- 1Institute of Medical Genetics, Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany
- 2Department of Neuropediatrics, University Children’s Hospital, Klinikum Oldenburg, Oldenburg, Germany
- 3Institute of Radiology and Neuroradiology, Evangelisches Krankenhaus Oldenburg, Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany
- 4Department of Pediatrics, Medizinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 5Institute of Human Genetics, University Hospital Tübingen, Tübingen, Germany
- 6Institute of Diagnostic and Interventional Radiology, Klinikum Oldenburg, Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany
Heterozygous pathogenic variants in ADAR have been associated with dyschromatosis symmetrica hereditaria, while biallelic pathogenic variants have been associated with Aicardi-Goutières syndrome 6 (AGS6). However, the heterozygous variant c.3019G>A, (p.Gly1007Arg) has been described to cause neurological manifestations, which resemble AGS6 and are associated with an upregulation of interferon-stimulated genes. We report a four-generation family with two symptomatic family members and five unaffected carriers of the heterozygous pathogenic ADAR variant c.3019G>A. The index (patient 1) manifested a gait disorder at three years of age (weakness in his legs, a tendency to fall and hyperreflexia), dyslalia, and mild cognitive developmental delay. A paternal half-brother (patient 4) to patient´s father (patient 2) presented with irritability and regression of previous skills at the age of 6 months after a fever reaction, following the second routine hexavalent vaccination at the age of 4 months. At 20 years of age, the patient was wheelchair-bound, had spasticity and severe global development delay. A blood test in both patients showed increased interferon signature with activation of type 1-interferon. Five asymptomatic carriers were identified in this family (age range 2–81 years of age) nearly all of them (except the 81-year old patient) showed a strong activation of type 1 interferon response in peripheral blood. Affected individuals had higher interferon signature than asymptomatic, underlining the possible role of interferon activation in disease mechanism. To our knowledge, this is the biggest family reported to date, encompassing a wide age-range of carriers, including an asymptotic carrier of advanced age (81 years of age).
Introduction
The adenosine deaminase acting on RNA gene (ADAR) codes for an enzyme that catalyses the conversion of adenosines to inosines in double-stranded RNA and thereby renders self RNA invisible to nucleic acid sensing receptors of the innate immune system (1). A reduced or absent ADAR activity is associated with an activation of interferon-stimulated genes due to aberrant sensing of self RNA (2, 3).
Heterozygous pathogenic variants in ADAR have been associated with dyschromatosis symmetrica hereditaria (DSH) and biallelic pathogenic variants have been associated with Aicardi-Goutières syndrome 6 (AGS6) (4, 5). DSH manifests with hyper- and hypopigmented macules on the dorsal aspect of hands and feet that appear in infancy or early childhood (5). Additionally, freckle-like macules are seen on the face. These macules are usually asymptomatic (5).
AGS is a type-1 interferonopathy that presents with variable neurological symptoms, mainly spasticity, dystonia, leukoencephalopathy associated with variable degrees of developmental delay and intellectual and physical disability (4). Onset has been described ranging from infancy to later childhood. Mostly, affected children develop age-appropriately before onset of disease. In some children, symptoms rapidly progress with a regression of previously acquired skills. Furthermore, cardiac valve involvement has been reported (6–8). Cranial MRI findings in ADAR-associated AGS include encephalopathy, brain atrophy, calcifications, bilateral striatal necrosis and globus pallidus signal alterations (4, 6, 8–11). Furthermore, AGS has been described as a type-1 interferonopathy with an up-regulation of type 1 interferon signalling, measured by the expression of interferon-stimulated genes (ISGs) (3, 4, 8).
The ADAR variant c.3019G>A, has been described to cause neurological manifestations, resembling AGS also in a heterozygous state (9). The variant has been shown to have a significant effect on editing, which is comparable to the inactive protein. It is assumed to exert a dominant negative effect by being catalytically inactive and competitively inhibiting the wildtype protein (4). Ultimately, the transcription of interferon stimulated genes (ISGs) is activated (3). To date, only few affected individuals have been described in literature. Clinical manifestation was very variable, presenting with or without DSH, spasticity, dystonia, rapid psychomotor regression and cognitive decline (6, 9, 12). In some patients, aortic valve sclerosis has been described (13). Furthermore, an association with upregulation of ISGs has been reported (6, 9). Symptoms are thought to be triggered by an infection, fever and possibly a vaccination in previously healthy children (6, 8).
Here we report a four-generational, non-consanguineous family with multiple carriers of the heterozygous pathogenic ADAR variant c.3019G>A with two affected and multiple asymptomatic individuals, highlighting incomplete penetrance and variability of neurological symptoms.
Methods
The index, patient 1, presented to our departments of neuropediatrics and medical genetics. Genome sequencing was performed in a diagnostic setting at the Institute of Medical Genetics and Applied Genomics (IMGAG) in Tuebingen using blood-derived genomic DNA. Library preparation was conducted using the TruSeq PCR-free library prep kit from Illumina. Paired-end sequencing (2x150 base pairs) was performed on an Illumina NovaSeq6000 sequencer. The sequence data was analysed using the megSAP pipeline (https://github.com/imgag/megSAP) and aligned to the GRCh38 reference genome. Phenotype-based filtering for rare variants (minor allele frequency ≤1% in Genome Aggregation Database (gnomAD, gnomad.broadinstitute.org) and ≤ 5 occurrences in an IMGAG in-house database) prioritized the pathogenic ADAR variant. Subsequent carrier testing in the parents and additional family members was done by Sanger sequencing and data analysis using the SEQPilot software from JSI medical systems. For patient 4, genome sequencing was performed as outlined above to exclude possible other disease-causing variants.
Expression of the ISGs IFI27, IFI44, IFI44L, IFIT1, ISG15, RSAD2 and SIGLEC1, normalized to GAPDH and HPRT1 in peripheral blood mononuclear cells were assessed. The relative gene expression (fold-change) was compared to the average gene expression of 10 healthy controls. Gene expression was analysed with quantitative RT-PCR (TaqMan) and calibrated. The mean values of at least three measurements were reported.
All medical information, genetic testing and further laboratory investigations were obtained in routine clinical care. Available clinical MRI data were re-assessed for findings typical of AGS. Written informed consent was obtained from each patient and/or their legal guardian.
Case description
Patient 1
The index patient (patient 1; Figure 1) developed age-appropriately until the age of three years. He then developed a gait disorder with weakness in his legs, a tendency to fall, dyslalia and mild cognitive developmental delay. Physical examination demonstrated hyperreflexia of lower extremities and bilateral Trendelenburg sign. The symptoms are consistent with AGS and in the AGS severity scale (14) he scored 10 points. No fever or infection preceded symptom development. Cerebral spinal fluid (CSF) neopterin was elevated (76 nmol/l, normal < 32 nmol/l). A cranial MRI showed minor signal elevation in T2-weighted images in the globus pallidus (Figure 2J) and periventricular white matter (Figure 2K), possibly consistent with myelination defects. MRI findings resembled previously reported alterations related to ADAR variants (6, 9). They were, however, subtle and thus relatively unspecific. There was no evidence of cerebral calcifications in susceptibility-weighted MRI images. Genome sequencing (GS) identified the heterozygous variant c.3019G>A, p.(Gly1007Arg) in ADAR ((ENST00000368474.9):c.[3019G>A];[=], p.[(Gly1007Arg)];[(=)]). No other possible disease-causing variants were identified. A blood test showed strong interferon signature with activation of type 1- interferon (multiple measurements, scores between 719 and 4623, normal value < 12,49). Additionally, increased expression of CD169+ and CD206+ was observed (see Supplementary Table 1 for more information). Echocardiographical examination of the heart was normal. At four years of age, the patient’s symptoms showed slow progression with progressive gait disorder and progressive leg weakness. A nerve conduction velocity test showed beginning demyelinating polyneuropathy in his legs. Due to progressive symptoms, a treatment with ruxolitinib (dosis twice daily 12,5mg) was initiated. Previously, a treatment option with ruxolitinib, a selective Janus kinase inhibitor has been described as effective in ADAR- TREX1- and IFIH1- related AGS (9, 15, 16). At five years of age, after half a year of treatment with ruxolitinib, neurological symptoms are stable.
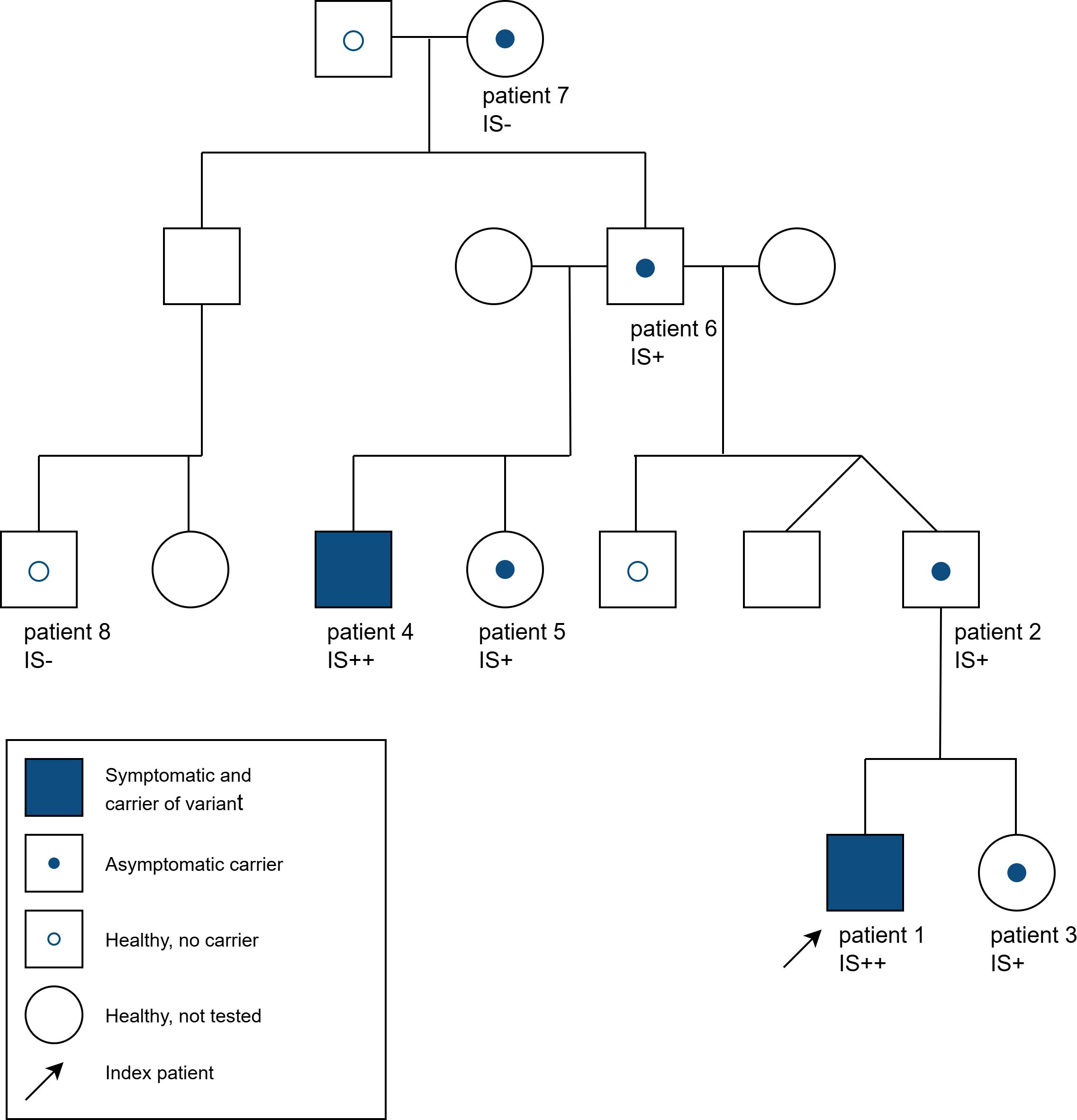
Figure 1. Pedigree of the family with various asymptomatic and symptomatic carriers of the heterozygous ADAR variant c.3019G>A and interferon signature (IS) in some family members [- = no activation, + = strong activation (score 60-200, normal value <12,49), ++ = very strong activation (score >200)].
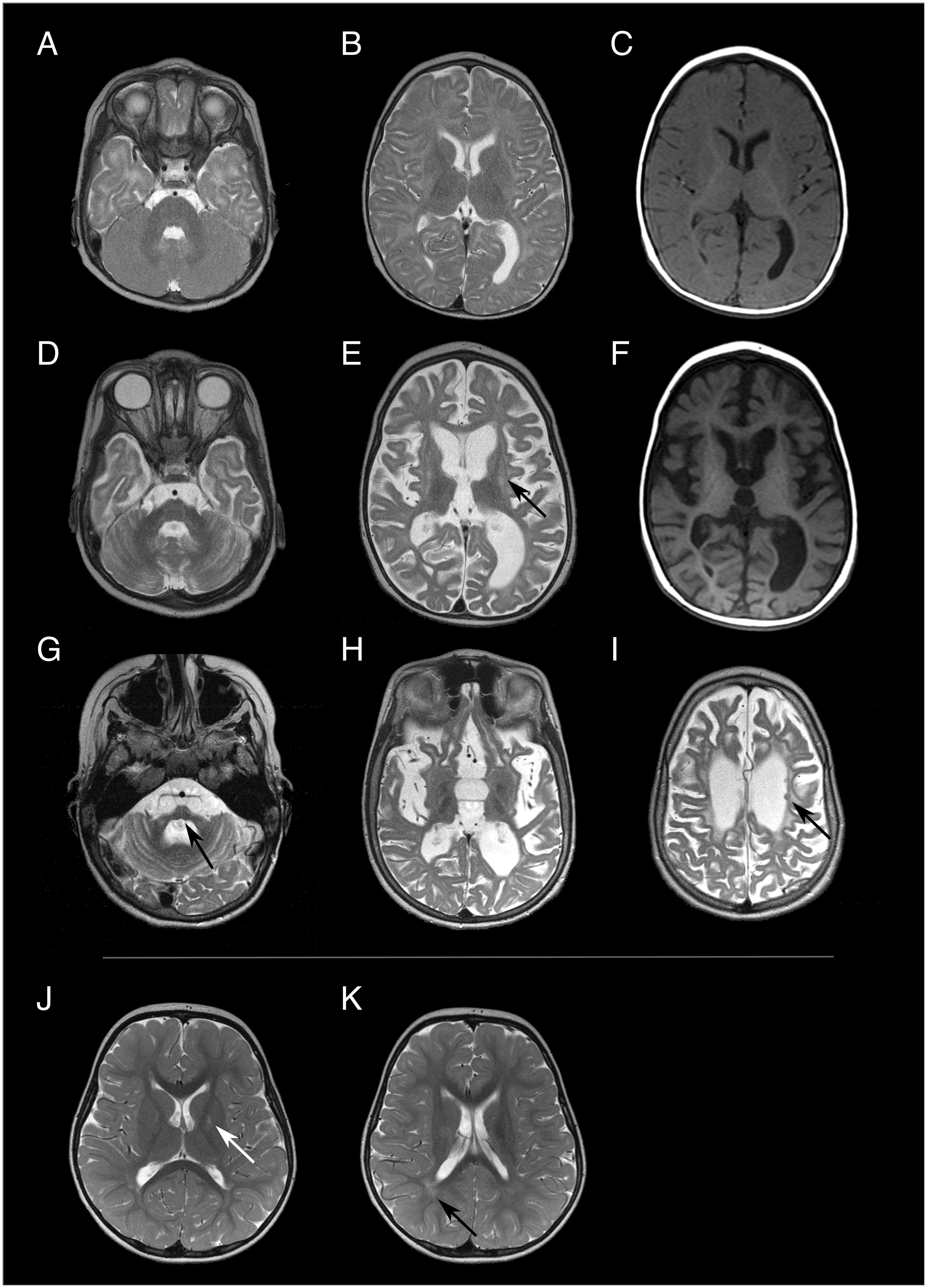
Figure 2. Brain MRI images of patient 4 [(A-C) at 8 months of age, (D-F) at 15 months of age, (G-I) at 10 years of age] and patient 1 [(J, K) at 3 years and 8 months of age]. Both patients exhibit different degrees and patterns of brain alterations. In the first MRI examination, patient 4 had a normal brain volume and the white matter signal was mainly compatible with delayed myelination both in T2w- (A, B) and T1-weighted (C) images. Only 8 months later signs of global atrophy were present, including volume loss and T2 hyperintensity of the striatum [arrow in (E) putamen]. Atrophy progressed to severe supra- and infratentorial volume loss [arrow in (G) pons] at 10 years of age. Few T2 hypointense nodular lesions were present in the periventricular white matter at all three time points [arrow in (I)], which could not be unambiguously identified as either focal calcifications or nodular heterotopia. Patient 1 only exhibited minor and relatively unspecific T2 hyperintense signal alteration in the globus pallidus [arrow in (J)] and the periventricular white matter [arrow in (K)] at 3 years and 8 months years of age.
Patients 2 and 3
The pathogenic ADAR variant was also identified in the index patient’s asymptomatic father (patient 2). Cranial MRI and neurological examination at 28 years of age had been reported as being normal (MRI images not available). Blood test showed an elevated interferon signature (score 110). The variant was also detected in the two years old sister of patient 1 (patient 3). She did not show any symptoms and EEG was age-appropriate. Multiple blood tests also showed activation of type 1 interferon (scores ranging from 83- 1026).
Patient 4
A paternal half-brother (patient 4) to the father (patient 2) presented at approximately 6 months of age after a fever reaction following the second routine hexavalent vaccination two months before. He developed restlessness, irritability, opisthotonus, regression of previous skills and spasticity. Physical examination at nine months of age showed muscular hypertonia of the extremities, weak reflexes and spontaneous Babinski sign. Cerebral spinal fluid (CSF) neopterin was elevated (305 nmol/l and 501nmol/l at 11 and 18 month of age respectively). The initial cranial MRI at the age of 8 months (Figures 2A–C) showed a normal brain volume. At the age of eight months, white matter signal was consistent with moderately delayed myelination, but could not be fully differentiated from potential initial leukoencephalopathy. Follow-up MRI, at the age of 15 months, (Figures 2D–F) exhibited generalized T2 signal elevation of the white matter, T2 hyperintensities in the putamen, dentate nucleus (striatum) and globus pallidus associated with pronounced volume loss as well as generalized brain atrophy. Brain atrophy further progressed (Figures 2G–I). All three available MRI examinations showed minor nodular hypointense lesions in T2-weighted images, which could represent minor parenchymal calcifications, but which were ambiguous in the available MRI data. At 20 years of age, the patient was wheelchair-bound and had severe global development delay, tetraparesis with spasticity, multiple joint contractures and hip luxation, scoliosis and inadequate nutrition. The symptoms are consistent with AGS and in the AGS severity scale (14) he scored 0 points.
Blood tests also showed a strong activation of type 1 interferon (score 1114). The familial heterozygous ADAR c.3019G>A variant was detected by Sanger sequencing and whole-genome sequencing showed no other variant that could be associated with his symptoms.
Patients 5, 6, 7 and 8
The sister (patient 5) of this second affected male (patient 4) in the family also carries the same ADAR variant. At 10 years of age, she did not show any symptoms. Measurement of type 1 interferon was not available.
The father of patient 2, 4 and 5 (patient 6) also carries the same pathogenic ADAR variant and shows strong activation of type 1 interferon (score 63) but was asymptomatic at age 59. The pathogenic ADAR variant was also detected in the mother of patient 6 (patient 7). At 81 years of age she shows no neurological symptoms and there was no detectable interferon signature (score <12,49). In a paternal cousin to patient 2 (patient 8) the heterozygous ADAR variant c.3019G>A was not detected and interferon measurements showed no detectable signature. To our knowledge, no one in the family showed any skin manifestations related to DSH. Patients 1 and 3 are of Caucasian-Turkish descent, the rest of the family are Caucasian. An overview of all available features of symptomatic and asymptomatic variant carriers is displayed in Table 1.
Discussion
We report a four generational family with multiple heterozygous carriers of the pathogenic ADAR variant c.3019G>A with two symptomatic family members and five unaffected carriers. Symptoms in the two affected individuals differed. While in patient 4, symptom onset was at around 6 months of age with rapid progression, patient 1 showed symptoms at 3 years of age and slow disease progression. Other family members were asymptomatic at various ages. Cranial MRI findings in both affected individuals were substantially different regarding both pattern and extent, with signal alterations in the globus pallidus being the only commonality. MRI findings were within the spectrum previously reported in ADAR-related AGS (6, 8, 9). Upregulation of type-1 interferon was shown in nearly all available family members (except patient 7), showing the highest elevation in the affected individuals. To our knowledge, the heterozygous ADAR pathogenic variant c.3019G>A has been described in 22 further cases (isolated and familiar), associated with AGS symptoms (6, 8, 9, 12, 13, 17–19).
Neurological symptoms described included dystonia, spasticity, tetraparesis, rigidity, irritability and regression of previous skills. Intellect was severely affected in some individuals and preserved in others. Age of onset (if known) spanned from 4–6 months to 17 years of age (8, 9, 13). This variability in symptom onset, disease progression, affected intellect and symptoms is also reflected in our family with the two affected individuals having variable disease manifestation. It has been reported, that symptoms developed after an infection and/or vaccination in previously age-appropriately developed children (8). This could also be observed in patient 4, where symptoms were thought to be triggered after a fever reaction, possibly in the context of a vaccination. For patient 1, no infect association was reported. Furthermore, DSH was present in the minority of described cases and not present in this family (9). Generally, DSH has rarely been described outside of Japan and China (4).
Inheritance of the ADAR c.3019G>A variant from asymptomatic or (mildly) symptomatic parents has been described in previous literature (6, 8, 12, 13). Paternal inheritance was assumed in two affected paternal half- sisters (father not available for testing). The father showed no ADAR-related symptoms (6). Kondo et al. described inheritance from a mother, who only presented with faint hypopigmented macules on the dorsal aspects of her fingers and no neurological symptoms (12). Rice et al. described subtle psychological features and basal ganglia calcification in a mother of an affected child (8). Furthermore, Tojo et al. described a father of an affected child harbouring the same pathogenic ADAR variant. He had DSH features from childhood on and in his early thirties he developed irritability, mental deterioration and severe aortic valve sclerosis (13). These examples show that later disease onset and significant intrafamilial variability is possible in ADAR-related disease (8). Additionally, Crow et al. described a 68-year-old asymptomatic individual with a homozygous pathogenic variants in RNASEH2A, associated with AGS (20). The family in this case report further undermines the strong intrafamilial variability and incomplete penetrance with the oldest family member showing no ADAR-related symptoms at the age of 81 years. Possible influencing factors could be genetic modifiers (protective or susceptibility alleles, epigenetic factors) and exposure to immunogens or other environmental triggers but exact pathomechanism and possible triggering factors warrant further research.
Previously, CSF neopterin has been reported as a valuable diagnostic tool in AGS patients (9). Elevated neopterin could also be observed in patient 1 and patient 4, underlining its possible diagnostic relevance.
In this family, nearly all carriers showed strong activation of type 1 interferon in blood. For patient 5 interferon signature was not available since the parents did not agree on conducting the measurement. This activation, however, was markedly stronger in affected individuals, highlighting the potential role as a disease biomarker and monitoring (8).
In prior literature, a mildly affected mother with ADAR c.3019G>A of a son with AGS related disease, showed an elevated interferon signature (scores between 25.743 and 12.836) (8). Some parental carriers of other recessive ADAR pathogenic variants (p.Pro193Ala and other) showed an elevated interferon signature (maximum score 22.5) (8). So far, no elevated interferon scores were reported from asymptomatic carriers of the ADAR c.3019G>A variant. We report four asymptomatic carriers with elevated interferon scores (score range 63 – 110), while the oldest carrier in this family did not present elevated scores. This is in accordance with the oldest carrier being asymptomatic and has been reported in other RNASEH2A –related AGS in an asymptomatic 68-year-old individual (20). However, we only conducted a single measurement and cannot exclude preanalytic or technical factors.
Cranial MRI showed signal alterations in the globus pallidus in both affected members of this family. While in patient 4 progressive cerebral atrophy could be seen, this was not detected in patient 1. Previously, MRI findings in affected individuals also detected changes in the basal ganglia with hypointensity of the striatum and pallidum (9). Furthermore, calcification and bilateral striatal necrosis have been reported in ADAR-related disease (6, 8). Generally, MRI findings tend to vary and some cases of normal cranial MRIs have been reported (8). In patient 1 bilateral striatal necrosis and calcification were not seen. In patient 4 possible calcifications could be seen. However, a CT-scan or susceptibility-weighted MRI images, where intracranial calcifications tend to be more visible, were not performed. In this family, available cranial MRI from one asymptomatic individual (patient 2) had been reported as a normal finding. Generally, limited information on MRI findings of asymptomatic carriers is available in literature. In one mildly affected parent described by Rice et al., 2017, white matter and cerebellar calcifications could be observed (8).
Patient 1 received ruxolitinib to halt disease progression. After half a year of treatment, his symptoms were stable. In previous literature treatment trials with levodopa, ruxolitinib and dexamethasone have been described (13). Treatment with levodopa has been described with variable efficacy (13). Treatment with ruxolitinib, a selective Janus kinase inhibitor, has been described as effective in some features of ADAR- TREX1- and IFIH1- related AGS by blocking interferon signalling (9, 15, 16). Especially treatment initiation in early stages of the disease has been considered important (15, 16).
Conclusion
The heterozygous pathogenic variant ADAR c.3019G>A shows incomplete penetrance in this family and might be causative for variable neurological symptoms in two affected family members. Other families have been described in which symptomatic children inherited the same variant from asymptomatic or (mildly) symptomatic parents (6, 8, 13). To our knowledge, this is the biggest family reported to date with a wide age-range of carriers and an asymptomatic carrier of advanced age (81 years of age). Furthermore, interferon profiles from nearly all carriers (except patient 5) in the family were conducted and, except for one, all were elevated. Affected individuals had higher interferon signature than asymptomatic, underlining the possible role of interferon activation in disease mechanism. This high intrafamiliar variability illustrates the diagnostic challenges and possibly underrecognized individuals and warrants further research into disease mechanism. A treatment option to halt disease progression with prednisolone or selective Janus kinase inhibitors has been suggested (6, 15). This makes early identification of symptomatic variant carriers even more important.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/clinvar/variation/39458/, VCV000039458.45.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
A-KB: Writing – original draft, Conceptualization, Data curation, Investigation. IM: Data curation, Supervision, Writing – review & editing. BS: Data curation, Visualization, Writing – original draft, Writing – review & editing. CW: Data curation, Writing – review & editing. KR: Conceptualization, Data curation, Methodology, Writing – review & editing. KG-H: Data curation, Writing – review & editing. LG: Supervision, Writing – review & editing. MO: Data curation, Writing – review & editing. MM: Supervision, Writing – review & editing. TH: Data curation, Writing – review & editing. ML-K: Data curation, Methodology, Writing – review & editing. G-CK: Data curation, Supervision, Writing – review & editing. M-PH: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the metabolic laboratory of Heidelberg University Hospital for providing neopterin concentrations in CSF.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1453496/full#supplementary-material
References
1. Schlee M and Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol. (2016) 16:566. doi: 10.1038/nri.2016.78
2. Liddicoat BJ, Chalk AM, and Walkley CR. ADAR1, inosine and the immune sensing system: distinguishing self from non-self. Wiley interdisciplinary reviews. RNA. (2016) 7:157–72. doi: 10.1002/wrna.1322
3. Rodero MP and Crow YJ. Type I interferon–mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J Exp Med. (2016) 213:2527. doi: 10.1084/jem.20161596
4. Rice GI, Kasher PR, Jenkinson EM, Bacino CA, Battini R, Bertini E, et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat Genet. (2012) 44:1243–8. doi: 10.1038/ng.2414
5. Hayashi M and Suzuki T. Dyschromatosis symmetrica hereditaria. J Dermatol. (2013) 40:336–43. doi: 10.1111/j.1346-8138.2012.01661.x
6. Livingston JH, Lin J, Dale RC, Gill D, Brogan P, Munnich A, et al. A type I interferon signature identifies bilateral striatal necrosis due to mutations inADAR1. J Med Genet. (2013) 51:76. doi: 10.1136/jmedgenet-2013-102038
7. Crow Y, Keshavan N, Barbet JP, Bercu G, Bondet V, Boussard C, et al. Cardiac valve involvement in ADAR-related type I interferonopathy. J Med Genet. (2019) 57:475. doi: 10.1136/jmedgenet-2019-106457
8. Rice G, Kitabayashi N, Barth M, Briggs T, Burton A, Carpanelli M, et al. Genetic, phenotypic, and interferon biomarker status in ADAR1-related neurological disease. Neuropediatrics. (2017) 48:166. doi: 10.1055/s-0037-1601449
9. Jones HF, Stoll M, Ho G, O&39;Neill D, Han VX, Paget S, et al. Autosomal dominant ADAR c.3019G>A (p.(G1007R)) variant is an important mimic of hereditary spastic paraplegia and cerebral palsy. Brain Dev (Tokyo 1979). (2022) 44:153–60. doi: 10.1016/j.braindev.2021.10.001
10. La Piana R, Uggetti C, Roncarolo F, Vanderver A, Olivieri I, Tonduti D, et al. Neuroradiologic patterns and novel imaging findings in Aicardi-Goutières syndrome. Neurology. (2016) 86:28–35. doi: 10.1212/WNL.0000000000002228
11. Uggetti C, La Piana R, Orcesi S, Egitto MG, Crow YJ, and Fazzi E. Aicardi-goutieres syndrome: neuroradiologic findings and follow-up. Am J Neuroradiol: AJNR. (2009) 30:1971–6. doi: 10.3174/ajnr.A1694
12. Kondo T, Suzuki T, Ito S, Kino M, Negoro T, and Tomita Y. Dyschromatosis symmetrica hereditaria associated with neurological disorders. J Dermatol. (2008) 35:662–6. doi: 10.1111/j.1346-8138.2008.00540.x
13. Tojo K, Sekijima Y, Suzuki T, Suzuki N, Tomita Y, Yoshida K, et al. Dystonia, mental deterioration, and dyschromatosis symmetrica hereditaria in a family withADAR1 mutation. Movement Disord. (2006) 21:1510–3. doi: 10.1002/mds.21011
14. Adang LA, Gavazzi F, Jawad AF, Cusack SV, Kopin K, Peer K, et al. Development of a neurologic severity scale for Aicardi Goutières Syndrome. Mol Genet Metab. (2020) 130:153. doi: 10.1016/j.ymgme.2020.03.008
15. Crow YJ, Shetty JJ, and Livingston JH. Treatments in aicardi–goutières syndrome. Dev Med Child Neuro. (2019) 62:42. doi: 10.1111/dmcn.14268
16. Frémond M, Hully M, Fournier B, Barrois R, Lévy R, Aubart M, et al. JAK inhibition in aicardi-goutières syndrome: a monocentric multidisciplinary real-world approach study. J Clin Immunol. (2023) 43:1436–47. doi: 10.1007/s10875-023-01500-z
17. Suzuki N, Suzuki T, Inagaki K, Ito S, Kono M, Fukai K, et al. Mutation analysis of the ADAR1 gene in dyschromatosis symmetrica hereditaria and genetic differentiation from both dyschromatosis universalis hereditaria and acropigmentatio reticularis. J Invest Dermatol. (2005) 124:1186–92. doi: 10.1111/j.0022-202X.2005.23732.x
18. Crow YJ, Chase DS, Lowenstein Schidt J, Szynkiewicz M, Forte GM, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet Part A. (2015) 167:296–312. doi: 10.1002/ajmg.a.36887
19. Di Lazzaro G, Graziola F, Sancesario A, Insalaco A, Moneta GM, Castelli E, et al. Movement disorders in ADAR1 disease: Insights from a comprehensive cohort. Parkinsonism Relat Disord. (2020) 79:100–4. doi: 10.1016/j.parkreldis.2020.08.039
Keywords: ADAR, c.3019G>A, Aicardi-Goutières syndrome, interferon signature, dystonia, spasticity, reduced penetrance
Citation: Bauer A-K, Marquardt I, Sundermann B, Wolf C, Raupach K, Grundmann-Hauser K, Gieldon L, Otterbach M, Maurer M, Haack T, Lee-Kirsch MA, Korenke G-C and Hitz M-P (2025) Case Report: Heterozygous ADAR c.3019G>A pathogenic variant associated with variable neurological symptoms and incomplete penetrance in a four-generational family. Front. Immunol. 16:1453496. doi: 10.3389/fimmu.2025.1453496
Received: 23 June 2024; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Kei Ikeda, Dokkyo Medical University, JapanReviewed by:
Masafumi Arima, Dokkyo Medical University, JapanAlain Lefèvre-Utile, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2025 Bauer, Marquardt, Sundermann, Wolf, Raupach, Grundmann-Hauser, Gieldon, Otterbach, Maurer, Haack, Lee-Kirsch, Korenke and Hitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann-Kathrin Bauer, YW5uLWthdGhyaW4uYmF1ZXIxQHVuaS1vbGRlbmJ1cmcuZGU=
 Ann-Kathrin Bauer
Ann-Kathrin Bauer Iris Marquardt
Iris Marquardt Benedikt Sundermann
Benedikt Sundermann Christine Wolf4
Christine Wolf4 Kathrin Grundmann-Hauser
Kathrin Grundmann-Hauser Tobias Haack
Tobias Haack