- Department of Periodontics, School and Hospital of Stomatology, China Medical University, Shenyang, China
Periodontitis, a chronic inflammatory disease, is characterized by irreversible bone resorption, persistent attachment loss, and even ultimate tooth loss. The individual quality of life and the public burden of health are greatly impacted by the destruction of periodontal tissue and the lack of effective treatment. Exploration into how the inflammatory and damaged structure changes into the healthy and regenerative tissue is one of the most fascinating subjects in this field. A novel approach is the application of a nanoparticle, extracellular vesicle, which originates from natural or engineered cells. To further exploit its full potential, scientists have conducted massive studies into its biological and functional mechanisms. This review provides an overview of current extracellular vesicle-based treatments on periodontal diseases. It begins with summarizing the history of periodontal regeneration. Then, this article takes a general overview of extracellular vesicle’s biological characteristics, structures and current achievements. After that, we discussed extracellular vesicle’s functions in periodontium. In addition, this study also embraces a diverse range of extracellular vesicle-based strategies, a general workflow for EVs’ periodontal application. Finally, the challenges and prospects of the extracellular-based therapy have been discussed.
1 Introduction
As a complex, chronic and inflammatory disease, periodontitis causes progressive loss of attachment, resorption of bone, and sometimes even loss of teeth (1). Epidemiological surveys have shown that 1.1 billion of severe periodontitis were detected globally in 2019 (2). Furthermore, recent data have indicated a strong association between periodontitis and several systemic conditions (3, 4), such as diabetes (5), colitis (6), cardiovascular disorders (7), rheumatoid arthritis (8), Alzheimer’s disease (9), and even pregnancy difficulties (10). Periodontitis patients require efficient treatments for better recovery and tissue regeneration (11).
Traditional periodontal treatments always focus on eliminating gingival plaque, controlling disease progression and restoring periodontal defects, through a sequence of operations involving tooth scaling, root planning, reconstructive surgery and regenerative surgery (12). However, these usual methods have limited efficacy and low predictability in clinical grounds. Achieving complete restoration of periodontium’s normal structure, function, and consistency remains a major challenge (13).
Extracellular vehicles (EVs) are natural particles secreted by all cells (14). Derived from distinct cells and biological fluids, EVs take active roles in intercellular communicating, material transferring, and even tissue regulating (15). In recent years, evidence has shown that EVs could be used to promote inflammation recovery and to facilitate tissue regeneration (16). And EVs are becoming a promising approach to improving periodontal treatment nowadays (17).
This article is the first extensive review aimed at summarizing all workflow of EVs in periodontal treatment with a broad objective. This review focuses on EV-based strategies for treating periodontitis and promoting periodontal regeneration. We begin with providing historical insights into periodontal regeneration, highlighting how evolving treatment philosophies are consistent with these concepts. After that, we discuss EVs functional mechanisms. Then, we explore the strategies of EV-based techniques in this field, generally summarize the knowledge and critically analyze the details in the utilization. Finally, we discuss the real challenges of EVs on biological mechanisms, engineering strategies, clinical translation and industrialization and the provide new perspectives for the near future.
2 Periodontium and periodontal regeneration
2.1 Periodontium
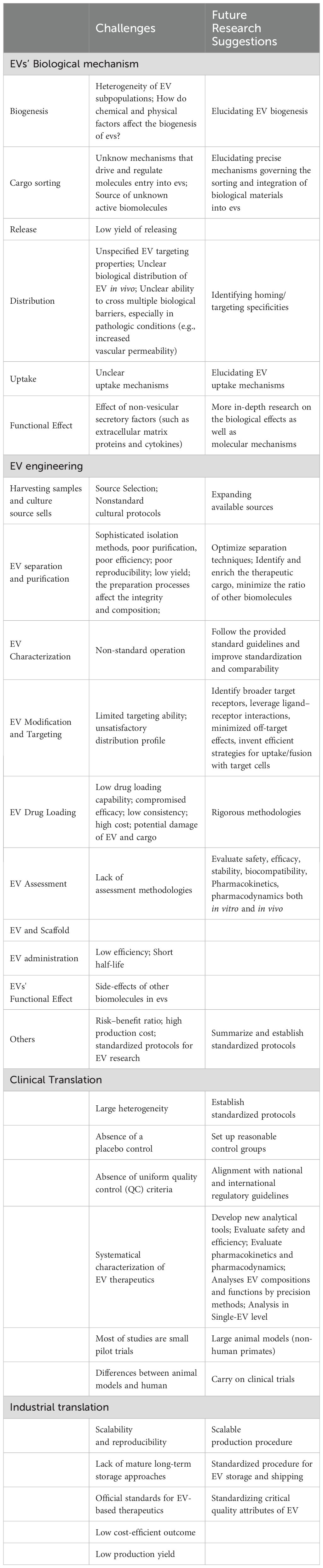
The certain term, periodontium, is used to describe the delicate structure that provides necessary support to maintain teeth in function and to protect them in health. As a hierarchical system, it consists of four principal components: gingiva, periodontal ligament, cementum, and alveolar bone (18). Covering root surface and alveolar bone in the outermost layer, gingiva, composed of epithelial and connective tissue, protects the other tissue components. Alveolar bone, the mineralized and porous tissue, supports the teeth structurally and functionally. Cementum is a calcified, avascular mesenchymal tissue that forms the outer covering of the anatomic root. And as to periodontal ligament, which continuous with the connective tissue of the gingiva and connects to the inner wall of the alveolar bone, is composed of a complex vascular and highly cellular connective tissue, and takes physical, formative and remodeling, nutritional, and sensory functions for the entire system. Although several diversities at the histological level, the four components integrate and function together as a single unit (19) (Figure 1).
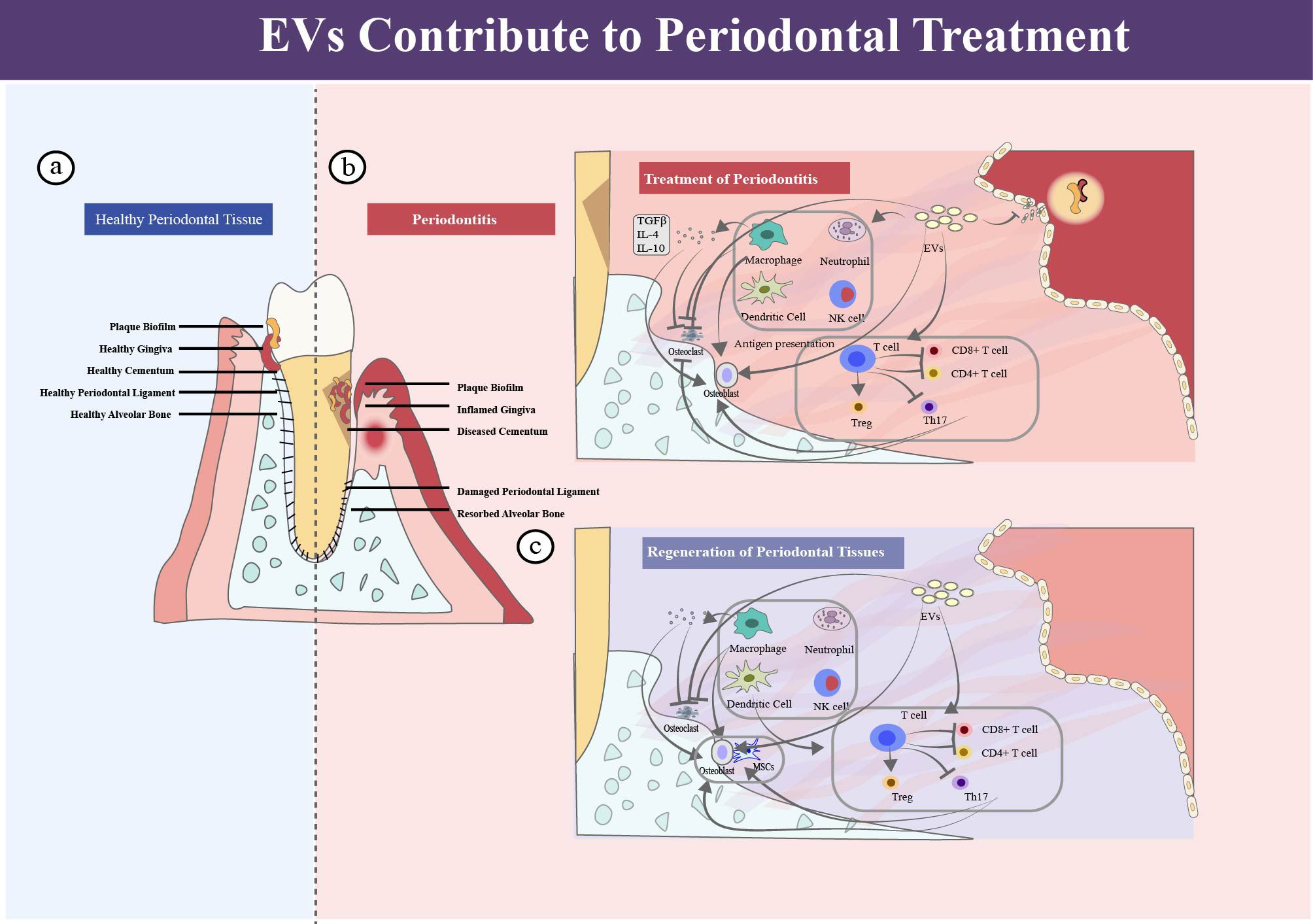
Figure 1. Periodontium, periodontitis and periodontal regeneration. (a) In healthy periodontal tissue, normal plaque biofilm exists in the gingival sulcus, and the four structures of gingiva, periodontal ligament, cementum, and alveolar bone present a healthy state. (b) Inflammation in periodontitis, and EVs contribute to periodontitis treatment. (c) EVs contribute to periodontal regeneration.
2.2 History of periodontal regeneration
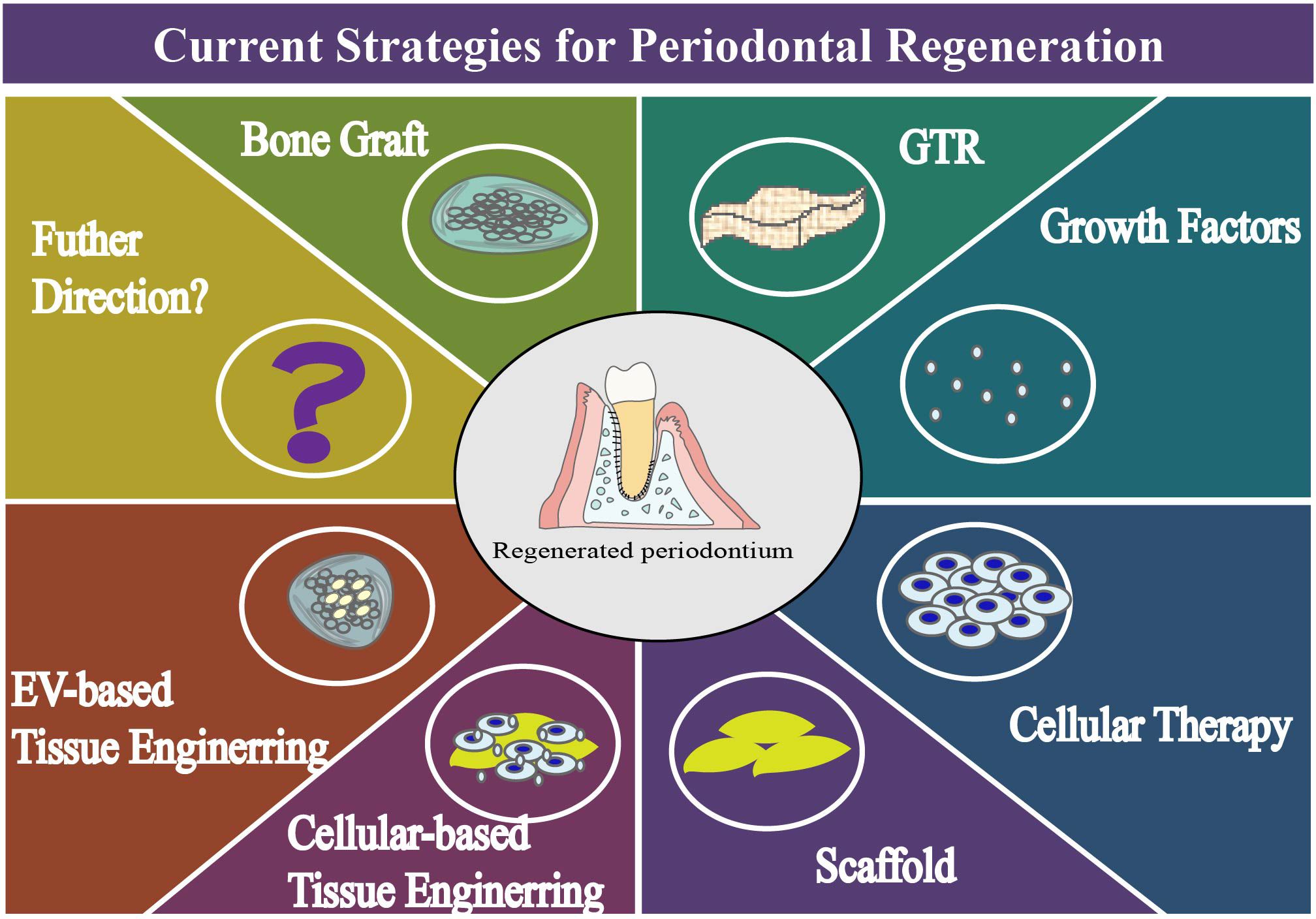
The restoration and regeneration of periodontal tissue have always been the goal of periodontal treatment. In history of the periodontal regeneration, scientists have made considerable efforts on a distinct range of subjects (Figure 2, Table 1).
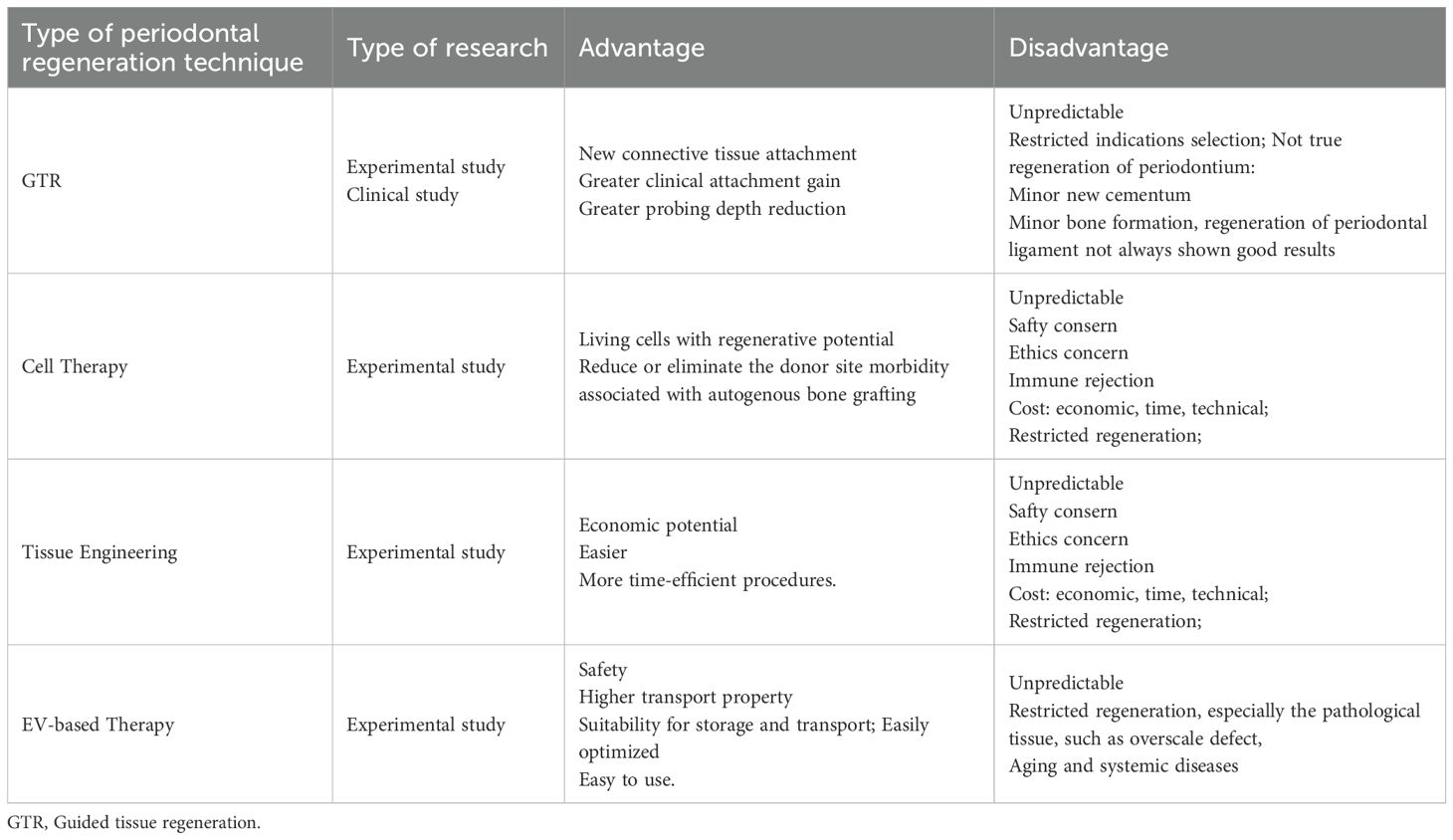
Development of guided tissue regeneration. We can read of things that happened 100 years ago, when people began their epic journey to periodontal regeneration (20). Before 1980s, long-term animal and clinical experience of periodontal tissue reconstruction has been accumulated through the application of bone graft materials, such as autogenous bone and allogeneic bone. At that time, Melcher discussed the healing potential of periodontal tissue and speculated on the interrelationship of four connective tissue cells in wound healing (21). Until 1980, with extraordinary significance, an array of experiments had been made by Neiman et al (22–24). In these original reports, periodontium was reacquired from defects of periodontitis, and a pioneering interpretation of the biological basis of regeneration was made from the experimental evidence, which was name guided tissue regeneration (GTR) (25). By placing a barrier which prevents epithelial cells from migrating along root surfaces, GTR enables the selective repopulation of connective tissue, leading to a positive outcome, such as reduced probing depth, increased clinical attachment and enhanced bone formation (26). After decades of clinical and scientific research, this procedure has been developed and validated, and has become a safe way to operate and a standard procedure of regenerate (27, 28). However, it’s the arguable outcome, limited predictability and restricted applied conditions hinder the potential of this technique.
Growth Factors, Gene Therapy, Cell Therapy and Tissue Engineering Strategy. 1980s is also a time to investigate the role of growth factors in promoting regeneration. Exploration of platelet-derived growth factor (PDGF) (29), insulin-like growth factors (30), enamel matrix derivative (31) in their efficacy, safety and controlled release method had been conducted in libraries. In 1993, Robert Langer and Joseph proposed the concept of tissue engineering and explained the biological and engineering principles of seed cells, scaffold materials and growth factors (32, 33). And in 2000, the gene delivery of growth factor was used for to root lining cells, and gene therapy for periodontal tissue engineering was studied at that time (34, 35). Then, the important discovery in 2004 was the extraction, characterization and culture of PDLSCs successfully (36). In the same year, BMSCs were used as auto-transplantation to promote periodontal regeneration in beagle dogs, which represented the cell therapy began its road in periodontology (37). It is expected that the implanted stem cells, with renewable potential, would promote regeneration, by directly and indirectly forming new bone tissue, periodontal membrane and cementum, through proliferation, differentiation and the release of bioactive molecules. In 2015, 3D-printed bioresorbable patient-specific polymer scaffold was investigated for periodontal reconstruction for the first time (38). The steady progress of culture technology, the gradual development of biological materials, and the practical applications of tissue engineering have resulted in endless explorations and exciting discoveries. Compared with other regenerative techniques, cell-therapy and tissue engineering merit further consideration because of the high investment of economy, the extra cost of time and the low efficiency of regeneration. Consequently, more rigorously designed studies are required in the regenerative field (39).
EV-based therapy and endogenous tissue engineering. Endogenous tissue engineering improves by leaps and bounds in recent decades opening up new opportunities to meet regenerative needs (11, 40). In 2019, mesenchymal stem cell exosomes were applied to promote periodontal ligament cell functions for tissue regeneration (41), and the functionally engineered extracellular vesicle were explored in 2020 (42). And the progress of the endogenous cells demonstrates the enormous potential of engineered tissue regeneration therapy.
3 Extracellular vesicle: a novel but promising approach to periodontal treatment
3.1 Overview of extracellular vesicle
Extracellular vesicles (EVs), particles with lipid bilayer, are released from cells and cannot replicate on their own. The umbrella term is recommended in the minimal information for studies of extracellular vesicles guideline in 2018 (43), which includes the several identified subtypes, such as exosomes, ectosomes, microvesicles, membrane vesicles and apoptotic bodies. Although the term “exosome” is largely used in articles, the limitation in isolation technique mixed the “exosome complex” and the true “exosomes” which derived from the endomembrane systems. To promote standardization without confusing readers, the name of EV is used in this review as the suggestion in the guideline, while the names used by the citation authors are retained when the literature is cited directly or indirectly (Figure 3).
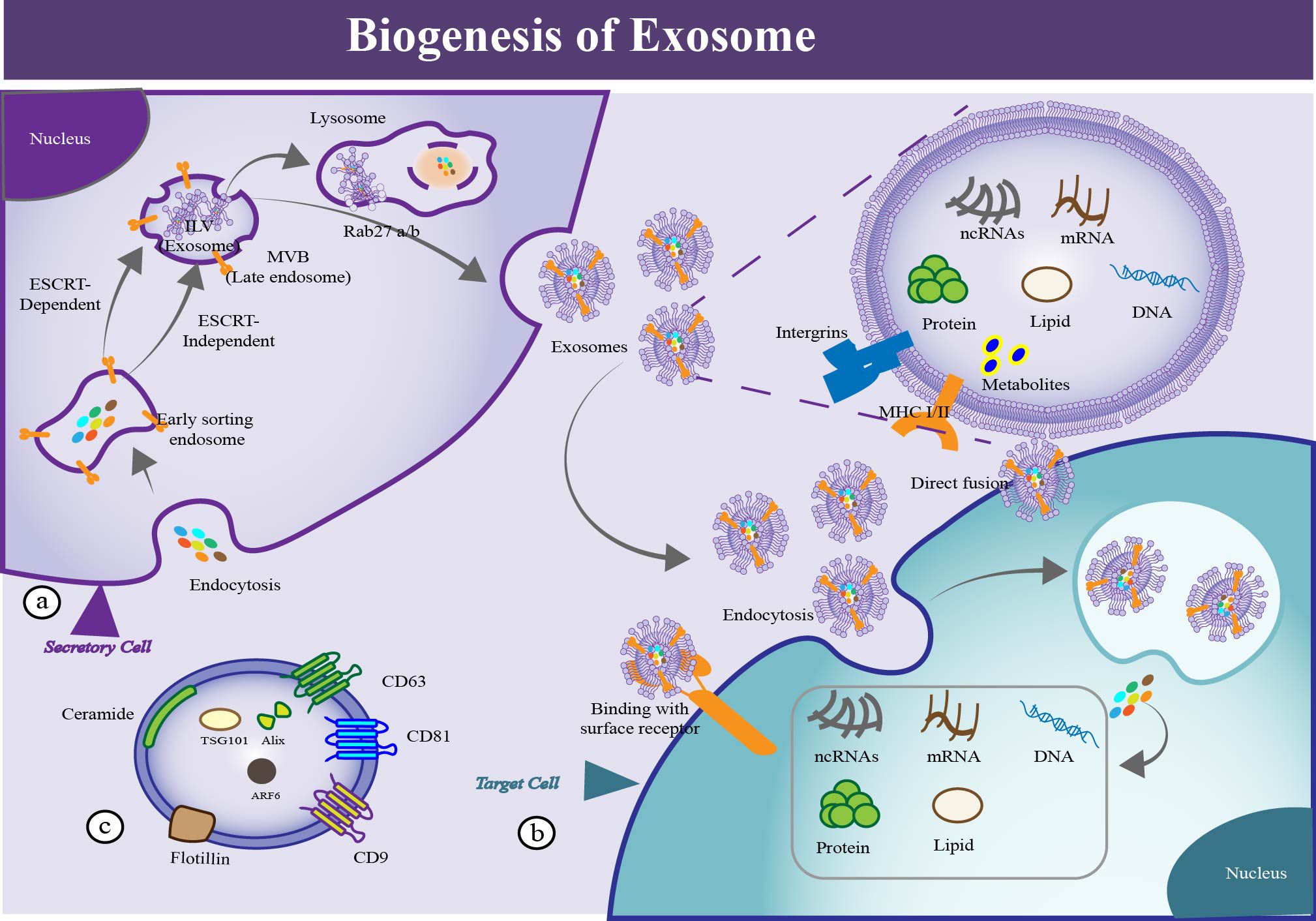
Figure 3. The process of EV biogenesis, uptake and identification. (a) Biogenesis of EVs. EVs generate from the sequential invagination of the plasma membrane, leading to the formation of intracellular multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs), and are released ultimately through MVB fusion to the plasma membrane and exocytosis. (b) Cellular uptake of EVs. Uptake of EVs by recipient cell via multiple mechanisms, such as macropinocytosis, phagocytosis, endocytosis, membrane fusion, gap junction-mediated transfer, exchange of cargo and receptor mediated signal transduction. By these means, protein, DNA, mRNA, ncRNA, metabolites and other molecules could be transported to the recipient cell. (c) Identification of EVs. Several proteins could be used as markers for EVs (CD9, CD81, CD63, flotillin, TSG101, ceramide, and Alix).
3.2 EVs' structures, components and functions
EVs are so tiny that they are difficult to be captured with human eyes or optical microscopes. Released from both prokaryotic and eukaryotic cells, EVs play an important role not only in maintaining structural stability but also in communicating among cells. They enjoy bilayer membrane lipids, comprising sphingolipids, phospholipids, phosphatidylinositol and mono-sialo-tetra-hexosylganglioside (14, 44). The extracellular domain of the membrane contains various adhesion molecules, that promote to connect with neighbor cells. In addition, EVs also contain proteins, both as surface structures and as cargo components. Proteomic analyses of EVs demonstrated that some of these proteins are general, but some are heterogeneous, ubiquitous and cell specific. For example, integrins are discovered on the surface of EVs that exert function on fusion with other cells. Cytoskeletal proteins, annexins, GTPases, tetraspanins, heat shock proteins, apoptosis proteins, and adhesion molecules contribute to synthesis, dynamics, sort and payload. Proteins can serve as indicators for their specificity in clinical diagnostics. Some proteins could also be used to isolate EVs subpopulations and characterize them for different proteins properties. Moreover, nucleic acids, including DNA, mRNA, noncoding RNA species, can affect the behavior and phenotype of receptor cells (14). But the precious mechanisms and available features of these molecules remain unknown.
EVs produce specific effects on receptor cells through multifarious mechanisms, such as entry of intact EV by clathrincoated pit, lipid raft, phagocytosis, caveola, micropinocytosis and receptor-mediated endocytosis, release of EV contents and signaling by direct fusion and direct binding (45). By these means, the therapeutic cargo or instantaneous signal are transferred to other cells, thereby delivering drug payload or triggering cellular responses. Literature reports highlighted that EVs could not only remove excess cellular components to maintain homeostasis, but also facilitate biological processes in cell, tissue and organ levels (46). They possess valuable properties, such as anti-inflammation (47), microbiome-modulation (48), immunomodulation (49) and tissue-regeneration, making them promising candidates for treating diseases (50) (Figure 2). Furthermore, participating actively in cell-cell communication, signal transduction and material transport, EVs perform a variety of functions in physiological, pathological and therapeutic circumstances (51, 52).
3.3 Advantages and disadvantages of EVs
3.3.1 Comparison between EVs and cell therapy in periodontal regeneration
The analyses illustrate that EVs are similar to their parent cells in some cases, and in several scenarios, they might be far more potent (53). Compared with cell therapy (Table 1), EVs gain advantages of: (1) Enhanced safety. They are safer than living cells with low immunogenicity. They produce fewer side effects and fewer possibility of tumor formation (54). (2) Superior transport capability. Their bilayer architecture endows them higher integrated ability into target cells. Also, they can be readily manipulated and genetically engineered, which provides excellent pharmacokinetic properties and tissue-targeting capacities. Released in a nano-size form, they are able to travel across capillaries and many small barriers freely, especially blood–brain barriers (55). (3) Easier to storage and transport. They can be kept for a long time without losing their characteristics. (4) Optimizable efficacy. Although the content of EVs is like that of their source cells, optimizing EVs in composition and characteristic can significantly improve their treatment efficiency and consequence. (5) Easier application. EVs not only could be directly injected, but also could combine with scaffold materials. Cell therapy can also enter the body by these means, but the condition requirements of living cells are much higher than that of the non-living EVs.
Disadvantages of EVs: A key disadvantage of EV-based application lies in their limited regeneration ability. For example, the regenerative performance of endogenous cells is weakened by the harmful effects of local and systemic factors, especially in inflammatory environments and aging individuals. Consequently, EVs exhibit reduced therapeutic efficacy. For further details on the application of EVs, please refer to the relevant review (52, 53) and see Table 1 for additional information.
3.3.2 Comparison between EVs and liposome in drug loading
Compared with the conventional carrier, liposomes, EVs do have some advantages (44, 56, 57). (1) EVs are safer than artificial liposomes. EVs are derived from natural cells with cellular lipids and negligible toxicity. (2) They have superior transport capability. They could travel across blood-brain barriers. (3) They could be manipulated in genetical level. EVs can be engineered in both active and passive approaches, while liposomes could only be regulated by the passive route, which provide additional possibilities for the source of content drugs encapsulated by EVs.
There are some drawbacks of EVs in drug loading. (1) EVs loading efficiency is limited by their intrinsically components. The cargoes with the natural EVs possess the highly heterogeneity and variability, which make the loading performance of EVs unstable to product and difficult to predict. In addition, this will also create obstacles for loading of multifunctional cargoes. (2) A lower degree of industrialization. Lower yield, high time- cost and effort-consuming restrain the application of EVs at the present time. (3) Poor reproducibility. Heterogeneity of EVs, non-standardized manufacturing process and numerous influencing factors make it difficult for EV derivatives to maintain consistency. (4) Unspecific targeting. Natural EVs lack the specific targeting and tissue-homing capacity. (5) Exogenous packaging technology may cause damage to the loaded drug.
3.4 New achievements of EVs
EVs’ use in scientific research and clinical application has made good progress due to their inherent properties as natural EVs, engineered capabilities as therapeutic vehicles and integrated cargoes as delivery systems (53). Over 50000 publication could be searched on EV’s topic and the trend continues dramatically. Currently, EVs have gained remarkable achievements: Engineered EVs treatment in Acute Liver Failure (58), EVs facilitate diabetic wound healing (59), EVs alleviate proinflammatory cascades in Alzheimer’s disease (60) and EVs’ impressive potential in anti-tumor immunity (61).
4 EVs perform valuable functions in periodontal treatment
EVs fulfill multiple functions in the periodontal field. Such as, EVs could inhibit inflammatory responses locally. They could regulate the remodeling of damaged tissue, such as cementum, periodontal ligament, alveolar bone tissue, extracellular matrix and vascularization. It is worth noting that EVs have an extensive impact on the periodontal regeneration (Figure 4).
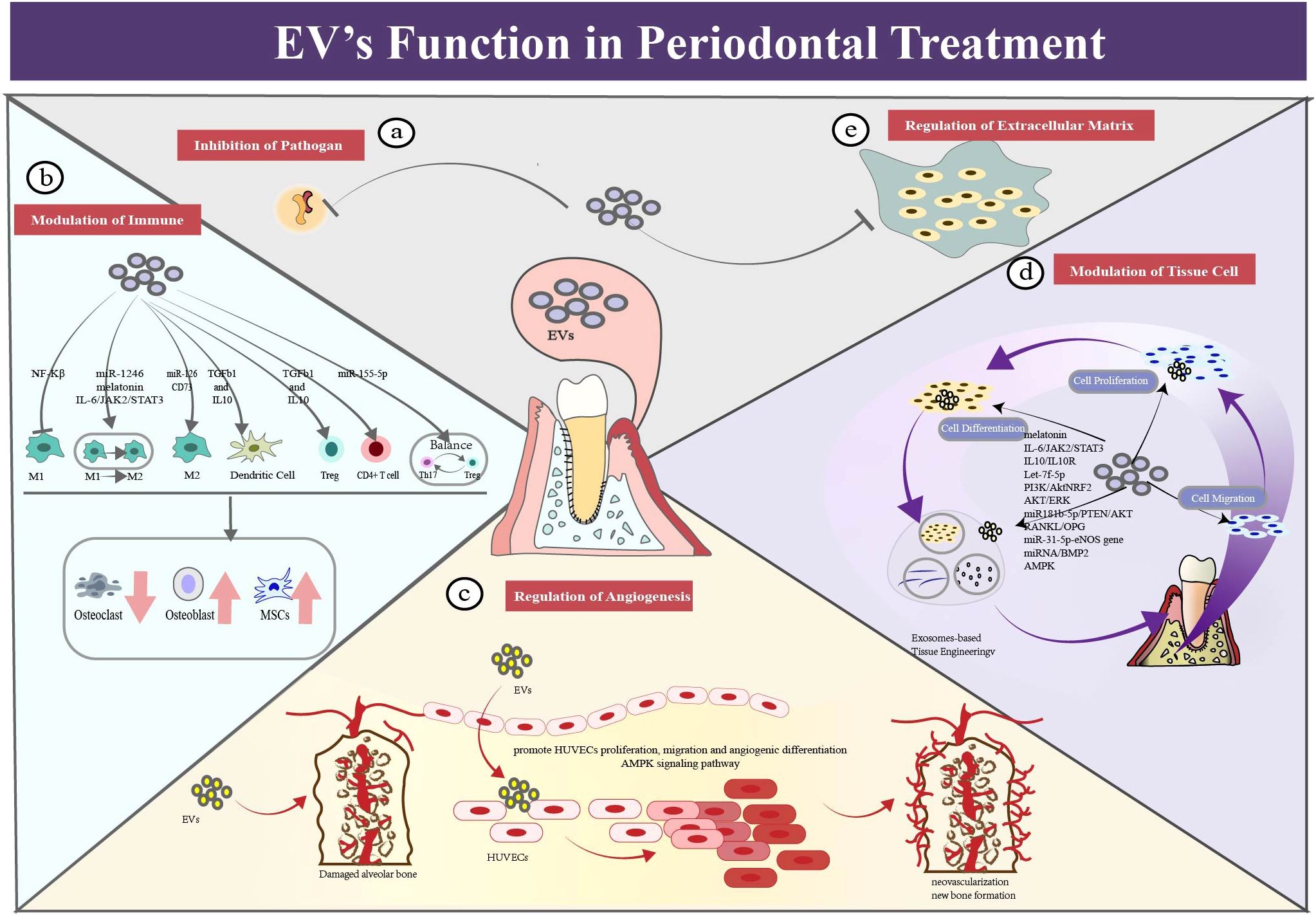
Figure 4. EV’s functions in periodontal therapy. (a) EVs exert a function of assisting in antimicrobial regulation. Exosome-like nanoparticles could serve as a potential therapeutic strategy for periodontitis. (b) EVs exert a function of immune modulation in periodontal therapy. EVs could regulate different phenotypes of macrophages to the anti-inflammation state. It can also regulate dendritic cells by cytokines (TGF-b and IL-10) delivery which alleviate bone resorption. EVs could function in regulating T cells, such as CD4+ T cell, Treg cells and Th17 cells. (c) EVs exert a function of regulating angiogenesis in periodontal therapy. EVs could promote migration, proliferation and differentiation of f human umbilical vein endothelial cells((HUVECs), which contributing the repair and regeneration of periodontium. (d) EVs exert a function of modulating activity of tissue cells in periodontal therapy. EVs could promote migration, proliferation and differentiation of tissue cells, which contributing the regeneration of periodontium. (e) EVs exert a function of modulating the extracellular matrix network, especially in the periodontal field.
4.1 EVs assist in antimicrobial regulation
EVs assist the regulation of local pathogens (62). For example, ginger extracellular vesicle-like nanoparticles (GELNs) were specifically absorbed by the periodontal pathogen, Porphyromonas gingivalis. (P. gingivalis) GELNs significantly reduced the harmful effects of P. gingivalis by binding to its heme-binding protein 35 on the bacterial surface and interacting with GELN cargo molecules such as phosphatidic acid and miRNA. This result demonstrated that the exosome-like nanoparticles could serve as a potential therapeutic strategy to prevent or treat chronic periodontitis in mouse models (63, 64). In the available literature, no evidence of using natural EVs directly for antibacterial application has been found.
4.2 EVs regulate immune inflammatory response
EVs can modulate macrophage polarization in local periodontal tissue, aiding in periodontal immune regulation (65–68). From healthy state to periodontitis condition, macrophages play distinct roles through phenotypic transformation. The study of Ru Wang (69) supported this notion by co-incubation of gingival marrow stem cell (GMSC)-derived exosomes with macrophages. The experiment showed significantly decreased level of M1 markers, decreased number of pro-inflammatory cells and reduced destruction of bone models. In addition, EVs have performed functions in other immune cells. This is supported by evidence from the Zhang Yong group. After routine injections of marrow stem cell(MSC)-exosomes, the periodontitis mice exhibited lower bone loss, better bone formation and fewer inflammatory cells compared with the control group (70), which indicated that exosomes could alleviate periodontitis by mediating of differentiation CD4+ T cells and relieving imbalance of Th17/Treg cells. Ya Zheng further discovered that periodontitis patients exhibited a Th17/Treg imbalance in their peripheral blood, with upregulation of Th17 cells or downregulation of Treg cells. They also found that the PDLSCs exosomes mitigated the inflammatory microenvironment via the regulatory network of Th17/Treg/miR-155-5p/SIRT1 (71). In addition, exosomes inhibited the maturation of dendric cells and the induction of Th17 effectors, but promoted the recruitment of T-regulatory cells, leading to the decline in resorptive cytokines and osteoclastic loss (71).
4.3 Modulation of periodontal tissue cells through EVs
EVs contribute to cementum regeneration. Cementum, a mineralized tissue covering tooth root surfaces, is a vital component of the periodontal tissue. Despite its importance, the specific mechanisms and effective strategies for cementum regeneration remain unclear. Previous studies have investigated the applications of growth factors or enamel matrix derivatives. Here, we present the evidence which supports EVs as a promising delivery vehicle to enhance the regenerative outcome (72–75). For example, Yi Zhao’s animal experiment demonstrated that exosomes from M2-polarized macrophage led to enhanced cementoblast mineralization (76). Additionally, a study of Shengnan Li et al. found that exosomes derived from human periodontal ligament stem cells (PDLSCs) promoted cementoblast activity via the PI3K/AKT signaling pathway, enhancing the migration, proliferation, and mineralization of OCCM-30 cells (77).
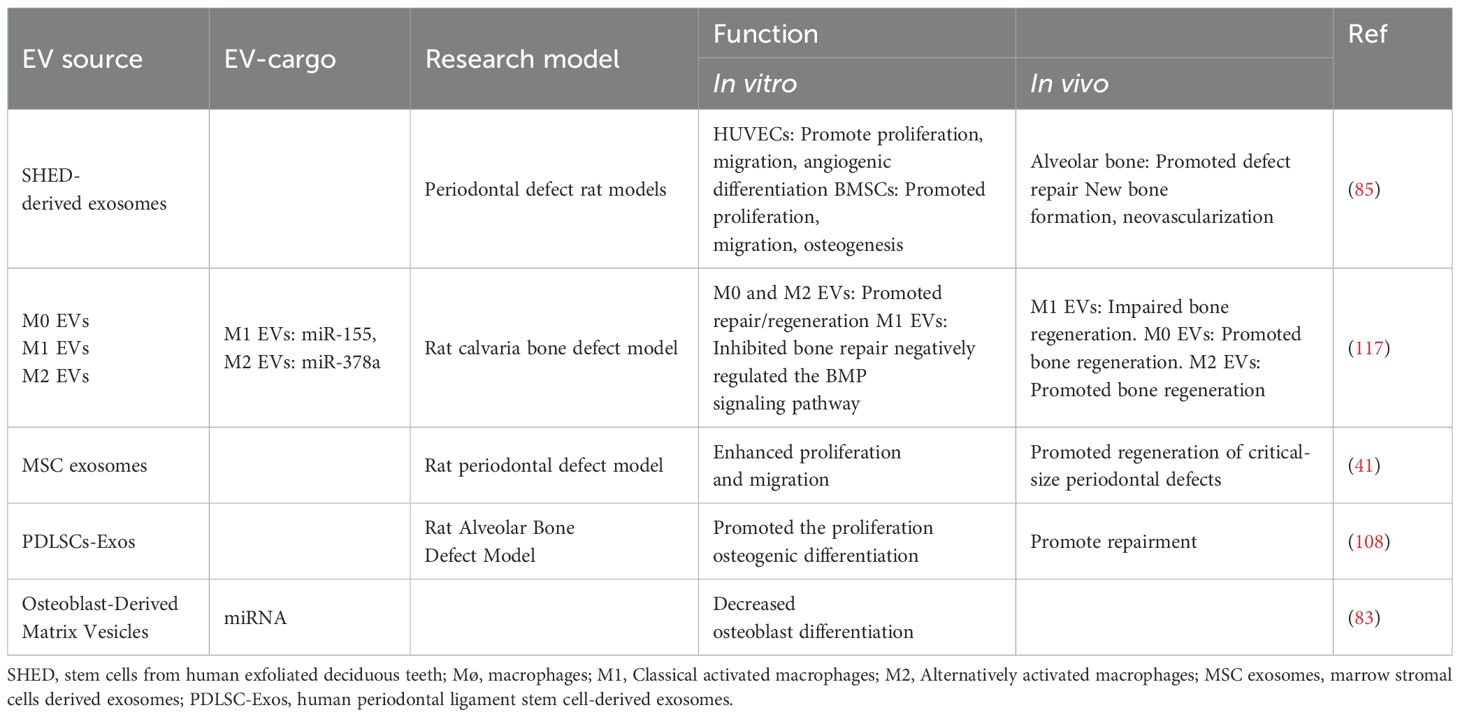
EVs regulate formation of periodontal ligament (67, 78–82). For example, human MSC-derived exosomes have been used to treat periodontal intrabony defects by local administration. Loaded onto a collagen sponge, rats in the exosome-treated group exhibited enhanced efficiency in repairing periodontal defects, with new bone and new periodontal membranes forming, accompanied by increased cell infiltration and promoted cell proliferation (72).
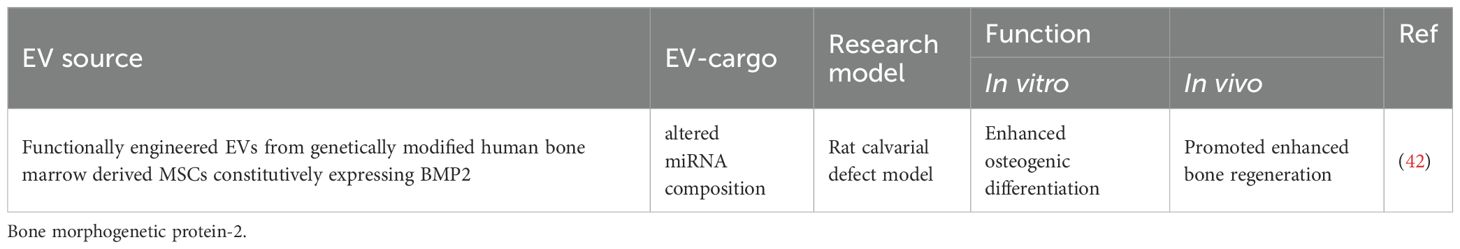
EVs could be used to regulate alveolar bone formation and absorption (41, 42, 50, 67, 75, 77, 81–92). Relevant theories can be referred to the review literature (93–95). EVs are expected to inhibit bone resorption and promote bone formation. Preclinical evidence has demonstrated that oral MSC-derived EVs could promote osteogenic repair in bone formation and periodontal defects (96–98). For example, MSC exosomes enhanced the migration and proliferation of periodontal ligament cells by activation of CD73-mediated adenosine receptor and stimulation of the pro-survival AKT and ERK signaling pathways (41). Combined with Matrigel or β-TCP, exosomes derived from h-PDLSCs enhanced bone formation in alveolar defects. That showed exosomes’ potential to restore the bone-forming ability of stem cells in the pro-inflammatory setting (50).
4.4 Modulation of extracellular matrix via EVs
EVs can modulate the extracellular matrix (ECM) network, especially in the periodontal field. ECM is a non-cellular three-dimensional structure, providing structural integrity and cellular regulation. As a highly dynamic network, ECM is essential to tissue maintenance, such as synthesis of substances and modification of chemicals (99). Deregulation of ECM structure is linked to diverse pathological conditions, such as osteoarthritis and periodontitis. It is also essential for tissue regeneration, that ECM could be tightly regulated instead. For example, derived from MSCs, functionally engineered EVs interacted with ECM proteins and peptides, leading to a fourfold increase in bone regeneration, compared with the control group in calvaria defect models (100).
4.5 Regulation of angiogenesis via EVs
EVs are considered as a novel approach to regulate angiogenesis (101). Blood vessels and lymphatic vessels serve their functions in resolving inflammation and promoting tissue regeneration. Beneath the gingival sulcular and junctional epithelium of periodontitis individuals, vascular networks undergo significant changes in microcirculation and vascular development. Alongside veins, nerves, and lymphatics, blood vessels carrying seed cells, growth factors and nutrients enter the interdental septa during periodontal regeneration process. For example, pre-treated with hypoxia, dental pulp stem cell-derived exosomes (DPSC-Exos) improved the proliferation, migration, and formation of human umbilical vein endothelial cells (HUVECs) and partially modified their proteome profile (42). Additionally, histological and immunohistochemical analysis showed that DPSC-Exos stimulated formation of new blood vessels in wound healing. In the vitro study, it was demonstrated that DPSC-Exos enhanced the ability of HUVECs to migrate, proliferate and regeneration through the Cdc42/p38 MAPK pathway (102). A comprehensive review has been published by Yunhao Qin (103).
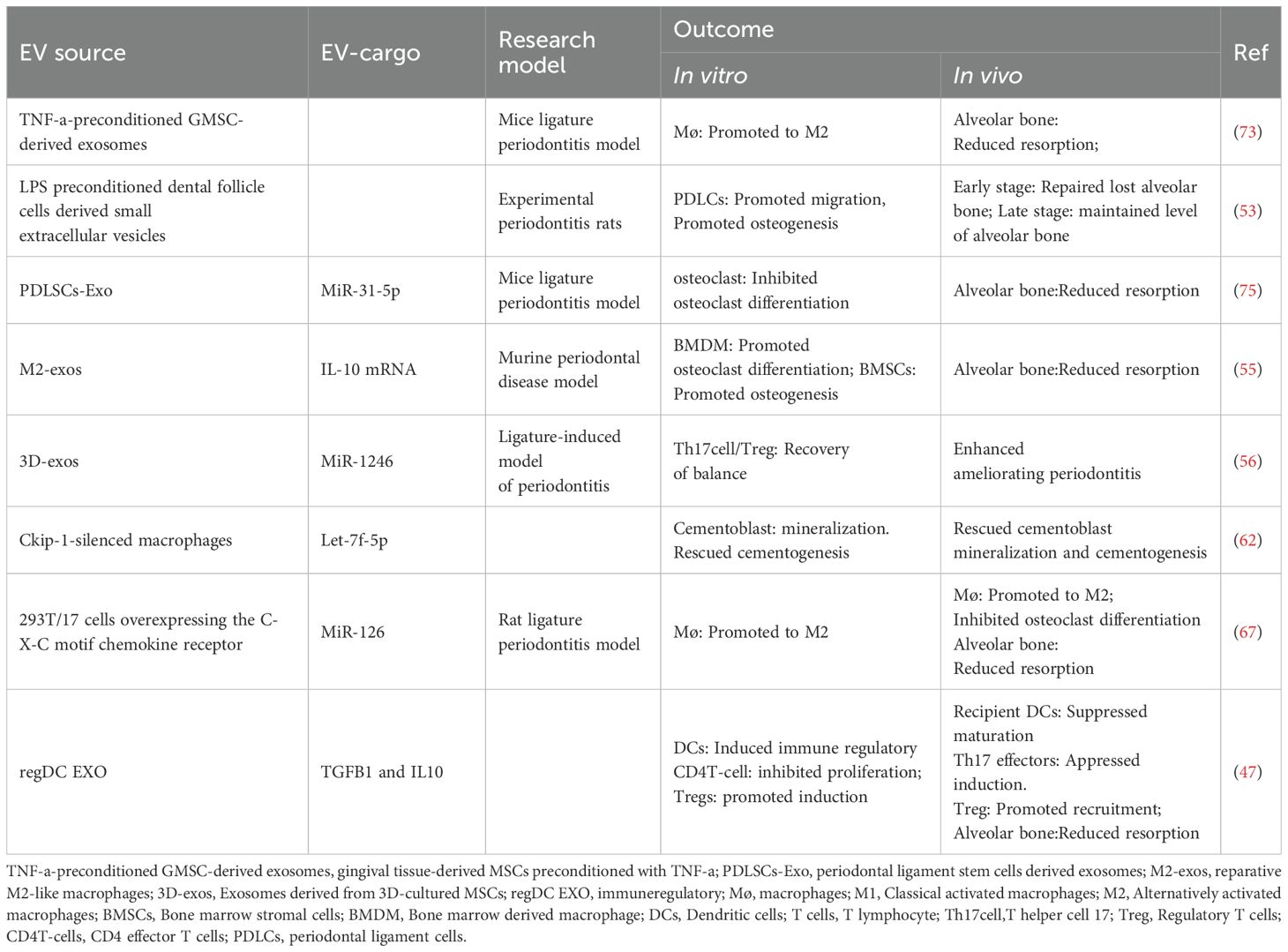
5 EV-based strategies for periodontal treatment
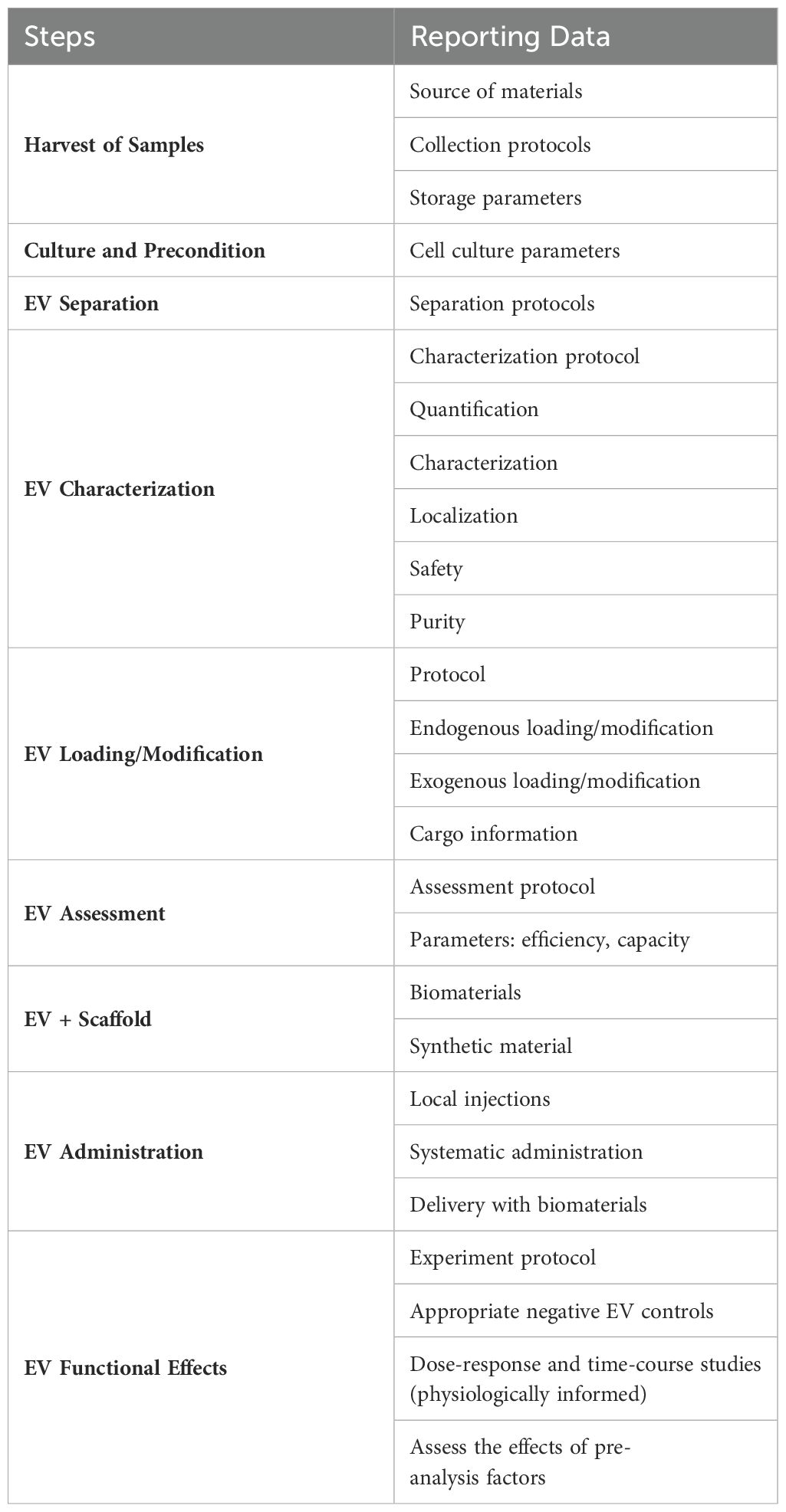
In this section, an overview EV-based strategies for periodontal treatment is provided. It is subdivided into seven parts according to the general workflow (104) (Figure 5) (Table 2).
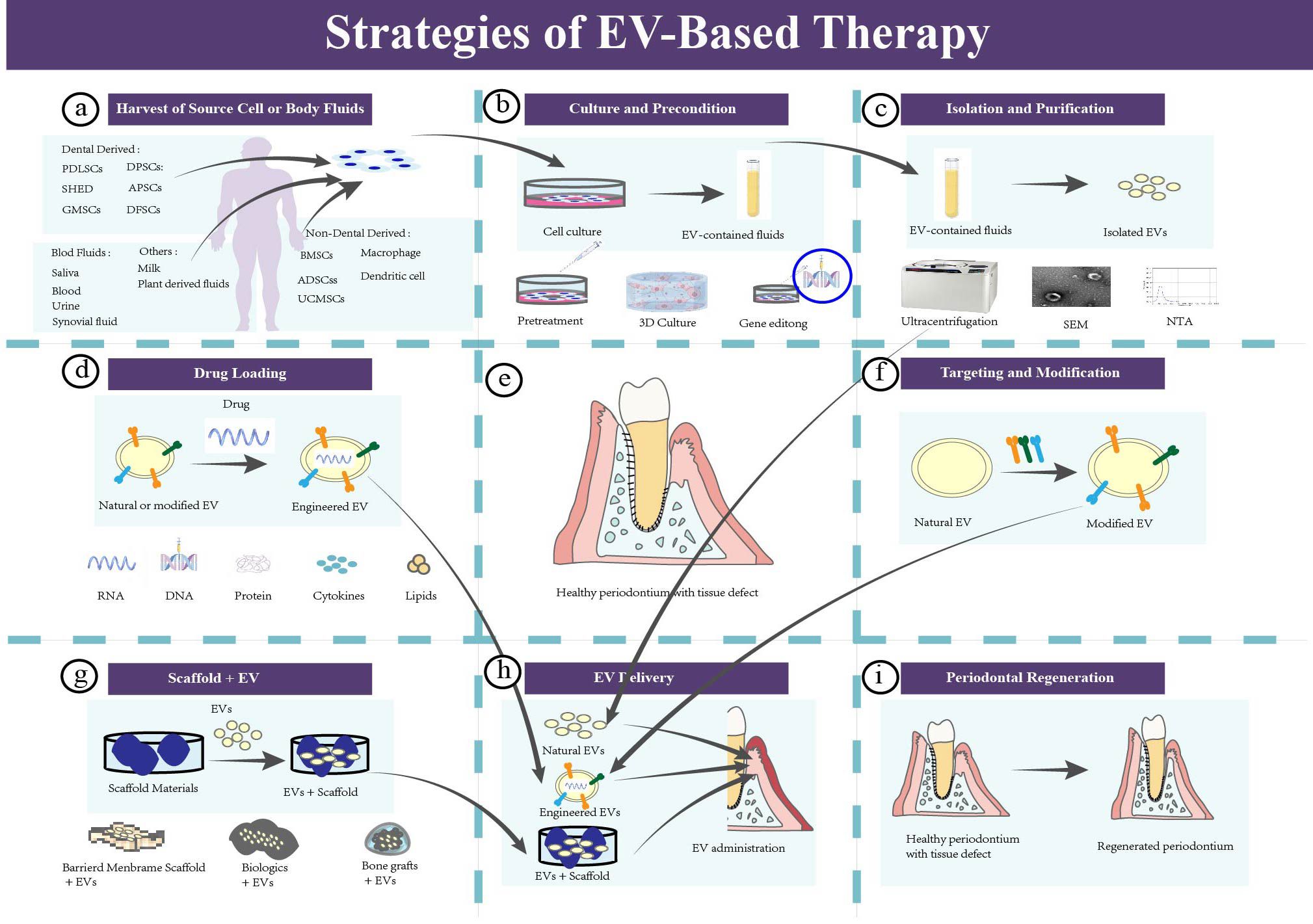
Figure 5. The strategies of EV-based therapy in periodontal regeneration. (a) Harvest of source cells or body fluids for isolating EVs. (b) Culture and pretreating the parent cells. Natural cells should be cultured for EVs insolation, and pretreating those parent cell could enhance the intrinsic properties of EVs. Common pretreatment methods include mechanical and physical pretreatment, chemical pretreatment, biological pretreatment, 3D culture, and gene editing. (c) Isolation, purification, and identification of EVs. (d) Drug loading of EVs. (e) Healthy periodontium with tissue defect. (f) Targeting and modification of EVs. (g) EVs-based tissue engineering strategy. (h) EVs delivery. Administration of EVs to the periodontal tissue defect. (i) Periodontal regeneration.
5.1 Sample harvesting
The initial step is sample harvesting for EV isolation. Rigorous preparation, based on complex demands and distinct sources, is crucial for designers to achieve optimal results and regeneration goals. At present, there is no international consensus on the standard scheme for EVs’ source selection, and researchers must refer to existing literature which has provided a series of clues for the consideration on this topic (17). Therefore, we have summarized the following lists to help think about the problem.
Source samples should provide appropriate characteristics for EVs:
1. Valuable functions
2. Tissue-homing property
3. Low immunogenic, low toxicity, no oncogenic considerations
4. Genetic stability, no host-cell impurities (pathogens, especially viruses)
5. Healthy donors
Source samples should provide a scalable and sustainable approach for EV harvesting:
1. Quality of EVs: stable, sustainable
2. Quantity of EVs: scalable
3. Manufacturing technology: practical, easy to harvest, to manipulate and to maintain
4. Cost effective
The selection of sample sources should avoid:
1. Highly invasive harvesting procedures
2. Highly challenging work
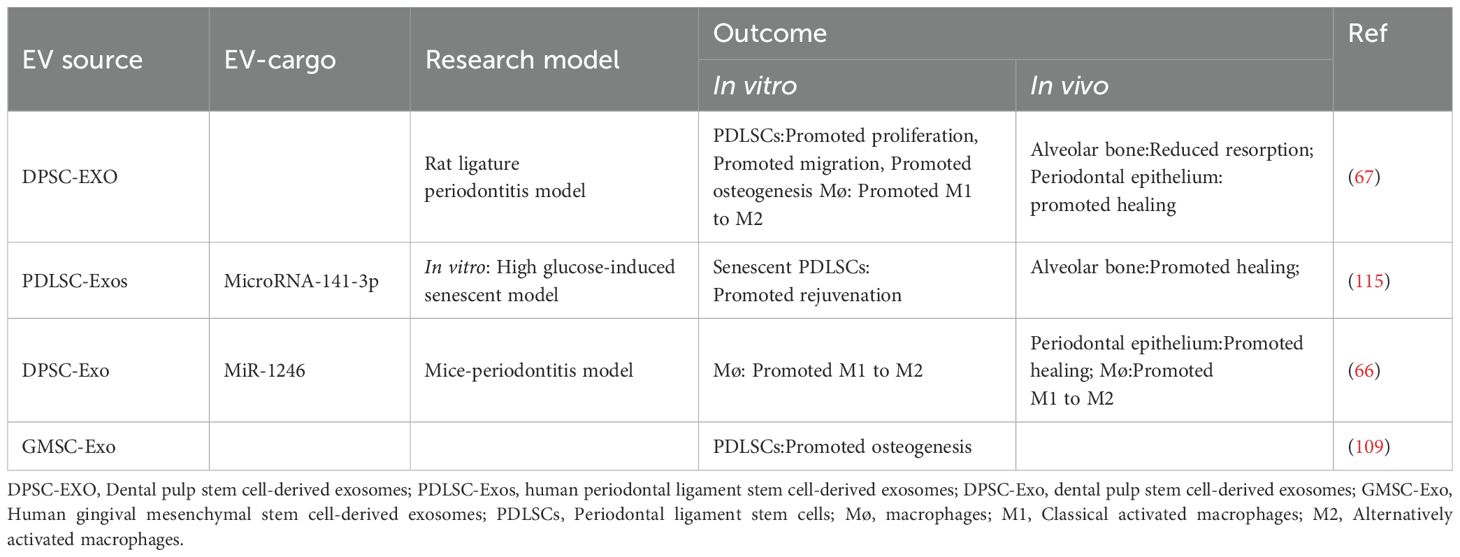
3. Unlikely to reach clinical implementation
4. Low-cost performance
5. Samples with pathological status and infection risk
To the first, source samples are required to provide EVs with appropriate characteristics (57, 105). The properties of EVs are firstly derived from inherent potential of their parental cells, such as tissue regulation capability, homing/targeting properties, immunogenic characteristic, genetic stability and anti-inflammatory potential (51). In the field of periodontal regeneration, it is common to use mesenchymal stem cells, for their self-renewal potential and multi-directional differentiation ability. Common stem cells include bone marrow stem cells (BMSCs) (106), adipose-derived stem cells (ADSCs) (107), and other mesenchymal stem cells, like umbilical cord stem cells (101). Also, oral tissue regeneration relies on various odontogenic stem cells, such as PDLSCs (108), DPSCs (102), dental follicle cells (109), stem cells from human exfoliated deciduous teeth (SHED) (110), GMSCs (109) and stem cells from the apical papilla (111). In addition, some immune cells have been studied for inflammation regulation and disease treatment, such as macrophages (78) and dendritic cells (112). Still another, plant cells, involving ginger (113) and onion (114), have been tested for more innovative and convenient options. For example, for enhancing periodontium formation, researchers could select PDLSCs, whose EVs have been indicated the properties of inhibiting osteoclast differentiation, reversing cell aging, promoting cell proliferation and facilizing inflammation recovery (50, 115).
Secondly, source samples should provide scalable and sustainable EVs. The harvesting of sample sources and the manufacturing of EV products must be scalable and repeatable, so that the quality, quantity, cost and efficacy could meet the needs of actual production and clinical application. For example, when engineering modification is required, specialized cell lines like 293T/17, known for their genetic editing capability, can be commonly applied (67). Then, blood, urine, saliva and milk, body fluids and solid tissue provide researchers with convenient sources for EVs.
Thirdly, researchers should avoid the selection of highly invasive samples, challenging manufacturing procedures, low cost-effective products, research projects with difficulty in clinical translation and parental tissue with risks in contamination (57). Genetic variability, epigenetic modification, environmental changes and pathological states could influence the functions of EVs. For example, exosomes from healthy periodontal ligament stem cells led to enhanced mineralized nodule formation and increased osteogenic indicators in the inflamed PDLSCs. Mechanistically, these exosomes inhibited the over-activation of the canonical Wnt pathway, thereby restoring the osteogenic potential of the inflamed PDLSCs (50).
5.2 Cell culture and pretreatment
Another key step is to culture and pretreat the obtained samples. Once the sample is collected, researchers need to culture, preprocess and store it for subsequent isolation and purification. In the complex process, the methodologic details should be recorded carefully. With guideline of Minimal Information for Studies of Extracellular Vesicles (MISEV) (116), a common basis for standardization had been set, which is crucial for quality control and outcome comparison. For example, when parent cells have been collected, characteristics of the cells and identification of cell lineage must be fully recorded. In addition, their medium components, culture conditions, stimulation and other treatments should be described in detail. Besides, if there are contaminations or infections, the specific situation and treatment operation should be carefully reported. Furthermore, the presence of apoptotic and dead cells greatly affects the characteristics of vesicles. Therefore, the percentage of these cells should also be faithfully recorded. Then, sometimes, natural EVs have been used in periodontal treatment (Tables 3, 4). For example, Shen, Z. extracted exosomes from DPSCs. Incorporated into chitosan hydrogel, these exosomes facilitated the transition of macrophages from a pro-inflammatory to an anti-inflammatory phenotype, and accelerated healing of the alveolar bone and periodontal epithelium in periodontitis-afflicted mice (66).
To enhance EVs properties and achieve high-performance products, pretreating parent cells is an important approach. Common pretreatment methods comprise culture regulation, biochemical stimulation and physical pretreatment (95), such as Tumor necrosis factor-α(TNF-a) (118), Lipopolysaccharide(LPS) (92), IL-4 (117), IL-10 (84), 3D culture (70), high glucose culture (119), hypoxia culture (120) and mechanical stretch (121). On the one hand, pretreating strategies preserve the original function and primary integrity. On the other hand, the produced EVs become more precise and more efficient for practical treatment (107, 122). However, the cells’ responses to the external stimuli might be variable, and the EVs’ properties are not fully defined, making it challenging to use this method accurately. For example, LPS-preconditioned dental follicle cell-derived sEV (L-D-sEV) promoted proliferation, migration, and differentiation of PDLSCs from periodontitis (p-PDLCs) in a dose-dependent and saturable manner. The L-D-sEV demonstrated a slight advantage over conventionally cultured dental follicle cell-derived sEV in enhancing p-PDLCs’ differentiation. Moreover, L-D-sEV leaded a partial reduction in the RANKL/OPG ratio and revealed advantageous in pathological models, through aiding the restoration of lost alveolar bone in the initial treatment phase and preserving alveolar bone levels in the later treatment phase (92).
In summary, standardized design of culture strategies and comprehensive documentation of practical protocols are very important for the rigor, reliability and repeatability of experimental research.
5.3 EVs' separation and characterization
Separation is a crucial step in the preparation of EVs. Expected to be scalable and robust, separation depends on EVs’ physiological characteristics such as size, density, charge, and surface structure, and different approaches determine the yield, specificity and purity of products. Common separation approaches consist of didderential ultra-centrifugation, density gradient centrifugation, precipitation, filter concentration, size-exclusion chromatography, immunoaffinity capture techniques, and microfluidic techniques (116). Among them, the most specific method is the immune-precipitation method, and the highest recovery rate of outer vesicles is the precipitation method. At present, the most widely used technique in literature is ultra-high-speed centrifugation. Different centrifugal forces are used to separate solutes according to their volume, mass and sedimentation coefficient. This method enjoys its advantages such as low cost, large quantity and large yield. But its low purity and heavy workload hinders the application of this technique. Researchers can improve the quality of EV through the combination of different approaches.
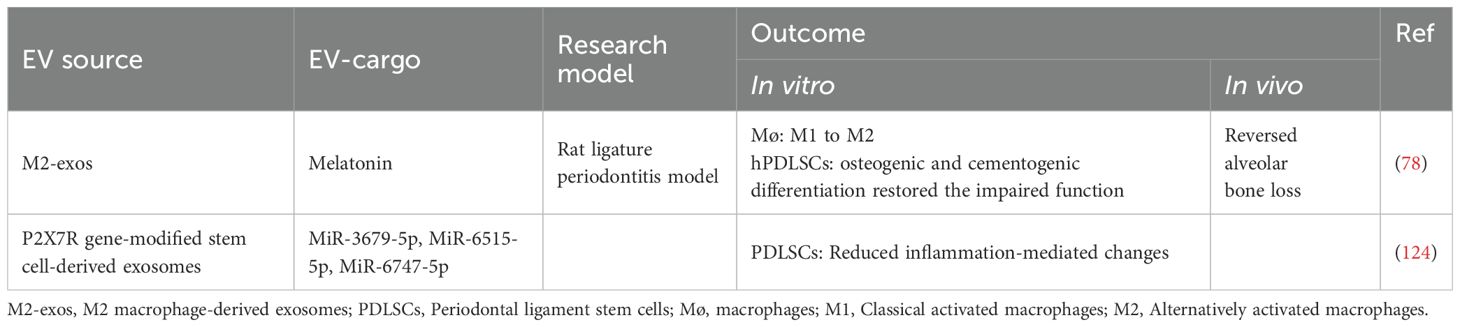
After the isolation procedure, researchers should characterize them to verify their existence, evaluate their quantity and identify their purity. Orthogonal methods are recommended, for no single technique is adequate for all needs. According to MISEV2024 (116), the quantitative measures and approximation estimate of EV source (e.g., number of secreting cells, volume of biofluid, mass of tissue) and the EV abundance should be made. The identification of components associated with EVs and the proportion of the non-vesicular, co-isolated components should be demonstrated. In detail, the total protein, total lipids, total RNA, EV morphology, protein composition, non-protein markers and localization of EV-associated components are required. These characteristic should be measured by Flow cytometry, PCR, Western Blot, Nanoparticle tracking analysis, Microscopy-based methods (123). Besides, the safety measurement of EV products is also in need, including their biocompatibility, immunogenicity, tumorigenicity and toxicity.
5.4 EVs' engineering and modification
Scientists are always exploring more stable, efficient and quantifiable strategies, especially at a genetical level. (Tables 5, 6) In parent cells, genetic engineering approaches may well modify RNA expression and protein production, thereby altering the contents and surface features of their EVs and changing their function. For example, Xin Huang etc. designed the M2-like macrophage-derived exosomes by the genetic engineering technique. To obtain permanent M2-like macrophages, they silenced the key gene, casein kinase 2 interacting protein-1 (Ckip-1), which promised not only a long-term specific characteristic, but also a promoted mineralization effect. They collected the exosomes from these engineered cells and measured their therapeutic capability on periodontal regeneration. These exosomes (sh-Ckip-1-EXO) rescued the cementoblast whose function had been suppressed by Porphyromonas gingivalis. At a molecular level, sh-Ckip-1-EXO enhanced the cementoblast mineralization and cementogenesis by delivering the key molecule of Let-7f-5p and silencing the inhibitor of cementum formation (75). Chun-Chieh Huang modified HMSCs genetically to carry out this exploration. HMSCs, with promoted osteoinductive effect, expressed BMP2 constitutively. For this reason, they are expected to produce exosomes with BMP2 protein and osteoinductive function. Then, in the calvarial defect models, these engineered HMSC exosomes indeed enhanced the bone regeneration. However, BMP2 protein was not detected as the constituent in these functionally vesicles. Further research showed that the exosomes, which enhanced the BMP2 signaling pathway without BMP2 protein, possessed alerted miRNA after gene modification. This result demonstrated that the effect of EVs had be improved by genetic modification of their original cells (42).
5.5 EVs for drug loading
Drug loading provides another choice for EV-based treatment. EVs have been explored as novel carriers for small molecules, proteins and nucleic acids delivery, in the light of their biological, structural and pharmacological characteristics (125) (Table 7).
Drug loading processes consist of endogenous (pre-secretory) loading strategies and exogenous (post-secretory) loading strategies (44). First, endogenous loading strategies involve modifying parental cells through gene editing and loading parental cells through co-incubation. By these means, EVs preserve their integrity when harvesting specific proteins, nucleic acids and drug molecules. Endogenous loading approaches also allow scientists for accurate prediction and stringent regulation. However, the primary limitation is the difficulty in effective loading (126). For all this, endogenous loading strategies have shown potential in application for periodontal regeneration. For example, exosomes containing miR-126 (CXCR4-miR126-Exo) were effectively created through the transient transfection of miR-126 into 293T/17 cells that overexpressed CXCR4. It increased the anti-inflammatory cytokines, decreased the bone resorption and ultimately halted the periodontitis advancement (127).
Secondly, to address the limitations of the endogenous methods, exogenous (post-secretory) loading approaches have been developed. Exogenous loading strategies incorporate proteins, nucleic acids, bioactive molecules and drugs into isolated EVs, offering simpler protocols, greater stability and continued scalability for large-scale production in efficient drug delivery systems (128). In the periodontal field, particularly applied techniques include co-incubation, electroporation, sonication, freeze–thaw cycles and extrusion (129). For example, melatonin, a crucial hormone-like substance, acts as an antioxidant in cellular physiology. It modulates tissue inflammation and regulates cell apoptosis under various pathophysiological conditions. Ya Cui and colleagues loaded melatonin into M2 macrophage-derived exosomes (Mel@M2-exos) to explore its application potential (78). Released in the periodontium, these Mel@M2-exos restored the osteogenic capacity of the inflammatory human periodontal ligament cells (hPDLSCs). Animal experiments demonstrated that Mel@M2-exos in gelatin methacryloyl hydrogels significantly accelerated periodontal bone in rats with ligation-induced periodontitis (78). Mahmoud Elashiry loaded dendritic-cell exosomes with TGF-B1 and IL-10 (112). Then, to explore their therapeutic effect, these exosomes were administrated locally and intravenously. Immunoregulatory DC exosomes (regDC EXO) were effectively taken up by DCs and T cells, protecting TGF-B1 and IL-10 and delivering them at the same time. As a result, the local alveolar bone resorption was inhibited, which illustrated that the exosomes, loaded with effective cytokines, could provide a promising outcome for periodontitis treatment.
The drug loading performance of EV needs to be evaluated by multiple indicators. Common parameters include safety, efficacy, biocompatibility, stability, pharmacokinetic parameters, pharmacodynamic parameters and loading parameters. It is very important to detect these indicators, because they can provide researchers with specific references in dosage, concentration and strategy design. For example, the encapsulation efficiency, parameter for exogenous loading, refers to ration of loaded fraction compared to the total loaded amount (104). In experimental studies and literature reports, researchers should report the specific operation steps of drug loading processes and evaluate specific loading and pharmacokinetic parameters in detail, to provide a truly valuable reference for the functional development of EV drug delivery.
5.6 EVs combine with scaffold
Scaffold materials provide a broader prospect for EVs applications. There are many advantages of combining EVs with scaffold in periodontal regeneration (130). To begin with, scaffold materials retain EVs in a specific location, especially in the periodontium. The multi-layered structure and the washout of saliva and gingival crevicular fluid can make those free EVs more easily to diffuse, thereby reducing their effectiveness. The application of scaffold materials can facilitate EVs’ retention in the periodontal pocket, preserve their bioactivity and reduce their risk in direct diffusion (131). Next, by encapsulating or carrying EVs in their ECM-like structure, scaffold materials might also control the concentration in local, achieve the sustained and controlled release in vivo and avoid the rapid clearance of EVs in circulation (92). Finally, this structure could provide the architectural restoration and biomechanical support for defective periodontal tissue (132). However, so far, the optimal solution for combining strategies still needs further study.
Hydrogel’s functions are different from the those of EVs in periodontal regeneration. Considering the biological mechanism, hydrogel is a common biological material in periodontal tissue regeneration engineering. It plays roles as scaffold, release-controlling material and ECM mimic. While EVs exert their functions by bioactive molecule delivery and information transport among cells. For example, EVs may well regulate bioactivities and metabolism of receptor cells. Those receptor cells, which take the role of “seed cells” and might be enhanced by EVs, rebuild a new tissue by their stimulated proliferation, migration and differentiation.
Many kinds of scaffold materials could be used as EV-carriers. Shen et al.’s (41) experiment used chitosan hydrogel for exosome-loading, Shi et al.’s (92) used gelatin hydrogel, Cui et al.’s (78) used GelMA hydrogel. Collagen has been applied in Tang et al.’s (92), Chew et al.’s (41) and Huang et al.’s (42) regenerative experiments, and it been proven to be beneficial for the final outcome. For example, Shen, Z. extracted exosomes from DPSCs. Incorporated into chitosan hydrogel, these exosomes facilitated the transition of macrophages from a pro-inflammatory to an anti-inflammatory phenotype, and accelerated healing of the alveolar bone and periodontal epithelium in periodontitis-afflicted mice (66). The theoretical reviews of scaffold materials for EVs can refer to articles of Ma et al. (133), Kasey S Leung et al. (134) and Wang et al. (73).
5.7 EVs' administration
Route of EV administration consists of local injection and systematic administration, which affects the efficiency of EV-based therapy.
First, injecting EVs is the simplest approach for researchers. In animal models, EVs are typically mixed with phosphate buffer solution and are directly injected into local tissue. This method may increase the bioavailability of EVs in complex periodontal tissue and circumvent the limitations of systemic administration. But this is a painful and invasive operation which may cause swelling, inflammation and tissue damage. Moreover, calculating appropriate dose of EVs, determining reasonable frequency of injection, controlling suitable concentrations in tissue, analyzing biological distribution and evaluating systematic clearance pose serious challenges to scientists. Despite these, the direct injection has become the most common way for its effectiveness and convenience according to the current literature. Numerous studies have demonstrated the efficacy of this strategy in periodontal treatment (67, 70, 84, 118, 127).
Secondly, through binding with biomaterials, EVs can be incorporated into periodontal tissue (135). For example, loaded onto a collagen sponge, human MSC exosomes were used to treat periodontal intrabony defects. Compared to the control group, rats with MSC exosomes showed improved periodontal defect repair, that is: enhanced new bone formation and promoted periodontal membranes regeneration, with increased cell infiltration and proliferation (41).
Thirdly, EVs can enter the body through the systemic route of administration, such as: intravenous administration, oral administration, etc. This approach has high clinical utility and eliminates the risk of local damage, and it is expected to target inflammatory tissue and exert therapeutic functions. However, studies have shown that systematically administered EVs accumulate rapidly in livers, spleens, lungs and kidneys. They are rapidly cleared in circulation, with insufficient concentrations in target tissue (136).
For example, Mahmoud Elashiry studied the relationship between the route of administration and the distribution in the body. Infused with radiolabeled IN-111, EXOs in intravenous route were slowly cleared in the mice’ bodies. These EXOs were absorbed into the systemic circulation such as spleen, but not to the injured maxillary site. Mice, using the local injection, showed 10-fold higher expression of radioactivity in inflamed lesion sites than the control group (112).
Although the potential to cross the physiological barriers renders EV promising biological agents, there are so many uncertainties in the administration process, such as short half-life, non-specific targeting and inefficiency of functional items. In the future, it is necessary to deal with these tackles, to add types of EVs, to increase more animal models, to make more rigorous design and operations, to establish more crucial parameters, to prevent off target effects and to provide more valuable evidence, for EVs’ application and transformation.
6 Challenges and prospects
EV-based treatment for periodontal tissue engineering is still in its infancy, but it is steadily growing and maturing. In fact, many challenges remain needed to be addressed (Table 8).
6.1 Research on the biological mechanism of EVs' periodontal treatment
Mechanisms in both the regeneration of periodontium and the biological functions EVs have long perplexed the researchers (137). Advanced knowledge on these processes is a prerequisite to develop effective therapies for periodontal regeneration. To the first, the exact mechanisms of biogenesis, sort, release and uptake of EVs have not been fully understood and require further investigation. It also mostly unknown how the EVs perform their functions in receptor cells and target tissue. A more comprehensive understanding will aid in developing suitable cultivation methods to alter EVs’ compositions and enhance their functions, to minimize off-target effects and improve targeted therapeutic approaches. Moreover, it would contribute to optimize their production process and enhance productivity (138). Secondly, although a conceptual model of periodontal regeneration has been proposed to guide experimental and clinical work, the specific mechanism of this process has not been firmly demonstrated. For example, the reason why seed cells are recruited can help to the regulation of migration and proliferation which improves therapeutic approaches in the end. The innate regenerative potential should be evaluated preciously for the selection of source sample of EVs. Therefore, researchers ought to investigate these scientific problems deeply, to provide more comprehensive insights into those controversial but important issues (139).
6.2 Optimize EV-based strategies
Lacking standardization of research processes impedes the development of EV-based strategies. Scientists could identify EVs formally when they begin their experiments. But there are some steps in the processes that people have no standard guideline. Their only way to conduct the experiment is to explore EVs based on their experience–protocols handed down from one generation of research assistants to another. This inconsistence complicates the comparing and referencing of EV-based results among different laboratories, putting obstacles in the way of collaborative work in this field. A systematic review and meta-analysis of preclinical trials illustrated that MSC-derived exosomes impressive potential for contributing to periodontal regeneration. But standardized and robust trials are required for the development of this technique (140). Therefore, to enhance the comparability and reproducibility of EV research, it is essential to explore a reasonable and standardized framework of EV-based therapy (141). And it is recommended that experiments should be designed, conducted and reported comprehensively according to guidelines for experimental knowledge bases and working process in the Minimal Information for Studies of Extracellular Vesicles (MISEV) of 2018 and 2024 (43, 116). A critical systematic review has been reported talking about of EV’s clinical trial. It highlighted the meticulous methodological reporting to enhance the successful clinical translation (142). To do so, the substitution of systematic protocols instead of personal work habits constitutes great options.
Other improvements rely on the safety and efficacy of EV engineering products. Researchers should focus on the systematic integration of research techniques, to improve the purity and yield, reduce the time cost and improve the efficacy of EV products in the future, as they are more suitable for periodontal application, as well as for clinical and industrial translation (143). More details could be learn from the systematic review of Van Delen etc. (144).
6.3 Explore EVs' clinical applications
A huge gap exists between the experimental research at present and the clinical requirements in the future. Firstly, absence of systematical characterization of EV therapies restricts the clinical transformation of EV. It is important to evaluate the safety and efficacy of the novel subcellular tool (145). Considered to enjoy high safety and low immunogenicity, EVs lack sufficient strong data and long-term evidence for these claims (143, 146). As the case stands, most of published reports remain small pilot trials. In its future work, the potential tumorigenicity and immunogenicity must be examined before clinical trials (147). As to efficacy, despite various attempts at the endogenous regeneration, these strategies often fail to demonstrate significant advantages in terms of the regenerative outcomes such as alveolar bone repair, periodontal ligament reconstruction and cementum regeneration. Further studies are required to develop new analytical tools, to evaluate safety and efficiency, to evaluate pharmacokinetics and pharmacodynamics, to analysis EV compositions and functions and to assess the functional effects of EV therapies. Secondly, inadequate uniform quality control (QC) criteria (17). Official research guidelines should provide standardized methods for more rigorous, more reproducible and more comparable research. In the scoping review of the literature of Clorinda Fusco in 2024, 40 studies about EVs as human therapies demonstrated that the large heterogeneity in EV experimental settings impeded the comparison across studies of the similar kind (143). Thirdly, there are regulatory challenges of EV-based strategies. Due to the high heterogeneity of natural EVs, complicated production processes, absent industrial specifications and lack of quality control guidelines, government regulatory is crucial to increase efficacy and promote safety (17). Next, most of the studies are small pilot trials (143), but clinical translation requires large animal model (non-human primates) experiments for more plausible conclusions. Furthermore, it is important for EV-based studies to set up reasonable control groups. Most of the current research literature lacks placebo controls, which are rigorous, especially for efficacy studies of biological agents.
6.4 Enhance EVs' industrial technology
There remains much to be done in EV manufacture to meet growing demands of industrial transformation (44). To the first, it’s a serious challenge to increase the scalability and reproducibility of EV production. Separation scalability, evaluation standardization, loading stability, modification reproducibility, standardization of functional experiments and mature of storage approaches are all technical problems to be overcome on the road to industrialization. Then, the low cost-efficient outcome undermines the confidence of researchers. In the future, close collaboration of biology, chemistry, engineering and periodontal medicine will be conducive to the progress of EV technology, thereby reducing the production cost, shortening the production time, improving the production efficiency and enhancing the therapeutic effect.
7 Conclusion
Collaborative efforts at EV-based treatment are bridging the gap between the ideal periodontium and the current outcome. In the future, inevitable integration of EV-based techniques and regenerative medicine may pave the way for the therapeutic development, and EVs indeed show considerable potential in the field of periodontology.
Author contributions
XZ: Writing – original draft, Writing – review & editing, Investigation, Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization. HG: Data curation, Methodology, Software, Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
AMPK: Adenosine monophosphate-activated protein kinase
ADSCs: Adipose tissue-derived stem cells
BMMΦ: Bone marrow macrophages
BMMSCs: Bone marrow stem cells
BMP2: Bone morphogenetic protein-2
CCL2: C-C motif chemokine ligand 2
CXCR4: C-X-C motif chemokine receptor 4
DCs: Dendritic cells
DPSCs: Dental pulp stem cells
ECM: Extracellular matrix
MMP-8: Extracellular matrix metalloproteinase-8
MMP-9: Extracellular matrix metalloproteinase-9
EV: Extracellular vesicle
FGF-2: Fibroblast growth factor-2
GELNs: Ginger extracellular vesicle-like nanoparticles
GMSC: Gingival marrow stem cell
GTR: Guided tissue regeneration
Th1: Helper T type 1
Th2: Helper T type 2
Th17: Helper T type 2
HBP35: Heme-binding protein 35
HUVECs: Human umbilical vein endothelial cells
IGF-1: Insulin like growth factor-1
IL-10: Interleukin-10
IL-12: Interleukin-12
IL-1b: Interleukin-1b
IL-8: Interleukin-8
MVBs: Intracellular multivesicular bodies
ILVs: Intraluminal vesicles
LPS: Lipopolysaccharide
MSC: Mesenchymal stem cells
NOS: Nitric oxide synthase
NF-κB: Nuclear transcription factor-κB
OPG: Osteoprotegerin
PDLSCs: Periodontal ligament stem cells
PDGF-BB: Platelet-derived growth factor-BB
PGE-2: Prostaglandin E2
RANK: Receptor activator for nuclear factor-κ
RANKL: Receptor-activator of nuclear factor kappa beta ligand
SHEDs: Stem cells from human exfoliated decid-uous teeth
TRAP: Tartrate resistant acid phosphatase
3D: Three-dimensional
TGF-β: Transforming growth factor-β
Treg: T-regulatory cells.
TCP: Tricalcium phosphate
TNF-a: Tumor necrosis factor-α
References
1. Loesche WJ and Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. (2001) 14:727–52. doi: 10.1128/CMR.14.4.727-752.2001
2. Chen MX, Zhong YJ, Dong QQ, Wong HM, and Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the global burden of disease study 2019. J Clin Periodontol. (2021) 48:1165–88. doi: 10.1111/jcpe.13506
3. Isola G, Polizzi A, Serra S, Boato M, and Sculean A. Relationship between periodontitis and systemic diseases: a bibliometric and visual study. Periodontol 2000. (2025). doi: 10.1111/prd.12621
4. Hajishengallis G and Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. (2021) 21:426–40. doi: 10.1038/s41577-020-00488-6
5. Lalla E and Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. (2011) 7:738–48. doi: 10.1038/nrendo.2011.106
6. Yamazaki K and Kamada N. Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal Immunol. (2024) 17:147–53. doi: 10.1016/j.mucimm.2023.11.006
7. Lim GB. Periodontal treatment reduces AF recurrence. Nat Rev Cardiol. (2024) 21:355. doi: 10.1038/s41569-024-01036-8
8. Koziel J and Potempa J. Pros and cons of causative association between periodontitis and rheumatoid arthritis. Periodontol 2000. (2022) 89:83–98. doi: 10.1111/prd.12432
9. Kittner SJ and Taylor BL. Oral health and brain health: cause, consequence, or confounding? Neurology. (2024) 102:e208089. doi: 10.1212/WNL.0000000000208089
10. Figuero E, Han YW, and Furuichi Y. Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontol 2000. (2020) 83:175–88. doi: 10.1111/prd.12295
11. Hynes K, Menicanin D, Gronthos S, and Bartold PM. Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. (2012) 59:203–27. doi: 10.1111/j.1600-0757.2012.00443.x
12. Ribeiro FV, Mehta JJ, Monteiro MF, Moore J, Casati MZ, and Nibali L. Minimal invasiveness in nonsurgical periodontal therapy. Periodontol 2000. (2023) 91:7–19. doi: 10.1111/prd.12476
13. Gao P, Kajiya M, Motoike S, Ikeya M, and Yang J. Application of mesenchymal stem/stromal cells in periodontal regeneration: Opportunities and challenges. Jpn Dent Sci Rev. (2024) 60:95–108. doi: 10.1016/j.jdsr.2024.01.001
14. Kalluri R and LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
15. He C, Zheng S, Luo Y, and Wang B. Exosome theranostics: biology and translational medicine. Theranostics. (2018) 8:237–55. doi: 10.7150/thno.21945
16. Wang X, Chen J, and Tian W. Strategies of cell and cell-free therapies for periodontal regeneration: the state of the art. Stem Cell Res Ther. (2022) 13:536. doi: 10.1186/s13287-022-03225-z
17. Miron RJ and Zhang Y. Understanding exosomes: Part 1-Characterization, quantification and isolation techniques. Periodontol 2000. (2023). 10.1111/prd.12520
18. Yu M, Luo D, Qiao J, Guo J, He D, Jin S, et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact Mater. (2022) 10:93–106. doi: 10.1016/j.bioactmat.2021.08.024
19. Newman MG, Takei H, Klokkevold PR, and Carranza FA. Carranza's Clinical Periodontology. (12th ed.) New York: Saunders. (2014).
20. Kumar J, Jain V, Kishore S, and Pal H. Journey of bone graft materials in periodontal therapy: a chronological review. J Dent Allied Sci. (2016) 5:30. doi: 10.4103/2277-4696.185195
21. Melcher AH. On the repair potential of periodontal tissues. J Periodontol. (1976) 47:256–60. doi: 10.1902/jop.1976.47.5.256
22. Nyman S, Gottlow J, Karring T, and Lindhe J. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. (1982) 9:257–65. doi: 10.1111/j.1600-051X.1982.tb02065.x
23. Caton J, Nyman S, and Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. (1980) 7:224–31. doi: 10.1111/j.1600-051X.1980.tb01965.x
24. Nyman S, Karring T, Lindhe J, and Plantén S. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. (1980) 7:394–401. doi: 10.1111/j.1600-051X.1980.tb02012.x
25. Karring T, Nyman S, Gottlow J, and Laurell L. Development of the biological concept of guided tissue regeneration–animal and human studies. Periodontol 2000. (1993) 1:26–35.
26. Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, and Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. (2014) 93:1212–21. doi: 10.1177/0022034514544301
27. Gottlow J, Nyman S, Lindhe J, Karring T, and Wennström J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. (1986) 13:604–16. doi: 10.1111/j.1600-051X.1986.tb00854.x
28. Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, and Ivanovski S. Tissue engineered constructs for periodontal regeneration: current status and future perspectives. Adv Healthc Mater. (2018) 7:e1800457. doi: 10.1002/adhm.201800457
29. Urist MR, DeLange RJ, and Finerman GA. Bone cell differentiation and growth factors. Sci (n Y NY). (1983) 220:680–6. doi: 10.1126/science.6403986
30. Lynch SE, Williams RC, Polson AM, Howell TH, Reddy MS, Zappa UE, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. (1989) 16:545–8. doi: 10.1111/j.1600-051X.1989.tb02334.x
31. Heijl L, Heden G, Svärdström G, and Ostgren A. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. (1997) 24:705–14. doi: 10.1111/j.1600-051X.1997.tb00253.x
32. Nakashima M and Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. (2003) 21:1025–32. doi: 10.1038/nbt864
34. Jin QM, Anusaksathien O, Webb SA, Rutherford RB, and Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. (2003) 74:202–13. doi: 10.1902/jop.2003.74.2.202
35. Giannobile WV, Lee CS, Tomala MP, Tejeda KM, and Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. (2001) 72:815–23. doi: 10.1902/jop.2001.72.6.815
36. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. (2004) 364:149–55. doi: 10.1016/S0140-6736(04)16627-0
37. Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. (2004) 75:1281–7. doi: 10.1902/jop.2004.75.9.1281
38. Rasperini G, Pilipchuk SP, Flanagan CL, Park CH, Pagni G, Hollister SJ, et al. 3D-printed bioresorbable scaffold for periodontal repair. J Dent Res. (2015) 94:153S–7S. doi: 10.1177/0022034515588303
39. Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, Freire M, et al. Potential for stem cell-based periodontal therapy. J Cell Physiol. (2016) 231:50–61. doi: 10.1002/jcp.v231.1
40. Chen FM, Zhang J, Zhang M, An Y, Chen F, and Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. (2010) 31:7892–927. doi: 10.1016/j.biomaterials.2010.07.019
41. Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH, et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. (2019) 89:252–64. doi: 10.1016/j.actbio.2019.03.021
42. Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, et al. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. (2020) 109:182–94. doi: 10.1016/j.actbio.2020.04.017
43. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
44. Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, and Zhou QA. Exosomes─nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS Nano. (2022) 16:17802–46. doi: 10.1021/acsnano.2c08774
45. Mulcahy LA, Pink RC, and Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. (2014) 4:3. doi: 10.3402/jev.v3.24641
46. Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. (2015) 161:1046–57. doi: 10.1016/j.cell.2015.04.042
47. Wang J, Jing J, Zhou C, and Fan Y. Emerging roles of exosomes in oral diseases progression. Int J Sci. (2024) 16:4. doi: 10.1038/s41368-023-00274-9
48. Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, et al. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. (2020) 95:1287–307. doi: 10.1111/brv.12608
49. Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, et al. Exosomes-Loaded Electroconductive Hydrogel Synergistically Promotes Tissue Repair after Spinal Cord Injury via Immunoregulation and Enhancement of Myelinated Axon Growth. Adv Sci (Weinh). (2022) 9:e2105586. doi: 10.1002/advs.202105586
50. Lei F, Li M, Lin T, Zhou H, Wang F, and Su X. Treatment of inflammatory bone loss in periodontitis by stem cell-derived exosomes. Acta Biomater. (2022) 141:333–43. doi: 10.1016/j.actbio.2021.12.035
51. Nuñez J, Vignoletti F, Caffesse RG, and Sanz M. Cellular therapy in periodontal regeneration. Periodontology 2000. (2019) 79:107–16. doi: 10.1111/prd.12250
52. Miron RJ, Estrin NE, Sculean A, and Zhang Y. Understanding exososmes: Part 3-therapeutic + diagnostic potential in dentistry. Periodontol 2000. (2024) 94:415–82. doi: 10.1111/prd.12557
53. Miron RJ, Estrin NE, Sculean A, and Zhang Y. Understanding exosomes: Part 2-Emerging leaders in regenerative medicine. Periodontol 2000. (2024) 94(1):257–414. doi: 10.1111/prd.12561
54. Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, et al. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Delivery Rev. (2020) 159:332–43. doi: 10.1016/j.addr.2020.04.004
55. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, and Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. (2011) 29:341–5. doi: 10.1038/nbt.1807
56. Zeng H, Guo S, Ren X, Wu Z, Liu S, and Yao X. Current strategies for exosome cargo loading and targeting delivery. Cells. (2023) 12:1416. doi: 10.3390/cells12101416
57. Herrmann IK, Wood MJA, and Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. (2021) 16:748–59. doi: 10.1038/s41565-021-00931-2
58. Lu YT, Chen TY, Lin HH, Chen YW, Lin YX, Le DC, et al. Small extracellular vesicles engineered using click chemistry to express chimeric antigen receptors show enhanced efficacy in acute liver failure. J Extracell Vesicles. (2025) 14:e70044. doi: 10.1002/jev2.70044
59. Wang K, He Q, Yang M, Qiao Q, Chen J, Song J, et al. Glycoengineered extracellular vesicles released from antibacterial hydrogel facilitate diabetic wound healing by promoting angiogenesis. J Extracell Vesicles. (2024) 13:e70013. doi: 10.1002/jev2.v13.11
60. Madhu LN, Kodali M, Upadhya R, Rao S, Somayaji Y, Attaluri S, et al. Extracellular vesicles from human-induced pluripotent stem cell-derived neural stem cells alleviate proinflammatory cascades within disease-associated microglia in alzheimer’s disease. J Extracell Vesicles. (2024) 13:e12519. doi: 10.1002/jev2.v13.11
61. Searles SC, Chen WS, Yee JD, Lee P, Lee CK, Caron C, et al. MAP kinase kinase 1 (MEK1) within extracellular vesicles inhibits tumour growth by promoting anti-tumour immunity. J Extracell Vesicles. (2024) 13:e12515. doi: 10.1002/jev2.v13.10
62. Subha D, AnuKiruthika R, Sreeraj H, and Tamilselvi KS. Plant exosomes: nano conveyors of pathogen resistance. Discov Nano. (2023) 18:146. doi: 10.1186/s11671-023-03931-4
63. Peng X, Luo Y, Yang L, Yang YY, Yuan P, Chen X, et al. A multiantigenic antibacterial nanovaccine utilizing hybrid membrane vesicles for combating pseudomonas aeruginosa infections. J Extracell Vesicles. (2024) 13:e12524. doi: 10.1002/jev2.v13.10
64. Sundaram K, Miller DP, Kumar A, Teng Y, Sayed M, Mu J, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. (2019) 21:308–27. doi: 10.1016/j.isci.2019.10.032
65. Alshahrani MY, Jasim SA, Altalbawy FMA, Bansal P, Kaur H, Al-Hamdani MM, et al. A comprehensive insight into the immunomodulatory role of MSCs-derived exosomes (MSC-exos) through modulating pattern-recognition receptors (PRRs). Cell Biochem Funct. (2024) 42:e4029. doi: 10.1002/cbf.v42.4
66. Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, et al. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. (2020) 5:1113–26. doi: 10.1016/j.bioactmat.2020.07.002
67. Qiao X, Tang J, Dou L, Yang S, Sun Y, Mao H, et al. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenesis in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomed. (2023) 18:4683–703. doi: 10.2147/IJN.S420967
68. Yi Q, Xu Z, Thakur A, Zhang K, Liang Q, Liu Y, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. (2023) 190:106733. doi: 10.1016/j.phrs.2023.106733
69. Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, et al. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. (2020) 81:106030. doi: 10.1016/j.intimp.2019.106030
70. Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Sci. (2021) 13:43. doi: 10.1038/s41368-021-00150-4
71. Zheng Y, Dong C, Yang J, Jin Y, Zheng W, Zhou Q, et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. (2019) 234:20662–74. doi: 10.1002/jcp.v234.11
72. Li S, Guan X, Yu W, Zhao Z, Sun Y, and Bai Y. Effect of human periodontal ligament stem cell-derived exosomes on cementoblast activity. Dis. (2023) 30(4):2511–22. doi: 10.1111/odi.14671
73. Wang T, Zhou Y, Zhang W, Xue Y, Xiao Z, Zhou Y, et al. Exosomes and exosome composite scaffolds in periodontal tissue engineering. Front Bioeng Biotechnol. (2023) 11:1287714. doi: 10.3389/fbioe.2023.1287714
74. Deng D, Li X, Zhang JJ, Yin Y, Tian Y, Gan D, et al. Biotin-avidin system-based delivery enhances the therapeutic performance of MSC-derived exosomes. ACS Nano. (2023) 17:8530–50. doi: 10.1021/acsnano.3c00839
75. Huang X, Deng Y, Xiao J, Wang H, Yang Q, and Cao Z. Genetically engineered M2-like macrophage-derived exosomes for P. gingivalis-suppressed cementum regeneration: From mechanism to therapy. Bioact Mater. (2024) 32:473–87. doi: 10.1016/j.bioactmat.2023.10.009
76. Zhao Y, Huang Y, Liu H, Tan K, Wang R, Jia L, et al. Macrophages with different polarization phenotypes influence cementoblast mineralization through exosomes. Stem Cells Int. (2022) 2022:4185972. doi: 10.1155/2022/4185972
77. Fu H, Sen L, Zhang F, Liu S, Wang M, Mi H, et al. Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway. J Nanobiotechnology. (2023) 21:466. doi: 10.1186/s12951-023-02214-5
78. Cui Y, Hong S, Xia Y, Li X, He X, Hu X, et al. Melatonin engineering M2 macrophage-derived exosomes mediate endoplasmic reticulum stress and immune reprogramming for periodontitis therapy. Adv Sci (Weinh). (2023) 10:e2302029. doi: 10.1002/advs.202302029
79. Sun J, Wang Z, Liu P, Hu Y, Li T, Yang J, et al. Exosomes derived from human gingival mesenchymal stem cells attenuate the inflammatory response in periodontal ligament stem cells. Front Chem. (2022) 10:863364. doi: 10.3389/fchem.2022.863364
80. Peng B, Wang L, Han G, and Cheng Y. Mesenchymal stem cell-derived exosomes: a potential cell-free therapy for orthodontic tooth stability management. Stem Cell Res Ther. (2024) 15:342. doi: 10.1186/s13287-024-03962-3
81. Gao J and Wu Z. M2 macrophage-derived exosomes enable osteogenic differentiation and inhibit inflammation in human periodontal ligament stem cells through promotion of CXCL12 expression. BMC Health. (2024) 24:1070. doi: 10.1186/s12903-024-04831-4
82. Lu J, Yu N, Liu Q, Xie Y, and Zhen L. Periodontal ligament stem cell exosomes key to regulate periodontal regeneration by miR-31-5p in mice model. Int J Nanomedicine. (2023) 18:5327–42. doi: 10.2147/IJN.S409664
83. Skelton AM, Cohen DJ, Boyan BD, and Schwartz Z. Osteoblast-derived matrix vesicles exhibit exosomal traits and a unique subset of microRNA: their caveolae-dependent endocytosis results in reduced osteogenic differentiation. Int J Mol Sci. (2023) 24:12770. doi: 10.3390/ijms241612770
84. Chen X, Wan Z, Yang L, Song S, Fu Z, Tang K, et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnology. (2022) 20:110. doi: 10.1186/s12951-022-01314-y
85. Wu J, Chen L, Wang R, Song Z, Shen Z, Zhao Y, et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. (2019) 5:3561–71. doi: 10.1021/acsbiomaterials.9b00607
86. He L, Zhang H, Zhao N, and Liao L. A novel approach in biomedical engineering: The use of polyvinyl alcohol hydrogel encapsulating human umbilical cord mesenchymal stem cell-derived exosomes for enhanced osteogenic differentiation and angiogenesis in bone regeneration. Int J Biol Macromol. (2024) 270:132116. doi: 10.1016/j.ijbiomac.2024.132116
87. Zhu Q, Tang Y, Zhou T, Yang L, Zhang G, Meng Y, et al. Exosomes derived from mesenchymal stromal cells promote bone regeneration by delivering miR-182-5p-inhibitor. Pharmacol Res. (2023) 192:106798. doi: 10.1016/j.phrs.2023.106798
88. Pu P, Wu S, Zhang K, Xu H, Guan J, Jin Z, et al. Mechanical force induces macrophage-derived exosomal UCHL3 promoting bone marrow mesenchymal stem cell osteogenesis by targeting SMAD1. J Nanobiotechnology. (2023) 21:88. doi: 10.1186/s12951-023-01836-z
89. Shimizu Y, Takeda-Kawaguchi T, Kuroda I, Hotta Y, Kawasaki H, Hariyama T, et al. Exosomes from dental pulp cells attenuate bone loss in mouse experimental periodontitis. J Periodontal Res. (2022) 57:162–72. doi: 10.1111/jre.12949
90. Hollý D, Klein M, Mazreku M, Zamborský R, Polák Š, Danišovič Ľ, et al. Stem cells and their derivatives-implications for alveolar bone regeneration: A comprehensive review. Int J Mol Sci. (2021) 22:11746. doi: 10.3390/ijms222111746
91. Trentini M, D’Amora U, Ronca A, Lovatti L, Calvo-Guirado JL, Licastro D, et al. Bone regeneration revolution: pulsed electromagnetic field modulates macrophage-derived exosomes to attenuate osteoclastogenesis. Int J Nanomedicine. (2024) 19:8695–707. doi: 10.2147/IJN.S470901
92. Shi W, Guo S, Liu L, Liu Q, Huo F, Ding Y, et al. Small extracellular vesicles from lipopolysaccharide-preconditioned dental follicle cells promote periodontal regeneration in an inflammatory microenvironment. ACS Biomater Sci Eng. (2020) 6:5797–810. doi: 10.1021/acsbiomaterials.0c00882
93. Huang X, Lan Y, Shen J, Chen Z, and Xie Z. Extracellular vesicles in bone homeostasis: emerging mediators of osteoimmune interactions and promising therapeutic targets. Int J Biol Sci. (2022) 18:4088–100. doi: 10.7150/ijbs.69816
94. Zhu F, Wang T, Wang G, Yan C, He B, and Qiao B. The exosome-mediated bone regeneration: an advanced horizon toward the isolation, engineering, carrying modalities, and mechanisms. Adv Healthc Mater. (2024) 13:e2400293. doi: 10.1002/adhm.202400293
95. Jiao Y, Liu Y, Du J, Xu J, Luo Z, Liu Y, et al. Advances in the study of extracellular vesicles for bone regeneration. Int J Mol Sci. (2024) 25:3480. doi: 10.3390/ijms25063480
96. Daneshvar A, Nemati P, Azadi A, Amid R, and Kadkhodazadeh M. M2 macrophage-derived exosomes for bone regeneration: a systematic review. Arch Biol. (2024) 166:106034. doi: 10.1016/j.archoralbio.2024.106034
97. Olaechea A, Benabdellah K, Vergara-Buenaventura A, Gómez-Melero S, Cafferata EA, Meza-Mauricio J, et al. Preclinical evidence for the use of oral mesenchymal stem cell-derived extracellular vesicles in bone regenerative therapy: a systematic review. Stem Cells Transl Med. (2023) 12:791–800. doi: 10.1093/stcltm/szad059
98. Zhou H, Qi YX, Zhu CH, Li A, and Pei DD. Mesenchymal stem cell-derived extracellular vesicles for treatment of bone loss within periodontitis in pre-clinical animal models: a meta-analysis. BMC Health. (2023) 23:701. doi: 10.1186/s12903-023-03398-w
99. Iwayama T, Bhongsatiern P, Takedachi M, and Murakami S. Matrix vesicle-mediated mineralization and potential applications. J Dent Res. (2022) 101:1554–62. doi: 10.1177/00220345221103145
100. Huang CC, Kang M, Shirazi S, Lu Y, Cooper LF, Gajendrareddy P, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. (2021) 126:199–210. doi: 10.1016/j.actbio.2021.03.030
101. Sun X, Mao Y, Liu B, Gu K, Liu H, Du W, et al. Mesenchymal stem cell-derived exosomes enhance 3D-printed scaffold functions and promote alveolar bone defect repair by enhancing angiogenesis. J Pers Med. (2023) 13:180. doi: 10.3390/jpm13020180
102. Li B, Liang A, Zhou Y, Huang Y, Liao C, Zhang X, et al. Hypoxia preconditioned DPSC-derived exosomes regulate angiogenesis via transferring LOXL2. Exp Cell Res. (2023) 425:113543. doi: 10.1016/j.yexcr.2023.113543
103. Qin Y, Sun R, Wu C, Wang L, and Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci. (2016) 17:712. doi: 10.3390/ijms17050712
104. Roerig J and Schulz-Siegmund M. Standardization approaches for extracellular vesicle loading with oligonucleotides and biologics. Small (weinh Bergstr Ger). (2023) 19:e2301763. doi: 10.1002/smll.202301763
105. Deng S, Cao H, Cui X, Fan Y, Wang Q, and Zhang X. Optimization of exosome-based cell-free strategies to enhance endogenous cell functions in tissue regeneration. Acta Biomater. (2023) 171:68–84. doi: 10.1016/j.actbio.2023.09.023
106. Wang H, Zhang X, Zhang W, and Sun H. The effects of exosomes originating from different cell sources on the differentiation of bone marrow mesenchymal stem cells into schwann cells. J Nanobiotechnology. (2024) 22:220. doi: 10.1186/s12951-024-02604-3
107. Li X, Fang S, Wang S, Xie Y, Xia Y, Wang P, et al. Hypoxia preconditioning of adipose stem cell-derived exosomes loaded in gelatin methacryloyl (GelMA) promote type H angiogenesis and osteoporotic fracture repair. J Nanobiotechnology. (2024) 22:112. doi: 10.1186/s12951-024-02342-6
108. Zhao Y, Gong Y, Liu X, He J, Zheng B, and Liu Y. The experimental study of periodontal ligament stem cells derived exosomes with hydrogel accelerating bone regeneration on alveolar bone defect. Pharmaceutics. (2022) 14:2189. doi: 10.3390/pharmaceutics14102189
109. Hu Y, Wang Z, Fan C, Gao P, Wang W, Xie Y, et al. Human gingival mesenchymal stem cell-derived exosomes cross-regulate the Wnt/β-catenin and NF-κB signalling pathways in the periodontal inflammation microenvironment. J Clin Periodontol. (2023) 50:796–806. doi: 10.1111/jcpe.13798
110. Fallah A, Hosseinzadeh Colagar A, Khosravi A, Mohammad-Hasani A, and Saeidi M. The role of natural exosomes from SHED-MSC in immunoregulation of M0/M1 polarized macrophage cells. Front Immunol. (2025) 16:1550280. doi: 10.3389/fimmu.2025.1550280
111. Nie Y, Meng W, Liu D, Yang Z, Wang W, Ren H, et al. Exosomes derived from apical papilla stem cells improve NASH by regulating fatty acid metabolism and reducing inflammation. Mol Med (camb Mass). (2024) 30:186. doi: 10.1186/s10020-024-00945-1
112. Elashiry M, Elashiry MM, Elsayed R, Rajendran M, Auersvald C, Zeitoun R, et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracell Vesicles. (2020) 9:1795362. doi: 10.1080/20013078.2020.1795362
113. Xie Q, Gu J, Sun Y, Hong J, Wang J, Li N, et al. Therapeutic potential of ginger exosome-like nanoparticles for alleviating periodontitis-induced tissue damage. Int J Nanomed. (2024) 19:11941–56. doi: 10.2147/IJN.S483091
114. Gomez N, James V, Onion D, and Fairclough LC. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): a systematic review. Respir Res. (2022) 23:82. doi: 10.1186/s12931-022-01984-0
115. Liu M, Chen R, Xu Y, Zheng J, Wang M, and Wang P. Exosomal miR-141-3p from PDLSCs alleviates high glucose-induced senescence of PDLSCs by activating the KEAP1-NRF2 signaling pathway. Stem Cells Int. (2023) 2023:7136819. doi: 10.1155/2023/7136819
116. Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12404
117. Kang M, Huang CC, Lu Y, Shirazi S, Gajendrareddy P, Ravindran S, et al. Bone regeneration is mediated by macrophage extracellular vesicles. Bone. (2020) 141:115627. doi: 10.1016/j.bone.2020.115627
118. Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T, Kou X, et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. (2021) 122:306–24. doi: 10.1016/j.actbio.2020.12.046
119. Li J, Guo Y, Chen YY, Liu Q, Chen Y, Tan L, et al. miR-124-3p increases in high glucose induced osteocyte-derived exosomes and regulates galectin-3 expression: A possible mechanism in bone remodeling alteration in diabetic periodontitis. FASEB J. (2020) 34:14234–49. doi: 10.1096/fj.202000970RR
120. Liu P, Qin L, Liu C, Mi J, Zhang Q, Wang S, et al. Exosomes Derived From Hypoxia-Conditioned Stem Cells of Human Deciduous Exfoliated Teeth Enhance Angiogenesis via the Transfer of let-7f-5p and miR-210-3p. Front Cell Dev Biol. (2022) 10:879877. doi: 10.3389/fcell.2022.879877
121. Wu Y, Qu F, Zhang Y, Song Y, Zhong Q, Huang Y, et al. Exosomes from Cyclic Stretched Periodontal Ligament Cells Induced Periodontal Inflammation through miR-9-5p/SIRT1/NF-κB Signaling Pathway. J Immunol. (2023) 210:2001–15. doi: 10.4049/jimmunol.2300074
122. Novello S, Pellen-Mussi P, and Jeanne S. Mesenchymal stem cell-derived small extracellular vesicles as cell-free therapy: perspectives in periodontal regeneration. J Periodontal Res. (2021) 56:433–42. doi: 10.1111/jre.12866
123. Sandira MI, Lim K, Yoshida T, Sajidah ES, Narimatsu S, Imakawa R, et al. Nanoscopic profiling of small extracellular vesicles via high-speed atomic force microscopy (HS-AFM) videography. J Extracell Vesicles. (2025) 14:e270050. doi: 10.1002/jev2.70050
124. Xu XY, Tian BM, Xia Y, Xia YL, Li X, Zhou H, et al. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med. (2020) 9:1414–30. doi: 10.1002/sctm.19-0418
125. Rädler J, Gupta D, Zickler A, and Andaloussi SE. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther: J Am Soc Gene Ther. (2023) 31:1231–50. doi: 10.1016/j.ymthe.2023.02.013
126. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, et al. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. (2019) 86:1–14. doi: 10.1016/j.actbio.2018.12.045
127. Luo H, Chen D, Li R, Li R, Teng Y, Cao Y, et al. Genetically engineered CXCR4-modified exosomes for delivery of miR-126 mimics to macrophages alleviate periodontitis. J Nanobiotechnology. (2023) 21:116. doi: 10.1186/s12951-023-01863-w
128. Wang J, Wang M, Jiang N, Ding S, Peng Q, and Zheng L. Emerging chemical engineering of exosomes as “bioscaffolds” in diagnostics and therapeutics. Genes Dis. (2023) 10:1494–512. doi: 10.1016/j.gendis.2022.10.020
129. Lee C, Kumar S, Park J, Choi Y, Clarissa EM, and Cho YK. Tonicity-induced cargo loading into extracellular vesicles. Lab Chip. (2024) 24:2069–79. doi: 10.1039/d3lc00830d
130. Tavelli L, Barootchi S, Rasperini G, and Giannobile WV. Clinical and patient-reported outcomes of tissue engineering strategies for periodontal and peri-implant reconstruction. Periodontol 2000. (2023) 91:217–69. doi: 10.1111/prd.12446
131. Huang CC, Kang M, Shirazi S, Lu Y, Cooper LF, Gajendrareddy P, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. (2021) 126:199–210. doi: 10.1016/j.actbio.2021.03.030
132. Aytac Z, Dubey N, Daghrery A, Ferreira JA, de Souza Araújo IJ, Castilho M, et al. Innovations in craniofacial bone and periodontal tissue engineering - from electrospinning to converged biofabrication. Int Mater Rev. (2022) 67:347–84. doi: 10.1080/09506608.2021.1946236
133. Ma Y, Brocchini S, and Williams GR. Extracellular vesicle-embedded materials. J Control Release: Off J Control Release Soc. (2023) :361:280–96. doi: 10.1016/j.jconrel.2023.07.059
134. Leung KS, Shirazi S, Cooper LF, and Ravindran S. Biomaterials and extracellular vesicle delivery: current status, applications and challenges. Cells. (2022) 11:2851. doi: 10.3390/cells11182851
135. Hu W, Wang W, Chen Z, Chen Y, and Wang Z. Engineered exosomes and composite biomaterials for tissue regeneration. Theranostics. (2024) 14:2099–126. doi: 10.7150/thno.93088
136. Kang M, Jordan V, Blenkiron C, and Chamley LW. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J Extracell Vesicles. (2021) 10:e12085. doi: 10.1002/jev2.12085
137. Li G, Chen T, Dahlman J, Eniola-Adefeso L, Ghiran IC, Kurre P, et al. Current challenges and future directions for engineering extracellular vesicles for heart, lung, blood and sleep diseases. J Extracell Vesicles. (2023) 12:e12305. doi: 10.1002/jev2.12305
138. Phan J, Kumar P, Hao D, Gao K, Farmer D, and Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. (2018) 7:1522236. doi: 10.1080/20013078.2018.1522236
139. Mu N, Li J, Zeng L, You J, Li R, Qin A, et al. Plant-derived exosome-like nanovesicles: current progress and prospects. Int J Nanomed. (2023) 18:4987–5009. doi: 10.2147/IJN.S420748
140. Zhou L, Cai W, Zhang Y, Zhong W, He P, Ren J, et al. Therapeutic effect of mesenchymal stem cell-derived exosome therapy for periodontal regeneration: a systematic review and meta-analysis of preclinical trials. J Orthop Surg Res. (2025) 20:27. doi: 10.1186/s13018-024-05403-6
141. Kim HI, Park J, Zhu Y, Wang X, Han Y, and Zhang D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med. (2024) 56:836–49. doi: 10.1038/s12276-024-01201-6
142. Mizenko RR, Feaver M, Bozkurt BT, Lowe N, Nguyen B, Huang KW, et al. A critical systematic review of extracellular vesicle clinical trials. J Extracell Vesicles. (2024) 13:e12510. doi: 10.1002/jev2.v13.10
143. Fusco C, De Rosa G, Spatocco I, Vitiello E, Procaccini C, Frigè C, et al. Extracellular vesicles as human therapeutics: a scoping review of the literature. J Extracell Vesicles. (2024) 13:e12433. doi: 10.1002/jev2.12433
144. Van Delen M, Derdelinckx J, Wouters K, Nelissen I, and Cools N. A systematic review and meta-analysis of clinical trials assessing safety and efficacy of human extracellular vesicle-based therapy. J Extracell Vesicles. (2024) 13:e12458. doi: 10.1002/jev2.12458
145. Das S, Lyon CJ, and Hu T. A panorama of extracellular vesicle applications: from biomarker detection to therapeutics. ACS Nano. (2024) 18:9784–97. doi: 10.1021/acsnano.4c00666
146. Bader J, Brigger F, and Leroux JC. Extracellular vesicles versus lipid nanoparticles for the delivery of nucleic acids. Adv Drug Delivery Rev. (2024) 215:115461. doi: 10.1016/j.addr.2024.115461
Keywords: extracellular vesicles, periodontitis, periodontitis treatment, periodontal regeneration, extracellular vesicle-based strategies
Citation: Zhang X, Gao H and Lin L (2025) The extracellular vesicle-based treatment: a developing strategy for periodontal diseases. Front. Immunol. 16:1480292. doi: 10.3389/fimmu.2025.1480292
Received: 13 August 2024; Accepted: 15 April 2025;
Published: 29 May 2025.
Edited by:
Zhenjia Wang, University at Buffalo, United StatesReviewed by:
Wan Nazatul Shima Shahidan, Universiti Sains Malaysia Health Campus, MalaysiaLei Hu, Capital Medical University, China
Copyright © 2025 Zhang, Gao and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Lin, bGlubGlAY211LmVkdS5jbg==
 Xuan Zhang
Xuan Zhang Li Lin
Li Lin