- 1Department of Infectious Diseases, Chengdu Fifth People’s Hospital, Chengdu, China
- 2Department of Medical Laboratory Technology, Chengdu Fifth People’s Hospital, Chengdu, China
Community-acquired Roseomonas mucosa sepsis can lead to significant morbidity and mortality if not diagnosed promptly. We report a case of a 59-year-old woman with community-acquired Roseomonas mucosa sepsis who presented with persistent fever progressing to septic shock, despite repeatedly negative host-response biomarker results. Initial metagenomic analysis of peripheral blood suggested Pseudomonas aeruginosa infection. However, a peripheral blood culture identified Roseomonas mucosa as the causative pathogen. She was cured after switching to meropenem according to blood cultures and antimicrobial susceptibility testing.
Introduction
Species of the genus Roseomonas have been reported to cause significant infections in immunocompromised patients, with bloodstream infections being the most common clinical manifestation (1–3). Due to their inherent resistance to β-lactams (including penicillin, piperacillin/tazobactam, and cephalosporins) and variable resistance to quinolones, early identification and targeted antimicrobial therapy are critical for improving clinical outcomes (3–5).
Sepsis, a life-threatening organ dysfunction caused by dysregulated host response to infection, remains a leading global cause of mortality (6). This clinical syndrome is distinguished from uncomplicated bloodstream infection by the presence of systemic inflammatory response accompanied by acute organ failure (7). Inflammatory biomarkers are commonly used to differentiate between distinct pathogenic conditions, indicate disease severity, guide treatment approaches, monitor therapeutic responses, and predict patient prognoses (8). The most common biomarkers of acute inflammation include procalcitonin (PCT), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR)—which are all typically elevated during inflammation and gradually decline after it is controlled (9).
Herein, we report a rare case of community-acquired Roseomonas mucosa (R. mucosa) sepsis where the patient consistently presented with normal PCT, CRP, and ESR levels. Despite peripheral blood metagenomic next-generation sequencing (mNGS) results suggesting that the infection was caused by Pseudomonas aeruginosa, a piperacillin-tazobactam treatment regimen subsequently proved to be ineffective, and the patient ultimately recovered after being switched to meropenem.
Case presentation
A 59-year-old woman was admitted to the infectious diseases ward of our hospital with recurrent fever lasting 4 days, wherein her highest body temperature reached 39.9°C. Prior to hospitalization, she developed fever, headaches, and fatigue. She had taken oral cefdinir for 2 days, but it proved ineffective. A post-admission physical examination revealed no significant abnormalities in the heart, lungs, or abdomen. She had been living with type 2 diabetes mellitus for 10 years, and had no prior history of hospitalization before the onset of her illness. She had not received any immunosuppressive therapy. She had been living in the city, had not traveled recently, and had no relevant history of contact with animals. Laboratory tests showed that her white blood cell (WBC), PCT, CRP, and ESR counts were all within normal range. Tests for respiratory adenovirus, rhinovirus, influenza A virus, influenza B virus, SARS-CoV-2, cytomegalovirus, rubella virus, Epstein-Barr virus, mycoplasma, and chlamydia were all negative, as were β-D-glucan (G) and galactomannan (GM) assays. Computed tomography (CT) did not reveal any signs of acute infection, and a cardiac ultrasound showed normal results. We administered piperacillin-tazobactam as an empirical treatment for bacteria on the first day of the patient’s hospitalization; however, she continued to experience recurrent high fever accompanied by chills and hypotension, with a Sequential Organ Failure Assessment (SOFA) score of 3. Despite this, her inflammatory markers (WBC, CRP, PCT, ESR, and interleukin 6) were within normal ranges, and blood cultures remained negative for 3 days.
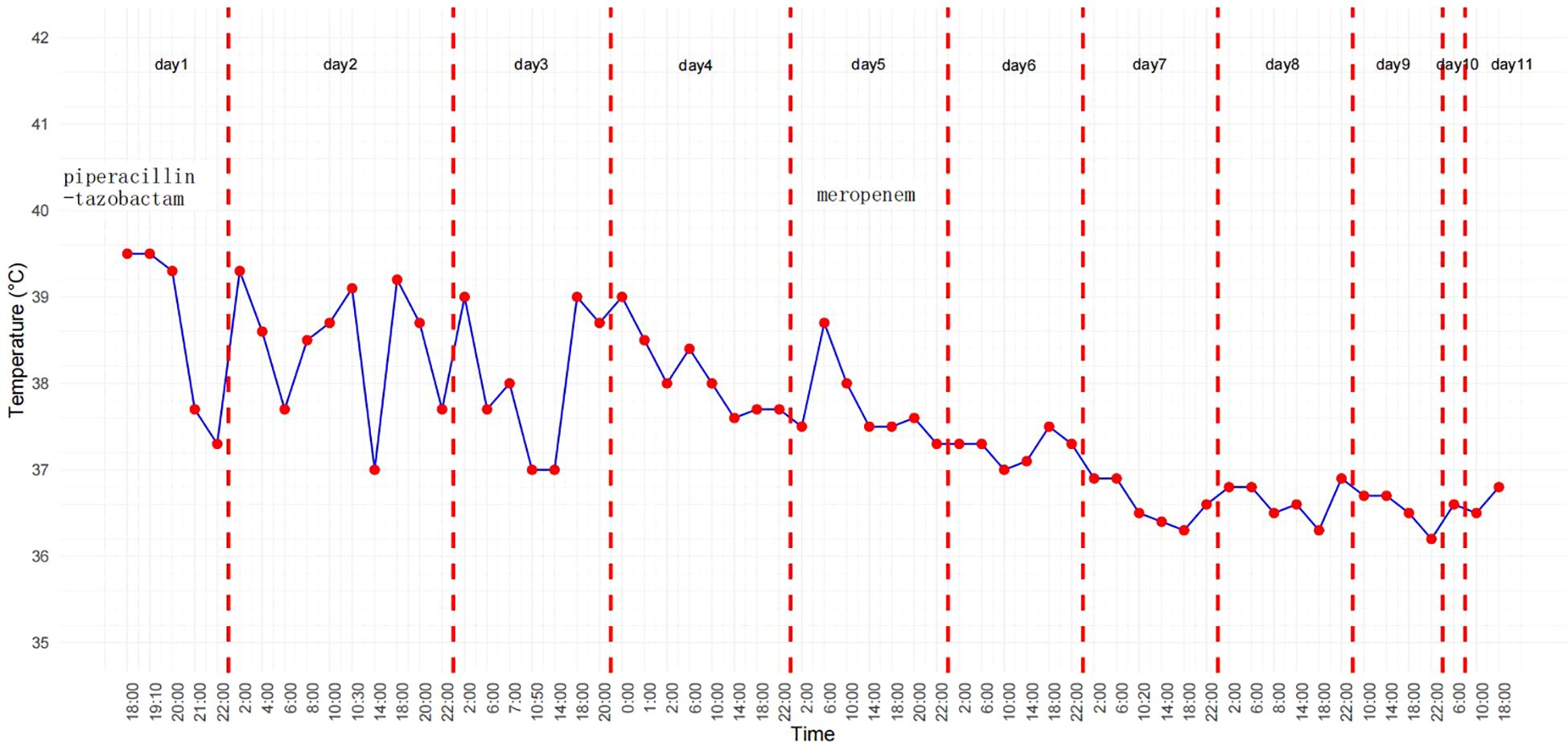
On the fourth day following her admission, we performed a peripheral blood mNGS test, which detected P. aeruginosa with 381 sequence reads. We considered the possibility that the antibiotic therapy regimen already administered had been insufficient, so we decided to continue it. However, the patient remained febrile, with an unchanged peak fever temperature, and displayed early signs of developing shock, as evidenced by a blood pressure drop to 84/42 mmHg. Therefore, on the fifth day, we changed her antibiotic regimen to meropenem. On the seventh day, blood culture test results suggested that the pathogen responsible for her infection was actually R. mucosa (Figure 1). This organism is resistant to piperacillin-tazobactam but sensitive to meropenem. The patient’s temperature began to decrease and her blood pressure returned to normal after her treatment regimen was switched. By the eighth day, her temperature had also normalized. After receiving meropenem for seven days, additional blood culture test results were also negative, indicating that the infection had been controlled. The temperature graph in Figure 2 visually presents the fever pattern of the patient throughout the treatment period. She was subsequently discharged from the hospital and followed up for 6 months, during which there was no recurrence of fever.
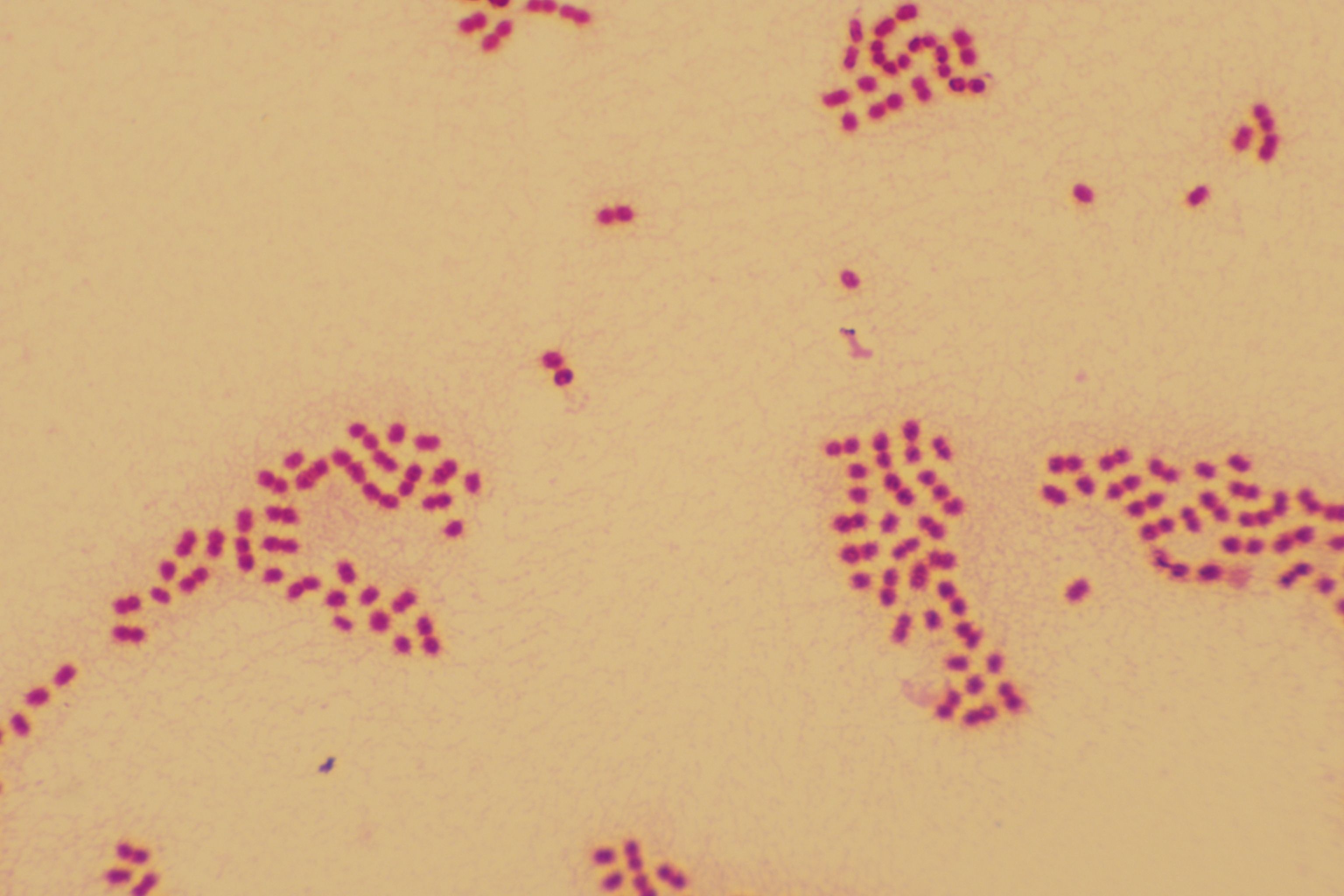
Figure 1. Gram stain (×1000), Roseomonas mucosa is a pink-pigmented, Gram-negative short rod bacterium.
Discussion and conclusion
Infections caused by Roseomonas spp. in humans are rare. However, they can infect immunocompromised patients, such as those with leukemia undergoing chemotherapy, active malignancies, peritonitis, diabetes, catheter-associated tuberculosis or pulmonary tuberculosis (2, 5, 10). In this case report, we describe a patient who suffered from septic shock caused by R. mucosa, but exhibited persistently normal host-response biomarkers, including PCT, CRP, and ESR, throughout the illness. This severe infection showed different results between mNGS tests and blood cultures. The patient was successfully treated with meropenem after undergoing antimicrobial susceptibility testing.
Species of the Roseomonas genus belong to the phylum Proteobacteria. They are pink-pigmented, slow-growing, non-fermentative, aerobic gram-negative coccobacilli, with a mortality rate of 3% in infected adult patients (11). Patients typically present with fever in 62.5% (45/72) of cases, and with sepsis in 27.8% (10/36) of cases (3). In general, R. mucosa is resistant to β-lactam antibiotics such as piperacillin-tazobactam, ampicillin, extended-spectrum cephalosporins, and colistin, but is fully susceptible to aminoglycosides, fluoroquinolones, and carbapenems (12, 13). Our patient presented with fever and septic shock, and antimicrobial susceptibility testing suggested significant resistance to piperacillin-tazobactam, intermediate resistance to levofloxacin, and high sensitivity to meropenem. In this case, R. mucosa caused sepsis in a patient with diabetes mellitus. We initially suspected that the source of infection was R. mucosa due to the presence of an itchy scratch on the patient’s skin one month prior to her admission to hospital. A small skin cut allowed the opportunistic pathogen to enter the immunocompromised host, ultimately causing sepsis. Immunocompromised patients with bloodborne infections should therefore always be thoroughly evaluated for history of skin damage. Early and accurate identification of the causative pathogen is crucial for effective treatment.
Sepsis is often accompanied by elevated levels of systemic inflammatory markers such as PCT and CRP (9, 14). Both of these markers are associated with a systemic host response (15). A systematic review and meta-analysis updated in 2018, including nine studies comparing the diagnostic accuracy of PCT vs CRP for sepsis, revealed a similar sensitivity for these two biomarkers (CRP: 0.80, 95% confidence interval [CI]: 0.63–0.90, PCT: 0.80, 95% CI: 0.69–0.87) but significantly lower specificity for CRP, at 0.61 (95% CI: 0.50–0.72), compared to PCT, at 0.77 (95% CI: 0.60–0.88) (16, 17). It is important to note that sepsis is a complex and heterogeneous condition. Patients may exhibit varying immune responses to the condition, leading to different levels of biomarker expression. Factors such as age, organ dysfunction, infection type, and comorbidities can all influence biomarker profiles. Dysregulated immune response in sepsis varies significantly among individuals. Some patients may not mount a robust inflammatory reaction, resulting in minimal elevation of typical biomarkers (18). As in this case, multiple negative tests for PCT and CRP did not suggest that the patient’s inflammatory response was mild or that the infection was under control. Conversely, the patient presented with septic shock, indicating severe infection. This may have been because the patient had been suffering from diabetes mellitus and associated chronic hyperglycemia—a complex clinical syndrome often accompanied by immunomodulatory dysfunction—for many years (19). Studies have found that long-term hyperglycemia can continuously activate the NF-kappaB pathway through oxidative stress, leading to elevated baseline levels of pro-inflammatory factors such as IL-6, which may blunt the magnitude of inflammatory response during acute infection (20). In the study by Al-Rashed F et al., it was also shown that monocytes from diabetic patients had impaired TLR4 signaling and reduced TNF-α release upon LPS stimulation, resulting in insufficient CRP synthesis (21).Another contributing factor may be the patient’s advanced age and immunosenescence, combined with delayed effective antimicrobial therapy during early infection, potentially leading to pathogen dissemination and progression to sepsis. Therefore, the diagnosis of sepsis should rely on a combination of clinical criteria, biomarkers, and imaging. The absence of specific biomarker elevation does not rule out sepsis, particularly in cases with atypical presentations or those in their early stages (22).
Recently, mNGS has emerged as a revolutionary tool for its use in pathogen detection. Compared to traditional blood cultures, mNGS is faster and more sensitive in pathogen detection (23, 24). However, owing to host DNA interference and database heterogeneity, mNGS may not be as effective as blood culture in terms of its specificity for identifying the causative pathogen. In our case, an mNGS test of the patient’s peripheral blood detected P. aeruginosa, whereas blood cultures detected R. mucosa. Based on the therapeutic failure of piperacillin-tazobactam treatment, we considered the actual etiological pathogen to be R. mucosa.
To the best of our knowledge, this is the first report in the literature of sepsis caused by R. mucosa with normal host response inflammatory biomarkers. In this case, blood cultures proved to be more accurate than mNGS. Therefore, it is crucial to thoroughly analyze mNGS reports in the context of each patient’s actual clinical condition.
Sepsis caused by R. mucosa is relatively rare, difficult to diagnose, and may progress rapidly. To improve patient prognoses, clinicians require use multiple tools to clarify its etiology as soon as possible, and appropriate antimicrobials must be selected rationally, with adequate screening for multiple potential pathogens.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee Chengdu Fifth People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XXM: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YL: Data curation, Investigation, Methodology, Writing – review & editing. XH: Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Chengdu Science and Technology Bureau Technology Innovation R&D Program (Grant no. 2024-YF05-01118-SN).
Acknowledgments
The authors are grateful to the patients and their families for providing valuable information on potential exposures and disease course. We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rihs JD, Brenner DJ, Weaver RE, Steigerwalt AG, Hollis DG, Yu VL. Roseomonas, is a new genus associated with bacteremia and other human infections. J Clin Microbiol. (1993) 31:3275–83. doi: 10.1128/jcm.31.12.3275-3283.1993
2. Dé I, Rolston KV, Han XY. Clinical significance of Roseomonas species isolated from catheter and blood samples: analysis of 36 cases in patients with cancer. Clin Infect Dis. (2004) 38:1579–84. doi: 10.1086/420824
3. Ioannou P, Mavrikaki V, Kofteridis DP. Roseomonas species infections in humans: a systematic review. J chemother (Florence Italy). (2020) 32:226–36. doi: 10.1080/1120009X.2020.1785742
4. Diesendorf N, Köhler S, Geißdörfer W, Grobecker-Karl T, Karl M, Burkovski A. Characterisation of Roseomonas mucosa isolated from the root canal of an infected tooth. BMC Res notes. (2017) 10:212. doi: 10.1186/s13104-017-2538-4
5. Wang CM, Lai CC, Tan CK, Huang YC, Chung KP, Lee MR, et al. Clinical characteristics of infections caused by Roseomonas species and antimicrobial susceptibilities of the isolates. Diagn Microbiol Infect disease. (2012) 72:199–203. doi: 10.1016/j.diagmicrobio.2011.11.013
6. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet (London England). (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
7. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer MC, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
8. Biron BM, Ayala A, Lomas-Neira JL. Biomarkers for sepsis: what is and what might be? biomark Insights. (2015) 10:7–17. doi: 10.4137/BMI.S29519
9. Hung SK, Lan HM, Han ST, Wu CC, Chen KF. Current evidence and limitation of biomarkers for detecting sepsis and systemic infection. Biomedicines. (2020) 8(11):494. doi: 10.3390/biomedicines8110494
10. Tsai SF, Chen CH, Shu KH, Wu MJ. Peritonitis caused by Roseomonas in a patient undergoing automated peritoneal dialysis: case report and literature review. Internal Med (Tokyo Japan). (2012) 51:1721–4. doi: 10.2169/internalmedicine.51.6737
11. Romano-Bertrand S, Bourdier A, Aujoulat F, Michon A-L, Masnou A, Parer S, et al. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin Microbiol infection. (2016) 22:737.e731–737. doi: 10.1016/j.cmi.2016.05.024
12. Kim YK, Moon JS, Song KE, Lee WK. Two cases of bacteremia due to roseomonas mucosa. Ann Lab Med. (2016) 36:367–70. doi: 10.3343/alm.2016.36.4.367
13. Boyd MA, Laurens MB, Fiorella PD, Mendley SR. Peritonitis and technique failure caused by Roseomonas mucosa in an adolescent infected with HIV on continuous cycling peritoneal dialysis. J Clin Microbiol. (2012) 50:3801–4. doi: 10.1128/JCM.01437-12
14. Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: a systematic review and meta-analysis. J Intensive Care. (2019) 7:22. doi: 10.1186/s40560-019-0374-4
15. Póvoa P, Coelho L, Dal-Pizzol F, Ferrer R, Huttner A, Morris AC, et al. How to use biomarkers of infection or sepsis at the bedside: guide to clinicians. Intensive Care Med. (2023) 49:142–53. doi: 10.1007/s00134-022-06956-y
16. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect diseases. (2013) 13:426–35. doi: 10.1016/S1473-3099(12)70323-7
17. Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem. (2019) 120:5852–9. doi: 10.1002/jcb.v120.4
18. Barichello T, Generoso JS, Singer M, Dal-Pizzol F. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care (London England). (2022) 26:14. doi: 10.1186/s13054-021-03862-5
19. Frydrych LM, Bian G, O’Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J leukocyte Biol. (2018) 104:525–34. doi: 10.1002/JLB.5VMR0118-021RR
20. Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. (2022) 12:542. doi: 10.3390/biom12040542
21. Al-Rashed F, Sindhu S, Arefanian H, Madhoun AA, Kochumon S, Thomas R, et al. Repetitive intermittent hyperglycemia drives the M1 polarization and inflammatory responses in THP-1 macrophages through the mechanism involving the TLR4-IRF5 pathway. Cells. (2020) 9:1892. doi: 10.3390/cells9081892
22. Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: time for a reappraisal. Crit Care (London England). (2020) 24:287. doi: 10.1186/s13054-020-02993-5
23. Gu W, Deng X, Lee M, Sucu YD, Arevalo S, Stryke D, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. (2021) 27:115–24. doi: 10.1038/s41591-020-1105-z
Keywords: Roseomonas mucosa, sepsis, inflammatory biomarkers, metagenomic next-generation sequencing, blood culture
Citation: Xu X, Li Y and Huang X (2025) Case Report: A rare case of community-acquired Roseomonas mucosa sepsis that presented with persistently normal host-response biomarkers. Front. Immunol. 16:1521161. doi: 10.3389/fimmu.2025.1521161
Received: 01 November 2024; Accepted: 25 March 2025;
Published: 10 April 2025.
Edited by:
Teresa Zelante, University of Perugia, ItalyReviewed by:
Attinder Chadha, University of California, San Francisco, United StatesValentino D’Onofrio, Ghent University, Belgium
Copyright © 2025 Xu, Li and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomei Xu, eGlhb21laXh1QGNkdXRjbS5lZHUuY24=
 Xiaomei Xu
Xiaomei Xu Ying Li1
Ying Li1