- 1Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, United States
- 2Division of Infectious Diseases and HIV Medicine, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 3Center for Clinical Research, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Background: People with HIV (PWH) are at higher risk of complications from acute COVID-19, but their risk of subsequent post-acute sequelae of SARS-CoV2 (PASC) remains unclear. Although vaccination is protective of PASC among survivors in the general population, its effectiveness in PWH has not been explored.
Methods: We used the TriNetX health research database to identify patients with and without HIV aged ≥18 years with confirmed SARS-CoV-2 between January 1, 2020 and July 20, 2023. We employed 1:1 propensity score matching to balance HIV and non-HIV cohorts based on demographics and key comorbidities. The primary outcomes accessed odds of PASC and mortality and secondary outcomes assessed odds of PASC and mortality by vaccination status. PASC was defined as new-onset conditions ≥ 28 days after COVID-19 diagnosis. We reported odd ratios (OR) of outcomes with 95% confidence intervals (CI), with statistical significance set at p < 0.05.
Results: Of 3,029,340 people with confirmed SARS-CoV-2 infection, 0.5% (n=13,214) were PWH, with 7.5% of PWH (n=989) vaccinated. After 28 days post-COVID-19, PWH had higher odds of mortality compared with their non-HIV counterparts (OR 1.22, 95% CI 1.06-1.40) and developing new-onset HTN (OR 1.18, 95% CI 1.03-1.36), heart disease (OR 1.35 95% CI 1.18-1.54), malignancy (OR 1.49, 95% CI 1.22-1.81), and mental disorders (OR 1.62, 95% CI 1.42-1.85). Furthermore, vaccinated PWH had significantly lower odds of death (OR 0.63, 95% CI 0.42-0.93) and new-onset PASC outcomes: DM (OR 0.65, 95% CI 0.43-0.99), heart disease (OR 0.58, 95% CI 0.4-0.85), mental disorders (OR 0.66, 95% CI 0.43-1.00), fatigue (OR 0.82, 95% CI 0.67-0.98), respiratory (OR 0.82, 95% CI 0.70-0.95) and gastrointestinal symptoms (OR 0.78, 95% CI 0.67-0.90).
Conclusion: HIV-positive status increased PASC odds, while COVID-19 vaccination reduced PASC and all-cause mortality risks in PWH.
1 Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus (SARS-CoV-2), remains a major global challenge, resulting in millions of confirmed cases and attributable deaths (1). Following the acute phase of SARS-CoV-2 infection, a significant proportion of survivors experience a constellation of persistent symptoms or new-onset health conditions collectively referred to as the post-acute sequelae of SARS-CoV-2 (PASC) or long COVID (2, 3). The true prevalence of PASC remains unknown; however, systematic reviews and meta-analyses of studies from the United States and globally suggest that 6% to 90% of individuals diagnosed with COVID-19 experience one or more PASC conditions (4–6). Frequently described manifestations of PASC include persistence after the acute illness of fatigue, malaise, myalgias, joint pains, loss of taste or smell, as well as cardiovascular, pulmonary, gastrointestinal, endocrine, mental health, and neurocognitive disorders (2, 3, 7–9–). These sequelae may follow asymptomatic, mild, or severe infection and may persist for many months, resulting in significant functional disability and reduction in the quality of life of survivors (2, 8–11).
The risk factors associated with PASC are incompletely understood; however, current evidence suggests that the occurrence and severity of PASC are influenced by the intensity of the initial acute illness (12), virus-specific factors such as the type of SARS-CoV-2 variant (13, 14), and the presence of premorbid risk factors, including older age, higher body mass index (BMI), cardiovascular disease, diabetes mellitus (DM), allergies, chronic lung disease, chronic kidney disease, and malignancy (15). People with HIV (PWH) have more severe COVID-19 symptoms and adverse outcomes (16) and HIV infection has been suggested as a potential risk factor for PASC; however, there is a dearth of supporting evidence to confirm this. Compared with the general population, people with HIV (PWH) tend to have a higher burden of comorbidities and predisposing risk factors at baseline (17, 18). Additionally, PWH are known to experience chronic residual systemic inflammation, immune activation, and antiretroviral treatment (ART)-induced mitochondrial toxicities even in well-treated HIV disease, which may amplify the “cytokine storm” in COVID-19 and increase the risk of PASC in PWH (17, 18). In a recent study by Peluso and colleagues (19), PWH recovering from COVID-19 had a 4-fold higher odds of developing PASC symptoms compared with their non-HIV counterparts. However, the study enrolled a small sample size (n=39 PWH and n=43 without HIV) and relied solely on persistent symptoms for the definition of PASC (19), thus warranting the need for larger and more systematic studies to examine the association between HIV status and the risk of PASC.
Due to the heterogeneity of the health conditions involved, current guidelines emphasize adopting a holistic and multidisciplinary approach in the management of PASC (20, 21). As the COVID-19 pandemic continues and the number of survivors increases, this is projected to incur considerable healthcare costs and loss in productivity for years to come (22). From a risk mitigation perspective, preventing COVID-19 infection is the most effective means of preventing PASC occurrence. Vaccination against COVID-19 is effective at preventing infection (23, 24) and reducing the severity of acute illness (15) and remains a priority public health intervention in controlling the pandemic. In a recent study, we showed that COVID-19 vaccination reduces the risk of PASC symptoms, new-onset health conditions, and all-cause mortality (25). However, no large-scale studies have systematically assessed the risk of PASC or the impact of vaccination on its occurrence among PWH.
The primary objectives of this study were to describe the association between HIV status and the risk of PASC and all-cause mortality by comparing PWH and their non-HIV counterparts in a large cohort in the United States. Additionally, as secondary objectives, we further assessed the effect of COVID-19 vaccination on the risk of PASC and all-cause mortality among vaccinated and unvaccinated PWH. Lastly, within the PWH population, we investigated the association between HIV disease indices (CD4 cell count, HIV viral load, and ART) and PASC.
2 Materials and methods
2.1 Data source
We used a large national health research network with data sourced from 69 health care organizations (HCO) within the United States (TriNetX, a global federated health research network with waiver from WCG IRB). We included any adult aged ≥18 years with a HCO encounter between January 1, 2020 and July 20, 2023 (last date of data access). TriNetX continuously aggregates clinical data directly from the electronic health records (EHRs) of participating HCOs. TriNetX provides de-identified data, transformed into a proprietary data schema, including an extensive data quality and accuracy assessment. TriNetX does not provide any identifiers on participating HCOs; however, a typical participating HCO includes a large academic health center with inpatient, outpatient, and specialty care services.
2.2 Patient selection, definitions and study outcomes
We first identified all adult patients with positive SARS-CoV-2 infection (COVID-19 International Classification of Diseases (ICD) 10th Revision codes, ICD-10: U07.1, J12.82, U07.2, or positive SARS-CoV-2 and related RNA test or positive Rapid Antigen test). We further categorized patient cohorts into those with previous HIV diagnosis (ICD-10: B20), and those without HIV. For all cohorts, we collected clinical data including patient demographics (age, sex, race and ethnicity), pre-existing comorbid conditions regarded as risk factors for COVID-19 severity (overweight and obesity, neoplasms, hypertension (HTN), heart disease, DM, chronic kidney disease, chronic obstructive lung disease and transplanted organ) COVID-19 vaccination status, and medication history. In addition, for PWH, we collected information on CD4 count, viral load (HIV-1 RNA) and antiretroviral treatment (ART).
The hypothesis of this study was that PWH with a history of COVID-19 are at higher risk of PASC when compared to propensity-matched population of non-HIV COVID-19 survivors. The primary outcomes were the odds of PASC and all-cause mortality compared by HIV status. The secondary outcomes were the odds of PASC and mortality among PWH, compared by COVID-19 vaccination history. PASC was defined using the criteria proposed by the United States Center for Disease Control and Prevention (CDC) as either the persistence of COVID-attributable symptoms or the occurrence of new-onset health conditions at least 28 days following the first COVID-19 diagnosis (26). We used the 28-day cutoff after the COVID-19 diagnosis to ensure that the PASC diagnosis captured did not include medical conditions patients might have had prior to their COVID-19 diagnosis.
For PASC symptoms, we selected the following commonly reported manifestations: respiratory symptoms, fatigue, headache, body ache, diarrhea/constipation and neurocognitive disfunction. Similarly, for new-onset conditions, we selected commonly reported outcomes, as follows: heart disease, HTN, DM, malignancy, thrombosis, thyroid disease, rheumatoid arthritis and mental disorders. We included all-cause mortality in all outcomes assessments as several PASC conditions (e.g., heart disease, thrombosis) are associated with high risk of mortality. All symptoms or conditions selected for inclusion in the study were based on healthcare provider entries of diagnoses and their corresponding International Classification of Diseases Tenth Revision (ICD-10) codes into the EHRs of patients. A full description of study definitions and variables used to query the TriNetX database and ICD-10 codes for specific diagnoses are provided in the Supplementary Materials.
Within the HIV cohort, we performed a secondary analysis to further assess outcomes based on degree of immunosuppression (CD4 < 200 cells/mm3 vs ≥ 200 cells/mm3), virologic suppression (HIV RNA < 200 copies/mL vs ≥ 200 copies/mL), ART class, and circulating SARS-CoV-2 variant. Since there was no information on SARS-CoV-2 variants in patient records, we used COVID-19 pandemic variant periods instead, as reported by the CDC (27): July 1, 2021 to November 30, 2021 for Delta variant and December 1, 2021 to July 20, 2023 for Omicron variant.
2.3 Statistical analyses
Characteristics of study cohorts were described using mean ± standard deviation for continuous variables and frequency and percentages for categorical variables. All analyses were conducted using TriNetX Advanced Analytics platform. To address potential confounders that could bias our results, cohorts were balanced using 1:1 greedy nearest-neighbor propensity score matching based on age, sex, race, ethnicity, BMI, and comorbid conditions (Table 1). For continuous data, we performed independent t-tests. For categorical data, we performed chi-square tests. For outcomes of interest, we calculated odds ratios (ORs) and 95% confidence intervals (CIs), with p < 0.05 considered statistically significant.
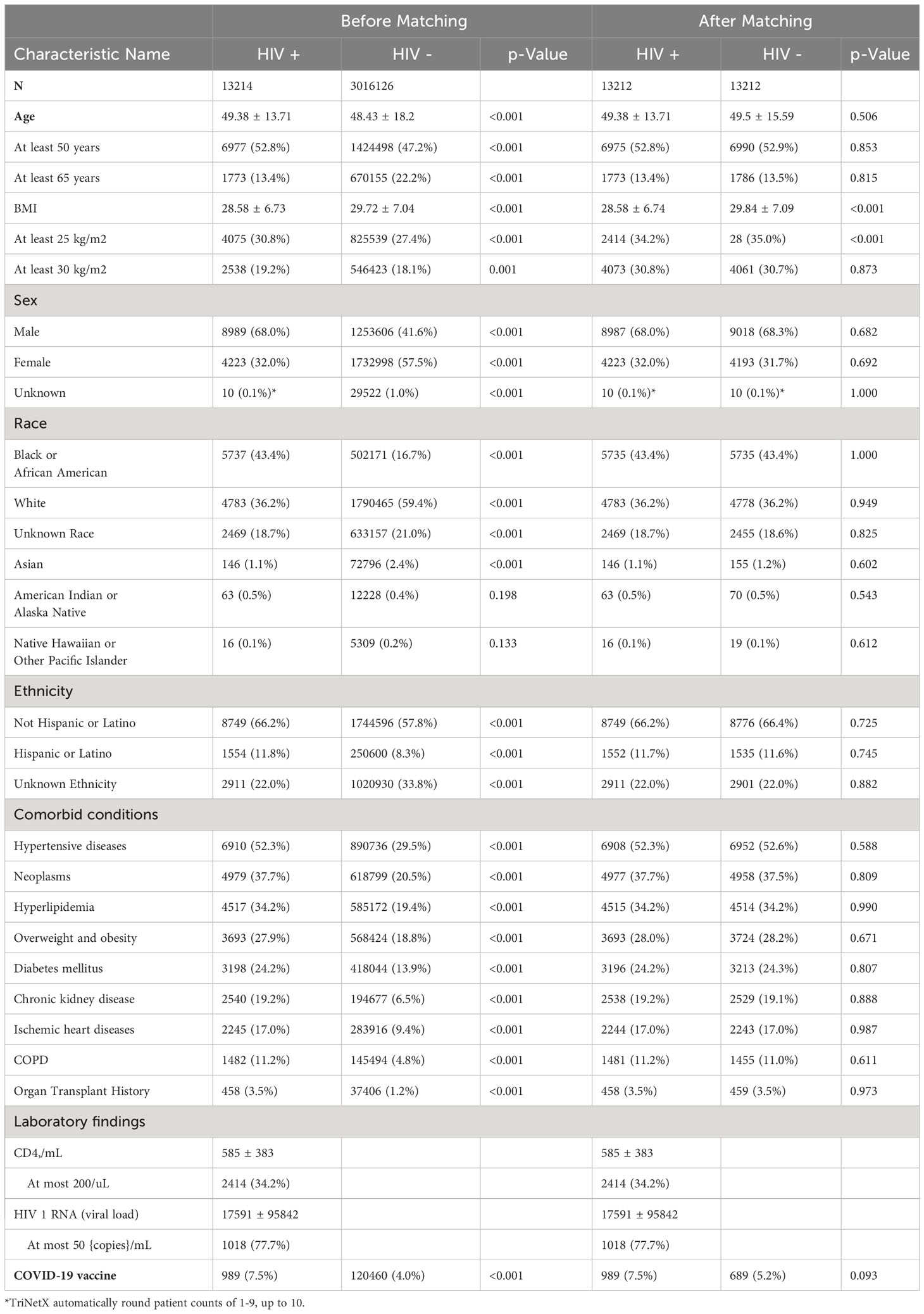
Table 1 Baseline characteristics between COVID-19 patients with or without a HIV positive diagnosis (HIV+, HIV-), both before and after propensity score matching.
2.4 Patient consent statement
The study was approved by the Institution Board Review committee at Case Western Reserve University/University Hospitals Cleveland Medical Center. Written informed consent was waived as data from the TriNetX system safeguards patient’s privacy by reporting deidentified data.
3 Results
3.1 Baseline characteristics of COVID-19 patients with and without a HIV diagnosis
Table 1 reports the baseline characteristics by HIV status before and after propensity score matching. Of 3,029,340 people with confirmed SARS-CoV-2 infection, 0.5% (n=13,214) were PWH, with 7.5% of PWH (n=989) having documented evidence of having received at least 1 dose of any COVID-19 vaccine. Compared to their non-HIV counterparts, PWH were older (mean age 49.38 ± 13.71years vs 48.43 ± 18.2 years, p < 0.001), had fewer females (32.0% vs 57.5%, p < 0.001) and a higher proportion of African Americans (43.3% vs 16.7%, p < 0.001), and Hispanics (11.8% vs 8.3%, p < 0.001). At baseline, PWH were more likely to have an underlying comorbidity, including a history of HTN (52.3% vs 29.5%, p < 0.001), cancer (37.7% vs 20.5%, p < 0.001), DM (36·9% vs 13.7%, p < 0.001), overweight or obesity (36·9% vs 18·7%, p < 0.001), hyperlipidemia (34.2% vs 19.4%, p < 0.001), overweight/obesity (27.9% vs 18.8%, p < 0.001), DM (24.2% vs 13.9%, p < 0.001), chronic kidney disease (19.2% vs 6.5%, p < 0.001), and ischemic heart disease (17.0% vs 9.4%, p < 0.001). Of PWH with available data, the mean CD4 count was 584.54 ± 383 cells/mm3 and 77.7% were virally suppressed (HIV-1 RNA < 50 copies/mL).
3.2 PASC outcomes in patients with and without HIV diagnosis
Table 2 displays the incidence of new-onset diagnosis or persistent symptoms captured at least 28 days after COVID-19 diagnosis. After propensity score matching, PWH had higher odds of death (OR 1.22, 95% CI 1.06-1.4; p = 0.006).The odds of developing new-onset HTN among PWH was 1.18 (95% CI 1.03, 1.36; p < 0.019), heart disease 1.35 (95% 1.18-1.54; p < 0.001), malignancy 1.49 (95% CI 1.22-1.81; p < 0.001), and mental disorders 1.62 (95% CI 1.42-1.85; p < 0.001). Similarly, the odds (OR) of persistent COVID-attributed symptoms remained significantly higher among PWH in a wide range organ systems: respiratory symptoms 1.41 (95% 1.33-1.50; p < 0.001), gastrointestinal symptoms 1.64 (95% CI 1.54-1.73; p < 0.001), headache 1.68 (95% CI 1.53-1.84; p < 0.001), fatigue 1.37 (95% CI, 1.26-1.49; p < 0·0001), body aches 1.41 (95% CI 1.25-1.6; p < 0.001), disturbances to smell and taste 1.43 (95%, 1-2.03; p = 0.049), cognitive impairment 1.43 (95%CI 1.11-1.86; p = 0.006), and abnormal movements 1.21 (95% CI 1.03, 1.43, p = 0.021). However, there was no significant association between odds of each new-onset PASC condition and CD4 count, HIV viremia or class of ART (Supplementary Materials).
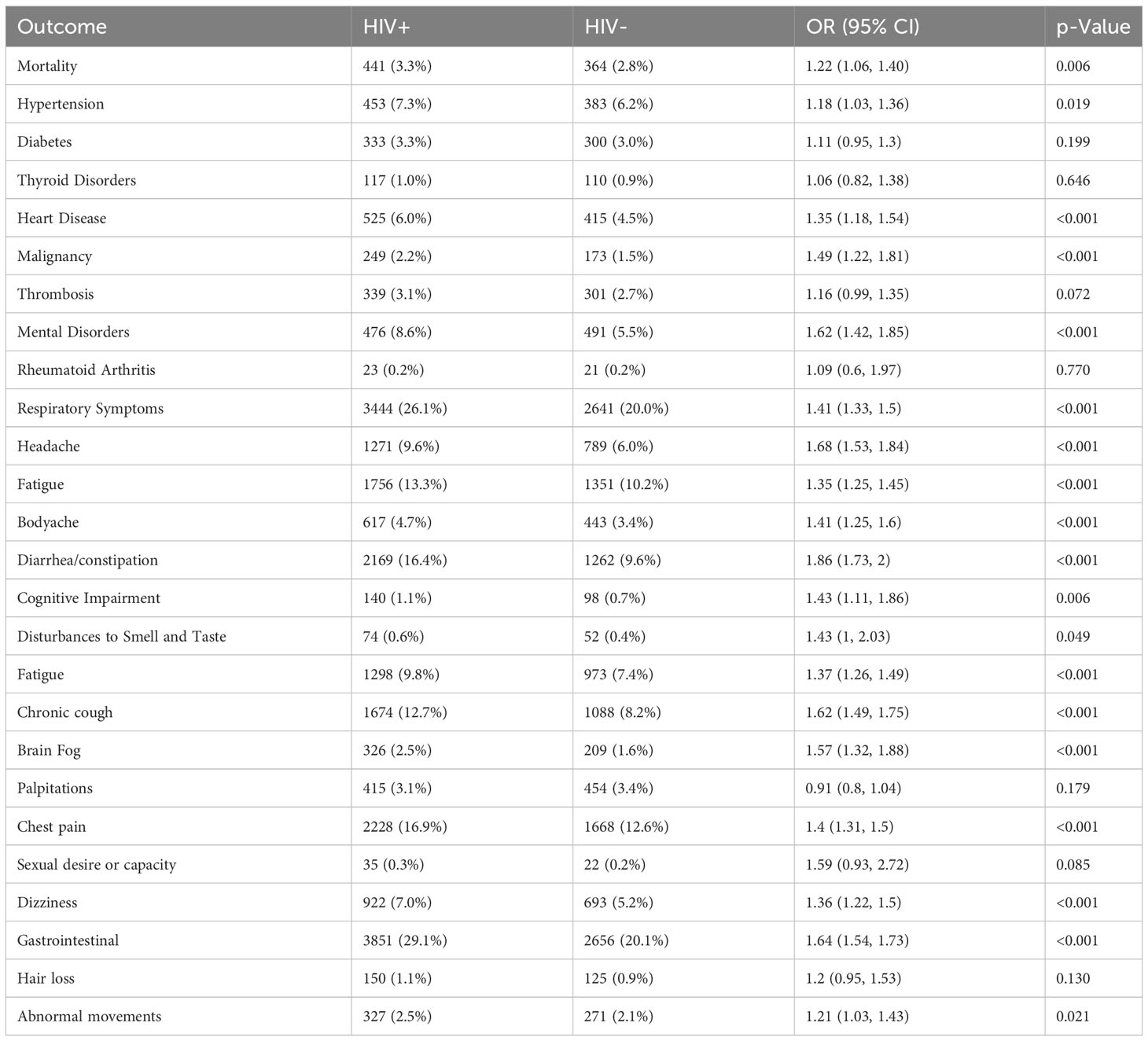
Table 2 PASC related outcomes between COVID-19 patients with or without a HIV positive diagnosis (HIV+, HIV-) after propensity score matching.
3.3 PASC outcomes in patients with HIV diagnosis by SARS-CoV-2 variant
As shown in Table 3, mortality was significantly higher in early pandemic (pre-Delta) period compared with later periods (OR 2.39, 95% CI 1.88-3.05; p < 0.001). The odds of new-onset PASC conditions was also significantly higher in the pre-Delta period: HTN 2.01 (95% CI 1.58-2.57; p < 0.001), DM 2.54 (95% CI 1.90-3.40; p < 0.001), thyroid disorders 2.29 (95% CI 1.39- 3.79; p < 0.001), heart disease 1.98 (95% CI 1.57-2.49; p < 0.001), malignancy 2.46 (95% CI 1.75- 3.45; p < 0.001), thrombosis 1.71 (95% CI 1.29-2.25; p < 0.001), and mental disorders 1.55 (95% CI 1.23-1.96; p < 0.001). There were also significant increases in the prevalence of symptoms among PWH in the pre-Delta compared to later periods: disturbances to smell and taste 3.02 (95% CI 1.61-5.66, p < 0.001), fatigue 1.62, 95% CI 1.4-1.88, p < 0.001), brain fog 1.60 (95% CI 1.2-2.15, p=0.001), palpitations 1.78 (95% CI: 1.38-2.3, p < 0.001), chest pain 1.47 (95% CI: 1.31-1.65, p <0.001), dizziness 1.49 (95% CI: 1.25-1.76, p < 0.001), gastrointestinal symptoms 1.49 (95% CI 1.36-1.64, p <0.001), and abnormal movements 1.61 (95% CI 1.21-2.14, p=0.001).
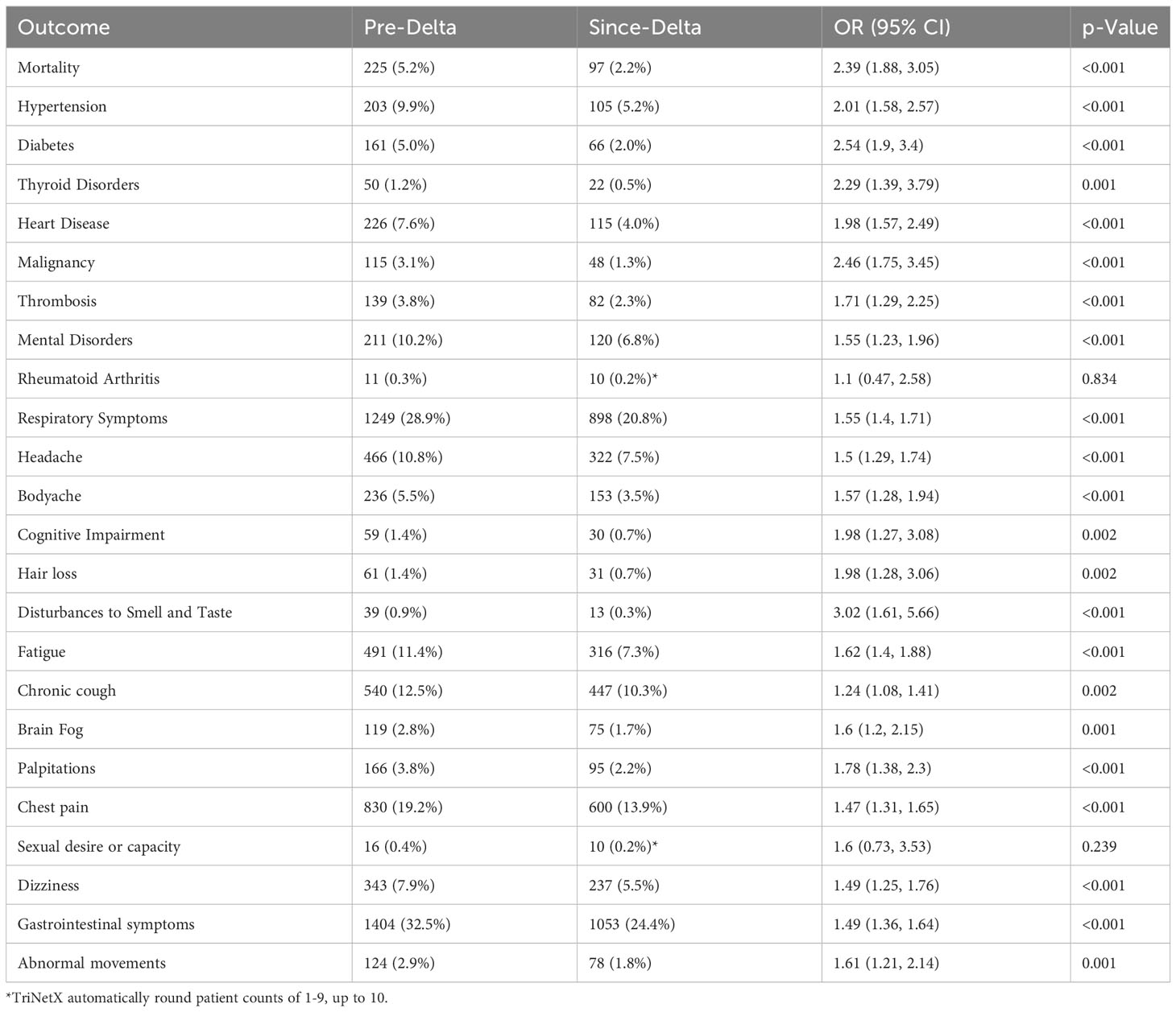
Table 3 PASC outcomes between pre- and since Delta variant COVID-19 patients with HIV positive diagnosis after propensity score matching.
3.4 PASC outcomes in vaccinated and unvaccinated patients with HIV diagnosis
The risk of PASC was compared between vaccinated and unvaccinated cohorts of PWH who had a prior diagnosis of COVID-19 (Table 4). Notably, the odds for mortality was 0.29 (95% CI 0.19-0.46, p <0.001), diabetes 0.65 (95% CI 0.43-0.99; p = 0.042), heart disease 0.58 (95% CI 0.4-0.85; p = 0.005), and mental disorders 0.66 (95% CI 0.43-1; p = 0.047). Similarly, the odds of persistent symptoms were significantly lower in the vaccinated PWH cohort versus unvaccinated PWH, as follows: respiratory symptoms 0.82 (95% CI 0.7-0.95; p = 0.008), gastrointestinal symptoms 0.78 (95% CI 0.67-0.90; p = 0.001), fatigue (OR: 0.81, 95% CI: 0.67-0.98, p = 0.033), chest pain 0.73 (95% CI 0.61-0.87; p = 0.001) and disturbances of smell and taste 0.47 (95% CI 0.22-1.01; p = 0.047).

Table 4 PASC related outcomes between vaccinated and unvaccinated COVID-19 patients with HIV positive diagnosis after propensity score matching.
4 Discussion
In this study from a large health network in the United States, we showed that compared to the general population, HIV infection was significantly associated with increased odds of experiencing PASC, as defined by either persistent COVID-attributable symptoms or developing new conditions at least 28 days after the initial COVID-19 diagnosis. We further showed COVID-19 vaccination had a protective effect against the development of new-onset conditions among PWH. Studies describing the association between HIV status and the emergence of PASC conditions or the impact of COVID-19 vaccination on PASC severity are limited. In an earlier study, we previously showed that vaccination significantly lowered the odds of PASC in vaccinated COVID-19 survivors in the general population (25), however, to the best of our knowledge, this is the first study to demonstrate the protective effect of COVID-19 vaccination against PASC among PWH. This is a crucial finding, as the management of PASC currently remains quite challenging and fragmented. With the approach to managing PASC likely to evolve with the accumulation of new evidence, our findings highlight the role of vaccination as an effective preventive approach to address a burgeoning public health problem.
The results of our study further showed that PASC manifestations were common among COVID-19 survivors even after propensity score matching and encompassed a wide range of heterogenous and overlapping clinical findings in multiple organ systems including hematolo-oncological disorders (malignancies and thrombosis), cardiovascular disorders (HTN and heart disease), respiratory symptoms, gastrointestinal disorders, neurocognitive impairment and mental health disorders. Among PWH, the odds of developing new medical conditions was lowest for HTN (1.18-fold increase) and highest for gastrointestinal symptoms (1.64-fold increase) and constitutional signs/symptoms related to general wellbeing such as headaches (1.68-fold increase). Similarly, the odds of new-onset heart disease and malignancy among PWH was similarly elevated significantly. However, contrary to other studies, there was no increased odds of developing DM, thyroid diseases or rheumatological disorders. Nonetheless, overall, these findings are consistent with findings from multiple studies, which have reported one or more new or persistent physical and/or mental health conditions in 6% to 90% of COVID-19 survivors up to one year after the initial acute infection (4–9, 28, 29).
The increased odds of malignancies among PWH after SARS-CoV-2 infection is an interesting finding that warrants further discussion. HIV is a well-recognized risk factor for both AIDS-defining and non-AIDS defining cancers despite successful treatment (30). However, although common molecular signaling pathways between SARS-CoV-2 and cancer have been noted (31), a clear association has not been established between the two entities. We therefore hypothesize that any potential role SARS-CoV-2 may play in increasing cancer risk in PWH may be a contributory rather than a primary effect, by amplifying the well-described mechanisms and pathways through which HIV is known to elevate the risk of both AIDS-defining and non-AIDS defining cancers (32). More research is needed to investigate this assertion.
The pathophysiologic mechanisms underlying PASC are poorly understood, however, current evidence suggests a prolonged systemic inflammation and an aberrant immune response as major contributing factors (2, 33, 34). Furthermore, in PWH specifically, it may be difficult to distinguish between the relative contributions of HIV-specific factors (i.e., HIV-associated gut dysfunction, immune activation, proinflammatory state and ART-related toxicities) (17, 18) from the effects of the intense systemic inflammatory response triggered by acute SARS-CoV-2 infection (34). Nonetheless, it is hypothesized that the binding of SARS-CoV-2 to the angiotensin-converting enzyme 2 (ACE2) receptor results in activation of the renin-angiotensin pathway which has been implicated in new-onset HTN, acute myocardial injury, cardiac arrhythmias and acute coronary artery events (2, 35). Other studies have suggested a more direct pathophysiologic mechanism through SARS-CoV-2-induced injury to cardiomyocytes, pneumocytes, endothelial cells, pancreatic islet cells and neurons (34, 35). This may lead to fibrosis, structural remodeling and prolonged end-organ dysfunction and unfavorable outcomes associated with PASC manifestations (34, 35). Despite these assertions, it remains unclear whether PASC manifestations are entirely attributable to the direct effects of SARS-CoV-2 infection, or whether infection with the virus results in unmasking or decompensation of pre-existing subclinical health conditions. It also remains unclear whether new-onset conditions that arise from SARS-CoV-2 infection are permanent or resolve over time.
Our findings further suggested that the odds of PASC may depend on the type of SARS-CoV-2 variants circulating in the population. Among PWH, infection with the wild-type variants earlier on in the pre-Delta phases of the pandemic was associated with 3-fold higher odds of mortality and a 2- to 3-fold higher odds of new-onset PASC conditions or persistent symptoms, compared with the Delta or Omicron variant pandemic period. These findings corroborate recent reports from large observational studies from the United Kingdom and Italy (13, 14). Several reasons could explain these observations. For example, while new mutations in Omicron sublineages confer greater replicative advantage (i.e., increased infectiousness), several studies have reported milder disease compared with Delta and prior variants, which could partly account for lower odds of PASC (36, 37). Another plausible explanation why PASC occurrence rates were lower with each successive wave of SARS-CoV-2 variant could be attributed in part to the protective effect of COVID-19 vaccination as it became more widely available for the population. More detailed studies are needed to confirm these findings.
Our study had a few methodological limitations worth discussing, which may affect the generalizability of our findings. First, people with asymptomatic or mild disease are less likely to seek medical care and therefore unlikely to have been captured in the database, leading to an underestimate of the true prevalence of PASC among COVID-19 survivors. Second, there are varying definitions of PASC and currently no standardized system of reporting symptoms or conditions. In addition, PASC documentation relies heavily on self-reports, which may have limited the accuracy of reporting in the EHR system. Third, we were unable to determine the type of COVID-19 vaccine and number of doses received by patients (i.e., first, second, or booster doses) both of which may have an impact on the occurrence and severity of PASC outcomes. Due to the nature of the database, a patients’ first or second dose may have been missed in the reporting (e.g., if a dose occurred outside a patients’ primary HCO, such as a pharmacy or mass vaccination event, and was not reported back), but included a record of a booster. We addressed this limitation in the EHR system by assuming that these patients have a first/second dose, and thus included them as a “vaccinated” patient. Fourth, we were able to assess onset and severity of symptoms, both which are known to be associated with HIV positive status as well as occurrence and severity of PASC. Fifth, in assessing incident PASC, we compared PWH with those without HIV. HIV positive status is itself is associated with several of the symptoms and comorbidities included in PASC, which may make it challenging to distinguish between complications related to SARS-CoV-2 and the progression of HIV. Another limitation of our study was the inability to evaluate the effect of COVID-19 reinfections on PASC, as reinfections are linked to an increased risk of PASC (38). This limitation stemmed from the lack of ICD-10 codes for distinguishing first COVID-19 infections from reinfections. Finally, being an observational study, causation cannot be inferred. Despite these limitations, however, major strengths of our study lie in the fact that we were able to demonstrate higher odds of PASC among PWH. Moreover, in addition to symptoms, we were able to carefully capture the emergence of new-onset health conditions following acute SARS-CoV-2 infection. Our findings may have implications outside of HIV as other clinical conditions known to be associated with chronic sustained inflammation, such as rheumatoid arthritis and systemic lupus erythematosus, may suffer from higher PASC and should be investigated separately.
In summary, our study showed that PWH have higher odds of PASC compared with their non-HIV counterparts. Importantly, we also demonstrated that prior COVID-19 vaccination was associated with significantly lower odds of all-cause mortality and was protective against the development of PASC among PWH. With the increase in the number of COVID-19 survivors, vaccination may offer an effective preventive strategy to address a burgeoning public health problem.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University Hospitals Cleveland Medical Center Institutional Review Board and the studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The TritNetX Research Network was used to obtained de-identified data.
Author contributions
GY: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing, Project administration, Visualization, Data curation. JP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. GM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication was made possible through funding support from the University Hospitals Cleveland Medical Center and the Clinical and Translational Science Collaborative of Cleveland (UL1TR002548) from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH Roadmap for Medical Research. The funders played no role in the designing, conduct and dissemination of the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1297195/full#supplementary-material
References
1. World Health Organization. COVID-19 Dashboard (2023). Available at: https://covid19.who.int/ (Accessed September 15, 2023).
2. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
3. Hope AA, Evering TH. Postacute sequelae of severe acute respiratory syndrome Coronavirus 2 infection. Infect Dis Clin North Am (2022) 36:379–95. doi: 10.1016/j.idc.2022.02.004
4. Global Burden of Disease Long COVID Collaborators, Wulf Hanson S, Abbafati C, Aerts JG, Al-Aly Z, Ashbaugh C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA (2022) 328:1604–15. doi: 10.1001/jama.2022.18931
5. Woodrow M, Carey C, Ziauddeen N, Thomas R, Akrami A, Lutje V, et al. Systematic review of the prevalence of long COVID. Open Forum Infect Dis (2023) 10:ofad233. doi: 10.1093/ofid/ofad233
6. O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine (2022) 55:101762. doi: 10.1016/j.eclinm.2022.101762
7. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw Open (2021) 4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568
8. Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract (2021) 75:e13746. doi: 10.1111/ijcp.13746
9. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature (2021) 594:259–64. doi: 10.1038/s41586-021-03553-9
10. Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ - The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res (2020) 9:1349. doi: 10.12688/f1000research.27287.1
11. Bowles KH, McDonald M, Barrón Y, Kennedy E, O’Connor M, Mikkelsen M. Surviving COVID-19 after hospital discharge: symptom, functional, and adverse outcomes of home health recipients. Ann Intern Med (2021) 174:316–25. doi: 10.7326/M20-5206
12. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun (2021) 12:6571. doi: 10.1038/s41467-021-26513-3
13. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet (2022) 399:2263–4. doi: 10.1016/S0140-6736(22)00941-2
14. Azzolini E, Levi R, Sarti R, Pozzi C, Mollura M, Mantovani A, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA (2022) 328:676–8. doi: 10.1001/jama.2022.11691
15. Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, et al. Risk factors for severe COVID-19 outcomes among persons aged ≥18 years who completed a primary COVID-19 vaccination series - 465 health care facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep (2022) 71:19–25. doi: 10.15585/mmwr.mm7101a4
16. Yendewa GA, Perez JA, Schlick K, Tribout H, McComsey GA. Clinical features and outcomes of coronavirus disease 2019 among people with human immunodeficiency virus in the United States: A multicenter study from a large global health research network (TriNetX). Open Forum Infect Dis (2021) 8:ofab272. doi: 10.1093/ofid/ofab272
17. Sax PE, Erlandson KM, Lake JE, Mccomsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis (2020) 71:1379–89. doi: 10.1093/cid/ciz999
18. Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation (2008) 118:e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624
19. Peluso MJ, Spinelli MA, Deveau TM, Forman CA, Munter SE, Mathur S, et al. Postacute sequelae and adaptive immune responses in people with HIV recovering from SARS-COV-2 infection. AIDS (2022) 36:F7–F16. doi: 10.1097/QAD.0000000000003338
20. Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl JP, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect (2022) 28:955–72. doi: 10.1016/j.cmi.2022.02.018
21. Centers for Disease Control and Prevention. Evaluating and caring for patients with post-COVID conditions: interim guidance: management (2021). Available at: https://stacks.cdc.gov/view/cdc/107146 (Accessed September 15, 2023).
22. McNaughton CD, Austin PC, Sivaswamy A, Fang J, Abdel-Qadir H, Daneman N, et al. Post-acute health care burden after SARS-CoV-2 infection: a retrospective cohort study. CMAJ (2022) 194:E1368–76. doi: 10.1503/cmaj.220728
23. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med (2020) 383:2603–15. doi: 10.1056/NEJMoa2034577
24. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med (2021) 384:403–16. doi: 10.1056/NEJMoa2035389
25. Zisis SN, Durieux JC, Mouchati C, Perez JA, McComsey GA. The protective effect of Coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: A multicenter study from a large national health research network. Open Forum Infect Dis (2022) 9:ofac228. doi: 10.1093/ofid/ofac228
26. Centers for Disease Control and Prevention. Post-COVID conditions: information for healthcare providers. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html (Accessed September 15, 2023).
27. Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (Accessed September 15, 2023).
28. Heesakkers H, van der Hoeven JG, Corsten S, Janssen I, Ewalds E, Simons KS, et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA (2022) 327:559–65. doi: 10.1001/jama.2022.0040
29. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet (2021) 398:747–58. doi: 10.1016/S0140-6736(21)01755-4
30. Deeken JF, Tjen-A-Looi A, Rudek MA, Okuliar C, Young M, Little RF, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis (2012) 55:1228–35. doi: 10.1093/cid/cis613
31. Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer (2021) 20:76. doi: 10.1186/s12943-021-01363-1
32. Isaguliants M, Bayurova E, Avdoshina D, Kondrashova A, Chiodi F, Palefsky JM. Oncogenic effects of HIV-1 proteins, mechanisms behind. Cancers (Basel) (2021) 13:305. doi: 10.3390/cancers13020305
33. Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, et al. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol (2021), 1–9. doi: 10.1007/s12016-021-08848-3
34. Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med (2022) 54:1473–87. doi: 10.1080/07853890.2022.2076901
35. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ (2021) 374:n1648. doi: 10.1136/bmj.n1648
36. Tegally H, Moir M, Everatt J, et al. Emergence of SARS-CoV-2 omicron lineages BA.4 and BA.5 in South Africa. Nat Med (2022) 28:1785–90. doi: 10.1038/s41591-022-01911-2
37. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA (2022) 327:583–4. doi: 10.1001/jama.2021.24868
Keywords: HIV, COVID-19, PASC, vaccination, comorbidities
Citation: Yendewa GA, Perez JA, Patil N and McComsey GA (2024) Associations between post-acute sequelae of SARS-CoV-2, COVID-19 vaccination and HIV infection: a United States cohort study. Front. Immunol. 15:1297195. doi: 10.3389/fimmu.2024.1297195
Received: 19 September 2023; Accepted: 08 January 2024;
Published: 22 January 2024.
Edited by:
Morgane Solis, Hôpitaux Universitaires de Strasbourg, FranceReviewed by:
Hang Su, Albert Einstein College of Medicine, United StatesJacques L. Tamuzi, Stellenbosch University, South Africa
Copyright © 2024 Yendewa, Perez, Patil and McComsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: George A. Yendewa, Z2F5N0BjYXNlLmVkdQ==
 George A. Yendewa
George A. Yendewa Jaime Abraham Perez3
Jaime Abraham Perez3 Nirav Patil
Nirav Patil Grace A. McComsey
Grace A. McComsey