- 1Control of Innate Immunity, Technology Research Association, Takamatsu, Kagawa, Japan
- 2Faculty of Pharmaceutical Sciences, Tokushima Bunri University, Tokushima, Japan
Immunotherapy is renowned for its capacity to elicit anti-infective and anti-cancer effects by harnessing immune responses to microbial components and bolstering innate healing mechanisms through a cascade of immunological reactions. Specifically, mammalian Toll-like receptors (TLRs) have been identified as key receptors responsible for detecting microbial components. The discovery of these mammalian Toll-like receptors has clarified antigen recognition by the innate immune system. It has furnished a molecular foundation for comprehending the interplay between innate immunity and its anti-tumor or anti-infective capabilities. Moreover, accumulating evidence highlights the crucial role of TLRs in maintaining tissue homeostasis. It has also become evident that TLR-expressing macrophages play a central role in immunity by participating in the clearance of foreign substances, tissue repair, and the establishment of new tissue. This macrophage network, centered on macrophages, significantly contributes to innate healing. This review will primarily delve into innate immunity, specifically focusing on substances targeting TLR4.
1 Introduction
The immune system is essential to protect the host from invading pathogens and abnormal cells such as cancer cells and is primarily classified as innate and acquired immunity. Innate immunity is an immediate general defense function that we are born with, while acquired immunity has the ability to learn, respond specifically, and memorize responses to specific pathogens. This memory system allows the host organism to respond more rapidly and effectively when exposed to the same or related antigens. In recent years, research has focused on the innate immune memory system, particularly macrophages. This research has not only shed new light on host defense mechanisms but also has significant implications for vaccination and allergy strategies (1–3).
The correlation between infection and remission of malignancy was initially observed in the 18th century (1, 2). Dr. William Corey, an American surgeon of the 18th century, is recognized as a trailblazer in this domain. In the 1890s, he demonstrated the regression of cancer in a patient who developed a bacterial infection following surgery for sarcoma. Intrigued by this observation, Dr. Coley administered live cultures of streptococci to induce erysipelas in cancer patients and assessed their response. He found that the antitumor effect was reliant on the bacterial toxin. Eventually, he combined toxins from a gram-positive bacterium (Streptococcus pyogenes) with those from a gram-negative bacterium (Serratia marcescens), naming it “Coley toxin” (also known as “mixed bacterial vaccine”) (3). Dr. Cawley and his daughter, Dr. Helen Cawley Notes, continued the pioneering efforts in treating cancer based on immune system function.
It has been demonstrated that innate immune responses to microorganisms can induce anti-tumor and anti-infective effects akin to those of Coley toxins, and a diverse array of immune-inducing bacterial-derived and other substances have been identified as “biological response modifiers (BRMs).”
1.1 Toll-like receptors
Cells of the innate immune system possess the ability to detect infectious agents through receptors that recognize characteristic components of pathogenic microorganisms. These components, known as pathogen-associated molecular patterns (PAMPs), exhibit high conservation and are encoded in the germ line, making them highly conserved across different species. Toll, initially recognized for its involvement in establishing the dorsal-ventral polarity in the Drosophila embryo (4), has also been found to participate in the innate immune response to fungi (5). In 1997, a mammalian homolog of Drosophila Toll was cloned (6), and to date, ten human molecules, known as Toll-like receptors (TLRs), have been confirmed (7, 8).
The discovery of TLRs in mammals has the potential to offer insights into the molecular basis of early host defense processes against microbial infections. Moreover, accumulating evidence suggests that TLRs play a diverse role in various biological processes. Like other pattern recognition receptors (PRRs), TLRs exhibit a repeated leucine-rich motif in their extracellular domain and a conserved intracellular motif known as the Toll/interleukin-1 receptor (TIR) domain, which initiates signal transduction. TLRs are type I transmembrane proteins.
The initial stages of Toll-like receptor (TLR) signaling involve the mediation of adaptor molecules, namely Myeloid differentiation factor 88 (MyD88), Toll-like receptor-associated activator of interferon (TRIF), MyD88 adaptor-like protein (MAL/TIRAP), Toll receptor-associated molecule (TRAM), and other adapter molecules, which interact with downstream components like NF-κB, JNK/p38 kinase, and interferon regulatory factors (IRF3, IRF5, and IRF7). Ultimately, TLR signaling triggers the expression of diverse transcripts, including cytokines and genes that are induced by interferon (IFN).
1.2 Role of TLRs in tissue homeostasis
The Toll-like receptors (TLRs) play a significant role in maintaining tissue homeostasis, beyond their primary function in host defense (9, 10). They are involved in recognizing various endogenous ligands released from dead cells in injured or infected tissues, termed damage-associated molecular patterns (DAMPs). These ligands include uric acid crystals, surfactant protein A, fibronectin (an extracellular matrix product), heparan sulfate, biglycan, fibrinogen, and hyaluronan oligosaccharides, as well as hyaluronan degradation products (11–19), which trigger TLR activation.
Wound healing is a complex process through which injured organs undergo repair (20). TLR activation can contribute to tissue damage correction, either positively or negatively, by recruiting inducible cells that release cytotoxic mediators or by triggering cytoprotective signals (21, 22). TLRs exhibit cytoprotective properties and prevent tissue injury under stress conditions in the lung and intestine. For instance, in bleomycin-induced lung injury, interactions between hyaluronic acid and TLR2/TLR4 signal the initiation of an inflammatory response, maintaining epithelial cell integrity, and promoting recovery from acute lung injury (19). In a model of intestinal injury induced by dextran sodium sulfate, TLR4 and MyD88 signaling are required for optimal proliferation and protection from apoptosis of the injured intestine. Additionally, activation of TLRs by commensal microflora is crucial for protecting against intestinal injury and associated mortality (23, 24). However, TLR4 has been shown to promote injury in ischemia-reperfusion experiments involving the liver, kidney, brain, and heart using TLR4 mutant or TLR4-deficient mice (25–28). In the central nervous system, TLRs coordinate protective responses to axonal injury and crush in the brain and spinal cord (29–31).
The involvement of TLRs in tissue and organ regeneration is evident in cases like the regeneration of the liver after partial hepatectomy. The regenerative response involves multiple biological functions, including cell proliferation, angiogenesis, extracellular matrix reconstruction, and epithelialization (32). TLRs also regulate compensatory proliferation of parenchymal cells after injury (24, 32, 33), induce cyclooxygenase, chemokines, vascular endothelial growth factor (VEGF), matrix metalloproteinases (23, 24, 34), and activate mesenchymal stem cells (35). Thus, TLRs play a pivotal role throughout the entire process of tissue repair and regeneration, significantly contributing to tissue homeostasis. During evolution, TLRs may have acquired dual roles in tissue homeostasis, involving the regulation of the body’s dynamics and the promotion of regenerative processes.
Recently, immune checkpoint inhibitors have been used in cancer therapy and have caused dynamic phenotypic changes to macrophages, and severe immune-related adverse events have been reported (36–38). This is thought to be due to the fact that checkpoint inhibitors change the polarity of macrophages from M2 to M1 (39–41). Although M1 and M2 macrophages differ in shape and properties, they both possess TLR receptor families, etc., and it has been suggested that their receptor ligands act to modulate the immune response (42, 43). Therefore, when using immune checkpoint inhibitors, it is important to activate macrophages via TLR4 in terms of maintaining homeostasis, i.e., to maintain a balance between M1 and M2 macrophages.
1.3 Maintenance of homeostasis by the macrophage network
Upon encountering external pathogens, macrophages activate intracellular signaling pathways through Toll-like receptors (TLRs) to initiate an immune response against the invading pathogen. Macrophages and dendritic cells, which are antigen-presenting cells, are known to express particularly high levels of TLRs (44, 45). While the precise origin of macrophages remains uncertain, phylogenetics suggest that they may have originated from protozoa with active phagocytic abilities, such as amoebae. Nevertheless, since phagocytic cells are present in all animals, from unicellular protists like amoebas to mammals, macrophages in humans likely play a critical role in maintaining homeostasis. Thus, stimulating TLRs expressed on macrophages and activating the network, which centers around macrophages, could significantly contribute to disease prevention and treatment and, consequently, promote homeostasis (46).
Recent research has identified at least two distinct tissue-endemic stromal macrophages in the steady-state lung. These macrophages exhibit unique transcriptional profiles and are spatially localized in the interstitium of bronchovascular bundles rather than within alveolar walls (47). In fact, it is now understood that most tissues harbor multiple macrophage populations localized to different microanatomical regions (47–49). Each of these populations differs in their developmental mode, replacement rate involving monocyte-derived cells, and self-renewal capacity. Moreover, each population may play a specific role in maintaining tissue homeostasis, responding to injury, and participating in tissue repair processes (43, 50–52).
Cells with TLR4 are abundant in the innate immune system and include macrophages and mucosal epithelial cells. Unlike all other cells, macrophages are systemically distributed and account for about half of the immune cells by weight (53). Macrophages are known to be migratory and active in migrating to lesions, processing not only invading foreign substances (bacteria, viruses, etc.) but also dead and senescent cells, degenerated proteins, oxidized lipids, AGEs, and other unwanted substances generated by the body, and are responsible for repair and regeneration of damaged tissue.
Macrophages (phagocytes) are ubiquitous in multicellular animals, but have been shown to play an important role in individual health even in those without a nervous system in early stages of evolution (54). In other words, in multicellular animals, there must have been a mechanism for maintaining individual integrity that predates the emergence of the nervous system. Based on our previous findings, we can predict the existence of a mechanism that maintains individual homeostasis through signal transduction between migrating macrophages. This macrophage-mediated signal transduction system has been proposed as the macrophage network hypothesis (46). Fujii et al. In a mouse model of pressure overload on the heart, kidney tissue macrophages were found to secrete M- CSF2 secretion and reported a network that activates cardiac tissue macrophages to increase cardiomyocyte (55). It is also speculated that the TLR4-mediated activation system acts as the first signal in macrophage network. In fact, Mizobuchi et al. have shown that orally administered LPS (lipopolysaccharide) induces membrane-bound CSF1 in peripheral blood leukocytes, which stimulates CSF1 receptors in brain microglia, and neuroprotective and anti-inflammatory effects have been shown in brain diabetes-induced mice (56). These reports suggest that systemic macrophages interact in mammals and that TLR4 signaling plays a role in their regulation.
1.4 Exogenous immunostimulants
1.4.1 LPS
LPS is a glycolipid present in the extracellular membrane of Gram-negative bacteria and is a known ligand that activates TLR4 at trace amounts of only a few pg/ml (57). LPS is released from Gram-negative bacteria as exosomes into the environment from survival as outer membrane vesicles, and dead Gram-negative bacteria readily release LPS (58, 59). Therefore, LPS is present wherever symbiotic Gram-negative bacteria are present on the mucosa of animals’ skin, oral cavity, airways, and intestinal tract, and it is thought that TLR4-mediated exchange is used as an information molecule from the symbiotic bacteria to and from the animal. LPS is known to induce antimicrobial peptides from Paneth cells in the small intestine (60), which contribute to the stabilization of intestinal bacteria, and to transmit information from tissue macrophages in the large intestine to neurons, which induce intestinal peristalsis (61).
Studies utilizing LPS have unveiled the importance of TLR ligands administered orally or transdermally in activating the macrophage network without inducing inflammatory reactions (56, 60, 62, 63). However, it is believed that oral and transdermal LPS administration activates the systemic macrophage network through mucosal macrophages. The involvement of the TNF superfamily and membrane-bound colony-stimulating factor 1 (mCSF1) in this macrophage network is suspected, although the specific mechanisms remain unclear.
Notably, animal experiments involving oral LPS administration have demonstrated its associations with various conditions, including cancer, Alzheimer’s disease, ulcers, viral infections, toxoplasma infections, allergies, hyperlipidemia, hypertension, diabetes, and hair growth. Clinical trials in humans have further revealed effects on cancer, atopy, diabetes, capillary growth, wound healing, and developmental disorders (64–70). Notably, recent findings suggest that oral LPS administration enhances the foreign body processing function of microglia, which are brain macrophages, providing a preventive effect against Alzheimer’s disease (56). These effects induced by oral LPS administration are likely a result of the indirect influence of cytokines, including membrane-bound types triggered by stimulation of innate immune sensor cells in the mucosal epithelium. These cytokines subsequently act on tissue macrophages in the juxtacrine and paracrine manner, contributing to the macrophage network.
Furthermore, sublingual administration of LPS has been shown to significantly enhance the efficacy of influenza vaccines and reduce mortality (71, 72). As a result, sublingual LPS administration holds promise in preventing and treating emerging infectious diseases, including those caused by novel coronavirus infections expected to arise in the future.
1.4.2 Fucoidan
Fucoidan, a sulfated polysaccharide abundant in the cell walls of brown algae and marine organisms, possesses a highly complex chemical composition that varies depending on the algal source, geographical location, and extraction process (1). The structural backbone of fucoidan consists of fucopyranose residues with repeated α-(1→3) bonds or L-fucopyranose residues with alternating α-(1→3) and α-(1→4) linkages. These fucosyl groups may be mono- or di-substituted with sulfate or acetate groups at C-2, C-4, and occasionally at C-3 (73, 74). Additionally, fucoidan structures may contain a variety of other monosaccharides (mannose-type), such as mannose, galactose, arabinose, xylose, glucose, uronic acid, and proteins, in addition to the fucosyl main chain (75).
Absorption studies with fucoidan (737 kDa) were conducted in rats. After administration, the concentration of fucoidan in the serum reached a maximum and absorbed fucoidan accumulated. Absorbed fucoidan accumulated in the kidneys. The accumulation of fucoidan in organs has also been demonstrated by the absorption of fucoidan in rats (76). In addition, observations of healthy volunteers who ingested or received fucoidan orally reported that some of the fucoidan was absorbed by endocytosis and was detected in serum and urine (77).
In recent years, fucoidan derived from algae has been the subject of intensive research due to its diverse biological activities and therapeutic potential. Fucoidan has shown interactions with TLR2 and TLR4 (78, 79), and various pharmacological effects have been reported, including antitumor, immunomodulatory, antiviral, antibacterial, antidiabetic, renoprotective, antioxidant, anti-inflammatory, and anticoagulant effects (80–82). Moreover, fucoidan has been investigated for its application in improving various pathological conditions, such as diabetes, hepatic lipidosis (fatty liver), liver damage, renal ischemia, abnormal blood coagulability, stem cell therapy, gastric ulcer, gout, bacterial and viral infections, and snakebite (73, 78, 79, 81–83). The wide range of biological activities makes fucoidan a potential candidate for immune response modulation, antibacterial and antiviral agents (81).
Developing standardized fucoidan supplements is a complex process, as factors such as raw materials, species, molecular weight, composition, structure, and route of administration significantly impact the efficacy of the compounds. Additionally, most of the reported activities are based on in vitro experiments or in vivo evaluations using laboratory animals. Care should be taken as different animal models may produce varying effects when evaluated in different contexts.
Despite the large number of studies on fucoidan, few clinical trials have been planned and conducted. In most cases, different cell lines and animal models have been used to study different types of fucoidan. This makes it difficult to determine the general mechanism of action for a particular type of fucoidan. There is also little information on the absorption, distribution, and excretion of fucoidan. Although the biological activities exhibited by fucoidan are fascinating, most of these studies have been conducted on relatively crude fucoidans, making it very difficult to determine the structure-activity relationship.
1.4.3 Vizantin
In 1956, Dr. Chisato Maruyama observed that there were few cancer patients in sanatoriums for tuberculosis and leprosy, which led to research on the application of extracts of Mycobacterium tuberculosis for cancer treatment (84). The prepared extract, called “Specific Substance Maruyama (SSM),” was a deproteinized extract primarily composed of lipoarabinomannan, a type of polysaccharide (85). Another example of a Mycobacterium tuberculosis-derived biological response modifier (BRM) is BCG-CWS, a cell wall skeletal preparation of Mycobacterium bovis bacillus Calmette-Guerin. BCG-CWS is largely nonpathogenic but retains the immunogenicity of tuberculosis (85). It is a peptidoglycan covalently bound to arabinogalactan and mycolic acid (86). BCG-CWS has been clinically used as a cancer immunotherapy (87, 88) but has not been approved by the Ministry of Health, Labour, and Welfare (MHLW). These biologically derived classical BRMs are considered “natural compounds” and are characterized as crude products containing multiple components, as they have not undergone full purification.
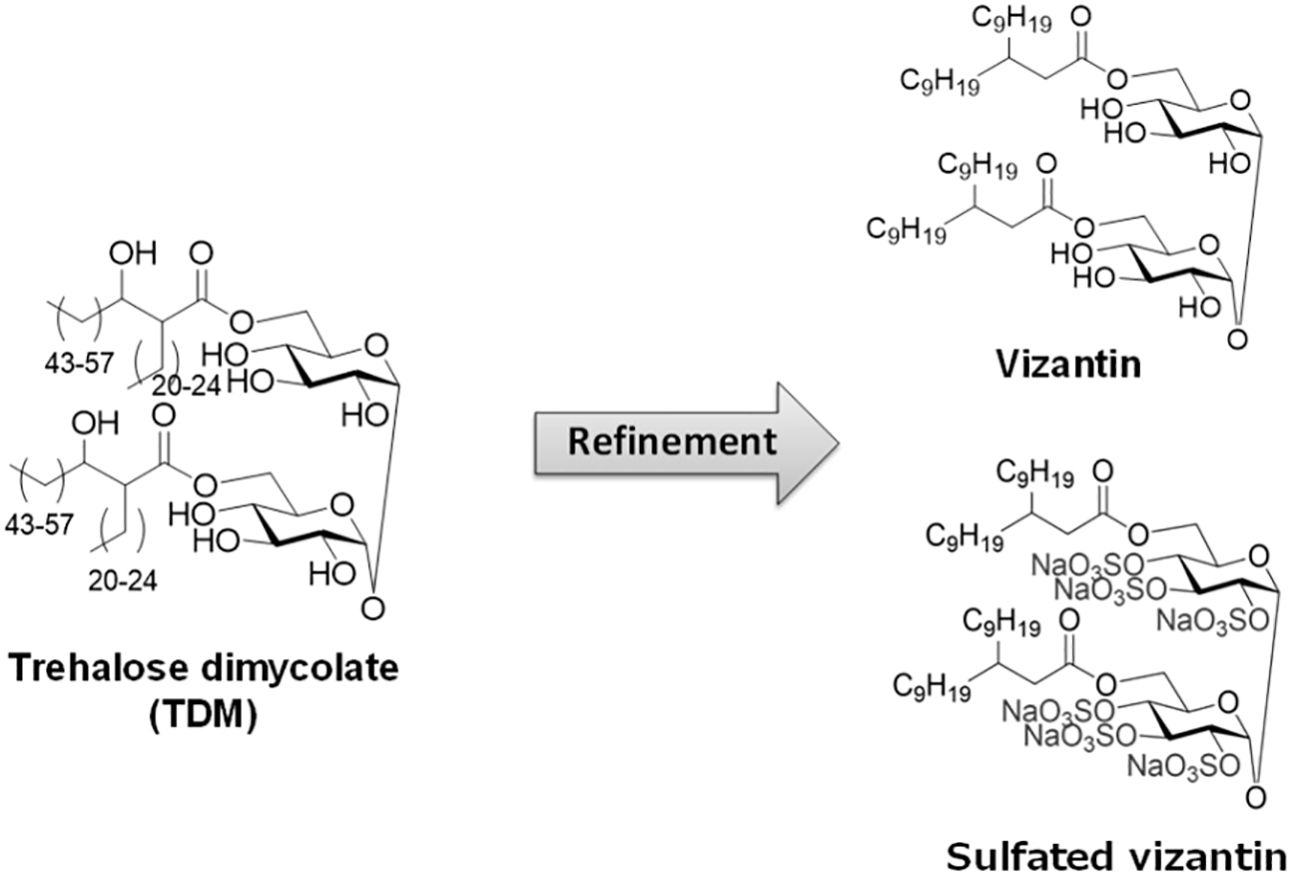
Focusing on Mycobacterium tuberculosis-derived BRMs, researchers developed Vizantin, a single-component immunostimulant, using trehalose dimycolate (TDM) present on the cell surface layer of M. tuberculosis as the lead compound (89). Vizantin is a trehalose diester consisting of two achiral β-branched fatty acids, 2-nonylundecanoic acid, fused to the hydroxyl groups at the 6, 6’ positions of trehalose. Additionally, researchers successfully created sulfated Vizantin by sulfating the hydroxyl group of the trehalose moiety, making it water-soluble (90) (Figure 1).
Oral administration of Vizantin to mice has been shown to prevent the settlement of melanoma cells in the lungs, effectively preventing lethality (89). Additionally, intravenous administration of Vizantin or sulfated Vizantin, which has improved water solubility, into the tail vein of mice has been found to prevent lethality caused by multidrug-resistant Pseudomonas aeruginosa infection. The mechanism behind this effect involves sulfated Vizantin acting on macrophages to form extracellular trapping nets (METs), which trap P. aeruginosa (91). Further analysis has revealed that sulfated Vizantin does not affect the growth of P. aeruginosa but reduces its swimming activity by disrupting the Che system, which is involved in flagellar motility. Moreover, sulfated Vizantin has been shown to inhibit biofilm formation by disrupting the glucosyltransferase production balance of mutans, the bacteria responsible for causing dental caries.
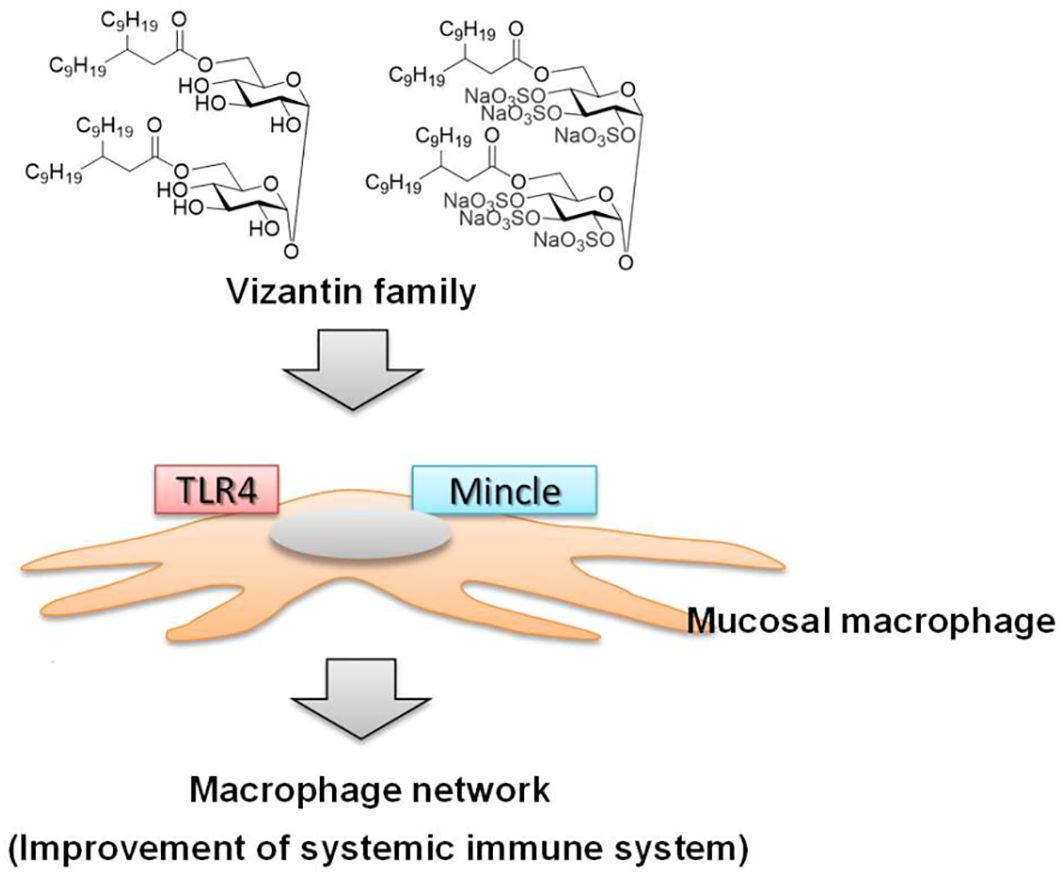
Vizantin is also a lead compound of Vizantin, and the glycosylation moiety (trehalose) of TDM, the lead compound of Vizantin, has been demonstrated to act on the Macrophage inducible C-type lectin (Mincle) receptor (92, 93). As Vizantin has a similar binding site to TDM, it is expected that Vizantin also acts on the Mincle receptor. Therefore, the diverse range of responses observed with Vizantin may be attributed to its action on both TLR4/MD-2 and Mincle receptors (Figure 2). As a result, it is thought to activate the macrophage network by acting on macrophages in the same way as LPS (Figure 2).
1.5 Endogenous immunostimulant
1.5.1 HSP70
Heat Shock Protein 70 (HSP70) is a protein that cells induce in response to environmental stimuli, such as stress and heat. Its role is crucial in maintaining protein folding and stability within cells, contributing to cell survival and function. HSP70 also plays a significant role in enhancing the antigen-presenting ability of antigen-presenting cells, like dendritic cells and macrophages. By binding to antigens on these cells, it facilitates the immune system’s recognition of foreign substances and pathogens, leading to an enhanced immune response.
The immune response triggered by HSP70 operates in a CD14-dependent manner through TLR2 and TLR4 receptors (94). This immune response activates cytotoxic T cells (CD8+ T cells), facilitating the elimination of abnormal cells, and regulates the production of inflammation-regulating cytokines and inflammatory cells. The production of cytokines that modulate inflammation and the activation of inflammatory cells are crucial for balancing the immune response. Consequently, HSP70 induced by hyperthermia and other therapies exhibits effects on various diseases, including neurodegenerative disorders like Alzheimer’s and Parkinson’s disease (95), metabolic syndrome (96), cancer (97, 98), and allergic conditions such as asthma (84, 99). These effects are attributed to the activation of the immune system through HSP70 expression, leading to enhanced natural healing capabilities. There are several types of HSPs, and their receptors are being analyzed for their relationship to immune activation, but there are still many unknowns.
2 Conclusion
Hippocrates once said, “Man has a hundred great physicians, and the great physician is the power of natural healing.” Today, while appropriate drugs are prescribed for various diseases, they do not cure the diseases themselves. Instead, the human body maintains its health through its own self-healing power. It is important not to overly rely on drugs but rather to strengthen our own immune system and rely on our natural healing abilities. This is especially crucial for dealing with emerging infectious diseases that may not be effectively addressed by individual vaccines or therapeutic agents. When such infectious diseases spread, measures like physical protection such as wearing masks and enhancing individual self-immunity become of utmost importance.
LPS, fucoidan, HSP, and vizantin, which have been discussed here, have been shown to enhance immunity by activating TLRs, especially TLR4, which are pattern recognition receptors. These substances contribute to the prevention and treatment of various diseases. Further research aims to better understand how these substances act on innate immunity, particularly how they influence the function of the macrophage network in maintaining a balanced immune response.
Author contributions
MO: Conceptualization, Writing – original draft. HY: Writing – review & editing. TK: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Control of Innate Immunity Collaborative Innovation Partnership. This research was supported by JSPS KAKENHI grant numbers 26293390, 26305034, 26670816, 15H05017, and 18H02657 and A-step AS2531332Q.
Acknowledgments
We gratefully acknowledge the work of past and present members of our laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garay RP, Viens P, Bauer J, Normier G, Bardou M, Jeannin JF, et al. Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol. (2007) 563:1–17. doi: 10.1016/j.ejphar.2007.02.018
2. Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. (2009) 9:57–63. doi: 10.1038/nrc2541
3. Tsung K, Norton JA. Lessons from coley's toxin. Surg Oncol. (2006) 15:25–8. doi: 10.1016/j.suronc.2006.05.002
4. Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. (1988) 52:269–79. doi: 10.1016/0092-8674(88)90516-8
5. Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. (1996) 86:973–83. doi: 10.1016/S0092-8674(00)80172-5
6. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. (1997) 388:394–7. doi: 10.1038/41131
7. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. (2003) 21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126
8. Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. (2004) 5:987–95. doi: 10.1038/ni1112
9. van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog Brain Res. (2009) 175:139–48. doi: 10.1016/S0079-6123(09)17509-X
10. Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. (2010) 49:1–9. doi: 10.1016/j.cyto.2009.08.010
11. Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R. TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol. (2005) 174:5016–23. doi: 10.4049/jimmunol.174.8.5016
12. Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. (2005) 52:2936–46. doi: 10.1002/art.21238
13. Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol. (2002) 168:5989–92. doi: 10.4049/jimmunol.168.12.5989
14. Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. 3rd, The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. (2001) 276:10229–33. doi: 10.1074/jbc.M100099200
15. Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. (2002) 168:5233–9. doi: 10.4049/jimmunol.168.10.5233
16. Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. (2005) 115:2223–33. doi: 10.1172/JCI23755
17. Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. (2001) 167:2887–94. doi: 10.4049/jimmunol.167.5.2887
18. Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. (2002) 195:99–111. doi: 10.1084/jem.20001858
19. Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. (2005) 11:1173–9. doi: 10.1038/nm1315
20. Xing Z, Afkhami S, Bavananthasivam J, Fritz DK, D'Agostino MR, Vaseghi-Shanjani M, et al. Innate immune memory of tissue-resident macrophages and trained innate immunity: Re-vamping vaccine concept and strategies. J Leukoc Biol. (2020) 108:825–34. doi: 10.1002/JLB.4MR0220-446R
21. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. (2008) 453:314–21. doi: 10.1038/nature07039
22. Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med (Berl). (2009) 87:125–38. doi: 10.1007/s00109-008-0426-z
23. Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. (2006) 131:862–77. doi: 10.1053/j.gastro.2006.06.017
24. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. doi: 10.1016/j.cell.2004.07.002
25. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. (2005) 201:1135–43. doi: 10.1084/jem.20042614
26. Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. (2007) 117:2847–59. doi: 10.1172/JCI31008
27. Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U.S.A. (2007) 104:13798–803. doi: 10.1073/pnas.0702553104
28. Oyama J, Blais C Jr., Liu X, Pu M, Kobzik L, Kelly RA, et al. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. (2004) 109:784–9. doi: 10.1161/01.CIR.0000112575.66565.84
29. Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. (2007) 102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x
30. Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. (2006) 26:12826–37. doi: 10.1523/JNEUROSCI.4937-05.2006
31. Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. (2007) 282:14975–83. doi: 10.1074/jbc.M607277200
32. Seki E, Tsutsui H, Iimuro Y, Naka T, Son G, Akira S, et al. Contribution of Toll-like receptor/myeloid differentiation factor 88 signaling to murine liver regeneration. Hepatology. (2005) 41:443–50. doi: 10.1002/(ISSN)1527-3350
33. Zhang Z, Schluesener HJ. Mammalian toll-like receptors: from endogenous ligands to tissue regeneration. Cell Mol Life Sci. (2006) 63:2901–7. doi: 10.1007/s00018-006-6189-1
34. Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U.S.A. (2005) 102:99–104. doi: 10.1073/pnas.0405979102
35. Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. (2007) 109:1422–32. doi: 10.1182/blood-2006-06-028704
36. Brom VC, Burger C, Wirtz DC, Schildberg FA. The role of immune checkpoint molecules on macrophages in cancer, infection, and autoimmune pathologies. Front Immunol. (2022) 13:837645. doi: 10.3389/fimmu.2022.837645
37. Deng Z, Loyher PL, Lazarov T, Li L, Shen Z, Bhinder B, et al. The nuclear factor ID3 endows macrophages with a potent anti-tumour activity. Nature. (2024) 626:864–73. doi: 10.1038/s41586-023-06950-4
38. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. (2020) 6:38. doi: 10.1038/s41572-020-0160-6
39. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
40. Beltraminelli T, De Palma M. Biology and therapeutic targeting of tumour-associated macrophages. J Pathol. (2020) 250:573–92. doi: 10.1002/path.5403
41. Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. (2015) 212:435–45. doi: 10.1084/jem.20150295
42. Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther. (2017) 19:245. doi: 10.1186/s13075-017-1447-1
43. McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci. (2013) 110:17253–8. doi: 10.1073/pnas.1308887110
44. Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. (2004) 430:257–63. doi: 10.1038/nature02761
45. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. (2010) 11:373–84. doi: 10.1038/ni.1863
46. Kohchi C, Inagawa H, Nishizawa T, Yamaguchi T, Nagai S, Soma G. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J Biosci Bioeng. (2006) 102:485–96. doi: 10.1263/jbb.102.485
47. Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T, et al. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol. (2017) 57:66–76. doi: 10.1165/rcmb.2016-0361OC
48. Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J, et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. (2017) 127:2904–15. doi: 10.1172/JCI75005
49. Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. (2019) 20:29–39. doi: 10.1038/s41590-018-0272-2
50. Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. (2017) 214:2387–404. doi: 10.1084/jem.20162152
51. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. (2019) 363:6432. doi: 10.1126/science.aau0964
52. Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. (2019) 129:2619–28. doi: 10.1172/JCI124615
53. Sender R, Weiss Y, Navon Y, Milo I, Azulay N, Keren L, et al. The total mass, number, and distribution of immune cells in the human body. Proc Natl Acad Sci U.S.A. (2023) 120:e2308511120. doi: 10.1073/pnas.2308511120
54. Steven JL. Metchnikoff on the comparative pathology of inflammation. Glasgow Med J. (1892) 38:195–205.
55. Fujiu K, Shibata M, Nakayama Y, Ogata F, Matsumoto S, Noshita K, et al. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med. (2017) 23:611–22. doi: 10.1038/nm.4326
56. Mizobuchi H, Yamamoto K, Yamashita M, Nakata Y, Inagawa H, Kohchi C, et al. Prevention of diabetes-associated cognitive dysfunction through oral administration of lipopolysaccharide derived from pantoea agglomerans. Front Immunol. (2021) 12:650176. doi: 10.3389/fimmu.2021.650176
57. Deng H, Maitra U, Morris M, Li L. Molecular mechanism responsible for the priming of macrophage activation. J Biol Chem. (2013) 288:3897–906. doi: 10.1074/jbc.M112.424390
58. Avila-Calderón ED, Ruiz-Palma MDS, Aguilera-Arreola MG, Velázquez-Guadarrama N, Ruiz EA, Gomez-Lunar Z, et al. Outer membrane vesicles of gram-negative bacteria: an outlook on biogenesis. Front Microbiol. (2021) 12:557902. doi: 10.3389/fmicb.2021.557902
59. Liu J, Kang R, Tang D. Lipopolysaccharide delivery systems in innate immunity. Trends Immunol. (2024) 45(4):274–87. doi: 10.1016/j.it.2024.02.003
60. Yokoi Y, Nakamura K, Yoneda T, Kikuchi M, Sugimoto R, Shimizu Y, et al. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci Rep. (2019) 9:2710. doi: 10.1038/s41598-019-39610-7
61. Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. (2014) 158:300–13. doi: 10.1016/j.cell.2014.04.050
62. Yamamoto K, Yamashita M, Oda M, Tjendana Tjhin V, Inagawa H, Soma GI. Oral administration of lipopolysaccharide enhances insulin signaling-related factors in the KK/ay mouse model of type 2 diabetes mellitus. Int J Mol Sci. (2023) 24:4619. doi: 10.3390/ijms24054619
63. Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. (2000) 1:113–8. doi: 10.1038/77783
64. Morishima A, Inagawa H. Clinical effects of orally administered lipopolysaccharide derived from pantoea agglomerans on Malignant tumors. Anticancer Res. (2016) 36:3747–51.
65. Nakai K, Kubota Y, Soma GI, Kohchi C. The effect of lipopolysaccharide-containing moisturizing cream on skin care in patients with mild atopic dermatitis. In Vivo. (2019) 33:109–14. doi: 10.21873/invivo.11446
66. Iguchi M, Inagawa H, Nishizawa T, Okutomi T, Morikawa A, Soma GI, et al. Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour). Chem Pharm Bull (Tokyo). (1992) 40:1004–6. doi: 10.1248/cpb.40.1004
67. Nakata K, Taniguchi Y, Yoshioka N, Yoshida A, Inagawa H, Nakamoto T, et al. A mixture of Salacia oblonga extract and IP-PA1 reduces fasting plasma glucose (FPG) and low-density lipoprotein (LDL) cholesterol levels. Nutr Res Pract. (2011) 5:435–42. doi: 10.4162/nrp.2011.5.5.435
68. Nakata Y, Kohchi C, Ogawa K, Nakamoto T, Yoshimura H, Soma GI. Effects of 3 months continuous intake of supplement containing Pantoea agglomerans LPS to maintain normal bloodstream in adults: Parallel double-blind randomized controlled study. Food Sci Nutr. (2018) 6:197–206. doi: 10.1002/fsn3.547
69. Morishima A, Inagawa H. Improvement in protracted wound healing by topical cream containing lipopolysaccharide derived from pantoea agglomerans. Anticancer Res. (2018) 38:4375–9. doi: 10.21873/anticanres.12739
70. Morishima A, Zhang R, Nagaoka T, Inagawa H. Useful cases of patients with developmental disorders improved by oral administration of LPS derived from pantoea agglomerans. Anticancer Res. (2020) 40:4755–62. doi: 10.21873/anticanres.14477
71. Fukasaka M, Asari D, Kiyotoh E, Okazaki A, Gomi Y, Tanimoto T, et al. A lipopolysaccharide from pantoea agglomerans is a promising adjuvant for sublingual vaccines to induce systemic and mucosal immune responses in mice via TLR4 pathway. PloS One. (2015) 10:e0126849. doi: 10.1371/journal.pone.0126849
72. Bandoro C, Runstadler JA. Bacterial lipopolysaccharide destabilizes influenza viruses. mSphere. (2017) 2:e00267–17. doi: 10.1128/mSphere.00267-17
73. Ale MT, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. (2011) 9:2106–30. doi: 10.3390/md9102106
74. Hentati F, Delattre C, Ursu AV, Desbrières J, Le Cerf D, Gardarin C, et al. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr Polym. (2018) 198:589–600. doi: 10.1016/j.carbpol.2018.06.098
75. Balboa EM, Conde E, Moure A, Falqué E, Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. (2013) 138:1764–85. doi: 10.1016/j.foodchem.2012.11.026
76. Nagamine T, Nakazato K, Tomioka S, Iha M, Nakajima K. Intestinal absorption of fucoidan extracted from the brown seaweed, Cladosiphon okamuranus. Mar Drugs. (2014) 13:48–64. doi: 10.3390/md13010048
77. Tokita Y, Nakajima K, Mochida H, Iha M, Nagamine T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci Biotechnol Biochem. (2010) 74:350–7. doi: 10.1271/bbb.90705
78. Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Mar Drugs. (2011) 9:1731–60. doi: 10.3390/md9101731
79. Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. (2008) 13:1671–95. doi: 10.3390/molecules13081671
80. Jeong J-W, Hwang SJ, Han MH, Lee D-S, Yoo JS, Choi I-W, et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol Cell Toxicol. (2017) 13:405–17. doi: 10.1007/s13273-017-0045-2
81. Myers SP, Mulder AM, Baker DG, Robinson SR, Rolfe MI, Brooks L, et al. Effects of fucoidan from Fucus vesiculosus in reducing symptoms of osteoarthritis: a randomized placebo-controlled trial. Biologics. (2016) 10:81–8. doi: 10.2147/BTT
82. Aleissa MS, Alkahtani S, Abd Eldaim MA, Ahmed AM, Bungău SG, Almutairi B, et al. Fucoidan ameliorates oxidative stress, inflammation, DNA damage, and hepatorenal injuries in diabetic rats intoxicated with aflatoxin B(1). Oxid Med Cell Longev. (2020) 2020:9316751. doi: 10.1155/2020/9316751
83. Wang Y, Xing M, Cao Q, Ji A, Liang H, Song S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar Drugs. (2019) 17:183. doi: 10.3390/md17030183
84. Suzuki F, Brutkiewicz RR, Pollard RB. Importance of Lyt 1+ T-cells in the antitumor activity of an immunomodulator, SSM, extracted from human-type Tubercle bacilli. J Natl Cancer Inst. (1986) 77:441–7.
85. Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin: involvement of toll-like receptors. Infect Immun. (2000) 68:6883–90. doi: 10.1128/IAI.68.12.6883-6890.2000
86. Azuma I, Ribi EE, Meyer TJ, Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. (1974) 52:95–101. doi: 10.1093/jnci/52.1.95
87. Hayashi A, Nishida Y, Yoshii S, Kim SY, Uda H, Hamasaki T. Immunotherapy of ovarian cancer with cell wall skeleton of Mycobacterium bovis Bacillus Calmette-Guérin: effect of lymphadenectomy. Cancer Sci. (2009) 100:1991–5. doi: 10.1111/j.1349-7006.2009.01271.x
88. Kodama K, Higashiyama M, Takami K, Oda K, Okami J, Maeda J, et al. Innate immune therapy with a Bacillus Calmette-Guérin cell wall skeleton after radical surgery for non-small cell lung cancer: a case-control study. Surg Today. (2009) 39:194–200. doi: 10.1007/s00595-008-3826-3
89. Yamamoto H, Oda M, Nakano M, Watanabe N, Yabiku K, Shibutani M, et al. Development of vizantin, a safe immunostimulant, based on the structure-activity relationship of trehalose-6,6'-dicorynomycolate. J Med Chem. (2013) 56:381–5. doi: 10.1021/jm3016443
90. Nakano M, Sakamoto K, Yamasaki N, Asano Y, Oda M, Takahashi H, et al. Development of a water soluble self-assembling analogue of vizantin. Chem Pharm Bull. (2024) 72:226–33. doi: 10.1248/cpb.c23-00716
91. Oda M, Kurosawa M, Yamamoto H, Domon H, Kimura T, Isono T, et al. Sulfated vizantin induces formation of macrophage extracellular traps. Microbiol Immunol. (2018) 62:310–6. doi: 10.1111/1348-0421.12589
92. Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. (2010) 184:2756–60. doi: 10.4049/jimmunol.0904013
93. Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. (2009) 206:2865–8. doi: 10.1084/jem.20092533
94. Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. (2002) 105:685–90. doi: 10.1161/hc0602.103617
95. Hunt AP, Minett GM, Gibson OR, Kerr GK, Stewart IB. Could heat therapy be an effective treatment for alzheimer's and parkinson's diseases? A narrative review. Front Physiol. (2019) 10:1556. doi: 10.3389/fphys.2019.01556
96. Faulkner SH, Jackson S, Fatania G, Leicht CA. The effect of passive heating on heat shock protein 70 and interleukin-6: A possible treatment tool for metabolic diseases? Temperature (Austin). (2017) 4:292–304. doi: 10.1080/23328940.2017.1288688
97. Glazer ES, Curley SA. The ongoing history of thermal therapy for cancer. Surg Oncol Clin N Am. (2011) 20:229–35. doi: 10.1016/j.soc.2010.11.001
98. Dai Q, Cao B, Zhao S, Zhang A. Synergetic thermal therapy for cancer: state-of-the-art and the future. Bioengineering. (2022) 9(9):474. doi: 10.3390/bioengineering9090474
Keywords: TLR4 ligands, macrophage, macrophage network, innate immunity, self-healing ability
Citation: Oda M, Yamamoto H and Kawakami T (2024) Maintenance of homeostasis by TLR4 ligands. Front. Immunol. 15:1286270. doi: 10.3389/fimmu.2024.1286270
Received: 31 August 2023; Accepted: 11 April 2024;
Published: 23 April 2024.
Edited by:
Soma Genichiro, Collaborative Innovation Partnership, JapanReviewed by:
Hisanori Domon, Niigata University, JapanHitoshi Hori, Tokushima University, Japan
Teruko Honda, Azabu University, Japan
Copyright © 2024 Oda, Yamamoto and Kawakami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masataka Oda, bXN0a29kYUBzaGl6ZW5tZW5la2kub3Jn
 Masataka Oda
Masataka Oda Hirofumi Yamamoto2
Hirofumi Yamamoto2 Takashige Kawakami
Takashige Kawakami