Abstract
Background:
The limited therapeutic options and inconsistent treatment efficacy of Intestinal Behçet’s disease complicate its management, with the absence of standardized guidelines further exacerbating the challenges faced by clinicians.
Case Presentation:
In this case report, we present a patient with refractory intestinal Behçet’s disease who experienced treatment failure with prior biologic agents. This case sheds light on managing this complex scenario with Janus kinase (JAK) inhibitors, the patient achieved mucosal healing after switching to Upadacitinib.
Conclusion:
This case underscores the potential of Upadacitinib as an effective alternative for managing difficult cases of intestinal Behçet’s disease.
Introduction
Intestinal Behçet’s disease is a rare autoimmune disorder characterized by recurrent intestinal ulcers, abdominal pain, and diarrhea. Traditional treatment options include corticosteroids, immunosuppressants, and biologic agents (1). However, due to the complex etiology and pathophysiology of this condition, many patients do not respond adequately to traditional therapies, resulting in cases that are classified as refractory (2). In recent years, upadacitinib, a novel Janus kinase (JAK) inhibitor, has demonstrated promising potential in the treatment of various autoimmune diseases. Its mechanisms of action involve the modulation of inflammatory pathways and it is currently employed in the management of inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, and psoriasis, among other autoimmune-related inflammatory conditions (3, 4). This emerging treatment option offers new hope for patients with challenging cases who have previously experienced limited success with standard therapies. Here, we present a male patient in whom upadacitinib successfully induced remission of Intestinal Behçet’s disease in steroid-dependent and resistant to anti-TNF-α. To the best of our knowledge, this is the first report of upadacitinib administration in childbearing age male patient of Intestinal Behçet’s disease, highlighting its potential role in managing this challenging condition and offering new hope for affected patients.
Case presentation
We present the case of a 24-year-old male diagnosed with intestinal Behçet’s disease. Four years prior, he experienced recurrent fever, oral ulcers and abdominal pain. Colonoscopy revealed a single, well-defined large ulcer in the ileocecal region. Despite undergoing diagnostic anti-tuberculosis treatment, his symptoms and ulcers did not improve. On August 13, 2021, the patient underwent laparoscopic partial colonic resection. Post-operative pathology indicated multiple occlusive small vein inflammations and lymphoid tissue aggregation were found on the mesentery side of the cecum and ascending colon (Figure 1). ultimately resulting in a diagnosis of intestinal Behçet’s disease, although no medical therapy was initiated at that time. One year after surgery, the patient was readmitted due to recurrence of right lower abdominal pain. Laboratory tests indicated significant inflammatory markers (c reactive protein=67.8mg/L, erythrocyte sedimentation rate=29mm/h, faecal calprotectin=989.10ug/G), along with anemia, while colonoscopy demonstrated a large, well-defined round ulcer adjacent to the anastomosis site (Figure 2A). Both ANAs (such as anti-dsDNA, nucleosome, histone, Sm, SS-A/Ro52, SS-A/Ro60, SS-B/La, CENP-B, Scl-70, AMA-M2, Jo-1, ribosomal-P and RNP) and ANCA are negative. The CTE showed significant uneven thickening of the intestinal wall in the lower right abdomen, obvious extravasation outside the serosa, and multiple small vessel shadows, which were significantly enhanced after enhancement (Figure 3).
Figure 1

Postoperative pathological manifestations, which indicated multiple occlusive small vein inflammations and lymphoid tissue aggregation were found on the mesentery side of the (A) cecum and ascending (B) colon.
Figure 2
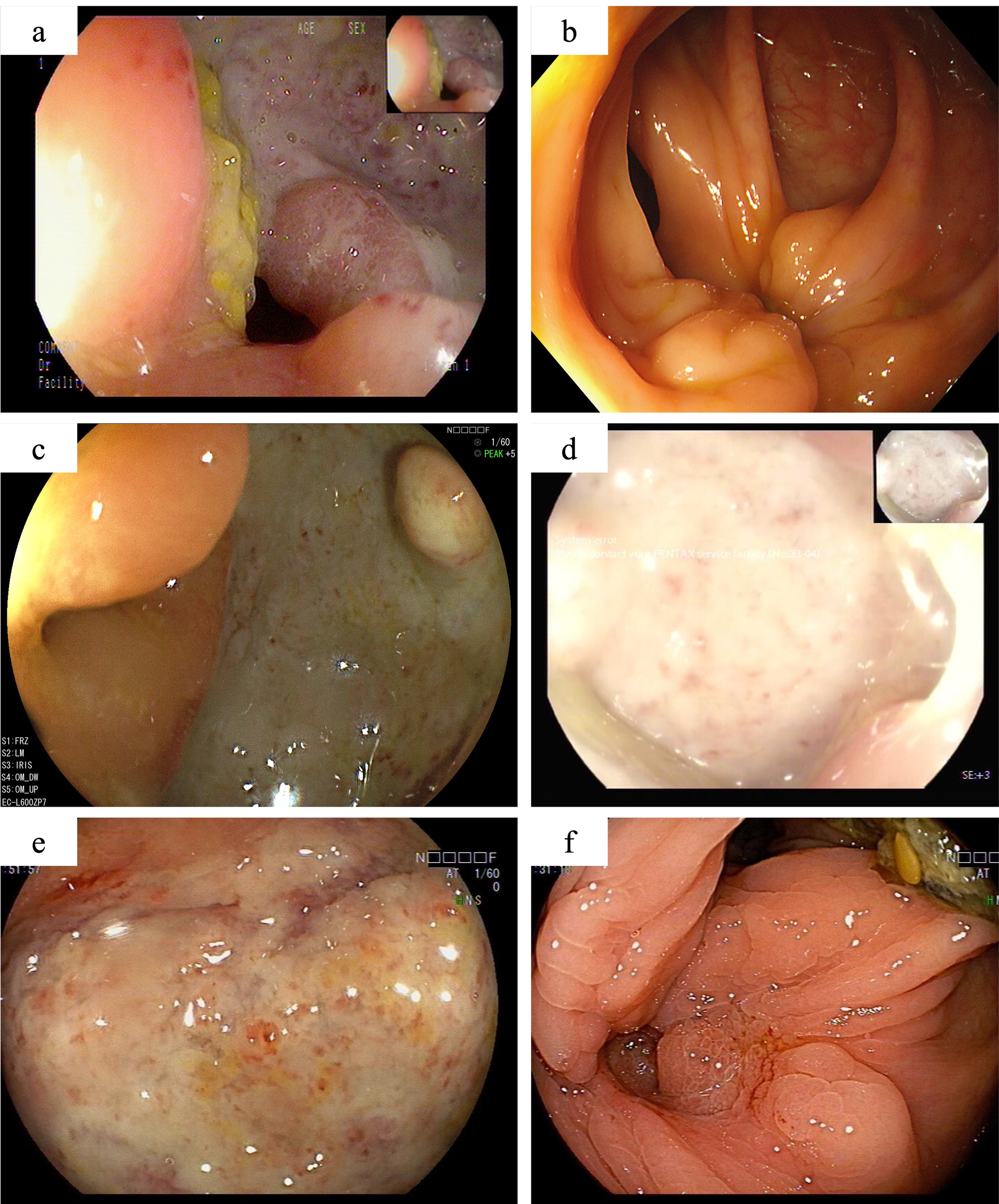
The colonoscopy findings of this patient. One year after partial colonic resection, colonoscopy demonstrated a large, well-defined round ulcer adjacent to the anastomosis site (A). Scar formation and mucosal aggregation can be seen near the anastomotic site with no ulcers are observed after induction treatment with Infliximab (B). Secondary unresponsive to Infliximab (C), with no significant improvement after intensified treatment. Colonoscopy revealed the appearance of large ulcers around the anastomotic site again (D). When switching to adalimumab combined with sulfasalazine treatment, there is still no sign of improvement in ulcers near the anastomotic site (E). After 12 weeks of oral administration of upadacitinib, colonoscopy showed complete healing of the ulcer (F).
Figure 3
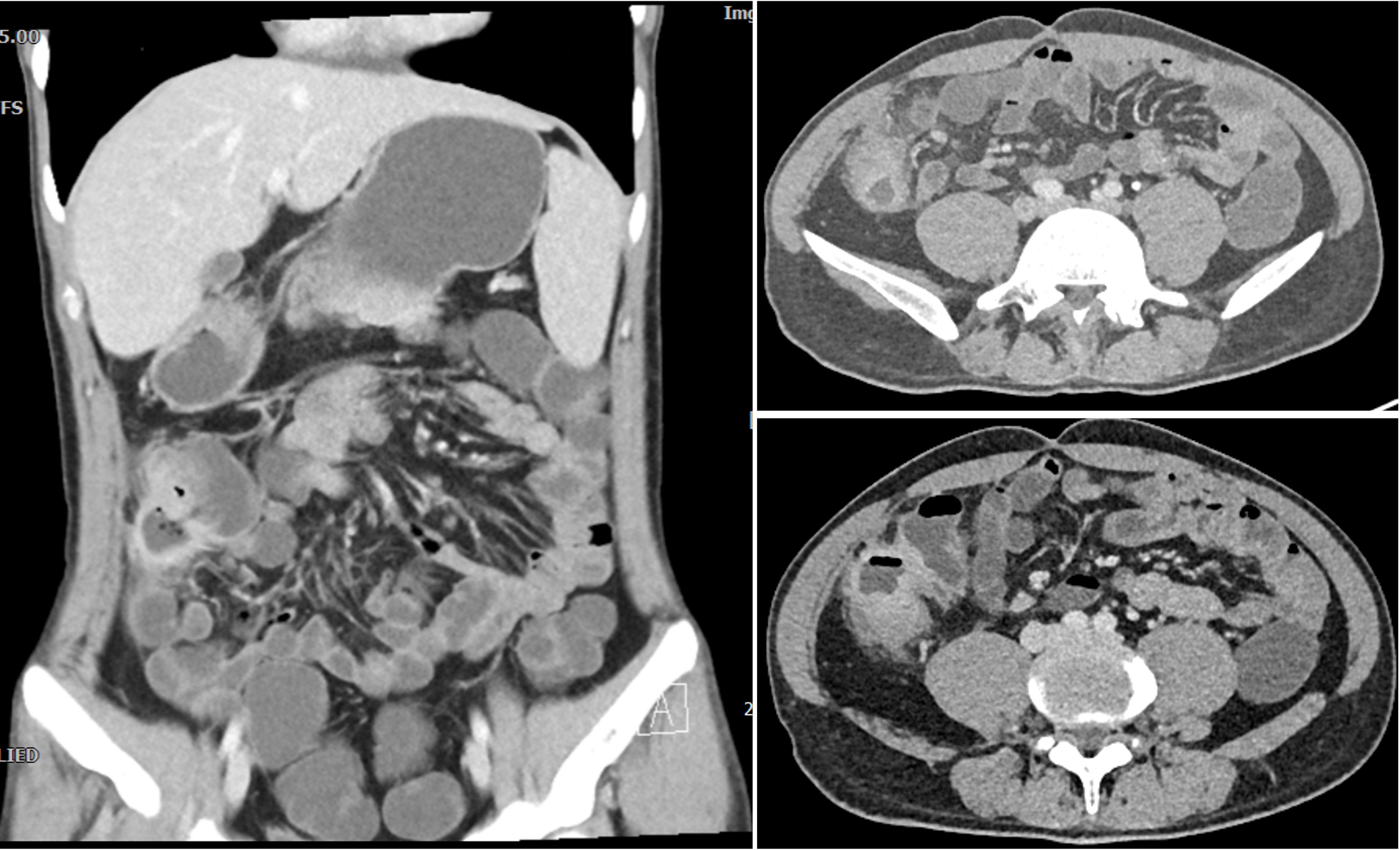
CTE manifestations of patients with recurrent abdominal pain one year after surgery. It revealed significant uneven thickening of the intestinal wall in the lower right abdomen, obvious extravasation outside the serosa, and multiple small vessel shadows, which were significantly enhanced after enhancement.
In the initial treatment phase, systemic corticosteroids were administered to quickly alleviate inflammation, and infliximab therapy was initiated as corticosteroid dose reduction began. After three months of treatment, the corticosteroids were successfully tapered, and the patient continued regular infliximab therapy. At the end of the induction phase, he reported significant relief from abdominal pain, normalization of inflammatory markers, and complete healing of the ulcers on follow-up endoscopy (Figure 2B). However, eight months later, the patient again experienced abdominal pain with colonoscopy revealing a recurrence of anastomotic ulcers (Figure 2C). Therapeutic drug monitoring (TDM) indicated an infliximab level of 4.3 μg/ml without the presence of anti-drug antibodies. Consequently, intensification of infliximab therapy with shortened intervals (from every 8 weeks to every 4 weeks) was recommended, but his condition did not improve (Figure 2D).
Subsequently, the patient underwent short-term systemic corticosteroid treatment before switching to a regimen of sulfasalazine combined with adalimumab in the rheumatology department to induce remission. Unfortunately, this treatment proved ineffective, with persistent abdominal pain, elevated inflammatory markers, and no significant improvement in colonoscopy findings (Figure 2E). After discussing the potential benefits and risks of alternative therapies, the patient and his parents consented to initiate upadacitinib treatment. Therefore, adalimumab was discontinued, and on February 21, 2024, the patient commenced treatment with 45 mg of upadacitinib daily. His abdominal pain symptoms rapidly improved, with no more oral ulcers nor fever appeared, inflammation indicators (c reactive protein, erythrocyte sedimentation rate and faecal calprotectin) returned to normal, anemia was corrected, and stool routine examination showed negative. By the 12-week follow-up, complete ulcer healing was noted (Figure 2F). The patient’s condition has remained stable, allowing him to return to normal daily activities, and the dosage of upadacitinib has been tapered to 30 mg for maintenance therapy. The patient experienced significant symptomatic improvement and a notable enhancement in quality of life, without the occurrence of severe adverse events up to now.
Discussion
Intestinal Behçet’s disease is a severe condition that significantly impacts patients’ quality of life due to its complex and often debilitating manifestations. Particularly when affecting the intestines, it presents significant therapeutic challenges due to the heterogeneous nature. Systemic glucocorticoids paired with other immunosuppressive agents, constitute the first line of treatment for Intestinal Behçet’s disease currently. In particular, the addition of anti-tumor necrosis factor inhibitors (anti-TNF) further controlled Behcet’s disease (5). Among them, corticosteroids can provide rapid symptom relief, their long-term use is often associated with a broad spectrum of adverse effects, including osteoporosis, cardiovascular issues, and metabolic disturbances. Immunosuppressants like azathioprine and methotrexate provide therapeutic benefits; however, concerns regarding their side effects and potential reproductive risks have led to a cautious approach. About 30% of such patients respond inadequately to treatment with anti-TNF while presenting with refractory and relapse inflammation (6). Additionally, issues like secondary loss of response further complicate their use, underscoring the urgent need for novel therapeutic strategies that can more effectively manage this condition. This variability emphasizes the need for personalized approaches to optimize therapeutic outcomes.
The intricate pathogenesis of Intestinal Behçet’s disease suggests a complex interplay of multiple immune pathways. Recent evidence suggests that the Janus kinase (JAK) signaling pathway may play a role in the disease process, with the JAK-STAT pathway emerging as a potential therapeutic target (7). Previous study (8) demonstrated the role of the JAK/STAT pathway in peripheral blood CD14+ monocyte and CD4+ T cells with whole genome gene expression analysis and confirmed our results with flow cytometric STAT3 analysis in peripheral blood of patients with Behçet’s disease. This hypothesis is reinforced by the successful application of JAK inhibitors, notably tofacitinib, a non-selective JAK inhibitor, which has demonstrated clinical and laboratory improvements in Intestinal Behçet’s disease patients (9, 10). Through inhibition of JAK, upadacitinib inhibits phosphorylation of downstream effector proteins, which consequently inhibits cytokine signaling for key pathways involved in inflammatory diseases (3). Furthermore, the JAK-1 targeting upadacitinib has been approved for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease (11). However, data regarding the use of selective JAK inhibitors, like upadacitinib, in this context remain scarce, presenting an area of active investigation.
Recent case reports have highlighted the efficacy and safety of upadacitinib in patients with systemic Behçet’s disease associated with other complications such as ankylosing spondylitis and uveitis (12, 13). However, no data is yet available on upadacitinib use in patients with intestinal Behçet’s disease in childbearing age. Our case report highlights the promising role of upadacitinib in a young patient with refractory intestinal Behçet’s disease, who had failed previous therapies including infliximab, sulfasalazine, and adalimumab. The favorable outcomes observed in this patient, along with previous case reports documenting the efficacy and safety of upadacitinib in systemic Behçet’s disease patients with comorbid conditions like ankylosing spondylitis and uveitis, suggest that upadacitinib has emerged as a promising treatment for refractory intestinal Behçet’s disease, particularly for patients who have not responded adequately to biologic therapies (Table 1). At the same time, previous reports suggest that upadacitinib may increase the risk of acne, herpes zoster, non-melanoma skin cancer, elevations in creatine phosphokinase levels, serious infections, major adverse cardiovascular events, venous thromboembolism and malignancies (14). We need to be vigilant about the safety issues of long-term use.
Table 1
| Country | Years, Sex | Complication | Previous treatments | Dosage of | Clinical outcome |
|---|---|---|---|---|---|
| Bulgaria (12) | 42, female | ankylosing spondylitis | corticosteroids | 15mg | signs having undergone substantial improvement or achieving complete resolution |
| China (13) | adolescent girl | Macular Edema, Behçet’s Uveitis | topical antiinflammatory drops, systemic corticosteroids, methotrexate, cyclosporine A, adalimumab and mycophenolate mofetil | 15mg | symptom-free for five months and has been receiving GC-free therapy |
| China (13) | a man in his thirties | Macular Edema, Behçet’s Uveitis | corticosteroids, methotrexate, cyclosporine A, mycophenolate mofetil and adalimumab | 15mg | Ocular inflammation was clinically inactive, visual acuity improved and the therapy has been well-tolerated after upadacitinib used for nine months |
| China (Present case) |
24, male | / | corticosteroids, infliximab, sulfasalazine, and adalimumab | 45mg for 12 weeks and 30mg for 13weeks | Symptom relief, mucosal healing |
Case reports of successful remission with upadacitinib in patients with Behçet’s disease.
Our findings contribute to the growing body of evidence supporting the use of selective JAK inhibitors in managing this difficult-to-treat condition. But the precise mechanisms underlying upadacitinib’s therapeutic effect in intestinal Behçet’s disease remain to be fully elucidated. Nonetheless, its targeted inhibition of JAK1 and JAK2, which are implicated in inflammatory pathways, may contribute to its ability to mitigate the excessive immune response seen in intestinal Behçet’s disease. The limitations of our case include the relatively short follow-up period and the solitary nature of the case. Further studies, including larger-scale clinical trials, are warranted to confirm the efficacy and safety of upadacitinib in intestinal Behçet’s disease and to define its optimal role in the therapeutic algorithm.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SS: Funding acquisition, Writing – original draft, Writing – review & editing. BX: Validation, Writing – review & editing. KW: Validation, Writing – review & editing. CQ: Formal analysis, Writing – review & editing. HS: Visualization, Writing – review & editing. JJ: Formal analysis, Writing – review & editing. QX: Formal analysis, Validation, Writing – review & editing. XL: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the youth program of Shaanxi Natural Science Foundation (No. 2022SF-195).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Manuelyan Z Butt E Parupudi S . Gastrointestinal Behcet's disease: Manifestations, diagnosis, and management. Dis Mon. (2024) 70:101674. doi: 10.1016/j.disamonth.2023.101674
2
He K Yan X Wu D . Intestinal behcet's disease: A review of the immune mechanism and present and potential biological agents. Int J Mol Sci. (2023) 24(9):8176. doi: 10.3390/ijms24098176
3
Mohamed MF Bhatnagar S Parmentier JM Nakasato P Wung P . Upadacitinib: Mechanism of action, clinical, and translational science. Clin Transl Sci. (2024) 17:e13688. doi: 10.1111/cts.13688
4
Loftus EV Jr Panes J Lacerda AP Peyrin-Biroulet L D'Haens G Panaccione R et al . Upadacitinib induction and maintenance therapy for crohn's disease. N Engl J Med. (2023) 388:1966–80. doi: 10.1056/NEJMoa2212728
5
Park J Cheon JH . Anti-tumor necrosis factor therapy in intestinal behcet's disease. Gut Liver. (2018) 12:623–32. doi: 10.5009/gnl17462
6
Miyazaki H Watanabe D Okamoto N Tokunaga E Ku Y Takenaka H et al . Efficacy and predictor of anti-TNFalpha agents in patients with intestinal Behcet's disease. BMC Gastroenterol. (2022) 22:149. doi: 10.1186/s12876-022-02221-0
7
Lee SB Hong HS Lee CK Lee BI Kim S Koh SJ et al . Real-world effectiveness and safety of adalimumab in Korean patients with intestinal Behcet’s disease: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter study. Korean J Intern Med. (2023) 38:661–71. doi: 10.3904/kjim.2022.394
8
Tulunay A Dozmorov MG Ture-Ozdemir F Yilmaz V Eksioglu-Demiralp E Alibaz-Oner F et al . Activation of the JAK/STAT pathway in Behcet's disease. Genes Immun. (2015) 16:170–5. doi: 10.1038/gene.2014.64
9
Liu J Hou Y Sun L Li C Li L Zhao Y et al . A pilot study of tofacitinib for refractory Behcet's syndrome. Ann Rheum Dis. (2020) 79:1517–20. doi: 10.1136/annrheumdis-2020-217307
10
Zou J Cai JF Ye JF Guan JL . Tofacitinib as an alternative therapy for refractory intestinal Behcet's syndrome. Ther Adv Musculoskelet Dis. (2022) 14:1759720X221124014. doi: 10.1177/1759720X221124014
11
Schwartz DM Kanno Y Villarino A Ward M Gadina M O'Shea JJ et al . JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discovery. (2017) 16:843–62. doi: 10.1038/nrd.2017.201
12
Kraev K Uchikov P Hristov B Kraeva M Basheva-Kraeva Y Popova-Belova S et al . Coexistence of ankylosing spondylitis and Behcet's disease: Successful treatment with upadacitinib. Immun Inflammation Dis. (2024) 12:e1242. doi: 10.1002/iid3.v12.4
13
Tao T He D Peng X Huang Z Su W . Successful remission with upadacitinib in two patients with anti-TNF-refractory macular edema associated with behcet's uveitis. Ocul Immunol Inflammation. (2023) 32(8):1897–900. doi: 10.1080/09273948.2023.2263557
14
Burmeister GR Cohen SB Winthrop KL Nash P Irvine AD Deodhar A et al . Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. (2023) 9(1):e002735. doi: 10.1136/rmdopen-2022-002735
Summary
Keywords
intestinal Behçet’s disease, upadacitinib, case report, mucosal healing, anti-TNF-refractory
Citation
Sha S, Xu B, Wang K, Qiao C, Shi H, Jiang J, Quan X and Liu X (2024) Case report: Successful remission with upadacitinib in a young patient with anti-TNF-refractory intestinal Behçet’s disease. Front. Immunol. 15:1483993. doi: 10.3389/fimmu.2024.1483993
Received
21 August 2024
Accepted
18 October 2024
Published
08 November 2024
Volume
15 - 2024
Edited by
Lazaros Ignatios Sakkas, University of Thessaly, Greece
Reviewed by
Zhang Ning, Capital Medical University, China
Shubhasree Banerjee, University of Pennsylvania, United States
Updates
Copyright
© 2024 Sha, Xu, Wang, Qiao, Shi, Jiang, Quan and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Liu, docliuxin126@xjtu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.