- 1Murdoch Children’s Research Institute and Department of Pediatrics, The University of Melbourne, Parkville, VIC, Australia
- 2Radboud Institute for Molecular Life Sciences RIMLS, Radboud University Medical Center, Nijmegen, Netherlands
- 3Department of Adolescent Medicine, Royal Children’s Hospital, Parkville, VIC, Australia
- 4Department of Medicine Austin Health, University of Melbourne, Heidelberg, VIC, Australia
- 5Department of Endocrinology, Austin Health, Melbourne, VIC, Australia
Sex differences in immune system development and response to pathogens has been well documented, with females exhibiting more favorable outcomes for certain infections but a higher incidence of autoimmune disease compared to males. At least some of these sex differences are mediated by sex hormones, which signal through sex hormone receptors to remodel the regulatory chromatin landscape of cells. Here, we summarize the current knowledge of how sex hormone receptors remodel chromatin structure and epigenetic marks in different contexts in humans. As the epigenome is fundamental to specifying cell identity and function, and reflects past exposures, epigenetic variation can influence cellular responses to future stimuli. This has implications for susceptibility to infection and complex inflammatory disease in a range of hormone therapy settings, including gender-affirming hormone therapy in transgender people. Therefore, profiling of epigenetic marks in the context of gender-affirming hormone therapy is an important unexplored field of research.
Introduction
Sexual dimorphism describes differences between males and females across various factors, including but not limited to behavior and immunity (1). Sex hormones are one contributor to this dimorphism, where males have higher testosterone and lower estrogen levels, while females have higher estrogen and lower testosterone levels, with age being a major factor in this ratio (2). Sex hormone signaling via sex hormone receptors is a major transcription pathway that influences cellular function, namely cytokine production, cell proliferation, and reactivity (3).
Sex differences in immune function between males and females are also influenced by genetics, with several key genes involved in immunity expressed on the X chromosome. This includes receptors such as TLR7, TLR8, and ACE2 (4, 5), as well as FOXP3, which controls regulatory T cell production (6). Further, the X-chromosome encodes 10% of all miRNAs, including miRNA-18 and 19 which are associated with sex-biased immune response (7, 8).
Functional programming of innate and adaptive immune cells depends on epigenetic remodeling, which alters the regulatory landscape of the genome and controls gene expression (9, 10). These changes influence immune cell identity and can predict how the cell will respond to exogenous stimuli (11). For example, T-cell exhaustion has been linked to epigenetic reprogramming that leads to changes in differentiation trajectory (12, 13). Autoimmune disease is associated with altered cytokine production and immune cell reactivity due to epigenetic changes that are caused by environmental influences, genetic variants, and medication (14). Additionally, certain infections, including SARS-CoV-2 and malaria, as well as immunizations, such as influenza and BCG vaccines, induce changes in the epigenomes of hematopoietic stem cells and monocytes (15–18).
This mini review will summarize recent data on sex hormone receptor-mediated epigenetic remodeling and immune function modulation, with implications for understanding immune function changes in transgender individuals following gender affirming hormone therapy (GAHT). Although not covered in this mini-review, the sex hormone progesterone also plays a vital role in immunity, particularly the promotion of maternal-fetal tolerance during pregnancy, through expanding regulatory T cells and regulation of reactivity of other immune cells (19).
Sex hormone receptor signaling
Sex hormones influence various physiological systems including neurological, reproductive, musculoskeletal, and immune systems (20, 21). Testosterone, which is a type of androgen, is associated with masculinizing effects, spermatogenesis, and is a modulator of immune response (7, 22). Estrogen is dominantly expressed in females, is associated with feminizing effects, and is a driver of several diseases such as cancer, and a promoter of immune function (7, 23).
Estrogen receptor
Estrogen receptors (ERs) -α and -ß are nuclear receptors encoded by the ESR1 and ESR2 genes, respectively (24). These receptors are transcriptional regulators that can activate or repress specific genes upon binding of a ligand, leading to changes in chromatin interactions (25, 26). Genomic signaling leads to a conformational change followed by the induction of receptor dimerization wherein the binding site affinity, and specificity of the receptor increase wherein the signaling ultimately influences the change of the gene expression profile (27). In contrast, non-genomic signaling can trigger multiple pathways, such as protein-kinase activation or phosphorylation of transcription factors and activate nuclear ERs to bind to the DNA (28, 29). In addition to binding regulatory elements in DNA, ER-α can also induce posttranslational modifications of proteins upon binding of specific ligands (30).
Androgen receptor
Androgen receptor (AR) is a nuclear receptor that is encoded by the AR gene on the X chromosome (31). Genomic AR signaling mediates transcriptional activity whereby the androgen-AR complex translocates to the nucleus, dimerizes, and binds to androgen-responsive elements (22) to enhance or repress nearby genes (32). Genomic signaling by AR is influenced by coregulators, which can enhance or inhibit transcriptional activity by facilitating chromatin remodeling and histone modifications (33). The non-genomic AR signaling pathway activates intracellular kinase cascades that benefit cell proliferation and survival through targeting plasma membrane proteins or receptors and can also activate phosphorylation pathways (34).
Epigenetics
Epigenetics literally means ‘above DNA’ and refers to the study of molecular interactions that influence DNA structure, compaction, and function (35). Epigenetic marks can be ‘written’, ‘erased’, and ‘read’ by specific nuclear proteins to regulate gene expression in a range of physiological processes (36, 37). Three major epigenetic modifications are (i) DNA methylation, where a methyl (-CH3) group is added to the cytosine nucleotide within a cytosine-guanine sequence (CpG dinucleotide) (38–40); (ii) histone post-translational modifications, such as acetylation and methylation (41); and (iii) non-coding RNAs, which are transcribed RNAs that are not translated into proteins, but can regulate gene expression through mediation of chromatin structure (42).
Most of the genome (over 95%) does not code for proteins. Much of this noncoding landscape plays an important role in regulating the transcription of coding genes (43). Indeed, hormone receptors such as ESR1 bind large swaths of the noncoding genome, influencing the activity of coding genes (44).
Epigenetic remodeling by estrogen receptors
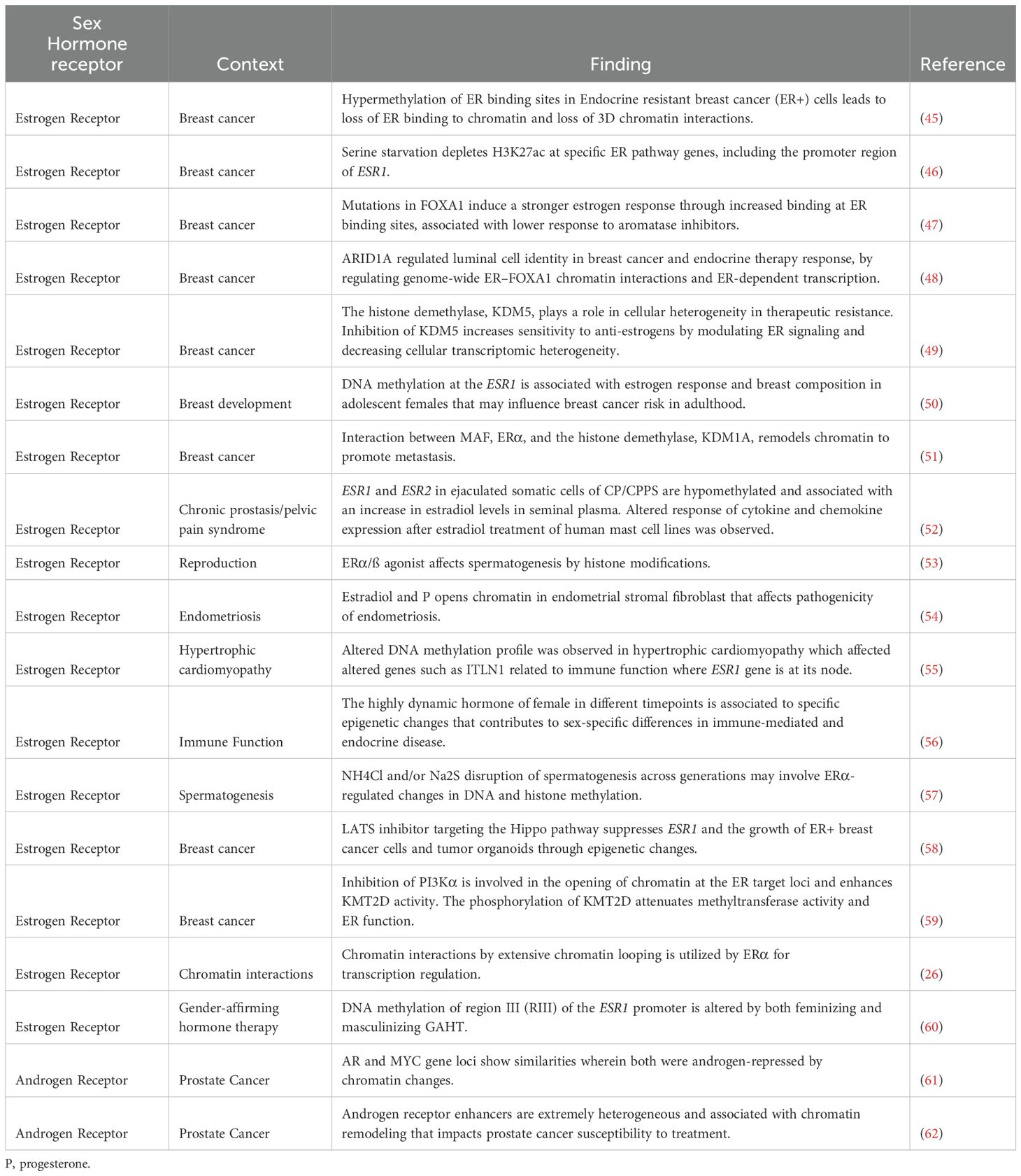
Changes in gene expression due to epigenetic remodeling via sex hormone receptors is observed in various diseases and physiological processes (Table 1). Both ER -α and -ß are expressed in more than 70% of all breast cancers, with estrogen signaling being a driver of carcinogenesis (63). Therefore, much of what we know about how the ER remodels chromatin is based on cancer studies (Table 1). These studies illustrate that sex hormone receptors can alter the 3D chromatin landscape, DNA methylation and histone post-translational modifications, by interacting with different chromatin modifiers (Table 1).
Epigenetic remodeling by ERs also plays a role in non-cancer settings, such as breast development, where the DNA methylation signature of adolescent girls is dependent on estrogen response and breast composition, which may influence breast cancer risk in adulthood (50). Hormonal changes in females and males during puberty were found to influence DNA methylation near predicted estrogen-responsive genes (56). In addition, disruption of DNA methylation at the ESR1 gene locus by endocrine-disrupting chemicals has been associated with decreased male fertility due to the decline of sperm quality (53, 57).
Epigenetic remodeling by androgen receptor
AR can also remodel chromatin, but these effects are much less studied than for ER. Most studies looked at the role of AR in prostate cancer, where the AR drives epigenetic heterogeneity at enhancers through AR binding sites, affecting response to therapy (62). There is a significant knowledge gap in our understanding of how these AR-mediated changes within the epigenome shape cellular function in other tissues and outside the prostate.
Sex differences in immunology
Sex differences are observed in immune responses to infections, with females generally exhibiting hyper reactivity than males (7). This is particularly evident in influenza infections and following influenza vaccination, where females produce higher neutralizing antibodies and inflammatory cytokines (64–66). Post-influenza vaccination, females show elevated levels of inflammatory markers such as leptin, or interleukin-receptor agonist (IL-1RA) (64, 67). Higher circulating estradiol concentrations in females reduce other proinflammatory cytokines like tumor necrosis factor (TNF)-α and chemokine ligand (CCL)-2, primarily mediated via the ERα, thereby lowering influenza-related morbidity and mortality (68). Conversely, males, influenced by the immunosuppressive effects of testosterone, generally exhibit weaker responses to influenza vaccination, with lower antibody production, especially in those with high serum testosterone concentrations (64, 66, 69). These sex differences extend to COVID-19, where males typically experience more severe outcomes, partly due to higher transmembrane protease, serine 2 or TMPRSS2 expression facilitating viral entry (70). Conversely, the effects of estradiol on angiotensin-converting enzyme 2 and angiotensin II receptor type 1 signaling in females reduce the severity of COVID-19 infection (71, 72). Males with long COVID cognitive symptoms show higher levels of the neuroinflammation-linked chemokine CCL11 compared to females (73), suggesting increased susceptibility to certain post-COVID neuroinflammatory effects. Together, these findings highlight the complex relationship between sex hormones and immune responses in viral infections.
In autoimmune disease, sexual dimorphism leads to stronger, estrogen-driven immune responses in females, increasing their overall susceptibility (74, 75). For example, in juvenile idiopathic arthritis (JIA), females are more susceptible to chronic inflammation with three to six females for every male patient are affected due to increased activation of immature neutrophil-related genes, leading to enhanced neutrophil activation that may impact treatment effectiveness, including responses to Interleukin (IL)-1 receptor antagonists (76). Similarly, females are more prone to developing multiple sclerosis (MS), with a 3:1 ratio compared to males, partly due to estrogen-enhanced IL-17 expression, which drives autoimmune pathogenesis by boosting pro-inflammatory cytokine production and T cell activity (75, 77, 78). Females also experience more severe skin inflammation, particularly in psoriasis, due to estrogen amplifying the inflammatory response by increasing cytokine production and immune cell activity (79). While genetic differences between males and females are a factor in this dimorphism, most differences are attributable to sex hormones receptors as well (80).
Sex hormones and immunity
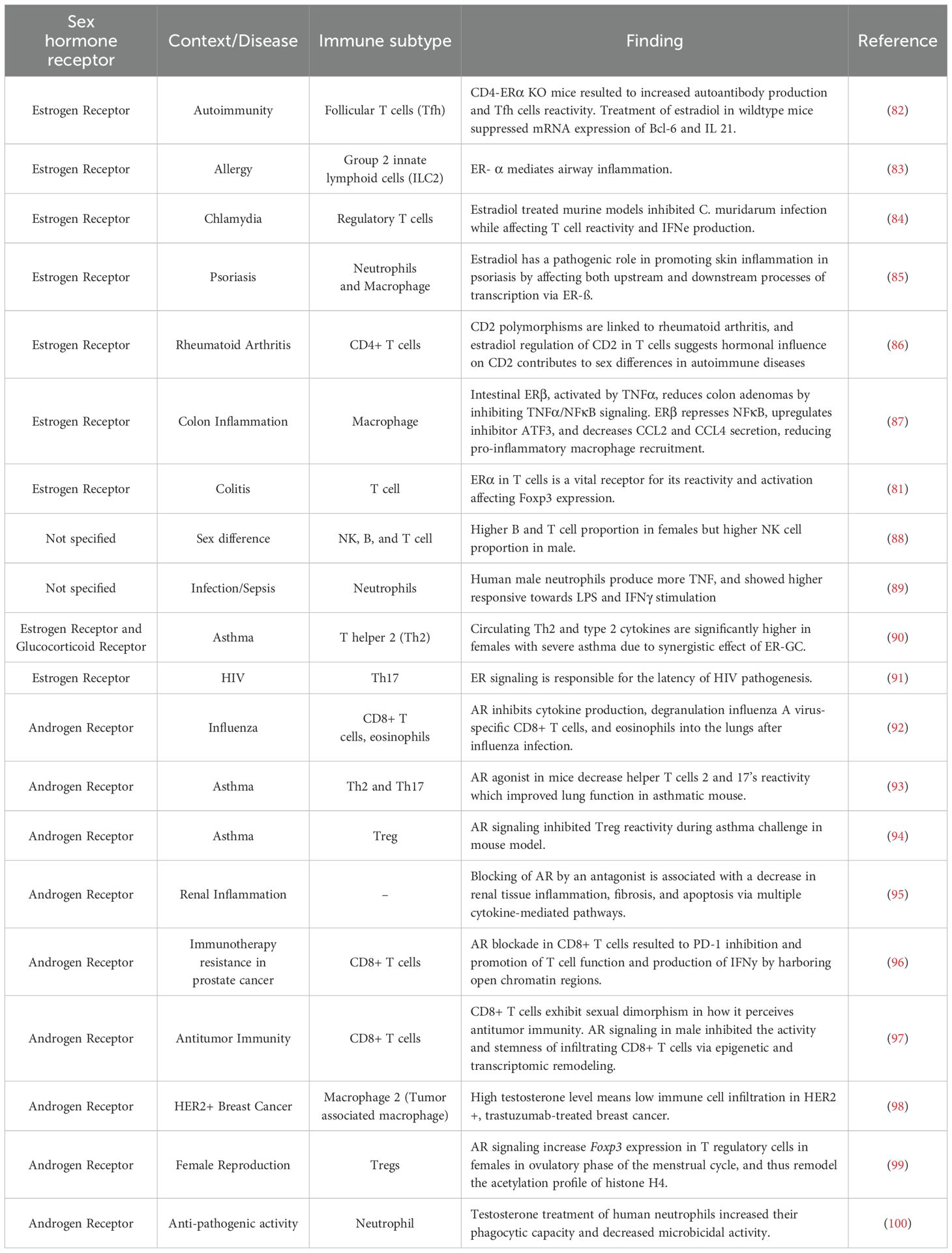
Sex hormone receptor signaling in immune cells influences various functions such as cell proliferation, reactivity, and overall function (81). Table 2 summarizes studies on how sex hormone signaling affects immune cells.
Estrogen receptor and immunity
CD4-ERα knockout (KO) female mouse model showed a mild autoimmune phenotype with increased autoantibody and follicular helper T cells (TFH) production (82), while polymorphisms in ER binding site affect rheumatoid arthritis by introducing a sex bias Cd2 expression to regulate T cell activation (86). Skin inflammation also depends on endogenous estradiol in mice where a psoriasis ER mouse KO increased IL-17A and IL-1ß production (85). In the context of infection, response to Chlamydia muridarum and hepatitis B virus (HBV) in mouse models were both influenced by polymorphisms in ESR1 (84, 101). The role of ERs in inflammation in the colon, liver, and airway is also evident in mouse studies where TNFα activates intestinal Er-β, while a reduction in ERα increases NF-kB activity through the liver receptor homolog (LRH-1) (87, 102). Additionally, ERα plays a role in allergy, elevating IL-33 release and ILC2-mediated airway inflammation upon allergen challenge (83).
In human studies, sex-based differences in immune cell composition are also observed where males have higher proportion of nature killer (NK) cells subsets, while females exhibit greater abundance of B cell subset (88). Male neutrophils also show higher TNF expression after lipopolysaccharide (LPS) stimulation, which is attributed to increased TLR4 expression in males which may influence sepsis response (89). In females with severe asthma, dual activation of ERα and glucocorticoid receptor synergistically enhances the production of circulating T helper (Th2) cells and type 2 cytokines (90). Finally, ESR1 has been identified to be an integral regulator in HIV-1 infection with females displaying lower inducible HIV-1 RNA reservoirs compared to males (91).
Androgen receptor and immunity
AR signaling has been reported to exert inhibitory actions in a number of immune responses (103). For example, in mouse models of influenza, testosterone inhibits influenza A virus (IAV) pathogenesis by systematically modulating CD8+ T cell reactivity (92). Similarly, in a mouse model of asthma, treatment with an allergen, Alterneria extract, decreases helper T cell reactivity, while the suppressive function of Tregs is promoted (93, 94). AR signaling also plays an important role in the inflammatory response in the kidney and liver. Treatment of male rats with flutamide, an AR antagonist, has been reported to systematically downregulate cytokines in renal fibrosis, while an increased AR expression was observed in mice during severe infection with HBV (95, 104). Sex-specific differences in anti-tumor immune responses in mice have been attributed, at least in part, to AR-mediated epigenetic remodeling of CD8+ T cells leading to lower reactivity and stemness in the tumor environment (97).
In human studies, testosterone reduces oxidative stress in neutrophils, increasing their phagocytic capacity while decreasing their microbicidal activity (100). AR signaling also increased Foxp3 expression in regulatory T cells of females during the ovulatory phase of the menstrual cycle, leading to changes in the acetylation profile of histone H4 (99). The importance of AR signaling is further highlighted by a study that showed that inhibition of AR activity in CD8+ T cells prevented T cell exhaustion and improved responsiveness to PD-1 targeted therapy (96). Finally, AR expression inversely correlated with the production of M2 tumor-associated macrophage, CD3+, and CD8+ T cell infiltration in trastuzumab-treated HER 2-positive breast cancer patients, suggesting a stronger role of AR in immune cells within cancer metastasis and proliferation (98).
This section mainly reviewed previous animal studies due to the scarcity of information on how sex hormone signaling affects functional human immune response in various contexts such as autoimmunity and other diseases. Several previous reviews have elucidated the role of sex hormone signaling in immune cells (7, 103, 105) but it is still unclear on how this signaling pathway affects immunity and mechanistically affects diseases associated with it. Overall, these studies highlight that both the ER and AR regulate a wide range of immune cell functions, explaining the sexual dimorphism in inflammation, cancer response, and disease susceptibility.
Role of epigenetic landscape in immune sex-differences
Epigenetic remodeling at promoters and distal regulatory elements is a key mechanism by which transcription factors establish immune lineages from hematopoietic stem cells (106). Further, exogenous stimuli such as microbial compounds and certain vaccines can induce new regulatory elements in differentiated immune cells, such as monocytes, through changes in histone modifications (107), or DNA methylation (108). Likewise, the tissue microenvironment shapes the epigenetic landscape in tissue resident macrophages (109) and T cells (110). As noted previously, several regulators of immune cell identity and function are located on the X chromosome. For example, in vitro-activated B cell subsets from adult and pediatric systemic lupus erythematosus (SLE) patients were found to exhibit disrupted X-chromosome inactivation (XCI), which is speculated to result from an increase in inflammatory cytokines and type I interferons (111). Further, UTX, a master epigenetic regulator that escapes XCI in both human and mouse models was found to control chromatin accessibility and gene expression patterns in NK cells (112). Therefore, sex-differences in immune cell phenotype and function can arise through epigenetic errors in X chromosome inactivation or epigenetic remodeling by sex hormone receptors.
Implications for gender affirming hormone therapy
At least 1.5 million people in the United States are transgender (~0.5%), of whom 90% have considered or are undergoing GAHT (113), with transgender women having a disproportionately higher rate of HIV infection (114). A cross-sectional study comparing transgender women and men receiving GAHT to cis-men and women, revealed that GAHT influences both the proportions in the circulation and the transcriptome of regulatory T (Treg) cells (115). Interestingly, sex hormones and chromosomes may collectively influence some cell proportions. Peckham et al. found that CD19+ CD27+ IgD- classical-switched memory B cells were sensitive to estrogen only in the XX karyotype, decreasing in transgender women following GAHT, but in post-menopausal cis-women following HRT, but not influenced by estrogen GAHT in transgender women (116). Collectively, these findings show that GAHT leads to a unique impact on different immune cell subtypes. The long-term effect of GAHT on the immune system has not been extensively studied which has clinical implications given the known sex-specific prevalence and risks in many inflammatory, infective and autoimmune diseases. To address this significant knowledge gap, we have longitudinally profiled the immune system of transgender women and transgender men newly commencing GAHT. A significant advantage to this approach is it allows us to dissect the contribution of sex hormone action relative to sex chromosomes in immune function, which is usually difficult to separate from the underlying genetic differences between males and females. Using longitudinal GAHT cohorts, we have shown that this therapy can influence epigenetic marks in blood cells affecting the immune response. A notable example of this is differentially methylated CpG site in the promoter of IL-21, which gained DNA methylation after 12 months of masculinizing GAHT, but lost DNA methylation after 12 months of feminizing GAHT (117). We further elucidated the effects of feminizing GAHT in the metabolome wherein a cyproterone-acetate specific decrease in glutamine levels, an important amino acid related to immune cell metabolism, has been observed (118). Altogether, these findings suggest that GAHT influences the changes within the immune cell in a transcriptional level. Recently, Lakshmikanth et al., showed that masculinizing GAHT increased chromatin accessibility at canonical NFkB binding sites in T and NK cells after 12 months of therapy together with the promotion of monocyte responsiveness, together with downstream upregulation of NF-kB and interferon-γ production in NK cells (119). Modulation of interferon signaling by masculinizing GAHT was previously reported, with a decline in IFN-I production by plasmacytoid dendritic cells through the regulation of TLR7/8 (120). This testosterone-associated reduction in IFN-I responses was in line with lower TLR7 responses in males compared to females (121). Therefore, approaches that study immune cell phenotypes, including membrane and nuclear receptors, and downstream chromatin remodeling will provide insights into how sex hormones signal in primary human cells, and potentially explain the contribution of sex hormones to sexual dimorphism in inflammation and development of complex immune diseases.
Conclusion
Epigenetic remodeling through sex hormone signaling may have wide-ranging impacts on the immune profile of transgender men and women. We highlight that most of our knowledge about how sex hormone receptors remodel chromatin come from cancer studies, and that human studies in the context of hormone change are warranted. Considering that AR and ERs are expressed in a range of tissues and immune cell types, changes in circulating sex hormone concentrations can influence cell responses in the circulation, as well as progenitor populations in the bone marrow. Based on the literature we presented, estrogen and testosterone do not simply promote or inhibit immune responses, but changes in their concentrations would lead to a unique immune cell phenotype in the context of GAHT. Profiling the effects of GAHT on epigenetic remodeling will not only provide insight into the role of sex hormones in immune function and the development of complex immune diseases, but will help inform the healthcare of transgender people on GAHT.
Author contributions
DC: Writing – original draft, Writing – review & editing. NN: Writing – original draft, Writing – review & editing. CL: Writing – original draft. KP: Funding acquisition, Writing – review & editing. RS: Funding acquisition, Writing – review & editing. RD: Funding acquisition, Writing – review & editing. MM: Funding acquisition, Writing – review & editing. AC: Funding acquisition, Writing – review & editing. BN: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. BN, AC, RD, and MM are supported by the Allen Distinguished Investigator program, a Paul G. Allen Frontiers Group advised program of the Paul G. Allen Family Foundation. BN is supported by an NHMRC (Australia) Investigator Grant (1173314). AC is supported by an NHMRC (Australia) Investigator grant (2008956). KP is supported by an NHMRC (Australia) Investigator Fellowship (2027186) as well as an NHMRC Clinical Trials and Cohort Study grant (2006529). Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI179004. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Murdoch Children’s Research Institute is supported by the Victorian Government’s Operational Infrastructure Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kleisner K, Tureček P, Roberts SC, Havlíček J, Valentova JV, Akoko RM, et al. How and why patterns of sexual dimorphism in human faces vary across the world. Sci Rep. (2021) 11:5978. doi: 10.1038/s41598-021-85402-3
2. Hammes SR, Levin ER. Impact of estrogens in males and androgens in females. J Clin Invest. (2019) 129:1818–26. doi: 10.1172/JCI125755
3. Hoffmann JP, Liu JA, Seddu K, Klein SL. Sex hormone signaling and regulation of immune function. Immunity. (2023) 56:2472–91. doi: 10.1016/j.immuni.2023.10.008
4. Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, et al. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. (2018) 3:eaap8855. doi: 10.1126/sciimmunol.aap8855
5. Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. (2020) 6:37. doi: 10.1038/s41420-020-0276-1
6. Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3(+) treg cells and gender bias in autoimmune diseases. Front Immunol. (2015) 6:493. doi: 10.3389/fimmu.2015.00493
7. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
8. Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays. (2011) 33:791–802. doi: 10.1002/bies.201100047
9. Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. (2012) 487:249–53. doi: 10.1038/nature11173
10. Kuznetsova T, Prange KHM, Glass CK, de Winther MPJ. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat Rev Cardiol. (2020) 17:216–28. doi: 10.1038/s41569-019-0265-3
11. Liotti A, Ferrara AL, Loffredo S, Galdiero MR, Varricchi G, Di Rella F, et al. Epigenetics: An opportunity to shape innate and adaptive immune responses. In: Immunology. Hoboken, New Jersey, United States: John Wiley and Sons Inc (2022). p. 451–70.
12. Belk JA, Daniel B, Satpathy AT. Epigenetic regulation of T cell exhaustion. Nat Immunol. (2022) 23:848–60. doi: 10.1038/s41590-022-01224-z
13. Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science. (2016) 354:1165–9. doi: 10.1126/science.aae0491
14. Surace AEA, Hedrich CM. The role of epigenetics in autoimmune/inflammatory disease. Front Immunol. (2019) 10:1525. doi: 10.3389/fimmu.2019.01525
15. Cheong JG, Ravishankar A, Sharma S, Parkhurst CN, Grassmann SA, Wingert CK, et al. Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell. (2023) 186:3882–3902.e24. doi: 10.1016/j.cell.2023.07.019
16. Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. (2018) 23:89–100 e5. doi: 10.1016/j.chom.2017.12.010
17. Nideffer J, Ty M, Donato M, John R, Kajubi R, Ji X, et al. Clinical immunity to malaria involves epigenetic reprogramming of innate immune cells. PNAS Nexus. (2024) 3:pgae325. doi: 10.1093/pnasnexus/pgae325
18. Wimmers F, Donato M, Kuo A, Ashuach T, Gupta S, Li C, et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell. (2021) 184:3915–3935 e21. doi: 10.1016/j.cell.2021.05.039
19. Motomura K, Miller D, Galaz J, Liu TN, Romero R, Gomez-Lopez N. The effects of progesterone on immune cellular function at the maternal-fetal interface and in maternal circulation. J Steroid Biochem Mol Biol. (2023) 229:106254. doi: 10.1016/j.jsbmb.2023.106254
20. Iguchi T, Sato T, Nakajima T, Miyagawa S, Takasugi N. New frontiers of developmental endocrinology opened by researchers connecting irreversible effects of sex hormones on developing organs. Differentiation. (2021) 118:4–23. doi: 10.1016/j.diff.2020.10.003
21. Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. (2020) 26:252–8. doi: 10.1038/s41591-020-0751-5
22. Linhares BL, Miranda EP, Cintra AR, Reges R, Torres LO. Use, misuse and abuse of testosterone and other androgens. In: Sexual Medicine Reviews. Amsterdam, Netherlands: Elsevier B.V (2022). p. 583–95.
23. Kumar RS, Goyal N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. (2021) 269:119091. doi: 10.1016/j.lfs.2021.119091
24. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. In: Advances in Protein Chemistry and Structural Biology. San Diego, California, United States: Academic Press Inc (2019). p. 135–70.
25. Arao Y, Korach KS. The physiological role of estrogen receptor functional domains. In: Essays in Biochemistry. London, England: Portland Press Ltd (2021). p. 867–75.
26. Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-α-bound human chromatin interactome. Nature. (2009) 462:58–64. doi: 10.1038/nature08497
27. Chakraborty S, Willett H, Biswas PK. Insight into estrogen receptor beta-beta and alpha-beta homo- and heterodimerization: A combined molecular dynamics and sequence analysis study. Biophys Chem. (2012) 170:42–50. doi: 10.1016/j.bpc.2012.09.002
28. Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. (2005) 19:833–42. doi: 10.1210/me.2004-0486
29. Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J. The many faces of estrogen signaling. In: Biochemia Medica. Zagreb, Croatia: Biochemia Medica, Editorial Office (2014). p. 329–42.
30. Treviño LS, Gorelick DA. The interface of nuclear and membrane steroid signaling. Endocrinol (United States). (2021) 162:1–14. doi: 10.1210/endocr/bqab107
31. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. (2016) 37:3–15.
32. Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. (2010) 42:813–27. doi: 10.1016/j.biocel.2009.11.013
33. Sawada T, Kanemoto Y, Kurokawa T, Kato S. The epigenetic function of androgen receptor in prostate cancer progression. Front Cell Dev Biol. (2023) 11. doi: 10.3389/fcell.2023.1083486
34. Leung JK, Sadar MD. Non-genomic actions of the androgen receptor in prostate cancer. Front Endocrinol. (2017) 8. doi: 10.3389/fendo.2017.00002
36. Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell. (2013) 152:1237–51. doi: 10.1016/j.cell.2013.02.014
37. Felsenfeld G. A brief history of epigenetics. Cold Spring Harbor Perspect Biol. (2014) 6. doi: 10.1101/cshperspect.a018200
38. Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacol. (2013) 38:23–38. doi: 10.1038/npp.2012.112
39. Penagos-Puig A, Furlan-Magaril M. Heterochromatin as an important driver of genome organization. Front Cell Dev Biol. (2020) 8. doi: 10.3389/fcell.2020.579137
40. Karagianni P, Amazit L, Qin J, Wong J. ICBP90, a novel methyl K9 H3 binding protein linking protein ubiquitination with heterochromatin formation. Mol Cell Biol. (2008) 28:705–17. doi: 10.1128/MCB.01598-07
41. Al Aboud NM, Tupper C, Jialal I. Genetics, Epigenetic Mechanism. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
42. Mangiavacchi A, Morelli G, Orlando V. Behind the scenes: How RNA orchestrates the epigenetic regulation of gene expression. Front Cell Dev Biol. (2023) 11. doi: 10.3389/fcell.2023.1123975
43. Mangiavacchi A, Morelli G, Orlando V. Behind the scenes: How RNA orchestrates the epigenetic regulation of gene expression. Front Cell Dev Biol. (2023) 11:1123975. doi: 10.3389/fcell.2023.1123975
44. Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. (2005) 122:33–43. doi: 10.1016/j.cell.2005.05.008
45. Achinger-Kawecka J, Valdes-Mora F, Luu PL, Giles KA, Caldon CE, Qu W, et al. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat Commun. (2020) 11:320. doi: 10.1038/s41467-019-14098-x
46. Li AM, He B, Karagiannis D, Li Y, Jiang H, Srinivasan P, et al. Serine starvation silences estrogen receptor signaling through histone hypoacetylation. Proc Natl Acad Sci U S A. (2023) 120. doi: 10.1073/pnas.2302489120
47. Arruabarrena-Aristorena A, Maag JLV, Kittane S, Cai Y, Karthaus WR, Ladewig E, et al. FOXA1 mutations reveal distinct chromatin profiles and influence therapeutic response in breast cancer. Cancer Cell. (2020) 38:534–550.e9. doi: 10.1016/j.ccell.2020.08.003
48. Xu G, Chhangawala S, Cocco E, Razavi P, Cai Y, Otto JE, et al. ARID1A determines luminal identity and therapeutic response in estrogen-receptor-positive breast cancer. Nat Genet. (2020) 52:198–207. doi: 10.1038/s41588-019-0554-0
49. Hinohara K, Wu HJ, Vigneau S, McDonald TO, Igarashi KJ, Yamamoto KN, et al. KDM5 histone demethylase activity links cellular transcriptomic heterogeneity to therapeutic resistance. Cancer Cell. (2018) 34:939–953.e9. doi: 10.1016/j.ccell.2018.10.014
50. Binder AM, Stiemsma LT, Keller K, Van Otterdijk SD, Mericq V, Pereira A, et al. Inverse association between estrogen receptor-α DNA methylation and breast composition in adolescent Chilean girls. Clin Epigenet. (2018) 10:534–50.e9. doi: 10.1186/s13148-018-0553-5
51. Llorente A, Blasco MT, Espuny I, Guiu M, Ballaré C, Blanco E, et al. MAF amplification licenses ERα through epigenetic remodelling to drive breast cancer metastasis. Nat Cell Biol. (2023) 25:1833–47. doi: 10.1038/s41556-023-01281-y
52. Nesheim N, Ellem S, Dansranjavin T, Hagenkötter C, Berg E, Schambeck R, et al. Elevated seminal plasma estradiol and epigenetic inactivation of ESR1 and ESR2 is associated with CP/CPPS. Oncotarget. (2018) 9:19623–39. doi: 10.18632/oncotarget.24714
53. Dumasia K, Kumar A, Deshpande S, Balasinor NH. Estrogen, through estrogen receptor 1, regulates histone modifications and chromatin remodeling during spermatogenesis in adult rats. Epigenetics. (2017) 12:953–63. doi: 10.1080/15592294.2017.1382786
54. Houshdaran S, Oke AB, Fung JC, Vo KC, Nezhat C, Giudice LC. Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PloS Genet. (2020) 16. doi: 10.1371/journal.pgen.1008601
55. Li X, Fan H, Song X, Song B, Liu W, Dong R, et al. DNA methylome and transcriptome profiling reveal key electrophysiology and immune dysregulation in hypertrophic cardiomyopathy. Epigenetics. (2023) 18. doi: 10.1080/15592294.2023.2195307
56. Thompson EE, Nicodemus-Johnson J, Kim KW, Gern JE, Jackson DJ, Lemanske RF, et al. Global DNA methylation changes spanning puberty are near predicted estrogen-responsive genes and enriched for genes involved in endocrine and immune processes. Clin Epigenet. (2018) 10. doi: 10.1186/s13148-018-0491-2
57. Han X, Zhang P, Shen W, Zhao Y, Zhang H. Estrogen receptor-related DNA and histone methylation may be involved in the transgenerational disruption in spermatogenesis by selective toxic chemicals. Front Pharmacol. (2019) 10. doi: 10.3389/fphar.2019.01012
58. Ma S, Tang T, Probst G, Konradi A, Jin C, Li F, et al. Transcriptional repression of estrogen receptor alpha by YAP reveals the Hippo pathway as therapeutic target for ER+ breast cancer. Nat Commun. (2022) 13:1061–77. doi: 10.1038/s41467-022-28691-0
59. Toska E, Castel P, Chhangawala S, Arruabarrena-Aristorena A, Chan C, Hristidis VC, et al. PI3K inhibition activates SGK1 via a feedback loop to promote chromatin-based regulation of ER-dependent gene expression. Cell Rep. (2019) 27:294–306.e5. doi: 10.1016/j.celrep.2019.02.111
60. Fernández R, Ramírez K, Gómez-Gil E, Cortés-Cortés J, Mora M, Aranda G, et al. Gender-affirming hormone therapy modifies the cpG methylation pattern of the ESR1 gene promoter after six months of treatment in transmen. J Sexual Med. (2020) 17:1795–806. doi: 10.1016/j.jsxm.2020.05.027
61. Fu B, Wang L, Jia T, Wei Z, Nama N, Liang J, et al. Androgen receptor and MYC transcriptomes are equilibrated in multilayer regulatory circuitries in prostate cancer. Prostate. (2023) 83:1415–29. doi: 10.1002/pros.v83.15
62. Kneppers J, Severson TM, Siefert JC, Schol P, Joosten SEP, Yu IPL, et al. Extensive androgen receptor enhancer heterogeneity in primary prostate cancers underlies transcriptional diversity and metastatic potential. Nat Commun. (2022) 13:7367–83. doi: 10.1038/s41467-022-35135-2
63. Scabia V, Ayyanan A, De Martino F, Agnoletto A, Battista L, Laszlo C, et al. Estrogen receptor positive breast cancers have patient specific hormone sensitivities and rely on progesterone receptor. Nat Commun. (2022) 13:3127. doi: 10.1038/s41467-022-30898-0
64. Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. (2014) 111:869–74. doi: 10.1073/pnas.1321060111
65. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PloS Pathog. (2011) 7:e1002149. doi: 10.1371/journal.ppat.1002149
66. Voigt EA, Ovsyannikova IG, Kennedy RB, Grill DE, Goergen KM, Schaid DJ, et al. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front Immunol. (2019) 10:180. doi: 10.3389/fimmu.2019.00180
67. Sampson OL, Jay C, Adland E, Csala A, Lim N, Ebbrecht SM, et al. Gonadal androgens are associated with decreased type I interferon production by plasmacytoid dendritic cells and increased IgG titres to BNT162b2 following co-vaccination with live attenuated influenza vaccine in adolescents. Front Immunol. (2024) 15:1329805. doi: 10.3389/fimmu.2024.1329805
68. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PloS Pathog. (2011) 7:e1002149. doi: 10.1371/journal.ppat.1002149
69. Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, Vom Steeg L, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines. (2019) 4:29–9. doi: 10.1038/s41541-019-0124-6
70. Okwan-Duodu D, Lim E-C, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduction Targeted Ther. (2021) 6:100–0. doi: 10.1038/s41392-021-00513-7
71. Li F, Boon ACM, Michelson AP, Foraker RE, Zhan M, Payne PRO. Estrogen hormone is an essential sex factor inhibiting inflammation and immune response in COVID-19. Sci Rep. (2022) 12:9462–2. doi: 10.1038/s41598-022-13585-4
72. Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. (2020) 18:369. doi: 10.1186/s12916-020-01851-z
73. Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee M-H, Wood J, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. (2022) 185:2452–2468.e16. doi: 10.1016/j.cell.2022.06.008
74. Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. (2015) 125:2187–93. doi: 10.1172/JCI78082
75. Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. (2001) 2:777–80. doi: 10.1038/ni0901-777
76. Prada-Medina CA, Peron JPS, Nakaya HI. Immature neutrophil signature associated with the sexual dimorphism of systemic juvenile idiopathic arthritis. J Leukoc Biol. (2020) 108:1319–27. doi: 10.1002/JLB.6MA0720-015RR
77. Milovanovic J, Arsenijevic A, Stojanovic B, Kanjevac T, Arsenijevic D, Radosavljevic G, et al. Interleukin-17 in chronic inflammatory neurological diseases. Front Immunol. (2020) 11:947–7. doi: 10.3389/fimmu.2020.00947
78. Voskuhl RR, Patel K, Paul F, Gold SM, Scheel M, Kuchling J, et al. Sex differences in brain atrophy in multiple sclerosis. Biol Sex Dif. (2020) 11:49–9. doi: 10.1186/s13293-020-00326-3
79. Wu H, Zeng L, Ou J, Wang T, Chen Y, Nandakumar KS. Estrogen acts through estrogen receptor-β to promote mannan-induced psoriasis-like skin inflammation. Front Immunol. (2022) 13:818173–3. doi: 10.3389/fimmu.2022.818173
80. Shepherd R, Cheung AS, Pang K, Saffery R, Novakovic B. Sexual dimorphism in innate immunity: the role of sex hormones and epigenetics. Front Immunol. (2021) 11:604000. doi: 10.3389/fimmu.2020.604000
81. Mohammad I, Starskaia I, Nagy T, Guo J, Yatkin E, Väänänen K, et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. (2018) 11:9415–5. doi: 10.1126/scisignal.aap9415
82. Kim DH, Park HJ, Park HS, Lee JU, Ko CM, Gye MC, et al. Estrogen receptor α in T cells suppresses follicular helper T cell responses and prevents autoimmunity. Exp Mol Med. (2019) 51:1–9. doi: 10.1038/s12276-019-0237-z
83. Cephus JY, Gandhi VD, Shah R, Brooke Davis J, Fuseini H, Yung JA, et al. Estrogen receptor-α signaling increases allergen-induced IL-33 release and airway inflammation. Allergy: Eur J Allergy Clin Immunol. (2021) 76:255–68. doi: 10.1111/all.14491
84. Gravitte A, Kintner J, Brown S, Cobble A, Kennard B, Hall JV. The hormonal environment and estrogen receptor signaling alters Chlamydia muridarum infection in vivo. Front Cell Infect Microbiol. (2022) 12. doi: 10.3389/fcimb.2022.939944
85. Adachi A, Honda T, Egawa G, Kanameishi S, Takimoto R, Miyake T, et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol. (2022) 150:909–919.e8. doi: 10.1016/j.jaci.2022.03.028
86. Fernandez Lahore G, Förster M, Johannesson M, Sabatier P, Lönnblom E, Aoun M, et al. Polymorphic estrogen receptor binding site causes Cd2-dependent sex bias in the susceptibility to autoimmune diseases. Nat Commun. (2021) 12:5565–77. doi: 10.1038/s41467-021-25828-5
87. Hases L, Indukuri R, Birgersson M, Nguyen-Vu T, Lozano R, Saxena A, et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. (2020) 492:54–62. doi: 10.1016/j.canlet.2020.06.021
88. Abdullah M, Chai P-S, Chong M-Y, Tohit ERM, Ramasamy R, Pei CP, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol. (2012) 272:214–9. doi: 10.1016/j.cellimm.2011.10.009
89. Aomatsu M, Kato T, Kasahara E, Kitagawa S. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Biophys Res Commun. (2013) 441:220–5. doi: 10.1016/j.bbrc.2013.10.042
90. Vijeyakumaran M, Jawhri MA, Fortunato J, Solomon L, Shrestha Palikhe N, Vliagoftis H, et al. Dual activation of estrogen receptor alpha and glucocorticoid receptor upregulate CRTh2-mediated type 2 inflammation; mechanism driving asthma severity in women? Allergy. (2023) 78:767–79. doi: 10.1111/all.15543
91. Das B, Dobrowolski C, Luttge B, Valadkhan S, Chomont N, Johnston R, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci. (2018) 115:E7795–804. doi: 10.1073/pnas.1803468115
92. Vom Steeg LG, Dhakal S, Woldetsadik YA, Park HS, Mulka KR, Reilly EC, et al. Androgen receptor signaling in the lungs mitigates inflammation and improves the outcome of influenza in mice. PloS Pathog. (2020) 16. doi: 10.1371/journal.ppat.1008506
93. Kalidhindi RSR, Ambhore NS, Balraj P, Schmidt T, Khan MN, Sathish V. Androgen receptor activation alleviates airway hyperresponsiveness, inflammation, and remodeling in a murine model of asthma. Am J Physiol - Lung Cell Mol Physiol. (2021) 320:L803–18. doi: 10.1152/ajplung.00441.2020
94. Gandhi VD, Cephus JY, Norlander AE, Chowdhury NU, Zhang J, Ceneviva ZJ, et al. Androgen receptor signaling promotes Treg suppressive function during allergic airway inflammation. J Clin Invest. (2022) 132. doi: 10.1172/JCI153397
95. Allam S, Elsakka EGE, Ismail A, Doghish AS, Yehia AM, Elkady MA, et al. Androgen receptor blockade by flutamide down-regulates renal fibrosis, inflammation, and apoptosis pathways in male rats. Life Sci. (2023) 323:121697–705. doi: 10.1016/j.lfs.2023.121697
96. Guan X, Polesso F, Wang C, Sehrawat A, Hawkins RM, Murray SE, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature. (2022) 606:791–6. doi: 10.1038/s41586-022-04522-6
97. Yang C, Jin J, Yang Y, Sun H, Wu L, Shen M, et al. Androgen receptor-mediated CD8+ T cell stemness programs drive sex differences in antitumor immunity. Immunity. (2022) 55:1268–1283.e9. doi: 10.1016/j.immuni.2022.05.012
98. van Rooijen JM, Qiu SQ, Timmer-Bosscha H, van der Vegt B, Boers JE, Schröder CP, et al. Androgen receptor expression inversely correlates with immune cell infiltration in human epidermal growth factor receptor 2-positive breast cancer. Eur J Cancer. (2018) 103:52–60. doi: 10.1016/j.ejca.2018.08.001
99. Walecki M, Eisel F, Klug J, Baal N, Paradowska-Dogan A, Wahle E, et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell. (2015) 26:2845–57. doi: 10.1091/mbc.E14-08-1323
100. Marin DP, Bolin AP, dos Santos Rde C, Curi R, Otton R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct. (2010) 28:394–402. doi: 10.1002/cbf.v28:5
101. Xie J, Ding Y, Li X, Pu R, Liu W, Li P, et al. Association of ESR1 gene polymorphisms with the susceptibility to Hepatitis B virus infection and the clinical outcomes. J Med Virol. (2023) 95. doi: 10.1002/jmv.28510
102. Lambrecht R, Delgado ME, Gloe V, Schuetz K, Plazzo AP, Franke B, et al. Liver receptor homolog-1 (NR5A2) orchestrates hepatic inflammation and TNF-induced cell death. Cell Rep. (2023) 42:113513–3. doi: 10.1016/j.celrep.2023.113513
103. Hoffmann JP, Liu JA, Seddu K, Klein SL. Sex hormone signaling and regulation of immune function. In: Immunity. Cambridge, Masachusetts, United States: Cell Press (2023). p. 2472–91.
104. Xu Y, Qi W, Wang X, Zhang Y, Han L, Shi J, et al. Signal transducer and activator of transcription 3 cooperates with androgen receptor/cell cycle-related kinase signalling pathway in the progression of hepatitis B virus infection and gender differences. J Viral Hepatitis. (2022) 29:569–78. doi: 10.1111/jvh.13699
105. Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang CY, McDonnell DP. Estrogen receptor signaling in the immune system. In: Endocrine Reviews. Washington, DC, United States: Endocrine Society (2023). p. 117–41.
106. Cedar H, Bergman Y. Epigenetics of haematopoietic cell development. Nat Rev Immunol. (2011) 11:478–88. doi: 10.1038/nri2991
107. Ostuni R, Piccolo V, Barozzi I, Polletti S, Termanini A, Bonifacio S, et al. Latent enhancers activated by stimulation in differentiated cells. Cell. (2013) 152:157–71. doi: 10.1016/j.cell.2012.12.018
108. Bannister S, Kim B, Domínguez-Andrés J, Kilic G, Ansell BRE, Neeland MR, et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci Adv. (2022) 8:eabn4002. doi: 10.1126/sciadv.abn4002
109. Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. (2014) 159:1312–26. doi: 10.1016/j.cell.2014.11.018
110. Luong TMH, Buggert M. Tissue-specific epigenomics of resident memory CD8+ T cells. Nat Rev Immunol. (2022) 22:533. doi: 10.1038/s41577-022-00767-4
111. Pyfrom S, Paneru B, Knox JJ, Cancro MP, Posso S, Buckner JH, et al. The dynamic epigenetic regulation of the inactive X chromosome in healthy human B cells is dysregulated in lupus patients. Proc Natl Acad Sci. (2021) 118:e2024624118. doi: 10.1073/pnas.2024624118
112. Cheng MI, Li JH, Riggan L, Chen B, Tafti RY, Chin S, et al. The X-linked epigenetic regulator UTX controls NK cell-intrinsic sex differences. Nat Immunol. (2023) 24:780–91. doi: 10.1038/s41590-023-01463-8
113. Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. (2017) 107:e1–8. doi: 10.2105/AJPH.2016.303578
114. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. (2013) 13:214–22. doi: 10.1016/S1473-3099(12)70315-8
115. Robinson GA, Peng J, Peckham H, Butler G, Pineda-Torra I, Ciurtin C, et al. Investigating sex differences in T regulatory cells from cisgender and transgender healthy individuals and patients with autoimmune inflammatory disease: a cross-sectional study. Lancet Rheumatol. (2022) 4:e710–24. doi: 10.1016/S2665-9913(22)00198-9
116. Peckham H, Radziszewska A, Sikora J, de Gruijter NM, Restuadi R, Kartawinata M, et al. Estrogen influences class-switched memory B cell frequency only in humans with two X chromosomes. J Exp Med. (2025) 222. doi: 10.1084/jem.20241253
117. Shepherd R, Bretherton I, Pang K, Mansell T, Czajko A, Kim B, et al. Gender-affirming hormone therapy induces specific DNA methylation changes in blood. Clin Epigenet. (2022) 14:14–24. doi: 10.1186/s13148-022-01236-4
118. Shepherd R, Angus LM, Mansell T, Arman B, Kim BW, Lange K, et al. Impact of distinct anti-androgen exposures on the plasma metabolome in feminizing gender-affirming hormone therapy. J Clin Endocrinol Metab. (2024) 109:2857–71. doi: 10.1210/clinem/dgae226
119. Lakshmikanth T, Consiglio C, Sardh F, Forlin R, Wang J, Tan Z, et al. Immune system adaptation during gender-affirming testosterone treatment. Nature. (2024) 633:155–64. doi: 10.1038/s41586-024-07789-z
120. Grünhagel B, Borggrewe M, Hagen SH, Ziegler SM, Henseling F, Glau L, et al. Reduction of IFN-I responses by plasmacytoid dendritic cells in a longitudinal trans men cohort. iScience. (2023) 26:108209. doi: 10.1016/j.isci.2023.108209
Keywords: epigenetics, sex hormones, estrogen, testosterone, estrogen receptor, gender-affirming hormonal therapy, gender, immunity
Citation: Celestra D, Nguyen NNL, Laberthonniere C, Pang KC, Saffery R, Davey RA, Mhlanga M, Cheung AS and Novakovic B (2025) Epigenetic remodeling by sex hormone receptors and implications for gender affirming hormone therapy. Front. Immunol. 16:1501959. doi: 10.3389/fimmu.2025.1501959
Received: 26 September 2024; Accepted: 17 April 2025;
Published: 08 May 2025.
Edited by:
Nils Landegren, Uppsala University, SwedenReviewed by:
Melissa Anne Cunningham, Medical University of South Carolina, United StatesMadhvi Menon, The University of Manchester, United Kingdom
Copyright © 2025 Celestra, Nguyen, Laberthonniere, Pang, Saffery, Davey, Mhlanga, Cheung and Novakovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boris Novakovic, Ym9yaXMubm92YWtvdmljQG1jcmkuZWR1LmF1
 Den Celestra
Den Celestra Nhi N. L. Nguyen
Nhi N. L. Nguyen Camille Laberthonniere2
Camille Laberthonniere2 Ken C. Pang
Ken C. Pang Richard Saffery
Richard Saffery Rachel A. Davey
Rachel A. Davey Musa Mhlanga
Musa Mhlanga Ada S. Cheung
Ada S. Cheung Boris Novakovic
Boris Novakovic