- 1Chinese Medicine Department, Songnan Town Community Health Service Center, Shanghai, China
- 2Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Institute of Dermatology, Shanghai Academy of Traditional Chinese Medicine, Shanghai, China
Background: The treatment of vitiligo is complex, and providing guidance based on lifestyle habits is a good option that has not been summarized or analyzed.
Objective: To elucidate the relationship between vitiligo and lifestyle factors.
Methods: Four databases (PubMed, Embase, Cochrane, and China National Knowledge Internet) were searched for articles published between 1980 and December 2022. Keywords such as smoking, drinking, exercise, diet, and sleep were used.
Results: Based on the search strategy, 875 relevant studies were retrieved, and 73 were included in this study, of which 41 studies with 8,542 patients with vitiligo were included in the meta-analysis. Vitamin C [mean difference (MD), −0.342; 95% confidence interval (CI), −1.090–0.407; p >0.05), folic acid (MD, −1.463; 95% CI, −7.133–4.208; p >0.05), and selenium (MD, 0.350; 95% CI, −0.687–1.387; p >0.05) levels did not differ between the groups. Vitamin E (MD, −1.408; 95% CI, −2.611–−0.206; p <0.05), vitamin B12 (MD, −0.951; 95% CI, −1.672–−0.275; p <0.05), copper (MD, −0.719; 95% CI, −1.185–−0.252, p <0.005), and zinc (MD, −0.642; 95% CI, −0.731–−0.554; p <0.001) levels were lower in the vitiligo group than in the control group. The serum iron level of the vitiligo group was significantly higher than that of the control group (MD, 1.181; 95% CI, 0.390–1.972; p <0.005). Finally, more participants in the vitiligo group smoked and drank alcohol than those in the control group.
Limitations: Most studies are from Eastern countries; thus, extrapolating these results to Western populations is questionable. The significant heterogeneity may be attributed to the different stages, types, duration, center settings, population registries, etc., which seriously impair the validity of the results.
Conclusions: Patients with vitiligo should reduce smoking and alcohol consumption and take appropriate vitamin E, B12, copper, and zinc supplements. However, vitamin C, vitamin D, selenium, iron, and folic acid supplements are unnecessary. Moreover, they should consider sun protection and avoid permanent hair dye use. Patients with vitiligo may experience sleep disturbances and sexual dysfunction, and these patients should seek help from a specialist if necessary.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier CRD42023480757.
Introduction
Vitiligo is an autoimmune skin disease associated with features such as chronic loss of melanocyte function and number and the formation of white patches or spots on the skin that impact one’s esthetic appearance. The disease affects approximately 0.1%–2% of people worldwide and profoundly impact patients’ quality of life.
Treatment options for vitiligo remain limited (1), as its pathogenesis remains unclear and may be related to oxidative stress, genetic, and environmental factors. Researchers have classified this disease as an autoimmune disease (2–5). Vitiligo is currently treated with narrow-band ultraviolet (UV) B-rays (UVB), 308-nm excimer lasers, calcium-regulated phosphatase inhibitors, glucocorticoids, Janus kinase inhibitors, surgical treatments, cosmetic covers, and others (6, 7). Owing to the limited treatment options for vitiligo, adopting a lifestyle approach to manage vitiligo symptoms and progression may be necessary. Although many studies have reported the influence of various lifestyle factors on vitiligo, no comprehensive literature review currently summarizes these factors to guide patients in their lifestyle choices. Therefore, in this study, we extensively reviewed the literature to summarize these findings for the first time. Our aim was to provide valuable information on vitiligo treatment and empower patients with better insights into managing their condition.
In this systematic review, we analyzed smoking, alcohol consumption, diet, exercise, light exposure, height, sleep, and permanent hair dye use data to provide more targeted, effective, and safe life coaching for patients suffering from this disfiguring disease. The summary of these studies may serve as a valuable resource for guiding future research in the field of vitiligo.
Materials and methods
We performed a systematic review and meta-analysis to assess the association between vitiligo and lifestyle. This study was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and registered with PROSPERO (CRD42023480757), an international registry of prospective systematic evaluations https://www.crd.york.ac.uk/PROSPERO/(Supplementary Tables S1, S2).
Data sources and searches
To explore the relationship between lifestyle and vitiligo, three reviewers (Xin Liang, Fei Guo, and Xin Li) systematically searched relevant publications from the EMBASE, PubMed, and Cochrane library electronic databases and the China National Knowledge Internet using the following keywords: “vitiligo,” “sports,” ‘‘smoking,’’ “alcohol consumption,” “insomnia,” “diet,” “vitamin C,” “vitamin D,” “vitamin E,” “vitamin B12,” “vitamin A,” “folic acid,” “zinc,” “copper,” “selenium,” “iron,” and “sunshine.” Our comprehensive search encompassed articles written in English, spanning from January 1980 to December 2022. Additionally, the references of the retrieved articles were manually scanned.
Inclusion and exclusion criteria
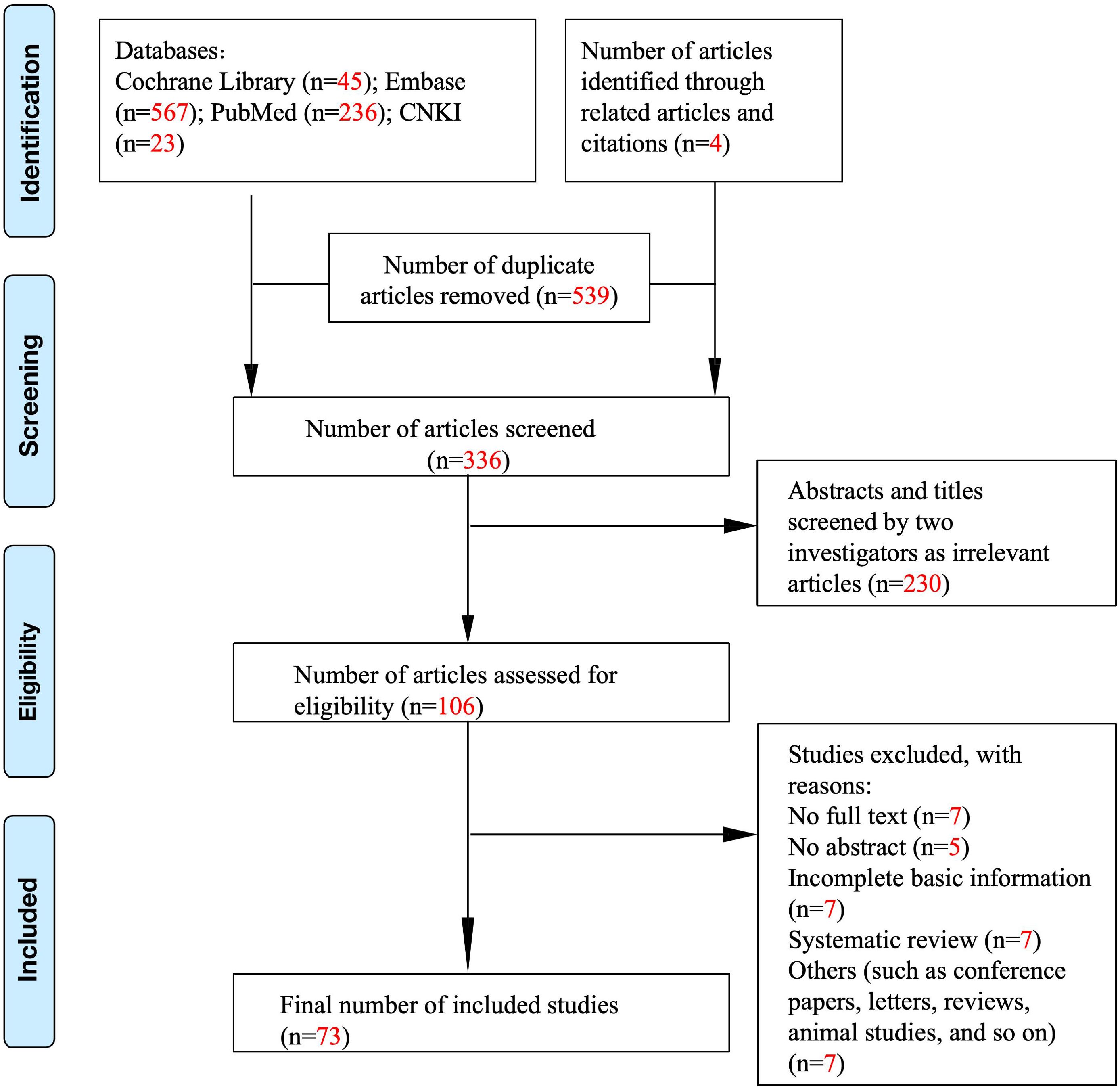
Studies were selected based on the following criteria: (1) randomized controlled trials and observational studies, (2) human studies only, (3) studies describing the relationship between vitiligo and lifestyle habits, and (4) studies assessing the impact of lifestyle habits on the course of vitiligo. The exclusion criteria were as follows: (1) animal studies and (2) inability to contact the corresponding author for data. Initially, 706 publications were included (Figure 1). After a manual review of the reference lists of the included studies, three additional articles were identified. Then, these studies were carefully reviewed. Finally, 73 studies were included in this article. Figure 1 shows a flowchart of the screening process.
Data extraction and quality assessment
Three reviewers, including the first author, independently checked the data within each selected study against a predetermined data extraction form, encompassing study, participant, and outcome characteristics. The Newcastle–Ottawa scale (8) was used to assess the study quality.
Data synthesis and analysis
All analyses were performed using Stata software. The weighted mean difference/standardized mean difference and corresponding 95% confidence intervals (CIs) were aggregated to assess the association between serum vitamin E, C, zinc, copper, B12, and folic acid levels and vitiligo. Heterogeneity was tested using the I2 statistic, with I2 >50% considered highly heterogeneous. A random-effects model was employed owing to the observed heterogeneity, and Egger’s test was used to assess publication bias. Finally, a sensitivity analysis was performed to explore the impact of potential sources of heterogeneity (Supplementary Figures S1-S4).
Results
Search results
A total of 875 articles were retrieved from PubMed, Cochrane Library, Embase databases, and China National Knowledge Infrastructure (CNKI) (Figure 1). In total, 539 duplicate items were excluded from further assessment. After screening the abstracts and titles, 106 studies remained. After a comprehensive review of the selected articles, 33 studies were excluded, and 73 studies were included in this systematic review, of which 41 studies with a total of 8,542 patients with vitiligo were finally included in the meta-analysis (Tables 1, 2).

Table 1. Characteristics of the included studies and the Newcastle–Ottawa Scale (NOS) Quality Assessment Table.

Table 2. Characteristics of the studies included in the meta-analysis and the Newcastle–Ottawa Scale (NOS) Quality Assessment Table.
Smoking
A meta-analysis of five studies (60–64) involving 552 patients indicated that the number of smokers in the vitiligo group was higher than in the control group [mean difference (MD), 1.240; 95% CI, 1.057–1.455; p <0.05; Table 3; Supplementary Figure S5). Additionally, a cohort study (15) involving 22,991,641 patients showed that smoking has an inhibitory effect on the development of vitiligo.
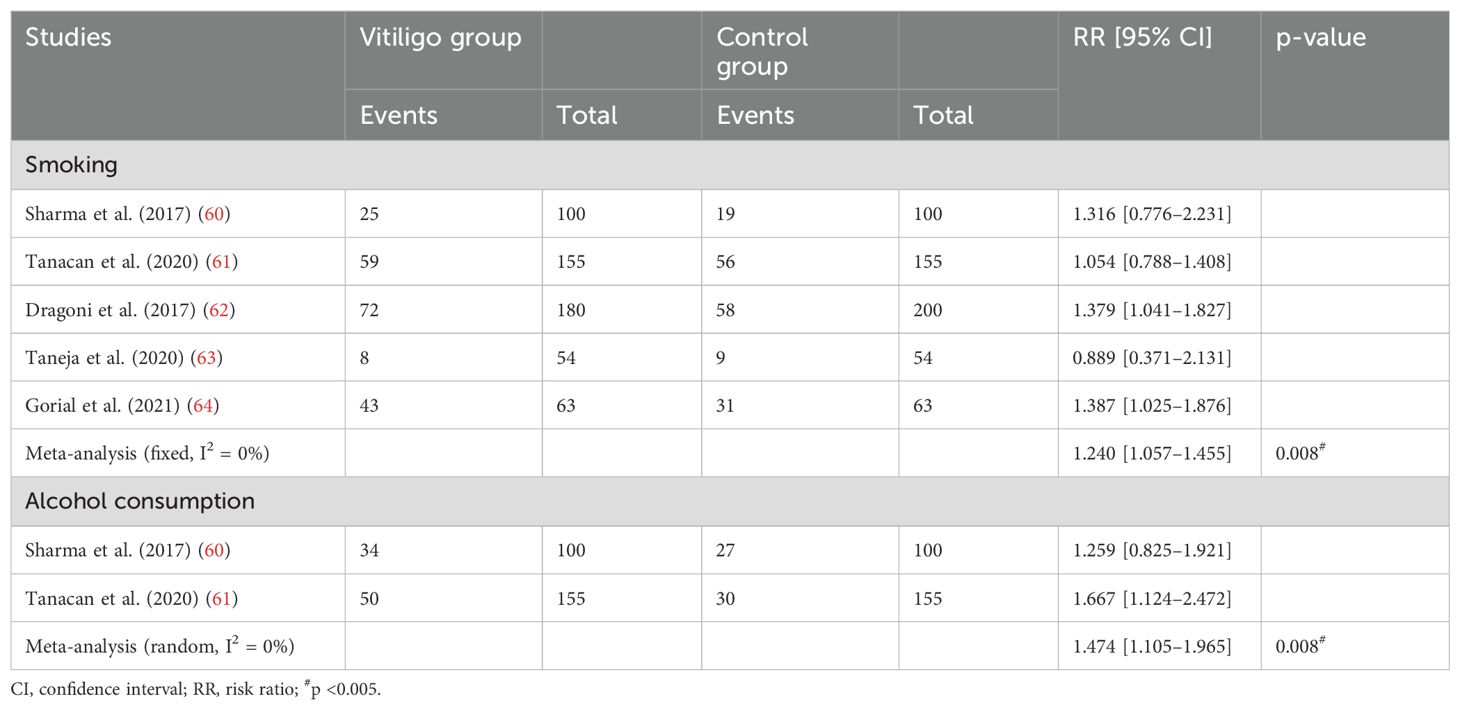
Table 3. The number of people with smoking and alcohol consumption habits between the vitiligo and control groups.
Alcohol consumption
We conducted a meta-analysis of two studies (60, 61) involving 255 patients, and the results showed that there was a significantly higher prevalence of alcohol dependence among vitiligo patients compared to the control group (MD, 1.474; 95% CI, 1.105–1.965; p <0.005; Table 4; Supplementary Figure S6).
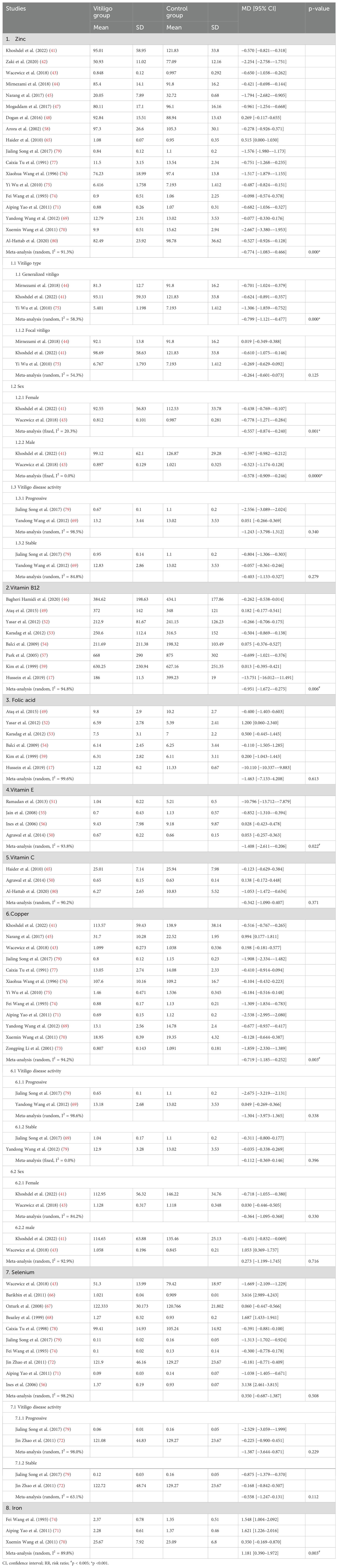
Table 4. Serum zinc, vitamin B12, folic acid, vitamin E, and vitamin C levels between the vitiligo and control groups.
Diet
Vitamin C
A meta-analysis of three studies (50, 65, 80) showed no significant differences in serum vitamin C levels between patients with vitiligo and controls (MD, 0.342; 95% CI, −1.090–0.407; p >0.05; Table 4; Supplementary Figure S7). Another study (32) concluded that patients with vitiligo had significantly lower vitamin C levels than the controls.
Vitamin B12 and folic acid
One study (9) suggested combining folic acid and vitamin B12 supplementation with sunlight-induced repigmentation to be more effective than vitamin or sunlight exposure alone. In addition, two case–control studies (17, 19) reported that serum folic acid and vitamin B12 levels were significantly lower in patients with vitiligo than in controls.
A cross-sectional study (20) and a cohort study (30) showed that elevated serum homocysteine (Hcy) and reduced serum vitamin B12 levels were significantly associated with vitiligo. However, another two studies (23, 31) reported no significant differences in vitamin B12 and folic acid levels between patients with vitiligo and controls. In addition, one study (25) showed that patients with vitiligo had no significant difference in Hcy and vitamin B12 levels compared to that in controls. In contrast, those with vitiligo had higher folic acid levels.
An evaluation of 33 patients treated for vitiligo did not reveal an association between vitamin B12 levels and improved repigmentation (27). Tjioe et al. (36) reported that adding vitamin B12 and folic acid did not provide any therapeutic benefit in treating patients with vitiligo. Memon et al. (39) concluded that the serum levels of vitamin B12 and folic acid significantly affected the course of vitiligo.
A meta-analysis of eight studies (17, 46, 49, 52–54, 57, 59) showed that vitamin B12 levels were significantly lower in patients with vitiligo than in controls (MD, −0.951; 95% CI, −1.672–−0.275; p <0.05; Table 4; Supplementary Figure S8). In contrast, a meta-analysis of six studies (17, 49, 52–54, 59) showed no significant difference in the folate levels between patients with vitiligo and controls (MD, −1.463; 95% CI, −7.133–4.208; p >0.05; Table 4; Supplementary Figure S9).
Vitamin D
A randomized controlled trial (18) showed that vitamin D treatment resulted in a more significant reduction in the extent and size of lesions in patients with vitiligo than in controls, suggesting that vitamin D plays a role in preventing the progression of active vitiligo. Another study (28) concluded that high-dose vitamin D3 therapy (35, 000 IU once daily) is safe and effective for patients with vitiligo. A retrospective cohort study (38) reported no significant association between vitamin D and any feature or treatment of vitiligo. Among the studies, two research papers (18, 28) that found vitamin D treatment effective for vitiligo included a total of 76 patients. Meanwhile, one cohort study (38) that deemed it ineffective included 297 patients.
Vitamin E
Patients with vitiligo who received oral antioxidants (Phyllanthus emblica, vitamin E, and carotenoids) had significantly milder repigmentation of the head, neck, and trunk, along with a higher level of disease stability, compared to the corresponding patients who did not receive oral antioxidants (24).
A study (26) evaluated the long-term treatment of children with facial vitiligo. The study employed a combination of approaches, including nutrition education, vitamin E, folic acid, multivitamin intake, and antioxidant cosmetics, as the primary treatment. Additionally, conventional therapies were used as part of the treatment protocol. A total of 91% of patients demonstrated lesion improvement.
A case–control study (32) concluded that the serum vitamin E levels were significantly lower in patients with vitiligo than in controls. In contrast, two other case–control studies (34, 37) reported no significant difference in blood vitamin E levels in patients with vitiligo compared to age-matched healthy controls. Akyol et al. (35) concluded that vitamin E prevented oxidative distress caused by Psoralen UVA rays (PUVA) treatment; however, it did not affect the clinical improvement of vitiligo lesions. A meta-analysis of four studies (50, 51, 55, 56) showed that the serum VE levels were significantly lower in patients with vitiligo than in controls (MD, −1.408; 95% CI, −2.611–−0.206; p <0.05; Table 4; Supplementary Figure S10).
Zinc
A retrospective cohort study reported no significant association between zinc and any feature or treatment associated with vitiligo (38). A randomized controlled trial showed that although the group that received oral zinc sulfate combined with topical corticosteroids responded better than the group that received topical corticosteroids alone, there was no statistical difference (29). Another study suggested that the average zinc level in the serum of patients with vitiligo was significantly lower (14). The meta-analysis of 18 studies (41–45, 47, 48, 58, 65, 69–71, 74–77, 79, 80) showed that the serum zinc levels were significantly lower in patients with vitiligo than in controls (MD, −0.774; 95% CI, −1.083–−0.466; p <0.001; Table 4; Supplementary Figure S11). Three studies showed that the serum zinc levels were significantly lower in patients with generalized vitiligo than in healthy controls (MD, −0.799; 95% CI, −1.121–−0.477; p <0.001; Table 4; Supplementary Figure S12), while the serum zinc levels in patients with localized vitiligo were not different from those in the control group (MD, −0.264; 95% CI, −0.601–−0.073; p >0.05; Table 4; Supplementary Figure S13). Both female (MD, −0.557; 95% CI, −0.874–−0.240; p <0.005; Table 4; Supplementary Figure S14) and male (MD, −0.578; 95% CI, −0.909–−0.246; p <0.001; Table 4; Supplementary Figure S15) patients with vitiligo had significantly lower serum zinc levels than those of healthy controls. However, the opposite was true for the serum zinc levels in patients with progressive (MD, −1.243; 95% CI, −3.789–1.312; p >0.05; Table 4; Supplementary Figure S16) and stable (MD, −0.403; 95% CI, −1.133–0.327; p >0.05; Table 4; Supplementary Figure S17) vitiligo.
Incompatible diet
Incompatible diets refer to incorrect combinations of food components in formulations, insufficient or excessive processing, inappropriate consumption amounts, and/or eating at incorrect times of the day and/or in the wrong seasons (11). A case–control study (11) concluded that the mean composite scores of two questionnaires for assessing incompatible dietary habits in patients with vitiligo were similar to those of controls. Additionally, a study (22) revealed that patients with vitiligo must avoid incompatible foods, as these are the most potent causative factors for vitiligo.
Total fat intake
A previous study (16) highlighted that the quantity of total fat consumed in the diet had a greater impact on the risk of vitiligo compared to specific subclasses of fat. The study suggested that a high-fat diet increases the risk of developing vitiligo.
Copper
The meta-analysis of 12 studies (41, 43, 45, 69–71, 73–77, 79) showed that the serum copper levels were significantly lower in patients with vitiligo than in controls (MD, −0.719; 95% CI, −1.185–−0.252; p <0.005; Table 4; Supplementary Figure S18). Whether progressive (MD, −1.304; 95% CI, −3.973–1.365; p >0.05; Table 4; Supplementary Figure S19) or stable (MD, −0.112; 95% CI, −0.369–0.146; p >0.05; Table 4; Supplementary Figure S20), and male (MD, 0.273; 95% CI, −1.199–1.745; p >0.05; Table 4; Supplementary Figure S21) or female (MD, −0.364; 95% CI, −1.095–0.368; p >0.05; Table 4; Supplementary Figure S22) patients, there were no significant differences in the serum copper levels in patients with vitiligo compared to those in controls.
Selenium
The meta-analysis of 10 studies (43, 56, 66–68, 71, 72, 74, 78, 79) indicated no significant difference in the serum selenium levels between patients with vitiligo and controls (MD, 0.350; 95% CI, −0.687–1.387; p >0.05; Table 4; Supplementary Figure S23). No significant difference was observed in the serum selenium level between the control group and the patients with progressive (MD, −1.387; 95% CI, −3.644–0.871; p >0.05; Table 4; Supplementary Figure S24) and stable (MD, −0.558; 95% CI, −1.247–0.131; p >0.05; Table 4, Supplementary Figure S25) vitiligo.
Iron
The meta-analysis of three studies (70, 71, 74) showed that serum iron levels were significantly higher in patients with vitiligo than in controls (MD, 1.209; 95% CI, 0.403–2.014; p <0.0055; Table 4; Supplementary Figure S26).
Exercise
No studies have examined the relationship between physical exercise and disease progression in patients with vitiligo.
Tanning ability
Women who had a painful burn/blistering skin reaction after 2 h of sun exposure as children/adolescents had a higher risk of vitiligo than those with no reaction or only redness after sun exposure. Women with strong tanning abilities had a higher risk of developing vitiligo compared to those without the ability to tan (10).
Sleeping
Two studies (12, 33) have examined the relationship between vitiligo and sleep disorders. One study indicated that patients with vitiligo reported significantly more sleep disturbances compared to that in controls, especially sleepwalking, nocturnal enuresis, nocturnal hallucinations, sleep fears, and nightmares. In addition, patients with vitiligo had statistically significant levels of nightmares, nocturnal hallucinations, and sleepwalking compared to those with other skin diseases; however, they did not have statistically significant levels of sleep phobias or nocturnal enuresis. Another study indicated that facial and neck vitiligo, vitiligo progression, and oral glucocorticoid use were risk factors for insomnia in patients with vitiligo.
Permanent hair dyes
The previous use of permanent hair dyes increased the risk of vitiligo. The association with vitiligo was more pronounced in those who had used hair dyes for a longer duration, initiated their use before the age of 30 years, and had a longer period of usage since their first use (21).
Height
A nationwide cohort study showed that height is positively associated with the risk of vitiligo in Korean adults. This association was stronger in the older adult population (age ≥65 years) (13).
Sexual dysfunction
In our previous study (96), we observed a higher risk of sexual dysfunction in patients with vitiligo, with the relationship being more prominent in women than in men.
Discussion
The importance of reviewing lifestyle habits (including smoking, alcohol consumption, diet, exercise, and sleep) lies in the provision of adjunctive measures for the treatment of vitiligo. Current treatments for vitiligo are abundant, with traditional approaches focusing on the autoimmune hypothesis through immunomodulatory and anti-inflammatory approaches. However, in recent years, new therapies, such as molecular targeted therapy, have become available (5–7). Despite the availability of various treatments, a few patients continue to experience unsatisfactory results with conventional therapies, and the effectiveness of newer treatments remains uncertain. This study carries several significant implications in this context.
We reviewed the smoking and alcohol consumption data of patients with vitiligo and observed that more patients with vitiligo smoked and drank alcohol compared to that in controls, which is contrary to the results of another cohort study (15), which suggested that smoking had a suppressive effect on the development of vitiligo. To date, there are no studies demonstrating the effect of smoking and alcohol consumption on the development of vitiligo.
Regarding diet, we focused on vitamins C, D, E, and B12, folic acid, zinc, copper, iron, selenium, incompatible diets, and total fatty acids. We observed no significant difference in the serum vitamin C levels between patients with vitiligo and controls, which refutes statements recommending vitamin C supplementation in patients with vitiligo. In addition, although the oxidative stress theory has been mentioned in studies on the pathogenesis of vitiligo, it is worth noting that vitamin C, as an antioxidant, may exert inhibitory effects on tyrosinase activity by inducing cytoplasmic acidification (95). This mechanism may contribute to a reduction in melanin content, offering a potential avenue for therapeutic intervention in vitiligo (58).
The results of this meta-analysis showed that vitamin B12 levels were low in patients with vitiligo. In contrast, the folic acid levels did not significantly differ from those of the controls. The folic acid and vitamin B12 levels are associated with homocysteine synthesis, inhibiting tyrosinase and reducing melanin production (81). Therefore, we recommend that vitamin B12 supplementation inhibits tyrosinase and reduces associated effects.
Decreased vitamin D levels play a role in the development of vitiligo by affecting Th1- and Th17-related immune responses (82). In contrast, studies have reported no role of circulating vitamin D in the pathogenesis of vitiligo. Although studies have suggested that vitamin D has a therapeutic effect on vitiligo, not all of these were single-drug studies, a few of these combined vitamin D with other therapeutic methods; therefore, the evidence for this is insufficient.
Our results suggested that vitamin E has a therapeutic effect on vitiligo; however, further studies are needed to confirm this hypothesis. Vitamin E, an antioxidant, inhibits tyrosinase activity, and its derivatives inhibit melanogenesis in epidermal melanocytes in vitro. In addition, vitamin E increases the expression of endosomal docking/fusion proteins, and melanosomes can be degraded within the lysosomal compartment by docking with lysosomes, decreasing the number of melanosomes (83, 84). Although the results of our meta-analysis identified low serum vitamin E levels in patients with vitiligo, these were inconclusive owing to the limited sample sizes and the observational nature of the included studies. Thus, whether vitamin E supplementation is beneficial for vitiligo needs to be confirmed in additional studies with larger sample sizes. Vitamin E supplementation is only appropriate for patients with vitamin E deficiency.
Copper, one of the trace elements, is a cofactor involved in the synthesis of melanin by tyrosinase and in the biosynthesis of superoxide dismutase, which plays an important role in protecting cells from oxidative stress (85, 86). Our study showed that patients with vitiligo had significantly lower serum copper levels than those of healthy controls. This indicates that copper may be involved in the pathogenesis of vitiligo, and further studies are needed to explain this.
Selenium is an essential immune nutrient for the human body, and the organic forms of selenium naturally exist in the human body: selenocysteine and selenoprotein. Glutathione peroxidase is the main selenium protein in the body, which helps control the excess production of free radicals at inflammatory sites (87). Our study did not find a difference in serum selenium between patients with vitiligo and healthy individuals. In the future, we can study the lesion site of vitiligo and observe whether the results have changed.
Toxic damage to melanocytes by redox-generated free radicals is one of the doctrines of the pathogenesis of vitiligo. Iron, an essential element for many important cellular functions in all organisms, catalyzes the formation of potentially toxic free radicals (88). Our research supports this view, although the results of two studies (31, 89) are diametrically opposed to ours.
Zinc is an antioxidant that inhibits apoptosis and may inhibit the apoptosis of melanocytes. Moreover, zinc plays an important role in the final stage of melanin formation. Therefore, zinc may have an important effect on vitiligo (43). The results of this meta-analysis showed low serum zinc levels in patients with vitiligo.
The association between incompatible diets and vitiligo remains controversial and poorly understood. High-fat diets are thought to increase the risk of vitiligo, and diets high in saturated fats have deleterious effects on macrophage phagocytosis and natural killer cell activity in autoimmune diseases. Moreover, it has been well established that high-fat diets can contribute to the development of various diseases and have detrimental effects on the lifespan of animals (16).
The benefits of gluten-free diets for vitiligo have been reported in only two cases. Although chronic physical exercise can change the balance of inflammation to an anti-inflammatory state and improve the structure and function of the endogenous antioxidant system and mitochondria (90), thus improving the clinical symptoms and quality of life of patients with vitiligo, observational and experimental studies are still lacking. Moreover, vitiligo has been consistently linked to sleep disorders, and extensive research indicates the presence of a cyclic relationship between vitiligo and depression, in which sleep deprivation may play a contributing role (12).
Women with a strong ability to tan reportedly present at a higher risk of developing vitiligo. This association may be attributed to various factors, including the direct impact of UV rays on DNA, leading to the upregulation of the tyrosinase gene, and the direct influence of UV rays on melanin cell membranes. These mechanisms contribute to the overall tanning response observed in individuals (91). Therefore, tyrosinase seems to be a key player in the tanning response (92). A recent study (93) published in The Lancet indicated that regions with a higher overall prevalence of vitiligo are located in South Asia, including India, Bangladesh, Nepal, Pakistan, and Bhutan. This may be related to the greater visibility of vitiligo in individuals with darker skin tones.
Permanent hair dye contains many chemicals, including phenols such as p-aminophenol and resorcinol (94). Phenols act as tyrosinase analogs and interfere with melanin production, which may be associated with an increased risk of vitiligo caused by permanent hair dye use (21).
A national cohort study in South Korea identified a significant association between height and an increased risk of vitiligo in Korean adults (13). However, data from other countries in Asia, Europe, and the United States is lacking. In the future, large cohort and mechanistic studies on the relationship between height and vitiligo should be conducted to further explore this phenomenon.
Limitations
This study had certain limitations. First, the available literature on the lifestyle habits of patients with vitiligo is relatively limited, resulting in insufficient evidence in certain areas. Second, the studies in this review were highly heterogeneous. Third, most of the studies were conducted in Asian and African countries, and there is a lack of data from studies conducted in Europe and the United States. Fourth, there were limited data available for the meta-analysis and bias assessment. Fifth, most of the data came from non-segmental vitiligo cases, and the evidence for segmental vitiligo was weak. Moreover, the limited data in this study could not conduct a more in-depth analysis of differences in age, sex, and disease stage, among others. Further research is still needed to confirm these associations and clarify the underlying mechanisms.
Conclusion
Our review of lifestyle habits in relation to vitiligo provides several guiding suggestions. Additionally, it is recommended that both normal individuals and those with vitiligo refrain from smoking and excessive alcohol consumption. We recommend that every patient with vitiligo undergo blood tests for vitamin E, vitamin B12, zinc, and copper. If the test results indicate low levels of these nutrients, supplementation should be considered under the guidance of a doctor. However, supplementation with vitamin C, vitamin D, selenium, and folic acid may not be necessary. Furthermore, patients with vitiligo avoid high-fat diets, as these have been associated with negative health effects. Given the increased risk of sleep disorders and sexual dysfunction in individuals with vitiligo, seeking specialized help from healthcare professionals in these areas is advisable if necessary. Additionally, sun protection is crucial, particularly for women with high tanning abilities, as exposure to UV rays can have implications for the development and progression of vitiligo. Finally, regardless of the presence or absence of vitiligo, it is recommended to avoid the use of permanent hair dyes due to their potential association with an increased risk of vitiligo.
These guiding suggestions aim to provide individuals with vitiligo with lifestyle recommendations that may help manage their condition and improve their overall wellbeing. However, it is important to consult healthcare professionals for personalized advice and treatment plans based on individual needs and circumstances.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XLia: Data curation, Methodology, Writing – original draft. FG: Data curation, Methodology, Writing – original draft. QF: Data curation, Writing – original draft. XC: Formal analysis, Writing – original draft. JW: Validation, Writing – original draft. JC: Investigation, Resources, Writing – original draft. FL: Project administration, Writing – original draft. YD: Visualization, Writing – original draft. YC: Supervision, Writing – review & editing. XLi: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Commission of Baoshan District, Shanghai Medical Health Project (Grant No. 21-E-33); the New round (2023–2025) Baoshan Medical Key (specialized) Department “Vitiligo, hair loss integrated traditional Chinese and Western medicine characteristic treatment clinic” (Grant No. BSZK-2023-A15); Li Bin Shanghai famous Chinese medicine studio grassroots workstation (Grant No. JCGZZ-2023078); 2023 Shanghai Traditional Chinese Medicine Specialty Capacity Construction “Traditional Chinese Medicine Dermatology” (Grant No. SQZBZK-23-25); the National Natural Science Foundation of Shanghai (Grant No. 19ZR1458700); the Key Discipline Construction Project of Shanghai’s Three Year Action Plan for Strengthening the Construction of Public Health System (Grant No. GWVI-11.1-24); High-Level Chinese Medicine Key Discipline Construction Project (Integrative Chinese and Western Medicine Clinic) of National Administration of TCM (Grant No. zyyzdxk-2023065); and Shanghai Three-Year Action Plan to Further Accelerate the Inheritance and Innovative Development of Chinese Medicine (2021–2023) [Grant No. ZY(2021-2023)-0302].
Conflict of interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1440705/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis of the serum zinc levels between patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 2 | Sensitivity analysis of the serum vitamin B12 levels between patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 3 | Sensitivity analysis of the serum copper levels between patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 4 | Sensitivity analysis of the serum selenium levels between patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 5 | Meta-analysis of smoking in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 6 | Meta-analysis of alcohol consumption in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 7 | Meta-analysis of the serum vitamin C levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 8 | Meta-analysis of the serum vitamin B12 levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 9 | Meta-analysis of the serum folic acid levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 10 | Meta-analysis of the serum vitamin E levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 11 | Meta-analysis of the serum zinc levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 12 | Meta-analysis of the serum zinc levels in patients with generalized vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 13 | Meta-analysis of the serum zinc levels in patients with localized vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 14 | Meta-analysis of the serum zinc levels in female patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 15 | Meta-analysis of the serum zinc levels in male patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 16 | Meta-analysis of the serum zinc levels in progressive patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 17 | Meta-analysis of the serum zinc levels in stable patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 18 | Meta-analysis of the serum copper levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 19 | Meta-analysis of the serum copper levels in progressive patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 20 | Meta-analysis of the serum copper levels in stable patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 21 | Meta-analysis of the serum copper levels in male patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 22 | Meta-analysis of the serum copper levels in female patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 23 | Meta-analysis of the serum selenium levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 24 | Meta-analysis of the serum selenium levels in progressive patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 25 | Meta-analysis of the serum selenium levels in stable patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Figure 26 | Meta-analysis of the serum iron levels in patients with vitiligo and controls 95% CI, 95% confidence interval.
Supplementary Table 2 | Search strategy.
Abbreviations
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GI, genital involvement; Hcy, homocysteine; MUFA, monounsaturated fatty acid; NOS, Newcastle–Ottawa Scale; PTH, parathormone; PUFA, polyunsaturated fatty acid; PUVA, psoralen ultraviolet A-ray; SFA, saturated fatty acid; UV, ultraviolet; UVB, ultraviolet B-rays.
References
1. Silverberg NB. The epidemiology of vitiligo. Curr Derm Rep. (2015) 4:36–43. doi: 10.1007/s13671-014-0098-6
2. Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. (1993) 100:816–22. doi: 10.1111/1523-1747.ep12476645
3. Spritz RA, Andersen GH. Genetics of vitiligo. Dermatol Clin. (2017) 35:245–55. doi: 10.1016/j.det.2016.11.013
4. Di Dalmazi G, Hirshberg J, Lyle D, Freij JB, Caturegli P. Reactive oxygen species in organ-specific autoimmunity. Auto Immun Highlights. (2016) 7:11. doi: 10.1007/s13317-016-0083-0
5. Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. (2017) 77:1–13. doi: 10.1016/j.jaad.2016.10.048
6. Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. (2020) 38:621–48. doi: 10.1146/annurev-immunol-100919-023531
7. Iannella G, Greco A, Didona D, Didona B, Granata G, Manno A, et al. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. (2016) 15:335–43. doi: 10.1016/j.autrev.2015.12.006
8. Stang A. Critical evaluation of the newcastle–ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
9. Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. (1997) 77:460–2. doi: 10.2340/000155555577460462
10. Lajevardi N, Wu S, Li W, Cho E, Qureshi AA. Vitiligo and associated pigmentation, sun exposure, and lifestyle factors in women. ” J Invest Dermatol. (2015) 135:S52.
11. Kulkarni M, Keny D, Potey AV, Tripathi RK. A cross-sectional study to assess the incompatible dietary behavior of patients suffering from skin diseases: A pilot study. J Ayurveda Integr Med. (2016) 7:113–8. doi: 10.1016/j.jaim.2016.06.001
12. Liu JW, Tan Y, Chen T, Liu W, Qian YT, Ma DL, et al. Location, spreading and oral corticosteroids are associated with insomnia in vitiligo patients: A case–control study. Clinical Cosmetic Investigational Dermatol. (2021) 14:971–80. doi: 10.2147/CCID.S322963
13. Lee YB, Kim HS. Height and risk of vitiligo: A nationwide cohort study. J Clin Med. (2021) 10:3958. doi: 10.3390/jcm10173958
14. Sanad EM, El-Fallah AA, Al-Doori AR, Salem RM. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthetic Dermatol. (2020) 13:S29–33.
15. Lee YB, Lee JH, Lee SY, Yu DS, Han KD, Park YG, et al. Association between vitiligo and smoking: A nationwide population-based study in Korea. Sci Rep. (2020) 10:6231. doi: 10.1038/s41598-020-63384-y
16. Derakhshandeh-Rishehri SM, Heidari-Beni M, Jaffary F, Askari G, Nilfroshzade M, Adibi N. Role of fatty acids intake in generalized vitiligo. Int J Prev Med. (2019) 10:52. doi: 10.4103/ijpvm.IJPVM_47_17
17. Hussein SM, Shehata H, El Zawahry YB, Soliman A, Rabie AA, Emam H, et al. Role of vitamin B12, folic acid and oxidative damages, in serum of patients with vitiligo. J Global Pharma Technol. (2019) 11:455–61.
18. Iraji F, Haftbaradaran E, Davashi S, Zolfaghari-Baghbaderani A, Bokaii-Jazi S. Comparing the improvement of unstable vitiligo in patients treated by topical PUVA-therapy alone, topical PUVA-therapy and oral vitamin D, and topical PUVA-therapy and oral vitamin D and vitamin B12. J Isfahan Med School. (2017) 34:1699–705.
19. Akhter QS, Sumi MN, Banu N. Estimation of serum vitamin B12, folic acid, homocysteine & ferritin levels in subjects with vitiligo. J Obstetrics Gynaecology Res. (2017) 43:52–3. doi: 10.1111/jog.13387
20. Dass S. Search for clinical and laboratory markers of severity and instability of vitiligo: A cross-sectional observational hospital based study. J Am Acad Dermatol. (2016) 74:AB230.
21. Wu S, Li WQ, Cho E, Harris JE, Speizer F, Qureshi AA. Use of permanent hair dyes and risk of vitiligo in women. Pigment Cell Melanoma Res. (2015) 28:744–6. doi: 10.1111/pcmr.2015.28.issue-6
22. Manisha T, Kumar MS, Reetu S. Epidemiological study of svitra (vitiligo) with special reference to viruddha ahara (incompatible diet). Int J Res Ayurveda Pharmacy. (2015) 6:662–6. doi: 10.7897/2277-4343.066123
23. Maryam G, Vahide L, Abbas F. Serum levels of vitamin B12, folic acid, and homocysteine in patients with vitiligo. Iranian J Dermatol. (2015) 18:45–50.
24. Colucci R, Dragoni F, Conti R, Pisaneschi L, Lazzeri L, Moretti S. Evaluation of an oral supplement containing Phyllanthus emblica fruit extracts, vitamin E, and carotenoids in vitiligo treatment. Dermatol Ther. (2015) 28:17–21. doi: 10.1111/dth.2015.28.issue-1
25. Khurrum H, Alghamdi K. Is there a real relationship between serum level of homocysteine and vitiligo? Pigment Cell Melanoma Res. (2014) 27:980. doi: 10.2310/7750.2013.13050
26. Kim SA, Cho S, Kwon SH, Park JT, Na JI, Huh CH, et al. Childhood facial vitiligo: how intractable is it? J Eur Acad Dermatol Venereol. (2015) 29:713–8. doi: 10.1111/jdv.12666
27. Araujo M, Avila P, Avila P, Araujo MF, Avila PF. The relation between vit B12 levels and vitiligos repigmentation. Pigment Cell Melanoma Res. (2014) 27:982. doi: 10.1111/pcmr.12292
28. Finamor DC, Sinigaglia-Coimbra R, Neves LC, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermato-Endocrinology. (2013) 5:222–34. doi: 10.4161/derm.24808
29. Yaghoobi R, Omidian M, Bagherani N. Original article title: “Comparison of therapeutic efficacy of topical corticosteroid and oral zinc sulfate-topical corticosteroid combination in the treatment of vitiligo patients: a clinical trial. BMC Dermatol. (2011) 11:7. doi: 10.1186/1471-5945-11-7
30. Silverberg J, Silverberg N. Serum homocysteine is associated with extent of vitiligo vulgaris. J Am Acad Dermatol. (2011) 64:AB142. doi: 10.1016/j.jaad.2010.09.582
31. Gonul M, Cakmak SK, Soylu S, Kilic A, Gul U, et al. Serum vitamin B12, folate, ferritin and iron levels in Turkish patients with vitiligo. Indian J Dermatol Venereol Leprol. (2010) 76:448. doi: 10.4103/0378-6323.66611
32. Khan R, Satyam A, Gupta S, Sharma VK, Sharma A, et al. Circulatory levels of antioxidants and lipid peroxidation in Indian patients with generalized and localized vitiligo. Arch Dermatol Res. (2009) 301:731–7. doi: 10.1007/s00403-009-0964-4
33. Mouzas O, Angelopoulos N, Papaliagka M, Tsogas P. Increased frequency of self-reported parasomnias in patients suffering from vitiligo. Eur J Dermatol. (2008) 18:165–8. doi: 10.1684/ejd.2008.0355
34. Agrawal D, Shajil EM, Marfatia YS, Begum R. Study on the antioxidant status of vitiligo patients of different age groups in Baroda. Pigment Cell Res. (2004) 17:289–94. doi: 10.1111/j.1600-0749.2004.00149.x
35. Akyol M, Celik VK, Ozcelik S, Polat M, Marufihah M, Atalay A. The effects of vitamin E on the skin lipid peroxidation and the clinical improvement in vitiligo patients treated with PUVA. Eur J Dermatol. (2002) 12:24–6.
36. Tjioe M, Gerritsen MJP. Treatment of vitiligo vulgaris with narrow band UVB (311nm) for one year and the effect of addition of folic acid and vitamin B12. Acta Derm Venereol. (2002) 82:369–72. doi: 10.1080/000155502320624113
37. Picardo M, Passi S, Morrone A, Grandinetti M, Di Carlo A, Ippolito F. Antioxidant status in the blood of patients with active vitiligo. Pigment Cell Res. (1994) 7:110–5. doi: 10.1111/j.1600-0749.1994.tb00034.x
38. Bashrahil B, Alzahrani Z, Nooh M, Alghamdi N, Alsolami H, Alturkistani R, et al. Association between vitamin D, zinc, and thyroid biomarker levels with vitiligo disease: A retrospective cohort study in a tertiary care center. Cureus. (2022) 14:e31774. doi: 10.7759/cureus.31774
39. Memon HS, Shah SMS, Nasreen S, Malik T, Izhar M, Shakilayousuf. Effect of vitamin b12 and folic acid in vitiligo patients. Pakistan J Med Health Sci. (2021) 15:1198–201.
40. Boisseau-Garsaud G, Garsaud P, Lejoly-Boisseau H, Robert M, Quist D, Arveiler B, et al. Increase in total blood antioxidant status and selenium levels in black patients with active vitiligo. Int J Dermatol. (2002) 41:640–2. doi: 10.1046/j.1365-4362.2002.01472.x
41. Khoshdel Z, Gholijani N, Niknam M, Rahmani N, Hemmati-Dinarvand M, Naghibalhossaini F, et al. Serum copper and zinc levels among Iranian vitiligo patients. Dermatol Pract Concept. (2022) 12:e2022140. doi: 10.5826/dpc.1204a140
42. Zaki AM, Nada AS, Elshahed AR, Abdelgawad NH, Jafferany M, Elsaie ML, et al. Therapeutic implications of assessment of serum zinc levels in patients with vitiligo: A patient controlled prospective study. Dermatol Ther. (2020) 33:e13998. doi: 10.1111/dth.13998
43. Wacewicz M, Socha K, Soroczyńska J, Niczyporuk M, Aleksiejczuk P, Ostrowska J, et al. Selenium, zinc, copper, Cu/Zn ratio and total antioxidant status in the serum of vitiligo patients treated by narrow-band ultraviolet-B phototherapy. J Dermatolog Treat. (2018) 29:190–5. doi: 10.1080/09546634.2017.1357797
44. Mirnezami M, Rahimi H. Serum zinc level in vitiligo: A case–control study. Indian J Dermatol. (2018) 63:227–30. doi: 10.4103/ijd.IJD_457_16
45. Narang I, Barman KD. Evaluation of serum levels of zinc and copper in vitiligo in pediatric patients. Pediatr Dermatol. (2017) 34:S87. doi: 10.4103/Pigmentinternational.Pigmentinternational_
46. Bagheri Hamidi A, Namazi N, Amoli MM, Amani M, Gholami M, Youssefian L, et al. Association of MTHFR C677T polymorphism with elevated homocysteine level and disease development in vitiligo. Int J Immunogenet. (2020) 47:342–50. doi: 10.1111/iji.12476
47. Mogaddam MR, Ardabili NS, Maleki N, Chinifroush MM, Fard EM. Evaluation of the serum zinc level in patients with vitiligo. Postepy Dermatol Alergol. (2017) 34:116–9. doi: 10.5114/ada.2017.67073
48. Dogan B, Bayram M, Karabacak E. Assessment of intraerythrocyte zinc levels in vitiligo patients. J Am Acad Dermatol. (2016) 74:AB230.
49. Ataş H, Cemil BÇ, Gönül M, Baştürk E, Çiçek E. Serum levels of homocysteine, folate and vitamin B12 in patients with vitiligo before and after treatment with narrow band ultraviolet B phototherapy and in a group of controls. J Photochem photobiology. B Biol. (2015) 148:174–80. doi: 10.1016/j.jphotobiol.2015.04.005
50. Agrawal S, Kumar A, Dhali TK, Majhi SK. Comparison of oxidant-antioxidant status in patients with vitiligo and healthy population. Kathmandu Univ Med J (KUMJ). (2014) 12:132–6. doi: 10.3126/kumj.v12i2.13660
51. Ramadan R, Tawdy A, Hay Abdel R, Rashed L, Tawfik D. The antioxidant role of paraoxonase 1 and vitamin E in three autoimmune diseases. Skin Pharmacol Physiol. (2013) 26:2–7. doi: 10.1159/000342124
52. Yasar A, Gunduz K, Onur E, Calkan M. Serum homocysteine, vitamin B12, folic acid levels and methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in vitiligo. Dis Markers. (2012) 33:85–9. doi: 10.1155/2012/540597
53. Karadag AS, Tutal E, Ertugrul DT, Akin KO, Bilgili SG. Serum holotranscobalamine, vitamin B12, folic acid and homocysteine levels in patients with vitiligo. Clin Exp Dermatol. (2012) 37:62–4. doi: 10.1111/j.1365-2230.2011.04142.x
54. Balci DD, Yonden Z, Yenin JZ, Okumus N. Serum homocysteine, folic acid and vitamin B12 levels in vitiligo. Eur J Dermatol. (2009) 19:382–3. doi: 10.1684/ejd.2009.0671
55. Jain D, Misra R, Kumar A, Jaiswal G. Levels of malondialdehyde and antioxidants in the blood of patients with vitiligo of age group 11-20 years. Indian J Physiol Pharmacol. (2008) 52:297–301.
56. Ines D, Sonia B, Riadh BM, Amel EG, Slaheddine M, Hamida T, et al. A comparative study of oxidant-antioxidant status in stable and active vitiligo patients. Arch Dermatol Res. (2006) 298:147–52. doi: 10.1007/s00403-006-0680-2
57. Park HH, Lee MH. Serum levels of vitamin B12 and folate in Korean patients with vitiligo. Acta Derm Venereol. (2005) 85:66–7. doi: 10.1080/00015550410021565
58. Arora PN, Dhillon KS, Rajan SR, Sayal SK, Das AL. Serum zinc levels in cutaneous disorders. Med J Armed Forces India. (2002) 58:304–6. doi: 10.1016/S0377-1237(02)80083-1
59. Kim SM, Kim YK, Hann SK. Serum levels of folic acid and vitamin B12 in Korean patients with vitiligo. Yonsei Med J. (1999) 40:195–8. doi: 10.3349/ymj.1999.40.3.195
60. Sharma YK, Bansal P, Menon S, Prakash N. Metabolic syndrome in vitiligo patients among a semi-urban Maharashtrian population: A case control study. Diabetes Metab Syndrome: Clin Res Rev. (2017) 11:S77–80. doi: 10.1016/j.dsx.2016.12.009
61. Tanacan E, Atakan N. Higher incidence of metabolic syndrome components in vitiligo patients: a prospective cross-sectional study. Anais Brasileiros Dermatologia. (2020) 95:165–72. doi: 10.1016/j.abd.2019.07.006
62. Dragoni F, Conti R, Cazzaniga S, Colucci R, Pisaneschi L, Naldi L, et al. No association between vitiligo and obesity: A case-control study. Med Princ Pract. (2017) 26:421–6. doi: 10.1159/000481436
63. Taneja K, Taneja J, Kaur C, Patel S, Haldar D. Lipid risk factors in vitiligo: homocysteine the connecting link? Clin Lab. (2020) 66:1987. doi: 10.7754/Clin.Lab.2020.200120
64. Gorial FI, Jehad SK, Taha SF, Tawfeeq AA. Presarcopenia in patients with vitiligo: A case control study. Mediterr J Rheumatol. (2021) 32:143–7. doi: 10.31138/mjr.32.2.143
65. Haider N, Islam MS, Al Maruf A, Shohag MH, Ali R, Mustafizur Rahman GKM, et al. Oxidative stress and antioxidant status in vitiligo patients. Dhaka Univ J Pharm Sci. (2010) 9:104–8.
66. Barikbin B, Kavand S, Yousefi M, Hedayati M, Saeedi M. No differences in serum selenium levels and blood glutathione peroxidase activities in patients with vitiligo compared with healthy control subjects. J Am Acad Dermatol. (2011) 64:444–5. doi: 10.1016/j.jaad.2010.03.011
67. Ozturk I, Batcioglu K, Karatas F, Hazneci E, Genc M. Comparison of plasma malondialdehyde, glutathione, glutathione peroxidase, hydroxyproline and selenium levels in patients with vitiligo and healthy controls. Indian J Dermatol. (2008) 53:106–10. doi: 10.4103/0019-5154.39577
68. Beazley WD, Gaze D, Panske A, Panzig E, Schallreuter KU. Serum selenium levels and blood glutathione peroxidase activities in vitiligo. Br J Dermatol. (1999) 141:301–3. doi: 10.1046/j.1365-2133.1999.02980.x
69. Yandong W, Xiuhua L, Xiaohong L, Qinghua F, Jie L. Determination of trace elements in serum of vitiligo patients in Daqing area. J Qiqihar Med Coll. (2012) 33:39–40.
70. Wang X. A correlative study on SOD and serum Zinc Copper Iron in patients with vitiligo. World Elemental Med (Quarterly Journal). (2011) 18:31–2.
71. Yao A, Aiping Y. Clinical analysis of nutritional status of trace elements in patients with vitiligo. China Higher Med Education. (2011) 7):145–6.
72. Zhao J, Jin Z, Wei L, Shiyuan L. Analysis of serum selenium concentration in patients with white paralysis. Chin J Leprosy Dermatol. (2011) 27:28–9.
73. Li Z, Zongping L, Meirong Z. Determination of trace element Cu in serum of patients with vitiligo. Shanghai J Prev Med. (2001) 13:239.
74. Wang F, Fei W, Hanqing X. Study on changes of some enzymes and microelements in serum and skin lesions of patients with white addiction wind. Chin J Dermatol venereology. (1993) 7:142–3.
75. Wu Yi, Yi W, Na H, Juchang L, Lin L. Determination of serum zinc and copper content in 70 patients with vitiligo in Guangxi. Chin J Dermatol Venereology. (2010) 24:722–3.
76. Wang X, Xiaohua W, Xiaodong C. Analysis of serum zinc and copper content in 48 patients with vitiligo. J Nantong Med College. (1996) 16:277.
77. Tu C, Caixia T, Xiran L, Feng Y. Determination of copper and zinc in serum and skin tissue fluid of patients with white fatigue. Chin J Dermatol venereology. (1991) 5:20–1.
78. Tu C, Caixia T, Xiran L, Haibo C. Analysis of selenium in 29 patients with vitiligo. J Dalian Med University. (1998) 20:29–31.
79. Song J, Jialing S, Ping Z, Lu Y. Observation on levels of trace elements and cytokines in patients with vitiligo. Chongqing Med Science. (2017) 46:1191–5.
80. Al-Hattab HH, Al-Hattab HH, Al-Joda BAN, Shaker M, Alhattab MK. Assessment of serum zinc and vitamin c as antioxidants in patients with vitiligo in Babylon province. Int J Pharm Res. (2020) 12:1636–41. doi: 10.31838/ijpr/2020.12.04.237
81. Shaker OG, El-Tahlawi SM. Is there a relationship between homocysteine and vitiligo? A pilot study. Br J Dermatol. (2008) 159:720–4. doi: 10.1111/j.1365-2133.2008.08712.x
82. Beyzaee AM, Goldust M, Patil A, Rokni GR, Beyzaee S, Goldust M, Patil A, Rokni GR, Beyzaee S. The role of cytokines and vitamin D in vitiligo pathogenesis. J Cosmet Dermatol. (2022) 21:6314–25. doi: 10.1111/jocd.v21.11
83. Kamei Y, Otsuka Y, Abe K. Comparison of the inhibitory effects of vitamin E analogues on melanogenesis in mouse B16 melanoma cells. Cytotechnology. (2009) 59:183–90. doi: 10.1007/s10616-009-9207-y
84. Choi B, Heo JH, Kwon HJ, Lee ES, Sohn S, Heo JH, Kwon HJ, Lee E-S, Sohn S. Tocotrienols enhance melanosome degradation through endosome docking/fusion proteins in B16F10 melanoma cells. Food Funct. (2013) 4:1481–8. doi: 10.1039/c3fo60289c
85. Muawia M HS, Modawe GA. Assessment of Serum Copper Level among Sudanese Patients with vitiligo. Sudan J Med Sci. (2020) 15:73–84. doi: 10.18502/sjms.v15i1.6707
86. Altobelli GG, Van Noorden S, Balato A, Cimini V. Copper/zinc superoxide dismutase in human skin: current knowledge. Front Med (Lausanne). (2020) 7:183. doi: 10.3389/fmed.2020.00183
87. Hariharan S, Dharmaraj S. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology. (2020) 28:667–95. doi: 10.1007/s10787-020-00690-x
88. Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev. (2007) 6:457–63. doi: 10.1016/j.autrev.2007.01.016
89. Mansur AT, Aydıngöz IE, Göktay F, Atalay S. Serum iron and ferritin levels in patients with vitiligo. Turk Derm. (2010) 44:153–5. doi: 10.4274/turkderm.44.153
90. de França E, Dos Santos RVT, Baptista LC, Da Silva MAR, Fukushima AR, Hirota VB, Martins RA, et al. Potential role of chronic physical exercise as a treatment in the development of vitiligo. Front Physiol. (2022) 13:843784. doi: 10.3389/fphys.2022.843784
91. Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. (1996) 63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x
92. Dunlap R, Wu S, Wilmer E, Cho E, Li WQ, Lajevardi N, Su M-Y, Jiang S, Luo L-F, Shi Y, Lei T-C, et al. Pigmentation traits, sun exposure, and risk of incident vitiligo in women. J Invest Dermatol. (2017) 137:1234–9. doi: 10.1016/j.jid.2017.02.004
93. Akl J, Lee S, Ju HJ, Parisi R, Kim JY, Jeon JJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study vitiligo: a systematic review and modelling study. Lancet Public Health. (2024) 9:e386–96. doi: 10.1016/S2468-2667(24)00026-4
94. Kim KH, Kabir E, Jahan SA. The use of personal hair dye and its implications for human health. Environ Int. (2016) 89-90:222–7. doi: 10.1016/j.envint.2016.01.018
95. Fang M, Su M-Y, Jian S, Luo L-F, Shi Y, Lei T-C. Intramelanocytic acidification plays a role in the antimelanogenic and antioxidative properties of vitamin C and its derivatives. Oxid Med Cell Longev. (2019) 2019:2084805. doi: 10.1155/2019/2084805
Keywords: lifestyle, systematic review, vitiligo, diet, exercise
Citation: Liang X, Guo F, Fan Q, Cai X, Wang J, Chen J, Liu F, Du Y, Chen Y and Li X (2024) Healthy lifestyle choices: new insights into vitiligo management. Front. Immunol. 15:1440705. doi: 10.3389/fimmu.2024.1440705
Received: 07 June 2024; Accepted: 16 October 2024;
Published: 18 November 2024.
Edited by:
Olga Simionescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Sorina Danescu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaAlina Mariana Avram, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Liang, Guo, Fan, Cai, Wang, Chen, Liu, Du, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, MTM2NjE5NTYzMjZAMTYzLmNvbQ==; Yan Chen, c255eWdoQDE2My5jb20=
†These authors have contributed equally to this work
 Xin Liang1†
Xin Liang1† Xiaoce Cai
Xiaoce Cai Jiao Wang
Jiao Wang Jiale Chen
Jiale Chen Xin Li
Xin Li