- 1Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
- 2Bone Marrow Transplant Program, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
Acute Myeloid Leukemia (AML) is the prototype of cancer genomics as it was the first published cancer genome. Large-scale next generation/massively parallel sequencing efforts have identified recurrent alterations that inform prognosis and have guided the development of targeted therapies. Despite changes in the frontline and relapsed standard of care stemming from the success of small molecules targeting FLT3, IDH1/2, and apoptotic pathways, allogeneic stem cell transplantation (alloHSCT) and the resulting graft-versus-leukemia (GVL) effect remains the only curative path for most patients. Advances in conditioning regimens, graft-vs-host disease prophylaxis, anti-infective agents, and supportive care have made this modality feasible, reducing transplant related mortality even among patients with advanced age or medical comorbidities. As such, relapse has emerged now as the most common cause of transplant failure. Relapse may occur after alloHSCT because residual disease clones persist after transplant, and develop immune escape from GVL, or such clones may proliferate rapidly early after alloHSCT, and outpace donor immune reconstitution, leading to relapse before any GVL effect could set in. To address this issue, genomically informed therapies are increasingly being incorporated into pre-transplant conditioning, or as post-transplant maintenance or pre-emptive therapy in the setting of mixed/falling donor chimerism or persistent detectable measurable residual disease (MRD). There is an urgent need to better understand how these emerging therapies modulate the two sides of the GVHD vs. GVL coin: 1) how molecularly or immunologically targeted therapies affect engraftment, GVHD potential, and function of the donor graft and 2) how these therapies affect the immunogenicity and sensitivity of leukemic clones to the GVL effect. By maximizing the synergistic action of molecularly targeted agents, immunomodulating agents, conventional chemotherapy, and the GVL effect, there is hope for improving outcomes for patients with this often-devastating disease.
Introduction
Acute Myeloid Leukemia (AML) is an aggressive malignancy characterized by hematopoietic stem cell and early myeloid progenitor developmental arrest and aberrant proliferation. Scientific advances over the past two decades have revealed that recurrent leukemia-associated mutations and cytogenetic abnormalities drive disease biology. With the advent of widely available massively parallel (“next generation”) sequencing (NGS) in the research and clinical setting, there is a growing understanding of how patterns of co-mutation inform prognosis and treatment selection at various treatment stages. Allogeneic stem cell transplantation (alloHSCT) is a cornerstone and standard treatment modality for patients with intermediate or higher risk AML in first complete remission (CR1) or beyond, and intensive research efforts have elucidated how the genomic profile of AML influences prognosis in this setting. Additionally, molecularly targeted therapies initially studied and approved in the front-line setting are increasingly being considered as maintenance or pre-emptive therapy in the post-transplantation period to mitigate ongoing risk of relapse, particularly in ultra-high risk genomic subgroups. In this review, we aim to provide an overview of our understanding of how targeted therapies intersect with the graft-versus-leukemia (GVL) effect after alloHSCT, which has long been considered the “modus operandi” of this often lifesaving intervention.
Genomics and prognosis
Since the publication of the first cancer genome, a cytogenetically normal AML, there has been ongoing interest in understanding the landscape of recurrent driver mutations in AML in order to inform prognosis (1). Prior to this, our understanding of the impact of genetic alterations on treatment outcomes was based on analysis of karyotypes, which we now know lacks the resolution to capture the full scope of pathogenic DNA-level alterations in AML (2–4). Initially, driver gene mutations were investigated in cytogenetically normal cases (5), with subsequent efforts focused on whole-genome and whole exome analysis in a broader set of 200 de novo AML cases (6). There was also a growing understanding of how cytogenetics and targeted sequencing approaches could be used in tandem to inform prognosis in clinical trial settings (7). Increasingly large datasets allowed for a more comprehensive assessment of gene mutation frequencies, co-mutation patterns, and development of distinct molecular subgroups that differ based on prognosis and leukemogenic pathways. A seminal example of this approach was the publication by Papaemmanuil and colleagues, who performed comprehensive diagnostic cytogenetic and molecular profiling in 1,540 patients who were enrolled in prospective trials of intensive therapy (8). By assessing driver mutations in 111 cancer genes, they found that 96% of patients had at least one driver mutation in one of 77 loci. Six genes (FLT3, NPM1, DNMT3A, NRAS, CEBPA, and TET2) were mutated in >10% of the cohort.
Incorporation of AML mutational profiles has led to the adoption of molecularly defined AML subgroups in the latest World Health Organization and International Consensus Classification of myeloid neoplasms classifications (9, 10) and continues to serve as the basis of the AML European Leukemia Net (ELN) risk stratification scheme (11). Most recently updated in 2022, high risk disease is characterized by mutations in TP53, secondary-ontogeny associated mutations, and a variety of high risk cytogenetic findings including complex and monosomal karyotypes which have long been known to portend dismal prognosis (12–15). In contrast, favorable risk is defined by mutated NPM1 without concomitant FLT3-ITD mutation, bZIP in-frame mutated CEBPA as well as core-binding factor leukemias (16–20). These risk groups are a useful simplification of highly complex effects of co-mutation, clonal hierarchy, and leukemic evolution. The outcomes of patients within these risk groups are variable, modified by co-mutations, baseline clinical variables, and emerging treatment strategies. Examples abound, including the impact of KIT mutations and secondary genetic lesions in core-binding factor leukemia, as well as the influence of age on FLT3-ITD mutated leukemia (21–23).
Measurable residual disease
Diagnostic mutations are known to impact outcomes in myeloid malignancies after alloHSCT, which is the preferred consolidative strategy in higher risk disease (24–27). Serial assessment of disease burden using measurable residual disease (MRD) testing over the course of therapy is now an established strategy in AML (28). MRD assessments may be performed using flow cytometric, RT-PCR, or NGS technologies, with the latter genetically focused technologies allowing for MRD assessments in a broader subset of AML cases (29). Molecular MRD has been evaluated for its prognostic utility in the post-up front therapy (30–37), pre-transplant (38–45), post-transplant (46, 47), and relapse/refractory settings (48), but NGS-MRD is not yet ready for widespread clinical adoption. Increasingly, MRD assessments are being studied to guide therapeutic decisions (49–54). We refer the reader to a recent review of MRD in acute myeloid leukemia for a comprehensive discussion on this topic (55).
Genomics and up-front treatment
Until recently, initial treatment for adults with AML consisted of intensive induction chemotherapy for fit patients and non-intensive therapy with DNA methyltransferase inhibitors (DNMTi, interchangeably referred to as hypomethylating agents [HMA]) for frail or elderly individuals. Intensive induction commonly consists of genomics-agnostic cytotoxic therapies such as the 7 + 3 regimen of an anthracycline and infusional cytarabine or FLAG (fludarabine, cytarabine, and G-CSF) based regimens. Non-intensive therapies have historically also been deployed in a genomics-agnostic manner and include the DNMTi azacitidine and decitabine.
Modern AML therapies (i.e., gilteritinib, ivosidenib with or without DNMTi, enasidenib, DNMTi/venetoclax and low-dose cytarabine/venetoclax combinations) with disease modifying capabilities have altered the prognostic implications of cytogenetics and genetics determined at the time of diagnosis, suggesting dynamic reassessment may be required in the context of therapy. The front-line treatment of older patients with AML has been revolutionized with the introduction of the BCL2 inhibitor venetoclax, which improved survival outcomes when combined with azacitidine in this patient population in the phase III VIALE-A trial (56). Just as the introduction of FLT3 inhibitors led to evolution in our understanding of the prognostic significance of mutation burden (in the form of allelic ratios) in FLT3-ITD, so too venetoclax may be altering the implications of splicing factor (secondary ontogeny-defining) mutations, which are considered adverse risk by 2022 ELN (16, 57).
Allogeneic stem cell transplantation
Outcomes in patients who relapse after alloHSCT are dismal. In a study of patients with acute nonlymphocytic leukemia relapsing after marrow transplantation, median disease-free survival (DFS) was 6 months (58). In a study of adults with relapsed AML after reduced intensity conditioning (RIC) alloHSCT, 2-year overall survival (OS) from relapse was 14% (59). In a study of MDS and secondary AML, median OS from relapse was 4.7 months with 2-year survival of 17.7% (60). Given these disappointing statistics, there has been great interest in identifying relapse-mitigating strategies. These strategies must consider the genomic profile of the leukemia as well as the complex immunologic landscape which includes the impact of any therapy on the donor immune system, the residual host immune system, and the immune microenvironment. In the remainder of this review, we will discuss innovations in conditioning regimens and post-transplant maintenance primarily through evidence from prospective studies. We will review whether the data suggest these therapies provide post-transplant disease control by cytoreduction or through GVL induction. Post-transplant pre-emptive or prophylactic cytoreductive therapy still has value if it can control MRD clone expansion in the early post-transplant period without causing excess toxicity or interfere with engraftment until the benefits of transplant GVL manifest. A long-standing goal in the alloHSCT field has been to decouple the beneficial anti-leukemic GVL effect after transplant from allo-immunity against non-leukemic tissue, or graft-vs-host disease (GVHD), since presence of GVHD, especially cGVHD, is almost universally associated with lower relapse rates, and even among patients who relapse after alloHSCT, durable remissions are rarely observed unless they subsequently develop GVHD (61). We will also review the emerging scientific data on these agents through a genomic and immunologic lens. Lastly, we will also highlight our limited, but evolving, understanding of how these relapse-prevention strategies impact the GVL effect.
Genomics and conditioning chemotherapy intensity
Intensive myeloablative conditioning (MAC) regimens offer the benefits of additional leukemic cytoreduction, and faster donor engraftment after transplant by creating more “marrow space” for engrafting cells. However, the potential relapse/engraftment benefit of MAC may be counterbalanced by an increase in regimen related toxicity and transplant-related mortality (TRM, also known as “non-relapse mortality, “NRM”). Scott and colleagues addressed these concerns in the BMT-CTN 0901 trial, a comparison of MAC vs RIC (or non-ablative conditioning) in patients with AML and MDS with less than 5% myeloblasts, with the hypothesis that the reduced intensity conditioning might mitigate TRM while preserving anti-leukemic efficacy of alloHSCT (62). Younger patients (≤65 years of age) in complete remission (CR) prior to alloHSCT were randomized in a 1:1 fashion to MAC or RIC. Among AML patients, relapse rates were significantly higher in the RIC arm compared to the MAC arm (48.3% vs 13.5% respectively, P <.001), while TRM was significantly higher in MAC vs RIC (15.8% vs 4.4%, P = .002). Overall survival was not significantly different between the arms but was numerically improved in the MAC arm. The significant difference in relapse in favor of MAC led to the early termination of this randomized trial, and established MAC as the preferred conditioning strategy in younger, fit patients with AML. The BMT-CTN 0502 trial established RIC alloHSCT as a treatment option in older, frailer patients not suitable for MAC alloHSCT (63).
In an attempt to broadly augment the RIC regimen, the phase II FIGARO trial compared combination fludarabine/amsacrine/cytarabine-busulfan to fludarabine-based RIC regimen (64). Among 244 patients, 43% of whom were older than 60 years of age, no difference in OS was detected (hazard ratio [HR] 1.05, 85% confidence interval [CI], 0.80 to 1.38). Though MRD positivity by flow-cytometry was linked to increased risk of relapse, the authors did not identify an interaction between MRD, conditioning intensity, and survival. In contrast, an NGS-MRD analysis by Hourigan and colleagues of the BMT CTN 0901 trial suggested that MAC can overcome the poor survival associated with persistent residual molecular disease as compared to RIC (42). Importantly, the 0901 trial focused on largely younger patients who were eligible for MAC. Another multicenter retrospective analysis of pre-transplant NGS-MRD in older patients largely undergoing RIC transplantation suggested that MRD is prognostic, but that risk is largely encoded at the time of diagnosis, with MRD lacking independent prognostic value within diagnostic risk groups (25).
The critical question is whether there is a RIC regimen that can overcome the poor risk tied to diagnostic genetics and pre-transplant MRD. Alternative methods include augmenting RIC with the addition of selective small molecule inhibitors, such as venetoclax (“RIC-Ven”) in high-risk myeloid malignancies (65). In a phase I study done at our center adding 7 days of venetoclax to standard RIC Flu/Bu2 (fludarabine 30 mg/m2 QD and busulfan 0.8 mg/kg BID) on day -5 to day -2, followed by matched related or unrelated donor unmanipulated PBSC with tacrolimus/methotrexate GVHD prophylaxis, we observed that the addition of venetoclax to the conditioning was well tolerated, and had no deleterious effects on engraftment, infection, or GVHD. After a median of 14.7 month follow up (range, 8.6-24.8 months), progression-free survival (PFS) was 12.2 months (95% CI, 6.0-not estimable) and median overall survival was not reached among survivors. We performed dynamic NGS-MRD surveillance to track MRD conversion and to detect early relapse. Among the 18 patients with pre-transplant MRD, 9 of the 18 patients who experienced conversion to MRD negativity after transplant remain in complete remission. The true benefit of adding venetoclax to RIC alloHSCT will require a randomized study to assess the impact on relapse risk and survival as compared to traditional RIC regimens.
The prevention of GVHD in the form of immunosuppression peri- and post- alloHSCT has evolved from single-agent strategies to combination strategies using a calcineurin inhibitor (CNI) such as cyclosporine or tacrolimus, in conjunction with methotrexate, mycophenolate mofetil (MMF), and/or sirolimus (66–71). Most recently, the combination of post-transplant cyclophosphamide (PTCy), CNI, and MMF have led to improvement in GVHD-free, relapse-free survival in patients undergoing HLA-matched RIC alloHSCT as compared to tacrolimus and methotrexate in the randomized phase III BMT CTN 1703 trial (72). The results of this important study have already led to a shift in the GVHD prevention paradigm after RIC transplantation across the world. Although the reduction in GVHD, especially cGVHD, associated with PTCy does not appear to be associated with a higher rate of relapse in this and other studies, it remains unknown whether the GVL effect is truly preserved after PTCy, or whether the lower relapse rates are mediated by alternative mechanisms, such as NK cells, or other activity (73). More research is needed to understand the functional impact (if any) of post-transplant cyclophosphamide on T-cell mediated GVL.
Immunology and limits of transplant and GVL
After the cytoreductive activity of conditioning chemotherapy and/or radiotherapy, the alloreactive donor immune system (largely T and potentially NK cells) mediates the anti-leukemic activity of alloHSCT which underpins the durable responses that are seen in a subset of AML patients. The presence of a GVL effect was first suggested by animal models, and then inferred from observations that patients with GVHD have reduced rates of, or delayed presentations of relapse as compared to patients who did not have GVHD (74–78). We refer the reader to the review from Sweeney and Vyas for a full discussion of the GVL effect, but note that additional lines of evidence for the presence of the GVL effect include the increased relapse rate after T-cell depleted transplantation, efficacy of RIC transplantation, efficacy of immunosuppression withdrawal and Donor Lymphocyte Infusions (DLI) as treatment for relapse, as well as loss of HLA antigens at relapse suggesting immune evasion mechanisms (61, 79–84).
Targeted therapy in the post-transplant setting
FLT3
The fms-related tyrosine kinase 3 gene (FLT3) encodes a receptor in the FMS family, and was originally implicated in leukemia biology in the early 1990s with northern blot analysis identifying high levels of expression in blasts (85). Several years later, internal tandem duplications (ITD) were identified in the FLT3 gene in acute myeloid leukemia, with subsequent identification of activating D835 mutations within the activating loop of FLT3 (86, 87). It is now estimated based on large patient datasets that FLT3 mutations are identified in ~30% of newly diagnosed AML (8, 88). There are now several multi-kinase and FLT3 inhibitors approved for the treatment of FLT3-mutated AML in the pre-alloHSCT setting, and there has been interest in studying these inhibitors in the peri-and post-transplant setting, with relevant agents including sorafenib, midostaurin, gilteritinib, and quizartinib. Crenolanib, a potent inhibitor with promise against resistance mutations, is being studied in a single-arm, phase II study (NCT01468467) as maintenance therapy after allo-HSCT for up to two years (89).
Sorafenib
Sorafenib is a multi-kinase inhibitor which was initially studied in leukemia cell lines as a potent inhibitor of FLT3 enzymatic and signaling activity (90). Chen and colleagues first showed the safety and tolerability of incorporating sorafenib therapy after alloHSCT in FLT3-ITD mutated AML in a phase I trial (91). In this study of 22 patients, sorafenib was initiated between days 45 and 120 after alloHSCT and continued in twelve 28-day cycles. One-year PFS was 85%. Pratz and colleagues next prospectively studied sorafenib in the peri-transplant period in 44 patients, 21 of whom started sorafenib pretransplant (92). Event free survival (EFS) at 2 years was 74%. Sorafenib was subsequently studied in phase II and III clinical trials, which confirmed its benefit. The SORMAIN phase II trial randomized 83 patients with FLT3-ITD+ AML undergoing alloHSCT in CR to receive sorafenib for 2 years versus placebo (93). The primary endpoint of relapse-free survival (RFS) was 53.3% with placebo compared to 85% with sorafenib, with a significant HR for relapse or death of 0.39 (95% CI, 0.18 to 0.85; log-rank P = .013) with median follow-up of 41.8 months. Of note, the median duration of therapy in the sorafenib group was 34.57 weeks, with side effects representing the most common reason for sorafenib discontinuation. Acute and chronic GVHD represented the most common grade 3+ adverse events, 76.8% in the sorafenib arm and 59.8% in the placebo arm. Other common grade 3+ adverse events in the sorafenib arm included infections in 26.2%, GI toxicity in 14.3%, and electrolyte derangements in 14.3% of patients. Sorafenib was studied in another randomized trial, a phase III study of 202 adult patients with FLT3-ITD+ AML who received alloHSCT and had hematopoietic recovery within day +60 who were randomized to sorafenib or non-maintenance control at 30-60 days post-alloHSCT (94). The original primary endpoint of 1-year cumulative incidence of relapse (CIR) in the intention-to-treat population was 7% in the sorafenib group and 24.5% in the control group (HR 0.25, 95% CI 0.11-0.57; p=0.0010). Long term follow-up showed significantly improved OS (72% sorafenib vs 55.9% placebo, HR 0.55, 95% CI 0.34-0.88; p=0·011) as well as improved leukemia-free survival (LFS), lower CIR, and no increase in NRM (95). These studies established 2-year sorafenib maintenance therapy as standard of care in FLT3-ITD mutated patients for long term disease control after transplant and is now recommended by the AML guideline of the National Comprehensive Cancer Network (NCCN) and ELN. As discussed below, sorafenib may act not only via cytoreduction, but via IL15-mediated enhancement of the GVL effect (96).
Midostaurin
Midostaurin is a multi-kinase inhibitor that was initially studied in humans as monotherapy in those not felt to be candidates for intensive induction, and is now approved in combination with cytotoxic chemotherapy in the up-front treatment of AML (97, 98). The post hoc efficacy analysis of midostaurin-maintenance in the RATIFY trial similarly did not confirm relapse risk reduction though in a non-transplant setting, thereby limiting the FDA label to its use in induction and consolidation phases of therapy. A phase II study investigated the addition of midostaurin to intensive chemotherapy, followed by alloHSCT and 1 year of single-agent maintenance (99). This study suggested EFS benefit to midostaurin, including in older patients. A subsequent prospective study, the RADIUS trial, of post-alloHSCT midostaurin in patients with hematologic recovery received midostaurin in twelve 4-week cycles. The primary endpoint of RFS at 18 months post-alloHSCT was numerically increased: 89% in the midostaurin arm compared to 76% in the standard of care arm. However, neither RFS nor OS was improved in a statistically significant manner in this negative trial (100). The most common grade 3+ adverse events in the midostaurin arm included hypertension and AST/ALT increase each in 13% of patients, as well as nausea in 10% of patients. Rates of GVHD were similar in the midostaurin (70%) compared to the standard of care arm (73%).
Gilteritinib
Gilteritinib is a selective FLT3 inhibitor which targets both ITD and tyrosine kinase domain (TKD) mutations, as well as exhibiting multikinase inhibitor activity against c-Kit and AXL, which has been implicated in resistance mechanisms to FLT3 inhibitors (101, 102). Approval in the relapse/refractory setting was based on the ADMIRAL trial, and a retrospective study evaluated gilteritinib in this patient population post-alloHSCT (103, 104). A follow-up to the ADMIRAL trial reported that 18 of 26 long-term survivors without relapse in the gilteritinib arm proceeded to alloHSCT, with 16 of 18 re-starting gilteritinib in the post-alloHSCT setting (105). In a report focusing on post-alloHSCT outcomes in the ADMIRAL trial, patients who resumed gilteritinib and had a CR had low relapse rates (20% for those in CRc, 0% for those in CR) (106). The unpublished phase III MORPHO study (BMT CTN 1506) randomized 356 patients to two years of gilteritinib (120mg per day) starting 30-90 days post-alloHSCT vs placebo in adults with FLT3-ITD AML in CR1. In this negative trial, there was no statistically significant difference in the primary endpoint of RFS or OS. However, there was a suggestion that gilteritinib had RFS benefit in MRD-positive patients (HR=0.515, 95% CI: 0.316, 0.838, p = 0.0065), in contrast to patients without detectable MRD (HR=1.213, 95% CI: 0.616, 2.387, p = 0.575) based on a prespecified subgroup analysis. Key adverse events included neutropenia (42.1% vs 15.8% in placebo) and chronic GVHD (52.2% vs 42.1% in placebo). It is unclear why MORPHO did not show benefit in the overall population, in contrast to the two positive studies for sorafenib. Possibilities include differences in TKI (with sorafenib having more salutary off-target effects), differential impacts on GVL possibly through IL15, insufficient power/sample size, or gilteritinib levels influenced by triazole use across different centers.
Quizartinib
Quizartinib has been studied in a phase II study as monotherapy in R/R FLT3-ITD+ AML and combined with ara-C in older patients with AML (107, 108). The QuANTUM-First randomized phase III trial evaluated quizartinib in patients with FLT3-ITD AML with or without alloHSCT, and a post-hoc analysis presented at EHA 2023 (Abstract S137) suggested benefit in those receiving alloHSCT (109). Sandmaier and colleagues conducted a phase I study of quizartinib maintenance after transplantation in 13 patients, reporting 1 relapse (110). Grade 1-2 QTc prolongation was seen in 54% of patients in this study, and none had grade 3 QTc prolongation or other cardiac toxicity.
GVL effects of FLT3 inhibition
FLT3 is a receptor tyrosine kinase which has established roles for the development of hematopoietic stem cells and early myeloid progenitors (111). There has been long-standing interest in understanding how mutated FLT3 alters leukocyte cell function and cytokine production with implications for relapse prediction (112, 113). Metzelder and colleagues concluded from retrospective data that sorafenib may synergize with the GVL effect based on differential rates of responses and outcome in relapse after alloHSCT as compared to relapses after chemotherapy (114). Tschan-Plessl and colleagues extended these observations by noting in their retrospective analysis that patients who developed chronic GVHD had a lower risk of developing sorafenib resistance (115). Immunophenotyping of three patients with FLT3-ITD mutated AML who relapsed after alloHSCT implicated a direct effect of sorafenib on immune function through CD8+PD1+ lymphocytes (116). Mathew and colleagues found that sorafenib exposure mediates reduced ATF4 expression leading to IRF7-IL15 axis activation, in turn leading to anti-AML lymphocytes with features suggesting longevity and metabolic reprogramming (96). Zhang and colleagues studied the more selective FLT3 inhibitor gilteritinib and its effects on lymphocytes, finding that gilteritinib exposure reduced co-inhibitory receptors on CD8+ T cells and up-regulated IL-15 expression (117). In total, there is growing data supporting the notion that FLT3 inhibition may have salutatory off-target effects on the donor graft which enhances the GVL effect, and in fact may become relevant as modulators of Chimeric Antigen Receptor (CAR) T cell therapies targeting FLT3 mutated AML (118, 119). Whether these salutary off-target effects are similar, or the extent to which they exist, across the different available FLT3 targeted therapies remains to be elucidated.
Unanswered questions and next steps for FLT3
A major unanswered question in this patient population is the utility of post-alloHSCT maintenance with FLT3 inhibitors in the setting of up-front FLT3 inhibitor use, particularly in the absence of detectable pre-transplant MRD. This is particularly salient as few patients (9 out of 83) in the SORMAIN trial were treated with up-front midostaurin as part of their induction regimen as is now standard of care. The MORPHO data strongly suggest that post-transplant gilteritinib maintenance provided benefit to the 50% of patients with detectable MRD either pre- or post-transplant compared to those without detectable MRD. Up-front inhibition of FLT3 may be enough to achieve MRD-negativity in a subset of patients. Subgroup analysis from SORMAIN and MORPHO differ in this regard. Exploratory analysis from SORMAIN suggested that patients with undetectable MRD before alloHSCT derive the most benefit from the multi-kinase TKI sorafenib. This is in contrast to the MORPHO study which suggested that only patients with MRD-positivity derived benefit from gilteritinib. Biologic rationales for both contradictory findings have been proposed. Patient and disease-related factors including prior treatment history (i.e., inclusion of FLT3 inhibitor with frontline induction chemotherapy; midostaurin approval was in 2017) and co-mutations contribute to the challenges in interpreting these studies. Ultimately, adequately powered prospective randomized trials in the relevant MRD subgroups will be required to address this important question. Further, while there is retrospective data and laboratory evidence for connections between FLT3 inhibition and the GVL effect, these findings are not clinically actionable at present. A key unanswered question is whether FLT3 inhibitors differ in any pro-GVL effect, and if so, what underlies that difference. One hypothesis includes the degree to which different FLT3-inhibitors exhibit multikinase activity, and activate the IRF7/IL-15 axis, but this needs to be more rigorously studied.
Lastly, while the RATIFY trial included patients with tyrosine kinase domain (TKD) FLT3 mutations, all of the randomized studies of FLT3-inhibitor maintenance after alloHSCT have focused on FLT3-ITD mutated AML. Thus, it is unclear whether patients with FLT3-TKD benefit from TKI maintenance post-transplantation, although MRD-positive patients may represent a subgroup at high risk of relapse who may derive particular benefit.
Take-home message:
● Sorafenib has demonstrated RFS and OS benefit in the post-transplantation maintenance setting and is a standard of care option for FLT3-ITD mutated AML.
● Patients with detectable FLT3 mutation at the pre-transplant stage benefit from pre-emptive post-alloHSCT gilteritinib therapy.
IDH1/2
Isocitrate Dehydrogenase (IDH) is a key enzyme in the citric acid cycle. Mutations in the gene coding for cytosolic IDH (IDH1) were first implicated in AML biology in 2009 when massively parallel sequencing identified IDH1 mutations associated with normal cytogenetic status (120). Subsequently, mutations in the mitochondrial IDH (IDH2) were identified in AML (121). The pathogenic mechanism in AML was revealed to be similar to that in gliomas, in which neomorphic activity leads to generation of the oncometabolite 2-hydroxyglutarate from alpha-ketoglutarate, which in turn inhibits alpha-ketoglutarate dependent dioxygenases which impacts histone demethylation and leads to impaired cell differentiation (122–126). Bill and colleagues have published data showing that persistence of IDH1 and IDH2 mutations at the time of transplantation is linked with increased risk of relapse, and that this association may be mutation-specific (43). However, these mutation-specific findings were based on a small cohort (33 patients with detectable IDH MRD) and must be interpreted with caution. Nonetheless, it is likely that the impact of NGS-MRD will depend on co-mutation patterns and possibly in a mutation-specific manner. This is illustrated by data presented by Gui and colleagues at the American Society of Hematology 2023 annual meeting (Abstract 424), showing that IDH2-MRD pre-transplant is prognostic for post-transplantation outcomes in the absence of co-mutated FLT3 or NPM1, but not in FLT3 or NPM1 co-mutated cases.
The mutant IDH1 inhibitor ivosidenib has been studied in a multicenter phase I trial as maintenance after alloHSCT (127). Ivosidenib was initiated between days 30 and 90 in 16 patients and continued for up to twelve 28-day cycles. In this study, the 2-year CIR was 19% and 2-year PFS and OS were 81% and 88%, respectively. Similarly, the mutant IDH2 inhibitor enasidenib was also studied as post-alloHSCT maintenance in a multi-center phase I trial in which 19 patients with myeloid malignancies received up to twelve 28-day cycles of treatment (128). Twelve-month CIR was 16% with 2-year PFS and OS of 69% and 74% respectively. Grade 3+ toxicities were rare in these trials, without any apparent increase in GVHD or infections. There is an ongoing phase I trial focusing specifically on enasidenib prophylactic maintenance in patients with IDH2-mutated AML (NCT03728335).
Unanswered questions and next steps for IDH1/2
An advantage of the IDH1 and IDH2 inhibitors in clinical use is their selectivity for the mutant enzyme, a property likely required for their use in humans given the central role these enzymes play in energy metabolism across tissues. Little is known about any off-target effects on the immune system or the GVL effect. However, data from Notarangelo and colleagues suggests that the D-2HG oncometabolite negatively impacts cytotoxic T cell activity via dysregulation of glucose metabolism and decreased IFN-gamma (129). An intriguing possibility is that post-transplant or peri-CAR-T inhibition of mutant IDH may ameliorate an inhibitory microenvironment mediated by 2-HG, allowing for maximal GVL effect. Other data suggests that IDH-mutated AML may be particularly susceptible to NK-based cellular therapies due to down-regulation of inhibitory HLA Class I proteins (130). These preclinical data highlight the importance of ongoing basic immunologic research to delineate the potentially differential impact IDH inhibitors may have on various arms of the immune system. This is of particular importance as combination therapy with hypomethylating agents may modulate the transcriptional and immunomodulatory effects of oncometabolite concentrations. Multiple resistance pathways may be involved in IDH-mutated AML, lending rationale to testing combination strategies (131).
Take-home message:
● Prophylactic IDH1/2 inhibitor maintenance therapy in the post-transplant setting has not yet been established, but the safety data and preliminary efficacy suggest that this is feasible.
● With the addition of venetoclax and IDH1/2 inhibitors to frontline older AML therapies, the role of maintenance therapy remains in question and consideration of co-mutations or potential for resistance might be necessary.
DNMT inhibitors
Parenteral DNA methyltransferase inhibitors (DNMTi) or hypomethylating agents (HMA) (azacitidine, decitabine) and more recently oral hypomethylating agents (azacitidine/CC-486, and cedazuridine/decitabine) have been extensively studied in the treatment of myeloid malignancies. In the transplant setting, hypomethylating agents have been studied in combination with traditional chemotherapy during conditioning, as maintenance post-alloHSCT, and in the post-alloHSCT relapse setting.
Conditioning: there are few randomized trials evaluating the efficacy of adding DNMTi to conditioning regimens. Xuan et al. report on a phase III trial of G-CSF, decitabine and busulfan/cyclophosphamide vs busulfan-cyclophosphamide in patients with MDS or secondary AML (132). The primary endpoint of this study was 2-year CIR, which was 10.9% in the G-CSF, decitabine, and busulfan-cyclophosphamide (Bu-Cy) arm compared to 24.8% in the control arm, with a statistically significant HR of 0.39 (95% CI 0·19-0·79; p=0·011). This finding builds on earlier phase studies adding decitabine to MAC Bu-Cy conditioning that demonstrated benefits in AML patients, even those transplanted with active disease (133, 134). In the non-randomized setting, there are studies which report on the addition of azacitidine to RIC for high risk MDS and for older patients with AML (CALGB 100801) (135), addition of 5-day decitabine to conditioning in patients with MDS and MPN (136), decitabine induction with MAC in high-risk patients (with infectious complications highlighted in this study) (137), and the use of 10-day decitabine with fludarabine and TBI (138).
Maintenance: Hypomethylating agents have been given preemptively after alloHSCT in cases of decreasing chimerism or MRD positivity or as prophylactic maintenance. Platzbecker and colleagues reported results of the RELAZA trial, in which patients were treated with azacitidine when donor CD34+ chimerism fell below 80% in patients with MDS and AML (139). In the RELAZA trial, 13 out of 20 patients (65%) still relapsed at a median of 231 days despite azacitidine being pre-emptively administered for dropping chimerism. A subsequent phase III trial randomized 187 patients to 32 mg/m2 of azacitidine given for 5 days every 28 days (up to 12 cycles) to no treatment in high-risk AML and MDS (140). A median of 4 treatment cycles of azacitidine were administered in the interventional arm, with approximately a quarter of patients finishing all planned cycles of azacitidine. The most common reasons for azacitidine maintenance discontinuation were relapse (47% of discontinuations), followed by toxicity (18%) and patient preference (15%). This trial was negative, with no statistically significant difference in RFS or OS. The toxicities in this trial are emblematic of the delicate balance between efficacy and tolerability in post-transplant maintenance, as 19.2% of patients experienced grade 3+ hematologic AE, compared with 2.3% grade 3+ hematologic AE in the control group. There were no major differences in GVHD between the azacitidine and control groups, with D100 cumulative incidence of grade 2-4 aGVHD of 25.5% with azacitidine and 28.7% in the control group (P = .73) and no difference in 1-year rates of chronic GVHD (25.8% in the azacitidine maintenance arm compared to 30.8% in the no maintenance arm). Oral formulations of DNMTi (i.e. oral azacitidine (CC-486) or oral decitabine/cedazuridine) result in lower peak plasma concentrations hence lower cumulative daily exposure, which may result in sustained epigenetic regulation compared to subcutaneous or intravenous formulations. Trials with these oral formulations for pre-emptive or prophylactic post-transplant maintenance are ongoing (refer to Table 1).
In contrast, Gao et al. found that combination rhG-CSF with minimal dose decitabine (5mg/m2 on days 1-5) was associated with far lower rates of cytopenias (8% grade 3/4 neutropenia, 15% grade 3/4 thrombocytopenia), but improved CIR (15% vs 38.3%, hazard ratio 0.32 (95% CI, 0.18 to 0.57; P <.01) as compared to no intervention in their phase II open-label, multicenter randomized trial (156). Whether this difference in relapse prevention and cytopenias between post-transplant maintenance with azacitidine and minimal dose decitabine + rhG-CSF relates to differences between the DNMTi, the rhG-CSF, or related to patient cohort or other trial related factors remains unknown. An intriguing possibility is that the addition of growth factor in the maintenance setting augments anti-leukemic activity of decitabine, with the salutatory incidental benefit of reduced myelosuppression. Interestingly, Gao et al. also noted increased NK, cytotoxic T-cell, and Tregs with G-CSF and minimal dose decitabine. Azacitidine has also been studied in combination with growth factor, specifically GM-CSF in combination with azacitidine in a phase II non-randomized study with 24-month RFS and OS of 47% and 57% respectively (144). There have been various other studies investigating decitabine and oral CC-486 in phase I/non-randomized settings (142, 146, 147). Additionally, there has been interest in combining hypomethylating agents with venetoclax (reviewed below), other small molecules, as well as DLI with or without other small molecules (145, 149, 157).
GVL Considerations: Goodyear and colleagues investigated the impact of azacitidine on T regulatory (Treg) cells as well as anti-tumor CD8+ T cells after alloHSCT. They found increased Tregs in the early post-transplant period (first three months), as well as preliminary evidence of T-cell response to tumor antigens (158). An analysis of the RICAZA trial further suggested a link between CD8+ T cell responses, azacitidine exposure, and relapse risk (141). Overall, the literature is mixed, with some studies suggesting augmentation of GVL effects (159, 160), while others suggest impairment of anti-leukemic activity (161, 162). It is likely that hypomethylating agents exert pleomorphic effects on residual recipient immune cells, the donor graft, and leukemic cells. Studies of decitabine with checkpoint inhibitors further suggest influence of the microenvironmental niche and cellular expression state-dependent clinical activity (163, 164). Further studies could deconvolute in more granular detail the effects of hypomethylating agents on various T cell and NK cell subsets, of particular interest in the context of multi-agent maintenance therapy. There are a number of prospective studies reporting the combination of DLI and DNMTi. Two of these report on the prophylactic use of DLI + DNMTi (145, 148) while others report this combination at the time of frank relapse after transplantation (165, 166).
Take-home message:
● Given the absence of convincing clinical trial data, there is currently no clear indication for single-agent DNMT inhibitors in subcutaneous or intravenous formulations in the prophylactic maintenance setting after alloHSCT (though pre-emptive use with rising MRD or dropping chimerism may be reasonable clinical scenarios).
Venetoclax
The oral, selective BCL-2 inhibitor venetoclax offers another therapeutic opportunity with broad activity, with demonstration of activity against NPM1, IDH1/2, splicing mutations, and now secondary-ontogeny cases (57, 167). Venetoclax with azacitidine is a new first-line therapeutic option for patients with AML ineligible for intensive chemotherapy based on the VIALE-A trial and it has also been studied in combination with low-dose ara-C and IDH inhibitors in the up-front setting (56, 168, 169). In the transplant setting, venetoclax has been studied in combination with RIC and MAC chemotherapy regimens in patients with high-risk myeloid malignancies (65, 170). The VIALE-T study is recruiting patients to study venetoclax with azacitidine maintenance versus standard best supportive care after alloHSCT (NCT04161885). We have recently reported our phase I single-center experience with prophylactic venetoclax plus azacitidine maintenance after RIC-Ven conditioning in 22 patients with high risk MDS/AML. Cytopenias were the most common adverse event but transient, and infections were uncommon (2 COVID19 infections and 2 non-COVID19 infections; all grade 1-2) (143). After a median follow-up of 25 months, the 2-year OS, PFS, NRM, and CIR rates were 67%, 59%, 0%, and 41%, respectively. We did not observe any significant impact on T cell expansion but did note delayed CD19+ B cell reconstitution in patients receiving maintenance therapy compared to a historical cohort that did not receive maintenance. Persistent NGS-MRD was associated with relapse, and dynamic monitoring suggested that day 28 and day 100 timepoints may be useful in detecting incipient relapse. A randomized study will be needed to determine the value of venetoclax-based prophylactic maintenance therapy, but our study demonstrates reasonable safety and supports further investigation of pre-emptive azacitidine/venetoclax maintenance in high risk MDS/AML patients with persistent MRD early post-transplant. This study is now enrolling patients to a separate cohort to test the safety and efficacy of an all-oral post-transplant prophylactic maintenance regimen with combination decitabine/cedazuridine and venetoclax (NCT03613532).
GVL considerations: given the central role of apoptosis in T cell development, BH3 mimetics have been studied to interrogate their effects on the immune system. Outside of the transplant setting, studies of patients with AML and CLL exposed to venetoclax have found limited impact on T and NK cells (171). However there may be a rationale for apoptosis targeting in the transplant setting, as the combination of venetoclax has also been suggested to increase cytotoxic T cell activity/GVL via reactive-oxygen species generation augmented by DNMTi-mediated AML immunoreactivity via the STING-cGAS pathway (172). NK cells may also play a role in GVL, with data supporting targeting mitochondrial apoptosis to augment NK cell immunotherapy (173). Ludwig and colleagues studied prolonged (several month) venetoclax exposure in a mouse model of bone-marrow transplantation (174). They found no change in T-cell subset proportions but did identify lower T cell numbers and evidence of apoptotic adaptation. They also identified transcriptional and signaling pathway changes which may have effects on T cell function and implications for the GVL effect. Their work builds on findings from Kohlapp and colleagues suggesting venetoclax augments immune-checkpoint mediated anti-tumor T-cell activity, as well as prior work by Carrington and colleagues that categorized BH3 mimetics as immunomodulatory agents (175, 176).
Take-home message:
● Despite concern for persistent myelosuppression or increased infection risk, the current preliminary data suggest reduced doses of venetoclax-azacitidine combinations can be safely delivered in the post-transplant setting.
● Dynamic MRD testing may identify patients that are likely to benefit the most from venetoclax-azacitidine post-transplant maintenance.
Novel/unapproved agents
TP53:
Mutations in TP53 portend dismal outcomes in AML. PRIMA-1 was identified in a screen of compounds inducing apoptosis in human cancer cell lines through restoration of p53 transcriptional function (177). APR-246 (an analog of PRIMA-1, also known as eprenetapopt) was first studied in humans in prostate cancer and refractory hematologic malignancies (178). Eprenetapopt was then studied in combination with Azacitidine in TP53-mutated MDS and AML (179, 180). It has also been studied in a phase I study in combination with azacitidine and venetoclax (181). In a phase II, multicenter open-label trial, eprenetapopt was combined with azacitidine as maintenance after alloHSCT in 33 patients with mutated TP53 MDS or AML (150). Patients received azacitidine for 5 days and eprenetapopt for 4 days in each 28-day cycle. Patients received up to 12 cycles. The primary outcome was RFS and safety. Median RFS was 12.5 months and 1-year RFS was 59.9% with a 1-year OS of 78.8%. Despite the initial enthusiasm with this phase II study, to our knowledge, there are no active clinical trials studying eprenetapopt as post-alloHSCT maintenance in TP53-mutated MDS or AML.
NPM1/KMT2A
Menin, a chromatin adaptor which interacts with MLL1/KMT2A and is implicated in the pathogenesis of NPM1-mutated AML, has been studied as a therapeutic target in both myeloid and lymphoid malignancies (182, 183). Menin inhibitors have been studied in early-phase clinical trials of KMT2A-rearranged or NPM1-mutated leukemia, with MEN1 mutations implicated in resistance mechanisms (184, 185). There is some data to suggest synergism with venetoclax (186). There are multiple phase I and II clinical trials studying menin inhibitors in combination with hypomethylating agents and conventional chemotherapy in patients with acute leukemias, including preliminary data in the post-transplant setting (NCT05360160, NCT05326516, NCT04067336, NCT04811560, NCT05453903, NCT05521087, NCT05153330). Issa and colleagues reported 3 patients treated with SNDX-5613 (revumenib) as post-transplantation maintenance in the AUGMENT-101 phase I trial, with long-term remissions in heavily pretreated patients (ASH 2022 Abstract #723). The on or off-target effect of menin inhibition on the donor immune system has not yet been elucidated.
RAS-pathway
There is considerable interest in targeting the RAS-pathway given its role in leukemic pathogenesis and as a resistance pathway after FLT3-inhibitor, IDH1/2-inhibitor, and venetoclax exposure (167, 187–189). Studies have evaluated the MEK inhibitor trametinib in combination with azacitidine and venetoclax in R/R AML with RAS-pathway mutations or in combination with an AKT inhibitor (190, 191). Selumetinib and binimetinib are MEK inhibitors studied in the relapsed/refractory setting (192, 193). Oral AKT inhibitors have also been studied as single agents (194). To our knowledge, none of these agents are currently under investigation in the post-transplant setting.
Other agents
Lenalidomide has been studied as post-alloHSCT maintenance in high-risk myeloid malignancies (155). A translational study of the HOVON-103 AML/SAKK30/10 study reported changes in microvascularization as well as T-cell mediated GVL (195). The immunomodulatory effect of lenalidomide after alloHSCT may not be limited to GVL, as there is data to suggest impact on T-cell trafficking to the gut with potential impact on GVHD (196). Other agents that have been studied after alloHSCT include pegylated IFN-2a (197) and the deacetylase inhibitor panobinostat (151).
Although data with mouse-double-minute-2 (MDM2) inhibitors in combination with chemotherapies or venetoclax have shown limited clinical activity in the setting of overt relapsed disease, renewed interest has been generated for its potential in the post-transplant setting based on preclinical data suggesting MDM2 inhibition increased TRAIL-receptor-1 and -2 MHC-II expression on leukemia cells (198, 199). These data prompted the development of a clinical trial (NCT05447663) to assess for safety and efficacy with post-transplant siremadlin (MDM2 inhibitor) prophylactic maintenance therapy with or without donor lymphocyte infusion in AML patients determined to be at high risk for relapse.
Chimeric antigen receptor therapy
CAR-T cell therapy is now used routinely in the care of patients with B-cell hematologic malignancies, and there is increasing interest in developing CAR constructs for use in myeloid malignancies. Challenges in the myeloid space include the identification of antigens which can be targeted on leukemic clones while sparing healthy hematopoietic stem cells and precursors. Recent advances in target identification and selection are showing promise in realizing this specificity, and we refer readers to a recent review summarizing this rapidly evolving field (200, 201). Yao and colleagues report a case utilizing donor CD123 CAR-T as part of reduced intensity conditioning prior to haploidentical transplantation in a highly aggressive leukemia relapsing post allogeneic transplantation (153). Anti-CLL1 CAR-T therapy may serve as a bridge to alloHSCT, and has been studied in early phase trials in both children and adults with relapse/refractory disease (202, 203).
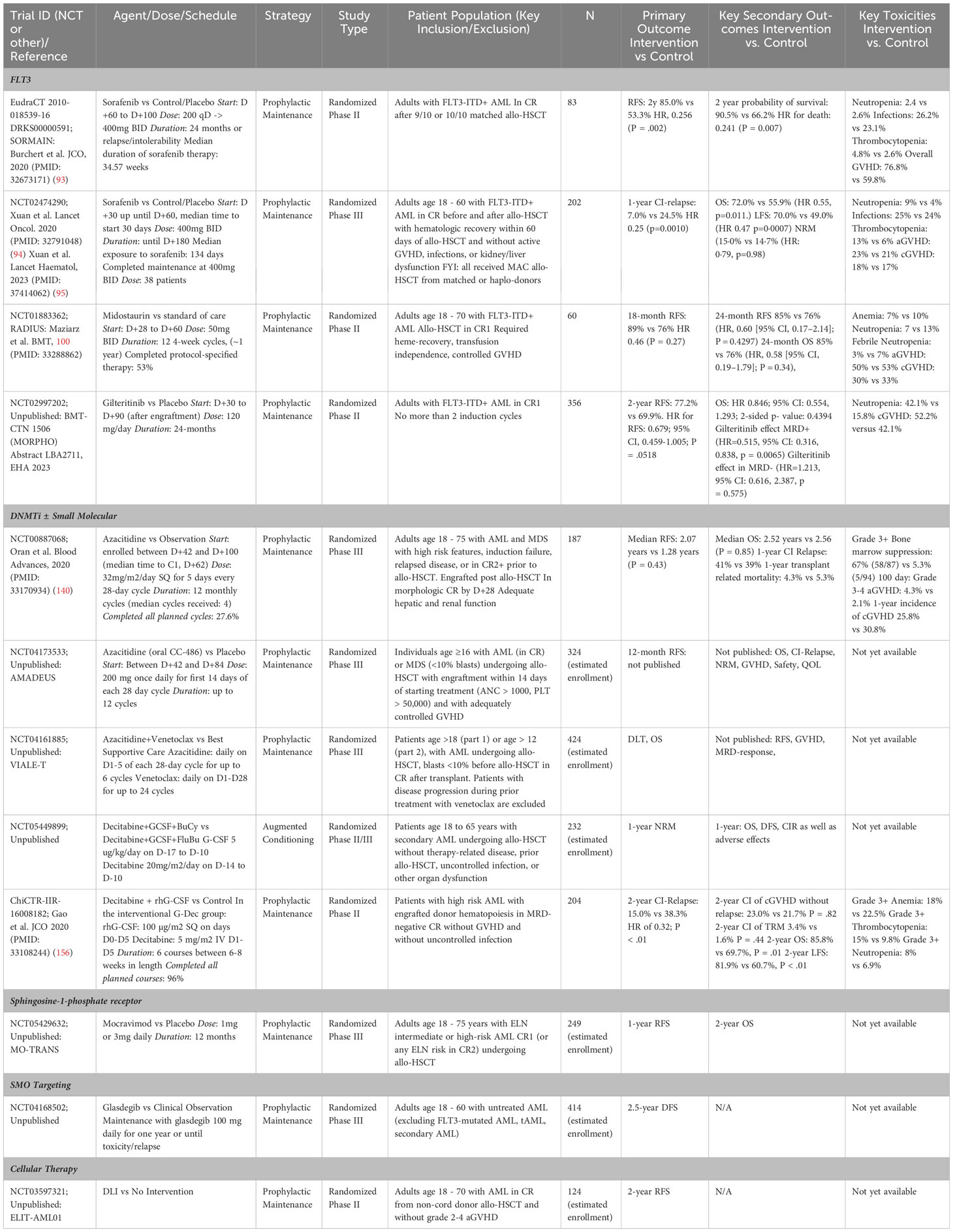
Table 1 summarizes non-randomized trials of conditioning augmentation, preemptive maintenance, and prophylactic maintenance, while Table 2 summarizes randomized clinical studies, both published and those registered at clinicaltrials.gov.
Conclusion
AML is no longer a singular disease entity, but rather is an increasingly complex condition that is subdivided into genomic subgroups with distinct sensitivity to traditional chemotherapy and novel agents that target a growing number of drivers of disease pathogenesis. For higher risk myeloid malignancies, allogeneic stem cell transplantation remains the only curative treatment modality through the graft-vs-leukemia effect. However, transplantation is not universally effective, and despite an expanding armamentarium of targeted agents and immune-based therapies for the treatment of AML, post-transplant relapse remains a significant challenge with near universal poor outcomes. Prevention of post-transplantation relapse with prophylactic or pre-emptive maintenance therapy has the potential to decrease relapse risk and improve overall survival. Maintenance therapy must prove anti-leukemic efficacy, yet balance amelioration of relapse with treatment toxicity, effects on quality-of-life, time-toxicity, and the impact on the donor graft.
The value of MRD is becoming more evident in the post-transplant setting. It may serve as a dynamic marker identifying those patients who would most benefit from relapse prevention strategies, while sparing patients at lower relapse risk from treatment-related toxicity. Because AML presents as a clonal disease with genomic alterations detected in routine clinical care, serial molecular MRD assessments are a modality that could be broadly deployed, informing relapse risk, and identify new therapeutic vulnerabilities that may not have been apparent at diagnosis. However, the genomic heterogeneity of AML complicates the routine incorporation of NGS-MRD into clinical practice. When interpreting NGS-MRD, persistent clonality can reflect a spectrum of biologically diverse reservoirs of disease with the possibility of distinct relapse risk and kinetics profiles. This is in contrast to other hematologic malignancies, such as those driven by the t(9;22) translocation, in which a single genetic lesion can be more easily correlated with leukemic burden and relapse risk. Deconvoluting the gene/phenotype-specific subtypes of persistent clonality will necessitate large-scale, multi-institutional, programmatic efforts to develop standardized definitions of clinically meaningful NGS-MRD for incorporation into prospective randomized trials powered for patient-centered outcomes. Efforts are underway through the FNIH, collaborative projects such as pre-MEASURE and MEASURE, and the ELN MRD Working Party to build the evidence base necessary for clinical adoption of NGS- MRD in AML (28, 45).
While significant advances in our understanding of leukemogenesis has led to development of gene/mutation-specific targeted agents, our understanding of the long-term impact of these agents on the normal/graft immune system is quite limited. Hematopoietic reconstitution after alloHSCT is a complex process with implications for recovery of counts sufficient to conduct daily life, for immunity against a wide array of microbes, and for surveillance against non-hematologic malignancy. For these reasons, correlative studies of immune reconstitution and function should be incorporated into studies of post-alloHSCT maintenance therapy. Targeted agents are often complicated by cytopenias, suggesting an impact on normal hematopoiesis. Whether there is a concomitant clinically relevant impact not only on cell number, but cell function is largely unknown. As new agents are developed in the pre-clinical setting, efforts should be made to study any impact on normal hematopoiesis and on immune effector function. We envision these parallel avenues of investigation leading to targeted agents which harness a growing understanding of the genomic pathobiology of leukemia with ever more nuanced understanding of the GVL effect to reduce the risk of relapse and improve outcomes for patients afflicted with this often-devastating disease.
Author contributions
HM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. VH: Investigation, Validation, Writing – review & editing. JG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under award number K08CA245209. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCI.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AML, acute myeloid leukemia; sAML, secondary AML; MDS, myelodysplastic syndromes; CMML, chronic myelomonocytic leukemia; JMML, juvenile myelomonocytic leukemia; MPAL, mixed-phenotype acute leukemia; MF, myelofibrosis; AE, adverse events; D+, days after stem cell infusion; qD, daily; BID, twice daily; TID, three times daily; PO, by mouth; SQ, subcutaneous; IV, intravenous; ECOG, eastern cooperative group performance status; CR, complete remission; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; ANC, absolute neutrophil count per uL; PLT, platelets per uL; GVHD, graft-vs-host disease cGVHD, chronic GVHD; aGVHD, acute GVHD; MTD, maximally tolerated dose; RP2D, recommended phase II dose; DLT, doselimiting toxicity; ORR, overall response rate; PFS, progression-free survival; EFS, event-free survival; RFS, relapse-free survival; DFS, disease-free survival; OS, overall survival; CI, cumulative incidence; NRM, non-relapse mortality; TRM, treatment-related mortality; GRFS, GVHD-free; relapse-free survival; VOD, vaso-occlusive disease; RIC, reduced intensity conditioning.
References
1. Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. (2008) 456:66–72. doi: 10.1038/nature07485
2. Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. (2002) 100:4325–36. doi: 10.1182/blood-2002-03-0772
3. Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. (1998) 92:2322–33. doi: 10.1182/blood.V92.7.2322
4. Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. (2010) 116:354–65. doi: 10.1182/blood-2009-11-254441
5. Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. (2008) 358:1909–18. doi: 10.1056/NEJMoa074306
6. Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. (2013) 368:2059–74. doi: 10.1056/NEJMoa1301689
7. Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. (2012) 366:1079–89. doi: 10.1056/NEJMoa1112304
8. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
9. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
10. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. (2022) 140:1200–28. doi: 10.1182/blood.2022015850
11. Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. (2022) 140:1345–77. doi: 10.1182/blood.2022016867
12. Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. (2015) 125:1367–76. doi: 10.1182/blood-2014-11-610543
13. Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. (2012) 119:2114–21. doi: 10.1182/blood-2011-08-375758
14. Schoch C, Haferlach T, Haase D, Fonatsch C, Löffler H, Schlegelberger B, et al. Patients with de novo acute myeloid leukaemia and complex karyotype aberrations show a poor prognosis despite intensive treatment: a study of 90 patients. Br J Haematol. (2001) 112:118–26. doi: 10.1046/j.1365-2141.2001.02511.x
15. Breems DA, Van Putten WLJ, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KBJ, Mellink CHM, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. (2008) 26:4791–7. doi: 10.1200/JCO.2008.16.0259
16. Döhner K, Thiede C, Jahn N, Panina E, Gambietz A, Larson RA, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. (2020) 135:371–80. doi: 10.1182/blood.2019002697
17. Tarlock K, Lamble AJ, Wang Y-C, Gerbing RB, Ries RE, Loken MR, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the Children’s Oncology Group. Blood. (2021) 138:1137–47. doi: 10.1182/blood.2020009652
18. Wakita S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. (2022) 6:238–47. doi: 10.1182/bloodadvances.2021004292
19. Schlenk RF, Benner A, Krauter J, Büchner T, Sauerland C, Ehninger G, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. (2004) 22:3741–50. doi: 10.1200/JCO.2004.03.012
20. Marcucci G, Mrózek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. (2005) 23:5705–17. doi: 10.1200/JCO.2005.15.610
21. Paschka P, Du J, Schlenk RF, Gaidzik VI, Bullinger L, Corbacioglu A, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG). Blood. (2013) 121:170–7. doi: 10.1182/blood-2012-05-431486
22. Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. (2006) 24:3904–11. doi: 10.1200/JCO.2006.06.9500
23. Shimony S, Fell GG, Chen EC, Tsai HK, Wadleigh M, Winer ES, et al. FLT3-ITD does not predict inferior prognosis in acute myeloid leukemia patients age ≥60 years. Blood Adv. (2023) 7:5354–8. doi: 10.1182/bloodadvances.2023009748
24. Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. (2017) 376:536–47. doi: 10.1056/NEJMoa1611604
25. Murdock HM, Kim HT, Denlinger N, Vachhani P, Hambley BC, Manning BS, et al. Impact of diagnostic genetics on remission MRD and transplantation outcomes in older AML patients. Blood. (2022) 139:3546–57. doi: 10.1182/blood.2021014520
26. Grimm J, Jentzsch M, Bill M, Goldmann K, Schulz J, Niederwieser D, et al. Prognostic impact of the ELN2017 risk classification in patients with AML receiving allogeneic transplantation. Blood Adv. (2020) 4:3864–74. doi: 10.1182/bloodadvances.2020001904
27. Jentzsch M, Bischof L, Ussmann J, Backhaus D, Brauer D, Metzeler KH, et al. Prognostic impact of the AML ELN2022 risk classification in patients undergoing allogeneic stem cell transplantation. Blood Cancer J. (2022) 12:170. doi: 10.1038/s41408-022-00764-9
28. Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL, et al. 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood. (2021) 138:2753–67. doi: 10.1182/blood.2021013626
29. Vedula RS, Lindsley RC. Measurement of residual disease in acute myeloid leukemia. Curr Hematol Malig Rep. (2017) 12:574–81. doi: 10.1007/s11899-017-0428-4
30. Jourdan E, Boissel N, Chevret S, Delabesse E, Renneville A, Cornillet P, et al. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood. (2013) 121:2213–23. doi: 10.1182/blood-2012-10-462879
31. Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA. (2015) 314:811–22. doi: 10.1001/jama.2015.9643
32. Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A, et al. Assessment of minimal residual disease in standard-risk AML. N Engl J Med. (2016) 374:422–33. doi: 10.1056/NEJMoa1507471
33. Boddu P, Jorgensen J, Kantarjian H, Borthakur G, Kadia T, Daver N, et al. Achievement of a negative minimal residual disease state after hypomethylating agent therapy in older patients with AML reduces the risk of relapse. Leukemia. (2018) 32:241–4. doi: 10.1038/leu.2017.285
34. Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, Al Hinai A, Zeilemaker A, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. (2018) 378:1189–99. doi: 10.1056/NEJMoa1716863
35. Short NJ, Zhou S, Fu C, Berry DA, Walter RB, Freeman SD, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: A systematic review and meta-analysis. JAMA Oncol. (2020) 6:1890–9. doi: 10.1001/jamaoncol.2020.4600
36. Maiti A, DiNardo CD, Wang SA, Jorgensen J, Kadia TM, Daver NG, et al. Prognostic value of measurable residual disease after venetoclax and decitabine in acute myeloid leukemia. Blood Adv. (2021) 5:1876–83. doi: 10.1182/bloodadvances.2020003717
37. Grob T, Sanders MA, Vonk CM, Kavelaars FG, Rijken M, Hanekamp DW, et al. Prognostic value of FLT3-internal tandem duplication residual disease in acute myeloid leukemia. J Clin Oncol. (2022) 41:756–65. doi: 10.1200/JCO.22.00715
38. Luskin MR, Carroll M, Lieberman D, Morrissette JJD, Zhao J, Crisalli L, et al. Clinical utility of next-generation sequencing for oncogenic mutations in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2016) 22:1961–7. doi: 10.1016/j.bbmt.2016.07.018
39. Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. (2018) 132:1703–13. doi: 10.1182/blood-2018-02-829911
40. Press RD, Eickelberg G, Froman A, Yang F, Stentz A, Flatley EM, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol. (2019) 94:902–12. doi: 10.1002/ajh.25514
41. Dillon R, Hills R, Freeman S, Potter N, Jovanovic J, Ivey A, et al. Molecular MRD status and outcome after transplantation in NPM1-mutated AML. Blood. (2020) 135:680–8. doi: 10.1182/blood.2019002959
42. Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. (2020) 38:1273–83. doi: 10.1200/JCO.19.03011
43. Bill M, Jentzsch M, Bischof L, Kohlschmidt J, Grimm J, Schmalbrock LK, et al. Impact of IDH1 and IDH2 mutation detection at diagnosis and in remission in patients with AML receiving allogeneic transplantation. Blood Adv. (2022) 7:436–44. doi: 10.1182/bloodadvances.2021005789
44. Loo S, Dillon R, Ivey A, Anstee NS, Othman J, Tiong IS, et al. Pre-transplant FLT3-ITD MRD assessed by high-sensitivity PCR-NGS determines post-transplant clinical outcome. Blood. (2022) 140:2407–11. doi: 10.1182/blood.2022016567
45. Dillon LW, Gui G, Page KM, Ravindra N, Wong ZC, Andrew G, et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA. (2023) 329:745–55. doi: 10.1001/jama.2023.1363
46. Heuser M, Heida B, Büttner K, Wienecke CP, Teich K, Funke C, et al. Posttransplantation MRD monitoring in patients with AML by next-generation sequencing using DTA and non-DTA mutations. Blood Adv. (2021) 5:2294–304. doi: 10.1182/bloodadvances.2021004367
47. Kim T, Moon JH, Ahn J-S, Kim Y-K, Lee S-S, Ahn S-Y, et al. Next-generation sequencing-based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood. (2018) 132:1604–13. doi: 10.1182/blood-2018-04-848028
48. Short NJ, Rafei H, Daver N, Hwang H, Ning J, Jorgensen JL, et al. Prognostic impact of complete remission with MRD negativity in patients with relapsed or refractory AML. Blood Adv. (2020) 4:6117–26. doi: 10.1182/bloodadvances.2020002811
49. Zhu H-H, Zhang X-H, Qin Y-Z, Liu D-H, Jiang H, Chen H, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. (2013) 121:4056–62. doi: 10.1182/blood-2012-11-468348
50. Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. (2018) 19:1668–79. doi: 10.1016/S1470-2045(18)30580-1
51. Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R, et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood. (2019) 134:935–45. doi: 10.1182/blood.2018886960
52. Yuan X-L, Tan Y-M, Shi J-M, Zhao Y-M, Yu J, Lai X-Y, et al. Preemptive low-dose interleukin-2 or DLI for late-onset minimal residual disease in acute leukemia or myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol. (2021) 100:517–27. doi: 10.1007/s00277-020-04326-6
53. Tiong IS, Dillon R, Ivey A, Teh T-C, Nguyen P, Cummings N, et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukaemia. Br J Haematol. (2021) 192:1026–30. doi: 10.1111/bjh.16722
54. Heitmann JS, Schlenk RF, Dörfel D, Kayser S, Döhner K, Heuser M, et al. Phase I study evaluating the Fc-optimized FLT3 antibody FLYSYN in AML patients with measurable residual disease. J Hematol Oncol. (2023) 16:96. doi: 10.1186/s13045-023-01490-w
55. Walter RB. Perspective on measurable residual disease testing in acute myeloid leukemia. Leukemia. (2023) 38:10–13. doi: 10.1038/s41375-023-02084-8
56. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
57. Senapati J, Urrutia S, Loghavi S, Short NJ, Issa GC, Maiti A, et al. Venetoclax abrogates the prognostic impact of splicing factor gene mutations in newly diagnosed acute myeloid leukemia. Blood. (2023) 142:1647–57. doi: 10.1182/blood.2023020649
58. Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA, et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol. (1989) 7:50–7. doi: 10.1200/JCO.1989.7.1.50
59. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. (2012) 119:1599–606. doi: 10.1182/blood-2011-08-375840
60. Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica. (2018) 103:237–45. doi: 10.3324/haematol.2017.168716
61. Kekre N, Kim HT, Thanarajasingam G, Armand P, Antin JH, Cutler C, et al. Efficacy of immune suppression tapering in treating relapse after reduced intensity allogeneic stem cell transplantation. Haematologica. (2015) 100:1222–7. doi: 10.3324/haematol.2015.129650
62. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. (2017) 35:1154–61. doi: 10.1200/JCO.2016.70.7091
63. Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW, et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from cancer and leukemia group B 100103 (Alliance for clinical trials in oncology)/blood and marrow transplant clinical trial network 0502. J Clin Oncol. (2015) 33:4167–75. doi: 10.1200/JCO.2015.62.7273
64. Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented reduced-intensity regimen does not improve postallogeneic transplant outcomes in acute myeloid leukemia. J Clin Oncol. (2021) 39:768–78. doi: 10.1200/JCO.20.02308
65. Garcia JS, Kim HT, Murdock HM, Cutler CS, Brock J, Gooptu M, et al. Adding venetoclax to fludarabine/busulfan RIC transplant for high-risk MDS and AML is feasible, safe, and active. Blood Adv. (2021) 5:5536–45. doi: 10.1182/bloodadvances.2021005566
66. Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. (1986) 314:729–35. doi: 10.1056/NEJM198603203141201
67. Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, et al. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. (1986) 68:119–25. doi: 10.1182/blood.V68.1.119.bloodjournal681119
68. Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. (1998) 92:2303–14.
69. Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. (2000) 96:2062–8.
70. Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. (2010) 16:937–47. doi: 10.1016/j.bbmt.2010.01.010
71. Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. (2014) 124:1372–7. doi: 10.1182/blood-2014-04-567164
72. Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med. (2023) 388:2338–48. doi: 10.1056/NEJMoa2215943
73. Maurer K, Ho VT, Inyang E, Cutler C, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. (2023) 7:3903–15. doi: 10.1182/bloodadvances.2023009791
74. Barnes DW, Corp MJ, Loutit JF, Neal FE. Treatment of murine leukaemia with X rays and homologous bone marrow; preliminary communication. Br Med J. (1956) 2:626–7. doi: 10.1136/bmj.2.4993.626
75. Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. (1977) 49:511–33. doi: 10.1182/blood.V49.4.511.bloodjournal494511
76. Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med. (1979) 301:597–9. doi: 10.1056/NEJM197909133011109
77. Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. (1979) 300:1068–73. doi: 10.1056/NEJM197905103001902
78. Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED, Seattle Marrow Transplant Team. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. (1981) 304:1529–33. doi: 10.1056/NEJM198106183042507
79. Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. (1994) 330:100–6. doi: 10.1056/NEJM199401133300204
80. Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. (2007) 25:4938–45. doi: 10.1200/JCO.2007.11.6053
81. Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. (2009) 361:478–88. doi: 10.1056/NEJMoa0811036
82. Toffalori C, Cavattoni I, Deola S, Mastaglio S, Giglio F, Mazzi B, et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood. (2012) 119:4813–5. doi: 10.1182/blood-2012-02-411686
83. Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation. Blood Adv. (2019) 3:2199–204. doi: 10.1182/bloodadvances.2019000445
84. Sweeney C, Vyas P. The graft-versus-leukemia effect in AML. Front Oncol. (2019) 9:1217. doi: 10.3389/fonc.2019.01217
85. Birg F, Courcoul M, Rosnet O, Bardin F, Pébusque MJ, Marchetto S, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. (1992) 80:2584–93. doi: 10.1182/blood.V80.10.2584.bloodjournal80102584
86. Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. (1996) 10:1911–8.
87. Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic Malignancies. Blood. (2001) 97:2434–9. doi: 10.1182/blood.V97.8.2434
88. Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. (2010) 116:5089–102. doi: 10.1182/blood-2010-04-261867
89. Galanis A, Ma H, Rajkhowa T, Ramachandran A, Small D, Cortes J, et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. (2014) 123:94–100. doi: 10.1182/blood-2013-10-529313
90. Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. (2007) 21:439–45. doi: 10.1038/sj.leu.2404508
91. Chen Y-B, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. (2014) 20:2042–8. doi: 10.1016/j.bbmt.2014.09.007
92. Pratz KW, Rudek MA, Smith BD, Karp J, Gojo I, Dezern A, et al. A prospective study of peritransplant sorafenib for patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic transplantation. Biol Blood Marrow Transplant. (2020) 26:300–6. doi: 10.1016/j.bbmt.2019.09.023
93. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. (2020) 38:2993–3002. doi: 10.1200/JCO.19.03345
94. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. (2020) 21:1201–12. doi: 10.1016/S1470-2045(20)30455-1
95. Xuan L, Wang Y, Yang K, Shao R, Huang F, Fan Z, et al. Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. (2023) 10:e600–11. doi: 10.1016/S2352-3026(23)00117-5
96. Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. (2018) 24:282–91. doi: 10.1038/nm.4484
97. Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. (2005) 105:54–60. doi: 10.1182/blood-2004-03-0891
98. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
99. Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. (2019) 133:840–51. doi: 10.1182/blood-2018-08-869453
100. Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. (2021) 56:1180–9. doi: 10.1038/s41409-020-01153-1
101. Lee LY, Hernandez D, Rajkhowa T, Smith SC, Raman JR, Nguyen B, et al. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. (2017) 129:257–60. doi: 10.1182/blood-2016-10-745133
102. Mori M, Kaneko N, Ueno Y, Yamada M, Tanaka R, Saito R, et al. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest New Drugs. (2017) 35:556–65. doi: 10.1007/s10637-017-0470-z
103. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
104. Terao T, Matsuoka K-I, Ueda H, Matsumura A, Matsubara C, Kondo K, et al. Early initiation of low-dose gilteritinib maintenance improves posttransplant outcomes in patients with R/R FLT3mut AML. Blood Adv. (2023) 7:681–6. doi: 10.1182/bloodadvances.2022008991
105. Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood. (2022) 139:3366–75. doi: 10.1182/blood.2021011583
106. Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Outcomes in patients with FLT3-mutated relapsed/refractory acute myelogenous leukemia who underwent transplantation in the phase 3 ADMIRAL trial of gilteritinib versus salvage chemotherapy. Transplant Cell Ther. (2023) 29:265.e1–265.e10. doi: 10.1016/j.jtct.2022.12.006
107. Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, et al. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. (2018) 132:598–607. doi: 10.1182/blood-2018-01-821629
108. Dennis M, Thomas IF, Ariti C, Upton L, Burnett AK, Gilkes A, et al. Randomized evaluation of quizartinib and low-dose ara-C vs low-dose ara-C in older acute myeloid leukemia patients. Blood Adv. (2021) 5:5621–5. doi: 10.1182/bloodadvances.2021005038
109. Erba HP, Montesinos P, Kim H-J, Patkowska E, Vrhovac R, Žák P, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 401:1571–83. doi: 10.1016/S0140-6736(23)00464-6
110. Sandmaier BM, Khaled S, Oran B, Gammon G, Trone D, Frankfurt O. Results of a phase 1 study of quizartinib as maintenance therapy in subjects with acute myeloid leukemia in remission following allogeneic hematopoietic stem cell transplant. Am J Hematol. (2018) 93:222–31. doi: 10.1002/ajh.24959
111. Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. (2002) 100:1532–42. doi: 10.1182/blood-2002-02-0492
112. Rickmann M, Macke L, Sundarasetty BS, Stamer K, Figueiredo C, Blasczyk R, et al. Monitoring dendritic cell and cytokine biomarkers during remission prior to relapse in patients with FLT3-ITD acute myeloid leukemia. Ann Hematol. (2013) 92:1079–90. doi: 10.1007/s00277-013-1744-y
113. Lau CM, Nish SA, Yogev N, Waisman A, Reiner SL, Reizis B. Leukemia-associated activating mutation of Flt3 expands dendritic cells and alters T cell responses. J Exp Med. (2016) 213:415–31. doi: 10.1084/jem.20150642
114. Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Götze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. (2012) 26:2353–9. doi: 10.1038/leu.2012.105
115. Tschan-Plessl A, Halter JP, Heim D, Medinger M, Passweg JR, Gerull S. Synergistic effect of sorafenib and cGvHD in patients with high-risk FLT3-ITD+AML allows long-term disease control after allogeneic transplantation. Ann Hematol. (2015) 94:1899–905. doi: 10.1007/s00277-015-2461-5
116. Lange A, Jaskula E, Lange J, Dworacki G, Nowak D, Simiczyjew A, et al. The sorafenib anti-relapse effect after alloHSCT is associated with heightened alloreactivity and accumulation of CD8+PD-1+ (CD279+) lymphocytes in marrow. PloS One. (2018) 13:e0190525. doi: 10.1371/journal.pone.0190525
117. Zhang Z, Hasegawa Y, Hashimoto D, Senjo H, Kikuchi R, Chen X, et al. Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2022) 57:775–80. doi: 10.1038/s41409-022-01619-4
118. Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, et al. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia. (2018) 32:1168–79. doi: 10.1038/s41375-018-0009-0
119. Giannakopoulou E, Lehander M, Virding Culleton S, Yang W, Li Y, Karpanen T, et al. A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo. Nat Cancer. (2023) 4:1474–90. doi: 10.1038/s43018-023-00642-8
120. Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. (2009) 361:1058–66. doi: 10.1056/NEJMoa0903840
121. Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. (2010) 28:2348–55. doi: 10.1200/JCO.2009.27.3730
122. Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. (2009) 462:739–44. doi: 10.1038/nature08617
123. Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. (2010) 17:225–34. doi: 10.1016/j.ccr.2010.01.020
124. Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. (2010) 18:553–67. doi: 10.1016/j.ccr.2010.11.015
125. Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S-H, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. (2011) 19:17–30. doi: 10.1016/j.ccr.2010.12.014
126. Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. (2012) 483:474–8. doi: 10.1038/nature10860
127. Fathi AT, Kim HT, Soiffer RJ, Levis MJ, Li S, Kim AS, et al. Multi-center phase I trial of Ivosidenib as Maintenance Treatment following Allogeneic Hematopoietic Cell Transplantation for IDH1-Mutated Acute Myeloid Leukemia. Clin Cancer Res. (2023) 29:2034–42. doi: 10.1158/1078-0432.CCR-23-0182
128. Fathi AT, Kim HT, Soiffer RJ, Levis MJ, Li S, Kim AS, et al. Enasidenib as maintenance following allogeneic hematopoietic cell transplantation for IDH2-mutated myeloid Malignancies. Blood Adv. (2022) 6:5857–65. doi: 10.1182/bloodadvances.2022008632
129. Notarangelo G, Spinelli JB, Perez EM, Baker GJ, Kurmi K, Elia I, et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science. (2022) 377:1519–29. doi: 10.1126/science.abj5104
130. Palau A, Segerberg F, Lidschreiber M, Lidschreiber K, Naughton AJ, Needhamsen M, et al. Perturbed epigenetic transcriptional regulation in AML with IDH mutations causes increased susceptibility to NK cells. Leukemia. (2023) 37:1830–41. doi: 10.1038/s41375-023-01972-3
131. Choe S, Wang H, DiNardo CD, Stein EM, de Botton S, Roboz GJ, et al. Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv. (2020) 4:1894–905. doi: 10.1182/bloodadvances.2020001503
132. Xuan L, Dai M, Jiang E, Wang Y, Huang F, Fan Z, et al. The effect of granulocyte-colony stimulating factor, decitabine, and busulfan-cyclophosphamide versus busulfan-cyclophosphamide conditioning on relapse in patients with myelodysplastic syndrome or secondary acute myeloid leukaemia evolving from myelodysplastic syndrome undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. (2023) 10:e178–90. doi: 10.1016/S2352-3026(22)00375-1
133. de Lima M, Ravandi F, Shahjahan M, Andersson B, Couriel D, Donato M, et al. Long-term follow-up of a phase I study of high-dose decitabine, busulfan, and cyclophosphamide plus allogeneic transplantation for the treatment of patients with leukemias. Cancer. (2003) 97:1242–7. doi: 10.1002/cncr.11184
134. Tang X, Valdez BC, Ma Y, Zhang Q, Qu C, Dai H, et al. Low-dose decitabine as part of a modified Bu-Cy conditioning regimen improves survival in AML patients with active disease undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2021) 56:1674–82. doi: 10.1038/s41409-021-01238-5
135. Vij R, Le-Rademacher J, Laumann K, Hars V, Owzar K, Shore T, et al. A phase II multicenter study of the addition of azacitidine to reduced-intensity conditioning allogeneic transplant for high-risk myelodysplasia (and older patients with acute myeloid leukemia): results of CALGB 100801 (Alliance). Biol Blood Marrow Transplant. (2019) 25:1984–92. doi: 10.1016/j.bbmt.2019.06.007
136. Cao Y-G, He Y, Zhang S-D, Liu Z-X, Zhai W-H, Ma Q-L, et al. Conditioning regimen of 5-day decitabine administration for allogeneic stem cell transplantation in patients with myelodysplastic syndrome and myeloproliferative neoplasms. Biol Blood Marrow Transplant. (2020) 26:285–91. doi: 10.1016/j.bbmt.2019.09.001
137. D’Angelo CR, Hall A, Woo KM, Kim K, Longo W, Hematti P, et al. Decitabine induction with myeloablative conditioning and allogeneic hematopoietic stem cell transplantation in high-risk patients with myeloid Malignancies is associated with a high rate of infectious complications. Leuk Res. (2020) 96:106419. doi: 10.1016/j.leukres.2020.106419
138. Cruijsen M, Hilberink JR, van der Velden WJFM, Jansen JH, Bär B, Schaap NPM, et al. Low relapse risk in poor risk AML after conditioning with 10-day decitabine, fludarabine and 2 Gray TBI prior to allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. (2021) 56:1964–70. doi: 10.1038/s41409-021-01272-3
139. Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. (2012) 26:381–9. doi: 10.1038/leu.2011.234
140. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. (2020) 4:5580–8. doi: 10.1182/bloodadvances.2020002544
141. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant. (2016) 22:385–90. doi: 10.1016/j.bbmt.2015.09.004
142. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant. (2018) 24:2017–24. doi: 10.1016/j.bbmt.2018.06.016
143. Garcia JS, Kim HT, Murdock HM, Ansuinelli M, Brock J, Cutler CS, et al. Prophylactic maintenance with venetoclax/azacitidine after reduced-intensity conditioning allogeneic transplant for high-risk MDS and AML. Blood Adv. (2024) 8:978–90. doi: 10.1182/bloodadvances.2023012120
144. Webster JA, Yogarajah M, Zahurak M, Symons H, Dezern AE, Gojo I, et al. A phase II study of azacitidine in combination with granulocyte-macrophage colony-stimulating factor as maintenance treatment, after allogeneic blood or marrow transplantation in patients with poor-risk acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS). Leuk Lymphoma. (2021) 62:3181–91. doi: 10.1080/10428194.2021.1948029
145. Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. (2019) 54:1815–26. doi: 10.1038/s41409-019-0536-y
146. Han S, Kim Y-J, Lee J, Jeon S, Hong T, Park G-J, et al. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol. (2015) 8:118. doi: 10.1186/s13045-015-0208-3
147. Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. (2015) 21:1761–9. doi: 10.1016/j.bbmt.2015.05.026
148. Zhang R, Wang L, Chen P, Gao X, Wang S, Li F, et al. Haematologic Malignancies with unfavourable gene mutations benefit from donor lymphocyte infusion with/without decitabine for prophylaxis of relapse after allogeneic HSCT: A pilot study. Cancer Med. (2021) 10:3165–76. doi: 10.1002/cam4.3763
149. Kalin B, van Norden Y, van Gelder M, Breems D, Maertens J, Jongen-Lavrencic M, et al. Panobinostat and decitabine prior to donor lymphocyte infusion in allogeneic stem cell transplantation. Blood Adv. (2020) 4:4430–7. doi: 10.1182/bloodadvances.2020002074
150. Mishra A, Tamari R, DeZern AE, Byrne MT, Gooptu M, Chen Y-B, et al. Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. (2022) 40:3985–93. doi: 10.1200/JCO.22.00181
151. Bug G, Burchert A, Wagner E-M, Kröger N, Berg T, Güller S, et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia. (2017) 31:2523–5. doi: 10.1038/leu.2017.242
152. Cooperrider JH, Fulton N, Artz AS, Larson RA, Stock W, Kosuri S, et al. Phase I trial of maintenance selinexor after allogeneic hematopoietic stem cell transplantation for patients with acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. (2020) 55:2204–6. doi: 10.1038/s41409-020-0925-2
153. Yao S, Jianlin C, Yarong L, Botao L, Qinghan W, Hongliang F, et al. Donor-derived CD123-targeted CAR T cell serves as a RIC regimen for haploidentical transplantation in a patient with FUS-ERG+ AML. Front Oncol. (2019) 9:1358. doi: 10.3389/fonc.2019.01358
154. Mo X-D, Wang Y, Zhang X-H, Xu L-P, Yan C-H, Chen H, et al. Interferon-α is effective for treatment of minimal residual disease in patients with t(8;21) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: results of a prospective registry study. Oncologist. (2018) 23:1349–57. doi: 10.1634/theoncologist.2017-0692
155. Pham B, Hoeg R, Krishnan R, Richman C, Tuscano J, Abedi M. Safety and tolerability of lenalidomide maintenance in post-transplant acute myeloid leukemia and high-risk myelodysplastic syndrome. Bone Marrow Transplant. (2021) 56:2975–80. doi: 10.1038/s41409-021-01444-1
156. Gao L, Zhang Y, Wang S, Kong P, Su Y, Hu J, et al. Effect of rhG-CSF combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: an open-label, multicenter, randomized controlled trial. J Clin Oncol. (2020) 38:4249–59. doi: 10.1200/JCO.19.03277
157. Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination lenalidomide and azacitidine: A novel salvage therapy in patients who relapse after allogeneic stem-cell transplantation for acute myeloid leukemia. J Clin Oncol. (2019) 37:580–8. doi: 10.1200/JCO.18.00889
158. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. (2012) 119:3361–9. doi: 10.1182/blood-2011-09-377044
159. Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K, et al. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid Malignancies. Blood Cancer J. (2014) 4:e197. doi: 10.1038/bcj.2014.14
160. Kwon Y-R, Kim HJ, Sohn M-J, Lim J-Y, Park K-S, Lee S, et al. Effects of decitabine on allogeneic immune reactions of donor lymphocyte infusion via activation of dendritic cells. Exp Hematol Oncol. (2020) 9:22. doi: 10.1186/s40164-020-00178-y
161. Schönefeldt C, Sockel K, Wehner R, Sopper S, Wolf D, Wermke M, et al. Azacytidine impairs NK cell activity in AML and MDS patients undergoing MRD-based pre-emptive treatment after allogeneic stem cell transplantation. Blood Cancer J. (2013) 3:e136. doi: 10.1038/bcj.2013.35
162. Stübig T, Badbaran A, Luetkens T, Hildebrandt Y, Atanackovic D, Binder TMC, et al. 5-azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity. Mediators Inflammation. (2014) 2014:418292. doi: 10.1155/2014/418292
163. Garcia JS, Flamand Y, Penter L, Keng M, Tomlinson B, Mendez LM, et al. Ipilimumab plus decitabine for patients with MDS or AML in post-transplant or transplant naïve settings. Blood. (2022) 141:1884–8. doi: 10.1182/blood.2022017686
164. Penter L, Liu Y, Wolff JO, Yang L, Taing L, Jhaveri A, et al. Mechanisms of response and resistance to combined decitabine and ipilimumab for advanced myeloid disease. Blood. (2023) 141:1817–30. doi: 10.1182/blood.2022018246
165. Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. (2013) 27:1229–35. doi: 10.1038/leu.2013.7
166. Ghobadi A, Choi J, Fiala MA, Fletcher T, Liu J, Eissenberg LG, et al. Phase I study of azacitidine following donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Leuk Res. (2016) 49:1–6. doi: 10.1016/j.leukres.2016.07.010
167. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. (2020) 135:791–803. doi: 10.1182/blood.2019003988
168. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. (2020) 135:2137–45. doi: 10.1182/blood.2020004856
169. Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. (2022) 386:1519–31. doi: 10.1056/NEJMoa2117344
170. Cao X-Y, Chen J-Q, Wang H, Ma W, Liu W-W, Zhang F-F, et al. Addition of venetoclax to myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in high-risk AML. Ann Med. (2023) 55:388–400. doi: 10.1080/07853890.2022.2164610
171. Teh CE, Peng H, Luo M-X, Tan T, Trussart M, Howson LJ, et al. Venetoclax treatment in patients with cancer has limited impact on circulating T and NK cells. Blood Adv. (2023) 7:2733–45. doi: 10.1182/bloodadvances.2022008221
172. Lee JB, Khan DH, Hurren R, Xu M, Na Y, Kang H, et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood. (2021) 138:234–45. doi: 10.1182/blood.2020009081
173. Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. (2022) 185:1521–1538.e18. doi: 10.1016/j.cell.2022.03.030
174. Ludwig LM, Hawley KM, Banks DB, Thomas-Toth AT, Blazar BR, McNerney ME, et al. Venetoclax imparts distinct cell death sensitivity and adaptivity patterns in T cells. Cell Death Dis. (2021) 12:1005. doi: 10.1038/s41419-021-04285-4
175. Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA. (2010) 107:10967–71. doi: 10.1073/pnas.1005256107
176. Kohlhapp FJ, Haribhai D, Mathew R, Duggan R, Ellis PA, Wang R, et al. Venetoclax increases intratumoral effector T cells and antitumor efficacy in combination with immune checkpoint blockade. Cancer Discove. (2021) 11:68–79. doi: 10.1158/2159-8290.CD-19-0759
177. Bykov VJN, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. (2002) 8:282–8. doi: 10.1038/nm0302-282
178. Lehmann S, Bykov VJN, Ali D, Andrén O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic Malignancies and prostate cancer. J Clin Oncol. (2012) 30:3633–9. doi: 10.1200/JCO.2011.40.7783
179. Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. (2021) 39:1584–94. doi: 10.1200/JCO.20.02341
180. Cluzeau T, Sebert M, Rahmé R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: A phase II study by the groupe francophone des myélodysplasies (GFM). J Clin Oncol. (2021) 39:1575–83. doi: 10.1200/JCO.20.02342
181. Garcia-Manero G, Goldberg AD, Winer ES, Altman JK, Fathi AT, Odenike O, et al. Eprenetapopt combined with venetoclax and azacitidine in TP53-mutated acute myeloid leukaemia: a phase 1, dose-finding and expansion study. Lancet Haematol. (2023) 10:e272–83. doi: 10.1016/S2352-3026(22)00403-3
182. Uckelmann HJ, Kim SM, Wong EM, Hatton C, Giovinazzo H, Gadrey JY, et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. (2020) 367:586–90. doi: 10.1126/science.aax5863
183. Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Invest. (2020) 130:981–97. doi: 10.1172/JCI129126
184. Issa GC, Aldoss I, DiPersio J, Cuglievan B, Stone R, Arellano M, et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature. (2023) 615:920–4. doi: 10.1038/s41586-023-05812-3
185. Perner F, Stein EM, Wenge DV, Singh S, Kim J, Apazidis A, et al. MEN1 mutations mediate clinical resistance to menin inhibition. Nature. (2023) 615:913–9. doi: 10.1038/s41586-023-05755-9
186. Carter BZ, Tao W, Mak PY, Ostermann LB, Mak D, McGeehan G, et al. Menin inhibition decreases Bcl-2 and synergizes with venetoclax in NPM1/FLT3-mutated AML. Blood. (2021) 138:1637–41. doi: 10.1182/blood.2021011917
187. Piloto O, Wright M, Brown P, Kim K-T, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. (2007) 109:1643–52. doi: 10.1182/blood-2006-05-023804
188. McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal selection with RAS pathway activation mediates secondary clinical resistance to selective FLT3 inhibition in acute myeloid leukemia. Cancer Discovery. (2019) 9:1050–63. doi: 10.1158/2159-8290.CD-18-1453
189. Wang F, Morita K, DiNardo CD, Furudate K, Tanaka T, Yan Y, et al. Leukemia stemness and co-occurring mutations drive resistance to IDH inhibitors in acute myeloid leukemia. Nat Commun. (2021) 12:2607. doi: 10.1038/s41467-021-22874-x
190. Ragon BK, Odenike O, Baer MR, Stock W, Borthakur G, Patel K, et al. Oral MEK 1/2 inhibitor trametinib in combination with AKT inhibitor GSK2141795 in patients with acute myeloid leukemia with RAS mutations: A phase II study. Clin Lymphoma Myeloma Leuk. (2019) 19:431–40.e13. doi: 10.1016/j.clml.2019.03.015
191. Desikan SP, Ravandi F, Pemmaraju N, Konopleva M, Loghavi S, Jabbour EJ, et al. A phase II study of azacitidine, venetoclax, and trametinib in relapsed or refractory acute myeloid leukemia harboring RAS pathway-activating mutations. Acta Haematol. (2022) 145:529–36. doi: 10.1159/000525566
192. Jain N, Curran E, Iyengar NM, Diaz-Flores E, Kunnavakkam R, Popplewell L, et al. Phase II study of the oral MEK inhibitor selumetinib in advanced acute myelogenous leukemia: a University of Chicago phase II consortium trial. Clin Cancer Res. (2014) 20:490–8. doi: 10.1158/1078-0432.CCR-13-1311
193. Maiti A, Naqvi K, Kadia TM, Borthakur G, Takahashi K, Bose P, et al. Phase II trial of MEK inhibitor binimetinib (MEK162) in RAS-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2019) 19:142–148.e1. doi: 10.1016/j.clml.2018.12.009
194. Konopleva MY, Walter RB, Faderl SH, Jabbour EJ, Zeng Z, Borthakur G, et al. Preclinical and early clinical evaluation of the oral AKT inhibitor, MK-2206, for the treatment of acute myelogenous leukemia. Clin Cancer Res. (2014) 20:2226–35. doi: 10.1158/1078-0432.CCR-13-1978
195. Brune MM, Stüssi G, Lundberg P, Vela V, Heim D, Manz MG, et al. Effects of lenalidomide on the bone marrow microenvironment in acute myeloid leukemia: Translational analysis of the HOVON103 AML/SAKK30/10 Swiss trial cohort. Ann Hematol. (2021) 100:1169–79. doi: 10.1007/s00277-021-04467-2
196. Tsubokura Y, Yoshimura H, Satake A, Nasa Y, Tsuji R, Ito T, et al. Early administration of lenalidomide after allogeneic hematopoietic stem cell transplantation suppresses graft-versus-host disease by inhibiting T-cell migration to the gastrointestinal tract. Immun Inflammation Dis. (2022) 10:e688. doi: 10.1002/iid3.688
197. Henden AS, Varelias A, Leach J, Sturgeon E, Avery J, Kelly J, et al. Pegylated interferon-2α invokes graft-versus-leukemia effects in patients relapsing after allogeneic stem cell transplantation. Blood Adv. (2019) 3:3013–9. doi: 10.1182/bloodadvances.2019000453
198. Ho JNHG, Schmidt D, Lowinus T, Ryoo J, Dopfer E-P, Gonzalo Núñez N, et al. Targeting MDM2 enhances antileukemia immunity after allogeneic transplantation via MHC-II and TRAIL-R1/2 upregulation. Blood. (2022) 140:1167–81. doi: 10.1182/blood.2022016082
199. Daver NG, Dail M, Garcia JS, Jonas BA, Yee KWL, Kelly KR, et al. Venetoclax and idasanutlin in relapsed/refractory AML: a non-randomized, open-label phase 1b trial. Blood. (2022) 141:1265–76. doi: 10.1182/blood.2022016362
200. Gottschlich A, Thomas M, Grünmeier R, Lesch S, Rohrbacher L, Igl V, et al. Single-cell transcriptomic atlas-guided development of CAR-T cells for the treatment of acute myeloid leukemia. Nat Biotechnol. (2023) 41:1618–32. doi: 10.1038/s41587-023-01684-0
201. Fetsch V, Zeiser R. Chimeric antigen receptor T cells for acute myeloid leukemia. Eur J Haematol. (2024) 112:28–35. doi: 10.1111/ejh.14047
202. Zhang H, Bu C, Peng Z, Li G, Zhou Z, Ding W, et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia. (2022) 36:2596–604. doi: 10.1038/s41375-022-01703-0
Keywords: AML, allogeneic stem cell transplant, graft-versus-leukemia, maintenance, targeted therapy, MRD, genetics
Citation: Murdock HM, Ho VT and Garcia JS (2024) Innovations in conditioning and post-transplant maintenance in AML: genomically informed revelations on the graft-versus-leukemia effect. Front. Immunol. 15:1359113. doi: 10.3389/fimmu.2024.1359113
Received: 20 December 2023; Accepted: 20 February 2024;
Published: 20 March 2024.
Edited by:
Robert Zeiser, University of Freiburg, GermanyReviewed by:
Vincenzo Maria Perriello, University of Perugia, ItalyMartin Sauer, Hannover Medical School, Germany
Copyright © 2024 Murdock, Ho and Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacqueline S. Garcia, amFjcXVlbGluZV9nYXJjaWFAZGZjaS5oYXJ2YXJkLmVkdQ==
 H. Moses Murdock
H. Moses Murdock Vincent T. Ho2
Vincent T. Ho2 Jacqueline S. Garcia
Jacqueline S. Garcia