- 1Department of Hepatic Surgery I (Ward I) Shanghai Eastern Hepatobiliary Surgery Hospital, Navy Medical University, Shanghai, China
- 2Department of Epidemiology, Faculty of Navy Medicine, Navy Medical University, Shanghai, China
- 3Key Laboratory of Biological Defense, Ministry of Education, Shanghai, China
- 4Laboratory of Medical Bioprotection, Navy Medical University, Shanghai, China
- 5The Second Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
Background and aims: Most Hepatocellular carcinoma (HCC) diagnoses occur at advanced stages precluding radical surgical resections. Conversion therapy offers a viable chance for patients with unresectable HCC (uHCC) to become eligible for curative surgery. Despite the application of various treatment modalities for conversion therapy, uncertainties persist regarding its efficacy. Consequently, we collected clinical data to evaluate the prognosis of TACE+TKI+ICI conversion therapy and compared it with the prognoses of other conversion therapies in the literature. We aimed to elucidate the potential superiority of triplet therapy as the optimal option among the existing conversion therapy regimens, by using this comprehensive analysis.
Methods: From January, 2019, to November, 2022, we collected data from 69 patients with HCC undergoing conversion therapy with the TACE+TKI+ICI triplet therapy. Ultimately, we analyzed data from 57 patients at BCLC Stages B and C in our study. We also conducted a comprehensive literature review on conversion therapy for uHCC by searching PubMed and Web of Science databases and gathered data from 9 studies comprising a total of 560 patients.
Results: The conversion and disease control rate (DCR) in our cohort reached 14.0% (95% CI, 9.4–18.6%) and 66.7% (95% CI, 60.5–72.9%), respectively. When compared to the conversion rates in the literature, the triplet therapy demonstrated significant benefits, underscoring the potential efficacy of the TACE+TKI+ICIs triplet therapy.
Conclusion: Our results presented improved conversion rates in patients with uHCC following TACE+TKI+ICI triplet therapy. However, overall survival (OS) and recurrence-free survival (RFS) were similar to those of other treatment modalities in the literature.
Clinical Trial Registration: ClinicalTrials.gov, identifier ChiCTR2400084896.
1 Introduction
Liver cancer ranks sixth in incidence among the global cancers and third in terms of mortality rates. Chinese patients represent 45.3% of newly diagnosed cases globally and 47.1% of the global liver cancer-related deaths (1). HCC, as the most common sort of liver cancer, the early stages of symptoms often are not typical due to the insidious onset and undefined epidemiological history of the disease that includes cases of HBV, HCV, or alcoholic liver cirrhosis (2, 3). Thus, 70% to 80% of patients with HCC are diagnosed at an advanced stage with surgical contraindications. The 5-year survival rate of these patients is less than 20% (4). Although radical surgical resection remains the primary treatment modality for HCC, only 20% to 30% of patients are eligible for resection (5–8). Therefore, conversion therapy provides an opportunity for curative resections in most cases. Studies have suggested that HCC, following conversion treatments such as transarterial chemoembolization (TACE), radiofrequency ablation, or radiotherapy reaches 5-year survival rates of 50–60% (9–11). Alternative conversion treatment approaches include portal vein embolization (PVE) and the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (12). These treatments enhance the eligibility of patients for radical resection.
The relatively low rates of initial curative resections for HCC have promoted research on systemic therapies, a field that has seen substantial progress. Significant advancements have been achieved in the use of anti-angiogenic inhibitors and immunotherapy. Sorafenib and lenvatinib are considered first-line treatments for patients with advanced HCC; however, their efficacy rates have not been optimal (13, 14). Encouragingly, the combination of targeted therapy and immunotherapy for advanced uHCC, such as the T+A regimen, has demonstrated a 67.2% 1-year survival rate and a median survival time of 11.2 months (15, 16). By contrast, the overall response rate (ORR) for the regimen of tyrosine kinase inhibitors (TKIs) combined with immune checkpoint inhibitors (ICIs) has reached 36% (17). Thus, the potential for improving outcomes with combination therapies is clear (18). Methods such as systemic therapy, local treatment, increasing future liver remnant (FLR)/standard liver volume (SLV), improving liver function, and antiviral therapy have all been studied and recommended for conversion therapy in the ‘Chinese Expert Consensus on Hepatocellular Carcinoma conversion Therapy (2021 Edition)’. However, in the clinical practice, some patients display suboptimal efficacy following systemic therapy. Therefore, we implemented a kind of treatment, adhering to the Chinese Primary Liver Cancer Diagnosis and Treatment Standard, using local TACE combined with systemic therapy for conversion therapy in patients with uHCC, followed by subsequent curative surgical resection.
2 Methods
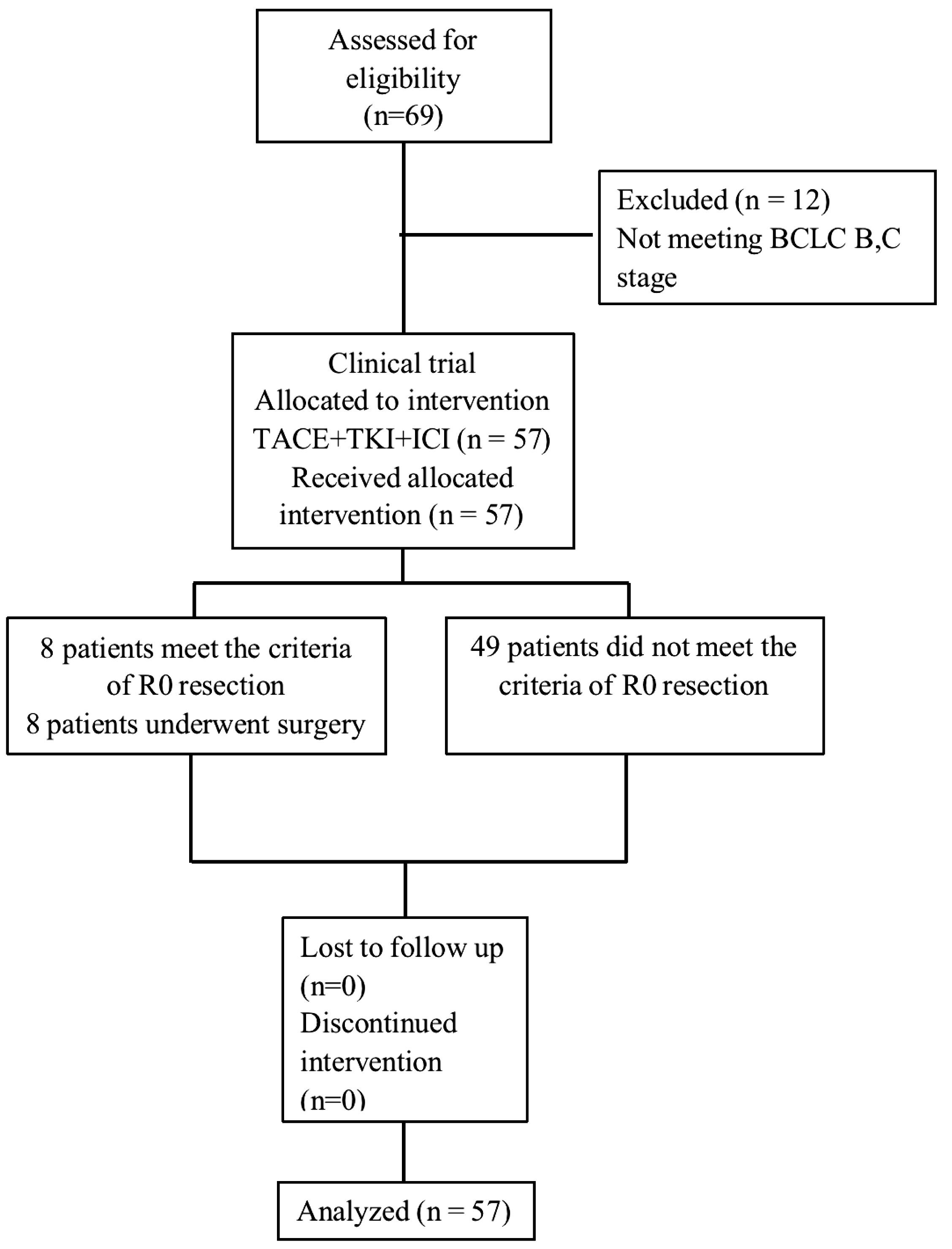
From 2019–01 to 2022-11, we enrolled 57 patients with BCLC stage B and C uHCC at the Shanghai Eastern Hepatobiliary Surgery Hospital. Following examinations and exclusion of contraindications, these patients received TACE for local lesion control, combined with first-line targeted therapy and immunotherapy. Their HCC diagnoses relied primarily on imaging evidence of well-defined focal lesions, with evaluation based on BCLC staging and CNLC guidelines to confirm the unresectable nature of the tumors, either with or without elevated tumor markers (AFP, AFP-L3, and PIVKA). We included individuals of either sex, and those with a history of hepatitis B and ECOG performance status of 0–1. In addition, we reviewed relevant literature following a comprehensive search on Pubmed, Web of Science and Embase databases including publications up until July 2022. We found 9 articles with precise matching analyses for conversion therapy. The therapeutic interventions included TACE, targeted therapy, and immune checkpoint inhibitors (ICIs) used both solely or jointly as conversion therapies for uHCC. Figure 1 shows the trial flow diagram and Supplementary Figure S1 shows the flowchart for retrieving the comparison of clinical trials. The Shanghai Eastern Hepatobiliary Surgery Hospital Ethics Committee reviewed and approved the study protocol (Ethics Approval Number: EHBHKY2023-K033-P001), and we obtained signed written informed consent forms from all enrolled patients. All authors had access to the study data and reviewed and approved the final manuscript.
2.1 Treatment plan
Following the assessment of overall health status and liver function (Child Pugh A or B) and the exclusion of contraindications to conversion therapy, all enrolled patients initially underwent TACE. Subsequently, in the absence of significant adverse effects, the patients received oral sorafenib at a dose of 0.25 g per day, or lenvatinib (8 mg–12 mg per day, with dosing based on body weight (<60 kg: 8 mg; ≥60 kg: 12 mg)) (13, 19, 20). The targeted oral medication was administered on the same day as the immunotherapy (every 3 weeks), using drugs such as camrelizumab (21) (200 mg, every 2 weeks) or sintilimab (200 mg, every 3 weeks). We conducted regular follow-up examinations for all enrolled patients, including complete blood counts, liver, kidney, thyroid, and cardiac function tests, tumor markers assessments, electrocardiogram, and a chest X-ray before each immunotherapy session. We assessed tumor changes via periodic imaging studies, with Computerized tomography (CT) or magnetic resonance imaging (MRI) scans conducted every 6 weeks, following the RECIST v1.1 criteria for tumor response evaluation and modified RECIST standards (22, 23).
2.2 Surgical procedure and standards
The preoperative assessment criteria were based on blood tests and imaging data to determine whether each patient met the R0 resection standards. According to the latest international guidelines, R0 resection is defined as a microscopically margin-negative resection, with no residual tumor cells detected at the resection margin (24). Preoperative contrast-enhanced CT was utilized to further evaluate the therapeutic response and confirm eligibility for R0 resection. Conditions not meeting R0 resection standards included a residual liver volume lower than 30% in non-cirrhotic individuals, lower than 40% in cirrhotic patients, a BCLC stage class B or C, and tumor recurrence within 12 months after the initial resection. The eligibility criteria for HCC surgical resection included the feasibility of an R0 resection leaving sufficient residual volume of functional liver. Additionally, intrahepatic lesions had to exhibit at least a partial response (PR) or stable disease (SD) for a minimum of 2 months, with no severe or persistent adverse effects from systemic therapy and no contraindications for hepatic resection.
The diagnosis and grading of postoperative liver dysfunction were based on criteria proposed by the International Study Group of Liver Surgery, including an increase in the international normalized ratio and postoperative hyperbilirubinemia occurring on or after the 5th postoperative day (25). We classified postoperative complications using the Clavien-Dindo system, which categorizes them based on their severity (26).
2.3 Tumor response and adverse reaction assessments
We assessed the tumor response using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) at regular intervals (typically every 4 to 8 weeks) using contrast-enhanced CT or MRI scans to categorize responses as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). A third-party review committee independently evaluated the assessment results. Objective response rate (ORR) was defined as the proportion of patients achieving CR or PR, with this assessment conducted at least 4 weeks after the initial fulfillment of response criteria. The disease control rate (DCR), encompassing CR, PR, and SD, served as a broader measure of the treatment’s overall control over the disease. We extracted adverse reaction information from the hospital electronic medical records database and evaluated the data based on the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0).
2.4 Postoperative management
After undergoing radical resection, patients returned for follow-up approximately 4–5 weeks postoperatively. We requested new preventive interventional therapies based on the pathological findings. Immunotherapy was administered continuously during this period, and targeted therapy was resumed 4–6 weeks after surgery. We re-examined the tumor marker levels before initiating the immunotherapy, with CT or MRI scans conducted every 9 weeks.
2.5 Data analysis
We analyzed all data using SPSS 18.0 software. OS was defined as the time from definitive diagnosis to death, and RFS was measured from the date of surgery to either the date of patient relapse or the time of patient death due to reasons other than an apparent relapse. We conducted a differential analysis of data using paired t-tests and exact Fisher tests. We generated Kaplan–Meier analysis OS curves, with log-rank test evaluating differences. Additionally, univariate and multivariate Cox regression analyses were performed to evaluate prognostic factors.
3 Results
3.1 Baseline characteristics
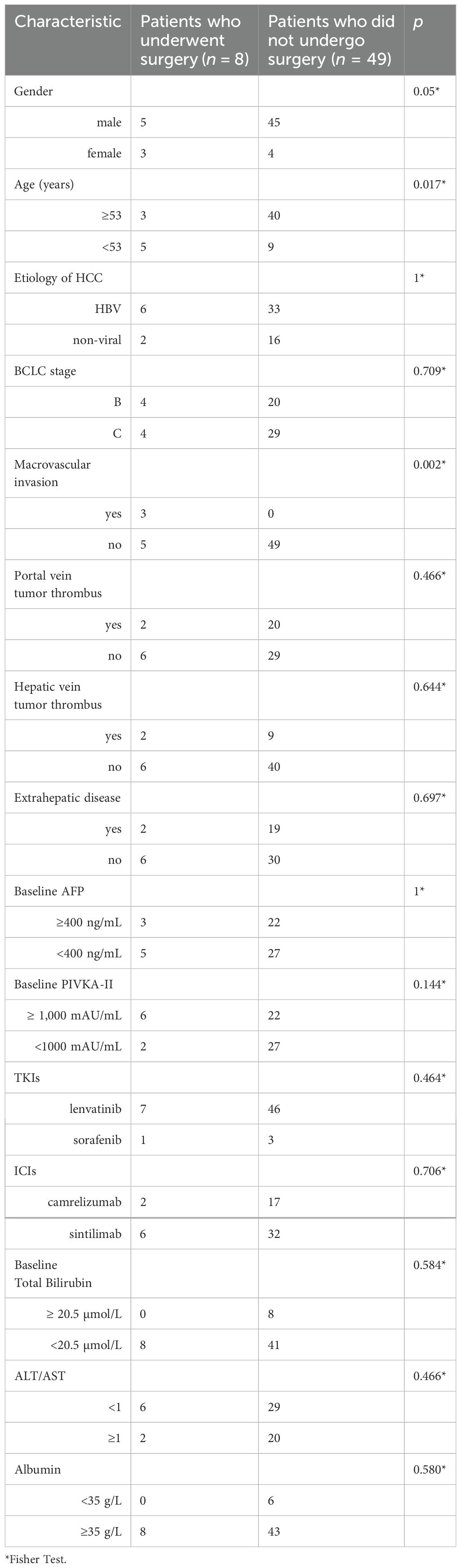
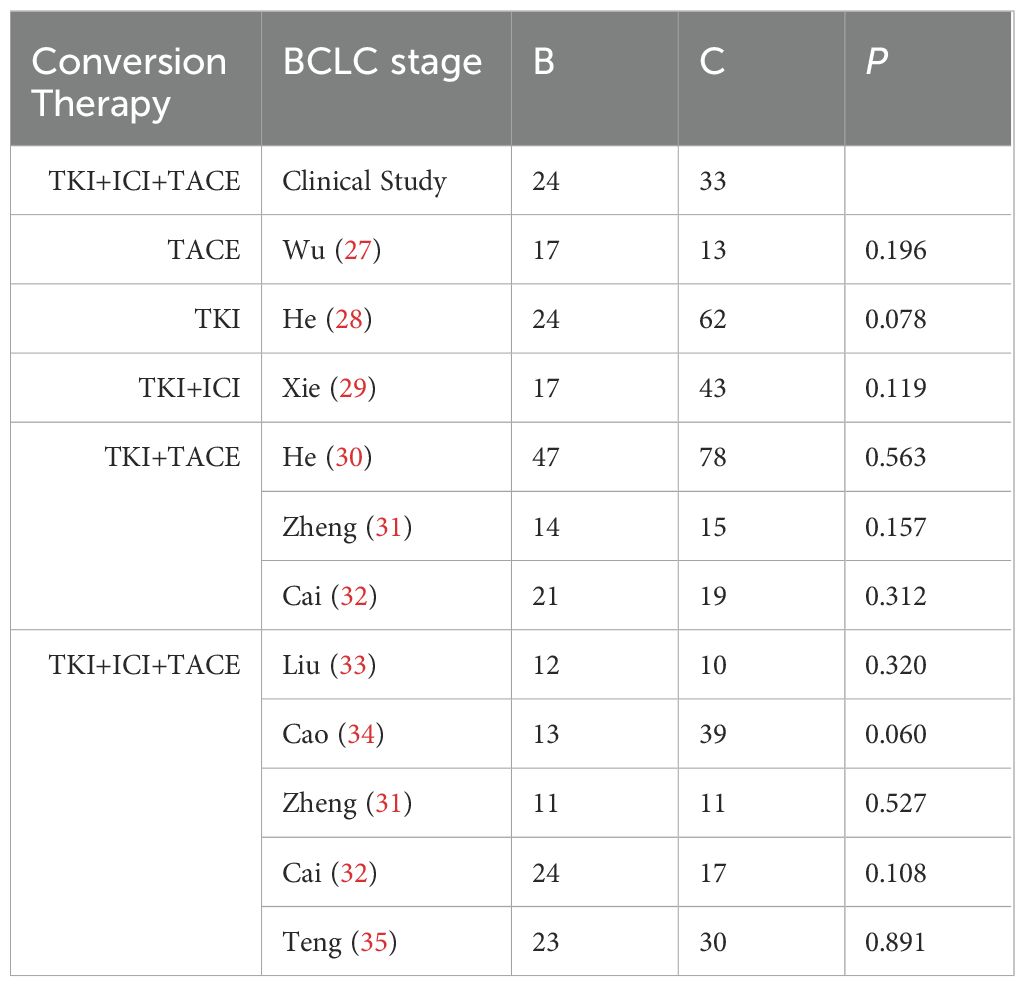
As of June 5, 2024, 69 patients underwent TACE+TKI+ICIs conversion therapy for initially diagnosed uHCC at the Shanghai Eastern Hepatobiliary Surgery Hospital. Among these cases, 57 were classified as BCLC B or C, and ultimately, 8 patients underwent radical resections for primary HCC. Table 1 summarizes the baseline characteristics of the 57 patients receiving systematic conversion therapy. Univariate Cox regression analyses on baseline variables yielded significances between AFP (P=0.029), albumin (P=0.021), and OS. After our multivariate analysis, we found significances between AFP (P=0.019), albumin (P=0.003) and OS, and between gender (P=0.043), abnormal prothrombin (PIVKA-II) (P=0.008), and RFS (Table 2). Patients who underwent surgical resections experienced a significant improvement in OS compared to those who did not (Figure 2), but we detected no significant differences in RFS (P>0.05). Additionally, we reviewed data from a subset of studies on HCC conversion therapy from PubMed, Web of Science and Embase. We performed a matching analysis based on BCLC staging and existing clinical data (Table 3). The analysis included data from various studies involving TACE, TKI, and ICIs, either single or joint. In total, 560 patients with BCLC B or C received conversion therapy, including 30 treated with TACE alone, 86 with TKI alone, 60 with a TKI+ICIs combination, 194 with TKI+TACE, and 190 with TKI+TACE+ICIs triplet therapy.
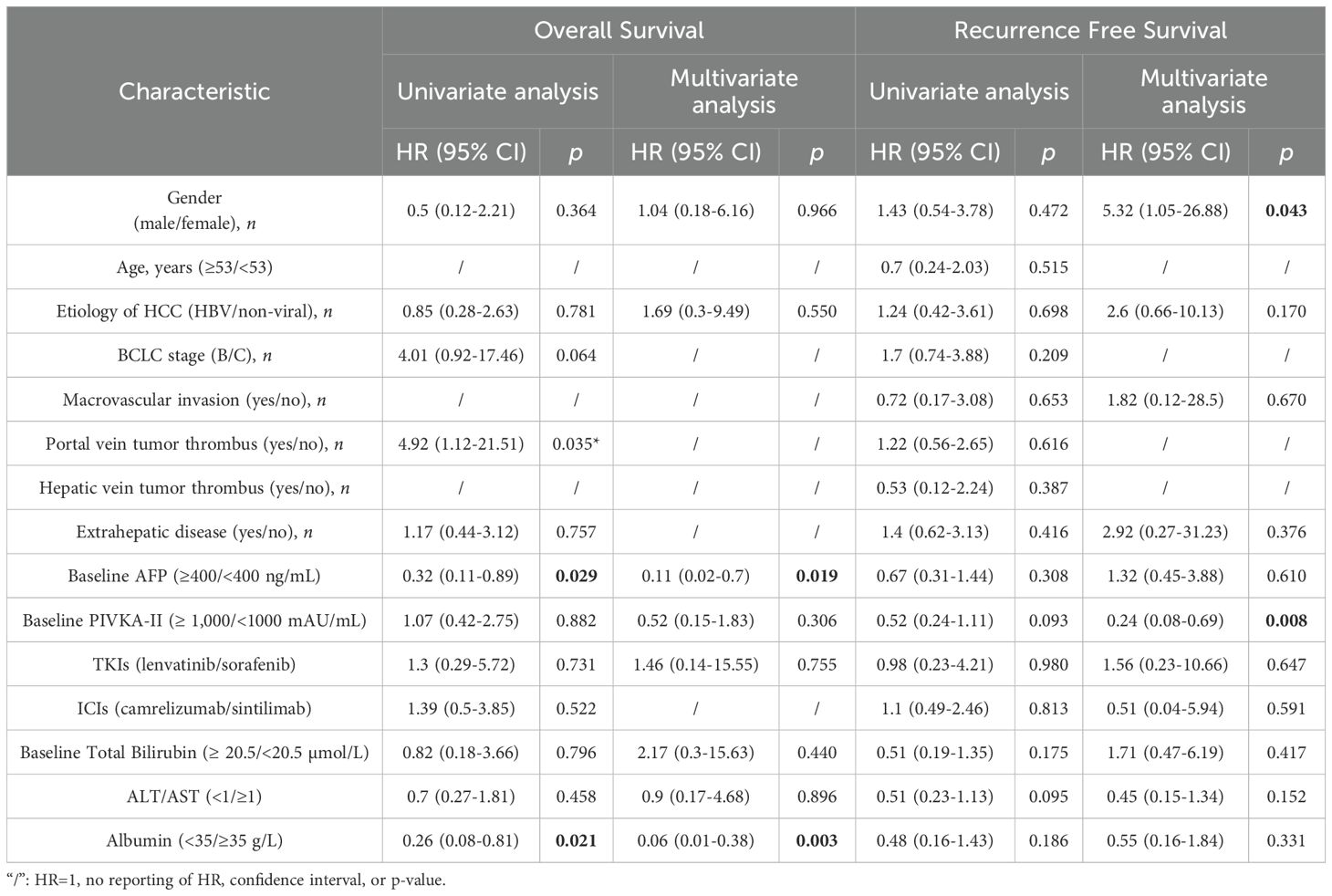
Table 2. Baseline characteristic univariate and multivariate Cox regression analysis with OS and RFS.
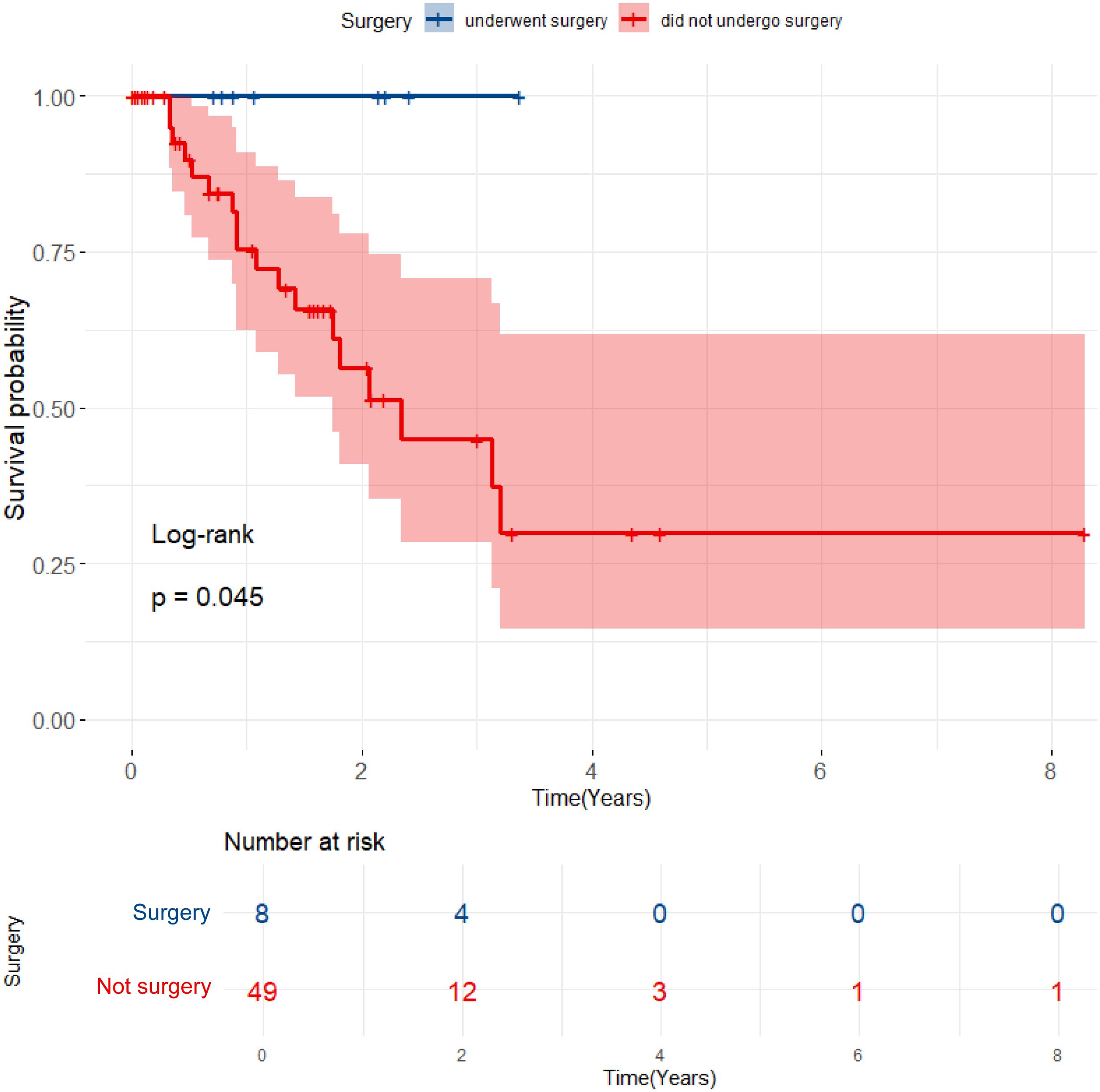
Figure 2. Kaplan-Meier curves for OS comparing patients who underwent surgical resection with those who did not.
3.2 TACE+TKI+ICI: conversion therapy for uHCC
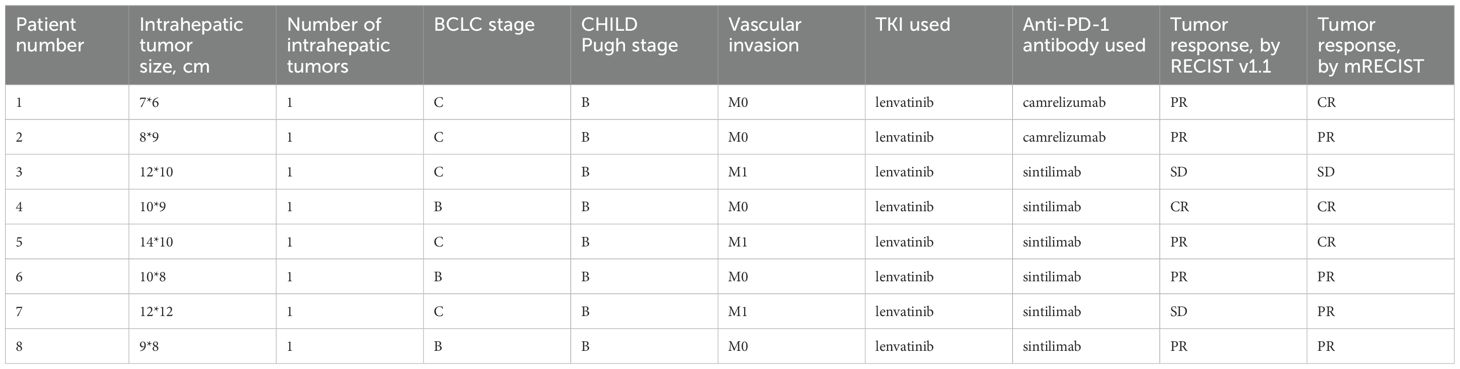
In our cohort of 57 patients with uHCC, according to the mRECIST criteria, the TKI+TACE+ICIs combination therapy achieved a conversion success rate of 14.0% (95% CI, 9.4–18.6%), with an ORR of 21.1% (95% CI, 15.7–26.5%), and a DCR of 66.7% (95% CI, 60.5–72.9%). Moreover, based on mRECIST version 1.1, the independent imaging data of surgical patients revealed significant differences before and after the conversion therapy, as evidenced by the contrast-enhanced CT scans of lesions before and after treatment (Supplementary Figure S2). The pathological results from patients undergoing primary surgical resections consistently demonstrated favorable conversion outcomes. Table 4 shows the baseline data of surgical patients.
3.3 TACE+TKI+ICI: a superior conversion therapy strategy for uHCC
Conversion therapies include TACE, TKI, TKI+ICI, TKI+TACE, and TKI+TACE+ICI, the conversion rate for the TKI+TACE+ICI therapy showed a distinct advantage after analyzing the conversion rates for other modalities (Figure 3A). However, in contrast to other studies, our clinical study finds that there are indistinct improvements in ORRs or DCRs (Figures 3B, C). A comparative analysis of our trial with other studies demonstrated the superiority of the TACE+TKI+ICI triplet therapy in terms of conversion rates for uHCC. However, the ORRs or DCRs of TKI+TACE+ICI in our trial not improve apparently compared with other researches.
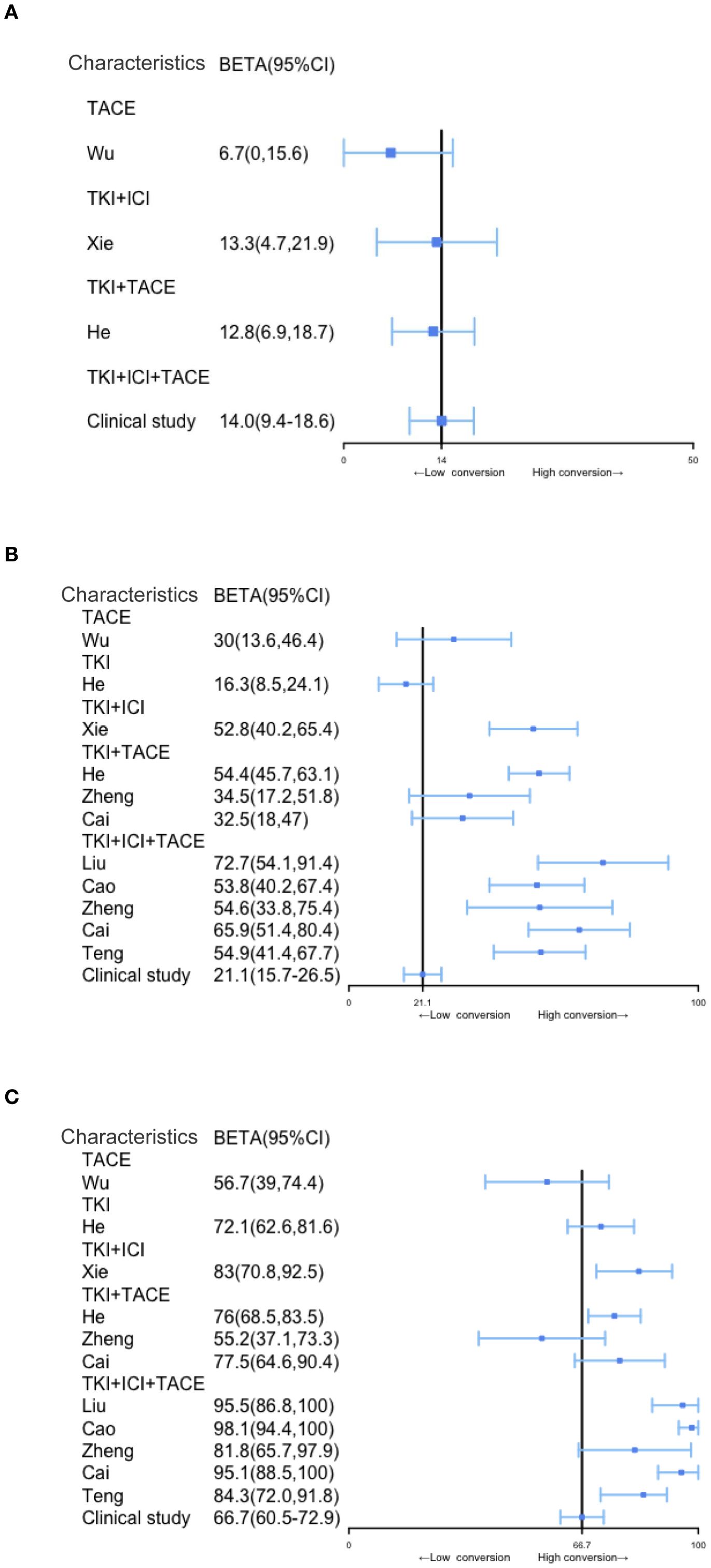
Figure 3. Comparison of conversion rates, ORRs, and DCRs in patients with uHCC undergoing different treatment modalities, including triple combination therapy. (A–C) Comparison of conversion rates, ORR and DCR among different conversion therapy modalities, the solid black line represents the clinical study.
3.4 TACE+TKI+ICI therapy exhibits a significant advantage in RFS for uHCC conversion therapy
According to our clinical trial, the follow-up periods ranged from 4 to 63.3 months, with a median follow-up time of 12.1 months. During the follow-ups, 24 patients (48.9%) experienced recurrences, and 18 patients (36.7%) succumbed to the disease. The median OS was 13.1 months (95% CI, 8.6–17.6), and the median RFS was 8.7 months (95% CI, 4.0–13.4). Interestingly, in our clinical study, RFS and OS demonstrated less pronounced improvements after the conversion therapy than those reported in the literature. However, the OSs and RFSs of studies on various conversion therapies have consistently shown that triplet therapy provides significantly longer survivals than other conversion treatment modalities (Figure 4).
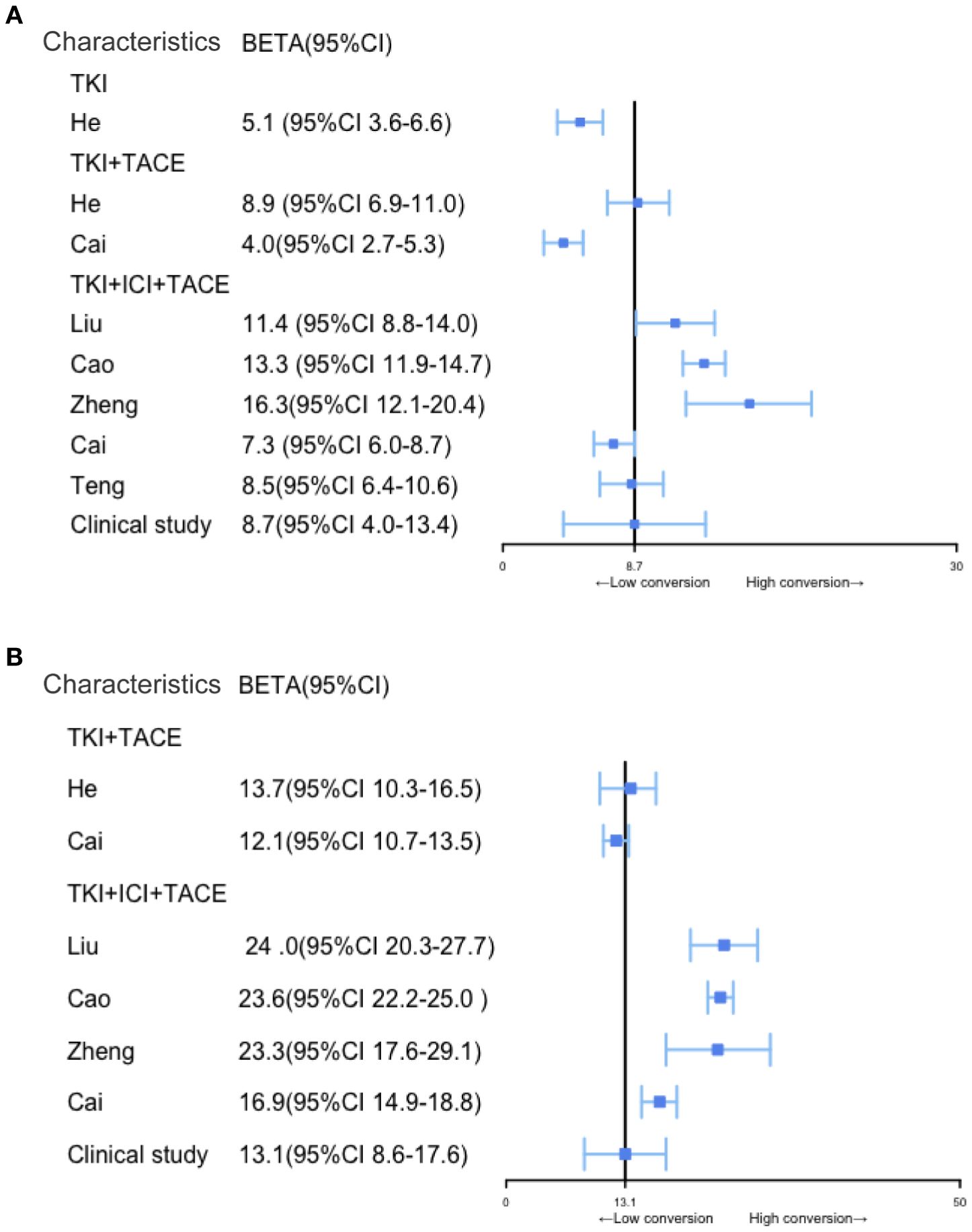
Figure 4. Comparison of RFS and OS among patients with BCLC stage B and C uHCC after different conversion therapy modalities. (A, B) Comparison of RFS and OS among different conversion therapy modalities, the solid black line represents the clinical study.
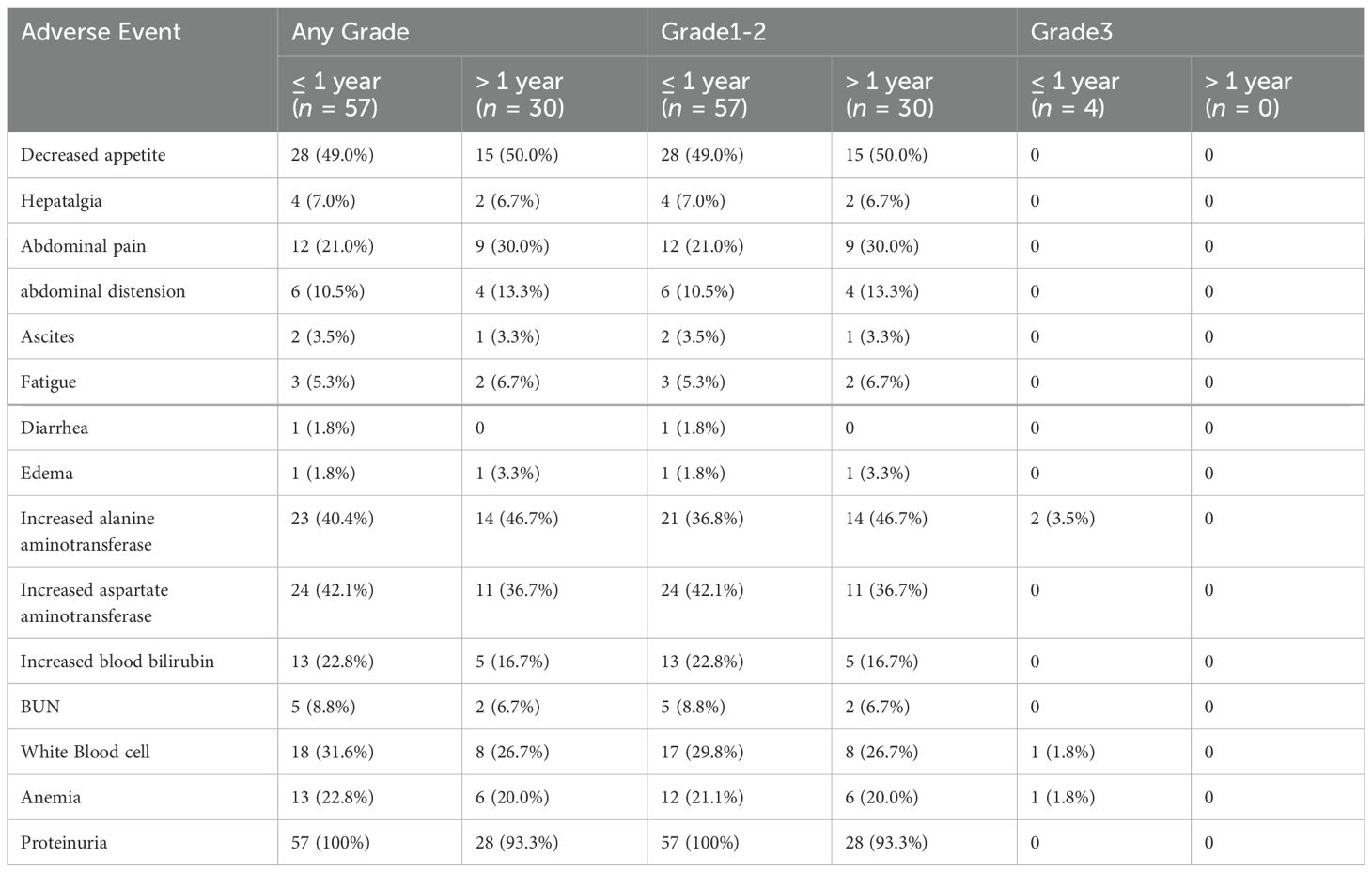
3.5 The adverse events with TACE+TKI+ICI therapy are manageable
In this clinical trial, we analyzed data from 57 adverse events (AEs) (100%) that occurred after triple therapy within 1 year. Grade 3 AEs occurred in 4 cases, including elevated AST in 2 patients (3.5%), leukopenia in 1 (1.8%), and severe anemia in 1 (1.8%). No adverse events exceeding grade 3 were observed, and the others were grade 2 or grade 1 AEs. After 1 year, 17 patients died, and the remaining patients had grade 2 or grade 1 AEs. We found no instances of grade 3 or above complications. Common AEs included decreased appetite, abdominal pain, elevated AST and/or elevated ALT levels, and leukopenia (Table 5). All AEs were assessed as mild and manageable during the follow-up periods.
4 Discussion
Advancements in the understanding of the molecular mechanisms of HCC, along with the continuous development of novel therapeutic strategies, have increased interest in the combined application of TACE, TKIs, and ICIs. This comprehensive treatment approach is an innovative strategy against HCC, but its underlying mechanisms and therapeutic efficacy require further exploration. We assessed the use of TACE to control uHCC tumors, combined with TKIs and ICIs to achieve the goal of conversion therapy and facilitate R0 resection. Drawing upon existing research and comparing various conversion therapy strategies, the TACE+TKI+ICI has demonstrated promising rates of successful conversion (36). TACE is currently the preferred local treatment for uHCC. Its primary mechanism involves embolizing the tumor-feeding arteries to achieve local ischemia and facilitate deep penetration of chemotherapy drugs, thereby achieving local tumor control. However, some patients may not achieve the desired outcomes when TACE is used as a single treatment (37). TKIs, particularly sorafenib and lenvatinib, act primarily by inhibiting the target tyrosine kinase pathways associated with tumor growth and angiogenesis, thereby halting tumor progression (38). ICIs, in particular PD-1 inhibitors, operate by restoring the suppressed T-cell function inhibited by tumors. However, there is variability in the response of patients to ICIs, probably due to differing tumor microenvironments (39). As an initial treatment, TACE directly damages tumor tissues, leading to the release of vast amounts of tumor antigens. This provides the necessary targets for ICIs to activate the immune system (40). Simultaneously, TKIs inhibit the reactive angiogenesis that may be triggered after TACE treatment, while also reducing the blood supply to the tumor (20). ICIs lift immune suppression, allowing T cells to effectively recognize and attack tumor cells. Moreover, the environment created by TACE and TKIs further enhances the immune response (41). As a result, the triplet therapy should synergistically facilitate effective conversion therapy for uHCC. Compared to single treatments, the triplet therapy with TACE+TKI+ICI seems to significantly improve conversion rates. Thus, a substantial proportion of patients undergoing this combined therapy should experience a reduction in tumor volume. Moreover, according to previous researches, the triplet therapy has demonstrated a clear advantage over monotherapy or doublet therapy in prolonging the OS and RFS of uHCC patients (42). According to the literature, conversion therapy of uHCC with triplet therapy leads to higher conversion rates in patients with BCLC stage A/B than in those with stage C; with stage A providing the best outcome, followed by stage B, and then stage C (43). However, in the clinical practice, the focus is primarily on stages B and C, and the conversion rate of patients with BCLC stage B is higher than that of patients with BCLC C. However, the triple conversion therapy did not demonstrate a significant improvement in RFS or OS compared to other treatment strategies. This may be attributed to the limited sample size and inconsistent follow-up durations. We aim to address these factors in future prospective randomized controlled trials. Meanwhile, some studies have found no significant differences in RFS among stages A, B, and C (44). Therefore, multicenter prospective studies are needed to explore the impact of BCLC staging on uHCC conversion therapy. Studies have shown increased risks of adverse events with the addition of treatment modalities; however, these are generally manageable. TACE+TKI+ICI therapy may induce some AEs, but most are mild and manageable. The most common AEs include hypertension, thyroid dysfunction, hepatic impairment, gastrointestinal reactions, and skin rashes (45). These reactions are mostly temporary and can be managed through medication adjustments or dose reductions. Compared with monotherapy, triplet therapy is more likely to result in adverse events such as neutropenia, hepatotoxicity, and embolic events (46). Therefore, the short-term management for patients involves frequent blood count monitoring and regular liver function tests during treatment, whereas the long-term management requires monitoring of cardiovascular risks and further lifestyle interventions. For patients initially deemed unsuitable for surgery, the triplet therapy facilitates tumor reduction and may also render some eligible for surgical resection. Therefore, the TACE+TKI+ICI strategy presents a novel and promising direction for HCC treatment. Of course, some studies have argument in triplet therapy. For example, Kudo’s study showed that although triplet therapy may theoretically provide strong efficacy; in actual clinical trials, the effects of dual therapy can be sufficient, and further increasing the treatment modalities (such as adding TACE or additional TKIs) may not necessarily bring additional benefits (46). Therefore, multicenter, prospective, randomized controlled trials are needed to investigate the therapeutic effects and AEs of triplet therapy versus monotherapy or dual therapy. Studies on the markers for predicting the therapeutic effect of triplet therapy are also needed. We plan to screen for effective efficacy markers in the research of triplet therapy in future studies.
This study has some limitations. First, the patients with uHCC diagnosed at Shanghai Eastern Hepatobiliary Surgery Hospital often present with relatively poor baseline conditions. Therefore, the achieved ORR and CR after combination therapy were not as satisfactory as anticipated. And the success rate of conversion is improved, but the OS and RFS were not particularly improved. This could also be due to the relatively small sample size in our study and differences in treatment modalities. Therefore, randomized controlled trials with large samples are needed to validate our findings. Additionally, our clinical study lacked direct comparisons between the various treatment modalities. Instead, we compared our data with previous relevant studies, the triplet therapy showed significant benefits. In our experience, the adverse effects of the TACE+TKI+ICI strategy were mostly manageable, and we believe these risks are acceptable given the benefits of the therapy. The short follow-up period is another limitation of our study. We plan to conduct multicenter randomized controlled trials with long-term follow-up to further evaluate the long-term prognosis and AEs of triplet therapy. Finally, the therapeutic drugs used in this study were not standardized enough. Triplet therapy can be administered with various medications determined according to the clinical experience of the physician prescribing it. Studies are needed to evaluate the efficacy and safety of different treatment methods.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shanghai Eastern Hepatobiliary Surgery Hospital, Navy Medical University, Shanghai, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CZ: Data curation, Investigation, Writing – review & editing. JY: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing – review & editing. NL: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Program of Shanghai Academic Research Leader (22XD1404800 to JY).
Acknowledgments
We thank the patients and their families.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1647890.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1451965/full#supplementary-material
Supplementary Figure 1 | Flowchart of the Literature studies selection process.
Supplementary Figure 2 | Contrast-enhanced CT scans of HCC lesions before and after treatment.
Abbreviations
uHCC, unresectable hepatocellular carcinoma; TACE, Transarterial Chemoembolization; TKIs, Tyrosine Kinase Inhibitors; ICIs, Immune Checkout Inhibitors; CR, complete response; PR, partial response; SD, stable disease; PD, disease progression; ORR, Overall Response Rate; DCR, Disease Control Rate; OS, Over Survival; RFS, Recurrence Free Survival; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
2. Guntipalli P, Pakala R, Kumari Gara S, Ahmed F, Bhatnagar A, Endaya Coronel MK, et al. Worldwide prevalence, genotype distribution and management of hepatitis C. Acta Gastroenterol Belg. (2021) 84:637–56. doi: 10.51821/84.4.015
3. Chidambaranathan-Reghupaty S, Fisher PB, and Sarkar D. Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res. (2021) 149:1–61. doi: 10.1016/bs.acr.2020.10.001
4. Zhong J-H, Peng N-F, You X-M, Ma L, Xiang X, Wang YY, et al. Tumor stage and primary treatment of hepatocellular carcinoma at a large tertiary hospital in China: A real-world study. Oncotarget. (2017) 8:18296–302. doi: 10.18632/oncotarget.15433
5. Benson AB, D’angelica MI, Abbott DE, Anaya DA, Anders R, Are C, et al. Hepatobiliary cancers. version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) . 19:541–65. doi: 10.6004jnccn.2021.0022
6. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma; 2019 edition). Liver Cancer. (2020) 9:682–720. doi: 10.1159/000509424
7. Forner A, Reig M, and Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
8. Kloeckner R, Galle PR, and Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. (2021) 73:137–49. doi: 10.1002/hep.31424
9. Lau WY, Ho SKW, Yu SCH, Lai ECH, Liew CT, and Leung TWT. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. (2004) 240:299–305. doi: 10.1097/01.sla.0000133123.11932.19
10. Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. (2016) 21:1442–9. doi: 10.1634/theoncologist.2016-0094
11. Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP, et al. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxf). (2018) 20:1109–18. doi: 10.1016/j.hpb.2018.06.1798
12. Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating liver partition and portal vein ligation for staged hepatectomy for unresectable hepatitis B virus-related hepatocellular carcinoma: A Single Center study of 45 patients. Ann Surg. (2020) 271:534–41. doi: 10.1097/SLA.0000000000002942
13. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
14. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
15. Finn RS and Cheng AL. Atezolizumab and bevacizumab in hepatocellular carcinoma [reply. N Engl J Med. (2020) 383:695. doi: 10.1056/NEJMc2021840
16. Kudo M. Combination immunotherapy with anti-VEGF/TKI for hepatocellular carcinoma: present and future perspective. Hepatobiliary Surg Nutr. (2021) 10:241–5. doi: 10.21037/hbsn-20-707
17. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
18. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib targets FGF Receptor 4 to enhance antitumor immune response of anti-programmed cell Death-1 in HCC. Hepatology. (2021) 74:2544–60. doi: 10.1002/hep.31921
19. Ni Y and Ye X. Apatinib for hepatocellular carcinoma. J Cancer Res Ther. (2019) 15:741–2. doi: 10.4103/jcrt.JCRT_400_19
20. Cheng A-L, Kang Y-K, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. (2009) 10:25–34. doi: 10.1016/S1470-2045(08)70285-7
21. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. (2020) 21:571–80. doi: 10.1016/S1470-2045(20)30011-5
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline. version 1.1. Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
23. Lencioni R and Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. (2010) 30:52–60. doi: 10.1055/s-0030-1247132
24. Vogel A, Chan SL, Dawson LA, Kelley RK, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2025) 36:491–506. doi: 10.1016/j.annonc.2025.02.006
25. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. (2011) 149:713–24. doi: 10.1016/j.surg.2010.10.001
26. Dindo D, Demartines N, and Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
27. Wu B, Zhou J, Ling G, Zhu D, and Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. (2018) 16:69. doi: 10.1186/s12957-018-1368-8
28. He M-K, Liang R-B, Zhao Y, Xu YJ, Chen HW, Zhou YM, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. (2021) 13:17588359211002720. doi: 10.1177/17588359211002720
29. Xie D, Sun Q, Wang X, Zhou J, Fan J, Ren Z, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med. (2021) 9:652. doi: 10.21037/atm-20-7037
30. He M, Li Q, Zou R, Shen J, Fang W, Tan G, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: A randomized clinical trial. JAMA Oncol. (2019) 5:953–60. doi: 10.1001/jamaoncol.2019.0250
31. Zheng L, Fang S, Wu F, Chen W, Chen M, Weng Q, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: A retrospective study. Front Mol Biosci. (2020) 7:609322. doi: 10.3389/fmolb.2020.609322
32. Cai M, Huang W, Huang J, Shi W, Guo Y, Liang L, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: A retrospective cohort study. Front Immunol. (2022) 13:848387. doi: 10.3389/fimmu.2022.848387
33. Liu J, Li Z, Zhang W, Lu H, Sun Z, Wang G, et al. Comprehensive treatment of trans-arterial chemoembolization plus lenvatinib followed by camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. (2021) 12:709060. doi: 10.3389/fphar.2021.709060
34. Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, et al. The efficacy of TACE combined with lenvatinib plus sintilimab in unresectable hepatocellular carcinoma: A multicenter retrospective study. Front Oncol. (2021) 11:783480. doi: 10.3389/fonc.2021.783480
35. Teng Y, Ding X, Li W, Sun W, and Chen J. A retrospective study on therapeutic efficacy of transarterial chemoembolization combined with immune checkpoint inhibitors plus lenvatinib in patients with unresectable hepatocellular carcinoma. Technol Cancer Res Treat. (2022) 21:15330338221075174. doi: 10.1177/15330338221075174
36. Pei Y, Li W, Wang Z, and Liu J. Successful conversion therapy for unresectable hepatocellular carcinoma is getting closer: A systematic review and meta-analysis. Front Oncol. (2022) 12:978823. doi: 10.3389/fonc.2022.978823
37. Hatanaka T, Yata Y, Naganuma A, and Kakizaki S. Treatment strategy for intermediate-stage hepatocellular carcinoma: transarterial chemoembolization, systemic therapy, and conversion therapy. Cancers (Basel). (2023) 15(6):1798. doi: 10.3390/cancers15061798
38. Kaneko S, Tsuchiya K, Yasui Y, Tanaka Y, Inada K, Ishido S, et al. Conversion surgery for hepatocellular carcinoma after tyrosine kinase inhibitor treatment. JGH Open. (2022) 6:301–8. doi: 10.1002/jgh3.12735
39. Chen J, Zhang D, and Yuan Y. Anti-PD-1/PD-L1 immunotherapy in conversion treatment of locally advanced hepatocellular carcinoma. Clin Exp Med. (2023) 23:579–90. doi: 10.1007/s10238-022-00873-6
40. Wang K, Wang C, Jiang H, Zhang Y, Lin W, Mo J, et al. Combination of ablation and immunotherapy for hepatocellular carcinoma: where we are and where to go. Front Immunol. (2021) 12:792781. doi: 10.3389/fimmu.2021.792781
41. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
42. Ke Q, Xin F, Fang H, Zeng Y, Wang L, and Liu J. The significance of transarterial Chemo(Embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: A systematic review. Front Immunol. (2022) 13:913464. doi: 10.3389/fimmu.2022.913464
43. Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, et al. Clinical features and outcomes of conversion therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel). (2023) 15(21):5221. doi: 10.3390/cancers15215221
44. Jia J, Ding C, Mao M, Gao F, Shao Z, Zhang M, et al. Pathological complete response after conversion therapy in unresectable hepatocellular carcinoma: a retrospective study. BMC Gastroenterol. (2024) 24:242. doi: 10.1186/s12876-024-03298-5
45. Liu W, Xie Z, Shen K, Jiang L, Liu C, Ge Y, et al. Analysis of the safety and effectiveness of TACE combined with targeted immunotherapy in the treatment of intermediate and advanced hepatocellular carcinoma. Med Oncol. (2023) 40:251. doi: 10.1007/s12032-023-02082-x
Keywords: hepatocellular carcinoma, TACE, anti-angiogenic therapy, anti-PD-1 antibody, conversion therapy
Citation: Wang Z, Zhang C, Yin J and Li N (2025) Conversion therapy with TACE, TKIs, and ICIs for unresectable BCLC stage B and C hepatocellular carcinoma. Front. Immunol. 16:1451965. doi: 10.3389/fimmu.2025.1451965
Received: 20 June 2024; Accepted: 26 May 2025;
Published: 10 June 2025; Corrected: 30 June 2025.
Edited by:
Zhanguo Zhang, Huazhong University of Science and Technology, ChinaReviewed by:
Win Topatana, Zhejiang University, ChinaIkhwan Rinaldi, RSUPN Dr. Cipto Mangunkusumo, Indonesia
Copyright © 2025 Wang, Zhang, Yin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, TGlwYXJpc2xpc2lAYWxpeXVuLmNvbQ==; Jianhua Yin, aGF3a3lqaDE2M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Zhuoran Wang
Zhuoran Wang Cunzhen Zhang1†
Cunzhen Zhang1† Jianhua Yin
Jianhua Yin Nan Li
Nan Li