- 1Department of Laboratory Medicine, Fujian Key Laboratory of Laboratory Medicine, Gene Diagnosis Research Center, Fujian Clinical Research Center for Clinical Immunology Laboratory Test, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 2Department of Laboratory Medicine, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Background: The varying individual responses to Pegylated interferon-α (Peg-IFNα) in patients with chronic hepatitis B (CHB) pose significant hurdles in treatment optimization, and the underlying mechanisms remain unclear.
Objective: We aimed to identify genetic polymorphisms influencing the efficacy of Peg-IFNα in patients with HBeAg-positive CHB, with the goal to predict Peg-IFNα response before treatment.
Methods: We employed an Asian Screening Array analysis involving 124 HBeAg-positive CHB patients treated with Peg-IFNα. We conducted assessment of immunological markers and mRNA expression of pivotal genes, establishing correlations with SNPs, functional genes of SNPs, and efficacy of Peg-IFNα therapy. In vitro experiments were performed to verify the functional involvement of the candidate SNPs.
Results: The G allele presented in rs2278420 and rs6509607 were significantly more common in patients who achieved a complete response (CR) compared to those who had a suboptimal response (SR), and linked to an increased rates of HBeAg loss following Peg-IFNα treatment (all p < 0.05). Additionally, the mRNA level of ZNF350 varied notably across different genotypes of both SNPs as determined by eQTL analysis, and showed higher expression in patients achieved a SR (all p < 0.05). In vitro investigations with IFNα stimulation showed that the mRNA level of SOCS3 was elevated in patients with rs2278420 genotype AA, similarly, mRNA levels of PKR, STAT2, SOCS1, SOCS3, PIAS1, PTPN6 and TRIM8 were heightened in patients with rs6509607 genotype AA compared to those with genotypes (AG+GG) (all p < 0.05).
Conclusion: The G allele of rs2278420 and rs6509607 were associated with mRNA level of ZNF350, with an increased probability of Peg-IFNα response in HBeAg-positive CHB patients, likely through the modulation of JAK-STAT signaling pathway.
1 Introduction
Chronic hepatitis B virus (HBV) infection remains a significant global health concern, affecting approximately 296 million individuals worldwide (1). Pegylated interferon-α (Peg-IFNα) and nucleos(t)ide analogues (NAs) are currently the mainstream therapies for chronic hepatitis B (CHB) (2). Despite their availability, only a minority of patients achieve the sustained virological response, and the underlying reasons for this variability in treatment outcomes remain largely unclear (3–5).
Recent studies have begun to uncover the role of single nucleotide polymorphisms (SNPs) in host genes in influencing both susceptibility to HBV infection and the individual differences in interferon response. For example, certain genotypes such as AT of rs77076061 and GG of rs1979262 have been identified as protective factors to HBsAg-positive patients, likely through influencing C19orf66 expression by alteration of transcription factors binding (6). The genotype CC of rs10838543 in the TRIM22 gene was associated with a beneficial response to PegIFN-α through the modulation of immunoinducible cytokines (7). The rs7519753 allele C was reported to be correlated to increased expression of the TP53BP2 protein, and associated with a higher probability of serum HBsAg loss in CHB patients following Peg-IFNα treatment (8). However, the genetic determinants that contribute to the therapeutic outcomes with Peg-IFNα and their underlying mechanisms remain partially understood, warranting further exploration.
Genome-wide association studies (GWAS), widely used to investigate complex traits genetics, disease biology, and new therapeutic development (9, 10), have recently incorporated an Asian Screening Array (ASA) program. This program includes East Asia’s largest whole-genome reference dataset, has newly identified over 120,000 clinically significant variants as well as drug efficacy-related variants, HLA regional variants, and GWAS susceptibility variants.
In this study, we conducted an ASA analysis on HBeAg-positive CHB patients who treated with Peg-IFNα, and identified two new loci (rs2278420 and rs6509607) associated with HBeAg loss on chromosome 19. Furthermore, these two SNPs were found to be associated with the regulation of IFNα signaling pathway in vitro. Additionally, through expression quantitative trait locus (eQTL) analysis and mRNA detection, we confirmed that the differential expression of ZNF350, a gene where rs2278420 is located on and 1.5 kb downstream of rs6509607, across different genotypes of SNPs and was associated with the varying efficacy of Peg-IFNα treatment. Our findings highlighted two new genetic biomarkers associated with the efficacy of PegIFN-α therapy, providing new ideas in guiding therapy selection for CHB patients.
2 Materials and methods
2.1 Subjects
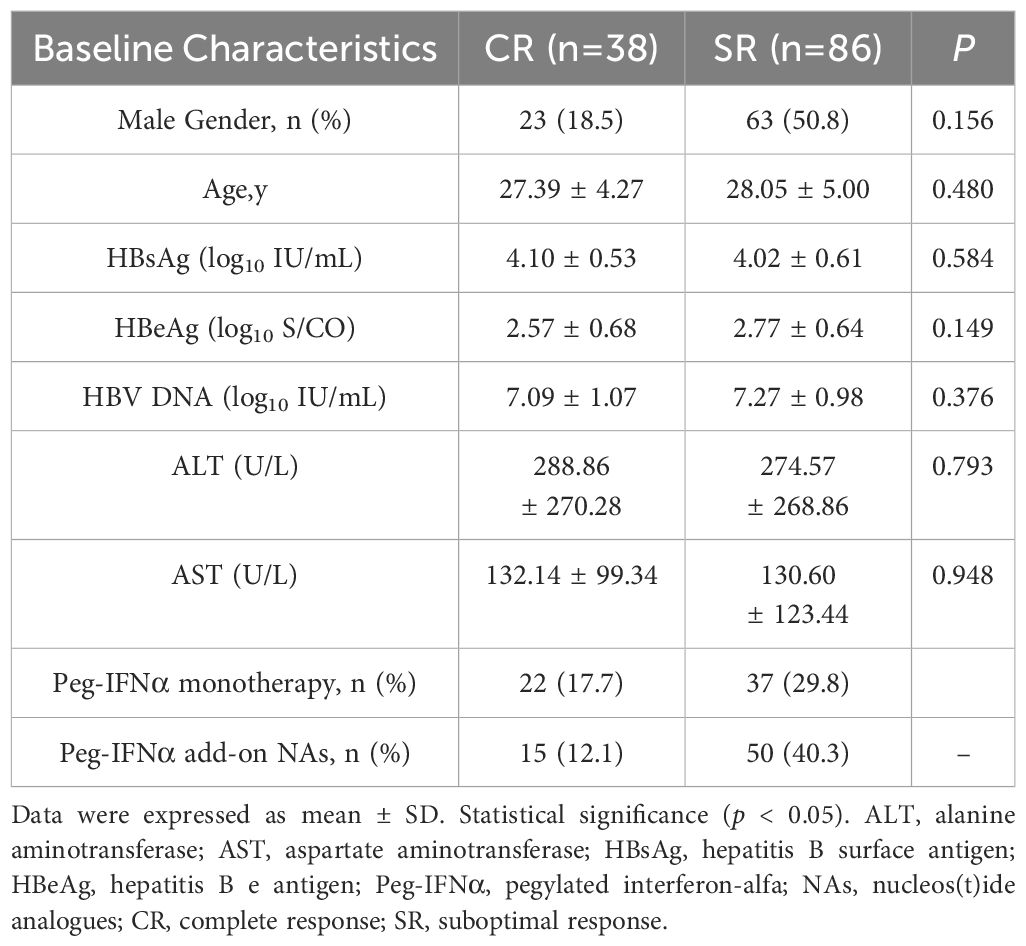
A total of 124 HBeAg-positive CHB patients treated with Peg-IFNα from the First Affiliated Hospital of Fujian Medical University were enrolled in this study. The inclusion criteria were as follows: i) a previous lack of any antiviral therapy; ii) age ≥ 16 years old; iii) HBsAg positive for more than 6 months; iv) HBeAg positive; v) HBV DNA ≥ 2000 IU/mL at baseline; vi) serum alanine aminotransferase (ALT) ≥ 40U/L; vii) patients were treated with Peg-IFNα for at least 48 weeks and subsequently observed until 72 weeks. Patients with the other infectious liver diseases or autoimmune diseases were excluded. Of these 124 patients, 63 patients were treated with Peg-IFNα monotherapy while the other 61 patients were received combination therapy of Peg-IFNα and NAs. All subjects were divided into a complete response (CR) group and a suboptimal response (SR) group. CR was simultaneously satisfied with HBV DNA persistently < 500 IU/mL, HBeAg loss, and ALT < 40 U/L, while patients who failed to meet either one of the criteria above were considered SR. For the cytological experiments, we enrolled 81 patients who were newly diagnosed with HBV infection and tested positive for HBeAg in physical examination center of the First Affiliated Hospital of Fujian Medical University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University.
2.2 Genotyping
Genomic DNA was extracted from 2mL of EDTA-anticoagulated peripheral blood using standard procedures of QIAamp DNA Mini Kit (QIAGEN, Germany). Genotyping of a total of 124 CHB patients were performed using high-throughput Infinium Asian Screening Array-24 (ASA) v1.0 Kit (Illumina, Inc., San Diego, CA, USA) and were detected by BioMiao Biological Technology (Beijing) Co., Ltd. All of the raw data was read by Illumina Genomstudio software. Sanger sequencing by BGI (Shanghai) Technology Co., Ltd was performed to identify the target SNPs of 81 HBV infected patients, and primers were designed by Oligo 7 software (Supplementary Table S1).
2.3 Measurement of serum HBV DNA, HBsAg, HBeAg and ALT
Serum HBsAg and HBeAg were quantified using the automated chemiluminescent microparticle immunology analyzer (Abott Architect-I4000, Chicago, IL, USA). Biochemical indicators such as Serum ALT and AST were detected by automatic biochemical analyzer ADVIA 2400 (Siemens, Munich, Germany). HBV DNA was detected with Roche LightCyCler480 (Roche Corporation, Basel, Switzerland) and quantitative real-time PCR method (Sansure Biotech Inc., Hunan, China).
2.4 eQTL analysis
To identify the target genes associated with SNPs, we performed the eQTL analysis through the GTEx database (https://gtexportal.org/), a project collected samples from non-diseased tissue sites. The correlations between SNPs and ZNF350 expression in the whole blood and other tissue of GTEx were analyzed through the “GTEx eQTL Calculator”.
2.5 Cell culture and stimulation
The EDTA-anticoagulated peripheral blood samples were collected from 81 patients who diagnosed with HBV infection firstly. Freshly isolated PBMCs were cultured on 96-well plates at 37 °C in a 5% CO2 incubator for 18 hours and subsequently incubated with IFNα (1,000 U/mL; Beyotime Biotechnology) for 6 hours. All cells were collected for RNA extraction using TRIzol Reagent (Thermo Fisher Scientific, USA).
2.6 Quantitative reverse transcription-polymerase chain reaction
RNA reverse transcription was performed with TransStart® Green qPCR Super Mix (TransGen Biotech, Beijing, China). The qPCR was performed with 2 plus SYBR Green PCR mix (Takara, Tokyo, Japan) on the ABI Step One Plus RT-PCR system (Applied Biosystems, CA, USA) with the program as follows: 30 s at 95 °C, then 40 cycles of 5 s at 95 °C and 30 s at 60 °C. Primers were designed by Oligo 7 software (Supplementary Table S1). The data of qRT-PCR were analyzed by the 2-ΔCt method.
2.7 Statistical analysis
Clinical indicators were represented as mean ± SD or indicated otherwise. The repeated measurement data were analyzed by generalized estimating equation. Student’s T test or Mann-Whitney U test was used for comparing two independent groups, and chi-square (χ2) test was used for categorical data. The correlation analysis was performed by the Pearson analysis. Data were analyzed by SPSS20.0 software (SPSS, Inc.) and figures were made by GraphPad Prism 6.0 (GraphPad Software, Inc.). P < 0.05 was considered statistically significant.
3 Results
3.1 Cohort characteristics and ASA association analysis
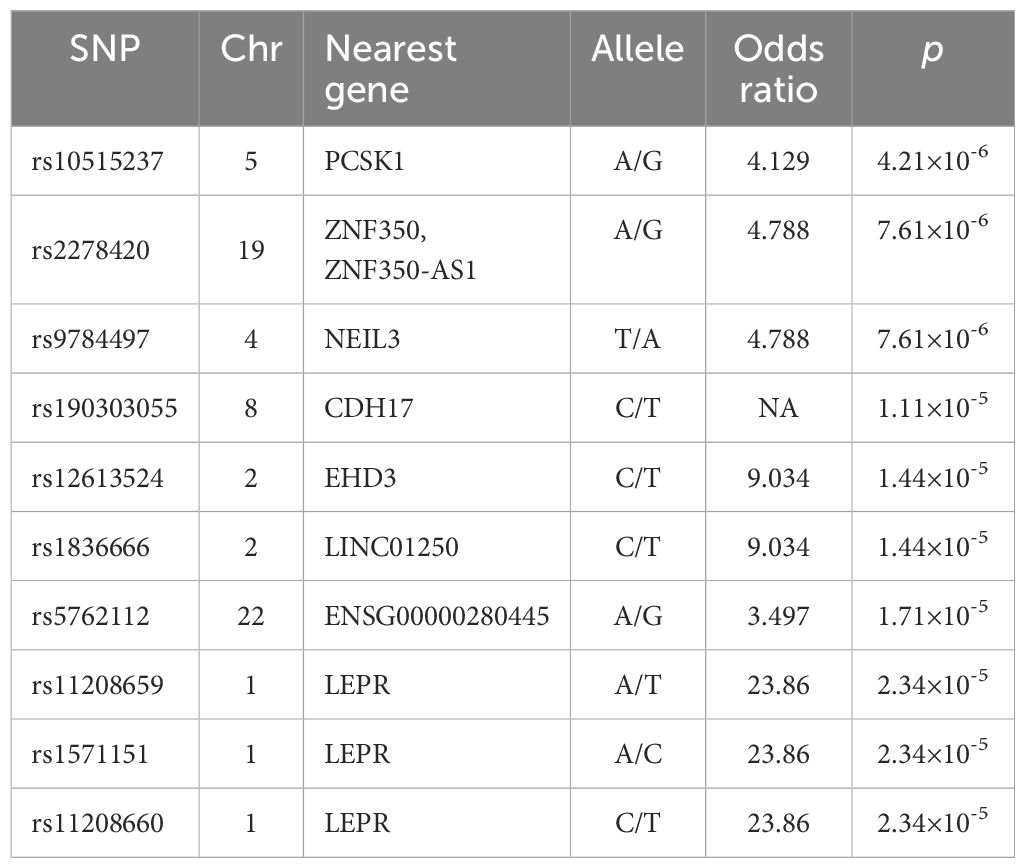
Previous studies demonstrated that baseline levels of HBsAg, HBeAg, HBV DNA, ALT and AST were associated with HBeAg or HBsAg loss in CHB patients following Peg-IFNα treatment (11, 12). However, in our study, there were no significant differences observed in the distribution of gender, age, and baseline levels of serological markers including HBsAg, HBeAg, HBV DNA, ALT, and AST between CR and SR groups (p > 0.05). General characteristics of the subjects were shown in Table 1. A total of 23,506 SNPs were found to be associated with the efficacy of Peg-IFNα in HBeAg-positive CHB patients (p < 0.05, Figure 1A). The top 10 SNPs and corresponding p-values were summarized in Table 2.
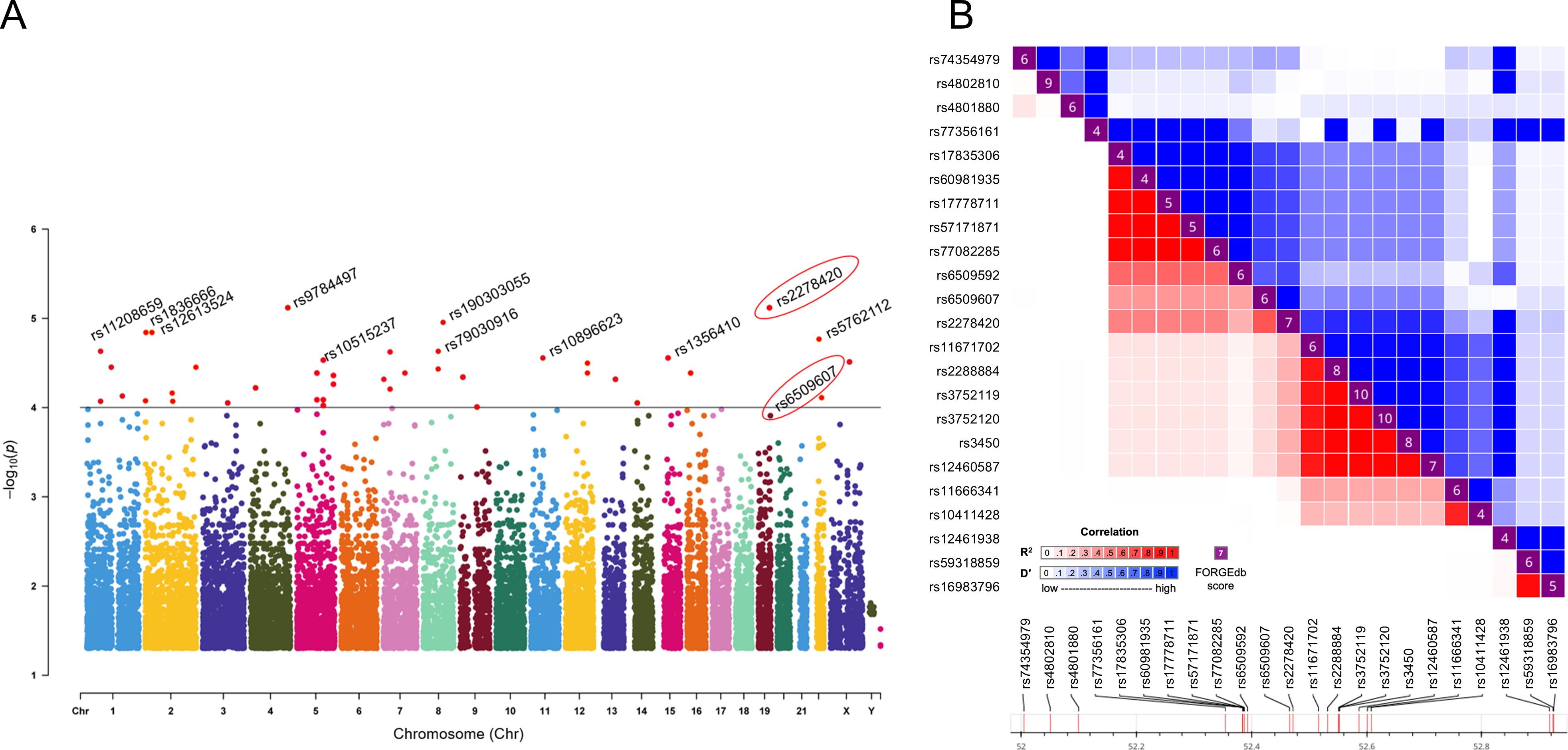
Figure 1. Rs2278420 and rs6509607 were associated with Peg-IFNα treatment efficacy. (A) The Manhattan plot showed the location of all significant SNPs in the human genome (UCSC hg37) and p-values for ASA analysis. The dots with p < 10-4 were all marked in red. (B) Linkage disequilibrium analysis showed D' and R2 for rs2278420 with other SNPs within 500 kb upstream and downstream of rs2278420.
3.2 rs2278420 and rs6509607 are significant loci associated with Peg-IFNα efficacy
The rs2278420, a missense variant, located on 5’-UTR of zinc finger protein 350 (ZNF350) and ZNF350 antisense RNA 1 (ZNF350-AS1), was found to be significantly associated with Peg-IFNα efficacy (p = 7.61×10-6, Table 2). We performed linkage disequilibrium (LD) analysis to sites within 500 kb upstream and downstream of rs2278420, the rs6509607 was found to be in LD with rs2278420 (D' = 1.0, R2 = 0.672, Figure 1B), which also had a relationship with Peg-IFNα efficacy (p=3.77×10-4, Table 3).
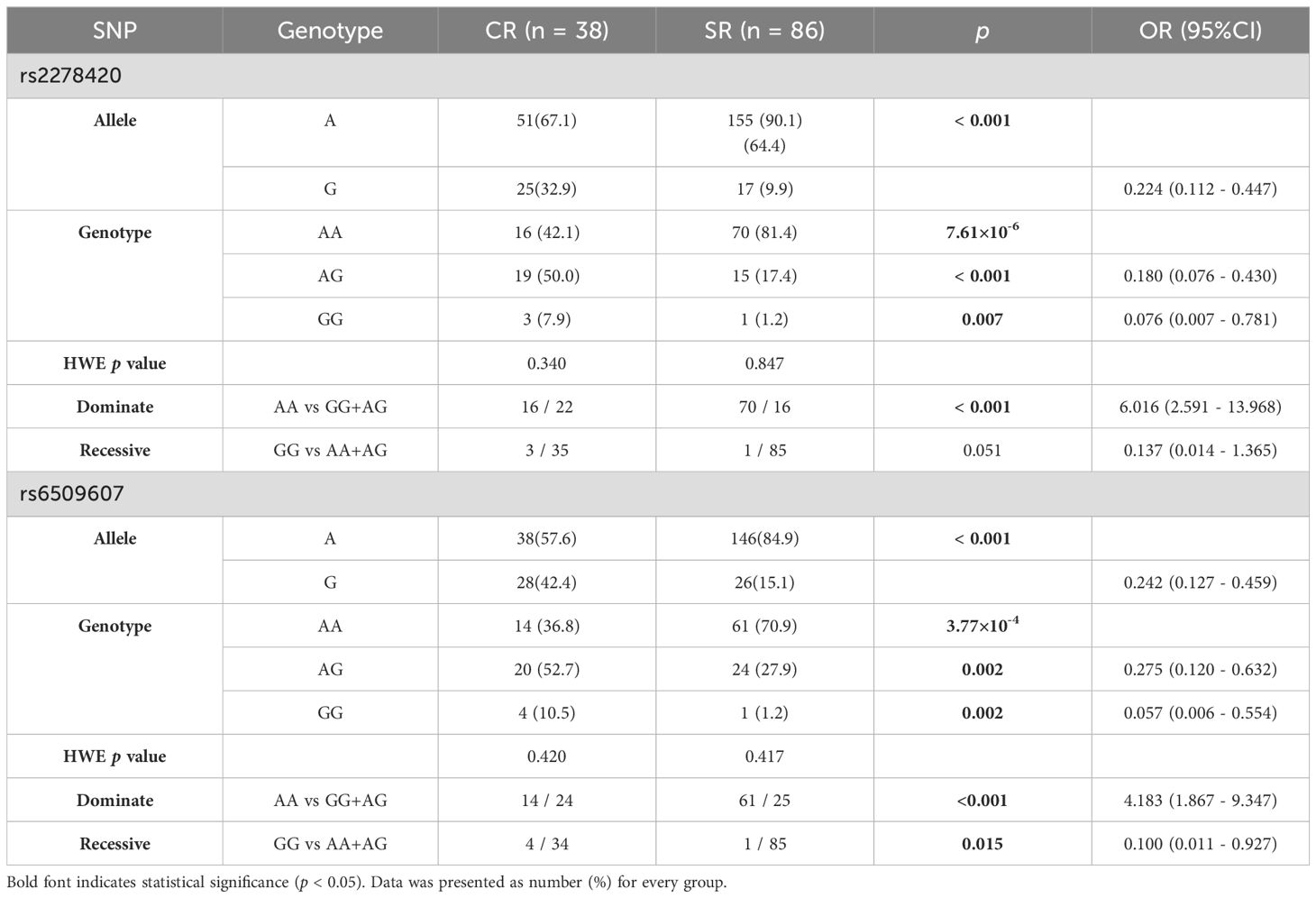
Table 3. Genotypes and alleles frequency of rs2278420 and rs6509607 in patients with Peg-IFNα treatment.
The allele A and the genotype AA of rs2278420 in SR group were more common compared to those in CR group (p < 0.001 and p = 7.61×10-6). Under the dominate model, the frequency of rs2278420 genotype AA was higher in SR group than that in CR group, and patients with genotype AA was found to have 6-fold increased risk of developing SR (p < 0.001, OR = 6.016, Table 3). For rs6509607, the genotype AA and the allele A were more prevalent in SR group compared to those in CR group (p = 3.77×10-4 and p < 0.001). The genotype AA was more likely to be observed in SR group under the dominate model. Likewise, the proportion of genotype GG was significantly higher in CR group under the recessive model (p < 0.001 and p = 0.015). Moreover, there was 4-fold increased risk of developing SR in patients with genotype AA (OR = 4.138, Table 3).
3.3 rs2278420 allele G and rs6509607 allele G are associated with HBeAg loss by Peg-IFNα treatment
The serum levels of HBV DNA, HBsAg, HBeAg and ALT, as well as the rates of HBeAg loss, were observed at specific time points over 72 weeks from the start of Peg-IFNα treatment in all HBeAg-positive CHB patients. The results showed that the levels of HBV DNA, HBsAg, HBeAg, and ALT decreased significantly as the treatment duration increased, but the trends in the decline of HBsAg differed between the two genotypes of both SNPs (all p <0.05, Supplementary Table S2). The mean concentration of HBeAg in patients with genotypes (AG+GG) was lower than those with genotype AA during Peg-IFNα treatment (p rs2278420 = 0.004, Figure 2C and p rs6509607 = 0.009, Figure 2G, Supplementary Table S2). From a temporal perspective, at 24, 36, 48, 60 and 72 weeks of Peg-IFNα treatment in patients with rs2278420 genotype (AG+GG), and at 4, 24, 36 and 48 weeks of Peg-IFNα treatment in patients with rs6509607 genotype (AG+GG), the mean concentration of serum HBeAg were significantly lower than those with genotype AA. (rs2278420: p 24W= 0.009, p 36W = 0.005, p 48W= 0.021, p 60W= 0.030 and p 72W= 0.019, Figures 2A–D; rs6509607: p 4W= 0.031, p 24W= 0.035, p 36W = 0.012 and p 48W = 0.032, Figures 2E–H).
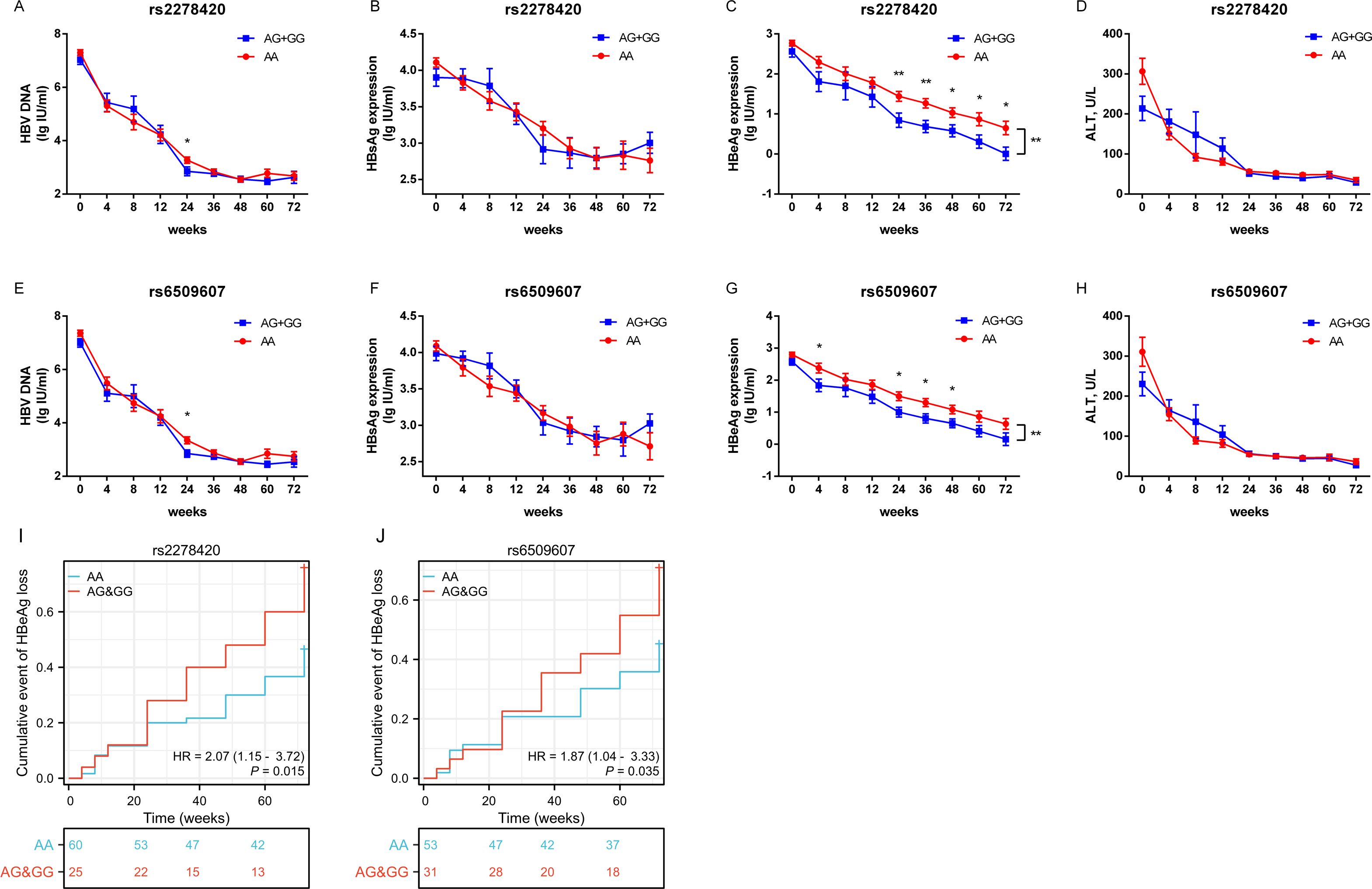
Figure 2. The allele G at both rs2278420 and rs6509607 was associated with HBeAg loss by Peg-IFNα treatment. Serum levels of (A, E) HBV DNA, (B, F) HBsAg, (C, G) HBeAg, and (D, H) ALT in rs2278420 (AG+GG) (blue)/AA (red) genotypes and rs6509607 (AG+GG) (blue)/AA (red) genotypes of all CHB patients at 0, 4, 8, 12, 24, 36, 48, 72 weeks since Peg-IFNα treatment. Generalized estimating equations were used to analyze the differences in overall changes of viral indicators between different genotypes, while t-tests were used to analyze the differences at a single time point between different genotypes. (I, J) The cumulative event of HBeAg loss was analyzed with cox regression analysis. Error bars, SEM. * p < 0.05, ** p < 0.01.
Furthermore, the cox regression model of HBeAg loss demonstrated that genotypes of rs2278420 and rs6509607 were independent factors to HBeAg loss (p = 0.015 and p = 0.035, Figures 2I, J). Patients with genotype (AG+GG) had respectively 2.07-fold and 1.87-fold increased probability of developing HBeAg loss (HR=2.07 and HR=1.87, Figures 2I, J). Which suggested that the allele G at both rs2278420 and rs6509607 might be related to HBeAg loss in CHB patients receiving Peg-IFNα therapy.
3.4 Genotypes of rs2278420 and rs6509607 are associated with ZNF350 mRNA expression
To investigate the potential functional genes related to rs2278420 and rs6509607, we conducted eQTL analysis based on GTEx database. The analysis revealed that rs2278420 allele G and rs6509607 allele A were significantly linked to increased ZNF350 mRNA levels, not only in the whole blood (p = 6.7×10-4 and p = 9.4×10-5 Figures 3A, B), but also in other organs such as liver (p = 0.0063 and p = 0.18) and thyroid (p = 2.2×10-7 and p = 5.3×10-7) according to GTEx data.
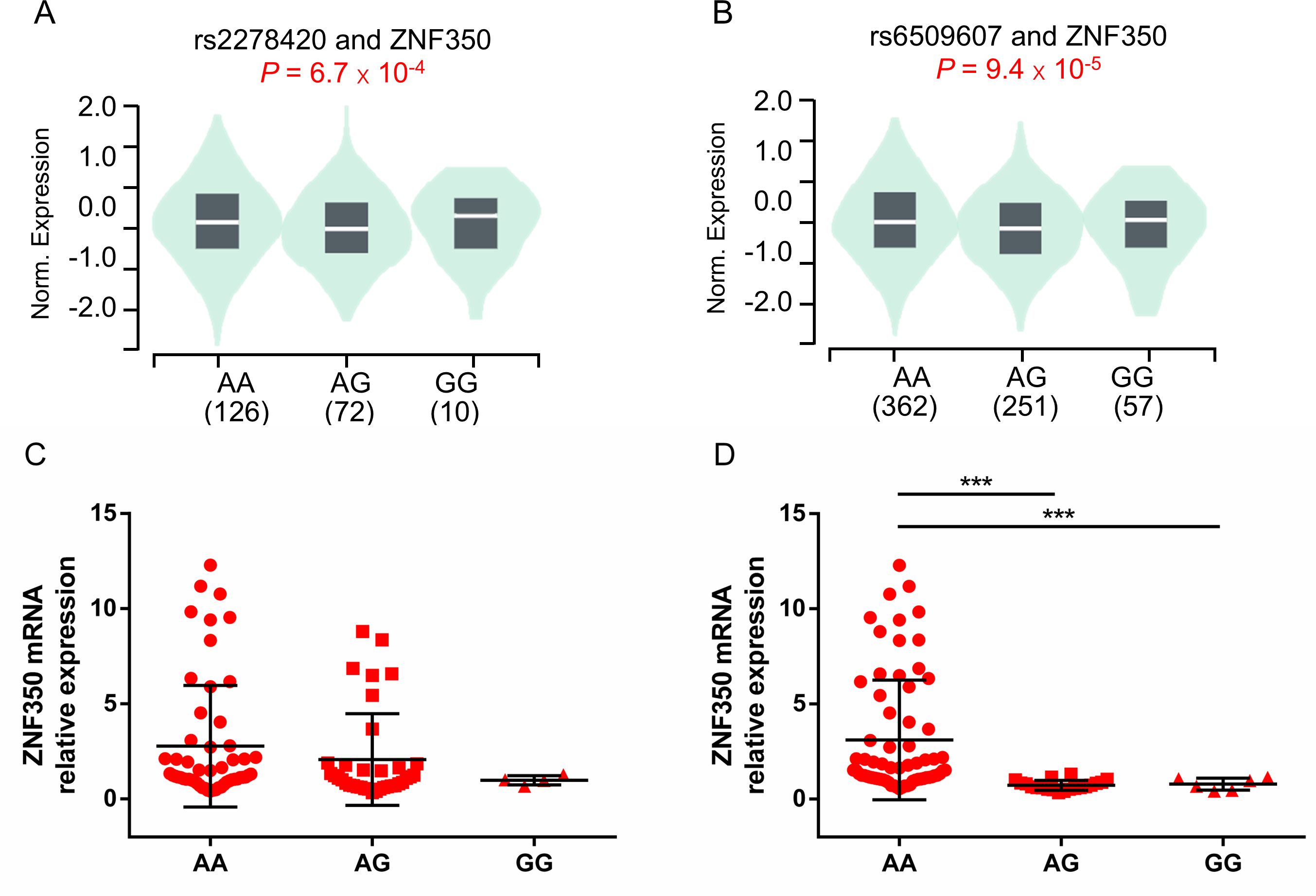
Figure 3. The comparison of ZNF350 mRNA expression with different genotypes of rs2278420 and rs6509607 in the whole blood. Comparison of whole blood ZNF350 mRNA levels in subjects with (A) different rs2278420 genotypes (AA, AG and GG) and (B) different rs6509607 genotypes (AA, AG and GG), results were shown from healthy samples of GTEx database. The expression of ZNF350 mRNA in AA, AG and GG genotype of (C) rs2278420 and (D) rs6509607 in HBeAg-positive HBV carriers. *** p < 0.001.
To observe whether this correlation between ZNF350 mRNA level and genotype exist in the presence of HBV, we randomly enrolled and genotyped 81 HBV carriers who were HBeAg positive, and detected the mRNA expression of ZNF350. There were 46 patients with AA, 31 patients with AG and 4 patients with GG genotype at rs2278420, while there were 59 patients with AA, 16 patients with AG and 6 patients with GG genotype at rs6509607. The results showed that the level of ZNF350 mRNA was higher in patients with genotype AA than those in patients with genotypes AG and GG at rs6509607 (p < 0.001 and p < 0.001, Figure 3D). Similar results were observed in rs2278420, but they were not statistically significant, probably due to the small number of patients with GG (p > 0.05, Figure 3C). The results suggested that the allele A at rs6509607 was associated with increased ZNF350 mRNA expression in individuals with HBV infection.
3.5 Lower ZNF350 mRNA expression is associated with CR in CHB patients treated with Peg-IFNα
To observe whether the expression of ZNF350 gene was associated with the efficacy of Peg-IFNα, the levels of biochemical indicators and ZNF350 mRNA from the whole blood of cohort patients were analyzed before treatment. The results showed that the level of ZNF350 mRNA in SR group was higher than that in CR group (p = 0.018, Figure 4A), and the correlation analysis demonstrated that the expression of ZNF350 mRNA were negatively correlated with serum levels of ALB, TP, AST, LDH, while positively associated with serum level of TBA (r ALB = -0.548, p = 0.002; r TP = -0.464, p = 0.011; r AST = -0.415, p = 0.025; r LDH = -0.328, p = 0.041; r TBA = 0.521, p = 0.006, Figures 4B–F).
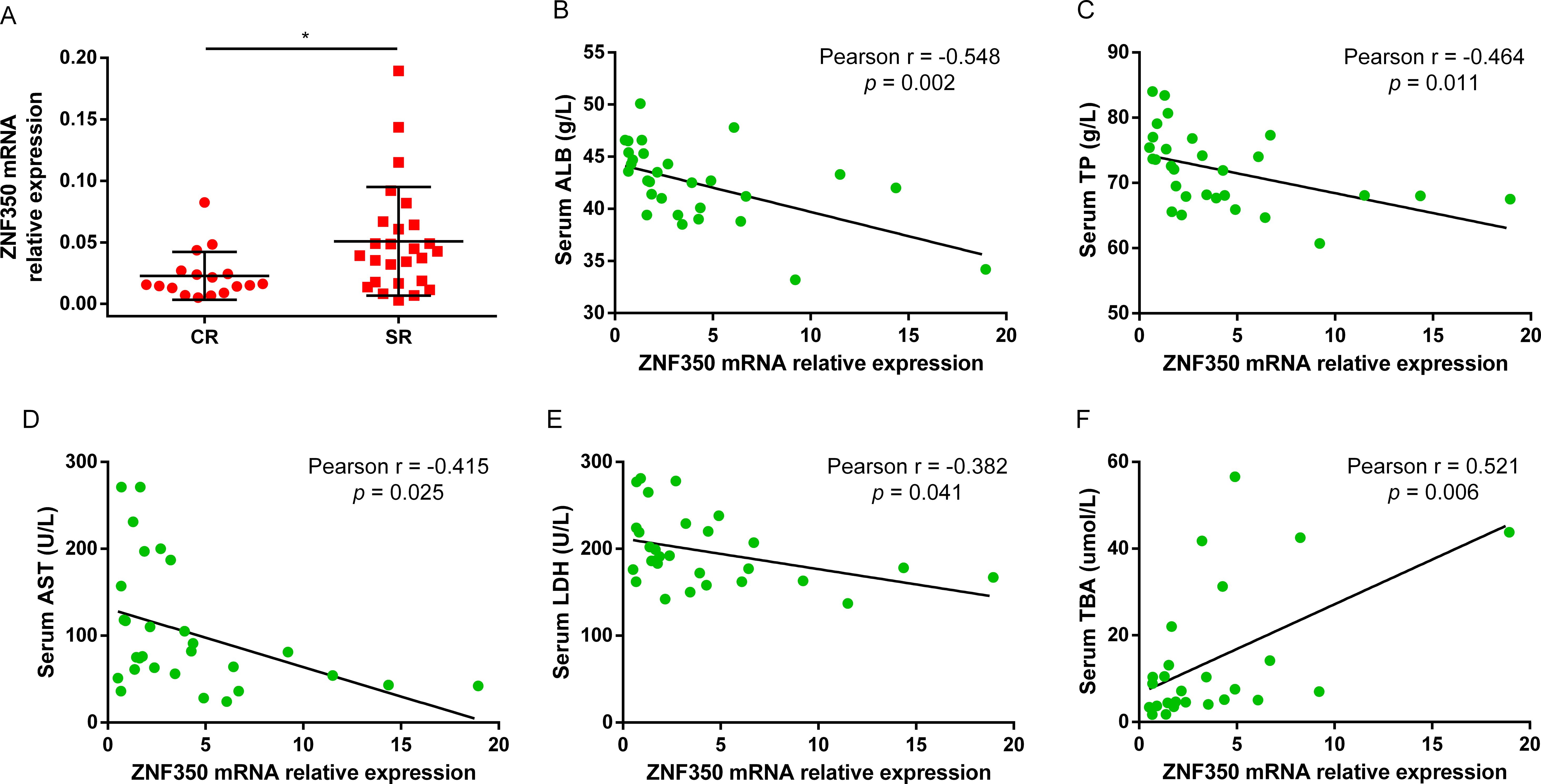
Figure 4. The mRNA level of ZNF350 was associated with CR of Peg-IFNα. (A) The mRNA expression of ZNF350 gene between CR group and SR group in CHB patients before Peg-IFNα treatment. (B-F) Correlation analysis between the level of ZNF350 mRNA and serum ALB, TP, AST, LDH and TBA. ALB, albumin; TP, total protein; AST, aspartate aminotransferase; LDH, lactic dehydrogenase; TBA, total bile acid. * p < 0.05.
3.6 The genotypes of rs2278420 and rs6509607 are associated with the regulation of IFNα signaling pathway
To explore the mechanism by which the antiviral effect of IFNα was affected by genotypes of rs2278420 and rs6509607, freshly isolated PBMCs from 81 patients enrolled above were cultured for 18 hours and subsequently incubated with IFNα for 6 hours. Finally, mRNA was extracted for detection. The mRNA level of SOCS3 gene in patients with rs2278420 genotype AA was higher than those in patients with genotypes (AG+GG) (p = 0.027, Figure 5A). The mRNA levels of PKR, STAT2, SOCS1, SOCS3, PIAS1, PTPN6, and TRIM8 genes in patients with rs6509607 genotype AA were higher than those in patients with genotypes (AG+GG). In contrast, the mRNA level of IFIT3 in patients with AA was lower than those in patients with (AG+GG) (p PKR = 0.029, p STAT2 = 0.013, p SOCS1 = 0.030, p SOCS3 = 0.030, p PIAS1 = 0.025, p PTPN6 = 0.003, p TRIM8 = 0.011, and p IFIT3 = 0.016, Figure 5B). Collectively, these results suggested that the IFNα signaling pathway might be modulated by the polymorphisms of rs2278420 and rs6509607, with the AA genotype likely playing a major role in negative regulation.
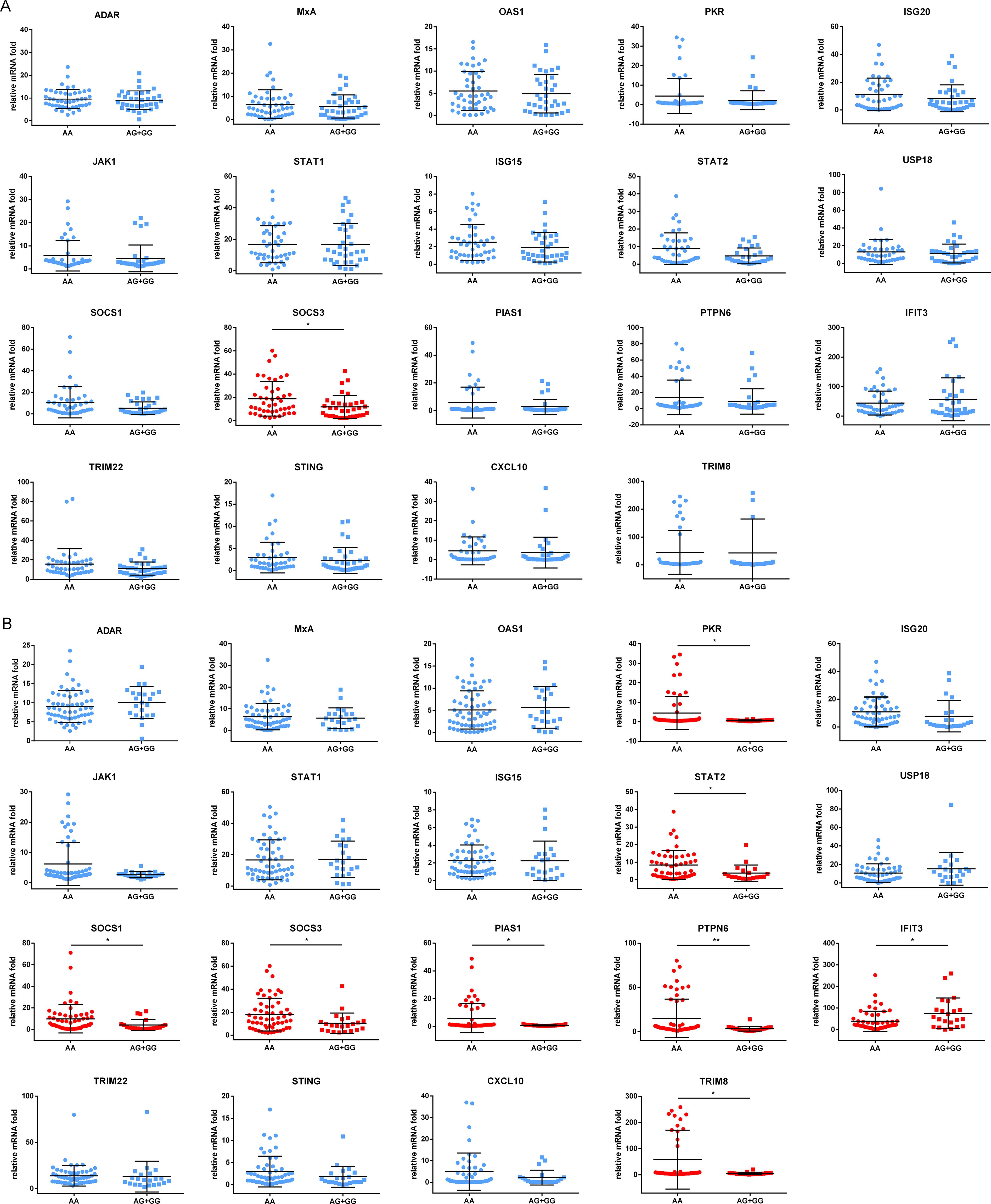
Figure 5. Expression of proteins induced by IFNα in different genotypes of rs2278420 and rs6509607. The peripheral blood mononuclear cells (PBMCs) of patients with (A) rs2278420 or (B) rs6509607 were cultured in vitro and stimulated with IFNα (1000U/L) for 6 hours, then the mRNA levels were detected. ADAR, Adenosine Deaminase; MxA, Myxoma Resistance Protein 1; OAS1, 2’-5’-Oligoadenylate Synthetase; PKR, Double Stranded RNA-activated Protein Kinase; ISG20, Interferon Stimulated Gene 20; JAK1, Janus-activated Kinase Janus 1; STAT1, Signal Transducer and Activator of Transcription1; USP18, Ubiquitin Specific Peptidase 18; SOCS1, Suppressor of Cytokine Signaling1; PIAS1, Protein Inhibitor of Activated Stat 1; PTPN6, Protein Tyrosine Phosphatase, Non-Receptor Type 6; IFIT3, Interferon Induced Protein with Tetratricopeptide Repeats; TRIM22, Tripartite Motif 22; STING, Stimulator of Interferon Genes Protein; CXCL10, C-X-C Motif Chemokine Ligand C-X-C 10. * p < 0.05, ** p < 0.01.
4 Discussion
To explain individual differences in susceptibility to HBV, disease development, clinical outcomes, and therapeutic efficacy, recent genetic studies have primarily focused on variants of HLA genes, Toll-like receptor pathway-related genes, cytokine genes, and interferon stimulated genes (ISGs) (13, 14). However, limited research has explored the correlation between single nucleotide polymorphisms (SNPs) and the effectiveness of antiviral therapy, with existing studies producing conflicting results (9, 15–17). The poor tolerance and limited efficacy of IFNα treatment necessitate the prediction of responsive patients prior to initiating therapy.
We found for the first time that ZNF350 gene polymorphisms were associated with the efficacy of Peg-IFNα in patients with HBeAg-positive CHB. ZNF350 belongs to Krüppel-associated box domain zinc finger proteins (KRAB-ZFPs) family, which is one of the largest families of transcriptional regulators in higher vertebrates. KRAB-ZFPs act as transcriptional repressors in repression of transposable elements (TEs) and as mediators of DNA methylation (18–20). ZNF350 interacts with a specific consensus sequence within intron 3 of the growth arrest and DNA damage inducible (GADD45) gene, which is induced by BRCA-1 and functioned as a transcriptional repressor (21, 22).
In this study, the lower level of ZNF350 mRNA was associated with complete response of Peg-IFNα in patients with HBeAg-positive CHB, along with higher serum level of ALB and lower serum level of TBA. The stability of the HBV covalently closed circular DNA (cccDNA) in non-dividing hepatocytes is a crucial factor to HBV persistence and limits the effectiveness of antiviral drugs. This residual of cccDNA can be broken down by hepatocyte regeneration, and elevated ALB level can serve as an indicator for monitoring hepatocyte regeneration (23–25). Conversely, abnormal accumulation of bile acids promoted HBV replication and inhibited Peg-IFNα response through impairing the functions of CD8+ T cells and NK cells (26). This correlation between ZNF350 mRNA and biochemical indicators such as ALB and TBA reinforced the finding that ZNF350 was a negative factor of Peg-IFNα response.
Our results based on the eQTL analysis revealed that genotypes of rs2278420 and rs6509607 were associated with ZNF350 mRNA level in the whole blood and several other tissues. The expression of ZNF350 might be organ-specific. In the whole blood, the allele G of rs2278420 was reported to be associated with higher ZNF350 mRNA expression, while in the artery-tibial, the allele A was reported to be associated with higher ZNF350 mRNA expression. We observed the same pattern in the artery-tibial in this study. This may be due to the non-disease populations of GTEx data, insufficient sample size, or different physiological states accompanying HBV infection.
The allele G of rs2278420 was reported to significantly influence individual susceptibility to breast cancer (27). In this study, the G allele in both rs2278420 and rs6509607 were found to be associated with a complete response to Peg-IFNα treatment in HBeAg-positive CHB patients, and related to a higher rate of HBeAg loss during Peg-IFNα therapy.
In exploring the mechanism by which gene polymorphisms were involved in the antiviral effect of IFNα, we found that many genes exhibited differential expression across different genotypes of rs6509607. This may be attributed to the variant expression of ZNF350 caused by different genotypes, as ZNF350 may be involved in the regulation of these genes' expression. Similarly, the rs2278420 genotype was related to SOCS3 mRNA level, possibly because the genotype also affected the properties of ZNF350. Although there was interferon stimulated gene (such as TRIM8) expressed higher in the AA genotype of rs6509607, it was primarily the negative regulatory genes of IFNα signaling pathway (such as SOCS1, SOCS3 and PIAS1) that were elevated in the AA genotype. In addition, at both SNPs of the AA genotype was the mRNA level of SOCS3 gene elevated, further indicating that the AA genotype may play a primary role in the negative regulation of IFNα signaling pathway. This also explained why patients with AA genotype of both SNPs had a lower rate of HBeAg loss.
rs2278420 is located not only on the ZNF350 gene, but also on ZNF350-AS1, and its linkage disequilibrium site, rs6509607, is also located on ZNF350-AS1. ZNF350-AS1, known as hepatocellular carcinoma associated transcript 3 (HCCAT3), is a lncRNA which potentially facilitating tumor growth in patients with HBV-related HCC (28). Systematic large-scale studies have shown that abnormal expression of antisense RNA (asRNA) is associated with tumorigenesis (29). AsRNAs could be powerful tools in antiviral and anticancer treatments (30). As mentioned above, asRNA can inhibit the transcription of target mRNA, but whether gene polymorphisms in asRNA affect its inhibitory activity remains unknown. In this study, we also explored the association between ZNF350-AS1 polymorphisms and its expression, but no statistical difference was found. Moreover, eQTL analysis did not show a correlation between ZNF350-AS1 expression and these two SNPs in peripheral blood or liver, but rs6509607 was found to have a strong correlation with ZNF350-AS1 expression in the testis and thyroid, so no further study was conducted.
In conclusion, our study reveals that polymorphisms in ZNF350 gene are associated with the efficacy of Peg-IFNα in patients with HBeAg-positive CHB, but it has not yet been fully explained how SNPs regulate IFNα signaling pathway. In the future, lager multicentric studies are needed and the mechanisms by which gene polymorphisms influence the efficacy of IFNα still require further investigation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GSE276479 (GEO).
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JG: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. SG: Data curation, Investigation, Writing – original draft. TC: Conceptualization, Methodology, Writing – original draft. YZ: Project administration, Supervision, Writing – original draft. YF: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft. QO: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Startup Fund for Scientific Research of Fujian Medical University (grant 2021QH1064), and the National Natural Science Foundation of China (grant 82372316, 82030063).
Acknowledgments
We thank other laboratory staff for technical assistance. We also thank all the patients who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1488055/full#supplementary-material
References
1. Hsu Y-C, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol. (2023) 20:524–37. doi: 10.1038/s41575-023-00760-9
2. Cornberg M, Lok AS-F, Terrault NA, Zoulim F. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. J Hepatol. (2020) 72:539–57. doi: 10.1016/j.jhep.2019.11.003
3. liver Eaftsot. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
4. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
5. Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2022). Zhonghua Gan Zang Bing Za Zhi. (2022) 30(12):1309–31. doi: 10.3760/cma.j.cn501113-20221204-00607
6. Liu N, Liu M, Yang J, Dong S, Yue M, Huang P, et al. Association of genetic polymorphisms in the C19orf66 gene and biochemical indices of HBV infected individuals in Yunnan. Front In Cell Infection Microbiol. (2023) 131180366. doi: 10.3389/fcimb.2023.1180366
7. Wang L, Lin N, Zhang Y, Guo S, Liu C, Lin C, et al. A novel TRIM22 gene polymorphism promotes the response to PegIFNα therapy through cytokine-cytokine receptor interaction signaling pathway in chronic hepatitis B. Microbiol Spectr. (2023) 11:e0224723. doi: 10.1128/spectrum.02247-23
8. Guan G, Zhang T, Ning J, Tao C, Gao N, Zeng Z, et al. Higher TP53BP2 expression is associated with HBsAg loss in peginterferon-α-treated patients with chronic hepatitis B. J Hepatol. (2024) 80:41–52. doi: 10.1016/j.jhep.2023.09.039
9. Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. (2017) 101:5–22. doi: 10.1016/j.ajhg.2017.06.005
10. Jia L, Li F, Wei C, Zhu M, Qu Q, Qin W, et al. Prediction of Alzheimer's disease using multi-variants from a Chinese genome-wide association study. Brain. (2021) 144:924–37. doi: 10.1093/brain/awaa364
11. Buster EHCJ, Hansen BE, Lau GKK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology. (2009) 137:2002–9. doi: 10.1053/j.gastro.2009.08.061
12. Zhang P-X, Tang Q-Q, Zhu J, Deng WY, Zhang ZH. Predictive models for functional cure in patients with CHB receiving PEG-IFN therapy based on HBsAg quantification through meta-analysis. Hepatol Int. (2024) 18:1110–21. doi: 10.1007/s12072-024-10666-6
13. Balagopal A, Thomas DL. Genomics on the road to functional cure of hepatitis B. J Hepatol. (2024) 80:6–7. doi: 10.1016/j.jhep.2023.11.002
14. Luo M, Dong C, Liang X, Na R, Zhou B, Huo J, et al. A genetic variant of CXCR4 predicts pegylated interferon-alpha treatment response in HBeAg-positive chronic hepatitis B patients. J Clin Microbiol. (2024) 62:e0139623. doi: 10.1128/jcm.01396-23
15. Jansen L, de Niet A, Makowska Z, Dill MT, van Dort KA, Terpstra V, et al. An intrahepatic transcriptional signature of enhanced immune activity predicts response to peginterferon in chronic hepatitis B. Liver Int. (2015) 35:1824–32. doi: 10.1111/liv.2015.35.issue-7
16. Lampertico P, Vigano M, Cheroni C, Facchetti F, Invernizzi F, Valveri V, et al. IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology. (2013) 57:890–6. doi: 10.1002/hep.25749
17. Luo M, Zhang L, Yang C, Zhou B, Hou J, Jiang DK. CXCL13 variant predicts pegylated-interferon α treatment response in HBeAg-positive chronic hepatitis B patients. J Med Virol. (2023) 95:e28963. doi: 10.1002/jmv.28963
18. Iyengar S, Farnham PJ. KAP1 protein: an enigmatic master regulator of the genome. J Biol Chem. (2011) 286:26267–76. doi: 10.1074/jbc.R111.252569
19. Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, et al. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. (1996) 10:2067–78. doi: 10.1101/gad.10.16.2067
20. Imbeault M, Helleboid P-Y, Trono D. KRAB zinc-finger proteins contribute to the evolution of gene regulatory networks. Nature. (2017) 543:550–4. doi: 10.1038/nature21683
21. Peng H, Begg GE, Schultz DC, Rux JJ, Rauscher FJ 3rd. A common DNA-binding site for SZF1 and the BRCA1-associated zinc finger protein, ZBRK1. . Cancer Res. (2002) 62(13):3773–81.
22. Zheng L, Pan H, Li S, Flesken-Nikitin A, Chen PL, Boyer TG, et al. Sequence-specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol Cell. (2000) 6:757–68. doi: 10.1016/S1097-2765(00)00075-7
23. Liu C, Wang L, Xu M, Sun Y, Xing Z, Zhang J, et al. Reprogramming the spleen into a functioning 'liver' in vivo. Gut. (2022) 71:2325–36. doi: 10.1136/gutjnl-2021-325018
24. Yuan L, Jiang J, Liu X, Zhang Y, Zhang L, Xin J, et al. HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation. Gut. (2019) 68:2044–56. doi: 10.1136/gutjnl-2018-316091
25. Allweiss L, Volz T, Giersch K, Kah J, Raffa G, Petersen J, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. (2018) 67:542–52. doi: 10.1136/gutjnl-2016-312162
26. Xun Z, Lin J, Yu Q, Liu C, Huang J, Shang H, et al. Taurocholic acid inhibits the response to interferon-α therapy in patients with HBeAg-positive chronic hepatitis B by impairing CD8+ T and NK cell function. Cell Mol Immunol. (2021) 18:461–71. doi: 10.1038/s41423-020-00601-8
27. Zhang N, Lu Y, Liu X, Yu D, Lv Z, Yang M. Functional evaluation of ZNF350 missense genetic variants associated with breast cancer susceptibility. DNA Cell Biol. (2018) 37:543–50. doi: 10.1089/dna.2018.4160
28. Yang F, Zhang L, Huo XS, Yuan JH, Xu D, Yuan SX, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. (2011) 54:1679–89. doi: 10.1002/hep.24563
29. Balbin OA, Malik R, Dhanasekaran SM, Prensner JR, Cao X, Wu YM, et al. The landscape of antisense gene expression in human cancers. Genome Res. (2015) 25:1068–79. doi: 10.1101/gr.180596.114
Keywords: hepatitis B virus, single nucleotide polymorphism, zinc finger protein 350, Peg-IFNα, therapeutic effect
Citation: Guo J, Guo S, Chen T, Zeng Y, Fu Y and Ou Q (2024) ZNF350 gene polymorphisms promote the response to Peg-IFNα therapy through JAK-STAT signaling pathway in patients with chronic hepatitis B. Front. Immunol. 15:1488055. doi: 10.3389/fimmu.2024.1488055
Received: 29 August 2024; Accepted: 21 October 2024;
Published: 07 November 2024.
Edited by:
Mohamed Mahdi, University of Debrecen, HungaryReviewed by:
Donglin Ding, Mayo Clinic, United StatesMarcel Schilling, German Cancer Research Center (DKFZ), Germany
Copyright © 2024 Guo, Guo, Chen, Zeng, Fu and Ou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qishui Ou, b3VxaXNodWlAZmptdS5lZHUuY24=; Ya Fu, aHp4ZnlAZmptdS5lZHUuY24=; Yongbin Zeng, emVuZ3lvbmdiaW5AZmptdS5lZHUuY24=
†ORCID: Shaoying Guo, orcid.org/0009-0001-5026-7614
 Jianhui Guo
Jianhui Guo Shaoying Guo1,2†
Shaoying Guo1,2† Tianbin Chen
Tianbin Chen Yongbin Zeng
Yongbin Zeng Ya Fu
Ya Fu Qishui Ou
Qishui Ou