- 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Molecular Imaging, Wuhan, China
- 3Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Immunotherapy, represented by immune checkpoint inhibitors (ICIs), is a major breakthrough in cancer treatment. Studies have reported that the use of ICIs is associated with an increase in the pulmonary artery to ascending aorta diameter (PAD/AoD) ratio. However, the impact of PAD/AoD ratio progression on the prognosis of patients is unclear.
Methods: This retrospective cohort study included patients with stage III or IV non-small cell lung cancer (NSCLC) treated with ICIs at the Wuhan Union Hospital between March 1, 2020, and September 1, 2022. The baseline and post-treatment PAD/AoD ratios of patients were evaluated through chest CT scans. The primary outcome of this study was overall survival (OS), while the secondary outcomes included progression-free survival (PFS), objective response rate (ORR) and disease control rate (DCR).
Results: The PAD/AoD ratio increased after the initiation of ICIs (from 0.75 to 0.78; P < 0.001). A total of 441 patients were divided into severe group (n=221) and non-severe group (n=220) according to the median increase of PAD/AoD ratio (1.06). Compared with the non-severe group, the severe group had a lower DCR (87.8% vs. 96.0%, P = 0.005) and ORR (87.5% vs. 96.0%, P = 0.063). Over the entire duration of follow-up (median 22.0 months), 85 (38.5%) patients in the severe group and 30 (7.3%) patients in the non-severe group died. An increased PAD/AoD ratio was associated with shorter PFS (Hazard ratio (HR): 1.48 [95% CI, 1.14 to 1.93]; P = 0.003) and OS (HR: 3.50 [95% CI, 2.30 to 5.30]; P < 0.001). Similar results were obtained across subgroups.
Conclusions: ICI treatment exacerbates an increase in the PAD/AoD ratio in patients with cancer, and greater increase in the PAD/AoD ratio was associated with a worse prognosis. PAD/AoD ratio could be a biomarker to stratify prognosis of NSCLC patients treated with ICIs.
Introduction
Different from traditional anti-tumor drugs that directly attack cancer cells, immune checkpoint inhibitors (ICIs) enhance the tumor-killing response mediated by CD8-positive T cells through blocking the programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways (1, 2). Lung cancer is the most common malignant tumor, with more than 2 million cases and nearly 1.8 million deaths worldwide every year, of which approximately 85% of patients are diagnosed with non-small cell lung cancer (NSCLC) (3, 4). Since 2015, nivolumab became the first PD-1 inhibitor approved by the Food and Drug Administration (FDA) for the treatment of NSCLC and achieved good efficacy (5). The use of ICIs in patients with NSCLC has continued to expand (6, 7). However, despite its impressive efficacy, not all patients with NSCLC respond to ICIs, and excessive immune response leads to the destruction of immune tolerance and the occurrence of immune-related adverse events (irAEs) (8–10). These include pulmonary and cardiovascular adverse effects (11–13). Previously, we performed a retrospective analysis of 1,458 patients with NSCLC and found that patients treated with ICIs had a higher incidence of cardiovascular events than patients treated with other anti-neoplastic therapies, such as chemotherapy and targeted therapies. Recently, two cases have been reported in which pulmonary arterial hypertension (PAH) developed after ICI treatment of (14, 15). Therefore, there is an urgent need for a non-invasive biomarker that can predict the efficacy of ICI treatment to determine which patients will benefit from ICIs and avoid unnecessary irAEs.
As a measurable imaging marker that can be obtained in chest computed tomography (CT) images, the pulmonary artery to ascending aorta diameter (PAD/AoD) ratio is highly correlated with the pulmonary arterial pressure obtained by right heart catheterization, and its increase is a potential feature of PAH (16–18). It is also closely related to the change of cardiac structure and function (19–21). Recent studies found that the use of ICIs is associated with the enlargement of PAD and a consequent increase in the PAD/AoD ratio (22, 23). However, the association between an increased PAD/AoD ratio and the clinical outcomes remains unclear.
Therefore, our study aims to elucidate the relationship between the progression of PAD/AoD ratio and the prognosis of patients with NSCLC treated with ICIs, thereby clarifying whether the PAD/AoD ratio can be used as a biomarker to stratify prognosis of NSCLC patients treated with ICIs.
Materials and methods
This retrospective cohort study was approved by the local ethics committee and the institutional review board of the Tongji Medical College of Huazhong University of Science and Technology (Institutional Review Board No. S054), and the study procedures were performed in compliance with Good Clinical Practice and the Declaration of Helsinki. The need for written informed consent was waived by the institutional review board.
Study design and patient selection
This was a retrospective study conducted at a single academic center. Consecutive NSCLC patients treated with ICIs initially at Wuhan Union Hospital from March 1, 2020 to September 1, 2022 were included in this study. All patients underwent at least two CT scans (before and after treatment).
The inclusion criteria were as follows: (1) patients diagnosed with stage III or IV NSCLC following the NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (24); (2) patients aged > 18 years; (3) patients had two or more chest CT scans; (3) patients received ICI treatment for more than 4 cycles. The exclusion criteria were as follows: (1) patients with incomplete or difficult-to-evaluate clinical or imaging data; (2) patients with other primary malignant tumors; (3) the time between two chest CT scans was less than three months.
Procedures
We retrospectively collected baseline data of patients from clinical cases, including age, gender, body mass index, diabetes, hypertension, smoking, hyperlipidemia, hemoglobin, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, type of ICIs, and stages of tumor. All baseline information was based on the results of the first hospitalization. Data related to hypertension, diabetes and hyperlipidemia included those diagnosed during hospitalization and use of related medications.
CT image acquisition
Imaging parameters were obtained from baseline and the most recent chest CT scan after initiation of ICIs. Two CT scans of all patients were acquired on a 128-slice CT scanner (Siemens Healthcare, Forchheim, Germany) using the same parameters. Patients were placed in a supine position and received sufficient breathing training before scanning. The scanning range was from the level of the upper thoracic inlet to below the costophrenic angle; the tube voltage was 120kVp and the tube current was automatically adjusted. All CT images were reconstructed using a standard soft convolution kernel with a slice thickness of 1 mm and an interval of 1 mm.
PAD/AoD ratio measurement and assessment
According to the method described by Wells et al. (25), the inner diameter of the main pulmonary artery (PAD) and the ascending aorta (AoD) at the same level were measured (Figure 1A). Two independent radiologists (L.B. and Y.L. with 26 and 20 years of thoracic imaging experience, respectively) manually evaluated the images at the PACS workstation, blinded to all the clinical information. Assessments were repeated after 30 days, and intra- and inter-observer agreements were calculated. Any disagreements were resolved by discussion and consensus. The progression of the PAD/AoD ratio was annualized according to the following formula: [(post-ICIs/pre-ICIs)/months] *12. Using the 1.06 as the cutoff value, the patients were divided into severe group (PAD/AoD ratio ≥ 1.06) and non-severe group (PAD/AoD ratio < 1.06) (Figures 1B–E). The cut-off value of 1.06 was based on the median of PAD/AoD ratio increase. The median is a common and practical cut-off value classification method, which can be found in continuous variables such as clinical indicators (26), laboratory indicators (27, 28), and imaging indicators (29), and pathological indicators (30, 31). Figure 1F depicts the PAD/AoD ratio before and after ICIs with standard deviation and data points.
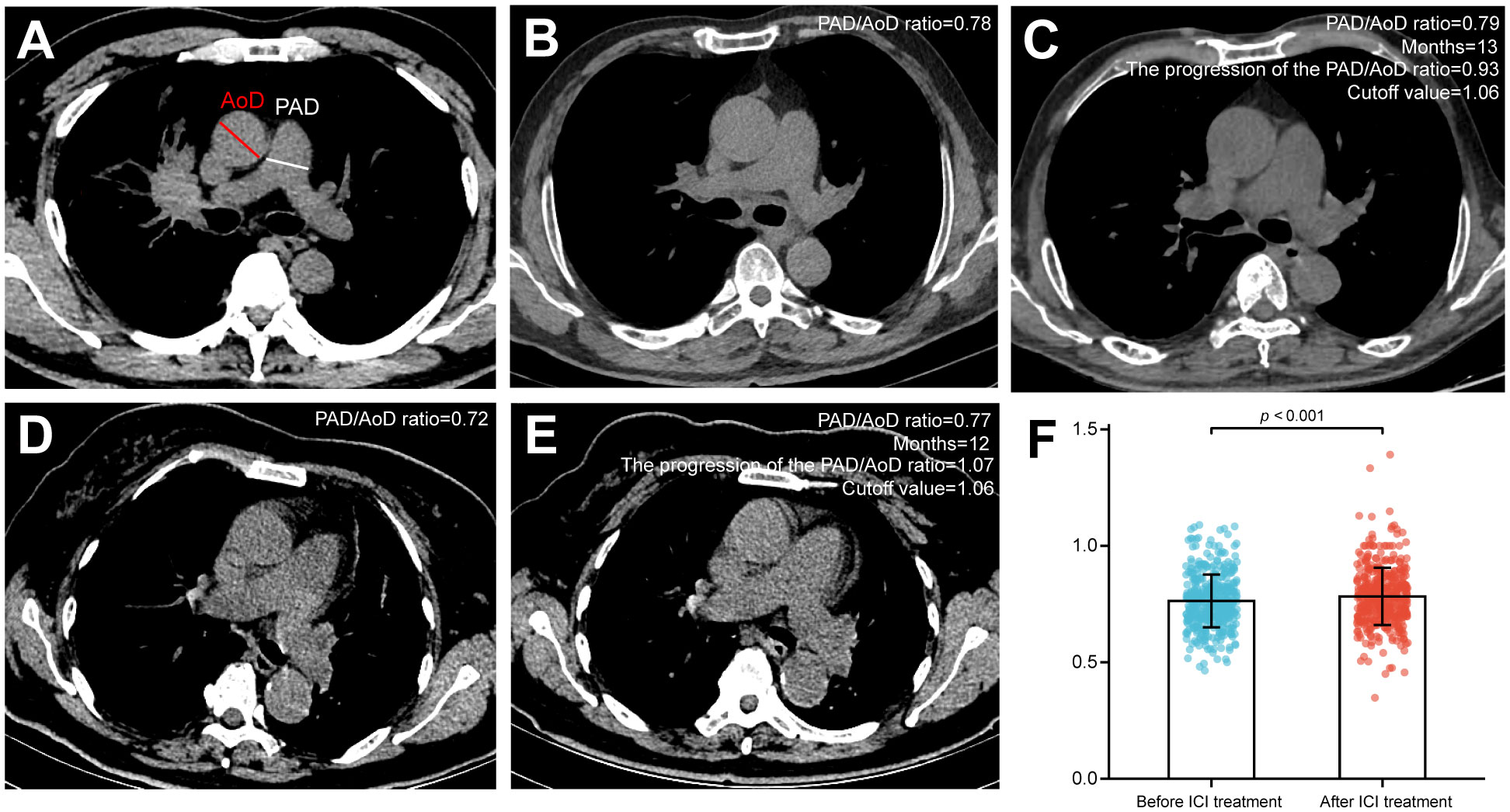
Figure 1 (A) Pulmonary artery diameter (PAD) and ascending aorta diameter (AoD) measured by chest CT scan. (B, C) The PAD/AoD ratio of a patient was 0.78 before immune checkpoint inhibitors (ICIs), which progressed to 0.79 after 13 months of ICI treatment. The progression of the PAD/AoD ratio was 0.93, which was less than the cutoff value (1.06), and this patient was included in the non-severe group. (D, E) The PAD/AoD ratio of a patient was 0.72 before ICIs, which progressed to 0.77 after 12 months of ICI treatment. The progression of the PAD/AoD ratio was 1.07, which was greater than the cutoff value (1.06), and this patient was included in the severe group. (F) Quantitation of the PAD/AoD ratio before and after ICIs. Data (n=441) were shown as means ± SD. Paired t-test was used to compare changes in the PAD/AoD ratio before and after ICIs.
Follow-up and end points
The required follow-up data was obtained through clinical cases information (including outpatient or inpatient information), imaging data (including CT and magnetic resonance (MR) images) and telephone follow-up. All patients were followed up until September 1, 2022 or until the patient death. The primary outcome of this study was overall survival (OS), defined as the time from ICI initiation to patient death from any cause. The secondary outcomes included progression-free survival (PFS), objective response rate (ORR) and disease control rate (DCR). The time from the patient starting ICI treatment to disease progression or death was expressed as PFS. Treatment efficacy was evaluated using complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) criteria. The ORR and DCR were calculated from the proportion of patients with CR and PR and from those with CR, PR and SD, respectively. The above definitions are based on New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) (32).
Statistical analysis
Continuous variables were presented as mean (SD) or median (IQR) and were compared using the t-test or the Wilcoxon rank sum test. Paired samples used paired t-test. Categorical variables were presented as counts (percent) and were compared using the Pearson χ2 test or the Fisher exact test. Baseline and post-treatment PAD/AoD ratios were compared by paired t-test. The log-rank test was used to compare the differences in PFS and OS between the severe and non-severe groups and the results were presented in the form of Kaplan-Meier survival curves. Univariable and multivariable Cox models were used to estimate hazard ratios for PFS and OS between the two groups. Variables identified as P < 0.1 in the univariable analysis were entered into the multivariable Cox regression model. Hazard ratios for PFS and OS were calculated for each subgroup using unstratified univariate Cox models with covariates of clinical interest. All statistical tests were two-tailed, with the P value level of significance being 0.05. The data were analyzed using SPSS software, version 26.0 (IBM, Chicago, IL, USA), and R software, version 4.3.0 (R Foundation).
Results
Patient characteristics
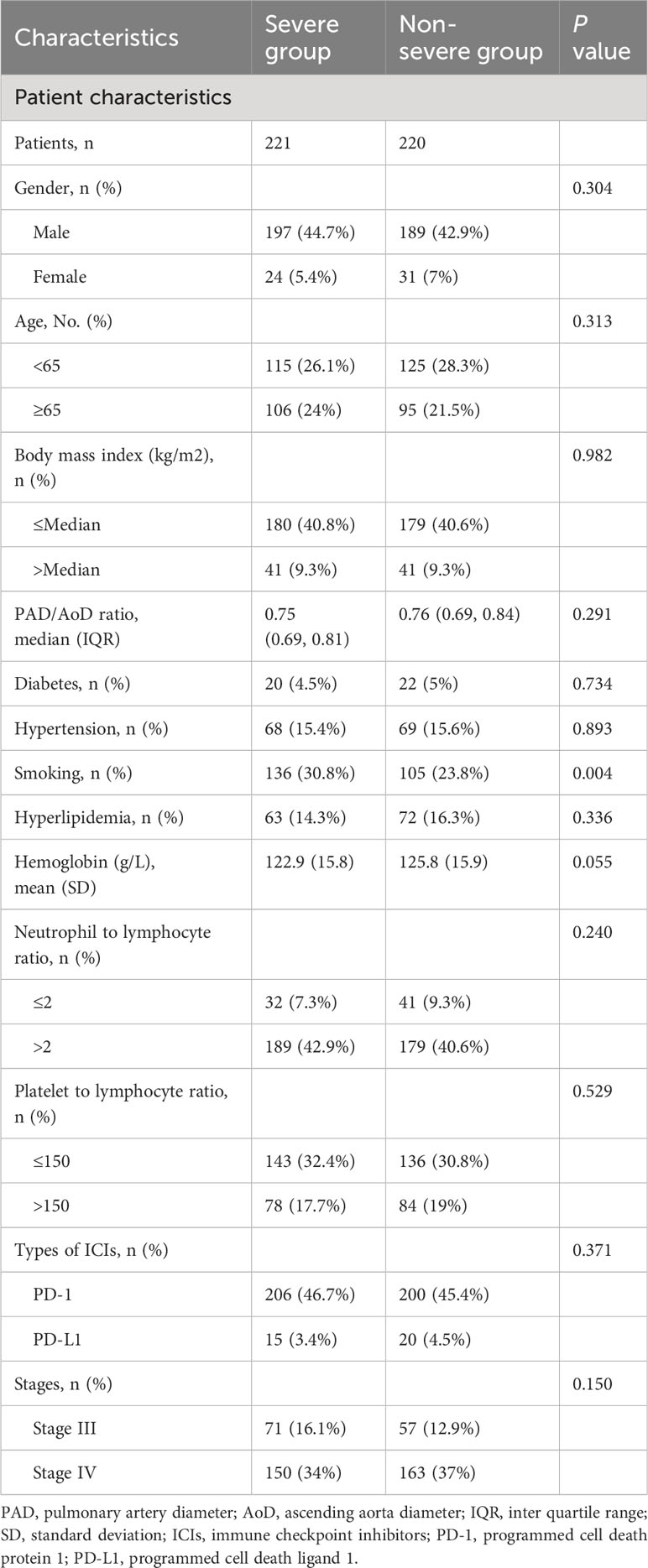
A total of 441 patients with NSCLC who received ICI treatment were included in this retrospective cohort study, including 221 patients with severe progression of PAD/AoD ratios and 220 patients with non-severe progression. The baseline demographic and clinical characteristics of the patients are shown in Table 1. The proportion of patients with a history of smoking in the severe group was higher than that in the non-severe group (30.8% vs. 23.8%, P = 0.004). With the exception of smoking history, there were no significant differences were observed in baseline characteristics between the two groups.
PAD/AoD ratio progression
During the entire follow-up period, the PAD/AoD ratio progressed from 0.75 (IQR, 0.69 to 0.82) before ICI treatment to 0.78 (0.71 to 0.85) after ICI treatment (P < 0.001, Figure 1F). In addition, we randomly selected 100 patients with NSCLC who receive non-ICI treatments at the same time and evaluated their PAD/AoD ratio before and after treatment to exclude the potential influence of factors other than ICI treatment. Baseline characteristics between ICI and non-ICI groups are summarized in Supplementary Table 1. There was no difference in baseline PAD/AoD ratio (0.75 vs. 0.76; P = 0.351) and the two CT scans intervals (12.0 months vs. 11.6 months; P = 0.279) between the two groups. The PAD/AoD ratio in the non-ICI group changed from 0.76 (IQR, 0.71 to 0.82) to 0.75 (IQR, 0.69 to 0.83) and was not statistically significant (P = 0.716; Supplementary Table 2). Intra-observer and inter-observer agreement for the PAD/AoD ratio measurement were both good at 0.96 (95% CI, 0.91 to 0.97) and 0.92 (95% CI, 0.87 to 0.95) respectively.
Tumor response
Tumor responses between the two groups are shown in Supplementary Table 3 and Supplementary Figure 1. The proportion of patients in the severe group with PR was 42.5%, with PD was12.2%, and with SD was 45.2%, compared with 51.4% (PR), 4.1% (PD), and 44.4% (SD) in the non-severe group. The DCR in the severe group was significantly lower compared to that in the non-severe group (87.8% vs. 96.0%, P = 0.005). At the same time, patients in the severe group also showed a tendency to have a lower ORR, although the difference was not statistically significant (87.5% vs. 96.0%, P = 0.063).
Survival analysis
During the median follow-up of 22.0 months (IQR 17.0, 29.0), 85 of 221 (38.5%) patients died in the severe group and 30 of 220 (7.3%) patients died in the non-severe group. Hazard ratios (HRs) for the severe group versus non-severe group were 1.48 (95% CI, 1.14 to 1.93; P = 0.003) for PFS and 3.50 (95% CI, 2.30 to 5.30; P < 0.001) for OS (Figure 2). Twenty-month PFS rates were 44.5% (95%CI, 38.4 to 51.6) versus 55.4% (95%CI, 49.1 to 62.6), and twenty-month OS rates were 63.2% (95%CI, 57.0 to 70.0) versus 89.7% (95%CI, 85.6 to 94.0). Median PFS was 14.0 (95%CI, 10.0 to not attained) and 36.0 (95%CI, 20.0 to not attained) months for the severe and non-severe groups, respectively. Due to insufficient follow-up time, the median OS of the two groups cannot be obtained. Instead, we plotted receiver operating characteristic (ROC) curves with OS at different time points and calculated the area under the curve (AUC) (Supplementary Figure 2). We constructed Kaplan-Meier curves for PFS and OS using the Youden index of 1-year, 1.5-year, and 2-year OS as the optimal cutoff value (Supplementary Figure 3), and obtained similar results as before.
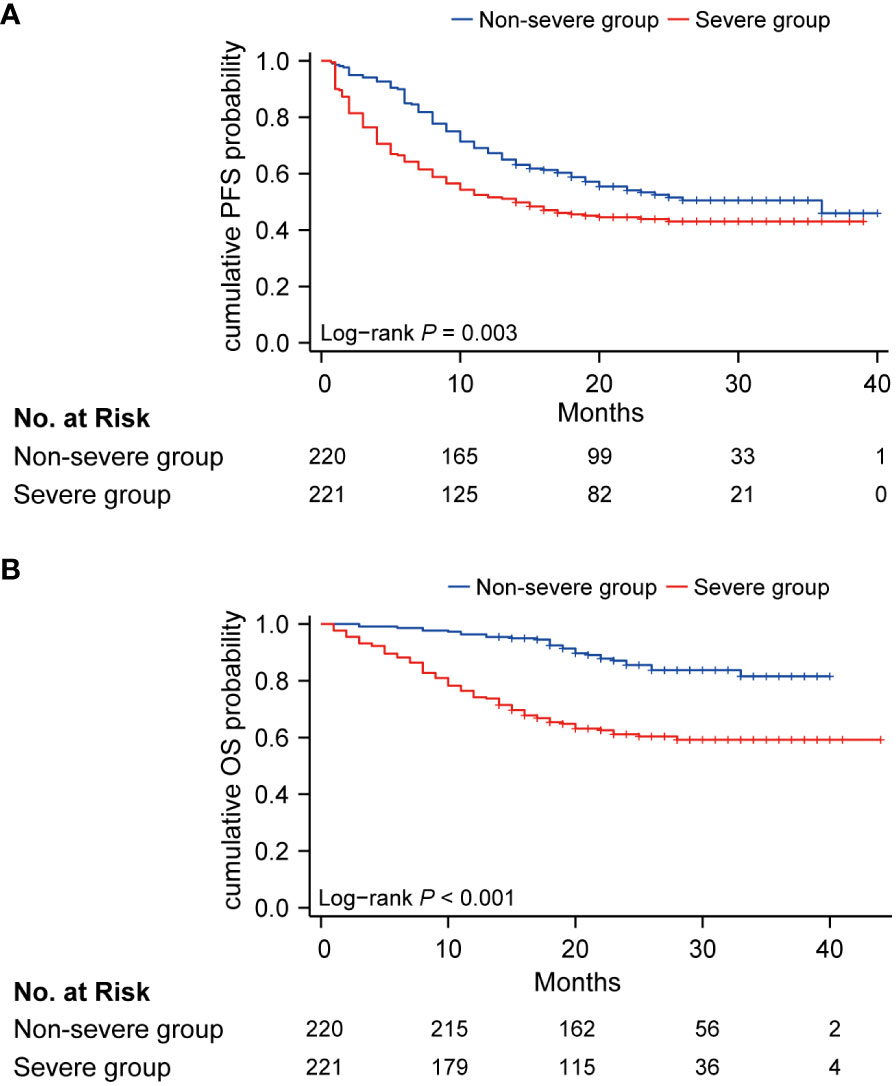
Figure 2 Kaplan-Meier curve of PFS (A) and OS (B) in non-severe group (blue) and severe group (red). PFS, progression-free survival; OS, overall survival.
Univariable and multivariable analyses
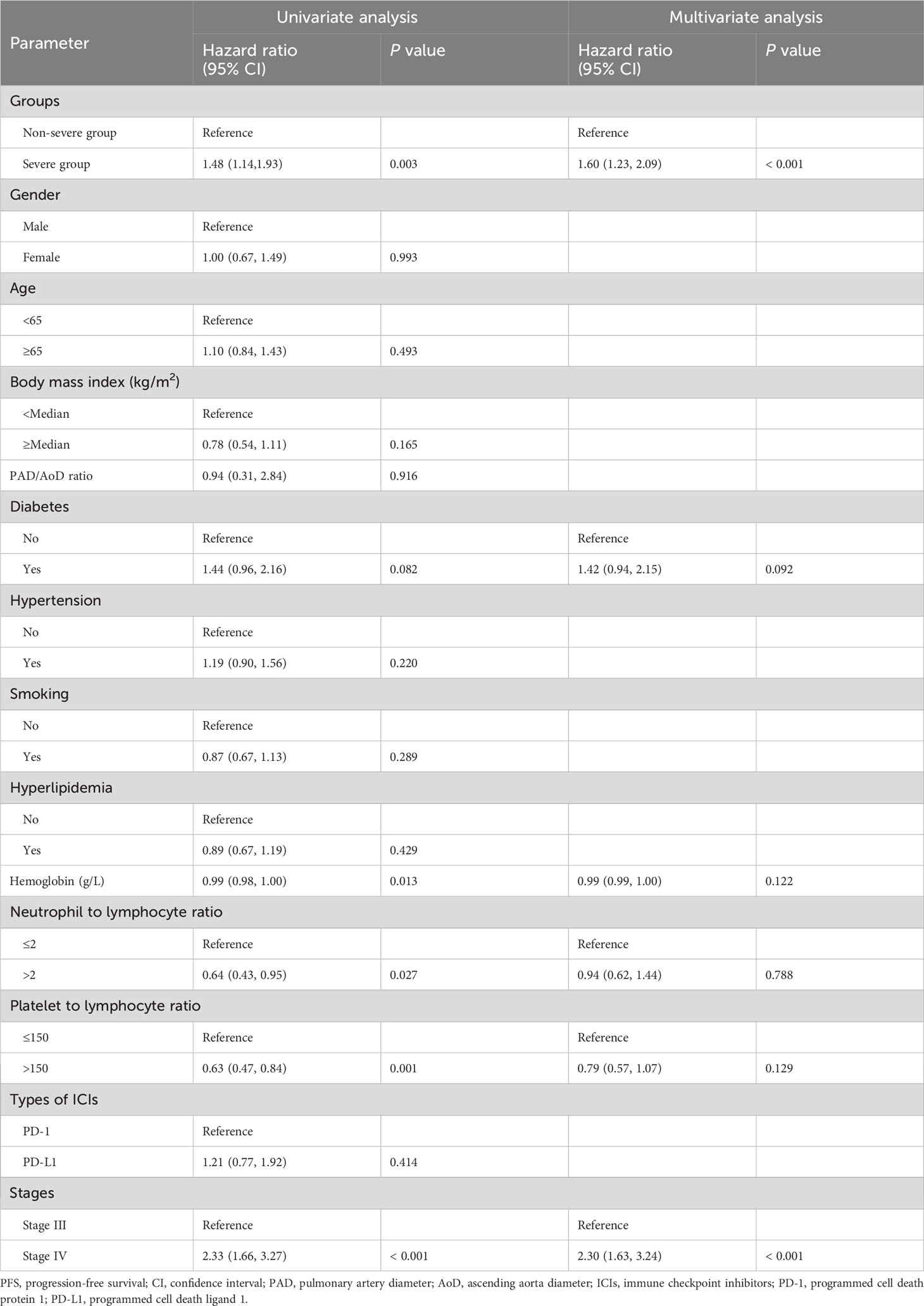
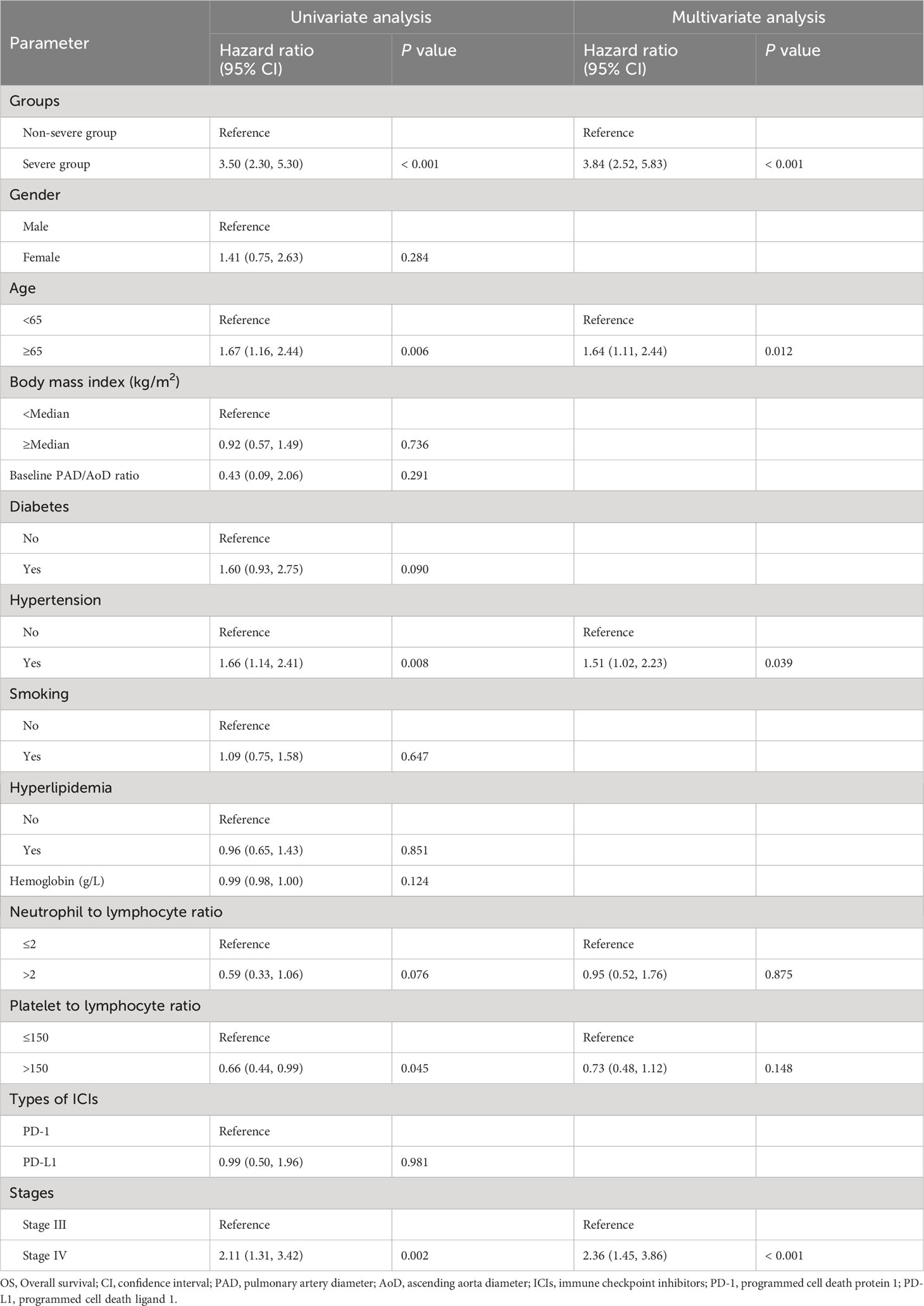
In univariable analysis for PFS, severe PAD/AoD progression, diabetes, hemoglobin, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and stage were identified as potential predictors and further included in the multivariable model. In the multivariable Cox regression model, severe PAD/AoD progression (HR, 1.60 [95%Cl, 1.23 to 2.09]; P < 0.001) and higher disease stage (HR, 2.30 [95%Cl, 1.63 to 3.24]; P < 0.001) were significant risk factors for reduced PFS (Table 2). Similarly, the final multivariable Cox regression model for OS showed that severe PAD/AoD progression (HR, 3.84 [95%Cl, 2.52 to 5.83]; P < 0.001), advanced age (HR, 1.64 [95%Cl, 1.11 to 2.44]; P = 0.012), hypertension (HR, 1.51 [95%Cl, 1.02 to 2.23]; P < 0.039) and advanced disease stage (HR, 2.36 [95%Cl, 1.45 to 3.86]; P < 0.001) were independent risk factors associated with the reduced OS (Table 3).
Subgroup analysis
Across subgroups based on baseline characteristics, a between-group difference in PFS (Figure 3) and OS (Figure 4) was consistently observed. In each subgroup, except for the subgroup of neutrophil to lymphocyte ratio ≤ 2, the severe group had a higher risk of shorter PFS than that in the non-severe group. However, in the OS analysis, the severe group presented a higher risk of shorter OS in all subgroups including the neutrophil to lymphocyte ratio ≤ 2 subgroup. Among patients with stage III disease, the severe group also had a tendency for higher risk of shorter PFS (HR, 1.64 [95%Cl, 0.87 to 3.11]) and OS (HR, 2.62 [95%Cl, 0.95 to 7.20]), although the difference was not statistically significant. PFS (HR, 2.06 vs. 1.40) and OS (HR, 6.76 vs. 3.16) in patients with obesity (body mass index ≥ Median) were more susceptible to PAD/AoD progression compared with patients without obesity (body mass index < Median), but the difference was not statistically significant.
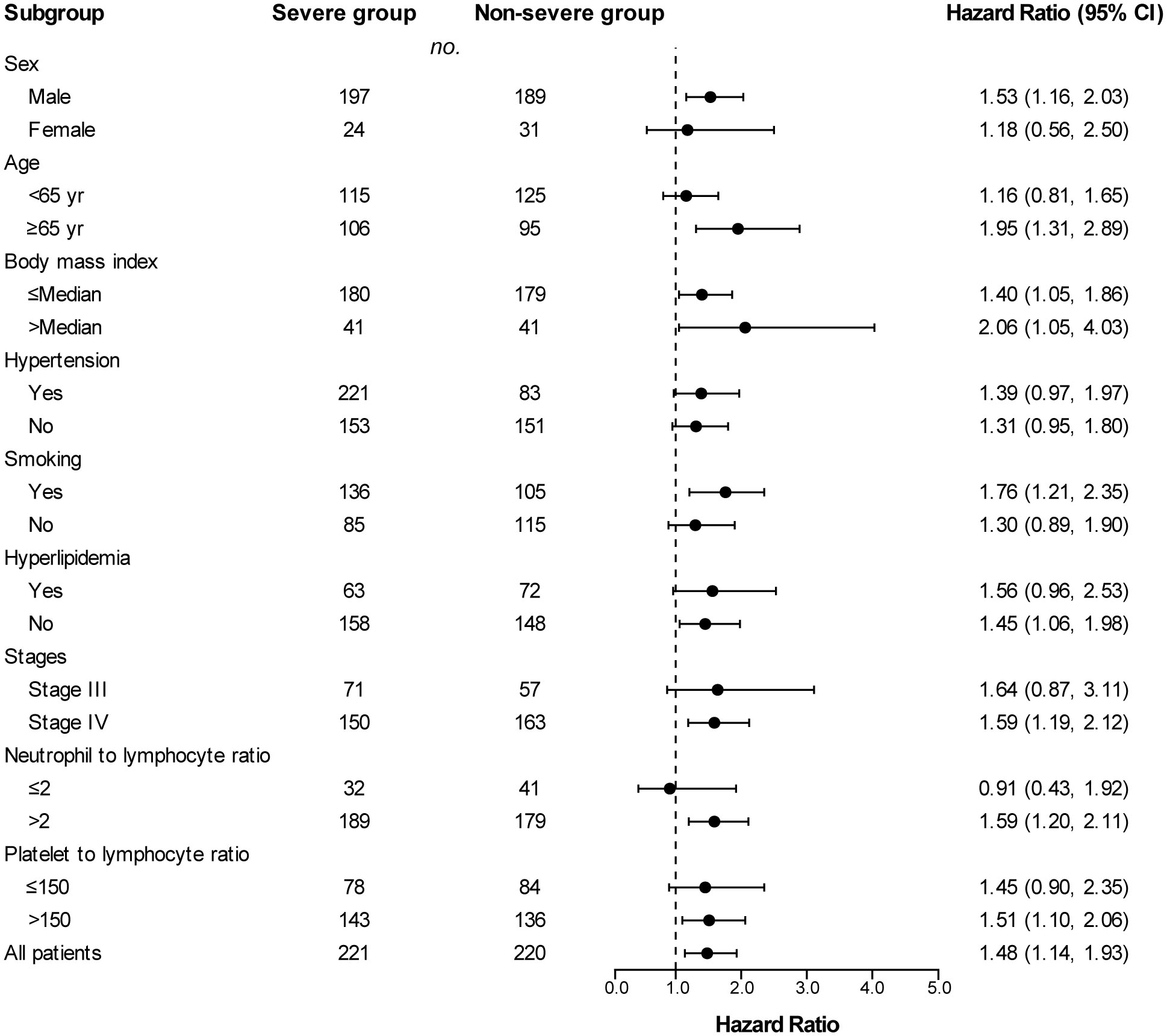
Figure 3 Subgroup analyses of progression-free survival between the severe and non-severe groups. Hazard ratios were derived from univariate cox model for each subgroup. Dashed line indicates Hazard ratio of 1.
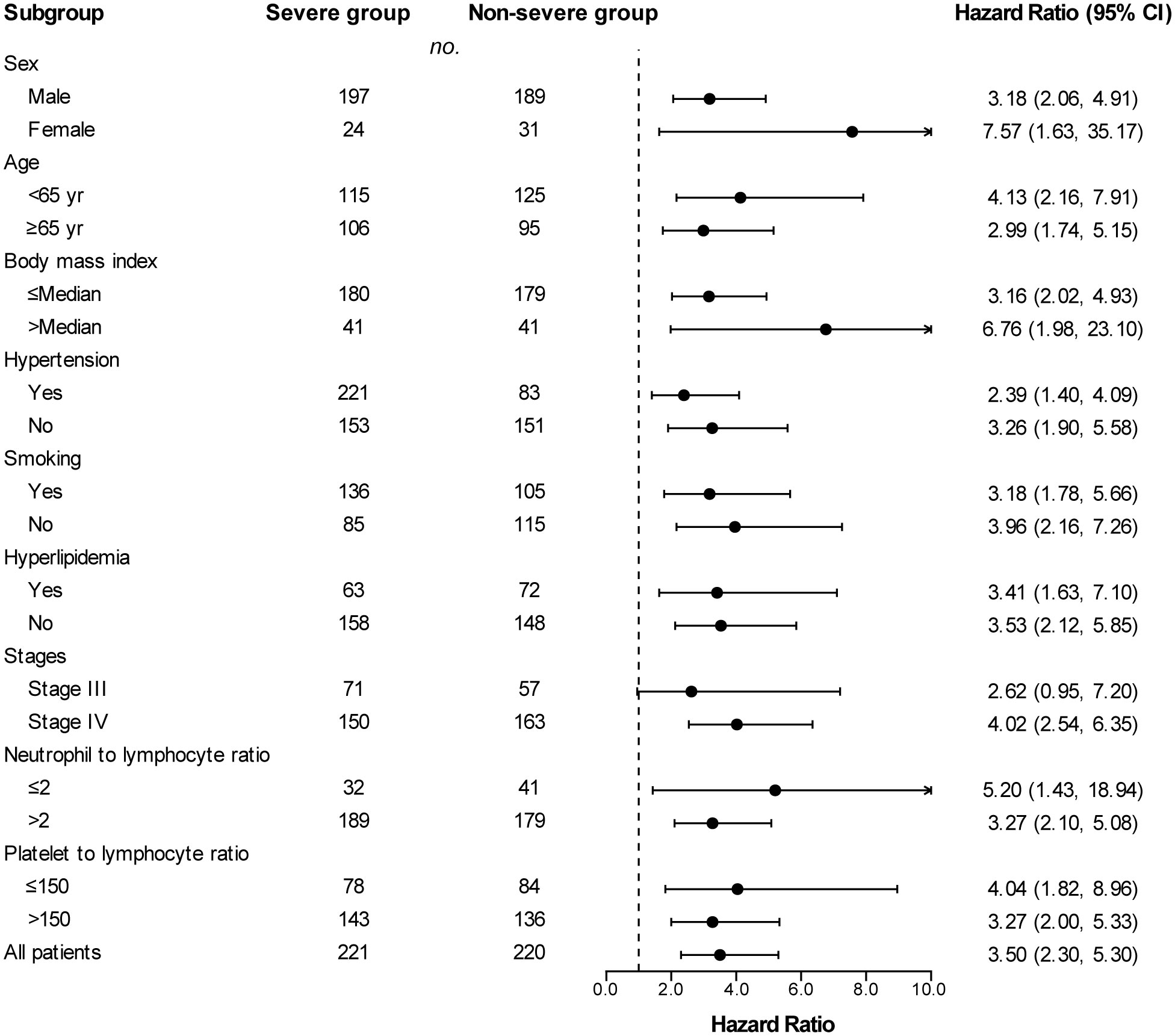
Figure 4 Subgroup analyses of overall survival between the severe and non-severe groups. Hazard ratios were derived from univariate Cox model for each subgroup. Dashed line indicates hazard ratio of 1.
Discussion
In our study, the PAD/AoD ratio in patients with NSCLC before and after ICI treatment was compared, and the prognosis of these patients was followed up. Meanwhile, patients with NSCLC treated with other anti-tumor drugs during the same period served as standard controls and negative results were obtained, which is consistent with previous studies (23). The increase of PAD/AoD ratio accelerated after the initiation of ICIs. Compared with the non-severe group, the severe group had shorter OS and PFS, and the proportion of ORR and DCR was also lower. These results indicate that the PAD/AoD ratio could affect patient prognosis and can be used as a measurable and objective biomarker to predict the efficacy of ICI treatment. The underlying mechanism may be through the effects of ICIs on the cardiopulmonary system.
ICIs regulate CD8-positive T lymphocytes and regulatory T (Treg) cells by targeting the PD-1, PD-L1 and CTLA-4 pathways, thereby restoring the body’s tumor-suppressed immune function and enabling the body to produce an efficient immune response to tumor cells. However, immune activation caused by over-regulation of T cell activity breaks local or systemic immune homeostasis, produces autoinflammatory lesions, and leads to specific or non-specific damage to tissues and organs, thereby triggering autoimmune diseases and acute or chronic irAEs (33). Single-cell RNA sequencing and mass cytometry has shown that T cells are the predominant cell type in both human and mouse atherosclerotic lesions (34, 35). Meanwhile, compared to the blood counterparts, the differentiation, activation, and exhaustion of T cells in plaques were more pronounced. These “off-target” T cells activated by ICIs may promote the development of vulnerable atherosclerotic plaques through perforin- and granzyme B-mediated apoptosis of macrophages, endothelial cells, and smooth muscle cells (36), eventually leading to the occurrence of cardiovascular events. PAH is an autoimmune inflammatory disease to some extent. Although the role of T lymphocytes in the pathogenesis of PAH is controversial, the impact of cellular immunity on its occurrence and development cannot be ignored (37). Clinical and preclinical studies have shown that the production of antibodies that inhibit the PD-1, PD-L1, and CTLA-4 checkpoints activate T cells and lead to Treg cell dysfunction, ultimately leading to the development of PAH (37–40). Similarly, researchers found that there were a large number of activated and clonally expanded CD4+ and CD8+ T cells infiltrating the myocardium, cardiac conduction system and tumor tissue of patients with myocarditis caused by ICI treatment, suggesting that both cardiomyocytes and tumors express antigens can be recognized by monoclonal T cells (13, 41, 42). Activated T cells attack tumors, leading to autoimmune myocarditis and inflammation of the cardiovascular system. In addition, ICIs also cause damage to the cardiovascular system through mechanisms such as humoral immunity and cytokine induction (41). Cortellino et al. (43) used fasting mimicking diet (FMD) treatment to reverse cardiac fibrosis, necrosis and hypertrophy caused by ICIs, while reducing the immune infiltration of CD3+ and CD8+ cells in myocardial tissue.
This is the first study to report the relationship between PAD/AoD ratio progression and prognosis after ICI treatment. The PAD/AoD ratio increased 0.03 (from 0.75 to 0.78) after initiating ICIs. Similar to our results, Fournel et al. (23) found that the PAD/AoD ratio increased 0.05 (from 0.82 to 0.87) after the use of nivolumab. Although there were differences in baseline PAD/AoD ratio compared with our study, we believe that this was due to ethnic differences. This view can be confirmed in the study of Nguyen-Thu et al. (21). In their study, 193 Asian patients with clinical indications for the assessment of cardiovascular disease (CVD) or the cardiac function who underwent both coronary CT angiography (CCTA) and echocardiography had the baseline PAD/AoD ratio of 0.76 ( ± 0.12), which was similar to our study. In addition, Fournel et al. (23) found that two cases of life-threatening acute pulmonary hypertension occurred after ICI treatment, and their post-treatment PAD/AoD ratio were both >1. In our study, 19 patients had a PAD/AoD ratio >1 before ICIs, and this number increased to 27 after ICI treatment. Previous studies have demonstrated that PAD/AoD ≥1 was highly associated with pulmonary hypertension (44). PAD and AoD reflect pulmonary arterial pressure and heart function (21, 25, 45, 46). The expansion of the PAD/AoD ratio is a comprehensive manifestation of cardiovascular dysfunction, such as pulmonary vascular inflammation and decreased right heart function, which leads to increased pulmonary arterial pressure and luminal dilation and/or reduced aortic pressure and luminal constriction. Shibata et al. (19) found that the higher PAD/AoD ratio was associated with a lower left ventricular (LV) reverse remodeling (LVRR) (Odds ratio (OR): 0.948 [95% CI, 0.908 to 0.990]; P = 0.016), and the presence of LVRR represented a better prognosis. In the study of Nguyen-Thu et al. (21), higher PAD/AoD ratio was a potential risk factor for higher left ventricular mass (LVM) (Beta: 67.27 [SE, 30.534]; P = 0.03), left atrial volume (LAV) (Beta: 34.23 [SE, 10.23]; P < 0.001), and early mitral inflow velocity to mitral annular early diastolic velocity ratio (E/e’) (Beta: 5.08 [SE, 2.47]; P = 0.04), which were associated with worse cardiac function. Excessive activation of the immune system by ICIs can lead to immune cell infiltration of the myocardium and myocardial oxidative stress, resulting in reduced cardiac function. Reduced left ventricular function leads to a reduction in aortic diameter. Although right ventricular function may be reduced by ICIs, pulmonary function may also be affected (47), leading to an increase in right ventricular afterload and subsequent increases in both pulmonary artery pressure and pulmonary artery diameter. An association between ICIs and PAD/AoD ratio progression has been observed previously. The PAD and PAD/AoD ratio of 59 patients with advanced NSCLC increased after treatment with nivolumab (23). In another study, Mylvaganam et al. (22) conducted a retrospective analysis of 24 patients received ICIs including PD-1, PD-L1, and CTLA-4 antibodies. Their results showed significant increases in both right ventricular free wall longitudinal systolic strain (RVfwLS) and PAD/AoD ratio over a median treatment period of less than 100 days, suggesting that ICIs are associated with right ventricular dysfunction and vascular changes. However, their study did not provide survival data that is crucial for cancer research. Compared to these two studies, the number of patients in our study was > 7-fold and > 18-fold higher, respectively. In our study, a total of 441 participants (excluding standard controls) were included and followed up for 22 months (median follow-up) to obtain patient tumor response (ORR and DCR) and survival data (PFS and OS).
There were a few limitations to this study. This study was retrospective and conducted at a single center. Differences in patient admission and examination times may lead to selection bias. However, this study includes one of the largest numbers of ICI-treated patients reported to date. Furthermore, although we performed a complete survival analysis of patients, data pertaining to irAEs including pulmonary hypertension and cardiovascular events were not collected. In addition, we did not conduct separate analyzes on different ICIs, and there may be differences in PAD/AoD ratio progression among different ICIs. It has been reported that 50% of patients identified with immune-related PAH were treated with nivolumab (48). However, we randomly collected real-world patients with NSCLC treated with different ICIs, and the results are hence considered more generalizable. Finally, the changes in PAD/AoD ratio were generally low in this study, and there was a lack of hemodynamic parameter data obtained from invasive right heart catheterization, and cardiac function tests to corroborate our results. Therefore, more comprehensive prospective multicenter studies with longer follow-up periods are still needed to verify our results in the future.
Conclusions
Our study found that PAD/AoD ratio progression accelerated after the initiation of ICIs, and greater increases were associated with worse clinical outcomes. The PAD/AoD ratio is expected to become an important indicator for evaluating the prognosis of patients treated with ICIs. Therefore, in clinical practice, we should routinely evaluate the PAD/AoD ratio in patients treated with ICIs to help early identify patients who could benefit from the treatment with ICIs versus those who could benefit from alternative treatment strategies and thereby guide clinicians in medication use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Tongji Medical College of Huazhong University of Science and Technology (Institutional Review Board No. S054). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is a retrospective study. Informed consent is not applicable in this study. The ethics committee approved the waiver of informed consent.
Author contributions
BG: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. YG: Writing – original draft, Writing – review & editing. JW: Writing – review & editing. WL: Writing – review & editing. GZ: Writing – review & editing. JS: Writing – review & editing. FP: Writing – review & editing. LY: Writing – review & editing. BL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grant from National Nature Science Foundation of China (No.82172034).
Acknowledgments
We thank Editage for language editing (www.editage.cn).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1302233/full#supplementary-material
References
1. Braun DA, Burke KP, Van Allen EM. Genomic approaches to understanding response and resistance to immunotherapy. Clin Cancer Res (2016) 22(23):5642–50. doi: 10.1158/1078-0432.CCR-16-0066
2. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
3. Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet (2021) 398(10299):535–54. doi: 10.1016/S0140-6736(21)00312-3
4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
5. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
6. Topalian SL, Forde PM, Emens LA, Yarchoan M, Smith KN, Pardoll DM. Neoadjuvant immune checkpoint blockade: A window of opportunity to advance cancer immunotherapy. Cancer Cell (2023) 41(9):1551–66. doi: 10.1016/j.ccell.2023.07.011
7. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer (2023) 22(1):40. doi: 10.1186/s12943-023-01740-y
8. Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int (2022) 71(2):169–78. doi: 10.1016/j.alit.2022.01.001
9. Curkovic NB, Johnson DB. Updates in toxicities associated with immune checkpoint inhibitors. Expert Rev Clin Immunol (2023) 19(9):1117–29. doi: 10.1080/1744666X.2023.2221434
10. Hu X, Wang L, Shang B, Wang J, Sun J, Liang B, et al. Immune checkpoint inhibitor-associated toxicity in advanced non-small cell lung cancer: An updated understanding of risk factors. Front Immunol (2023) 14:1094414. doi: 10.3389/fimmu.2023.1094414
11. Young A, Bluestone JA. At the heart of immune checkpoint inhibitor-induced immune toxicity. Cancer Discovery (2021) 11(3):537–9. doi: 10.1158/2159-8290.CD-21-0091
12. Spagnolo P, Chaudhuri N, Bernardinello N, Karampitsakos T, Sampsonas F, Tzouvelekis A. Pulmonary adverse events following immune checkpoint inhibitors. Curr Opin Pulm Med (2022) 28(5):391–8. doi: 10.1097/MCP.0000000000000895
13. Zito C, Manganaro R, Ciappina G, Spagnolo CC, Racanelli V, Santarpia M, et al. Cardiotoxicity induced by immune checkpoint inhibitors: what a cardio-oncology team should know and do. Cancers (Basel) (2022) 14(21):5403. doi: 10.3390/cancers14215403
14. Zhang J, Zhang S, Xu S, Zhang X, Li J, Ji Z, et al. Pulmonary arterial hypertension induced by immune checkpoint inhibitor combined therapy in a patient with intrahepatic cholangiocarcinoma: A case report. Iran J Immunol (2023) 20(2):240–6. doi: 10.22034/iji.2023.96898.2464
15. Glick M, Baxter C, Lopez D, Mufti K, Sawada S, Lahm T. Releasing the brakes: a case report of pulmonary arterial hypertension induced by immune checkpoint inhibitor therapy. Pulm Circ (2020) 10(4):2045894020960967. doi: 10.1177/2045894020960967
16. Sirajuddin A, Mirmomen SM, Henry TS, Kandathil A, Kelly AM, King CS, et al. ACR appropriateness criteria® Suspected pulmonary hypertension: 2022 update. J Am Coll Radiol (2022) 19(11s):S502–s12. doi: 10.1016/j.jacr.2022.09.018
17. Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging (1999) 14(4):270–8. doi: 10.1097/00005382-199910000-00007
18. Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology (2010) 254(2):609–16. doi: 10.1148/radiol.09090548
19. Shibata N, Hiraiwa H, Kazama S, Kimura Y, Araki T, Mizutani T, et al. Clinical effect of pulmonary artery diameter/ascending aorta diameter ratio on left ventricular reverse remodeling in patients with dilated cardiomyopathy. Circ J (2022) 86(7):1102–12. doi: 10.1253/circj.CJ-21-0786
20. Cuttica MJ, Bhatt SP, Rosenberg SR, Beussink L, Shah SJ, Smith LJ, et al. Pulmonary artery to aorta ratio is associated with cardiac structure and functional changes in mild-to-moderate COPD. Int J Chron Obstruct Pulmon Dis (2017) 12:1439–46. doi: 10.2147/COPD.S131413
21. Nguyen-Thu H, Ohyama Y, Taketomi-Takahashi A, Nguyen-Cong T, Sumiyoshi H, Nakamura T, et al. Pulmonary artery diameter (PAD) and the pulmonary artery to aorta ratio (PAD/AAD) as assessed by non-contrast cardiac CT: the association with left ventricular (LV) remodeling and the LV function. Internal Med (2022) 61(12):1809–15. doi: 10.2169/internalmedicine.8605-21
22. Mylvaganam R, Avery R, Goldberg I, Makowski C, Kalhan R, Villaflor V, et al. Adverse effects of immune checkpoint inhibitor therapies on right ventricular function and pulmonary arterial dilatation. Pulm Circ (2021) 11(1):2045894021992236. doi: 10.1177/2045894021992236
23. Fournel L, Boudou-Rouquette P, Prieto M, Hervochon R, Guinet C, Arrondeau J, et al. Nivolumab increases pulmonary artery pressure in patients treated for non-small cell lung cancer. Cancer Chemotherapy Pharmacol (2020) 86(4):497–505. doi: 10.1007/s00280-020-04142-9
24. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(5):497–530. doi: 10.6004/jnccn.2022.0025
25. Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med (2012) 367(10):913–21. doi: 10.1056/NEJMoa1203830
26. Gómez-Sánchez L, Rodríguez-Sánchez E, Ramos R, Marti-Lluch R, Gómez-Sánchez M, Lugones-Sánchez C, et al. The association of dietary intake with arterial stiffness and vascular ageing in a population with intermediate cardiovascular risk-A MARK study. Nutrients (2022) 14(2):244. doi: 10.3390/nu14020244
27. Van De Bruaene A, Delcroix M, Pasquet A, De Backer J, De Pauw M, Naeije R, et al. Iron deficiency is associated with adverse outcome in Eisenmenger patients. Eur Heart J (2011) 32(22):2790–9. doi: 10.1093/eurheartj/ehr130
28. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev (2015) 41(10):971–8. doi: 10.1016/j.ctrv.2015.10.003
29. Sharshar M, Kaido T, Shirai H, Okumura S, Yao S, Miyachi Y, et al. Impact of the preoperative bone mineral density on the outcomes after resection of pancreatic cancer. Surg Today (2020) 50(7):757–66. doi: 10.1007/s00595-019-01954-y
30. Cappuzzo F, Tallini G, Finocchiaro G, Wilson RS, Ligorio C, Giordano L, et al. Insulin-like growth factor receptor 1 (IGF1R) expression and survival in surgically resected non-small-cell lung cancer (NSCLC) patients. Ann Oncol (2010) 21(3):562–7. doi: 10.1093/annonc/mdp357
31. Han M, Wang S, Fritah S, Wang X, Zhou W, Yang N, et al. Interfering with long non-coding RNA MIR22HG processing inhibits glioblastoma progression through suppression of Wnt/β-catenin signalling. Brain (2020) 143(2):512–30. doi: 10.1093/brain/awz406
32. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
33. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
34. Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res (2018) 122(12):1675–88. doi: 10.1161/CIRCRESAHA.117.312513
35. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med (2019) 25(10):1576–88. doi: 10.1038/s41591-019-0590-4
36. Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, et al. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation (2013) 127(9):1028–39. doi: 10.1161/CIRCULATIONAHA.112.001347
37. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. Bmj (2018) 360:j5492. doi: 10.1136/bmj.j5492
38. Tomaszewski M, Bebnowska D, Hrynkiewicz R, Dworzynski J, Niedzwiedzka-Rystwej P, Kopec G, et al. Role of the immune system elements in pulmonary arterial hypertension. J Clin Med (2021) 10(16):3757. doi: 10.3390/jcm10163757
39. Tamosiuniene R, Manouvakhova O, Mesange P, Saito T, Qian J, Sanyal M, et al. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circ Res (2018) 122(12):1689–702. doi: 10.1161/CIRCRESAHA.117.312058
40. Sada Y, Dohi Y, Uga S, Higashi A, Kinoshita H, Kihara Y. Non-suppressive regulatory T cell subset expansion in pulmonary arterial hypertension. Heart Vessels (2016) 31(8):1319–26. doi: 10.1007/s00380-015-0727-4
41. Li X, Peng W, Wu J, Yeung SJ, Yang R. Advances in immune checkpoint inhibitors induced-cardiotoxicity. Front Immunol (2023) 14:1130438. doi: 10.3389/fimmu.2023.1130438
42. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol (2019) 280:163–75. doi: 10.1016/j.ijcard.2019.01.038
43. Cortellino S, Quagliariello V, Delfanti G, Blazevits O, Chiodoni C, Maurea N, et al. Fasting mimicking diet in mice delays cancer growth and reduces immunotherapy-associated cardiovascular and systemic side effects. Nat Commun (2023) 14(1):5529. doi: 10.1038/s41467-023-41066-3
44. Shen Y, Wan C, Tian P, Wu Y, Li X, Yang T, et al. CT-base pulmonary artery measurement in the detection of pulmonary hypertension: a meta-analysis and systematic review. Med (Baltimore) (2014) 93(27):e256. doi: 10.1097/MD.0000000000000256
45. Chimura M, Ohtani T, Tsukamoto Y, Kioka H, Katsimichas T, Onishi T, et al. Ratio of pulmonary artery diameter to ascending aortic diameter and severity of heart failure. J Heart Lung Transplant (2018) 37(11):1341–50. doi: 10.1016/j.healun.2018.07.006
46. Toya T, Nagatomo Y, Kagami K, Yukino M, Yasuda R, Namba T, et al. Computed tomography-measured pulmonary artery to aorta ratio and EUTOS score for detecting dasatinib-induced pulmonary arterial hypertension. Int J Cardiovasc Imaging (2019) 35(8):1435–42. doi: 10.1007/s10554-019-01548-2
47. Guo X, Chen S, Wang X, Liu X. Immune-related pulmonary toxicities of checkpoint inhibitors in non-small cell lung cancer: Diagnosis, mechanism, and treatment strategies. Front Immunol (2023) 14:1138483. doi: 10.3389/fimmu.2023.1138483
Keywords: pulmonary artery diameter, ascending aorta diameter, immune checkpoint inhibitors, non-small cell lung cancer, prognosis
Citation: Gong B, Li Y, Guo Y, Wang J, Liu W, Zhou G, Song J, Pan F, Yang L and Liang B (2024) The impact of pulmonary artery to ascending aorta diameter ratio progression on the prognosis of NSCLC patients treated with immune checkpoint inhibitors. Front. Immunol. 15:1302233. doi: 10.3389/fimmu.2024.1302233
Received: 26 September 2023; Accepted: 15 January 2024;
Published: 29 January 2024.
Edited by:
Sang T. Kim, Yale University, United StatesReviewed by:
Lars Michel, Essen University Hospital, GermanyVincenzo Quagliariello, Istituto Nazionale Tumori-IRCCS-Fondazione G. Pascale, Italy
Copyright © 2024 Gong, Li, Guo, Wang, Liu, Zhou, Song, Pan, Yang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Liang, eGllaGVsYkBzaW5hLmNvbQ==; Lian Yang, eWFuZ2xpYW5AaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Bingxin Gong1,2†
Bingxin Gong1,2† Jing Wang
Jing Wang Guofeng Zhou
Guofeng Zhou Feng Pan
Feng Pan Bo Liang
Bo Liang