- 1Department of Liver Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 2Department of Pathology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
Background: The prognosis for unresectable intrahepatic cholangiocarcinoma (ICC) is poor and the efficacy of traditional chemotherapy remains unsatisfactory. Hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX) is effective in patients with unresectable ICC. In this study, we determined the preliminary clinical efficacy and safety of lenvatinib plus durvalumab combined with FOLFOX-HAIC in patients with untreated, unresectable ICC.
Materials and methods: Between July 2021 and July 2023, patients with unresectable ICC who initially received lenvatinib plus durvalumab combined with FOLFOX-HAIC at the Sun Yat-Sen University Cancer Center (SYSUCC) were reviewed for eligibility. Efficacy was evaluated by tumor response rate and survival, and safety was assessed by the frequency of key adverse events (AEs).
Results: A total of 28 eligible patients were enrolled. The objective response rates (ORRs) based on mRECIST and RECIST 1.1 criteria were 65.2% and 39.1%, respectively. The median OS was 17.9 months (95% CI, 5.7–30.1) and the median PFS was 11.9 months (95% CI, 6.7–17.1). Most patients (92.9%) experienced adverse events (AEs), whereas 46.5% (13/28) experienced grade 3 or 4 AEs.
Conclusion: Lenvatinib plus durvalumab combined with FOLFOX-HAIC showed promising antitumor activity and manageable AEs in patients with treatment-naive unresectable ICC. This regimen may be suitable as a novel first-line treatment option for this patient population.
Introduction
Cholangiocarcinoma is a heterogeneous spectrum of highly aggressive adenocarcinomas that arise from the biliary epithelium. It is categorized as intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) (1). ICC is the second most common primary liver cancer, accounting for up to 20% of all liver malignancies. Over the past 40 years, the incidence of ICC has increased more than 140% (2). Surgical resection is the only curative treatment; however, most patients are diagnosed with advanced disease when clinical symptoms appear hence lose the opportunity for surgery (3). The prognosis of the patients with unresectable ICC is very poor, with a median survival of 2.5–7.5 months in the absence of treatment (4).
Gemcitabine plus cisplatin (GC) is the first-line systemic chemotherapy recommended for unresectable biliary tract cancer; however, its survival benefits are limited as it has a median overall survival (OS) of only 11.7 months (5). Compared with systemic chemotherapy, hepatic arterial infusion chemotherapy (HAIC) enables the delivery of high concentrations of chemotherapeutic drugs directly into the liver with fewer systemic side effects (6, 7). Previous studies have indicated that HAIC has higher tumor control rates compared with systemic chemotherapy for advanced ICC (8, 9). The FOLFOX regimen has shown considerable efficacy and is recommended as a palliative treatment option for advanced ICC (10, 11). In a previous study, we demonstrated that FOLFOX-HAIC was safe and effective for treating unresectable ICC (12), which was consistent with the results of Cai et al. (13). This indicates the promising therapeutic effect of FOLFOX-HAIC for advanced ICC. Based on this evidence, FOLFOX-HAIC may be an effective treatment for advanced ICC.
Lenvatinib is a novel oral multikinase inhibitor that exerts antiangiogenic and direct antitumor effects (14). Based on the results of the REFLECT study, lenvatinib has been recommended as the first-line standard treatment for advanced hepatocellular carcinoma (HCC) by guidelines such as NCCN, ESMO, and CSCO (15). Recently, several studies have reported that lenvatinib exhibits remarkable efficacy in advanced bile duct cancer (BTC) (16–18).
Recently, the TOPAZ-1 trial showed that durvalumab, a programmed cell death 1 ligand 1 (PD-L1) antibody, combined with chemotherapy provides a significant survival benefit in the patients with locally advanced or metastatic BTC. Therefore, it is one of the preferred first-line regimens for advanced BTC (19).
In recent years, combination therapy with lenvatinib, PD-1/PD-L1 antibody, and FOLFOX-HAIC demonstrated significant efficacy in advanced HCC, which not only showed an improved objective response rate (ORR), but also significantly prolonged patient survival (20). However, the efficacy of this treatment in unresectable ICC has rarely been reported. In this study, we examined the efficacy and safety of lenvatinib, durvalumab, and FOLFOX-HAIC as first-line treatment for unresectable ICC.
Materials and methods
This study was conducted according to the ethical guidelines of the Declaration of Helsinki and approved by the Ethics Committee (B2020-318-01) of Sun Yat-Sen University Cancer Center (SYSUCC; Guangzhou, China).
Patients
Between July 2021 and July 2023, patients diagnosed with ICC who received lenvatinib, durvalumab, and FOLFOX-HAIC treatment in the Department of Liver Surgery of SYSUCC were reviewed for eligibility. The eligibility criteria were as follows: (1) ICC confirmed by clinical or histopathological evidence; (2) assessed by at least two experienced hepatobiliary surgeons as unsuitable for surgical resection; (3) 18 years or older and less than 75 years old; (4) adequate hepatic and renal function; (5) Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0–1; and (6) at least one measurable target lesion that could be evaluated by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) and modified RECIST (mRECIST). Patients were excluded based on the following criteria: (1) previously received antitumor treatment; (2) history of other malignant tumors; (3) incomplete medical information; and (4) loss to follow-up.
Treatment procedure
HAIC was performed every three weeks as described previously (21). Briefly, a microcatheter was placed in the main feeding hepatic artery and the following regimen was infused: 135 mg/m2 oxaliplatin from hour 0 to 3 on day 1; 400 mg/m2 leucovorin from hour 3 to 4.5 on day 1; 400 mg/m2 5-fluorouracil bolus from hour 4.5 to 6.5 on day 1; and 2400mg/m2 5-fluorouracil over 46 h from day 1 to day 3. Lenvatinib (The UK, Eisai Europe Co. Ltd.) was administered orally every day with a dose of 8 mg. Durvalumab was administered intravenously with a dose of 1000 mg every three weeks. The first administration of lenvatinib and durvalumab was within 7 days of HAIC initiation. Lenvatinib and durvalumab treatment continued after HAIC was discontinued until tumor progression or intolerable adverse reactions occurred.
Data collection
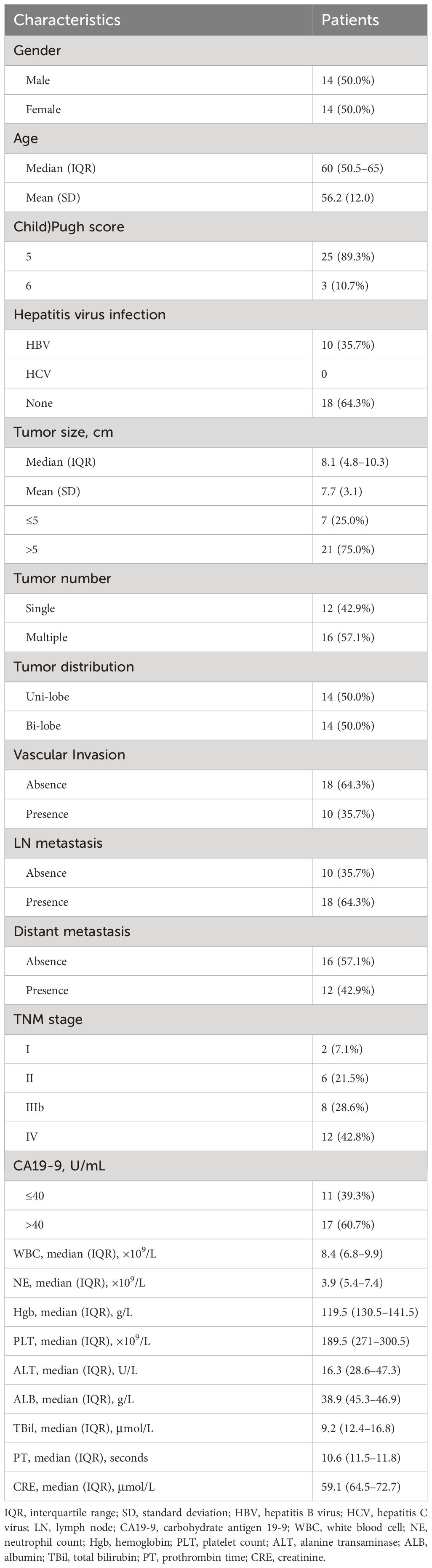
All clinical baseline characteristics of the eligible patients were collected from medical records filed at SYSUCC, including gender, age, Child–Pugh score, the status of hepatitis virus infection, tumor size, tumor number, tumor distribution, vascular invasion, lymph node (LN) metastasis, distant metastasis, tumor–node–metastasis (TNM) stage, carbohydrate antigen 19-9 (CA19-9), white blood cell count, neutrophil (NE) count, hemoglobin (HGB), platelet (PLT) count, alanine transaminase (ALT), serum albumin levels (ALB), total bilirubin, prothrombin time, and creatinine (CRE).
Follow-up and survival analyses
Each follow-up visit included a medical history taking, physical examination, laboratory tests, and contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) examination. The initial follow-up appointment was administered 6–8 weeks (2 cycles of HAIC) after the first treatment. This study was followed until November 1, 2023.
OS was defined as the interval from the date of the initial administration of HAIC to death from any cause. PFS was defined as the interval from the initial administration of HAIC to tumor progression or death from any cause, whichever occurred first. ORR was defined as the proportion of patients achieving either a complete response (CR) or a partial response (PR), which was maintained for at least four weeks from the first radiological confirmation. The disease control rate (DCR) was defined as the ORR plus the percentage of patients with stable disease (SD). Tumor response was assessed using Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) and modified RECIST (mRECIST) (22, 23). Adverse events (AEs) were evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events version (NCI-CTCAE) 4.03.
Statistical analysis
Normally distributed variables were described as the mean ± standard error (SE) and nonnormal distributions were described as the median and interquartile range (IQR). Categorical variables were compared using the Pearson χ2 test or Fisher’s exact test. Continuous parametric variables were compared by the unpaired Student’s t-test, whereas for continuous nonparametric variables, the Mann–Whitney U test was used. The Kaplan–Meier method was used for survival analysis and the log-rank test was used for survival curve differences. Forward LR-based univariate and multivariate Cox regression analyses were used to identify independent predictive variables. A two-tailed p < 0.05 was considered statistically significant. All data analyses were performed using SPSS software, version 24.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Between July 2021 and July 2023, 28 patients who met the inclusion criteria and agreed to be treated with lenvatinib, durvalumab, and FOLFOX-HAIC were included in this study. The clinical characteristics of these patients are shown in Table 1.
The population consisted of 14 males and 14 females, ranging in age from 30 to 73 years. Among the patients, all had Child– Pugh class A liver function, 10 (35.7%) had hepatitis B infection, the maximum tumor diameter was 10.3 cm, 16 (57.1%) had multiple lesions, 10 (35.7%) had macrovascular invasion, 18 (64.3%) had hilar LN metastasis, and 12 (42.9%) had distant metastasis.
Tumor response and patient survival
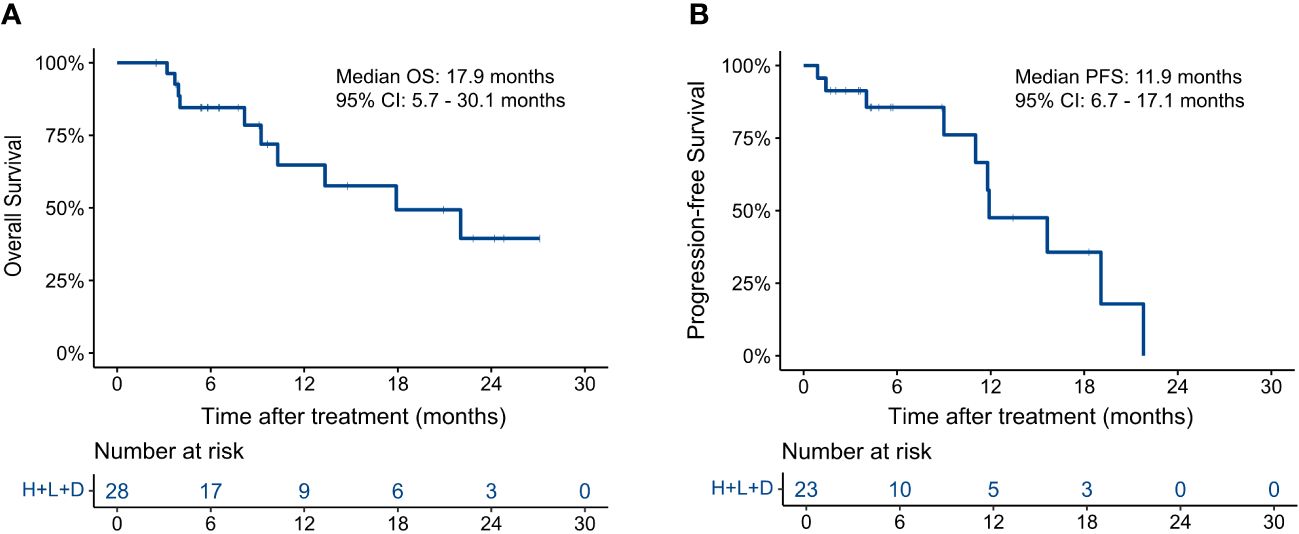
A total of 74 HAIC cycles were performed during the study, with a median of 2.5 cycles (range, 1 to 4 cycles). At the end of follow-up, 10 patients had died (10/28, 35.7%), and 10 had disease progression (10/23, 43.5%). As shown in Figure 1, the median OS was 17.9 months (95% CI, 5.7–30.1) and the median PFS was 11.9 months (95% CI, 6.7–17.1). The 3-, 6-, and 12-month OS rates were 100.0%, 84.5%, and 64.8%, respectively. The 3-, 6-, and 12-m PFS rates were 91.3%, 85.6%, and 47.6%, respectively.
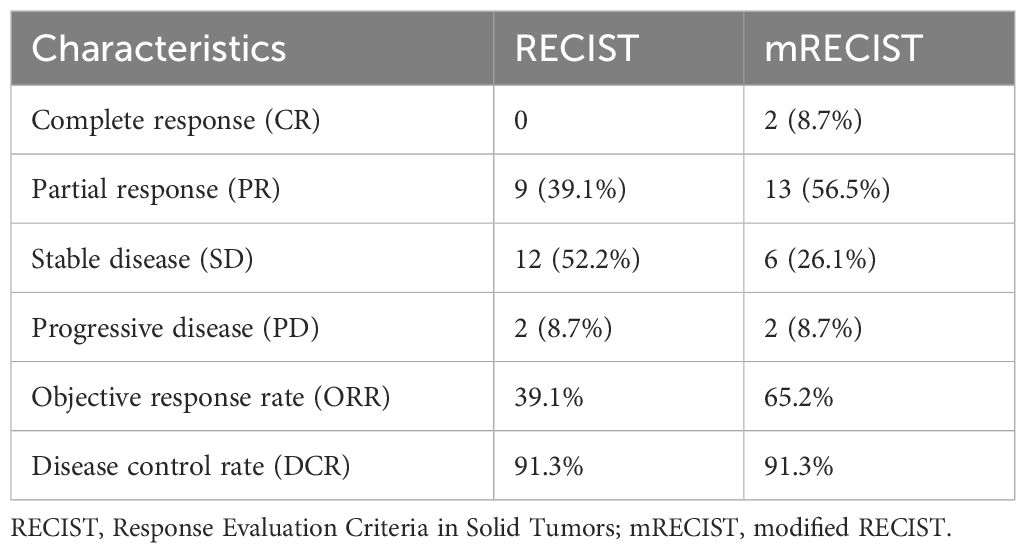
The tumor response outcomes are shown in Table 2. Of the 28 enrolled patients, 23 were evaluable for tumor response assessment. The ORRs based on the RECIST 1.1 and mRECIST criteria were 39.1% and 65.2%, respectively. Two patients (2/23, 8.7%) achieved a CR based on the mRECIST criteria and the DCR was 91.3%. The change in the intrahepatic target lesion size of the patients is shown in Figure 2. As shown in Figure 3, three patients exhibited tumor shrinkage or downstaging after treatment and eventually underwent radical liver tumor resection. Eight patients remained on treatment at the time of the follow-up cut-off. The median time to response (TTR) was 2.1 months (range, 1.6–4.2 months). The median duration of treatment was 5.8 months (range, 0.9–24.8 months).
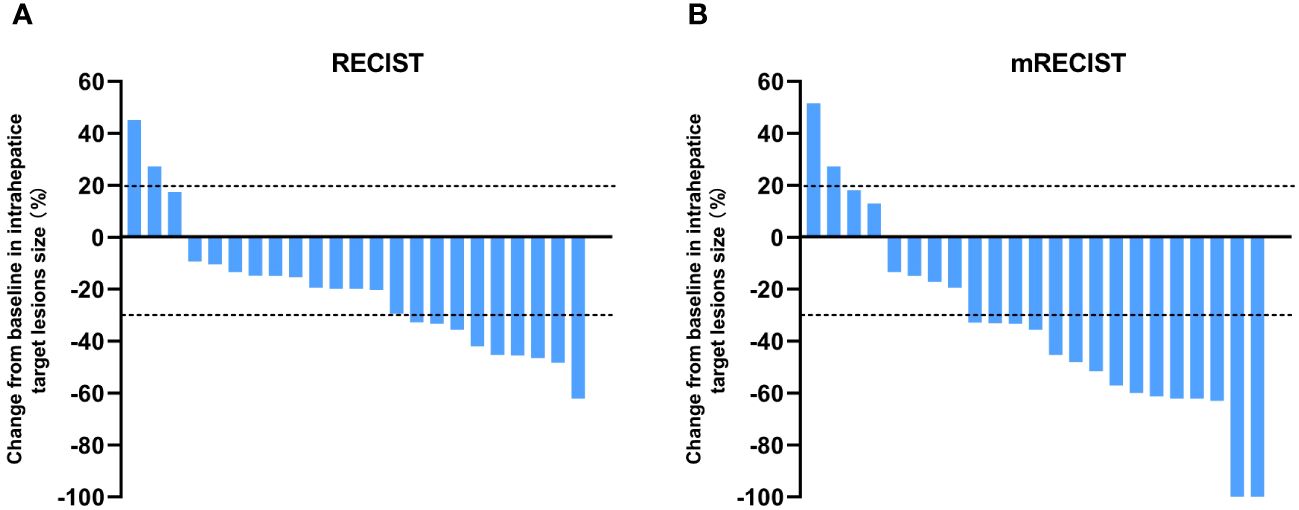
Figure 2 Best percentage changes from baseline in the size of the intrahepatic target lesions of patients receiving lenvatinib plus durvalumab combined with FOLFOX-HAIC. (A) RECIST1.1 was used to evaluate the image measurements before and after treatment. (B) Evaluated using mRECIST in patients with image measurements before and after treatment.
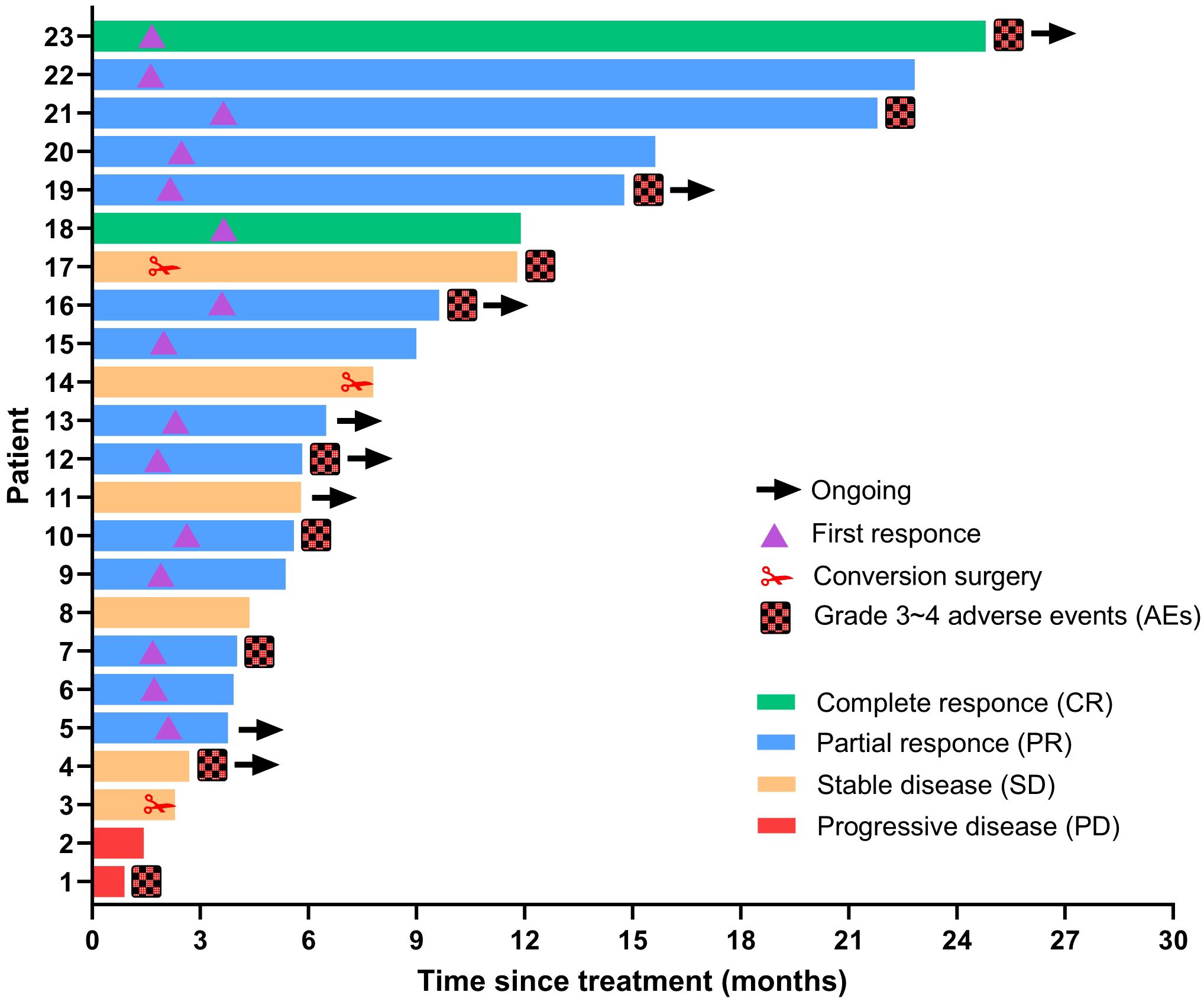
Figure 3 Duration of treatment and response assessments using mRECIST. The length of each bar represents the duration of treatment for each patient.
The prognostic factors of all clinical variables were analyzed by a univariate analysis, which showed that tumor response based on mRECIST (p = 0.038) and cycles of HAIC (p = 0.037) were significant risk factors for patient OS. CA19-9 (p = 0.075), vascular invasion (p = 0.077), and ALB (p = 0.065) appeared to exhibit a trend toward a difference; however, the p-value was not statistically significant, possibly because of the small sample size. Univariate analysis for PFS showed that tumor response based on RECIST (p = 0.043) and mRECIST (p = 0.039), as well as cycles of HAIC (p = 0.016), were significant risk factors. A multivariate Cox proportional analysis revealed that the cycles of HAIC (p = 0.016) were significant and an independent prognostic factor of PFS (Supplementary Table 1).
The OS of responders based on RECIST 1.1 appeared to be better than that of the non-responders, but there was no statistical difference (p = 0.053, HR = 0.151, 95% CI 0.017-1.314). The PFS of responders based on RECIST 1.1 was significantly better than that of nonresponder (p = 0.017, HR = 0.111, 95% CI 0.013–0.934). Moreover, the OS (p = 0.018, HR = 0.163, 95% CI 0.029–0.901) and PFS (p = 0.019, HR = 0.039, 95% CI 0.029–0.909) of responders based on the mRECIST criteria were better than those of the non-responders (Supplementary Figure 1).
Adverse events and safety
There was one treatment-related death in this study. A 71-year-old female was diagnosed with bronchiectasis, type 2 diabetes, a history of cerebral infarction, and cholecystolithiasis. The patient developed a severe pulmonary infection after one cycle of FOLFOX-HAIC, lenvatinib plus durvalumab treatment, which was considered the main cause of death.
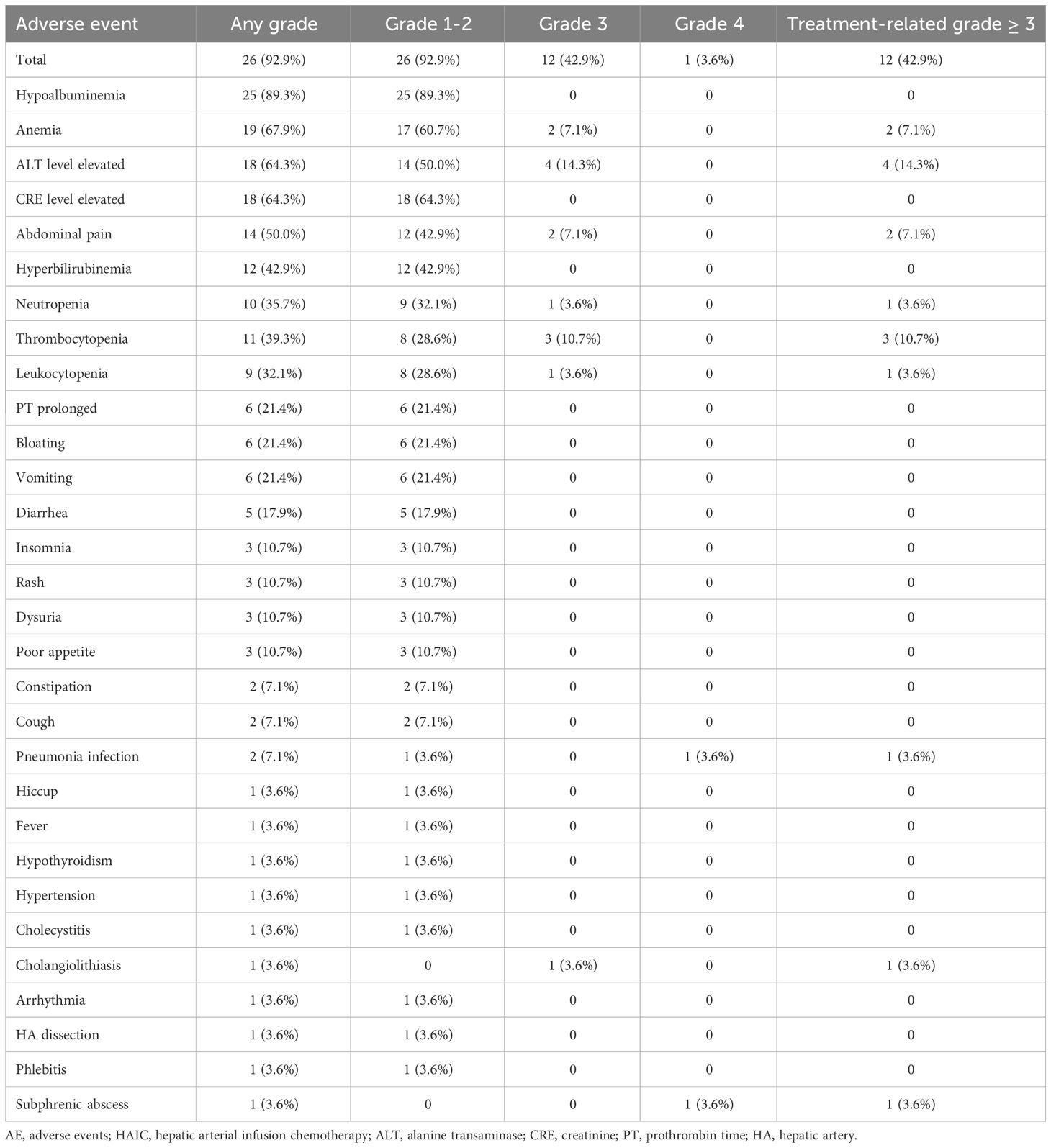
At the time of the first treatment cycle, three patients had dose modification of the HAIC agent because of hepatic dysfunction. No patients had dose modification because of AEs during subsequent treatment cycles. As shown in Table 3, the frequency of key AEs of all grades was 92.9% (26 AEs in 28 patients). Treatment-related AEs (TRAEs) are shown in Table 3. The most common grade 1–2 AEs included hypoalbuminemia (89.3%), anemia (67.9%), elevated ALT (64.3%), elevated CRE (64.3%), and pain (50.0%). The most common grade 3–4 AEs were elevated ALT (5.8%), anemia (7.1%), and pain (7.1%). Any grade liver dysfunction in most patients, such as hypoalbuminemia, elevated ALT, and hyperbilirubinemia, was primarily mild to moderate and returned to normal following treatment. Furthermore, specific abdominal pain associated with the HAIC of oxaliplatin occurred in 14 (50.0%) patients, but most pain was mild and tolerable. Severe pain was rare and can be relieved quickly by suspending the oxaliplatin infusion or the use of spasmolytics, after which drug administration continued.
Discussion
Our team has been focusing on the potential of FOLFOX-HAIC for the treatment of primary liver cancer, including HCC and ICC. We previously reported that adjuvant HAIC after hepatectomy reduces the risk of recurrence in HCC patients with microvascular invasion (MVI) (21), and preoperative neoadjuvant HAIC can improve the prognosis of patients with potentially resectable BCLC A/B HCC (24). Furthermore, we examined its efficacy and application prospects in unresectable ICC and found that FOLFOX-HAIC exhibited good efficacy and safety for treating unresectable ICC (12). With significant advances in systemic therapy, particularly the extensive use of PD-1/PD-L1 inhibitors in HCC, the continued evaluation of FOLFOX-HAIC along with lenvatinib and durvalumab for the treatment of unresectable ICC is warranted.
The preliminary results of this retrospective study indicated that the triple therapy of lenvatinib and durvalumab combined with FOLFOX-HAIC has a high ORR (39.1%, RECIST; 65.2%, mRECIST) and a considerable median OS (17.9 months) and PFS (11.9 months) in unresectable ICC with acceptable safety profiles. This indicates this novel triple therapy may yield satisfactory efficacy and favorable survival of patients with treatment-naive unresectable ICC.
TKIs synergize with PD-1/PD-L1 inhibitors in liver cancer by targeting VEGFR (25, 26). The high response rate and improved survival observed in our study may be attributed to the synergistic effect of FOLFOX-HAIC, lenvatinib, and durvalumab. HAIC reduces tumor burden by maintaining high concentrations of chemotherapeutic drugs in the tumor. Conversely, immunogenic cell death induced by chemotherapy enhances the antitumor effects of ICIs (27, 28). Lenvatinib can enhance the antitumor activity of durvalumab by inhibiting immunosuppressive cells, such as Tregs, and by promoting the infiltration of immune T cells into the tumor microenvironment (29, 30). Moreover, lenvatinib along with durvalumab may overcome resistance to FOLFOX by disrupting the hypoxic microenvironment within tumors through normalizing the tumor vasculature (31). Therefore, triple therapy with lenvatinib, durvalumab, and HAIC rapidly reduces tumor burden, prolongs systemic treatment response, and further prolongs the long-term survival of patients.
Of note, patients in this study had a median PFS of 11.9 months, which is relatively close to the median OS of 17.9 months. This may be the result of the following: 1) some patients did not return to our center after treatment; thus, we could not assess tumor treatment response. As a result, some patients only had OS data but not PFS data, which introduced some bias in the PFS analysis; 2), ICC often infiltrated along the bile duct because of its high invasiveness, which could rapidly lead to biliary obstruction after progression and cause serious complications, such as obstructive jaundice and pancreatitis. This resulted in a rapid deterioration and death, which may partially explain why the OS is close to the PFS.
In the present study, although nearly all (92.9%) patients experienced varying degrees of AEs, the incidence of severe AEs was similar to that of other studies (8, 18). Leukocytopenia is a common AE associated with chemotherapy (32). In the present study, 32.1% (9/28) of the patients had different degrees of leukocytopenia, of which only 3.6% (1/28) had grade 3–4 AEs. In the TOPAZ-1 trial, the gemcitabine plus cisplatin (GC) regimen along with durvalumab yielded an incidence of grade 3–4 AEs as high as 75.7% (19). However, the incidence of grade 3–4 AEs in our study was only 46.5% (13/28), which was lower compared with that in the TOPAZ-1 trial. This may be because: First, compared with systemic chemotherapy, as a type of local treatment, HAIC directly injected chemotherapeutic drugs into the hepatic artery resulted in a higher local drug concentration and lower extrahepatic non-target drug concentration, effectively reducing AEs, and improving treatment tolerance. Second, our study included only patients with ICC, whereas the TOPAZ-1 study also included patients with ECC and gallbladder cancer, which had a higher incidence of complications and adverse reactions. Overall, the present study used the FOLFOX regimen, whereas the TOPAZ-1 study adopted the GC regimen, so different chemotherapy regimens may also partially explain the differences in AE rates.
There are several limitations to this study. First, this is a retrospective, single-center study; therefore, an unknown selection bias can inevitably be ruled out, which reduces the generalizability of the study. Second, the small sample size and lack of a control group decreased the reliability of the evidence and increased the comparison error. Thus, further studies in a larger population are needed with a control group. Finally, the relatively short follow-up time may reduce the certainty of the observed effectiveness and influence the estimation of ORR, PFS, and OS.
Conclusion
In summary, FOLFOX-HAIC combined with lenvatinib plus durvalumab showed promising antitumor activity and manageable AEs in patients with treatment-naive unresectable ICC. Although the value of HAIC as a standard of care for unresectable ICC may be limited, this study indicates that this drug combination may be suitable as a novel first-line treatment option for this patient population; however, it should be confirmed in large-scale prospective randomized clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was conducted according to the ethical guidelines of the Declaration of Helsinki and approved by the Ethics Committee (B2020-318-01) of Sun Yat-Sen University Cancer Center (SYSUCC; Guangzhou, China). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RZ: Writing – original draft. JZ: Writing – original draft. ZM: Data curation, Writing – review & editing. XX: Software, Writing – review & editing. WW: Writing – review & editing. SL: Data curation, Writing – review & editing. RG: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (No. 82303879, No. 82203002, No. 82172579); Science and Technology Planning Project of Guangzhou (No. 2023A04J1777, No. 2023A04J2137); Fostering Program for NSFC Young Applicants (Tulip Talent Training Program) of Sun Yat-sen University Cancer Center (No. TTP-SYSUCC-202313, No. TTP-SYSUCC-202208); Clinical Trials Project (5010 Project) of Sun Yat-sen University (No. 5010-2017009, No. 5010-2023001).
Acknowledgments
The authors acknowledge and express their deepest gratitude to the participants of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1397827/full#supplementary-material
Supplementary Figure S1 | Survival analysis of patients with tumor response and non-response. (A) Overall survival and (B) Progression-free survival of patients with tumor response and non-response based on the RECIST 1.1 criteria; (C) Overall survival and (D) Progression-free survival of patients with tumor response and non-response based on the mRECIST criteria.
References
1. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. (2021) 397:428–44. doi: 10.1016/S0140-6736(21)00153-7
2. Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. Ca-Cancer J Clin. (2023) 73:198–222. doi: 10.3322/caac.21759
3. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastro Hepat. (2020) 17:557–88. doi: 10.1038/s41575-020-0310-z
4. Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: A large-scale observational study. Gut Liver. (2009) 3:298–305. doi: 10.5009/gnl.2009.3.4.298
5. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
6. Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer. (2012) 1:62–70. doi: 10.1159/000342402
7. Obi S, Sato S, Kawai T. Current status of hepatic arterial infusion chemotherapy. Liver Cancer. (2015) 4:188–99. doi: 10.1159/000367746
8. Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma A phase 2 clinical trial. JAMA Oncol. (2020) 6:60–7. doi: 10.1001/jamaoncol.2019.3718
9. Kasai K, Kooka Y, Suzuki Y, Suzuki A, Oikawa T, Ushio A, et al. Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2014) 21:3638–45. doi: 10.1245/s10434-014-3766-7
10. Nehls O, Klump B, Arkenau HT, Hass HG, Greschniok A, Gregor M, et al. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Brit J Cancer. (2002) 87:702–4. doi: 10.1038/sj.bjc.6600543
11. Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. (2021) 22:690–701. doi: 10.1016/S1470-2045(21)00027-9
12. Li SH, Deng M, Wang QX, Mei J, Zou JW, Lin WP, et al. Transarterial infusion chemotherapy with FOLFOX could be an effective and safe treatment for unresectable intrahepatic cholangiocarcinoma. J Oncol. (2022) 2022. doi: 10.1155/2022/2724476
13. Cai ZY, He CB, Zhao CY, Lin XJ. Survival comparisons of hepatic arterial infusion chemotherapy with mFOLFOX and transarterial chemoembolization in patients with unresectable intrahepatic cholangiocarcinoma. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.611118
14. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
15. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Tar. (2020) 5. doi: 10.1038/s41392-020-00264-x
16. Shi CY, Li YL, Yang C, Qiao L, Tang LK, Zheng YT, et al. Lenvatinib plus programmed cell death protein-1 inhibitor beyond first-line systemic therapy in refractory advanced biliary tract cancer: A real-world retrospective study in China. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.946861
17. Xie LL, Huang JZ, Wang LL, Ren WR, Tian H, Hu AH, et al. Lenvatinib combined with a PD-1 inhibitor as effective therapy for advanced intrahepatic cholangiocarcinoma. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.894407
18. Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Tar. (2023) 8. doi: 10.1038/s41392-023-01317-7
19. Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.4_suppl.378
20. Li S, Mei J, Wang Q, Zhao R, Lu L, Deng M, et al. Comparison of anti–PD-L1 with anti–PD-1 antibodies in the combination of HAIC and lenvatinib for the treatment of unresectable hepatocellular carcinoma. J Clin Oncol. (2023) 41:509. doi: 10.1200/JCO.2023.41.4_suppl.509
21. Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: A multicenter, phase III, randomized study. J Clin Oncol. (2023) 41:1898. doi: 10.1200/JCO.22.01142
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
24. Li SH, Zhong C, Li Q, Zou JW, Wang QX, Shang CZ, et al. Neoadjuvant transarterial infusion chemotherapy with FOLFOX could improve outcomes of resectable BCLC stage A/B hepatocellular carcinoma patients beyond Milan criteria: An interim analysis of a multi-center, phase 3, randomized, controlled clinical trial. J Clin Oncol. (2021) 39. doi: 10.1200/JCO.2021.39.15_suppl.4008
25. Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. (2020) 71:1247–61. doi: 10.1002/hep.30889
26. Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. (2020) 72:307–19. doi: 10.1016/j.jhep.2019.09.025
27. Lesterhuis WJ, Punt CJA, Hato SV, Eleveld-Trancikova D, Jansen BJH, Nierkens S, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. (2011) 121:3100–8. doi: 10.1172/JCI43656
28. Zhu HZ, Shan YQ, Ge K, Lu J, Kong WC, Jia CK. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol. (2020) 43:1203–14. doi: 10.1007/s13402-020-00552-2
29. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. (2014) 515:563. doi: 10.1038/nature14011
30. Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. (2016) 7. doi: 10.1038/ncomms12624
31. He MK, Li QJ, Zou RH, Shen JX, Fang WQ, Tan GS, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion A randomized clinical trial. JAMA Oncol. (2019) 5:953–60. doi: 10.1001/jamaoncol.2019.0250
Keywords: unresectable intrahepatic cholangiocarcinoma, hepatic arterial infusion chemotherapy, lenvatinib, durvalumab, FOLFOX
Citation: Zhao R, Zhou J, Miao Z, Xiong X, Wei W, Li S and Guo R (2024) Efficacy and safety of lenvatinib plus durvalumab combined with hepatic arterial infusion chemotherapy for unresectable intrahepatic cholangiocarcinoma. Front. Immunol. 15:1397827. doi: 10.3389/fimmu.2024.1397827
Received: 08 March 2024; Accepted: 25 April 2024;
Published: 10 May 2024.
Edited by:
Mithun Rudrapal, Vignan’s Foundation for Science, Technology and Research, IndiaReviewed by:
Xiaoli Yang, The Affiliated Hospital of Southwest Medical University, ChinaSamiksha Garse, DY Patil Deemed to be University, India
Copyright © 2024 Zhao, Zhou, Miao, Xiong, Wei, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongping Guo, Z3VvcnBAc3lzdWNjLm9yZy5jbg==; Wei Wei, d2Vpd2VpQHN5c3VjYy5vcmcuY24=; Shaohua Li, bGlzaGFvaEBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work and share first authorship
 Rongce Zhao
Rongce Zhao Jing Zhou2†
Jing Zhou2† Rongping Guo
Rongping Guo