- 1Department of Neurology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Neuroimmunology, Shandong Provincial Key Medical and Health Laboratory of Neuroimmunology, Jinan, China
- 2College of First Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Autoimmune diseases encompass a wide range of disorders characterized by disturbed immunoregulation leading to the development of specific autoantibodies, which cause inflammation and multiple organ involvement. However, its pathogenesis remains unelucidated. Furthermore, the cumulative medical and economic burden of autoimmune diseases is on the rise, making these diseases a ubiquitous global phenomenon that is predicted to further increase in the coming decades. Coumarins, a class of aromatic natural products with benzene and alpha-pyrone as their basic structures, has good therapeutic effects on autoimmune diseases. In this review, we systematically highlighted the latest evidence on coumarins and autoimmune diseases data from clinical and animal studies. Coumarin acts on immune cells and cytokines and plays a role in the treatment of autoimmune diseases by regulating NF-κB, Keap1/Nrf2, MAPKs, JAK/STAT, Wnt/β-catenin, PI3K/AKT, Notch and TGF-β/Smad signaling pathways. This systematic review will provide insight into the interaction of coumarin and autoimmune diseases, and will lay a groundwork for the development of new drugs for autoimmune diseases.
1 Introduction
Autoimmune diseases (AIDs) are inflammatory disorders caused by immune dysfunction and loss of immune tolerance, leading to the recognition of self-antigens by the body’s immune system (1, 2). Currently, more than 80 AIDs have been identified, including rheumatoid arthritis, type 1 diabetes mellitus, and psoriasis (3). AIDs can occur at any age and are particularly more prevalent in women than in men. It is estimated that 8% to 10% of population worldwide is afflicted by AIDs (4). Autoimmunity and autoimmune diseases have been increasing dramatically in many parts of the world in recent years, possibly due to changes in our exposure to environmental factors. Current evidence suggests that major changes in our food, exogenous substances, air pollution, infections, personal lifestyles, stress, and climate change are responsible for these increases (5). Autoimmune diseases have a devastating impact on individuals and caregivers in our society, and a large amount of healthcare utilization leads to high public and private costs, and current projections suggest that they will become more prominent diseases in the future (6). In particular, AIDs pose a major challenge to the public health system, which is second only to cancer and cardiovascular diseases, due to their long cycle and susceptibility to relapse (7–10). Therefore, it is of great importance and urgency to find effective methods for the prevention and treatment of AIDs. Coumarins are a class of aromatic natural products with benzene and alpha-pyrone as its basic structure, which are widely found in Umbelliferae, Brassicaceae, Asteraceae, Leguminosae, Orchidaceae (11). Coumarins can be divided into simple coumarins, furanocoumarins, pyranocoumarins and others based on the chemical structures (12). Accumulating studies have shown that coumarins possess a variety of pharmacological activities such as anti-tumor, anti-inflammatory, and anti-osteoporosis (13). Nowadays, coumarins have been gaining more attention from investigators due to its excellent biological activities in AIDs. Here, we review the latest research data on coumarins for the treatment of AIDs with the aim of understanding the pharmacological mechanisms of coumarins and developing novel agents for the treatment of AIDs.
2 Pathophysiology of autoimmune diseases
The mechanism by which the immune system prevents pathogens from attacking the organism is very complex. it can remove senescent cells and immune complexes from the body through various immune cells (such as macrophages, dendritic cells (DCs), T-lymphocytes, B-lymphocytes, etc.), and at the same time, it can recognize its own tissues and cells as its “self”, thus forming immune tolerance. Immune tolerance is defined as a state in which immunologically active cells are unable to produce specific immune effector cells and specific antibodies when exposed to antigenic substances, thus failing to execute a normal immune response (9, 14). In some cases, autoimmune tolerance is disrupted and the absence of immune tolerance induces the immune system to produce autoantibodies in response to self-antigens. Antigen presenting cells (APCs) present autoantigens to T cells with the participation of major histocompatibility complex (MHC) molecules (15). T helper cells are stimulated by MHC II and release different cytokines that can directly trigger macrophages (MP), monocytes, and B cells (16). T cells control the immune response by influencing the mixture of interleukins produced. B cells produce antibodies against their own molecules that react with accessible cells and directly or indirectly mediate damage (17). When the immune system produces a strong and sustained immune response against its own tissues and cells, leading to cellular destruction or tissue damage and clinical symptoms, it can lead to AIDs (18). Broadly speaking, AIDs are diseases caused by the immune response of the immune system against its own components. All diseases caused by dysfunctions of the autoimmune system can be referred to as AIDs.
Genetic, epigenetic, and environmental factors (hormones, nutrition, drugs, microbiota, apoptosis, and others) are predisposing factors for autoimmunity (19). Although AIDs are considered rare, epidemiologic data show that nearly 3-5% of the population suffers from type 1 diabetes (T1D) and autoimmune thyroid diseases (20). According to clinical manifestations, AIDs can be categorized into two categories: systemic AIDs and organ-specific AIDs (3, 21). Systemic AIDs are those in which immune response causes pathological damage to multiple organs and tissues throughout the body, mainly including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjögren′s syndrome (SS) and others. Organ-specific AIDs refer to patients whose lesions are generally confined to a specific organ and caused by an autoimmune response against the particular organ. It mainly includes Hashimoto’s thyroiditis (HT), Graves’ Disease (GD), myasthenia gravis (MG) and others.
The imbalance of immune cells activation and regulation caused by the failure of lymphocyte self-tolerance mechanisms is considered to be a major driver of the progression of human AIDs (22). The production of autoantibodies is a key event in the development of AIDs. Under the influence of T cells or innate triggers, self-tolerance is first interrupted, and the B-cell response leads to systemic autoimmunity and the production of pathogenic autoantibodies, which are the main immune abnormality in AIDs (23). Expansion of self-reactive T cells is a biomarker of many AIDs, which is essential in the orchestration of innate and adaptive immune responses and in the induction of tissue damage. Among them, CD4+ T cells make important contributions by secreting various cytokines, chemokines and cell-cell interactions. IL-17-producing CD4+ T cells (Th 17 cells) are the core of the disease pathogenesis. When activated by antigen presenting cells (APCs), CD4+ T cells differentiate into different cell lines with unique functions, including helper T (Th) 1, Th2, Th17, and regulatory T (Treg) cells, each of which secretes its own set of cytokines (24). A balance is required to maintained between Th cell activation and Treg cells-mediated inhibition to maintain effective immune homeostasis. Disruption of this balance lead to lymphocytes generating an immune response and/or producing antibodies against their own cells and tissues (25).
The T cell subsets involved in the inflammatory response are mainly Th1 and Th17. Th1 cells are generated from CD4+ T lymphocytes activated by interleukin (IL)-12 through the STAT4 signaling pathway and the transcription factor T-bet, mainly secreting cytokines, such as IL-2, IL-12, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), and participate in the cellular immune response (26). Th2 cells are induced by IL-4 through the STAT6 signaling pathway and the transcription factor GATA-3, mainly secreting IL-4, IL-5, IL-6, IL-10 and IL-21 to participate in the immune response. Th1 plays a certain role in inhibiting the activation of Th2, and the two regulate and constrain each other, putting the body in a dynamic balance of cellular immunity and humoral immunity (27). Th 17 cells are one of the most predominant pathogenic cells among Th cells, and their main function is to secrete cytokines IL-17A, IL-17F, and IL-22. Their activation and proliferation require multiple transcription factors (such as NF-κB, STAT3) and specific cytokines (such as transforming growth factor-β (TGF-β), IL-6, IL-23) (28, 29). Treg cells, as an important factor in the maintenance of immune tolerance by the organism, can regulate the stable state of lymphocytes. Under the induction of the specific transcriptional regulator Forkhead box protein P3 (Foxp3), they exert anti-inflammatory effects by releasing anti-inflammatory cytokines such as IL-10 and TGF-β (30).
Recent studies have confirmed the role of immune cells and cytokines in AIDs. For example, multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system, characterized by a positive correlation between the imbalance of the Th17/Treg ratio and the severity of MS symptoms (31, 32). Similarly, inflammatory bowel disease (IBD) and SLE are AIDs characterized by elevated levels of pro-inflammatory cytokines IL-1 and LTB4 (33). Furthermore, it is known that psoriasis is caused by activation of the IL-23/Th17 cytokine axis (34, 35). In UC patients, the increasement of IL-1, IL-6 and TNF-α are observed. In addition, elevated levels of IL-6 can also be observed in patients with T1D, RA and psoriasis (36). Studies have confirmed that Th17 cells are critical for the severity of collagen-induced arthritis and RA (37). Similarly, IL-17 and TNF-α-induced increase in intestinal barrier permeability can promote the development of Crohn’s disease (CD), ulcerative colitis (UC), and MS (38, 39).
AIDs are the result of a combination of genetic predisposition and environmental factors. Genome-wide association studies (GWASs) has been widely used to identify susceptibility genes for AIDs and has identified many relevant mutations in T cells, including Single Nucleotide Polymorphism (SNPs) in IL-23R, IL-17A/F, IL-21, JAK2, STAT2, CARD9, CCR6, and others (40). In addition, epigenetic mechanisms influence the development of many AIDs under the influence of environmental factors. M6A-modified regulatory factors can be involved in T cell-mediated autoimmune diseases. It was found that the m6A-modifying demethylase ALK-BH5 promotes IFN-γ and CXCL2 mRNA stability in CD4+ T cells, which in turn enhances CD4+ T cell pathogenicity in experimental autoimmune encephalomyelitis (EAE), whereas the demethylase FTO does not function (41). In experimental autoimmune uveitis, the presence of METTL3 in autoreactive Th17 attenuates Th17 pathogenicity by enhancing ASH1L mRNA stability to reduce IL-17 and IL-23 receptor expression (42). However, in psoriasis, T cell-specific deletion of ALKBH5 instead exacerbates skin inflammation (43). Epigenetic modifications regulate the body’s inflammatory response and immune response at multiple levels through DNA methylation, histone acetylation, and microRNAs, while the DNA sequence remains unchanged (44). For example, due to the reduced expression of H3K4 methyltransferase Ash1L, Tregs in RA patients express low levels of Foxp3 while Ash1L can enhances TGF-β/Smad signaling promotes Treg differentiation, inhibits histone deacetylase 1 (HDAC1), and reduces histone deacetylation of Foxp3 (45).
3 Clinical status of coumarins in autoimmune disorders
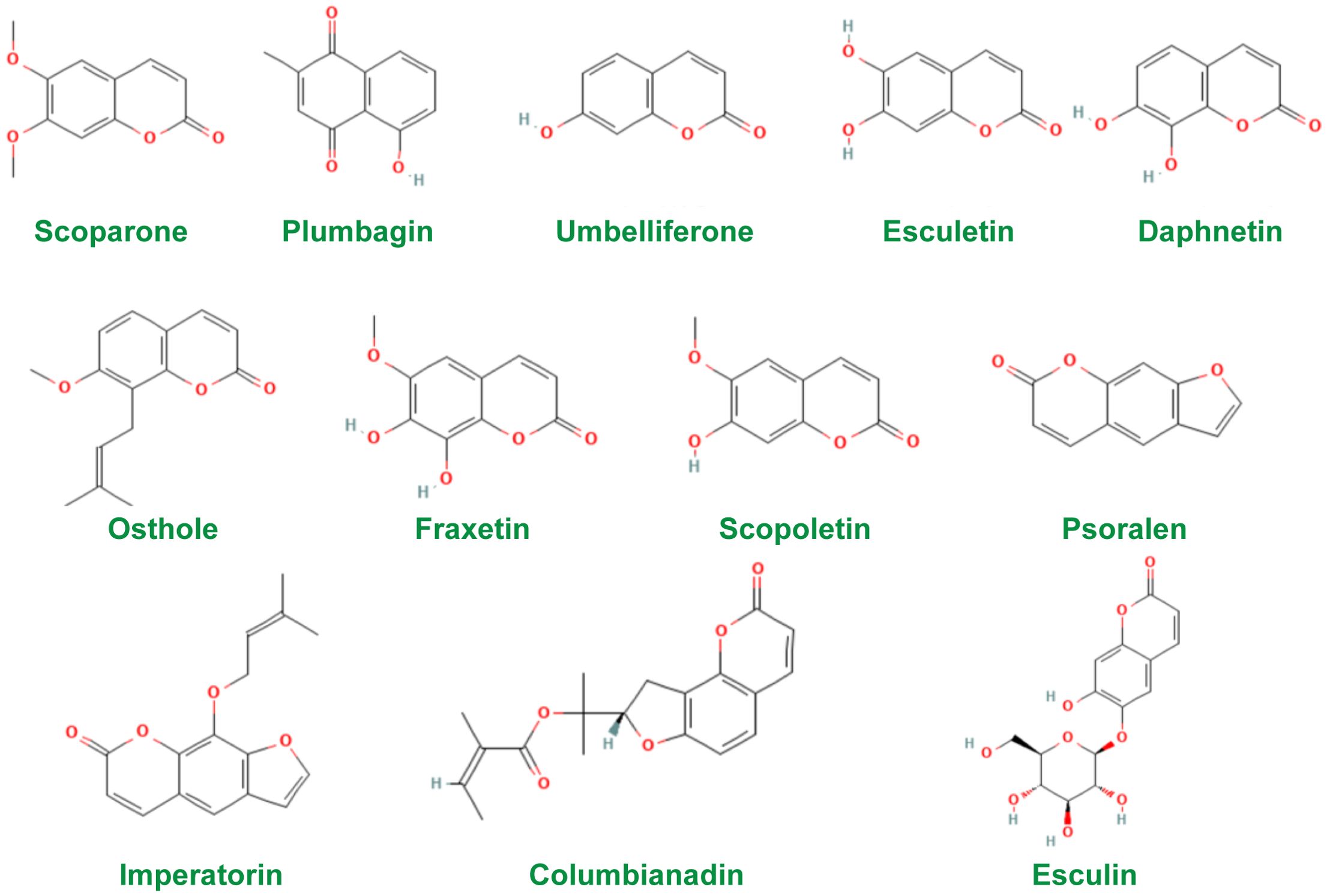
Coumarins are a class of natural compounds widely found in nature (46). Modern pharmacological and clinical studies have shown that coumarins have pharmacological effects such as anti-tumor (47), anti-inflammatory (48), anti-osteoporosis (49), cardiovascular and neuroprotection (50), anti-bacterial (51), anti-tuberculosis (52), and photosensitization (53). In addition, coumarins are effective in the treatment of several AIDs in studies. Therefore, coumarins need more attention. The chemical structures of the constituents were screened using the PubChem database (http://pubchem.ncbi.nlm.nih.gov) and the structures of the most widely studied coumarins are given in Figure 1.
Given its immunomodulatory activity, coumarin has become a pharmacological tool for the treatment of various AIDs. Currently, coumarin combinations are used in the treatment of skin and AIDs such as psoriasis (54). Furanocoumarins are a class of natural plant photosensitizers. Studies have confirmed that furanocoumarins can increase the body’s sensitivity to long-wave ultraviolet light (53). PUVA, a combination of psoralen (P) and ultraviolet A (UVA), is increasingly being used to treat chronic plaque-type psoriasis and chronic palmoplantar psoriasis, and has become a second-line therapy for patients with moderate to severe psoriasis (55–61). In the treatment of severe chronic atopic dermatitis, PUVA therapy provides better short- and long-term efficacy than UV therapy alone (62–64). PUVA is also safe and effective in the treatment of cutaneous T-cell lymphoma (65). And, PUVA therapyis effective in patients with cutaneous T-cell lymphoma (CTCL) complicated by ankylosing spondylitis (AS) (66). In patients with alopecia areata (AA), dilutions of psoralen were applied to the patient’s scalp, and hair regrowth was observed in 6 of 9 patients after up to 10 weeks of treatment (67). Also, PUVA is effective in patients with AA (68, 69). In clinical practice, coumarins have been used regularly in the treatment of vitiligo. Psoralen and bergapten can increase the tolerance of human skin to radiation and produce hyperpigmentation when exposed to ultraviolet light (70). In addition, in a clinical study evaluating the photochemotherapeutic properties of bergapten microcrystalline formulations, the data results showed that bergapten was almost completely free of phototoxic and drug intolerance reactions, and that other side effects, such as severe erythema, itching, and nausea, were seen only rarely. Bergapten may be used as a photochemical therapy (PUVA) as an important alternative therapy (71). A study investigated the distribution of bergapten in the skin following oral administration of the drug. Bergapten concentrations in the skin following single and multiple oral doses of the drug were measured at healthy and psoriatic sites in 10 patients with psoriasis. The results showed that after oral administration of bergapten, accumulation levels were higher in the more external layers of the skin, the drug had a high affinity for the stratum corneum, and drug concentrations were similar in healthy and psoriasis sites, suggesting that lesions did not affect the distribution of the drug in the skin (72).
4 Coumarins act on immune cells and cytokines in autoimmune disorders
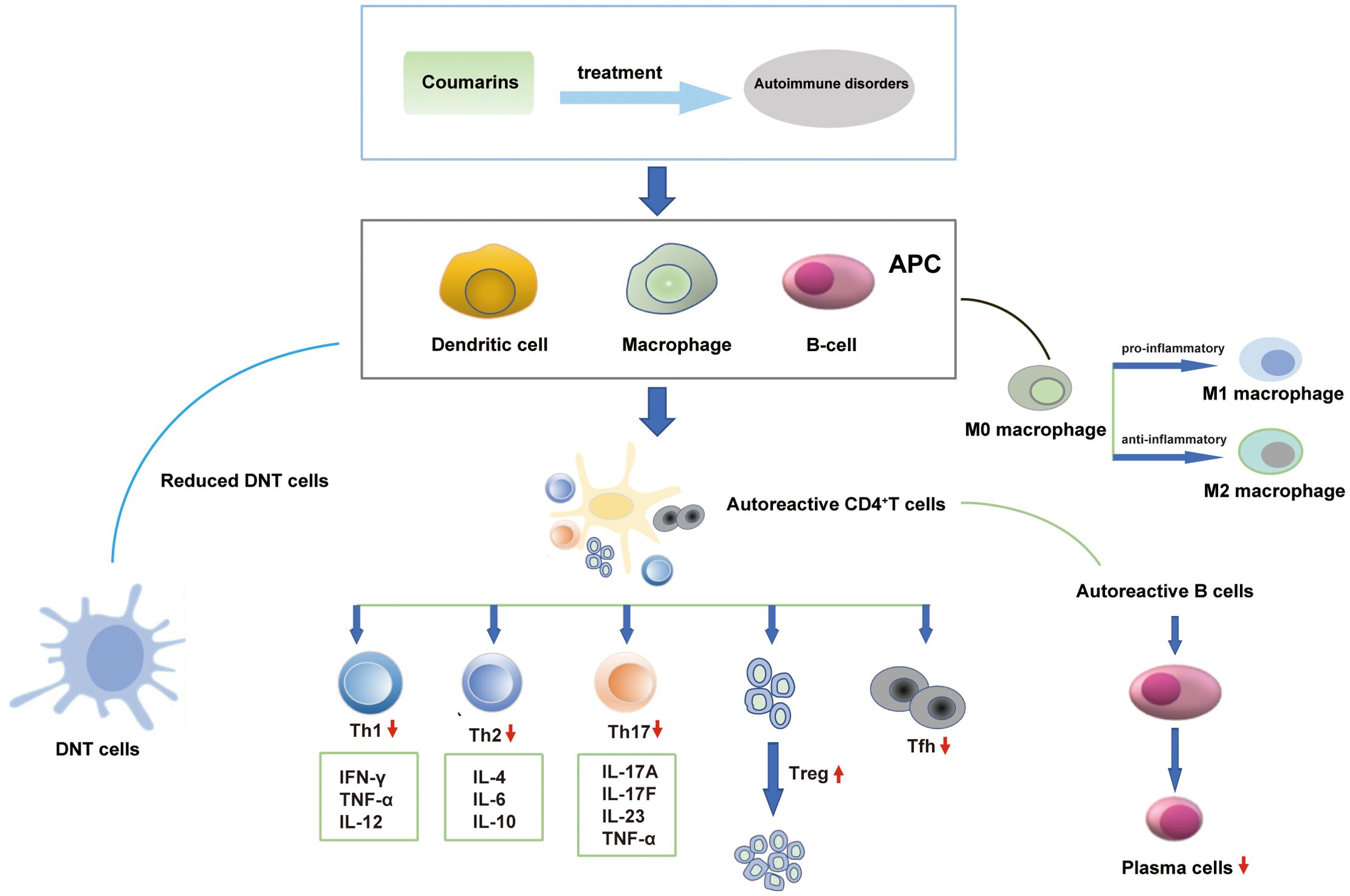
AIDs have been categorized into several types and more than 80 such diseases have been identified. Coumarins can act on immune cells and cytokines to exert beneficial effects on AIDs. The regulatory effects of coumarins on significant immune cells and cytokines are shown in Figure 2.
RA is a chronic, systemic autoimmune disease with symmetrical, erosive, inflammation occurring in multiple joints as its main clinical manifestation. CD83 is a dendritic cell marker that belongs to the immunoglobulin superfamily and is closely associated with autoimmune diseases (73). TNF-β, which mediates a variety of inflammatory and immunostimulatory responses (74). Sterol regulatory element‐binding protein 1 (SREBP1), a transcription factor, is a major regulator of genes that control cellular lipid homeostasis (75). Synergistic treatment with Imperatorin and β‐sitosterol significantly up-regulated the expression levels of TNF-β, CD83, and SREBP1 in peripheral blood CD4+ T cells, which improved the severity of arthritis in collagen-induced arthritis (CIA) rats (76). In addition, imperatorin inhibits cell proliferation and induces apoptosis in RA fibroblast-like synoviocytes (RA-FLSs) cells through mitochondrial/caspase-mediated signaling pathways (77). Migration inhibitory factor (MIF) is a pleiotropic inflammatory cytokine important in both innate and adaptive immune responses, and studies have demonstrated that elevated levels of MIF expression are observed in synovial tissues of RA patients compared to healthy individuals (78). Isopsoralen has been shown to ameliorate RA by targeting macrophage MIF, as evidenced by a significant decrease in serum production of IL-6, IL-1β, and cartilage oligomeric matrix protein (COMP) but an increase in IL-10 production in CIA mice (79).
SLE is a chronic autoimmune disease in which 75% to 80% of patients have skin manifestations, such as erythema of the cheeks, rashes, and skin ulcers (80). Double-negative (DN) T cells are defined by the lack of CD4 and CD8 and the ability to produce pro-inflammatory cytokines, such as IFN-γ, which have been implicated in the pathogenesis of SLE in humans and mice (81). Umbelliferone reduced DN T cells, plasma cells, IFN-γ+CD4+ T cells, and T follicular helper cells (CD3+TCRβ+CD4+CXCR5+PD1+) and increased the percentage of Treg cells in lupus nephritis MRL/lpr mice (82).
MS is a common clinical neuroimmune disease, which occurs in young and middle-aged people between the ages of 20 and 40, with more female patients than male patients, strong relapses, and a high disability rate (83). EAE is the classical animal model of MS. Different coumarins including daphnetin, plumbagin, umbelliferone, and osthole can treat EAE. Daphnetin attenuates EAE by up-regulating Th2 and Treg cells and inhibiting Th1 and Th17 cells, as evidenced by increased expression of anti-inflammatory cytokines and transcription factors (IL-4, IL-10, IL-33, GATA3, Foxp3), and decreased pro-inflammatory cytokines and transcription factors (IL-17, TNF-α, IFN-γ, STAT4, T-bet, STAT3, ROR-γt) production (84). In addition, daphnetin reduced pro-inflammatory cytokines, including IL-17, IFN-γ, IL-6, IL-12a, and IL-23a, in brain tissues of EAE mice. Heme oxygenase-1 (HO-1) is a typical antioxidant and anti-inflammatory factor. The study confirmed the ability of daphnetin to inhibit IL-1β, IL-6, and TNF-α production and significantly elevate HO-1 levels in lipopolysaccharide (LPS)-stimulated mouse BV2 microglial cells (85). A study looking at the effects of plumbagin on EAE found that plumbagin inhibited the differentiation, maturation, and function of human monocyte-derived DCs, as well as inhibited Th1 and Th17 cell polarization (decreasing the expression levels of IL-6, IL-1β, and IL-23), and promoted Th2 cell polarization (up-regulating the expression level of IL-4) (86). Umbelliferone attenuates clinical symptoms in EAE mice by inhibiting the activation of autoreactive T cells, suppressing Th1 cell polarization, and increasing the level of Foxp3+ regulatory T cells (87). The effects of osthole on EAE have also been reported. Osthole augments the therapeutic efficiency of neural stem cells and inhibits the reduction of nerve growth factor (NGF) and the elevation of IFN-γ in EAE mice (88, 89).
Psoriasis is primarily a refractory disease mediated by T-lymphocytes with a combination of genetic and environmental effects (90). Daphnetin treatment inhibits the proliferation and inflammatory response of human HaCaT keratinocytes and ameliorates imiquimod (IMQ)-induced psoriasis-like skin injury in mice and attenuates the IMQ-induced upregulation of inflammatory cytokines, including IL-6, IL-23A, and IL-17A (91). Treatment of psoriasis mice with an ointment containing osthole was found to reduce the secretion of TNF-α, IL-12, IL-17, and IL-23 in the skin of mice (92).
UC is a chronic non-specific inflammatory bowel disease, with lesions mainly located in the mucous membrane and submucosa. The main clinical manifestations include abdominal pain, diarrhea, and bloody stools (93). Treatment with decursin and decursinol inhibitthe production of IL-6, TNF-α, cyclooxygenase (COX)-2, hypoxia inducible factor (HIF)-1a, and prostaglandin E2 (PGE2) in colonic tissues of UC mice induced by dextran sulfate sodium (DSS) (94). Decursinol angelate ameliorates DSS-induced colitis by modulating Th17 cell responses, which is reflected in its ability to reduce the mRNA level of RORγt in Th17 cells and the expression of IL-17 in CD4+ T cells (95). In addition, plumbagin reduces the expression of circulating inflammatory monocytes (CD14+/CD16+) and cytokines (TNF-α and IFN-γ) in UC mice (96). Osthole treatment down-regulated the levels of pro-inflammatory Th1-associated cytokines (TNF-α) and Th17-associated cytokines (IL-17) and up-regulated the levels of anti-inflammatory Th2-associated cytokines (IL-4 and IL-10) (97). Another study confirmed that daphnetin can improve UC by regulating Treg/Th17 balance (98). In addition, the therapeutic effects of esculin, bergapten, esculetin and scoparone have been reported in UC (99–102).
Type 1 diabetes mellitus (T1DM) is an autoimmune disease in which pancreatic β cells are destroyed, resulting in an absolute lack of insulin (103). Umbelliferone increases the number of Foxp3+ regulatory T cells, thereby alleviating the severity of type 1 diabetes (104). In addition, imperatorin acts as a Takeda G-protein-coupled receptor 5 (TGR5) and G-protein-coupled receptor 119 (GPR119) agonist, inducing glucagon-like peptide (GLP-1) secretion and lowering blood glucose levels in type 1 diabetic rats through activation of TGR5 and GPR119 (105).
Chronic prostatitis (CP) is a common and intractable genitourinary chronic inflammatory disease in young and middle-aged men, characterized by slow onset, stubbornness, recurrent episodes, and intractability. The autoimmune response caused by the imbalance of CD4+ T cell differentiation was found to be an important etiological factor of CP. 4‐methylumbelliferone could reduce the severity of experimental autoimmune prostatitis (EAP) in experimental EAP mice by significantly decreasing the proportion of Th1 cells (106).
5 Coumarins targeting various signaling pathways
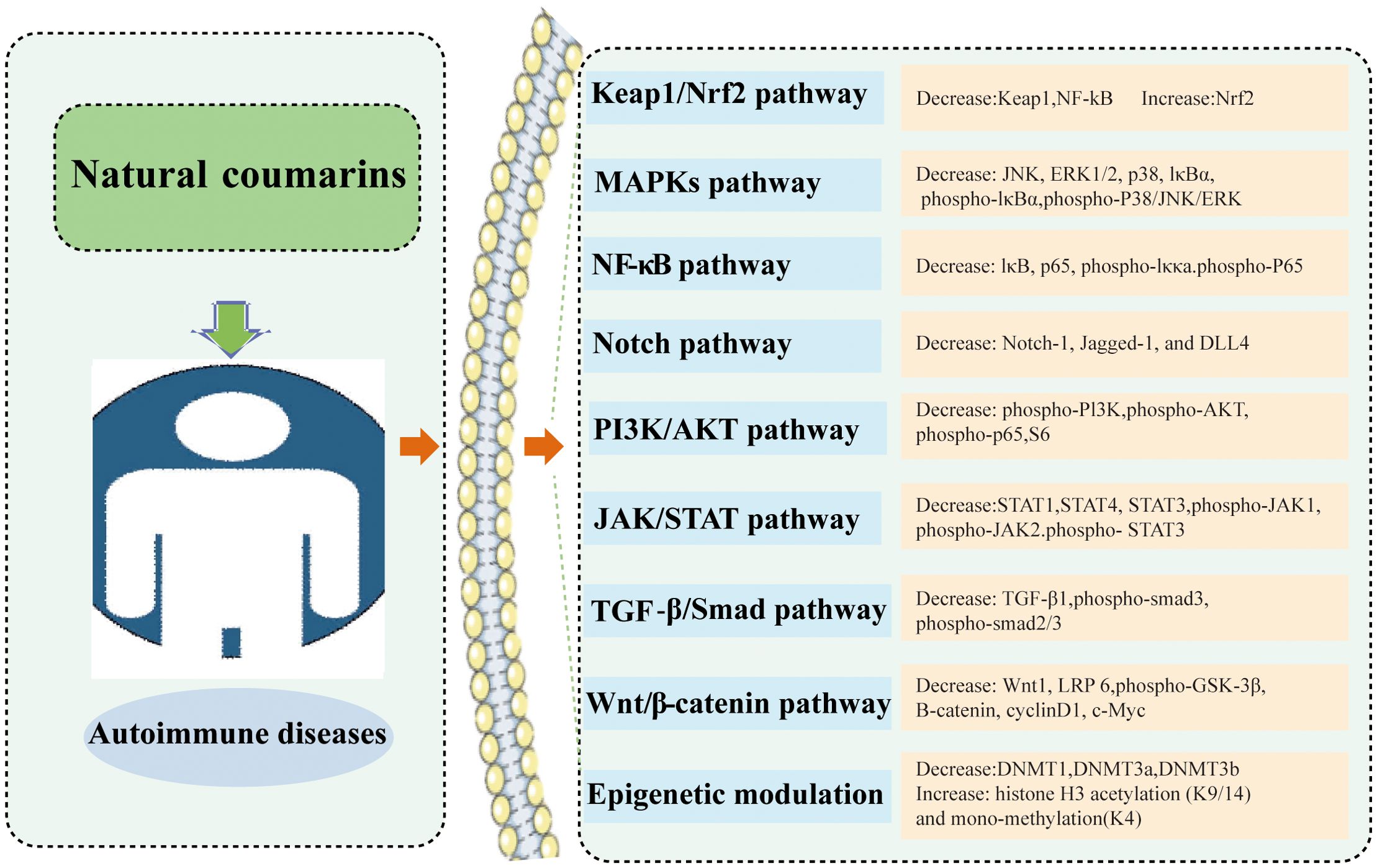
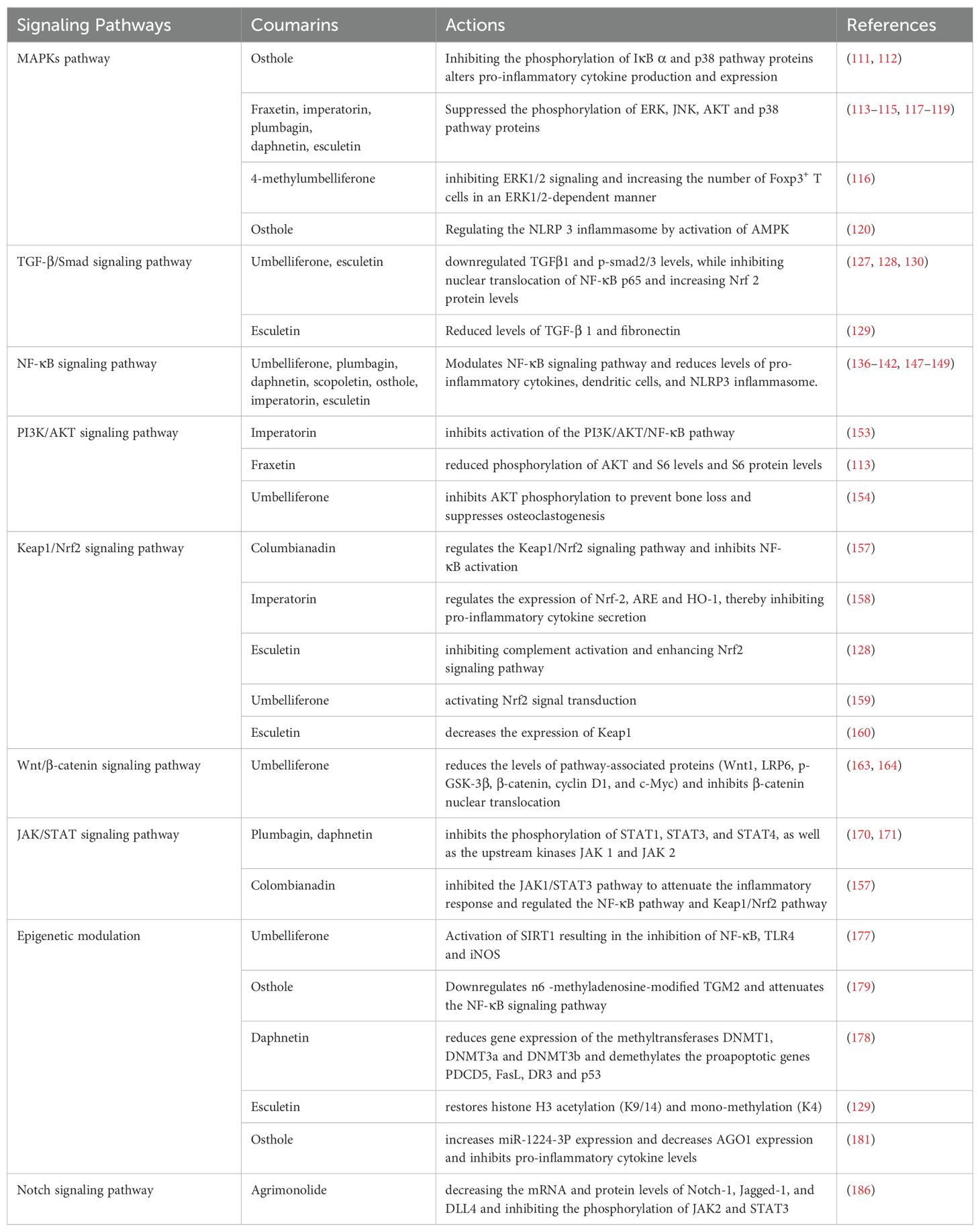
Coumarin-like chemicals are notable for their anti-tumor properties. In recent years, accumulated studies have shown that coumarins exhibit promising immune regulatory effects in AIDs. Each type of coumarin targets different immune cells, thus triggering a large number of different intracellular signaling pathways, ultimately regulating the host’s immune response. The modulation of several signaling pathways leads to alterations in the expression of pro-inflammatory genes, which ultimately lead to an improvement in immune environment. To date, most of the mechanistic studies have been conducted in animal experiments. Many mechanistic studies have been conducted in animal and cell experiments. The action of coumarins in AIDs are summarized in Figure 3 and Table 1.
5.1 Mitogen-activated protein kinases (MAPKs) pathway
The family of MAPKs includes several subfamilies such as c-Jun n-terminal kinase (JNK), p38 MAPK, and extracellular signal-regulated kinases (ERK), which can regulate proliferation, differentiation, apoptosis, or survival, cellular activities such as inflammation and innate immunity (107, 108). There is connectivity and relative independence between different signaling pathways in the MAPK family. The c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and p38 kinase are activated by environmental stress and inflammatory signals, whereas extracellular signal-regulated kinase (ERK1/2/5) is mainly activated by growth factor receptors and some cytokine receptors. Once activated, MAPK will phosphorylate different proteins, acting as other kinase translation regulators and transcription factors, leading to cellular responses (109). Signals can be transmitted from the cell surface to the nucleus by activated MAPK to increase the expression of relevant inflammatory genes and promote the secretion of a variety of inflammatory factors, such as COX-2, PGE2, Monocyte chemoattractant protein-1 (MCP-1), IL-1β, IL-6, and TNF-α (110). Several studies have reported the inhibitory effects of coumarins on JNK, ERK1/2, and p38. These inhibitory effects lead to a reduction in the expression and release of pro-inflammatory mediators (IL-1β, IL-6, COX-2, MCP-1, e.g.). For example, osthole inhibited the expression of p38 MAPK, COX-2, inducible nitric oxide synthase (iNOS), and IκB α in LPS-induced RAW 264.7 cells and decreased the levels of NO, PGE2, TNF-α, and IL-6. On this basis, in DSS-induced UC mice, osthole decreased the expression of NF-κB p65 and p-IκB α in colonic tissues (111). Similarly, osthole significantly inhibited the phosphorylation of p38, which was induced by 2,4,6-Trinitrobenzenesulfonic acid (TNBS) in mice or by LPS in Raw264.7 cells, and strongly inhibited IL-1β, IL-6, COX-2, and MCP-1. Interestingly, the inhibition by protein kinase A (PKA) partially reversed the suppressive effects of osthole on p38 phosphorylation in LPS-stimulated cells (112). In endometriotic animal models and cells (End1/E6E7 and VK2/E6E7), fraxetin reduced endometriotic lesions by inhibiting P38/JNK/ERK phosphorylation, inducing apoptosis, and generating reactive oxygen species (ROS) (113). Imperatorin was able to attenuate symptoms associated with a mouse model of psoriasiform dermatitis by inhibiting the phosphorylation of ERK, JNK, and AKT. Meanwhile, the inhibitory effects of imperatorin on cell responses and signaling could be reversed by a PKA inhibitor, suggesting that cAMP/PKA is involved in the anti-inflammatory effects of imperatorin (114). In addition, inhibition of p38 activation by imperatorin has been reported (115). 4-methylumbelliferone (4-MU) inhibits ERK 1/2 signaling and increases the number of Fox P3+ T cells in an ERK1/2-dependent manner, thereby inhibiting hyaluronan synthesis to restore immune tolerance in autoimmune insulitis (116). Additionally, other coumarins, such as plumbagin, daphnetin, and esculetin have been shown to reduce inflammation by interfering with the MAPKs pathway (117–119).
It is noteworthy that the beneficial effects of coumarins may also be associated with increased signaling in the AMPKs pathway. For example, the anti-RA activity of osthole requires the involvement of AMPK phosphorylation activation. Osthole can regulate NLRP3 inflammasome by activating AMPK. This result was also reverse-validated by the experimental application of the AMPK inhibitor compound C, which blocked the activation of AMPK by osthole and also attenuated the positive effect of osthole on inflammasome activation, which was manifested as increased protein levels of NLRP3, CAS1, ASC and IL-1β (120).
5.2 Transforming growth factor beta/small mother against decapentaplegic (TGF-β/Smad) signaling pathway
The TGF-β/Smad signaling pathway is involved in many cellular processes (121). TGF-β is a multifunctional cytokine consisting of three isoforms, TGF-β1, TGF-β2, and TGF-β3, which is widely expressed in different types of cells and tissues, with TGF-β1 being the major isoform. TGF-β can negatively regulate immune cell proliferation, differentiation, and activation and plays an important role in suppressing immunity and inflammation (122, 123). Smad proteins, on the other hand, are signal transducers of intracellular TGF-β and mediate most of the functions of TGF-β (124). One of the mechanisms by which coumarins can modulate the immune response is through direct inhibition of the TGF-β/Smad signaling pathway. During diabetes, the expression of TGF-b is increased in the kidney, which leads to further deterioration of nephropathy (125). The circulating level of TGF-b1 is one of the important markers for predicting diabetes-related renal injury (126). Umbelliferone reduces Renal damage in type 1 diabetic rats by decreasing the levels of TGF-β1 in Renal tissue and circulation (127). In the MRL/lpr mouse model, esculetin significantly down-regulated the levels of TGFβ1 and p-smad3 in renal tissues, as well as significantly inhibited the nuclear translocation of NF-κB p65 and increased the level of Nrf2 protein in the nucleus, which had a significant therapeutic effect on murine lupus nephritis (128). In addition, esculetin treatment protects against the increase in expression of TGF-β1and fibronectin in type I diabetic rat kidney and hence shows efficacy in attenuating glomerulosclerosis (129). Another study reported that umbelliferone and esculetin could inhibit the activation of TGF-smad signal, which showed that they could down-regulate the secretion of fibronectin in HK2 cells stimulated by TGF-β1 and inhibit smad2/3 phosphorylation, thus playing a beneficial role in rats with type 1 diabetic nephropathy (130).
5.3 Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway
NF-κB is an important intracellular transcription factor that regulates the expression of a wide range of genes and plays a key regulatory role in a variety of biological processes including inflammatory response, cell proliferation, apoptosis, and cell infiltration (131). NF-κB is phosphorylated by IκB kinase upon cellular stimulation by chemical or mechanical signals and subsequently degraded via the ubiquitin-proteasome system. After IκB degradation, NF-κB dimers detached from IκB are activated by translation and enter the nucleus to participate in transcription (132–134). Activated NF-κB regulates the production of inflammatory factors such as TNF-α, COX-2, and PGE2 in the nucleus, and participates in and mediates a variety of immune responses and inflammatory reactions in the body (135). Different coumarins including umbelliferone, plumbagin, daphnetin, scopoletin, osthole, imperatorin, and esculetin all inhibit NF-κB pathways. In FLS of RA, umbelliferone and scopoletin counteract RA by binding to and inhibiting tyrosine kinases in RA-FLS and subsequently inhibiting NF-κB (136). Furthermore, umbelliferone ameliorates RA induced by complete Freund’s adjuvant by inhibiting the NF-κB signaling pathway in osteoclast differentiation (137). In a mouse model of EAE, scopoletin attenuates DCs activation through inhibition of the NF-κB signaling pathway and significantly reduces central nervous system (CNS) inflammation and demyelination in EAE mice (138). The inhibitory effect of daphnetin on NF-κB activation has been reported in various autoimmune disease models (psoriasis mice, EAE mice, NZB/WF1 SLE mice) (139–141). Plumbagin has been shown to reduce the levels of TNF-α, IL-6 and matrix metalloproteinases (MMPs) in RA mouse cells by inhibiting NF-κB activation, and its mechanism of action is related to the inhibition of IκB and NF-κB activation as well as the entry of p65 into the cell nucleus (142). Furthermore, in patients, IL-1β plays a pathogenic role in the evolution of IgA nephropathy (143), and serum levels of IL 18 are elevated in IgA nephropathy patients (144). Mature IL-1β and IL-18 are produced by active caspase-1 from NLRP3 inflammasome from their respective precursors pro- IL-1β and pro IL-18 (145, 146). In a mouse model of progressive IgA nephropathy, osthole blocked the activation of NF-kB and NLRP3 inflammasome, thereby improving renal function and blocking progressive renal lesions (147). IL-1β A study observing the effects of esculetin on skin inflammation in psoriasis mice found that esculetin inhibited the activation of the NF-κB signaling pathway, including inhibiting the phosphorylation of IKKα and P65 in psoriatic skin (148). In addition, the inhibitory effect of imperatorin on NF-κB has been reported in mice with UC (149).
5.4 Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway
The PI3K/AKT pathway is an important signaling pathway in the body, consisting of two protein kinases, PI3K and AKT, which are involved in the phosphorylation of NF-κB p65 and nuclear translocation, and contribute to the production of inflammatory mediators (150, 151). Overall, the PI3K/AKT pathway activates the signaling pathway upon stimulation of the corresponding upstream signals, which in turn directs the downstream signaling substances as well as the cytosolic nucleus to make the corresponding response, and further regulates the phenomena of cell autophagy, apoptosis, and inflammation release, which ultimately affects the development of diseases (152). It is hypothesized that the beneficial effects of coumarins may be related to the inhibition of signaling pathways in the PI3K/AKT pathway. It was confirmed that imperatorin significantly inhibited the activation of the PI3K/AKT/NF-κB pathway by inhibiting the phosphorylation levels of PI3K, AKT, and p65 in the ectopic endometrium tissue, thereby significantly inhibiting the growth and ameliorate the histopathological features of ectopic endometrium in experimental endometriosis rats (153). Another study reported that fraxetin significantly reduced phosphorylation of AKT and S6 levels and S6 protein levels in End1/E6E7 and VK2/E6E7 cells (endometriotic epithelial cell lines) (113). Furthermore, umbelliferone prevents LPS-induced bone loss and inhibits RANKL-induced osteoclastogenesis by inhibiting AKT phosphorylation (154).
5.5 Kelch-like-ech-associated protein 1-nuclear factor E2-related factor 2 (Keap1/Nrf2) signaling pathway
Kelch-like-ech-associated protein 1 (Keap1)-nuclear factor E2-related factor 2 (Nrf2) pathway is closely related to oxidative stress and inflammation in various organs and systems of the body, and is considered as the therapeutic target of many organ protection. Nrf2 is the main regulator of cell antioxidant response, and its activity is precisely regulated by the negative regulatory protein Keap1. The antioxidant effect of Nrf2 was inhibited by the interaction with Keap1 (155, 156). The imbalance of Keap1/Nrf2 transcription activity is related to the pathogenesis of many diseases. Keap1/Nrf2 axis has become the most important regulator of intracellular homeostasis and plays an important role in the occurrence and development of many chronic diseases. Some studies have reported the regulatory effects of coumarin on Nrf2 and Keap1. For example, in the collagen-induced RA mouse model, columbianadin can play an anti-RA role by regulating inflammation and oxidative stress, and its mechanism includes inhibiting the expression of Keap1 at mRNA and protein levels, increasing the expression of Nrf2 mRNA in CIA mice, regulating Keap1/Nrf2 signaling pathway in CIA mice, and inhibiting the activation of NF-κB (157). In addition, imperatorin has been proven to interfere with the expression of Nrf2 in the colon of rats with UC induced by TNBS, and inhibit the secretion of TNF-α and IL-6 by regulating the expressions of Nrf-2, ARE and HO-1, thus alleviating the symptoms of UC (158). Umbelliferone can also alleviate UC induced by DSS by inhibiting inflammation, which is related to activating Nrf2 signal transduction (159). Other studies have reported that esculetin can treat lupus nephritis in mice by inhibiting complement activation and enhancing Nrf2 signaling pathway (128). In addition, the inhibition of esculetin on Keap1 activation has also been reported. In a study, it was reported that esculetin can reduce the expression of Keap1 in aorta of hyperinsulinemia combined with T1DM rats, and has a protective effect on vascular function (160).
5.6 Wngless-type/beta-catenin (Wnt/β-catenin) pathway
The Wnt/β-catenin signaling pathway, also known as the Canonical Wnt signaling pathway, is a conserved signaling axis (161). The Wnt/β-catenin pathway consists of four segments: the extracellular signaling, membrane segment, cytoplasmic segment, and nuclear segment. Extracellular signaling is mainly mediated by Wnt proteins, among which are Wnt3a, Wnt1, and Wnt5a. The cytosolic fragment mainly contains the Wnt receptor Frizzled and low-density lipoprotein receptor-related protein (LRP5/6). The cytoplasmic fraction mainly consisted of β-catenin, Dishevelled (DVL), glycogen synthase kinase-3β (GSK-3β), AXIN, adenomatous polyposis coli (APC) protein, and casein kinase-1 (CK-1). Nuclear segments mainly include β-catenin translocated to the nucleus, T-cell factor/lymphoid enhancer factor family (TCF/LEF), and β-catenin downstream target genes such as MMPs and c-Myc (162). Coumarins can alter the Wnt/β-catenin pathway along multiple steps in the signaling cascade. Umbelliferone reduces Wnt1 protein levels, activates GSK-3β kinase by blocking GSK-3β (Ser9) phosphorylation, and reduces the protein level and nuclear translocation of β-catenin (163). Furthermore, in FLS from RA rats, Umbelliferone could reduce the activation of the Wnt/β-catenin pathway by restoring GSK-3β activity, reducing the levels of pathway-associated proteins (e.g., Wnt1, LRP6, p-GSK-3β (Ser 9), β-catenin, cyclin D1, and c-Myc), and inhibiting β-catenin nuclear translocation (164).
5.7 Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway
The JAK-STAT pathway is a signaling pathway from the cell membrane to the nucleus and is critical in apoptosis, proliferation and differentiation, body immune function, and inflammatory response (165, 166). The JAK-STAT pathway consists of JAK-associated receptors, JAK, and STAT (167). Among them, the Janus kinase family is a class of non-receptor-type protein tyrosine kinases including JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) (168). STAT is a class of cytosolic proteins, located downstream of JAK, including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6 (169). These molecules contribute to the inflammatory process and, by inference, their inhibition represents a therapeutic target for the reduction of inflammation. Thus, the mechanism by which coumarins can exert immunomodulatory effects may be the inhibition of these molecules. Plumbagin significantly inhibited the phosphorylation of STAT1, STAT4, and STAT3, as well as the upstream kinases JAK1 and JAK2, resulting in a reduction in the number of CD4+ T-lymphocytes and pro-inflammatory cytokines in mice with experimental autoimmune encephalomyelitis, which ameliorated the locomotor dysfunction and body weight loss of mice (170). Similar results were observed in LPS-induced Caco-2 cells, where daphnetin inhibited the phosphorylation of JAK2 and STAT3 proteins (171). In addition, in mice models of CIA, colombianadin was able to exert anti-RA effects by modulating immune and inflammatory responses, and its mechanism of action included decreasing the phosphorylation levels of JAK1 and STAT3 in the ankle joints of mice with CIA as well as the STAT3 mRNA expression, suggesting that colombianadin attenuates inflammatory responses by inhibiting the JAK1/STAT3 pathway. It is worth mentioning that columbianadin also inhibited the protein expression of P65, P50, and phosphorylated IκBα in the ankle joints of mice, inhibited the expression of Keap1 at the mRNA and protein levels, and increased the expression of Nrf2 at the mRNA level in CIA mice (157).
5.8 Epigenetic modulation
More and more studies show that epigenetic modification can regulate the inflammatory response and immune response through DNA, histone, transcriptional, and post-transcriptional levels (44, 172). Indeed, a series of studies have reported the existence of coumarin-induced epigenetic modifications leading to gene activation or silencing in the absence of changes in DNA sequence (173–176). A novel point of coumarins in cellular control is their ability to modulate modular epigenetic mechanisms such as DNA methylation, histone modifications, and posttranscriptional regulation of microRNAs, thereby regulating immune cell activation and differentiation. Among various coumarins, umbelliferone has been shown to be a strong activator of Silent information regulator 1 (SIRT1), leading to down-regulation of gene and protein expression of TLR4, NF-κB, and iNOS signaling factors, as well as decreasing the levels of TNF-α, IL-6, MPO, and VCAM-1 in the colon, resulting in a potent anti-inflammatory effect in acetic acid-induced UC rats (177). It is reported that esculetin can attenuate the decrease in histone H3 acetylation (K9/14) and mono-methylation (K4) in the kidney of rats with type I diabetic nephropathy induced by streptozotocin (STZ) (129). In addition, daphnetin had a demethylating effect on the proapoptotic genes PDCD5, FasL, DR3, and p53 in CIA rat synovial cells, and decreased the gene expression of the methyltransferases DNMT1, DNMT3a, and DNMT3b (178). In a study, osthole downregulated n6-methyladenosine-modified TGM2 to exert its additive effect with methotrexate and suppress the proliferation, migration, and invasion of RA-FLSs by attenuating NF-κB signaling pathway, resulting in the suppression of RA progression (179).
MicroRNAs are small and non-coding regulatory RNAs that can regulate the translocation and/or degradation of messenger RNAs (180). The regulatory effect of coumarin on microRNAs was also reported. It is reported that osthole can increase the expression of microRNA-1224-3p (miR-1224-3p) and decrease the expression of AGO1 in HUM-iCell-s010 RA cells, and decrease the levels of IL-6 and IL-1β in these cells. This discovery suggests that osthole may have the potential to treat RA by regulating the expression of miR-1224- 3 P and AGO 1 and reducing the level of proinflammatory cytokines (181).
5.9 Other pathways
The Notch signaling pathway is a conserved and important mechanism for maintaining immune homeostasis by regulating cell differentiation and modulating inflammation (182). In mammals, the pathway includes ligands (e.g., Jagged1, Jagged2, Delta1, Delta3, and Delta4), Notch receptors (Notch1-4), and downstream signaling components (183, 184). Aberrant activation of the Notch signaling pathway disrupts Th17/Treg cell homeostasis (185). Agrimonolide was able to correct the imbalance of Th17/Treg cells by significantly decreasing the mRNA and protein levels of Notch-1, Jagged-1, and DLL4, as well as inhibiting the phosphorylation of JAK2 and STAT3, which effectively attenuated the symptoms of weight loss and hematochezia, decreased the expression of inflammatory cytokines, and repaired intestinal mucosal barrier in UC mice (186). The Hedgehog (HH) pathway is critical for embryonic development and homeostatic maintenance of many adult tissues and organs. It is also associated with certain functions of the innate and adaptive immune system (187). HH, including sonic hedgehog (SHH), Indian hedgehog (IHH), and desert hedgehog (DHH) (188). FLSs are the main effector cells responsible for synovitis and joint destruction in RA. Studies have shown that the SHH signaling pathway is involved in the aberrant activation of RA-FLSs, and inhibition of the SHH pathway reduces the proliferation and migration of RA-FLSs (189). Therefore, the Hedgehog signaling pathway may be one of the pathways of coumarins for the treatment of autoimmune diseases, and it also provides a reference for the further development and utilization of coumarins.
6 Conclusions
Therapeutic agents available for the treatment of AIDs are limited, and there are certain shortcomings, such as dosage, route of administration, and bioavailability. Some therapeutic drugs have varying degrees of side effects. Given these limitations, we reviewed various coumarins that shown promising efficacy in AIDs such as T1DM, UC, SLE, RA, MS. Coumarin can regulate inflammatory cytokines such as IL-4, IL-6, IL-10, IL-17, IL-23, TNF-α, and IFN-γ, as well as related signaling pathways in immune cells, including JAK-STAT, Wnt/β-catenin, PI3K-AKT, TGF-β/Smad, MAPKs, Keap1/Nrf2, Notch and NF-κB pathways. The review provides new evidence for the discovery of effective and safe new drugs for AIDs.
Author contributions
YL: Visualization, Writing – original draft. G-QW: Resources, Supervision, Writing – review & editing. Y-BL: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by grants from the Academic promotion programme of Shandong First Medical University (2019QL013) and the Nature Science Foundation of Shandong (ZR2023MH127).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stathopoulou C, Nikoleri D, Bertsias G. Immunometabolism: an overview and therapeutic prospects in autoimmune diseases. Immunotherapy. (2019) 11:813–29. doi: 10.2217/imt-2019-0002
2. Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin Cancer Biol. (2020) 64:1–12. doi: 10.1016/j.semcancer.2019.06.001
3. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med. (2015) 278:369–95. doi: 10.1111/joim.2015.278.issue-4
4. Alexander T, Bondanza A, Muraro PA, Greco R, Saccardi R, Daikeler T, et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transplant. (2014) 50:173–80. doi: 10.1038/bmt.2014.251
5. Xu Q, Ni J-J, Han B-X, Yan S-S, Wei X-T, Feng G-J, et al. Causal relationship between gut microbiota and autoimmune diseases: a two-sample mendelian randomization study. Front Immunol. (2022) 12. doi: 10.3389/fimmu.2021.746998
6. Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. (2023) 80:102266. doi: 10.1016/j.coi.2022.102266
7. Jiang SH, Stanley M, Vinuesa CG. Rare genetic variants in systemic autoimmunity. Immunol Cell Biol. (2020) 98:490–9. doi: 10.1111/imcb.12339
8. Li R, Sun X, Liu X, Yang Y, Li Z. Autoimmune diseases in China. Adv Immunol China - Part A. (2019) 144:173–216. doi: 10.1016/bs.ai.2019.09.002
9. Wu Y-X, Jin S-H, Cui J. Autophagy and immune tolerance. Autophagy: Biol Dis. (2019) 1206:635–65. doi: 10.1007/978-981-15-0602-4_28
10. Fernandes JC. Therapeutic application of antibody fragments in autoimmune diseases: current state and prospects. Drug Discovery Today. (2018) 23:1996–2002. doi: 10.1016/j.drudis.2018.06.003
11. Feng D, Zhang A, Yang Y, Yang P. Coumarin-containing hybrids and their antibacterial activities. Archiv der Pharmazie. (2020) 353:e1900380. doi: 10.1002/ardp.201900380
12. Annunziata F, Pinna C, Dallavalle S, Tamborini L, Pinto A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int J Mol Sci. (2020) 21:4618. doi: 10.3390/ijms21134618
13. Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM. Recent advancements of coumarin-based anticancer agents: an up-to-date review. Bioorganic Chem. (2020) 103:104163. doi: 10.1016/j.bioorg.2020.104163
14. Kumar P, Saini S, Khan S, Surendra Lele S, Prabhakar BS. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell Immunol. (2019) 339:41–9. doi: 10.1016/j.cellimm.2018.09.008
15. Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. (2015) 15:203–16. doi: 10.1038/nri3818
16. Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM. T cell responses: naïve to memory and everything in between. Adv Physiol Educ. (2013) 37:273–83. doi: 10.1152/advan.00066.2013
17. Katsuyama T, Tsokos GC, Moulton VR. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol. (2018) 9:1088. doi: 10.3389/fimmu.2018.01088
18. Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. (2006) 6:823–35. doi: 10.1038/nri1957
19. Bolon B. Cellular and molecular mechanisms of autoimmune disease. Toxicologic Pathol. (2011) 40:216–29. doi: 10.1177/0192623311428481
20. Mariani SM. Genes and autoimmune diseases - a complex inheritance. MedGenMed: Medscape Gen Med. (2004) 6:18. doi: 10.1046/j.1344-3941.2002.00054.x
21. Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. (2009) 33:197–207. doi: 10.1016/j.jaut.2009.09.008
22. Rose NR. Mechanisms of autoimmunity. Semin liver Dis. (2002) 22:387–94. doi: 10.1055/s-2002-35708
23. Ye Y, Liu M, Tang L, Du F, Liu Y, Hao P, et al. Iguratimod represses B cell terminal differentiation linked with the inhibition of PKC/EGR1 axis. Arthritis Res Ther. (2019) 21:92. doi: 10.1186/s13075-019-1874-2
24. Astry B, Venkatesha SH, Moudgil KD. Involvement of the IL-23/IL-17 axis and the Th17/Treg balance in the pathogenesis and control of autoimmune arthritis. Cytokine. (2015) 74:54–61. doi: 10.1016/j.cyto.2014.11.020
25. Aslani S, Mahmoudi M, Karami J, Jamshidi AR, Malekshahi Z, Nicknam MH. Epigenetic alterations underlying autoimmune diseases. Autoimmunity. (2016) 49:69–83. doi: 10.3109/08916934.2015.1134511
26. Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Sci (New York N.Y.). (1993) 260:547–9. doi: 10.1126/science.8097338
27. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. (2005) 6:1123–32. doi: 10.1038/ni1254
28. Zhu X, Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. (2020) 21:8011. doi: 10.3390/ijms21218011
29. Schmidt T, Luebbe J, Paust H-J, Panzer U. Mechanisms and functions of IL-17 signaling in renal autoimmune diseases. Mol Immunol. (2018) 104:90–9. doi: 10.1016/j.molimm.2018.09.005
30. Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. (2011) 23:282–92. doi: 10.1016/j.smim.2011.04.003
31. Jamshidian A, Shaygannejad V, Pourazar A, Zarkesh-Esfahani S-H, Gharagozloo M. Biased Treg/Th17 balance away from regulatory toward inflammatory phenotype in relapsed multiple sclerosis and its correlation with severity of symptoms. J Neuroimmunology. (2013) 262:106–12. doi: 10.1016/j.jneuroim.2013.06.007
32. Etesam Z, Nemati M, Ebrahimizadeh MA, Ebrahimi HA, Hajghani H, Khalili T, et al. Altered expression of specific transcription factors of Th17 (RORγt, RORα) and Treg lymphocytes (FOXP3) by peripheral blood mononuclear cells from patients with multiple sclerosis. J Mol neuroscience: MN. (2016) 60:94–101. doi: 10.1007/s12031-016-0789-5
33. Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. (2002) 21:495–505. doi: 10.1080/07315724.2002.10719248
34. Pan H-F, Li X-P, Zheng SG, Ye D-Q. Emerging role of interleukin-22 in autoimmune diseases. Cytokine Growth Factor Rev. (2013) 24:51–7. doi: 10.1016/j.cytogfr.2012.07.002
35. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. (2013) 34:174–81. doi: 10.1016/j.it.2012.11.005
36. Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. (2002) 13:357–68. doi: 10.1016/S1359-6101(02)00027-8
37. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. (2014) 13:668–77. doi: 10.1016/j.autrev.2013.12.004
38. Ray K. PARP inhibition protective against alcoholic steatohepatitis and NASH. Nat Rev Gastroenterol Hepatol. (2016) 14:3–3. doi: 10.1038/nrgastro.2016.186
39. Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. (2018) 141:1900–16. doi: 10.1093/brain/awy131
40. Kochi Y. Genetics of autoimmune diseases: perspectives from genome-wide association studies. Int Immunol. (2016) 28:155–61. doi: 10.1093/intimm/dxw002
41. Zhou J, Zhang X, Hu J, Qu R, Yu Z, Xu H, et al. m (6)A demethylase ALKBH5 controls CD4(+) T cell pathogenicity and promotes autoimmunity. Sci Adv. (2021) 7:eabg0470. doi: 10.1126/sciadv.abg0470
42. Zhao L, Liu Y, Ma B, Liu X, Wei R, Nian H. METTL3 inhibits autoreactive Th17 cell responses in experimental autoimmune uveitis via stabilizing ASH1L mRNA. FASEB J. (2023) 37:e22803. doi: 10.1096/fj.202201548R
43. Yuan L, Chen S, Ding K, Wang X, Lv W, Liu Y, et al. The m6A modification of Il17a in CD4+ T cells promotes inflammation in psoriasis. Exp Dermatol. (2023) 33:e14879. doi: 10.1111/exd.14879
44. Liu C, Xu J, Chen Y, Guo X, Zheng Y, Wang Q, et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature. Environ Health. (2015) 14:65. doi: 10.1186/s12940-015-0052-5
45. Xia M, Liu J, Liu S, Chen K, Lin H, Jiang M, et al. Ash1l and lnc-Smad3 coordinate Smad3 locus accessibility to modulate iTreg polarization and T cell autoimmunity. Nat Commun. (2017) 8:15818. doi: 10.1038/ncomms15818
46. Santos Junior CM, Silva SMC, Sales EM, Velozo E, dos Santos EKP, Canuto GAB, et al. Coumarins from Rutaceae: Chemical diversity and biological activities. Fitoterapia. (2023) 168:105489. doi: 10.1016/j.fitote.2023.105489
47. Yin S, Liu H, Wang J, Feng S, Chen Y, Shang Y, et al. Osthole induces apoptosis and inhibits proliferation, invasion, and migration of human cervical carcinoma HeLa cells. Evidence-Based Complementary Altern Med. (2021) 2021:1–7. doi: 10.1155/2021/8885093
48. Lee Y, Hyun C-G. Anti-Inflammatory effects of psoralen derivatives on RAW264.7 cells via regulation of the NF-κB and MAPK signaling pathways. Int J Mol Sci. (2022) 23:5813. doi: 10.3390/ijms23105813
49. Huang Y, Liao L, Su H, Chen X, Jiang T, Liu J, et al. Psoralen accelerates osteogenic differentiation of human bone marrow mesenchymal stem cells by activating the TGF−β/Smad3 pathway. Exp Ther Med. (2021) 22:940. doi: 10.3892/etm.2021.10372
50. Lee S-E, Kim J-H, Lim C, Cho S. Neuroprotective effect of Angelica gigas root in a mouse model of ischemic brain injury through MAPK signaling pathway regulation. Chin Med. (2020) 15:101. doi: 10.1186/s13020-020-00383-1
51. Tan N, Yazıcı-Tütüniş S, Bilgin M, Tan E, Miski M. Antibacterial activities of pyrenylated coumarins from the roots of prangos hulusii. Molecules. (2017) 22:1098. doi: 10.3390/molecules22071098
52. Reddy DS, Kongot M, Kumar A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis. (2021) 127:102050. doi: 10.1016/j.tube.2020.102050
53. Richard EG. The science and (lost) art of psoralen plus UVA phototherapy. Dermatologic Clinics. (2020) 38:11–23. doi: 10.1016/j.det.2019.08.002
54. Calzavara-Pinton P, Arisi M, Tonon F, Calzavara-Pinton I, Venturini M, Rossi M. Bath-PUVA still represents a valuable treatment option for the subsets of psoriatic patients who are not eligible to or rejecting systemic treatments and are not responsive to NB-UVB phototherapy. Photodermatology Photoimmunology Photomedicine. (2022) 39:351–6. doi: 10.1111/phpp.12846
55. Li Y, Cao Z, Guo J, Li Q, Zhu W, Kuang Y, et al. Assessment of efficacy and safety of UV-based therapy for psoriasis: a network meta-analysis of randomized controlled trials. Ann Med. (2022) 54:159–69. doi: 10.1080/07853890.2021.2022187
56. Berneburg M, Herzinger T, Rampf J, Hoetzenecker W, Guenova E, Meisner C, et al. Efficacy of bath psoralen plus ultraviolet A (PUVA) vs. system PUVA in psoriasis: a prospective, open, randomized, multicentre study. Br J Dermatol. (2013) 169:704–8. doi: 10.1111/bjd.2013.169.issue-3
57. Khanna N, Nazli T, Siddiqui K, Kalaivani M, Rais ur R. A non-inferiority randomized controlled clinical trial comparing Unani formulation & psoralen plus ultraviolet A sol in chronic plaque psoriasis. Indian J Med Res. (2018) 147:66–72. doi: 10.4103/ijmr.IJMR_249_16
58. Aggarwal K, Khandpur S, Khanna N, Sharma VK, Pandav CS. Comparison of clinical and cost-effectiveness of psoralen + ultraviolet A versus psoralen + sunlight in the treatment of chronic plaque psoriasis in a developing economy. Int J Dermatol. (2013) 52:478–85. doi: 10.1111/j.1365-4632.2012.05692.x
59. Sivanesan SP, Gattu S, Hong J, Chavez-Frazier A, Bandow GD, Malick F, et al. Randomized, double-blind, placebo-controlled evaluation of the efficacy of oral psoralen plus ultraviolet A for the treatment of plaque-type psoriasis using the Psoriasis Area Severity Index score (improvement of 75% or greater) at 12 weeks. J Am Acad Dermatol. (2009) 61:793–8. doi: 10.1016/j.jaad.2009.04.053
60. Hofer A, Fink-Puches R, Kerl H, Quehenberger F, Wolf P. Paired comparison of bathwater versus oral delivery of 8-methoxypsoralen in psoralen plus ultraviolet: A therapy for chronic palmoplantar psoriasis. Photodermatol Photoimmunol Photomed. (2006) 22:1–5. doi: 10.1111/j.1600-0781.2006.00196.x
61. Frankel AJ, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Bebo BF, et al. Treatment of psoriasis in patients with hepatitis C: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. (2009) 61:1044–55. doi: 10.1016/j.jaad.2009.03.044
62. Tzaneva S, Kittler H, Holzer G, Reljic D, Weber M, Hönigsmann H, et al. 5-Methoxypsoralen plus ultraviolet (UV) A is superior to medium-dose UVA1 in the treatment of severe atopic dermatitis: a randomized crossover trial. Br J Dermatol. (2010) 162:655–60. doi: 10.1111/j.1365-2133.2009.09514.x
63. Der-Petrossian M, Seeber A, Hönigsmann H, Tanew A. Half-side comparison study on the efficacy of 8-methoxypsoralen bath-PUVA versus narrow-band ultraviolet B phototherapy in patients with severe chronic atopic dermatitis. Br J Dermatol. (2000) 142:39–43. doi: 10.1046/j.1365-2133.2000.03239.x
64. Prinz B, Michelsen S, Pfeiffer C, Plewig G. Long-term application of extracorporeal photochemotherapy in severe atopic dermatitis. J Am Acad Dermatol. (1999) 40:577–82. doi: 10.1016/S0190-9622(99)70440-8
65. Morita A, Tateishi C, Muramatsu S, Kubo R, Yonezawa E, Kato H, et al. Efficacy and safety of bexarotene combined with photo(chemo)therapy for cutaneous T-cell lymphoma. J Dermatol. (2020) 47:443–51. doi: 10.1111/1346-8138.15310
66. Akay BN, Sanli H, Kutlay S. Treatment of ankylosing spondylitis by extracorporeal photochemotherapy given for mycosis fungoides. JCR: J Clin Rheumatol. (2011) 17:278–80. doi: 10.1097/RHU.0b013e3182287f59
67. Behrens-Williams SC, Leiter U, Schiener R, Weidmann M, Peter RU, Kerscher M. The PUVA-turban as a new option of applying a dilute psoralen solution selectively to the scalp of patients with alopecia areata. J Am Acad Dermatol. (2001) 44:248–52. doi: 10.1067/mjd.2001.110060
68. Mohamed Z, Bhouri A, Jallouli A, Fazaa B, Kamoun MR, Mokhtar I. Alopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralen. J Eur Acad Dermatol Venereology. (2005) 19:552–5. doi: 10.1111/j.1468-3083.2005.01226.x
69. Tan L, Hsia Chan M, An Tan D, See Lee J, Chong W-S. Effectiveness of paint psoralen and ultraviolet-A in alopecia areata – Our experience in the national skin center. Indian J Dermatol. (2020) 65:199–203. doi: 10.4103/ijd.IJD_400_18
70. Costa Martins JE, Pozetti GL, Sodré M. Effects of psoralen and bergapten on irradiated skin. Int J Dermatol. (1974) 13:124–8. doi: 10.1111/j.1365-4362.1974.tb01781.x
71. Tanew A, Ortel B, Rappersberger K, Hönigsmann H. 5-Methoxypsoralen (Bergapten) for photochemotherapy. Bioavailability, phototoxicity, and clinical efficacy in psoriasis of a new drug preparation. J Am Acad Dermatol. (1988) 18:333–8. doi: 10.1016/S0190-9622(88)70048-1
72. Zucchi A, Raho E, Marconi B, Nicoli S, Santini M, Allegra F, et al. Plasma and skin concentration of 5-methoxypsoralen in psoriatic patients after oral administration. J Invest Dermatol. (2001) 117:379–82. doi: 10.1046/j.0022-202x.2001.01419.x
73. Kristensen A-M, Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen K, Junker P, Østergaard M, et al. Expression of soluble CD83 in plasma from early-stage rheumatoid arthritis patients is not modified by anti-TNF-α therapy. Cytokine. (2017) 96:1–7. doi: 10.1016/j.cyto.2017.02.017
74. Buhrmann C, Shayan P, Aggarwal BB, Shakibaei M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res Ther. (2013) 15:R202. doi: 10.1186/ar4393
75. Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology — divergent pathophysiology. Nat Rev Endocrinol. (2017) 13:710–30. doi: 10.1038/nrendo.2017.91
76. Guo Q, Li L, Zheng K, Zheng G, Shu H, Shi Y, et al. Imperatorin and β-sitosterol have synergistic activities in alleviating collagen-induced arthritis. J Leukocyte Biol. (2020) 108:509–17. doi: 10.1002/JLB.3MA0320-440RR
77. Zhai K-F, Duan H, Chen Y, Khan GJ, Cao W-G, Gao G-Z, et al. Apoptosis effects of imperatorin on synoviocytes in rheumatoid arthritis through mitochondrial/caspase-mediated pathways. Food Funct. (2018) 9:2070–9. doi: 10.1039/C7FO01748K
78. Liu M, Hu C. Association of MIF in serum and synovial fluid with severity of knee osteoarthritis. Clin Biochem. (2012) 45:737–9. doi: 10.1016/j.clinbiochem.2012.03.012
79. Han Y, Wang J, Li S, Li Y, Zhang Y, Zhang R, et al. Isopsoralen ameliorates rheumatoid arthritis by targeting MIF. Arthritis Res Ther. (2021) 23:243. doi: 10.1186/s13075-021-02619-3
80. Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatology. (2020) 59:v4–v11. doi: 10.1093/rheumatology/keaa379
81. Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol (Baltimore Md.: 1950). (2008) 181:8761–6. doi: 10.4049/jimmunol.181.12.8761
82. Suarez-Fueyo A, Tsokos MG, Kwok S-K, Maeda K, Katsuyama E, Lapchak PH, et al. Hyaluronic acid synthesis contributes to tissue damage in systemic lupus erythematosus. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.02172
83. Schafflick D, Xu CA, Hartlehnert M, Cole M, Schulte-Mecklenbeck A, Lautwein T, et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat Commun. (2020) 11:247. doi: 10.1038/s41467-019-14118-w
84. Soltanmohammadi A, Tavaf MJ, Zargarani S, Yazdanpanah E, Sadighi-Moghaddam B, Yousefi B, et al. Daphnetin alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating Th2 and regulatory T cells. Acta neurobiologiae experimentalis. (2022) 82:273–83. doi: 10.55782/ane-2022-026
85. Wang D, Zhu B, Liu X, Han Q, Ge W, Zhang W, et al. Daphnetin ameliorates experimental autoimmune encephalomyelitis through regulating heme oxygenase-1. Neurochemical Res. (2020) 45:872–81. doi: 10.1007/s11064-020-02960-0
86. Zhang K, Ge Z, Da Y, Wang D, Liu Y, Xue Z, et al. Plumbagin suppresses dendritic cell functions and alleviates experimental autoimmune encephalomyelitis. J Neuroimmunology. (2014) 273:42–52. doi: 10.1016/j.jneuroim.2014.05.014
87. Kuipers HF, Rieck M, Gurevich I, Nagy N, Butte MJ, Negrin RS, et al. Hyaluronan synthesis is necessary for autoreactive T-cell trafficking, activation, and Th1 polarization. Proc Natl Acad Sci. (2016) 113:1339–44. doi: 10.1073/pnas.1525086113
88. Chen X, Pi R, Zou Y, Liu M, Ma X, Jiang Y, et al. Attenuation of experimental autoimmune encephalomyelitis in C57 BL/6 mice by osthole, a natural coumarin. Eur J Pharmacol. (2010) 629:40–6. doi: 10.1016/j.ejphar.2009.12.008
89. Gao Z, Wen Q, Xia Y, Yang J, Gao P, Zhang N, et al. Osthole augments therapeutic efficiency of neural stem cells–based therapy in experimental autoimmune encephalomyelitis. J Pharmacol Sci. (2014) 124:54–65. doi: 10.1254/jphs.13144FP
90. Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis. Drugs. (2021) 81:1751–62. doi: 10.1007/s40265-021-01612-z
91. Gao J, Chen F, Fang H, Mi J, Qi Q, Yang M. Daphnetin inhibits proliferation and inflammatory response in human HaCaT keratinocytes and ameliorates imiquimod-induced psoriasis-like skin lesion in mice. Biol Res. (2020) 53:48. doi: 10.1186/s40659-020-00316-0
92. Murayama MA, Zhang Y, Wang Q, Sun S, Jiang L. The therapeutic effect of glycyrrhizic acid compound ointment on imiquimod-induced psoriasis-like disease in mice. PloS One. (2023) 18:e0290637. doi: 10.1371/journal.pone.0290637
93. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis. Lancet. (2017) 389:1756–70. doi: 10.1016/S0140-6736(16)32126-2
94. Oh S-R, Ok S, Jung T-S, Jeon S-O, Park J-M. Jung, J.-w.; Ryu, D.-S., Protective effect of decursin and decursinol angelate-rich Angelica gigas Nakai extract on dextran sulfate sodium-induced murine ulcerative colitis. Asian Pacific J Trop Med. (2017) 10:864–70. doi: 10.1016/j.apjtm.2017.08.017
95. Thapa B, Pak S, Kwon H-J, Lee K. Decursinol angelate ameliorates dextran sodium sulfate-induced colitis by modulating type 17 helper T cell responses. Biomolecules Ther. (2019) 27:466–73. doi: 10.4062/biomolther.2019.004
96. Pile JE, Navalta JW, Davis CD, Sharma NC. Interventional effects of plumbagin on experimental ulcerative colitis in mice. J Natural Products. (2013) 76:1001–6. doi: 10.1021/np3008792
97. Khairy H, Saleh H, Badr AM, Marie M-AS. Therapeutic efficacy of osthole against dinitrobenzene sulphonic acid induced-colitis in rats. Biomedicine Pharmacotherapy. (2018) 100:42–51. doi: 10.1016/j.biopha.2018.01.104
98. Ji J, Ge X, Chen Y, Zhu B, Wu Q, Zhang J, et al. Daphnetin ameliorates experimental colitis by modulating microbiota composition and Treg/Th17 balance. FASEB J. (2019) 33:9308–22. doi: 10.1096/fj.201802659RR
99. Witaicenis A, Seito LN, da Silveira Chagas A, de Almeida LD, Luchini AC, Rodrigues-Orsi P, et al. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. (2014) 21:240–6. doi: 10.1016/j.phymed.2013.09.001
100. Yum S, Jeong S, Lee S, Kim W, Nam J, Jung Y. HIF-prolyl hydroxylase is a potential molecular target for esculetin-mediated anti-colitic effects. Fitoterapia. (2015) 103:55–62. doi: 10.1016/j.fitote.2015.03.013
101. Adakudugu EA, Ameyaw EO, Obese E, Biney RP, Henneh IT, Aidoo DB, et al. Protective effect of bergapten in acetic acid-induced colitis in rats. Heliyon. (2020) 6:e04710. doi: 10.1016/j.heliyon.2020.e04710
102. Witaicenis A, Seito LN, Di Stasi LC. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chemico-Biological Interact. (2010) 186:211–8. doi: 10.1016/j.cbi.2010.03.045
103. Ruegsegger GN, Creo AL, Cortes TM, Dasari S, Nair KS. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Invest. (2018) 128:3671–81. doi: 10.1172/JCI120843
104. Kuipers HF, Nagy N, Ruppert SM, Sunkari VG, Marshall PL, Gebe JA, et al. The pharmacokinetics and dosing of oral 4-methylumbelliferone for inhibition of hyaluronan synthesis in mice. Clin Exp Immunol. (2016) 185:372–81. doi: 10.1111/cei.12815
105. Wang L-Y, Cheng K-C, Li Y, Niu C-S, Cheng J-T, Niu H-S. The dietary furocoumarin imperatorin increases plasma GLP-1 levels in type 1-like diabetic rats. Nutrients. (2017) 9:1192. doi: 10.3390/nu9111192
106. Chen J, Meng J, Jin C, Mo F, Ding Y, Gao X, et al. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy for chronic prostatitis. Prostate. (2021) 81:1078–90. doi: 10.1002/pros.v81.14
107. Kim EK, Choi E-J. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. (2015) 89:867–82. doi: 10.1007/s00204-015-1472-2
108. Amini J, Beyer C, Zendedel A, Sanadgol N. MAPK is a mutual pathway targeted by anxiety-related miRNAs, and E2F5 is a putative target for anxiolytic miRNAs. Biomolecules. (2023) 13:544. doi: 10.3390/biom13030544
109. Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine. (2009) 48:161–9. doi: 10.1016/j.cyto.2009.08.002
110. Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. (2008) 60:261–310. doi: 10.1124/pr.107.00106
111. Fan H, Gao Z, Ji K, Li X, Wu J, Liu Y, et al. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine. (2019) 58:152864. doi: 10.1016/j.phymed.2019.152864
112. Sun W, Cai Y, Zhang X-X, Chen H, Lin Y-D, Li H. Osthole pretreatment alleviates TNBS-induced colitis in mice via both cAMP/PKA-dependent and independent pathways. Acta Pharmacologica Sin. (2017) 38:1120–8. doi: 10.1038/aps.2017.71
113. Ham J, Park W, Song J, Kim HS, Song G, Lim W, et al. Fraxetin reduces endometriotic lesions through activation of ER stress, induction of mitochondria-mediated apoptosis, and generation of ROS. Phytomedicine. (2024) 123:155187. doi: 10.1016/j.phymed.2023.155187
114. Tsai Y-F, Chen C-Y, Lin IW, Leu Y-L, Yang S-C, Syu Y-T, et al. Imperatorin alleviates psoriasiform dermatitis by blocking neutrophil respiratory burst, adhesion, and chemotaxis through selective phosphodiesterase 4 inhibition. Antioxidants Redox Signaling. (2021) 35:885–903. doi: 10.1089/ars.2019.7835
115. Lin W, Chen G, Mao Y, Ma X, Zhou J, Yu X, et al. Imperatorin inhibits proliferation, migration, and inflammation via blocking the NF-κB and MAPK pathways in rheumatoid fibroblast-like synoviocytes. ACS Omega. (2022) 7:29868–76. doi: 10.1021/acsomega.2c02766
116. Nagy N, Kaber G, Johnson PY, Gebe JA, Preisinger A, Falk BA, et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J Clin Invest. (2015) 125:3928–40. doi: 10.1172/JCI79271
117. Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S. Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radical Biol Med. (2016) 99:352–63. doi: 10.1016/j.freeradbiomed.2016.08.026
118. Zhang Y, Qu L, Sun Y, Lin Y, Zeng J, He L, et al. Daphnetin contributes to allergen-induced Th2 cytokine expression and type 2-immune responses in atopic dermatitis and asthma. Food Funct. (2022) 13:12383–99. doi: 10.1039/D2FO02518C
119. Wang S-K, Chen T-X, Wang W, Xu L-L, Zhang Y-Q, Jin Z, et al. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-кB and MAPKs pathway in vitro and in vivo. J Ethnopharmacology. (2022) 296:115489. doi: 10.1016/j.jep.2022.115489
120. Jiang X, Lu Z, Zhang Q, Yu J, Han D, Liu J, et al. Osthole: A potential AMPK agonist that inhibits NLRP3 inflammasome activation by regulating mitochondrial homeostasis for combating rheumatoid arthritis. Phytomedicine. (2023) 110:154640. doi: 10.1016/j.phymed.2022.154640
121. Xin X, Cheng X, Zeng F, Xu Q, Hou L. The role of TGF-β/SMAD signaling in hepatocellular carcinoma: from mechanism to therapy and prognosis. Int J Biol Sci. (2024) 20:1436–51. doi: 10.7150/ijbs.89568
122. Yu Y, Feng X-H. TGF-β signaling in cell fate control and cancer. Curr Opin Cell Biol. (2019) 61:56–63. doi: 10.1016/j.ceb.2019.07.007
123. Ma T-T, Meng X-M. TGF-β/Smad and renal fibrosis. Renal Fibrosis: Mech Therapies. (2019) pp:347–64. doi: 10.1007/978-981-13-8871-2_16
124. Budi EH, Duan D, Derynck R. Transforming growth factor-β receptors and smads: regulatory complexity and functional versatility. Trends Cell Biol. (2017) 27:658–72. doi: 10.1016/j.tcb.2017.04.005
125. Jin Y, Shi Y, Zou Y, Miao C, Sun B, Li C. Fenugreek prevents the development of STZ-induced diabetic nephropathy in a rat model of diabetes. Evidence-Based complementary Altern medicine: eCAM. (2014) 2014:259368. doi: 10.1155/ecam.v2014.1
126. Ibrahim S, Rashed L. Estimation of transforming growth factor-beta 1 as a marker of renal injury in type II diabetes mellitus. Saudi Med J. (2007) 28:519–23. doi: 10.1016/j.revmed.2006.12.012
127. Garud MS, Kulkarni YA. Attenuation of renal damage in type I diabetic rats by umbelliferone – a coumarin derivative. Pharmacol Rep. (2017) 69:1263–9. doi: 10.1016/j.pharep.2017.06.014
128. Zhang Y, Li Z, Wu H, Wang J, Zhang S. Esculetin alleviates murine lupus nephritis by inhibiting complement activation and enhancing Nrf2 signaling pathway. J Ethnopharmacology. (2022) 288:115004. doi: 10.1016/j.jep.2022.115004
129. Surse VM, Gupta J, Tikoo K. Esculetin induced changes in Mmp13 and Bmp6 gene expression and histone H3 modifications attenuate development of glomerulosclerosis in diabetic rats. J Mol Endocrinol. (2011) 46:245–54. doi: 10.1530/JME-10-0154
130. Sen Z, Weida W, Jie M, Li S, Dongming Z, Xiaoguang C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. (2019) 57:385–95. doi: 10.1016/j.phymed.2018.12.045
131. Efferth T, Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Medicinal Res Rev. (2021) 41:3023–61. doi: 10.1002/med.21842
132. Ding S, Lu G, Wang B, Xiang J, Hu C, Lin Z, et al. Astilbin activates the reactive oxidative species/PPARγ pathway to suppress effector CD4+ T cell activities via direct binding with cytochrome P450 1B1. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.848957
133. Zou S, Shen X, Tang Y, Fu Z, Zheng Q, Wang Q. Astilbin suppresses acute heart allograft rejection by inhibiting maturation and function of dendritic cells in mice. Transplant Proc. (2010) 42:3798–802. doi: 10.1016/j.transproceed.2010.06.031
134. Di T-T, Ruan Z-T, Zhao J-X, Wang Y, Liu X, Wang Y, et al. Astilbin inhibits Th17 cell differentiation and ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via Jak3/Stat3 signaling pathway. Int Immunopharmacol. (2016) 32:32–8. doi: 10.1016/j.intimp.2015.12.035
135. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. (2011) 21:103–15. doi: 10.1038/cr.2010.178
136. Chen Q, Zhou W, Huang Y, Tian Y, Wong SY, Lam WK, et al. Umbelliferone and scopoletin target tyrosine kinases on fibroblast-like synoviocytes to block NF-κB signaling to combat rheumatoid arthritis. Front Pharmacol. (2022) 13. doi: 10.3389/fphar.2022.946210
137. Wu G, Nie W, Wang Q, Hao Y, Gong S, Zheng Y, et al. Umbelliferone ameliorates complete freund adjuvant–induced arthritis via reduction of NF-κB signaling pathway in osteoclast differentiation. Inflammation. (2021) 44:1315–29. doi: 10.1007/s10753-021-01418-x
138. Zhang F, Zhang Y, Yang T, Ye Z-Q, Tian J, Fang H-R, et al. Scopoletin suppresses activation of dendritic cells and pathogenesis of experimental autoimmune encephalomyelitis by inhibiting NF-κB signaling. Front Pharmacol. (2019) 10:863. doi: 10.3389/fphar.2019.00863
139. Peng S, Cheng L, Wu Q, Li Y, Ran L, Wang W, et al. A modified hyaluronic acid–based dissolving microneedle loaded with daphnetin improved the treatment of psoriasis. Front Bioengineering Biotechnol. (2022) 10. doi: 10.3389/fbioe.2022.900274
140. Wang D, Lu Z, Zhang H, Jin SF, Yang H, Li YM, et al. Daphnetin alleviates experimental autoimmune encephalomyelitis via regulating dendritic cell activity. CNS Neurosci Ther. (2016) 22:558–67. doi: 10.1111/cns.2016.22.issue-7
141. Li M, Shi X, Chen F, Hao F. Daphnetin inhibits inflammation in the NZB/W F1 systemic lupus erythematosus murine model via inhibition of NF-κB activity. Exp Ther Med. (2017) 13:455–60. doi: 10.3892/etm.2016.3971
142. Shu C, Chen J, Lv M, Xi Y, Zheng J, Xu X. Plumbagin relieves rheumatoid arthritis through nuclear factor kappa-B (NF-κB) pathway. Bioengineered. (2022) 13:13632–42. doi: 10.1080/21655979.2022.2081756
143. Hahn WH, Cho BS, Kim SD, Kim SK, Kang S. Interleukin-1 cluster gene polymorphisms in childhood IgA nephropathy. Pediatr Nephrol. (2009) 24:1329–36. doi: 10.1007/s00467-009-1146-5
144. Shi B, Ni Z, Cao L, Zhou M, Mou S, Wang Q, et al. Serum IL-18 is closely associated with renal tubulointerstitial injury and predicts renal prognosis in IgA nephropathy. Mediators Inflammation. (2012) 2012:1–9. doi: 10.1155/2012/728417
145. Schroder K, Tschopp J. The inflammasomes. Cell. (2010) 140:821–32. doi: 10.1016/j.cell.2010.01.040
146. Chen M, Wang H, Chen W, Meng G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int Immunopharmacol. (2011) 11:549–54. doi: 10.1016/j.intimp.2010.11.025
147. Moura IC, Hua K-F, Yang S-M, Kao T-Y, Chang J-M, Chen H-L, et al. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-κB/NLRP3 pathway. PloS One. (2013) 8:e77794. doi: 10.1371/journal.pone.0077794
148. Chen Y, Zhang Q, Liu H, Lu C, Liang C-L, Qiu F, et al. Esculetin ameliorates psoriasis-like skin disease in mice by inducing CD4+Foxp3+ Regulatory T cells. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.02092
149. Liu M, Zhang G, Zheng C, Song M, Liu F, Huang X, et al. Activating the pregnane X receptor by imperatorin attenuates dextran sulphate sodium-induced colitis in mice. Br J Pharmacol. (2018) 175:3563–80. doi: 10.1111/bph.v175.17
150. Park C, Jeong J-W, Lee D-S, Yim M-J, Lee J, Han M, et al. Sargassum serratifolium extract attenuates interleukin-1β-induced oxidative stress and inflammatory response in chondrocytes by suppressing the activation of NF-κB, p38 MAPK, and PI3K/Akt. Int J Mol Sci. (2018) 19:2308. doi: 10.3390/ijms19082308
151. Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. (2020) 28:400–9. doi: 10.1016/j.joca.2020.02.027
152. Vara JÁ.F, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. (2004) 30:193–204. doi: 10.1016/j.ctrv.2003.07.007
153. Ma T, Liu P, Wei J, Zhao M, Yao X, Luo X, et al. Imperatorin alleviated endometriosis by inhibiting the activation of PI3K/Akt/NF-κB pathway in rats. Life Sci. (2021) 274:119291. doi: 10.1016/j.lfs.2021.119291
154. Kwak SC, Baek JM, Lee CH, Yoon K-H, Lee MS, Kim J-Y. Umbelliferone prevents lipopolysaccharide-induced bone loss and suppresses RANKL-induced osteoclastogenesis by attenuating Akt-c-Fos-NFATc1 signaling. Int J Biol Sci. (2019) 15:2427–37. doi: 10.7150/ijbs.28609
155. Kiani BH, Kayani WK, Khayam AU, Dilshad E, Ismail H, Mirza B. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep. (2020) 47:6321–36. doi: 10.1007/s11033-020-05669-z
156. Dong Y, Chen H, Gao J, Liu Y, Li J, Wang J. Bioactive ingredients in chinese herbal medicines that target non-coding RNAs: promising new choices for disease treatment. Front Pharmacol. (2019) 10. doi: 10.3389/fphar.2019.00515
157. Chen S, Wang Y, Zhang L, Han Y, Liang C, Wang S, et al. Therapeutic effects of columbianadin from Angelicae Pubescentis radix on the progression of collagen-induced rheumatoid arthritis by regulating inflammation and oxidative stress. J Ethnopharmacology. (2023) 316:116727. doi: 10.1016/j.jep.2023.116727
158. Luo M, Luo Y. Imperatorin relieved ulcerative colitis by regulating the Nrf-2/ARE/HO-1 pathway in rats. Inflammation. (2020) 44:558–69. doi: 10.1007/s10753-020-01353-3
159. Wang C, Liu J, Su H, Zhou Y, Feng G. The effect of 7-hydroxycoumarin on dextran sulfate sodium-induced ulcerative colitis. Basic Clin Pharmacol Toxicol. (2019) 124:749–9. doi: 10.1111/bcpt.2019.124.issue-6
160. Kadakol A, Malek V, Goru SK, Pandey A, Bagal S, Gaikwad AB. Esculetin attenuates alterations in Ang II and acetylcholine mediated vascular reactivity associated with hyperinsulinemia and hyperglycemia. Biochem Biophys Res Commun. (2015) 461:342–7. doi: 10.1016/j.bbrc.2015.04.036
161. Hu H-H, Cao G, Wu X-Q, Vaziri ND, Zhao Y-Y. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. (2020) 60:101063. doi: 10.1016/j.arr.2020.101063
162. Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. (2017) 169:985–99. doi: 10.1016/j.cell.2017.05.016
163. Cai L, Zhou M-Y, Hu S, Liu F-Y, Wang M-Q, Wang X-H, et al. Umbelliferone inhibits migration, invasion and inflammation of rheumatoid arthritis fibroblast-like synoviocytes and relieves adjuvant-induced arthritis in rats by blockade of Wnt/β-Catenin signaling pathway. Am J Chin Med. (2022) 50:1945–62. doi: 10.1142/S0192415X22500835
164. Cai L, Zong P, Zhou M-Y, Liu F-Y, Meng B, Liu M-M, et al. 7-Hydroxycoumarin mitigates the severity of collagen-induced arthritis in rats by inhibiting proliferation and inducing apoptosis of fibroblast-like synoviocytes via suppression of Wnt/β-catenin signaling pathway. Phytomedicine. (2022) 94:153841. doi: 10.1016/j.phymed.2021.153841
165. Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. (2020) 80:106210. doi: 10.1016/j.intimp.2020.106210
166. O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. (2015) 66:311–28. doi: 10.1146/annurev-med-051113-024537
167. Li H-X, Zhao W, Shi Y, Li Y-N, Zhang L-S, Zhang H-Q, et al. Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumor Biol. (2015) 36:8671–8. doi: 10.1007/s13277-015-3534-8
168. Kim H-O. Development of JAK inhibitors for the treatment of immune-mediated diseases: kinase-targeted inhibitors and pseudokinase-targeted inhibitors. Arch Pharmacal Res. (2020) 43:1173–86. doi: 10.1007/s12272-020-01282-7
169. Zhou Q, Ren Q, Jiao L, Huang J, Yi J, Chen J, et al. The potential roles of JAK/STAT signaling in the progression of osteoarthritis. Front Endocrinol. (2022) 13. doi: 10.3389/fendo.2022.1069057
170. Tansey MG, Jia Y, Jing J, Bai Y, Li Z, Liu L, et al. Amelioration of experimental autoimmune encephalomyelitis by plumbagin through down-regulation of JAK-STAT and NF-κB signaling pathways. PloS One. (2011) 6:e27006. doi: 10.1371/journal.pone.0027006
171. He Z, Liu J, Liu Y. Daphnetin attenuates intestinal inflammation, oxidative stress, and apoptosis in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3 signaling pathway. Environ Toxicol. (2023) 38:2132–42. doi: 10.1002/tox.23837
172. Zhao L, Zhang M, Bai L, Zhao Y, Cai Z, Yung KKL, et al. Real-world PM2.5 exposure induces pathological injury and DNA damage associated with miRNAs and DNA methylation alteration in rat lungs. Environ Sci pollut Res. (2022) 29:28788–803. doi: 10.1007/s11356-021-17779-7
173. Yamayoshi A, Matsuyama Y, Kushida M, Kobori A, Murakami A. Novel photodynamic effect of a psoralen-conjugated oligonucleotide for the discrimination of the methylation of cytosine in DNA. Photochem Photobiol. (2014) 90:716–22. doi: 10.1111/php.2014.90.issue-3
174. Kadakol A, Goru SK, Malek V, Gaikwad AB. Esculetin ameliorates vascular perturbation by intervening in the occupancy of H2BK120Ub at At1, At2, Tgfβ1 and Mcp1 promoter gene in thoracic aorta of IR and T2D rats. Biomedicine Pharmacotherapy. (2017) 95:1461–8. doi: 10.1016/j.biopha.2017.09.067
175. Yao L, Yang Y, He G, Ou C, Wang L, Liu K. Global proteomics deciphered novel-function of osthole against pulmonary arterial hypertension. Sci Rep. (2018) 8:5556. doi: 10.1038/s41598-018-23775-8
176. Nadhan R, Vaman JV, Sengodan SK, Hemalatha SK, Chellappan N, Sadasivan S, et al. BRCA1 promoter hypermethylation in human placenta: a hidden link with β-hCG expression. Carcinogenesis. (2020) 41:611–24. doi: 10.1093/carcin/bgz117
177. Abdel-Wahab BA, Alkahtani SA, Alqahtani AA, Hassanein EHM. Umbelliferone ameliorates ulcerative colitis induced by acetic acid via modulation of TLR4/NF-κB-p65/iNOS and SIRT1/PPARγ signaling pathways in rats. Environ Sci pollut Res. (2022) 29:37644–59. doi: 10.1007/s11356-021-18252-1
178. Shu K, Kuang N, Zhang Z, Hu Z, Zhang Y, Fu Y, et al. Therapeutic effect of daphnetin on the autoimmune arthritis through demethylation of proapoptotic genes in synovial cells. J Trans Med. (2014) 12:287. doi: 10.1186/s12967-014-0287-x
179. Lin X, Chen J, Tao C, Luo L, He J, Wang Q. Osthole regulates N6-methyladenosine-modified TGM2 to inhibit the progression of rheumatoid arthritis and associated interstitial lung disease. MedComm. (2023) 4:e219. doi: 10.1002/mco2.v4.2
180. Ambros V. The functions of animal microRNAs. Nature. (2004) 431:350–5. doi: 10.1038/nature02871
181. Zhang W, Li Z, Dai Z, Chen S, Guo W, Wang Z, et al. GelMA hydrogel as a promising delivery system for osthole in the treatment of rheumatoid arthritis: targeting the miR-1224-3p/AGO1 axis. Int J Mol Sci. (2023) 24:13210. doi: 10.3390/ijms241713210
182. Mirandola L, Comi P, Cobos E, Martin Kast W, Chiriva-Internati M, Chiaramonte R. Notch-ing from T-cell to B-cell lymphoid Malignancies. Cancer Lett. (2011) 308:1–13. doi: 10.1016/j.canlet.2011.05.009
183. Garcia EG, Veloso A, Oliveira ML, Allen JR, Loontiens S, Brunson D, et al. PRL3 enhances T-cell acute lymphoblastic leukemia growth through suppressing T-cell signaling pathways and apoptosis. Leukemia. (2020) 35:679–90. doi: 10.1038/s41375-020-0937-3
184. Paiva RA, Sousa AGG, Ramos CV, Ávila M, Lilue J, Paixão T, et al. Self-renewal of double-negative 3 early thymocytes enables thymus autonomy but compromises the β-selection checkpoint. Cell Rep. (2021) 35:108967. doi: 10.1016/j.celrep.2021.108967
185. Marquez-Exposito L, Rodrigues-Diez RR, Rayego-Mateos S, Fierro-Fernandez M, Rodrigues-Diez R, Orejudo M, et al. Deletion of delta-like 1 homologue accelerates renal inflammation by modulating the Th17 immune response. FASEB J. (2020) 35:e21213. doi: 10.1096/fj.201903131R
186. Jiang J, Sheng Y, Zheng Z, Qin F, Jiang B. Agrimonolide mitigated DSS-induced colitis by modulating the balance between Treg and Th17 cells through the suppression of the Notch and JAK2/STAT3 signaling pathways. Heliyon. (2024) 10:e33803. doi: 10.1016/j.heliyon.2024.e33803
187. Ballester A, Guijarro A, Bravo B, Hernández J, Murillas R, Gallego MI, et al. Hedgehog signalling modulates immune response and protects against experimental autoimmune encephalomyelitis. Int J Mol Sci. (2022) 23:3171. doi: 10.3390/ijms23063171
188. Tam A, Osei ET, Cheung CY, Hughes M, Yang CX, McNagny KM, et al. Hedgehog signaling as a therapeutic target for airway remodeling and inflammation in allergic asthma. Cells. (2022) 11:3016. doi: 10.3390/cells11193016
Keywords: coumarins, autoimmune diseases, anti-inflammatory, MAPKs, NF-κB, epigenetic modulation
Citation: Li Y, Wang G-q and Li Y-b (2024) Therapeutic potential of natural coumarins in autoimmune diseases with underlying mechanisms. Front. Immunol. 15:1432846. doi: 10.3389/fimmu.2024.1432846
Received: 14 May 2024; Accepted: 14 October 2024;
Published: 31 October 2024.
Edited by:
Feng Dong, AbbVie, United StatesReviewed by:
Saeed Mohammadi, Golestan University of Medical Sciences, IranVirginia Flores-Morales, Universidad Autonoma de Zacatecas, Mexico
Copyright © 2024 Li, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-bin Li, MTM4NjQwMDY5MzNAMTYzLmNvbQ==; Guan-qing Wang, d2FuZ2d1YW5xaW5nMjAyMUAxMjYuY29t
 Yan Li1,2
Yan Li1,2 Guan-qing Wang
Guan-qing Wang Yan-bin Li
Yan-bin Li