- 1Dept. of Molecular Immunology, Ruhr University Bochum, Bochum, Germany
- 2Systems-oriented Immunology and Inflammation Research Group, Helmholtz Centre for Infection Research, Braunschweig, Germany
- 3Institute of Molecular and Clinical Immunology, Otto-von-Guericke University, Magdeburg, Germany
- 4Department of Experimental Immunology, Helmholtz Centre for Infection Research, Braunschweig, Germany
Macroautophagy (hereafter called autophagy) is an ancient catabolic process that delivers bulky cargo to lysosomal degradation. The autophagic pathway is regulated by autophagy-related (ATG) proteins that govern the formation of double-membraned vesicles called autophagosomes. Autophagy has been shown to be important for T cell survival and proliferation. However, all studies performed so far used genetic models, in which deletion of an essential Atg gene occurs at early stages of thymic T cell development, raising the question whether developmental defects account for the phenotypes observed in mature T cells. Especially regarding CD4+ T helper cells, little is known about the function of autophagy in specific subsets. Therefore, we generated mice that lack Atg5, an essential component of the core autophagy machinery, in activated CD4+ T cells using OX40-Cre mice. As expected, thymic T cell development was unaffected in these mice. Despite impaired CD4+ T cell activation, Atg5ΔOX40 mice developed lymphadenopathy and exhibited increased T cell numbers, pointing to a defect in immune regulation. Accordingly, frequencies of Foxp3+ regulatory T (Treg) cells were decreased. While activation-induced cell death and in vitro suppressive activity of Treg cells were not affected, ATG5 deficiency in CD4+ T cells led to increased anti-tumor responses against melanoma. In conclusion, our data suggest that ATG5 is crucial for the functional properties of CD4+ T cells and the homeostasis of Treg cells.
1 Introduction
Unwanted organelles, protein aggregates, or even intracellular pathogens are degraded via the lysosomal pathway by an ancient catabolic process called autophagy. The bulky cargo to be degraded is initially enclosed in double-membraned vesicles known as autophagosomes (1, 2). The autophagic machinery consists of two ubiquitin-like conjugation systems, in which first the ubiquitin-like molecule ATG12 is conjugated to ATG5 (3). The ATG5–12 complex, together with ATG16L1, associates with nascent autophagosomes and acts as an E3 ligase to conjugate the ubiquitin-like molecule ATG8 to the lipid phosphatidyl-ethanolamine (4). As part of the core autophagic machinery, the ATG8 protein family participates in the elongation of the nascent autophagosome and closure of the double membrane vesicle, which finally fuses with a lysosome to degrade the cargo (5). Despite being a conserved process among species, only one ATG8 protein exists in yeasts, while six different proteins, namely LC3A, LC3B, LC3C, GABARAP, GABARAPL1, and GATE-16, can be found in mammals (3).
CD4+ T helper (Th) cells are a central part of adaptive immunity. They orchestrate the adaptive immune response via membrane-bound co-stimulatory ligands and the secretion of soluble cytokines. In order to cope with the challenges of extracellular and intracellular pathogens, they differentiate into various effector subsets such as Th1, Th2, and Th17 cells that tailor the immune response accordingly (6). Besides their effector function, T cells are also fundamental for the regulation of the immune response. A subset of CD4+ T cells, characterized by the expression of the transcription factor Foxp3, employs immunosuppressive mechanisms to dampen excessive responses of the immune system (7, 8). Expression of inhibitory receptors like CTLA-4, IL-2 sequestration, or production of inhibitory cytokines can be found among the mechanisms used by Treg cells to suppress the immune system (9, 10). Treg cells play an essential role in the maintenance of immune homeostasis, and a misbalance in the ratio of Treg cells to the effector arm of the immune system causes or favors the rise of pathologies. For instance, excessive Treg cells impair immunosurveillance of tumor cells and can give rise to cancer (11). In fact, tumor cells recruit Treg cells via the Neuropilin1 (Nrp1)-VEGF axis to generate an immunosuppressive environment (12). Contrariwise, defects in the suppressive function of the Treg cells or reduction in their numbers result in autoimmune disease as observed in the human IPEX syndrome and the murine scurfy mutation, both characterized by non-functional Foxp3 (13–15), or upon depletion of Treg cells (16, 17).
T cell development and naïve T cell homeostasis are biological processes that critically rely on autophagy (reviewed in (18, 19)). Previously, we and others have shown the importance of autophagy in the maintenance of the immune homeostasis by Treg cells (20–23). Furthermore, it was demonstrated that autophagy has an essential role in the formation of a protective CD8+ memory population (24, 25). Here, we created a novel conditional knockout mouse line to investigate the function of autophagy in activated CD4+ T cells. In line with Treg cells having an activated phenotype, we confirm the importance of autophagy for Treg homeostasis, phenotype, and function. Moreover, we show that autophagy is important for the proper activation of conventional CD4+ Th cells. Despite impaired CD4+ T cell activation, Atg5 deficiency in activated CD4+ T cells led to an enhanced anti-tumor response.
2 Materials and methods
2.1 Mice
Atg5fl/fl and OX40-Cre mice were described previously (26, 27) and have been kindly provided by Dr. Mizushima and bought from The Jackson Laboratory, respectively. Atg5ΔOX40 mice were generated by crossing Atg5fl/fl with OX40-Cre mice. To test for OX40-Cre activity, OX40-Cre mice were crossed with ROSAmT/mG mice [Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J], which have been described previously (28). All mice were kept under specific pathogen-free conditions in the animal facility of the Helmholtz Center for Infection Research (HZI), Braunschweig (29). Animal experiments and breeding were performed following the guidelines of local and national authorities.
2.2 B16 melanoma model
The B16 tumor model was performed as previously described (30). Briefly, 2 x 106 B16 melanoma cells were subcutaneously injected into the right flank of the mice, and tumor growth was monitored by measuring the orthogonal tumor diameters. Mice were sacrificed if the mean tumor diameter reached 12 mm.
2.3 Flow cytometric analyses
For surface marker staining, cells were resuspended in 100 μl FACS buffer (2% BSA in PBS) and incubated in the presence of the respective antibodies for 15 minutes at 4 °C in the dark. Afterwards, cells were washed twice with 500 μl FACS buffer and analyzed using an LSRII flow cytometer (BD Biosciences). Before surface staining, in the case of cell population analyses (excluding viability determinations), dead cells were excluded by incubating the cell suspension with LIVE/DEAD® Fixable Blue Dead Cell Stain staining (LifeTechnologies) for 30 minutes at 4˚C in the dark and subsequently washed twice with PBS; afterwards Fc receptors were blocked by 15 minutes incubation with Fc-block (CD16/32) in FACS buffer at 4˚C and washed with FACS buffer. For intracellular proteins, staining was performed using Foxp3 Staining Buffer Set (Miltenyi, #130-093-142) according to the manufacturer’s instructions. Antibodies used were: CD4-FITC (RM4-5, eBiosciences), CD4-PacificBlue (L3T4, BioLegend), CD8-PECy7 (53-6.7, Biolegend), CD25-PE-Cy7 (PZ61, BD Pharmigen), CD44-APC (IM7, Biolegend), CD62L-PerCPCy5.5 (MEL-14, eBioscience), CD134(OX40)-PE (OX86, Biolegend), CD134(OX40)-APC (OX86, Biolegend), CTLA-4-PE (UC10-4P9, eBioscience), Foxp3-AlexaFluor488 (FJK-16s, eBioscience), GITR-PECy7 (DTA-1, eBioscience), GITR-PE (DTA-1, BD Pharmigen), Helios-PE (22F6, Biolegend) and Nrp1-PE (3E12, Biolegend). Samples were acquired on LSR Fortessa or LSR II (Becton, Dickinson and Company) and analyzed by FlowJo software (Tristar).
2.4 Activation-induced cell death assay
Cells isolated from peripheral lymph nodes of Atg5ΔOX40 and Atg5fl/fl control mice were seeded in primary T cell medium, i.e. RPMI1640 supplemented with 10% FCS (PAA Laboratories), 50 μM β-mercaptoethanol, 50 μg/ml each of penicillin and streptomycin, 1% non-essential amino acids and 1 mM sodium pyruvate (all from Life technologies) and stimulated with Concanavalin A (1.5 μg/ml) and IL-2 (100 ng/ml). After 72 hours, cells were re-stimulated with pre-coated anti-CD3 (10 μg/ml), PMA (100 ng/ml) + Ionomycin (1μg/ml), CD95L (100 ng/ml), or left untreated for additional 24 hours. Subsequently, cells were stained with 7-AAD (Biolegend), CD4, and CD134 antibodies to determine cell viability in a Cytoflex LX (Beckman Coulter) flow cytometer.
2.5 In vitro Treg suppression assay
CD4+ CD25− conventional T cells (Tcon) and CD4+ CD25+ regulatory T cells (Treg) were isolated from mouse spleen using the mouse CD4+CD25+ Regulatory T Cell Isolation Kit, (#130-091-041, Miltenyi). Tcon cells were stained with Cell Trace Violet according to the manufacturer´s protocol (C34557, ThermoFisherScientific). Afterwards, Tcon and Treg cells were co-cultured in primary T cell medium and stimulated using T Cell Activation/Expansion Kit, mouse (130-093-627, Miltenyi) supplemented with IL-2 (100 ng/ml). Treg: Tcon cells were seeded in the indicated ratios. After 72 hours, cells were harvested and stained with CD4-FITC (RM4-5, eBiosciences) and CD25-PE (Miltenyi) and analyzed for proliferation in a Cytoflex LX (Beckman Coulter).
2.6 Immunoblotting
OX40- and OX40+ cells were purified using a CD4+ CD25+ regulatory T cell isolation kit for magnetic separation (130-091-041; Miltenyi Biotec) according to the manufacturer’s protocol, with the modification that the anti-OX40-PE (#119410, BioLegend) was used instead of the anti-CD25-PE provided in the kit. Cells were lysed by incubation in TPNE buffer (1x PBS add 300 mM NaCl, 1 mM EDTA, 1% Triton X-100) supplemented with protease inhibitors for 20 minutes on ice. After centrifugation, protein concentration was determined by BCA assay (ThermoFisher Scientific). Protein lysates were loaded onto 12% SDS-polyacrylamide gels for ATG5 or 15% SDS-polyacrylamide gels for LC3, blotted onto PVDF membrane (GE Healthcare), and detected by chemiluminescence. Antibodies used for Western blotting: ATG5 (12994S, Cell Signaling Technology), LC3A/B (4801S, Cell Signaling Technology), Beta-Actin (66009-1-lg, Proteintech).
2.7 Statistical analysis
Statistical analyses were performed by Mann-Whitney, one-way ANOVA (Tukey’s multiple comparison post-test), or two-way ANOVA (Dunnett´s multiple comparison test) tests to determine statistical significance using GraphPad Prism (GraphPad Software Inc.). Standard error of the mean (SEM) is represented as error bars in the graphs.
3 Results
3.1 Cre recombinase driven by the Tnfrsf4 promoter deletes in activated conventional CD4+ T cells and Treg cells
OX40 is a member of the tumor necrosis factor receptor superfamily (TNFRS4, CD134) and is a secondary co-stimulatory molecule that is expressed mainly by activated T cells to prolong their activation and to sustain their proliferation (31). The Tnfrsf4 promoter has been used to drive Cre expression in activated CD4+ T cells and Foxp3+ Treg cells (27, 32). Crossing OX40-Cretg/wt mice with Rosa26-mT/mGfl/wtOX40-Cretg/wt mice (Figure 1A), we verified that OX40-driven Cre expression is detectable in CD3+ T cells, but not CD19+ B cells (Figure 1B). Interestingly, we detected some Cre activity in Gr-1+ cells (Figure 1B), which is consistent with the fact that activated Th1 cells express this marker (33). Comparing T cell lineages, OX40-Cre was largely inactive in CD8+ and active in CD4+ T cells, with particularly high recombination efficiency in Foxp3+ CD4+ T cells (Figure 1C).
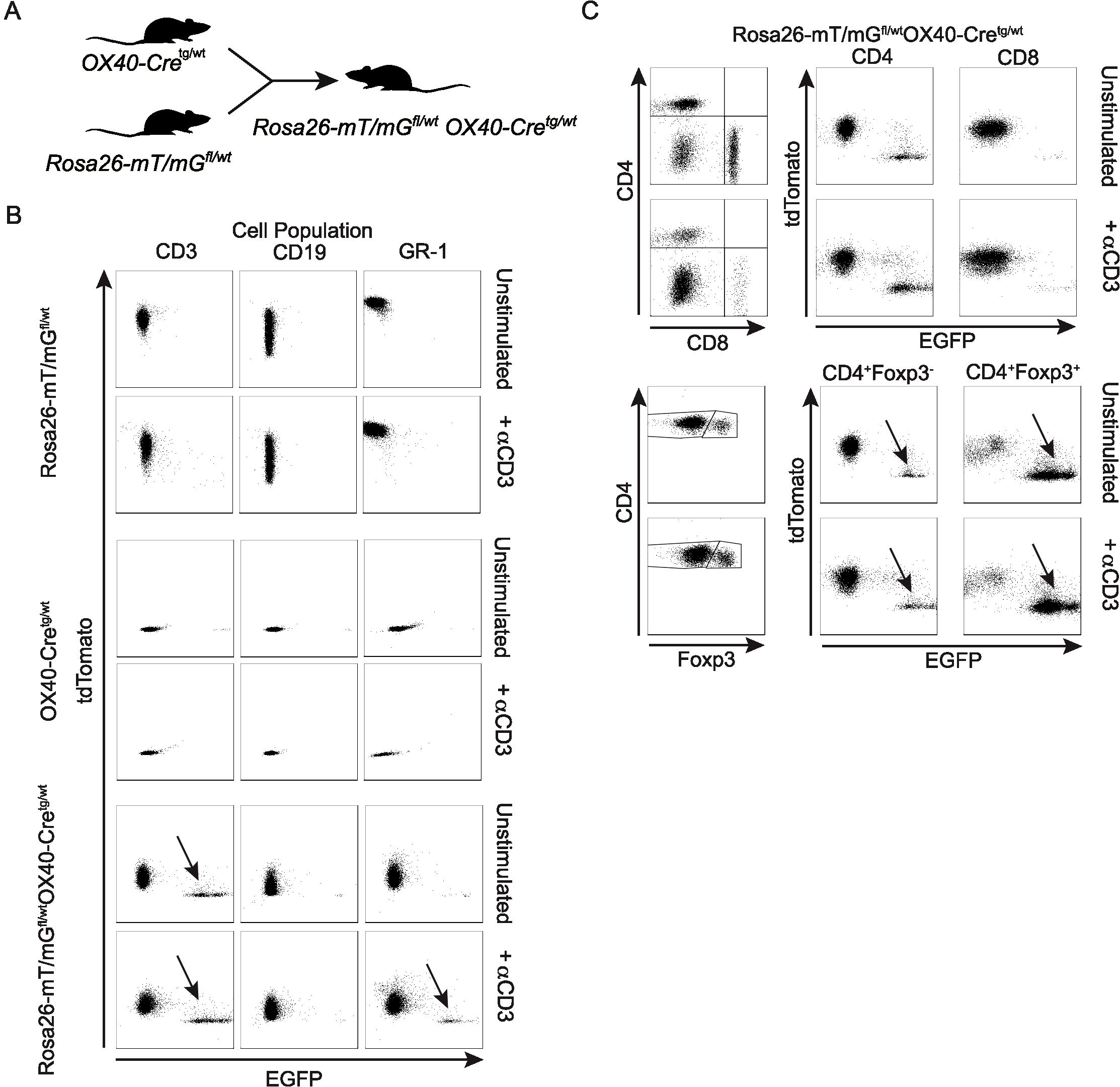
Figure 1. Characterization of the OX40-Cre deleter line. (A) Scheme describing the generation of Rosa26-mT/mGfl/wtOX40-Cretg/wt mice. (B) Dot plots of tdTomato and EGFP expression within unstimulated or anti-CD3-stimulated CD3+, CD19+ and GR-1+ cells from Rosa26-mT/mGfl/wt, OX40-Cretg/wt and Rosa26-mT/mGfl/wtOX40-Cretg/wt mice. (C) Dot plots of tdTomato and EGFP expression within unstimulated or anti-CD3-stimulated CD4+, CD8+ and CD4+Foxp3+ cells from Rosa26-mT/mGfl/wtOX40-Cretg/wt mice.
3.2 Autophagy-deficiency in Atg5ΔOX40 mice affects mostly CD3+ T cells and does not perturb thymocyte development
To investigate the role of autophagy in activated CD4+ T cells, OX40-Cretg/wt mice were bred with Atg5fl/fl mice (26) to generate Atg5ΔOX40 mice (Figure 2A). Deletion of ATG5 in OX40-expressing CD4+ T cells was confirmed by immunoblotting (Figure 2B). In line with ATG5 deficiency, LC3 conjugation to phosphatidylethanolamine was strongly impaired as LC3-I but hardly any LC3-II was detected in OX40-positive CD4+ T cells (Figure 2C). Consistent with the lack of Cre activity in the thymus, T cell development was not affected in Atg5ΔOX40 mice since both the frequencies of thymocyte populations (Figure 2D) and the thymic cellularity (Figure 2E) were comparable to control mice. While the cellularity was only marginally affected in the spleen, the effect of autophagy-deficiency in OX40-expressing cells of Atg5ΔOX40 mice resulted in a significant expansion of cell numbers in peripheral lymph nodes (pLN) (Figures 2F, G). Although not statistically significant, there was a tendency towards a higher cellularity in mesenteric LN (mLN) (data not shown). Accordingly, the lymph nodes of Atg5ΔOX40 mice were enlarged, consistent with lymphadenopathy (Figure 2H).
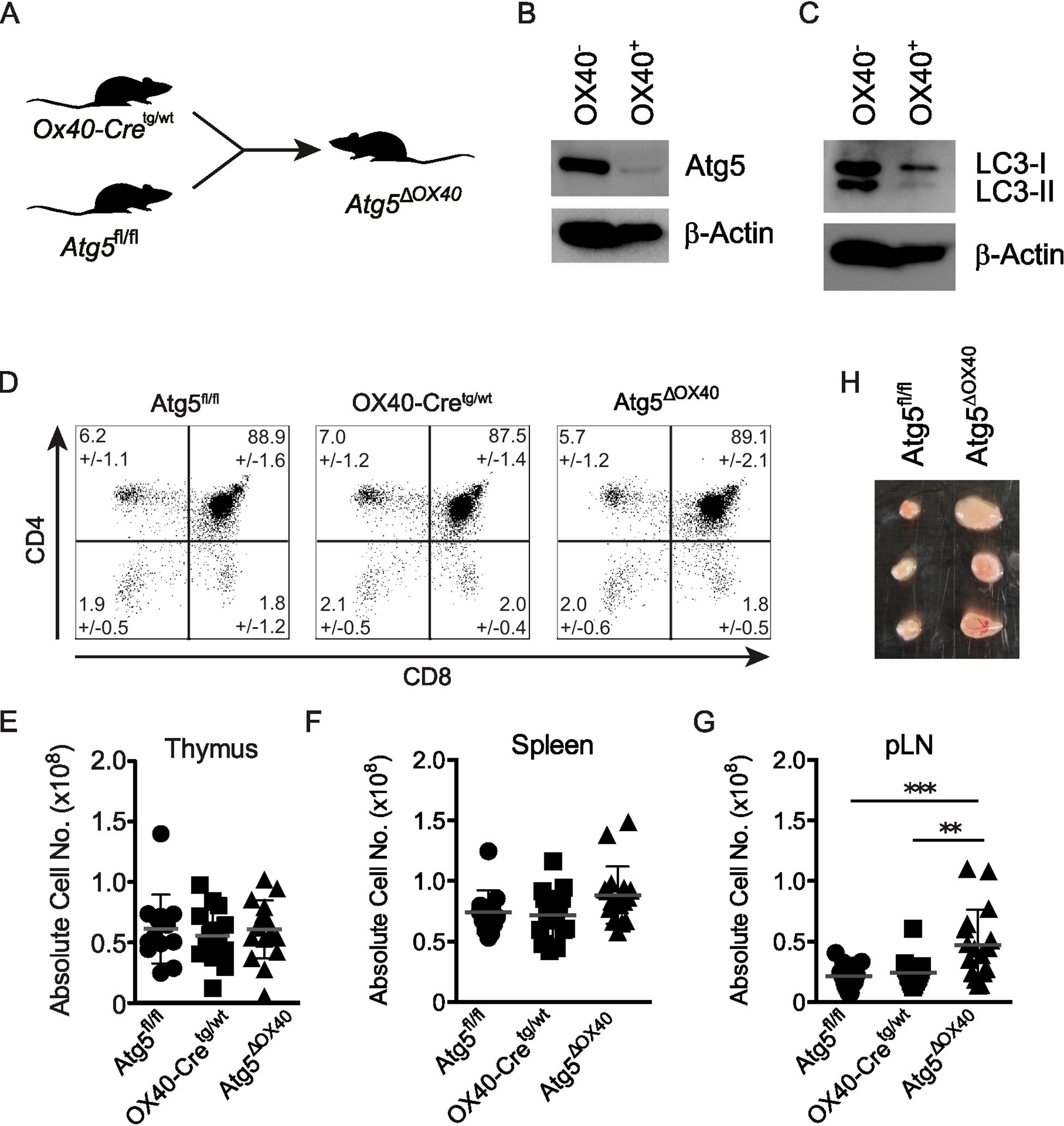
Figure 2. No gross alterations of T cell development in Atg5ΔOX40 mice. (A) Scheme describing the generation of Atg5ΔOX40 mice. (B, C) Immunoblot of ATG5 and LC3 expression in OX40-negative and OX40-positive cells from Atg5ΔOX40 mice. Actin was used as a loading control. (D) Dot plots of thymic CD4- and CD8-expressing cells in Atg5fl/fl, OX40-Cre and Atg5ΔOX40 mice. Mean frequency +/-SD are given; n=10-12. The one-way ANOVA and Tukey’s multiple comparison tests were used for statistical analysis. (E-G) Absolute cell numbers of thymi (E), spleen (F) and pLN (G) from the indicated mice. Mean and SD are shown; n=12-16. (H) Representative examples of lymph nodes from Atg5ΔOX40 and control mice. **p < 0.01 and ***p < 0.001.
When CD4+ and CD8+ T cell populations were analyzed, Atg5ΔOX40 mice exhibited a reduction in the frequencies of both subsets (Figures 3A, B). However, the absolute numbers of helper and cytotoxic T cells were not significantly altered, although there was a slight tendency towards an enlarged T cell compartment (Figure 3C). This suggests that other cell populations were expanded in Atg5ΔOX40 mice, which were found to be mostly CD19+ B cells (Figure 3D). In summary, Atg5ΔOX40 mice exhibit normal T cell development but increased cellularity within peripheral lymph nodes, suggesting a lower threshold for the activation of the immune system.
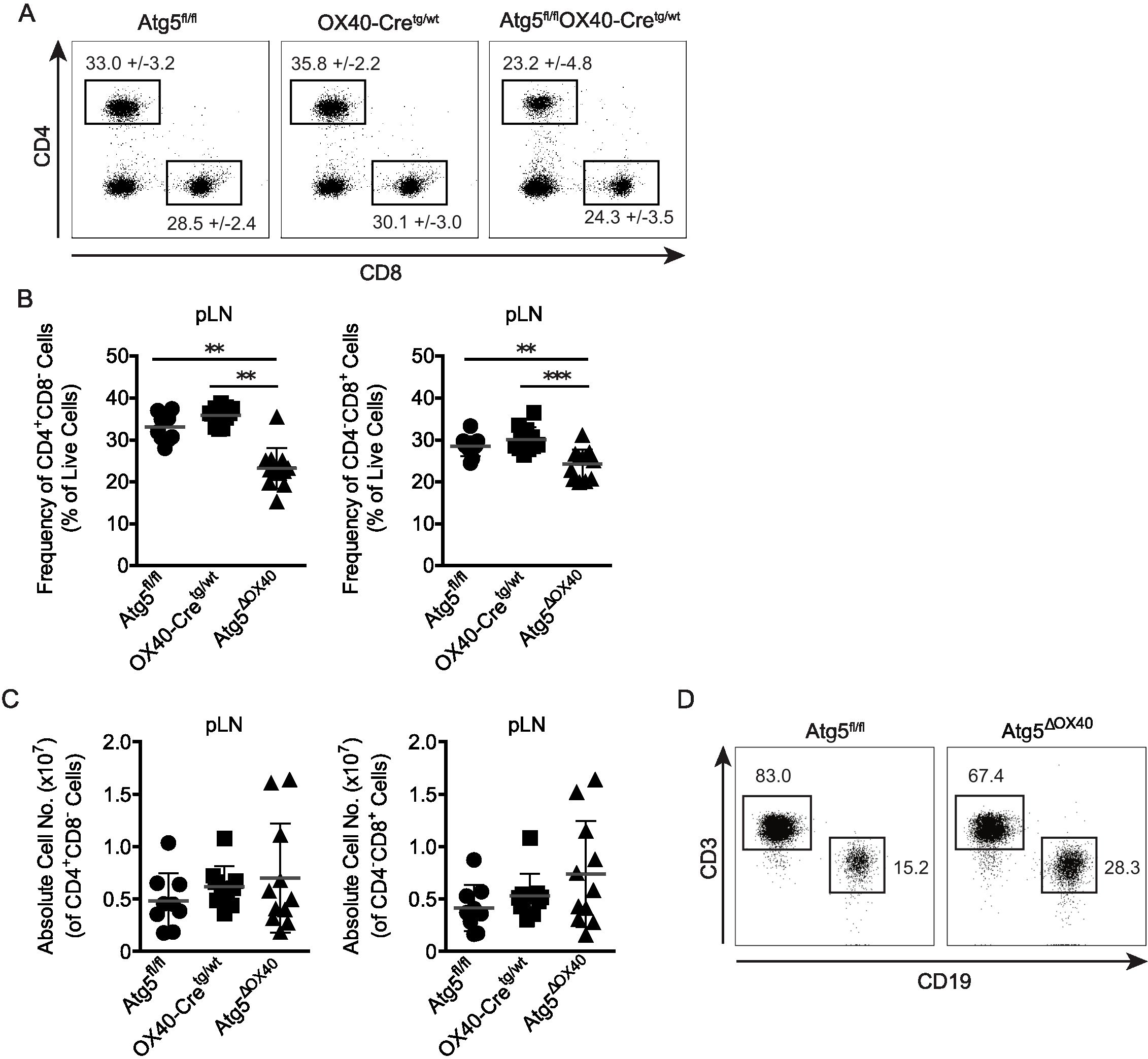
Figure 3. Characterization of the CD4+ and CD8+ T cell compartment in Atg5ΔOX40 mice. (A) Dot plots of CD4+ and CD8+ cell populations from pLN of the indicated mice. (B) Graphical presentation of frequencies of CD4+ and CD8+ cells. Mean frequency +/-SD are given; n=10-12. (C) Graphical presentation of absolute CD4+ and CD8+ cell numbers in pLN of the indicated mice. Mean and SD are shown; n=9-11. (D) Dot plots of CD3+ T cells vs. CD19+ B cells in pLN of Atg5fl/fl and Atg5ΔOX40 mice. **p < 0.01 and ***p <0.001.
3.3 Peripheral CD4+ T cells of Atg5ΔOX40 mice have an activation defect
The enhanced cellularity described above might result from enhanced activation of effector cells or impaired regulatory mechanisms. In that respect, autophagy has on the one hand been connected to activation of T cells (34), but has also been shown to down-modulate TCR-induced signaling (35). To dissect between these two possibilities, we injected anti-CD3 antibodies into Atg5ΔOX40 and control mice to activate T cells in vivo. We observed an increased cellularity in secondary lymphoid organs of Atg5ΔOX40 mice, which was most prominent in pLN (Figure 4A). Accordingly, the absolute numbers of CD4+ and CD8+ T cells were increased in Atg5ΔOX40 mice upon anti-CD3 injection (Figure 4B). Thus, autophagy deficiency in CD4+ T cells appears not to affect T cell proliferation in general.
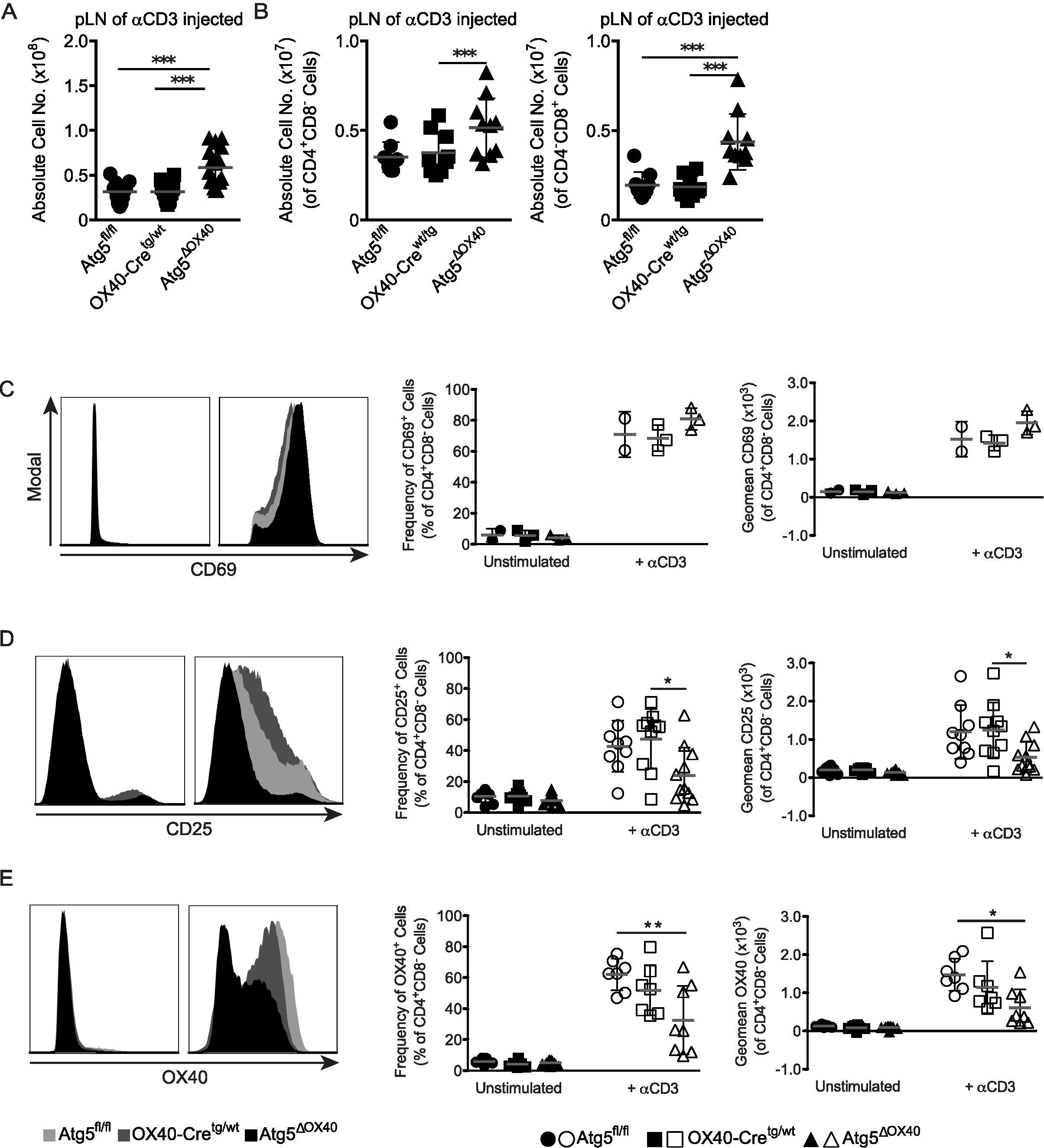
Figure 4. Lymphadenopathy and activation defect in peripheral T cells of Atg5ΔOX40 mice. (A) Absolute cell numbers of pLN from anti-CD3 injected mice. Mean and SD are indicated; n=12-16. (B) Absolute cell numbers of CD4+ cells and CD8+ cells from pLN of anti-CD3 injected mice. Mean and SD are shown; n=12-16. (C) Histograms of CD69 expression by CD4+ T cells in pLN, as well as graphs showing the corresponding frequency and geomean. Mean and SD are shown; n=2-3. (D) Histograms of CD25 expression by CD4+ T cells in pLN, as well as graphs showing the corresponding frequency and geomean. Mean and SD are shown; n=9-11. (E) Histograms of OX40 expression by CD4+ T cells in pLN, as well as graphs showing the corresponding frequency and geomean. Mean and SD are shown; n=7-9. *p < 0.05; **p < 0.01 and ***p < 0.001.
Subsequently, we investigated the activation status of CD4+ T cells by analyzing the expression of the activation markers CD69, CD25, and OX40 in mice injected with anti-CD3 or left untreated. T cell activation was not impaired in general since CD69 expression was normal (Figure 4C). In contrast, the late activation markers CD25 and OX40 exhibited impaired induction in CD4+ T cells of stimulated Atg5ΔOX40 mice (Figures 4D, E). Although OX40 expression by CD4+ T cells was reduced in OX40-Cretg/wt mice compared to Atg5fl/fl mice due to the knock-in of Cre into the Tnfrsf4 locus, OX40 induction was even further reduced in Atg5ΔOX40 mice (Figure 4E).
Taken together, although the immune system appears to be activated in Atg5ΔOX40 mice, ATG5 deficiency in T helper cells impairs CD4+ T cell activation. Therefore, the increased cellularity in the secondary lymphoid organs alludes to an altered threshold for immune cell activation, potentially due to deficient Treg suppression.
3.4 Loss of autophagy in Treg cells of Atg5ΔOX40 mice causes reduced Treg cell numbers
Treg cells are an essential part of peripheral tolerance in the immune system. We had a closer look again at the Rosa26-mT/mGfl/wtOX40-Cretg/wt mice and compared the EGFP-positive with the EGFP-negative cell population from pLN. Interestingly, the former had a much higher expression of CD25 both in unstimulated and anti-CD3 stimulated mice and a higher expression of OX40 in stimulated mice, which is consistent with a Treg cell phenotype (Figure 5A). Indeed, most CD4+Foxp3+ Treg cells of Rosa26-mT/mGfl/wtOX40-Cretg/wt mice were EGFP + suggesting that OX40-dependent loss of autophagy affects especially Treg cells (Figure 1C). CD4+Foxp3+ Treg frequencies and numbers were reduced in peripheral lymphoid organs of both unstimulated and stimulated Atg5ΔOX40 mice (Figures 5B, C). Although several OX40 knockout studies have shown that OX40 expression is crucial for Treg development and function and that these mice have severely reduced mature Treg numbers (36–38), the effect seen in Atg5ΔOX40 mice was independent of reduced OX40 expression, since OX40-Cretg/wt mice did not show reduced Treg numbers in peripheral lymphoid organs (Figures 5B, C). Furthermore, when investigating functional Treg markers, the percentage of CTLA-4-expressing Treg cells was increased in peripheral lymphoid organs of Atg5ΔOX40 mice (Figure 5D). Analyzing additional Treg markers, we found that Nrp-1 expression was reduced in Treg cells from peripheral lymphoid organs of unstimulated and especially of stimulated Atg5ΔOX40 mice compared to Treg cells from control mice (Figure 5D).
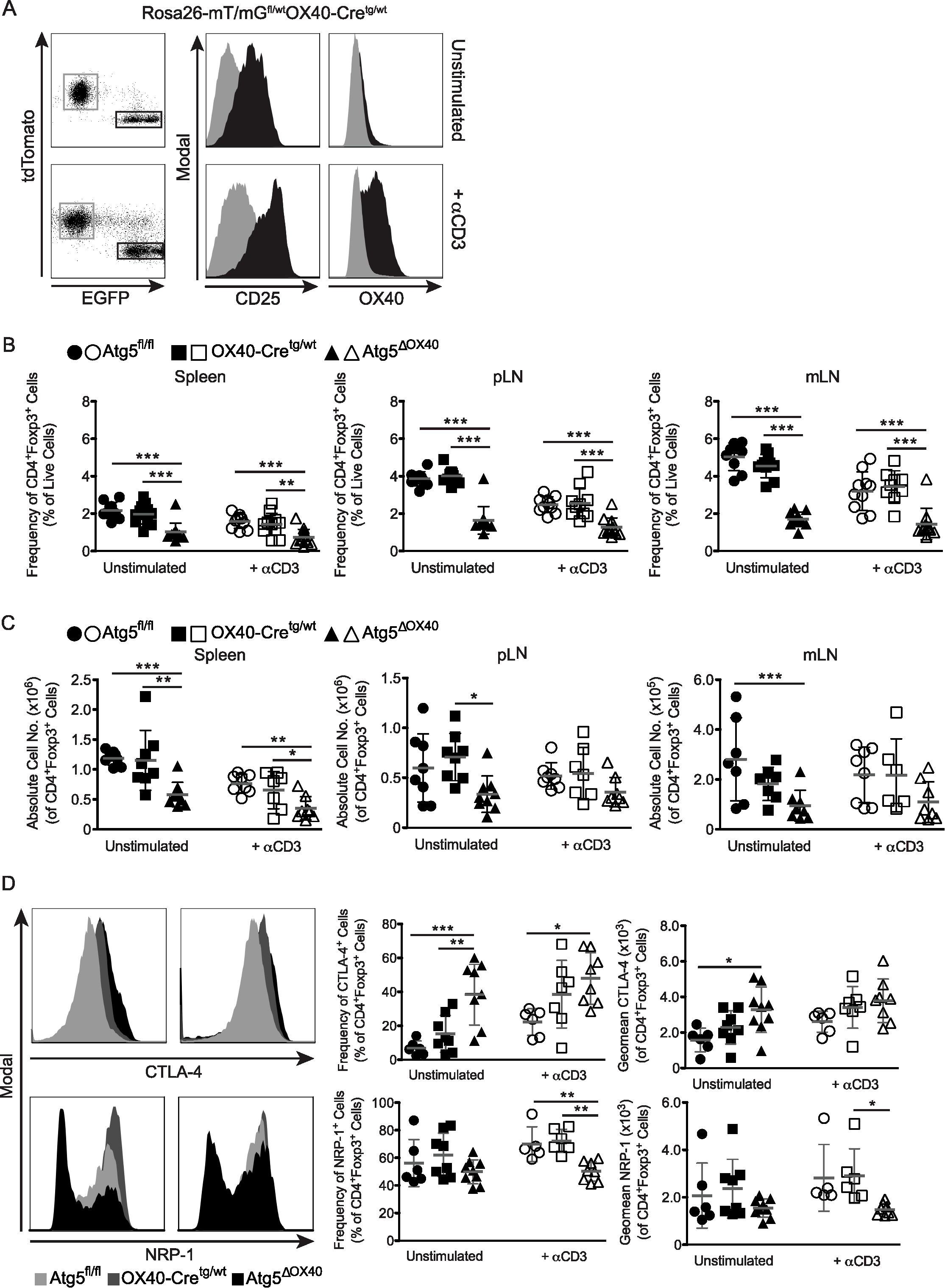
Figure 5. Effect of autophagy-deficiency on Treg cells in Atg5ΔOX40 mice. (A) CD25 and OX40 expression within EGFP- (grey) and EGFP+ (black) cells from peripheral lymph nodes of Rosa26-mT/mGfl/wtOX40-Cretg/wt mice stimulated with anti-CD3 or left untreated. (B) Frequencies and (C) absolute cell numbers of CD4+Foxp3+ Treg cells in the indicated organs from unstimulated and anti-CD3 stimulated mice. Mean and SD are shown; (A) n=9–13 and (B) n=6-9. Statistical analysis was performed with the one-way ANOVA and Tukey’s multiple comparison tests. (D) Histograms and graphs depicting CTLA-4- and Nrp-1-expression by Treg cells. Unstimulated mice are shown in the left panels and anti-CD3 treated animals are depicted in the right panels. Mean and SD are shown; CTLA-4: n=6-9, Nrp-1: n=5-9. *p < 0.05; **p < 0.01 and ***p < 0.001.
3.5 Atg5-deficient OX40-positive T cells are susceptible to AICD and have suppressive activity in vitro
Atg5 has been connected to the regulation of life and death of T cells (20, 22, 34). Therefore, we tested susceptibility to activation-induced cell death (AICD) in OX40-negative and OX40-positive T cells. To this end, CD4+ T cells were purified, activated in vitro and subsequently, after a short culture period, stimulated with anti-CD3, PMA plus ionomycin, or recombinant CD95L to induce cell death. While CD95L was the most potent death stimulus, no significant differences between OX40-negative and OX40-positive T cells and the two genotypes were observed (Figure 6A). We conclude that differences in cell death susceptibility did not account for the disturbed immune homeostasis in Atg5ΔOX40 mice.
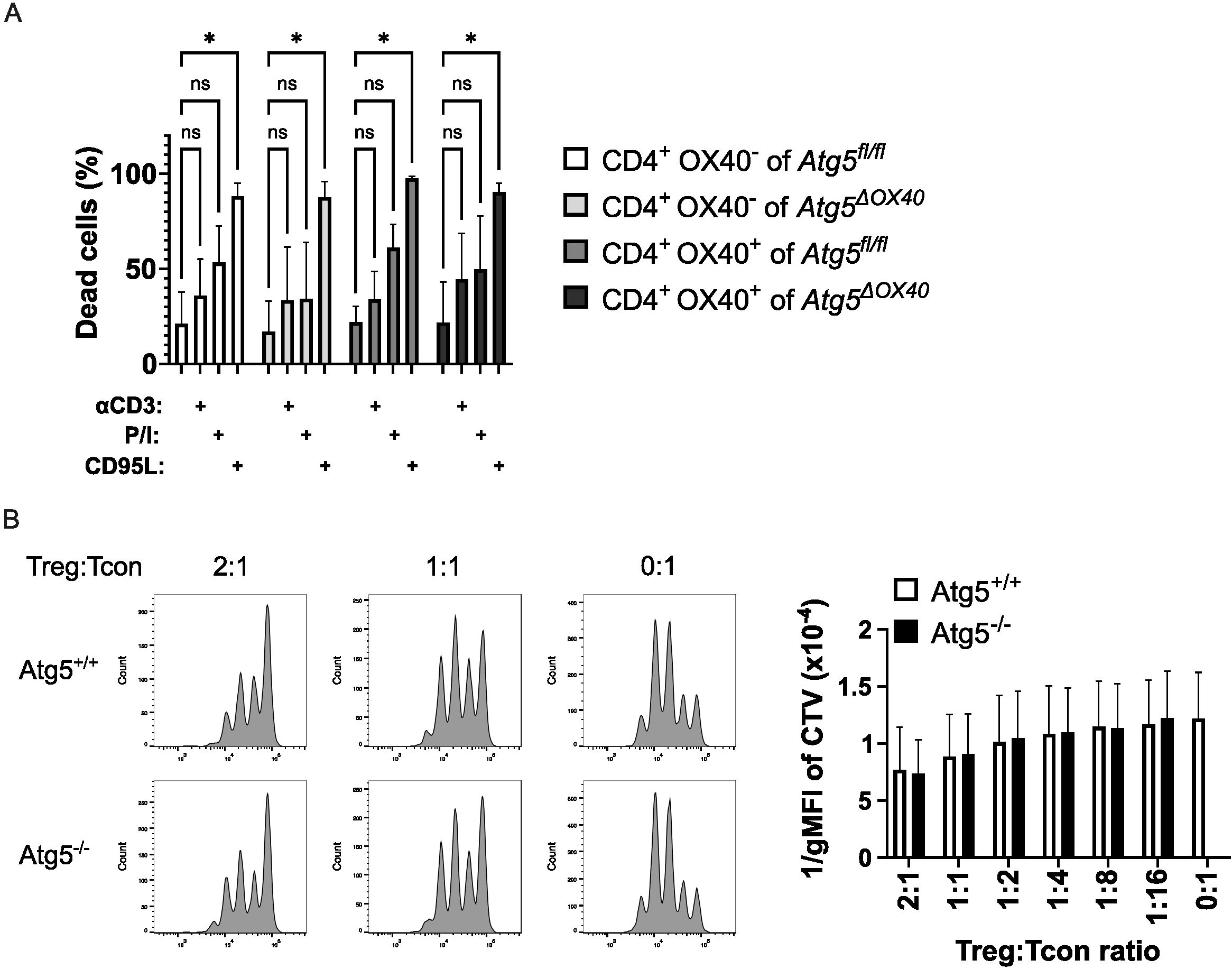
Figure 6. Unaltered T cell death and Treg suppressive capacity. (A) CD4+ T cells were activated with concanavalin A and IL-2 for 3 days and subsequently stimulated with anti-CD3, PMA/ionomycin, or CD95L. Cell death was measured with 7AAD and flow cytometry. The data shown is from three independent experiments. The two-way ANOVA Dunnett´s multiple comparison test was used for statistical analysis. (B) In vitro suppression assay comparing Treg cells from Atg5ΔOX40 and control mice. Wild-type conventional T cells were labeled with cell trace violet (CTV) and incubated at the indicated ratios with Treg cells of the respective genotype. Cells were analyzed by flow cytometry. Representative dot plots are shown on the left; the right panel shows the summary of three independent experiments. *p < 0.05.
Next, we tested the suppressive capacity of Treg cells from Atg5ΔOX40 and control mice. Conventional wild-type T cells were labeled with cell trace violet, activated via CD3 plus CD28, and incubated with Treg cells of conditional knockout or control mice. No differences in Treg suppressive activity could be detected in this in vitro setting (Figure 6B). Since Treg activity might be different in vitro versus in vivo, we went on to test Atg5-deficient Treg cells in Atg5ΔOX40 mice in vivo.
3.6 Loss of autophagy in Treg cells of Atg5ΔOX40 mice improves anti-tumor responses
Nrp-1 has been described as a marker for thymus-derived Treg cells (39, 40), but is also known to play a role in the migration of Treg cells to VEGF-expressing tumors, and this results in increased tumor growth due to Treg cells suppressing anti-tumoral responses (12). When injecting Atg5ΔOX40 and control mice with B16 tumor cells, tumor growth was significantly delayed in Atg5ΔOX40 mice (Figure 7A). CD4+Foxp3+ Treg cell frequencies were substantially reduced in all peripheral lymphoid organs analyzed as well as in the tumor (Figure 7B), whereas CD4+Foxp3- conventional T cell frequencies were only slightly reduced in draining (dLN) and control lymph nodes (cLN) (Figure 7C). Furthermore, an increased frequency of intratumoral CD8+ cytotoxic T cells was seen in Atg5ΔOX40 compared to control mice (Figure 7D), similar to what has been reported for mice in which Nrp-1 expression was deleted (12). In turn, the frequencies of CD8+ cytotoxic T cells were decreased in dLN and spleen, suggesting migration of these cells from the secondary lymphoid tissue towards the tumor. Lastly, intratumoral CD62L+CD44- naïve CD4+ T cell frequencies were reduced, whereas activated CD62L-CD44+ cell frequencies were increased in these mice (Figure 7E). CD62L/CD44 populations of CD4+ T cells derived from dLN, cLN, and spleen were normal (Figure 7E and data not shown). CD8+ T cells had decreased frequencies of CD62L+CD44- naïve cells and increased frequencies of CD62L-CD44+ activated cells (Figure 7E). Furthermore, whereas in dLN and cLN the CD62L+CD44+ memory cell frequencies were increased, this cell population was decreased in intratumoral CD8+ T cells (Figure 7E and data not shown). In summary, our data indicate that autophagy is a critical process for the activation of T cells and supports previous findings that have illustrated the importance of this biological process for the homeostasis and function of Treg cells.
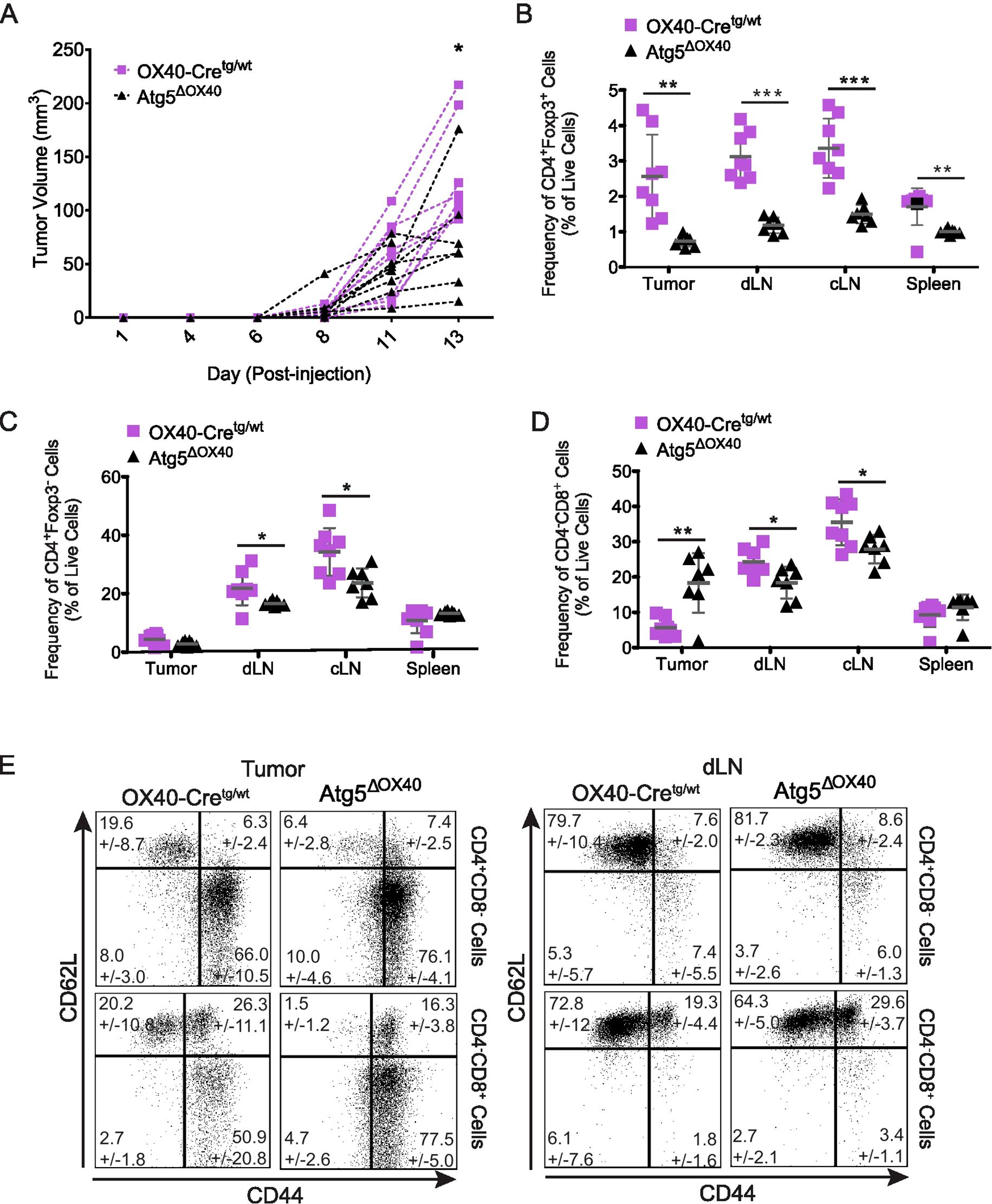
Figure 7. Enhanced anti-tumor response in Atg5ΔOX40 mice. (A) B16 tumor growth curves in individual mice. Atg5ΔOX40 mice are indicated as black triangles and OX40-Cre control mice are depicted as violet squares. (B-D) Frequency of CD4+Foxp3+ (B) and CD4+Foxp3- (C) and CD4-CD8+ (D) cell populations in the tumor, draining lymph nodes (dLN), control lymph node (cLN) and spleen. (E) Dot plots of CD62L and CD44 expressing CD4+Foxp3- and CD4+Foxp3+ cell populations from the tumor and dLN, mean frequency +/- SD are shown. One of two experiments is depicted where n=7-8. *p < 0.05; **p < 0.01 and ***p < 0.001.
4 Discussion
Although the potential roles of autophagy in the activation of T cells have been investigated, published results are not conclusive. It was shown that T cells upregulate autophagy upon activation and that autophagy degrades proteins in stimulated cells, rather than performing organelle degradation as seen in unstimulated cells, in order to aid the synthesis of products necessary for cells when activated (41, 42). Most previous Atg-deletion studies have used Lck-Cre or Cd4-Cre mice, in which thymocyte frequencies and numbers were perturbed due to Atg gene deletion in thymocytes during development, resulting in increased endoplasmatic reticulum and mitochondrial mass in naïve T cells reported by most studies (43–48). These mice also exhibited both reduced CD4+ and CD8+ T cell frequencies and numbers in the periphery. However, this reduction in peripheral T cells might be due to the impaired T cell development observed in these mice. Indeed, frequencies and absolute numbers of CD4+ T cells were not affected in mice, in which Atg5 deletion was driven by dLck-Cre (49), where Cre expression is driven by the distal Lck promoter that is active in peripheral T cells, but not thymocytes. In contrast, naïve and central memory CD8+ T cells were reduced in these mice (49). To investigate autophagy specifically in CD4+ T cells, we generated Atg5ΔOX40 mice, in which Cre expression is driven by the Tnfrsf4 (OX40) promoter that is active selectively in activated CD4+ T cells (27, 32). As expected, T cell development appeared unperturbed in Atg5ΔOX40 mice, allowing us to address the function of autophagy in peripheral T cells in an unbiased manner.
TAX1BP1, an autophagy receptor protein involved in selective autophagy, is critical to enable the metabolic transition of activated T cells and to sustain the proliferation of these cells, providing key amino acids for mTOR activation (50). Furthermore, previous studies reported normal CD25 and CD69 expression by activated autophagy-deficient T cells, and the current view is that proliferation and survival of T cells, but not the activation process per se, is autophagy-dependent (43, 45–47). Interestingly, T cells from Atg5ΔOX40 mice failed to upregulate the late activation markers CD25 and OX40 upon anti-CD3 stimulation in vivo, while the early activation marker CD69 was normally induced upon stimulation. Since OX40/OX40L signaling supports continued proliferation and clonal expansion of CD4+ T cells via maintenance of anti-apoptotic proteins and ensures their effector functions (36, 38, 51–54), these results suggest that autophagy is important for sustained CD4+ T cell activation.
Autophagy is important for the survival of T cells, including Treg cells (20, 22, 34). One mechanism is via reducing the expression of death-promoting molecules such as caspases (20, 55). Another mechanism is via mitophagy, since autophagy-deficient T cells accumulate mitochondria, which leads to enhanced production of reactive oxygen species (56). Next to survival, autophagy regulates Treg cell stability by influencing mTORC1 signaling and c-Myc expression (20). Both affect the metabolism of the cell and switch from OXPHOS, on which Treg cells usually rely, towards aerobic glycolysis, which impairs Foxp3 expression (57, 58). While the studies discussed so far mostly used Atg5 or Atg7 deletion to impair autophagy, deletion of other core autophagy proteins such as Atg16l1, Vps34 (21, 45, 59) and novel components of the pathway such as ZFP91 and RGS1 (60, 61) support the view that autophagy – and not non-autophagy-related functions of these molecules - regulates Treg survival, stability, and function. Importantly, this connection has been demonstrated for human Treg cells as well (60, 62).
The expansion of cells in peripheral lymph nodes and the increase of B cells in the Atg5ΔOX40 mouse line point towards a dysregulation of the immune system. Since Atg5 has been connected to cell death regulation in T cells (20, 22, 34), impaired deletion of activated effector T cells could explain this phenotype. However, we did not detect any differences in activated T cells regardless of OX40 expression in T cells from Atg5ΔOX40 and control mice. This, together with the reduced cell frequencies and numbers of CD4+Foxp3+ Treg cells, suggests an insufficient capacity to maintain the immune homeostasis by Treg cells. Surprisingly, the expression of CTLA-4 by Treg cells of Atg5ΔOX40 mice was increased. CTLA-4/CD152 is constitutively expressed by CD4+CD25high Treg cells and is important for their suppressive function (63, 64). Thus, Treg cells might upregulate CTLA-4 in Atg5ΔOX40 mice to compensate for the reduced number of Treg cells present in these mice. Several studies have demonstrated that loss of autophagy proteins such as ATG5, ATG7, or ATG16L1 in Treg cells results in impaired homeostasis and functioning of these cells (20–23). Therefore, it is most likely that the defects in autophagy - and not non-canonical functions of ATG proteins - in Treg cells are the basis of the spontaneous inflammation described in those mice. Since OX40 is expressed in Treg cells and we have detected that OX40-driven Cre expression is particularly high in CD4+Foxp3+ cells, it is plausible that the Treg cells of the Atg5ΔOX40 mouse line have also functional deficiencies, explaining the rise of the spontaneous phenotype of immune system activation in these mice. While we were not able to demonstrate a functional impairment of Treg cells from Atg5ΔOX40 mice in a conventional in vitro assay, the better anti-tumor response in the B16 model strongly supports the notion that autophagy competence is important for Treg function.
Nrp-1 expression was reduced by Treg cells in Atg5ΔOX40 mice, which led us to investigate Treg cells in a tumor setting in these mice. Using a B16 melanoma model, we observed a more active anti-tumor response by CD8+ T cells in Atg5ΔOX40 mice compared to control mice. Next to reduced Treg cell numbers, the diminished Nrp-1 expression by Treg cells could cause a deficit in the migration of Treg cells to the tumor. Even the increased CTLA-4 expression by Treg cells was not sufficient to suppress the anti-tumor response in Atg5ΔOX40 mice injected with B16 tumor cells. Therefore, probably due to both reduced Nrp-1 expression and reduced Treg cell numbers, tumor growth was reduced in these mice. Thus, inhibition of autophagy in T cells might be a suitable therapeutic option for cancer treatment. Here, we showed that impairing autophagy selectively in CD4+ T cells is sufficient to increase anti-tumor responses by CD8+ T cells. Furthermore, it was shown that autophagy-deficient CD8+ T cells adopt an effector memory T cell phenotype, resulting in anti-tumor immunity (65). Therefore, targeting an autophagy inhibitor to T cells might be a promising approach in cancer therapy.
In conclusion, our study shows the relevance of autophagy in T cell activation and makes a distinction between the importance of this biological process in mature conventional and regulatory T cells. Furthermore, it points to autophagy in regulatory T cells as a potential target for anti-tumor therapies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Landesamt für Verbaucherschutz und Lebensmittelsicherheit ethical Committee, Niedersachsen. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CP-S: Formal analysis, Investigation, Writing – original draft. AB: Formal analysis, Investigation, Writing – original draft. CB: Data curation, Methodology, Formal analaysis, Investigation, Vizualization, Writing – review & editing. KK: Formal analysis, Investigation, Writing – review & editing. MS: Formal analysis, Investigation, Writing – review & editing. JH: Resources, Supervision, Writing – review & editing. IS: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. AB was supported by the President’s Initiative and Networking Fund of the Helmholtz Association of German Research Centers (HGF) under contract number VH-GS-202. This study was funded via the Fritz Thyssen Stiftung, Deutsche Forschungsgemeinschaft (SCHM1586/3-1 and SCHM1586/3-2), and the Helmholtz program Metabolic Dysfunction.
Acknowledgments
We thank Christopher Brennsohn, Sabrina Schumann, and Christian Kozowsky for their excellent technical assistance and David Dettbarn for mouse husbandry. We are very thankful to Dr. Lothar Groebe, Dr. Marcus Peters, and Petra Bonowitz for performing cell sorting. We are grateful to Drs. Noboru Mizushima and Siegfried Weiss for providing Atg5 flox and ROSAmT/mG mice, respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dikic I and Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. (2018) 19:349–64. doi: 10.1038/s41580-018-0003-4
2. Mizushima N, Yoshimori T, and Ohsumi Y. The role of atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. (2011) 27:107–32. doi: 10.1146/annurev-cellbio-092910-154005
3. Klionsky DJ and Schulman BA. Dynamic regulation of macroautophagy by distinctive ubiquitin-like proteins. Nat Struct Mol Biol. (2014) 21:336–45. doi: 10.1038/nsmb.2787
4. Hanada T, Noda NN, Satomi Y, Ichimura Y, Fujioka Y, Takao T, et al. The atg12-atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J Biol Chem. (2007) 282:37298–302. doi: 10.1074/jbc.C700195200
5. Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, and Elazar Z. Lc3 and gate-16/gabarap subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. (2010) 29:1792–802. doi: 10.1038/emboj.2010.74
6. Kara EE, Comerford I, Fenix KA, Bastow CR, Gregor CE, McKenzie DR, et al. Tailored immune responses: novel effector helper T cell subsets in protective immunity. PloS Pathog. (2014) 10:e1003905. doi: 10.1371/journal.ppat.1003905
7. Fontenot JD, Gavin MA, and Rudensky AY. Foxp3 programs the development and function of cd4+Cd25+ Regulatory T cells. Nat Immunol. (2003) 4:330–6. doi: 10.1038/ni904
8. Hori S, Nomura T, and Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. (2003) 299:1057–61. doi: 10.1126/science.1079490
9. Josefowicz SZ, Lu LF, and Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. (2012) 30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623
10. Vignali DA, Collison LW, and Workman CJ. How regulatory T cells work. Nat Rev Immunol. (2008) 8:523–32. doi: 10.1038/nri2343
11. Facciabene A, Motz GT, and Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. (2012) 72:2162–71. doi: 10.1158/0008-5472.CAN-11-3687
12. Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, et al. Neuropilin 1 deficiency on cd4+Foxp3+ Regulatory T cells impairs mouse melanoma growth. J Exp Med. (2012) 209:2001–16. doi: 10.1084/jem.20111497
13. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (Ipex) is caused by mutations of foxp3. Nat Genet. (2001) 27:20–1. doi: 10.1038/83713
14. Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. (2001) 27:68–73. doi: 10.1038/83784
15. Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. (2001) 27:18–20. doi: 10.1038/83707
16. Kim JM, Rasmussen JP, and Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. (2007) 8:191–7. doi: 10.1038/ni1428
17. Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, et al. Selective depletion of foxp3+ Regulatory T cells induces a scurfy-like disease. J Exp Med. (2007) 204:57–63. doi: 10.1084/jem.20061852
18. Bronietzki AW, Schuster M, and Schmitz I. Autophagy in T-cell development, activation and differentiation. Immunol Cell Biol. (2015) 93:25–34. doi: 10.1038/icb.2014.81
19. Clarke AJ and Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. (2019) 19:170–83. doi: 10.1038/s41577-018-0095-2
20. Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. (2016) 17(3):277–85. doi: 10.1038/ni.3365
21. Kabat AM, Harrison OJ, Riffelmacher T, Moghaddam AE, Pearson CF, Laing A, et al. The autophagy gene atg16l1 differentially regulates treg and th2 cells to control intestinal inflammation. eLife. (2016) 5:e12444. doi: 10.7554/eLife.12444
22. Plaza-Sirvent C, Zhao B, Bronietzki AW, Pils MC, Tafrishi N, Schuster M, et al. A central role for atg5 in microbiota-dependent foxp3(+) rorgammat(+) treg cell preservation to maintain intestinal immune homeostasis. Front Immunol. (2021) 12:705436. doi: 10.3389/fimmu.2021.705436
23. Le Texier L, Lineburg KE, Cao B, McDonald-Hyman C, Leveque-El Mouttie L, Nicholls J, et al. Autophagy-dependent regulatory T cells are critical for the control of graft-versus-host disease. JCI Insight. (2016) 1:e86850. doi: 10.1172/jci.insight.86850
24. Puleston DJ, Zhang H, Powell TJ, Lipina E, Sims S, Panse I, et al. Autophagy is a critical regulator of memory cd8(+) T cell formation. eLife. (2014) 3:e03706. doi: 10.7554/eLife.03706
25. Xu X, Araki K, Li S, Han JH, Ye L, Tan WG, et al. Autophagy is essential for effector cd8(+) T cell survival and memory formation. Nat Immunol. (2014) 15:1152–61. doi: 10.1038/ni.3025
26. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. (2006) 441:885–9. doi: 10.1038/nature04724
27. Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, and Killeen N. Thymic ox40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. (2009) 182:4581–9. doi: 10.4049/jimmunol.0900010
28. Muzumdar MD, Tasic B, Miyamichi K, Li L, and Luo L. A global double-fluorescent cre reporter mouse. Genesis. (2007) 45:593–605. doi: 10.1002/dvg.20335
29. Stehr M, Greweling MC, Tischer S, Singh M, Blocker H, Monner DA, et al. Charles river altered schaedler flora (Crasf) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab Anim. (2009) 43:362–70. doi: 10.1258/la.2009.0080075
30. Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of foxp3+ Regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. (2010) 70:7788–99. doi: 10.1158/0008-5472.CAN-10-1736
31. Gramaglia I, Weinberg AD, Lemon M, and Croft M. Ox-40 ligand: A potent costimulatory molecule for sustaining primary cd4 T cell responses. J Immunol. (1998) 161:6510–7. doi: 10.4049/jimmunol.161.12.6510
32. Kim JK, Klinger M, Benjamin J, Xiao Y, Erle DJ, Littman DR, et al. Impact of the tcr signal on regulatory T cell homeostasis, function, and trafficking. PloS One. (2009) 4:e6580. doi: 10.1371/journal.pone.0006580
33. Yamanouchi S, Kuwahara K, Sakata A, Ezaki T, Matsuoka S, Miyazaki J, et al. A T cell activation antigen, ly6c, induced on cd4+ Th1 cells mediates an inhibitory signal for secretion of il-2 and proliferation in peripheral immune responses. Eur J Immunol. (1998) 28:696–707. doi: 10.1002/(SICI)1521-4141(199802)28:02<696::AID-IMMU696>3.0.CO;2-N
34. Pua HH, Dzhagalov I, Chuck M, Mizushima N, and He YW. A critical role for the autophagy gene atg5 in T cell survival and proliferation. J Exp Med. (2007) 204:25–31. doi: 10.1084/jem.20061303
35. Paul S and Schaefer BC. Selective autophagy regulates T cell activation. Autophagy. (2012) 8:1690–2. doi: 10.4161/auto.21581
36. Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, and Croft M. The ox40 costimulatory receptor determines the development of cd4 memory by regulating primary clonal expansion. J Immunol. (2000) 165:3043–50. doi: 10.4049/jimmunol.165.6.3043
37. Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples tcr signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. (2014) 15:473–81. doi: 10.1038/ni.2849
38. Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, et al. Distinct roles for the ox40-ox40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. (2004) 172:3580–9. doi: 10.4049/jimmunol.172.6.3580
39. Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced foxp3+ T reg cells. J Exp Med. (2012) 209:1723–42. doi: 10.1084/jem.20120914
40. Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. (2012) 209:1713–22. doi: 10.1084/jem.20120822
41. Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, and Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. (2010) 185:7349–57. doi: 10.4049/jimmunol.1000576
42. Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, et al. Autophagy is induced in cd4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. (2006) 177:5163–8. doi: 10.4049/jimmunol.177.8.5163
43. Jia W, Pua HH, Li QJ, and He YW. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. J Immunol. (2011) 186:1564–74. doi: 10.4049/jimmunol.1001822
44. McLeod IX, Zhou X, Li QJ, Wang F, and He YW. The class iii kinase vps34 promotes T lymphocyte survival through regulating il-7ralpha surface expression. J Immunol. (2011) 187:5051–61. doi: 10.4049/jimmunol.1100710
45. Parekh VV, Wu L, Boyd KL, Williams JA, Gaddy JA, Olivares-Villagomez D, et al. Impaired autophagy, defective T cell homeostasis, and a wasting syndrome in mice with a T cell-specific deletion of vps34. J Immunol. (2013) 190:5086–101. doi: 10.4049/jimmunol.1202071
46. Pua HH, Guo J, Komatsu M, and He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. (2009) 182:4046–55. doi: 10.4049/jimmunol.0801143
47. Stephenson LM, Miller BC, Ng A, Eisenberg J, Zhao Z, Cadwell K, et al. Identification of atg5-dependent transcriptional changes and increases in mitochondrial mass in atg5-deficient T lymphocytes. Autophagy. (2009) 5:625–35. doi: 10.4161/auto.5.5.8133
48. Willinger T and Flavell RA. Canonical autophagy dependent on the class iii phosphoinositide-3 kinase vps34 is required for naive T-cell homeostasis. Proc Natl Acad Sci U.S.A. (2012) 109:8670–5. doi: 10.1073/pnas.1205305109
49. Murera D, Arbogast F, Arnold J, Bouis D, Muller S, and Gros F. Cd4 T cell autophagy is integral to memory maintenance. Sci Rep. (2018) 8:5951. doi: 10.1038/s41598-018-23993-0
50. Whang MI, Tavares RM, Benjamin DI, Kattah MG, Advincula R, Nomura DK, et al. The ubiquitin binding protein tax1bp1 mediates autophagasome induction and the metabolic transition of activated T cells. Immunity. (2017) 46:405–20. doi: 10.1016/j.immuni.2017.02.018
51. Croft M. Control of immunity by the tnfr-related molecule ox40 (Cd134). Annu Rev Immunol. (2010) 28:57–78. doi: 10.1146/annurev-immunol-030409-101243
52. McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. Cd4(+)Cd25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced tnf receptor. Immunity. (2002) 16:311–23. doi: 10.1016/S1074-7613(02)00280-7
53. Pippig SD, Pena-Rossi C, Long J, Godfrey WR, Fowell DJ, Reiner SL, et al. Robust B cell immunity but impaired T cell proliferation in the absence of cd134 (Ox40). J Immunol. (1999) 163:6520–9. doi: 10.4049/jimmunol.163.12.6520
54. Rogers PR, Song J, Gramaglia I, Killeen N, and Croft M. Ox40 promotes bcl-xl and bcl-2 expression and is essential for long-term survival of cd4 T cells. Immunity. (2001) 15:445–55. doi: 10.1016/S1074-7613(01)00191-1
55. Kovacs JR, Li C, Yang Q, Li G, Garcia IG, Ju S, et al. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. (2012) 19:144–52. doi: 10.1038/cdd.2011.78
56. Jia W and He YW. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. J Immunol. (2011) 186:5313–22. doi: 10.4049/jimmunol.1002404
57. Jeong J, Choi YJ, and Lee HK. The role of autophagy in the function of cd4(+) T cells and the development of chronic inflammatory diseases. Front Pharmacol. (2022) 13:860146. doi: 10.3389/fphar.2022.860146
58. Shi H and Chi H. Metabolic control of treg cell stability, plasticity, and tissue-specific heterogeneity. Front Immunol. (2019) 10:2716. doi: 10.3389/fimmu.2019.02716
59. Norton EG, Chapman NM, Shi H, Meng X, Huang H, Kc A, et al. Vps34-orchestrated lipid signaling processes regulate the transitional heterogeneity and functional adaptation of effector regulatory T cells. PloS Biol. (2025) 23:e3003074. doi: 10.1371/journal.pbio.3003074
60. Flynn AL, Gans J, Escobedo J, Zhu C, Florescu AM, Shankara S, et al. Rgs1 modulates autophagic and metabolic programs and is a critical mediator of human regulatory T cell function. J Immunol. (2023) 211:1656–68. doi: 10.4049/jimmunol.2200402
61. Wang A, Ding L, Wu Z, Ding R, Teng XL, Wang F, et al. Zfp91 is required for the maintenance of regulatory T cell homeostasis and function. J Exp Med. (2021) 218:e20201217. doi: 10.1084/jem.20201217
62. Wang N, Yuan J, Karim MR, Zhong P, Sun YP, Zhang HY, et al. Effects of mitophagy on regulatory T cell function in patients with myasthenia gravis. Front Neurol. (2020) 11:238. doi: 10.3389/fneur.2020.00238
63. Read S, Malmstrom V, and Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of cd25(+)Cd4(+) regulatory cells that control intestinal inflammation. J Exp Med. (2000) 192:295–302. doi: 10.1084/jem.192.2.295
64. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by cd25(+)Cd4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. (2000) 192:303–10. doi: 10.1084/jem.192.2.303
Keywords: autophagy, ATG5, CD4 T cell, regulatory T cell, T cell activation
Citation: Plaza-Sirvent C, Bessen C, Bronietzki AW, Klages K, Schuster M, Huehn J and Schmitz I (2025) Loss of ATG5 impairs CD4+ T cell activation and promotes anti-tumor responses. Front. Immunol. 16:1284391. doi: 10.3389/fimmu.2025.1284391
Received: 28 August 2023; Accepted: 22 August 2025;
Published: 04 September 2025.
Edited by:
Jin Wang, Houston Methodist Research Institute, United StatesReviewed by:
Panayotis Verginis, University of Crete, GreeceFrancesc Marti, Department of Surgery/Transplant University of Kentucky, United States
Copyright © 2025 Plaza-Sirvent, Bessen, Bronietzki, Klages, Schuster, Huehn and Schmitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ingo Schmitz, aW5nby5zY2htaXR6QHJ1Yi5kZQ==
†Present address: Katjana Klages, Department of Science Policy and Internationalisation, DSMZ - German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany
Marc Schuster, Miltenyi Biotec, Bergisch Gladbach, Germany
 Carlos Plaza-Sirvent
Carlos Plaza-Sirvent Clara Bessen
Clara Bessen Alisha W. Bronietzki
Alisha W. Bronietzki Katjana Klages
Katjana Klages Marc Schuster
Marc Schuster Jochen Huehn
Jochen Huehn Ingo Schmitz
Ingo Schmitz