- 1Experimental Teaching Management Center, Chongqing Medical University, Chongqing, China
- 2Department of Rheumatology and Immunology, Hainan Hospital of Chinese People's Liberation Army of China (PLA) General Hospital, Sanya, Hainan, China
- 3Department of Cardiovascular, Hainan Hospital of Chinese People’s Liberation Army of China (PLA) General Hospital, Sanya, Hainan, China
Background: Despite establishing an association between gut microbiota and spondyloarthritis (SpA) subtypes, the causal relationship between them remains unclear.
Methods: Gut microbiota data were obtained from the MiBioGen collaboration, and SpA genome-wide association study (GWAS) summary data were obtained from the FinnGen collaboration. We conducted a two-sample Mendelian randomization (MR) analysis using the inverse-variance-weighted method supplemented with four additional MR methods (MR-Egger, weighted median, simple mode, and weighted mode). Pleiotropy and heterogeneity were also assessed. Reverse MR analysis was used to detect reverse causal relationships.
Results: We identified 23 causal links between specific gut microbiota taxa and SpA levels. Of these, 22 displayed nominal causal associations, and only one demonstrated a robust causal connection. Actinobacteria id.419 increased the risk of ankylosing spondylitis (AS) (odds ratio (OR) = 1.86 (95% confidence interval (CI): 1.29–2.69); p = 8.63E−04). The family Rikenellaceae id.967 was associated with a reduced risk of both AS (OR = 0.66 (95% CI: 0.47–0.93); p = 1.81E−02) and psoriatic arthritis (OR = 0.70 (95% CI: 0.50–0.97); p = 3.00E−02). Bacillales id.1674 increased the risk of AS (OR = 1.23 (95% CI: 1.00–1.51); p = 4.94E−02) and decreased the risk of enteropathic arthritis (OR = 0.56 (95% CI: 0.35–0.88); p = 1.14E−02). Directional pleiotropy, or heterogeneity, was not observed. No reverse causal associations were observed between the diseases and the gut microbiota.
Conclusion: Our MR analysis suggested a genetic-level causal relationship between specific gut microbiota and SpA, providing insights into the underlying mechanisms behind SpA development mediated by gut microbiota.
1 Introduction
Spondyloarthritis (SpA) is a group of rheumatic diseases characterized primarily by sacroiliitis and accompanied by musculoskeletal and extra-articular manifestations. It encompasses several subtypes, including ankylosing spondylitis (AS), enteropathic arthritis (EA), psoriatic arthritis (PsA), reactive arthritis, undifferentiated SpA, and juvenile SpA (1).
The aetiology and pathogenesis of SpA remain unknown, with substantial evidence indicating a connection between intestinal and articular inflammation (2–4). The investigation of the gut-joint axis has become a significant domain in unraveling the pathogenesis of SpA (5). However, studies investigating gut microbiota dysbiosis in patients with SpA have produced inconsistent results, and the key bacteria identified vary between studies (6–15). Previous studies have failed to elucidate the causal association between gut microbiota and the development of SpA (16, 17), necessitating further investigation.
Mendelian randomization (MR) is a method utilized to infer causal associations between instrumental variables, typically single-nucleotide polymorphisms (SNPs) (18). By utilizing natural genetic variations associated with a specific exposure factor, MR simulates the effects of randomized controlled trials and infers the causal impact of the exposure factor on a specific outcome (19). SNPs are randomly assigned prior to the occurrence of disease, thereby enabling the elimination of potential confounding factors and reverse causality influences (20). A recent study using MR examined the potential causal association between six bacteria and AS, but no significant causal associations were found (21). However, considering the growing evidence supporting the significant role of the gut microbiota in the development of SpA, there is still no consensus regarding the specific taxonomic group of gut microbiota that has the greatest impact on SpA. Additionally, it remains unclear whether there is a mutual influence of gut bacteria among different subtypes of SpA, highlighting the need for further research to clarify this aspect.
Our research focuses on investigating the causal association between the gut microbiota and three types of SpA: AS, PsA, and EA. Another objective is to identify shared bacteria among these three diseases. This approach identifies gut bacteria that have a causal relationship with SpA, providing new avenues for future prevention and treatment of SpA.
2 Methods
2.1 Study design
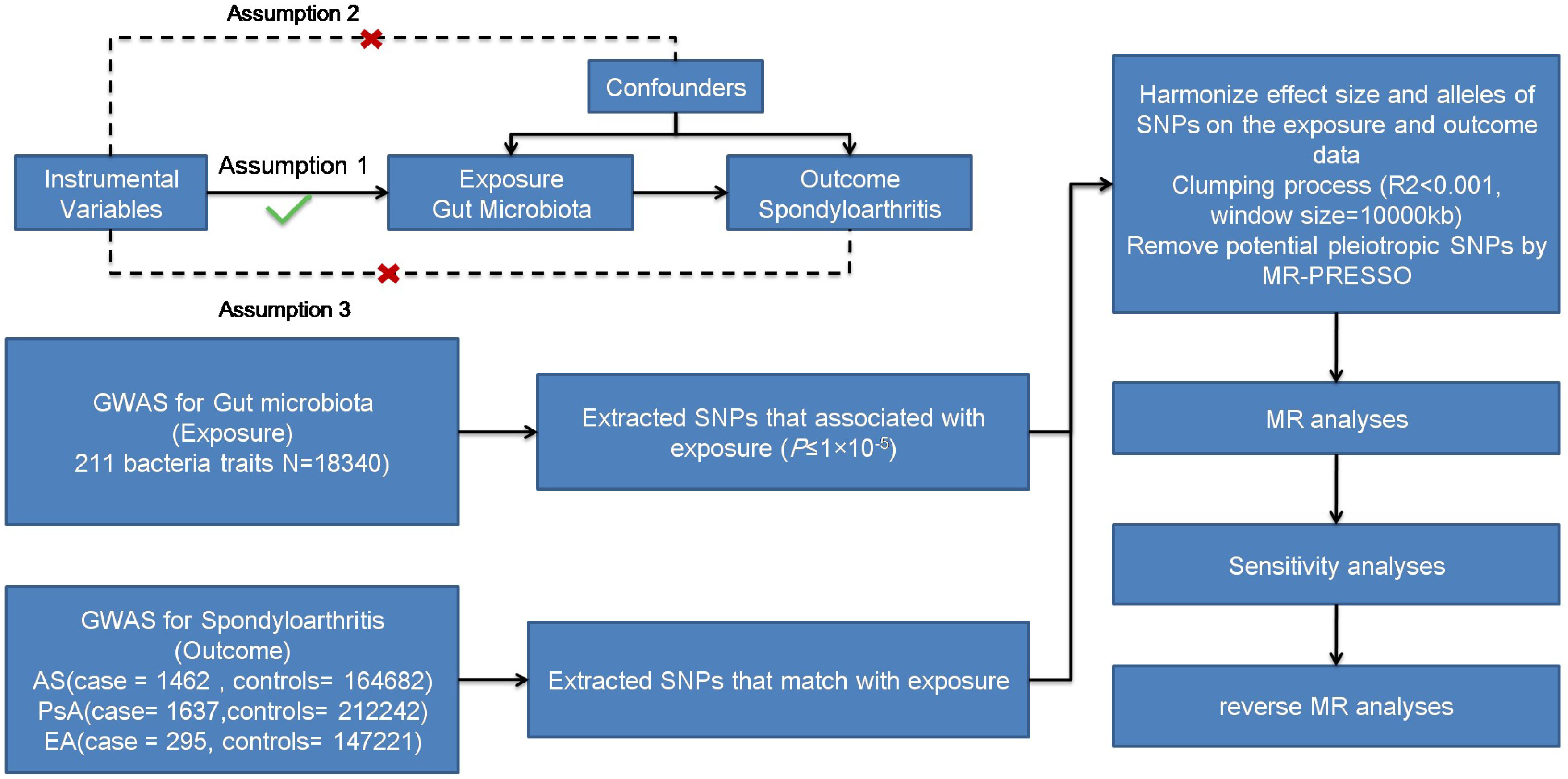
We aimed to examine the causal association between gut microbiota and different subtypes of SpA (AS, PsA, and EA) using a two-sample MR approach. Figure 1 illustrates the schematic diagram of the study design. MR studies must adhere to three key assumptions for reliable and valid results. Firstly, the first assumption is that genetic variants are used as instrumental variables for the exposure of interest. These instrumental variables should be strongly associated with the exposure but not directly associated with confounding factors or the outcome. Secondly, the instrumental variables should be independent of confounding factors that could bias the causal relationship. Lastly, the outcome variable should be solely associated with the exposure factor, without direct influence from other variables.
2.2 Data sources
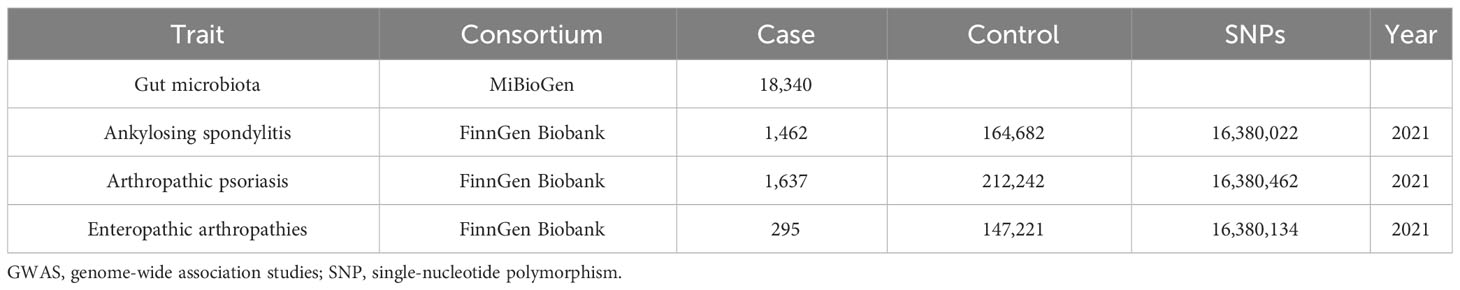
Genome-wide association study (GWAS) data concerning human gut microbiota, which served as the exposure variable, were obtained from the MiBioGen study. This study involved a total of 18,340 individuals from 24 cohorts and recorded 211 taxonomic units (131 genera, 35 families, 20 orders, 16 phyla, and nine classes), as well as 122,110 SNPs (22). GWAS summary data for the different subtypes of SpA (AS, PsA, and EA) were obtained from the FinnGen Biobank. Patients with AS (21), PsA (23), and EA were selected using ICD-10 diagnostic codes. Table 1 provides detailed data. Ethical approval is not required for this study as all summary-level data have already obtained ethical approval in previous GWAS studies.
2.3 Genetic instruments selection
To ensure robustness and accuracy while adhering to the assumptions necessary for MR analysis, a quality check was performed on the SNPs to obtain valid instruments (Figure 1). Due to the strict threshold (p < 5.0 × 10−8), only a few instrumental variables were obtained. To increase the number of instrumental variables, a threshold of p < 1.0 ×10−5 was adopted (24, 25). Palindromic SNPs and SNPs not present in the outcomes (AS, PsA, and EA) were excluded from the instrumental variables. We utilized the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/phenoscanner, accessed on 7 October 2022) to exclude confounding factors reported in the literature, including body mass index (BMI) and smoking (26). Linkage disequilibrium was eliminated using a threshold of r2 < 0.001 and a clumping distance of 10,000 kb. The basic formula for the F-statistic in MR analysis is: F = (r2/(1−r2)) * ((N−k−1)/k), where r2 is the proportion of variance in the exposure variable explained by the instrumental variables, N is the sample size, and k is the number of instrumental variables used in the analysis. The F-statistic was calculated for all the instrumental variables, with a value of > 10 considered sufficient and unbiased (27, 28).
2.4 MR analysis
MR analysis employed five methods, including inverse variance weighting (IVW), MR-Egger, weighted median, simple mode, and weighted mode, to infer causal relationships (29–31). IVW was the primary method, with the other four serving as supplementary approaches. Estimates were presented using odds ratios (ORs) along with their respective 95% confidence intervals (CIs). The study also investigated reverse causality, with SpA considered the exposure and gut microbiota the outcome. The procedures for the reverse and regular MR analyses were identical.
2.5 Sensitivity analysis
Heterogeneity in IVW and MR Egger was detected by calculating Cochran’s Q statistic and its corresponding p-value. The evaluation of pleiotropy, a potential source of heterogeneity, was performed using the MR-Egger intercept test. The leave-one-out analysis method was utilized to identify and eliminate potential outliers.
2.6 Statistical analysis
To ensure a more rigorous interpretation of the causal relationship between bacterial species and health outcomes, the Bonferroni correction was applied to address the issue of increased false-positive results when conducting multiple hypothesis tests for different levels of bacterial classification. In our study, since statistical tests were conducted for 131 genera, 35 families, 20 orders, 16 classes, and nine phyla, the significance level was adjusted to 0.05/131 (3.8 × 10−4) for genera, 0.05/35 (1.4 × 10−3) for families, 0.05/20 (2.5 × 10−3) for orders, 0.05/16 (3.1 × 10−3) for classes, and 0.05/9 (5.5 × 10−3) for phyla after applying the Bonferroni correction (32). MR results with p-values lower than the adjusted significance level were considered statistically significant, while MR estimates with p-values less than 0.05 were considered nominally significant (31). All analyses were conducted using the TwoSample MR package (version 0.5.6) in R 4.0.2 (31, 33).
3 Results
3.1 Selection of instrumental variables
Overall, 1,784 unique SNPs were identified as the final instrumental variables, which were associated with exposure at p-values < 1E−5 (Supplementary Table S1). They were classified into five different levels of bacterial classification. There were 54 instrumental variables in phyla, 181 instrumental variables in classes, 14 instrumental variables in orders, 313 instrumental variables in families, and 1,222 instrumental variables in genera. The F-statistics range from 16.91 to 58.15, and all the F-values are greater than 10.
3.2 Mendelian randomization analysis
Using the IVW method, we discovered significant associations between the nine microbial taxa and AS. The results indicate that the class Actinobacteria id.419, order Bacillales id.1674, genus Enterorhabdus id.820, and genus Ruminococcaceae NK4A214 group id.11358 may increase the risk of AS. By contrast, the families Lactobacillaceae id.1836 and Rikenellaceae id.967, and the genera Anaerotruncus id.2054, Howardella id.2000, and Oscillibacter id.2063 were negatively associated with AS (Figure 2). Supplementary Table S2 displays the IVW results as well as the results using the four complementary methods (MR-Egger, weighted median, simple mode, and weighted mode). After using Bonferroni correction, the Actinobacteria id.419 class (OR = 1.86 (95% CI: 1.29–2.69); p = 8.63E−04) remained a risk factor for AS. In the reverse MR analysis, no causal association was found between AS exposure variable and gut microbiota as an outcome (Supplementary Table S3).
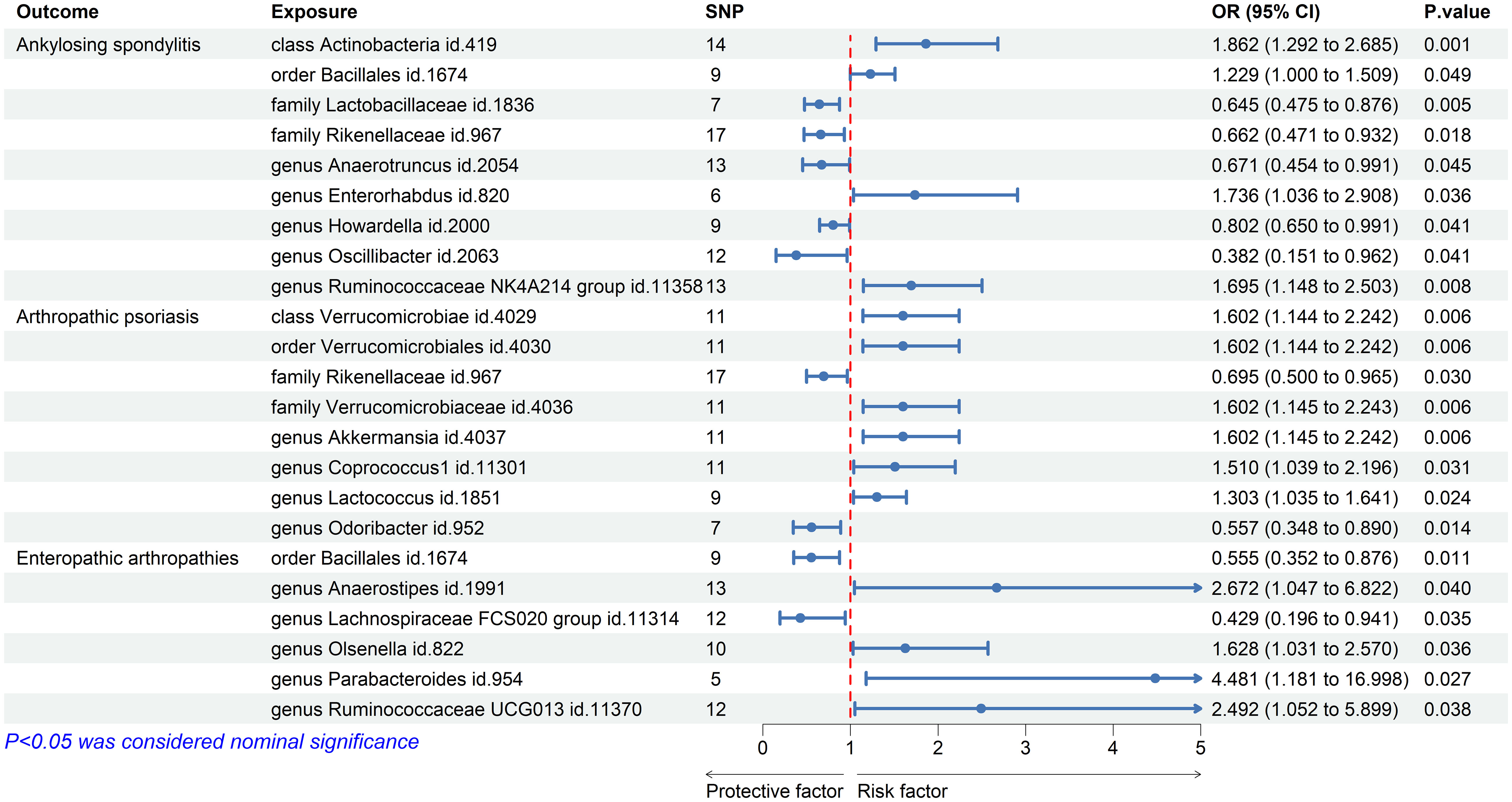
Figure 2 Forest plot of gut microbiota taxa associated with spondyloarthritis by inverse variance weighting.
The results of IVW showed that the class Verrucomicrobia id.4029, the order Verrucomicrobiales id.4030, the family Verrucomicrobiaceae id.4036, and the genera Akkermansia id.4037, Coprococcus1 id.11301, and Lactococcus id.1851 were associated with an increased risk of PsA, whereas the family Rikenellaceae id.967 and the genus Odoribacter id.952 were associated with a decreased risk of PsA (Figure 2; Supplementary Table S2). However, after Bonferroni correction, these microbial taxa did not show any significant causal effect on PsA. In the reverse MR analysis, no causal association was found between PsA as an exposure variable and gut microbiota as an outcome (Supplementary Table S3).
We also used the IVW method to evaluate the causal associations between gut microbiota and EA. The results indicated that the genera Anaerostipes id.1991, Olsenella id.822, Parabacteroides id.954, and Ruminococcaceae UCG013 id.11370 were associated with an increased risk of EA, whereas the order Bacillales id.1674 and the genus Lachnospiraceae FCS020 group id.11314 were associated with a decreased risk (Figure 2; Supplementary Table S2). However, after Bonferroni correction, these microbial taxa did not show significant causal effects on EA. In the reverse MR analysis, the genera Parabacteroides id.954 and Lachnospiraceae FCS020 showed nominal reverse causal relationships with EA; however, these associations disappeared after Bonferroni correction.
The MR results are also presented as scatterplots, and the causal associations of gut microbiota on the risks of AS (Supplementary Figure S1), PsA (Supplementary Figure S2), and EA (Supplementary Figure S3) are illustrated. Supplementary Figures S4-S6 indicate the forest plot results for the bacteria in terms of AS, PsA, and EA, respectively.
The causal associations between the gut microbiota taxa identified in our study and the risks of AS, PsA, and EA were visualized using a heatmap (Figure 3). The Rikenellaceae id.967 family showed a reduced risk of both AS (OR = 0.66 (95% CI: 0.47–0.93); p = 1.81E−02) and PsA (OR = 0.70 (95% CI: 0.50–0.97); p = 3.00E−02), while the order Bacillales id.1674 increased the risk of AS (OR = 1.23 (95% CI: 1.00–1.51); p = 4.94E−02) and decreased the risk of EA (OR = 0.56 (95% CI: 0.35–0.88); p = 1.14E−02).
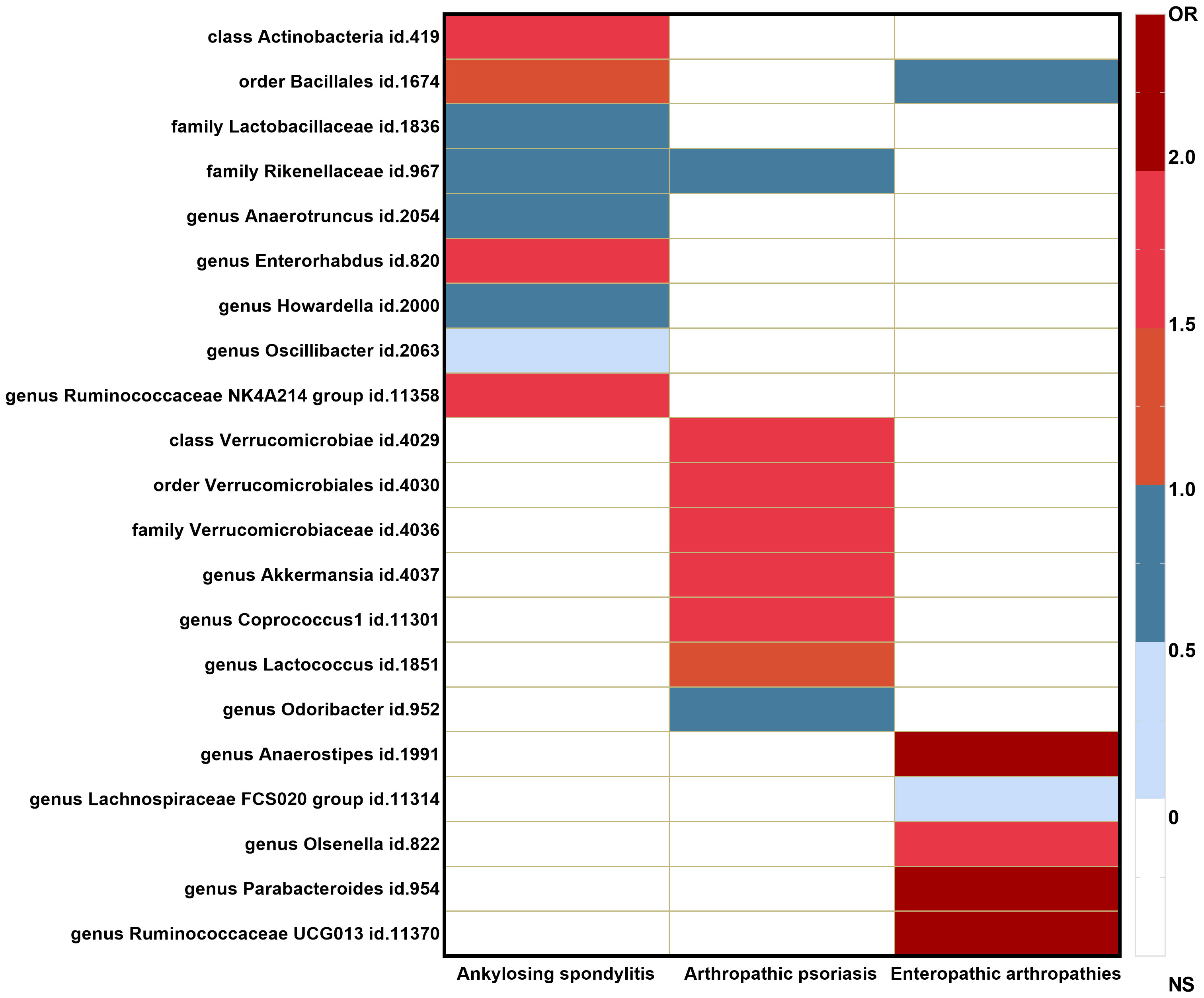
Figure 3 Heatmap of GM taxa causally associated with ankylosing spondylitis, psoriatic arthritis, and enteropathic arthritis.
3.3 Sensitivity analysis
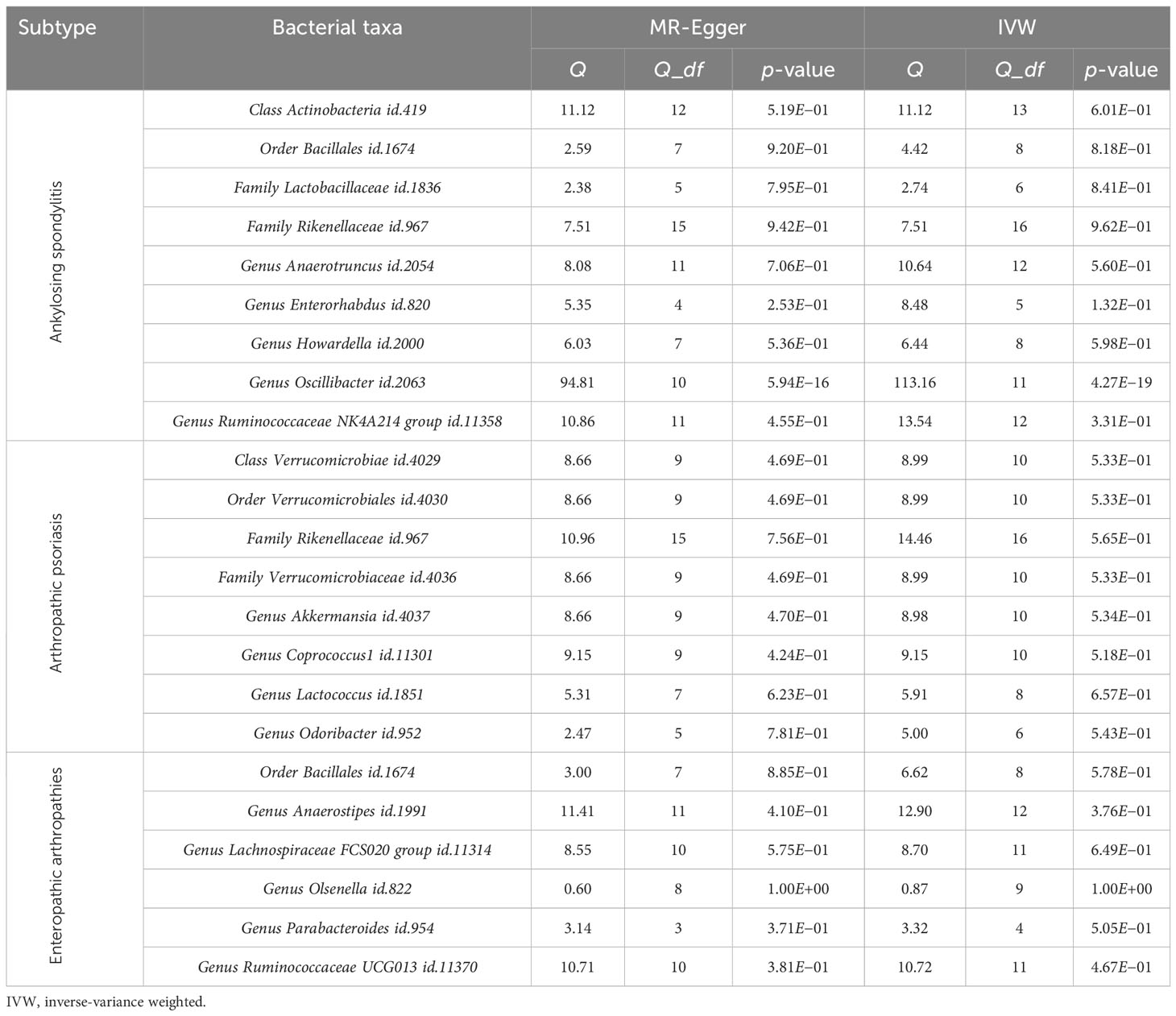
Cochran’s Q test was used to assess heterogeneity, and MR-Egger and IVW tests were conducted as well. Except for the genus Oscillibacter id.2063, which showed heterogeneity in the MR analysis, all other p-values were greater than 0.05 (Table 2).
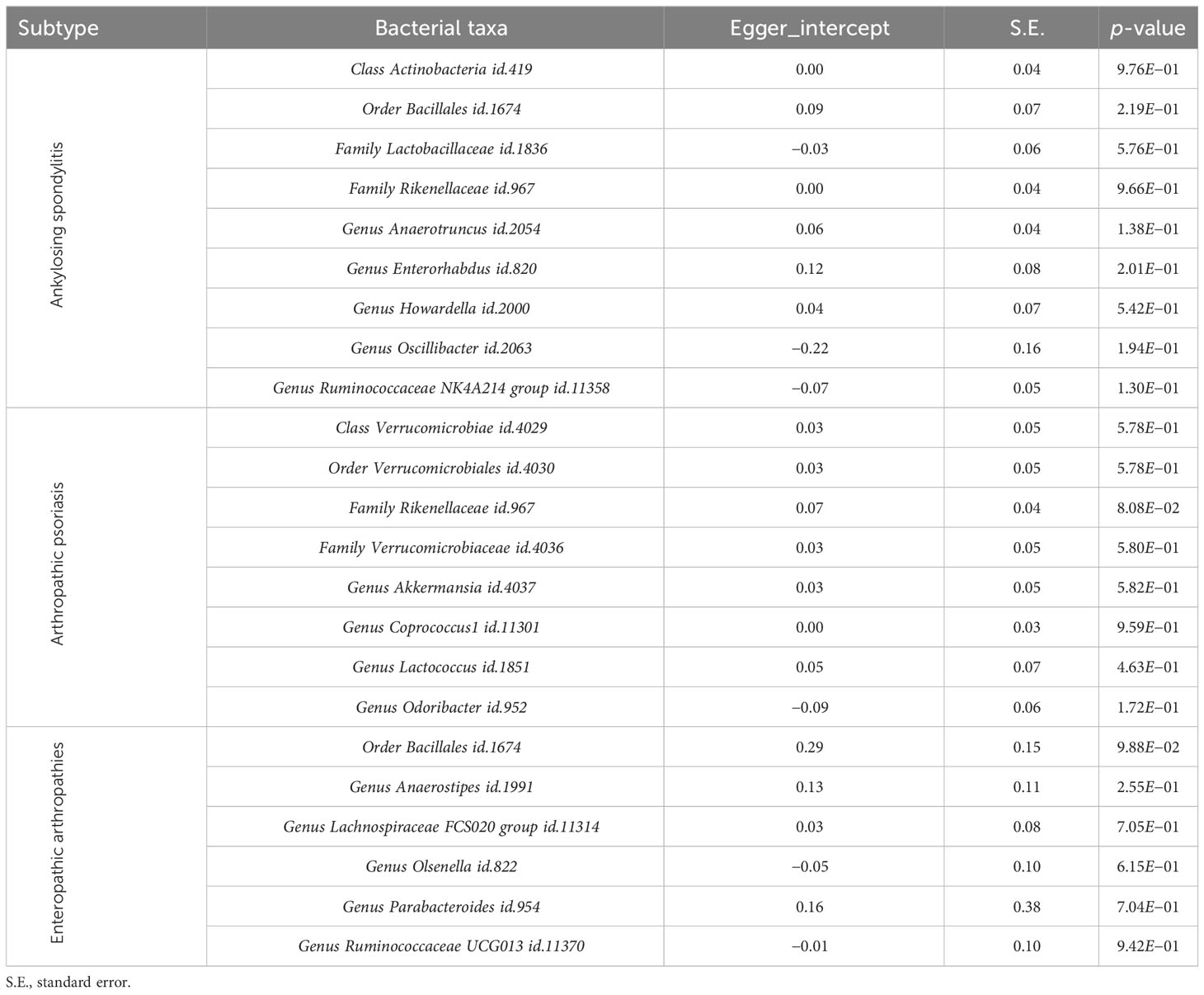
The Egger intercept was used to evaluate the horizontal pleiotropy, and the results showed no evidence of horizontal pleiotropy (Table 3). The leave-one-out results of the above bacteria for AS, PsA, and EA are shown in Supplementary Figures S7-S9, respectively.
4 Discussion
Our study is the first to identify the causal effects of gut microbiota on various subtypes of SpA. Actinobacteria id.419 increased the risk of AS, while Rikenellaceae id.967 protected against both AS and PsA. Bacillales id.1674 increased the risk of AS but decreased the risk of EA. These findings shed light on the potential role of the gut microbiota in influencing different SpA subtypes.
Our study revealed a robust causal relationship wherein the Actinobacteria id.419 class remained significantly associated with an increased risk of AS, even after adjusting for multiple comparisons using Bonferroni correction. Patients with AS have a higher abundance of Actinobacteria in their gut compared to healthy individuals (10, 34). Another study proposed that Actinobacteria may regulate the ubiquitination of IκB-α, activate the NF-kB signaling pathway, and promote the accumulation of inflammatory factors, thereby contributing to the progression of AS (35). Our study further reveals a causal relationship between Actinobacteria and the onset of AS, suggesting that Actinobacteria may be implicated as a potential cause of AS.
In our investigation of the causal relationship between various SpA subtypes and gut bacteria, we discovered that the Rikenellaceae id.967 family exhibited a potential protective effect against AS and PsA, albeit with nominal significance. Notably, previous studies have reported an elevated abundance of Rikenellaceae in terminal ileum biopsy samples from patients with AS (36), as well as a positive correlation between psoriasis-like pathological features and Rikenellaceae (37). Based on these findings, we hypothesize that the increased presence of Rikenellaceae may offer some degree of protection against disease pathogenesis but may also coincide with heightened disease activity. Interestingly, the order Bacillales id.1674 was associated with a reduced risk of EA. This finding aligns with suggestions that Bacillus species can alleviate enterococcal spondylitis (“kinky back”) in poultry (38). However, we observed an increased risk of AS associated with the order Bacillales id.1674, suggesting that the same bacterium may exert opposing effects on different subtypes of SpA. This intriguing discrepancy warrants further investigation to better understand the complex interplay between Bacillales id.1674 and different SpA subtypes.
The potential link between SpA and gut microbiota has garnered increasing research attention in recent years (39–41). For instance, approximately 7% of patients with AS also have inflammatory bowel disease (IBD), while 10–50% of patients with IBD eventually develop SpA (42). HLA-B27 transgenic rats reared under germ-free conditions do not exhibit arthritic symptoms (43) until they are colonized by bacteria (44). Three hypotheses have been proposed to explain this, including the “arthritogenic peptide theory”, the “migration of mucosal cells to the joints theory”, and the “gut bacteria-driven theory” (45). Analyzing the correlation between SNPs in gut bacteria and susceptibility genes for SpA, such as HLA-B27 and IL-23/IL-17 pathway genes, is indeed a meaningful and valuable area of study (46). Several meta-analyses suggest that susceptibility genes, such as TNF-α polymorphisms (rs769178) (47), the SNPs (rs11209026, rs1004819, rs10489629, rs11465804, rs1343151, rs11209032, rs1495965, rs7517847, and rs2201841) of IL-23R (48), and the SNPs (rs27044, rs10050860, rs2287987, rs17482078, rs26653, rs30187, rs27037, rs27980, and rs27582) of endoplasmic reticulum aminopeptidase 1 (49), could influence the susceptibility to ankylosing spondylitis in the total population. In our study, it should be emphasized that the selected SNPs used in the MR analysis did not include the susceptibility gene SNPs mentioned in the aforementioned literature. Because our study did not specifically investigate the correlation between the selected SNPs and the susceptibility genes mentioned, we were unable to provide an exhaustive analysis of all the susceptibility genes involved in this matter. Further in-depth research is required to explore this relationship in future studies.
Our study represents the first attempt to establish a causal association between gut microbiota and SpA, including its subtypes, thus offering potential candidate bacteria for future functional investigations. Nevertheless, our study has several limitations. First, due to the stringent threshold (p < 5.0 × 10−8) required for instrumental variables, we adopted a relatively lenient threshold (p < 1.0 × 10−5) to screen for instrumental variables. This may have introduced some potential bias. Second, the study population is predominantly of European descent, and therefore the findings cannot be generalized to other ethnicities. Third, the EA subtype of SpA had a relatively small number of cases, based on its strict definition criteria. Consequently, future analyses using GWAS summary data from larger sample sizes are required to enhance confidence in our results. Fourth, current research on gut microbiota mainly focuses on bacteria; however, other types of gut microorganisms may also contribute significantly. In our study, we employed the MR-Egger intercept to detect pleiotropy. While these methods enhance the reliability of causal relationships in MR analysis, they do not conclusively rule out the presence of pleiotropy, as mentioned in our manuscript. Therefore, we recognize the significance of accounting for other biases and limitations in MR analysis and interpreting the findings with caution.
5 Conclusion
Through MR analysis, we conducted a comprehensive investigation of the causal association of 211 gut microbiota taxa on SpA. We ultimately identified 23 causal effects, including 22 nominal and one strong causal relationship. Specifically, the class Actinobacteria id.419 showed a significant association with an increased risk of AS. Our findings provide potential biomarkers and therapeutic targets for the progression of SpA.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. Individual-level data was obtained from the FinnGen biobank (https://finngen.gitbook.io/documentation).
Ethics statement
The requirement of ethical approval was waived by The ethics committee of the Hainan Branch of People’s Liberation Army General Hospital for the studies on humans because Ethical approval is not required for this study, as all summary-level data has already obtained ethical approval in previous GWAS studies. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. The human samples used in this study were acquired from gifted from another research group.
Author contributions
JT: Writing – original draft, Data curation, Funding acquisition, Resources. SM: Data curation, Writing – original draft. LF: Data curation, Writing – review & editing. SF: Writing – review & editing. XL: Writing – review & editing, Formal Analysis, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are highly grateful to the participants for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1284466/full#supplementary-material
References
1. Alamanos Y, Pelechas E, Voulgari PV, Drosos AA. Incidence of spondyloarthritis and its subtypes: a systematic review. Clin Exp Rheumatol (2021) 39:660–7. doi: 10.55563/clinexprheumatol/yycy0o
2. Sternes PR, Brett L, Phipps J, Ciccia F, Kenna T, Guzman E, et al. Distinctive gut microbiomes of ankylosing spondylitis and inflammatory bowel disease patients suggest differing roles in pathogenesis and correlate with disease activity. Arthritis Res Ther (2022) 24(1):163. doi: 10.1186/s13075-022-02853-3
3. Qaiyum Z, Lim M, Inman RD. The gut-joint axis in spondyloarthritis: immunological, microbial, and clinical insights. Semin Immunopathol (2021) 43:173–92. doi: 10.1007/s00281-021-00845-0
4. Ashrafi M, Kuhn KA, Weisman MH. The arthritis connection to inflammatory bowel disease (IBD): why has it taken so long to understand it? RMD Open (2021) 7(1):e001558. doi: 10.1136/rmdopen-2020-001558
5. Gracey E, Dumas E, Yerushalmi M, Qaiyum Z, Inman RD, Elewaut D. The ties that bind: skin, gut and spondyloarthritis. Curr Opin Rheumatol (2019) 31:62–9. doi: 10.1097/BOR.0000000000000569
6. Yin J, Sternes PR, Wang M, Song J, Morrison M, Li T, et al. Shotgun metagenomics reveals an enrichment of potentially cross-reactive bacterial epitopes in ankylosing spondylitis patients, as well as the effects of TNFi therapy upon microbiome composition. Ann Rheum Dis (2020) 79:132–40. doi: 10.1136/annrheumdis-2019-215763
7. Zhang L, Han R, Zhang X, Fang G, Chen J, Li J, et al. Fecal microbiota in patients with ankylosing spondylitis: Correlation with dietary factors and disease activity. Clin Chim Acta (2019) 497:189–96. doi: 10.1016/j.cca.2019.07.038
8. Huang R, Li F, Zhou Y, Zeng Z, He X, Fang L, et al. Metagenome-wide association study of the alterations in the intestinal microbiome composition of ankylosing spondylitis patients and the effect of traditional and herbal treatment. J Med Microbiol (2020) 69:797–805. doi: 10.1099/jmm.0.001107
9. Zhou C, Zhao H, Xiao X-Y, Chen B, Guo R-J, Wang Q, et al. Metagenomic profiling of the pro-inflammatory gut microbiota in ankylosing spondylitis. J Autoimmun (2020) 107:102360. doi: 10.1016/j.jaut.2019.102360
10. Klingbergg E, Magnusson MK, Strid H, A Deminger, Ståhl A, Sundin J, Simrén M, et al. A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin. Arthritis Res Ther (2019) 21(1):248. doi: 10.1186/s13075-019-2018-4
11. Cardoneanu A, Cozma S, Rezus C, Petrariu F, Burlui AM, Rezus E. Characteristics of the intestinal microbiome in ankylosing spondylitis. Exp Ther Med (2021) 22(1):676. doi: 10.3892/etm.2021.10108
12. Breban M, Tap J, Leboime A, Said-Nahal R, Langella P, Chiocchia G, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis (2017) 76:1614–22. doi: 10.1136/annrheumdis-2016-211064
13. Regner EH, Ohri N, Stahly A, Gerich ME, Fennimore BP, Ir D, et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res Ther (2018) 20(1):149. doi: 10.1186/s13075-018-1639-3
14. Di Paola M, Cavalieri D, Albanese D, Sordo M, Pindo M, Donati C, et al. Alteration of fecal microbiota profiles in juvenile idiopathic arthritis. Associations with HLA-B27 allele and disease status. Front Microbiol (2016) 7:1703. doi: 10.3389/fmicb.2016.01703
15. Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol (2015) 67:128–39. doi: 10.1002/art.38892
16. Breban M, Beaufrère M, Glatigny S. Intestinal dysbiosis in spondyloarthritis - chicken or egg? Curr Opin Rheumatol (2021) 33:341–7. doi: 10.1097/BOR.0000000000000800
17. Mauro D, Ciccia F. Gut dysbiosis in Spondyloarthritis: Cause or effect? Best Pract Res Clin Rheumatol (2019) 33:101493. doi: 10.1016/j.berh.2020.101493
18. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA (2017) 318:1925–26. doi: 10.1001/jama.2017.17219
19. Swanson SA, Tiemeier H, Ikram MA, Hernán MA. Nature as a trialist?: deconstructing the analogy between Mendelian randomization and randomized trials. Epidemiology (2017) 28:653–59. doi: 10.1097/EDE.0000000000000699
20. Smith GD, Ebrahim. S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32:1–22. doi: 10.1093/ije/dyg070
21. Yang M, Wan X, Zheng H, Xu K, Xie J, Yu H, et al. No evidence of a genetic causal relationship between ankylosing spondylitis and gut microbiota: A two-sample Mendelian randomization study. Nutrients (2023) 15:1057. doi: 10.3390/nu15041057
22. Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet (2021) 53:156–65. doi: 10.1038/s41588-020-00763-1
23. Cao Z, Li Q, Li Y, Wu J. Causal association of leisure sedentary behavior with arthritis: A Mendelian randomization analysis. Semin Arthritis Rheum (2023) 59:152171. doi: 10.1016/j.semarthrit.2023.152171
24. Lv WQ, Lin X, Shen H, Liu HM, Qiu X, Li BY, et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J Cachexia Sarcopenia Muscle (2021) 12:1860–70. doi: 10.1002/jcsm.12788
25. Su M, Tang Y, Kong W, Zhang S, Zhu T. Genetically supported causality between gut microbiota, gut metabolites and low back pain: a two-sample Mendelian randomization study. Front Microbiol (2023) 14:1157451. doi: 10.3389/fmicb.2023.1157451
26. Farran Y, Reveille J, Hwang M. Environmental risks for spondyloarthropathies. Rheum Dis Clin North Am (2022) 48:813–26. doi: 10.1016/j.rdc.2022.06.004
27. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet (2018) 27:3641–49. doi: 10.1093/hmg/ddy271
28. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol (2011) 40:740–52. doi: 10.1093/ije/dyq151
29. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37:658–65. doi: 10.1002/gepi.21758
30. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol (2016) 40:597–608. doi: 10.1002/gepi.21998
31. Luo M, Cai J, Luo S, Hong X, Xu L, Lin H, et al. Causal effects of gut microbiota on the risk of chronic kidney disease: a Mendelian randomization study. Front Cell Infect Microbiol (2023) 13:1142140. doi: 10.3389/fcimb.2023.1142140
32. Li N, Wang Y, Wei P, Min Y, Yu M, Zhou G, et al. Causal effects of specific gut microbiota on chronic kidney diseases and renal function—A two-sample Mendelian randomization study. Nutrients (2023) 15:360. doi: 10.3390/nu15020360
33. Wei Y, Huang L, Liu C, Qi M. Causal relationship between Gut Microbiota and Obstructive sleep apnea. Arch Gerontol Geriatrics (2023) 113:105052. doi: 10.1016/j.archger.2023.105052
34. Wang L, Wang Y, Zhang P, Song C, Pan F, Li G, Peng L, et al. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin Arthritis Rheum (2022) 52:151925. doi: 10.1016/j.semarthrit.2021.11.002
35. Wen C, Zheng Z, Shao T, Liu L, Xie Z, Chatelier EL, et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol (2017) 18:142. doi: 10.1186/s13059-017-1271-6
36. Costello M-E, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, et al. Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol (2015) 67:686–91. doi: 10.1002/art.38967
37. Lu W, Deng Y, Fang Z, Zhai Q, Cui S, Zhao J, et al. Potential role of probiotics in ameliorating psoriasis by modulating gut microbiota in imiquimod-induced psoriasis-like mice. Nutrients (2021) 13:2010. doi: 10.3390/nu13062010
38. Medina Fernández S, Cretenet M, Bernardeau M. In vitro inhibition of avian pathogenic Enterococcus cecorum isolates by probiotic Bacillus strains. Poult Sci (2019) 98:2338–46. doi: 10.3382/ps/pey593
39. Lyu X, Chen J, Gao X, Yang J. Emerging story of gut dysbiosis in spondyloarthropathy: From gastrointestinal inflammation to spondyloarthritis. Front Cell Infect Microbiol (2022) 12:973563. doi: 10.3389/fcimb.2022.973563
40. Gracey E, Vereecke L, McGovern D, Fröhling M, Schett G, Danese S, et al. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol (2020) 16:415–33. doi: 10.1038/s41584-020-0454-9
41. Wendling D, Guillot X, Prati C, Miceli-Richard C, Molto A, Lories R, et al. Effect of gut involvement in patients with high probability of early spondyloarthritis: data from the DESIR cohort. J Rheumatol (2020) 47:349–53. doi: 10.3899/jrheum.181326
42. Gill T, Asquith M, Brooks SR, Rosenbaum JT, Colbert RA. Effects of HLA-B27 on gut microbiota in experimental spondyloarthritis implicate an ecological model of dysbiosis. Arthritis Rheumatol (2018) 70:555–65. doi: 10.1002/art.40405
43. Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernández-Sueiro JL, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med (1994) 180:2359–64. doi: 10.1084/jem.180.6.2359
44. Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest (1996) 98:945–53. doi: 10.1172/JCI118878
45. Speca S, Dubuquoy L. Chronic bowel inflammation and inflammatory joint disease: Pathophysiology. Joint Bone Spine (2017) 84:417–20. doi: 10.1016/j.jbspin.2016.12.016
46. Díaz-Peña R, Castro-Santos P, Durán J, Santiago C, Lucia A. The genetics of spondyloarthritis. J Pers Med (2020) 10:151. doi: 10.3390/jpm10040151
47. Hu N, Chen X, Wang S, Yuan G, Wang Q, Shu H, et al. The association of polymorphisms in TNF and ankylosing spondylitis in common population: a meta-analysis. Eur Spine J (2021) 30:1402–10. doi: 10.1007/s00586-021-06845-w
48. Xia Y, Liang Y, Guo S, Yu J-G, Tang M-S, Xu P-H, et al. Association between cytokine gene polymorphisms and ankylosing spondylitis susceptibility: a systematic review and meta-analysis. Postgrad Med J (2018) 94:508–16. doi: 10.1136/postgradmedj-2018-135665
Keywords: spondyloarthritis, gut microbiota, Mendelian randomization, causal effect, genome wide association study
Citation: Tang J, Mo S, Fan L, Fu S and Liu X (2024) Causal association of gut microbiota on spondyloarthritis and its subtypes: a Mendelian randomization analysis. Front. Immunol. 15:1284466. doi: 10.3389/fimmu.2024.1284466
Received: 28 August 2023; Accepted: 18 January 2024;
Published: 08 February 2024.
Edited by:
Matthew Stoll, University of Alabama at Birmingham, United StatesReviewed by:
Merry-Lynn McDonald, University of Alabama at Birmingham, United StatesHemant K Tiwari, University of Alabama at Birmingham, United States
Copyright © 2024 Tang, Mo, Fan, Fu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihui Fu, eGlhb3hpYW8wOTE1QDEyNi5jb20=; Xiaofei Liu, eGlhb2ZlaWxpdTE5ODZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jun Tang1†
Jun Tang1† Shihui Fu
Shihui Fu Xiaofei Liu
Xiaofei Liu