendricksen K Smit LA de Feijter JM et al . Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the nabucco trial. Nat Med (2020) 26(12):1839–44. doi: 10.1038/s41591-020-1085-z
30
Bañares R Albillos A Rincón D Alonso S González M Ruiz-del-Arbol L et al . Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: A meta-analysis. Hepatol (Baltimore Md) (2002) 35(3):609–15. doi: 10.1053/jhep.2002.31354
31
Slim K Nini E Forestier D Kwiatkowski F Panis Y Chipponi J . Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
32
Liu N Zhou Y Lee JJ . Ipdfromkm: reconstruct individual patient data from published kaplan-meier survival curves. BMC Med Res Method (2021) 21(1):111. doi: 10.1186/s12874-021-01308-8
33
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol (2021) 134:178-89. doi: 10.1016/j.jclinepi.2021.03.001
34
Goubet AG Silva CAC De Melo LL Gazzano M Lebacle C Thibault C et al . Bacteria-specific cxcl13-producing follicular helper T cells are putative prognostic markers to neoadjuvant pd-1 blockade in muscle-invasive urothelial carcinoma. J Clin Oncol (2022) 40(6):535. doi: 10.1200/JCO.2022.40.6_suppl.535
35
Szabados B Rodriguez-Vida A Durán I Crabb SJ van der Heijden MS Pous AF et al . Toxicity and surgical complication rates of neoadjuvant atezolizumab in patients with muscle-invasive bladder cancer undergoing radical cystectomy: updated safety results from the abacus trial. Eur Urol Oncol (2021) 4(3):456–63. doi: 10.1016/j.euo.2020.11.010
36
Koshkin VS Natesan D Zhang L Oh DY Porten SP Meng M et al . Phase ii trial of escalating doses of neoadjuvant atezolizumab for patients with non-metastatic urothelial carcinoma ineligible for cisplatin-based neoadjuvant chemotherapy. J Clin Oncol (2021) 39(6_suppl):442–. doi: 10.1200/JCO.2021.39.6_suppl.442
37
Guercio BJ Pietzak EJ Brown S Chen J-F Peters V Regazzi AM et al . Neoadjuvant nivolumab (N) +/- ipilimumab (I) in cisplatin-ineligible patients (Pts) with muscle-invasive bladder cancer (Mibc). J Clin Oncol (2022) 40(6_suppl):498–. doi: 10.1200/JCO.2022.40.6_suppl.498
38
Grivas P Yin J Koshkin VS Cole S Jain RK Dreicer R et al . Pre0807: A phase ib feasibility trial of neoadjuvant nivolumab (N) without or with lirilumab (L) in cisplatin-ineligible patients (Pts) with muscle-invasive bladder cancer (Mibc). J Clin Oncol (2021) 39(15):4518. doi: 10.1200/JCO.2021.39.15_suppl.4518
39
Wei XX McGregor BA Lee RJ Gao X Kilbridge KL Preston MA et al . Durvalumab as neoadjuvant therapy for muscle-invasive bladder cancer: preliminary results from the bladder cancer signal seeking trial (Blasst)-2. J Clin Oncol (2020) 38(6):507. doi: 10.1200/JCO.2020.38.6_suppl.507
40
Martinez Chanza N Carnot A Barthélémy P Casert V Staudacher L Van Den Brande J et al . Avelumab as the basis of neoadjuvant regimen in platinum-eligible and -ineligible patients with nonmetastatic muscle-invasive bladder cancer: aura (Oncodistinct-004) trial. J Clin Oncol (2022) 40(16_suppl):4517–. doi: 10.1200/JCO.2022.40.16_suppl.4517
41
Van Dorp J Suelmann BBM Mehra N Van Montfoort M Van Dijk N Hendricksen K et al . Lba31 high- vs low-dose pre-operative ipilimumab and nivolumab in locoregionally advanced urothelial cancer (Nabucco cohort 2). Ann Oncol (2021) 32:S1305–S6. doi: 10.1016/j.annonc.2021.08.2107
42
Grande E Guerrero F Puente J Galante I Duran I Dominguez M et al . Dutreneo trial: A randomized phase ii trial of durvalumab and tremelimumab versus chemotherapy as a neoadjuvant approach to muscle-invasive urothelial bladder cancer (Mibc) patients (Pts) prospectively selected by an interferon (Inf)-gamma immune signature. J Clin Oncol (2020) 38(15_suppl):5012. doi: 10.1200/JCO.2020.38.15_suppl.5012
43
Gao J Navai N Alhalabi O Siefker-Radtke A Campbell MT Tidwell RS et al . Neoadjuvant pd-L1 plus ctla-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med (2020) 26(12):1845–51. doi: 10.1038/s41591-020-1086-y
44
Xing N Han S Jiang J Xu W Shi B Ping H et al . 703p camrelizumab in combination with gemcitabine plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Ann Oncol (2021) 32:S714. doi: 10.1016/j.annonc.2021.08.099
45
Rose TL Harrison MR Deal AM Ramalingam S Whang YE Brower B et al . Phase ii study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer. J Clin Oncol Off J Am Soc Clin Oncol (2021) 39(28):3140–8. doi: 10.1200/jco.21.01003
46
Lin T Li K Fan J Wang S Yu D Xu T et al . Interim results from a multicenter clinical study of tislelizumab combined with gemcitabine and cisplatin as neoadjuvant therapy for patients with ct2-T4an0m0 mibc. J Clin Oncol (2022) 40(16_suppl):4580. doi: 10.1200/JCO.2022.40.16_suppl.4580
47
Kaimakliotis H Albany C Hoffman-Censits J Trabulsi E Kelly WK Picus J et al . Pd52-03 a multicenter phase 1b/2 study of neoadjuvant pembrolizumab and cisplatin chemotherapy for muscle invasive urothelial cancer. J Urol (2019) 201(Supplement 4):e924–e5. doi: 10.1097/01.JU.0000556959.45525.89
48
Gupta S Gibb E Sonpavde GP Gupta S Maughan BL Agarwal N et al . Biomarker analysis and updated clinical follow-up from blasst-1 (Bladder cancer signal seeking trial) of nivolumab, gemcitabine, and cisplatin in patients with muscle-invasive bladder cancer (Mibc) undergoing cystectomy. J Clin Oncol (2022) 40(6_suppl):528. doi: 10.1200/JCO.2022.40.6_suppl.528
49
Funt SA Lattanzi M Whiting K Al-Ahmadie H Quinlan C Teo MY et al . Neoadjuvant atezolizumab with gemcitabine and cisplatin in patients with muscle-invasive bladder cancer: A multicenter, single-arm, phase ii trial. J Clin Oncol (2022) 40(12):1312–22. doi: 10.1200/jco.21.01485
50
Cathomas R Petrausch U Hayoz S Schneider M Schardt JA Seiler R et al . Perioperative chemoimmunotherapy with durvalumab (Durva) in combination with cisplatin/gemcitabine (Cis/gem) for operable muscle-invasive urothelial carcinoma (Miuc): preplanned interim analysis of a single-arm phase ii trial (Sakk 06/17). J Clin Oncol (2020) 38(6_suppl):499. doi: 10.1200/JCO.2020.38.6_suppl.499
51
Thibault C Elaidi R Vano YA Rouabah M Braychenko E Helali I et al . Open-label phase ii to evaluate the efficacy of neoadjuvant dose-dense mvac in combination with durvalumab and tremelimumab in muscle-invasive urothelial carcinoma: nemio. Bull du Cancer (2020) 107(5s):eS8–eS15. doi: 10.1016/s0007-4551(20)30281-2
52
Kaimakliotis HZ Adra N Kelly WK Trabulsi EJ Lauer RC Picus J et al . Phase ii neoadjuvant (N-) gemcitabine (G) and pembrolizumab (P) for locally advanced urothelial cancer (Lauc): interim results from the cisplatin (C)-ineligible cohort of gu14-188. J Clin Oncol (2020) 38(15_suppl):5019. doi: 10.1200/JCO.2020.38.15_suppl.5019
53
Han S Ji Z Jiang J Fan X Ma Q Hu L et al . Neoadjuvant therapy with camrelizumab plus gemcitabine and cisplatin for patients with muscle-invasive bladder cancer: A multi-center, single-arm, phase 2 study. Cancer Med (2023) 12(11):12106–17. doi: 10.1002/cam4.5900
54
Szabados B Kockx M Assaf ZJ van Dam PJ Rodriguez-Vida A Duran I et al . Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur Urol (2022) 82(2):212–22. doi: 10.1016/j.eururo.2022.04.013
55
Takahashi T . Comments on "Survival after neoadjuvant/induction combination immunotherapy versus combination platinum-based chemotherapy for locally advanced (Stage iii) urothelial cancer". Int J Cancer (2022) 151(10):1847–8. doi: 10.1002/ijc.34206
56
Forde PM Chaft JE Smith KN Anagnostou V Cottrell TR Hellmann MD et al . Neoadjuvant pd-1 blockade in resectable lung cancer. New Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
57
Rozeman EA Blank CU Van Akkooi ACJ Kvistborg P Fanchi L Van Thienen JV et al . Neoadjuvant ipilimumab + Nivolumab (Ipi+Nivo) in palpable stage iii melanoma: updated data from the opacin trial and first immunological analyses. J Clin Oncol (2017) 35(15):9586. doi: 10.1200/JCO.2017.35.15_suppl.9586
58
Forde PM Spicer J Lu S Provencio M Mitsudomi T Awad MM et al . Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
59
Schmid P Park YH Munoz-Couselo E Kim SB Sohn J Im SA et al . Pembrolizumab (Pembro) plus chemotherapy (Chemo) as neoadjuvant treatment for triple negative breast cancer (Tnbc): preliminary results from keynote-173. J Clin Oncol (2017) 35:556. doi: 10.1200/JCO.2017.35.15_suppl.556
60
Broderick SR . Adjuvant and neoadjuvant immunotherapy in non - small cell lung cancer. Thorac Surg Clinics (2020) 30(2):215. doi: 10.1016/j.thorsurg.2020.01.001
61
Owen D Chaft JE . Immunotherapy in surgically resectable non-small cell lung cancer. J Thorac Dis (2018) 10(Suppl 3):S404–s11. doi: 10.21037/jtd.2017.12.93
62
Liu J Blake SJ Yong MCR Harjunpaa H Ngiow SF Takeda K et al . Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discovery (2016) 6(12):1382–99. doi: 10.1158/2159-8290.cd-16-0577
63
Powles T van der Heijden MS Castellano D Galsky MD Loriot Y Petrylak DP et al . Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (Danube): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1574–88. doi: 10.1016/s1470-2045(20)30541-6
64
Iyer G Tully CM Zabor EC Bochner BH Dalbagni G Herr HW et al . Neoadjuvant gemcitabine-cisplatin plus radical cystectomy-pelvic lymph node dissection for muscle-invasive bladder cancer: A 12-year experience. Clin genitourinary Cancer (2020) 18(5):387–94. doi: 10.1016/j.clgc.2020.02.014
65
Rosenberg JE Hoffman-Censits J Powles T van der Heijden MS Balar AV Necchi A et al . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet (London England) (2016) 387(10031):1909–20. doi: 10.1016/s0140-6736(16)00561-4
66
Sharma P Retz M Siefker-Radtke A Baron A Necchi A Bedke J et al . Nivolumab in metastatic urothelial carcinoma after platinum therapy (Checkmate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. doi: 10.1016/s1470-2045(17)30065-7
67
Massard C Gordon MS Sharma S Rafii S Wainberg ZA Luke J et al . Safety and efficacy of durvalumab (Medi4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol (2016) 34(26):3119–25. doi: 10.1200/jco.2016.67.9761
68
Powles T Park SH Voog E Caserta C Valderrama BP Gurney H et al . Maintenance avelumab + Best supportive care (Bsc) versus bsc alone after platinum-based first-line (1l) chemotherapy in advanced urothelial carcinoma (Uc): javelin bladder 100 phase iii interim analysis. J Clin Oncol (2020) 38(18_suppl):LBA1–LBA. doi: 10.1200/JCO.2020.38.18_suppl.LBA1
69
Zargar H Shah JB van Rhijn BW Daneshmand S Bivalacqua TJ Spiess PE et al . Neoadjuvant dose dense mvac versus gemcitabine and cisplatin in patients with ct3-4an0m0 bladder cancer treated with radical cystectomy. J Urol (2018) 199(6):1453–9. doi: 10.1016/j.juro.2017.12.062
70
Peyton CC Tang D Reich RR Azizi M Chipollini J Pow-Sang JM et al . Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol (2018) 4(11):1535–42. doi: 10.1001/jamaoncol.2018.3542
71
Van Der Heijden MS Gupta S Galsky MD Derleth CL Lee S Kataria RS et al . Study ev-302: A two-arm, open-label, randomized controlled phase 3 study of enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated advanced urothelial carcinoma (Auc) (Trial in progress). J Clin Oncol (2022) 40(6_suppl):TPS589–TPS. doi: 10.1200/JCO.2022.40.6_suppl.TPS589
72
Hoimes CJ Bedke J Loriot Y Nishiyama H Fang X Kataria RS et al . Keynote-B15/ev-304: randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab versus chemotherapy in cisplatin-eligible patients with muscle-invasive bladder cancer (Mibc). J Clin Oncol (2021) 39(15_suppl):TPS4587–TPS. doi: 10.1200/JCO.2021.39.15_suppl.TPS4587
73
Eriksson V Holmlund J Wiberg E Johansson M Huge Y Alamdari F et al . Adverse events during neoadjuvant chemotherapy for muscle invasive bladder cancer-a Swedish retrospective multicentre study of a clinical database. Trans Androl Urol (2022) 11(8):1105–15. doi: 10.21037/tau-22-78
74
Frenel JS Le Tourneau C O'Neil B Ott PA Piha-Paul SA Gomez-Roca C et al . Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase ib keynote-028 trial. J Clin Oncol (2017) 35(36):4035–41. doi: 10.1200/jco.2017.74.5471
75
Necchi A Joseph RW Loriot Y Hoffman-Censits J Perez-Gracia JL Petrylak DP et al . Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: post-progression outcomes from the phase ii imvigor210 study. Ann Oncol (2017) 28(12):3044–50. doi: 10.1093/annonc/mdx518
76
Grunewald CM Niegisch G Albers P . Using circulating tumor DNA to guide adjuvant therapy in bladder cancer: imvigor010 and imvigor011. Eur Urol Focus (2022) 8(3):646–7. doi: 10.1016/j.euf.2022.04.001
77
Bellmunt J Hussain M Gschwend JE Albers P Oudard S Castellano D et al . Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (Imvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(4):525–37. doi: 10.1016/s1470-2045(21)00004-8
Summary
Keywords
PD-1 inhibitor, programmed cell death protein 1 inhibitor, programmed death-ligand 1 inhibitor, muscle invasive bladder cancer, neoadjuvant, complication
Citation
Huang S, Huang Y, Li C, Liang Y, Huang M, Luo R and Liang W (2024) Efficacy and safety of neoadjuvant PD-1 inhibitors or PD-L1 inhibitors for muscle invasive bladder cancer: a systematic review and meta-analysis. Front. Immunol. 14:1332213. doi: 10.3389/fimmu.2023.1332213
Received
02 November 2023
Accepted
18 December 2023
Published
09 January 2024
Volume
14 - 2023
Edited by
Jesse Haramati, University of Guadalajara, Mexico
Reviewed by
Karim Amrane, Morlaix Hospital, Morlaix, France
Bedeir Ali-El-Dein, Mansoura University, Egypt
Xiaobin Gu, First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2024 Huang, Huang, Li, Liang, Huang, Luo and Liang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Liang, Liangwm22@icloud.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.