- 1Department of Neurology, Hangzhou Third People’s Hospital, Hangzhou, Zhejiang, China
- 2State key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Disease, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 3Department of Medical Microbiology and Parasitology, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Objective: Reports on central nervous system (CNS) infection caused by varicella-zoster virus (VZV) reactivation are increasing, but its pathogenesis remains unclear, which causes delayed diagnosis and treatment. Some studies suggested that hypercoagulability is involved in the pathogenesis of CNS infection of VZV. This study investigated the coagulation parameters of herpes zoster (HZ) and their correlations with the VZV-related CNS infection, and provided a reference for the early diagnosis and treatment.
Methods: We selected 123 consecutive patients, including 95 HZ cases and 28 VZV meningitis (VZVM) cases hospitalized due to HZ. Forty-seven patients who underwent physical examination in our hospital were used as Health controls (HCs) group. The coagulation parameters of the three groups were measured and compared, and the correlation between coagulation function parameters and CNS infection was analyzed by Logistic regression. the expression of coagulation factor in cerebrospinal fluid (CSF) proteomics of 28 VZVM patients and 11 HZ patients were analyzed.
Results: Compared with HCs group, plasma Fibrinogen (Fib) and D-dimer (DD) levels in HZ and VZVM group were significantly increased (P <0.01), while there were no significant differences in other parameters (P > 0.05). There was also no significant difference in the levels of coagulation parameters between the HZ and VZVM groups (P > 0.05). Proteomic analysis of CSF revealed that there was no difference in the expression levels of Fib, Antithrombin III (AT-III), and coagulation factors VII, IX, X, XI in the HZ and VZVM patients (P > 0.05). The expression levels of coagulation factors XII and XIIIa were higher in VZVM patients than those in HZ patients (P < 0.05 and P < 0.01, respectively).
Conclusion: In HZ and VZVM patients, a hypercoagulable state was observed with increased Fib and DD levels. However, hypercoagulation was not a risk factor for CNS infection, and there was no significant correlation between the elevated level and the severity of disease.
Introduction
Herpes zoster (HZ) is a skin infectious disease caused by the reactivation of latent varicella-zoster virus (VZV) in the human body. It is characterized by vesicles and rashes of the skin in the neurodistributive area, often accompanied by pain (1). Although HZ is a self-limiting skin disease, it can cause neurological complications such as postherpetic neuralgia (PHN) and cranial neuritis. In severe cases, central nervous system (CNS) infection can occur, such as meningitis, encephalitis, myelitis, cerebellar encephalitis and other complications, and even lead to serious complications such as death, increasing the burden on families and society (2–6). However, early identification and treatment of HZ patients with CNS infection mostly achieved good prognosis. Therefore, the early diagnosis and treatment is extremely important.
However, the mechanism of VZV reactivation leading to CNS infection has not been fully clarified, which may be related to direct viral infection, sustained inflammatory response, vasculitis, hypercoagulability, etc. Some studies have also found that viremia occurs in approximately 50% of the cases of HZ in immunocompetent adults, which is believed to be an important component of the pathogenesis of CNS complications in HZ (7–9). Currently, vasculitis is postulated as the most likely pathophysiological mechanism of VZV-related CNS infection (10–12). Vasculitis can cause coagulation dysfunction, thrombosis, and vice versa. Previous studies have also found that virus infection can activate hemolytic fibrinolytic system, lead to changes in coagulation function, microthrombus, and even pulmonary embolism, disseminated intravascular coagulation (DIC) and other life-threatening complications (13–15).
Human type 3 herpesvirus VZV can cause chickenpox and shingles after infection. However, it is still unclear whether the changes in coagulation functions in HZ patients and the changes in the plasma coagulation function parameters are risk factors for VZV-related CNS infection. Although some scholars believe that hypercoagulability is involved in the pathophysiological mechanism of VZV-related CNS infections, there are few studies on coagulation function in those patients. Therefore, this study examined coagulation factors in HZ and VZVM patients to assess their correlation with CNS infection, aiming to provide initial evidence of coagulation dysfunction’s role in HZ-CNS infection and support early diagnosis and treatment.
Materials and methods
Subjects
A total of 123 consecutive patients with shingles who were admitted to our hospital from January 2020 to January 2023 were selected, including 28 VZV meningitis (VZVM) cases and 95 HZ cases according to clinical manifestations of shingles patients and routine cerebrospinal fluid, biochemical and VZV DNA detection, and 47 health controls (HCs) from physical examination program in our hospital. Our study has been approved by the Ethics Committee of Hangzhou Third people’s Hospital (NO.2021KA013). All procedures were conducted in accordance with the Helsinki Declaration.
Inclusion criteria: 1). Age >18; 2). All patients had skin erythema, vesicles, blisters and other clinical manifestations, which met the diagnostic criteria of HZ/VZV meningitis (16); 3). Within 2 weeks of onset; 4). Diagnosed with shingles for the first time and had not used anticoagulants in the last 2 weeks. The diagnosis of shingles is made by a dermatologist and the diagnosis of VZV meningitis is made by a neurologist.
Exclusion criteria: 1). zoster sine herpete; 2). lactating or pregnant women; 3). infections caused by pathogens other than VZV.
Methods
Data collection
We collected the general information of the patients, including ages, genders, clinical characteristics (mainly including the courses of diseases, the sites of herpes, treatments, etc.), comorbidity, and previous medical records.
Test of plasma coagulation function
Five mL of venous blood was collected from each enrolled patient and control subject on the morning of the second day of admission by anticoagulant vessels (containing 0.2ml, 0.11mol/L sodium citrate anticoagulant) under aseptic conditions, thoroughly mixed, left at normal temperature (processed within 4 hours). Plasma was collected by centrifugation at 3000r/min for 15 minutes. Levels of fibrinogen (Fib) and D-dimer (DD), prothrombin time PT), active partial thromboplastin time (APTT), thrombin time (TT) were measured on the automatic coagulation analyzer CS-5100 (Sysmex Corporation, Japan) in accordance with the operating procedures.
Routine and biochemical tests of cerebrospinal fluid
According to the clinical manifestations, lumbar puncture was performed within 48 hours after admission for patients with suspected VZV-related CNS infection with the consent of the patients or their families, and 6 mL cerebrospinal fluid was obtained with sterile test tubes. White blood cell count, glucose, protein, chlorine, adenosine deaminase (ADA), lactate dehydrogenase (LDH) and VZV DNA concentrations were determined.
Cerebrospinal fluid proteomics
CSF level of 8 coagulation proteins (Fibrinogen, Antithrombin-III, Coagulation factor VII, IX, X, XI, XII, XIIIa) were extracted from previously collected CSF proteomics dataset of the same 28 VZV meningitis and 11 herpes zoster (HZ) patients (17). Briefly, the proteins from CSF samples analyzed by mass spectrometry using data-independent acquisition (DIA) method for quantitation. Relative protein intensity in each sample was log2 transformed prior to statistical analysis.
Statistical analysis
SPSS 20.0 software (Chicago, IL, USA) was used for data processing and statistical analysis. Normal distribution data of measurement data were expressed as mean ± standard deviation (x ± SD), and non-normal distribution data were expressed as M (Q1 ~ Q3). One-way analysis of variance (ANOVA) was used to compare the mean of multiple groups; S-N-K method was used to test the comparison between two groups; counting data were expressed by frequency and percentage; Chi-square test or Fisher exact test was used for comparison between groups. Univariate and multivariate logistic regression analysis were performed to identify independent risk factors, and Pearson analysis was used for correlation analysis. P< 0.05 (two-tailed) was considered statistically significant.
Results
Baseline characteristics of patients
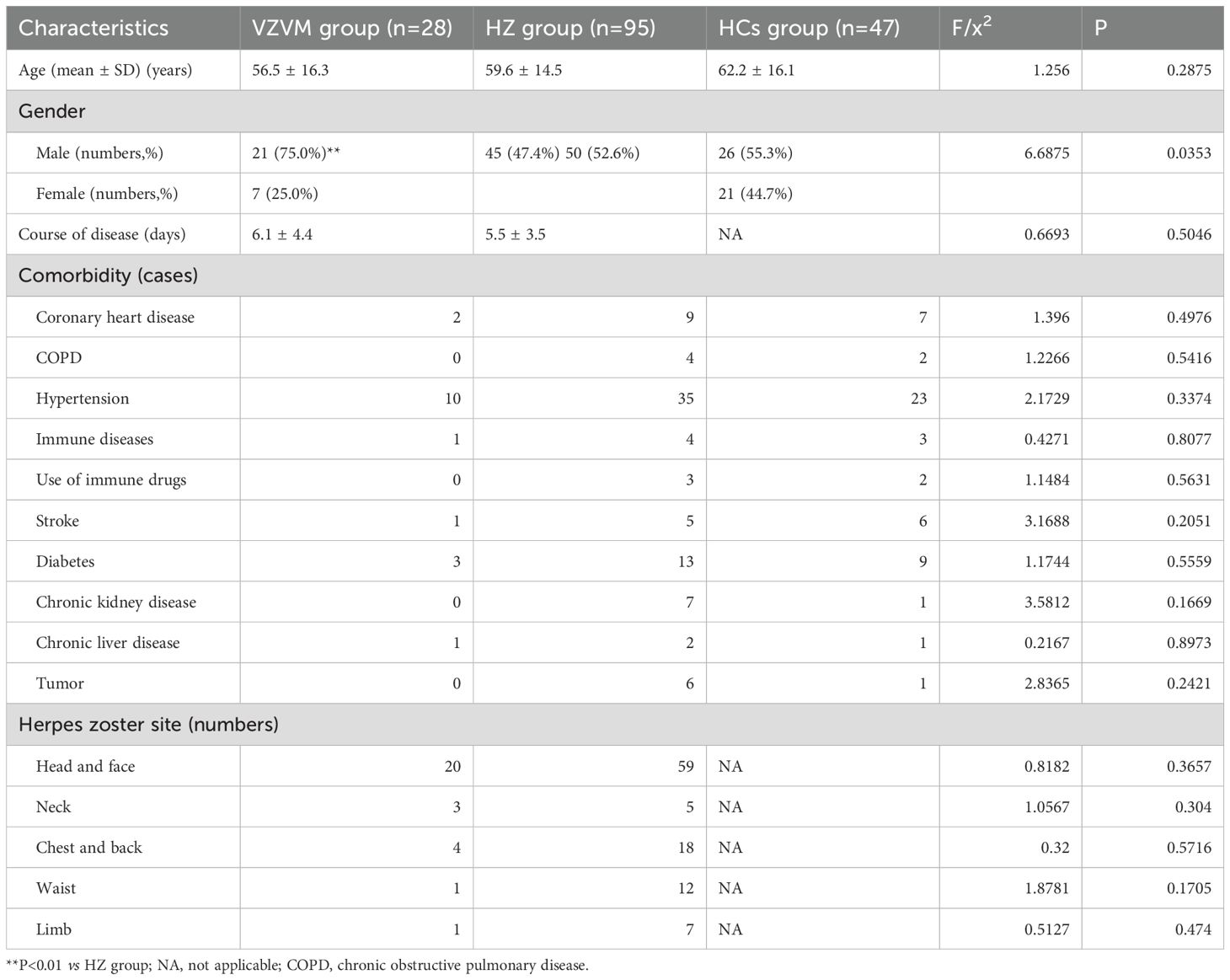
According to the inclusion/exclusion criteria, 95 HZ patients were included: 45 males and 50 females, with ages 23 to 88 years (59.6 ± 14.5 years). There were 28 patients with VZVM: 21 males and 7 females, with ages from 24 to 88 years (56.5 ± 16.3). The HCs group consisted of 47 cases: 26 males and 21 females, with ages from 24 to 88 years (62.2 ± 16.1 years). There was no significant difference (P>0.05) in age and comorbidity (hypertension, diabetes, stroke, coronary heart disease, tumor, chronic obstructive pulmonary disease, immune diseases, immune drug use, etc.) among the three groups except gender (Table 1).
In the HZ group, shingle were found in 59 cases located on the head and face, 5 on the neck, 18 on the chest and back, 12 on the waist and 7 on the limbs. Among the 28 VZVM patients, 20 on the head and face, 3 on the neck, 4 on the chest and back, 1 on the waist and 1 on the limbs. There was no difference in shingle sites between the two groups (P>0.05). The proportion of males in VZVM group was significantly higher than the HZ group (P<0.01).
Comparison of coagulation parameters among three groups
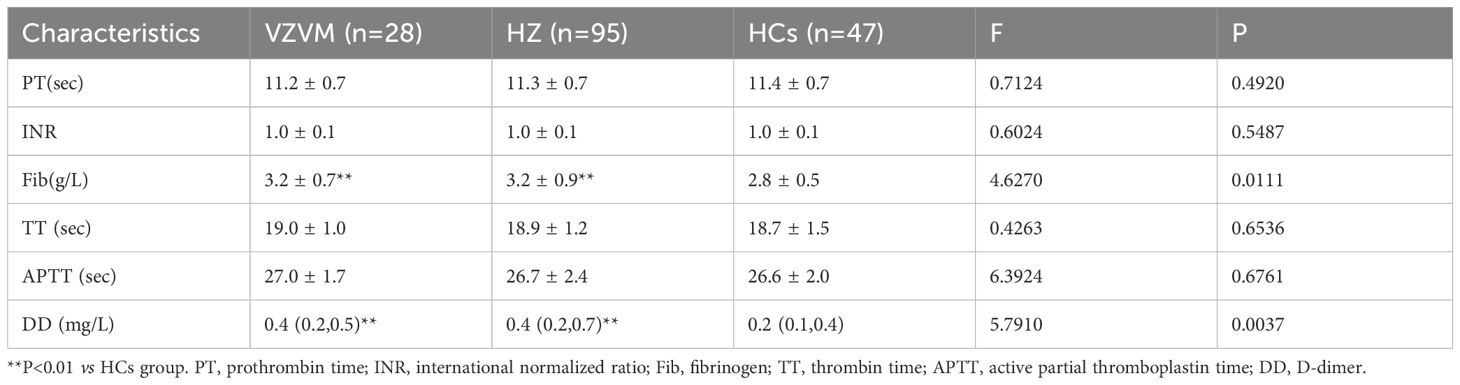
There were significantly increased levels of circulating Fib and DD in HZ and VZVM patients compared to the HCs (F=4.627, P<0.01; F=5.791, P<0.01), but there were no significant differences in PT, APTT, INR and TT levels among the three groups (P>0.05), as shown in Figure 1, Table 2. Inter-group comparison showed that there was no significant difference in coagulation parameters between the HZ group and the VZVM group (P>0.05).
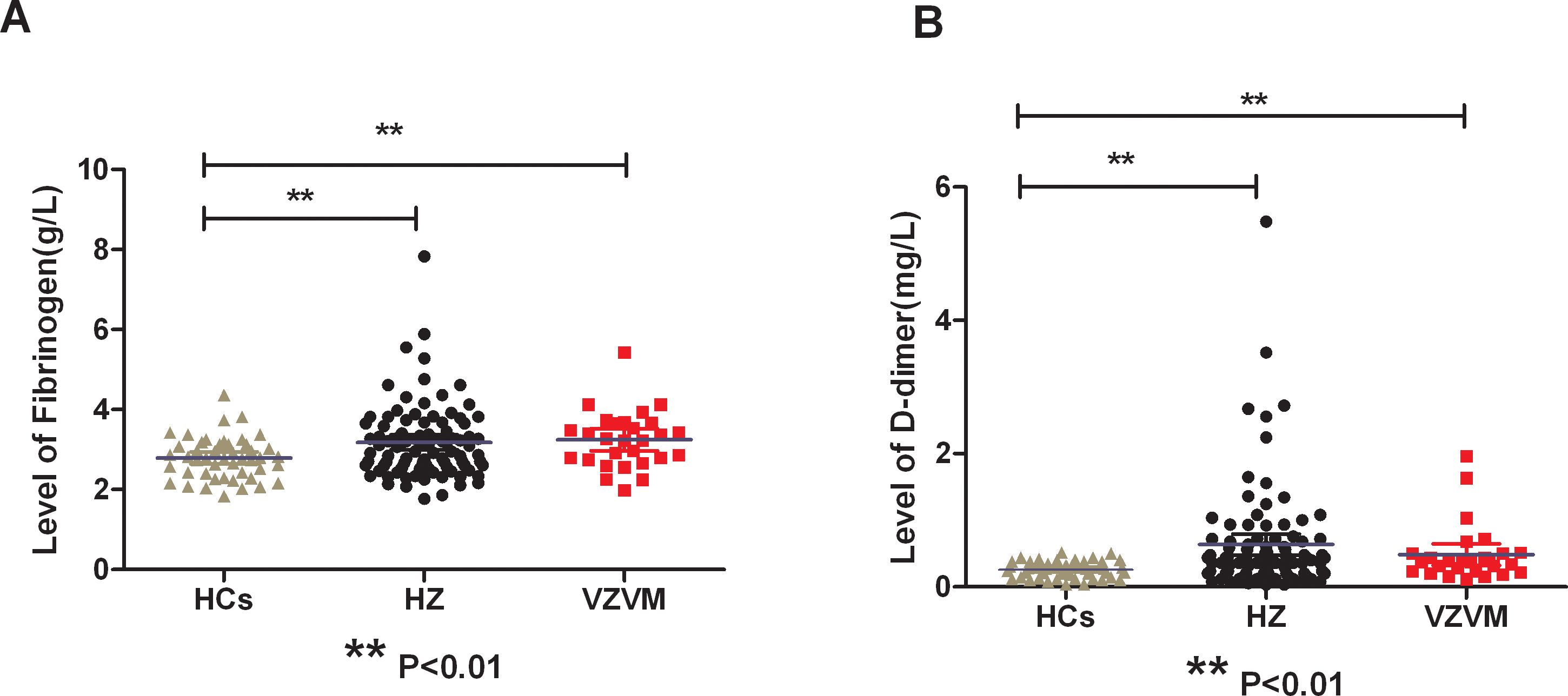
Figure 1. Comparison of plasma Fib (A) and DD (B) levels among three groups. HCs, health controls; HZ, herpes zoster; VZVM, varicella-zoster virus meningitis. ** P<0.01.
Multivariate logistic regression analysis
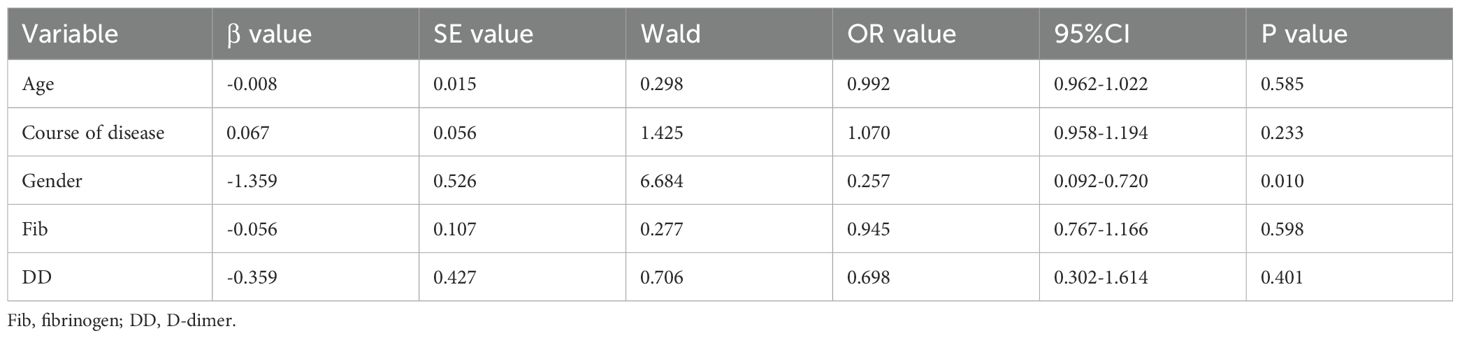
Multivariate logistic regression analysis was performed with the occurrence of meningitis as the dependent variable, and the factors with P<0.5 in univariate analysis (age, course of disease, gender, Fib, DD) as the independent variables (Table 3). Male was the independent risk factor for VZV meningitis in HZ patients. However, coagulation function parameters and other factors were not independent risk factors (P>0.05).
Correlation analysis of plasma coagulation function and CSF parameters in VZVM patients
All 28 VZVM patients and 11 of the 95 HZ patients completed lumbar puncture and CSF examination. In patients with VZVM group, the white blood cell counts and protein content in CSF were all higher than the normal range, but the levels of glucose, chlorine, ADA and LDH in CSF were in the normal range. There was no significant correlation between blood coagulation parameters and the levels of white blood cell counts, protein and glucose in CSF (P>0.05).
Comparison of coagulation factors in CSF proteomes in 28 VZVM patients and 11 HZ patients
Previously reported CSF proteomics data for 28 VZVM and 11 HZ patients (17) was reinterpreted to focus on coagulation markers. Compared with HZ patients, there were no differences in the expression levels of Fib, AT-III and coagulation factor VII, IX, X, XI in VZVM patients (P>0.05, Figure 2), but the expression levels of coagulation factor XII and XIIIa in VZVM patients were significantly higher than those in HZ patients (P<0.05, P<0.01, Figure 3).
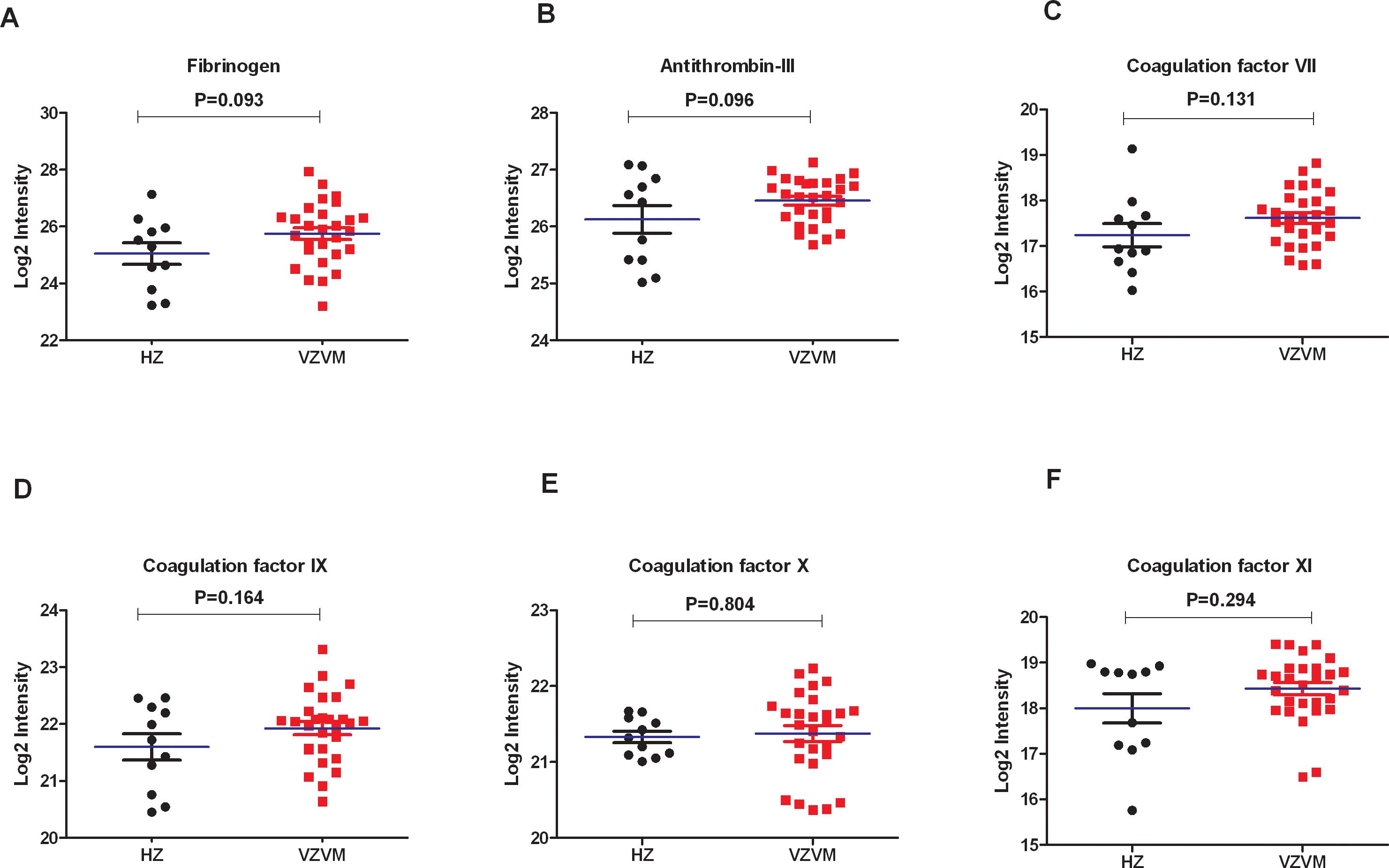
Figure 2. The expression levels of Fib (A), antithrombin III (B) and coagulation factor VII (C), IX (D), X (E), XI (F) in CSF between HZ and VZVM groups.
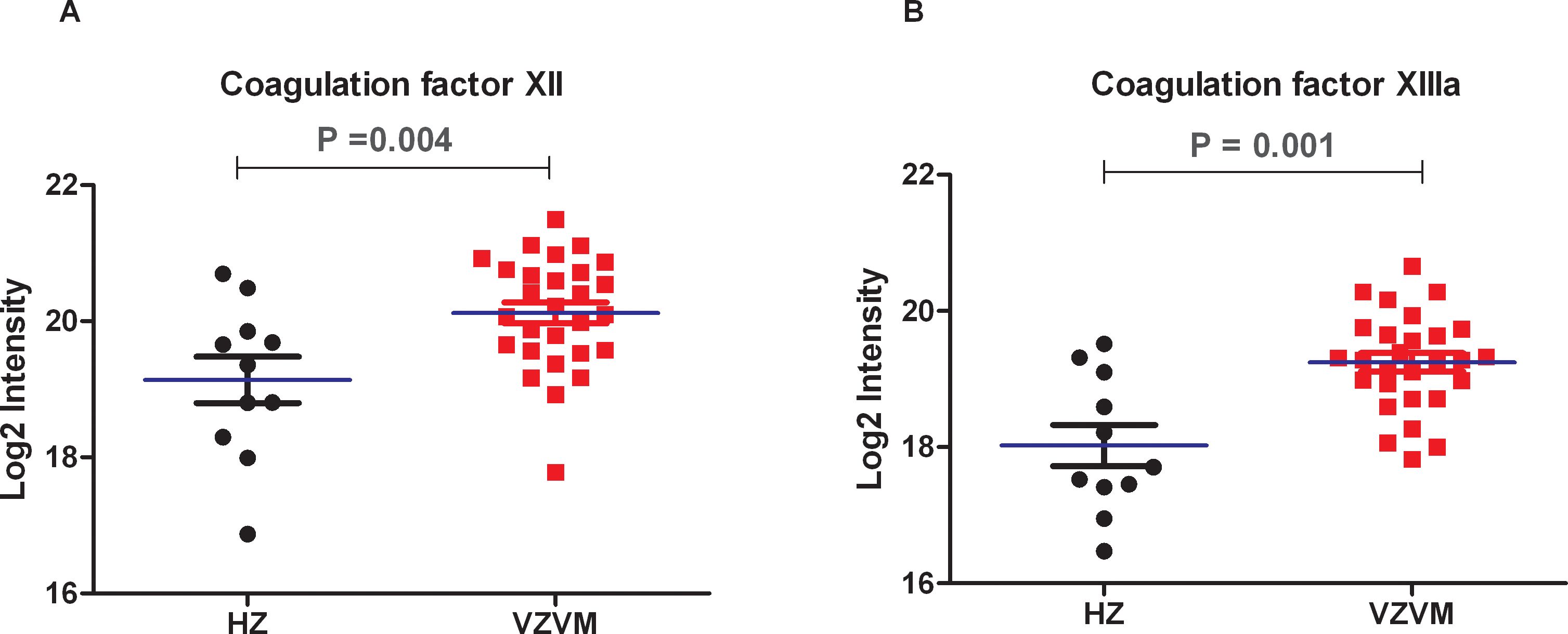
Figure 3. The expression levels of coagulation factors XII (A) and XIIIa (B) in VZVM group and HZ group.
Discussion
Herpes zoster is caused by reactivated VZV, mostly in middle-aged and elderly people. With global rising of HZ incidence, 20% to 30% of people suffer from HZ in their lifetime (18, 19), and 50% of people with HZ will have a concomitant VZV viremia (7, 8). Moreover, the patients may develop various complications upon onset, including lethal CNS complications. In the case of HZ, early and timely treatment can significantly reduce the incidence of complications. Older age, diabetes mellitus, immunosuppression, and treatment delay might serve as risk factors for CNS infection in HZ patients (10, 11, 20). Kim et al. (21) found that the incidence of VZV-related meningitis in male was significantly higher than that of female. In agreement, male gender was independently associated with an increased risk of meningitis in our study.
Viral invasion can trigger homeostatic disorders within the host, including changes in coagulation function, and even lead to severe complications such as pulmonary embolism and life-threatening DIC (13–15). The parameters of coagulation function include APTT, PT, TT, Fib and DD, etc. Monitoring coagulation biomarkers which correlate with disease severity can inform therapeutic strategies. Limb et al. (22) first reported a case of amputation in an adult male with shingles complicated by pneumonia and peripheral venous thrombosis in 2009. Since then, deep vein thrombosis has been confirmed repeatedly in HZ patients (23–25), while pulmonary embolism can also be complicated in shingles patients (26–28), and the risk of stroke is significantly increased (29, 30). These results indicate that VZV infection can also lead to changes in blood coagulation function in patients. In this study, we found plasma Fib and DD levels were significantly increased in patients with HZ and meningitis as compared to HCs. As a liver-derived coagulation protein, fibrinogen (Fib) primary substrate for both hemostasis and thrombosis, and serves as a principal determinant of coagulation capacity (31, 32). In addition, Fib also binds to platelet membrane glycoprotein IIb/IIIa to mediate platelet aggregation, thus affecting blood viscosity. Increasing Fib level will lead to increased fibrin leveland therefore creats a hypercoagulable state (33). DD is one of the degradation products of fibrin and fibrinogen after activation of coagulation-fibrinolytic system, and is a marker reflecting fibrinolytic activity in vivo. The increase of plasma DD often occurs in patients with venous thrombosis, infection, hypercoagulable state, etc. (34, 35). This study reveals that acute herpes zoster infection is characterized by enhanced coagulation functions, compensatory fibrinolysis activation, and consequent disruption of hemostatic balance, resulting in a prothrombotic state. However, our study found that although the plasma Fib and DD levels in patients with VZV meningitis were higher than those in the control group, there was no significant difference in all coagulation function parameters compared with HZ patients. Further analysis showed that abnormal coagulation function was not an independent risk factor for meningitis in patients with shingles. Therefore, we speculate that hypercoagulability is not the pathophysiological mechanism of VZV meningitis, which needed to be validated in further studies.
CSF composition changes resulting from microbial or macromolecular infiltration correlate with CNS microenvironment alterations, serving as potential lesion biomarkers. Proteomics provides comprehensive protein quantification in biological systems, aiding pathophysiological understanding. Proteomic analyses of brain or CSF in normal and diseased states, helps to identify disease-associated proteins with diagnostic, therapeutic and prognostic relevance (36–38). Our previous study (17) on CSF proteomics in patients with VZV meningitis also found that the expression of CXCL10, IL-1RN, MPO, PRTN3 and other proteins which reflecting inflammation and immune cell activation were significantly increased. Ramachandran et al. have also found inflammatory markers elevated in the CSF during VZV-related meningitis, including interferon gamma, interleukins IL-6, IL-8, IL-10, IL-17F, IL-1RA, chemokines CXCL-9, CXCL-10, CCL-2 and G-CSF (39). Such inflammatory response is closely related to the coagulation system, and there is mutual regulation between the two systems (40). The inflammatory response of the brain can lead to increased clotting function, and anticoagulation can reduce the damage of the inflammatory response (41, 42). It is likely that the observed upregulation of coagulation factors XII and XIIIa in the CSF of VZV meningitis patients is more consistent with an inflammatory response causing BBB permeability rather than with intrinsic coagulation pathway activation. Therefore, based on the results of this study, we speculate that hypercoagulable state is not involved in the pathogenesis of VZV meningitis. The pathogenesis of VZV meningitis may be different from that of VZV encephalitis, and further comparative studies are needed to clarify it in the future. A retrospective study by Kim et al. to identify risk factors for CNS infection in 578 acute HZ patients, including 24 cases of HZ-related meningitis, in addition to discovering that males are a risk factor for VZVM, they also found that meningitis were more common in patients who had shingles in the craniocervical dermatomes, skin lesions on craniocervical distribution is also a risk factor for subsequent meningitis, and they pointed out that hematogenous invasion of virus may play a role in CNS VZV infections (21). According to studies by Satyaprakash and Kennedy et al. (7, 8), approximately 50% of herpes zoster patients have viremia, which is an important pathophysiological mechanism for the concurrent CNS infection in HZ patients. Is the inflammatory response in VZVM patients caused by viremia? It is possible that the more severe cases of HZ with meningitis are among those 50% of HZ patients who have a viremia. In future studies, we will consider testing for viremia by measuring VZV DNA in the blood of HZ and VZVM patients by PCR technology.
A limitation of this study is the relatively small cohort of VZV meningitis patients, particularly the limited number of HZ cases who underwent diagnostic lumbar puncture for CSF analysis, which may have introduced bias in our findings. Moreover, the absence of longitudinal data tracking individual patients’ pre- and post-treatment progression limits our ability to establish prognostic indicators for clinical outcomes. In the future, larger scale and multi-center studies are needed to further explore the effects of coagulation screening indicators on clinical outcomes and the need for preventive antithrombotic therapy.
Conclusions
Reactivation of VZV can lead to changes in coagulation function in the host. Both HZ and VZV meningitis patients have hypercoagulable internal environment, mainly characterized by elevated fibrinogen and D-dimer, but such environment does not seem to be a risk factor for CNS infection. Disruption of the BBB in patients with VZV meningitis may lead to changes in partial coagulation parameters, but the pathogenesis of CNS infection in HZ may not be related to hypercoagulability. Further large-scale, multi-center, controlled studies are needed to clarify it in the future, and simultaneously detect viremia to verify our hypothesis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Hangzhou Third People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YRY: Conceptualization, Data curation, Investigation, Project administration, Writing – original draft. JG: Data curation, Formal Analysis, Investigation, Writing – original draft. HL: Data curation, Formal Analysis, Investigation, Writing – review & editing. YW: Formal Analysis, Methodology, Software, Writing – review & editing. ZS: Formal Analysis, Funding acquisition, Supervision, Writing – review & editing. DP: Formal Analysis, Funding acquisition, Investigation, Writing – review & editing. YXY: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge the work was supported by grants from the Key Projects of Hangzhou Health Science and Technology Plan (ZD20210010), the Guidance Project of Hangzhou Science Plan (agriculture and social development) (20211231Y034), the Construction Fund of Medical Key Disciplines of Hangzhou (2020-2024), National Key Research and Development Program (2022YFA1303801), Chinese National Natural Science Foundation (U20A200603, 82370612), Independent Foundation of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases (zz202311).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Andrei G, Snoeck R. Advances and perspectives in the management of varicella-zoster virus infections. Molecules (Basel Switzerland). (2021) 26:1132. doi: 10.3390/molecules26041132
2. Kennedy PGE. The spectrum of neurological manifestations of varicella-zoster virus reactivation. Viruses. (2023) 15:1663. doi: 10.3390/v15081663
3. Hass RM, Sturgill FL, Young NP. Sensory and motor neurologic presentations of herpes zoster (Shingles). Mayo Clin Proc. (2023) 98:1128–30. doi: 10.1016/j.mayocp.2023.03.009
4. Bricout H, Haugh M, Olatunde O, Prieto RG. Herpes zoster-associated mortality in Europe: a systematic review. BMC Public Health. (2015) 15:466. doi: 10.1186/s12889-015-1753-y
5. Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. (2017) 96:656–63.
6. Maher MD, Douglas VP, Douglas KAA, Collens SI, Gilbert AL, Torun N, et al. Clinical and neuroradiologic characteristics in varicella zoster virus reactivation with central nervous system involvement. J Neurol Sci. (2022) 437:120262. doi: 10.1016/j.jns.2022.120262
7. Satyaprakash AK, Tremaine AM, Stelter AA, Creed R, Ravanfar P, Mendoza N, et al. Viremia in acute herpes zoster. J Infect Dis. (2009) 200:26–32. doi: 10.1086/599381
8. Kennedy PGE, Grose C. Insights into pathologic mechanisms occurring during serious adverse events following live zoster vaccination. J Virol. (2025) 99:e0181624. doi: 10.1128/jvi.01816-24
9. Levin MJ, Cai GY, Lee KS, Rouphael NG, Mehta AK, Canniff J, et al. Varicella-zoster virus DNA in blood after administration of herpes zoster vaccine. J Infect Dis. (2018) 217:1055–9. doi: 10.1093/infdis/jix653
10. Yan Y, Yuan Y, Wang J, Zhang Y, Liu H, Zhang Z. Meningitis/meningoencephalitis caused by varicella zoster virus reactivation: a retrospective single-center case series study. Am J Transl Res. (2022) 14:491–500.
11. Hung CH, Chang KH, Kuo HC, Huang CC, Liao MF, Tsai YT, et al. Features of varicella zoster virus myelitis and dependence on immune status. J Neurol Sci. (2012) 318:19–24. doi: 10.1016/j.jns.2012.04.017
12. Nagel MA, Niemeyer CS, Bubak AN. Central nervous system infections produced by varicella zoster virus. Curr Opin Infect Dis. (2020) 33:273–8. doi: 10.1097/QCO.0000000000000647
13. Subramaniam S, Scharrer I. Procoagulant activity during viral infections. Front Biosci (Landmark edition). (2018) 23:1060–81. doi: 10.2741/4633
14. Yang Y, Tang H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell Mol Immunol. (2016) 13:432–42. doi: 10.1038/cmi.2016.1
15. Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. (2021) 113:45–57. doi: 10.1007/s12185-020-03029-y
16. Werner RN, Nikkels AF, Marinović B, Schäfer M, Czarnecka-Operacz M, Agius AM, et al. European consensus-based (S2k) guideline on the management of herpes zoster-guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 1: diagnosis. JEADV. (2017) 31:9–19. doi: 10.1111/jdv.13995
17. Liu H, Wang J, Zhang Y, Gu J, Wang Y, Yan Y, et al. Cerebrospinal fluid proteomics in meningitis patients with reactivated varicella zoster virus. Immun Inflammation Dis. (2023) 11:e1038. doi: 10.1002/iid3.1038
18. Zhang Z, Liu X, Suo L, Zhao D, Pan J, Lu L. The incidence of herpes zoster in China: A meta-analysis and evidence quality assessment. Hum Vaccin Immunother. (2023) 19:2228169. doi: 10.1080/21645515.2023.2228169
19. Rosamilia LL. Herpes zoster presentation, management, and prevention: A modern case-based review. Am J Clin Dermatol. (2020) 21:97–107. doi: 10.1007/s40257-019-00483-1
20. Carey RAB, Chandiraseharan VK, Jasper A, Sebastian T, Gujjarlamudi C, Sathyendra S, et al. Varicella zoster virus infection of the central nervous system - 10 year experience from a tertiary hospital in south India. Ann Indian Acad Neurol. (2017) 20:149–52. doi: 10.4103/aian.AIAN_484_16
21. Kim SH, Choi SM, Kim BC, Choi KH, Nam TS, Kim JT, et al. Risk factors for aseptic meningitis in herpes zoster patients. Ann Dermatol. (2017) 29:283–7. doi: 10.5021/ad.2017.29.3.283
22. Limb J, Binning A. Thrombosis associated with varicella zoster in an adult. Int J Infect Dis. (2009) 13:e498–500. doi: 10.1016/j.ijid.2009.02.007
23. Maity PK, Chakrabarti N, Mondal M, Patar K, Mukhopadhyay M. Deep vein thrombosis: A rare signature of herpes zoster. J Assoc Physicians India. (2014) 62:72–4.
24. Srivastava T. Nagpal K.Herpes zoster meningoencephalitis complicated with peripheral vascular disease: an uncommon presentation of a common disease. Scand J Infect Dis. (2014) 46:716–8. doi: 10.3109/00365548.2014.926022
25. Dogan M, Acikgoz M, Bora A, Başaranoğlu M, Oner AF. Varicella-associated purpura fulminans and multiple deep vein thromboses: a case report. J Nippon Med Sch. (2009) 76:165–8. doi: 10.1272/jnms.76.165
26. Salvotti F, Trapletti S, Chiarini G, Castellano M, Muiesan ML. Atypical varicella-Zoster virus reactivation: A case report. Eur J Case Rep Intern Med. (2023) 10:3945. doi: 10.12890/2023_003945
27. Khan R, Yasmeen A, Pandey AK, Saffar KA, Narayanan SR. Cerebral venous thrombosis and acute pulmonary embolism following varicella infection. Eur J Case Rep Intern Med. (2019) 6:1171. doi: 10.12890/2019_001171
28. Sahra S, Jahangir A, Gavica MC, Mobarakai N, Jahangir A. First case report of pulmonary embolism with Zoster Sine Herpete. Respir Med Case Rep. (2021) 33:101462. doi: 10.1016/j.rmcr.2021.101462
29. Horev A, Horev A, Gordon-Irshai A, Gordon M, Andre N, Ifergane G. Herpes zoster and long-term vascular risk: a retrospective cohort study. Sci Rep. (2023) 13:2364. doi: 10.1038/s41598-023-29667-w
30. Lu P, Cui L, Zhang X. Stroke risk after varicella-zoster virus infection: a systematic review and meta-analysis. J Neurovirol. (2023) 29:449–59. doi: 10.1007/s13365-023-01144-0
31. Weisel JW, Litvinov RI. Fibrin formation, structure and properties. Subcell Biochem. (2017) 82:405–56. doi: 10.1007/978-3-319-49674-0_13
32. Wolberg AS. Fibrinogen and fibrin: synthesis, structure, and function in health and disease. J Thromb Haemost. (2023) 21:3005–15. doi: 10.1016/j.jtha.2023.08.014
33. Nurden AT. Association of fibrinogen-bound glycoprotein IIb-IIIa complexes on the activated platelet surface. J Lab Clin Med. (1996) 128:7–8. doi: 10.1016/s0022-2143(96)90107-9
34. Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. The D-Dimer test in combination with a decision rule for ruling out deep vein thrombosis in primary care: diagnostic technology update. Br J Gen Pract. (2012) 62:e393–395. doi: 10.3399/bjgp12X641645
35. Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and Meta-analysis. BMJ. (2013) 346:f2492. doi: 10.1136/bmj.f2492
36. Bader JM, Geyer PE, Müller JB, Strauss MT, Koch M, Leypoldt F, et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer's disease. Mol Syst Biol. (2020) 16:e9356. doi: 10.15252/msb.20199356
37. Dayon L, Cominetti O, Affolter M. Proteomics of human biological fluids for biomarker discoveries: technical advances and recent applications. Expert Rev Proteomics. (2022) 19:131–51. doi: 10.1080/14789450.2022.2070477
38. Torretta E, Arosio B, Barbacini P, Capitanio D, Rossi PD, Moriggi M, et al. Novel insight in idiopathic normal pressure hydrocephalus (iNPH) biomarker discovery in CSF. Int J Mol Sci. (2021) 22:8034. doi: 10.3390/ijms22158034
39. Ramachandran PS, Wilson MR, Catho G, Blanchard-Rohner G, Schiess N, Cohrs RJ, et al. Meningitis caused by the live varicella vaccine virus: metagenomic next generation sequencing, immunology exome sequencing and cytokine multiplex profiling. Viruses. (2021) 13:2286. doi: 10.3390/v13112286
40. Chapman J. Coagulation in inflammatory diseases of the central nervous system. Semin Thromb Hemost. (2013) 39:876–80. doi: 10.1055/s-0033-1357482
41. Shavit-Stein E, Aronovich R, Sylantiev C, Gera O, Gofrit SG, Chapman J, et al. Blocking thrombin significantly ameliorates experimental autoimmune neuritis. Front Neurol. (2019) 9:1139. doi: 10.3389/fneur.2018.01139
Keywords: herpes zoster, central nervous system, varicella-zoster virus, coagulation function, infection
Citation: Yuan Y, Gu J, Liu H, Wang Y, Sun Z, Pan D and Yan Y (2025) Coagulation functions and factors correlated with central nervous system infection in herpes zoster patients. Front. Immunol. 16:1511901. doi: 10.3389/fimmu.2025.1511901
Received: 16 October 2024; Accepted: 23 April 2025;
Published: 09 May 2025.
Edited by:
Julie Olson, University of Minnesota Twin Cities, United StatesReviewed by:
Charles Grose, The University of Iowa, United StatesSofia Jordão, Hospital Pedro Hispano, Portugal
Copyright © 2025 Yuan, Gu, Liu, Wang, Sun, Pan and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongxing Yan, eXl4aW5nMjBAMTI2LmNvbQ==; Dongli Pan, cGFuZG9uZ2xpQHpqdS5lZHUuY24=; Zeyu Sun, emV5dXN1bkB6anUuZWR1LmNu
†ORCID: Dongli Pan, orcid.org/0000-0002-0421-6242
 Yanrong Yuan1
Yanrong Yuan1 Zeyu Sun
Zeyu Sun Dongli Pan
Dongli Pan Yongxing Yan
Yongxing Yan